Abstract
Background
The blood-brain barrier (BBB) is a major limiting factor for drug delivery in brain tumors. Laser interstitial thermal therapy (LITT) disrupts the peritumoral BBB. In this study, we examine survival in patients with recurrent glioblastoma (GBM) treated with LITT followed by low-dose doxorubicin, a potent anti-neoplastic drug with poor BBB permeability.
Methods
Forty-one patients with recurrent GBM were enrolled; thirty patients were evaluable. Participants underwent LITT followed by 6 weekly doxorubicin treatments starting within one week (Early Arm) or at 6–8 weeks (Late Arm) after LITT. The overall survival (OS), local progression-free survival (PFS), and any PFS were compared to historical controls treated with bevacizumab salvage therapy (n = 50) or LITT with standard BBB-permeable salvage therapy (n = 28). Cox proportional-hazards models examined the contribution of age, gender, MGMT promoter status, and IDH-mutation status on any PFS and OS. Adverse events were also cataloged.
Results
The Late Arm and all patients (Early Arm + Late Arm) demonstrated significant improvement in OS compared to historical controls treated with bevacizumab (p < 0.001) and LITT with standard salvage therapy (p < 0.05). No significant difference in any PFS was observed between either arm and historical controls. Low-dose doxorubicin was well tolerated with comparable adverse event rates between the arms.
Conclusions
Low-dose doxorubicin given after LITT is well tolerated and correlated with higher OS compared to historical controls treated with bevacizumab or LITT with standard salvage chemotherapy. A larger study is needed to further characterize survival and progression patterns.
Keywords: bevacizumab, doxorubicin, GBM, glioblastoma, glioma topic: recurrent glioblastoma, Laser interstitial thermal therapy (LITT), recurrent GBM, salvage therapy
Key Points.
LITT combined with low-dose doxorubicin is associated with longer overall survival in recurrent GBM compared to historical controls.
Low-dose doxorubicin is safe for patients, even with extended (>6 weeks) dosing.
Importance of the Study.
The blood-brain barrier (BBB) poses a significant challenge to central nervous system (CNS) drug delivery in glioblastoma (GBM). Overcoming this barrier will be key for the development of new therapies. MRI-guided LITT is a means to achieve both tumor cytoreduction and local BBB disruption. This study investigates the combination of LITT and systemic administration of doxorubicin, a BBB-impermeant chemotherapy drug, as a treatment approach for recurrent GBM. Our findings demonstrate that this combination therapy is well tolerated and is associated with improved overall survival when compared to historical controls. Further studies of this therapeutic approach for recurrent GBM are needed.
Recurrent glioblastoma (GBM) remains devastatingly lethal with a median overall survival (OS) of 9.3 months and only 76% of patients alive at 6 months after initial therapy.1 Many phase II and phase III studies examining novel treatments have failed to demonstrate statistically meaningful survival benefits.2–8 Recurrent GBM tends to arise from microscopic infiltrative disease extending beyond the contrast-enhancing margin of the primary tumor.9 This study aims to target microscopic infiltrative disease within the peritumoral region to improve OS and delay local disease progression.
The blood-brain barrier (BBB) severely limits drug delivery to the central nervous system (CNS).10 This includes adjuvant anti-neoplastic agents directed at residual microscopic disease. Doxorubicin has been shown to potently inhibit growth of glioma cells in vitro and in vivo.11,12 In contrast to temozolomide (TMZ), doxorubicin has negligible BBB permeability.13–15 Improving peritumoral BBB permeability is critical for increasing the peritumoral concentrations of impermeant drugs like doxorubicin to target the microscopic infiltrative disease responsible for the majority of disease recurrences.16–18
MRI-guided laser interstitial thermal therapy (LITT) has been shown to induce BBB disruption and improve CNS drug delivery. LITT is a minimally invasive technique for selective thermal ablation of brain tissue.19–21 In animal models of glioma, LITT was demonstrated to induce BBB disruption, and LITT plus adjuvant doxorubicin increased survival in brain tumor-bearing mice compared to either intervention alone.22,23 In patients with recurrent GBM, we recently reported that LITT induced peritumoral BBB disruption as evidenced by elevated vascular transfer constant (Ktrans) of gadolinium and serum levels of neuron-specific enolase for up to 6 weeks after the procedure.24,25 Sustained increase in contrast permeability of BBB following LITT has also been reported to last up to 6 months after the acute phase.26 While post-LITT CNS drug concentrations are not known, this suggests a potential therapeutic window of improved delivery of otherwise BBB-impermeant therapeutic agents like doxorubicin.21,24 It remains unknown whether LITT combined with doxorubicin results in improved local progression-free survival (PFS) or overall survival in patients with recurrent GBM.
In this follow-up Phase II study, we combined LITT therapy with doxorubicin for the management of recurrent GBM. Patients were treated with LITT therapy and randomized to either early doxorubicin administration within 1 week after LITT (Early Arm), or to the late doxorubicin administration at 6-8 weeks after LITT (Late Arm). Detailed assessments of OS, PFS, and adverse event rates were assessed and compared against historical controls.
Methods
Patients
The study protocol was approved by the Internal Review Board at the Washington University in St. Louis School of Medicine. Written informed consent was obtained from each participant prior to any trial-related activities. Forty-one adult patients (≥18 years old) with bevacizumab naïve GBM WHO grade IV who had unequivocal radiographic evidence of tumor recurrence were screened for eligibility (Table 1) in this phase II trial (ClinicalTrials.gov identifier number NCT01851733). Patients with rare GBM variants or suspected secondary GBM that developed from a lower grade tumor were eligible. Inclusion criteria for recurrent tumors included tumor size ≤3 cm in the largest dimension, at least 12 weeks after completion of radiotherapy with concurrent TMZ or if pseudo-progression was excluded by PET-scan, a KPS score greater or equal to 60, a candidate for MRI-guided LITT, sufficient cardiac, bone marrow, and hepatic functions, and no prior cytotoxic chemotherapy within 2–4 weeks (2 weeks for vincristine, 3 weeks for procarbazine, and 4 weeks for other cytotoxic chemotherapies). Patients with prior doxorubicin or bevacizumab exposure were specifically excluded (See Supplementary Material 1 for full study inclusion and exclusion criteria).
Table 1.
Patient Characteristics
| All | Late arm | Early arm | p | |
|---|---|---|---|---|
| n | 30 | 16 | 14 | |
| Age at discovery(mean) | 57.3 | 56.8 | 57.9 | 0.75 |
| Gender | ||||
| Male (n,%) | 19 (63%) | 8 (50%) | 11 (79%) | 0.11 |
| Female (n, %) | 11 (37%) | 8 (50%) | 3 (21%) | |
| Race | ||||
| Caucasian (white, non-Hispanic) | 30 (100%) | 16 (100%) | 14 (100%) | - |
| KPS at enrollment | ||||
| mean KPS score (SD) | 84 (±9) | 81 (±10) | 88 (±7) | 0.07 |
| Steroids pre-LITT | ||||
| on Dexamethasone (n,%) | 7 (23%) | 5 (31%) | 2 (14%) | 0.27 |
| Tumor | ||||
| WHO grade IV (n,%) | 30 (100%) | 16 (100%) | 14 (100%) | - |
| Frontline/initial surgery | ||||
| Gross total (n,%) | 23 (77%) | 12 (75%) | 11 (79%) | 0.82 |
| Subtotal (n,%) | 3 (10%) | 1 (6%) | 2 (14%) | 0.46 |
| Biopsy (n,%) | 4 (13%) | 3 (19%) | 1 (7%) | 0.35 |
| MGMTp status | ||||
| Unmethylated | 16 (53%) | 7 (44%) | 9 (64%) | 0.26 |
| Methylated | 11 (37%) | 7 (44%) | 4 (29%) | 0.39 |
| Indeterminate | 3 (10%) | 2 (12%) | 1 (7.1%) | 0.63 |
| IDH1 status | ||||
| Wildtype | 28 (93%) | 14 (88.5%) | 14 (100%) | 0.17 |
| Mutated | 0 (0%) | 0 (0%) | 0 (0%) | - |
| Unknown | 2 (7%) | 2 (12.5%) | 0 (0%) | 0.17 |
Demographic and tumor characteristics of analyzed individuals of the Late Arm (doxorubicin 6 weeks after LITT) or Early Arm (doxorubicin a week after LITT). P values reflect between group comparisons using unpaired t-tests for continuous variables and chi-square tests between categorical values. Both groups received identical median doses of doxorubicin (n = 6). Additional details available in Supplementary Table 1.
Trial Design and Treatments
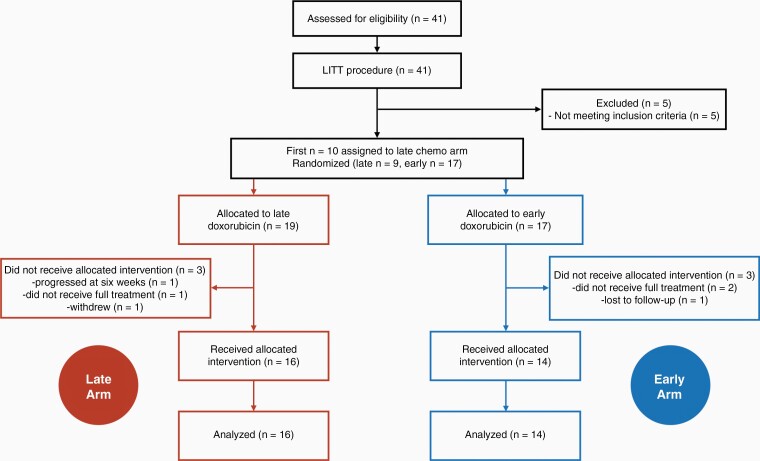
The main objective of this Phase II clinical trial was to determine local PFS, any PFS, and OS in patients who receive 6 doses of 20 mg/m2 IV weekly doxorubicin beginning within 72 hours (Early Arm) or 6–8 weeks (Late Arm) after LITT as compared to historical controls treated with LITT followed by standard salvage therapy with TMZ/lomustine (CCNU) or bevacizumab. As described previously,24 the first 10 patients were assigned to the Late (doxorubicin treatment) Arm with the remainder randomized at a 2:1 ratio to either the Early Arm or Late Arm (Figure 1). Five patients did not meet inclusion criteria. A further three individuals did not receive the allocated intervention in each arm (six total). Patients must have received at least 2 doses of doxorubicin to be evaluable.
Figure 1.
Study design. A total of 41 participants were divided into the Late Arm (doxorubicin 6 weeks after LITT) or Early Arm (doxorubicin a week after LITT) as follows: the first 10 participants were assigned to the Late Arm, then the next 30 were randomized at a ratio of 2:1 to either the Early or Late Arms. Evaluable patients must have received at least 2 doses of doxorubicin.
MRI-Guided Laser Thermal Ablation Therapy
LITT is a minimally invasive laser surgery currently approved by the FDA for interstitial thermal treatment of brain lesions.21,24,27–30 In this procedure, a small incision is made, followed by a small burr hole into the skull, through which a laser probe is inserted and guided by MRI to a tumor mass where it delivers ablative thermal energy. LITT can raise internal temperature at the lesion core up to 70°C, resulting in coagulative necrosis, which decreases to a temperature of 40-45°C in the peritumoral region.27
Historical Controls
Study arms were compared to a cohort of fifty (n = 50) recurrent GBM historical controls treated using bevacizumab as stipulated in the original study protocol and a cohort of twenty-eight (n = 28) recurrent GBM historical controls treated with LITT followed by standard chemotherapy including TMZ and CCNU as well as bevacizumab. Among historical controls treated with bevacizumab, inclusion criteria required that patients had progressed following definitive radiotherapy and concurrent TMZ followed by adjuvant TMZ, had received up to one prior salvage chemotherapy regimen, and no prior cytotoxic chemotherapy within 4-6 weeks (4 weeks for TMZ or 6 weeks for PCV/BCNU). Similar to our cohort, patients with prior bevacizumab exposure were excluded.31 Historical LITT controls were patients who underwent LITT for a recurrent GBM followed by standard of care chemotherapy. Like our study patients, no more than 2 prior recurrences were permitted and the recurrent glioma must not carry a IDH1 mutation (IDHR132H) per immunohistochemistry (IHC).32 Tumor volume/size and IDH1 mutation status were not previously reported for the bevacizumab control group.31
Statistical Analysis
Two group t-tests were used for demographic comparisons between continuous variables in the Late and Early Arms. Chi-square tests were used for comparisons between categorical variables. For both arms, OS was defined as the months from the date of intervention (LITT) to date of death. Likewise, local PFS was defined using modified RANO criteria33 relative to the date of LITT, with a further requirement of disease progression within 3 cm of the outer rim of the LITT site. For both study arms and historical controls treated with LITT, any PFS was defined as the months from intervention (date of LITT) to date of progression from any cause or death, with alive patients without progression censored at the last follow-up. In contrast for the bevacizumab control group, OS and PFS were calculated relative to date of bevacizumab treatment.31
Kaplan-Meier (KM) curves for OS and PFS were generated that provide unadjusted survival estimates for the patients and across arms. Differences between arms were determined by log-rank tests. The median OS and PFS (95% CI) for each arm were calculated. Cox proportional-hazards models were used to evaluate the relationship of the age at discovery, sex, MGMT promoter methylation status, and IDH1-mutation status per IHC vs. survival for the study arms vs. historical LITT controls. Unfortunately, MGMT promoter methylation status and IDH1 mutation status were not available for the historical bevacizumab controls.31 The proportionality assumption was tested by adding a time-dependent covariate for each variable. The variables with p < 0.20 from univariate models were considered in the multivariate model. All statistical tests were two-sided using an α = 0.05 level of significance. Survival curves were generated using Matlab (MathWorks®) using the matSurv function,34 and independently verified using SAS Version 9.4 (Cary, NC); SAS was also used to perform all statistical modeling.
Results
Patient Characteristics
Forty-one bevacizumab-naïve patients with suspected recurrent GBM were enrolled (Figure 1). Five patients (1002, 1003, 1006, 1008, and 1011) were excluded for failing to meet the inclusion criteria when a needle biopsy immediately prior to LITT failed to demonstrate histologic recurrent GBM. Patient 1015 (Late Arm) developed multifocal progression noted on the week-6 MRI scan and was removed from the study. Patient 1021 (Late Arm) withdrew. Patient 1028 (Early Arm), 1030 (Early Arm), and 1031 (Late Arm) missed their treatment window and were taken off the study. Patient 1039 (Early Arm) was lost to follow-up. In total, thirty patients (sixteen—Late Arm, fourteen—Early Arm) were evaluable for the study (Table 1). The majority (77%) underwent a frontline gross total resection. All recurrent tumors were approximately 3 cm or less at the longest dimension and varied in their anatomical localization (Supplementary Table 1). Both study arms received an identical median of six doses of doxorubicin. The control groups had comparable characteristics (Table 2, Supplementary Table 2).
Table 2.
Historical Control Characteristics
| Bevacizumab | LITT | |
|---|---|---|
| n | 50 | 28 |
| Age at treatment (mean) | 61.4 | 61.3 |
| Gender | ||
| Male (n,%) | 33 (66%) | 17 (61%) |
| Female (n, %) | 17 (34%) | 11 (39%) |
| Race | ||
| Caucasian (white, non-Hispanic) | Not reported | 22 (79%) |
| median KPS score | 80 | 90 |
| Steroids pre-intervention | ||
| on Dexamethasone (n,%) | 34 (68%) | 10 (36%) |
| Tumor | ||
| WHO grade IV (n,%) | 50 (100%) | 28 (100%) |
| Frontline/initial surgery | ||
| Gross total (n,%) | 19 (38%) | 18 (64%) |
| Subtotal (n,%) | 15 (30%) | 5 (18%) |
| Biopsy or unclear (n,%) | 16 (32%) | 5 (18%) |
| MGMTp status | ||
| Unmethylated | Not reported | 13 (46%) |
| Methylated | Not reported | 12 (43%) |
| Indeterminate | Not reported | 3 (11%) |
| IDH1 status | ||
| Wildtype | Not reported | 24 (86%) |
| Mutated | Not reported | 0 (0%) |
| Unknown | Not reported | 3 (14%) |
Demographic and tumor characteristics of the two control groups. Bevacizumab controls reflected historical controls available in the literature (31). The LITT control group were historical controls with recurrent GBM who lacked an IDH1 mutation. Additional details available in Supplementary Table 2.
Overall Survival
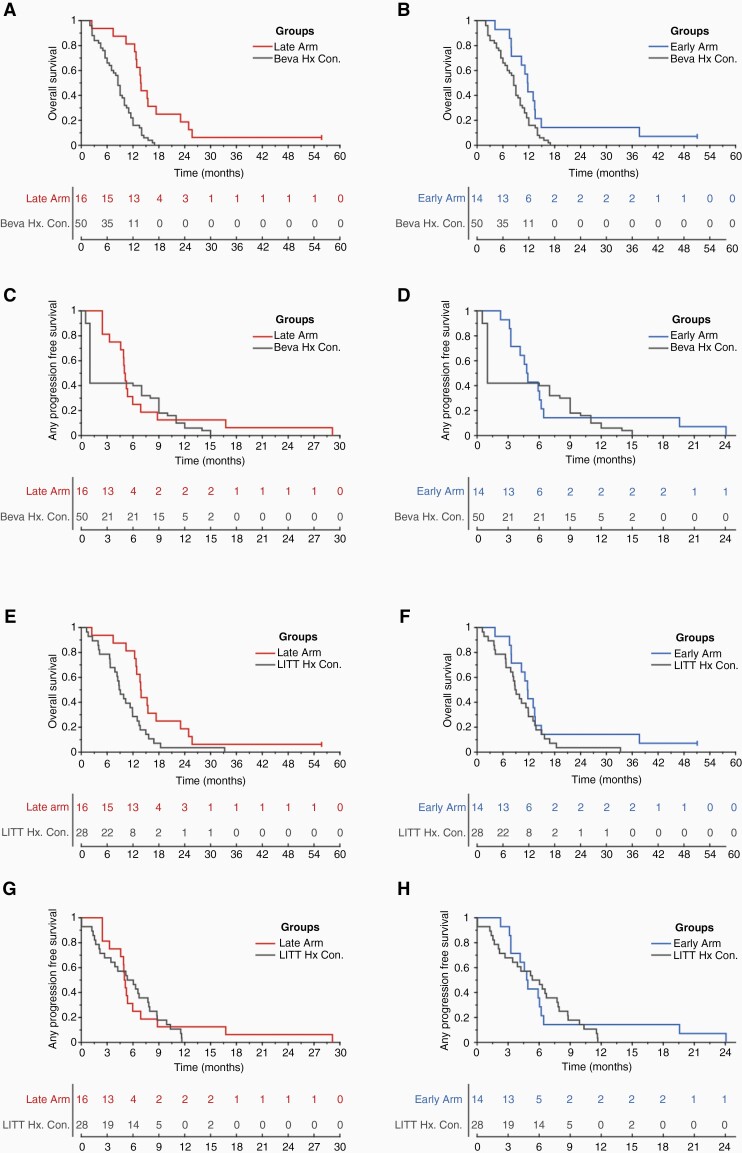
A significant improvement in OS was observed between historical bevacizumab controls and the Late Arm (p < 0.001; Figure 2A), the Early Arm (p < 0.05; Figure 2B), and the combined Arms (p < 0.001; Supplementary Figure 1E). Similarly, a significant improvement in OS was detected between historical LITT controls and the Late arm (p < 0.05; Figure 2E), and the combined Arms (p < 0.05; Supplementary Fig 1G), but not the Early Arm alone (Figure 2F). No difference in OS was observed comparing between the Late Arm and the Early Arm (Supplementary Figure 1C). Comparable results were obtained when including the three study patients who received a single dose of doxorubicin (Supplementary Material 2).
Figure 2.
Survival curves. Overall survival between the bevacizumab historical controls and (A) Late Arm or (B) Early Arm. Progression-free survival between the bevacizumab historical controls and the (C) Late Arm or (D) Early Arm. Progression in study arms was defined as any progression relative to intervention (See Methods). Overall survival between the LITT historical controls and the (E) Late Arm or (F) Early Arm. Finally, progression-free survival between the LITT historical controls and the (G) Late Arm or (H) Early Arm. No censoring in any progression-free survival comparisons. Two participants still living at 60 months (n = 1 in the Late Arm, n = 1 in the Early Arm) were censored at the last follow-up for overall survival comparisons.
The median OS for the Late Arm was 13.6 months (95% CI [12.2 17.1]) with a 94% probability of survival at 6 months. Median OS for the Early Arm was 11.6 months (95% CI [7.8 13.3]) with 93% probability of survival at 6 months. Median OS for the combined Arms was 13.0 months (95% CI: 11.5-14.7) with a 93% probability of survival at 6 months. Median OS for historical controls treated with LITT was 8.9 months (95% CI [6.6 11.7]) with a 79% probability of survival at 6 months.
Univariate Cox modeling mirrored these results with a significant improvement in OS between historical LITT controls and the Late Arm (p = 0.021, HR 0.47 (95% CI [0.24 0.89])) and the combined Arms (p = 0.023, HR 0.53 (95% CI [0.31 0.92])). No difference in OS was observed between the Early Arm and historical LITT controls (p = 0.25). Multivariate modeling noted patients with methylated tumors had better OS even after accounting for group status (late arm and combined arms; See Supplementary Material 3). However, no contribution of age at discovery, sex, or IDH1 (IDHR132H) mutation status (wild-type vs. unknown) was observed for any Arm vs controls.
Progression-Free Survival
For the Late Arm, the local PFS at 6 months was 31% (95% CI: 11–54%) and the median local PFS was 5.1 (95% CI: 3.2–6.8) months. For the Early Arm, the local PFS at 6 months was 36% (95% CI: 13–59%) and the median local PFS was 5.8 (95% CI: 4.1–6.3) months. No difference in local PFS was observed comparing between the Late Arm and the Early Arm (Supplementary Figure 1A). Local PFS was unavailable for historical controls treated with LITT.
No difference in any PFS was also observed between arms (Supplementary Figure 1B), or between either arm and historical controls treated with bevacizumab (Figure 2C, D) or LITT (Figure 2G, H). For the Late and Early Arms, the rate of any PFS at 6 months was 25% (95% CI: 8–47%) and 29% (95% CI: 9–52%), while median any PFS was 5.0 (95% CI: 3.2–5.9) and 4.8 (95% CI: 3.2–6.1) months, respectively. When both arms were combined (Late+Early), any PFS at 6 months was 27% (95% CI: 13–43%) and median PFS was 4.9 (95% CI: 4.5–5.9) months (Supplementary Figure 1D, F), compared to historical controls treated with LITT followed by standard salvage therapy with PFS at 6 months of 46.4% (95% CI: 28–63%) and median any PFS of 5.6 (95% CI: 2.7–7.6) months.
Univariate Cox modeling also demonstrated no difference in any PFS between historical controls treated with LITT and the Late Arm, the Early Arm, or the combined Arms (all p > 0.05). Likewise, no group difference in any PFS between either the Late Arm, Early Arm, or combined arms and historical LITT controls was observed in multivariate models controlling for MGMT promoter status (See Supplementary Material 3).
Finally, three of fourteen patients in the Early Arm demonstrated distal prior to local progression. In contrast, this was observed in only one patient in the Late Arm. However, given the small sample sizes, this was not statistically significant.
Adverse Events
Both study arms were well tolerated with comparable event rates between the study arms (Table 3). Headache, nausea, and fatigue were the most common grade 1 events. The majority of grade 3 events were hematologic leukopenia or neutropenia. A minority also reported grade 3 fatigue (one event in each arm). Two grade 4 neutropenia events were observed in the Early Arm. Two grade 4 events unrelated to the study were also observed in the Early Arm (bacterial meningitis complicated by ventriculomegaly prior to doxorubicin dosing). There were no grade 5 events.
Table 3.
Adverse Events
| Event category | All | Late arm | Early arm |
|---|---|---|---|
| Eye disorders | 7 | 1 | 6 |
| Gastrointestinal disorders | 31 | 18 | 13 |
| General/Constitutional | 31 (3) | 12 (1) | 20 (2) |
| Infectious | 7 (2) | 4 (1) | 3 (1) |
| Injury/Falls | 9 | 7 | 2 |
| Hematologic | 23 (11) | 13 (5) | 10 (6)a |
| Immune system | 1 | 0 | 1 |
| Nutrition/Metabolism | 7 (2) | 4 (1) | 3 (1) |
| Musculoskeletal and connective tissue | 5 (2) | 3 (1) | 2 (1) |
| Nervous system | 37 (6) | 11 (4) | 26 (2)b |
| Psychiatric | 15 | 7 | 8 |
| Renal | 2 | 0 | 2 |
| Respiratory | 5 | 2 | 3 |
| Skin/Cutaneous | 9 | 7 | 2 |
| Vascular disorder | 2 (1) | 0 | 2 (1)c |
Summary of adverse events by category, grouped by study arm. Number of grade 3 or higher events are included in paracenteses. There were no grade 5 events. A given participant may have multiple events (e.g. neutropenia and leukopenia). Two grade 4 events observed in the Early Arm were determined related to the studya and two unrelated to the studyb,c
aTwo participants developed grade 4 neutropenia, one of whom requiring dose delay
bTwo weeks post-LITT, a patient presented to the emergency department with acute altered mental status, nausea, and somnolence. Acute interval ventriculomegaly was discovered on imaging prompting the placement of a left-sided external ventriculostomy drain. Subsequent CSF evaluation identified an incidental acute bacterial meningitis which was ultimately managed with antibiotics. As the patient had not received the chemotherapy for the study, it was determined unrelated to the study
c Two weeks post-LITT, a patient presented to the emergency department with acute altered mental status, nausea, and somnolence. Acute interval ventriculomegaly was discovered on imaging prompting the placement of a left-sided external ventriculostomy drain. Subsequent CSF evaluation identified an incidental acute bacterial meningitis which was ultimately managed with antibiotics. As the patient had not received the chemotherapy for the study, it was determined unrelated to the study.
Discussion
LITT is a minimally invasive surgical tool that simultaneously cytoreduces tumors while improving local CNS permeability through peritumoral BBB disruption. Doxorubicin is a potent anti-neoplastic agent whose efficacy in brain tumors is hampered by its limited CNS penetration. In this study, a low weekly dose of 20 mg/m2 IV doxorubicin was associated with significant improvement in OS when coupled with LITT for patients with recurrent GBM compared to historical controls treated bevacizumab alone or with LITT followed by standard nondoxorubicin, BBB-permeant salvage chemotherapy. One caveat is that tumor size and molecular profile were not reported and therefore could not be controlled in the historical bevacizumab controls. Recurrent GBM tumors that are amenable to LITT are generally smaller (<=3-4cm) potentially biasing the results. This is why comparison to historical LITT controls is critical. Historical LITT controls also permitted controlling for other prognostic factors such as IDH1 mutation status and MGMTp methylation status. No significant difference in PFS was observed; however, a higher frequency of patients in the Late Arm developed distal prior to local progression. Finally, low dose weekly doxorubicin was well tolerated between both arms.
Since there is no consensus standard treatment for recurrent GBM, many ongoing studies examine immunotherapy,35 novel agents targeting tumor sensitivity to alkylating agents,36 and targeting specific molecular profiles, among others. Salvage therapies, particularly for patients with MGMT promoter methylation, include CCNU or procarbazine combination therapies and a TMZ rechallenge.37–39 Side effects for these salvage therapies can be significant37,38 with common severe hematologic toxicity, resulting in a delay in chemotherapy in upwards of 30% and cessation in over 10% of patients.37 Here we demonstrate weekly low dose doxorubicin improved OS with only 8 participants (22% of 36 evaluable) developing adverse grade III/IV hematological events. Of those, 7 (19%) required dose delay and 1 (3%) required dose modification. Notably, three evaluable participants (8%) had a doxorubicin cessation for nonhematologic adverse events unlikely related to the study, including stroke and infection.
This study specifically examined local vs. distal recurrence by calculating local and any (i.e., neuraxis) PFS at 6 months. This primary study endpoint was not met with low dose doxorubicin demonstrating only comparable local PFS at 6 months to recurrent GBM historically treated with bevacizumab. Bevacizumab is a steroid-sparing agent known to improve PFS but provide no benefit in OS.40–42 Often given in conjunction with salvage chemotherapy, recurrent GBM treated with bevacizumab demonstrate a PFS at 6 months between ~30–40%31,42 compared to less than 20% for salvage chemotherapy alone.42 Poor CNS penetration of doxorubicin distal to the LITT site likely explains the lower any PFS compared to local PFS observed in our cohort.
LITT has been shown to disrupt the peritumoral BBB, although the duration of clinically relevant BBB disruption is unknown. In an animal model, LITT triggered an increase in BBB permeability permitting passage of molecules the side of immunoglobin for up to one month after LITT.22 Our previous human study also demonstrated a transient elevation in serum BSE levels peaking 1–3 weeks after LITT, before returning to baseline by 6 weeks.24 BSE is a 78 kDa γ-homodimer43 compared to 0.54 kDa mass of doxorubicin. BBB permeability to comparably smaller molecules may persist beyond 6 weeks. This is supported by recent work demonstrating sustained contrast enhancement 6 months after LITT using an approximately 0.6 kDa contrast agent.26 Our previous work using dynamic contrast enhanced (DCE) MRI, which used an approximately 1kDa agent, revealed an early peak immediately after LITT followed by a gradual loss of signal over the subsequent 4 weeks.24 The lack of sensitivity for sub-kDa permeability changes may explain the difference in results.
If LITT results in prolonged permeability to sub-kDa molecules as suggested by Morris and colleagues,26 then the arm with the greater duration of therapy should have improved OS. Such a model assumes minimal added efficacy of doxorubicin over LITT in the short term. In this study, both arms underwent a median 6 doses of doxorubicin, however, the Late Arm was distributed over a longer interval due to the 6 week pause immediately following LITT. The Early Arm was comparably shorter, as doxorubicin was started immediately after LITT. Indeed, counter to the original hypothesis, improvements in OS were most pronounced in the Late Arm, where chemotherapy was started 6 weeks after LITT. Early low dose doxorubicin (Early Arm) did not provide additional tumor control beyond LITT. This suggests that patients may further benefit from extension (beyond 6 doses) of weekly low-dose doxorubicin.
Early low dose doxorubicin may also alter tumor immunogenicity by an immunomodulatory role by suppressing innate and adaptive immune cell proliferation.44 In this model, early low dose doxorubicin may suppress initial neutrophil and secondary macrophage infiltrate, leading to blunted BBB breakdown and long-term permeability.45 In contrast, delaying doxorubicin may allow early immune infiltrate to further augment BBB breakdown and longer-term permeability.
Other possible mechanisms may be related to more broad-ranging peritumoral alterations induced by LITT, of which altered BBB permeability is only one result. Here, key is the extent of alterations beyond the LITT margin into the peritumoral space, where temperatures of 40–45°C are observed.27 Supraphysiological temperatures may induce a host of inflammatory, epigenetic, and/or additional cytoarchitectural changes beyond increased BBB permeability alone. These changes to the peritumoral micro-environment may then further augment late doxorubicin treatment. Indeed, MGMTp status had a significant effect on OS even after controlling for group status in multivariate models, supporting a synergistic contribution of an alkylating agent like doxorubicin.
Our results support a model of LITT-mediated augmentation of adjunctive therapy via either improved peritumoral BBB permeability or extent of peritumoral alterations after LITT. However, selection of the optimal therapy to couple with LITT remains an open question. Several ongoing clinical trials are currently testing various LITT-combination therapies, including LITT combined with immune checkpoint inhibitors such as pembrolizumab (NCT02311582) and avelumab (NCT03341806).
Our study did not demonstrate a difference in PFS between the study arms and our historical control group. This lack of improvement in PFS may suggest an alternative prognostic factor not fully accounted for. By comparing with the historical LITT controls, IDH1 mutation status, gross tumor volume, tumor locations, and number of recurrences were directly controlled. Furthermore, age, frontline surgery, and MGMTp status were all comparable between cohorts. Nonetheless, a secondary, yet unidentified factor may be driving the group differences in overall survival and warrants continued investigation. There are several additional limitations of this study including its underpowered sample size to examine differences in local progression between arms and the lack of local vs. distal PFS information in the historical controls. The spatial distribution and longitudinal measurements of doxorubicin concentrations in the CSF following LITT remain unknown. A better understanding of doxorubicin concentrations, particularly at the tumor margin, may help determine the optimal duration and number of doses of doxorubicin to achieve better survival outcomes.
Nevertheless, results of this trial suggest that combining LITT with a wider class of BBB-impermeant anti-neoplastic agents is a safe and viable therapeutic strategy in recurrent GBM.
Supplementary material
Supplemental material is available at Neuro-Oncology Advances online.
Trial Registration: ClinicalTrials.gov NCT01851733
Funding
This work was supported by Siteman Cancer Center and the Foundation for Barnes-Jewish Hospital Cancer Frontier Fund (Team Science Award to E.C.L, D.D.T. and J.L.C.)
Conflict of Interest. M.G.C. receives research funding from Neoimmune Tech and Orbus Pharma Research.
E.C.L. is a consultant for Monteris Medical, E15, Acera, Alcyone, Intellectual Ventures, Medtronic Inc., Neurolutions, Osteovantage, Pear Therapeutics, Inc., Sante Ventures, and Microbot; E.C.L owns equity in Neurolutions, General Sensing, Osteovantage, Pear Therapeutics, Face to Face Biometrics, Immunovalent, Caeli Vascular, Acera, Sora Imaging Solutions, Inner Cosmos. D.D.T. has received research funding and/or personal honoraria from Monteris, Novocure, Merck, Novartis, Sarepta, Lacerta, Northwest Bio, Stemline, and Celldex. Remaining authors declare no conflict of interest.
Authorship Statement. Writing the manuscript: O.H.B., A.Y.Z. Study design: A.H.K., E.C.L., D.D.T., J.L.C., J.S.S. Study implementation: A.H.K., E.C.L., D.D.T., J.L.C., J.H., M.C., T.M.J., G.A., G.T., J.S.S., Data analysis: O.H.B., A.Y.Z., W.A.L., A.E.S., J.L., R.N. Manuscript revision: W.A.L and A.E.S. Critical manuscript review: All authors.
References
- 1. Wong ET, Gautam S, Malchow C, Lun M, Pan E, Brem S. Bevacizumab for recurrent glioblastoma multiforme: a meta-analysis. J Natl Compr Canc Netw. 2011;9(4):403–407. [DOI] [PubMed] [Google Scholar]
- 2. Westphal M, Ylä-Herttuala S, Martin J, et al. ; ASPECT Study Group . Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(9):823–833. [DOI] [PubMed] [Google Scholar]
- 3. Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 5. Wang Y, Pan L, Sheng XF, Chen S, Dai JZ. Nimotuzumab, a humanized monoclonal antibody specific for the EGFR, in combination with temozolomide and radiation therapy for newly diagnosed glioblastoma multiforme: First results in Chinese patients. Asia Pac J Clin Oncol. 2016;12(1):e23–e29. [DOI] [PubMed] [Google Scholar]
- 6. Weller M, Butowski N, Tran DD, et al. ; ACT IV trial investigators . Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. [DOI] [PubMed] [Google Scholar]
- 7. Ursu R, Carpentier A, Metellus P, et al. Intracerebral injection of CpG oligonucleotide for patients with de novo glioblastoma-A phase II multicentric, randomised study. Eur J Cancer. 2017;73:30–37. [DOI] [PubMed] [Google Scholar]
- 8. Marenco-Hillembrand L, Wijesekera O, Suarez-Meade P, et al. Trends in glioblastoma: outcomes over time and type of intervention: a systematic evidence based analysis. J Neurooncol. 2020;147(2):297–307. [DOI] [PubMed] [Google Scholar]
- 9. Lemée JM, Clavreul A, Menei P. Intratumoral heterogeneity in glioblastoma: don’t forget the peritumoral brain zone. Neuro Oncol. 2015;17(10):1322–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7(1):41–53. [DOI] [PubMed] [Google Scholar]
- 11. Stan AC, Casares S, Radu D, Walter GF, Brumeanu TD. Doxorubicin-induced cell death in highly invasive human gliomas. Anticancer Res. 1999;19(2A):941–950. [PubMed] [Google Scholar]
- 12. Lesniak MS, Upadhyay U, Goodwin R, Tyler B, Brem H. Local delivery of doxorubicin for the treatment of malignant brain tumors in rats. Anticancer Res. 2005;25(6B):3825–3831. [PMC free article] [PubMed] [Google Scholar]
- 13. Warren KE, Patel MC, McCully CM, Montuenga LM, Balis FM. Effect of P-glycoprotein modulation with cyclosporin A on cerebrospinal fluid penetration of doxorubicin in non-human primates. Cancer Chemother Pharmacol. 2000;45(3):207–212. [DOI] [PubMed] [Google Scholar]
- 14. Higham C, Murphy RF, Bhaumik S, Dunleavy K, Wilson WH, Widemann BC. Plasma and cerebrospinal fluid pharmacokinetics of doxil after intravenous administration in adults with primary CNS lymphoma [abstract]. J Clin Oncol. 2017;35:15_suppl:e14067–e14067. [Google Scholar]
- 15. Patel M, McCully C, Godwin K, Balis FM. Plasma and cerebrospinal fluid pharmacokinetics of intravenous temozolomide in non-human primates. J Neurooncol. 2003;61(3):203–207. [DOI] [PubMed] [Google Scholar]
- 16. Stewart DJ, Lu K, Benjamin RS, et al. Concentration of vinblastine in human intracerebral tumor and other tissues. J Neurooncol. 1983;1(2):139–144. [DOI] [PubMed] [Google Scholar]
- 17. Stewart DJ, Richard MT, Hugenholtz H, et al. Penetration of VP-16 (etoposide) into human intracerebral and extracerebral tumors. J Neurooncol. 1984;2(2):133–139. [DOI] [PubMed] [Google Scholar]
- 18. Holodny AI, Nusbaum AO, Festa S, Pronin IN, Lee HJ, Kalnin AJ. Correlation between the degree of contrast enhancement and the volume of peritumoral edema in meningiomas and malignant gliomas. Neuroradiology. 1999;41(11):820–825. [DOI] [PubMed] [Google Scholar]
- 19. Rahmathulla G, Recinos PF, Kamian K, Mohammadi AM, Ahluwalia MS, Barnett GH. MRI-guided laser interstitial thermal therapy in neuro-oncology: a review of its current clinical applications. Oncology. 2014;87(2):67–82. [DOI] [PubMed] [Google Scholar]
- 20. Thomas JG, Rao G, Kew Y, Prabhu SS. Laser interstitial thermal therapy for newly diagnosed and recurrent glioblastoma. Neurosurg Focus. 2016;41(4):E12. [DOI] [PubMed] [Google Scholar]
- 21. Patel B, Yang PH, Kim AH. The effect of thermal therapy on the blood-brain barrier and blood-tumor barrier. Int J Hyperthermia. 2020;37(2):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salehi A, Paturu MR, Patel B, et al. Therapeutic enhancement of blood-brain and blood-tumor barriers permeability by laser interstitial thermal therapy. Neurooncol Adv. 2020;2(1):vdaa071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sabel M, Rommel F, Kondakci M, Gorol M, Willers R, Bilzer T. Locoregional opening of the rodent blood-brain barrier for paclitaxel using Nd:YAG laser-induced thermo therapy: a new concept of adjuvant glioma therapy? Lasers Surg Med. 2003;33(2):75–80. [DOI] [PubMed] [Google Scholar]
- 24. Leuthardt EC, Duan C, Kim MJ, et al. Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. Plos One. 2016;11(2):e0148613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tran D, Leuthardt E, Shimony J, Kim A, Ansstas G, Campian J. ACTR-82. Laser Interstitial Thermal Therapy (LITT) Of Recurrent Glioblastoma (GBM) Induces Temporary Disruption Of The Peritumoral Blood Brain Barrier (BBB) and may improve efficacy of chemotherapy with poor CNS penetration. Neuro Oncol. 2017;19(suppl.):vi18. [Google Scholar]
- 26. Morris SA, Rollo M, Rollo P, et al. Prolonged blood-brain barrier disruption following laser interstitial ablation in epilepsy: a case series with a case report of postablation optic neuritis. World Neurosurg. 2017;104:467–475. [DOI] [PubMed] [Google Scholar]
- 27. Hawasli AH, Ray WZ, Murphy RK, Dacey RG Jr, Leuthardt EC. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for subinsular metastatic adenocarcinoma: technical case report. Neurosurgery. 2012;70(2 Suppl Operative):332–337; discussion 338. [DOI] [PubMed] [Google Scholar]
- 28. Hawasli AH, Bagade S, Shimony JS, Miller-Thomas M, Leuthardt EC. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for intracranial lesions: single-institution series. Neurosurgery. 2013;73(6):1007–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hawasli AH, Kim AH, Dunn GP, Tran DD, Leuthardt EC. Stereotactic laser ablation of high-grade gliomas. Neurosurg Focus. 2014;37(6):E1. [DOI] [PubMed] [Google Scholar]
- 30. Mohammadi AM, Hawasli AH, Rodriguez A, et al. The role of laser interstitial thermal therapy in enhancing progression-free survival of difficult-to-access high-grade gliomas: a multicenter study. Cancer Med. 2014;3(4):971–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chamberlain MC, Johnston SK. Salvage therapy with single agent bevacizumab for recurrent glioblastoma. J Neurooncol. 2010;96(2):259–269. [DOI] [PubMed] [Google Scholar]
- 32. Kamath AA, Friedman DD, Akbari SHA, et al. Glioblastoma treated with magnetic resonance imaging-guided laser interstitial thermal therapy: safety, efficacy, and outcomes. Neurosurgery. 2019;84(4):836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chinot OL, Macdonald DR, Abrey LE, Zahlmann G, Kerloëguen Y, Cloughesy TF. Response assessment criteria for glioblastoma: practical adaptation and implementation in clinical trials of antiangiogenic therapy. Curr Neurol Neurosci Rep. 2013;13(5):347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Creed JH, Gerke TA, Berglund AE. MatSurv: survival analysis and visualization in MATLAB. J Open Source Soft. 2020;5(46):1830. [Google Scholar]
- 35. Medikonda R, Dunn G, Rahman M, Fecci P, Lim M. A review of glioblastoma immunotherapy. J Neurooncol. 2021;151(1):41–53. [DOI] [PubMed] [Google Scholar]
- 36. Pilié PG, Gay CM, Byers LA, O’Connor MJ, Yap TA. PARP inhibitors: extending benefit beyond BRCA-mutant cancers. Clin Cancer Res. 2019;25(13):3759–3771. [DOI] [PubMed] [Google Scholar]
- 37. Schmidt F, Fischer J, Herrlinger U, Dietz K, Dichgans J, Weller M. PCV chemotherapy for recurrent glioblastoma. Neurology. 2006;66(4):587–589. [DOI] [PubMed] [Google Scholar]
- 38. Carvalho BF, Fernandes AC, Almeida DS, et al. Second-line chemotherapy in recurrent glioblastoma: a 2-cohort study. Oncol Res Treat. 2015;38(7-8):348–354. [DOI] [PubMed] [Google Scholar]
- 39. Weller M, Tabatabai G, Kästner B, et al. ; DIRECTOR Study Group . MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: the DIRECTOR trial. Clin Cancer Res. 2015;21(9):2057–2064. [DOI] [PubMed] [Google Scholar]
- 40. Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 41. Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wick W, Gorlia T, Bendszus M, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 2017;377(20):1954–1963. [DOI] [PubMed] [Google Scholar]
- 43. Haque A, Ray SK, Cox A, Banik NL. Neuron specific enolase: a promising therapeutic target in acute spinal cord injury. Metab Brain Dis. 2016;31(3):487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wehner R, Bitterlich A, Meyer N, et al. Impact of chemotherapeutic agents on the immunostimulatory properties of human 6-sulfo LacNAc+ (slan) dendritic cells. Int J Cancer. 2013;132(6):1351–1359. [DOI] [PubMed] [Google Scholar]
- 45. Yang C, Hawkins KE, Doré S, Candelario-Jalil E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol. 2019;316(2):C135–C153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.