Abstract
Purpose of review
We describe the recent advances in rehabilitation nutrition, which is especially important for disabled or frail older individuals.
Recent findings
Recent evidence pertaining to rehabilitation nutrition conducted in rehabilitation wards and acute care hospitals has been accumulating. The combination of rehabilitation nutrition and rehabilitation pharmacotherapy is important for eliciting higher functions. The 2020 update of the clinical practice guidelines for rehabilitation nutrition provides a weak recommendation for enhanced nutritional care for patients with cerebrovascular disease, hip fracture, cancer, or acute illness who are undergoing rehabilitation. Rehabilitation nutritional care process and the International Classification of Functioning, Disability and Health-Dietetics are used to implement high-quality rehabilitation nutrition. Aggressive nutrition therapy incorporates the daily energy expenditure plus daily energy accumulation to increase body weight and muscle mass. Preventing and treating sarcopenic dysphagia should include iatrogenic sarcopenia prevention and aggressive nutrition therapy. The diagnosis criteria for respiratory sarcopenia and sarcopenic respiratory disability have been established.
Summary
The International Association of Rehabilitation Nutrition and Total Nutrition Therapy Rehabilitation program may contribute to international expansion of rehabilitation nutrition. Improving evidence–practice gaps in rehabilitation nutrition and increasing national health insurance coverage of aggressive nutrition therapy and rehabilitation nutrition teams are warranted.
Keywords: aggressive nutrition therapy, clinical practice guideline, iatrogenic sarcopenia, rehabilitation nutritional care process, sarcopenic respiratory disability
INTRODUCTION
Rehabilitation nutrition is a new area of research and clinical practice that has developed over the past decade mainly due to the work of the Japanese Association of Rehabilitation Nutrition members. Rehabilitation nutrition involves (1) holistic evaluation by the International Classification of Functioning, Disability and Health (ICF), and the presence and cause of nutritional disorders, sarcopenia, and excessive or deficient nutritional intake; (2) rehabilitation nutrition diagnosis and setting rehabilitation nutrition goals; and (3) improvements in nutritional status, sarcopenia, and frailty using nutritional care management in consideration of rehabilitation and rehabilitation in consideration of nutrition to achieve the highest body functions (e.g., swallowing function, activities, and participations) and quality of life (QOL) in disabled or frail older individuals [1▪,2]. Malnutrition is a cause of frailty and disability, including sarcopenic dysphagia [3▪▪] and respiratory sarcopenic disability [4]; therefore, nutritional management can improve frailty and disability. Indeed, nutrition has become necessary knowledge for physical therapists [5,6].
This review aims to summarize the recent developments in rehabilitation nutrition, such as the current evidence, clinical practice guidelines, rehabilitation nutritional care process, ICF-Dietetics, aggressive nutrition therapy, sarcopenic dysphagia, respiratory sarcopenia, and sarcopenic respiratory disability.
Box 1.
no caption available
RECENT EVIDENCE IN REHABILITATION NUTRITION
Recent evidence in rehabilitation nutrition from rehabilitation wards has accumulated. Convalescent rehabilitation wards can provide program rehabilitation therapy up to 3 h per day based on national health insurance coverage in Japan. Assignment of registered dietitians to convalescent rehabilitation wards is mandatory or effort mandatory from 2020. Sarcopenic obesity diagnosed based on the presence of both sarcopenia and a high body fat percentage is associated with lower activities of daily living (ADL) and a lower rate of home discharge [7,8]. Malnutrition was found to be associated with poor improvement after poststroke cognitive impairment [9]. An elevated creatinine-based estimated glomerular filtration rate and low hemoglobin level are associated with risk of sarcopenia, dysphagia, and lower ADL after stroke [10,11]. Moreover, a systematic review and meta-analysis showed that nutritional status was associated with decreased functionality and that the prevalence of sarcopenia was 40–76% in patients undergoing geriatric rehabilitation [12].
Rehabilitation nutrition increase patient functions in the rehabilitation ward. An improvement in the hemoglobin level was associated with faster recovery of ADL and a shorter hospital stay in patients with anemic stroke [13]. Muscle mass gain was associated with better recovery of ADL after sarcopenic stroke [14]. Frequent and individualized nutritional support improved nutritional status, physical function, and dysphagia after stroke [15]. The chair-stand exercise was associated with improved swallowing function, ADL, and length of hospital stay in patients with dysphagic stroke [16], whereas a whey protein-based nutritional formula enriched with leucine and vitamin D improved physical performance and function in older sarcopenic patients [17].
Recent evidence in rehabilitation nutrition has also been reported from acute care hospitals. Less energy intake at 1 week after hospitalization was an independent risk factor for mortality, difficult at-home discharge, and pneumonia recurrence in older patients with pneumonia [18]. Nutritional improvement was associated with improvements in ADL and dysphagia in malnourished patients with pneumonia [19]. Energy intake affected femur muscle thickness on the nonparalysis side in older patients after stroke [20]. Individualized intensive nutritional treatment improved the ADL of older stroke patients at risk of malnutrition [21]. Moreover, a systematic review and meta-analysis showed that exercise and nutritional intervention were effective in improving frailty and physical function in hospitalized older patients [22]. The recent evidence indicates the importance of rehabilitation nutrition in acute care hospitals, although assignment of registered dietitians to acute care wards is not mandatory in Japan.
A combination of rehabilitation nutrition and rehabilitation pharmacotherapy is also important for eliciting higher functions. Rehabilitation pharmacotherapy aims to help frail older or disabled people achieve the highest level of function and QOL [23▪]. For example, a sodium–glucose cotransporter 2 inhibitor resulted in the development of malnutrition and sarcopenia in type 2 diabetes patients [24], and anticholinergic and sedative drug burden was associated with poorer performance of ADL and postural balance in stroke patients [25]. Moreover, decreased use of potentially inappropriate medications during hospitalization can improve ADL [26]. Combination of anamorelin administration with rehabilitation nutrition might lead to better function and QOL in patients with cachectic cancer in Japan [27].
CLINICAL PRACTICE GUIDELINES FOR REHABILITATION NUTRITION
The 2020 update of the clinical practice guidelines for rehabilitation nutrition for patients with cerebrovascular disease, hip fracture, cancer, or acute illness was published by the Japanese Association of Rehabilitation Nutrition [28▪▪]. Enhanced nutritional care is weakly recommended for patients with cerebrovascular disease, hip fracture, cancer, or acute illness who are undergoing rehabilitation. In these clinical practice guidelines, enhanced nutritional care refers to all types of nutritional care in addition to standard nutritional care, such as regular hospital/institution diets or individual habitual diets.
PROCESS OF REHABILITATION NUTRITIONAL CARE AND INTERNATIONAL CLASSIFICATION OF FUNCTIONING, DISABILITY AND HEALTH DIETETICS
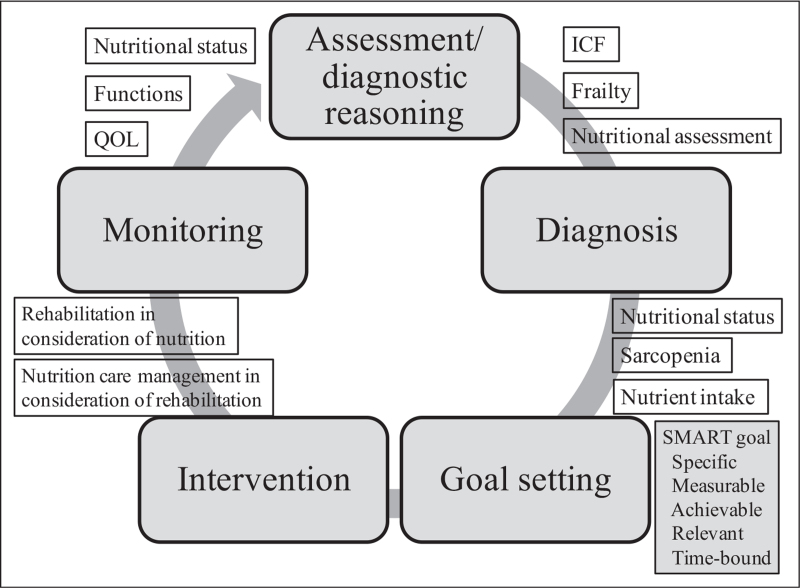
The process of rehabilitation nutritional care is a systematic problem-solving method used to assess nutritional status, sarcopenia, and nutrient intake in disabled or frail elderly people [1▪,2]. Rehabilitation nutritional care involves five steps: rehabilitation nutrition assessment and diagnostic reasoning, rehabilitation nutrition diagnosis, rehabilitation nutrition goal setting, rehabilitation nutritional intervention, and rehabilitation nutrition monitoring (Fig. 1). Assessment of rehabilitation nutrition includes ICF, frailty, and nutritional assessments. Rehabilitation nutrition diagnosis is divided into three major categories with 15 subitems: nutritional status (undernutrition, overnutrition, at risk of malnutrition [undernutrition, overnutrition], lack of nutrients, excess nutrients, not applicable), sarcopenia (sarcopenia, decreased muscle mass, decreased muscle strength and/or physical performance, not applicable), and excess and/or insufficient nutrient intake (excess nutrient intake, insufficient nutrient intake, prediction of excess nutrient intake, prediction of insufficient nutrient intake, not applicable).
FIGURE 1.
Process of rehabilitation nutritional care. The process of rehabilitation nutritional care has five steps: rehabilitation nutrition assessment and diagnostic reasoning, rehabilitation nutrition diagnosis, rehabilitation nutrition goal setting, rehabilitation nutritional intervention, and rehabilitation nutrition monitoring. Goal setting using the SMART concept in both nutrition and rehabilitation before intervention is important. SMART, specific, measurable, achievable, relevant, and time-bound.
The most important step in the process of rehabilitation nutritional care is rehabilitation nutrition goal setting. Goal setting should be performed in accordance with the concept of specific, measurable, achievable, relevant, and time-bound (SMART) [1▪,2]. For example, nutritional improvement and ADL independence are not SMART goals. In contrast, gaining 1 kg body weight within 1 month, consuming three meals of pureed food orally, and weaning from tube feeding within 2 weeks are all SMART goals. Both nutritional and rehabilitation goals should be set prior to intervention.
Rehabilitation nutritional intervention includes nutritional care management in consideration of rehabilitation and rehabilitation in consideration of nutrition. Nutritional care management and rehabilitation programs are interdependent, and each should be conducted with awareness of the interventions of the other. Rehabilitation nutrition monitoring includes assessments of nutritional status, functions, and QOL.
The ICF-Dietetics, developed by the Dutch Association of Dietitians, is another method for rehabilitation nutrition. Approximately 900 dietetics-related categories were added to the ICF to form the ICF-Dietetics [29▪]. Implementation of the ICF-Dietetics requires a holistic perspective of functioning with greater attention on participation and environmental factors, which have been overlooked in traditional nutritional care management [29▪]. However, the ICF-Dietetics is used in only 7 out of 56 (12.5%) hospitals in Jeddah, Saudi Arabia [30] and is not used in most hospitals in Japan. A qualitative study of the ICF-Dietetics addressed six implementation objectives: (1) provide information to other professional groups, (2) provide training on the ICF-Dietetics coding system, (3) integrate the ICF-Dietetics into an electronic health information system, (4) apply the ICF-Dietetics for goal setting and evaluation, (5) establish a learning environment, and (6) define individual goals for change [31]. Both rehabilitation nutritional care and the ICF-Dietetics should be used more widely in clinical practice.
AGGRESSIVE NUTRITION THERAPY
Aggressive nutrition therapy is defined as nutritional care management in which the daily energy requirement is established according to the daily energy expenditure and the daily energy accumulation [32▪]. Daily energy requirement equals daily energy expenditure in well-nourished individuals to maintain body weight. The aim in obese individuals wanting to lose body weight is to reduce the daily energy requirement to less than the daily energy expenditure. Similarly, the target daily energy requirement should be higher than the daily energy expenditure in undernourished individuals wanting to gain body weight and muscle mass. Aggressive nutrition therapy is performed in malnourished and/or sarcopenic individuals to increase body weight and muscle mass.
There are two key questions regarding the implementation of aggressive nutrition therapy. The first is ‘Should nutrition status and/or muscle status be improved?’ The answer is yes primarily to avoid complications related to malnutrition and muscle wasting. From a rehabilitation nutrition perspective, the answer is yes if improvement of malnutrition and/or sarcopenia leads to improved functions and QOL. For example, aggressive nutrition therapy does not improve functions or QOL in the case of malnourished and/or sarcopenic patients with cervical spinal cord injury at a higher position on the spinal cord and complete quadriplegia, with the exception of cases of pressure ulcer prevention in severely undernourished patients. The second key question is ‘Can nutrition status and/or muscle status be improved?’ Improving malnutrition and/or sarcopenia is difficult in patients with terminal-stage disease, severe inflammation, or refeeding syndrome or a risk thereof. If the answers to these two key questions are both yes, aggressive nutrition therapy will be indicated.
The level of daily energy accumulation is determined by nutrition goal setting. The amount of energy accumulation per 1 kg body weight is generally 7500 kcal [32▪]. If the nutrition goal is to gain 1 kg of body weight in 1 month, the hypothetical daily energy accumulation would be 250 kcal, which is 7500 kcal divided by 30 days. However, adjustment of nutritional provision via regular monitoring of rehabilitation nutrition is necessary. Moreover, a combination of aggressive nutrition therapy and resistance training must be performed to increase body weight via muscle gain instead of fat gain.
SARCOPENIC DYSPHAGIA
Sarcopenic dysphagia is dysphagia due to sarcopenia in both generalized skeletal muscles and swallowing-related muscles and is excluded if whole-body sarcopenia is absent [3▪▪]. Lower laryngeal movement and enlargement of the pharyngeal cavity have been observed in sarcopenic patients [33]. The prevalence of sarcopenic dysphagia in inpatients who require dysphagia rehabilitation was 32% [34]. Moreover, the prevalence of sarcopenic dysphagia in older inpatients with pneumonia was 81% [35]. Therefore, sarcopenic dysphagia is quite common in older patients with dysphagia, and both clinical and research interests in sarcopenic dysphagia are growing.
Recent studies have investigated swallowing muscle mass and function in patients with sarcopenic dysphagia. Geniohyoid muscle mass was independently associated with maximum tongue pressure and tongue muscle mass [36]. Digastric muscle mass and intensity were independent factors for sarcopenic dysphagia [37]. Geniohyoid muscle mass was significantly associated with the severity of dysphagia after salvage surgery for head and neck cancer [38]. Older patients with sarcopenic dysphagia had weaker pharyngeal contractility, a greater rate of upper esophageal sphincter dysfunction [39], a longer duration of submental muscle activity, and a higher amplitude of the submental muscles during swallowing [40].
Diagnosis of sarcopenic dysphagia is mainly performed using a reliable and validated diagnostic algorithm for sarcopenic dysphagia, developed by the Japanese Working Group on Sarcopenic Dysphagia [41]. In a scoping review of sarcopenic dysphagia, 10 papers used this diagnostic algorithm [41]. The presence of whole-body sarcopenia and dysphagia and the absence of other potentially causative diseases of the dysphagia are necessary to diagnose sarcopenic dysphagia. An additional diagnostic criterion is the strength of the swallowing muscles, which is assessed by tongue pressure. A tongue pressure < 20 kPa is considered to indicate probable sarcopenic dysphagia, whereas a tongue pressure of ≥ 20 kPa or that cannot be measured is considered to indicate possible sarcopenic dysphagia.
Prevention of iatrogenic sarcopenia can lead to prevention of sarcopenic dysphagia. Iatrogenic sarcopenia is sarcopenia caused by the activities of doctors, nurses, or other healthcare workers in healthcare facilities [1▪,2]. The three causes of iatrogenic sarcopenia are inappropriately low activity levels, inappropriate nutritional care management, and iatrogenic diseases including polypharmacy. For example, among inpatients with aspiration pneumonia with a nil per os status, only 5.3% were prescribed ≥ 20 kcal/kg, and only 6.4% were prescribed ≥ 1.0 g/kg amino acids on day 7 of nil per os[42]. Moreover, the 25th percentile of daily energy intake in inpatients who require dysphagia rehabilitation was 648 kcal [34]. Iatrogenic sarcopenia due to inappropriate nutritional management is quite common and should be avoided in acute care hospitals.
Aggressive nutrition therapy combined with dysphagia rehabilitation may further improve sarcopenic dysphagia. An energy intake of approximately 35 kcal/kg/day based on ideal body weight (IBW) along with dysphagia rehabilitation was suggested for sarcopenic dysphagia in a position paper [3▪▪]. An energy intake of ≥ 30 kcal/kg/day and protein intake of ≥ 1.2 g/kg/day based on IBW increased tongue strength in older adults with sarcopenia [43]. Furthermore, providing ≥ 30 kcal/kg/day energy based on IBW improved swallowing function more compared with providing < 30 kcal/kg/day energy in patients with sarcopenic dysphagia [44]. A scoping review of sarcopenic dysphagia treatment showed that six of eight case reports used rehabilitation nutrition to improve body weight, swallowing function, and ADL [41]. A combination of aggressive nutrition therapy and dysphagia rehabilitation performed by an interdisciplinary rehabilitation nutrition team may be useful for treating sarcopenic dysphagia.
RESPIRATORY SARCOPENIA AND SARCOPENIC RESPIRATORY DISABILITY
The Japanese Working Group of Respiratory Sarcopenia defined respiratory sarcopenia as whole-body sarcopenia and low respiratory muscle mass, along with poor respiratory muscle strength and/or respiratory function [4]. Kera et al. defined respiratory sarcopenia according to a cut-off value of the peak expiratory flow rate of 1 standard deviation below the mean [45]. However, whole-body sarcopenia and respiratory muscle mass are also important for defining and diagnosing respiratory sarcopenia. Indeed, whole-body sarcopenia was associated with reduced diaphragmatic muscle thickness and respiratory function in older individuals [46]. The parameter respiratory muscle function was correlated with reduced handgrip strength and functional impairment but not with lean mass [47].
The diagnostic criteria for respiratory sarcopenia were developed in the same way as the diagnostic criteria for sarcopenic dysphagia [4]. Definite respiratory sarcopenia is diagnosed in patients with whole-body sarcopenia and low respiratory muscle mass, along with poor respiratory muscle strength and/or respiratory function, after excluding obvious causative respiratory diseases. Probable respiratory sarcopenia is diagnosed when whole-body sarcopenia with low respiratory muscle strength and/or deteriorated respiratory function are identified, after excluding obvious causative respiratory diseases. Patients with potentially causative respiratory diseases are diagnosed with possible respiratory sarcopenia.
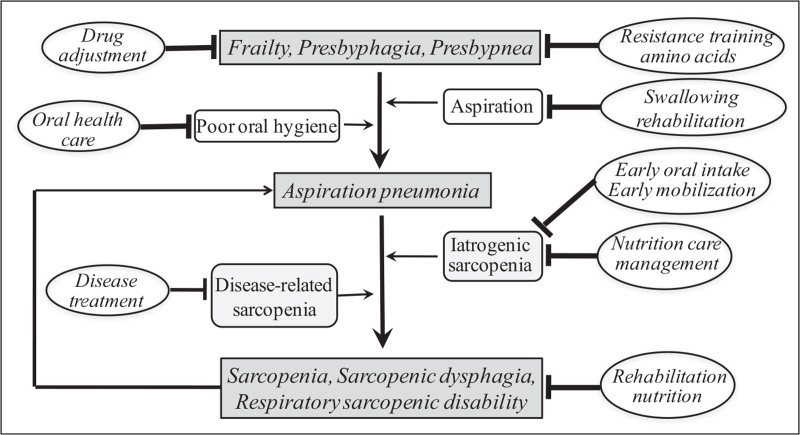
The Japanese Working Group of Respiratory Sarcopenia also proposed definitions for sarcopenic respiratory disability and ‘presbypnea,’ a novel term combining presby- (geriatric) and -pnea (respiratory) [4]. Sarcopenic respiratory disability is a disability with deterioration of respiratory function due to respiratory sarcopenia. Functional disability is defined by a modified Medical Research Council grade of 2 (slower walking pace compared with people of the same age due to breathlessness or the need to stop for breath when walking at own pace on a level surface) or higher. Presbypnea is defined as a decline in respiratory function with aging. Minor respiratory functional disability in presbypnea is defined as a modified Medical Research Council grade of 1 (shortness of breath when hurrying or walking straight uphill). Older people with frailty, presbyphagia (decline in swallowing function due to aging) or presbypnea may prevent aspiration pneumonia by resistance training, amino acid supplementation, or drug adjustment. However, if they suffer from aspiration pneumonia, they are at risk of developing whole-body sarcopenia, sarcopenic dysphagia, and respiratory sarcopenic disability due to disease-related sarcopenia or iatrogenic sarcopenia (Fig. 2).
FIGURE 2.
Mechanism of sarcopenic dysphagia and respiratory sarcopenic disability in aspiration pneumonia. Aspiration pneumonia may be prevented by resistance training, amino acid supplementation, or drug adjustment in older people with frailty, presbyphagia, or presbypnea. Older people with aspiration pneumonia who are hospitalized in acute care hospitals are at risk of developing whole-body sarcopenia, sarcopenic dysphagia, and respiratory sarcopenic disability due to disease-related sarcopenia and iatrogenic sarcopenia. Rehabilitation nutrition can improve whole-body sarcopenia, sarcopenic dysphagia and respiratory sarcopenic disability.
Leucine, β-hydroxy-β-methylbutyrate (HMB), and protein have been shown to increase muscle mass. Leucine supplementation is recommended for older people with sarcopenia to increase muscle mass in an umbrella review of systematic reviews and meta-analyses [48]. Leucine binds to the proteins Sestrin and LeuRS, which increases the activity of mammalian target of rapamycin complex 1 (mTORC1) and promotes protein synthesis. Moreover, leucine inhibits the protein degradation pathway of the ubiquitin-proteasome system. HMB increases mTORC1 activity in the same way as leucine. Recent systematic review showed that HMB supplementation significantly increased fat-free mass in older people in older people [49]. Furthermore, supplementation of 3 g of HMB was beneficial in improving muscle strength and body composition, especially in bedridden or frail older people [50]. On the other hand, recommendation for protein supplementation to increase muscle mass was weaker than for leucine [48].
CONCLUSION
The concept of and evidence for rehabilitation nutrition, aggressive nutrition therapy, sarcopenic dysphagia, respiratory sarcopenia, and sarcopenic respiratory disability have evolved over the past decade. Rehabilitation nutrition research from countries other than Japan is needed to develop a worldwide consensus on rehabilitation nutrition. The International Association of Rehabilitation Nutrition (https://sites.google.com/site/jsrhnt/international-association-of-rehabilitation-nutrition-iarn) and Total Nutrition Therapy Rehabilitation program developed by the Japanese Association of Rehabilitation Nutrition in 2019 may contribute to international expansion of rehabilitation nutrition. In addition, improving evidence–practice gaps in rehabilitation nutrition and reimbursement by national health insurance for aggressive nutrition therapy and rehabilitation nutrition teams are warranted.
Acknowledgements
None.
Financial support and sponsorship
This work was supported by a grant from JSPS KAKENHI (grant number 19H03979).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪.Nagano A, Nishioka S, Wakabayashi H. Rehabilitation nutrition for iatrogenic sarcopenia and sarcopenic dysphagia. J Nutr Health Aging 2019; 23:256–265. [DOI] [PubMed] [Google Scholar]; This review described iatrogenic sarcopenia, sarcopenic dysphagia, and rehabilitation nutritional care process in detail.
- 2.Kakehi S, Wakabayashi H, Inuma H, et al. Rehabilitation nutrition and exercise therapy for sarcopenia. World J Mens Health 2021; 39:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3▪▪.Fujishima I, Fujiu-Kurachi M, Arai H, et al. Sarcopenia and dysphagia: position paper by four professional organizations. Geriatr Gerontol Int 2019; 19:91–97. [DOI] [PubMed] [Google Scholar]; This position paper was reported by the Japanese Society of Dysphagia Rehabilitation, the Japanese Association of Rehabilitation Nutrition, the Japanese Association on Sarcopenia and Frailty, and the Society of Swallowing and Dysphagia of Japan.
- 4.Nagano A, Wakabayashi H, Maeda K, et al. Respiratory sarcopenia and sarcopenic respiratory disability: concepts, diagnosis, and treatment. J Nutr Health Aging 2021; 25:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berner P, Bezner JR, Morris D, Lein DH. Nutrition in physical therapist practice: tools and strategies to act now. Phys Ther 2021; 101:zab061. [DOI] [PubMed] [Google Scholar]
- 6.Berner P, Bezner JR, Morris D, Lein DH. Nutrition in physical therapist practice: setting the stage for taking action. Phys Ther 2021; 101:zab062.doi: 10.1093/ptj/pzab062. [DOI] [PubMed] [Google Scholar]
- 7.Yoshimura Y, Wakabayashi H, Nagano F, et al. Sarcopenic obesity is associated with activities of daily living and home discharge in postacute rehabilitation. J Am Med Dir Assoc 2020; 21:1475–1480. [DOI] [PubMed] [Google Scholar]
- 8.Matsushita T, Nishioka S, Taguchi S, et al. Sarcopenic obesity and activities of daily living in stroke rehabilitation patients: a cross-sectional study. Healthcare 2020; 8:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsutsumiuchi K, Wakabayashi H, Maeda K, Shamoto H. Impact of malnutrition on poststroke cognitive impairment in convalescent rehabilitation ward inpatients. Eur Geriatr Med 2021; 12:167–174. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura Y, Wakabayashi H, Nagano F, et al. Elevated creatinine-based estimated glomerular filtration rate is associated with increased risk of sarcopenia, dysphagia, and reduced functional recovery after stroke. J Stroke Cerebrovasc Dis 2021; 30:105491. [DOI] [PubMed] [Google Scholar]
- 11.Yoshimura Y, Wakabayashi H, Nagano F, et al. Low hemoglobin levels are associated with sarcopenia, dysphagia, and adverse rehabilitation outcomes after stroke. J Stroke Cerebrovasc Dis 2020; 29:105405. [DOI] [PubMed] [Google Scholar]
- 12.Wojzischke J, van Wijngaarden J, van den Berg C, et al. Nutritional status and functionality in geriatric rehabilitation patients: a systematic review and meta-analysis. Eur Geriatr Med 2020; 11:195–207. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimura Y, Wakabayashi H, Shiraishi A, et al. Hemoglobin improvement is positively associated with functional outcomes in stroke patients with anemia. J Stroke Cerebrovasc Dis 2021; 30:105453. [DOI] [PubMed] [Google Scholar]
- 14.Nagano F, Yoshimura Y, Bise T, et al. Muscle mass gain is positively associated with functional recovery in patients with sarcopenia after stroke. J Stroke Cerebrovasc Dis 2020; 29:105017. [DOI] [PubMed] [Google Scholar]
- 15.Shimazu S, Yoshimura Y, Kudo M, et al. Frequent and personalized nutritional support leads to improved nutritional status, activities of daily living, and dysphagia after stroke. Nutrition 2021; 83:111091. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura Y, Wakabayashi H, Nagano F, et al. Chair-stand exercise improves poststroke dysphagia. Geriatr Gerontol Int 2020; 20:885–891. [DOI] [PubMed] [Google Scholar]
- 17.Rondanelli M, Cereda E, Klersy C, et al. Improving rehabilitation in sarcopenia: a randomized-controlled trial utilizing a muscle-targeted food for special medical purposes. J Cachexia Sarcopenia Muscle 2020; 11:1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shirado K, Wakabayashi H, Maeda K, et al. Impact of energy intake at one week after hospitalization on prognosis for older adults with pneumonia. J Nutr Health Aging 2020; 24:119–124. [DOI] [PubMed] [Google Scholar]
- 19.Uno C, Maeda K, Wakabayashi H, et al. Nutritional status change and activities of daily living in elderly pneumonia patients admitted to acute care hospital: a retrospective cohort study from the Japan Rehabilitation Nutrition Database. Nutrition 2020; 71:110613. [DOI] [PubMed] [Google Scholar]
- 20.Kokura Y, Kato M, Taniguchi Y, et al. Energy intake during the acute phase and changes in femoral muscle thickness in older hemiplegic inpatients with stroke. Nutrition 2020; 70:110582. [DOI] [PubMed] [Google Scholar]
- 21.Otsuki I, Himuro N, Tatsumi H, et al. Individualized nutritional treatment for acute stroke patients with malnutrition risk improves functional independence measurement: a randomized controlled trial. Geriatr Gerontol Int 2020; 20:176–182. [DOI] [PubMed] [Google Scholar]
- 22.Han CY, Miller M, Yaxley A, et al. Effectiveness of combined exercise and nutrition interventions in prefrail or frail older hospitalised patients: a systematic review and meta-analysis. BMJ Open 2020; 10:e040146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23▪.Kose E, Wakabayashi H. Rehabilitation pharmacotherapy: a scoping review. Geriatr Gerontol Int 2020; 20:655–663. [DOI] [PubMed] [Google Scholar]; This review described the concept of rehabilitation pharmacotherapy and discuss its importance from the perspective of polypharmacy, the effect of drugs on disability and disease, nutritional status and ADL.
- 24.Yasuda M, Iizuka K, Kato T, et al. Sodium-glucose cotransporter 2 inhibitor and sarcopenia in a lean elderly adult with type 2 diabetes: a case report. J Diabetes Investig 2020; 11:745–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogawa Y, Nibe F, Ogawa R, Sakoh M. Anticholinergic and sedative drug burden and functional recovery after cerebrovascular accident: a retrospective descriptive study. Prog Rehabil Med 2020; 5:20200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kose E, Hirai T, Seki T, Yasuno N. The impact of decreasing potentially inappropriate medications on activities of daily living in a convalescent rehabilitation setting. Int J Clin Pharm 2021; 43:577–585. [DOI] [PubMed] [Google Scholar]
- 27.Wakabayashi H, Arai H, Inui A. The regulatory approval of anamorelin for treatment of cachexia in patients with nonsmall cell lung cancer, gastric cancer, pancreatic cancer, and colorectal cancer in Japan: facts and numbers. J Cachexia Sarcopenia Muscle 2021; 12:14–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28▪▪.Nishioka S, Aragane H, Suzuki N, et al. Clinical practice guidelines for rehabilitation nutrition in cerebrovascular disease, hip fracture, cancer, and acute illness: 2020 update. Clin Nutr ESPEN 2021; 43:577–585. [DOI] [PubMed] [Google Scholar]; These clinical practice guidelines were developed by the Japanese Association for Rehabilitation Nutrition. These clinical practice guidelines were made based on 4 to 9 randomized control trials up to April 2020 for cerebrovascular disease, hip fracture, cancer, and acute illnesses.
- 29▪.Gäbler G, Coenen M, Lycett D, Stamm T. Towards a standardized nutrition and dietetics terminology for clinical practice: an Austrian multicenter clinical documentation analysis based on the International Classification of Functioning, Disability and Health (ICF)-Dietetics. Clin Nutr 2019; 38:791–799. [DOI] [PubMed] [Google Scholar]; Many ICF-Dietetics categories were used in the ICF component body function, while very few categories were used in the ICF component participation and environmental factors.
- 30.Alkhaldy AA, Allahyani MN, Alghamdi NA, et al. Status of nutrition care process implementation in hospitals in Jeddah, Saudi Arabia. Clin Nutr ESPEN 2020; 36:53–59. [DOI] [PubMed] [Google Scholar]
- 31.Gäbler G, Coenen M, Fohringer K, et al. Towards a nationwide implementation of a standardized nutrition and dietetics terminology in clinical practice: a preimplementation focus group study including a pretest and using the consolidated framework for implementation research. BMC Health Serv Res 2019; 19:920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32▪.Nakahara S, Takasaki M, Abe S, et al. Aggressive nutrition therapy in malnutrition and sarcopenia. Nutrition 2021; 84:111109. [DOI] [PubMed] [Google Scholar]; This review described why, what and how to perform aggressive nutrition therapy in conjunction with rehabilitation nutritional care process.
- 33.Miyashita T, Kikutani T, Nagashima K, et al. The effects of sarcopenic dysphagia on the dynamics of swallowing organs observed on videofluoroscopic swallowing studies. J Oral Rehabil 2020; 47:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wakabayashi H, Takahashi R, Murakami T. The prevalence and prognosis of sarcopenic dysphagia in patients who require dysphagia rehabilitation. J Nutr Health Aging 2019; 23:84–88. [DOI] [PubMed] [Google Scholar]
- 35.Miyauchi N, Nakamura M, Nakamura I, Momosaki R. Effect of early versus delayed mobilization by physical therapists on oral intake in patients with sarcopenic dysphagia after pneumonia. Eur Geriatr Med 2019; 10:603–607. [DOI] [PubMed] [Google Scholar]
- 36.Mori T, Wakabayashi H, Ogawa N, et al. The mass of geniohyoid muscle is associated with maximum tongue pressure and tongue area in patients with sarcopenic dysphagia. J Nutr Health Aging 2021; 25:356–360. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa N, Wakabayashi H, Mori T, et al. Digastric muscle mass and intensity in older patients with sarcopenic dysphagia by ultrasonography. Geriatr Gerontol Int 2021; 21:14–19. [DOI] [PubMed] [Google Scholar]
- 38.Hashida N, Shamoto H, Maeda K, Wakabayashi H. Impact of geniohyoid and masseter muscle masses on dysphagia after salvage surgery and radiotherapy in head and neck cancer. Sci Rep 2021; 11:2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kunieda K, Fujishima I, Wakabayashi H, et al. Relationship between tongue pressure and pharyngeal function assessed using high-resolution manometry in older dysphagia patients with sarcopenia: a pilot study. Dysphagia 2021; 36:33–40. [DOI] [PubMed] [Google Scholar]
- 40.Sakai K, Nakayama E, Rogus-Pulia N, et al. Submental muscle activity and its role in diagnosing sarcopenic dysphagia. Clin Interv Aging 2020; 15:1991–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakabayashi H, Kishima M, Itoda M, et al. Diagnosis and treatment of sarcopenic dysphagia: a scoping review. Dysphagia 2021; 36:523–531. [DOI] [PubMed] [Google Scholar]
- 42.Maeda K, Murotani K, Kamoshita S, et al. Nutritional management in inpatients with aspiration pneumonia: a cohort medical claims database study. Arch Gerontol Geriatr 2021; 95:104398. [DOI] [PubMed] [Google Scholar]
- 43.Nagano A, Maeda K, Koike M, et al. Effects of physical rehabilitation and nutritional intake management on improvement in tongue strength in sarcopenic patients. Nutrients 2020; 12:3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimizu A, Fujishima I, Maeda K, et al. Nutritional management enhances the recovery of swallowing ability in older patients with sarcopenic dysphagia. Nutrients 2021; 13:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kera T, Kawai H, Hirano H, et al. Definition of respiratory sarcopenia with peak expiratory flow rate. J Am Med Dir Assoc 2019; 20:1021–1025. [DOI] [PubMed] [Google Scholar]
- 46.Deniz O, Coteli S, Karatoprak NB, et al. Diaphragmatic muscle thickness in older people with and without sarcopenia. Aging Clin Exp Res 2021; 33:573–580. [DOI] [PubMed] [Google Scholar]
- 47.Martínez-Arnau FM, Buigues C, Fonfría-Vivas R, Cauli O. Respiratory muscle strengths and their association with lean mass and handgrip strengths in older institutionalized individuals. J Clin Med 2020; 9:2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gielen E, Beckwée D, Delaere A, et al. Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: an umbrella review of systematic reviews and meta-analyses. Nutr Rev 2021; 79:121–147. [DOI] [PubMed] [Google Scholar]
- 49.Lin Z, Zhao Y, Chen Q. Effects of oral administration of beta-hydroxy beta-methylbutyrate on lean body mass in older adults: a systematic review and meta-analysis. Eur Geriatr Med 2021; 12:239–251. [DOI] [PubMed] [Google Scholar]
- 50.Costa Riela NA, Alvim Guimarães MM, Oliveira de Almeida D, Araujo EMQ. Effects of beta-hydroxy-beta-methylbutyrate supplementation on elderly body composition and muscle strength: a review of clinical trials. Ann Nutr Metab 2021; 77:16–22. [DOI] [PubMed] [Google Scholar]