Abstract
Objective.
Advanced robotic lower limb prostheses are mainly controlled autonomously. Although the existing control can assist cyclic movements during locomotion of amputee users, the function of these modern devices is still limited due to the lack of neuromuscular control (i.e. control based on human efferent neural signals from the central nervous system to peripheral muscles for movement production). Neuromuscular control signals can be recorded from muscles, called electromyographic (EMG) or myoelectric signals. In fact, using EMG signals for robotic lower limb prostheses control has been an emerging research topic in the field for the past decade to address novel prosthesis functionality and adaptability to different environments and task contexts. The objective of this paper is to review robotic lower limb Prosthesis control via EMG signals recorded from residual muscles in individuals with lower limb amputations.
Approach.
We performed a literature review on surgical techniques for enhanced EMG interfaces, EMG sensors, decoding algorithms, and control paradigms for robotic lower limb prostheses.
Main results.
This review highlights the promise of EMG control for enabling new functionalities in robotic lower limb prostheses, as well as the existing challenges, knowledge gaps, and opportunities on this research topic from human motor control and clinical practice perspectives.
Significance.
This review may guide the future collaborations among researchers in neuromechanics, neural engineering, assistive technologies, and amputee clinics in order to build and translate true bionic lower limbs to individuals with lower limb amputations for improved motor function.
Keywords: robotic lower limb protheses, neural–machine interface, EMG, gait and balance, human motor control
1. Introduction
A human controlling a prosthetic limb as if it were their own biological limb has fascinated biomedical researchers for many decades [1–6]. At the center of this idea is a direct link between the human nervous system and the prosthesis actuators, allowing for commands from the user to the prosthetic limb. Electromyography (EMG) provides an additional way to decode peripheral efferent signals from muscles in the residual limb [7]. EMG signals are common control signals for powered upper limb prostheses and have been in use for over 50 years [8]. In contrast, EMG control of robotic lower limb prostheses is still in its infancy. This is largely because: (a) motorized, robotic lower limb prostheses have only been practical as devices in the past decade, and (b) autonomous control of robotic lower limb prostheses has been sufficient to support basic locomotive activities in amputee users [9–15].
The time is ripe to develop myoelectric control of lower limb prostheses to maximally restore motor function of individuals with lower limb amputations. The mechatronics of robotic lower limb prostheses have become more mature, practical, and accessible [15–17], yet these modern, robotic devices are still limited in function, partly because the devices are preprogramed autonomous machines [10, 11, 18–22] unable to directly take user input. Current autonomous prosthesis controllers are sufficient to actively assist cyclic stepping motions in predictable environments (e.g. a clean floor in clinics), however they are inadequate to actively assist versatile daily tasks that require coordination with user intent (e.g. anticipatory postural adjustments in standing or walking, performing leisure activities) [23, 24]. They also do not provide adaptation to varying, unconstructed environments and task contexts (e.g. change of load carriage or walking on uneven terrains) [4, 25]. While increasingly complex autonomous control designs are being developed to incrementally address these draw-backs, myoelectric control, on the other hand, can be a simple and viable solution to resolve these limitations because the human motor control system is highly flexible and adaptable to changing tasks and environments.
Publications on EMG control of robotic lower-limb prostheses have started to emerge and accumulate in the last decade [4, 5, 26–29]. Decoding algorithms and control frameworks have significantly advanced since the early foundational EMG control analysis [1, 30, 31]. These pioneering studies explored different EMG decoding algorithms and control frameworks, brought forth novel functionality in robotic prosthetic legs which cannot be easily achieved by autonomous control, and showed feasibility and promise in amputee testing. However, none of these existing methods have been adopted by commercial robotic prostheses so far. As myoelectric control of robotic lower-limb prostheses is a growing topic of interest in the field, and review of the related literature has been very limited, there is a pressing need to summarize existing methods on this topic, understand challenges facing translation to the community, and highlight their potential applications and future directions.
Hence, this paper aims to summarize the literature related to EMG control of robotic lower-limb prostheses and highlight existing challenges, potential solutions, and opportunities related to its widespread clinical implementation. One goal of this review is to emphasize the need for more fundamental research on the neuromechanics of lower-limb amputees using neurally controlled prostheses. A second goal is to highlight the need for innovations in neural–machine interfacing technologies in lower-limb prostheses. Lastly, we hope to inspire more collaborations across disciplines to further our understanding on the potential and limitations of EMG control of robotic lower-limb prostheses, compared to current autonomous control. To address our goals, we first review the different surgical approaches/muscle nerve configurations and how they could influence EMG control. We then summarize the current methods for measuring EMG and the existing EMG control paradigms. Finally, we address current opportunities for EMG control to improve autonomous Prosthesis control. The resulting knowledge may provide a novel control framework for robotic lower-limb prostheses, shared by both autonomy and humans, to maximize the mobility of individuals with lower-limb amputations in the future.
2. Literature review
Considerations of the biological configuration of residual muscles, existing sensor technology and current control strategies will be needed to advance the field of EMG prosthesis control. This section reviews the current state of each of those areas to provide a full perspective of the state of EMG control in lower-limb prosthetics. We start with residual muscle configurations to summarize existing amputation procedures and how they could impact EMG residual muscle signal quality. Section 2.2 reviews current methods for measuring residual muscle EMG inside the prosthetic socket. Section 2.3 summarizes current EMG control paradigms, in which we focus on supervisory control (i.e. hierarchical combination of an EMG decoder for locomotion mode recognition with state-machine-based autonomous control) and direct control (i.e. continuous EMG control of prosthetic joint mechanics). Within each control paradigm, we layout considerations/approaches as well as evaluation methods and reported results. Tables 1 and 2 in the appendix provide additional information about study methods and controller information for the reviewed studies.
2.1. Amputated muscle/nerve configuration
The configuration of the muscle–nerve attachment (i.e. to bone or tendon) in the residual limb determines how existing biological signaling pathways can be used for prosthetic feedback and neuromuscular control [32–34]. Many factors, such as the cause of amputation (e.g. traumatic or dysvascular), residual limb length and shape, and subsequent muscle atrophy, can influence existing number of motor units, proprioceptors, and afferent neurons, which alter muscle fiber function and quality [35–38]. The type of surgical technique used for limb amputation is crucial for preservation of muscle tone and length, motor unit recruitment, and proprioception. For example, residual muscles must be stabilized to either muscle or bone at appropriate tensions [39, 40] because insufficient or too high tension can lead to atrophy, contractures, and/or pain. These resulting issues can affect residual muscle activity [39–41] and the quality of EMG signals in prosthesis control.
Traditional surgical techniques for lower limb amputations have had little evolution and do not consider the neural interface for prosthesis control [42]. The most common surgery for transtibial and transfemoral amputees discards distal tissue around the amputation site and fixes isolated muscle bellies through a combination of myodesis and myoplasty [39, 43, 44]. Myoplasty sews opposing muscle groups together while myodesis attaches muscles directly to bone [39]. The nerves are transected and positioned in soft tissue away from scar tissue, the incision, or areas subject to prosthetic socket irritation, with the goal of minimizing painful neuromas [39]. Residual muscles after traditional amputation surgery can still be activated by the brain and spinal cord, but the EMG patterns during walking are often different from the patterns in physically intact humans [45–47].
Recently, novel surgical techniques have been developed that consider the human–machine interface of a powered lower limb prosthesis. The goal of the surgical techniques is not just to reshape the residual limb, but to improve the neural interface for adaptable, reliable neuromuscular control of lower limb prostheses in dynamic real-world environments.
One surgical technique, the agonist–antagonist myoneural interface (AMI), attempts to use the body’s natural mechanisms for proprioceptive feedback to enhance prosthetic control and embodiment. In the AMI, surgeons reconnect agonist–antagonist residual muscle pairs to restore reciprocal muscle function [48]. When the agonist contracts, the mechanical linkage stretches the antagonist and vice versa. Such reciprocal contractions engage length and force receptors in both muscle-tendon units, resulting in a more natural sensation of position and velocity for improved motor control of residual muscles [32, 48, 49]. One individual who received the AMI procedure produced more isolated contractions of antagonist residual muscles and improved stability in gait-related tasks when using an EMG controlled two-degree-of-freedom prosthetic ankle compared to amputees without the AMI [32]. The individual also exhibited reflexive prosthesis motions indicating a higher level of embodiment. In order to produce this antagonist mechanical linkage for individuals who have already received a ‘traditional’ amputation surgery, a regenerative neural interface has been proposed to implement AMI through the use of targeted muscle reinnervation (TMR) and muscle grafts [50, 51].
TMR is a surgical technique that aims to restore neuromuscular control sources in amputees by transferring residual nerves to muscles that are no longer biomechanically functional [33, 34, 52]. The reinnervated muscles act as a biological amplifier to restore EMG recording sites for the missing joint control [33, 34]. For example, the tibial nerve branch for transfemoral amputees can be connected to the semitendinosis and the common peroneal nerve branch can be inserted into the long head of the biceps femoris [53]. The EMG signals from these reinnervated thigh muscles can convey neuromuscular control signals for the prosthetic ankle joint. Combining peripheral nerve surgery with EMG based control strategies for prosthetics has led to more coordinated control of multi-jointed prosthetic devices. The majority of studies using TMR have been on individuals with upper-limb amputations [33, 54, 55], but there is increasing focus on shifting to individuals with lower-limb amputations [53].
2.2. Neural interfaces
One of the most critical aspects of neural prosthesis control is the accurate and robust sensing of neuromuscular activity (i.e. the control input). The limb–socket interface of lower-limb prostheses, which are subject to weight bearing forces, sweat accumulation, changes in limb volume, make EMG sensing challenging (discussed more in section 3). A majority of existing studies on myoelectric control of lower limb prostheses have used bipolar surface EMG electrodes to record neuromuscular control signals. One major challenge with this approach is the attachment of EMG electrodes within the prosthetic socket or liner for reliable EMG recordings without compromising socket suspension or user comfort. One study developed several socket-EMG interfaces that integrated commercial EMG electrodes on the prosthesis socket directly [56]. Additionally, fluctuations in residual limb volume over time can compromise reliable skin contact with the sensor inside the socket. Recent studies have used low profile, neonatal EMG sensors within the prosthetic socket for successful myoelectric prosthesis ankle control [57, 58]. A novel prosthetic liner with embedded dome electrodes and conductive textile fibers [59] can ease the sensor placement and wire management and yield reliable skin-electrode contact. Furthermore, new flexible, low-profile EMG sensors [60] have the potential to be fabricated directly within the prosthetic liner, ensuring comfort and reducing skin contact problems that arise from limb volume fluctuations.
Another challenge with myoelectric control of lower limb prostheses can be the placement of bipolar EMG electrodes to target specific residual muscles. Although the volume of lower-limb muscles is relatively large compared to those in the upper limbs, identifying specific muscles is often challenged by atrophy of residual musculature and lack of knowledge on amputation procedure. Fite et al used principal component analysis of surface EMG to reduce the effect of variation in measured residual muscle activity caused by differences in sensor placement across days [27]. This provided some success at standardizing the myoelectric signals for prosthetic control.
Implantable EMG sensor interfaces can mitigate limitations accompanying bipolar surface electrodes. Wireless intramuscular EMG sensors have been developed recently to transmit muscle activity signals from residual muscles to the prosthesis without any transcutaneous leads [61–66]. This interface has significant potential to target specific residual muscles not reachable with surface EMG and could be surgically implanted in parallel with other surgical procedures such as osseointegration or nerve reinnervation. These invasive neural interfaces have been, however, primarily tested in upper limb amputees to date. We are aware of only one study that implanted wireless intramuscular EMG sensors in lower-limb amputees for prosthesis control [64]. Another promising technology is high-density, flexible surface EMG. It may provide more information and greater resolution of residual muscle activations for prosthesis control. High-density EMG was first used with amputees to confirm reinnervation of residual muscles [67, 68]. Other studies have used high-density EMG to remove motion artifacts [69, 70] in walking and measure muscular activity from ankle flexors/extensors and invertors/evertors for prosthetic control [71]. High-density EMG shows significant potential for future prosthesis control development through integration of individual motor unit activations with prosthetic control, targeting specific muscle locations easily, and removing artifacts caused by movement.
2.3. EMG control paradigms
Current commercialized robotic prosthetic legs (e.g. PowerKnee™, Össur, Iceland; EmPower, Otto bock, Germany) do not rely on active neuromuscular human input for control but instead use onboard kinetic/kinematic sensing to drive autonomous controllers for pre-programmed activities [72]. These commercial devices employ finite-state machines to adjust knee and/or ankle joint impedance or position the joints based on predefined states such as the gait phase (e.g. swing and stance) and locomotion mode (e.g. stair ascent and level-ground walking) [10, 11, 18]. Transitions between gait phases can be triggered by measurements of intrinsic sensors (e.g. a load cell or motion sensor) in the prosthesis, while transitions between locomotion modes often requires input from the human user (e.g. specific body motions measured by sensors) [14, 73–75]. Existing autonomous control approaches are sufficient to assist amputees walking in well-defined environments, but they are inadequate for unconstrained tasks that require dynamic user intent and/or adaptation with varying environments (e.g. trail hiking, jumping, catching objects). These limitations have sparked interest in the research community to develop neural/EMG control that might improve adaptability and versatility of robotic lower-limb prostheses. There have been two prominent approaches to integrate amputee users’ efferent neural signals (i.e. EMG signals) for lower-limb prosthesis control in the current literature: supervisory EMG control and direct EMG control (figures 1 and 2).
Figure 1.

Supervisory EMG control paradigm for robotic lower-limb prosthesis. In supervisory EMG control, EMG signals and gait events are used to classify the user’s locomotion mode (such as level-ground walking, stair ascent/descent, ramp ascent/descent). The classifier’s decision determines transitions between the predefined finite-states and thus the specified low-level control (e.g. impedance control) for prosthesis operation associated with the identified locomotion mode.
Figure 2.

Direct EMG control paradigm for robotic lower-limb prosthesis. In direct EMG control, the magnitude of of EMG signals recorded from antagonistic residual muscles directly and continuously modulate the prosthesis joint dynamics. Various control laws can be used to continuously map EMG activity to ankle control torque to drive prosthesis dynamics. For example, EMG magnitude of residual ankle antagonistic muscles (u1 and u2) can activate an EMG-driven musculoskeletal model to estimate intended ankle control torque.
2.3.1. Supervisory EMG control
In current commercial robotic lower limb prostheses, transitions between locomotion modes are achieved manually, which is cumbersome. Instead, supervisory EMG control automatically recognizes the user’s locomotion mode by EMG pattern recognition (figure 1). By monitoring EMG patterns, prostheses can hierarchically adjust low-level autonomous control (e.g. finite state machine) to switch control based on recognized locomotion modes [2, 4, 29, 76, 77]. Essentially, supervisory EMG controllers are built upon autonomous locomotion-mode-dependent prosthesis control, where the joint mechanics in each mode are dominated by the low-level autonomous control. In this manner, the supervisory EMG controller acts as a part of a finite-state machine and it adjusts the mechanics of prosthesis joints only at the locomotion mode transitions.
2.3.1.1. Input signals
In past studies, researchers used EMG signals recorded from residual limb muscles as neural inputs for locomotion mode recognition [4, 29, 78–81]. EMG electrodes are typically placed on the residual limb based on intact muscle anatomical location, palpation, and visual inspection of EMG signals [11, 29, 82]. Because EMG pattern recognition classified the locomotion mode based on multi-channel signal pattern, cross-talk in EMG recordings did not significantly influence classification performance. Within the existing studies, as many as nine EMG electrodes on a residual thigh [4] or four electrodes on a residual shank [80] were used. Groups also experimented with augmenting classifier inputs with muscles above the amputation level (e.g. the gluteus maximus on transfemoral amputees or the thigh muscles of transtibial amputees) [4, 83, 84]. However, adding sensors to intact muscles requires additional sensors outside the prosthetic socket, increasing the complexity for daily use and sensor setup. Another group found TMR surgery on a transfemoral amputee enhanced myoelectric control information recorded from reinnervated residual muscles, improving prosthesis control [29, 85]. The participants with TMR surgery had around a 40% error reduction rate during virtual movements compared to the amputee participants without TMR [29]. Furthermore, pattern recognition that combined EMG signals with intrinsic mechanical measures (neuromuscular–mechanical fusion) further improved the accuracy and reliability of locomotion mode recognition [26, 73, 85]. This fusion-based approach outperformed the algorithm that solely used EMG or only used mechanical measurements as system inputs [26]. With this, a source selection study showed EMG signals were essential for accurate prediction of user locomotion mode transitions compared to mechanical measurements alone [78].
2.3.1.2. EMG feature extraction and phase-dependent EMG pattern classification
EMG pattern recognition has been widely used for upper limb prosthesis control [3, 8, 86–89], but adjustments are necessary for lower-limb prosthesis control. For control of upper-limb prosthesis movements (e.g. hand open), the human user must attempt the hand motion and hold the posture of the phantom hand. During this period, the EMG signals are considered to be stationary, i.e. the distribution of the stochastic signals does not change, and therefore the EMG activation pattern is consistent for the classifier to identify the user intended motion for continuous prosthesis control. In contrast, EMG signals in the lower limbs during walking are non-stationary over a full gait cycle. As a result, a different EMG pattern recognition strategy is necessary for lower limb prosthesis control compared to upper limb prosthesis control. If we assume that: (a) gait EMG is quasi-stationary within a gait phase, and (b) EMG patterns recorded from residual muscles are different between locomotion modes, but consistent within the same mode, then a phase-dependent EMG pattern recognition strategy can be implemented. This approach has been enacted with a system consisting of multiple pattern classifiers, each corresponding to a gait phase [4] (figure 1).
In each phase, the pattern recognition includes feature extraction, dimension reduction (optional), pattern classification, and post processing of classification decisions (optional). Feature extraction is an important step for accurate pattern classification. Selected features from the input data sources should maximally extract information that can distinguish between locomotion modes (classes). Focusing on EMG features, time domain (e.g. number of zero crossings, mean absolute value, and slope sign change) [26, 29, 80, 90] and frequency domain (e.g. medium frequency [76], bi-spectrum [77]) features have been used previously. Additionally, adding autoregression coefficients for EMG features can account for potential signal degradation, fatigue, and motion artifacts [29, 73, 91–93]. Groups have also used dimension reduction techniques, such as principal component analysis, to reduce the dimension of feature vectors and prevent model overfitting [79, 83, 92, 94]. Other feature/source reduction methods explored in lower-limb prosthesis control include sequential forward and backward selection and minimum-redundancy-maximum-relevance algorithms [78]. The extracted features were fed to a pattern classifier for locomotion mode recognition. A variety of commonly used classifiers have been used, e.g. artificial neural networks, support vector machine (SVM), linear discriminant analysis (LDA), quadratic discriminant analysis (QDA), and dynamic Bayesian networks (DBNs). DBN can be combined with LDA to provide time history and feed forward information to the classifier [92]. The DBN model predicts the future locomotion model, while LDA labels the previous stride. This structure is especially helpful with EMG inputs because the classifier can re-learn EMG patterns over time or across multiple training sessions. Finally, a post-processing method, such as majority vote [26], has been considered to further reduce classification errors, but increased the system delay for real-time applications.
2.3.1.3. EMG pattern recognition based prosthesis control
In practice, EMG or data fusion-based decoders require model training before applying them to real-time prosthesis control. Model training requires collection of labeled training data (i.e. input data with class labels), followed by establishing the parameters in the classifiers. Collecting enough training data for multiple conditions, such as sit-to-stand, ramps, stairs, and level-ground walking across multiple speeds could take hours, on top of time needed for tuning/customizing prosthesis control parameters for each user [29]. In addition, daily recollection of EMG training data for each individual user is required for reliable performance [29, 78]. A means for efficient and automatic training data collection could minimize users’ time and effort to calibrate the system [95].
During real-time operation, the trained EMG classifier estimates the locomotion mode, which triggers task state transitions in the finite-state machine for robotic leg control (figure 1). One challenge is EMG pattern recognition approaches are sensitive to EMG signal variability caused by disturbances (like motion artifacts and electrode location shifts over time/multiple sessions) or physiological changes (such as muscle fatigue) [80, 93, 96–99], which threatens the reliability of the supervisory EMG control system and user safety. Beyond re-training the pattern classifier, other solutions have been proposed to improve the robustness of locomotion mode recognition system. For example, classifiers with redundant EMG sensors can detect abnormal signals, reject corrupted EMG channels, and only select viable EMG signals and mechanical sensor inputs for robust performance [96]. Adaptive pattern recognition, which can update the parameters in the classifier while using it in real-time, can be another promising solution to allow for robust classifiers better equipped to handle real-world settings [92, 100]. Another challenge for supervisory EMG control is the definition of timing to trigger the switch for low-level prosthesis control mode [101]. Although EMG pattern recognition provides real-time decisions regarding the user’s locomotion mode, the low-level controller parameter only updates at one critical timing that is defined for each type of task transition. For example, Huang et al defined the critical timing for transitions from level-ground walking to stair ascent at the prosthesis foot toe-off before stepping on the staircase to ensure a smooth and safe switch of walking terrain in amputee users [26]. Table 1 in the appendix summarizes existing literature related to EMG-based locomotion mode recognition and supervisory control with detailed approaches used in each study. Note we only included the studies that tested the system on individuals with lower-limb amputations in this table.
2.3.1.4. Performance/evaluation metrics
The performance of supervisory EMG control systems is typically evaluated by classification error/accuracy rate, the confusion matrix during steady state activity, and/or prediction accuracy of task transition and prediction time. The reported accuracy rate ranges from 75% to 99%. Usually task transition can be predicted accurately before the defined critical timings [26, 77, 92, 93]. The most common classification error is between ramps and level-ground walking [29, 92, 102]. However, how these engineering performance metrics influence the amputee user’s locomotion performance is unclear. Zhang et al systematically studied the influence of errors and delays in supervisory EMG control of robotic knee prostheses on human walking stability [101, 103]. The research found that not all errors disturb measured dynamic stability and the user’s perceived walking stability; it depends on the timing and cumulated mechanical work change around the prosthesis knee joint. The group also suggested a range of timing for switching prosthesis control mode that ensures user safety during terrain transitions [101].
Supervisory EMG controllers are inherently autonomous finite-state-machine-based controllers where the low-level autonomous control law dominates the joint mechanics. Even though the EMG signals are included in the control algorithm, the EMG control only functions during locomotion mode (i.e. state) transitions and the approach is inadequate to enable the prosthesis to assist tasks that have not been preprogramed in the low-level control. This approach is also problematic for tasks that do not readily conform to the autonomous finite-state-based controller (e.g. dancing, sports activities).
2.3.2. Direct EMG control
While most lower-limb prosthesis controllers measure prosthesis activity or human muscle activity to inform a state prediction for autonomous control, direct EMG control uses active and continuous input from the human user muscle activity to determine prosthesis dynamics. Thus, direct EMG control mimics the biological neural control pathway in an intact musculoskeletal system. The efferent neural signals (EMG) of the residual agonist–antagonist muscle pairs are used to directly modulate prosthesis joint mechanics (i.e. impedance, angle, and/or torque) (figure 2). The prosthesis joint mechanics can be determined by the human feedforward neural output. This method has shown increasing success in improving various activity performance and postural control in a recent study [58]. Note that direct EMG control here is defined as a myoeletric control method for powered prostheses, which follows antagonistic muscle function around a joint for movement control. Therefore, non-biomimicry mappings of EMG signals to joint mechanics, such as neural networks, are not discussed in this section.
2.3.2.1. EMG decoding methods and control
The inputs of the decoder for direct EMG control are the EMG signals recorded from residual antagonistic muscles. Most commonly, the magnitude of the EMG signals proportionally increases a prosthetic joint parameter [5, 104]. One challenge of this approach is that the antagonist residual muscle sometimes inadvertently contracts as the amputee intends to activate the agonist muscle only, causing a certain level of involuntary co-activation [27, 45]. Involuntary co-activation limits the ability of amputees to access portions of the control input space like isolated joint flexion or extension. To avoid this problem, one approach incorporates an EMG classifier in the direct EMG control scheme to identify the isolated intended joint motion (e.g. flexion vs extension) first [94]. This approach has been evaluated on individuals with amputations in the sitting position, but not during walking. Another solution first transforms the multiple-channel EMG inputs via principal component analysis [27] or non-negative matrix factorization [71] to obtain the isolated ‘motor primitive’ representing the voluntary control for each studied motion. These decoding algorithms may help amputees with involuntary co-activation [45]. However, it remains to be seen whether amputees are capable of generating more isolated residual muscle contractions given sufficient training.
A large portion of work has used impedance control laws, where neuromuscular activity modulate one or multiple joint impedance parameters (i.e. set stiffness, equilibrium position, etc). Initial efforts with impedance control used residual muscle activity to proportionally modulate equilibrium velocity (i.e. rate of change of the equilibrium point) [27, 94]. Subsequent studies incorporated relative co-activity from residual muscles to additionally modulate the set stiffness value in the impedance control law [27, 28, 48, 71]. These studies have shown the ability for amputees to volitionally modulate both stiffness and position of the prosthesis, showing promise for its commercial use. Another common output of the decoder is joint torque. While using the impedance control law described above, Fite et al also incorporated additional torque gain terms for the residual thigh flexors/extensors to proportionally generate control torque for a prosthetic knee [104]. This allowed the direct torque terms to be weighted depending on phase of gait (stance vs. swing) and for the impedance control to be more responsible for limb kinematics during the swing. Using a pneumatically actuated prosthetic ankle, Huang et al used EMG magnitude of the residual gastrocnemius (GAS) to proportionally modulate plantar-flexor torque [5, 105], and this control method has been extended to two agonist–antagonistic residual muscles to control both dorsi- and plantar-flexor torques [57, 58].
Musculoskeletal models are another possible means of EMG decoding method for direct EMG control (figure 2). The EMG magnitude, extracted from EMG signals of residual agonist–antagonist muscles, activates a virtual musculoskeletal model (similar to a human biological joint) to estimate the missing joint mechanics. However, this type of control has only been tested with a virtual ankle joint for transtibial amputees in a sitting position [81] and with able-bodied individuals walking with a bent-knee adapter [106].
2.3.2.2. Activity evaluation
The gold-standard task to evaluate direct EMG control paradigms in the literature has been locomotion. For transfemoral amputees, direct EMG control has been tested for over-ground walking [27, 107] and stair ascent [104]. These preliminary studies with an individual amputee showed potential for amputees to adapt residual thigh muscles to control prosthetic knee torque during cyclic movements like walking. For transtibial amputees, direct EMG control has been tested in over-ground walking using the residual GAS [5, 105]. Amputees successfully adapted their residual muscle activation once given feedback of the prosthetic ankle state. Clites et al incorporated multiple residual muscles into prosthesis control for stair ascent and descent for transtibial amputees [48]. Direct EMG control was also tested in stair ascent/descent and over-ground walking on a transtibial amputee after receiving an AMI surgery [48]. The AMI recipient demonstrated restored reflexive muscle activity and improved prosthesis embodiment, compared to amputees without AMI procedures using direct EMG control.
One of the benefits for direct EMG control is that it is not constrained to rhythmic locomotor tasks. Instead, direct EMG enables prosthesis assistance for a variety of activities in daily living. Unfortunately, there has been limited work to understand amputees’ ability to use direct EMG control for other daily activities. Rogers et al demonstrated the ability for EMG control of a novel powered ankle prosthesis to augment rock climbing in a person with transtibial amputation [108]. One preliminary study investigated direct EMG control use during situations with expected perturbations [57]. This study showed a transtibial amputee could produce anticipatory postural adjustments on an EMG-controlled prosthetic ankle to significantly improve stability after a perturbation. They also studied the ability for a transtibial amputee to control a variety of standing postural control tasks like quiet standing on firm and compliant surfaces as well as load transfer tasks [58]. The results demonstrated the ability for an amputee to significantly improve bilateral EMG activation synchronization and standing postural control with direct EMG control of a prosthesis ankle after extended, guided training with a physical therapist. These aforementioned activities have never been demonstrated by autonomous or EMG supervisory prosthesis control. The existing designs of direct EMG control discussed in this section are summarized in table 2 in the appendix along with critical study components (i.e. measured muscle activity, EMG decoding methods, control parameters, level of amputation, and activity used for evaluation).
3. Current challenges and opportunities
3.1. Questions from motor control perspective and future research directions
The existing literature has shown scattered ideas for using EMG signals to control the robotic lower limb prostheses, from using EMG to switch the prosthesis control mode (supervisory control) to using EMG for continuous control of joint torque (direct control). Despite promising pilot results, the future design of EMG control, in our opinion, should be guided by a systematic framework, built upon theory or mechanistic approaches. We argue that if the goal of EMG control of robotic prostheses is to enable intuitive prosthesis use and bionic function, human motor control theory is a necessary framework to consider.
Internal models have been one of the established theoretical frameworks to interpret human motor control, although the detailed interpretations of the motor representations and applied computational models vary across groups [109–113]. Here, we adopted the framework reported by Frith et al (figure 3), which we use to examine the abnormalities of motor control in different patient populations, including amputees. The key to proficient motor performance is the establishment of a relationship between motor commands and actual system state (i.e. internal models). Note the state hereafter means the human motor control system state as distinguished from the state in a finite-state machine. Awareness of discrepancies among the desired state (related to the goal of the system), actual state, and predicted state enables the update of internal models for improved motor performance via repetitive practice. When biological muscles and skeletons are placed with artificial actuators and machines, the original internal models are disrupted and need to be updated (re-learned) to capture the new relationship between motor commands (including EMG signals of residual muscles) and system state (including state of prosthesis) (figure 3). Hence, we use this framework of human motor control to guide our discussion on open questions and future research opportunities in EMG-based lower limb prosthesis control.
Figure 3.
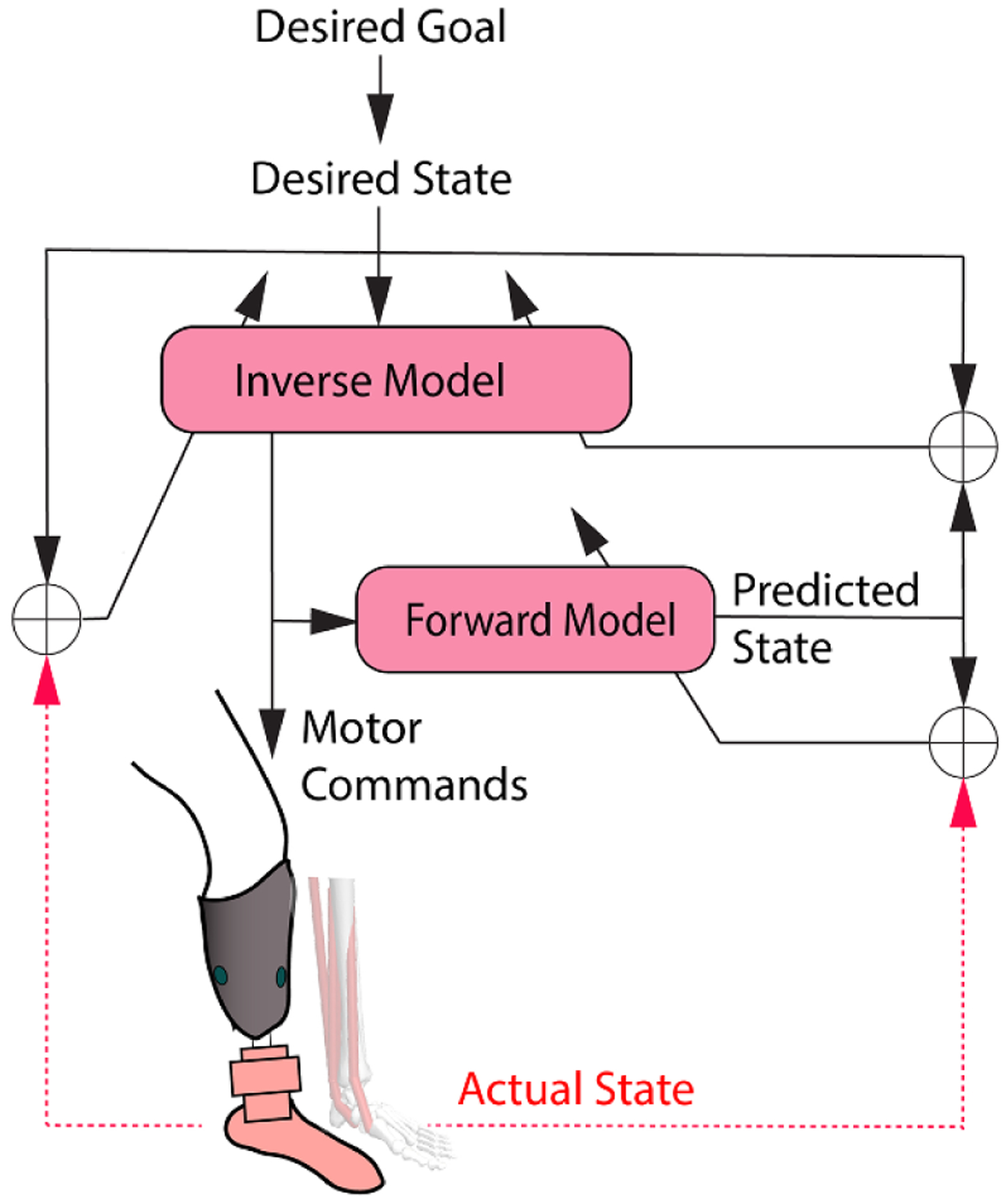
A human motor control framework (adopted from the framework reported in [109] to guide future research in myoelectric control of robotic lower limb prostheses. The actual state of the distal limb is disrupted after limb amputation (red dashed lines). When motor commands (EMG signals of residual muscles) are used to drive a robotic prosthetic limb, humans need to adapt internal model control parameters (the inverse model and forward model) via repetitive motor practice to minimize errors between the desired state and the predicted state, between the desired state and the actual state, and between the predicted state and actual state. This review presents the framework as a means to facilitate future research improving an amputee’s capability to produce appropriate motor commands (residual muscle EMG activity) and control the robotic prosthesis.
3.1.1. Are lower limb amputees capable of producing needed muscle activity (motor commands in figure 3) sufficiently to learn appropriate internal models for prosthesis control?
In order to apply this motor control framework in figure 3, we must begin by characterizing the possible control inputs that can be used to learn new internal models, post-amputation, for prosthesis control. Because EMG signals are the source of control, answering this question is the key to the success of neural prosthesis control. Many existing studies assume activity of residual muscles is similar to activity of intact muscles. However, this assumption is not necessarily true, evidenced by studies that found abnormalities in residual muscle activation patterns during walking [46, 47]. When asked to voluntarily coordinate the activation of residual antagonistic ankle muscles (i.e. the residual tibialis anterior (TA) and GAS), transtibial amputees showed large variation in their capability to reach certain levels of coactivation [45]. One amputee demonstrated an extensive capability in co-activation of the residual TA and GAS, while some amputees could only activate one muscle at a time. In the latter case, designing a direct EMG prosthesis controller requiring flexible coactivation of antagonistic muscles to function is probably not suitable for these individuals. Similarly, limited co-activation patterns among residual muscles may constrain the number of movement classes distinguishable by an EMG pattern recognition-based decoder.
One fundamental unanswered question is what causes the abnormality and large inter-individual variations in activation and coordination of residual muscles in individuals with limb loss. As previously discussed, surgical techniques and altered peripheral nerve/muscle configurations due to limb amputations can be contributors. In addition, the lack of sensory feedback and discontinued use of the amputated limb causes changes in the motor control system and motor representations, which may modify the feedforward motor commands over time. Evidence providing greater insight into these possibilities has been limited. Further research efforts in understanding the alteration of physiology in residual muscles and the peripheral and central nervous system after limb amputation may unveil the cause of abnormality and variations in coactivation of residual muscles. The results would help find solutions (e.g. a new combination of surgical techniques, implants [114], efferent and afferent neural interfaces) to further improve EMG control (enriched neural information, improved reliability, and voluntary controllability) and determine appropriate and practical EMG decoding designs.
3.1.2. Can lower limb amputees adapt and learn how to produce appropriate EMG activation for prosthesis control (i.e. updates of internal models in figure 3)?
EMG decoding methods might influence the capability of amputees to adapt and reliably use EMG controlled robotic prostheses. Previously, there have been two basic concepts in designing EMG decoders. One uses humans’ adaptability to learn how to use an EMG decoder with a biomimetic and straightforward mapping, such as proportional EMG control [5]. In this case, when errors between desired and actual prosthesis states occur (see figure 3), amputees can learn to adjust the activation level of residual muscles to achieve an intended motion. Another design is based on machine learning algorithms with the hope that artificial intelligence can adapt to the human’s existing EMG activation pattern without requirement of human adaptation, such as EMG pattern recognition [4, 26, 80, 84, 91]. However, such a design might limit human adaptation and learning. For example, in the supervised EMG control, when EMG pattern recognition error happens, users may not even sense the error between desired and actual state of the prosthesis as observed in our previous study [103]. Even if the users can sense the error, how to modify residual muscles’ activity to correct errors is not straightforward to the users because the mapping from residual muscle pattern to movement classes is a black box. Determining appropriate characteristics in EMG decoder design (e.g. continuous vs discrete, black box vs white box mapping) that enable human adaptation will be important for future prosthesis technology. It may even lead to the merging of the two design concepts, leveraging both machine adaptation and human adaptation for faster and more robust EMG prosthesis control.
To our knowledge, in-depth studies examining the systematic training of lower limb amputees in using EMG controlled lower limb prostheses are missing from the literature. These types of studies are needed to understand the potential and limits of EMG control for restoring the motor function of individuals with lower-limb amputations. Collaborations between researchers in physical therapy and engineering are needed to develop effective training methods and protocols that enhance human adaptation in using EMG controlled prostheses for activities of daily living. This line of research could also open many exciting opportunities to address questions related to amputee motor control and learning in gait and posture. For example, exploring adaptations in muscle activation patterns after training in using EMG prosthesis control may reveal neuroplasticity of motor control mechanisms in amputees. Human motor control models [110, 113, 115–117] may explain variations of training effects across individuals and identify potential factors (such as physiological constraints or peripheral nerve injuries) that limit adaptability in using EMG controlled lower limb prostheses. These factors can be also used to predict an individual amputee’s capability for using an EMG controlled prosthesis for improved motor function in the future.
3.1.3. Does EMG control of robotic prosthetic legs increase mental load?
Walking, the most common daily activity involving lower limbs, requires little cognitive effort in able-bodied adults [118]. The question often facing researchers, when designing EMG controlled robotic prosthetic legs, is whether the amputee user has to ‘think’ about how to activate residual muscles and ‘pay attention’ to prosthesis joint motion at all times, which is undesirable because it increases the user’s cognitive load. Additional mental load could be detrimental to postural stability and balance confidence in walking [119, 120], especially for individuals with lower limb amputations since they have already reported the need to ‘concentrate on every step’ [119] without neuromuscular control.
Though cognitive load has been quantified for the lower limb amputee population [121], we are unaware of any research quantifying effects of lower-limb prosthesis control approaches (particularly EMG control approaches) on cognitive load. Supervisory EMG control avoids the need for continuous neuromuscular control [4, 26, 29] and may have advantages in reducing cognitive load. The EMG decoder is discrete and only acts during task mode transitions. Therefore, it does not impose additional mental load to amputees in using neuromuscular control most of the time in walking. For direct EMG control, extra mental processes while learning how to use EMG to operate a prosthesis joint during task performance are initially needed [5, 58, 107]. However, mental load may reduce after training. In addition, as direct EMG prosthesis control mimics human neuromuscular control mechanisms for the biological limb, we postulate that long-term use of direct EMG control may restore original motor pathways for missing limb control and normalize the mental workload needed in lower limb amputees in walking. Testing this hypothesis would enhance our understanding of how training alters human–machine performance in the long run. To achieve this aim, future research needs to identify methodologies and novel technologies (e.g. mobile EEG [122, 123]) that can quantify human cognitive load in locomotion beyond traditional dual task paradigms [124] or questionnaires [125].
3.1.4. Does restoration of haptics and proprioception of prosthetic limbs further assist feedforward neuromuscular control of robotic legs?
This question is motivated by the human motor control framework in figure 3, in which the feedback of actual limb state is necessary but is missing in individuals with lower-limb amputations. In general, the actual lower-limb state is fed back via haptics sensation in the foot and proprioception in lower limbs. Therefore, we discuss these sensory modality individually.
Haptic sensation is important for humans to interact with environments, such as object manipulation via hands [126], identifying terrain type [127], and proper foot placement [128]. For individuals with lower-limb amputations, haptic sensation is reduced with current prosthesis technology. Although haptic sensation of prosthesis foot contact can be received via the residual limb within the socket interface or residual bones via osseointegration [129], the sensation lacks spatial resolution to directly map plantar foot contact areas with the ground. Recent technologies in afferent nerve stimulation [130–132] aim to evoke haptic sensation of the missing feet in individuals with lower limb amputations. A case study has shown promising preliminary results in improving gait stability, energetic efficiency, and cognitive load even with a passive prosthesis [131]. Coupling novel afferent interfaces with feedforward neuromuscular control for closed-loop prosthesis operation has not been fully demonstrated yet. Understanding the effects of haptic sensation of a prosthetic foot on the ability of amputees to coordinate residual muscle activity for EMG prosthesis control is an exciting area for future work.
Proprioception (feedback of joint position, muscle force, and movement) also plays a critical role in human movement control. Artificial afferent nerve stimulations or targeted sensory reinnervation [133] seldomly evoke proprioceptive sensations. The innovative AMI procedure (discussed previously) combined with muscle stimulation has been the most promising method to evoke proprioception so far [48]. A patient case study has shown more normative activity (reduced coactivation and tonic activation) of residual muscles in a transtibial amputee with AMI surgery, partially due to increased proprioception.
For individuals who have no access to the AMI procedure, can they be aware of and predict prosthesis motion? Based on the human motor control framework (figure 3), training of amputees in direct EMG control of a prosthesis itself may aid the sense of prosthesis motion. This is because estimation of the actual motor system state (such as limb movement and position) depends not only on the afferent feedback, but also from the stream of efferent movement commands (efference copy) to the residual muscles (figure 3) [109, 112]. This may explain why amputees can still sense the ‘movement’ the missing limb, even though the peripheral receptors no longer exist. The perception of missing joint movement gradually diminishes due to internal model updates. When the function of efferent motor commands (EMG signals) is restored for prosthetic joint control, through practice, the internal models could be re-built. Therefore, we postulate that through direct EMG prosthesis control and sufficient training of amputees in learning internal models (relationship between EMG commands and action of prosthesis), perhaps amputees could regain awareness and prediction of prosthetic limb movement/position, even without artificial proprioceptive feedback or visual feedback of the prosthetic limb. Additional research is needed to test this hypothesis.
3.2. Current challenges from a clinical practice perspective and potential solutions
Since prostheses are assistive devices for daily use, we also want to highlight the research and technology development needed towards making EMG control clinically viable.
3.2.1. Are EMG signals too noisy for daily practice?
Physical disturbances within the prosthetic socket can affect the interface between the skin and the surface EMG electrodes (such as humidity, shift of electrode contact, motions and collision). These disturbances elicit variations in time and frequency components of EMG recordings, interfering with EMG control [134, 135]. One study reported that socket pressure was highly associated with EMG activity of residual muscles [136]. Unfortunately, systematic investigation of the key factors of socket design and fit that affect EMG signals of residual limb muscles is lacking. Maintenance of the EMG interface is critical for successful application of EMG-based prosthesis control in everyday settings. Implantable EMG sensors [61, 63, 137], flexible and stretchable EMG sensors and sensor arrays [69, 138], and new prosthesis attachment methods (e.g. osseointegration [129]), provide potential to eliminate complications caused by physical disturbances of EMG interfaces within a socket and promote EMG-based prosthetic control for daily use. In fact, some exciting feasibility study of implantable EMG sensors for lower limb prosthesis control has already been carried out on a lower limb amputee [64], which shows improve robustness in EMG recordings for real-time prosthesis control compared to surface EMG recordings.
EMG signals are random processes often deemed noisy and unreliable for prosthesis control. We argue that the reliability of EMG decoders depends on what and how information in the EMG signals is extracted. As reviewed, information of EMG signals used for prosthesis control has been extracted by various features (e.g. mean absolute value, number of zero crossings, median frequency of the power spectrum density), estimated from the signals in a time window. Some features are sensitive to the aforementioned physical or physiological disturbances, while other features can be more resilient to these disturbances [99]. Identifying reliable EMG features more responsive to user movement intent and less sensitive to noise and disturbances can further improve the robustness of EMG decoders. One promising feature is the firing rate of motor units captured by high-density surface EMG recordings [139, 140]. This feature counts the number of motor unit action potentials per time bin, which is less influenced by signal noises or magnitude and frequency drift. It is a promising method to address the reliability of EMG decoding methods. It is not hard to imagine that a soft, high-density EMG grid could be built into the prosthesis liner for prosthesis control in the future. Another potential method is deep learning, which can automatically learn features for accurate and reliable classification. This method has been explored in EMG pattern recognition for upper limb motion classification recently [141], and can be extended into EMG signals in lower limbs. In addition, various random signal processing techniques and fault-tolerance mechanisms [134] can be explored in the future to address the robustness of EMG control of robotic prostheses.
3.2.2. Are EMG controlled prostheses safe to use?
The failure of lower-limb prosthesis control might lead to falls and injuries in lower-limb amputees. Understanding the amputee user’s safety when relying on an EMG controlled prosthesis is essential to evaluate the device’s practical value. Previous studies have investigated effects of EMG pattern recognition errors selecting locomotion mode [101, 103] and identified a set of critical pattern recognition decision errors that disturb the user’s walking stability and perceived stability. Future work should focus on how to eliminate these critical errors to ensure the user’s balance and safety. For continuous, direct EMG control, the error correction and tolerance become the responsibility of the human motor control system [142]. Future research may focus on how to train individuals with lower-limb amputations to calibrate the forward model (figure 3) for error tolerance and correction when performing tasks with direct EMG control of a robotic prosthetic leg.
3.2.3. What are the benefits and limitations of EMG prosthesis control, compared to existing autonomous prosthesis control, for daily prosthesis use?
Understanding the benefits and limitations of EMG prosthesis control, compared to the existing autonomous approach, is necessary for future clinical translation. In terms of function, robotic machines are good at tasks in known contexts with precision and fast feedback control rates but lack adaptability and flexibility. On the other hand, human motor control systems are slow and have large variation in movement output but are highly adaptable to deal with varied environments and versatile activities. This view is applicable to autonomous control versus EMG-based control of robotic lower limb prostheses. Existing autonomous controllers are very reliable for biomechanically well-established, stereotypical tasks (such as walking). However, they are inadequate to handle unstructured daily environments and activities that cannot be easily predicted or pre-programmed. On the other hand, EMG control of lower-limb prostheses enables versatile prosthesis function adaptive to various contexts, but control can be limited by the lack of accuracy and capability of amputees to produce needed EMG control signals. One way to explore the benefits and limits of the two approaches in the future is via task allocation [143]. We can classify whether a task can be achieved by only one method or both autonomous and human neuromuscular control; in the latter case, additional research should compare the two methods on system design complexity and the user’s task performance. The gained knowledge could guide future design of robotic lower-limb prosthesis control, shared by both autonomous and human motor controllers.
In terms of utility and user acceptance, we should also consider evaluating both autonomous and EMG control methods by measuring the user’s learning rate, cognitive workload, trust in the robotic prosthesis, satisfaction, and sense of embodiment. To our knowledge, these user-centered evaluations have not been systematically quantified and reported, even on existing autonomous prosthesis control schemes. Collaboration with researchers in cognitive ergonomics and clinical outcome measurements are needed to evaluate the user’s acceptance and utility of various controllers for robotic lower limb prostheses.
While the challenges and opportunities we have discussed here are important, this is not an exhaustive list. Several factors, such as device cost, limited prosthesis reimbursement, and power requirements stand as challenges in the path translating new prosthesis technology to end users. The introduction of EMG control will likely face these challenges as well. However, the benefits of EMG control to prosthesis function, reviewed here, demonstrates the value of continued investment in its development. Further, these challenges likely do not all need to be addressed before this control can begin to be introduced in new lower-limb prosthetic technology.
Acknowledgments
We thank Dr I-Chieh Lee for her feedback on this paper. We thank Noah Rubin for editing the paper. We thank N I H (EB024570, HD043730, HD101285) and N S F (1954587, 1926998, 1835317) for supporting our research.
Appendix
Table 1.
Supervisory EMG control review summary.
| Authora | Muscles recordedb | Classifier typec | EMG features | Windowing/prediction time | Training | Control type | Amputation type | Activity |
|---|---|---|---|---|---|---|---|---|
| Au et al [81] | TA, GAS, SOL | Neural network | Pre-processed EMG | Predictions at 0.5 Hz | Back propagation method with pre-processed EMG as input | None; offline classification only | Transtibial | Trajectory tracking sitting |
| Jin et al [76] | ADDL, TFL, RF, VL, VM, BF, SM, ST | Distribution of different muscle features | Mean absolute value, waveform length, mean square value, zero tangent number, median power frequency | Entire gait cycle | 3–5 times along walkway for each condition | None | Transfemoral | Level-ground walking at varying speeds, stairs, and ramps |
| Huang et al [4] | GME , GMA , SAR, RF, VL, VM, GRA, BFL, BFS, SEM, ADM | LDA and neural network | 3rd order autoregression coefficients, mean absolute value, zero crossings, waveform length, number of slope sign changes and root mean square | Range from 50 to 150 ms EMG window, 200 ms phase window | Ten walking trials of all activities with own prosthesis | None; offline classification only | Transfemoral | Level-ground walking, stepping over and obstacle, stairs, ipsilateral and contralateral turning, and standing |
| Au et al [11] | GASL, GASM, TA | Neural network | Variance of EMG signals | 100 ms EMG window | Matching imagined ankle position to virtual ankle | Impedance control | Transtibial (bilateral) | Level-ground walking, stair descents |
| Ha et al [94] | Quadriceps, hamstring | LDA, QDA | Not provided | Not provided | 100 s of knee flexion/extension visualizations | Online impedance control (prosthesis next to participant) | Transfemoral | Virtual tracking tasks (sitting) |
| Huang et al [26] | GME, GMA, SAR, RF, VL, VM, ST, GRA, BFL, BFS, ADM | SVM, LDA | Zero crossings, signal direction change, mean absolute value, waveform length | 150 ms sliding EMG window prediction | 15 times each activity | None; online classification | Transfemoral | Walking, stair/ramp ascent and descent, stepping over obstacles (and transitions) |
| Simon et al [144] | SM, SAR, TFL, ADM, GRA, RF, VL, BFL | Two LDA classifiers | Mean absolute value, zero crossings, waveform length, slope sign change number, five-count majority vote | 250 ms overlapping | Ten sit-to-stand trials | Impedance control | Transfemoral | Sitting down, walking and transition between them, repositioning prosthesis while sitting |
| Hargrove et al [29] | Two reinnervated muscle segments, proximal BF, RF, VL, VM, SAR, GRA, ADM, TFL | DBN | Not provided | Not provided | Offline, 20 reps of locomotor circuit | Impedance control | Transfemoral | Level-ground walking, ramps, stairs and outside stairs |
| Young et al [145] | ST, BF, TFL, VM, SAR, ADM, GRA | Two LDA models | Mean absolute value, waveform length, zero crossings, slope sign change, 1st two coefficients of 3rd order autoregressive model | 300 ms before heel contact and toe off | Offline, 20 reps of two locomotor circuits, train classifier on three subjects test 1 as novel user. Then add 5–10 min of level-ground walking for novel user to train classifier. | None; offline classification | Transfemoral | Level-ground walking, ramps, stairs |
| Zhang et al [78] | RF, VL, VM, TFL, BFL, BFS, ST, ADM | SVM | Zero crossings, slope change number, mean absolute value, waveform length | EMG window 150 ms decision time between 45 and 28 ms | Offline training, at least five reps of 30 s of data per condition | None; online classification | Transfemoral | Ramp/stair ascent, descent, level-ground walking, sitting, standing |
| Tkach et al [91] | TA, PL, GASL, GASM | LDA | Mean absolute value, zero crossings, slope sign change, and waveform length | 250 ms window | 12 trials of each ambulation mode (stairs and ramps), and 12 trials of level-ground walking | None; offline classification only | Transtibial | Level-ground walking, stairs, ramps, and transitions |
| Tkach et al [84] | TA, PL, GASL, GASM, VM, VL, RF, BF | LDA | Six coefficients from 6th order autoregressive model, mean absolute value, zero crossings, slope sign change, waveform length | 250 ms prediction window 50 ms overlap | 18 s for data for each movement class (one-DOF up to three-DOF, including no movement flexion, rotation, in/eversion) | None; Offline classification only | Transtibial | Virtual tasks for varying DOF ankle movements (rotation, flexion, in/eversion) |
| Hargrove et al [146] | ST, SAR, TFL, ADM, GRA, VM, RF, VL, BFL | LDA | Mean absolute value, zero crossings, slope sign change, waveform length | 250 ms window, decisions every 50 ms | EMG for virtual cases, four reps of 3 s for each movement, no feedback provided | Online impedance control and virtual avatar feedback | Transfemoral | Knee flexion/extension, ankle plantar flexion/dorsiflexion,internal/external tibial/femoral rotation, relaxation |
| Miller et al [80] | TA, GASM, VL, BF | LDA and SVM | Mean absolute value, variance, wavelength, number slope sign changes, zero crossings | Three subwindows with varying times based on heel strike and toe off | Six trials each activity, additional trials for different walking speeds | None; offline classification only | Transtibial | Level-ground walking three speeds, ramp/stair ascent/descent |
| Du et al [97] | SAR, RF, VL, VM, GRA, BFL, ST, BFS, | Adaptive algorithm: EBA and learning from test data | Absolute value, slope sign changes, waveform length, zero crossings | 160ms sliding window | 15–10 trials of each activity | None; offline classification only | Transfemoral | Level-ground walking, ramps, stairs and their transitions |
| Zhang et al [147] | RF, VL, VM, BFL, SAR, ST, ADM | Not provided | Not provided | Not provided | Offline training with ten reps of walking level-ground and ramp course | Impedance control | Transfemoral | Standing, level-ground walking ramp ascent/descent |
| Young et al [148] | ST, BF, TFL, RF, VL, VM, SAR, ADM, GRA | DBN | Mean absolute value, waveform length, zero crossings, slope sign change, two autoregressive coefficients | Tested 0–450 ms windows with 20 ms sliding window | Offline training with 20 reps of locomotor circuit | None; offline classification | Transfemoral | Level-ground walking, ramps, stairs |
| Hargrove et al [85] | ST, BF, TFL, RF, VL, VM, SAR, ADM, GRA | LDA + DBN | Not provided | Not provided | Offline training, 20 reps of locomotor circuit, level-ground walking at variable speeds, stopping/starting/turning | Impedance control | Transfemoral | Walking, stairs, ramps |
| Spanias et al [149] | ST, ADM, TFL, RF | DBN | Mean absolute value, waveform length, zero crossings, slope sign changes, six autoregressive coefficients | 300 ms window | 20 reps of locomotion circuit | None; offline classification only | Transfemoral | Level-ground walking, ramp/straight ascent/descent, turn around |
| Spanias et al [102] | ST, BF, TFL, RF, VL, VM, SAR, ADM, GRA | LDA with log-likelihood | Mean absolute value, waveform length, zero crossings, slope sign change, 1st two coefficients of 3rd order autoregressive model | 300 ms before toe off and heel strike | Offline training with 20 reps of locomotor circuit | Impedance control | Transfemoral | Level-ground walking, ramps, stairs |
| Liu et al [100] | RF, VL, VM, BFL, SM, TFL, BFS | EBA, TSVM | Absolute value, signal length, slope sign changes, zero crossings | 160 ms window avg processing time 45 ms | Four 1 min trials of each locomotion mode collected in separate training session | EBA online impedance control | Transfemoral | Level-ground walking, ramps, stairs, and their transitions |
| Spanias et al [92] | Pairs of electrodes over RF, TFL, ST, ADM | Eight DBN classifiers + LDA | Mean absolute value, waveform length, zero crossings, slope sign changes, six autoregressive coefficients from 6th order model PCA and ULDA to features totals | Transition modes 300 ms (210 ms before gait event and 90 ms after gait event) | Offline training with 1st session data across locomotor modes. Online training 2nd session through forward prediction. | Impedance control | Transfemoral | Level-ground walking, ramps, stairs over multiple days |
| Hussain et al [77] | GM, GASL, GASM, TFL, RF, VL, VM, BFL, SOL, TA | SVM and LDA | Bispectrum for high order frequency spectrum/non-Gaussian info, RMS, zero crossings, histogram, integrated EMG, sum of square integral, waveform length, mean/median frequency, autoregression, and reduced with PCA | Non-overlapping 150 ms-300 ms windows | Offline training five trials for each walking mode | None; offline classification only | Transtibial and transfemoral | Slow, steady state, and fast walking, ramps |
Authors are listed in order of year published.
Muscle abbreviations: adductor magnus (ADM), adductor longus (ADDL), biceps femoris (BF), biceps femoris long head (BFL), biceps femoris short head (BFS), extensor digitorum brevis (EDB), gastrocnemius (GAS), gastrocnemius medialis (GASM), gastrocnemius lateralis (GASL), gluteus maximus (GMA), gluteus medius (GME), gracilis (GRA), peroneus longus (PL), rectus femoris (RF), sartorius (SAR), semimembranosus (SM), semitendinosus (ST), soleus (SOL), tensor fasciae latae (TFL), tibialis anterior (TA), vastus lateralis (VL), vastus medialis (VM).
Classifier abbreviations: support vector machine (SVM), neural network (NN), linear discriminant analysis (LDA), quadratic descriminant analysis (QDA), dynamic Bayesian network (DBN), entropy-based adaption (EBA), transductive support vector machine (TSVM).
Table 2.
Direct EMG control review summary.
| Authora | Muscleb | EMG decoding method | Modulated control parameters | Amputation type | Activity |
|---|---|---|---|---|---|
| Ha et al [94] | Quadriceps, hamstring (unspecified) | Envelope (2 Hz cutoff), 20% MVC threshold QDA | Reference angular velocity | Transfemoral | Virtual tracking task (sitting) |
| Hoover et al [107] | VL, VM, RF, BF | Envelope (5–10 Hz cutoff). | Flexion/extension torque | Transfemoral | Level-ground walking |
| Hoover et al [104] | BF, RF, ST, VL, VM | Envelope (2.5 Hz cutoff) | Flexion/extension torque | Transfemoral | Stair ascent |
| Dawley et al [27] | Quadriceps, hamstring, (unspecified) | Initial processing not described, principal component analysis (flexion/extension) | Reference angular velocity, joint stiffness | Transfemoral | Level-ground walking |
| Wang et al [28] | GAS (unspecified head) | Low-pass filtered (15 Hz), rectified envelope (200 ms moving average window) | Plantar flexor torque gain (push-off only) | Transtibial | Level-ground walking |
| Alcaide-Aguirre et al [150] | TA | Envelope (10 Hz cutoff) | Virtual object acceleration | Transtibial | Virtual tracking task (sitting) |
| Chen et al [151] | TA, GAS (unspecified head) | Envelope (2.5 Hz cutoff), PCA (flexion/extension) | Reference angular velocity, joint stiffness | Transtibial | Virtual target hitting (sitting) |
| Huang et al [105] | GASL | Envelope (2 Hz cutoff) | Pneumatic artificial muscle force | Transtibial | Level-ground walking |
| Huang et al [5] | GASM or GASL | Envelope (2 Hz cutoff) | Pneumatic artificial muscle force | Transtibial | Level-ground walking |
| Huang et al [152] | TA, GASM or GASL | Envelope (2 Hz cutoff) | Virtual object position | Transtibial | Virtual ballistic target hitting (sitting) |
| Clites et al [48] | TA, GASL, TP, PL | Envelope (100 ms moving average window) | Flexion/extension torque | Transtibial | Virtual target hitting, stair ascent/descent, obstacle walking |
| Fleming et al [23] | TA, GASL | Envelope (2 Hz cutoff) | Virtual spring stiffness | Transtibial | Virtual balancing inverted pendulum (sitting) |
| Huang et al [45] | TA, GASL | Envelope (2 Hz cutoff) | Virtual cursor position | Transtibial | Virtual control input space filling (sitting) |
| Dimitrov et al [71] | TA, GASM, GASL | Envelope (5 Hz cutoff), non-negative matrix factorization (125 ms windows) | Equilibrium angle, joint stiffness | Transtibial | Target hitting (standing), walking (with passive device) |
| Fleming et al [58] | TA, GASL | Envelope (2 Hz cutoff) | Pneumatic artificial muscle force | Transtibial | Quiet standing (vision, no vision, foam and firm surfaces), Sit-to-stand, load transfer. |
Authors are listed in order of year published.
Muscle abbreviations: biceps femoris (BF), gastrocnemius (GAS), gastrocnemius medialis (GASM), gastrocnemius lateralis (GASL), peroneus longus (PL), rectus femoris (RF), semitendinosus (ST), soleus (SOL), tibialis anterior (TA), vastus lateralis (VL), vastus medialis (VM), tibialis posterior (TP).
Data availability statement
No new data were created or analyzed in this study.
References
- [1].Horn G 1972. Electro-control: an EMG-controlled A/K prosthesis Med. Biol. Eng 10 61–73 [DOI] [PubMed] [Google Scholar]
- [2].Peeraer L, Aeyels B and van der perre G 1990. Development of EMG-based mode and intent recognition algorithms for a computer-controlled above-knee prosthesis J. Biomed. Eng 12 178–82 [DOI] [PubMed] [Google Scholar]
- [3].Englehart K and Hudgins B 2003. A robust, real-time control scheme for multifunction myoelectric control IEEE Trans. Biomed. Eng 50 848–54 [DOI] [PubMed] [Google Scholar]
- [4].Huang H, Kuiken TA and Lipschutz RD 2009. A strategy for identifying locomotion modes using surface electromyography IEEE Trans. Biomed. Eng 56 65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Huang S, Wensman JP and Ferris DP 2015. Locomotor adaptation by transtibial amputees walking with an experimental powered prosthesis under continuous myoelectric control IEEE Trans. Neural Syst. Rehabil. Eng 24 573–81 [DOI] [PubMed] [Google Scholar]
- [6].Ferris DP and Schlink BR 2017. Robotic devices to enhance human movement performance Kinesiol. Rev 6 70–77 [Google Scholar]
- [7].Merletti R and Parker PJ 2004. Electromyography: Physiology, Engineering, and Non-Invasive Applications (New York: Wiley; ) [Google Scholar]
- [8].Scheme E and Englehart K 2011. Electromyogram pattern recognition for control of powered upper-limb prostheses: state of the art and challenges for clinical use J. Rehabil. Res. Dev 48 643. [DOI] [PubMed] [Google Scholar]
- [9].Martinez-Villalpando EC and Herr H 2009. Agonist-antagonist active knee prosthesis: a preliminary study in level-ground walking J. Rehabil. Res. Dev 46 361. [PubMed] [Google Scholar]
- [10].Sup F, Bohara A and Goldfarb M 2008. Design and control of a powered transfemoral prosthesis Int. J. Robot. Res 27 263–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Au S, Berniker M and Herr H 2008. Powered ankle-foot prosthesis to assist level-ground and stair-descent gaits Neural Netw. 21 654–66 [DOI] [PubMed] [Google Scholar]
- [12].Lambrecht B and Kazerooni H 2009. Design of a semi-active knee prosthesis IEEE Int. Conf. on Robotics and Automation (Kobe, Japan, 12–17 May) 639–45 [Google Scholar]
- [13].Hitt J, Bellman R, Holgate M, Sugar T and Hollander K 2007. The sparky (spring ankle with regenerative kinetics) project: design and analysis of a robotic transtibial prosthesis with regenerative kinetics ASME 2007 Int. Design Engineering Technical Conf. and Computers and Information in Engineering Conf. (Las Vagas, NV) 1587–96 [Google Scholar]
- [14].Sup F, Varol HA, Mitchell J, Withrow TJ and Goldfarb M 2009. Preliminary evaluations of a self-contained anthropomorphic transfemoral prosthesis IEEE ASME Trans. Mechatron 14 667–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Azocar AF, Mooney LM, Hargrove LJ and Rouse EJ 2018. Design and characterization of an open-source robotic leg prosthesis 2018 7th IEEE Int. Conf. on Biomedical Robotics and Biomechatronics (Biorob) Enschede, Netherlands (IEEE) pp 111–8 [Google Scholar]
- [16].Lenzi T, Cempini M, Hargrove L and Kuiken T 2018. Design, development, and testing of a lightweight hybrid robotic knee prosthesis Int. J. Robot. Res 37 953–76 [Google Scholar]
- [17].Elery T, Rezazadeh S, Nesler C, Doan J, Zhu H and Gregg RD 2018. Design and benchtop validation of a powered knee-ankle prosthesis with high-torque, low-impedance actuators 2018 IEEE Int. Conf. on Robotics and Automation (ICRA) Brisbane, QLD, Australia (IEEE) pp 2788–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu M, Zhang F, Datseris P and Huang H 2014. Improving finite state impedance control of active transfemoral prostheses using Dempster–Shafer state transition rules J. Intell. Robot. Syst 76 461–74 [Google Scholar]
- [19].Lawson BE, Varol HA, Huff A, Erdemir E and Goldfarb M 2013. Control of stair ascent and descent with a powered transfemoral prosthesis IEEE Trans. Neural Syst. Rehabil. Eng 21 466–73 [DOI] [PubMed] [Google Scholar]
- [20].Zheng H and Shen X 2015. Design and control of a pneumatically actuated transtibial prosthesis J. Bionic Eng 12 217–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Elery T, Rezazadeh S, Nesler C and Gregg RD 2020. Design and validation of a powered knee-ankle prosthesis with high-torque, low-impedance actuators IEEE Trans. Robot 36 1649–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wen Y, Si J, Brandt A, Gao X and Huang HH 2020. Online reinforcement learning control for the personalization of a robotic knee prosthesis IEEE Trans. Cybern 50 2346–56 [DOI] [PubMed] [Google Scholar]
- [23].Fleming A, Huang S and Huang H 2019. Proportional myoelectric control of a virtual inverted pendulum using residual antagonistic muscles: toward voluntary postural control IEEE Trans. Neural Syst. Rehabil. Eng 27 1473–82 [DOI] [PubMed] [Google Scholar]
- [24].Legro MW, Reiber GE, Czerniecki JM and Sangeorzan BJ 2001. Recreational activities of lower-limb amputees with prostheses J. Rehabil. Res. Dev 38 319–26 [PubMed] [Google Scholar]
- [25].Brandt A, Wen Y, Liu M, Stallings J and Huang HH 2017. Interactions between transfemoral amputees and a powered knee prosthesis during load carriage Sci. Rep 7 14480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Huang H, Zhang F, Hargrove LJ, Dou Z, Rogers DR and Englehart KB 2011. Continuous locomotion-mode identification for prosthetic legs based on neuromuscular–mechanical fusion IEEE Trans. Biomed. Eng 58 2867–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dawley JA, Fite KB and Fulk GD 2013. EMG control of a bionic knee prosthesis: exploiting muscle co-contractions for improved locomotor function 2013 IEEE 13th Int. Conf. on Rehabilitation Robotics (ICORR) Seattle, WA, USA (IEEE) pp 1–6 [DOI] [PubMed] [Google Scholar]
- [28].Wang J, Kannape OA and Herr HM 2013. Proportional EMG control of ankle plantar flexion in a powered transtibial prosthesis 2013 IEEE 13th Int. Conf. on Rehabilitation Robotics (ICORR) Seattle, WA, USA (IEEE) pp 1–5 [DOI] [PubMed] [Google Scholar]
- [29].Hargrove LJ, Simon AM, Young AJ, Lipschutz RD, Finucane SB, Smith DG and Kuiken TA 2013. Robotic leg control with EMG decoding in an amputee with nerve transfers New Engl. J. Med 369 1237–42 [DOI] [PubMed] [Google Scholar]
- [30].Donath M. Research Thesis. Massachusetts Institute of Technology; 1974. Proportional EMG Control for Above Knee Pros-Theses. [Google Scholar]
- [31].Triolo RJ, Nash D and Moskowitz GD 1988. The identification of time series models of lower extremity EMG for the control of prostheses using box-Jenkins criteria IEEE Trans. Biomed. Eng 35 584–94 [DOI] [PubMed] [Google Scholar]
- [32].Clites TR, Herr HM, Srinivasan SS, Zorzos AN and Carty MJ 2018. The Ewing amputation: the first human implementation of the agonist–antagonist myoneural interface Plast. Reconstr. Surg. Glob. Open 6 e1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kuiken TA et al. 2009. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms JAMA 301 619–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Souza JM, Fey NP, Cheesborough JE, Agnew SP, Hargrove LJ and Dumanian GA 2014. Advances in transfemoral amputee rehabilitation: early experience with targeted muscle reinnervation Curr. Surg. Rep 2 51 [Google Scholar]
- [35].Tintle LSM, Keeling CJJ, Shawen LSB, Forsberg LJA and Potter MBK 2010. Traumatic and trauma-related amputations: part I: general principles and lower-extremity amputations JBJS 92 2852–68 [DOI] [PubMed] [Google Scholar]
- [36].Isakov E, Burger H, Gregorič M and Mariňccek Č 1996. Stump length as related to atrophy and strength of the thigh muscles in trans-tibial amputees Prosthet. Orthot. Int 20 96–100 [DOI] [PubMed] [Google Scholar]
- [37].Robinson V, Sansam K, Hirst L and Neumann V 2010. Major lower limb amputation—what, why and how to achieve the best results? Orthop. Trauma 24 276–85 [Google Scholar]
- [38].Sherk VD, Bemben MG and Bemben DA 2010. Interlimb muscle and fat comparisons in persons with lower-limb amputation Arch. Phys. Med. Rehabil 91 1077–81 [DOI] [PubMed] [Google Scholar]
- [39].Smith D 2004. General principles of amputation surgery Atlas of Amputations and Limb Deficiencies: Surgical, Prosthetic, and Rehabilitation Principles 3rd edn (Rosemont, IL: American Academy of Orthopaedic Surgeons; ) pp 21–30 [Google Scholar]
- [40].Ranz EC, Wilken JM, Gajewski DA and Neptune RR 2017. The influence of limb alignment and transfemoral amputation technique on muscle capacity during gait Comput. Methods Biomech. Biomed. Eng 20 1167–74 [DOI] [PubMed] [Google Scholar]
- [41].Pasquina PF, Bryant PR, Huang ME, Roberts TL, Nelson VS and Flood KM 2006. Advances in amputee care Arch. Phys. Med. Rehabil 87 34–43 [DOI] [PubMed] [Google Scholar]
- [42].Brown BJ, Iorio ML, Klement M, Conti Mica MR, El-Amraoui A, O’Halloran P and Attinger CE 2014. Outcomes after 294 transtibial amputations with the posterior myocutaneous flap Int. J. Low Extreme Wounds 13 33–40 [DOI] [PubMed] [Google Scholar]
- [43].Eidt J and Kalapatapu V 2010. Lower extremity amputation: techniques and results Rutherford’s Vascular Surg. 2 Rutherford R (Philadelphia, PA: Saunders/Elsevier; ) [Google Scholar]
- [44].Murdoch G 1968. Knee-disarticulation amputation Bull. Prosthet. Res 10 14–18 [Google Scholar]
- [45].Huang S and Huang H 2018. Voluntary control of residual antagonistic muscles in transtibial amputees: reciprocal activation, coactivation, and implications for direct neural control of powered lower limb prostheses IEEE Trans. Neural Syst. Rehabil. Eng 27 85–95 [DOI] [PubMed] [Google Scholar]
- [46].Huang S and Ferris DP 2012. Muscle activation patterns during walking from transtibial amputees recorded within the residual limb-prosthetic interface J. Neuroeng. Rehabil 9 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wentink EC, Prinsen EC, Rietman JS and Veltink PH 2013. Comparison of muscle activity patterns of transfemoral amputees and control subjects during walking J. Neuroeng. Rehabil 10 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Clites TR, Carty MJ, Ullauri JB, Carney ME, Mooney LM, Duval J-F, Srinivasan SS and Herr HM 2018. Proprioception from a neurally controlled lower-extremity prosthesis Sci. Transl. Med 10 eaap8373. [DOI] [PubMed] [Google Scholar]
- [49].Clites TR, Carty MJ, Srinivasan S, Zorzos AN and Herr HM 2017. A murine model of a novel surgical architecture for proprioceptive muscle feedback and its potential application to control of advanced limb prostheses J. Neural Eng 14 036002. [DOI] [PubMed] [Google Scholar]
- [50].Srinivasan S, Vyas K, McAvoy M, Calvaresi P, Khan OF, Langer R, Anderson DG and Herr H 2019. Polyimide electrode-based electrical stimulation impedes early stage muscle graft regeneration Front. Neurol 10 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Srinivasan S, Carty MJ, Calvaresi PW, Clites TR, Maimon BE, Taylor CR, Zorzos AN and Herr H 2017. On prosthetic control: a regenerative agonist–antagonist myoneural interface Sci. Robot 2 eaan2971. [DOI] [PubMed] [Google Scholar]
- [52].Souza JM, Cheesborough JE, Ko JH, Cho MS, Kuiken TA and Dumanian GA 2014. Targeted muscle reinnervation: a novel approach to postamputation neuroma pain Clin. Orthop. Relat. Res 472 2984–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kuiken TA, Barlow AK, Hargrove L and Dumanian GA 2017. Targeted muscle reinnervation for the upper and lower extremity Tech. Orthop 32 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Miller LA, Lipschutz RD, Stubblefield KA, Lock BA, Huang H, Williams TW, Weir RF and Kuiken TA 2008. Control of a six degree of freedom prosthetic arm after targeted muscle reinnervation surgery Arch. Phys. Med. Rehabil 89 2057–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gart MS, Souza JM and Dumanian GA 2015. Targeted muscle reinnervation in the upper extremity amputee: a technical roadmap J. Hand Surg. Am 40 1877–88 [DOI] [PubMed] [Google Scholar]
- [56].Hefferman GM, Zhang F, Nunnery MJ and Huang H 2015. Integration of surface electromyographic sensors with the transfemoral amputee socket: a comparison of four differing configurations Prosthet. Orthot. Int 39 166–73 [DOI] [PubMed] [Google Scholar]
- [57].Fleming A and Huang HH 2019. Proportional myoelectric control of a powered ankle prosthesis for postural control under expected perturbation: a pilot study 2019 IEEE 16th Int. Conf. on Rehabilitation Robotics (ICORR) (IEEE) pp 899–904 [DOI] [PubMed] [Google Scholar]
- [58].Fleming A, Huang S, Buxton E, Hodges F and Huang HH 2021. Direct continuous electromyographic control of a powered prosthetic ankle for improved postural control after guided physical training: a case study Wearable Technol. 2 E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Reissman T, Halsne E, Lipschutz R, Miller L and Kuiken T 2018. A novel gel liner system with embedded electrodes for use with upper limb myoelectric prostheses PLoS One 13 e0198934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yeon SH et al. 2019. Flexible dry electrodes for EMG acquisition within lower extremity prosthetic sockets 2020 8th IEEE RAS/EMBS Int. Conf. for Biomedical Robotics and Biomechatronics (Biorob) (IEEE) pp 1088–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Dewald HA, Lukyanenko P, Lambrecht JM, Anderson JR, Tyler DJ, Kirsch RF and Williams MR 2019. Stable, three degree-of-freedom myoelectric prosthetic control via chronic bipolar intramuscular electrodes: a case study J. Neuroeng. Rehabil 16 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Vaskov AK. et al. Surgically implanted electrodes enable real-time finger and grasp pattern recognition for prosthetic hands. medRxiv. 2020 doi: 10.1109/tro.2022.3170720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ortiz-Catalan M, Brånemark R, Håkansson B and Delbeke J 2012. On the viability of implantable electrodes for the natural control of artificial limbs: review and discussion Biomed. Eng. Online 11 1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sigurðardóttir J. Research Thesis. Reykjavík University; 2015. EMG as a control parameter in lower limb prosthetics: surface vs implanted electrodes. [Google Scholar]
- [65].Salminger S. et al. Long-term implant of intramuscular sensors and nerve transfers for wireless control of robotic arms in above-elbow amputees. Sci. Robot. 2019;4:eaaw6306. doi: 10.1126/scirobotics.aaw6306. [DOI] [PubMed] [Google Scholar]
- [66].Merrill DR, Lockhart J, Troyk PR, Weir RF and Hankin DL 2011. Development of an implantable myoelectric sensor for advanced prosthesis control Artif. Organs 35 249–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhou P, Lowery MM, Englehart KB, Huang H, Li G, Hargrove L, Dewald JPA and Kuiken TA 2007. Decoding a new neural machine interface for control of artificial limbs J. Neurophysiol 98 2974–82 [DOI] [PubMed] [Google Scholar]
- [68].Huang H, Zhou P, Li G and Kuiken TA 2008. An analysis of EMG electrode configuration for targeted muscle reinnervation based neural machine interface IEEE Trans. Neural Syst. Rehabil. Eng 16 37–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Schlink BR, Nordin AD and Ferris DP 2020. Comparison of signal processing methods for reducing motion artifacts in high-density electromyography during human locomotion IEEE Open J. Eng. Med. Biol 1 156–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Schlink BR and Ferris DP 2019. A lower limb phantom for simulation and assessment of electromyography technology IEEE Trans. Neural Syst. Rehabil. Eng 27 2378–85 [DOI] [PubMed] [Google Scholar]
- [71].Dimitrov H, Bull A and Farina D 2020. Real-time interface algorithm for ankle kinematics and stiffness from electromyographic signals IEEE Trans. Neural Syst. Rehabil. Eng 28 1416–27 [DOI] [PubMed] [Google Scholar]
- [72].Au SK and Herr HM 2008. Powered ankle-foot prosthesis IEEE Robot. Autom. Mag 15 52–59 [Google Scholar]
- [73].Young AJ, Simon AM, Fey NP and Hargrove LJ 2014. Intent recognition in a powered lower limb prosthesis using time history information Ann. Biomed. Eng 42 631–41 [DOI] [PubMed] [Google Scholar]
- [74].Liu Z, Lin W, Geng Y and Yang P 2017. Intent pattern recognition of lower-limb motion based on mechanical sensors IEEE/CAA J. Autom. Sin 4 651–60 [Google Scholar]
- [75].Varol HA and Goldfarb M 2007. Real-time intent recognition for a powered knee and ankle transfemoral prosthesis 2007 IEEE 10th Int. Conf. on Rehabilitation Robotics (IEEE) pp 16–23 [Google Scholar]
- [76].Jin D, Yang J, Zhang R, Wang R and Zhang J 2006. Terrain identification for prosthetic knees based on electromyographic signal features Tsinghua Sci. Technol 11 74–79 [Google Scholar]
- [77].Hussain T, Iqbal N, Maqbool HF, Khan M, Awad MI and Dehghani-Sanij AA 2020. Intent based recognition of walking and ramp activities for amputee using sEMG based lower limb prostheses Biocybern. Biomed. Eng 40 1110–23 [Google Scholar]
- [78].Zhang F and Huang H 2013. Source selection for real-time user intent recognition toward volitional control of artificial legs IEEE J. Biomed. Health Inf 17 907–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Joshi D and Hahn ME 2016. Terrain and direction classification of locomotion transitions using neuromuscular and mechanical input Ann. Biomed. Eng 44 1275–84 [DOI] [PubMed] [Google Scholar]
- [80].Miller JD, Beazer MS and Hahn ME 2013. Myoelectric walking mode classification for transtibial amputees IEEE Trans. Biomed. Eng 60 2745–50 [DOI] [PubMed] [Google Scholar]
- [81].Au SK, Bonato P and Herr H 2005. An EMG-position controlled system for an active ankle-foot prosthesis: an initial experimental study 9th Int. Conf. on Rehabilitation Robotics (ICORR 2005) (IEEE) pp 375–9 [Google Scholar]
- [82].Nakamura BH and Hahn ME 2017. Myoelectric activation pattern changes in the involved limb of individuals with transtibial amputation during locomotor state transitions Arch. Phys. Med. Rehabil 98 1180–6 [DOI] [PubMed] [Google Scholar]
- [83].Hu B, Rouse E and Hargrove L 2018. Fusion of bilateral lower-limb neuromechanical signals improves prediction of locomotor activities Front. Robot. AI 5 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Tkach D, Lipschutz RD, Finucane SB and Hargrove LJ 2013. Myoelectric neural interface enables accurate control of a virtual multiple degree-of-freedom foot-ankle prosthesis 2013 IEEE 13th Int. Conf. on Rehabilitation Robotics (ICORR) (IEEE) pp 1–4 [DOI] [PubMed] [Google Scholar]
- [85].Hargrove LJ, Young AJ, Simon AM, Fey NP, Lipschutz RD, Finucane SB, Halsne EG, Ingraham KA and Kuiken TA 2015. Intuitive control of a powered prosthetic leg during ambulation: a randomized clinical trial JAMA 313 2244–52 [DOI] [PubMed] [Google Scholar]
- [86].Oskoei MA and Hu H 2008 Support vector machine-based classification scheme for myoelectric control applied to upper limb IEEE Trans. Biomed. Eng 55 1956–65 [DOI] [PubMed] [Google Scholar]
- [87].Schultz AE and Kuiken TA 2011. Neural interfaces for control of upper limb prostheses: the state of the art and future possibilities PM&R 3 55–67 [DOI] [PubMed] [Google Scholar]
- [88].Madusanka D, Wijayasingha L, Gopura R, Amarasinghe Y and Mann G 2015. A review on hybrid myoelectric control systems for upper limb prosthesis 2015 Moratuwa Engineering Research Conf. (Mercon) (IEEE) pp 136–41 [Google Scholar]
- [89].Jiang N, Rehbaum H, Vujaklija I, Graimann B and Farina D 2013. Intuitive, online, simultaneous, and proportional myoelectric control over two degrees-of-freedom in upper limb amputees IEEE Trans. Neural Syst. Rehabil. Eng 22 501–10 [DOI] [PubMed] [Google Scholar]
- [90].Xie H-B, Huang H, Wu J and Liu L 2015. A comparative study of surface EMG classification by fuzzy relevance vector machine and fuzzy support vector machine Physiol. Meas 36 191. [DOI] [PubMed] [Google Scholar]
- [91].Tkach D and Hargrove LJ 2013. Neuromechanical sensor fusion yields highest accuracies in predicting ambulation mode transitions for trans-tibial amputees 2013 35th Annual Int. Conf. of the IEEE Engineering in Medicine and Biology Society (EMBC) (IEEE) pp 3074–7 [DOI] [PubMed] [Google Scholar]
- [92].Spanias J, Simon A, Finucane S, Perreault E and Hargrove LJ 2018. Online adaptive neural control of a robotic lower limb prosthesis J. Neural Eng 15 016015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Simon AM, Ingraham KA, Spanias JA, Young AJ, Finucane SB, Halsne EG and Hargrove LJ 2016. Delaying ambulation mode transition decisions improves accuracy of a flexible control system for powered knee-ankle prosthesis IEEE Trans. Neural Syst. Rehabil. Eng 25 1164–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Ha KH, Varol HA and Goldfarb M 2010. Volitional control of a prosthetic knee using surface electromyography IEEE Trans. Biomed. Eng 58 144–51 [DOI] [PubMed] [Google Scholar]
- [95].Zhang X, Wang D, Yang Q and Huang H 2012. An automatic and user-driven training method for locomotion mode recognition for artificial leg control Annual Int. Conf. IEEE Eng. Med. Biol. Soc. vol 2012 pp 6116–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Huang H, Zhang F, Sun YL and He H 2010. Design of a robust EMG sensing interface for pattern classification J. Neural Eng 7 056005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Du L, Zhang F, He H and Huang H 2013. Improving the performance of a neural–machine interface for prosthetic legs using adaptive pattern classifiers 2013 35th Annual Int. Conf. of the IEEE Engineering in Medicine and Biology Society (EMBC) (IEEE) pp 1571–4 [DOI] [PubMed] [Google Scholar]
- [98].Doud J and Walsh J 1995. Muscle fatigue and muscle length interaction: effect on the EMG frequency components Electromyogr. Clin. Neurophysiol 35 331. [PubMed] [Google Scholar]
- [99].Tkach D, Huang H and Kuiken TA 2010. Study of stability of time-domain features for electromyographic pattern recognition J. Neuroeng. Rehabil 7 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Liu M, Zhang F and Huang HH 2017. An adaptive classification strategy for reliable locomotion mode recognition Sensors 17 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zhang F, Liu M and Huang H 2015. Investigation of timing to switch control mode in powered knee prostheses during task transitions PLoS One 10 e0133965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Spanias JA, Perreault EJ and Hargrove LJ 2015. Detection of and compensation for EMG disturbances for powered lower limb prosthesis control IEEE Trans. Neural Syst. Rehabil. Eng 24 226–34 [DOI] [PubMed] [Google Scholar]
- [103].Zhang F, Liu M and Huang H 2015. Effects of locomotion mode recognition errors on volitional control of powered above-knee prostheses IEEE Trans. Neural Syst. Rehabil. Eng 23 64–72 [DOI] [PubMed] [Google Scholar]
- [104].Hoover CD, Fulk GD and Fite KB 2012. Stair ascent with a powered transfemoral prosthesis under direct myoelectric control IEEE/ASME Trans. Mechatron 18 1191–200 [Google Scholar]
- [105].Huang S, Wensman JP and Ferris DP 2014. An experimental powered lower limb prosthesis using proportional myoelectric control J. Med. Device 8 024501 [Google Scholar]
- [106].Wu S-K, Waycaster G and Shen X 2011. Electromyography-based control of active above-knee prostheses Control Eng. Pract 19 875–82 [Google Scholar]
- [107].Hoover CD, Fulk GD and Fite KB 2012. The design and initial experimental validation of an active myoelectric transfemoral prosthesis J. Med. Device 6 011005 [Google Scholar]
- [108].Rogers EA, Carney ME, Yeon SH, Clites TR, Solav D and Herr HM 2020. An ankle-foot prosthesis for rock climbing augmentation IEEE Trans. Neural Syst. Rehabil. Eng 29 41–51 [DOI] [PubMed] [Google Scholar]
- [109].Frith CD, Blakemore S-J and Wolpert DM 2000. Abnormalities in the awareness and control of action Phil. Trans. R. Soc. B 355 1771–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Wolpert DM 1997. Computational approaches to motor control Trends Cogn. Sci 1 209–16 [DOI] [PubMed] [Google Scholar]
- [111].Kawato M 1999. Internal models for motor control and trajectory planning Curr. Opin. Neurobiol 9 718–27 [DOI] [PubMed] [Google Scholar]
- [112].Todorov E 2004. Optimality principles in sensorimotor control Nat. Neurosci 7 907–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Shadmehr R and Mussa-Ivaldi FA 1994. Adaptive representation of dynamics during learning of a motor task J. Neurosci 14 3208–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Hall PT et al. 2020. Fully implanted prostheses for musculoskeletal limb reconstruction after amputation: an in vivo feasibility study Ann. Biomed. Eng 49 1012–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Shadmehr R and Krakauer JW 2008. A computational neuroanatomy for motor control Exp. Brain Res 185 359–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Todorov E and Jordan MI 2002. Optimal feedback control as a theory of motor coordination Nat. Neurosci 5 1226–35 [DOI] [PubMed] [Google Scholar]
- [117].Thoroughman KA and Shadmehr R 1999. Electromyographic correlates of learning an internal model of reaching movements J. Neurosci 19 8573–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Abernethy B, Hanna A and Plooy A 2002. The attentional demands of preferred and non-preferred gait patterns Gait Posture 15 256–65 [DOI] [PubMed] [Google Scholar]
- [119].Miller WC, Deathe AB, Speechley M and Koval J 2001. The influence of falling, fear of falling, and balance confidence on prosthetic mobility and social activity among individuals with a lower extremity amputation Arch. Phys. Med. Rehabil 82 1238–44 [DOI] [PubMed] [Google Scholar]
- [120].Horak FB 2006. Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing 35 ii7–ii11 [DOI] [PubMed] [Google Scholar]
- [121].Geurts A, Mulder TW, Nienhuis B and Rijken R 1991. Dual-task assessment of reorganization of postural control in persons with lower limb amputation Arch. Phys. Med. Rehabil 72 1059–64 [PubMed] [Google Scholar]
- [122].Bradford JC, Lukos JR, Passaro A, Ries A and Ferris DP 2019. Effect of locomotor demands on cognitive processing Sci. Rep 9 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Jacobsen NSJ, Blum S, Witt K and Debener S 2020. A walk in the park? Characterizing gait-related artifacts in mobile EEG recordings Eur. J. Neurosci In Press [DOI] [PubMed] [Google Scholar]
- [124].Plummer P and Eskes G 2015. Measuring treatment effects on dual-task performance: a framework for research and clinical practice Front. Hum. Neurosci 9 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Powell LE and Myers AM 1995. The activities-specific balance confidence (ABC) scale J. Gerontol. A 50 M28–M34 [DOI] [PubMed] [Google Scholar]
- [126].Kim K and Colgate JE 2012. Haptic feedback enhances grip force control of sEMG-controlled prosthetic hands in targeted reinnervation amputees IEEE Trans. Neural Syst. Rehabil. Eng 20 798–805 [DOI] [PubMed] [Google Scholar]
- [127].Wan AH, Wong DW, Ma CZ, Zhang M and Lee WC 2016. Wearable vibrotactile biofeedback device allowing identification of different floor conditions for lower-limb amputees Arch. Phys. Med. Rehabil 97 1210–3 [DOI] [PubMed] [Google Scholar]
- [128].Rokhmanova N and Rombokas E 2019. Vibrotactile feedback improves foot placement perception on stairs for lower-limb prosthesis users 2019 IEEE 16th Int. Conf. on Rehabilitation Robotics (ICORR) (IEEE) pp 1215–20 [DOI] [PubMed] [Google Scholar]
- [129].Hebert JS, Rehani M and Stiegelmar R 2017. Osseointegration for lower-limb amputation: a systematic review of clinical outcomes JBJS Rev. 5 e10. [DOI] [PubMed] [Google Scholar]
- [130].Charkhkar H, Shell CE, Marasco PD, Pinault GJ, Tyler DJ and Triolo RJ 2018. High-density peripheral nerve cuffs restore natural sensation to individuals with lower-limb amputations J. Neural Eng 15 056002. [DOI] [PubMed] [Google Scholar]
- [131].Petrini FM et al. 2019. Sensory feedback restoration in leg amputees improves walking speed, metabolic cost and phantom pain Nat. Med 25 1356–63 [DOI] [PubMed] [Google Scholar]
- [132].Pan L, Vargas L, Fleming A, Hu X, Zhu Y and Huang HH 2020. Evoking haptic sensations in the foot through high-density transcutaneous electrical nerve stimulations J. Neural Eng 17 036020. [DOI] [PubMed] [Google Scholar]
- [133].Hebert JS, Olson JL, Morhart MJ, Dawson MR, Marasco PD, Kuiken TA and Chan KM 2013. Novel targeted sensory reinnervation technique to restore functional hand sensation after transhumeral amputation IEEE Trans. Neural Syst. Rehabil. Eng 22 765–73 [DOI] [PubMed] [Google Scholar]
- [134].Zhang X and Huang H 2015. A real-time, practical sensor fault-tolerant module for robust EMG pattern recognition J. Neuroeng. Rehabil 12 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Sensinger JW, Lock BA and Kuiken TA 2009. Adaptive pattern recognition of myoelectric signals: exploration of conceptual framework and practical algorithms IEEE Trans. Neural Syst. Rehabil. Eng 17 270–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Hong JH and Mun MS 2005. Relationship between socket pressure and EMG of two muscles in trans-femoral stumps during gait Prosthet. Orthot. Int 29 59–72 [DOI] [PubMed] [Google Scholar]
- [137].Pasquina PF et al. 2015. First-in-man demonstration of a fully implanted myoelectric sensors system to control an advanced electromechanical prosthetic hand J. Neurosci. Methods 244 85–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Myers AC, Huang H and Zhu Y 2015. Wearable silver nanowire dry electrodes for electrophysiological sensing RSC Adv. 5 11627–32 [Google Scholar]
- [139].Sartori M, Yavuz US and Farina D 2017. In vivo neuromechanics: decoding causal motor neuron behavior with resulting musculoskeletal function Sci. Rep 7 13465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Dai C and Hu X 2020. Finger joint angle estimation based on motoneuron discharge activities IEEE J. Biomed. Health Inf 24 760–7 [DOI] [PubMed] [Google Scholar]
- [141].Atzori M, Cognolato M and Müller H 2016. Deep learning with convolutional neural networks applied to electromyography data: a resource for the classification of movements for prosthetic hands Front. Neurorobot 10 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Shadmehr R, Smith MA and Krakauer JW 2010. Error correction, sensory prediction, and adaptation in motor control Annu. Rev. Neurosci 33 89–108 [DOI] [PubMed] [Google Scholar]
- [143].Price HE 1985. The allocation of functions in systems Hum. Factors 27 33–45 [Google Scholar]
- [144].Simon AM, Fey NP, Ingraham KA, Young AJ and Hargrove LJ 2013. Powered prosthesis control during walking, sitting, standing, and non-weight bearing activities using neural and mechanical inputs 2013 6th Int. IEEE/EMBS Conf. on Neural Engineering (NER) (IEEE) pp 1174–7 [Google Scholar]
- [145].Young AJ, Simon AM, Fey NP and Hargrove LJ 2013. Classifying the intent of novel users during human locomotion using powered lower limb prostheses 2013 6th Int. IEEE/EMBS Conf. on Neural Engineering (NER) (IEEE) pp 311–4 [Google Scholar]
- [146].Hargrove LJ, Simon AM, Lipschutz R, Finucane SB and Kuiken TA 2013. Non-weight-bearing neural control of a powered transfemoral prosthesis J. Neuroeng. Rehabil 10 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Zhang F, Liu M, Harper S, Lee M and Huang H 2014. Engineering platform and experimental protocol for design and evaluation of a neurally-controlled powered transfemoral prosthesis J. Vis. Exp 89 e51059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Young A, Kuiken T and Hargrove L 2014. Analysis of using EMG and mechanical sensors to enhance intent recognition in powered lower limb prostheses J. Neural Eng 11 056021. [DOI] [PubMed] [Google Scholar]
- [149].Spanias JA, Simon AM, Ingraham KA and Hargrove LJ 2015. Effect of additional mechanical sensor data on an EMG-based pattern recognition system for a powered leg prosthesis 2015 7th Int. IEEE/EMBS Conf. on Neural Engineering (NER) (IEEE) pp 639–42 [Google Scholar]
- [150].Alcaide-Aguirre RE, Morgenroth DC and Ferris DP 2013. Motor control and learning with lower-limb myoelectric control in amputees J. Rehabil. Res. Dev 50 687–98 [DOI] [PubMed] [Google Scholar]
- [151].Chen B, Wang Q and Wang L 2014. Promise of using surface EMG signals to volitionally control ankle joint position for powered transtibial prostheses 2014 36th Annual Int. Conf. of the IEEE Engineering in Medicine and Biology Society (IEEE) pp 2545–8 [DOI] [PubMed] [Google Scholar]
- [152].Huang S and Huang H 2018. Voluntary control of residual antagonistic muscles in transtibial amputees: feedforward ballistic contractions and implications for direct neural control of powered lower limb prostheses IEEE Trans. Neural Syst. Rehabil. Eng 26 894–903 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study.


