Abstract
The dodecapeptide angiotensin-(1–12) [Ang-(1–12)] functions as an intracrine/paracrine substrate for local production of angiotensin II. We developed a reliable and specific radioimmunoassay (RIA) method for the measurement of Ang-(1–12) in human plasma and urine using an affinity purified antibody fraction directed towards the C-terminus of the human Ang-(1–12) sequence. The RIA method was applied to quantify the Ang-(1–12) in plasma and urine collected from thirty-four human subjects (29 treated with antihypertensive medicines and 5 untreated patients). Plasma Ang-(1–12) level was significantly higher (P < 0.05) in patients with systolic blood pressure ≥140 mm Hg (n=10) compared to the group with systolic blood pressure <140 mm Hg (n=24). No significant difference (P = 0.22) was found in spot urine between the groups. Our study also shows that the polyclonal antibody neutralizes the cleavage sites of the human Ang-(1–12) from recombinant human chymase (rhChymase) and serum angiotensin converting enzyme (ACE) mediated Ang II generating hydrolysis. Overall, this newly developed RIA method is reliable and applicable to accurately quantify the Ang-(1–12) level in clinical samples (plasma and urine). Further, our in vitro neutralization study suggests that the anti-Ang-(1–12)-antibody might be used as an in vivo therapeutic agent for preventing Ang-(1–12)/Ang II-mediated hypertension and organ damage.
Keywords: Angiotensin-(1–12), Angiotensin I, Angiotensin II, Angiotensinogen, Renin-Angiotensin System, Radioimmunoassay, Hypertension
Introduction
The renin-angiotensin system (RAS) regulates blood pressure, fluid balance and homeostasis (1–3). Recent research revealed that the RAS biochemical cascade is not a linear renin-dependent pathway but instead a complex system where separate circulating and tissue-based enzymes and proteins are implicated in the generation of biologically active angiotensin peptides (4, 5). Adding to the biotransformation complexity, a differential processing of angiotensinogen (AGT) through a non-renin pathway leads to the production of angiotensin II (Ang II) from an extended form of angiotensin I (Ang I) named angiotensin-(1–12) [Ang-(1–12)] in rodents and humans (6–11). This alternate substrate, with a predominant tissue expression, acts as an intracrine and paracrine precursor for direct Ang II generation by the action of chymase rather than angiotensin converting enzyme (ACE) (6–8). Ang-(1–12) is ubiquitous in rodent cardiovascular tissues and has also been identified in the human heart (6). New studies from this laboratory suggest that Ang-(1–12) may be a novel biomarker of hypertensive heart and vascular disease (12). The absence of a specific assay for the measurement of the human form of Ang-(1–12) has impeded further progress into this research area. A limiting factor in the extrapolation of tools used in rodent Ang-(1–12) studies is the existence of a differential amino acid sequence in the C-terminus of the dodecapeptide between rodents and humans (13). This study reports the characterization of polyclonal antibodies directed toward the human amino acid composition of the C-terminus of the Ang-(1–12) peptide and a reliable radioimmunoassay (RIA) procedure development for the detection and quantification of the Ang-(1–12) peptide in human biological fluids (plasma and urine). This newly developed RIA method was applied, for the first time, to uncover potential contributions that higher circulating and urinary Ang-(1–12) levels have in relation to human cardiovascular disease, particularly in those individuals with elevated systemic blood pressure and pulse pressure.
Materials and Methods
Chemicals and Reagents
All custom-made angiotensin peptides [human Ang-(1–12), Ang I, Ang-(1–9), Ang II, and Ang-(1–7); purity >98%] were purchased from GenScript USA Inc. (Piscataway, NJ). Human plasma angiotensinogen protein (purity >95%) was purchased from Athens Research and Technology (Athens, GA). Radioactive 125Iodine [125I] was purchased from PerkinElmer Life and Analytical Sciences, Inc. (Waltham, Massachusetts). The polyclonal antibody against the C-terminus of full length human Ang-(1–12) sequence was generated and affinity purified by PrimmBiotech Inc., Cambridge, MA (http://www.primmbiotech.com/) according to our specifications. All other chemicals used in this study were of analytical grade and were obtained from Sigma (St. Louis, MO) and Fisher Scientific (Atlanta, GA).
Custom Polyclonal Antibody Production
A polyclonal antibody against C-terminus of the human Ang-(1–12) sequence [DRVYIHPFHLVI] was developed in collaboration with PrimmBiotech, Inc. (Cambridge, MA; http://www.primmbiotech.com/) using a standard protocol. Briefly, New Zealand white rabbits (n=4) were immunized on day 0, 21, 28, 35, 43 and 48 (six injections) with synthetic human Ang-(1–12) peptide conjugated to a carrier protein OVA (ovalbumin) at the N-terminus. On day 50, all rabbits were sacrificed, serum was separated, and the polyclonal antibody was first purified using an affinity column [CNBr-Sepharose + human Ang-(1–12) peptide]. The immuno purification of depleted serum was further depleted using a second affinity column (CNBr-Sepharose + mixture of Ang I and Ang II) to remove the Ang I and Ang II cross-reacting polyclonal antibodies. The purified polyclonal antibody fraction was further characterized and used in RIA measurements.
Characterization of Anti-Ang-(1–12) Affinity Purified Polyclonal Antibody Fraction
Antibody concentration and functional titers were performed using serial dilutions of the affinity purified antibody fraction directed towards the C-terminus of Ang-(1–12) sequence produced in rabbits (rabbit #1 to rabbit #4) to determine the highest titer 125 dilution that can bind ~30–40% of the radiolabeled human 125I-Ang-(1–12) tracer (specific activity 3,900 cpm/fmol). The antibody titer was determined in RIA buffer containing 50 mM phosphate buffer saline, 25 mM EDTA, 0.5% triton X-100, 0.05% sodium azide and 0.5% BSA (pH 7.4). Affinity purified antibodies (rabbit #1 to rabbit #4) were serially diluted (1:100 to 1:1000) in RIA buffer and titer assays were performed in duplicate or quadruplicate. Briefly, 50 μL of each diluted antibody was mixed with a highly purified 125 radiolabeled I-Ang-(1–12) trace [~6–8 fmoles trace per RIA tube] in 200 μL RIA buffer. After mixing the contents, the tubes were incubated overnight (20–22 hours at 4°C). At the end of incubation period, 50 μL of 1% γ-globulin was added, mixed, and the bound/free [B/Bo] forms of Ang-(1–12) were then separated by precipitating the bound fraction with 200 μL of 23% polyethylene glycol (P EG). After 15 min of incubation in ice, the bound [B] and unbound [Bo] traces were separated by centrifuging the tubes at in the precipitate was measured in a Packard Cobra II Auto-Gamma Counter. Data were analyzed to calculate the percent of B/Bo ratio. We found that the highest dilution of affinity purified antibody fraction from rabbit #4 (IgG 0.52 mg/mL) that recognizes around 30–40% of the radiolabeled human 125I-Ang-(1–12) C-terminus epitope was 1:400. We used 1:400 dilution of the rabbit #4 antibody in all RIA experiments (analytical validations tests, cross-reactivity, recovery, parallelism assays and quantification of Ang-(1–12) in human plasma/urine).
Calibration Curve and Cross-reactivity Assays
A standard calibration curve was prepared using nine different concentrations of the synthetic non-radiolabeled Ang-(1–12) standard ranging from 0 – 75 ng/0.2 mL RIA buffer. The amount of 125I-Ang-(1–12) was fixed to determine the specificity of the antibody for human Ang-(1–12) sequence. Briefly, a total 200 μL of RIA buffer containing 50 μL of antibody (1:400 dilution of IgG concentration 0.52 mg/mL), 50 μL of 125I-Ang-(1–12) [6–8 fmoles/tube, purity ≥99%] and 50 μL of different concentrations of non-radiolabeled human Ang-(1–12) peptide [0 to 75 ng/0.2 mL RIA tube in duplicate, purity ≥98.5%] were combined and the RIA tubes were incubated overnight for 20–22 hours at 4°C. At the end of the incubation period, the RIA tubes were processed as described above to separate the bound-ligand [B] from unbound [Bo], and counted in a Packard Cobra II Auto-Gamma Counter. The binding data were analyzed and graphed using a statistical program (GraphPad Prism 8). To examine the cross-reactivity of this polyclonal antibody with closely related angiotensin peptides and purified human plasma AGT, several fold higher concentrations of angiotensin peptides (7.5 ng/0.2 mL RIA tube) or AGT protein (15 μg/0.2 mL RIA tube, with or without C18 Sep-Pak) were used; higher than the endogenous levels reported in human plasma and urine (Table 2). Briefly, in a total 200 μL of RIA buffer we added 50 μL of antibody (1:400 dilution), 50 μL of 125I-Ang-(1–12) [6–8 fmoles/tube] and 50 μL (7.5 ng per RIA tube) of each non-radiolabeled smaller angiotensin peptides [Ang I, DRVYIHPFHL; Ang-(1–9), DRVYIHPFH; Ang II, DRVYIHPF; and Ang-(1–7); DRVYIHP] or purified human AGT protein (15 μg per RIA tube with or without Sep-Pak) and after mixing the contents, the binding tubes were incubated overnight (20–22 hours at 4°C) and processed as described above. The percent cross-reactivity was calculated as the ratio of observed values of the Ang-(1–12) to the closely related small-sized angiotensin peptides or AGT, multiplied by 100.
Table 2:
Endogenous level of angiotensin peptides in human plasma and urine of healthy individuals, prehypertension, hypertension and diseased subjects.
| Angiotensin level [References] | Plasma | Urine |
|---|---|---|
| Angiotensinogen [(34, 37)] | 28 to 71 μg/mL | 1 to 126 ng/mL |
| Angiotensin I [(14, 27, 31, 32, 45)] | 25–143 pg/mL | 123 to 293 pg/mL |
| Angiotensin-(1–9) [(15, 17)] | 0.5 to 53 pg/mL | No report |
| Angiotensin II [(19, 24, 25, 27, 31–33)] | 7 to 272 pg/mL | 9 to 80 pg/mL |
| Angiotensin-(1–7) [(25–28, 33, 45)] |
5 to 69 pg/mL | 56 to 234 pg/mL |
Verified levels of angiotensinogen protein and angiotensin peptides reported in plasma and urine samples obtained from healthy, diseased and treated subjects were compiled from published references. Angiotensinogen protein and angiotensin peptide levels in human samples were measured by various methods, ELISA/RIA/LC-MS.
Collection and Processing of Human Blood and Urine for RIA
To optimize the sample collection and processing conditions to accurately measure the human Ang-(1–12) levels in plasma and urine, 5 adult volunteers (ages 20–55 years, 2 females and 3 males) were recruited after signing an informed consent document approved by the Wake Forest University Medical Center Institutional Review Board (IRB number BG05–516). Venous blood was rapidly collected in pre-chilled tubes containing 5 mM EDTA in the absence and in the presence of a cocktail of inhibitors [1, 10-ortho-phenanthroline (0.5 mM); p-hydroxymercuribenzoate (1 mM); pepstatin A (125 μM)]. Blood tubes were centrifuged at 3,000 g for 10 min to separate the plasma and 500 μL aliquots of plasma were stored at −80°C until Ang-(1–12) measurement by RIA. Spot urine was also collected from the same individuals and 1 mL aliquots were immediately stored at −80°C for Ang-(1–12) measurement by RIA. Considering the limit of detection, a minimum 250 μL of plasma and 500 μL of spot-urine from antihypertensive treated human patients are needed for a reliable single RIA measurement of Ang-(1–12).
Blood and spot urine were also collected from 34 patients (5 females), attending the Trinity Hypertension Research Institute (Carrollton, TX) between February and September 2019 (Sterling IRB number 7175-HAPunzi). Patient provided 7 mL of peripheral venous blood in the inhibitor cocktail described above and 20 mL spot urine for measurements of Ang-(1–12). Clinical and demographic data were obtained from all participants and the average of three consecutive blood pressure determinations (spaced three – five minutes apart) were obtained prior to the blood draw with a mercury sphygmomanometer (Table 4). Twenty nine of 34 patients were on antihypertensive medications while five others were untreated.
Table 4:
Clinical, demographic, plasma and urinary Ang-(1–12) data of 34 hypertensive patients.
| Variable | Systolic Pressure (≥140 mm Hg) | Systolic Pressure (<140 mm Hg) |
|---|---|---|
| Gender (Male/Female) | 7/3 | 22/2 |
| Age (Years) | 63 ± 2 | 64 ± 3 |
| Body weight (kg) | 91 ± 4 | 100 ± 6 |
| Body mass index (kg/m2) | 30.35 ± 1.45 | 32.51 ± 1.33 |
| Systolic blood pressure (mm Hg) | 147 ± 3*** | 128 ± 2 |
| Diastolic blood pressure (mm Hg) | 89 ± 3** | 82 ± 2 |
| Pulse pressure (mm Hg) | 55 ± 2** | 47 ± 2 |
| Heart Rate (beats/min) | 71 ± 2 | 75 ± 3 |
| Plasma angiotensin-(1–12) (ng/mL) | 2.09 ± 0.14* | 1.65 ± 0.11 |
| Urinary angiotensin-(1–12) (ng/mL) | 11.09 ± 1.38 | 18.70 ± 3.89 |
| Antihypertensive Medications (Yes/No) | (9/1) | 20/4 |
Values are Mean ± SEM
P < 0.05
P < 0.01
P < 0.0001 (≥140 mm Hg systolic blood pressure vs <140 mm Hg systolic pressure).
Solid-phase Extraction (SPE)
Samples (500 μL of plasma or 1 mL of urine) were thawed on ice and diluted to 3 mL with 0.1% HFBA (heptafluorobutyric acid). Normal human urine pH ranges from 4.0 (acidic) to 8.0 (alkaline). In 1 mL of urine samples, 500 μL of 1N HCl was added to ensure the pH of the urine was in acidic range at the time of loading to the column. Urine samples were acidified to prevent metabolism. S amples were then centrifuged at 28,000 g for 5 min and supernatants were transferred into pre-chilled tubes for SP E. SPE columns (Sep-Pak C18, 200 mg silica sorbent, 3cc Vac Cartridge, Waters, Milford, MA) were first activated with 5 mL of a methanol:HFBA mixture (80%:0.1%) and then washed with 5 mL of 0.1% HFBA. To concentrate the peptides, 3 mL of sample (28,000 g post-centrifuged supernatant) were applied to the activated SPE column and let them flow-through by gravity. The column was washed sequentially with 5 mL of chilled 0.1% HFBA followed by 5 mL of ultra-pure MilliQ water. After MilliQ washing, the peptides were eluted from the column with 3 mL of methanol:HFBA mixture (80%:0.1%) into a glass tube containing 0.1 mL of 1% BSA. Eluted samples were equally divided into two 12 × 75 mm conical polypropylene tubes and evaporated to dryness in a SpeedVac (Eppendorf Vacufuge plus, Hauppauge, NY). The dried tubes were sealed and stored at 4°C until Ang-(1–12) measurement by RIA. We employed solid phase extraction to concentrate the Ang-(1–12) using a C18 cartridge (SPE column) to avoid any cross-reactivity with the human AGT protein. The C18 column can retain only low molecular weight molecules. High molecular weight proteins (such as AGT, ~50 kDa) flush out quickly from the SPE column after washing. To check whether any AGT retained by SPE column, a known amount the purified human plasma AGT (15 μg) was passed through the C18 Sep-Pak column and processed as described above for the cross-reactivity assays.
Ang-(1–12) RIA Measurement
The dried human sample tubes containing Ang-(1–12) were placed in ice and processed for RIA measurement. The dried RIA tubes were reconstituted with 0.1 mL of RIA buffer containing 50 mM phosphate buffer saline, 25 mM EDTA, 0.5% triton X-100, 0.05% sodium azide and 0.5% BSA (pH 7.4). Next, highly purified radiolabeled human 125I-Ang-(1–12) trace [purity ≥99%; ~6–8 fmol/RIA tube] and 50 μL of affinity purified human anti-Ang-(1–12) antibody (1:400 dilution) were added and incubated for 20–22 hours at 4°C. At the end of the incubation period, the RIA tubes were processed as described above to separate the bound/free [B/Bo] forms of Ang-(1–12). The radioactivity corresponding to the antibody-bound antigen in the precipitate was measured in a Packard Cobra II Auto-Gamma Counter. The amount of Ang-(1–12) in the human plasma and urine extracts were estimated from a calibration curve for synthetic human Ang-(1–12) peptide (purity >98.5%) standard plotted ranging from 0 – 75 ng/0.2 mL per RIA tube. All RIA experiments were done using freshly iodinated and highly purified 125I-Ang-(1–12) trace (<30 days). In addition to purity check on HPLC, the recovery of the radiolabeled Ang-(1–12) trace was also checked using a Sep-Pak column in each RIA experiment and normalized accordingly with percent recovery of the trace. Blank tubes, substituted plasma, and urine samples with 0.1% HFBA, were prepared accordingly and readings were subtracted from the samples.
Radiolabeling of Human Ang-(1–12) Peptide and HPLC Purification
For the RIA and ligand-binding assays, the tyrosine at the 4th position of human Ang-(1–12) sequence was radiolabeled with 125I using oxidant chloramine-T and purified on a C18 column by HPLC in our laboratory as previously described (8). Briefly, 10 μL of 1 mM human Ang-(1–12) peptide was added to 20 μL of PBS and 10 μL of 1 mCi of Na[125I] (Perkin-Elmer, Waltham, MA). The iodination reaction was started by adding 10 μL of chloramine-T solution (10–15 mg/10 mL) to the mixture for 30 sec. The reaction was stopped by adding 50 μL of sodium bisulfate solution (30 mg/10 mL MilliQ water). The iodinated Ang peptide was separated from free Na[125I] by passing the mixture through an activated C18 SPE column. The SPE eluted iodinated Ang-(1–12) peptide was further purified by HPLC using a linear gradient from 10% to 50% mobile phase B at a flow rate of 0.35 mL/min at 32° C. The solvent system consisted of 0.1% phosphoric acid (mobile phase A) and 80% acetonitrile/0.1% phosphoric acid (mobile phase B). The eluted 125I-Ang-(1–12) fractions were monitored by an in-line flow-through gamma detector (BioScan Inc., Washington, DC) and also monitored by in-line flow-through Shimadzu SPD UV detector (215 nm) to check the un-labeled Ang-(1–12) impurity. Products were identified by comparison of retention times of synthetic [125I] standard Ang-(1–12) peptide. The radiolabeled 125I-Ang-(1–12) peptide corresponding to the peak retention time was collected on a fraction collector and the data were analyzed with Shimadzu LCSolution (Kyoto, Japan) acquisition software. In RIA, we have used a highly purified 125I-Ang-(1–12) (purity ≥99%) that was free of un-labeled Ang-(1–12) as the radiolabeled purified fraction was also monitored by an in-line flow-through UV detector (215 nm).
Recovery, Linearity and Parallelism Assays
Analytical validations of RIA were demonstrated by examining the recovery after spiking the known concentration of Ang-(1–12) in plasma, and linearity between different amounts of samples were used for quantification and parallelism assays. The recovery was tested by spiking 0 to 15 ng of non-radiolabeled Ang-(1–12) into 250 μL of pooled plasma containing inhibitors. Ang-(1–12) was extracted from plasma using C18 columns and processed as described above to quantify the Ang-(1–12) from the Ang-(1–12) standard calibration curve. Recovery (%) was calculated as the difference between theoretical and measured values of Ang-(1–12) in pooled plasma samples with or without the added standard Ang-(1–12).
Ang-(1–12) was quantified using different volumes of pooled plasma and urine samples to determine the linearity. In this assay, Ang-(1–12) was extracted using C18 column using different volumes of pooled plasma (100, 250, 500 and 750 μL) and pooled urine (100, 200, 300, 400, 500 and 600 μL) and quantified as described above. The validity of the Ang-(1–12) RIA was demonstrated by parallelism between the series of Ang-(1–12) standards (0 to 75 ng/0.2 mL RIA tube) and serial dilutions (1:4 to 1:40) of pooled plasma/urine extracts. Ang-(1–12) was extracted from each diluted sample and quantified using polyclonal antibody, as described above. The standard curve was plotted using the same y and x axis with the same scale to demonstrate the parallelism of serially diluted pooled plasma and urine.
Human Ang-(1–12)-Polyclonal Antibody Neutralization Assay
For antigen-antibody in vitro neutralization assay, the radiolabeled human 125I-Ang-(1–12) antigen was pre-mixed with or without affinity purified polyclonal antibody fraction and then exposed to rhChymase or rat serum ACE. The radiolabeled 125I-Ang II product formation from Ang-(1–12) by rhChymase/ACE was analyzed as previously described by us (6–8). Briefly, the 125I-Ang-(1–12) [1 nmol/L; specific activity 3,900 cpm/fmol] in a total 200 μL of PBS (pH 7.4) was pre-adsorbed with or without affinity purified polyclonal antibody fraction (2 μg) for 5 min and then incubated with rhChymase (0.325 μg/mL) for 30 min or 10 μL of rat serum for 60 min at 37oC. Excluding the ACE inhibitor (lisinopril), we added all other RAS inhibitors (chymostatin for chymase, MLN-4760 for ACE2 and SCH39370 for neprilysin; each 50 μM) and peptidase inhibitors (amastatin, bestatin, benzyl succinate and p-chloromercuribenzoate; each 50 μM) in rat serum reaction tubes. At the end of incubation time, the reactions were stopped by adding equal volume of 1% phosphoric acid, mixed well, centrifuged at 28,000 g for 20 min and injected on the HPLC. The metabolic product (125I-Ang II) was separated from 125I-Ang-(1–12) substrate on a C18 column using a linear gradient from 10% to 50% mobile phase B at a flow rate of 0.35 mL/min at 32oC. The solvent system consisted of 0.1% phosphoric acid (mobile phase A) and 80% acetonitrile/0.1% phosphoric acid (mobile phase B). The eluted 125I-Ang II product was monitored by an in-line flow-through gamma detector (BioScan Inc., Washington, DC). Products were identified by comparison of retention of synthetic [125I] standard peptides and data were analyzed with the Shimadzu LCSolution (Kyoto, Japan) acquisition software. Experiments were performed three or more times.
Statistical Analysis
Experiments were repeated three or more times. Quantification of Ang-(1–12) by RIA in human samples was done in triplicate or quadruplicate tubes. Data were analyzed using GraphPad PRISM 8.0 software (San Diego, CA) and are presented as mean ± SE.
Results and Discussion
Radiolabeled Human Ang-(1–12) for RIA
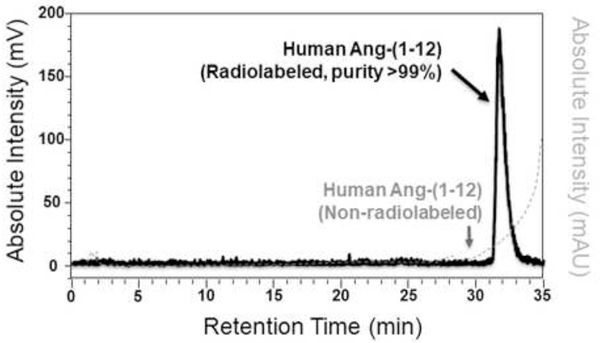
Human Ang-(1–12) peptide was radiolabeled with 125I and purified by reverse-phase high performance liquid chromatography (HPLC) in our laboratory as documented in various publications by us, elsewhere (6–8). As shown in Figure 1, the purity of the radiolabeled human 125I-Ang-(1–12) peptide was ≥99% and was free of un-labeled Ang-(1–12). The purity of radiolabeled 125I-Ang-(1–12) was routinely checked on the HPLC to confirm that the 125I-Ang-(1–12) peptide did not degrade at the time it was used for RIA method validations and human Ang-(1–12) measurements.
Figure 1.
HPLC chromatogram of purified radiolabeled human 125I-Ang-(1–12). HPLC chromatogram shows the purity (>99%) of 125I-Ang-(1–12). Human Ang-(1–12) was radiolabeled with 125I using oxidant chloromine-T and purified on a C18 column by HPLC. A highly purified 125I-Ang-(1–12) fraction that was free of un-labeled Ang-(1–12) and used in this RIA measurement.
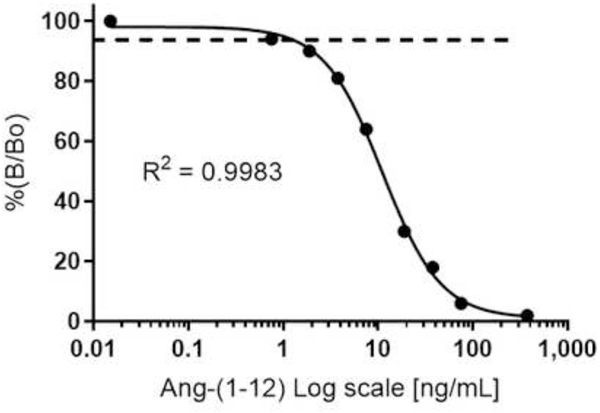
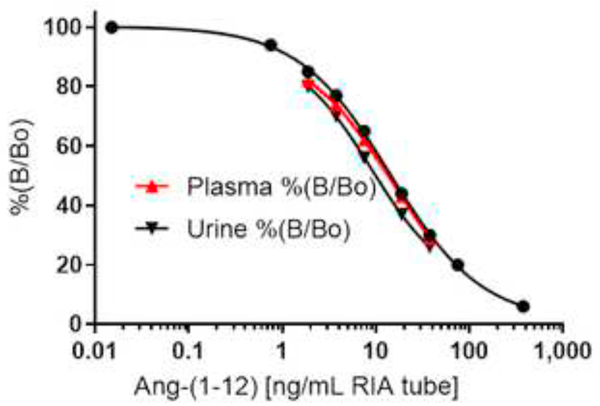
Calibration Curve and Detection Limit
Using a highly pure synthetic human Ang-(1–12) peptide (purity ≥98.5%) and radiolabeled 125I-Ang-(1–12) (purity ≥99%), calibration curves were established for Ang-(1–12) standard ranging from 0 – 75 ng/0.2 mL RIA tube (Figure 2). The horizontal line at 95% is the limit of detection. The lower limit of detection is defined as the lowest point of the calibration curve with a precision of coefficient of variation (CV) <10%. The limit of detection is defined as the lowest concentration of Ang-(1–12) peptide that can give a signal at least three times higher than the RIA tube having no cold standard peptide (0 ng/mL). We found that the lowest detection limit of this affinity purified polyclonal antibody fraction for the human Ang-(1–12) peptide was 0.15 ng/0.2 mL per RIA tube (0.75 ng/mL).
Figure 2.
Human Ang-(1–12) standard calibration curve. The standard human Ang-(1–12) calibration curve ranges from 0 to 375 ng/mL RIA buffer (log scale). Ang-(1–12) level in human plasma and spot urine are representative of averages (Mean ± SE) of triplicate and quadruplicate assays.
Anti-Ang-(1–12) Polyclonal Antibody Specificity and Cross-reactivity
Our custom-made and affinity purified polyclonal antibody fraction (rabbit #4) binding assay reveals that the C-terminus directed human Ang-(1–12) antibody has a high specificity for only the human Ang-(1–12) sequence. The anti-Ang-(1–12) polyclonal antibody shows negligible cross-reactivity with closely related smaller angiotensin peptides [Ang I, Ang-(1–9), Ang II and Ang-(1–7)] in RIA buffer (Table 1). The polyclonal antibody also shows negligible cross-reactivity with human AGT proteins (0% with Sep-Pak vs 3% without Sep-Pak) at concentration 15 μg/0.2 mL per RIA tube. The endogenous concentrations of closely related smaller angiotensin peptides were reported in the range of 0.5 pg/mL to 272 pg/mL or AGT (28 to 71 μg/mL) in human plasma and urine samples from healthy individuals and hypertensive subjects measured by various methods, ELISA/RIA/LC-MS (Table 2) (14–33). Compared to the verified endogenous level of related angiotensin peptides reported in plasma from healthy and diseased subjects, we used several fold higher concentrations of closely related small-sized angiotensin peptides (7.5 ng/0.2 mL per RIA tube) or human AGT (15 μg/0.2 mL per RIA tube) for cross-reactivity testing. Even at this high concentration, the closely related angiotensin peptides or AGT showed negligible cross-reactivity (≤3%, Table 1). Further, our Sep-Pak data suggests that the AGT protein has not been retained by the C18 column and it was removed after sequentially washing the column with HFBA and ultra-pure MilliQ water. Specificity and cross-reactivity results clearly show that the C-terminus anti-Ang-(1–12) polyclonal antibody is highly specific and recognizes only the human Ang-(1–12) sequence. Closely related angiotensin peptides and human AGT have negligible cross-reactivity in the RIA measurement of Ang-(1–12) in human plasma and urine samples using this anti-Ang-(1–12) polyclonal antibody.
Table 1:
Specificity and cross-reactivity of human Ang-(1–12) polyclonal antibody (rabbit #4) with angiotensin peptides and human angiotensinogen (AGT) protein.
| Human Angiotensin Peptides | % Cross-Reactivity |
|---|---|
| Angiotensin-(1–12) | 100% |
| Angiotensin I | 3% |
| Angiotensin-(1–9) | 0% |
| Angiotensin II | 0% |
| Angiotensin-(1–7) | 0% |
| AGT, human (with C18 Sep-Pak) | 0% |
| AGT, human (without C18 Sep-Pak) | 3% |
To confirm the cross-reactivity of the affinity purified polyclonal antibody fraction (rabbit #4) with other closely related angiotensin peptides [Ang I, Ang-(1–9), Ang II and Ang-(17)], several fold higher amounts than the physiological level of angiotensin peptides (7.5 ng/0.2 mL RIA tube) were used. Cross-reactivity was tested for with or without C18 Sep-Pak human AGT protein (15 μg/0.2 mL RIA tube). Percent cross-reactivity is defined as the ratio of observed values of the Ang-(1–12) to the angiotensin peptides, multiplied by 100. Details of the cross-reactivity analysis are described in Methods section. Cross-reactivity assays were done in triplicate or quadruplicate tubes.
Analytical Validations of RIA
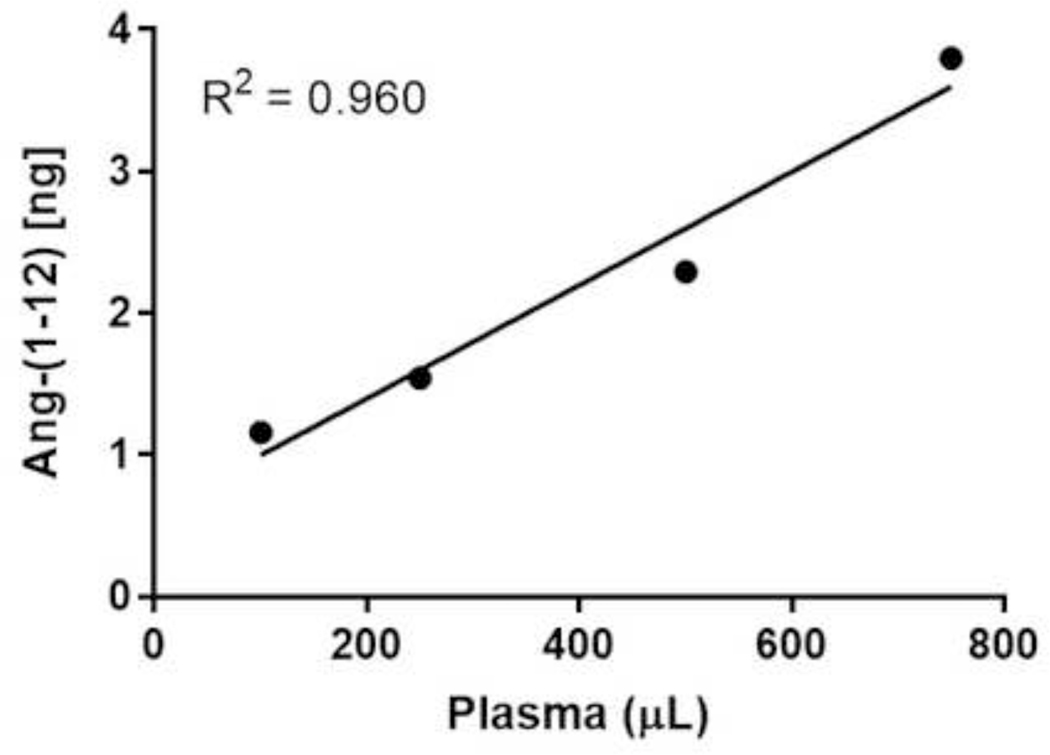
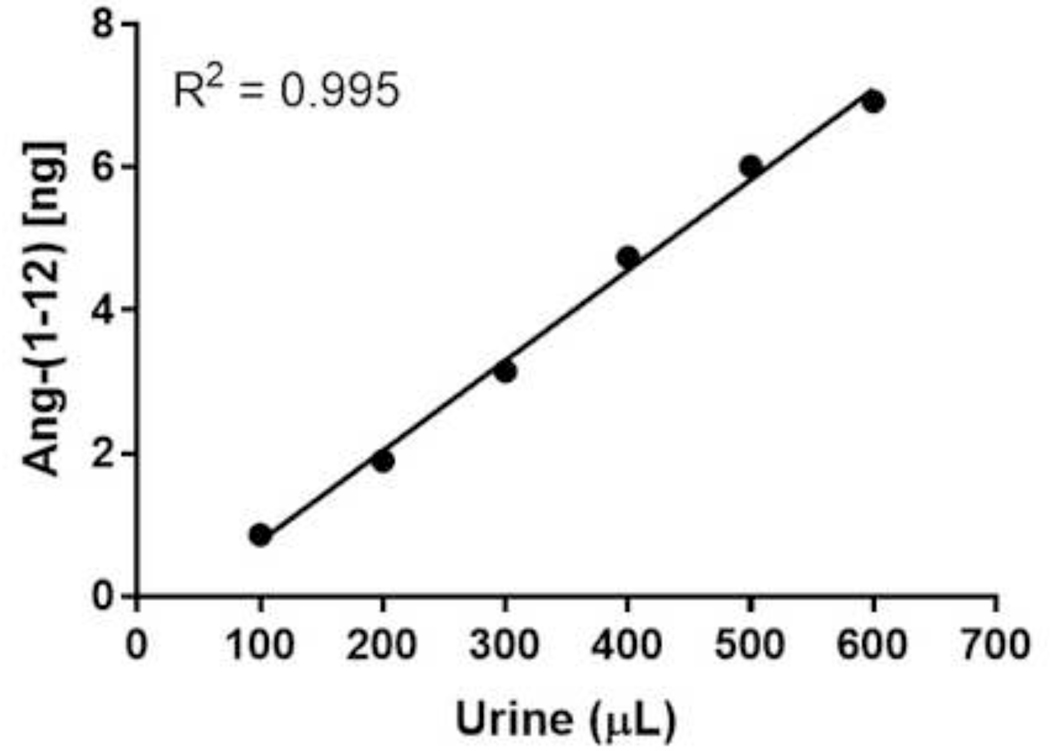
The RIA described here was analytically sensitive, linear, accurate, precise and reproducible for the quantification of Ang-(1–12) peptide in blood and urine extract. The assay was validated by determining the spiked recovery, linearity between the different amount of human fluids used for quantification, parallelism, intra- and interassay variability. Assay validation experiments demonstrated a ≥97% recovery when known amounts of Ang-(1–12) (0 – 15 ng/0.2 mL per RIA tube) were spiked to the human pooled plasma samples (Table 3). Complete recovery of the Ang-(1–12) added to the plasma samples, suggests that the Ang-(1–12) fragment is stable in the conditions used for RIA measurements. RIA assays show linearity (R2 = 0.960 and 0.995, respectively) when different volumes of pooled plasma (100 μL to 750 μL) and urine (100 μL to 600 μL) samples were used for Ang-(1–12) quantification (Figure 3 and 4). The displacement curves showed parallelism between the serially diluted pooled plasma/urine against the standard Ang-(1–12) (Figure 5). These analytical tests validate that the newly developed RIA method is reliable to recognize the Ang-(1–12) fragment.
Table 3:
Addition and recovery tests of Ang-(1–12) supplemented to human plasma (pooled samples).
| Pooled Plasma (+Spiking, ng/tube) | Theoretical Value (ng/tube) | Measured Value (ng/tube) | Recovery (%) |
|---|---|---|---|
| Plasma alone | 1.77 | ||
| +0.75 ng/tube | 2.52 | 2.58 | 102% |
| +1.5 ng/tube | 4.09 | 3.48 | 106% |
| +3.75 ng/tube | 5.54 | 5.59 | 101% |
| +7.5 ng/tube | 9.31 | 9.74 | 105% |
| +15.0 ng/tube | 16.85 | 16.33 | 97% |
Ang-(1–12) was measured by RIA in pooled plasma [(+)Inhibitors] alone and after spiking with a known amount (0.75, 1.5, 3.75, 7.5 and 15.0 ng/tube) of standard Ang-(1–12) in each tube having 250 μL of plasma. Recovery (%) was calculated as the difference between theoretical and measured values of Ang-(1–12) in pooled plasma samples with or without the added standard Ang-(1–12). Details of recovery assays are described in Methods section.
Figure 3.
Linearity assay of pooled plasma. Ang-(1–12) was quantified after extracting the different volumes (100, 250, 500 and 750 μL) of pooled plasma samples. Plasma peptides were extracted using a C18 Sep-Pak column. The eluted samples were dried and RIA was performed using a highly purified radiolabeled 125I-Ang-(1–12) and affinity purified polyclonal antibody fraction (1:400 dilution). The RIA of different volumes of plasma sample were performed in quadruplicate.
Figure 4.
Linearity as s ay of pooled s pot urine. Ang-(1–12) was quantified after extracting the different volumes (100, 200, 300, 400, 500 and 600 μL) of pooled spot urine samples. P ooled urine peptides were extracted using C18 Sep-Pak column. The eluted samples were dried and RIA was performed using a highly purified radiolabeled 125I-Ang-(1–12) and affinity purified polyclonal antibody fraction (1:400 dilution). The RIA of different volumes of pooled urin esample were performed in quadruplicate.
Figure 5.
Parallelism assay of serially diluted pooled plasma and spoturine. Parallelism assay between the series of Ang-(1–12) standards (0 to 375 ng/mL RIA tube) and serial dilutions (1:4 to 1:40) of pooled plasma/urine samples and Ang-(1–12) were extracted using C18 Sep-Pak column. The eluted samples were dried and RIA was performed using a highly purified radiolabeled 125I-Ang-(1–12) and affinity purified polyclonal antibody fraction (1:400 dilution). The RIA of serial diluted pooled plasma and urine samples were performed in quadruplicate.
Intra- and Inter-assay Variation
The percentages of coefficient (% CV) of intra- and inter-assay variation ranged between 3.4%–13.9% and 1.0%–10.7%, and for the spot urine samples between 3.4%–18.9% and 1.8%–15.3%, respectively.
Plasma and Urine Ang-(1–12) Measurements
As documented in the Methods section, this affinity purified antibody fraction shows very high specificity towards human Ang-(1–12) when compared to the closely related smaller angiotensin peptides with distinctly different amino acids at the C-terminal end. Furthermore, the anti-Ang-(1–12) antibody does not cross-react with any closely related angiotensin peptides [Ang I, Ang-(1–9), Ang II and Ang-(1–7)] at endogenous concentrations reported in human plasma and urine (14, 33–37).
Using this polyclonal antibody, the assay conditions for Ang-(1–12) measurements were identified in individual blood samples drawn from five adult volunteers (3 males and 2 females, age range 20–55 years) and collected with and without a previously reported inhibitor cocktail (4, 38, 39). Similarly, collected spot urine samples were processed in the presence and absence of HCl (described in Methods section). The endogenous Ang-(1–12) levels were quantified from a linear calibration standard plot ranging from 0 – 75 ng/0.2 mL per RIA tube of synthetic human Ang-(1–12) peptide (Figure 2). Endogenous Ang-(1–12) was identified in all human plasma and spot urine samples collected with or without the addition of an inhibitor cocktail (for blood) or 1N HCl (for urine), respectively. Further analysis demonstrated the need for the collection of blood in the presence of protease inhibitors as plasma Ang-(1–12) concentrations averaged 38% higher in the presence of the inhibitor cocktail compared to the no inhibitor-spiked samples. Similarly, in urine collected and processed in HCl, the Ang-(1–12) concentrations were 22% higher compared to samples processed without HCl.
After optimizing the sample collection and processing conditions, this newly developed RIA method was applied to the quantification of Ang-(1–12) levels in peripheral venous blood samples obtained in the presence of inhibitor cocktail and spot urine processed in the presence of HCl (described in the Methods section) from 34 hypertensive patients (29 patients were treated with antihypertensive medications and 5 patients remained untreated). For analyses, we divided the patients into uncontrolled hypertension (irrespective of treatment), defined by a systolic pressure ≥140 mm Hg, and controlled hypertension, defined by a systolic pressure <140 mm Hg. Circulating Ang-(1–12) was detected in all venous samples (n = 34) with a range between 0.72 ng/mL and 2.87 ng/mL. We found that plasma Ang-(1–12) levels were significantly higher in uncontrolled hypertensive compared to those with controlled hypertension (Mean ± SE, 2.09 ± 0.14 ng/mL and 1.65 ± 0.11 ng/mL, respectively; P < 0.05) (Table 4). As illustrated in Table 4, urinary Ang-(1–12) spot levels are several orders of magnitude higher than the values found in venous blood. Urinary Ang-(1–12) levels, almost 10-fold higher than those recorded in the plasma, were modestly lower in patients with systolic pressure ≥140 mm Hg when compared to patients with systolic pressure <140 mm Hg (11.09 ± 1.38 ng/mL and 18.70 ± 3.89 ng/mL, respectively; P = 0.22). As expected, systolic blood pressure (147 ± 3 and 128 ± 2 mm Hg, respectively; P < 0.0001), diastolic blood pressure (89 ± 3 and 82 ± 2 mm Hg, respectively; P < 0.01) and pulse pressure (55 ± 2 and 47 ± 2 mm Hg, respectively; P < 0.01) were significantly higher in patients with uncontrolled hypertension when compared to patients with controlled hypertension (Table 4). Plans for a large prospective study are underway, in which we will include normotensive control, untreated, and anti-hypertensive medication treated patients.
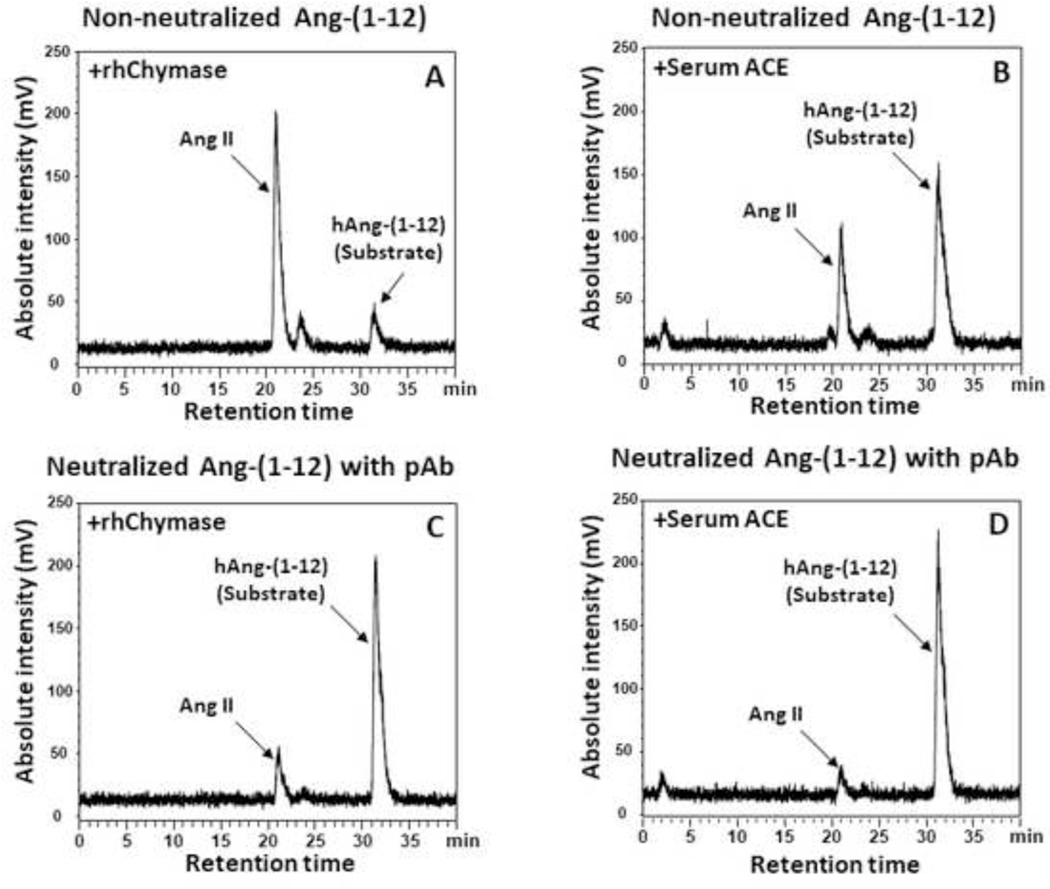
Neutralization Effect of Ang-(1–12) Polyclonal Antibody
Our in vitro study shows that the cleavage sites of the Ang-(1–12) sequence was neutralized by the polyclonal antibody and significantly decreased the rhChymase and ACE-mediated Ang II generation from human Ang-(1–12) substrate (Figure 6). Analysis of the HPLC chromatograms show that the rhChymase and ACE-mediated Ang II generation from Ang-(1–12) was decreased to 17% and 7% in the presence of the antibody compared to 81% and 35% (without antibody), respectively. These studies clearly demonstrate that the polyclonal antibody against the human Ang-(1–12) sequence blocks the cleavage sites of ACE and chymase that are primarily responsible for Ang II generation from Ang-(1–12) in circulation and cardiac tissue, respectively. These findings further show that our polyclonal antibody is highly specific in recognizing the human Ang-(1–12) sequence.
Figure 6.
Neutralization of Ang-(1–12) with polyclonal antibody. In vitro incubation of human 125 I-Ang-(1–12) [1 nmol/L] with or without affinity purified polyclonal antibody fraction (2 μg) and then rh Chymase (0.325 μg/mL) for 30 min or rat serum ACE (10 μL) of or 60 min at 37°C in PBS (pH 7.4). Reactions were stopped by 1% phosphoric acid, mixed well, centrifuged at 28,000 g for 20 min and injected on C 18 HPLC column. The metabolic product (125I-Ang II) was separated from I-Ang-(1–12) substrate on C18 column using a linear gradient from 10% to 50% mobile phase B at a flow rate of 0.35 m L/min at 32°C. The HPLC chromatogram results are representative of three or more separate experiments.
An emergent robust literature implicates the Ang-(1–12)/chymase axis as a critical pathway for direct Ang II production and as a potential biomarker of tissue-dependent Ang II pathological functions. The absence of a precise and sensitive method for direct Ang-(1–12) quantification in human biological fluids is now circumvented by the generation of specific polyclonal Ang-(1–12) antibodies expressing minimal, if any, cross-reactivity with downstream angiotensin peptides. In an in vitro study, Ang-(1–12) has been reported as the primary metabolic product generated from the exogenous angiotensin-(1–14) substrate in rat aortic tissue by liquid chromatography-mass spectrometry (LC-MS) methods (40). We also quantified Ang-(1–12) levels in human plasma using a LC-MS. The Ang-(1–12) amounts detected by LC-MS was relatively lower compared to the same samples quantified by RIA method (unpublished results). Since the quantification of the Ang-(1–12) standard synthetic peptide (purity >98.5%) is relatively consistent in the standard solution compared to plasma, we believe LC-MS analysis is associated with higher matrix effect of our target analyte in plasma that might compromise its sensitivity and selectivity, thus reducing the accuracy, precision, and robustness of its application for our analysis. We have validated, in previous studies, the higher sensitivity of RIA over LC-MS for measurements of cardiac tissue Ang-(1–12) (41). As detailed by Chappell (2016), the quantification of angiotensin peptides by LC-MS methods yield values that are not consistent among studies (42).
Human AGT protein concentrations are in the range of μg/mL in plasma and ng/mL in urine (34–37). Studies show that AGT concentrations measured in the plasma of healthy volunteers by ELISA ranges from 28 to 71 μg/mL (Table 2) (34). Based on the Ang-(1–12) concentration detected in the plasma samples from patients with systolic pressure <140 (averaged 1.65 ± 0.11 ng/mL, +inhibitors cocktail, Table 4) by RIA method, the ratio of Ang-(1–12) to the plasma AGT level reported in healthy volunteers (Table 2) ranged from 0.002% to 0.006%. However, the plasma Ang-(1–12) level was around 12 to 66-fold higher when compared to the Ang I level reported in normal human male subjects (25–143 pg/mL plasma, Table 2) (14). The data reported by Reddy et al. (2020) from the plasma of patients with the acute respiratory distress syndrome (ARDS) documents plasma Ang-(1–12) median concentrations in ARDS patients measured by LC-MS were 0.05 ng/mL (interquartile range 0.05 – 0.64) in non-survivors and 0.05 ng/mL (interquartile range 0.05 – 1.97) in survivors (43). These values are within the range of the data reported in our study. Circulating and urinary RAS components (including angiotensin peptides metabolizing enzymes and substrates) are altered in hypertensive and renal-diseased subjects (1, 25, 26, 30, 44–48). Influence of gender, genetic and ethnicity-related variability on plasma and urinary angiotensin peptides levels were also reported (25, 32, 33). Higher levels of the Ang-(1–7) were found in the plasma of men compared to women (25, 32). Angiotensin peptide analysis in an Afro-Caribbean population showed that Ang II levels were comparable between genders, while urinary Ang-(1–7) levels were greater in females (33). The much higher concentrations of Ang-(1–12) in the patients with elevated systolic pressure (≥140 mm Hg) compared to Ang I in plasma and urine (Table 2) suggests that the Ang-(1–12) may serve as a primary substrate for Ang II generation through a non-renin dependent mechanism. Its role as a hypertensive biomarker or as a discriminating element in the selection of pharmacotherapy regimens has now become possible. Evidence that plasma Ang-(1–12) may serve as a biomarker of ARDS morbidity further strengthen a rationale for investigating this peptide as a sensitive discriminative biomarker of cardiovascular disease.
Conclusions
RIA is a highly reliable antibody-based method used in pre-clinical and clinical diagnostic settings for quantification of specific metabolic biomarkers. To date, there have been no specific and reliable methods to quantify human Ang-(1–12) in human biological fluids. The commercially available ELISA kit by Peninsula Laboratories/BMA Biomedicals, Switzerland (Product number S-1343) is also not suitable to quantify human Ang-(1–12) in biological samples because the antibody shows significant cross reactivity (13%) with the Ang-(1–9) sequence and has not been tested for cross reactivity with human AGT protein. Detailed studies dealing with the expression and function of Ang-(1–12) as an Ang II forming substrate in humans are lacking while no studies had being undertaken to evaluate whether or not this alternate Ang II-forming, novel substrate may be a better marker for the progression of cardiovascular disease and/or chronic kidney disease compared to plasma renin activity (PRA) or even Ang II. This is important since abnormal PRA values are found in less than 20% of the hypertensive population (49). Urinary AGT has been reported to be a marker of renal RAS expression and its concentration in human urine correlates with blood pressure (50, 51). Detection of Ang-(1–12) in human urine suggests processing of AGT into the dodecapeptide. Characterization of Ang-(1–12) in human urine will now allow for the determination of whether both AGT and the dodecapeptide are comparable biomarkers of renal disease, and to what extent, if any, the current commercially available AGT ELISA assay show cross-reactivity with Ang-(1–12).
For the first time, we have generated and characterized a polyclonal antibody against the C-terminus of the human Ang-(1–12) sequence to advance the clinical viability of Ang-(1–12) as a cardiovascular disease biomarker. We identify for the first time the presence of Ang-(1–12) in the blood and urine in human subjects and demonstrate significantly higher concentrations of this Ang II-forming substrate in patients with elevated systolic pressure (≥140 mm Hg). The absence of corresponding changes in the much higher concentrations of Ang-(1–12) in urine of patients with high systolic pressure further implicates a differential regulation of the substrate expression between the circulation and kidneys. Earlier, we showed that kallikrein or a kallikrein-like enzyme might be responsible for the release of Ang-(1–12) fragment from the angiotensinogen protein (13, 41, 52). Increases in plasma AGT levels are associated with essential hypertension (53).
Acknowledgment
This work was supported by a grant from the National Heart, Lung and Blood Institute of the NIH (P01 HL-051952).
Footnotes
Ethical Approval
This study was approved by the bioethics committee of the Wake Forest Medical Center (IRB number BG05-516) and by Sterling IRB Review Board (Sterling IRB number 7175-HAPunzi).
Disclosure
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ferrario CM, Chappell MC, Dean RH, Iyer SN. Novel angiotensin peptides regulate blood pressure, endothelial function, and natriuresis. J Am Soc Nephrol. 1998;9(9):1716–22. [DOI] [PubMed] [Google Scholar]
- 2.Ferrario CM, Trask AJ, Jessup JA. Advances in biochemical and functional roles of angiotensin-converting enzyme 2 and angiotensin-(1–7) in regulation of cardiovascular function. Am J Physiol Heart Circ Physiol. 2005;289(6):H2281–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao T, Feng Y. The (pro)renin receptor and body fluid homeostasis. Am J Physiol Regul Integr Comp Physiol. 2013;305(2):R104–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brosnihan KB, Chappell MC. Measurement of Angiotensin Peptides: HPLC-RIA. Methods Mol Biol. 2017;1527:81–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang T, Xu C. Physiology and Pathophysiology of the Intrarenal Renin-Angiotensin System: An Update. J Am Soc Nephrol. 2017;28(4):1040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad S, Simmons T, Varagic J, Moniwa N, Chappell MC, Ferrario CM. Chymase-dependent generation of angiotensin II from angiotensin-(1–12) in human atrial tissue. PLoS One. 2011;6(12):e28501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad S, Varagic J, Westwood BM, Chappell MC, Ferrario CM. Uptake and metabolism of the novel peptide angiotensin-(1–12) by neonatal cardiac myocytes. PLoS One. 2011;6(1):e15759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmad S, Wei CC, Tallaj J, Dell’Italia LJ, Moniwa N, Varagic J, et al. Chymase mediates angiotensin-(1–12) metabolism in normal human hearts. J Am Soc Hypertens. 2013;7(2):128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrario CM, Ahmad S, Varagic J, Cheng CP, Groban L, Wang H, et al. Intracrine angiotensin II functions originate from noncanonical pathways in the human heart. Am J Physiol Heart Circ Physiol. 2016;311(2):H404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagata S, Kato J, Sasaki K, Minamino N, Eto T, Kitamura K. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun. 2006;350(4):1026–31. [DOI] [PubMed] [Google Scholar]
- 11.Chappell MC. Nonclassical renin-angiotensin system and renal function. Compr Physiol. 2012;2(4):2733–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrario CM, Groban L, Iyer S, Ahmad S, Wright KN, Burnett J. Human angiotensin-(1–12) [Ang-(1–12)] is a hypertension and cardiac disease biomarker. The FASEB Journal. 2020.
- 13.Ferrario CM, Ahmad S, Nagata S, Simington SW, Varagic J, Kon N, et al. An evolving story of angiotensin-II-forming pathways in rodents and humans. Clin Sci (Lond). 2014;126(7):461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lijnen PJ, Amery AK, Fagard RH. Endogenous angiotensin I concentration in human plasma. J Lab Clin Med. 1978;92(3):353–62. [PubMed] [Google Scholar]
- 15.Lawrence AC, Evin G, Kladis A, Campbell DJ. An alternative strategy for the radioimmunoassay of angiotensin peptides using amino-terminal-directed antisera: measurement of eight angiotensin peptides in human plasma. J Hypertens. 1990;8(8):715–24. [DOI] [PubMed] [Google Scholar]
- 16.Campbell DJ, Kladis A. Simultaneous radioimmunoassay of six angiotensin peptides in arterial and venous plasma of man. J Hypertens. 1990;8(2):165–72. [DOI] [PubMed] [Google Scholar]
- 17.Johnson H, Kourtis S, Waters J, Drummer OH. Radioimmunoassay for immunoreactive [des-Leu10]-angiotensin I. Peptides. 1989;10(3):489–92. [DOI] [PubMed] [Google Scholar]
- 18.Miller JA, Anacta LA, Cattran DC. Impact of gender on the renal response to angiotensin II. Kidney Int. 1999;55(1):278–85. [DOI] [PubMed] [Google Scholar]
- 19.Schulz A, Jankowski J, Zidek W, Jankowski V. Absolute quantification of endogenous angiotensin II levels in human plasma using ESI-LC-MS/MS. Clin Proteomics. 2014;11(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jalil JE, Palomera C, Ocaranza MP, Godoy I, Roman M, Chiong M, et al. Levels of plasma angiotensin-(1–7) in patients with hypertension who have the angiotensin-I-converting enzyme deletion/deletion genotype. Am J Cardiol. 2003;92(6):749–51. [DOI] [PubMed] [Google Scholar]
- 21.Merrill DC, Karoly M, Chen K, Ferrario CM, Brosnihan KB. Angiotensin-(1–7) in normal and preeclamptic pregnancy. Endocrine. 2002;18(3):239–45. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Li J, Hao P, Chen W, Meng X, Li H, et al. Imbalance between angiotensin II and angiotensin-(1–7) in human coronary atherosclerosis. J Renin Angiotensin Aldosterone Syst. 2016;17(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schindler C, Ferrario CM, Jatzke C, Ahner K, Brosnihan KB, Bramlage P, et al. Characterization of vascular reactivity in dorsal hand veins after oral rosiglitazone treatment in healthy subjects. Int J Clin Pharmacol Ther. 2008;46(1):30–9. [DOI] [PubMed] [Google Scholar]
- 24.Xu Z, Xu B, Xu C. Urinary Angiotensinogen as a Potential Biomarker of Intrarenal Renin-angiotensin System Activity in Chinese Patients with Chronic Kidney Disease. West Indian Med J. 2014;63(5):436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrario CM, Jessup JA, Smith RD. Hemodynamic and hormonal patterns of untreated essential hypertension in men and women. Ther Adv Cardiovasc Dis. 2013;7(6):293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrario CM, Martell N, Yunis C, Flack JM, Chappell MC, Brosnihan KB, et al. Characterization of angiotensin-(1–7) in the urine of normal and essential hypertensive subjects. Am J Hypertens. 1998;11(2):137–46. [DOI] [PubMed] [Google Scholar]
- 27.Ferrario CM, Smith RD, Brosnihan B, Chappell MC, Campese VM, Vesterqvist O, et al. Effects of omapatrilat on the renin-angiotensin system in salt-sensitive hypertension. Am J Hypertens. 2002;15(6):557–64. [DOI] [PubMed] [Google Scholar]
- 28.Nussberger J, Brunner DB, Nyfeler JA, Linder L, Brunner HR. Measurement of immunoreactive angiotensin-(1–7) heptapeptide in human blood. Clin Chem. 2001;47(4):726–9. [PubMed] [Google Scholar]
- 29.Hildebrand D, Merkel P, Eggers LF, Schluter H. Proteolytic processing of angiotensin-I in human blood plasma. PLoS One. 2013;8(5):e64027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basu R, Poglitsch M, Yogasundaram H, Thomas J, Rowe BH, Oudit GY. Roles of Angiotensin Peptides and Recombinant Human ACE2 in Heart Failure. J Am Coll Cardiol. 2017;69(7):805–19. [DOI] [PubMed] [Google Scholar]
- 31.Hermann K, Rittweger R, Ring J. Urinary excretion of angiotensin I, II, arginine vasopressin and oxytocin in patients with anaphylactoid reactions. Clin Exp Allergy. 1992;22(9):845–53. [DOI] [PubMed] [Google Scholar]
- 32.Reyes-Engel A, Morcillo L, Aranda FJ, Ruiz M, Gaitan MJ, Mayor-Olea A, et al. Influence of gender and genetic variability on plasma angiotensin peptides. J Renin Angiotensin Aldosterone Syst. 2006;7(2):92–7. [DOI] [PubMed] [Google Scholar]
- 33.Cohall DH, Scantlebury-Manning T, James S, Hall K, Ferrario CM. Reninangiotensin-aldosterone system gender differences in an Afro-Caribbean population. J Renin Angiotensin Aldosterone Syst. 2015;16(3):539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katsurada A, Hagiwara Y, Miyashita K, Satou R, Miyata K, Ohashi N, et al. Novel sandwich ELISA for human angiotensinogen. Am J Physiol Renal Physiol. 2007;293(3):F956–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobori H, Alper AB Jr., Shenava R, Katsurada A, Saito T, Ohashi N, et al. Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension. 2009;53(2):344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saito T, Urushihara M, Kotani Y, Kagami S, Kobori H. Increased urinary angiotensinogen is precedent to increased urinary albumin in patients with type 1 diabetes. Am J Med Sci. 2009;338(6):478–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juretzko A, Steinbach A, Hannemann A, Endlich K, Endlich N, Friedrich N, et al. Urinary Angiotensinogen and Renin Excretion are Associated with Chronic Kidney Disease. Kidney Blood Press Res. 2017;42(1):145–55. [DOI] [PubMed] [Google Scholar]
- 38.Kohara K, Tabuchi Y, Senanayake P, Brosnihan KB, Ferrario CM. Reassessment of plasma angiotensins measurement: effects of protease inhibitors and sample handling procedures. Peptides. 1991;12(5):1135–41. [DOI] [PubMed] [Google Scholar]
- 39.Chappell MC. Biochemical evaluation of the renin-angiotensin system: the good, bad, and absolute? Am J Physiol Heart Circ Physiol. 2016;310(2):H137–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bujak-Gizycka B, Olszanecki R, Suski M, Madek J, Stachowicz A, Korbut R. Angiotensinogen metabolism in rat aorta: robust formation of proangiotensin-12. J Physiol Pharmacol. 2010;61(6):679–82. [PubMed] [Google Scholar]
- 41.Ferrario CM, VonCannon J, Ahmad S, Wright KN, Roberts DJ, Wang H, et al. Activation of the Human Angiotensin-(1–12)-Chymase Pathway in Rats With Human Angiotensinogen Gene Transcripts. Front Cardiovasc Med. 2019;6:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chappell MC. Reply to “Letter to the editor: Angiotensin quantification by mass spectrometry”. Am J Physiol Heart Circ Physiol. 2016;310(3):H454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy R, Asante I, Liu S, Parikh P, Liebler J, Borok Z, et al. Circulating angiotensin peptides levels in Acute Respiratory Distress Syndrome correlate with clinical outcomes: A pilot study. PLoS One. 2019;14(3):e0213096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrario CM. Cardiac remodelling and RAS inhibition. Ther Adv Cardiovasc Dis. 2016;10(3):162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luque M, Martin P, Martell N, Fernandez C, Brosnihan KB, Ferrario CM. Effects of captopril related to increased levels of prostacyclin and angiotensin-(1–7) in essential hypertension. J Hypertens. 1996;14(6):799–805. [DOI] [PubMed] [Google Scholar]
- 46.Umemura S, Nyui N, Tamura K, Hibi K, Yamaguchi S, Nakamaru M, et al. Plasma angiotensinogen concentrations in obese patients. Am J Hypertens. 1997;10(6):629–33. [DOI] [PubMed] [Google Scholar]
- 47.Ola MS, Alhomida AS, Ferrario CM, Ahmad S. Role of Tissue Renin-angiotensin System and the Chymase/angiotensin-( 1–12) Axis in the Pathogenesis of Diabetic Retinopathy. Curr Med Chem. 2017;24(28):3104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun W, Feng Y, Yao XD, Xu YF, Peng B, Liu M, et al. Urinary angiotensinogen is elevated in patients with nephrolithiasis. Biomed Res Int. 2014;2014:349602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manosroi W, Williams GH. Genetics of Human Primary Hypertension: Focus on Hormonal Mechanisms. Endocr Rev. 2018. [DOI] [PMC free article] [PubMed]
- 50.Kobori H, Navar LG. Urinary Angiotensinogen as a Novel Biomarker of Intrarenal Renin-Angiotensin System in Chronic Kidney Disease. Int Rev Thromb. 2011;6(2):108–16. [PMC free article] [PubMed] [Google Scholar]
- 51.Kobori H, Urushihara M, Xu JH, Berenson GS, Navar LG. Urinary angiotensinogen is correlated with blood pressure in men (Bogalusa Heart Study). J Hypertens. 2010;28(7):1422–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wei CC, Chen Y, Powell LC, Zheng J, Shi K, Bradley WE, et al. Cardiac kallikrein-kinin system is upregulated in chronic volume overload and mediates an inflammatory induced collagen loss. PLoS One. 2012;7(6):e40110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caulfield M, Lavender P, Newell-Price J, Kamdar S, Farrall M, Clark AJ. Angiotensinogen in human essential hypertension. Hypertension. 1996;28(6):1123–5. [DOI] [PubMed] [Google Scholar]