Abstract
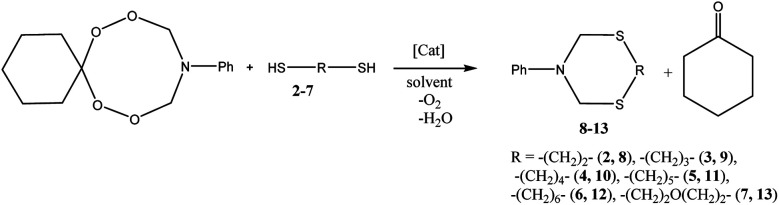
Co(OAc)2-catalyzed ring transformation reaction of 10-aryl-7,8,12,13-tetraoxa-10-azaspiro[5.7]tridecanes with α,ω-dithiols (ethane-1,2-, propane-1,3-, butane-1,4-, pentane-1,5-, and hexane-1,6-dithiols, 3,6-dioxaoctane-1,8-dithiol) giving 3-aryl-1,5,3-dithiazacyclanes was studied.
Co(OAc)2-catalyzed ring transformation reaction of 10-aryl-7,8,12,13-tetraoxa-10-azaspiro[5.7]tridecanes with α,ω-dithiols giving 3-aryl-1,5,3-dithiazacyclanes was studied.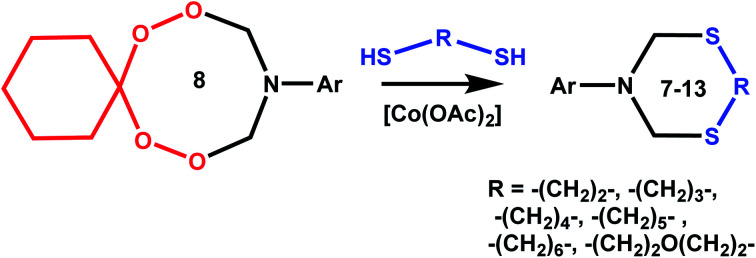
Cyclic peroxides attract attention for their antimalarial,1 antibacterial,2 and antitumor3 activities. Among numerous cyclic peroxides, heteroatomic cyclic peroxides occupy a special place owing to their high biological activities.4 The methods of synthesis of heteroatom-containing cyclic peroxides are limited. Recently,5–10 nitrogen- and sulfur-containing cyclic di- and triperoxides with antitumor activity have been synthesized.5–9 The development of efficient methods for the preparation of new cyclic hetero-di(tri)peroxides5–10 promotes active investigation of their transformations. It was shown that the reduction of silatriperoxycycloalkanes with PPh3 affords siladiperoxycycloalkanes;11 the reaction of spiro{adamantane-[2,3′]-(pentaoxacane)} with o-phenylenediamine results in the synthesis of benzodioxazocine.5 The implemented conversion of pentaoxacane with o-phenylenediamine to benzodioxazocine5 suggests that cyclic N-containing peroxides can be involved in reactions with binucleophilic reagents, in particular α,ω-dithiols, to give new heterocycles. In contrast to the previously described methods of synthesis5–10 and transformation of the peroxide ring,5,11 this work for the first time discusses the method of catalytic conversion of tetraoxazaspirotridecane to dithiazacycloalkanes.
It was shown by preliminary experiments that the reaction of 10-phenyl-7,8,12,13-tetraoxa-10-azaspiro[5.7]tridecane 1 with ethane-1,2-dithiol 2 does not proceed without a catalyst. The reaction of azadiperoxide 1 with ethane-1,2-dithiol 2 catalyzed by Sm(NO3)3·6H2O, H2SO4 or BF3·Et2O in THF as a solvent affords 3-phenyl-1,5,3-dithiazepane 8 in 10–15% yield (Scheme 1, Table 1). It was found that the yield of 3-phenyl-1,5,3-dithiazepane12 is affected by the nature of the catalyst. When the reaction is carried out in a polar solvent (MeOH) in the presence of catalytic amounts of Sm(NO3)3·6H2O, H2SO4 or BF3·Et2O, the yield of the target product 8 increases to 30%. In the presence of the Co(OAc)2 catalyst, the yield of heterocycle 8 is 85%. When AlCl3 or CuCl catalysts are used, the yields of heterocycle 8 are 55% and 75%, respectively (Table 1). Under these conditions, cyclohexanone is formed and O2 is released (Scheme 1). All reactions were carried out at room temperature for 20 h.
Scheme 1. Ring transformation reaction of 10-phenyl-7,8,12,13-tetraoxa-10-azaspiro[5.7]tridecane with α,ω-dithiols.
Effect of the catalyst and solvent nature on the yield of 3-phenyl-1,5,3-dithiazacyclanes (∼20 °C, 20 h).
| No. | Compound | [Cat] | Solvent | Yield, % |
|---|---|---|---|---|
| 1 | 8 | AlCl3 | THF | 45 |
| 2 | 8 | AlCl3 | MeOH | 55 |
| 3 | 8 | Co(OAc)2 | THF | 79 |
| 4 | 8 | Co(OAc)2 | MeOH | 85 |
| 5 | 8 | BF3·OEt2 | THF | 15 |
| 6 | 8 | BF3·OEt2 | MeOH | 30 |
| 7 | 8 | CuCl | THF | 68 |
| 8 | 8 | CuCl | MeOH | 75 |
| 9 | 8 | H2SO4 | THF | 13 |
| 10 | 8 | H2SO4 | MeOH | 25 |
| 11 | 8 | Sm(NO3)3·6H2O | THF | 10 |
| 12 | 8 | Sm(NO3)3·6H2O | MeOH | 20 |
| 13 | 8 | — | THF | — |
| 14 | 8 | — | MeOH | — |
| 15 | 9 | Co(OAc)2 | MeOH | 87 |
| 16 | 10 | Co(OAc)2 | MeOH | 79 |
| 17 | 11 | Co(OAc)2 | MeOH | 83 |
| 18 | 12 | Co(OAc)2 | MeOH | 89 |
| 19 | 13 | Co(OAc)2 | MeOH | 91 |
A probable pathway to the synthesis of 3-phenyl-1,5,3-dithiazepane 8 from 10-phenyl-7,8,12,13-tetraoxa-10-azaspiro[5.7]tridecane 1 includes13 coordination of the peroxide oxygen atom to the central atom of the catalyst, nucleophilic addition of ethane-1,2-dithiol to the resulting carbocation,14,15 and the subsequent ring closure giving heterocycle 8 (Scheme 2).
Scheme 2. Probable synthesis mechanism for 3-phenyl-1,5,3-dithiazepane 8.
Under conditions including 5 mol% of Co(OAc)2, 20 °C, MeOH, and 20 h, 10-phenyl-7,8,12,13-tetraoxa-10-azaspiro[5.7]tridecane 1 was allowed to react with propane-1,3- 3, butane-1,4- 4, pentane-1,5- 5, and hexane-1,6-dithiols 6, which furnished the corresponding 3-phenyl-1,5,3-dithiaazacycloalkanes169–12 in 83–89% yields (Table 1). The ring transformation reaction of azadiperoxide 1 with 3,6-dioxa-1,8-octanedithiol 7 (monooxa derivative is shown in the scheme) under the conditions described above resulted in the synthesis of 6-phenyl-1,11-dioxa-4,8-dithia-6-azacyclotridecane1612 in 91% yield (Scheme 1).
The discovered ring transformation reaction of azadiperoxide 1 with ethane-1,2-dithiol 2 was also carried out for 10-aryl-7,8,12,13-tetraoxa-10-azaspiro[5.7]tridecanes 14–24, which produced 3-aryl-1,5,3-dithiazepanes1225–35 in 76–90% yields (Scheme 3).
Scheme 3. Ring transformation reaction of 10-aryl-7,8,12,13-tetraoxa-10-azaspiro[5.7]tridecanes with ethane-1,2-dithiol.
In conclusion, we demonstrated that on treatment with α,ω-alkanedithiols and the Co(OAc)2 catalyst, azadiperoxides are converted to N-aryl-substituted 1,5,3-dithiazamacroheterocycles in high yields.
Conflicts of interest
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
The reported study was funded by RFBR according to the research project No. 20-33-90002/20.
Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra09758f
Notes and references
- Slack R. Jacobine A. Posner G. Med. Chem. Commun. 2012;3:281. doi: 10.1039/C2MD00277A. [DOI] [Google Scholar]
- Vil' V. Yaremenko I. Ilovaisky A. Terent'ev A. Molecules. 2017;22:1881. doi: 10.3390/molecules22111881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D. Liu J. Nat. Prod. Bioprospect. 2013;3:161. doi: 10.1007/s13659-013-0042-7. [DOI] [Google Scholar]; Crespo-Ortiz M. P. Wei M. Q. Antitumor Activity of Artemisinin and Its Derivatives: From a Well-Known Antimalarial Agent to a Potential Anticancer Drug. J. Biomed. Biotechnol. 2012:257597. doi: 10.1007/s13659-013-0042-7. [DOI] [Google Scholar]
- Tu Y. Nat. Med. 2011;17:1217. doi: 10.1038/nm.2471. [DOI] [PubMed] [Google Scholar]
- Tyumkina T. V. Makhmudiyarova N. N. Kiyamutdinova G. M. Meshcheryakova E. S. Bikmukhametov K. Sh. Abdullin M. F. Khalilov L. M. Ibragimov A. G. Dzhemilev U. M. Tetrahedron. 2018;74:1749. doi: 10.1016/j.tet.2018.01.045. [DOI] [Google Scholar]
- Makhmudiyarova N. N. Ishmukhametova I. R. Dzhemileva L. U. Tyumkina T. V. D'yakonov V. A. Ibragimov A. G. Dzhemilev U. M. RSC Adv. 2019;9:18923. doi: 10.1039/C9RA02950H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhmudiyarova N. N. Rakhimov R. Sh. Tyumkina T. V. Meshcheryakova E. S. Ibragimov A. G. Dzhemilev U. M. Russ. J. Org. Chem. 2019;5:620. doi: 10.1134/S1070428019050075. [DOI] [Google Scholar]
- Makhmudiyarova N. N. Shangaraev K. R. Dzhemileva L. U. Tuymkina T. V. Mescheryakova E. S. D'yakonov V. A. Ibragimov A. G. Dzhemilev U. M. RSC Adv. 2019;9:29949. doi: 10.1039/C9RA06372B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhmudiyarova N. N. Ishmukhametova I. R. Dzhemileva L. U. D'yakonov V. A. Ibragimov A. G. Dzhemilev U. M. Molecules. 2020;25:1874. doi: 10.3390/molecules25081874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhmudiyarova N. N. Ishmukhametova I. R. Ibragimov A. G. Dhzemilev U. M. Dokl. Chem. 2020;492:93. doi: 10.1134/S001250082036001X. [DOI] [Google Scholar]
- Makhmudiyarova N. N. Ishmukhametova I. R. Ibragimov A. G. Russ. J. Org. Chem. 2020;10:1495. [Google Scholar]
- Murzakova N. N. Prokof'ev K. I. Tyumkina T. V. Ibragimov A. G. Russ. J. Org. Chem. 2012;48:588. doi: 10.1134/S1070428012040215. [DOI] [Google Scholar]
- (a) Oda S. Franke J. Krishce M. J. Chem. Sci. 2016;7:136. doi: 10.1039/C5SC03854E. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Vojacek S. Beese K. Alhalabi Z. Swyter S. Bodtke A. Schulzke C. C. Jung M. Sippl W. Link A. Arch. Pharm. 2017;350:e1700097. doi: 10.1002/ardp.201700097. [DOI] [PubMed] [Google Scholar]
- Wellmar U. J. Heterocyclic Chem. 1998;35:1531. doi: 10.1002/jhet.5570350653. [DOI] [Google Scholar]
- Krohn K. Cludius-Brandt S. Synthesis. 2010;8:1344. doi: 10.1055/s-0029-1218658. [DOI] [Google Scholar]
- Makhmudiyarova N. N. Mudarisova L. V. Meshcheryakova E. S. Ibragimov A. G. Dzhemilev U. M. Tetrahedron. 2015;71:259. doi: 10.1016/j.tet.2014.11.064. [DOI] [Google Scholar]
- Makhmudiyarova N. N. Khatmullina G. M. Rakhimov R. Sh. Meshcheryakova E. S. Ibragimov A. G. Dzhemilev U. M. Tetrahedron. 2016;72:3277. doi: 10.1016/j.tet.2016.04.055. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.