Abstract
Fractalkine (CX3CL1) is the first described chemokine that can exist either as a soluble protein or as a membrane-bound molecule. Both forms of fractalkine can mediate adhesion of cells expressing its receptor, CX3CR1. This activity, together with its expression on endothelial cells, suggests that fractalkine might mediate adhesion of leukocytes to the endothelium during inflammation. Fractalkine is also highly expressed in neurons, and its receptor, CX3CR1, is expressed on glial cells. To determine the biologic role of fractalkine, we used targeted gene disruption to generate fractalkine-deficient mice. These mice did not exhibit overt behavioral abnormalities, and histologic analysis of their brains did not reveal any gross changes compared to wild-type mice. In addition, these mice had normal hematologic profiles except for a decrease in the number of blood leukocytes expressing the cell surface marker F4/80. The cellular composition of their lymph nodes did not differ significantly from that of wild-type mice. Similarly, the responses of fractalkine−/− mice to a variety of inflammatory stimuli were indistinguishable from those of wild-type mice.
The trafficking of blood leukocytes is mediated primarily by two major classes of molecules: cell adhesion molecules, including selectins and integrins, and chemotactic factors, such as the chemokines (4). Chemokines (chemotactic cytokines) are low-molecular-weight, secreted proteins that are defined by their ability to induce directed migration (chemotaxis) of cells expressing an appropriate chemokine receptor(s) (28). The ability of individual chemokines to effect cell migration in vivo is well established (reviewed in reference 28), and recent data suggest that different chemokines have different functions in vivo. For example, some chemokines are required for constitutive trafficking of immune cells through lymphoid tissues (11, 12), whereas others recruit effector cells to sites of infection (7) or regulate immune responses (15).
Chemokines may be involved at various stages of the cell recruitment process. First, some chemokines can activate integrins on the surface of leukocytes, resulting in the firm adhesion of these cells to the vascular endothelium (6). Second, chemokines are thought to participate in directing the migration of cells once they exit the vasculature, perhaps by establishing a concentration gradient within that tissue. A third mechanism of chemokine action was immediately suggested by the discovery of fractalkine (1), a multidomain molecule that includes a chemokine region, a mucin-like stalk, and an 18-amino-acid stretch of hydrophobic residues that is predicted to span the cell membrane. The ability of fractalkine to be presented on the cell surface suggests that this chemokine might directly mediate cell-cell interactions. The expression of fractalkine on endothelial cells (13) and its upregulation by proinflammatory mediators such as tumor necrosis factor and interleukin-1 (1) further suggest that fractalkine might mediate adhesion of leukocytes expressing its receptor to inflamed endothelium. Indeed, T cells and monocytes which express that receptor, CX3CR1, adhere to monolayers of fractalkine-expressing HEK293 cells under static conditions (1). Furthermore, cells expressing CX3CR1 also bind to fractalkine-coated slides under flow conditions via a mechanism that is independent of CX3CR1 signaling and integrin activation (10, 17).
Fractalkine can also exist as a soluble, 95-kDa glycoprotein that results from cleavage of the full-length protein at a dibasic motif (Thr-Arg-Arg-Gln) situated adjacent to the membrane-binding domain. In vitro, this soluble form of human fractalkine has potent chemoattractant activity for the same cell types that adhere to monolayers expressing the membrane-bound form (1). Soluble fractalkine also induces cell adhesion but, unlike the membrane-bound form, does so by an integrin-dependent mechanism that requires signaling through CX3CR1 (14). The biologically relevant actions of these two forms of fractalkine are not yet known.
Murine fractalkine has 67% identity at the amino acid level with the human protein and has the same general structural features as the human protein (29), which was recently assigned the name CX3CL1, according to the new nomenclature system for human chemokines (31). However, unlike human fractalkine, the soluble murine form of fractalkine may be chemotactic to both neutrophils and T cells in vitro (27). The highest expression of murine fractalkine is seen in the brain (27, 29). Although fractalkine was initially reported to be expressed by glial cells in mouse brain (27), subsequent studies of rat and mouse brain indicated that the primary source of fractalkine is neurons (16, 30), particularly in the olfactory bulb, cerebral cortex, and hippocampus. By contrast, CX3CR1 is primarily expressed in astrocytes and microglia throughout the brain (16, 25). Recently, it was demonstrated that fractalkine can induce microglial cell migration and activation (16, 23) and that it can inhibit Fas-mediated microglial cell death in vitro (2). Together, these data suggest that neurons might communicate with glial cells through this ligand-receptor pair, thereby assisting in the formation of neuronal networks or controlling cell survival.
Organs other than the brain that express murine fractalkine include the intestine, kidney, heart, brain, lung, skeletal muscle, and pancreas (1). The expression in the intestine suggests a possible role of fractalkine in mucosal immune responses. Epithelial cells of the small and large intestine have been identified as sources of human fractalkine, and levels of this chemokine are increased in Crohn's disease (24). The human fractalkine receptor CX3CR1 is expressed in the intraepithelial lymphocytes found in the epithelial lining of the gut (24). This expression of fractalkine and CX3CR1 in adjacent cell types suggests that one function of fractalkine might be to control the position and number of lymphocytes near the epithelium in both healthy and inflamed intestinal tissue.
The cell types and organs that express fractalkine suggest several hypothetical functions for this chemokine, including possible roles in neuronal network organization or survival, adherence of leukocytes to endothelium, and the initiation of contact between dendritic cells and lymphocytes. To directly investigate biologic requirements for fractalkine, we generated fractalkine-deficient (fractalkine−/−) mice by targeted gene disruption. Here, we report that these mice develop normally and have normal migration of leukocytes to lymphoid tissue and peripheral sites in several models of inflammation.
MATERIALS AND METHODS
Generation of fractalkine−/− mice.
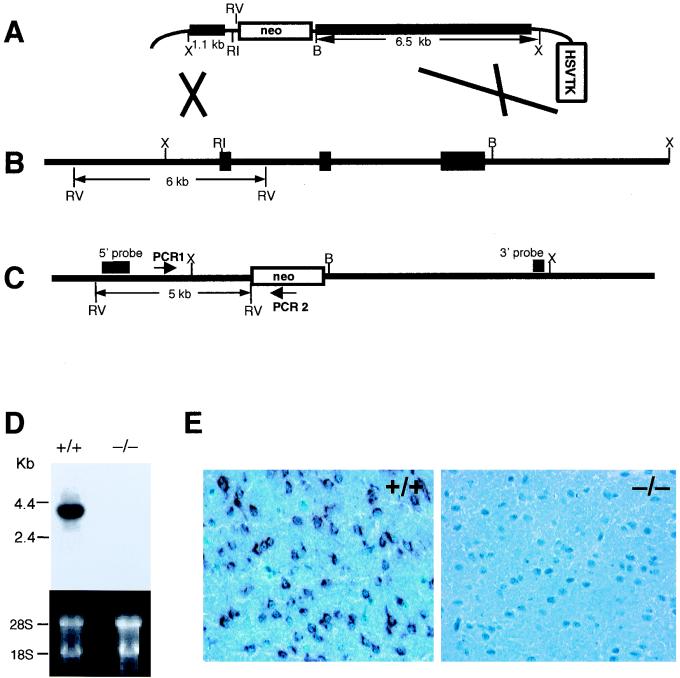
Three bacteriophage P1 clones containing the genomic copy of the mouse fractalkine gene were isolated from a mouse 129/ola embryonic stem (ES) cell genomic library (Incyte Genomics, Inc., St. Louis, Mo.) using PCR primers corresponding to the murine fractalkine gene (5′-ACAGACGCTTCTGTGCTGA-3′ and 5′-TCCAAAGCAAGGTCTTCCA-3′). Two overlapping fragments containing the mouse gene were identified by Southern blotting of EcoRI- and XbaI-digested P1 plasmid DNA using a 211-bp PCR-generated probe labeled with [32P]dCTP (3,000 Ci/mmol; Amersham Pharmacia Biotech, Piscataway, N.J.) by random priming (Megaprime DNA Labeling System; Amersham Pharmacia Biotech). These fragments were subcloned into pBluescript (Stratagene, La Jolla, Calif.) and analyzed by DNA sequencing and restriction enzyme analysis. A DNA vector designed to remove the entire coding region of the fractalkine gene was constructed. This targeting vector (Fig. 1A) was electroporated into 129SvEv-derived ES cells, and colonies resistant to both ganciclovir and G418 were picked and expanded for DNA analysis. DNAs from these colonies were screened for targeted fractalkine genes by a PCR-based strategy using one primer, PCR1 (5′-CCAGGCTGGCTATGGTCCAACTG-3′), corresponding to a region upstream of the fractalkine DNA and another primer, PCR2 (5′-TGGCGGACCGCTATCAGGAC-3′), corresponding to the neomycin resistance gene (neo). Of 1,774 ES cell clones analyzed, 6 yielded the 1.5-kb diagnostic PCR amplification product. The predicted structures of the targeted fractalkine loci (Fig. 1C) in the PCR-positive cells were confirmed by Southern blotting using probes prepared from DNA located 5′ and 3′ to the fractalkine locus. Cells from several correctly targeted ES cell lines were injected into C57BL/6 blastocysts to generate chimeric mice. Fractalkine-heterozygous (+/−) offspring were identified by a PCR-based screening strategy using three oligonucleotide primers corresponding to the region of homology, the neo gene, and the deleted region of the fractalkine gene. These mice were interbred to generate fractalkine-null (−/−) mice.
FIG. 1.
Gene targeting of fractalkine. (A) Targeting vector. Thick lines represent regions of homology to fractalkine. The restriction enzyme sites used to subclone these regions are indicated; RV, EcoRV; RI, EcoRI; B, BglI; X, XbaI. (B) Wild-type fractalkine locus. Black rectangles represent exons; transcription is from left to right. The EcoRV fragment diagnostic of the wild-type locus (6 kb) is shown. (C) fractalkine targeted locus. The positions of the oligonucleotide primers (PCR1 and PCR2) used to screen targeted ES cells are indicated, as is the EcoRV fragment diagnostic of the targeted locus. The arrow indicates the transcriptional direction of neo. (D) Northern blot analysis of fractalkine RNA from the brain of a wild-type (+/+) mouse and a fractalkine−/− mouse. The ethidium bromide-stained gel is shown to demonstrate equal loading of RNA samples. (E) Immunohistochemical analysis of fractalkine in the brain of a wild-type and a fractalkine−/− mouse. Dark-staining regions represent cells producing fractalkine protein; neurons of the wild-type mouse are stained, but not those from the fractalkine−/− mouse.
Histochemistry, immunohistochemistry, and in situ hybridization.
Tissues were either fresh frozen for cryosection or fixed, processed for paraffin sections, and stained with hematoxylin and eosin. For immunostaining, fresh frozen sections were fixed with acetone. A monoclonal antifractalkine antibody was a generous gift from John Abrams (DNAX). Antibody binding was amplified using a Vectastain Elite ABC kit and detected with a diaminobenzidine substrate kit (Vector Laboratories, Burlingame, Calif.). Hematoxylin was used to counterstain the sections. In situ hybridization was carried out as described (22). Sense and antisense 33P-labeled RNA probes were transcribed using T7 or T3 polymerase (Roche Molecular Biochemicals, Indianapolis, Ind.) from a plasmid containing fractalkine cDNA from nucleotides 1786 to 2299.
Mice.
Mice were generally used between 6 and 12 weeks of age. Age- and gender-matched control mice of the same 129SvEv and C57BL/6 mixed genetic background were either bred in-house or purchased from Jackson Labs (Bar Harbor, Maine). All experiments with animals were conducted according to the Schering-Plough guidelines for animal care.
Experimental peritonitis.
Mice were injected intraperitoneally with 1.5 ml of thioglycolate (Microbiology Systems, Cockeysville, Md.). At 25 and 72 h postinjection, the contents of the peritoneum were lavaged by injection of 8 ml of phosphate-buffered saline (PBS). Total cells were determined from an aliquot of the lavage from each of five mice per experimental group. Smears of the cells were also dried, fixed with acetone, and stained with Protocol Hema Solutions I and II (Biochemical Sciences, Swedesboro, N.J.) according to the manufacturer's specifications. Differential counts were determined by microscopic analysis.
DTH.
To determine delayed-type hypersensitivity (DTH), mice were injected subcutaneously at two sites on the dorsal flank with 0.1 ml of complete Freund's adjuvant containing 100 μg of keyhole limpet hemocyanin (KLH). Seven days postimmunization, the mice were injected in the subplantar region of the hind paw with 200 μg of KLH in 25 μl of PBS. Calipers were used to measure paw thickness at 24-h intervals postchallenge. Ten mice were used in each experimental group, and the experiment was done three times.
Flow cytometric analysis.
Cells from the epithelium and lamina propria of the intestinal mucosa were prepared as described previously (8). These cells were blocked with 5 μg of Fc Block (PharMingen, San Diego, Calif.) per ml, 300 μg of mouse immunoglobulin G (Pierce, Rockford, Ill.) per ml, and 10% rat serum (Pierce) and then stained with phycoerythrin- or fluorescein isothiocyanate-conjugated monoclonal antibodies directed against CD4, CD8α, CD8β, CD3, CD11c, CD45, B220, Gr-1, NK1.1, T-cell receptors alpha and beta (TCRαβ), and TCRγδ (PharMingen). Cells were gated on CD45, and data were collected on a FACScan (Becton Dickinson, San Jose, Calif.) and analyzed using CellQuest software. The total cell number for each population was calculated based on total cells recovered, percent CD45 cells, and percent CD45-gated cells staining with the various antibodies.
DSS-induced enterocolitis.
Ten fractalkine−/− mice and 10 age- and gender-matched wild-type control mice were provided with water containing either 3.5 or 5% dextran sulfate sodium (DSS) for 7 days. The mice were weighed daily and examined for the presence of blood in the perianal area. After 7 days of treatment, the DSS-containing water was replaced with regular water for an additional 21 days. The animals were weighed daily for the first 7 days and once a week thereafter. All experimental mice were fasted overnight on day 27. On the following morning, the cecum together with the adjacent pieces of jejunum and proximal colon were collected in addition to the distal colon and mesenteric lymph nodes. Ten mice were used in each experimental group. This experiment was done three times.
Challenge with Listeria monocytogenes.
Cultures of L. monocytogenes (strain EGD) were grown as described previously (9). Briefly, bacteria were grown overnight in Trypticase soy broth, and the concentration of the culture was determined by spectrophotometric analysis. Bacteria were suspended in sterile, nonbacteriostatic saline at 105 CFU/ml, and equal volumes (0.1 ml) were injected into the lateral tail veins of experimental mice. At 48 h postinfection, spleens were collected and strained through a 40-μl nylon mesh in PBS containing 0.1% Triton X-100. Serial dilutions of this homogenate were spread onto Trypticase soy agar plates. Colonies were counted the following day. For survival experiments, 10 wild-type and 8 fractalkine−/− mice were used. For CFU counts, 10 mice per group were used. Statistical significance was assessed using Student's t test.
RESULTS AND DISCUSSION
Analysis of fractalkine expression in wild-type mice.
We first used Northern blot analysis to investigate fractalkine expression in 19 different mouse tissues. As reported previously by others, very high levels of fractalkine expression were seen in the brain and spinal cord, with lower levels seen in kidney, large and small intestine, lung, uterus, ovary, and adrenal gland. On prolonged exposure of the autoradiograph, fractalkine expression was detected in almost all tissues (data not shown).
Generation of fractalkine−/− mice.
A gene-targeting vector (Fig. 1A) was constructed using fractalkine genomic DNA. This vector was designed so that after its recombination with the fractalkine locus (Fig. 1B), the entire coding region of CX3C would be excised (Fig. 1C). Intercrosses of fractalkine+/− mice derived from chimeras yielded wild-type (fractalkine+/+), fractalkine+/−, and fractalkine−/− mice in the expected Mendelian ratio of 1:2:1. The fractalkine−/− mice appeared healthy and were fertile. To determine if any abnormalities would become apparent in older mice, 10 fractalkine−/− mice were allowed to reach 18 months of age. No overt abnormalities were observed.
Analysis of fractalkine RNA and fractalkine protein in gene-targeted mice.
To test whether the fractalkine−/− mice were able to produce fractalkine RNA, we performed Northern blot analysis of mRNA prepared from brains of wild-type and fractalkine−/− mice. fractalkine RNA was readily detected in wild-type brains, but was undetectable in brains of fractalkine−/− mice (Fig. 1D).
We next performed immunohistologic analysis of brain sections taken from wild-type and fractalkine−/− mice, using an antibody directed against fractalkine. In wild-type mice, no immunoreactive protein was seen in glial cells, although it was abundant in neurons (Fig. 1E). These data are in contrast to the fractalkine expression in glial cells that was originally reported but consistent with more recent reports of neuronal fractalkine expression in the mouse (30) and rat (23, 25). No fractalkine was detected in the brains of fractalkine−/− mice.
Histologic and flow cytometric analyses of fractalkine−/− mice.
To determine whether the absence of fractalkine results in abnormalities of any major tissue (most of which express fractalkine), we examined formalin-fixed tissue sections of fractalkine−/− mice by light microscopy. No inflammation or developmental abnormalities were noted in lung, kidney, brain, heart, thymus, stomach, small and large intestine, spine, lymph nodes, or liver. Thus, fractalkine is not required for the development of these organs.
Previous reports have revealed that CX3CR1 is expressed on leukocytes (13, 18, 19). To determine whether the interaction of fractalkine with CX3CR1 is required for the development or proliferation of any major hematopoietic lineages, we performed a complete blood count on fractalkine−/− mice. This analysis did not reveal statistically significant differences between fractalkine−/− mice and wild-type mice with regard to the total white blood cell count, red blood cell number, hematocrit, or percentage or absolute number of neutrophils, lymphocytes, monocytes, eosinophils, and basophils (Table 1).
TABLE 1.
Blood cell dataa
| Measureb | Mean value ± SD in:
|
P | |
|---|---|---|---|
| Wild-type mice | fractalkine−/− mice | ||
| WBC (103/μl) | 4.5 ± 2.5 | 4.2 ± 0.5 | 0.75 |
| RBC (103/μl) | 9.3 ± 0.5 | 9.8 ± 0.4 | 0.06 |
| Hematocrit (%) | 40.9 ± 1.5 | 42.1 ± 2.0 | 0.33 |
| Platelets (103/μl) | 780 ± 215 | 792 ± 59 | 0.91 |
| Neutrophils (%) | 20.6 ± 15.2 | 13.9 ± 5.1 | 0.38 |
| Lymphocytes (%) | 73.2 ± 15.9 | 81.4 ± 5.3 | 0.30 |
| Monocytes (%) | 4.0 ± 1.2 | 3.2 ± 0.4 | 0.21 |
| Eosinophils (%) | 1.7 ± 1.3 | 1.1 ± 0.3 | 0.36 |
| Basophils (%) | 0.3 ± 0.2 | 0.2 ± 0.3 | 0.24 |
| Absolute neutrophils (10/μl) | 118 ± 141 | 57 ± 22 | 0.37 |
| Absolute lymphocytes (10/μl) | 303 ± 78 | 339 ± 49 | 0.41 |
| Absolute monocytes (10/μl) | 20.2 ± 17.6 | 13.4 ± 2.4 | 0.41 |
| Absolute eosinophils (10/μl) | 7.1 ± 5.0 | 4.9 ± 1.5 | 0.38 |
| Absolute basophils (10/μl) | 1.4 ± 0.7 | 0.8 ± 1.1 | 0.24 |
Data are for six males per group.
WBC, white blood cells; RBC, red blood cells.
Flow cytometric analyses were performed on peripheral blood as well as spleen and lymph nodes using antibodies directed against the cell surface markers F4/80 (monocytes), Mac-1 (several myeloid-derived cellular subsets), CD3 (mature T cells), CD11c (dendritic cells), B220 (B cells), and Pan NK (NK cells). The only significant and reproducible difference seen in fractalkine−/− mice was a significant decrease in F4/80-expressing blood monocytes (Table 2). Taken together, these results demonstrate that fractalkine is not required for normal development of any major leukocyte subset and that these cells migrate normally to the tissues examined.
TABLE 2.
Flow cytometric analysis of leukocytes
| Tissue | Marker | Mean % of leukocytesa ± SD in:
|
P | |
|---|---|---|---|---|
| Wild-type mice | fractalkine−/− mice | |||
| Blood | F4/80 | 18.2 ± 2.1 | 11.5 ± 0.8 | 0.01b |
| Mac-1 | 37.5 ± 2.1 | 38.8 ± 0.6 | 0.37 | |
| CD11c | 3.4 ± 0.28 | 3.5 ± 1.1 | 0.15 | |
| Pan NK | 10.2 ± 2.3 | 7.4 ± 2.8 | 0.91 | |
| B220 | 40.0 ± 0.2 | 44.0 ± 7.7 | 0.53 | |
| CD3 | 39.0 ± 3.9 | 30.7 ± 6.0 | 0.19 | |
| Lymph nodes | F4/80 | 0.3 ± 0.11 | 0.5 ± 0.06 | 0.14 |
| Mac-1 | 0.6 ± 0.2 | 0.6 ± 0.08 | 0.87 | |
| CD11c | 1.6 ± 0.21 | 1.6 ± 0.12 | 0.60 | |
| Pan NK | 1.5 ± 0.07 | 1.4 ± 0.12 | 0.87 | |
| B220 | 19.4 ± 5.7 | 20.4 ± 3.4 | 0.80 | |
| CD3 | 79.6 ± 5.3 | 78.7 ± 3.3 | 0.83 | |
| Spleen | F4/80 | 6.6 ± 0.2 | 7.5 ± 0.9 | 0.28 |
| Mac-1 | 3.5 ± 0.01 | 5.0 ± 1.3 | 0.21 | |
| CD11c | 5.9 ± .92 | 6.5 ± 0.61 | 0.40 | |
| Pan NK | 6.4 ± 1.6 | 6.7 ± 0.91 | 0.79 | |
| B220 | 56.4 ± 4.6 | 59.3 ± 0.7 | 0.34 | |
| CD3 | 36.2 ± 5.6 | 31.6 ± 1.9 | 0.26 | |
The values refer to the percentage of CD45+ leukocytes in the tissue having the indicated cell surface marker.
The significant difference in blood F4/80 cells between wild-type and fractalkine−/− mice was seen in each of three separate experiments with three mice per group.
Response to thioglycolate.
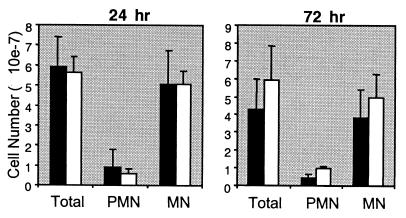
As the decrease in circulating F4/80-expressing monocytes was the only abnormality seen in unchallenged fractalkine−/− mice, we next investigated the requirement for fractalkine in inflammatory responses. Injection of thioglycolate into the peritoneum of mice induces an inflammatory response characterized by an influx of mononuclear cells into the peritoneum at 24 h postchallenge. Chemokines are required for this recruitment (3, 5, 20, 21). To determine whether fractalkine is required for this influx, we challenged both wild-type and fractalkine−/− mice with thioglycolate and counted the number of inflammatory cells in the peritoneal exudate. There were no significant differences in the number of total cells or mononuclear cells between the two groups of mice at either 24 h (Fig. 2A) or 72 h (Fig. 2B) postchallenge. Thus, at least in this model, fractalkine is not required for the recruitment of mononuclear cells to the peritoneum.
FIG. 2.
Peritoneal exudate cells following elicitation with thioglycolate at (A) 24 h and (B) 72 h. Solid bars, wild-type mice; open bars, fractalkine−/− mice. Total cells, polymorphonuclear cells (PMN), and mononuclear cells (MN) are shown. The standard error of the mean is indicated. Differences between wild-type and fractalkine−/− mice were not statistically significant.
DTH.
To determine if fractalkine is required for a T-lymphocyte-mediated DTH response, an experiment was performed in which mice were immunized by a subcutaneous injection of KLH and challenged 7 days later by injection of KLH into the footpad. Measurements of paw swelling in the challenged mice did not reveal significant differences between fractalkine−/− and wild-type mice (Fig. 3), indicating that fractalkine−/− mice have no gross defect in T-cell-mediated immune responses.
FIG. 3.
DTH response to KLH. Mice were immunized subcutaneously and challenged in the foot pad. The paw thickness following challenge with either antigen (squares) or vehicle only (diamonds) is shown.
Analysis of small intestine.
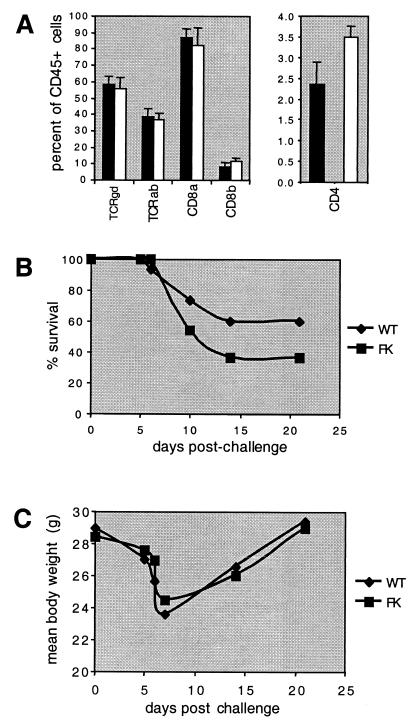
The expression of fractalkine and CX3CR1 by intestinal epithelial cells and intraepithelial lymphocytes (24), respectively, suggests that fractalkine might mediate physiologic trafficking of lymphocytes to the intestine. To test this possibility, we prepared lymphocytes from the epithelium and lamina propria and analyzed various cell lineages by flow cytometry. Of several T-cell lineages analyzed, the only difference between wild-type and fractalkine−/− mice was a significant and reproducible increase in the percentage of CD4+ cells in the gut epithelium (Fig. 4A). An increase in CD4+ CD8+ cells was also seen, but this difference was not statistically significant. No differences were seen in the leukocyte populations in the lamina propria. These results demonstrate that fractalkine is not required for the normal, physiologic trafficking of most leukocytes to the small intestine.
FIG. 4.
Analysis of small intestine. (A) Flow cytometric analysis of CD4-expressing intraepithelial lymphocytes. Error bars indicate standard deviation from the mean. One of two representative experiments is shown. (B) Survival following treatment with 5% DSS for 7 days. One of three experiments is shown. No consistent difference between wild-type (WT) and fractalkine−/− (FK) mice was seen among these experiments. (C) Weight loss following 5% DSS treatment. Mean body weights are indicated. Error bars are omitted for clarity.
To investigate the requirement for fractalkine during intestinal inflammation, we induced experimental colitis in mice by providing them with water containing DSS for 7 days. The DSS-containing water was then replaced with regular water for 3 weeks, during which time the mice were visually monitored and their weights were recorded. In some experiments, the survival of fractalkine−/− mice was slightly lower than that of wild-type mice during the early phase of the experiment (Fig. 4B), but this difference was not statistically significant or seen in all experiments. Moreover, no marked differences between wild-type and fractalkine−/− mice were seen during the weight loss or weight gain phases of the experiment (Fig. 4C). Thus, fractalkine is not required for the induction of enterocolitis or for recovery of mice from the acute phase of the disease.
Response of fractalkine−/− mice to L. monocytogenes.
Monocytes express CX3CR1 and migrate in response to soluble fractalkine. As the number of circulating F4/80-positive cells was decreased in fractalkine−/− mice, we considered the possibility that fractalkine might mediate recruitment of monocytes or macrophages to infected tissue. To test whether macrophage function was impaired in fractalkine−/− mice, we infected them with L. monocytogenes. No significant differences between wild-type and fractalkine−/− mice were seen in their abilities to survive relatively low doses (3 × 103) of listeriae over 2 weeks (Fig. 5A). We also performed an analysis of splenic CFU at 48 h postinfection. This time point was chosen because clearance of this facultative intracellular bacterial pathogen 48 h postinfection is mediated primarily by activated macrophages (reviewed in reference 26). Analysis of the listeria CFU present in spleens of infected mice did not reveal statistically significant differences between wild-type and fractalkine−/− mice (Fig. 5B). Thus, fractalkine is not required for macrophage function in this model.
FIG. 5.
Response to L. monocytogenes. Mice were challenged intravenously with either 3 × 103 CFU for survival experiments (A) or 104 CFU for analysis of splenic bacteria 48 h later (B). Solid bars and circles, wild-type mice; open bars and circles, fractalkine−/− mice. Error bars represent standard deviation from the mean. The difference in CFU between the two groups was not statistically significant.
In summary, we have generated fractalkine−/− mice and demonstrated that they do not produce fractalkine RNA or fractalkine protein in the brain. Despite the absence of this protein, fractalkine−/− mice do not have histologic abnormalities in any major organs (including the brain), and hematopoietic lineages in blood and lymphoid tissue are essentially normal. Likewise, fractalkine−/− mice do not exhibit any overt behavioral abnormalities. These preliminary findings are similar to data described by Jung et al., who used targeted gene disruption to generate mice lacking CX3CR1 (19). Those investigators were unable to demonstrate differences between CX3CR1−/− mice and wild-type mice with regard to monocyte recruitment, dendritic cell differentiation and migration, or their microglial response to facial nerve injury. Thus, the biological role of fractalkine remains an enigma, and additional studies will be required to uncover any biologic requirement for this structurally fascinating chemokine and its receptor.
ACKNOWLEDGMENTS
We thank Susan Abbondanzo, Petronio Zalamea, Channa Young, Margaret Monahan, and Linda Hamilton for excellent technical assistance and Albert Zlotnik and Fernando Bazan for helpful discussions and for the murine fractalkine cDNA.
The first two authors contributed equally to this work.
REFERENCES
- 1.Bazan J F, Bacon K B, Hardiman G, Wang W, Soo K, Rossi D, Greaves D R, Zlotnik A, Schall T J. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 2.Boehme S A, Lio F M, Maciejewski-Lenoir D, Bacon K B, Conlon P J. The chemokine fractalkine inhibits Fas-mediated cell death of brain microglia. J Immunol. 2000;165:397–403. doi: 10.4049/jimmunol.165.1.397. [DOI] [PubMed] [Google Scholar]
- 3.Boring L, Gosling J, Chensue S W, Kunkel S L, Farese R V, Jr, Broxmeyer H E, Charo I F. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Investig. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butcher E C, Picker L J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 5.Cacalano G, Lee J, Kikly K, Ryan A M, Pitts-Meek S, Hultgren B, Wood W I, Moore M W. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. . (Erratum, 270:365, 1995.) [DOI] [PubMed] [Google Scholar]
- 6.Campbell J J, Hedrick J, Zlotnik A, Siani M A, Thompson D A, Butcher E C. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 7.Cook D N, Beck M A, Coffman T M, Kirby S L, Sheridan J F, Pragnell I B, Smithies O. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science. 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 8.Cook D N, Prosser D M, Forster R, Zhang J, Kuklin N A, Abbondanzo S J, Niu X D, Chen S C, Manfra D J, Wiekowski M T, Sullivan L M, Smith S R, Greenberg H B, Narula S K, Lipp M, Lira S A. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- 9.Cook D N, Smithies O, Strieter R M, Frelinger J A, Serody J S. CD8+ T cells are a biologically relevant source of macrophage inflammatory protein-1 alpha in vivo. J Immunol. 1999;162:5423–5428. [PubMed] [Google Scholar]
- 10.Fong A M, Robinson L A, Steeber D A, Tedder T F, Yoshie O, Imai T, Patel D D. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med. 1998;188:1413–1419. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forster R, Mattis A E, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 12.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 13.Foussat A, Coulomb-L'Hermine A, Gosling J, Krzysiek R, Durand-Gasselin I, Schall T, Balian A, Richard Y, Galanaud P, Emilie D. Fractalkine receptor expression by T lymphocyte subpopulations and in vivo production of fractalkine in human. Eur J Immunol. 2000;30:87–97. doi: 10.1002/1521-4141(200001)30:1<87::AID-IMMU87>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 14.Goda S, Imai T, Yoshie O, Yoneda O, Inoue H, Nagano Y, Okazaki T, Imai H, Bloom E T, Domae N, Umehara H. CX3C-chemokine, fractalkine-enhanced adhesion of THP-1 cells to endothelial cells through integrin-dependent and -independent mechanisms. J Immunol. 2000;164:4313–4320. doi: 10.4049/jimmunol.164.8.4313. [DOI] [PubMed] [Google Scholar]
- 15.Gu L, Tseng S, Horner R M, Tam C, Loda M, Rollins B J. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature. 2000;404:407–411. doi: 10.1038/35006097. [DOI] [PubMed] [Google Scholar]
- 16.Harrison J K, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara R K, Streit W J, Salafranca M N, Adhikari S, Thompson D A, Botti P, Bacon K B, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci USA. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haskell C A, Cleary M D, Charo I F. Molecular uncoupling of fractalkine-mediated cell adhesion and signal transduction: rapid flow arrest of CX3CR1-expressing cells is independent of G-protein activation. J Biol Chem. 1999;274:10053–10058. doi: 10.1074/jbc.274.15.10053. [DOI] [PubMed] [Google Scholar]
- 18.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall T J, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 19.Jung S, Aliberti J, Graemmel P, Sunshine M J, Kreutzberg G W, Sher A, Littman D R. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurihara T, Warr G, Loy J, Bravo R. Defects in macrophage recruitment and host defense in mice lacking the CCR2 chemokine receptor. J Exp Med. 1997;186:1757–1762. doi: 10.1084/jem.186.10.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuziel W A, Morgan S J, Dawson T C, Griffin S, Smithies O, Ley K, Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci USA. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lugo D, Roberts J, Pintar J. Analysis of proopiomelanocortin gene expression during prenatal development of the rat pituitary gland. Mol Endocrinol. 1989;3:1313–1324. doi: 10.1210/mend-3-8-1313. [DOI] [PubMed] [Google Scholar]
- 23.Maciejewski-Lenoir D, Chen S, Feng L, Maki R, Bacon K B. Characterization of fractalkine in rat brain cells: migratory and activation signals for CX3CR-1-expressing microglia. J Immunol. 1999;163:1628–1635. [PubMed] [Google Scholar]
- 24.Muehlhoefer A, Saubermann L J, Gu X, Luedtke-Heckenkamp K, Xavier R, Blumberg R S, Podolsky D K, MacDermott R P, Reinecker H C. Fractalkine is an epithelial and endothelial cell-derived chemoattractant for intraepithelial lymphocytes in the small intestinal mucosa. J Immunol. 2000;164:3368–3376. doi: 10.4049/jimmunol.164.6.3368. [DOI] [PubMed] [Google Scholar]
- 25.Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N, Kume T, Akaike A, Satoh M. Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS Lett. 1998;429:167–172. doi: 10.1016/s0014-5793(98)00583-3. [DOI] [PubMed] [Google Scholar]
- 26.North R J, Dunn P L, Conlan J W. Murine listeriosis as a model of antimicrobial defense. Immunol Rev. 1997;158:27–36. doi: 10.1111/j.1600-065x.1997.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 27.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo J A, Vath J, Gosselin M, Ma J, Dussault B, Woolf E, Alperin G, Culpepper J, Gutierrez-Ramos J C, Gearing D. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–617. doi: 10.1038/42491. . (Erratum, 389:100.) [DOI] [PubMed] [Google Scholar]
- 28.Rollins B J. Chemokines. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 29.Rossi D L, Hardiman G, Copeland N G, Gilbert D J, Jenkins N, Zlotnik A, Bazan J F. Cloning and characterization of a new type of mouse chemokine. Genomics. 1998;47:163–170. doi: 10.1006/geno.1997.5058. [DOI] [PubMed] [Google Scholar]
- 30.Schwaeble W J, Stover C M, Schall T J, Dairaghi D J, Trinder P K, Linington C, Iglesias A, Schubart A, Lynch N J, Weihe E, Schafer M K. Neuronal expression of fractalkine in the presence and absence of inflammation. FEBS Lett. 1998;439:203–207. doi: 10.1016/s0014-5793(98)01384-2. [DOI] [PubMed] [Google Scholar]
- 31.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]