Abstract
This study aims to assess the effect of green waste compost (GWC), biochar (BC) and humic acid (HA) amendments of an alkaline heavy metal-contaminated soil. In this study, amendments with GWC, GWC + BC and GWC + HA were applied to the heavy metal-contaminated soil in four application rates (0, 1, 2 and 5%), and was aimed at substantially mitigating the bioavailability of heavy metals for pakchoi cabbage from the sewage irrigation soils. The addition of different ratios of amendments can increase the pH of the soil by 0.11–0.30 units and also increase the organic matter content by 3.1–35.1%. The concentration of available arsenic (As), cadmium (Cd), zinc (Zn) and copper (Cu) in the CaCl2 extract was decreased effectively by all the amendments, except for the increase in the available concentration of As by compost–humic acid (T8) in the soil. Compared with the control, the CaCl2 extractable Cd was decreased by 33–48% after the addition of different ratios of amendments in the soil. Moreover, by increasing the content of compost and compost–biochar in combinations, easily exchangeable fractions of As, Cd, Zn and Cu were decreased, while the oxidation fraction and residual fractions were increased. When the soil amendments were applied, fresh weight of the root and shoot increased by 29–63% and 39–85%, respectively. Cd concentration in the roots and shoots of the pakchoi cabbage decreased by 21–44% and 26–53%, respectively, after adding different ratios of amendments. All the amendments were effective in reducing the Cd, Zn and Cu uptake by the roots and shoots of the pakchoi cabbage, and simultaneously reduce the absorption of As in the roots of pakchoi cabbage. As soil amendments, GWC alone or GWC + BC/GWC + HA application can significantly reduce the heavy metal levels in pakchoi cabbage while increasing the biomass production and higher application rate is more effective than the lower application rate.
The concentration of available arsenic (As), cadmium (Cd), zinc (Zn) and copper (Cu) in CaCl2 extract was effectively decreased by all the amendments, except the available concentration of As was increased by compost–humic acid (T8) in soil.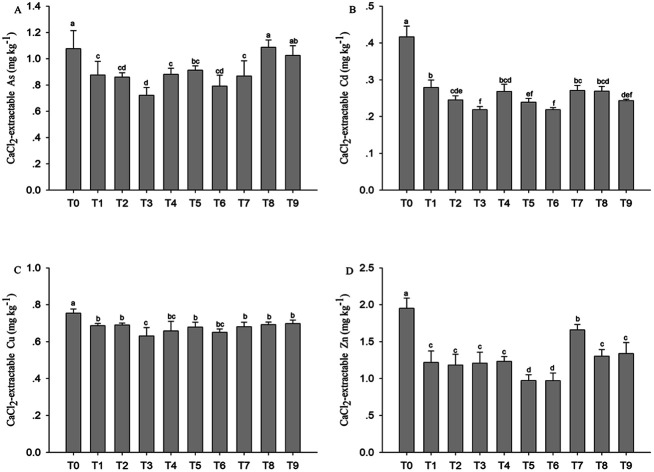
1. Introduction
Mining, fertilization and sewage irrigation lead to the accumulation of heavy metals in agricultural soil, which makes China one of the most widely and severely polluted countries in the world with the heavy metals.1–3 In China, more than 36 million hectares of farmland are polluted by numerous heavy metals,1 of which more than 10 million hectares are polluted by cadmium (Cd).4 In the past few decades, more than 100 tons of Cd has been accumulated into the soil through sewage irrigation, resulting in severe Cd pollution in China.2 In addition, arsenic (As), copper (Cu) and zinc (Zn) also exist in the soil, although the quantities and polluted areas are lower than those of Cd. Excessive heavy metal can damage human health, and Cd and As are the most typical and representative elements, which can induce a variety of adverse effects, including non-carcinogenic and carcinogenic risks.5,6
Vegetables are the most important components of food intake worldwide. Leafy vegetables excessively accumulate heavy metals as compared to other food crops, and the consumption of leafy vegetables contaminated with heavy metals is a major pathway of exposure for humans.7,8 In general, 70% of the total Cd accumulation in humans comes from leafy vegetables.9 Therefore, it is a very important task to reduce excessive toxic heavy metals in vegetables, particularly leafy vegetables.
Globally, the use of organic amendments to minimize the heavy metal mobility and bioavailability in contaminated soils has increased rapidly.3,10–12 Compared with other soil remediation agents, compost, biochar and humic acid have their own advantages. Biochar can persist in the soil for hundreds or even thousands of years.13 It has highly porous structure, alkali and cation exchange capacity, contains a large number of carboxyl and hydroxyl groups, which can reduce the bioavailability of heavy metals, thus reducing plant absorption and food chain transfer.10,14–16 However, biochar can not only immobilize heavy metals, but simultaneously immobilize essential nutrients for plants, leading to the deficiency of Ca, P, and N in plants, thus affecting the plant growth.17 Compost is a cost-effective method given the low price of residues and by-products that can be used as soil amendments.18 The turnover of organic carbon in the compost can be combined with heavy metals in a compost-treated soil to stabilize heavy metals.19,20 Green waste compost (GWC), which has a low metal concentration and abundant organic matter, has been used as organic mulch and plant growth medium in recent years.21,22 Humic acids exhibited high microbiological stability in the studied contaminated soils, with almost 95% of organic-C being resistant to decomposition.18 Humic acid (HA) is the main component of organic matter, which can promote the mineral nutrition absorption and plant growth.23 In the heavy metal-contaminated soil, it was found that humic acid can immobilize Zn and Pb (increased proportion of the residual fraction), thus decreasing heavy metal extractable fractions.18 GWC, BC and HA have been researched widely to restore soil in recent years. In order to reinforce the effects of three effective soil repair agent, GWC, BC, and HA can be mixed among each other thoroughly to improve each other's properties.
In 2012, the production of GWC in Beijing was 2.14 × 105 tons per year, which increased year by year. The use of GWC for soil heavy metal amendment can achieve the recycling mechanism of “reduction”, “recycling” and “reuse” of GWC. Abundant supply of GWC can better balance the relationship between the remediation effect and cost control. Therefore, in this study, we investigated the effects of GWC, GWC + BC and GWC + HA in different ratios on the mobility and availability of metals aimed at (1) evaluating the changes in the physicochemical properties of the soil amended by amendments; (2) examining the effect of amendments on the availability of arsenic (As), cadmium (Cd), zinc (Zn) and copper (Cu) in the contaminated soil by the CaCl2-extraction procedure; (3) using sequential extraction procedures to evaluate the efficacy of various combinations of amendments as immobilizing agents to reduce the mobility of these elements in soil; and (4) the effects of amendments on the growth and heavy metal accumulation of pakchoi cabbage.
2. Materials and methods
2.1. Experimented materials
The soil source of this study is Laohekou town, Xiong'an (115°42′18′′ E; 38°49′45′′ N). The site is located in the North China Plain, which was irrigated with river sewage in the last century, resulting in the farmland soil pollution. First, the top litter on the surface was removed and 0–20 cm soil was collected. The soil was room-dried at 25 °C for 4 weeks, sieved to <2 mm, followed by the removal of the animal and plant debris. The collected soil was a tidal soil with a total Cd concentration of 4.19 mg kg−1 (Table 1), significantly exceeding the Ministry of Ecology and Environment of the People's Republic of China limit (Cd 0.6 mg kg−1, pH > 7.5; GB 15618-2018).
Selected physical and chemical properties of the raw materials used for the pot experimenta.
| Properties | Units | Soil | Compost | Biochar | Humic acid |
|---|---|---|---|---|---|
| pH | — | 7.92 ± 0.02 | 8.38 ± 0.03 | 8.13 ± 0.02 | 5.68 ± 0.03 |
| EC | μS cm−1 | 331 ± 12 | 920 ± 12 | 225 ± 10 | 342 ± 9 |
| Cd | mg kg−1 | 4.19 ± 0.05 | ND | ND | ND |
| As | mg kg−1 | 24.7 ± 0.54 | 3.67 ± 0.16 | 2.88 ± 0.11 | 3.07 ± 0.11 |
| Cr | mg kg−1 | 41.7 ± 0.32 | 16.60 ± 0.81 | 7.25 ± 0.56 | 26.88 ± 0.52 |
| Fe | mg kg−1 | 20 861 ± 659 | 3767 ± 30 | 932 ± 19 | 2779 ± 42 |
| Pb | mg kg−1 | 388 ± 4.3 | 11.45 ± 0.34 | 13.29 ± 0.84 | 18.85 ± 0.32 |
| Zn | mg kg−1 | 243 ± 5.23 | 37.07 ± 1.53 | 15.61 ± 0.51 | 12.95 ± 0.28 |
| Cu | mg kg−1 | 196 ± 1.27 | 12.20 ± 0.57 | 5.50 ± 0.32 | 13.12 ± 0.11 |
ND = below the limit of detection.
Three soil amendments were utilized in this study including green waste compost, fruit charcoal biochar and humic acid. The green waste compost used in the experiment was from a garden waste absorption center in Beijing. The raw materials were mainly pruning residues or litter of wax, willow, weeds and other plants. After crushing, the addition of microbial agents, and primary and secondary fermentation, they are completely decomposed under aerobic conditions. Fig. 1 shows the surface morphology and chemical compositions of GWC examined by a field emission electron microscope (FEI Nova NanoSEM 450) equipped with an energy dispersive spectrometer (EDS). The biochar used in the experiment is fruit charcoal (pyrolyzed for 5 h under high temperature anaerobic condition at 500 °C), which is provided by Shaanxi Yixin Bioenergy CO., Ltd. Shaanxi Province, China. Humic acid is provided by Dezhou Chemical Products Co., Ltd. Shandong Province, China. The detailed characteristics of three soil amendments, such as the pH and total elemental concentrations, are presented in Table 1.
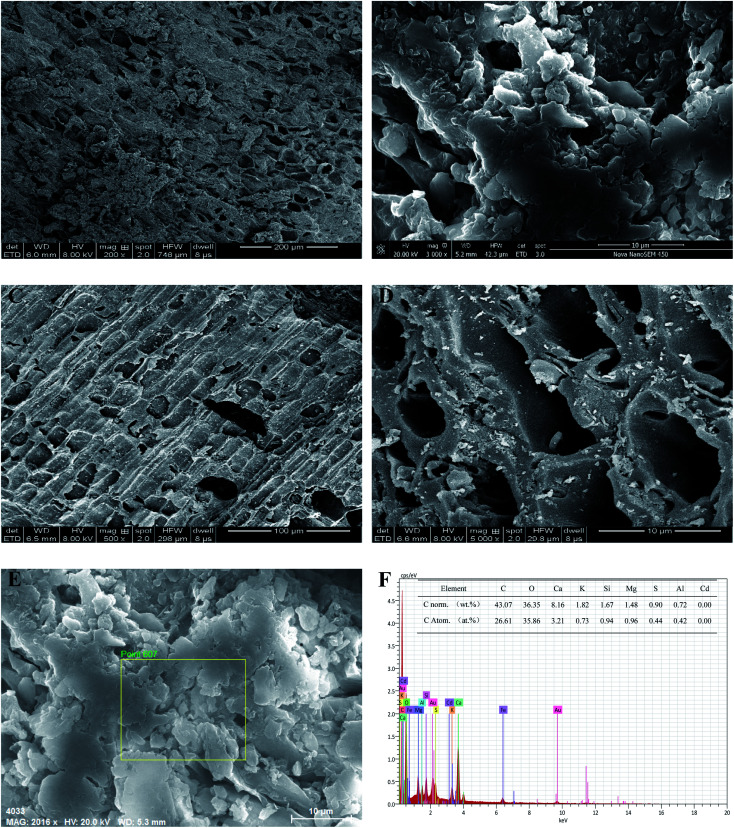
Fig. 1. SEM photomicrograph of GWC (A and B) and BC (C and D), EDS analyses of GWC (E and F).
2.2. Experiment design
In order to investigate the effect of GWC, GWC + BC and GWC + HA combinations on the mobility and availability of heavy metals in the soil, different ratios of amendments were thoroughly mixed with the contaminated soil (Table 2).
Treatments of experiments.
| Treatments | Description of treatments | Treatment assembly |
|---|---|---|
| T0 | 3000 g soil (control) | S |
| T1 | 2970 g soil + 30 g compost (1% w/w) | SG1 |
| T2 | 2940 g soil + 60 g compost (2% w/w) | SG2 |
| T3 | 2850 g soil + 150 g compost (5% w/w) | SG5 |
| T4 | 2970 g soil + (24 g compost + 6 g biochar) (1% w/w) | SGB1 |
| T5 | 2940 g soil + (48 g compost + 12 g biochar) (2% w/w) | SGB2 |
| T6 | 2850 g soil + (120 g compost + 30 g biochar) (5% w/w) | SGB5 |
| T7 | 2970 g soil + (24 g compost + 6 g humic acid) (1% w/w) | SGH1 |
| T8 | 2940 g soil + (48 g compost + 12 g humic acid) (2% w/w) | SGH2 |
| T9 | 2850 g soil + (120 g compost + 30 g humic acid) (5% w/w) | SGH5 |
After 2 weeks of soil balance, pakchoi cabbage seedlings (Jingyan Yinong (Beijing) Seed Sci-tech Co, Ltd., China) were planted from May 7, 2019 to June 17, 2019 (40 days). The pakchoi cabbage seed was planted in the polluted soil. When the pakchoi cabbage grew upto 10 cm, three seedlings with uniform growth were kept. According to the need of the pakchoi cabbage, water was added three times a week to maintain the soil water holding capacity of 60% (w/w), and grown to harvest in a greenhouse at 20–30 °C.
2.3. Soil sampling and analysis
After the pakchoi cabbage harvest, the soil was taken from each pot and its physical–chemical properties were measured. The pH value of the soil was measured by an automatic pH meter in a 1 : 5 (w/v, soil/water) soil water suspension. The content of the organic matter was determined by the hydrothermal method.
The HF–HClO4–HNO3 method was used to determine the total heavy metals in the soil samples.24 Heavy metal availability in every soil treatment was evaluated via CaCl2 extraction. With this four-step sequential extraction, the mobilities of As, Cd, Zn and Cu in the soil samples were evaluated.25 Four metal fractions namely: exchangeable fraction (acid soluble/exchangeable, 0.11 M HOAc), reduction fraction (Fe and Mn bound oxides and hydroxides, 0.5 M NH2OH), oxidation fraction (organic matter-bound, NH4OAc), residual fraction (aqua regia) were determined.
2.4. Plant sampling and analysis
The pakchoi cabbage was harvested after 40 days. First, it was washed with distilled water, and then immersed in 20 mM Na2EDTA for 15–20 min to eliminate heavy metals from the root surface. Finally, it was cleaned with distilled water, and the surface moisture was removed and weighed. In order to determine the heavy metal content in roots and shoots, samples were prepared by microwave digestion, and the content was determined by inductively coupled plasma mass spectrometry (ICP-MS).
2.5. Statistic analysis
The data were checked by the one-way analysis of variance (ANOVA) with the LSD test at significant level of P < 0.05 by the SPSS (version 22.0) software. A two-way analysis of variance was conducted to test the effects of the type and rate of organic amendment on the root heavy metals, shoot heavy metals and availability of heavy metals in the soil. Sigmaplot software v. 12.5 was used for graphical work. Correlations between different parameters were established using Pearson's correlation coefficients for SPSS (version 22.0).
3. Results
3.1. The scanning electron microscopy with energy dispersive spectroscopy (SEM-EDS) analysis of GWC
The scanning electron microscopy with energy dispersive spectroscopy (SEM-EDS) analysis of GWC is shown in Fig. 1. Fig. 1A and B are the SEM images of GWC before and after comminution. Fig. 1C and D are the SEM images of BC before and after comminution. The surface of BC and GWC is uneven, with an irregular pore structure and a certain amount of mesopores on the channel, which is conducive to the intercalation coordination of metal ions. According to the EDS (Fig. 1E and F) analysis, C and O are the important elements of GWC, with the mass ratio of C and O being 43.07% and 36.35%, respectively. These elements can form alkyl and aromatic structures in the GWC. In addition, GWC also contains nutrients such as K, Ca, Mg, S and Fe, but No Cd was found by the EDS analysis.
3.2. Properties of soil treated with or without amendments
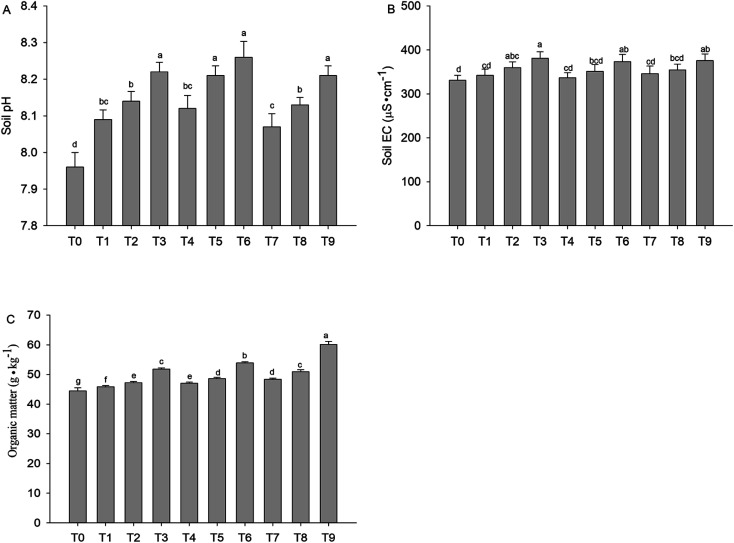
The pH value, EC value and organic matter content of the contaminated soil changed obviously after adding different proportion of the compost–biochar and compost–humic acid (Fig. 2). Compost and biochar are alkaline, while humic acid is acidic. Because the proportion of the compost is larger than that of biochar and humic acid, the pH value of all the treated soil increases. GWC, GWC + BC and GWC + HA increased the soil pH by 0.13–0.26, 0.16–0.30 and 0.11–0.25 units, respectively. Simultaneously, the EC value of the soil was also increased by increasing the soil amendment amount. Compared with the unamended soil, organic matter increased significantly with the addition of different remediation materials. GWC, GWC + BC and GWC + HA increased the soil organic matter by 3.1–16.5%, 5.7–21.1% and 8.8–35.1%, respectively. Among them, the combination of GWC and HA is the most conducive for increasing the organic matter content because humic acid is the main component of the organic matter. In addition, according to the Chinese soil environmental quality standard (GB 15618-2018), the contaminated soil was heavily polluted (Table 1).
Fig. 2. pH, EC and organic matter changes after treatment with various amendments. Error bars are the SD of the means (n = 3) and (p < 0.05).
3.3. Changes of available Cd, Zn, Cu and As in the soil after the pakchoi cabbage harvest
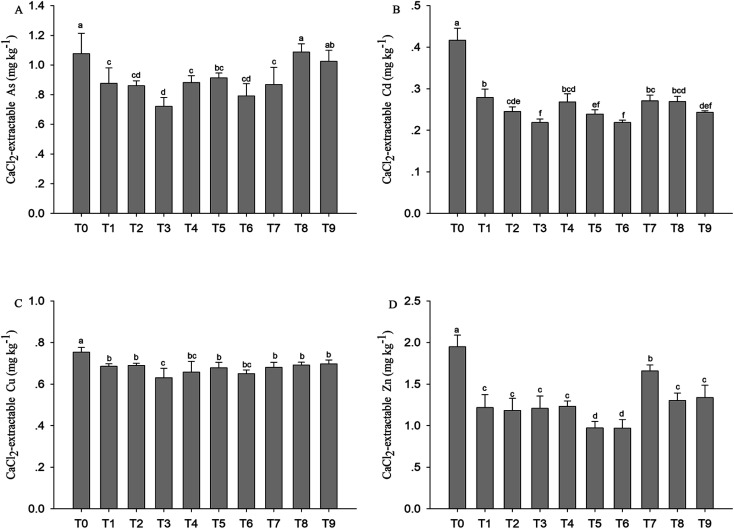
The effect of GWC, GWC + BC and GWC + HA on the availability of As, Cd, Zn and Cu in the contaminated soil was measured by CaCl2 extraction (Fig. 3). The available concentration of each heavy metal is far lower than its total concentration. Compared to the untreated soil, all the modifiers effectively reduced the content of available Cd, Zn and Cu in the soil. Compared with the control, CaCl2 extractable Cd and Zn in the soil decreased by 33–48%, 36–48%, 35–42% and 38–39%, 37–50%, 15–33%, when the application amounts of the compost, compost–biochar and compost–humic acid were 1%, 2% and 5%, respectively. The reduction rate of the effective state of Cu is less than that of Cd and Zn for all the amendments. The application of compost and compost–biochar can effectively reduce the available concentration of As in the soil; however, different from the compost and compost–biochar, the change of availability of As was increased by compost–humic acid (T8) in the soil. The results of two-way ANOVA (Table 6) test showed that the amount of organic amendments had a significant effect on the content of available of heavy metals (P < 0.01), while the types of organic amendments had significant effects only on the available Zn and As (P < 0.01). There was significant interaction between the types and rates of organic amendments on the available Zn content (P < 0.05) of the soil, but there was no significant interaction on the available Cd, Cu and As content in the soil.
Fig. 3. Concentration of available heavy metals of soil with different proportion amendments. Error bars are the SD of the means (n = 3) and (p < 0.05).
The two-way ANOVA results on effects of type and rate of organic amendment on roots heavy metals, shoots heavy metals and available of heavy metals in soil.
| Heavy metals concentration | Type of organic amendment | Rate of organic amendment | Type of organic amendment × rate of organic amendment | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Roots heavy metals | ||||||
| Cd | 10.84 | <0.01 | 218.97 | <0.01 | 1.95 | 0.11 |
| Zn | 236.68 | <0.01 | 832.54 | <0.01 | 67.43 | <0.01 |
| Cu | 114.59 | <0.01 | 4268.09 | <0.01 | 78.94 | <0.01 |
| As | 45.41 | <0.01 | 586.30 | <0.01 | 32.36 | <0.01 |
| Shoots heavy metals | ||||||
| Cd | 1.05 | 0.37 | 129.45 | <0.01 | 0.32 | 0.92 |
| Zn | 2.14 | 0.14 | 724.13 | <0.01 | 9.72 | <0.01 |
| Cu | 17.05 | <0.01 | 2112.61 | <0.01 | 19.71 | <0.01 |
| As | 58.12 | <0.01 | 39.11 | <0.01 | 8.21 | <0.01 |
| Available of heavy metals in soil | ||||||
| Cd | 1.94 | 0.17 | 194.04 | <0.01 | 0.81 | 0.57 |
| Zn | 15.84 | <0.01 | 81.94 | <0.01 | 2.96 | 0.03 |
| Cu | 1.92 | 0.17 | 20.77 | <0.01 | 1.32 | 0.29 |
| As | 6.39 | <0.01 | 10.98 | <0.01 | 2.40 | 0.06 |
3.4. Sequential extraction of Cd, Zn, Cu and As
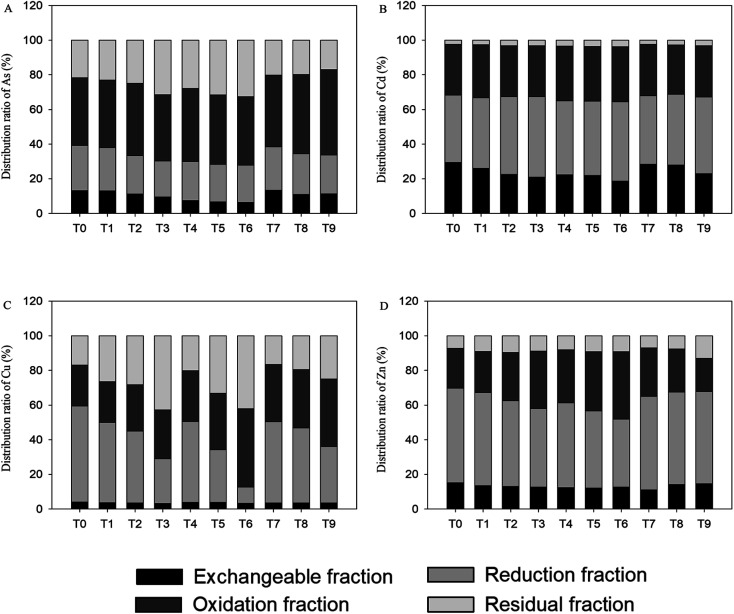
The effects of sequential extraction on the As, Cd, Zn and Cu contents in the contaminated soil by the addition of amendments with different ratios are summarized in Fig. 4. From the results of sequential extraction for Cd, after the incorporation of amendments, easily exchangeable fraction was reduced remarkably with remarkable increase in reduction fraction, in which the easily exchangeable fraction decreased gradually and the reduction fraction also increased gradually with the increase of addition of the amendments. The oxidation fraction and residual fraction also increased slightly with the increase in the content of amendments. The easily exchangeable fraction decreased from 29.4% to 20.9% in the T3 treatment and from 29.4% to 18.7% in the T6 treatment. The effect of GWC and the GWC + BC combination is found to be better than that of GWC + HA, and greater the amount of amendments, the more obvious is the effect. For Zn, the easily exchangeable fraction and reduction fraction decreased with the addition of amendments, and the oxidation fraction and residual fraction increased with the increase in the content of amendments. For Cu, it has a strong combination in all soil samples due to its over 95% of the total content in reduction fraction, oxidation fraction and residual fraction. Compared to the control, with the increase in the content of amendments, the easily exchangeable fraction decreased slightly, the reduction fraction decreased significantly, and the oxidation fraction and residual fraction also increased significantly. For As, the GWC and GWC + BC combination decreased the easily exchangeable fraction and reduction fraction, increased the residual fraction, and the trend was more obvious with the increase in the additional amount of the amendments. The GWC + HA combination initially increased and then decreased the easily exchangeable fraction, reduced the reduction fraction and residual fraction, and increased the oxidation fraction.
Fig. 4. Speciation of heavy metal in soils amended with different proportion amendments.
3.5. Growth and heavy metal accumulation of the pakchoi cabbage
The yield in the pakchoi cabbage under all the treatments was significantly higher than the control, and the pakchoi cabbage yield increased by 30–65% (Table 3). Compared to the control, the fresh weight of the root increased by 29–63% and fresh weight of the shoot increased by 39–85%. The selected heavy metal limit in leafy vegetables set by the State Environmental Protection Agency (SEPA, China) is given in Table 4. All the amendments were effective in reducing the Cd uptake by the pakchoi cabbage, and the greater the amount of amendments added, the more obvious the effects were (Table 3). The Cd concentration in the roots of the pakchoi cabbage was reduced by 31–44%, 24–38%, and 21–33% with GWC, GWC + BC and GWC + HA, respectively. The Cd concentration in the shoots of the pakchoi cabbage was reduced by 27–51%, 31–48%, and 26–53% with GWC, GWC + BC and GWC + HA, respectively. The Cd concentrations in the pakchoi cabbage under all the treatments were still above the permissible limits of SEPA (0.2 mg kg−1). The application of amendments also decreased the concentrations of Zn and Cu in the pakchoi cabbage compared to the control. The Zn and Cu concentrations in the pakchoi cabbage under all the treatments were below the permissible limits of SEPA (20 mg kg−1 and 10 mg kg−1). Among the amendments, GWC was found to be the most effective in reducing the Zn uptake by roots (34–36%), and GWC + BC was the most effective in reducing the Zn uptake by the shoots (14–30%). For the amendments, the mean concentration of Cu in the roots and shoots decreased by 36–60% and 42–67%, respectively. Although all the treatments reduced the absorption of As by roots, the concentration of As in the roots of the pakchoi cabbage under all the treatments was still above the permissible limits of SEPA, China (0.5 mg kg−1). All the amendments had no significant effect on the concentration of As in the shoots of the pakchoi cabbage, and the content was far lower than 0.5 mg kg−1. The results of the two-way ANOVA (Table 6) test showed that the types and rates of organic amendments had significant (P < 0.01) effects on the heavy metals present in the roots of the pakchoi cabbage. The rates of organic amendments also had a significant effect on the heavy metals present in the shoots of the pakchoi cabbage, while the types of organic amendments only had significant effects on the contents of Cu and As, but had no significant effects on Cd and Zn. There was no significant interaction between the types and rates of organic amendments on the Cd content of the roots and shoots of the pakchoi cabbage, but it was significant for the Zn, Cu and As contents.
Fresh weight and absorption of heavy metals by pakchoi cabbage under different levels of amendmentsa.
| Treatments | Root fresh weight (g) | Root heavy metal (mg kg−1 FW) | Shoot fresh weight (g) | Shoot heavy metal (mg kg−1 FW) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cd | Zn | Cu | As | Cd | Zn | Cu | As | |||
| T0 | 2.25 ± 0.14d | 0.51 ± 0.03a | 14.43 ± 0.13a | 10.18 ± 0.04a | 1.91 ± 0.09a | 35 ± 3e | 0.71 ± 0.06a | 11.26 ± 0.23a | 6.67 ± 0.17a | 0.08 ± 0.007d |
| T1 | 3.15 ± 0.11c | 0.35 ± 0.01d | 9.55 ± 0.04e | 4.77 ± 0.01de | 1.05 ± 0.04c | 46 ± 2d | 0.51 ± 0.04b | 9.96 ± 0.27b | 2.96 ± 0.04e | 0.06 ± 0.002f |
| T2 | 3.29 ± 0.14bc | 0.34 ± 0.01d | 9.58 ± 0.44e | 5.01 ± 0.01c | 0.96 ± 0.04cd | 52 ± 3c | 0.41 ± 0.04de | 8.56 ± 0.01f | 3.50 ± 0.17c | 0.07 ± 0.004e |
| T3 | 4.12 ± 0.09a | 0.28 ± 0.01f | 9.27 ± 0.03e | 4.06 ± 0.01f | 0.60 ± 0.05g | 56 ± 4ab | 0.35 ± 0.03f | 7.89 ± 0.20h | 2.23 ± 0.04g | 0.09 ± 0.002cd |
| T4 | 3.25 ± 0.13bc | 0.38 ± 0.02bc | 12.00 ± 0.01bc | 6.47 ± 0.08b | 1.57 ± 0.04b | 52 ± 2c | 0.49 ± 0.03bc | 9.67 ± 0.05c | 3.84 ± 0.20b | 0.07 ± 0.002ef |
| T5 | 3.42 ± 0.14b | 0.35 ± 0.01d | 11.83 ± 0.01c | 4.89 ± 0.01cd | 0.87 ± 0.10de | 56 ± 2ab | 0.42 ± 0.02de | 8.31 ± 0.17g | 3.28 ± 0.01d | 0.09 ± 0.011c |
| T6 | 4.37 ± 0.11a | 0.31 ± 0.01c | 10.14 ± 0.45d | 4.99 ± 0.41 cd | 0.82 ± 0.05ef | 57 ± 2a | 0.37 ± 0.02ef | 7.91 ± 0.02h | 2.46 ± 0.02f | 0.10 ± 0.002b |
| T7 | 3.14 ± 0.14c | 0.40 ± 0.01b | 12.25 ± 0.01b | 4.13 ± 0.02f | 0.73 ± 0.07f | 51 ± 2c | 0.53 ± 0.04b | 9.18 ± 0.02d | 3.83 ± 0.09b | 0.09 ± 0.002c |
| T8 | 3.29 ± 0.12bc | 0.36 ± 0.01 cd | 10.41 ± 0.01d | 4.63 ± 0.04e | 0.78 ± 0.03ef | 53 ± 2bc | 0.44 ± 0.03cd | 8.88 ± 0.01e | 3.14 ± 0.06fe | 0.11 ± 0.003ab |
| T9 | 4.17 ± 0.32a | 0.34 ± 0.01d | 11.99 ± 0.01bc | 4.87 ± 0.01cd | 0.76 ± 0.01f | 58 ± 2a | 0.38 ± 0.01f | 8.19 ± 0.01i | 2.78 ± 0.05g | 0.11 ± 0.004a |
Different letters in the same column indicate significant differences (P < 0.05).
China limits of the selected heavy metals in leafy vegetables.
| Element | Leafy vegetables limit | Detail |
|---|---|---|
| Cd | 0.2 mg kg−1 | GB2762-2017 |
| As | 0.5 mg kg−1 | GB2762-2017 |
| Cu | 10 mg kg−1 | GB15199-1994 |
| Zn | 20 mg kg−1 | GB13106-1991 |
4. Discussion
4.1. Effects of amendments on soil chemical characteristics
Results of this study demonstrated that the soil properties were obviously enhanced by the application of amendments, including the increase in the pH, EC and organic matter content, and greater the application amount, more obvious the effect (Fig. 2). The EC value was significantly increased by GWC, GWC + BC and GWC + HA, but the EC cannot cause saline conditions. Increasing soil pH and organic matter, which is beneficial for heavy metal retention.3,9,12,26 With the increase in the soil pH, available forms of heavy metals are transformed into hydroxide precipitation, which combines with carbonate, thus reducing the mobility and biological toxicity effect of the heavy metals in the soil.6,27 The most significant factor affecting the mobility of Pb and As in the soil is the increase in the soil pH value caused by the biochar.3,6,28 In general, the soil with a higher organic matter content can reduce the concentration of Pb, Cu and Zn in the plants than the soil with a lower organic matter content.26 Average changes in the Cd and Zn concentrations in pakchoi cabbage shoots showed significantly negative variation in relation to the soil organic matter content (Table 5).
Pearson's correlation coefficients between the parametersa.
| pH | Organic matter | Available As | Available Cd | Available Zn | Available Cu | Shoot As | Shoot Cd | Shoot Zn | Shoot Cu | |
|---|---|---|---|---|---|---|---|---|---|---|
| pH | 1.000 | |||||||||
| Organic matter | 0.706* | 1.000 | ||||||||
| Available As | −0.501 | 0.004 | 1.000 | |||||||
| Available Cd | −0.907** | −0.554 | 0.622 | 1.000 | ||||||
| Available Zn | −0.855** | −0.316 | 0.513 | 0.843** | 1.000 | |||||
| Available Cu | −0.746* | −0.296 | 0.821** | 0.849** | 0.716* | 1.000 | ||||
| Shoot As | 0.451 | 0.780** | 0.304 | −0.272 | −0.059 | −0.063 | 1.000 | |||
| Shoot Cd | −0.951** | −0.676* | 0.502 | 0.957** | 0.829** | 0.762* | −0.397 | 1.000 | ||
| Shoot Zn | −0.942** | −0.706* | 0.491 | 0.926** | 0.738* | 0.710* | −0.526 | 0.961** | 1.000 | |
| Shoot Cu | −0.867** | −0.593 | 0.553 | 0.948** | 0.809** | 0.812** | −0.289 | 0.928** | 0.856** | 1.000 |
Significance between the parameter are indicated by *p < 0.05, **p < 0.01 level.
4.2. Effects of amendments on the availability of Cd, Zn, Cu and As
According to Fig. 1 (SEM), the surface of BC and GWC has an irregular pore structure, in which the micropores and mesopores can adsorb the heavy metals.29 Different mechanisms of BC dominate the sorption of As (complexation and electrostatic interactions), Cd and Pb (complexation, cation exchange, and precipitation).30 The adsorption of Cu2+, Zn2+ and Cd2+ on HA has a significant electrostatic interaction.31 In this study, all the amendments significantly reduced the concentration of available Cd, Zn and Cu in the contaminated soil, and GWC and GWC + BC were more beneficial to reduce the metal mobility (especially for Cd and Zn) (Fig. 3). The solubility and mobility of Cd and Zn were significantly negatively correlated with the soil pH (Table 5). The interaction of biochar and compost increased the soil pH, thus reducing the content of extractable metal containing CaCl2.32,33 The addition of compost produced from maize straw, sewage sludge and biochar to the soil significantly reduced the contents of mobile Cu and Cd in the soil.34 In the first year, the concentration of CaCl2 extractable Cd and Cu in the soil decreased by 57.9% and 63.8%, respectively. In the third year, 5% of the corn straw biochar reduced the CaCl2 extractable Cd and Cu by 53.6% and 66.8%, respectively.35 GWC and GWC + BC can effectively reduce the available concentration of As in the soil, while GWC + HA (T8) can increase the availability of As. It was found that the presence of humic acid promoted the formation of a humic acid iron water complex and prevented the formation of iron hydroxide, thus increasing the availability and mobility of As in the tailings.36
4.3. Effects of amendments on the speciation distribution of Cd, Zn, Cu and As
The inactivation of heavy metals is usually evaluated by two methods: morphological analysis of heavy metals and absorption of heavy metal during plant growth.37,38 The existing form of heavy metals in soil is an important indicator of their migration, transformation and bioavailability. The internal properties of heavy metals, physical and chemical properties of the soil (pH, organic matter, etc.) and environmental factors (moisture, foreign heavy metals, etc.) all affect the distribution of heavy metals in the soil.11,16,32 Different proportions of all amendments can reduce the exchangeable fraction of Cd, Zn and Cu, and increase the oxidation fraction and residual fraction (Fig. 4). The increase in pH will promote the immobilization of Cd and Zn.11,12,27 The pH value of soil is increased with the addition of amendments, which reduced the exchangeable fraction and promote the immobilization of Cd and Zn. With the application of rice husk-derived biochar and steel slag, the pH value of soil increases. Calcium carbonate and oxides on the surface of rice husk-derived biochar and steel slag tend to form CdCO3 and Cd-oxide compounds, which cause the adsorption and precipitation of CdCO3 and reduce the exchangeability of Cd in the polluted soil.9,39 Biochar and compost increase the content of the organic matter in the soil, and enhance the adsorption of heavy metals and organic pollutants.11,40 It is reported that the low content of the soil organic matter and the weak binding capacity of soil Cd may lead to the reduction or even absence of the Cd residual fraction in the soil.41,42 Compost and biochar (particularly 3% biochar) reduced the water-soluble and exchangeable components of heavy metals, and increased the residual fraction of the treated soil. Compared to the untreated control the residual amounts of Sb, As, Cr and Ni increased by 48, 56, 66 and 68%, respectively, in the treated soil.43 The interaction of oxygen-containing groups on the surface of biochar with As results in the formation of As carbonate, which reduces the exchangeability of As in the soil.39,44 In addition, Ca2+, Mg2+ and other cations in the soil exchange with the heavy metals, which increases the concentrations of reduction fraction, oxidation fraction and residual fraction of the heavy metals but decreases the easily exchangeable fraction.27,45 In addition, by reducing the mobile and exchangeable heavy metals while increasing the soluble nutrients, green waste compost–biochar is more efficient in reducing soil toxicity.11,46,47 In general, compared to the control, different proportions of GWC and GWC + BC significantly improved As, Cd, Zn and Cu from the effective fraction to ineffective fraction (Fig. 4).
4.4. Effects of amendments on the pakchoi cabbage growth and heavy metal accumulation
The biomass of the pakchoi cabbage was significantly increased by adding GWC, GWC + BC and GWC + HA. The EDS patterns (Fig. 1) demonstrated that some nutrient elements such as K, Ca, Mg, S and Fe were observed in GWC, thus promoting the growth of the pakchoi cabbage. One of the main objectives of adding compost to the soil is to improve the nutritional levels of plants when growing in low-fertility soils.11,12,48 Biochar contains numerous hydroxyl, carboxyl and aromatic groups, which increase the ion exchange point and may affect the absorption of nutrients by plants.8,33 Therefore, the addition of GWC to the soil can significantly enhance the Miscanthus yield, but the addition of biochar will not.49 Humic acid contains a lot of organic matter and improves the ability of nitrogen fixation, phosphorus decomposition (promote the transformation of soil organic phosphorus to available phosphorus) and potassium fixation in the soil, so humic acid promotes the absorption of nitrogen, phosphorus and potassium by the plants and is conducive to the growth of plants.38 The effect of GWC + BC and GWC + HA on the biomass of pakchoi cabbage is slightly better than that of GWC. The reason is that the compost usually cannot provide all the nutrients for the plant growth, so compost–biochar or compost–humic acid can help it overcome the nutrient limitation.16,50 However, because GWC, GWC + BC and GWC + HA can vividly enhance the pakchoi cabbage yields, harvestable amounts of heavy metals were only significantly reduced by the amendments, which reduced the shoot heavy metal levels to the maximum. The accumulation of heavy metals in the plants is different with soil properties, amendment types, plant species and heavy metal pollutants. The concentrations of heavy metals in the plants were positively correlated with the bioavailability of the metal measured via numerous extraction methods and soil extractants (such as CaCl2, NH4NO3, DTPA).8,11,20,33 The bioavailability of Cd, Zn and Cu in the soil was significantly positively correlated with the content of heavy metals in the shoot of the pakchoi cabbage (Table 5). The results showed that the bioavailability of heavy metals in the soil decreased with the addition of GWC, GWC + BC and GWC + HA, and the concentration of heavy metals in the shoots and roots of the plants was also decreased significantly (Table 3).
By increasing the soil alkalinity and organic matter content resulted in greater decrease in the heavy metal uptake of the plant.8,9,26 GWC, GWC + BC and GWC + HA increased the soil pH and organic matter content significantly, resulting in the reduction of Cd, Zn and Cu concentration in the root and shoot of the pakchoi cabbage, respectively. Biochar has a high pH value and increases the organic matter contents in the soil, resulting in 38%, 39%, 25% and 17% reduction in the average concentrations of Cd, Pb, Cu and Zn in plant tissues.26 The application of biochar produced from the waste materials significantly reduced the Cd concentrations in the shoots of the Chinese cabbage plants by 34% to 80%.7 All the amendments were effective in reducing the Cd uptake by the pakchoi cabbage; however, the Cd concentrations in the shoot of the pakchoi cabbage under all the treatments were still above the permissible limits of SEPA, China (0.2 mg kg−1). The application of the hardwood biochar decreased the concentrations of Zn and Cu, in cilantro by 37.1% and 42.5%, respectively as compared to the control.10 The application of all the amendments decreased the concentrations of Zn and Cu in pakchoi cabbage, which was below the permissible limits of SEPA (20 mg kg−1 and 10 mg kg−1). The application of low-dose biochar and biochar combined with a silicate fertilizer reduced the As level in grain (brown rice) by 14–16%, but did not decrease the As level in rice straw and root.51 Although the concentration of As in the shoots of the pakchoi cabbage was not significantly affected by all treatments, which was due to the small changes in As mobility/phytoavailability in the amended soil, the As content of the shoots was far lower than the permissible limits of SEPA, China (0.5 mg kg−1). The application of amendments significantly reduced the uptake of heavy metals by pakchoi cabbage, which might be another reason for enhancing the biomass production as compared to the control.52 Leafy vegetables have a high transfer rate of heavy metals,10 and GWC, GWC + BC and GWC + HA efficiently inhibit these heavy metal uptake, thereby reducing the daily intake and thus health risks.
In our study, the types and rates of organic amendments had significant effects on the content of available heavy metals in the soil and the accumulation of heavy metals in the plants. The cost of biochar and humic acid production is higher than that of the garden waste compost. It costs 631.49 US dollars to produce 540 kg biochar, which is thousands of US dollars per ton.53 This will directly affect the remediation effect and cost control. Therefore, it is necessary to further adjust the types and rates ratio of organic amendments to achieve economic and effective effect.
5. Conclusions
This study provides an effective and inexpensive material-GWC for the in situ amendment of the multi metal contaminated agricultural soils. The addition of different ratios of the amendments has the potential to reduce Cd, Zn and Cu accumulation in the pakchoi cabbage and promote the pakchoi cabbage yield by 30–65%, which may provide a feasible option for the safe cultivation of heavy metal contaminated soil. Applying GWC individually and in combination of GWC + BC, GWC + HA to the contaminated soil could increase the soil pH and organic matter content, which would reduce the heavy metal mobility and decrease in the available concentration of the heavy metals. Compared to the control, all the amendments could improve the stability of As, Cd, Zn and Cu from the effective fraction to ineffective fraction. However, in order to balance the relationship between the remediation effect and cost control, it is still necessary to further adjust the additive ratio between GWC, BC and HA, so as to reduce the accumulation of heavy metals in pakchoi cabbage and reduce the cost as much as possible.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by the Beijing Municipal Natural Science Foundation program “Microbial mechanism of lignin degradation and isolation of dominant strain during green waste composting” (Grant No. 6202021) and the Beijing Municipal Education Commission for their financial support through Innovative Transdisciplinary Program “Ecological Restoration Engineering”.
References
- Duan Q. Lee J. Liu Y. Chen H. Hu H. Bull. Environ. Contam. Toxicol. 2016;97:303–309. doi: 10.1007/s00128-016-1857-9. [DOI] [PubMed] [Google Scholar]
- Shi T. Zhang Y. Gong Y. Ma J. Wei H. Wu X. Zhao L. Hou H. Chemosphere. 2019;230:136–143. doi: 10.1016/j.chemosphere.2019.04.208. [DOI] [PubMed] [Google Scholar]
- Fresno T. Morenojimenez E. Zornoza P. Penalosa J. M. J. Environ. Manage. 2018;205:142–150. doi: 10.1016/j.jenvman.2017.09.069. [DOI] [PubMed] [Google Scholar]
- Zhao F. J. Ma Y. Zhu Y. G. Tang Z. Mcgrath S. P. Environ. Sci. Technol. 2015;49:750–759. doi: 10.1021/es5047099. [DOI] [PubMed] [Google Scholar]
- Johri N. Jacquillet G. Unwin R. BioMetals. 2010;23:783–792. doi: 10.1007/s10534-010-9328-y. [DOI] [PubMed] [Google Scholar]
- Yang X. Igalavithana A. D. Oh S.-E. Nam H. Zhang M. Wang C.-H. Kwon E. E. Tsang D. C. W. Ok Y. S. Sci. Total Environ. 2018;640–641:704–713. doi: 10.1016/j.scitotenv.2018.05.298. [DOI] [PubMed] [Google Scholar]
- Khan K. Y. Ali B. Cui X. Feng Y. Stoffella P. J. Pan F. Tang L. Yang X. J. Agric. Sci. 2016;8:23–36. [Google Scholar]
- Kamran M. Malik Z. Parveen A. Zong Y. Abbasi G. H. Rafiq M. T. Shaaban M. Mustafa A. Bashir S. Rafay M. J. Environ. Manage. 2019;250:1–12. doi: 10.1016/j.jenvman.2019.109500. [DOI] [PubMed] [Google Scholar]
- Bashir S. Salam A. Chhajro M. A. Fu Q. Khan M. J. Zhu J. Shaaban M. Kubar K. A. Ali U. Hu H. Int. J. Phytorem. 2018;20:1221–1228. doi: 10.1080/15226514.2018.1448364. [DOI] [PubMed] [Google Scholar]
- Khan A. Z. Ding X. Khan S. Ayaz T. Khan M. A. Chemosphere. 2020;244:1–10. doi: 10.1016/j.chemosphere.2019.125543. [DOI] [PubMed] [Google Scholar]
- Li S. Sun X. Liu Y. Li S. Zhou W. Ma Q. Zhang J. Environ. Sci. Pollut. Res. 2020;27:9979–9986. doi: 10.1007/s11356-019-07533-5. [DOI] [PubMed] [Google Scholar]
- Zhan F. Zeng W. Yuan X. Li B. Li T. Zu Y. Jiang M. Li Y. Environ. Sci. Pollut. Res. 2019;26:7743–7751. doi: 10.1007/s11356-018-04079-w. [DOI] [PubMed] [Google Scholar]
- Leng L. Huang H. Li H. Li J. Zhou W. Sci. Total Environ. 2018;647:210–222. doi: 10.1016/j.scitotenv.2018.07.402. [DOI] [PubMed] [Google Scholar]
- Beesley L. Moreno-Jimenez E. Gomez-Eyles J. L. Harris E. Robinson B. Sizmur T. Environ. Pollut. 2011;159:3269–3282. doi: 10.1016/j.envpol.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Ahmad M. Yong S. O. Rajapaksha A. U. Lim J. E. Kim B.-Y. Ahn J.-H. Lee Y. H. Al-Wabel M. I. Lee S.-E. Lee S. S. J. Hazard. Mater. 2016;301:179–186. doi: 10.1016/j.jhazmat.2015.08.029. [DOI] [PubMed] [Google Scholar]
- Ali A. Guo D. Jeyasundar P. G. S. A. Li Y. Xiao R. Du J. Li R. Zhang Z. Ecotoxicol. Environ. Saf. 2019;184:1–9. doi: 10.1016/j.ecoenv.2019.109635. [DOI] [PubMed] [Google Scholar]
- Rees F. Germain C. Sterckeman T. Morel J.-L. Plant Soil. 2015;395:57–73. doi: 10.1007/s11104-015-2384-x. [DOI] [Google Scholar]
- Clemente R. Bernal M. Chemosphere. 2006;64:1264–1273. doi: 10.1016/j.chemosphere.2005.12.058. [DOI] [PubMed] [Google Scholar]
- Juang K. W. Ho P. C. Yu C. H. Environ. Sci. Pollut. Res. 2011;19:1696–1708. doi: 10.1007/s11356-011-0684-0. [DOI] [PubMed] [Google Scholar]
- Awasthi M. K. Wang Q. Chen H. Liu T. Awasthi S. K. Duan Y. Varjani S. Pandey A. Zhang Z. J. Soils Sediments. 2019;19:3883–3897. doi: 10.1007/s11368-019-02277-8. [DOI] [Google Scholar]
- Zhang L. Sun X. Waste Manage. 2016;48:115–126. doi: 10.1016/j.wasman.2015.11.032. [DOI] [PubMed] [Google Scholar]
- Gong X. Li S. Sun X. Wang L. Cai L. Zhang J. Wei L. Sci. Hortic. 2018;236:186–191. doi: 10.1016/j.scienta.2018.03.051. [DOI] [Google Scholar]
- Zhang L. Sun X. Y. Tian Y. Gong X. Q. Sci. Hortic. 2014;176:70–78. doi: 10.1016/j.scienta.2014.06.021. [DOI] [Google Scholar]
- Carignan R. Tessier A. Geochim. Cosmochim. Acta. 1988;52:1179–1188. doi: 10.1016/0016-7037(88)90271-2. [DOI] [Google Scholar]
- Shen Y. Zhao L. Meng H. Hou Y. Zhou H. Wang F. Cheng H. Liu H. Waste Manage. 2016;34:578–583. doi: 10.1177/0734242X16640063. [DOI] [PubMed] [Google Scholar]
- Chen D. Liu X. Bian R. Cheng K. Zhang X. Zheng J. Joseph S. Crowley D. Pan G. Li L. J. Environ. Manage. 2018;222:76–85. doi: 10.1016/j.jenvman.2018.05.004. [DOI] [PubMed] [Google Scholar]
- He Y. Lin H. Jin X. Dong Y. Luo M. Ecotoxicol. Environ. Saf. 2020;198:1–9. doi: 10.1016/j.ecoenv.2020.110660. [DOI] [PubMed] [Google Scholar]
- Xing Y. Kouping L. Kim M. Lei C. Hu G. Qiuyue W. Liu X. Shen L. Huagang H. Zhengqian Y. Hailong W. J. Soils Sediments. 2017;17:751–762. doi: 10.1007/s11368-017-1731-3. [DOI] [Google Scholar]
- Dąbrowski A. Adv. Colloid Interface Sci. 2001;93:135–224. doi: 10.1016/S0001-8686(00)00082-8. [DOI] [PubMed] [Google Scholar]
- Li H. Dong X. Da Silva B. De Oliveira M. Chen Y. Ma L. Q. Chemosphere. 2017;178:466–478. doi: 10.1016/j.chemosphere.2017.03.072. [DOI] [PubMed] [Google Scholar]
- Wei L. Li J. Xue M. Wang S. Zhao Q. Bioresour. Technol. 2019;291:1–9. doi: 10.1016/j.biortech.2019.121868. [DOI] [PubMed] [Google Scholar]
- Liang J. Yang Z. Tang L. Zeng G. Yu M. Li X. Wu H. Qian Y. Li X. Luo Y. Chemosphere. 2017;181:281–288. doi: 10.1016/j.chemosphere.2017.04.081. [DOI] [PubMed] [Google Scholar]
- Zhang Z. Solaiman Z. Meney K. Murphy D. Rengel Z. J. Soils Sediments. 2013;13:140–151. doi: 10.1007/s11368-012-0571-4. [DOI] [Google Scholar]
- Gondek K. Mierzwahersztek M. Kopec M. J. Environ. Manage. 2018;210:87–95. doi: 10.1016/j.jenvman.2018.01.023. [DOI] [PubMed] [Google Scholar]
- Li H. Ye X. Geng Z. Zhou H. Guo X. Zhang Y. Zhao H. Wang G. J. Hazard. Mater. 2016;304:40–48. doi: 10.1016/j.jhazmat.2015.10.048. [DOI] [PubMed] [Google Scholar]
- Wang S. Mulligan C. N. Chemosphere. 2008;74:274–279. doi: 10.1016/j.chemosphere.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Cao X. Ma L. Q. Chen M. Singh S. P. Harris W. G. Environ. Sci. Technol. 2002;36:5296–5304. doi: 10.1021/es020697j. [DOI] [PubMed] [Google Scholar]
- Haibin Z. Haibo M. Lixin Z. Yujun S. Yueqing H. Bioresour. Technol. 2018;258:279–286. doi: 10.1016/j.biortech.2018.02.086. [DOI] [PubMed] [Google Scholar]
- Gu J. Zhou H. Tang H. Yang W. Zeng M. Liu Z. Peng P. Liao B. Ecotoxicol. Environ. Saf. 2019;171:451–459. doi: 10.1016/j.ecoenv.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Puga A. P. De Abreu C. A. Melo L. C. A. Beesley L. J. Environ. Manage. 2015;159:86–93. doi: 10.1016/j.jenvman.2015.05.036. [DOI] [PubMed] [Google Scholar]
- Daixia Y. Xin W. Can C. Bo P. Changyin T. Chemosphere. 2016;152:196–206. doi: 10.1016/j.chemosphere.2016.01.044. [DOI] [PubMed] [Google Scholar]
- Wang Y. Xu Y. Li D. Tang B. Man S. Jia Y. Xu H. Sci. Total Environ. 2018;621:1057. doi: 10.1016/j.scitotenv.2017.10.121. [DOI] [PubMed] [Google Scholar]
- Jaoude L. A. Castaldi P. Nassif N. Pinna M. V. Garau G. Sci. Total Environ. 2020;711:1–10. doi: 10.1016/j.scitotenv.2019.134511. [DOI] [PubMed] [Google Scholar]
- Ahmad M. Sang S. L. Lim J. E. Lee S. E. Ju S. C. Moon D. H. Hashimoto Y. Yong S. O. Chemosphere. 2014;95:433–441. doi: 10.1016/j.chemosphere.2013.09.077. [DOI] [PubMed] [Google Scholar]
- Guangming Z. Haipeng W. Jie L. Shenglian G. Lu H. Piao X. Yuanyuan L. Yujie Y. Xiaoxiao H. Yan H. RSC Adv. 2015;5:34541–34548. doi: 10.1039/C5RA04834F. [DOI] [Google Scholar]
- Beesley L. Morenojimenez E. Gomezeyles J. L. Environ. Pollut. 2010;158:2282–2287. doi: 10.1016/j.envpol.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Karami N. Clemente R. Morenojimenez E. Lepp N. W. Beesley L. J. Hazard. Mater. 2011;191:41–48. doi: 10.1016/j.jhazmat.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Ciadamidaro L. Madejón P. Camacho F. Boy E. F. Madejón E. Soil Sci. 2017;181:487–493. doi: 10.1097/SS.0000000000000186. [DOI] [Google Scholar]
- Hartley W. Dickinson N. M. Riby P. Lepp N. W. Environ. Pollut. 2009;157:2654–2662. doi: 10.1016/j.envpol.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Medina E. Paredes C. Pérez-Murcia M. D. Bustamante M. A. Moral R. Bioresour. Technol. 2009;100:4227–4232. doi: 10.1016/j.biortech.2009.03.055. [DOI] [PubMed] [Google Scholar]
- Jin W. Wang Z. Sun Y. Wang Y. Bi C. Zhou L. Zheng X. Ecotoxicol. Environ. Saf. 2020;189:1–8. doi: 10.1016/j.ecoenv.2019.109928. [DOI] [PubMed] [Google Scholar]
- Khan M. A. Ding X. Khan S. Brusseau M. L. Khan A. Nawab J. Sci. Total Environ. 2018;636:810–817. doi: 10.1016/j.scitotenv.2018.04.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akom M. Fanyin-Martin A. Oti-Boateng C. Otoo E. Dawoe E. J. Agric. Environ. Sci. 2020;9:2334–2412. [Google Scholar]