Abstract
A total of 204 nonrepetitive isolates of group A streptococci (GAS), including 107 randomly collected between 1992 and 1995 and 66 and 31 consecutively collected in 1997 and 1998, respectively, from a university hospital in southern Taiwan were examined to determine the prevalence and mechanisms of erythromycin resistance among these isolates. Resistance to erythromycin was detected in 129 isolates (63.2%) by the agar dilution test. Of these, 42 isolates (32.6%) were assigned to the constitutive macrolide, lincosamide, and streptogramin B resistance (cMLS) phenotype, and all carried the ermB gene; 4 (3.1%) were assigned to the inducible MLS resistance (iMLS) phenotype, and all harbored the ermTR gene; and 83 (64.3%) were erythromycin resistant but susceptible to clindamycin (M phenotype), and all possessed the mefA gene. Distributed by years, the rates of erythromycin resistance and different phenotypes were 61.7% (53.0% cMLS, 6.1% iMLS, and 40.9% M phenotype) between 1992 and 1995, 62.1% (12.2% cMLS and 87.8% M phenotype) in 1997, and 71.0% (9.1% cMLS and 90.9% M phenotype) in 1998. Pulsed-field gel electrophoresis showed that all but 2 cMLS isolates were clonal in origin, and 17 clones were detected among the M-phenotype isolates. These results indicate that the high incidence and increasing rate of erythromycin-resistant GAS in southern Taiwan are due to the prevalence of multiple M-phenotype clones and that clindamycin may be the drug of choice for the treatment of infections with GAS in penicillin-hypersensitive patients in this area.
Erythromycin has been the drug of choice for the treatment of infections with group A streptococci (GAS) in penicillin-hypersensitive patients (5, 9). The prevalence of erythromycin-resistant strains among GAS remains quite low in most parts of the world (1, 5, 7, 14, 18); however, widespread erythromycin resistance has been reported in Finland (15, 27), Italy (6, 31), Spain (21, 22), and Taiwan (2, 12).
Two major mechanisms account for erythromycin resistance in GAS: target site modification and active efflux of macrolides (16, 17, 28). Target site modification is the most common and extensively investigated mechanism of erythromycin resistance among gram-positive bacteria (16, 17, 32). It is mediated by Erm methylases, which methylate 23S rRNA and induce ribosomal modification, leading to loss of binding to macrolide, lincosamide, and streptogramin B (MLS) antibiotics (16, 32). Expression of MLS resistance in gram-positive cocci can be either constitutive or inducible (16, 17, 33, 34). Two classes of methylase genes have been described for GAS: the ermB gene (16) and the recently described ermTR gene (25). The ermB gene class has been that most commonly found in streptococci (16), while the ermTR gene has been found to be predominant among MLS-resistant GAS isolates in Finland (15) and Canada (7). Macrolide efflux in GAS is effected by a membrane protein encoded by the mefA gene (4). Strains with mefA have been shown to be resistant to 14- and 15-membered macrolides but to still be susceptible to 16-membered MLS antibiotics (M phenotype) (4). A predominance of the mefA gene among erythromycin-resistant GAS isolates in Spain (21, 22) and Sweden (13) has been reported.
High rates of erythromycin resistance among streptococci, including GAS, in Taiwan have been recognized since the mid-1990s (2, 12, 35). The mechanisms underlying erythromycin resistance are still not clear. The purposes of this study were to (i) investigate the mechanisms responsible for erythromycin resistance in clinical isolates of GAS in southern Taiwan and (ii) determine whether the prevalence of erythromycin resistance was due to clonal spreading of resistant strains.
MATERIALS AND METHODS
Bacterial strains.
A total of 204 nonrepetitive isolates of GAS from the Department of Pathology, National Cheng Kung University Hospital, a 900-bed teaching hospital in southern Taiwan, were examined. Among these isolates, 107 were randomly collected between 1992 and 1995, and 66 and 31 were consecutively collected in 1997 and 1998, respectively. No evidence of GAS outbreaks was noted during the study period. All isolates were identified by colony morphology, bacitracin susceptibility, and the pyrrolidonyl arylamidase test (24), and their identities were confirmed by the latex agglutination technique (Oxoid, Basingstoke, United Kingdom). All isolates were stored at −70°C in Todd-Hewitt medium (Difco Laboratories, Detroit, Mich.) with 15% glycerol until testing.
Susceptibility testing.
The MICs of erythromycin and clindamycin (Sigma Chemical Co., St. Louis, Mo.) were determined by the agar dilution method according to the recommendations of the National Committee for Clinical Laboratory Standards (20). Mueller-Hinton agar supplemented with 5% sheep blood was used. The antibiotics were incorporated into the agar in serial twofold concentrations as follows: erythromycin, 0.013 to 128 μg/ml, and clindamycin, 0.013 to 128 μg/ml. The bacterial inocula, containing approximately 1 × 104 to 3 × 104 CFU, were applied to the plates with a Steers replicator. The plates were incubated at 35°C in ambient air for 20 to 24 h. Streptococcus pneumoniae ATCC 49619 was used as a control.
Determination of erythromycin resistance phenotypes.
The resistance phenotypes of erythromycin-resistant GAS isolates were determined by the double-disk test with erythromycin and clindamycin (Becton Dickinson, Cockeysville, Md.) disks as described previously (26). Blunting of the growth inhibition zone around clindamycin in the area between the two disks indicated an inducible type of MLS resistance (iMLS), and resistance to both disks indicated a constitutive type of MLS resistance (cMLS). The M phenotype was characterized by resistance to erythromycin and susceptibility to clindamycin, with no blunting of the growth inhibition zone around clindamycin.
Detection of erythromycin resistance genes.
Detection of erythromycin resistance genes in the erythromycin-resistant GAS isolates by PCR was performed with oligonucleotide primer pairs specific for ermB (29), ermTR (15), and mefA (29). The PCR mixture, PCR conditions, and electrophoresis of PCR products were as described previously (15, 29). The expected sizes of the PCR products were 640 bp for ermB, 348 bp for mefA, and 530 bp for ermTR.
PFGE.
Pulsed-field gel electrophoresis (PFGE) was carried out to determine the clonal characteristics of the erythromycin-resistant isolates with a contour-clamped homogeneous electric field system (Pulsaphor plus; Pharmacia LKB Biotechnology, Uppsala, Sweden) as described previously (3). The genomic DNAs were prepared as described by Piggot et al. (23) and were digested overnight with 10 U of SmaI (New England Biolabs, Beverly, Mass.). DNA was electrophoresed through a 1% agarose gel in Tris-borate-EDTA (TBE) buffer at 190 V for 30 h, with pulse times ranging from 5 to 35 s. The DNA bands were visualized by staining of the gel with ethidium bromide and were photographed. Bacteriophage lambda DNA concatemers (Gibco BRL, Gaithersburg, Md.) were used as size standards.
RESULTS
Prevalence of erythromycin-resistant isolates.
Of the 204 GAS isolates, 129 (63.2%) were resistant to erythromycin (MIC, ≥1 μg/ml). Distributed by year, the prevalence rates of the erythromycin-resistant isolates were 61.7% (66 of 107 isolates) between 1992 and 1995, 62.1% (41 of 66 isolates) in 1997, and 71.0% (22 of 31 isolates) in 1998.
Resistance phenotypes.
Resistance phenotypes of the erythromycin-resistant isolates were determined according to the results of double-disk tests. Among the 129 erythromycin-resistant isolates, 83 (64.3%) had an M phenotype. They were all resistant to erythromycin (MIC, 2 to 32 μg/ml) but susceptible to clindamycin (MIC, 0.03 to 0.13 μg/ml). The frequencies of the M-phenotype isolates were 40.9% between 1992 and 1995, 87.8% in 1997, and 90.9% in 1998 (Table 1). Overall, 42 isolates (32.6%) had a cMLS phenotype, and all of them showed high-level resistance to erythromycin (MIC, ≥128 μg/ml) and clindamycin (MIC, ≥128 μg/ml). The frequencies of the cMLS isolates were 53.0% between 1992 and 1995, 12.2% in 1997, and 9.1% in 1998 (Table 1). Only four of 66 erythromycin-resistant isolates (6.1%) collected between 1992 and 1995 had an iMLS phenotype. All of them showed intermediate resistance to erythromycin (MIC, 4 μg/ml) and were susceptible to clindamycin (MIC, 0.06 to 0.25 μg/ml).
TABLE 1.
Distribution of resistance genes and phenotypes among 129 erythromycin-resistant GAS isolates
| Yr (no. of resistant isolates) | Resistance phenotype | No. (%) of isolates with the indicated phenotype | MIC (μg/ml) of:
|
Genotypea
|
|||
|---|---|---|---|---|---|---|---|
| Erythromycin | Clindamycin | ermB | mefA | ermTR | |||
| 1992–1995 (66) | cMLS | 35 (53.0) | ≥128 | ≥128 | + | − | − |
| iMLS | 4 (6.1) | 4 | 0.06–0.25 | − | − | + | |
| M | 27 (40.9) | 2–32 | 0.03–0.13 | − | + | − | |
| 1997 (41) | cMLS | 5 (12.2) | ≥128 | ≥128 | + | − | − |
| iMLS | 0 (0) | ||||||
| M | 36 (87.8) | 2–32 | 0.03–0.13 | − | + | − | |
| 1998 (22) | cMLS | 2 (9.1) | ≥128 | ≥128 | + | − | − |
| iMLS | 0 (0) | ||||||
| M | 20 (90.9) | 2–32 | 0.03–0.13 | − | + | − | |
+, gene was present; −, gene was absent.
Erythromycin resistance genes.
Erythromycin resistance genes in the 129 erythromycin-resistant isolates were detected by PCR. All 42 cMLS isolates were positive with the primers specific for ermB, whereas all 84 M-phenotype isolates were positive for the mefA gene (Table 1). All four iMLS isolates were positive with the primers specific for ermTR.
PFGE.
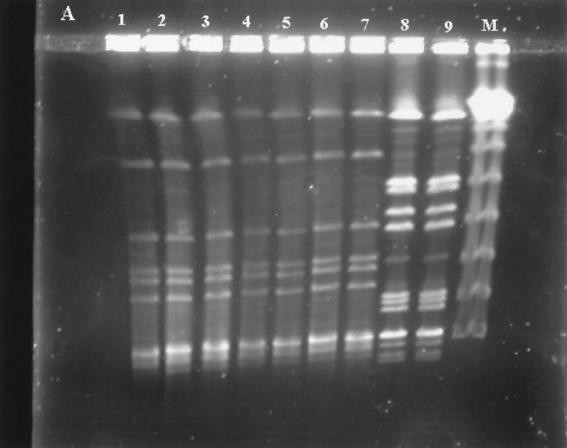
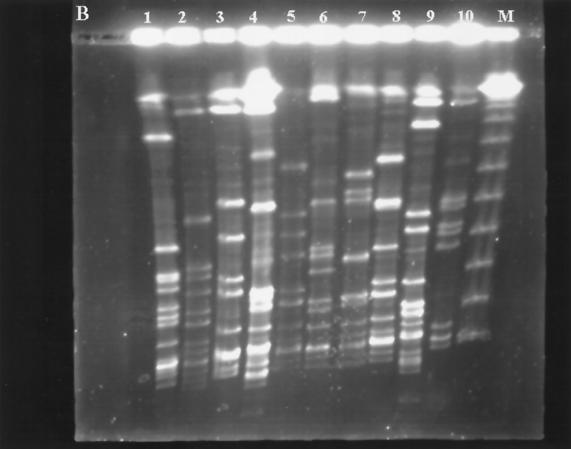
The clonal characteristic study of the erythromycin-resistant isolates was performed with PFGE. The results are partially shown in Fig. 1 and summarized in Table 2. Two PFGE patterns were found in the 42 cMLS isolates. Pattern A was predominant and found in 40 isolates (95.2%), while pattern J was found in only 2 isolates (4.8%), both of which were collected in 1997. Seventeen different PFGE patterns were found in the 84 M-phenotype isolates. Among the 17 PFGE patterns, pattern M was the most common one, being found in 19 (22.9%) of the 84 M-phenotype isolates. This pattern was found to be prevalent among erythromycin-resistant GAS in 1997, when 13 of 36 M-phenotype isolates (36.1%) showed this pattern. Few patterns were prevalent among the M-phenotype isolates collected between 1992 and 1995 (patterns C, L, and Q) and in 1998 (patterns B, D, and M), and none of them was predominant. Two PFGE patterns were found in the four iMLS isolates (Table 2).
FIG. 1.
PFGE profiles of SmaI-digested genomic DNAs from 19 isolates of erythromycin-resistant GAS. (A) Two PFGE patterns in cMLS isolates: seven isolates have pattern A (lanes 1 to 7), and two isolates have pattern J (lanes 8 and 9). Lane M contains a lambda ladder (Gibco BRL) which served as a molecular size marker. (B) Lanes 1 and 2, two PFGE profiles (patterns N and T) in iMLS isolates; lanes 3 to 10, eight PFGE profiles (patterns B, C, I, L, M, O, Q, and S) in eight representative M-phenotype isolates. Lane M contains a lambda ladder.
TABLE 2.
Distribution of PFGE patterns by resistance phenotype and collection year among 129 erythromycin-resistant GAS isolates
| Yr and phenotype | No. of isolates | No. (%) of isolates with the following PFGE pattern:
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | ||
| 1992–1995 | ||||||||||||||||||||||
| cMLS | 35 | 35 (100) | ||||||||||||||||||||
| iMLS | 4 | 2 (50.0) | 2 (50.0) | |||||||||||||||||||
| M | 27 | 4 (14.8) | 1 (3.7) | 1 (3.7) | 1 (3.7) | 1 (3.7) | 1 (3.7) | 5 (18.5) | 2 (7.4) | 2 (7.4) | 1 (3.7) | 6 (22.2) | 1 (3.7) | 1 (3.7) | ||||||||
| 1997 | ||||||||||||||||||||||
| cMLS | 5 | 3 (60.0) | 2 (40.0) | |||||||||||||||||||
| M | 36 | 3 (8.3) | 1 (2.8) | 1 (2.8) | 1 (2.8) | 5 (13.9) | 6 (16.7) | 1 (2.8) | 2 (5.6) | 13 (36.1) | 1 (2.8) | 1 (2.8) | 1 (2.8) | |||||||||
| 1998 | ||||||||||||||||||||||
| cMLS | 2 | 2 (100) | ||||||||||||||||||||
| M | 20 | 5 (25.0) | 4 (20.0) | 1 (5.0) | 2 (10.0) | 3 (15.0) | 1 (5.0) | 4 (20.0) | ||||||||||||||
| Total isolates | 129 | |||||||||||||||||||||
| cMLS | 42 | 40 (95.2) | 2 (4.8) | |||||||||||||||||||
| iMLS | 4 | 2 (50.0) | 2 (50.0) | |||||||||||||||||||
| M | 83 | 8 (9.6) | 5 (6.0) | 5 (6.0) | 3 (3.6) | 1 (1.2) | 7 (8.4) | 1 (1.2) | 10 (12.0) | 2 (2.4) | 8 (9.6) | 19 (22.9) | 2 (2.4) | 1 (1.2) | 7 (8.4) | 1 (1.2) | 2 (2.4) | 1 (1.2) | ||||
DISCUSSION
A high incidence of erythromycin-resistant GAS in Taiwan has been recognized since the mid-1990s, when 37 to 60% of GAS strains were found to be resistant to erythromycin (2, 12). The present study indicates that the resistance rate is still increasing in Taiwan. Rates of resistance have been reported to range from 10 to 47% in European countries (6, 13, 15, 21, 22, 27, 31). Although up to 70% of GAS isolates in Japan were resistant to erythromycin in the late 1970s and early 1980s (19), the country has experienced a rapid decline of erythromycin resistance in GAS with decreased consumption of erythromycin (8). The resistant strains have almost disappeared at present. As many as 71% of the GAS isolates in 1998 were resistant to erythromycin in this study, indicating that Taiwan has become a country with the highest prevalence of erythromycin-resistant GAS in the world.
In the present study, the rates of prevalence of M-phenotype isolates among erythromycin-resistant GAS were 40.9% between 1992 and 1995, 87.8% in 1997, and 90.9% in 1998, whereas those of cMLS isolates declined from 53.0% between 1992 and 1995 to 9.1% in 1998. The rates of prevalence of M-phenotype isolates in 1997 and 1998 in this study are similar to that reported in Spain and Sweden (90%) (13, 21, 22) and are higher than those recently reported in Finland (60%) (15), Canada (70%) (7), and Italy (50%) (10, 30). The data indicate that macrolide efflux mediated by the mefA gene has replaced target site modifications mediated by the erm genes as the most common mechanism responsible for erythromycin resistance among GAS isolates in Taiwan in a short time. Furthermore, because of an extremely high incidence of M-phenotype isolates among GAS and because these isolates are susceptible to clindamycin, our study suggests clindamycin as the drug of choice for the treatment of infections with GAS in penicillin-hypersensitive patients in Taiwan.
A novel erm gene, ermTR, was recently identified in an erythromycin-resistant clinical isolate of GAS in Finland (25). The nucleotide sequence of ermTR is 82.5% identical to that of ermA (25). MLS resistance in GAS with ermTR is usually expressed inducibly but occasionally is expressed constitutively (7, 10, 15). The ermTR gene has been found to be predominant among iMLS isolates in Finland (100%) (15) and Canada (100%) (7) since its discovery and has also been found, although less commonly (60% of iMLS isolates), in Italy (9). Only four iMLS isolates collected between 1992 and 1995 were found in this study, and all of them harbored the ermTR gene. The incidence of our iMLS isolates among erythromycin-resistant GAS is far lower than that reported in Finland (38%) (25), Canada (28%) (7), and Italy (31%) (6). This finding indicates that iMLS GAS are rare in Taiwan and that most of them harbor the ermTR gene.
As shown by PFGE, almost all cMLS isolates in this study were clonal in origin. This result appears to be in accordance with studies indicating that erm determinants are usually located on chromosomes in streptococci, although they are often associated with conjugative transposons (11). In contrast, PFGE showed the polyclonal nature of the M-phenotype isolates. The spread of the mefA gene in multiple clones has also been found in Italy (30) and Spain (22). Conducting conjugation experiments, Kataja et al. (15) demonstrated that the mefA gene could be transferred from M-phenotype isolates of GAS to erythromycin-susceptible GAS and Enterococcus faecalis isolates; however, no extrachromosomal DNA was detectable in these isolates by plasmid analysis. The authors thus speculated that the mefA gene might reside on a chromosomal conjugative transposon with a high transfer frequency. Whether the mefA gene in our M-phenotype isolates is also located on the chromosome is not known. However, since the emergence and prevalence of multiple clones of M-phenotype isolates occurred within a very short period in Taiwan, it is likely that in addition to the dissemination of resistance strains and transfer by chromosomal conjugative transposons, the spread of resistance plasmids might also have occurred among our GAS isolates. Further studies are needed to confirm the mechanisms of mefA transfer among these isolates.
In conclusion, our study showed that macrolide efflux mediated by the mefA gene has replaced target site modification mediated by the ermB gene as the most common mechanism responsible for erythromycin resistance in GAS in southern Taiwan. Almost all cMLS isolates carrying the ermB gene were from a single clone, whereas the spread of the mefA gene among M-phenotypes isolates occurred in multiple clones. Since M-phenotype isolates are prevalent in southern Taiwan and all are susceptible to clindamycin, our study also suggests the use of clindamycin as the drug of choice for the treatment of infections with GAS in penicillin-hypersensitive patients in this area.
ACKNOWLEDGMENTS
This work was partially supported by a grant (DOH 88-TD-1001) from the Department of Health, the Executive Yuan, Taipei, Taiwan, and by a grant (NCKUH-88-042) from the National Cheng Kung University Hospital, Tainan, Taiwan.
REFERENCES
- 1.Barry A L, Fuchs P C, Brown S D. Macrolide resistance among Streptococcus pneumoniae and Streptococcus pyogenes isolates from out-patients in the USA. J Antimicrob Chemother. 1997;40:139–140. doi: 10.1093/jac/40.1.139. [DOI] [PubMed] [Google Scholar]
- 2.Chang S C, Chen Y C, Luh K T, Hsieh W C. Macrolide resistance of common bacteria isolated from Taiwan. Diagn Microbiol Infect Dis. 1995;23:147–154. doi: 10.1016/0732-8893(95)00197-2. [DOI] [PubMed] [Google Scholar]
- 3.Chu G, Vollrath D, Davis R W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986;234:1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- 4.Clancy J, Petitpas J, Dib-Hajj F, Yuan W, Cronan M, Kamath A V, Bergeron J, Retsema J A. Molecular cloning and functional analysis of a novel macrolide-resistance determinant, mefA, from Streptococcus pyogenes. Mol Microbiol. 1996;22:867–879. doi: 10.1046/j.1365-2958.1996.01521.x. [DOI] [PubMed] [Google Scholar]
- 5.Coonan K M, Kaplan E L. In vitro susceptibility of recent North American group A streptococcal isolates to eleven oral antibiotics. Pediatr Infect Dis J. 1994;13:630–635. doi: 10.1097/00006454-199407000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Cornaglia G, Ligozzi M, Mazzariol A, Masala L, Cascio G L, Orefici G, Fontana R the Italian Surveillance Group for Antimicrobial Resistance. Resistance of Streptococcus pyogenes to erythromycin and related antibiotics in Italy. Clin Infect Dis. 1998;27(Suppl. 1):S87–S92. doi: 10.1086/514908. [DOI] [PubMed] [Google Scholar]
- 7.De Azavedo J C S, Yeung R H, Bast D J, Duncan C L, Norgia S B, Low D E. Prevalence and mechanisms of macrolide resistance in clinical isolates of group A streptococci from Ontario, Canada. Antimicrob Agents Chemother. 1999;43:2144–2147. doi: 10.1128/aac.43.9.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujita J, Murono K, Yoshikawa M, Murai T. Decline of erythromycin resistance of group A streptococci in Japan. Pediatr Infect Dis J. 1994;13:1075–1078. doi: 10.1097/00006454-199412000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Gerber M A. Antibiotic resistance in group A streptococci. Pediatr Clin North Am. 1995;42:539–551. doi: 10.1016/s0031-3955(16)38978-7. [DOI] [PubMed] [Google Scholar]
- 10.Giovanetti E, Montanari M P, Mingoia M, Varaldo P E. Phenotypes and genotypes of erythromycin-resistant Streptococcus pyogenes strains in Italy and heterogeneity of inducibly resistant strains. Antimicrob Agents Chemother. 1999;43:1935–1940. doi: 10.1128/aac.43.8.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horaud T, De Cespedes G, Clermont D, David F, Delbos F. Variability of chromosomal genetic elements in streptococci. In: Dunny G M, Cheary P P, McKay L L, editors. Genetics and molecular biology of streptococci, lactococci, and enterococci. Washington, D.C.: American Society for Microbiology; 1991. pp. 16–20. [Google Scholar]
- 12.Hsueh P R, Chen H M, Huang A H, Wu J J. Decreased activity of erythromycin against Streptococcus pyogenes in Taiwan. Antimicrob Agents Chemother. 1995;39:2239–2242. doi: 10.1128/aac.39.10.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jasir A, Schalén C. Survey of macrolide resistance phenotypes in Swedish clinical isolates of Streptococcus pyogenes. J Antimicrob Chemother. 1998;41:135–137. doi: 10.1093/jac/41.1.135. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan, E. L. Recent evaluation of antimicrobial resistance in β-hemolytic streptococci. Clin. Infect. Dis. 24(Suppl. 1):S89–S92. [DOI] [PubMed]
- 15.Kataja J, Huovinen P, Skurnik M, Seppälä H the Finnish Study Group for Antimicrobial Resistance. Erythromycin resistance genes in group A streptococci in Finland. Antimicrob Agents Chemother. 1999;43:48–52. doi: 10.1128/aac.43.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leclercq R, Courvalin P. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother. 1991;35:1267–1272. doi: 10.1128/aac.35.7.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leclercq R, Courvalin P. Intrinsic and unusual resistance to macrolide, lincosamide, and streptogramin antibiotics in bacteria. Antimicrob Agents Chemother. 1991;35:1273–1276. doi: 10.1128/aac.35.7.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopardo H A, Venuta M E, Vidal P, Rosaenz L, Corthey C, Farinati A, Couto E, Sarachian B, Sparo M, Kaufman S, De Mier C A, Gubbay L, Schilingo V, Villaverde P. Argentinian collaborative study on prevalence of erythromycin and penicillin susceptibility in Streptococcus pyogenes. The Argentinian Streptococcus Study Group. Diagn Microbiol Infect Dis. 1997;29:29–32. doi: 10.1016/s0732-8893(97)00070-9. [DOI] [PubMed] [Google Scholar]
- 19.Maruyama S, Yoshioka H, Fujita K, Takimoto M, Satake Y. Sensitivity of group A streptococci to antibiotics: prevalence of resistance to erythromycin in Japan. Am J Dis Child. 1979;133:1143–1145. doi: 10.1001/archpedi.1979.02130110051007. [DOI] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing; ninth informational supplement. M100-S9. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 21.Perez-Trallero E, Urbieta M, Montes M, Ayestaran I, Marimon J M. Emergence of Streptococcus pyogenes strains resistant to erythromycin in Gipuzkoa, Spain. Eur J Clin Microbiol Infect Dis. 1998;16:25–31. doi: 10.1007/BF01584359. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Trallero E, Marimon J M, Montes M, Orden B, de Pablos M. Clonal differences among erythromycin-resistant Streptococcus pyogenes in Spain. Emerg Infect Dis. 1999;5:235–240. doi: 10.3201/eid0502.990207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piggot P J, Amjad M, Wu J J, Sandoval H, Castro J. Genetic and physical maps of Bacillus subtilis 168. In: Harwood C R, Cutting S M, editors. Molecular biology methods for Bacillus. West Sussex, England: John Wiley & Sons Ltd.; 1990. pp. 493–543. [Google Scholar]
- 24.Ruoff K L. Streptococcus. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 299–307. [Google Scholar]
- 25.Seppälä H, Skurnik M, Soini H, Roberts M C, Huovinen P. A novel erythromycin resistance methylase gene (ermTR) in Streptococcus pyogenes. Antimicrob Agents Chemother. 1998;42:257–262. doi: 10.1128/aac.42.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seppälä H, Nissinen A, Yu Q, Huovinen P. Three different phenotypes of erythromycin-resistant Streptococcus pyogenes in Finland. J Antimicrob Chemother. 1993;32:885–891. doi: 10.1093/jac/32.6.885. [DOI] [PubMed] [Google Scholar]
- 27.Seppälä H, Klaukka T, Vuopio-Varkila J, Muotiala A, Helenius H, Lager K, Huovinen P the Finnish Study Group for Antimicrobial Resistance. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441–446. doi: 10.1056/NEJM199708143370701. [DOI] [PubMed] [Google Scholar]
- 28.Sutcliffe J, Tait-Kamardt A, Wondrack L. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob Agents Chemother. 1996;40:1817–1824. doi: 10.1128/aac.40.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutcliffe J, Grebe T, Tait-Kamardt A, Wondrack L. Detection of erythromycin-resistant determinants by PCR. Antimicrob Agents Chemother. 1996;40:2562–2566. doi: 10.1128/aac.40.11.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valisena S, Falci C, Mazzariol A, Cornaglia G, Cocuzza C E, Nicoletti P, Rescaldani R, Fontana R. Molecular typing of erythromycin-resistant Streptococcus pyogenes strains with the M phenotype isolated in Italy. Eur J Clin Microbiol Infect Dis. 1999;18:260–264. doi: 10.1007/s100960050274. [DOI] [PubMed] [Google Scholar]
- 31.Varaldo P E, Debbia E A, Nicoletti G, Pavesio D, Ripa S, Schito G C, Tempera G the Artemis-Italy Study Group. Nationwide survey in Italy of treatment of Streptococcus pyogenes pharyngitis in children: influence of macrolide resistance on clinical and microbiological outcomes. Clin Infect Dis. 1999;29:869–873. doi: 10.1086/520451. [DOI] [PubMed] [Google Scholar]
- 32.Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother. 1995;39:577–585. doi: 10.1128/AAC.39.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisblum B. Insights into erythromycin action from studies of its activity as an inducer of resistance. Antimicrob Agents Chemother. 1995;39:797–805. doi: 10.1128/aac.39.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weisblum B. Inducible resistance to macrolides, lincosamides and streptogramin type B antibiotics: the resistance phenotype, its biological diversity, and structural elements that regulate expression—a review. J Antimicrob Chemother. 1985;16(Suppl. A):63–90. doi: 10.1093/jac/16.suppl_a.63. [DOI] [PubMed] [Google Scholar]
- 35.Wu J J, Lin K Y, Hsueh P R, Liu J W, Pan H I, Sheu S. High incidence of erythromycin-resistant streptococci in Taiwan. Antimicrob Agents Chemother. 1997;41:844–846. doi: 10.1128/aac.41.4.844. [DOI] [PMC free article] [PubMed] [Google Scholar]