Abstract
Three new thymol derivatives, 7-formyl-9-isobutyryloxy-8-hydroxythymol (1), 7,9-di-isobutyryloxy-8,10-dehydrothymol (2) and 2α-methoxyl-3β-methyl-6-methylol-2,3-dihydrobenzofuran (3), along with five known ones (4–8), were isolated from the aerial parts of the invasive plant Ageratina adenophora. Their structures were elucidated by extensive spectroscopic analysis and they were all isolated from the aerial part of A. adenophora for the first time. These compounds, except 8, selectively showed in vitro antimicrobial activity against three Gram-(+) and two Gram-(−) bacterial strains. In particular, compounds 1 and 5 showed notable in vitro antimicrobial activity against all five bacterial strains with IC50 values ranging from 3.9 to 15.6 μg mL−1, as compared to reference compound kanamycin sulfate with a MIC value 1.9–3.9 μg mL−1. Compounds 1 and 5 were further revealed to show in vitro cytotoxic activity against three tested human tumor (MCF-7, NCI-H460 and HeLa) cell lines, with IC50 values ranging from 7.45 to 28.63 μM. Compounds 7 and 8 selectively showed slight but detectable in vitro cytotoxicity toward MCF-7 and NCI-H460 cell lines, with IC50 values 44.65–83.19 μM. No cytotoxic effects were detected in the bioassay of the other four thymol derivatives. The present results provide new data to support that the aerial parts of A. adenophora are a rich source of bioactive chemicals valuable in medicinal applications.
Eight thymol derivatives including three new ones (1–3) were obtained from the aerial parts of Ageratina adenophora, with most of them, in particular 1 and 5, showing notable in vitro antimicrobial and cytotoxic activity.
Introduction
Ageratina adenophora (Sprengel) King & Robinson (synonym: Eupatorium adenophorum Sprengel) is a perennial, herbaceous invasive plant, native to Mexico and Costa Rica.1 As a well-known invasive species, this plant has successfully invaded more than thirty countries and regions in tropical and temperate zones of the world, including America, Australia, Europe, India, South Africa, Southeast Asia, and the southwest part of China.2,3 At its invasion places, the rapid spread of A. adenophora has caused serious economic losses to agriculture, forestry and livestock, and intensely damaged the local ecosystem and the original biodiversity.4,5
In nature, A. adenophora is seldom attacked by microorganisms and insects. This suggests a rich defense related to bioactive chemicals, that possibly might also be pharmaceutically valuable, would exist in this plant. Previously, some literature reported that this plant was used in Nigeria and India as traditional or folk herb medicine for treatment of many human diseases, like fever, diabetes, inflammation, etc.6,7 To date, some terpenoids, flavonoids, phenylpropanoids, coumarins, sterols and alkaloids have been reported from this plant,8–10 with part of them exhibiting phytotoxic,11 allelopathic,12,13 antifungal14 and antifeedant15 activities. In our recent study, some bioactive compounds, including phytotoxic phenolics,16 antifungal monoterpenes17 and antibacterial quinic acid derivatives,18 were also revealed from the roots or aerial parts of this plant. In continuation of the work to clarify those potentially new and bioactive chemicals in the aerial parts of A. adenophora, eight thymol derivatives including three new (1–3) and five known (4–8) ones are here further obtained (Fig. 1). We herein report the isolation and structural elucidation of these compounds, as well as describe their in vitro antimicrobial activity against five bacteria and cytotoxicity toward three human cancer cell lines.
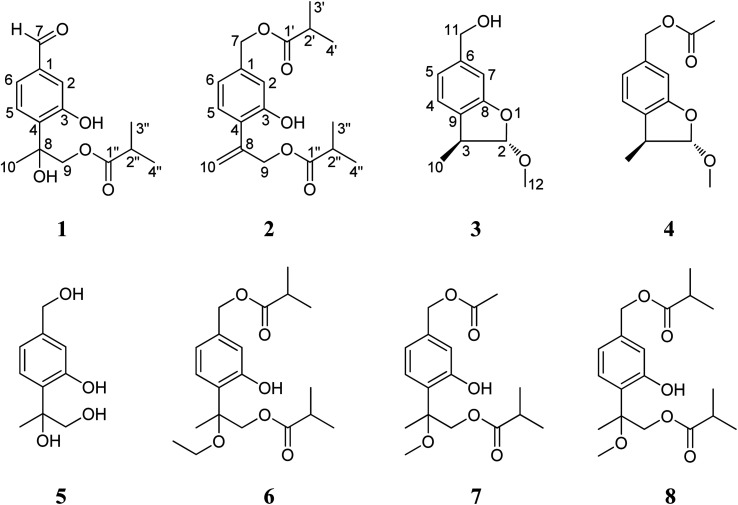
Fig. 1. Chemical structures of compounds 1–8.
Results and discussion
The petroleum ether- and EtOAc-soluble fractions of the ethanol extract of the aerial parts of Ageratina adenophora (Spreng.) were isolated and purified by repeated column chromatography (CC) and HPLC to afford the three new (1–3) and five known (4–8) thymol derivatives. By comparing their NMR and MS data with those reported in literatures, the five known compounds were identified as 2α-methoxyl-3β-methyl-6-(acetyl-O-methyl)-2,3-dihydrobenzofuran (4),19 7,8,9-trihydroxythymol (5),20 7,9-di-isobutyryloxy-8-ethoxythymol (6),21 7-acetoxy-9-isobutyryloxy-8-methoxythymol (7)21 and 7,9-di-isobutyryloxy-8-methoxythymol (8).21
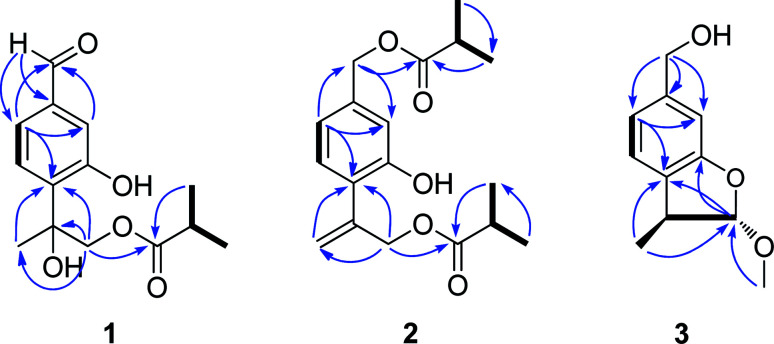
Compound 1 was isolated as a yellow oil and determined to have a molecular formula C14H18O5 on the basis of HR-ESI-MS data (m/z 289.1056 [M + Na]+, calcd. for C14H18O5Na, 289.1052). The IR absorptions at 3424 and 1695 cm−1 revealed the presence of hydroxyl and carbonyl groups in the molecule. The existence of three methyl groups was revealed by 1H NMR spectrum which provided methyl signals at δH 1.66 (3H, s), 1.12 (3H, s) and 1.13 (3H, s). The three aromatic proton signals at δH 7.34 (1H, d, J = 1.2 Hz), 7.18 (1H, d, J = 8.4 Hz), and 7.35 (1H, dd, J = 8.4, 1.2 Hz) were indicative of a typical pattern of 1,3,4-trisubstituted phenyl group.22 The 13C NMR (DEPT) spectra (Table 2), coupled with HSQC spectral analysis, revealed the presence of a formyl group at δC 191.9 (C-7), a carbonyl group at δC 177.7 (C-1′′), an oxymethylene group at δC 70.4 (C-9), a methine group at δC 33.9 (C-2′′), and an oxygenated quaternary carbon at δC 77.7 (C-8). Taken together these spectral data and the molecular formula into consideration, the existence of two hydroxyl groups could further be deduced. In the 1H–1H COSY spectrum, significant correlation signals for two proton systems were displayed, one at C-5 through C-6 and the other at C-3′′ through C-4′′ (Fig. 2), corresponding to structure fragments of –CH(5)–CH(6)– and CH3(3′′)–CH(2′′)–CH3(4′′), respectively. In the HMBC spectrum, the exhibition of correlation signals of H-7 (δH 9.92) with C-2 and C-6, and of H-2, H-6 with C-7 (δC 191.9) evidenced the connection of C-7 with C-1. The HMBC correlations of Me-10, H-9 with C-4 supported the connection of C-8 with C-4. The HMBC correlations of Me-3′′, Me-4′′ with C-1′′ supported the connection of C-1′′ with C-2′′. The HMBC correlations of H-9 with C-1′′ supported the ester bond linkage of C-1′′ with C-9. Further consideration of the substitution pattern of the aromatic ring and the other NMR data led to the assignment of the two hydroxyl groups at C-3 and C-8, respectively. Based on these above spectroscopic analyses, we can unambiguously establish the planner structure of 1 as depicted in Fig. 1. However, it is presently difficult to determine the configuration of C-8. Thus, the structure of 1 was elucidated as 7-formyl-9-isobutyryloxy-8-hydroxythymol.
The 13C (DEPT) NMR spectral data (δ in ppm) for compounds 1, 2 and 3.
| Position | 1a | 2a | 3b |
|---|---|---|---|
| 1 | 137.4 (C) | 138.2 (C) | — |
| 2 | 118.9 (CH) | 115.5 (CH) | 115.5 (CH) |
| 3 | 157.1 (C) | 153.7 (C) | 44.4 (CH) |
| 4 | 132.5 (C) | 125.1 (C) | 124.7 (CH) |
| 5 | 127.0 (CH) | 129.6 (CH) | 120.8 (CH) |
| 6 | 120.5 (CH) | 119.3 (CH) | 143.3 (C) |
| 7 | 191.9 (CH) | 65.5 (CH2) | 109.3 (CH) |
| 8 | 77.7 (C) | 141.9 (C) | 159.3 (C) |
| 9 | 70.4 (CH2) | 65.5 (CH2) | 131.8 (C) |
| 10 | 25.8 (CH3) | 116.8 (CH2) | 18.9 (CH3) |
| 11 | — | — | 65.2 (CH2) |
| 12 | — | — | 56.2 (CH3) |
| 1′ | — | 176.9 (C) | — |
| 2′ | — | 34.0 (CH) | — |
| 3′ | — | 18.8 (CH3) | — |
| 4′ | — | 18.8 (CH3) | — |
| 1′′ | 177.7 (C) | 177.8 (C) | — |
| 2′′ | 33.9 (CH) | 34.0 (CH) | — |
| 3′′ | 18.8 (CH3) | 18.9 (CH3) | — |
| 4′′ | 18.8 (CH3) | 18.8 (CH3) | — |
Recorded at 150 MHz in CDCl3.
Recorded at 100 MHz in CD3OD.
Fig. 2. Key HMBC ( ) and 1H–1H COSY (
) and 1H–1H COSY ( ) correlations of 1, 2 and 3.
) correlations of 1, 2 and 3.
Compound 2 was assigned the molecular formula C18H24O5, as deduced from ESI-MS and HR-ESI-MS data. Comparative analysis of the spectroscopic data and literature precedents21,23,24 supported 2 to be also a thymol derivative. Careful comparison of 1H and 13C NMR data (Tables 1 and 2) of 2 with those of known compound 8 revealed the major differences of the two compounds that the resonances for Me-10, Me-11 and the sp3 quaternary carbon C-8 in compound 8 were replaced by signals [δH 5.28 (1H, Ha-10), 5.47 (1H, Hb-10); δC 141.6 (C-8), 116.8 (C-10)] for a terminal double bond in 2. In the HMBC spectrum, significant correlation signals of H2-10 to C-4 and C-9 were observed, which confirmed the location of the terminal double bond between C-8 and C-10. The 1H–1H COSY spectrum and HMBC correlations (Fig. 2) of Me-3′ and Me-4′ with C-1′, of Me-3′′ and Me-4′′ with C-1′′, of H-7 with C-1′, and of H-9 with C-1′′, evidenced the ester bond linkages of C-7 and C-9 with an individual isobutyryl group, respectively. The presence of diagnostic proton signals at δH 6.92 (1H, d, J = 1.2 Hz), 7.07 (1H, d, J = 7.6 Hz), and 6.84 (1H, dd, J = 7.6, 1.2 Hz) supported the appearance of the core 1,3,4-trisubstituted phenyl moiety. The HMBC correlations of H-2 and H-6 with C-7, of H-10 and H-9 with C-4, and of H-5 with C-8 confirmed the connections of C7 with C-1, C-4 with C-8, and the location of a hydroxyl group at C-3. Therefore, compound 2 was determined as 7,9-di-isobutyryloxy-8,10-dehydrothymol.
The 1H NMR spectral data (δ in ppm, J in Hz) for compounds 1, 2 and 3.
| Position | 1a | 2a | 3b |
|---|---|---|---|
| 2 | 7.34 (1H, d, 1.2) | 6.92 (1H, d, 1.2) | 5.21 (1H, d, 2.0) |
| 3 | — | — | 3.15 (1H, brq, 7.2) |
| 4 | — | — | 7.12 (1H, d, 7.6) |
| 5 | 7.18 (1H, d, 8.4) | 7.07 (1H, d, 7.6) | 6.87 (1H, brd, 7.6) |
| 6 | 7.35 (1H, dd, 8.4, 1.2) | 6.84 (1H, dd, 7.6, 1.2) | — |
| 7 | 9.92 (1H, s) | 5.06 (2H, s) | 6.79 (1H, brs) |
| 9 | 4.47 (1H, d, 12.0) | 4.73 (2H, s) | — |
| 4.33 (1H, d, 12.0) | |||
| 10 | 1.66 (3H, s) | 5.47 (1H, d, 1.2) | 1.23 (3H, d, 7.2) |
| 5.28 (1H, d, 1.2) | |||
| 11 | — | — | 4.53 (2H, s) |
| 12 | — | — | 3.47 (3H, s) |
| 2′ | — | 2.62 (1H, sept, 7.2) | — |
| 3′ | — | 1.20 (3H, d, 7.2) | — |
| 4′ | — | 1.20 (3H, d, 7.2) | — |
| 2′′ | 2.57 (1H, sept, 7.2) | 2.62 (1H, sept, 7.2) | — |
| 3′′ | 1.12 (3H, d, 7.2) | 1.20 (3H, d, 7.2) | — |
| 4′′ | 1.13 (3H, d, 7.2) | 1.20 (3H, d, 7.2) | — |
Recorded at 600 MHz in CDCl3.
Recorded at 400 MHz in CD3OD.
The molecular formula of compound 3 was determined as C11H14O3 by the HR-EI-MS, due to a quasi-molecular ion peak at m/z 194.0938 (calcd. for C11H14O3, 194.0937). The IR absorption at 3448 cm−1 revealed the presence of hydroxyl group in the molecule. The 1H and 13C (DEPT) NMR spectra displayed closely related signals with those of 2α-methoxyl-3β-methyl-6-(acetyl-O-methyl)-2,3-dihydrobenzofuran,19 a known thymol compound which was also obtained as compound 4 in the present study. Careful comparison of their NMR spectral data indicated the major difference that the spectroscopic resonances for the acetoxy group connected to C-11 in 4 were absent in 3, suggesting that the acetoxy group located at C-11 in 4 was replaced by a free hydroxyl group in 3. Accordingly, we can preliminarily establish the structure of compound 3 as shown in Fig. 1, and this deduction was further well supported by 1H–1H COSY and HMBC analysis (Fig. 2). Furthermore, the presented coupling constant of H-2 (J2,3 = 2.0 Hz) and the NOE correlation between H-2 and H3-10 in the NOESY spectrum supported the β-orientation of Me-10 and the α-orientation of the methoxy group at C-2.19,25 Therefore, compound 3 was assigned as 2α-methoxyl-3β-methyl-6-methylol-2,3-dihydrobenzofuran.
Among these thymol derivatives, 1–3 are new compounds that are here reported from nature for the first time. Compound 3 is structurally characterized with a dihydrobenzofuran skeleton and this type of monoterpene is rare in natural products.25 Compound 5 was previously only reported from plant Eupatorium fortunei and this is the first time for it being isolated from A. adenophora.20 The other four known compounds 4, 6, 7 and 8 are here also isolated and identified from the aerial parts of A. adenophora for the first time.
Aimed to explore their potential and undiscovered pharmacological activity of these isolated thymol derivatives, compounds 1–8 were evaluated for their in vitro antimicrobial activity by testing their MIC values, using a bioassay method as previously we used and described.26,27Table 3 lists the results obtained for these compounds on the viability of five tested bacterial strains, compared to kanamycin sulfate (KS) as a reference compound. Among them, compounds 1 and 5 were found to be strongly active against all the five assayed microorganisms with MIC values ranging from 3.9 to 15.6 μg mL−1, which were comparable to the positive control KS (MICs = 1.9 to 3.9 μg mL−1). Compounds 2, 3, 4, 6 and 7 only selectively showed antibacterial activity (MIC 31.3–62.5 μg mL−1) against three tested Gram-(+) bacteria, i.e. S. aureus, B. cereus and B. thuringiensis. While no antibacterial activity was detected for compound 8 toward all the five tested bacterial strains. From the result, it seems to show that the existence of both the hydroxyl group at C-3 and C-8 would be important for this group of thymol compounds to display their antibacterial potentials.
MIC values of compounds 1–8 in μg mL−1 against five bacterial strainsa.
| Compounds | Staphylococcus aureus | Bacillus cereus | Bacillus thuringiensis | Escherichia coli | Salmonella enterica |
|---|---|---|---|---|---|
| 1 | 7.8 | 3.9 | 7.8 | 15.6 | 15.6 |
| 2 | >100 | 62.5 | >100 | >100 | >100 |
| 3 | 31.3 | 31.3 | 62.5 | >100 | >100 |
| 4 | 31.3 | 62.5 | 62.5 | >100 | >100 |
| 5 | 7.8 | 7.8 | 15.6 | 15.6 | 15.6 |
| 6 | 62.5 | 62.5 | 62.5 | >100 | >100 |
| 7 | >100 | 62.5 | 62.5 | >100 | >100 |
| 8 | >100 | >100 | >100 | >100 | >100 |
| KS | 1.9 | 3.9 | 3.9 | 3.9 | 3.9 |
KS = kanamycin sulfate.
Compounds 1–8 were further tested for their in vitro cytotoxicity against human cancer MCF-7, HeLa and NCI-H460 cell lines, using a microdilution titre technique as recently we described.28 The resulting IC50 values are displayed in Table 4, compared to Adriamycin as positive control. Compounds 1 and 5 were found to show strong or moderate cytotoxicity against all the three tested cancer cell lines, with IC50 values ranging from 7.45 to 28.63 μM. Compounds 7 and 8 showed slight but detectable cytotoxicity toward MCF-7 and NCI-H460 cell lines, with IC50 values ranging from 44.65 to 83.19 μM. While, no obvious cytotoxic activity was detected for the other compounds in this bioassay. Comparison of the chemical structures and the cytotoxic activity of these compounds indicated that the existence of both the hydroxyl group at C-3 and C-8 would be important for this group of thymol derivatives to fully display their cytotoxic potentials.
Cytotoxic activity of compounds 1–8 (IC50, μM)a.
| Compounds | MCF-7 | NCI-H460 | HeLa |
|---|---|---|---|
| 1 | 7.45 ± 0.22 | 8.32 ± 0.21 | 9.45 ± 0.46 |
| 2–4 | >100 | >100 | >100 |
| 5 | 11.54 ± 0.86 | 15.67 ± 1.03 | 28.63 ± 1.93 |
| 6 | >100 | >100 | >100 |
| 7 | 44.65 ± 4.08 | 52.74 ± 5.16 | >100 |
| 8 | 83.19 ± 6.55 | 77.42 ± 5.06 | >100 |
| Adriamycin | 0.78 ± 0.06 | 1.12 ± 0.05 | 0.54 ± 0.04 |
Values represent mean ± SD (n = 3) based on three individual experiments.
As a well-known invasive plant, A. adenophora has attracted much attention of scientists to investigate its invasion mechanisms. Since that this plant is accumulating a huge biomass at its invasion areas, to explore the potential utilization of A. Adenophora is gradually also concentrated and emphasized by some researchers. Up to date, phytochemical studies have indicated that structurally diverse chemicals exist in this invasive species. Our present findings further support that the aerial part tissue of this plant is rich in bioactive natural products valuable to be explored for medicinal usage. It is interesting to note that compound 5 was reported as a natural product capable of strongly inhibiting the growth of Microcystis aeruginosa,20 suggesting that these thymol derivatives, at least for compound 5, might have some allelopathic potential to contribute the invasion success of A. adenophora. Noteworthily, for all these thymol derivatives identified from A. adenophora, the carbon C-7 (or C-11 in compounds 3 and 4) is generally appeared as an oxygenated carbon. While for those thymol compounds reported from some other Eupatorium plants, such as thymol compounds from E. fortunei and E. cannabinum,22–24 usually exhibited at C-7 is a methyl group (an unoxygenated carbon). Thus, the general oxygenation extent of thymol compounds at C-7 might have some chemotaxonomic significance for plants in Adenophora and (or) Eupatorium genus. Furthermore, it is evident that compounds 3 and 4 contain a typical dihydrobenzofuran skeleton. Dihydrobenzofuran type monoterpene is rare in nature and the discovery of dihydrobenzofuran type new compound 3 suggests that some so far undiscovered rare monoterpenes would still exist in the aerial parts of A. adenophora worthy of further investigation.
Materials and methods
General experimental procedures
UV spectra were recorded in MeOH on a PerkinElmer Lambda 35 UV-vis spectrophotometer. IR spectra (KBr) were recorded on a Bruker Tensor 27 spectrophotometer in cm−1. 1H (600 MHz and 400 MHz), 13C (150 MHz and 100 MHz), and 2D NMR spectra were recorded in CDCl3 and CD3OD on a Bruker DRX-400 instrument and a Bruker AVANCE 600 instrument with TMS as an internal standard. HR-ESI-MS data were obtained on a Water Q-TOF Premier mass spectrometer and HR-EI-MS data were obtained on a Finigan MAT 95XP mass spectrometer. ESIMS were collected on an MDS SCIEX API 2000 LC/MS/MS instrument. Preparative HPLC was conducted using a P3000 HPLC pump and a UV3000 UV-VIS Detector with a Fuji-C18 column (10 μm–100A). For column chromatography (CC), silica gel (200–300 mesh, Qingdao Marine Chemical Inc., Peoples Republic of China), YMC ODS-A (50 μm, YMC Co. Ltd., Japan) were used, and Sephadex LH-20 (Pharmacia Fine Chemical Co. Ltd.) were used. Fractions were monitored by TLC, and spots were visualized by heating the silica gel plates sprayed with 10% H2SO4 in ethanol.
Plant materials
The aerial part material of Ageratina adenophora (Spreng.) were collected in a suburb of Kunming, Yunnan province, P. R. China, in July 2009, and authenticated by Prof. Fu-Wu Xing, South China Botanical Garden, Chinese Academy of Sciences. A voucher specimen (no. 20090702) was deposited at the Laboratory of Phytochemistry at the South China Botanical Garden, Chinese Academy of Sciences.
Extraction and isolation
The air-dried and powdered aerial part material of A. adenophora (10 kg) were extracted with 95% aqueous ethanol at room temperature for three times (3 × 20 L, each for 24 h). The ethanol extracts were next combined and concentrated in vacuo, with the resulting residue suspended in water and sequentially extracted with petroleum ether (3 × 3 L) and EtOAc (3 × 3 L). The petroleum ether and EtOAc layers were evaporated in vacuo to yield petroleum-soluble fraction (93.1 g) and EtOAc-soluble fraction (80.0 g), respectively.
The petroleum ether fraction (93.1 g) was subjected to silica gel CC (1000 mm × 120 mm i.d.), eluting with a gradient of CHCl3–MeOH (100 : 0 to 90 : 10, v/v) to give twelve fractions (P1–P12) after pooled according to their TLC profiles. Fraction P6 (20.6 g), obtained by elution with CHCl3–MeOH (98 : 2, v/v), was applied to silica gel CC (800 × 75 mm i.d.) eluted with a gradient of petroleum ether–acetone (100 : 0 to 80 : 20, v/v) to obtain nine fractions (P6-1–P6-9). Fraction P6-4 (7.4 g) was subjected to silica gel CC (800 × 50 mm i.d.) eluted with petroleum–acetone (300 : 1 to 100 : 5) in a gradient to obtain sub-fractions P6-4-4–P6-4-9, of which sub-fraction P6-4-4 (600.0 mg) was separated by preparative HPLC (flow rate 8 mL min−1) using MeOH–H2O (70 : 30, v/v) to afford compound 6 (tR = 37.5 min, 2.4 mg). Fraction P6-7 (6.2 g) was applied to an ODS CC using MeOH–H2O (30 : 70 to 90 : 10) to obtain sub-fractions P6-7-1–P6-7-12. Subsequently sub-fraction P6-7-8 (19.2 mg) was purified by Sephadex LH-20 CC (1500 mm × 25 mm i.d.) eluted with CHCl3–MeOH (20 : 80, v/v) to afford compounds 2 (2.1 mg) and 8 (2.5 mg), and sub-fraction P6-7-3 (930.0 mg) was purified by a Sephadex LH-20 CC using acetone to afford compound 7 (25.1 mg). Fraction P6-8 (340.0 mg) was subjected to Sephadex LH-20 CC and preparative HPLC (flow rate 8 mL min−1) using MeOH–H2O (45 : 55 to 50 : 50, v/v) as mobile phase to afford compound 1 (tR = 48.0 min, 6.7 mg). Fraction P6-9 (600.0 mg) was subjected to silica gel CC eluted with petroleum–acetone (100 : 1 to 90 : 10, v/v) in gradient to obtain sub-fractions P6-8-1 and P6-8-2, and sub-fraction P6-8-2 (45.5 mg) was further purified by an ODS CC using MeOH–H2O (65 : 35, v/v) as eluent to afford compound 4 (3.0 mg).
The EtOAc-soluble fraction (80.0 g) was subjected to silica gel CC (1000 mm × 120 mm i.d.) using a gradient of CHCl3–MeOH (95 : 5 to 60 : 40, v/v) to give ten fractions (E1–E10). Fraction E6 (12.0 g), obtained by elution with CHCl3–MeOH (85 : 15, v/v), was subjected to silica gel CC eluted with CHCl3–MeOH (40 : 1 to 9 : 1, v/v) in gradient to obtain sub-fractions E6-1–E6-8. Subfraction E6-2 (213.1 mg) was further applied to ODS CC eluted with MeOH–H2O (20 : 80 to 90 : 10, v/v) to afford compound 3 (7.0 mg). Fraction E6-5 (2.7 g) was subjected to ODS CC using MeOH–H2O (10 : 90 to 70 : 30, v/v) to afford five sub-fractions (E6-5-1–E6-5-5), of which sub-fraction E6-5-1 (312.0 mg) was further purified by Sephadex LH-20 CC using MeOH to afford compound 5 (30.0 mg).
7-Formyl-9-isobutyryloxy-8-hydroxythymol (1)
Yellowness oil; [α]20D −2.0 (c 0.65, CHCl3); IR (KBr) νmax 3424, 1695, 1612, 1579 cm−1; UV (MeOH) λmax (log ε) nm: 222 (4.11), 260 (3.88), 317 (3.36); 1H (CDCl3, 600 MHz) and 13C NMR (CDCl3, 150 MHz) data: see Tables 1 and 3; ESI-MS m/z 571 [2M + K]+, 555 [2M + Na]+, 567 [2M + Cl]−, 289 [M + Na]+, 301 [M + Cl]−, 265 [M − H]−; HR-ESI-MS m/z 289.1056 [M + Na]+ (calcd. for C14H18O5Na, 289.1052).
7,9-Di-isobutyryloxy-8,10-dehydrothymol (2)
Yellowness oil; [α]20D 0.0 (c 0.20, CH3OH); IR (KBr) νmax 3438, 1733, 1677 cm−1; UV (MeOH) λmax (log ε) nm: 205 (4.34), 285 (3.42); 1H (CDCl3, 600 MHz) and 13C NMR (CDCl3, 100 MHz) data: see Tables 1 and 3; ESI-MS m/z 343 [M + Na]+, 359 [M + K]+; HR-ESI-MS m/z 343.1526 [M + Na]+ (calcd for C18H24O5Na, 343.1521).
2α-Methoxyl-3β-methyl-6-methylol-2,3-dihydrobenzofuran (3)
Yellowness oil; [α]20D 2.8 (c 0.16, CH3OH); IR (KBr) νmax 3448, 1596, 1496, 1455 cm−1; UV (MeOH) λmax (log ε) nm: 204 (4.09), 279 (3.32); 1H (CD3OD, 400 MHz) and 13C NMR (CD3OD, 100 MHz) data: see Tables 1 and 3; ESI-MS m/z 195 [M + H]+, 217 [M + Na]+, 289 [M + Na]+, 423 [2M + Cl]−; HR-EI-MS m/z 194.0938 (calcd. for C11H14O3, 194.0937).
Antimicrobial activity
Antimicrobial activity of these eight thymol compounds were evaluated, using 96-well plates, by a method based on a microdilution titre technique with modification in determination of the minimum inhibitory concentration (MIC) values.26 Before the assay, proper amounts of the tested compounds and kanamycin sulfate (positive control) were dissolved in methanol to prepare their 1.0 mg mL−1 sample solutions and the positive control solution, respectively, and resazurin (indicator) was dissolved in distilled water to prepare 100 μg mL−1 indicator solution for the assay. In the test, 100 μL indicator solution (resazurin, 100 μg mL−1) was first placed into each of the sterility control wells (11th column) on the 96 well plates, and about 7.5 mL indicator solution was mixed with 5 mL test organism (106 cfu mL−1) followed by transferring (100 μL, each) to growth control wells (12th column) and all test wells (1–10th column). Then, each of 100 μL of the sample solutions (1.0 mg mL−1 of tested compounds in methanol) and the positive control solution (1.0 mg mL−1 of kanamycin sulfate in methanol) as well as the negative control sample (pure MeOH) were allied to the wells in the 1st column of the plates. In each plate, up to six samples along with a positive control and a negative control samples were applied. Once all samples and controls were properly applied to the 1st column of wells on the plate, half of the homogenized content (100 μL) from these wells was then parallel transferred to the 2nd column of wells, and each subsequent well was treated similarly (doubling dilution) up to the 10th column, followed by discarding the last 100 μL aliquot. Finally, the plates were incubated at 37 °C for 5–6 h until the color of growth control change to pink. The lowest concentration for each tested compound at which color change occurred was recorded as its primary MIC value. The average of primary values from three individual tests were calculated and that was taken as the final MIC value for each of the test compounds.27 A total of five microorganisms including three Gram-(+) bacteria (Staphylococcus aureus, Bacillus cereus and Bacillus thuringiensis) and two Gram-(−) bacterial species (Escherichia coli and Salmonella enterica) were used in the bioassay. The resulting MIC values of the tested compounds were listed in Table 3.
Cytotoxic assay
The cytotoxic activity of compounds 1–8 against three human tumor (MCF-7, NCI-H460 and Hela) cell lines were assayed by using 96 well plates according to a MTT method as we used previously.28 In brief, the cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum in a humidified atmosphere with 5% CO2 at 37 °C. Each well of 96-well cell culture plates was seeded with 100 μL adherent cells (5 × 104 cell per mL) and placed in an atmosphere with 5% CO2 at 37 °C for 24 h to form a monolayer on the flat bottoms. Subsequently, in each well, the supernatant was removed and 100 μL fresh medium and 100 μL medium containing one of the test compounds was added. Then the plate was incubated in 5% CO2 atmosphere at 37 °C. After 3 days, 20 μL MTT at concentration 5 mg mL−1 in DMSO was added into each well and incubated for 4 h. Carefully, the supernatant in each well was removed and 150 μL DMSO was added. Then the plate was vortex shaken for 15 min to dissolve blue formazan crystals. The OD (optical density) value of each well was tested on a Genois microplate reader (Tecan GENios, Männedorf, Switzerland) at 570 nm. All the tests were conducted by three individual experiments and adriamycin was applied as a positive control. In a test, for each of the tumor cell lines, each of the test compounds was set at concentrations 50, 25, 12.5, 6.25, 3.125, 1.5625 μg mL−1. The inhibitory rate of tumor cell growth was calculated by the formula: inhibition rate (%) = (ODcontrol − ODtreated)/ODcontrol × 100%, and the IC50 values were calculated by SPSS 16.0 statistic software. The three tumor cell lines were purchased from the Kunming Institute of Zoology, CAS. The resulting IC50 values listed in Table 4 were based on three individual experiments and represented as means ± standard deviation (SD).
Conclusions
Eight thymol derivatives, including three new (1–3) and five known ones (4–8), were obtained from the aerial parts of the invasive plant Ageratina adenophora. Their structures were elucidated by extensive spectroscopic analysis and they were all isolated from the aerial parts of A. Adenophora for the first time. Compounds 1 and 5 were found to show obviously in vitro antimicrobial activity against all the five tested bacterial strains with IC50 values ranging from 7.8 to 15.6 μg mL−1. Other thymol compounds, except 9, only selectively showed detectable in vitro antimicrobial activity toward three tested Gram-(+) bacteria. Compounds 1 and 5 were further revealed to show in vitro cytotoxic activity against human tumor MCF-7, NCI-H460 and HeLa cell lines, with IC50 values ranging from 7.45 to 28.63 μM. Compounds 7 and 8 selectively showed slight in vitro cytotoxicity toward MCF-7 and NCI-H460 cell lines. These data supported that the aerial parts of A. Adenophora are rich in potential bioactive natural products valuable to be developed in medicinal field.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This research project was financially supported by the National Natural Science Foundation of China (30970453, 31270406), and the Natural Science Foundation of Guangdong Province, China (2019A1515011236, 2014A030313742).
Electronic supplementary information (ESI) available. See DOI: 10.1039/d0ra08885d
References
- Qiang S. J. Wuhan Bot. Res. 1998;16:366–372. [Google Scholar]
- Wan F.-H. Liu W.-X. Guo J.-Y. Qiang S. Li B.-P. Wang J.-J. Yang G.-Q. Niu H.-B. Gui F.-R. Huang W.-K. Sci. China: Life Sci. 2010;53:1291–1298. doi: 10.1007/s11427-010-4080-7. [DOI] [PubMed] [Google Scholar]
- Sun X.-Y. Lu Z.-H. Sang W.-G. J. For. Res. 2004;15:319–322. doi: 10.1007/BF02911018. [DOI] [Google Scholar]
- Wan F.-H. Guo J.-Y. Wang D.-H. Biodiversity Sci. 2002;10:119–125. [Google Scholar]
- Wang R. Wang Y.-Z. Divers. Distrib. 2006;12:397–408. doi: 10.1111/j.1366-9516.2006.00250.x. [DOI] [Google Scholar]
- Awah F. M. Uzoegwu P. N. Ifeonu P. Oyugi J. O. Rutherford J. Yao X. Fehrmann F. Fowke K. R. Eze M. O. Food Chem. 2012;131:1279–1286. doi: 10.1016/j.foodchem.2011.09.118. [DOI] [Google Scholar]
- Ahluwalia V. Sisodia R. Walia S. Sati O. P. Kumar J. Kundu A. J. Pestic. Sci. 2014;87:341–349. [Google Scholar]
- Yan Q.-S. Yang J. Li H.-M. Cao A.-C. Chen Q.-H. Wen Y.-Q. He L. J. Beijing Norm. Univ. 2006;42:70–73. [Google Scholar]
- Li Y.-M. Li Z.-Y. Ye M. J. Yunnan Agric. Univ. 2008;23:42–46. [Google Scholar]
- He L. Hou J. Gan M. L. Shi J. G. Chantrapromma S. Fun H. K. Williams I. D. Sung H. H. Y. J. Nat. Prod. 2008;71:1485–1488. doi: 10.1021/np800242w. [DOI] [PubMed] [Google Scholar]
- Zheng G. Jia Y. Zhao X. Zhang F. Luo S. Li S. Li W. Chemoecology. 2012;22:131–138. doi: 10.1007/s00049-012-0105-y. [DOI] [Google Scholar]
- Yang G.-Q. Wan F.-H. Liu W.-X. Zhang X.-W. Allelopathy J. 2006;18:237–245. [Google Scholar]
- Zhao X. Zheng G.-W. Niu X.-M. Li W.-Q. Wang F.-S. Li S.-H. J. Agric. Food Chem. 2009;57:478–482. doi: 10.1021/jf803023x. [DOI] [PubMed] [Google Scholar]
- Liu X. Ouyang C. Li Y. Yang D. Fang W. Yan D. Guo M. Cao A. Wang Q. J. Plant Dis. Prot. 2016;123:163–170. doi: 10.1007/s41348-016-0022-3. [DOI] [Google Scholar]
- Shi W. Luo S. Li S. Chin. J. Chem. 2012;30:1331–1334. doi: 10.1002/cjoc.201200279. [DOI] [Google Scholar]
- Zhou Z.-Y. Liu W.-X. Pei G. Ren H. Wang J. Xu Q.-L. Xie H.-H. Wan F.-H. Tan J.-W. J. Agric. Food Chem. 2013;61:111792–111799. doi: 10.1021/jf400876j. [DOI] [PubMed] [Google Scholar]
- Xu Q.-L. Zhang M. Zhou Z.-Y. Liu W.-X. Wan F.-H. Wang H.-F. Tan J.-W. Phytochem. Lett. 2014;9:123–126. doi: 10.1016/j.phytol.2014.05.004. [DOI] [Google Scholar]
- Zhang M. Liu W.-X. Zheng M.-F. Xu Q.-L. Wan F.-H. Wang J. Lei T. Zhou Z.-Y. Tan J.-W. Molecules. 2013;18:14096–14104. doi: 10.3390/molecules181114096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B. Dong L.-M. Xu Q.-L. Zhang X. Zhang Q. Liu W.-B. Tan J.-W. Phytochem. Lett. 2018;24:67–70. doi: 10.1016/j.phytol.2018.01.012. [DOI] [Google Scholar]
- Pham T. N. Pham H. D. Dang D. K. Duong T. T. Le T. P. Q. Nguyen Q. D. Tien D. N. Nat. Prod. Res. 2019;33:1345–1348. doi: 10.1080/14786419.2018.1476511. [DOI] [PubMed] [Google Scholar]
- Dong L.-M. Zhang M. Xu Q.-L. Zhang Q. Luo B. Luo Q.-W. Liu W.-B. Tan J.-W. Molecules. 2017;22:592. doi: 10.3390/molecules22040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Li J. Wang H. Jin D.-Q. Chen H. Xu J. Ohizumi Y. Guo Y. Phytochem. Lett. 2014;7:190–193. doi: 10.1016/j.phytol.2013.12.004. [DOI] [Google Scholar]
- Tori M. Ohara Y. Nakashima K. Sono M. J. Nat. Prod. 2001;64:1048–1051. doi: 10.1021/np0101191. [DOI] [PubMed] [Google Scholar]
- Chen J.-J. Tsai Y.-C. Hwang T.-L. Wang T.-C. J. Nat. Prod. 2011;74:1021–1027. doi: 10.1021/np100923z. [DOI] [PubMed] [Google Scholar]
- Jiang H. X. Liu Q. Gao K. Nat. Prod. Res. 2008;22:937–941. doi: 10.1080/14786410701642888. [DOI] [PubMed] [Google Scholar]
- Wang J. Ren H. Xu Q.-L. Zhou Z.-Y. Wu P. Wei X.-Y. Cao Y. Chen X.-X. Tan J.-W. Food Chem. 2015;168:623–629. doi: 10.1016/j.foodchem.2014.07.105. [DOI] [PubMed] [Google Scholar]
- Rahman M. M. Gray A. I. Phytochemistry. 2005;66:1601–1606. doi: 10.1016/j.phytochem.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Ouyang J.-K. Dong L.-M. Xu Q.-L. Wang J. Liu S.-B. Qian T. Yuan Y.-F. Tan J.-W. RSC Adv. 2018;8:40483–40489. doi: 10.1039/C8RA08894B. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.