Dear Editor,
The SARS-CoV-2 Delta (B.617.2) variant has rapidly replaced the Alpha variant to become dominant in many European countries including Spain1. The Delta variant, which accumulates several Spike (S) mutations that increase virus binding to ACE2 (i.e. L452R and P681R), appears to outperform Alpha in terms of replication efficiency in the respiratory tract,2 allegedly resulting in higher viral loads and enhanced transmissibility.3 , 4 Interestingly, Ong et al.5 in a cohort of adults among which Delta variant cases were underrepresented (8%), reported comparable SARS-CoV-2 Delta and Alpha RNA loads in nasopharyngeal specimens (NP) at the time of SARS-CoV-2 infection RT-PCR diagnosis. This observation clearly contrasts with data reported in other studies9 [6], [7], [8], [9] which consistently show higher viral RNA loads for the Delta variant in the upper respiratory tract. None of the above studies9 [6], [7], [8], [9] seemingly matched participants infected with the Delta or Alpha variant for presence of one or more of the following parameters, all with a potential impact on results: presence or absence of COVID-19 symptoms, demographics, time to testing from symptoms onset (or index case contact in asymptomatic subjects) or vaccination status. To gain further insight into this issue, we conducted a retrospective, observational study with a convenience sample of 545 participants of whom 295 (149 male; 231, adults; median age, 39 years; range, 1–93) and 250 (125 male; 128 adults; median age 22 years; range, 0–96) had been infected by the Alpha and Delta variants, respectively, as documented by whole-genome sequencing,10 variant-specific RT-PCR (SARS-CoV-2 PCR Variant, Ascires, Sistemas Genómicos, Valencia, Spain), or inferred by S-gene target failure (SGTF) in the RT-PCR for the Alpha variant.10 All cases due to the Alpha variant occurred in unvaccinated participants between February-May 2021, whereas 51/250 represented SARS-CoV-2 Delta breakthrough infections in fully vaccinated adults (diagnosed at least after 15 days after vaccine schedule completion) between May and July 2021. The study was approved by the INCLIVA Research Ethics Committee, and informed consent was waived due to its retrospective nature. SARS-CoV-2 RNA viral loads in NP were estimated by using the TaqPath COVID-19 Combo Kit (Thermo Fisher Scientific, MS, USA) calibrated to the AMPLIRUN® TOTAL SARS-CoV-2 RNA Control (Vircell SA, Granada, Spain).10
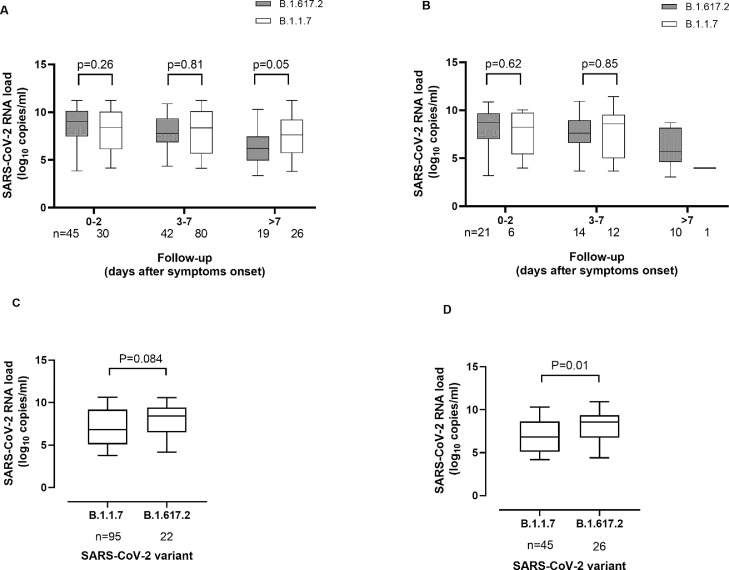
We initially compared NP viral RNA loads in COVID-19 non-vaccinated individuals stratified by age (children, ≤18 years/adults) and time since symptoms onset (arbitrarily defined as 0–2, 3–7, or ≥ 8 days). As shown in Fig. 1 , a trend towards higher viral RNA loads was seen for the Delta variant in both adults and children within 2 days after symptoms onset (P = 0.27 and P = 0.26, respectively), but not afterwards. Nonetheless, a wide range of viral RNA loads (3.-≥ 11.0 log10 copies/ml) was observed in individuals in comparison groups. In participants asymptomatic at the time of RT-PCT testing, SARS-CoV-2 RNA loads were notably higher for the Delta than the Alpha variant, in both adults (median, 1.5 log10 higher) and children (median, 1.7 log10 higher) (P = 0.08 and P = 0.01, respectively).
Fig. 1.
Initial SARS-CoV-2 RNA loads in nasopharyngeal specimens from unvaccinated individuals infected by either Alpha (B.1.1.7) or Delta (B.1.617.2) variants. The Alpha lineage was confirmed by whole-genome sequencing in 108 cases, whereas in the remaining 230 it was inferred by S-gene target failure (SGTF) in the RT-PCR, as more than 95% of SGTFs detected in the Clínico-Malvarrosa Health Department belonged to that lineage within the timeframe of specimen collection (not shown). The Delta lineage was confirmed by whole-genome sequencing (n = 138) or variant-specific RT-PCR (n = 61). Whisker-plots of SARS-CoV-2 RNA loads in NP by time of sampling relative to symptoms onset are displayed separately for adults (A) and children (B). Whisker-plots of SARS-CoV-2 RNA loads in NP for asymptomatic participants are also shown separately for adults (C) and children (D). P values are shown for comparisons across groups.
Note that asymptomatic individuals in comparison groups were matched for time elapsed from diagnosis of the index case to RT-PCR testing (within 7 days, as per protocol). Unfortunately, no data were available regarding as to how asymptomatic infections evolved over time.
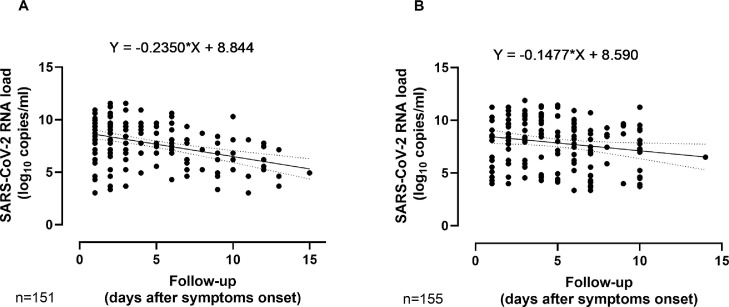
As can be seen in Fig. 2 , the slope of the regression line best fitting SARS-CoV-2 RNA load decay in COVID-19 patients was slightly higher overall for the Delta than for the Alpha variant, irrespective of participant age (not shown). Although speculative, the above data could be interpreted as suggesting that peak viral load in NP may be reached earlier in individuals infected by the Delta variant than the Alpha variant: even before symptoms onset, which favors increased transmissibility. In our view the data did not support a more extended period of viral shedding in the upper respiratory tract for the Delta variant, as previously suggested.7
Fig. 2.
Linear regression analysis of initial SARS-CoV-2 RNA loads from individuals infected with COVID by the Delta (A) and Alpha (B) variants according to time of sampling since symptoms onset. Regression lines best fitting SARS-CoV-2 RNA load decay are depicted.
We next compared SARS-CoV-2 Delta RNA loads in NP from non-vaccinated (n = 128) and fully vaccinated adults (n = 51) with Comirnaty® (n = 27), Spikevax® (n = 9), Janssen® (n = 10), or Vaxzebria® (n = 5). Time since full-dose vaccination was 51 days (range, 14 – 177). Overall, SARS-CoV-2 RNA load was found to be higher in non-vaccinated (n = 128; median, 8.1 log10 copies/ml; range, 3.4–11.6) than vaccinated individuals (n = 51; median, 7.8 log10 copies/ml; range 3.0–11.2), although without reaching statistical significance (P = 0.31). When considering patients with COVID-19 for the analysis (matched for time since symptoms onset: median, 3 days in both groups), a clear trend (P = 0.12) towards higher viral RNA loads was observed in non-vaccinated individuals (8.1 log10 copies/ml; range, 3.3–11.6) compared with vaccinated participants (median, 7.4 log10 copies/ml; range, 3.3–11.2).
In contrast, asymptomatic vaccinated and unvaccinated individuals displayed rather similar viral RNA loads (median, 8.7 log10 copies/ml; range, 3–0–10.9, and median, 8.4 log10 copies/ml; range, 4.0–10.6; P = 0.85). Vaccinated and unvaccinated participants were matched for sex (P = 0.1, but not age, P = < 0.01). Contradictory data9 , 8 have been published on this issue, which are likely explained by between-study differences regarding time of RT-PCR testing.
Finally, we showed that initial SARS-CoV-2 RNA loads in NP for the prototypical B.1.617.2 variant (n = 138) and recently emerged subvariants (n = 47; AY.4, n = 29, AY.12, n = 9, AY.5, n = 5, AY.9, n = 3 and AY.3, n = 1) were quite similar when considered in combination (median, 8.7 log10 copies/ml; range, 4.6–11.6 vs. median, 8.4 log10 copies/ml; range, 4.0–12.1; P = 0.20). Comparison groups were matched for sex (P = 0.61), age (P = 0.34), development of COVID-19 (P = 0.44), time to NP sampling for both symptomatic and asymptomatic cases (P = 0.80) and vaccination status (P = 0.20). Of note, no B.1.617.2 subvariants seemed to carry additional spike mutations (K417N, Y145H and A222V) than might confer partial resistance to vaccine-elicited neutralizing antibodies. In summary, we found substantially higher NP initial viral loads for the Delta than the Alpha variant, in particular during the asymptomatic phase of the infection. Together with the overall lack of significant differences observed between viral loads in vaccinated and unvaccinated Delta-infected participants, this may help explain the epidemiological behavior of the Delta variant.
Financial support
This work received no public or private funds.
CRediT authorship contribution statement
Rosa Costa: Methodology, Validation, Writing – original draft. Beatriz Olea: Methodology, Validation, Writing – original draft. María Alma Bracho: Methodology, Validation, Writing – original draft. Eliseo Albert: Methodology, Validation, Writing – original draft. Paula de Michelena: Methodology, Validation, Writing – original draft. Cecilia Martínez-Costa: Methodology, Validation, Writing – original draft. Fernando González-Candelas: Methodology, Validation, Conceptualization, Writing – original draft. David Navarro: Conceptualization, Supervision, Writing – original draft.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgments
We are grateful to all personnel working in the Clínico-Malvarrosa Health Department and at Clinic University Hospital, in particular those at the Microbiology laboratory, for their commitment in the fight against COVID-19. Eliseo Albert holds a Juan Rodés Contract (JR20/00011) from the Carlos III Health Institute. Estela Giménez holds a Juan Rodés Contract (JR18/00053) from the Carlos III Health Institute
References
- 1.Data on SARS-CoV-2 variants in the EU/EEA. https://www.ecdc.europa.eu/en/publications-data/data-virus-variants-covid-19-eueea1. Accessed October 21, 2021.
- 2.Mlcochova P., Kemp S.A., Dhar M.S., Papa G., Meng B., Ferreira I.A.T.M., et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature. 2021 doi: 10.1038/s41586-021-03944-y. Sep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell F., Archer B., Laurenson-Schafer H., Jinnai Y., Konings F., Batra N., et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Eurosurveillance. 2021;26(24) doi: 10.2807/1560-7917.ES.2021.26.24.2100509. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teyssou E., Delagrèverie H., Visseaux B., Lambert-Niclot S., Brichler S., Ferre V., et al. The Delta SARS-CoV-2 variant has a higher viral load than the Beta and the historical variants in nasopharyngeal samples from newly diagnosed COVID-19 patients. J Infect. 2021;83(4):e1–e3. doi: 10.1016/j.jinf.2021.08.027. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong S.W.X., Chiew C.J., Ang L.W., Mak T.M., Cui L., Toh M.P.H.S., et al. Clinical and virological features of SARS-CoV-2 variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta) Clin Infect Dis. 2021:ciab721. doi: 10.1093/cid/ciab721. Aug 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y., Chen R., Hu F., Lan Y., Yang Z., Zhan C. Transmission, viral kinetics and clinical characteristics of the emergent SARS-CoV-2 Delta VOC in Guangzhou, China. EClinicalMedicine. 2021;40 doi: 10.1016/j.eclinm.2021.101129. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo C.H., Morris C.P., Sachithanandham J., Amadi A., Gaston D., Li M., et al. Infection with the SARS-CoV-2 delta variant is associated with higher infectious virus loads compared to the alpha variant in both unvaccinated and vaccinated individuals. medRxiv. 2021 doi: 10.1093/cid/ciab986. Aug 20:2021.08.15.21262077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riemersma K.K., Grogan B.E., Kita-Yarbro A., Halfmann P., Kocharian A., Florek K.R., et al. Shedding of infectious SARS-CoV-2 despite vaccination. medRxiv. 2021 doi: 10.1101/2021.07.31.21261387. when the delta variant is prevalent - Wisconsin, july 2021. August 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanquart F., Abad C., Ambroise J., Bernard M., Cosentino G., Giannoli J.M., et al. Characterisation of vaccine breakthrough infections of SARS-CoV-2 Delta and Alpha variants and within-host viral load dynamics in the community, France, June to July 2021. Eurosurveillance. 2021;26(37) doi: 10.2807/1560-7917.ES.2021.26.37.2100824. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Costa R., Bueno F., Giménez E., Bracho A., Albert E., Carretero D., et al. Initial viral load and decay kinetics of SARS-CoV-2 lineage B.1.1.7 in the upper respiratory tract of adults and children. J Infect. 2021;83(4):496–522. doi: 10.1016/j.jinf.2021.08.015. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]