Abstract
Background
The role of children and young people (CYP) in transmission of SARS-CoV-2 in household and educational settings remains unclear. We undertook a systematic review and meta-analysis of contact-tracing and population-based studies at low risk of bias.
Methods
We searched 4 electronic databases on 28 July 2021 for contact-tracing studies and population-based studies informative about transmission of SARS-CoV-2 from 0 to 19 year olds in household or educational settings. We excluded studies at high risk of bias, including from under-ascertainment of asymptomatic infections. We undertook multilevel random effects meta-analyses of secondary attack rates (SAR: contact-tracing studies) and school infection prevalence, and used meta-regression to examine the impact of community SARS-CoV-2 incidence on school infection prevalence.
Findings
4529 abstracts were reviewed, resulting in 37 included studies (16 contact-tracing; 19 population studies; 2 mixed studies). The pooled relative transmissibility of CYP compared with adults was 0.92 (0.68, 1.26) in adjusted household studies. The pooled SAR from CYP was lower (p = 0.002) in school studies 0.7% (0.2, 2.7) than household studies (7.6% (3.6, 15.9) . There was no difference in SAR from CYP to child or adult contacts. School population studies showed some evidence of clustering in classes within schools. School infection prevalence was associated with contemporary community 14-day incidence (OR 1.003 (1.001, 1.004), p<0.001).
Interpretation
We found no difference in transmission of SARS-CoV-2 from CYP compared with adults within household settings. SAR were markedly lower in school compared with household settings, suggesting that household transmission is more important than school transmission in this pandemic. School infection prevalence was associated with community infection incidence, supporting hypotheses that school infections broadly reflect community infections. These findings are important for guiding policy decisions on shielding, vaccination school and operations during the pandemic.
Background
The role of children and young people (CYP) in transmission of SARS-CoV-2 remains unclear, in both households and child-specific settings, such as schools and nurseries.1 Observations of low incidence of symptomatic infection in CYP early in the pandemic led to assumptions that they played a very limited role in infection or transmission. This view has been challenged by the recognition that high proportions of asymptomatic infections in CYP led to low ascertainment of infections in this age-group,1 particularly when testing capacity was limited. Findings from some large contact-tracing studies (contact-tracing studies)2 have suggested CYP do play an important role in household transmission. In educational settings, whilst outbreaks have been reported in day-care nurseries,3 schools4, 5, 6 and school-like residential camps,7 , 8 a number of population-based school studies have found evidence of limited transmission especially between children.9 , 10 It remains unclear the extent to which cases and outbreaks in schools reflect transmission in schools or the wider community.
Epidemiological studies that can provide useful information about transmission with the lowest risk of bias include contact-tracing studies with active follow-up and testing of all contacts regardless of symptoms and population-based studies which test all members of the population regardless of symptoms. Population-based studies are informative about prevalence across age-groups and risk factors for infection, and may provide information about clustering or timing of infection within a setting (e.g. households or schools). Studies have shown that children under 10–12 years have lower susceptibility to SARS-CoV-2 infection than adults, although the risk in teenagers appears to be closer to young adults.11 However CYP also tend to have the highest social mixing rates across society, including during the pandemic,12 and transmission is a complex interaction of viral properties, susceptibility, social mixing and population age structures. For these reasons, studies of incidence of symptomatic infection in CYP provide a weak basis for inference around children's role in transmission.11
Over 18 months into the COVID-19 pandemic, there are only now sufficient data to allow meta-analysis of relevant data only including studies at low risk of bias. Existing systematic reviews are now outdated, including only data from early in the pandemic,13, 14, 15, 16, 17, 18 and are critically biased by their inclusion of studies which systematically under-ascertained asymptomatic infections in CYP. A large literature has since been published, including several population-based studies of CYP within schools.9 , 10 Many of these date from late 2020 or early 2021 when schools had extensive mitigation measures in place that are hypothesized to reduce transmission within schools, as does reducing attendance during periods of hybrid in-person and online learning, yet data on the effects of such measures are lacking.19 , 20
We undertook a systematic review and meta-analysis of high quality epidemiological studies published during the first 18 months of the pandemic (Jan 2020- July 2021) to answer the following questions: (a) To what extent do CYP under 20 years of age transmit SARS-CoV-2 to other CYP and to adults in household and child-specific (e.g. educational) settings?; (b) how does transmission differ between household and educational settings?; and (c) is community infection incidence associated with prevalence of or transmission of infection within educational settings?
Methods
The search was undertaken using a protocol registered with Prospero registry (CRD42021222276).
Search strategy
We searched four electronic databases (PubMed; medRxiv; COVID-19 Living Evidence database; Europe PMC) to 28 July 2021. The search terms for PubMed were ("COVID-19” [Text Word] OR "2019-nCoV" [Text Word] OR "SARS-CoV-2” [Text Word]) AND ("child*" [All Fields] OR "infant*" [All Fields]) AND ("disease transmission, infectious" [MeSH Terms] OR "epidemiology" [MeSH Terms] OR "schools" [MeSH Terms]) with terms for other databases shown in Appendix Table 1 .
Table 1.
Study characteristics.
| Authors | Source | Site | Dates | Virus/ variant | Case identification | Study type | Setting and exposure | N | Age of CYP | Testing | Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blaisdell et al. | PubMed | USA | June-August 2020 | NS | Population | Contact-tracing | Four residential summer school camps for children and staff. Mixture of outdoor and indoor activities. Approximately 75% of usual enrolment. | 1022 attendees from 41 US states (642 children, 380 staff); 1006 tested (98%). Attended from 44 to 62 days. 3 primary cases and 41 contacts (30 children, 11 staff) | 7–18y | RT-PCR (swab site not stated) before arrival, on arrival and at 4 and 9 days | 3 attendees (0.3%) (2 staff, 1 child) tested positive after arrival and their cohorts (n = 41 contacts) isolated for 8–14d, being released after 2 negative tests. No secondary cases in contacts in 30 contacts of child primary and 11 contacts of the 2 adult primary cases. |
| Varma et al. | Professional | USA | Period 1 9 Oct-20 Nov; Period 2: 6–18 Dec 2020 | NS | A) Population and B) Infection | A) Surveillance & B) Contact tracing | A) Surveillance: Routine testing of a random sample CYP attending public schools in New York City; 12 Oct-20 Nov: 26% of CYP attended 1–3 days per week with remainder learning online; all schools closed 19Nov-6 Dec and only elementary schools reopened in Dec; B) Routine public health data from city database and contact-tracing. Contacts quarantined for 14 days. | A) Surveiillance in schools: 10–20% of each school selected: Period 1: n = 60,783 CYP (41% of eligible consent), Period 2: n = 34,556 CYP (61% of eligible consented); B) Contact-tracing: 2231 cases (child & adult) linked with schools and their 36,423 school-based contacts identified across entire period. | 5–14y | RT-PCR (NP swab): A) Monthly testing for all schools with some schools moving to weekly in November and all primary schools weekly in Dec. B) RT-PCR testing of contacts of identified cases. Proportion of contacts identified and tested not stated - mean 16.2 contacts per case tested | A) Surveillance: Prevalence: Period 1 12Oct-20Nov: 0–4y 0.45% (1/223) 5–14y 0.28%(148/52,050) 15–24y 0.28%(24/8600); Period 2: 7–18Dec: 0–4y 1.61%(1/62) 5–14y 0.77%(257/33,330) 15–24y 0.69%(8/1164). B) Contact tracing: 191/36,423 = 0.5% contacts tested positive. Of these 132 cases (69%) had information to allow assessment of transmission: 67 (51%) staff-to-staff, 36 (27%) from staff-to-student, 18 (14%) student-to-staff, and 11 (8%) from student-to-student |
| Park et al. | Handsearch | South Korea | 20 Jan-27 Mar 2020 | NS | Infection | Contact-tracing | Households. National Korea Centers for Disease Control contact-tracing database used. High quality testing, tracing and isolation system. | 10,962 index cases (29 (0.5%) aged 0–9y, 124 (2.2%) 10–19y) and 10,592 HH contacts (57 for 0–9y index; 231 for 10–19y index). Data on HH contacts only used, as all HH contacts routinely tested while other contacts tested if symptomatic. | 0–19y | RT-PCR (swab site not stated) | SAR for 0–9y index: 5.3%(1.3, 13.7; 3/57). SAR for 10–19y index: 18.6%(14.0, 24.0; 43/231). Compared with 10.5% (889/8440) in 20–59 year olds. |
| Schoeps et al. | medRxiv | Germany | 17 Aug-16 Dec 2020 | NS | Infection | Contact-tracing | K-12 schools in 1 state (Rhineland-Palatinate): FTF. Data from school reopening in August 2020 through to lockdown on 16 Dec 2020 | Population: 1492 schools, 406,607 schoolchildren & 144,245 children < 6 years in day-care. 784 index cases notified; information on contacts available on 441 index cases (346 students, 91 staff, 20 unknown) with 14,591 contacts of whom 13,005 were tested contacts. | 3–18y | Public health notification of PCR+cases (NP swab) linked to educational institutions; all close contacts offered PCR testing routinely - 89% of contacts (87% of child contacts) were PCR-tested (13,005 contacts). | When restricted to PCR-tested contacts (441 index cases & 13,005 contacts), overall SAR was 1•51 (1•30–1•73); SAR from children 99/10,716=0.92(0.75–1.12). These 99 secondary cases occurred in 53 clusters of 3 cases or more; SAR from teachers 91/2858=3.18(2.57–3.90); transmission from teacher index was greater than from child index IRR 4.4 p<0.001; calculated each teacher index resulted in 0.5 secondary cases, whereas there was only 1 teacher secondary for 25 child indexes. |
| Hu et al. | medRxiv then published | China (Hunan) | 13 Jan-2 April 2020 | NS | Infection | Contact-tracing | Households in Hunan province | 1178 index cases (61 aged 0–14y) and 15,648 close contacts (1706 aged 0–14y): 471 secondary cases | Children & adults: child age <15y | Hunan Province CDC dataset: all contacts quarantined for 14 days and tested regardless of symptoms | Age-related transmission could be examined in 461 index cases (25 0–14y). Unadjusted OR for secondary infection from 0 to 14yo 0.33(0.04, 2.83) compared with 15–64yo, however small numbers of index children (25/461=5%). In adjusted general linear models, this association was again not significant (0.28(0.04, 2.04). |
| Dattner et al. | medrxiv then published | Israel | 17 Mar-3 May 2020 | NS | Population | Contact-tracing | 637 HH in Bnei Brak, Israel where all HH members were tested. Note 51% of population <20y. | 3353 (1809 adults and 1544 children 0–19y) | 0–19y | RT-PCR (site not stated) all HH contacts; Serology IgG in 130/637HH | Joint PCR & serology transmission mode: Relative susceptibility of <20y compared with adults was 43% (31%, 55%) and relative transmissibility/infectivity 63%(37,88). Positive PCR: excluding index cases, 44% of adults were infected compared to 25% of the children. Serology positive: <20y= 34% (141/417), adults= 48% (137/288) |
| Yoon et al. | medrxiv then published | South Korea | 20 May-31 July 2020 | NS | Infection | Contact-tracing | National school surveillance data from test-trace system. Schools resumed FTF learning in 4 steps from 20 May (Year 12 only) through to 8 June. Efficient test-trace system with testing of all contacts | 44 index children and >13,100 contacts attending 38 schools/EYS: 6 EYS(4-5y), 17 primary school(7-12y), 6 middle school (13-15y) and 15 high school (16-18y). Contacts: 875 YES, 3374 primary, 1525 middle and 6255 high school. All contacts tested;% contacts participating not stated however tested mean 297 contacts per index | 4–18y | RT-PCR (swab, siting not stated) | SAR (children and adults) from child index cases: total 1/13,100: EYS 0%(0/875), primary 0.03% (1/3374), middle and high 0% (0/7780). Identified source for 29/44 child index cases: 79%(23) infected by family members. |
| Li et al. | medrxiv then published | China (Wuhan) | 2 Dec 2019–18 Apr 2020 | NS | Infection | Contact-tracing | Retrospective regional data from Wuhan Center for Disease Control and Prevention system. | 29,578 primary cases in 29,405 HH and 57,581 HH contacts. Test data were available for 48,962 contacts (85%; data missing for remainder & unclear if tested or not; all HH contacts tested after 2 Feb but not before). For HH with a single primary case, there were 24,985 index cases (327 were <20y (1.3%)) and 52,822 contacts. Note that non-tested contacts were assumed to be negative | 0–19y | RT-PCR (swab site not stated) | SAR for primary cases <20y 5.8%(4.3, 7.7; 46/793). Unconditional GEE models suggested lower transmissibility for <20y (OR 0•66 (0•48–0•90) compared with >=60y) whereas conditional chain-binomial models suggested higher infectivity for <20y (OR 1•58 (1.28,1.95) compared with >=60y |
| Laxminarayan et al. | medrxiv then published | India | 5 Mar-June 2020 | NS | Infection | Contact-tracing | Community and HH CTS of state national surveillance-identified positive cases in Andhra Pradesh and Tamil Nadu | Index cases 6063 <18y + 78,866 adults; contacts 57,415 <18y + 507,476 adults. All recruited contacts tested. 20% of reported cases included and 19% of traced contacts participated | <18y | RT-PCR (site not stated). All contacts were quarantined for 14 days and PCR-tested at least once during quarantine. | SAR= 7.2% (4110/57,415) from 0 to 17y and 7.4%(37,479/507,476) for 18 plus. |
| Larosa et al. | Professional | Italy | 1 Sep-15 Oct 2020 | NS | Infection | Contact-tracing | Schools and early years settings in Reggio Emilia province after reopening of schools. Schools reopened 15 Sep, very largely FTF although some large schools operated 50% hybrid teaching if classrooms don't allow distancing | 48 index cases (43 children, 5 staff) identified in 41 classes of 36 schools; 1198/1200 contacts tested (99.8%; 994 children, 204 staff) | 0–19y | RT-PCR - swab, site not stated. Cases identified through routine public health systems. Included all cases noted to have connection with schools in 48H before symptoms/test. Contacts tested once each. | 38 secondary cases in 9 clusters amongst children (SAR = 3.8%, 38/994) and no secondary cases amongst teachers. Overall school SAR from child+adult index cases 3.2% (38/1198). No secondary cases amongst children in early years settings. SAR from children only calculable for primary schools (only child index cases n = 14): 0.4%(1/266) |
| Macartney et al. | Professional | Australia | 4 July - 18 Dec 2020: Term3 (4 July-25 Sep), Term 4 (26 Sep-18 Dec). | WT; no VOC detected | Infection | Contact-tracing | State-wide surveillance of cases identified attending schools in New South Wales while infectious. Schools fully open FTF; 88% attendance Term 3 and 4. | RT-PCR. Term 3: 39 primary cases (32 students, 7 staff) and 3641 contacts: 95% of contacts tested. Term 4: 10 primary cases (9 students, 1 staff) and 1098 contacts (99% contacts tested) | 3–18y | RT-PCR (Np swab). Note serology also conducted on small numbers - not reported here. | TERM 3: 33 secondary cases (28 stent, 5 staff) - SAR=0.9% (33/3641). EYS: 6 primary cases (2 children, 4 staff): overall SAR 1.7% (13/754); SAR from 2 child primary cases: SAR to children 0% (0/58), SAR to adults 0% (0/11)Primary schools:13 primary cases (11 children, 2 staff) in 12 schools: SAR from child primary: SAR to children 0.3% (2/643) SAR to adults 0% (0/76)Secondary schools: 20 primary cases (19 student, 1 staff): overall SAR 1.1%(27/2466) - 19 student primary in 16 schools: SAR to students 1.27%(26/2045), SAR to adults 0.4% (1/226). TERM 4: 13 secondary cases (12 student, 1 staff) occurred in 4 settings (2 primary, 2 EYS) - overall SAR 1.2% (13/1098).EYS: 4 primary child cases (no adult) resulted in 4 secondary cases (3 children, 1 adult). SAR from child index: child 0.8% (3/393) adult 1.3% (1/79)Primary: 3 primary cases (2 children, 1 staff) in 3 schools: 9 secondary children, 0 secondary staff cases. SAR from child index: child 0.4% (1/269) adult 0% (0/33)Secondary: 3 primary children in 3 schools: 0 secondary cases in 199 student and 43 staff contacts. |
| Kim et al. | PubMed | South Korea | 20 Jan-6-Apr 2020 | NS | Infection | Contact-tracing | HH contact-tracing study of all confirmed cases ≤18 years in South Korea | First 107 index cases ≤18y identified nationally and their 248 HH members (defined as close contacts; mean 4.3 per child) | <18y | RT-PCR (site not stated) of all contacts (100%); quarantined for 14D | 41/248 (16.5%) were positive but 40 of these were assessed to likely have the same initial exposure as the child therefore removed from total contact number. O 1 definite secondary case was identified from index<19y – SAR = 1/208=0.48 (reported in paper as 0.4 using total contact number) |
| Verberk et al | medRxiv; data obtained from authors | Netherlands & Belgium | Apr-December 2020 | WT; recruitment before VOC circulating | Infection | Contact-tracing | HH in Utrecht or Antwerp recruited through a positive index case in HH with 2 or more members. Households approached after positive PCR test in one member; not designed to be representative of broader population | 272 Households recruited. Interim data in the preprint provided on first 117 HH. Data provided by authors on 39 index cases aged 0–18y and their 131 HH contacts. | 0–18y | RT-PCR (nasopharyngeal) and serology IgG of all HH members at baseline (median Day 5 after index diagnosis) and repeated if symptomatic or for all participants at D21. Secondary infection defined as PCR or seropositive | Preprint findings: overall SAR 27.9% (95%-CI: 22.7–33.8%); SAR highest from parent to child (36.1%) and lowest from child to parent (15.7%). Data supplied by authors: infections from 39 index children: SAR for 0–11y 4.3% (2/47) and 12–18y 17.9% (15/84) |
| Brandal et al. | PubMed | Norway | 28 Aug-11 Nov 2020 | NS | Infection | Contact-tracing | Primary schools in 2 counties with highest prevalence | 13 child index cases identified during period; 292 contacts (234 child; 58 adults). Contact participation was 73% child & 78% adult. | 5–13y | RT-PCR on saliva: Cases were PCR+ & attended school within 48 h of sample/symptom; 2 saliva RT-PCR for all contacts: immediate and at 10 days of isolation | All child index cases except 1 had HH members who tested positive before child. SAR from child index cases = 0.9%(2/234) for children and 1.7% (1/58) for adults |
| Reukers et al. | medRxiv then published | Netherlands | Mar-May 2020 | NS | Infection | Contact-tracing | Households in Utrecht region: all HH with a positive adult and <18 h in HH were contacted to recruit entire HH; studied within 24 hrs of recruitment;% of eligible indexes not stated | 55 HH: 242 participants (55 adult index cases, 187 contacts (70 children 1-11y, 46 adolescents 12-17y). Entire households participated. | 1–17y | RT-PCR (NP and oral swabs) and serology for entire HH 3 times - on Days 1, 14–21 and 28–42. Participation rate for contacts not stated but implied to be 100% | In 1/55 HH the primary case was an adolescent and not the index adult. No secondary cases in 17HH and 100% secondary infections in 11 HH. Overall SAR 43%(33,53): lower risk of infection for 1–11yo compared with adults in adjusted models. Adjusted SAR 1–11y 35%(24,46), 12–18y 41%(27,56) and 18yplus 51%(39,63). Transmission/susceptibility model: susceptibility compared to adults: 1–11y 0.67(0.40,1.1) 12–17y 0.93(0.51, 1.7). Transmissibility compared with adults: 1–11y 0.73(0.04, 2.6) 12–17y 2.7(0.98,5.6) |
| Lyngse et al. | medRxiv | Denmark | 25 Aug 2020–10 Feb 2021 | NS | Infection/ Population | Contact-tracing | Danish population register linked with national testing database, including all contact-tracing data. Reconstructed HH and identified transmission chains using time data. 73% of national primary cases included. | 66,311 primary cases (36,388 aged 0-19y) and 213,576 HH contacts (148,724 aged 0–19y). 89% of HH contacts tested | <20y | RT-PCR (swab site not stated) | SAR from primary aged 0–5y 22%(3313/14,306), 5–10y 39%(5960/15,263), 10–15y 43%(8908/20,596) 15–20y 51% (12,440/24,197) compared with 52.3% (72,761/139,177) aged 20y plus. Adjusted OR for transmission from index aged 0–5y 1.11(1.03,1.19), 5–10y 0.95(0.90, 1.0), 10–15y 0.82(0.78,0.85), 15–20y 0.70(0.67,0.72) compared with 30–35yo. |
| Telle et al. | medRxiv then published | Norway | 1 March 2020–1 Jan 2021 | NS | Infection/ Population | Contact-tracing | Norwegian Population Registry linked with all national COVID testing databases including test and trace. Included all HH with children <20y and a single identifiable index case. 3 million of the Norwegian population of 5.4million were tested during study period. | 7548 single index cases (1498 <=16y; 200<7y, 517 7-12y, 781 13-16y) and their HH, including 26,991 individuals (14,808 <20y and 12,184 adults). Testing of contacts within 14D varied with index age: 92% 0-6y, 88% 7-12y, 87% 13-16y and 60-70% for 17 plus. | 0–16y (17–19y not reported as contact testing <85%) | RT-PCR (swab site not stated) of all contacts regardless of symptoms (after April 2020) | SAR within 14d: SAR was highest from 0 to 6y and from parents to both children and adults. SAR from children: index 0–6y 23%(18,30) to children and 29%(24–34) to parents; index 7–12y 12%(10,15) to children and 21%(19,24) to parents; index 13–16y 15%(13,18) to children and 18%(16,21) to parents. SAR from parents: 24%(23,25) to children and 38%(36,40) to other parents. |
| Hoehl et al. | Handsearch for R1; medRxiv (Shenk et al.) for R2&3 | Germany | R1: 18 Jun-10 Sep 2020R2: 18 Jan-Feb 11 2021R3: 17 May-June 11 2021, | R1: NSR2: WT dominant, alpha emergingR3: alpha dominant | Population | Surveillance | SAFE KiDS study Rounds 1-3. Representative sample of 50 daycare centres (R1), 47 centres (R2) and 46 centres (R3) in state of Hesse (1% of facilities in Hesse). 30 individuals (children and staff) per facility invited for weekly home testing. R1 was low community incidence with wild type virus; R2 was high incidence, R3 was moderate incidence | R1: 1235 participants from 50 centres (859 children; 376 staff). Total of 13,273 swabs tested (56% oral). Median 6 samples per child and 7 per staff member.R2: 47 centres with 577 children and 334 staff providing 1 or more swabs.R3: 46 centres with 756 children and 226 staff providing 1 or more swabs | 3 months to 8y | RT-PCR weekly (buccal and anal swabs from each participant weekly). Buccal only R3. Only buccal data included here | R1: 2 positive from 2 staff members (2/376). No positive swabs from children (0/9057 swabs in 859 children). R2: 2 positive in children (2/577) and 0 staff (0/334). All S-gene positive i.e. unlikely to be alpha variantR3: 0 children or staff positive |
| Kriemler et al | medRxiv then published | Switzerland | 1–11 Dec 2020 | NS | Population | Surveillance | 14 invited primary and secondary schools from high prevalence areas of Zurich: a subset of the 55 schools participating in Ulyte et al. | 641/1299 (49%) of invited children participated, from 67 classes | 6–16y | RT-PCR oral swab: participants tested twice 1 week apart. | positive RT-PCR in 1 child = 0.2%(0,1.1); no evidence of clustering in classes |
| Theuring et al. | medRxiv | Germany | 2–16 Nov 2020 | NS | Population | Surveillance | 24 randomly selected schools in Berlin as per Hommes et al. 1 class from each school and their HH members. FTF teaching till 16 Dec | N = 1119 (352 students (177 primary, 175 secondary), 142 staff and 625 HH members). Mean 65% eligible children participated | 8–18y | RT-PCR - oral and NP combined swabs- on all participants (98.6% students, 100% staff and 99.5% HH). Serology on dried blood spots. Participants in 8 classes with positive cases were retested after 1 week. | Prevalence: 2.7%(1.2, 5.0) in students (6/177 primary, 3/175 secondary) and 0.7%(0.0, 3.9) in staff (1/142); 8/24 classes had 1 or 2 cases, with none >2. HH prevalence: 2.3(1.3, 3.8) = 14 cases in 9 HH. 3/9 HH had positive students in the study but origin of infection unclear. Seropositivity in 2.0%(0.8, 4.1) students and 1.4%(0.6, 2.7) of staff; 8 classes with a positive test were retested after 1 week (after variable quarantine): 1 student and 1 staff were positive but judged not to be school related. |
| Thielecke et al. | medRxiv then published | Germany | 28 Sep-2 Oct 2020 | NS | Population | Surveillance | 12 randomly selected kindergartens from >2700 in Berlin. FTF | N = 720: 155 children, 78 staff, 487 HH members.% of eligible participating not stated. | 1–6y | RT-PCR (combined oral and NP swabs) and serology IgG on dried blood spots | None of 701 PCR samples was positive; no children, nil HH and 1 staff were seropositive . |
| Hoch et al. | medRxiv then published | Germany | Time 1: 15 Jun-26 July; Time 2: 7 Sep-1 Nov 2020 | NS | Population | Surveillance | Sentinel surveillance in 5 randomly selected primary schools & 6 kindergartens in Munich over two 6-week periods. FTF | 3169 total swabs over 12 weeks: overall 2149 children (1065 Wks1–6; 1084 wks 7–12), 1020 staff. N = 527 serology samples from staff.% of eligible recruited not stated | 1–11y | Weekly RT-PCR (oral swab) testing on 20 randomly selected children and 5 staff from each institution each week. Serology IgG on staff only | Time 1: All swabs and serology negative. Time 2: 2 positive PCR from 1 primary school (1 child; 1 teacher), all serology negative |
| Lubke et al. | medRxiv | Germany | 10 June −7 July 2020 | NS | Population | Surveillance | Representative sample of 115 daycare facilities in Dusseldorf, North Rhine-Westphalia. Representative across social deprivation in the city. 115 facilities selected from 314 respondents of 364 invited. Schooling resumed 8 June. Routine twice weekly testing of participating children and staff. | 115 daycare facilities with 5210 participants (3955 children, 1255 staff). Participation by children was 60% of total attending children. 94.6% provided at least 1 sample. | 2–6y | RT-PCR (saliva) - twice weekly for 4 weeks. | Prevalence: children 0.03% (1/3955), staff 0% 0/1255 |
| Espenhain et al. | medRxiv | Denmark | 3 rounds: R1 May 2020; R2 August 2020; R3 Oct - Dec 2020, with two subrounds defined as October and December 2020 | NS | Population | Surveillance | Nationally representative community survey, linked with national COVID-19 testing database and routine health administrative data. | R1: 2512 (48% participation), nil 12–17y; R2: 7015 (39%) of whom 1492 aged 12–17y(31% participation); R3: 18,161 (26%) participants of whom 5631 aged 12–17y (20% participation). 1244 families had a child and at least one parent tested. | 12–17y | Serology IgG | Seroprevalence: August 12–17y 0.9%(0.2, 2.0), 18–39y 2.8%(2.2, 3.6); October 12–17y 2.8%(1.6,4.5) 18/39y 3.3%(2.6,4.1); December 12–17y 6.4%(3.8,10) 18–39y 5.2%(4.0, 6.6). Of families with at least 1 child and 1 parent tested, 6.4%(79/1244) had at least 1 seropositive family member: 21/79 families had both child and parent(s) positive, 19 families only child positive and 39 families only parent(s) positive. |
| Doron et al. | medRxiv | USA | 16 Sept −31 Dec 2020. Three periods Baseline Week 1 (mid Sept); Period 2 week 6–13 (1 Oct to 20 Nov) and Period 3 Weeks 15–18 (7–31 Dec 2020). | NS | Population | Surveillance | Massachusetts educational settings through Wellesley schools: early-years to Grade 12 in 10 schools (7 primary schools, 1 preschool and 1 middle (G6–8)and 1 high schools (G9–12)). Baseline screening offered to all staff and students in week 1. Subsequent weekly screening offered to all staff and to students from middle and high schools from start of hybrid learning in week 6. | 921 eligible staff (10 schools) and 2403 eligible students: depending on week, participation 58–77% students and 73–83% staff | 11–18y | RT-PCR (saliva): Baseline then weekly RT-PCR (pooled, then confirmatory) | 126 positive cases amongst enrolled students and staff: 37 identified through screening program and 89 identified through outside tests (e.g. public health system). Including all cases: Week 1 baseline: students positive 0.03% (1/3596); staff 0.01% (2/1005); Weeks 6–13: students: 1.7% (42/2403) staff 2.6% (24/921); Wk 15–18: student 1.8% (43/2403) staff 1.2% (11/921) . Concluded in-school clusters and therefore transmission was rare |
| ONS SIS | Professional | UK | Round 1: 3–19 Nov 2020; Round 2: 2–10 Dec 2020; (Round 3 not undertaken due to school closures) Round 4: 15–31 March 2021; Round 5: 5–21 May 2021 | R1: NSR2: alpha emergingR4: alpha dominantR5: delta dominant by late May | Population | Surveillance | Oversampling of schools in high prevalence areas of England. | Round 1: 105 schools (63 secondary, 42 primary) in 14 local authorities (64% high prevalence, 36% low prevalence); n = 9662 (students: primary 2137 secondary 3099; staff primary 1068 secondary 3054) - participation 17% students 51% staff. Round 2: 121 schools (41 primary, 80 secondary) in 15 local authorities: n = 5114 staff, 7089 pupils. Participation 15% students 40% staff. Note: 7751(4429 students 3322 staff) participated in both rounds. Round 4: 137 schools in 15 local authorities: data reported on 7156 secondary students and 2645 staff (17% of eligible students; 29% staff). Round 5: 57 primary and 85 secondary schools: 4207 primary and 8297 secondary students & 1348 primary and 2637 secondary staff (estimated response rate 25% primary & 17% secondary students) | 4–19y | RT-PCR (NP swab); serology IgG on all participating students and staff in participating schools | Round 1: PCR+ child: primary school 0.89%(0.54, 1.39) secondary 1.48%(1.10, 1.98), PCR+ staff: primary 0.75%(0.32, 1.47) secondary 1.47%(1.08, 1.97). Higher proportions of students and staff tested positive in higher prevalence areas: students low prevalence: primary 0%(0, 0.7) secondary 1.12%(0.62, 1.19), high prevalence: primary 1.18%(0.71, 1.83) secondary 1.73%(1.18, 2.43). No infections identified in 47/105 schools, 29 had 1 positive case and 28% had 2–5 cases. Round 2 PCR+: child primary 0.94%(0.44,1.76) secondary 1.22%(0.60, 2.2), staff primary 0.99%(0.37, 2.12) 1.64%(1.1, 2.33). No positive cases in 46% of primary and 37% of secondary. Seropositivity data from Round 1: positive students primary 7.7%(5.9, 9.8) secondary 11.0%(8.8, 13.5). Seropositivity in Round 2: primary 9.05% (7.33, 11.0), secondary 13.45% (11.67, 15.4) Round 4: PCR+ 0.34%(0.16, 0.63) of secondary students (primary too low to be reported) and 0.19% (0.04, 0.58) of staff. Round 5: PCR+ 0.65% (0.27, 1.29) primary and 0.05% (0.01, 0.18) secondary students. |
| House et al. | Professional | UK | 26 Apr 2020–15 Feb2021 R1: 26 Ap-1 Sep 2020:; R2: 1 Sep-15 Nov 2020; R3: 15 Nov 2020–1 Jan 2021; R4: 1 Jan-15 Feb 2021 | R1: WT R2: WTR3: alpha emergingR4: alpha dominant | Population | Surveillance | National longitudinal HH population surveillance study (ONS COVID-19 Infection Survey): weekly testing of a nationally representative set of households in England. Analyses limited to HH <7 persons.R1: schools closed, low prevalenceR2: high prevalence, schools openR3: high prevalence, schools mainly openR4: schools closed, high prevalence | Total across rounds 371,420 individuals (29,793 <12y, 20,091 12–16y) in 181,710 HH: 19,548 positive cases of which 7151 were consistent with B.1.1.7 variant. Numbers of participants increased across tranches (T1 89,624; T2 293,570; T3 315,187; T4 329,532). Longitudinal attrition <5%. Initially 20,000 HH approached in April 2020 and 51% of approached HH participated. An additional 5000 HH per week have been approached since mid 2020 however 14% of approached HH have agreed to participate since July 2020. Approx 90% of eligible individuals in participating HH are tested. | 2–16y | RT-PCR weekly (NP and oral swab) | Bayesian transmission probability models estimated susceptible-infectious transmission probabilities including infectivity and external force of infection by age, based upon first case within each HH. Found relative transmissibility not significantly different to adults for 2–11y for each tranche, with 12–16y having significantly lower transmissibility in T3 (RR 0.7) but not in other tranches. The relative external exposure compared with adults was significantly higher for 2–11y for T3 (RR 1.4) and for 12–16y for T2 and T3 (RR 1.64 and 2.35 respectively). |
| Villani et al | PubMed | Italy | 21 Sep-4 Dec 2020, in 3 periods: 21 Sep-12 Oct; 19 Oct-13 Nov; 16 Nov-4 Dec | NS | Population | Surveillance | Schools: 2 K12 schools in Rome | 1083 students and 168 staff: 96.5–100% student participation by age | 3–18y | RT-PCR: oral swabs: 3 monthly samples all participants | 13 positive students & 3 staff across 3 rounds (3431 samples). Positive Round 1: 1/1099, Round 2: 12/1075; Round 3: 3/1257. Using the participant N of students as swab number for each round, prevalence in children was R1: 1/1083, R2: 9/1083 and R3: 3/1083 (swab numbers for students not given). Only 2 classrooms had >=1 positive (2 students; 1 with student and staff member). Note 2 +students were siblings. Prevalence of 0.1, 1.1 and 0.2% was lower than background for age |
| Hommes et al. | medRxiv then published | Germany | 11–19 Jun 2020 | NS | Population | Surveillance | 24 randomly selected schools in Berlin; FTF teaching reopened 28 April but 15% of teaching virtual in primary and 50% in secondaries. | n = 535: 192 primary and 192 secondary students and 150 school staff.65% of students participated | 8–18y | RT-PCR- oral and NP combined swabs- plus dried blood spot serology on all participants | 1 positive case identified in 16yo: prevalence 0.5% for secondary and no teachers. Positive IgG in 7 students (1.8%) and no teachers: 3 clustered in one secondary class. |
| Kirsten et al. | medRxiv (as Armann et al.) then published as Kirsten et al. | Germany | Time 1 25 May-30 June 2020; Time 2: 15 Sep-13 Oct 2020 | NS | Population | Surveillance | 13 secondary schools in eastern Saxony. School recruitment not stated. Schools reopened FTF 18 May and then late August after summer break | T1: 1538 students (76% participation) & 507 teachers; T2: 1334 students (87% of T1) & 445 teachers | 12–19y | Serology IgG | Seroprevalence T1: 12 positive (11 students, 1 teacher) = 0.6%; T2: 12 positive (11 students, 1 teacher). Positives in 7/13 schools, with maximum of 4 in any school. |
| Ulyte et al. | R1 & 2: medrxiv then publishedR3: medrxiv | Switzerland | R1: 16 Jun-9 July 2020R2: 26 Oct-19 Nov 2020R3: 15 Mar - 16 April 2021 | R1 & 2: NSR3: alpha dominant | Population | Surveillance | Ciao Corona study (3 rounds): Primary and secondary schools in Zurich; 55 randomly selected schools (55/156 invited), 275 classes; FTF learning at all rounds | R1 n = 2603; R2 n = 2552. R3: n = 2487, including 250 newly enrolled children. Retention was 84% from R1-R2 and 88% from R2-R3. | 6–16y | Serology IgG | R1 seropositive = 74/2496. R2 seropositive = 173/2503. Modelled seroprevalence R1 2.4%(1.4,3.6); R2 new seropositive 4.5%(3.2, 6.0); positive R1&2 7.8%(6.2, 9.5). No clear age differences across schools. Clustering of >=3 cases slightly higher than expected from chanceR3: Raw data: 447 positive out of 2483 tests: modelled seroprevalence 16.4% (12.1, 19.5). Clustering of >=3 cases slightly higher than expected from chance |
| Willeit et al. | medRxiv then published | Austria | Time 1: 28 Sep-22 Oct 2020; Time 2: 10–16 Nov 2020 | NS | Population | Surveillance | Random sample of 6% of all Austrian primary & secondary schools =250. 60 students per school invited (across all classes). Random sample of teachers. Fully FTF. Note schools closed 16 Nov due to national lockdown | T1: 10,156 samples from 243 schools participating (97.2% of schools; no data on% children participating) n = 8934 students & 1222 teachers; T2: 3745 samples from 88 schools (reduced due to lockdown). Median 40 children and 6 teachers per school. N = 3295 students & 450 teachers | 6–16y | RT-PCR (gargle specimens) | T1: prevalence students 0.4%(0.3, 0.5) teachers 0.6%(0.3, 1.3); 0 cases in 209/243 schools, 1 in 28 schools and 2 in 6 schools. T2: children 1.5%(1.1, 2.0) teachers 0.4%(0.1, 1.8). 0 cases in 52/88 schools, 1 in 23, 2 in 10 and 3 cases in 4 schools. No significant difference in prevalence in primary versus secondary. in regression analyses, social deprivation and community prevalence predicted school prevalence. 100% increase in community prevalence increased odds of school prevalence by 66% (OR 1.66(1.39,1.99) |
| Ladhani et al. sKIDSs | Professional | UK | June-Dec 2020: RT-PCR June-July. Serology round 1 June, round 2 July, round 3 Nov-Dec 2020; | R1: WTR2: WT dominant, alpha emerging | Population | Surveillance | English primary schools (across all regions) and early years settings after reopening of schools June 2020 (SKIDS study (Rounds 1 & 2)). Schools all FTF. Note alpha variant predominant for Round 4. | RT-PCR: Round 1: 11 966 participants (6727 students, 4628 staff, and 611 with unknown staff or student status) in 131 schools had 40 501 swabs taken: . Serology: 45 schools (816 students, 209 staff) recruited. 95% participant recruitment. | 4–12y | RT-PCR (NP swab) and Serology IgG | Round 1: RT-PCR: 1 student and 5 staff positive during 4 weeks: estimated incidence rate/wk student 4•1 (0•1–22•8), staff: 12•5(1•5–45•0) per 100 000. Seropositive: Round 1: children 11•2%(7•9,15•1) staff 15.2%(11.9,18.9). Seropositivity was not clustered (in model after adjustment) by school for children but was for staff. Seropositivity was not associated with school attendance during lockdown (children or staff). Round 2: 74% participation: children 10.4% staff 13.1% - only 5 seroconversions (staff & children) between rounds. Round 3: 54.2% participation for children: 8.6% of children and 11.2% of staff. |
| Jordan et al. | Professional | Spain | 29- Jun - 31 July 2020 | NS | Population | Surveillance (prospective) with contact-tracing | Children and staff in 22 summer schools in Barcelona over 2–5 weeks. Attended 40 h/week. Note additional data on children identified through symptom-based screening (Recruitment Pathway 2) not included here. | 5240 samples from 1905 participants in 22 camps (45% of recruited camps) 1509 children and 396 adults; 9 child and 3 adult primary cases identified through screening. 89 close contacts of the 9 child cases identified and tested. 90% of contacts participated. | 3–15y | RT-PCR saliva samples. Prospective weekly testing of all children; contacts tested at 0,7,14 days. nd serology IgG: all children at time 0; contacts at 0 and 5 weeks. | PCR+: 12 /5240 over 5 weeks (5/580 nasopharyngeal validation tests were positive): 9/1509 children = 0.6%. SAR from 9 child index = 1.1% (1/89). SAR from adult index was 1.6% (1/63) |
| Fontanet et al. | medRxiv then published | France | 28–30 April 2020 | NS | Population | Surveillance | 6 French primary schools in a city that had previously experienced an outbreak in the local high-school. Data included here from the primary schools; the single high school data not included as this was a single institution outbreak and data were not population-based | 510 children (49% of eligible/invited) and 42 teachers (82% of invited) provided samples. Also 641 parents of children and 119 other HH members provided samples. | 6–11y | Serology IgG | Seropositivity in 8.8%(45/510) of primary school children, 7.1(3/42)% of teachers, 11.9%(76/641) of parents and 11.8%(14/119) other HH members. Seroprevalence did not vary significantly by age. Note 61% of parents of an infected pupil were seropositive compared with 6.9% of parents of non-infected parents), suggesting transmission occurred primarily within households. 44% of seropositive children <12y were asymptomatic. |
| Ladhani et al. sKIDSsPLUS | medRxiv | UK | R1: 22 Sep-17 Oct 2020R2: 3–17 Dec 2020R3: 23 Mar-21 April 2021 | R1: WTR2: WT dominant, alpha emergingR3: alpha dominant, delta emerging | Population | Surveillance | sKIDsPLUS study of 18 secondary schools purposively recruited across England, aligned with sKIDs study of primary schools also included here. Round 4 - undertaken immediately after schools reopened after lengthy lockdown (1 Jan to 7 March 2021). Schools all FTF. Note alpha variant predominant for Round 4. | R1: 893 students, 861 staffR2: 893 students, 873 staffR3: 1094 students and 792 staff. | 11–18y | RT-PCR (NP swab) and Serology IgG. Data provided for various assays - the Abbott assay data were used consistently across R1–3 and therefore used here. | PCR data only provided for Round 3: Positive in 0.18% (2/1094) children and 0/792 staff. Clustering was not significant (p = 0.1) for school infections in Round 3. Serology data provided for Rounds 1–3: Serology data provided for Rounds 1–3: R1: seropositive student 12.8% (114/893) staff 9.2% (79/861); R2: 13.1% student (117/893) 13.4% staff (117/873); Round 3: students 22.1% (227/1029), staff 19.5% (150/771). |
| Lachassinne et al. | Professional | France | 4 Jun-3 July 2020 | NS | Population | Surveillance | Early years setting: recruited children and staff who attended daycare during national lockdown (15 Mar-9 May 2020) as parents were essential workers; recruited from 22 early years settings in Paris region. All children invited to participate and recruitment ceased once planned N achieved. Also studied parental serology. | Recruited the first 327 children agreed to participate, along with 197 daycare staff i.e. 100% of recruited were tested. | 0.5–4y | RT-PCR nasal swabs. Stool samples also collected but data not examined here. Serology Ig & IgM. | Seropositivity in 4.3% (14/327) children and 17.7% (4/197) staff. The 14 seropositive children came from 13 daycare centres - i.e. no evidence of clustering of infection. 55% (6/11) of seropositive children had a seropositive parent compared with 14% (22/149) of seronegative children. PCR - 0/197 nasal swabs were positive. Found no evidence of transmission within daycare centres in this high risk group. Concluded most children were infected from household contacts. |
Oral = oropharyngeal.
NP= nasopharyngeal.
R=Round.
Brackets () show 95% CI.
Variant: NS = not stated; likely original or wild-type virus. VOC = variant of concern. WT = wild type (original) virus.
We defined children and young people as being < 20 years of age, but note that different studies used different age-ranges across childhood. We did not limit studies by date or language. The reference lists of identified relevant reviews were checked for additional likely studies. Studies were also identified through other systematic reviews and the professional networks of the authors.
Eligibility
We searched for contact-tracing studies and community incidence studies to answer questions a) and b), and school incidence or prevalence studies to answer question c). We included published or unpublished reports of studies of SARS-CoV-2 infection of the following types:
-
a.
Contact-tracing studies informative about transmission from primary or index cases aged 0–19 years separately to adult index cases and which identified and tested all contacts regardless of symptoms
-
b.Population-based studies that were either:
-
i.longitudinal incidence studies in any setting which reported or modelled transmission chains between 0 and 19 year olds and others
-
ii.studies of prevalence or incidence in 0–19 year olds in child-specific settings (e.g. day-care, nurseries or schools) using either longitudinal or cross-sectional designs
-
i.
We only included studies which identified SARS-CoV-2 infection through RT-PCR on oral or nasal samples or through established serological methods. We did not include studies which used less well validated methods such as rapid antigen tests, stool samples21 or wastewater methods.
We excluded studies of transmission from single individuals or within single institutions; modeling studies that did not provide observational data; studies of vertical transmission; systematic reviews; studies only of school staff; and biological studies of transmission dynamics such as viral load, viral shedding or aerosolization. We excluded ecological level studies of the impact of school opening or closing on community transmission as this has been examined in a separate review.22
We excluded studies judged to be at critical risk of bias relating to inadequate ascertainment of asymptomatic infections in CYP. We, therefore, excluded:
-
1.
contact-tracing studies which only tested symptomatic contacts, tested low proportions of recruited contacts or provided insufficient information to judge completeness of contact testing.
-
2.
population studies where infection was identified only by testing of symptomatic individuals or recruitment from clinical settings
-
3.
non-representative population studies due to limited sampling of the target population e.g. where testing was only performed in low proportions of participants
Study selection
Titles and abstracts of identified studies were reviewed for potential eligibility by one researcher (RV). Those potentially eligible were retrieved in full-text and reviewed independently by 2 researchers (RV and CW or OM) for eligibility and quality.
Outcomes and data extraction
Outcomes of interest were:
-
1.
From contact-tracing studies: secondary attack rates (SAR) by age of index cases (<18–20 years compared adults) in contact-tracing studies. SAR by age of contact, SAR from adult index cases and effect estimates for adjusted transmission models from CYP were also extracted where data allowed.
-
2.From population-based studies:
-
a.School studies: prevalence or seroprevalence of SARS-CoV-2 infection and presence of clustering (frequency of occurrence of >2 cases) of infection within settings. We also extracted data on school attendance (see below under meta-regression)
-
b.Longitudinal incidence studies: effect estimates for transmission models from CYP aged 0–19 years.
-
a.
Data from each study were extracted to a spreadsheet and checked for accuracy by four reviewers (RV, JC, CW and JW). Source of data in each study are shown in Appendix Table 2 . We approached authors for further data where necessary.
Table 2.
Moderators of prevalence and seroprevalence in school studies.
| PCR prevalence | Seroprevalence | |||
|---|---|---|---|---|
| Age | Odds ratios (95% CI) | p | Odds ratios (95% CI) | p |
| 0–19 years (reference) | 1 | – | 1 | – |
| Early years ≤7 years | 0.245 (0.030, 2.000) | 0.189 | – | – |
| Children 5–12 years | 0.649 (0.207, 2.034) | 0.458 | 1.567 (0.228, 10.773) | 0.648 |
| Adolescents 12–19 years | 1.433 (0.429, 4.787) | 0.559 | 1.185 (0.178, 7.877) | 0.860 |
| Community SARS-CoV-2 14 day incidence per 100,000 population (continuous) | ||||
| Contemporary with study | 1.003 (1.001, 1.004) | <0.001 | 1.001 (0.999, 1.003) | 0.307 |
| Month previous to study | 1.003 (1.001, 1.006) | 0.008 | 1.005 (1.000, 1.007) | 0.038 |
| Two months previous to study | 1.001 (0.997, 1.005) | 0.591 | 1.005 (1.002, 1.008) | 0.003 |
| School attendance (% in face-to-face learning) | 1.001 (0.982, 1.021) | 0.908 | 1.020 (0.977, 1.066) | 0.375 |
| PCR source | ||||
| Swab (nasopharyngeal or oropharyngeal) | 1 | – | ||
| Saliva or gargle | 1.54 (0.49, 4.84) | 0.456 | – | |
Quality and bias evaluation
Methodological quality was independently assessed by two authors (RV and CW) using a score adapted from previously published quality assessment tools23, 24, 25, 26 for prevalence, cohort and case-control studies (see Appendix for details and Appendix Tables 3 and 4). Only studies of high and medium quality at low risk of bias were included in these analyses.
Data synthesis and analysis
Studies were included in random effects meta-analyses and meta-regressions using a multilevel framework. This accounted for many studies collecting multiple rounds of data collection over time or for studies providing data for CYP age-groups (e.g. primary or secondary students). Analyses used the metafor package in R, using log-transformed proportions.
For contact-tracing studies, meta-analyses were undertaken of secondary attack rate (SAR) from index children grouped by setting, age of index child and age of contact. Meta-analysis comparing SAR from child index cases with SAR from adult index cases was undertaken first using raw SAR data and then using estimates of relative transmissibility from adjusted transmission models where data were provided.
For school population-based studies, we first undertook separate meta-analyses of studies providing prevalence and seroprevalence data grouped by age-group. We then used meta-regression to examine associations of school prevalence with:
-
1.
Community 14-day incidence of SARS-CoV-2 across the study period and for the one and two months prior (see Appendix Table 5 for data and sources).
-
2.
School attendance (% face-to-face) in each study (Appendix Table 6). Attendance was measured at the measurement-round level as this varied within a study over time.
We also undertook a post-hoc analysis to examine whether the use of nasopharyngeal or oral swab compared with saliva or gargle sample influenced estimates.
Role of the funding source
No funding obtained for these analyses.
Ethics
Ethics permission not required for these secondary analyses of published data.
Results
The PRISMA flow diagram is shown in Fig. 1 . Titles and abstracts of 4511 articles were reviewed from electronic databases. Two additional studies were identified through searching citation lists and 16 through professional networks. 336 were assessed in full-text and 89 articles were judged potentially eligible. 45 studies (46 articles) were excluded as being at critical risk of bias (see Appendix Table 7). Characteristics of the 37 included studies (described in 43 articles, some of which describe later rounds of a study) are shown in Table 1.
Fig. 1.
FLOW diagram.
Sixteen studies were contact-tracing studies (6 school;27, 28, 29, 30, 31, 32, 33 10 household2 , 34, 35, 36, 37, 38, 39, 40, 41, 42), 2 provided both contact-tracing and population data (both school studies43 , 44) and 19 were population studies (17 in educational settings;9 , 10 , 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59 2 were national community surveillance surveys60 , 61).
Twenty-four studies were high quality (13 population; 10 contact-tracing and 1 study providing both data) and 13 studies were medium quality (6 population, 6 contact-tracing and 1 study providing both data). Of the 43 articles reporting the 37 studies, 26 (60%) were published, 11 (26%) were preprints and 6 (14%) were government or university reports.
Eight studies were from Germany, 4 from the UK, 3 from South Korea and the USA, 2 each from China, France, Switzerland, Denmark, Italy and Norway, one included data from both the Netherlands and Belgium, and 1 study each from Netherlands, Austria, Israel, India, Spain, and Australia.
Thirty-one studies (84%) were undertaken before November 2020 and involved the wild-type virus, although only 2 explicitly reported this; 6 (16%) studies included rounds with the alpha variant emerging (1) or dominant (5), with 2 (5%) also including rounds in which the delta variant was emerging.
Contact-tracing studies (household and school)
Eighteen studies provided data on secondary infection or attack rates (SAR) from child index cases, including five large regional2 , 31 , 32 , 35 , 37 and five national34 , 38 , 41 , 62 , 63 studies. Fifteen (8 household;2 , 34 , 35 , 37, 38, 39 , 41 , 63 7 school27, 28, 29, 30, 31, 32, 33 , 44) provided sufficient data to include in meta-analyses of secondary attack rates.
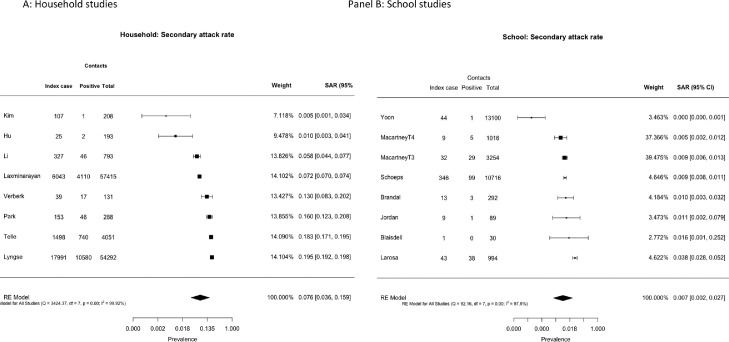
Forest plots of SAR from child index cases to all-age contacts are shown in Fig. 2 separately by setting. The pooled estimates of SAR were 7.6% (3.6, 15.9) for household studies (panel A), significantly higher than the pooled estimate for school studies of 0.7% (0.2, 2.7) (panel B) (difference QM (df=1) = 9.325, p = 0.0023).
Fig. 2.
Secondary attack rates from child index cases to all contacts for (A) household studies and (B) school contact-tracing studies.
Transmission from child index cases by age of contacts could be assessed in 4 school studies and 1 household study (Appendix Fig. 1). Pooled SAR to child contacts was not different to that to adult contacts (p = 0.45).
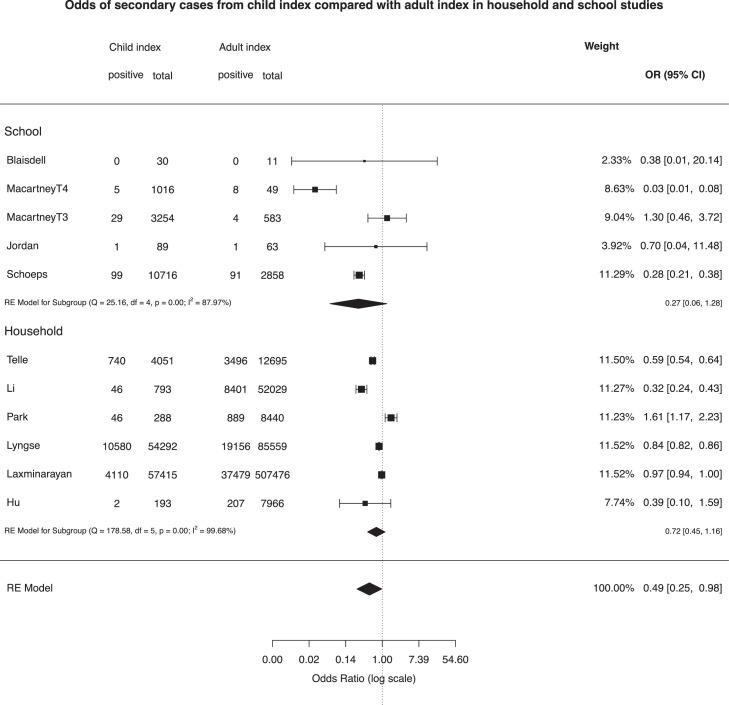
Odds of being a secondary case (of any age) from a child index compared with an adult index case were calculated from 11 rounds of data (6 household, 5 school; see Fig. 3 ). Across all studies, pooled risk of transmission was lower from child index cases than adults (OR 0.49 (0.25, 0.98); in sub-group analyses the OR was 0.27 (0.06,1.28) for school studies and 0.72 (0.45, 1.16) for household studies, all with high heterogeneity.
Fig. 3.
Odds of being a secondary case from child compared with adult index cases.
Two studies could not be included in the meta-analyses. Varma et al. undertook a large school contact-tracing study from New York City43 and reported that the overall school SAR from CYP and adults was 0.5%; of the 69% of secondary cases for which a source of infection could be identified, 51% were staff-to-staff, 27% staff-to-student, 14% student-to-staff, and 8% from student-to-student. Espenhain et al.61 used data from 4 rounds of a Danish nationally representative community survey to examine transmission in 1244 households with resident adolescents. They reported that, in 73% of families with at least one seropositive family member, only the parent(s) or the child were seropositive, concluding that transmission between generations was uncommon.
Adjusted household transmission models
Six studies examined transmission from CYP to household members using adjusted transmission models accounting for a range of factors including individual exposure histories, potential tertiary transmission, poverty and the age-structure of populations. Two studies used nationally representative data from England60 and Denmark,41 and four were contact-tracing studies (from China,35 , 37 Israel36 and the Netherlands40).
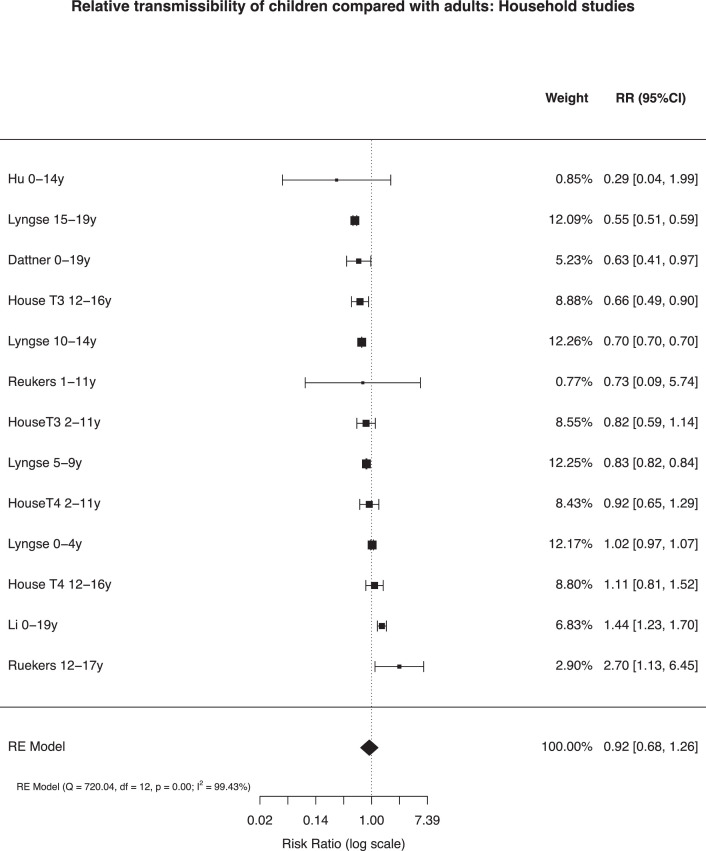
House et al.60 used longitudinal weekly PCR testing from a very large representative national sample of English household64 to estimate susceptible-infectious transmission probabilities from models in four periods from April 2020 to February 2021 across low and high prevalence, schools being reopened and the emergence of the alpha (B.1.1.7) variant in late 2020. They found transmissibility did not differ by age. However they did observe that the risk of bringing infection into household (relative external exposure) was higher amongst 12–16y than for adults although these included periods of national lockdown for adults whilst all children continued to attend full-time schooling. A Dutch contact-tracing study similarly concluded there were no differences in transmissibility between children and adults,40 whilst a large national Danish study41 and an Israeli contact-tracing study36 found lower relative transmissibility in children and young people compared to adults. Two contact-tracing studies from China found that, whilst in unadjusted analyses infected children generated fewer secondary cases than adults, adjusted models showed no difference,35 or higher infectivity.37
Multilevel random-effects meta-analysis of relative transmissibility from CYP compared with adults included 13 estimates from 6 studies with total person-observations from 127,822
CYP and 1526,117 adults (Fig. 4 ). The pooled relative transmissibility from CYP was 0.92 (0.68, 1.26) compared with adults, with high heterogeneity (99.43%). Data did not allow sub-group analyses by age of child.
Fig. 4.
Relative transmissibility of children and adolescents compared with adults in adjusted household models
Note: Analysis includes the last two periods from House et al. and estimates by age from other studies.
School prevalence studies
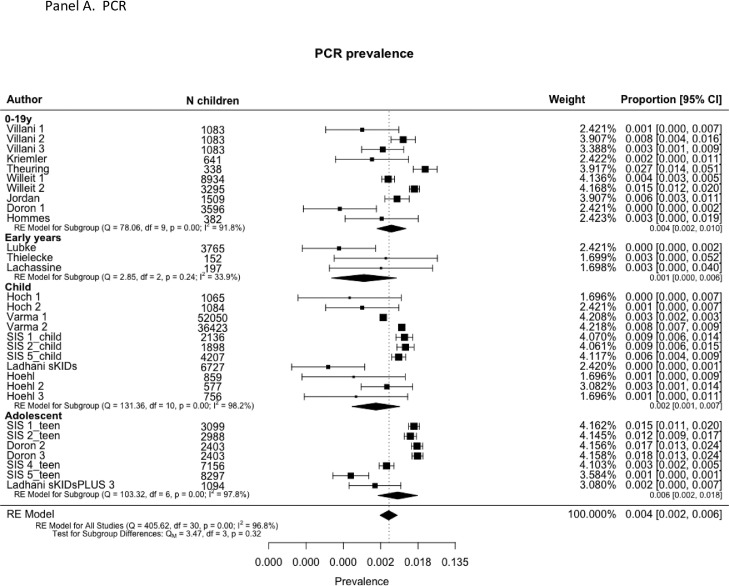
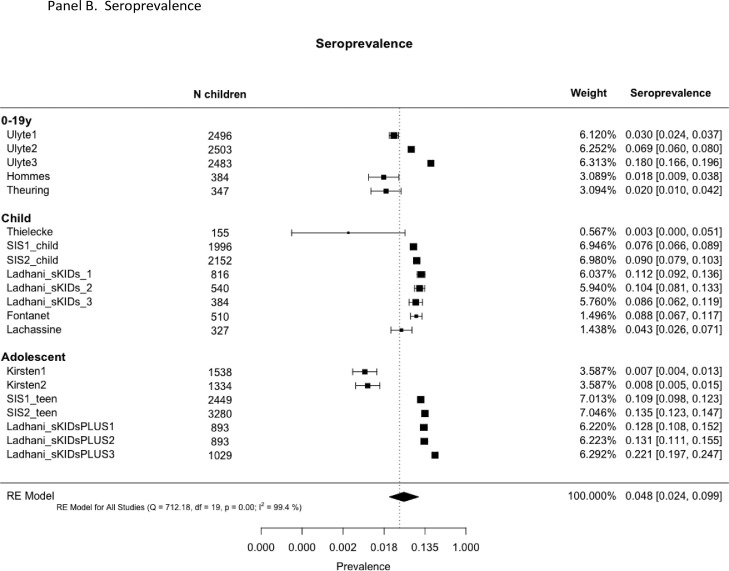
Infection prevalence in schools or nurseries was measured in 16 studies (31 rounds of observations; total 161,280 child-observations) and antibody prevalence was measured in 9 studies (20 rounds; 26,509 child-observations). Some provided data for single age-groups (e.g. early-years, primary or secondary students) while others provided cross age-group data. In the main analyses, we used overall estimates where they exist and estimates by age-group where the former were not provided.
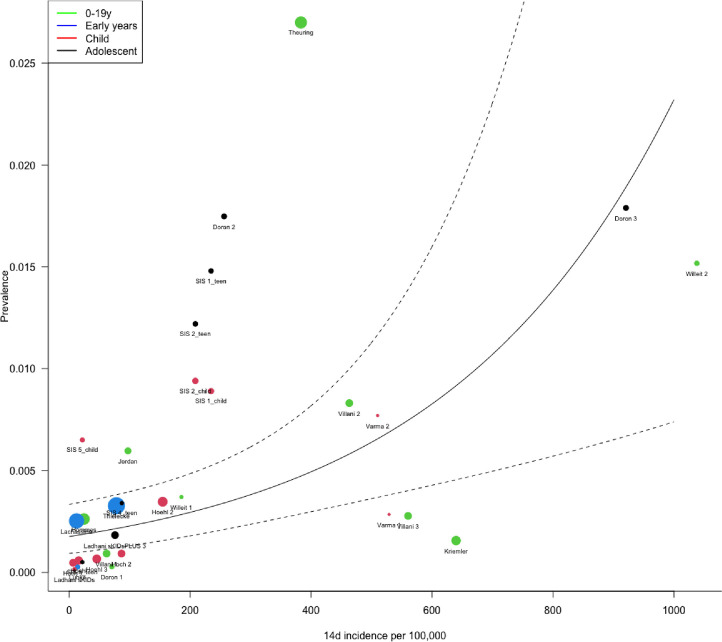
Forest plots of PCR prevalence and seroprevalence by age are shown in Fig. 5 . Meta-regression models are shown in Table 2. Pooled infection (PCR) prevalence across all studies was 0.4% (0.2, 0.6), not significantly different by age-group (p = 0.32). Prevalence was also associated with contemporary community 14-day incidence (OR 1.003 (1.001, 1.004), p<0.001) and prevalence in the month prior to the study (OR 1.003 (1.001, 1.006), p = 0.008) but not with 2 months prior. PCR prevalence was not associated with school attendance rate nor PCR source. Plot of predicted school prevalence by 14-day incidence is shown across age-groups in Fig. 6 .
Fig. 5.
. Prevalence and seroprevalence of SARS-CoV-2 infection in schools by age-group: (A) PCR prevalence and (B) Seroprevalence.
Fig. 6.
Plot of predicted prevalence and 95% CI in school studies by community 14-day incidence of SARS-CoV-2 infections per 100,000.
Pooled seroprevalence across all studies was 4.8% (2.4, 9.9), with no significant difference by age-group. Seroprevalence was associated with community incidence in the month and two months prior to the study, but not with contemporary incidence. Seroprevalence was not associated with school attendance.
No school studies fitted adjusted transmission models. Only two studies undertook a detailed analysis of clustering; Ulyte et al.9 , 65 reported that clusters of ≥3 cases occurred in 7 of 129 classes in Round 2 and 24 of 119 in Round, more than the 4 and 17 classes expected by chance respectively. A very large school contact-tracing study by Schoeps et al.28 reported that 83% of 784 school index cases led to no secondary cases. All other studies reported no evidence of clustering of infections (i.e. > 3–5 infections per class) within schools.10 , 46 , 47 , 51, 52, 53, 54, 55, 56 , 59 , 66 , 67 Other observations supporting limited transmission in schools were calculations showing that where direction of transmission was available, the majority appeared to be from adults to children28 , 43 , 49 , 51 , 68 or that origins of transmission chains were outside schools;47 and observations that virus prevalence in school children and teachers was lower than in the local community at the time despite higher levels of testing within schools.43 , 52 , 53 , 67 Seroprevalence studies, however, reported similar antibody prevalence amongst students and teachers54 , 67 , 68 or adults in the local community.9 , 67 , 68
The association of school prevalence with community infection rates was examined in two school studies, both of which reported positive associations.43 , 56 Only one study examined associations of prevalence with social deprivation, reporting a positive association.56
Discussion
We report the first findings relating to SARS-CoV-2 transmission from CYP through meta-analysis of studies with low risk of bias. Meta-analysis of household studies which undertook adjusted transmission analyses showed no difference in relative transmissibility between CYP and adults (OR 0.92 (0.68, 1.26)), although meta-analysis of unadjusted secondary attack rates suggested that transmission from CYP was lower than from adults, although with wide confidence intervals. There are a number of sources of potential bias in the unadjusted analyses, including low numbers of child index cases as well as differential transmission from children across generations of spread within households, and it is likely that these analyses under-estimate relative transmissibility. These findings suggest that, within households, CYP play a role in transmission that is to similar but not higher than adults. The only study to examine external force of infection suggests CYP play a role in bringing infection into the house when schools are open, but this included periods when the country was in lockdown whilst schools remained fully open.60
We found a striking difference in transmission from CYP across different settings, with the pooled SAR from CYP index cases in household studies (7.6%) being 10-fold higher than in school studies (0.7%), despite a similar quantity and quality of evidence in both settings. We were unable to draw conclusions about transmissibility from CYP compared with adults in educational settings, due to wide confidence intervals and lack of studies reporting adjusted analyses. We found no evidence that transmission differed from CYP index cases to contacts of differing ages. Similar to our findings, other studies have concluded that household settings have higher transmission potential than other settings such as schools.17 , 18 This disparity may reflect differences in the duration and intensity of social mixing within schools compared with households, with more prolonged, intense and intimate contacts between children and siblings or parents within households carrying a greater risk of transmission.69 Our findings may also reflect the successful operation of NPI mitigations within schools in markedly reducing transmission.70 This observation is supported by findings from some of the included school studies, including a lower prevalence in schools than in surrounding communities and the lack of notable clustering of infection within classrooms, even when local prevalence was high. Lack of clustering is supported by a number of studies not included in our review for quality reasons including a national study from Luxembourg.71 There may, however, be systematic bias that might contribute to lower transmission in school compared with household studies. For example, CYP who are known to be infected or are contacts of positive cases are usually excluded from school but would be included within household studies. However, a substantial proportion of infected CYP are likely to be asymptomatic and, therefore, unlikely to be absent from school.10 Biases related to relatively low numbers of CYP index cases, adequacy of contact-tracing and validity of PCR or serology testing in CYP apply equally to both school and household studies.
Our meta-regression findings that local community incidence was positively associated with school infection prevalence, as was incidence in the month prior, whereas seroprevalence was only associated with historical community incidence, show the inter-dependence of schools with their localities with respect to infection levels. Ismail et al.72 reported the risk of an outbreak increased by 72% for every five cases per 100 000 population increase in community incidence, whilst Willeit et al.56 reported that the odds of testing positive in schools were 1.64 (1.38, 1.96) for a two-fold higher community incidence. Our findings support the hypothesis that school infections predominantly reflect community infection levels, although our analysis could not attribute causality.
Our review included a number of studies undertaken when the prevalence of variants with higher transmissibility (e.g. alpha or B.1.1.7 variant) was rising or dominant, although most studies preceded this. No contact-tracing studies were included of transmission related to the delta variant although two school prevalence studies included data collection whilst delta infection was rising. Our findings therefore cannot be assumed to apply to periods when delta was predominant. However, whilst the delta variant has substantially higher overall transmissibility, and the prevalence of delta infection in children has been high at a time when adult populations had high vaccination coverage, there is no evidence of variant-specific differential transmission between children and adults. It is possible that the differential in transmission between school and household settings is lower for the higher transmissibility variants such as delta or omicron than reported here, although the higher transmissibility of the delta variant appears not to be setting-specific.
Limitations
Our data are subject to a number of limitations. Potential biases in school studies have been discussed above. RT-PCR studies may under-estimate infection in children compared with serology,36 and different seroassays may provide differing results. Many of the included studies, however, combined findings from both PCR and serology,10 , 31 , 32 , 39 , 40 , 44 , 47 , 48 , 54 , 67 or undertook repeated PCR measures40 , 44 , 45 , 49, 50, 51 , 53 , 60 Importantly, though, these issues are likely to be similar across both contact-tracing and population studies and, therefore, would not alter the notable differences we found by setting.
Contact-tracing studies are open to bias due to missed testing of contacts, although we only included those who planned routine testing of all contacts and who achieved a high proportion of contacts tested. Low numbers of child index cases and their contacts in some studies may also be a source of bias. Population studies may be biased by higher participation by higher socioeconomic status groups and also as some studies specifically excluded those with recent contacts or symptoms.50
We conducted multi-level analyses accounting for the nesting of multiple rounds of data-collection within single studies. Some of the smaller meta-analyses, however, may have been overly influenced by studies with many rounds of testing. Meta-regression analyses are conducted at study rather than individual level and are, therefore, subject to ecological biases and cannot infer causality.
Our findings relate largely to the original/Wuhan virus and the alpha variant and it is unclear how generalisable they will be to the delta or other variants. Paucity of data meant we were unable to compare transmissibility from CYP between the Wuhan and alpha variants. Additionally all data precede widespread vaccination of adults and no studies included populations of teenagers who had been vaccinated. Our data were largely limited to high-income countries and there is an urgent need for similar studies from low-and-middle-income countries.
Conclusions and implications
We found no difference in transmission of SARS-CoV-2 from CYP compared with adults within household settings. Secondary attack rates were markedly lower in school compared with household settings and there was little clustering of infections within schools, suggesting that household transmission is more high risk than school transmission in this pandemic.
School infection prevalence was associated with community infection incidence in the month before and during the study, with seroprevalence associated with historical community infections, supporting hypotheses that school infections broadly reflect community infections. These findings are important for guiding policy decisions on school operations during the pandemic. With appropriate mitigations, school infections can be limited and face-to-face learning is feasible, even at times of moderate to high community prevalence and in the presence of variants with higher transmissibility.
Our findings support a potential role for vaccination of CYP, if proven safe, in reducing transmission within households. Where countries go on to achieve very high levels of adult vaccination, this will focus transmission amongst the unvaccinated, increasing the relative importance of transmission amongst CYP.
Our findings largely relate to SARS-CoV-2 transmission from children before highly transmissible variants such as delta or omicron became predominant and this work needs replication once sufficient data are available from periods dominated by other variants. A number of other gaps in our knowledge remain about transmission from CYP, particularly relating to potential age-differences between younger and older children, and effectiveness of various NPIs, especially face masks, to reduce transmission in child-specific settings. Detailed population studies are required which link households and schools and use a combination of repeated PCR and serology testing to assess the risk of infection and direction of transmission across settings.
Contributions
RV and CB conceptualised the paper and led the writing of the manuscript,RV undertook the searches, contributed to data extraction and quality assessment and undertook the meta-analyses. . CW, OM, JC and JW contributed to eligibility assessment, data extraction and quality assessment. GMT and CB contributed to planning the analyses. All authors contributed to writing and editing of the manuscript.
Funding
No funding obtained.
Declaration of Competing Interests
All authors declare no competing interests.
Acknowledgments
We thank Kjetil Telle, Norwegian Institute of Public Health, and Marieke de Hoog, University Medical Center Utrecht, for providing additional data for their studies included here. We also thank Semina Michalopoulou and Zainab Dedat for checking the accuracy of data extraction.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2021.12.026.
Appendix. Supplementary materials
References
- 1.Flasche S., Edmunds W.J. The role of schools and school-aged children in SARS-CoV-2 transmission. Lancet Infect Dis. 2021;21(3):298–299. doi: 10.1016/S1473-3099(20)30927-0. Epub 2020/12/08PubMed PMID: 33306982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laxminarayan R., Wahl B., Dudala S.R., Gopal K., Mohan C., Neelima S., et al. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science. 2020:eabd7672. doi: 10.1126/science.abd7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okarska-Napierala M., Mandziuk J., Kuchar E. SARS-CoV-2 cluster in nursery, Poland. Emerg Infect Dis. 2021;27(1) doi: 10.3201/eid2701.203849. Epub 2020/10/10PubMed PMID: 33035153PubMed Central PMCID. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fontanet A., Tondeur L., Grant R., et al. SARS-CoV-2 infection in schools in a northern French city: a retrospective serological cohort study in an area of high transmission, France, January to April 2020. Euro Surveill. medRxiv preprint server. 2021;26(15) doi: 10.2807/1560-7917.ES.2021.26.15.2001695. Published E-Pub Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres J.P., Pinera C., De La Maza V., Lagomarcino A.J., Simian D., Torres B., et al. SARS-CoV-2 antibody prevalence in blood in a large school community subject to a Covid-19 outbreak: a cross-sectional study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa955. Epub 2020/07/11PubMed PMID: 32649743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein-Zamir C., Abramson N., Shoob H., Libal E., Bitan M., Cardash T., et al. A large COVID-19 outbreak in a high school 10 days after schools' reopening, Israel, May 2020. Euro Surveill. 2020;25(29) doi: 10.2807/1560-7917.ES.2020.25.29.2001352. Epub 2020/07/29PubMed PMID: 32720636; PubMed Central PMCID: PMCPMC7384285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pray I.W., Gibbons-Burgener S.N., Rosenberg A.Z., Cole D., Borenstein S., Bateman A., et al. COVID-19 outbreak at an overnight summer school retreat - Wisconsin, July-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(43):1600–1604. doi: 10.15585/mmwr.mm6943a4. Epub 2020/10/30PubMed PMID: 33119558PubMed Central PMCID: PMCPMC7640998 Journal Editors form for disclosure of potential conflicts of interest. No other potential conflicts of interest were disclosed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szablewski C.M., Chang K.T., Brown M.M., Chu V.T., Yousaf A.R., Anyalechi N., et al. SARS-CoV-2 transmission and infection among attendees of an overnight camp - Georgia, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(31):1023–1025. doi: 10.15585/mmwr.mm6931e1. Epub 2020/08/08PubMed PMID: 32759921PubMed Central PMCID: PMCPMC7454898 Journal Editors form for disclosure of potential conflicts of interest. No potential conflicts of interest were disclosed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ulyte A., Radtke T., Abela I.A., Haile S.R., Berger C., Huber M., et al. Clustering and longitudinal change in SARS-CoV-2 seroprevalence in school children in the canton of Zurich, Switzerland: prospective cohort study of 55 schools. BMJ. 2021;372 doi: 10.1136/bmj.n616. n616. Epub 2021/03/19PubMed PMID: 33731327; PubMed Central PMCID: PMCPMC7966948 at www.icmje.org/coi_disclosure.pdf and declare: support from Swiss School of Public Health (SSPH+), Swiss Federal Office of Public Health, private funders, funds of the cantons of Switzerland (Vaud, Zurich, and Basel), institutional funds of universities, and University of Zurich Foundation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladhani S.N., Baawuah F., Beckmann J., Okike I.O., Ahmad S., Garstang J., et al. SARS-CoV-2 infection and transmission in primary schools in England in June-December 2020 (sKIDs): an active, prospective surveillance study. Lancet Child Adolesc Health. 2021 doi: 10.1016/S2352-4642(21)00061-4. Epub 2021/03/20PubMed PMID: 33740430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viner R.M., Mytton O.T., Bonell C., Melendez-Torres G.J., Ward J., Hudson L., et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.4573. Epub 2020/09/26PubMed PMID: 32975552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jarvis C., Munday J., Gimma A., Wong K., Van Zandvoort K., Funk S., et al. London School of Tropical Medicine and Hygiene; 2021. Social contacts in the UK from the CoMix Social Contact Survey: Report For Survey Week 61. 1 June 2021. Report No. [Google Scholar]
- 13.Suk J.E., Vardavas C., Nikitara K., Phalkey R., Leonardi-Bee J., Pharris A., et al. The role of children in the transmission chain of SARS-CoV-2: a systematic review and update of current evidence. medRxiv. 2020 doi: 10.1101/2020.11.06.20227264. 2020.11.06.20227264. [DOI] [Google Scholar]
- 14.Gaythorpe KAM, Bhatia S., Mangal T., Unwin HJ, Imai N, et al. Children’s role in the COVID-19 pandemic: a systematic review of early surveillance data on susceptibility, severity, and transmissibility. Scientific reports. 2021;11(1):13903. doi: 10.1038/s41598-021-92500-9. Published E-Pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu W., Li X., Dozier M., He Y., Kirolos A., Lang Z., et al. What is the evidence for transmission of COVID-19 by children in schools? A living systematic review. medRxiv. 2020 doi: 10.1101/2020.10.11.20210658. 2020.10.11.20210658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spielberger B.D., Goerne T., Geweniger A., Henneke P., Elling R. Intra-household and close-contact SARS-CoV-2 transmission among children - a systematic review. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.613292. 613292Epub 2021/04/27PubMed PMID: 33898355; PubMed Central PMCID. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madewell Z.J., Yang Y., Longini I.M., Jr., Halloran M.E., Dean N.E. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(12) doi: 10.1001/jamanetworkopen.2020.31756. e2031756-ePubMed PMID: 33315116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson H.A., Mousa A., Dighe A., Fu H., Arnedo-Pena A., Barrett P., et al. SARS-CoV-2 setting-specific transmission rates: a systematic review and meta-analysis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2021:ciab100. doi: 10.1093/cid/ciab100. PubMed PMID: 33560412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnaratne S., Pfadenhauer L.M., Coenen M., Geffert K., Jung-Sievers C., Klinger C., et al. Measures implemented in the school setting to contain the COVID-19 pandemic: a rapid scoping review. Cochr Database Syst Rev. 2020;(12) doi: 10.1002/14651858.CD013812. PubMed PMID: CD013812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stuart E.A., Dowdy D.W. Evidence-based COVID-19 policy-making in schools. Nat Med. 2021 doi: 10.1038/s41591-021-01585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haag L., Blankenburg J., Unrath M., Grabietz J., Kahre E., Galow L., et al. Prevalence and transmission of SARS-CoV-2 in childcare facilities: a longitudinal study. medRxiv. 2021 doi: 10.1101/2021.04.16.21255616. 2021.04.16.21255616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh S., Chowdhury A., Braithwaite V., et al. Do school closures and school reopenings affect community transmission of COVID-19? A systematic review of observational studies. BMJ open. 2021;11(8):e053371. doi: 10.1136/bmjopen-2021-053371. Published E-Pub Aug 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joanna Briggs Institute; Adelaide, South Australia: 2017. Checklist For Prevalence studies: The Joanna Briggs Institute Critical Appraisal Tools For Use in JBI Systematic Reviews. [Google Scholar]
- 24.Munn Z., Moola S., Lisy K., Riitano D., Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015;13(3):147–153. doi: 10.1097/XEB.0000000000000054. Epub 2015/09/01PubMed PMID: 26317388. [DOI] [PubMed] [Google Scholar]
- 25.Loney P.L., Chambers L.W., Bennett K.J., Roberts J.G., Stratford P.W. Critical appraisal of the health research literature: prevalence or incidence of a health problem. Chronic Dis Can. 1998;19(4):170–176. Epub 1999/02/24. PubMed PMID: 10029513. [PubMed] [Google Scholar]
- 26.Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., et al. JBI Manual for Evidence Synthesis. JBI; 2020. Systematic reviews of etiology and risk. Aromataris E, Munn Z, editors. [Google Scholar]
- 27.Blaisdell L.L., Cohn W., Pavell J.R., Rubin D.S., Vergales J.E. Preventing and mitigating SARS-CoV-2 transmission - four overnight camps, Maine, June-August 2020. MMWR Morb Mort Wkly Rep. 2020;69(35):1216–1220. doi: 10.15585/mmwr.mm6935e1. PubMed PMID: 32881850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoeps A., Hoffmann D., Tamm C., Vollmer B., Haag S., Kaffenberger T., et al. COVID-19 transmission in educational institutions August to December 2020 in Germany: a study of index cases and close contact cohorts. medRxiv. 2021 doi: 10.1101/2021.02.04.21250670. 2021.02.04.21250670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoon Y., Kim K.R., Park H., Kim S., Kim Y.J. Stepwise school opening and an impact on the epidemiology of COVID-19 in the children. J Korean Med Sci. 2020;35(46):e414. doi: 10.3346/jkms.2020.35.e414. Epub 2020/12/02. doi: 10.3346/jkms.2020.35.e414. PubMed PMID: 33258334; PubMed Central PMCID: PMCPMC7707922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larosa E., Djuric O., Cassinadri M., Cilloni S., Bisaccia E., Vicentini M., et al. Secondary transmission of COVID-19 in preschool and school settings in northern Italy after their reopening in September 2020: a population-based study. Euro Surveill. 2020;25(49) doi: 10.2807/1560-7917.ES.2020.25.49.2001911. PubMed PMID: 33303065; PubMed Central PMCID: PMCPMC7730487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Centre for Immunisation Research and Surveillance or NCIRS . National Centre for Immunisation Research and Surveillance; Sydney, Australia: 2020. COVID-19 in Schools and Early Childhood Education and Care Services – the Term 3 experience in NSW. 21 October 2020. Report No. [Google Scholar]
- 32.National Centre for Immunisation Research and Surveillance or NCIRS . National Centre for Immunisation Research and Surveillance; Sydney, Australia: 2021. COVID-19 in Schools and Early Childhood Education and Care Services – the Term 4 experience in NSW. 9 March 2021Report No. [Google Scholar]
- 33.Brandal L.T., Ofitserova T.S., Meijerink H., Rykkvin R., Lund H.M., Hungnes O., et al. Minimal transmission of SARS-CoV-2 from paediatric COVID-19 cases in primary schools, Norway, August to November 2020. Euro surveill: Bull Eur Malad Trans = Eur Commun Dis Bull. 2021;26(1) doi: 10.2807/1560-7917.ES.2020.26.1.2002011. 2002011PubMed PMID: 33413743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park Y.J., Choe Y.J., Park O., Park S.Y., Kim Y.M., Kim J., et al. Contact tracing during coronavirus disease outbreak, South Korea, Emerg Infect Dis. 2020;26(10) doi: 10.3201/eid2610.201315. Epub 2020/07/17PubMed PMID: 32673193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu S., Wang W., Wang Y., Litvinova M., Luo K., Ren L., et al. Infectivity, susceptibility, and risk factors associated with SARS-CoV-2 transmission under intensive contact tracing in Hunan, China. Nat Commun. 2021;12(1):1533. doi: 10.1038/s41467-021-21710-6. Epub 2021/03/23. doiPubMed PMID: 33750783; PubMed Central PMCID: PMCPMC7943579 Seqirus, and H.Y. has received research funding from Sanofi Pasteur, GlaxoSmithKline, Yichang HEC Changjiang Pharmaceutical Company, and Shanghai Roche Pharmaceutical Company. None of those research funding is related to COVID-19. All other authors report no competing interests. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dattner I., Goldberg Y., Katriel G., Yaari R., Gal N., Miron Y., et al. The role of children in the spread of COVID-19: using household data from Bnei Brak, Israel, to estimate the relative susceptibility and infectivity of children. PLoS Comput Biol. 2021;17(2) doi: 10.1371/journal.pcbi.1008559. e1008559-ePubMed PMID: 33571188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li F., Li Y.-.Y., Liu M.-.J., Fang L-Q, Dean N.E., Wong G.W.K., et al. Household transmission of SARS-CoV-2 and risk factors for susceptibility and infectivity in Wuhan: a retrospective observational study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(20)30981-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim J., Choe Y.J., Lee J., Park Y.J., Park O., Han M.S., et al. Role of children in household transmission of COVID-19. Arch Dis Child. 2020 doi: 10.1136/archdischild-2020-319910. Epub 2020/08/10PubMed PMID: 32769089. [DOI] [PubMed] [Google Scholar]
- 39.Verberk J., de Hoog M., Westerhof I., Van Goethem S., Lammens C., Ieven M., et al. Transmission of SARS-CoV-2 within households: a prospective cohort study in the Netherlands and Belgium – Interim results. medRxiv. 2021 doi: 10.1101/2021.04.23.21255846. 2021.04.23.21255846. [DOI] [Google Scholar]
- 40.Reukers D.F.M., van Boven M., Meijer A., Rots N., Reusken C., Roof I., et al. High infection secondary attack rates of SARS-CoV-2 in Dutch households revealed by dense sampling. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab237. Epub 2021/04/07PubMed PMID: 33822007; PubMed Central PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyngse F.P., Mølbak K., Træholt Frank K., Nielsen C., Skov R.L., Kirkeby C.T. Association between SARS-CoV-2 transmission risk, viral load, and age: a nationwide study in danish households. medRxiv. 2021 doi: 10.1101/2021.02.28.21252608. 2021.02.28.21252608. [DOI] [Google Scholar]
- 42.Telle K., Jørgensen S.B., Hart R., Greve-Isdahl M., Kacelnik O. Secondary attack rates of COVID-19 in Norwegian families: a nation-wide register-based study. Eur J Epidemiol. 2021:1–8. doi: 10.1007/s10654-021-00760-6. PubMed PMID: 34036466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varma J.K., Thamkittikasem J., Whittemore K., Alexander M., Stephens D.H., Arslanian K., et al. COVID-19 infections among students and staff in New York City public schools. Pediatrics. 2021 doi: 10.1542/peds.2021-050605. [DOI] [PubMed] [Google Scholar]
- 44.Jordan I., de Sevilla M.F., Fumado V., Bassat Q., Bonet-Carne E., Fortuny C., et al. Transmission of SARS-CoV-2 infection among children in summer schools applying stringent control measures in Barcelona, Spain. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoehl S., Kreutzer E., Schenk B., Westhaus S., Foppa I., Herrmann E., et al. Longitudinal testing for respiratory and gastrointestinal shedding of SARS-CoV-2 in day care centres in Hesse, Germany. Clin Infect Dis. 2021 doi: 10.1093/cid/ciaa1912. Epub 2021/01/04PubMed PMID: 33388748; PubMed Central PMCID: PMCPMC7799213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kriemler S., Ulyte A., Ammann P., Peralta G.P., Berger C., Puhan M.A., et al. Surveillance of acute SARS-CoV-2 infections in school children and point-prevalence during a time of high community transmission in Switzerland. Front Pediatr. 2021;9 doi: 10.3389/fped.2021.645577. Epub 2021/04/03PubMed PMID: 33796490; PubMed Central PMCID: PMCPMC8007924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Theuring S., Thielecke M., van Loon W., Hommes F., Hülso C., von der Haar A., et al. SARS-CoV-2 infection and transmission in school settings during the second wave in Berlin, Germany: a cross-sectional study. medRxiv. 2021 doi: 10.1101/2021.01.27.21250517. 2021.01.27.21250517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thielecke M., Theuring S., van Loon W., Hommes F., Mall M.A., Rosen A., et al. SARS-CoV-2 infections in kindergartens and associated households at the start of the second wave in Berlin, Germany - a cross sectional study. Eur J Public Health. 2021:ckab079. doi: 10.1093/eurpub/ckab079. PubMed PMID: 33956945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoch M., Vogel S., Kolberg L., Dick E., Fingerle V., Eberle U., et al. Weekly SARS-CoV-2 sentinel surveillance in primary schools, kindergartens, and nurseries, Germany, June‒November 2020. Emerg Infect Dis. 2021;27(8) doi: 10.3201/eid2708.204859. Epub 2021/06/05PubMed PMID: 34087088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lübke N., Schupp A.-.K., Bredahl R., Kraus U., Hauka S., Andrée M., et al. Screening for SARS-CoV-2 infections in daycare facilities for children in a large city in Germany. medRxiv. 2021 doi: 10.1101/2021.02.26.21252510. 2021.02.26.21252510. [DOI] [Google Scholar]
- 51.Doron S., Ingalls R.R., Beauchamp A., Boehm J., Boucher H.W., Chow L.H., et al. Weekly SARS-CoV-2 screening of asymptomatic students and staff to guide and evaluate strategies for safer in-person learning. medRxiv. 2021 doi: 10.1101/2021.03.20.21253976. 2021.03.20.21253976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Office for National Statistics (ONS); England: 2020. COVID-19 Schools Infection Survey Round 1, England. November 202017 Dec 2020. Report No. [Google Scholar]
- 53.Villani A., Coltella L., Ranno S., Bianchi di Castelbianco F., Murru P.M., Sonnino R., et al. School in Italy: a safe place for children and adolescents. Ital J Pediatr. 2021;47(1):23. doi: 10.1186/s13052-021-00978-w. Epub 2021/02/0410.1186/s13052-021-00978-w. PubMed PMID: 33531046; PubMed Central PMCID: PMCPMC7851807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hommes F., van Loon W., Thielecke M., Abramovich I., Lieber S., Hammerich R., et al. SARS-CoV-2 infection, risk perception, behaviour and preventive measures at schools in Berlin, Germany, during the early post-lockdown phase: a cross-sectional study. Int J Environ Res Public Health. 2021;18(5) doi: 10.3390/ijerph18052739. Epub 2021/04/04PubMed PMID: 33800392; PubMed Central PMCID: PMCPMC7967466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kirsten C., Unrath M., Lück C., Dalpke A.H., Berner R., Armann J. SARS-CoV-2 seroprevalence in students and teachers: a longitudinal study from May to October 2020 in German secondary schools. BMJ Open. 2021;11(6) doi: 10.1136/bmjopen-2021-049876. Epub 2021/06/12PubMed PMID: 34112645; PubMed Central PMCID: PMCPMC8193693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willeit P., Krause R., Lamprecht B., Berghold A., Hanson B., Stelzl E., et al. Prevalence of RT-qPCR-detected SARS-CoV-2 infection at schools: first results from the Austrian School-SARS-CoV-2 prospective cohort study. Lancet Reg Health - Europe. 2021;5 doi: 10.1016/j.lanepe.2021.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fontanet A., Tondeur L., Grant R., Temmam S., Madec Y., Bigot T., et al. SARS-CoV-2 infection in schools in a northern French city: a retrospective serological cohort study in an area of high transmission, France, January to April 2020. Euro Surveill. 2021;26(15) doi: 10.2807/1560-7917.ES.2021.26.15.2001695. Epub 2021/04/17PubMed PMID: 33860747; PubMed Central PMCID: PMCPMC8167414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lachassinne E., de Pontual L., Caseris M., Lorrot M., Guilluy C., Naud A., et al. SARS-CoV-2 transmission among children and staff in daycare centres during a nationwide lockdown in France: a cross-sectional, multicentre, seroprevalence study. Lancet Child Adolesc Health. 2021;5(4):256–264. doi: 10.1016/S2352-4642(21)00024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ladhani S.N., Ireland G., Baawuah F., Beckmann J., Okike I.O., Ahmad S., et al. Emergence of SARS-CoV-2 Alpha (B.1.1.7) variant, infection rates, antibody seroconversion and seroprevalence rates in secondary school students and staff: active prospective surveillance, December 2020 to March 2021, England. medRxiv. 2021 doi: 10.1101/2021.07.14.21260496. 2021.07.14.21260496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.House T., Pellis L., Pouwels K.B., Bacon S., Eidukas A., Jahanshahi K., et al. Inferring risks of coronavirus transmission from community household Data2021 April 01, 2021: [arXiv:2104.04605 p.]. Available from: https://ui.adsabs.harvard.edu/abs/2021arXiv210404605H. [DOI] [PMC free article] [PubMed]
- 61.Espenhain L., Tribler S., Jørgensen C.S. Holm Hansen C, Wolff Sönksen U, Ethelberg S. Prevalence of SARS-CoV-2 antibodies in Denmark 2020: results from nationwide, population-based sero-epidemiological surveys. medRxiv. 2021 doi: 10.1101/2021.04.07.21254703. 2021.04.07.21254703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoon Y., Kim K.-.R., Park H., Sy Kim, Kim Y.-.J. Stepwise school opening online and off-line and an impact on the epidemiology of COVID-19 in the pediatric population. medRxiv. 2020 doi: 10.1101/2020.08.03.20165589. 2020.08.03.20165589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Telle K., Jørgensen S.B., Hart R., Greve-Isdahl M., Kacelnik O. Secondary attack rates of COVID-19 in Norwegian families: a nation-wide register-based study. medRxiv. 2021 doi: 10.1101/2021.03.06.21252832. 2021.03.06.21252832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Office for National Statistics (ONS); England: 2021. COVID-19 Infection Survey: Methods and Further Information. 26 March 2021. Report No. [Google Scholar]
- 65.Ulyte A., Radtke T., Abela I.A., Haile S.R., Ammann P., Berger C., et al. Evolution of SARS-CoV-2 seroprevalence and clusters in school children from June 2020 to April 2021 reflect community transmission: prospective cohort study Ciao Corona. medRxiv. 2021 doi: 10.1101/2021.07.19.21260644. 2021.07.19.21260644. [DOI] [PubMed] [Google Scholar]
- 66.Thielecke M., Theuring S., van Loon W., Hommes F., Mall M.A., Rosen A., et al. SARS-CoV-2 infections in kindergartens and associated households at the start of the second wave in Berlin, Germany – a cross sectional study. medRxiv. 2020 doi: 10.1101/2020.12.08.20245910. 2020.12.08.20245910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.COVID-19 Schools Infection Survey Rounds 2 to 5. England: office for National Statistics (ONS), 2021 July 2021. Report No.
- 68.Fontanet A., Grant R., Tondeur L., Madec Y., Grzelak L., Cailleau I., et al. SARS-CoV-2 infection in primary schools in northern France: a retrospective cohort study in an area of high transmission. medRxiv. 2020 doi: 10.1101/2020.06.25.20140178. 2020.06.25.20140178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mossong J., Hens N., Jit M., Beutels P., Auranen K., Mikolajczyk R., et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008;5(3):e74. doi: 10.1371/journal.pmed.0050074. Epub 2008/03/28PubMed PMID: 18366252; PubMed Central PMCID: PMCPMC2270306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lessler J., Grabowski M.K., Grantz K.H., Badillo-Goicoechea E., Metcalf C.J.E., Lupton-Smith C., et al. Household COVID-19 risk and in-person schooling. Science. 2021;372(6546):1092–1097. doi: 10.1126/science.abh2939. Epub 2021/05/01. doi: 10.1126/science.abh2939. PubMed PMID: 33927057; PubMed Central PMCID: PMCPMC8168618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mossong J., Mombaerts L., Veiber L., Pastore J., Coroller G.L., Schnell M., et al. SARS-CoV-2 transmission in educational settings during an early summer epidemic wave in Luxembourg. BMC Infect Dis. 2021;21(1):417. doi: 10.1186/s12879-021-06089-5. Epub 2021/05/06PubMed PMID: 33947340; PubMed Central PMCID: PMCPMC8093902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ismail S.A., Saliba V., Lopez Bernal J., Ramsay M.E., Ladhani S.N. SARS-CoV-2 infection and transmission in educational settings: a prospective, cross-sectional analysis of infection clusters and outbreaks in England. Lancet Infect Dis 2021;21(3):344–53. Epub 2020/12/08. doi: 10.1016/S1473-3099(20)30882-3. PubMed PMID: 33306981. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.