Summary
Background
Vaccines against COVID-19 are a powerful tool to control the current SARS-CoV-2 pandemic. A thorough description of their immunogenicity among people living with HIV (PLWHIV) is necessary. We aimed to assess the immunogenicity of the mRNA-1273 vaccine among PLWHIV.
Methods
In this prospective cohort, adult PLWHIV outpatients were enrolled during the Italian vaccination campaign. Enrolment was allowed irrespective of ongoing combination antiretroviral therapy (ART), plasma HIV viral load and CD4+ T cell count. A two-dose regimen of mRNA-1273, with administrations performed 28 days apart, was employed. The primary outcomes were anti-spike (anti-S) antibody titres and neutralising antibody activity, assessed 28 days after completing the vaccination schedule. A convenient sample of individuals not affected by HIV was also collected to serve as control (referred as healthy-donors, HDs).
Findings
We enrolled 71 PLWHIV, mostly male (84·5%), with a mean age of 47 years, a median CD4+ T cell count of 747·0 cells per µL and a median HIV viral load <50 copies/mL. COVID-19-experienced PLWHIV displayed higher anti-S antibody titres (p=0·0007) and neutralising antibody activity in sera (p=0·0007) than COVID-19-naïve PLWHIV. When stratified according to CD4+ T cell count (<350 cells/μL, 350-500 cells/μL, >500 cells/μL), anti-S antibody titres (6/71, median 2173 U/mL [IQR 987-4109]; 7/71, 5763 IU/mL [IQR 4801->12500]; 58/71, 2449 U/mL [IQR 1524-5704]) were not lower to those observed among HDs (10, median 1425 U/mL [IQR 599-6131]). In addition, neutralising antibody activity, stratified according to the CD4+ T cell count (6/71, median 1314 [IQR 606-2477]; 7/71, 3329 IU/mL [IQR 1905-10508]; 58/71, 1227 U/mL [IQR 761-3032]), was like those displayed by HDs (10, median 2112 U/mL [IQR 719-8889]).
Interpretation
In our cohort of PLWHIV with well-controlled ART, stable viral suppression and robust CD4+ T cell count, inoculation with mRNA-1273 vaccine given 4 weeks apart produced detectable humoral immune response, similar to individuals without HIV infection, supporting vaccination in PLWHIV.
Funding
This study was partially supported by Italian Ministry of Health Ricerca Corrente 2021, by Intesa San Paolo COVID-19 emergency 2020 funds, and by Fondazione Cariplo Grant (INNATE-CoV).
Keywords: PLWHIV, SARS-CoV-2, COVID-19, vaccination, mRNA-1273
Research in context.
Evidence before this study
Vaccination against COVID-19 has proved to be an effective tool to curb-out mortality and morbidity related to SARS-CoV-2 infection. The mRNA-1273 vaccine has shown to be safe and effective, but data are mainly focused on immunocompetent individuals. In certain populations (e.g., solid organ transplant recipients) vaccine efficacy can be severely hampered. HIV infection can reduce both magnitude and durability of vaccine-induced immune response and some individuals with HIV might require additional doses of vaccine. We searched PubMed, Scopus and Embase platforms for studies published in English up to August 31, 2021, that assessed outcomes for people living with HIV (PLWHIV) after vaccination against COVID-19 using the search terms “HIV” AND “COVID-19” OR “SARS-CoV-2” AND “vaccination”. We found one study assessing safety and immunogenicity of the adenovirus-based vaccine ChAdOx1 nCoV-19, thus an adenovirus-base vaccine, and three studies that involved PLWHIV vaccinated with mRNA vaccines. Among them, only one study enrolled nine PLWHIV who received the mRNA-1273 vaccine, showing values of anti-spike antibodies comparable to those observed among individuals without HIV. One study identified lower absolute IgG and pseudovirus neutralization titres post BNT162b2 vaccination among PLWHIV, while another one did not find differences by HIV status post ChAdOx1 nCoV-19 vaccination, although CD4+ T cell counts of enrolled patients were all >350/µL.
Added value of this study
Our data show, in a large and well-defined cohort of PLWHIV on suppressive combination antiretroviral therapy and with good CD4+ T cell counts, the presence of humoral immune response to the mRNA-1273 vaccine, providing not only anti-spike antibodies titres but also neutralising antibody activity. These data strengthen the fact that the mRNA-1273 vaccine is immunogenic in PLWHIV on combination antiretroviral therapy with stable viral suppression and good CD4+ T cell counts during early follow-up. Further data are needed to test vaccine effectiveness, durability of the humoral response, assess the cellular immune response, and confirm these results also in those who are viraemic or display very low CD4+ T cell counts.
Implications of all the available evidence
This study confirms that the mRNA-1273 vaccine can elicit a detectable humoral immune response in PLWHIV and emphasize the fact that this population should receive immunization against COVID-19.
Alt-text: Unlabelled box
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic keeps taking a heavy toll globally, with more than 200,000,000 confirmed cases and 4,000,000 deaths reported by the World Health Organization at the time of writing.1 Unprecedented effort towards public health interventions have been made, and massive vaccination campaigns against coronavirus disease 2019 (COVID-19) have been launched by a growing number of countries, some using vaccines developed employing the novel mRNA technology.2,3
BNT162b2 and mRNA-1273 are the two recently approved mRNA-based vaccines against COVID-19, both have shown excellent safety and efficacy in the registration studies.4 The mRNA-1273 vaccine has displayed a 94·1% efficacy at preventing COVID-19 illness; the phase 3 randomized, observer-blinded, placebo-controlled trial, assessing efficacy and safety of the compound, included 179 (0·6%) participants with HIV infection, which were categorized as having risk for severe COVID-19, based on the Centers for Disease Control and Prevention (CDC) criteria available at the time of trial design.3 Indeed, large studies suggest that people living with HIV (PLWHIV), particularly those with low CD4+ T cell counts or untreated HIV infection, might have a more severe clinical course compared to HIV-negative individuals.5 On these bases, vaccination was one of the strategies recommended by national and international HIV societies for PLWHIV to protect PLWHIV from severe illness. This population was given different levels of priority throughout distinct campaigns across Europe.6
However, only a limited number of studies have investigated responses to mRNA-based COVID-19 vaccines in PLWHIV, and previous reports showing that certain vaccines against pandemic H1N1 influenza induce suboptimal responses in PLWHIV may raise concern over immunogenicity.7 Indeed, combination antiretroviral therapy (ART) reduces but does not fully eliminate HIV-induced inflammation and immune activation, suggesting that some immune defects may persist despite fully suppressive antiretroviral therapy. However, effective control of viremia with ART has been shown to improve responsiveness to routine vaccines, especially when ART is started early during HIV infection.8 A recent publication has highlighted how the adenovirus-based ChAdOx1 nCoV-19 (AZD1222) vaccine is likely to be safe and immunogenic against PLWHIV with COVID-19.9 In this study the authors did not find differences according to the HIV status, although all the enrolled patients displayed CD4+ T cell counts >350/µL. Promising results have been shown also for the mRNA-vaccines, with the detection of anti-spike (anti-S) antibody titres, neutralising antibody activity and cellular immune responses after vaccination of PLWHIV with BNT162b2 or mRNA-1273.10,11 Interestingly, Levy and colleagues in their study on individuals vaccinated with BNT162b2, reported lower absolute IgG and pseudovirus neutralization titres post immunisation among PLWHIV compared to healthy donors (HDs). Only a small study has currently investigated the response to mRNA-1273, in a very limited number of individuals (nine) by measuring only anti-S antibody titres, which resulted comparable to those observed in non-HIV-infected individuals.12
With the present study, we assessed the capacity of the mRNA-1273 vaccine to induce effective antibody responses in PLWHIV. We evaluated the anti-S titres and the neutralising antibody activity in PLWHIV on ART who received two doses of mRNA-1273 vaccine, 28 days after the completion of vaccination schedule.
Methods
Study design and participants
In this study, we evaluated a cohort of PLWHIV, and a group of healthy donors vaccinated with mRNA-1273 vaccine. The PLWHIV cohort comprised individuals with HIV who were stable on ART under routine follow-up at the Infectious Diseases Unit of the IRCCS Ospedale Maggiore Policlinico in Milan, Italy, and received mRNA-1273 vaccination according to the schedule of attendance in the context of the Italian national vaccination program. This plan delivered COVID-19 vaccination since February 2021 to high-risk patients, which included PLWHIV. Inclusion criteria for the study were age above 18 years and a positive diagnosis of HIV infection. HDs were a group of healthcare workers without underlying chronic diseases or receiving immunosuppressive drugs, who received mRNA-1273 vaccine in the same timeframe. Written informed consent was obtained from all participants, and the study was done in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. The study protocol (286_2021) was approved by the INMI “Lazzaro Spallanzani” Ethics Committee (Roma, Italy), responsible for the evaluation of studies assessing the efficacy of drugs against SARS-CoV-2 in the Italian territory.
Clinical procedures
Blood samples from PLWHIV in care at the Infectious Diseases Unit of the IRCCS Ospedale Maggiore Policlinico in Milan, Italy, who received COVID-19 vaccination with mRNA-1273 and were enrolled in the study, were collected before the first vaccine shot (T0), before the second shot administered 28 days later (T1a) and 28 days after the second shot (T1b). At T0 participants completed a questionnaire collecting demographic and clinical variables. Blood samples from HDs were collected at the same time points.
Laboratory procedures
Anti-SARS-CoV-2 nucleocapsid antibodies (total Ig) and anti-SARS-CoV-2 spike RBD (total Ig) were measured using Roche kits (Elecsys anti-SARS-CoV-2 and Elecsys anti-SARS-CoV-2 S respectively) on Roche Cobas e801 (Roche Diagnostics, Monza Italy). Both tests are based on ElectroChemiLuminescent Immuno Assay (ECLIA).
Human embryonic kidney (293TN and 293TN hACE2) cell lines (System Bioscience, cat#LV900A-1) were cultured in Dulbecco's modified Eagle medium (DMEM, GIBCO) supplemented with 10% heat-inactivated FBS, 100 U/mL penicillin (Euroclone), 0·1 mg/mL streptomycin (Euroclone), 1X MEM non-essential amino acids (GIBCO), 2 mM L-glutamine (Euroclone), 0·11 g/L sodium pyruvate (GIBCO). Cells were maintained at 37°C in a 5% CO2 humidified incubator. Testing for mycoplasma was carried out using MycoAlertTM Mycoplasma Detection Kit (Lonza). HEK 293TN hACE2 cells were derived from HEK 293TN after transduction with a lentiviral vector coding for human ACE2 gene and Hygromycin resistance as previously described.13
VSVΔG/ffLUC/GFP dual reporter seed particles were previously described.14 Briefly, to amplify the original stock, 2 × 106 HEK 293TN were seeded onto a 100 mm petri dish in DMEM and 24 hours later transfected with 2·4 μg of pcDNA3.1 VSV-G glycoprotein encoding plasmid. 24 hours later, the supernatant was removed, and cells were inoculated with a 100-fold dilution of the original seed particle stock (in DMEM with 2% FBS), for 1 hour at 37°C. Fresh DMEM with 2% FBS was then added to cells. 48 hours later supernatant was collected, filtered through a PES 0·45 μm filter, aliquoted and stored at -80°C.
Production of SARS-CoV-2 VSV-pseudotyped particles (VSVΔG/ffLUC/GFP pseudotyped with SARS-CoV-2 Del-D614G glycoprotein) was carried out as previously described.14 Briefly, 5 × 106 HEK 293 TN were seeded in a 150mm petri dish in DMEM and 24 hours later cells were transfected with 12 μg of pcDNA3.1 SARS-CoV-2-Del-D614G S glycoprotein encoding plasmid. Twenty-four hours later, the supernatant was removed, and cells were inoculated with a 100-fold dilution of seed particles (in DMEM with 2% FBS) for 1 hour at 37°C. After, plates were washed three times with PBS, one time with DMEM 2% FBS and covered with new DMEM 2% FBS. 24 hours later, the supernatant was collected and filtered through a PES 0·45 μm filter. Subsequently, cleared supernatants were ultracentrifuged for 2h at 80000g (Beckman, swinging-rotor SW 32Ti) and concentrated in PBS. Concentrated stocks were stored at -80°C.
Titration of pseudoparticles was carried out using HEK 293TN hACE2. Briefly, 25000 HEK 293TN hACE2 cells were seeded in each well of a 24 wells plate. 24 hours later, supernatant was removed, and cells were inoculated with serial 3-fold dilutions of the SARS-CoV-2 VSV-pseudotyped particles. After 24 hours, the percentage of GFP+ cells was determined by flow cytometry and the viral titre of the stock was calculated.
Neutralising antibody activity in sera was determined using HEK 293TN hACE2. Cells were seeded at 10000 cells/well in 100 µL DMEM into white 96 well-plates. The next day, sera were serially diluted in PBS in 96-well plates, to obtain 7 dilution points plus not treated control and SARS-CoV-2 VSV-pseudotyped particles were preincubated with sera dilutions for 1 hour at 37°C. SARS-CoV-2 VSV-pseudotyped particles preincubated with sera were used to infect HEK 293TN hACE2 (MOI=0.1) in triplicate.
After 16 hours, luminescence assay was performed to determine the levels of infection for each dilution point. Bright-Glo™ Luciferase Assay System (Promega) was used following manufacturer's instructions to develop a luminescence signal, which was measured through Tecan Luminometer. Obtained relative luciferase units were normalized to not treated (PBS) controls and dose-response curves were generated by nonlinear regression curve fitting to calculate ND50.
Outcomes
The primary outcome of interest in this cohort was the immunogenicity profile of mRNA-1273 in PLWHIV. Here, we present antibody responses, in terms of anti-S total immunoglobulins, assessed 28 days after completion of vaccination schedule, and neutralising antibody activity, assessed 28 days after the first vaccine dose (T1a) and 28 days after the second vaccine dose (T1b). Further analyses (e.g., T-cell responses) and timepoints will be published when available.
Statistical analysis
Quantitative variables were described as mean and standard deviation (SD) or medians and inter-quartile ranges (IQR). Categorical variables were presented as frequencies and percentages. Mann–Whitney U test and Kruskal-Wallis test were employed to assess differences in anti-S antibodies and neutralising antibody activity across groups. Differences in neutralising antibody activity between T1a and T1b time points were assessed by Wilcoxon matched-pairs signed-rank test. The association between continuous variables was assessed with Spearman's rank correlation coefficients (rho) and by fitting linear regression models after log-transformation of dependent variables. Being this study an exploratory analysis, formal sample size estimation a priori was not performed. Analyses were performed with Prism 9 (GraphPad Software 2021) and Stata 17 (StataCorp. 2021).
Results
In the study period, 71 PLWHIV receiving mRNA-1273 prime and boost vaccinations 4 weeks apart were enrolled. Participants were mostly male (60, 84·5%), with a mean age of 47 years (standard deviation SD 8). All participants self-reported white ethnicity and all were receiving suppressive ART with a median CD4+ T cell count of 747·0 cells per µL (IQR 593-942). All 71 PLWHIV completed the vaccination schedule. Demographics and clinical characteristics of enrolled PLWHIV are displayed in Table 1. Among the 10 HDs, men were 7 (70%) and the mean age was 58 years (SD 8). All 10 HDs received mRNA-1273 prime and boost vaccinations 4 weeks apart and completed the vaccination schedule.
Table 1.
Demographics and clinical characteristics of PLWHIV vaccinated with mRNA-1273. Data are expressed as n (%) or median (IQR) except when stated otherwise.
| Variable | PLWHIV (n=71) |
|---|---|
| Sex | |
| Male | 60 (84·5%) |
| Female | 11 (15·5) |
| Age, (years)* | 47 (13) |
| On antiretroviral therapy | 71 (100%) |
| InSTI | 55 (77·5%) |
| NNRTI | 9 (12·3%) |
| PI | 10 (14%) |
| NRTI Backbone | 64 (90·1%) |
| TDF-TAF/FTC-3TC | 44 (62%) |
| ABC/FTC-3TC | 12 (17%) |
| 3TC-FTC | 8 (11·3%) |
| Plasma HIV viral load, (copies/mL) | <50·0§ |
| CD4+ T cell count per µL | 747 (593-942) |
| <350 per µL | 6 (8·4%) |
| 350-500 per µL | 7 (9·8%) |
| >500 per µL | 58 (81·7%) |
| CD4+ T cell count percentage | 34 (25-42) |
| CD4+ T/CD8 cells ratio | 0·84 (0·49-1·2) |
| CD4+ T/CD8 ratio >=1 | 32 (45%) |
| CD4+ T/CD8 ratio >=0·4 | 57 (80·3%) |
| Optimal immunologic response† | 29 (40·8) |
| HCV-Ab positive | 5 (7%) |
| HBsAg positive | 2 (2·8%) |
| Years since diagnosis | 4 (1·5-12) |
| CD4+ T cell count per µL at nadir | 278 (82-404) |
| Plasma HIV viral load at zenith | 115150 (30424-219644) |
| AIDS diagnosis | 19 (26·8%) |
| CMV IgG positive | 38 (53·5%) |
| SARS-CoV-2 anti-N Ig positive | 9 (12·3%) |
PLWHIV, people living with HIV; NRTIs, nucleoside reverse transcriptase inhibitors; InSTIs, integrase strand transfer inhibitors; NNRTIs, non-nucleoside reverse transcriptase inhibitors; PIs, protease inhibitors; HCV, hepatitis C virus; HBsAg, hepatitis B surface antigen; CMV, cytomegalovirus; anti-N, anti-nucleocapsid protein.
mean (standard deviation).
All viral loads were lower than 50 RNA copies per mL of plasma except for 5 patients who had, respectively, 2930000, 201, 176, 93 and 85 copies per mL.
Optimal immunologic response was defined as: CD4+ T/CD8 ratio≥1 plus CD4+ T≥500cells/µL plus CD4+ T%≥30%.
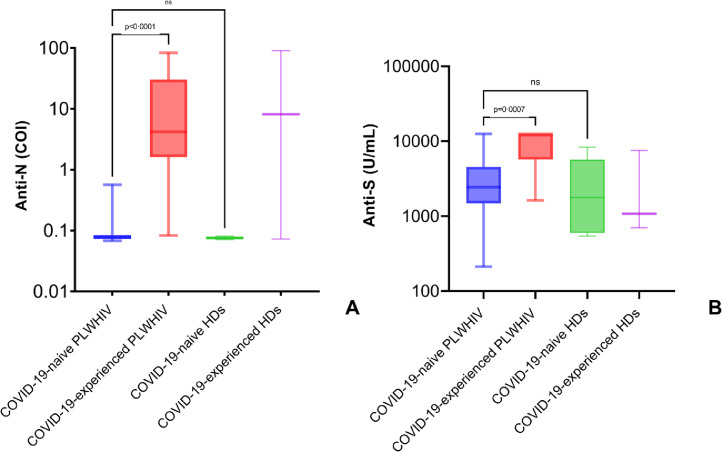
Nine PLWHIV (12·7%) and 2 HDs (20%) had a history of COVID-19, confirmed by anti-SARS-CoV-2 nucleocapsid antibodies (anti-N) titres assessed at T0. As expected, anti-N antibodies were detectable only in COVID-19-experienced PLWHIV (p<0·0001, median 4·19 COI IQR [1·61-30·18]) and not in COVID-19-naïve PLWHIV (median 0·08 COI IQR [0·07-0·08]), and their titres were similar to those observed in COVID-19-experienced HDs (p=0·9, median 8·16 COI IQR [0·07-90·20]) (Figure 1A).
Figure 1.
Serological response to infection by SARS-CoV-2 and vaccination with mRNA-1273 in people living with HIV.
SARS-CoV-2 anti-N total Ig at enrolment (A) and anti-S antibodies 28 days after completion of vaccination schedule (B) in people living with HIV. Comparison with healthy donors. Box and whiskers showing median values, 95% CIs and min to max. Statistics were calculated using Kruskal-Wallis test with Dunn's multiple comparisons test. Exact numbers of participants for each group are indicated in the corresponding results paragraph.
COI= Cut-Off Index; PLWHIV=people living with HIV; HDs=healthy donors.
All (N=71) participants living with HIV as well as all (N=10) healthy donors included in our cohort achieved positive anti-S antibody titres at T1b. Anti-S antibodies were higher in COVID-19-experienced PLWHIV (p=0·0007, median 12500 U/mL IQR [5704->12500]) than in COVID-19-naïve PLWHIV (median 2437 U/mL IQR [1485-4526]) but did not differ to those observed in COVID-19-experienced HDs (p=0·09, median 1077 U/mL IQR [702-7551]) (Figure 1B). Univariate analysis was performed to assess the association between demographic, clinical and virological variables at T0 and anti-S antibodies titres at T1b. Overall, only CD4+ T cell count groups and having positive anti-N serology were factors associated with anti-S antibodies titres (Table 2).
Table 2.
Association between clinical and serologic variables of PLWHIV and anti-S antibody titres at 28 days since the second dose of mRNA-1273 vaccine.
| Variable | Subjects (n) | Anti-S1 (U/mL) median (IQR) | p-value |
|---|---|---|---|
| Sex* | 0·55 | ||
| Male | 60 | 2746 (1601-6015) | |
| Female | 11 | 2247 (1510-5763) | |
| Age (years)° | 0·46 | ||
| <35 | 17 | 2948 (2105-5349) | |
| 35-50 | 22 | 2644 (1445-5763) | |
| 50-60 | 22 | 2077 (1073-5111) | |
| >60 | 10 | 2915 (1924 -9454) | |
| Antiretroviral therapy° | 0·88 | ||
| NRTI | 63 | 2474 (1566-5763) | |
| InSTI | 54 | 2467 (1546-5171) | |
| NNRTI | 9 | 2683 (1170-12500) | |
| PI | 9 | 2999 (670-6358) | |
| Plasma HIV viral load§ | 71 | ||
| (slope; 95% CI) | (0·66; 0·35,1·26) | 0·20 | |
| CD4+ T cell count per µL° | 0·02 | ||
| <350 per µL | 6 | 2173 (987-4109) | |
| 350-500 per µL | 7 | 5763 (4801-12500) | |
| >500 per µL | 58 | 2449 (1524-5704) | |
| CD4+ T cell count percentage§ | 71 | ||
| (slope; 95% CI) | (0·99; 0·97,1·01) | 0·31 | |
| CD4+ T/CD8 ratio >=1* | 0·14 | ||
| No | 39 | 2948 (1877-6086) | |
| Yes | 32 | 2195 (1492-4478) | |
| CD4+ T/CD8 ratio >=0·4* | 0·68 | ||
| No | 14 | 3746 (1031-6146) | |
| Yes | 57 | 2474 (1575-5111) | |
| Optimal immunologic response†,* | 0·06 | ||
| No | 42 | 3174 (1836-6157) | |
| Yes | 29 | 2143 (1445-3361) | |
| HCV-Ab* | 0·38 | ||
| Negative | 59 | 2490 (1575-5349) | |
| Positive | 5 | 6701 (2644-9274) | |
| HBsAg* | 0·21 | ||
| Negative | 62 | 2702 (1601-6015) | |
| Positive | 2 | 1474 (1071-1877) | |
| Years since diagnosis§ | 69 | ||
| (slope; 95% CI) | (0·99; 0·97,1·01) | 0·38 | |
| (CD4+ T cell count per µL)[2] at nadir§ | 69 | ||
| (slope; 95% CI) | (1·00; 0·89,1·13) | 0·96 | |
| Plasma HIV viral load at zenith§ | 69 | ||
| (slope; 95% CI) | (1·03; 0·73,1·46) | 0·86 | |
| AIDS diagnosis* | 0·50 | ||
| No | 31 | 2579 (1794-6086) | |
| Yes | 19 | 2721 (1031-5349) | |
| CMV IgG* | 0·96 | ||
| Negative | 5 | 2771 (2474-3696) | |
| Positive | 38 | 2475 (1510-5763) | |
| Anti-N* | <0·001 | ||
| Negative | 61 | 2437 (1485-4526) | |
| Positive | 9 | 12500 (5704->12500) |
CI, confidence interval; NRTIs, nucleoside reverse transcriptase inhibitors; InSTIs, integrase strand transfer inhibitors; NNRTIs, non-nucleoside reverse transcriptase inhibitors; PIs, protease inhibitors; HCV, hepatitis C virus; HBsAg, hepatitis B surface antigen; CMV, cytomegalovirus; anti-N, anti-nucleocapsid protein.
Optimal immunologic response was defined as: CD4+ T/CD8 ratio≥1 plus CD4+ T≥500cells/µL plus CD4+ T%≥30%. Statistics calculated using
Mann–Whitney U test,
Kruskal-Wallis test or
Spearman's rank correlation coefficients.
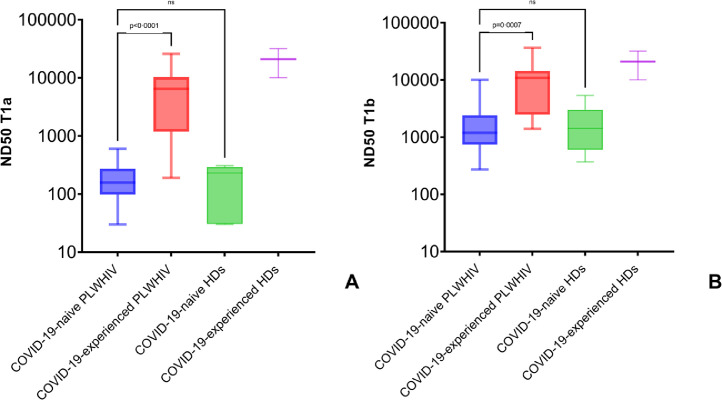
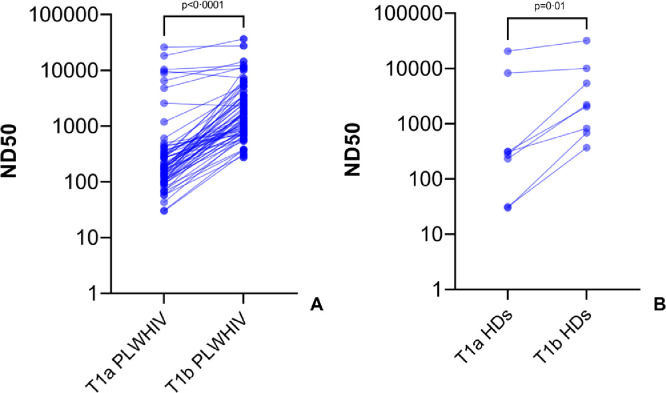
All (N=71) participants living with HIV as well as all (N=10) healthy donors included in our cohort showed neutralizing activity of antibody in sera. At T1a we observed that the activity was higher in COVID-19-experienced PLWHIV (median 6481 IQR [1188-10346]) than in COVID-19-naïve PLWHIV (p<0·0001, median 157 IQR [98-273]), but was not different to those observed in COVID-19-experienced HDs (p=0·09, median 1077 IQR [702-7551]) (Figure 2A). The same results were confirmed at T1b, where we observed a higher neutralising antibody activity in COVID-19-experienced PLWHIV (median 10888 IQR [2478-14416]) compared to COVID-19-naïve PLWHIV (p=0·0007, median 1192 IQR [742-2421]). Again, the ND50 calculated for COVID-19-experienced PLWHIV at T1b was comparable to the ND50 obtained in COVID-19-experienced HDs (p=0·99, median 20959 U/mL IQR [10060-31857]) (Figure 2B). As expected, the neutralising activity of antibodies elicited by vaccination increased between T1a and T1b in both PLWHIV (p<0·0001, median 182 [IQR 102-332] vs 1567 [IQR 789-3531]) and HDs (p=0·01, median 290 [IQR 81-6265] vs 2112 [IQR 719-8889]) (Figure 3A-3B). As with anti-S antibodies, only CD4+ T cell count groups and anti-N positive serology were associated with neutralising antibody activity (Table 3).
Figure 2.
Neutralising antibody activity elicited by vaccination with mRNA-1273 in people living with HIV.
Neutralising antibody activity (ND50) elicited by vaccination with mRNA-1273 in people living with HIV at time of second vaccine shot (A) and 28 days since completion of vaccination schedule (B). Comparison with healthy donors. Box and whiskers showing median values, 95% CIs and min to max. Statistics were calculated using Kruskal-Wallis test with Dunn's multiple comparisons test. Exact numbers of participants for each group are indicated in the corresponding results paragraph.
PLWHIV=people living with HIV; HDs=healthy donors.
Figure 3.
Increase in neutralising antibody activity during vaccination schedule with mRNA-1273.
Matched values of neutralising antibody activity elicited by vaccination with mRNA-1273 at time of second vaccine shot (T1a) and 28 days since completion of vaccination schedule (T1b) in people living with HIV (A) and HDs (B). Before-after graph with matched individuals, each point represents a single participant. Statistics calculated using Wilcoxon match signed-rank test.
PLWHIV=people living with HIV; HDs=healthy donors.
Table 3.
Association between clinical and serologic variables of PLWHIV and neutralising antibody activity (ND50) since the second dose of mRNA-1273 vaccine.
| Variable | Subjects (n) | Neutralising antibody activity (ND50) | p-value |
|---|---|---|---|
| Sex* | 0·50 | ||
| Male | 60 | 1513 (789-3531) | |
| Female | 11 | 1225 (700-2516) | |
| Age (years)° | 0·48 | ||
| <35 | 17 | 1608 (924-2897) | |
| 35-50 | 22 | 1752 (719-4291) | |
| 50-60 | 22 | 1041 (741-1609) | |
| >60 | 10 | 2492 (877-5228) | |
| Antiretroviral therapy° | 0·36 | ||
| NRTI | 65 | 1567 (769-3531) | |
| InSTI | 55 | 1419 (771-3437) | |
| NNRTI | 8 | 525 (130-5158) | |
| PI | 10 | 1154 (363-3335) | |
| Plasma HIV viral load§ | 71 | ||
| (slope; 95% CI) | (0·81; 0·38,1·71) | 0·57 | |
| CD4+ T cell count per µL° | 0·03 | ||
| <350 per µL | 6 | 1314 (606-2477) | |
| 350-500 per µL | 7 | 3329 (1905-10508) | |
| >500 per µL | 58 | 1227 (761-3032) | |
| CD4+ T cell count percentage§ | 71 | ||
| (slope; 95% CI) | (0·98; 0·96,1·00) | 0·11 | |
| CD4+ T/CD8 ratio >=1* | 0·94 | ||
| No | 39 | 1828 (807-5142) | |
| Yes | 32 | 1119 (756-1903) | |
| CD4+ T/CD8 ratio >=0·4* | 0·55 | ||
| No | 14 | 1588 (935-3561) | |
| Yes | 57 | 1250 (771-3437) | |
| Optimal immunologic response†,* | 0·07 | ||
| No | 42 | 1824 (826-5142) | |
| Yes | 29 | 1159 (745-1827) | |
| HCV-Ab* | 0·88 | ||
| Negative | 59 | 1567 (771-3437) | |
| Positive | 5 | 2516 (675-4291) | |
| HBsAg* | 0·32 | ||
| Negative | 62 | 1598 (771-3501) | |
| Positive | 2 | 861 (719-1004) | |
| Years since diagnosis§ | 71 | ||
| (slope; 95% CI) | (0·98; 0·96,1·01) | 0·23 | |
| (CD4+ T cell count per µL)[2] at nadir§ | 71 | ||
| (slope; 95% CI) | (0·99; 0·86,1·14) | 0·89 | |
| Plasma HIV viral load at zenith§ | 71 | ||
| (slope; 95% CI) | (1·09; 0·72,1·64) | 0·68 | |
| AIDS diagnosis* | 0·83 | ||
| No | 31 | 1567 (864-3437) | |
| Yes | 19 | 1393 (674-3501) | |
| CMV IgG* | 0·84 | ||
| Negative | 5 | 935 (741-2137) | |
| Positive | 38 | 1406 (719-3157) | |
| Anti-N* | <0·001 | ||
| Negative | 9 | 1192 (742-2421) | |
| Positive | 61 | 10888 (2478-14416) |
CI, confidence interval; NRTIs, nucleoside reverse transcriptase inhibitors; InSTIs, integrase strand transfer inhibitors; NNRTIs, non-nucleoside reverse transcriptase inhibitors; PIs, protease inhibitors; HCV, hepatitis C virus; HBsAg, hepatitis B surface antigen; CMV, cytomegalovirus; anti-N, anti-nucleocapsid protein.
Optimal immunologic response was defined as: CD4+ T/CD8 ratio≥1 plus CD4+ T≥500cells/µL plus CD4+ T%≥30%. Statistics calculated using
Mann–Whitney U test,
Kruskal-Wallis test or
Spearman's rank correlation coefficients.
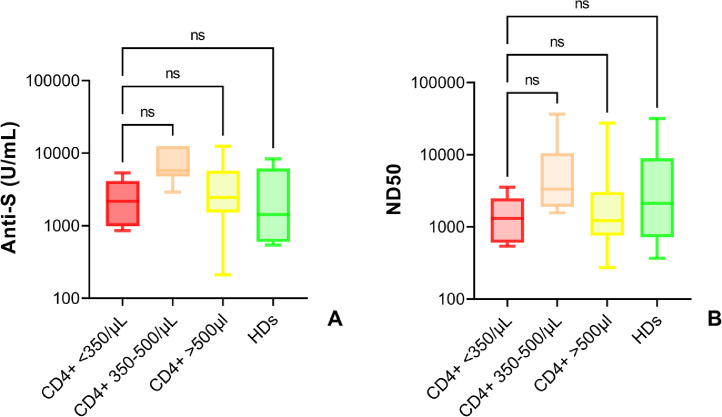
When stratified according to the CD4+ T cell count (<350 cells/μL, 350-500 cells/μL, >500 cells/μL) at the time of vaccination, we observed that this parameter did not influence vaccine-induced antibody response. Indeed, anti-S antibody titres in PLWHIV (6/71, median 2173 U/mL [IQR 987-4109]; 7/71, 5763 IU/mL [IQR 4801->12500]; 58/71, 2449 U/mL [IQR 1524-5704]) were not different to those observed among HDs (median 1425 U/mL [IQR 599-6131]) (Figure 4A). Similarly, the ND50 was also comparable in all the PLWHIV groups at T1b (6/71, median 1314 [IQR 606-2477]; 7/71, 3329 IU/mL [IQR 1905-10508]; 58/71, 1227 U/mL [IQR 761-3032]) as well as in HDs (median 2112 U/mL [IQR 719-8889]) (Figure 4B).
Figure 4.
Serologic responses elicited in people living with HIV stratified according to the CD4+ T cell count.
Anti-S antibodies (A) and neutralising antibody activity (B) 28 days after completion of vaccination schedule with mRNA-1273 in people living with HIV, stratified according to CD4+ T cell count (<350 cells/μL, 350-500 cells/μL, >500 cells/μL). Comparison with healthy donors. Box and whiskers showing median values, 95% CIs and min to max. Statistics calculated using Kruskal-Wallis test with Dunn's multiple comparisons test. Exact numbers of participants for each group are indicated in Table 2 and Table 3.
COI= Cut-Off Index; PLWHIV=people living with HIV; HDs=healthy donors.
Correlations between continuous variables are depicted in supplementary figure 1. Nadir CD4+ T cell count showed a positive correlation with current CD4+ T cell count, CD4+ T cell percentage and CD4+ T/CD8+ ratio at time of enrolment (rho: 0·59, 0·48 and 0·38, respectively). Moderate correlations were also highlighted between anti-N antibody titres and anti-S antibody titres and neutralising antibody activity at T1a and T1b (rho: 0·29, 0·36 and 0·36, respectively). Of note, a strong correlation between anti-S antibody titres and antibodies neutralizing activity both at T1a (slope: 2·8 activity times per ten-fold anti-S increase, 95%CI 1·9-4·3) and T1b (4·7, 95%CI 3·0-7·2) was observed (supplementary figure 2) among PLWHIV COVID-19-naïve.
Discussion
The results of our cohort study show that the vaccination with mRNA-1273 vaccine given 4 weeks apart produced equivalent immune responses, assessed in terms of anti-S antibody titres and neutralising antibody activity, in PLWHIV, who are well controlled on ART, with stable viral suppression and good CD4+ T cell, counts, compared with a similar adult population without HIV infection. Higher anti-S antibody titres and neutralising antibody activity are observed in PLWHIV with previous SARS-CoV-2 infection compared to those naïve for COVID-19. None of the demographic and clinical variables analysed, with the exception of a previous SARS-CoV-2 infection, had a significant impact on the humoral immunologic responses assessed in our study.
Our data agree with those provided by Frater and colleagues,9 that highlighted how PLWHIV who are on effective ART with suppressed viral loads and high CD4+ T cell counts (>350 cells per μL) do not have diminished humoral responses to the ChAdOx1 nCoV-19 prime-boost vaccine. In the case of our cohort, no differences were seen also in PLWHIV with CD4+ T cell count below 350 cells per μL, even though the sample size of this subgroup was limited. Notably, our study is the first where PLWHIV receiving the COVID-19 vaccine were stratified for the CD4+ T cell count at the time of vaccination. Works focusing on the response to mRNA-based COVID-19-vaccines among PLWHIV are provided in two Americans and one Israeli cohorts. In the first one, 12 PLWHIV were vaccinated with BNT162b2, showing values of anti-S antibodies, neutralising antibody activity and cellular immune responses, comparable to those observed in healthy donors.10 Of note, 11 of 12 PLWHIV in this study were African American, and none had a previous SARS-CoV-2 infection. In the second one, five PLWHIV received BNT162b2 and nine mRNA-1273, but only anti-S antibody titres were assessed, with values comparable to those reported in the HIV-uninfected population.12 In the third one, 143 PLWHIV were vaccinated with BNT162b2, with anti-S antibodies and neutralising antibodies detected in 98% and 97% of participants, respectively.11 Of note, only three patients in this study had a current CD4+ T cell count <200 cells per µL, but all of them developed high levels of anti-spike antibodies and neutralising antibodies in response to immunization. Moreover, the timeframe of blood sampling was not homogenous. Indeed, anti-S antibodies titre was assessed on average 18 days after the second dose, whereas neutralising antibody activity was ascertained on average 26 days after the second dose. In our study, serology and neutralization assays were performed the same day after vaccination, thus giving a precise description of the neutralizing activity of the determined antibody titre.
To understand the immunization results obtained in our cohort, immunologic determinants to SARS-CoV-2 infection in PLWHIV should be considered. Alrubayyi et al., in their preprint, characterized the humoral and SARS-CoV-2-specific T cell responses in this population, showing that the overall magnitude of SARS-CoV-2-specific T cell responses was associated with the size of the naïve CD4+ T cell pool and the CD4+/CD8+ T cell ratio.15 Instead, the magnitude of cellular immune responses only showed a weak correlation with anti-N antibody titres but not with anti-S antibody titres. In our cohort the majority of patients have an optimal immunologic response and a high CD4+ T/CD8+ ratio, therefore adequate SARS-CoV-2-specific T cell responses are expected and are currently under evaluation. It remains to be verified whether in the setting of vaccination, without the immunologic perturbances caused by COVID-19, humoral and cellular immune responses correlate to each other.
It is worthy of note the fact that all the PLWHIV enrolled in our study achieved detectable humoral responses. It is possible to speculate that this result could be related to the viral suppression achieved in almost all patients. Indeed, several studies have highlighted how B cells taken from patients with high levels of HIV plasma viremia are defective in their proliferative responses to various stimuli and HIV-associated premature exhaustion of B cells may contribute to poor antibody responses in infected individuals.16,17 Furthermore, early initiation of ART in PLWHIV has been associated with a better functional profile in memory B-cell responses to HIV and non-HIV antigens and control of HIV viremia has been linked with the normalization of activated B cell subsets, which allows age-dependent accumulation of resting memory B cells.8,18
The greatest limitation of our study is represented by the small sample size and the limited number of severely immunocompromised PLWHIV. Indeed, only six participants have less than 350 CD4+ T cells per μL, with the lowest count per μL recorded being 180, and the majority have achieved an OIR (optimal immunologic response, CD4+ T/CD8 ratio≥1 plus CD4+ T≥500cells/µL plus CD4+ T%≥30%) (54/71, 80·4%). Nonetheless, we did not find a lower response in the <350 cells/ μL group nor a monotonic trend across CD4+ T cell groups. Moreover, our cohort reflects the characteristics of the majority of HIV outpatients in Italy: receiving ART, with effective viro-suppression and good CD4+ T cell values, thus being a realistic benchmark for real-life practice. Two other great limits of our study are the absence of a concomitant analysis of the cellular immune responses and the short follow-up. We are therefore working on a follow-up study assessing both humoral and cellular immune response at 3, 6 and 12 months after vaccination. Lack of individuals with un-suppressed HIV replication in our cohort prevents us from evaluating the effect of HIV uncontrolled infection on vaccine response. However, our data demonstrate that HIV infection per se does not limit the initial antibody response to the mRNA-1273 vaccine. Numerous studies have shown the effect of ART on vaccine-induced immunity against other pathogens (e.g., measles, polio and rabies) in HIV-infected patients. Similarly, the use of effective ART could explain the observed similarities between PLWH and HD individuals in our study. Finally, we did not assess the impact of vaccination on CD4+ T cell count and HIV viral load. A previous study by Levy et al. found, in their cohort of PLWHIV vaccinated with BNT162b2, a decrease in CD4+ T cell count between baseline levels and those measured following the first and second vaccine administration, as well as four months after the second shot.11 Of note, in one patient enrolled in the study with stable viral suppression and adequate drug plasma values we observed a transient increase in HIV viral load,19 suggesting that as already described in the literature with other vaccines,20,21 immunization in PLWHIV can induce transient increases in HIV-RNA.
Acknowledging the small sample size, the findings of our observations do not support dose or schedule adjustment in the vaccination of PLWHIV on ART with a well-controlled disease (stable undetectable viremia). We cannot exclude that lower immune responses to vaccination can be found in PLWHIV with very low CD4+ T cell count (e.g., below 200 cells per μL), as observed for other vaccines.22,23 It is reassuring that, at least in our observation time, CD4+ T cell count did not affect the antibody response to vaccination. Moreover, the higher anti-S antibody titres and neutralising antibody activity observed among COVID-19-experienced PLWHIV, suggest that also in this population vaccination can boost the immunity obtained through natural infection. The study was not planned to assess vaccine efficacy in PLWHIV, nonetheless, the immune responses observed are comparable to those highlighted in people without HIV infection, where mRNA-1273 has shown high efficacy in preventing COVID-19 and thus we could speculate an equal efficacy in preventing COVID-19 among PLWHIV. Nonetheless, specific studies designed to verify efficacy among PLWHIV are needed to confirm this assumption.
Several pieces of evidence suggest that duration of seroprotection after vaccination is shorter in PLWHIV compared to uninfected individuals.24 Future research should aim to assess the kinetics of humoral response, to compare it with those displayed by individuals without HIV infection and verify the need for additional vaccine shots, and inquiry the cellular arm of the immune system. Moreover, further data should be provided regarding the immune response in the subset of PLWHIV with severe depletion of CD4+ T cell (e.g., below 200 cells per μL), which are also those who may benefit the most from vaccination, and those with uncontrolled viral infection (i.e., those viremic). Finally, the impact of COVID-19 vaccination on HIV viral load and CD4+ T cell count in patients with stable viral suppression should be verified.
Contributors
AL, GB, AB, AG, RDF and LM were involved in conceptualisation, data curation, supervision, method design, writing, and reviewing the manuscript. AG, AB, RDF and LM were involved in funding acquisition. AL, GB, AB, SL, AM and MM enrolled participants and collected demographic and clinical variables. FC, MO and PB performed serologic analyses. ML provided the construct for pseudoparticles realization. GB, LD, MC, SS, GG, ES, RG, RDF and LM performed neutralisation analyses and reviewed the results, AL and DC performed statistical analyses. AL, DC, AB and LM had access to and verified the underlying study data. All authors critically reviewed and approved the final version. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Declaration of interests
All other authors declare no competing interests.
Acknowledgments
Data sharing
Anonymised participant-level data will be made available, upon requests directed to the corresponding author. Proposals will be reviewed and approved by the competing Ethical Committee, and investigators, on the basis of scientific merit. After approval of a proposal, data can be shared through a secure online platform after signing a data access agreement.
Acknowledgements
The authors are grateful to Simone Villa for the help provided in data analyses and to the data study coordinators (Rosaria Bianco, Valeria Pastore, Valentina Ferroni) and nurses (Claudio Colella, Alessandra Beltrami, Virginia Caccialanza, Eliana Chitani) involved in data collection and blood samples collection. We thank the volunteers who participated in this study.
Funding
This study was partially supported by Italian Ministry of Health Ricerca Corrente 2021 and Grant Ricerca Finalizzata GR 2018-12365699, by Intesa San Paolo COVID-19 emergency 2020 funds, and by Fondazione Cariplo (INNATE-CoV).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2021.100287.
Appendix. Supplementary materials
References
- 1.WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data. https://covid19.who.int/(accessed Aug 13, 2021).
- 2.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lombardi A, Bozzi G, Ungaro R, et al. Mini Review Immunological Consequences of Immunization With COVID-19 mRNA Vaccines: Preliminary Results. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.657711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambrosioni J, Blanco JL, Reyes-Urueña JM, et al. Overview of SARS-CoV-2 infection in adults living with HIV. Lancet HIV. 2021;8:e294–e305. doi: 10.1016/S2352-3018(21)00070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EACS Statement 15 January 2021 | EACSociety. https://www.eacsociety.org/home/eacs-statement-15-january-2021/(accessed Aug 13, 2021).
- 7.Tebas P, Frank I, Lewis M, et al. Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS. 2010;24:2187–2192. doi: 10.1097/QAD.0b013e32833c6d5c. [DOI] [PubMed] [Google Scholar]
- 8.Muema DM, Macharia GN, Hassan AS, et al. Control of Viremia Enables Acquisition of Resting Memory B Cells with Age and Normalization of Activated B Cell Phenotypes in HIV-Infected Children. J Immunol. 2015;195:1082–1091. doi: 10.4049/jimmunol.1500491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frater J, Ewer KJ, Ogbe A, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV. 2021;19:1–12. doi: 10.1016/S2352-3018(21)00103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woldemeskel BA, Karaba AH, Garliss CC, et al. The BNT162b2 mRNA Vaccine Elicits Robust Humoral and Cellular Immune Responses in People Living with HIV. Clin Infect Dis. 2021:1–14. doi: 10.1093/cid/ciab648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy I, Wieder – Finesod A, Litchevsky V, et al. Immunogenicity and safety of the BNT162b2 mRNA Covid-19 vaccine in people living with HIV-1. Clin Microbiol Infect. 2021 doi: 10.1016/j.cmi.2021.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruddy JA, Boyarsky BJ, Bailey JR, et al. Safety and antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in persons with HIV. AIDS. 2021 doi: 10.1097/QAD.0000000000003017. published online July 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Notarbartolo S, Ranzani V, Bandera A, et al. Integrated longitudinal immunophenotypic, transcriptional, and repertoire analyses delineate immune responses in patients with COVID-19. Sci Immunol. 2021;6:eabg5021. doi: 10.1126/sciimmunol.abg5021. [DOI] [PubMed] [Google Scholar]
- 14.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alrubayyi A, Gea-Mallorquí E, Touizer E, et al. Characterization of humoral and SARS-CoV-2 specific T cell responses in people living with HIV. bioRxiv Prepr Serv Biol. 2021 doi: 10.1101/2021.02.15.431215. published online Feb 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moir S, Ho J, Malaspina A, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J Exp Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moir S, Malaspina A, Ogwaro KM, et al. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc Natl Acad Sci U S A. 2001;98:10362–10367. doi: 10.1073/pnas.181347898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moir S, Buckner CM, Ho J, et al. B cells in early and chronic HIV infection: Evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood. 2010;116:5571–5579. doi: 10.1182/blood-2010-05-285528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozzi G, Lombardi A, Ludovisi S, et al. Transient increase in plasma HIV RNA after COVID-19 vaccination with mRNA-1272. Int J Infect Dis. 2021;113:125–126. doi: 10.1016/j.ijid.2021.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Günthard HF, Wong JK, Spina CA, et al. Effect of influenza vaccination on viral replication and immune response in persons infected with human immunodeficiency virus receiving potent antiretroviral therapy. J Infect Dis. 2000;181:522–531. doi: 10.1086/315260. [DOI] [PubMed] [Google Scholar]
- 21.Kolber MA, Gabr AH, De la Rosa A, et al. Genotypic analysis of plasma HIV-1 RNA after influenza vaccination of patients with previously undetectable viral loads. Aids. 2002;16:537–542. doi: 10.1097/00002030-200203080-00004. [DOI] [PubMed] [Google Scholar]
- 22.Kojic EM, Kang M, Cespedes MS, et al. Immunogenicity and Safety of the Quadrivalent Human Papillomavirus Vaccine in HIV-1-Infected Women. Clin Infect Dis. 2014;59:127–135. doi: 10.1093/cid/ciu238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vardinon N, Handsher R, Burke M, Zacut V, Yust I. Poliovirus Vaccination Responses in HIV-Infected Patients: Correlation with T4 Cell Counts. J Infect Dis. 1990;162:238–241. doi: 10.1093/infdis/162.1.238. [DOI] [PubMed] [Google Scholar]
- 24.Kerneis S, Launay O, Turbelin C, Batteux F, Hanslik T, Boelle P-Y. Long-term Immune Responses to Vaccination in HIV-Infected Patients: A Systematic Review and Meta-Analysis. Clin Infect Dis. 2014;58:1130–1139. doi: 10.1093/cid/cit937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.