Abstract
There is emergent need for in vitro models which are physiologically correct, easy to reproduce, and mimic characteristic functionalities of desired tissue, organ, or diseases state for ophthalmic drug screening, as well as disease modeling. To date, a variety of in vitro models have been developed for the applications ranging from 2D cell culture-based monolayers, multilayer, or co-culture models, to 3-dimensional (3D) organoids, 3D printed and organ on chip systems. Each model has its own pros and cons. While simple models are easier to create, and faster to reproduce, they lack recapitulation of the complex framework, functionalities, and properties of tissues or their subunits. Recent advancements in technologies and integration with tissue engineering and involvement of microfluidic systems have offered novel platforms which can better mimic the in vivo microenvironment, thus possessing potential in transformation of ophthalmic drug development. In this review we summarize existing in vitro ocular models while discussing applicability, drawbacks associated with them, and possible future applications.
Keywords: cell culture, ocular, alternative models, stem cell, organoids, 3D printing, organ on a chip, corneal, retinal
I. INTRODUCTION
The human eyeball is a complex organ having specialized compartments with distinct functions and physiology. It has an anterior segment and a posterior segment. The anterior cavity/segment extends from cornea to lens, comprising conjunctiva, iris, aqueous humor, and ciliary body, and the posterior segment/cavity extends from back portion of lens to the retina (Fig. 1a).1 There are various ocular barriers in the anterior and posterior cavities of human eye which control and regulate transport of fluids solutes, as well as administered drugs/formulations. Anterior static barriers (tight junctions present in corneal epithelial layers), dynamic barriers (drainage, induced lacrimation, tear film, conjunctival blood, and blinking) and metabolic enzymes (esterases, ketone reductases, peptidases, and CYP-450)2 are involved in preventing topically administered therapeutics from reaching the site of action, which leads to reduced bioavailability. Additionally, the posterior segment impedes drug delivery by efflux pumps (present on the membranes of retinal cells), static barriers such as sclera, blood capillary endothelial cells, retinal pigment epithelium (RPE), and other barriers which are dynamic (i.e., blood and lymph circulation).3
FIG. 1:
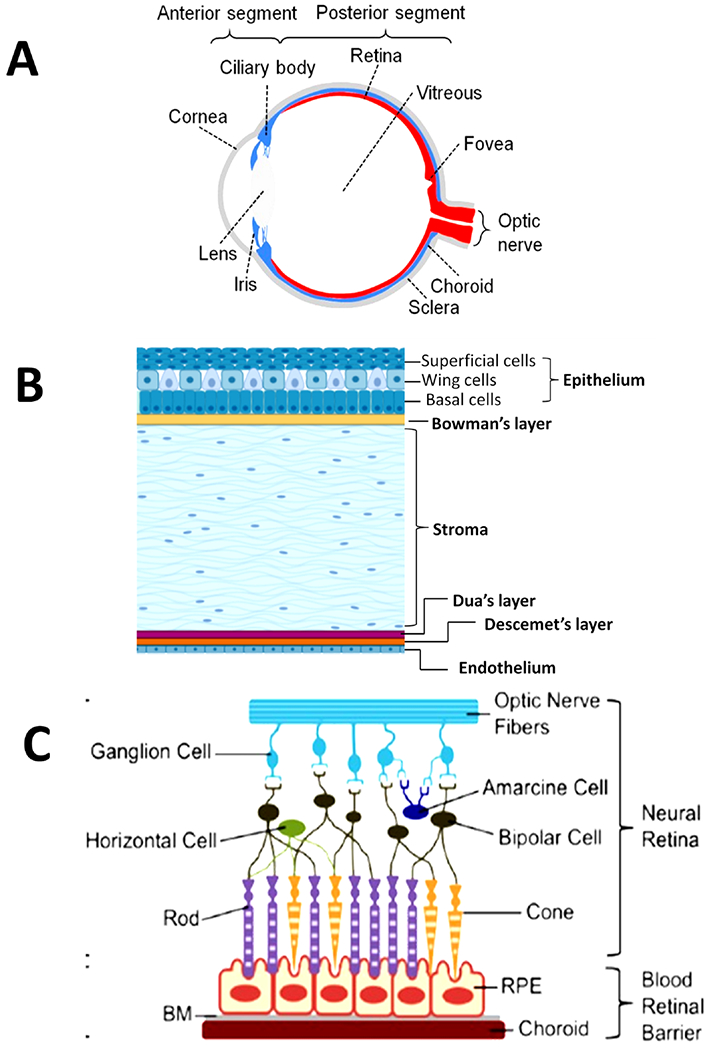
Schematic diagram of the human eye showing its anatomy. (a) Whole eye showing anterior and posterior segments (reprinted from Chuang et al. with permission from Yale Journal of Biology and Medicine, copyright 2017). (b) Cornea with 6 layers. (c) Distribution of various types of cells in retina and blood retinal barrier (Figure reproduced from White et al. under a Creative Commons license).
In 1940, the FDA approved in vivo Draize eye testing for acute ocular toxicity studies. In the Draize test, 0.1 mL/0.1 g solid examination substance was applied on one eye (cornea and conjunctival sac) of conscious New Zealand rabbit (at least 6 rabbits/test) for about 72 h while the other untreated eye served as control. The animals were observed for degree of irritation on the cornea, conjunctiva, and iris. This allowed the classification of the chemicals into categories ranging from nonirritant to highly irritant.4 However, owing to structural, physiological, and biochemical differences in the anatomy of the human and rabbit eyes, various differences in sensitivity to irritants were observed. Corneas of humans are thicker, have higher tear production, higher blinking rate, as well as ocular surface sensitivity in comparison to rabbits.5 Further, rabbits have a nictitating membrane and larger conjunctival sacs assisting the elimination of administered drugs from the surface of eye. Therefore, various modifications were made to the draize testing method.6 Besides rabbits, several other animals such as monkeys, rats, mouse, and zebra fish have also been used while developing ocular disease models, understanding molecular mechanisms, and testing formulations.7–10 Nevertheless, in recent years, ethical, business related such as time and cost, scientific concerns (i.e., reproducibility) and legal issues associated with animals have demanded alternative in vitro protocols. Recently, to reduce the experimental usage of live animals, ex vivo organotypic models were developed. Ex vivo studies involving ocular tissues isolated from various animals such as bovine, porcine, rabbits, and chicken have been reported in the literature.11–14 These models involved the use of enucleated eyes while the isolated organ maintained its biochemical and physiological functions. These models generally combine the quantitative measurements of permeability of test substances across the cornea with histological analysis.4 However, in vitro models were always warranted as they offered advantages of being cost effective, reproducible, and easy to design. In this article we discuss the existing in vitro ocular models designed for ocular disease modeling, drug development, and delivery purposes.
II. MORPHOLOGY OF HUMAN EYE
A. Cornea
The avascular and transparent cornea is the outermost part of eyeball and protects intraocular tissues by serving as barrier to chemical and mechanical insults. It is comprised of 6 layers (corneal epithelium, Bowman’s layer, stroma, Dua’s layer, Descemet layer, and endothelium) as shown in Fig. 1b, with an average horizontal diameter, vertical diameter and radius of curvature of 11.5 mm, 10.5 mm, and 7.7 mm, respectively.3,15 Corneal epithelium is the outermost layer beneath which lies a tough acellular collagen layer, Bowman’s layer. The stroma comprises of 90% of corneal thickness and provides strength and transparency to the cornea due to specific arrangement of its 200–250 collagenous lamellae, crystalline expressing keratocytes and proteoglycans throughout the corneal surface.16 The innermost layer of the cornea is comprised of polygonal and flattened cells known as endothelium. It is separated from stroma by two layers: Type VI collagen-rich pre-Descemet layer/Dua’s layer and 5–15 μm thick Descemet’s membrane. The endothelium plays a crucial role in protecting the cornea from swelling, as well as maintaining regular arrangement of collagen matrix by extracting water from the stroma.5
B. Sclera and Conjunctiva
The sclera is a tough, poorly vascularized, fibrous, and white exterior coating of the eye. It is about 670 ± 80 μm thick, protective in nature and acts as a noncorneal route of absorption of drug molecules.17 The conjunctiva is the thin clear membrane, with average thickness of 195 ± 32 μm, covering front and inner surface of the eye and the eyelids.18 The primary function of conjunctiva is to keep the front surface of eye and inner surface of eyelids lubricated and moistened. It also serves as protective barrier and helps in adherence of tear film. It is permeable to many drugs, has many blood vessels, and plays an important role in noncorneal absorption of large, hydrophilic drug molecules (poorly absorbed by corneal route).19
C. Retina
The retina is about 250 μm thick, innermost layer of the eye, and surrounds the vitreous region. Retina receives light focused by the lens, converts it to neuronal signals, and sends signals for visual recognition to the brain. It is comprised of photoreceptors (rods, cones), neurons (bipolar, ganglion, amacrine, horizontal cells) and interplexiform neurons (Fig. 1c).20 Light sensitive photoreceptor cells (rods and cones) perform phototransduction to transform light photons into a neuronal signal. This signal is then transferred to bipolar cells and to ganglion cells via synapse, and finally transmitted from the eye to the brain. Other retinal cells (e.g., horizontal cells, amacrine cells) are accountable for lateral interactions, modification integration, and transmission of signal from photoreceptors towards ganglion cells.21 There are 3 layers in blood–retinal barrier (BRB): the retinal pigmental epithelium (RPE) with its tight junctions, Bruch’s membrane (BM) in the middle, and the innermost layer of vascular endothelia (retinal capillary endothelium).20,22 The RPE controls the diffusion of drugs, transport of nutrients as well as solutes, from the choroid towards the retina. It is also responsible for phagocytosis of retinal waste, produced along with support to photoreceptors. The BRB has a very crucial role maintaining homeostasis of retina.23
III. IN VITRO CORNEA MODELS
Transcorneal absorption is the major route for absorption during topical application. Corneal epithelium acts as arate limiting barrier causing about 60% of total trans-epithelial electrical resistance (TEER).3 Hence, corneal models are widely applicable in permeation studies, absorption studies, and ocular toxicity assays of various drugs. Cell lines are very important in vitro systems having wide application in medical research, with a in preclinical studies, cancer research,24–26 formulation testing,27–30 permeation studies,31 and drug discovery. Several cell culture-based corneal models involving monolayers of corneal epithelial cells, multilayers (stratified epithelium), co-cultures (epithelial cells and stromal cells), corneal tissues, and three-dimensional (3D) corneal equivalents have been reported.3
A. Corneal Epithelial Models
1. Cell-Based Models
Primary cultures of human corneal epithelial cells (HCE) have been used to investigate cytotoxicity of commonly used preservatives (e.g., benzalkonium chloride, chlorobutanol in ophthalmic formulations).32 Tripathi et al. studied effects of hydrogen peroxide exposure on HCE cells.33,34 Scott et al. discussed the potential of epithelial models in identifying non-irritating substances from mild irritants.35 Han et al. used HCE cells suspended in cross-linked fibrin/fibrinogen gel to create bioengineered ocular surface for potential transplantation/replacement tissue.36 Ramesh et al. discussed various steps that brought expansion of epithelial stem cells obtained from limbal biopsy and their transplantation into diseased cornea for corneal surface reconstruction.37 Various researchers have used an HCE cell-based model for evaluating various types of ophthalmic formulations. Hakkarainen et al. demonstrated acute cytotoxic effects of excipients like benzalkonium chloride (BAK), polysorbate 80 (PS80), and macrogolglycerol hydroxystearate (MGHS40) and various marketed ocular formulations (containing BAK, PS80, and MGHS40) on transformed HCE cells and established that HCE cells could efficiently serve as a model to investigate cytotoxicity of pharmaceuticals. They observed no acute cytotoxicity by formulations containing excipients like MGSH40 and PS80 however, concentration and time dependent toxicity in BAK preserved formulations.38 Chuanlong et al., developed cyclosporin A nanomicelles and performed in vitro toxicity and uptake studies, and investigated the mechanism of internalization of nanoparticles in the HCE cells.39 Scheurer et al. used corneal limbal epithelial cells and human conjunctival cells (HCJC) to investigate the toxicity of various buffers used in ophthalmic formulations.40 They incubated cells with various concentrations of borate, citrate, phosphate, and tris-HCL buffers for different durations. They observed that HCJE cells were more sensitive to higher concentrations of buffers as compared to HCE. However, they observed time-dependent decreases in viability of both the cell types on exposure to higher concentration of buffers.40 Ragaranjan et al. used transformed human HCE (HCE-T) models to determine the protective effects of artificial tear formulations on metabolic activities of cells after exposure to desiccation. During their studies they exposed cells to the formulations for 30 minutes, followed by desiccation for 5 minutes at 37°C and 45% relative humidity. They then observed cellular metabolic activities of the cells before and after desiccation stress and evaluated desiccation protection potential of the eye drop formulations.41 Bucolo et al. exposed rabbit corneal epithelial cells to 1 mM H2O2 to induce oxidative stress followed by treatment with taurine and sodium hyaluronate to determine osmoprotective and antioxidant effects of taurine.42 While monolayer cultures offered advantages of faster testing, they did not completely model the corneal epithelium. Hoffman et al. demonstrated importance of selecting suitable model while performing toxicity studies. They observed false positive endpoints in their in vitro studies while using HCE-T cells monolayer cultures. On evaluating same formulations during in vivo studies and multilayered cultures of transformed HCE cells, no adverse effects on cell proliferation and viability were observed.43 Stratified epithelium was developed by growing human corneal limbal cells on acellular amniotic membrane (AM) by air lifting techniques which promoted barrier functions by reducing intercellular spaces in superficial cells.44 Nagai et al. tested corneal toxicity of dexamethasone nano-dispersions on (HCE-T) cells.45 Primary cell line-based models were easy to develop with applications ranging from permeability, transport studies to toxicity, biology as well as drug/gene delivery studies. However, there were limitations such as low availability of cornea donors and inability to represent the whole corneal barriers.
2. Corneal Equivalents
Various researchers have developed corneal equivalents for drug screening (permeation/efficacy). Alaminos et al. developed a complete biological substitute for rabbit cornea by tissue engineering. They seeded endothelial cells on the base of porous inserts followed by culturing keratocytes entrapped in fibrin, agarose-based gel, and corneal epithelial cells on the scaffold. They observed stratification in epithelial layer (air lifting technique), proliferation in stromal keratocytes and pattern in endothelial layer.46 Xiaodong et al. used YIGSR-modified dendrimers as cross linkers in collagen gels to promote adhesion and proliferation of HCE in tissue engineering.47 Another successful attempt to reconstruct complete cornea was performed by Griffith et al.48 They created a complete corneal equivalent using immortalized HCE, human stromal and human endothelial cells. The tissue matrix comprised of collagen-chondroitin sulfate cross-linked using glutaraldehyde (0.02–0.04%). The corneal equivalent was created by sandwiching stromal cells (mixed with the substrate) between layers of epithelial cells at top and endothelial at the bottom. The corneal equivalents were observed to be comparable to human corneas in physical (morphology, transparency) and physiological functions (marker and gene expression, ion and fluid transport). Reichl et al. engineered organotypic corneal equivalent consisting all the 3 layers and demonstrated its applicability as in vitro model for prediction of drug absorption/permeability through human corneas. Corneal constructs were created on collagen- coated polycarbonate filters by seeding immortalized endothelial cells. Corneal fibroblasts were then casted on confluent endothelial layer, followed by seeding of epithelial cells on the top. The culture were maintained for 3 weeks and were tested for trans-corneal permeation of pilocarpine, befunolol, and hydrocortisone49,50 and data were compared to porcine eye (excised porcine cornea, porcine cornea construct). Similarly, several other researchers designed corneal equivalents using human,51 bovine,52,53 rabbit54 or porcine cells55 and demonstrated their potential in corneal wound healing,56 transplantation, and drug screening.57 The ability to reconstruct stratified multilayered epithelium embarked the usage of corneal equivalents as interesting tools in drug permeation and toxicology studies. Apparently, these multilayered, multicellular in vitro models could not account for various factors such as tear turn over, blinking rate, tear film, and aqueous humor, thus lacking the complexity of the native organ.
There are various commercially available human corneal equivalents. EpiOcular from MatTek used human epidermal keratocytes (source: neonatal human foreskin) cultured on polycarbonate membrane. This model is widely used as substitute to Draize test in testing for irritants in ocular drug development.58,59 Similarly, other corneal equivalents models were developed (Clonetics by Lonza and Skinethic HCE by Episkin). Skinethic HCE was in vitro testing model based on transformed human corneal keratinocytes and was obtained by culturing cells on inert polycarbonate filter in an air–liquid interface. This model was structurally and morphologically similar to human corneal tissue, possessed stratified epithelium, was validated for solid and liquid chemicals testing by various laboratories.60 A Clonetics model was also functional for assessing corneal penetration, transepithelial permeability, and hydrolysis studies of various ocular drugs.61 Furthermore, MatTek developed another in vitro organotypic model called EpiCorneal which was based on primary human corneal epithelial cells. The model had highly differentiated multilayered epithelium containing tight junctions possessing barrier properties comparable to that of native human cornea. The model had applicability in testing permeability and safety of various pharmaceutical drugs.62 Although these in vitro cell-based models are less complex, have applicability in permeation and toxicity evaluation, they have shortcomings (e.g., variability in permeation data depending upon the species used and lack of arrangement for hormonal, neural and immune influences, as well as factors like tear fluid, blinking and aqueous humor) leading to need for more realistic models.
B. Retinal Models
1. Retinal Epithelium Models
Various animal-based primary retinal epithelial cell models have been reported.63–66 However, these models are associated with species-related variations, hence use of human retinal epithelium models was encouraged. Cultures of human retinal pigmental epithelial (RPE) cells have been developed to study bi-direction transport of glutathione,67 uptake of N5-methyltetrahydrofolate,68 and transport of lactate and protons.69 In vitro cultures of RPE have also been investigated to determine transport of verapamil. It was reported that organic cations (e.g., quindine, quinacrine, and pyrilamine) reduce uptake, while cationic drugs like diltiazem, propranolol, and timolol inhibit uptake of verapamil.70 To overcome the limited supply of human donor eye cells, various immortalized/secondary cell lines with epithelial characterization, fast differentiation properties, and barrier functions were established and utilized by various researchers.71–74 Hellinen et al. generated pigmented retinal cell model by re-pigmenting human ARPE-19 cell lines (regular non-pigmented continuosly growing cell lines).75 They incubated melanosomes from porcine RPE with ARPE-19, leading to internalization of melanosomes. Melanosome uptake of various drugs was analyzed. They observed lowered uptake in diclofenac and methotrexate (low melanin-binders) and higher uptake in chloroquin and propranolol (high melanin-binders). It was reported that pigmentation models have potential to study different retinal conditions, toxicology, and drug development.75 Bennis et al. established human stem cell-derived RPE cell models (hESC-RPE) and reported that hESC-RPE possess RPE specific morphology, molecular characterization, and expression of pathways (leading to stabilized epithelial morphology) while lacking expression for photoreceptor cells and protection from oxidative stress.76 Researchers used ARPE-19 cell lines during in vitro studies to evaluate effects of vascular endothelial growth factors (VEGF) inhibitor based formulations (e.g., thermo-reversible gel of Sunitinib nanoparticles)77 and poly(lactic co-glycolic acid)-based nanoparticles of Axitinib38 for age-related macular degeneration (AMD). Cell-based assays such as cytotoxicity, scratch assay, and cell uptake were used to evaluate safety and efficacy of sunitinib nanoparticles-based gels. Hellinen et al. investigated permeability of various drugs (having lipophilicities/logD7.4 values varying from −5.1 to 1.92 across monolayers of several RPE immortalized cell lines such as ARPE19, ARPE19mel (re-pigmented ARPE19), and LEPI (derived from ARPE-19); primary cell lines (hfRPE) and hESC-RPE cells. They observed comparable resistance to drug efflux in hESC-RPE, LEPI cells, and bovine RPE-choroid, thus considered these cell lines models as helpful tools in ocular drug development.78 Spencer et al. co-cultured human RPE cells with endothelial cells for 4 weeks to observe the interactions between these two cell types responsible for maintaining homeostasis. They observed that co-culture enhanced phagocytic activity of RPE cells, expression of anti-angiogenic receptors,s and growth factors in both types of cells.79 Cai et al. designed co-culture model of RPE and human Bruch’s membrane to demonstrate the crosstalk between them and understand effects of structural changes in the matrix on attachment, proliferation behavior of RPE cells.80 As RPE constitutes outer BRB, it controls the transport of nutrients across the choroid; hence Palanisamy et al.,81 established co-culture models of RPE/Buch’s membrane and choroid layers. They investigated effects of VEGF and anti-VEGF bevacizumab on the models, thus demonstrating the potential of such models in identifying and screening of various small molecules for drug delivery and toxicity analysis.81 RPE models have been applicable in various toxicity studies, drug screening, permeability studies, and disease modeling. Associated limitations were restricted supply of primary donors, changes in morphology, characteristics of cells under variable isolation protocols, and culture conditions which affect their barrier functions.82
2. Retinal Endothelial Models
In some severe conditions such as AMD, chronic retinal diseases, and diabetic retinopathy, breakdown of inner BRB occurs, leading to edema and vision loss. Hence, various retinal capillary endothelial (RCE) models were developed to understand the mechanistic pathway of diseases and factors affecting permeability of therapeutic molecules across retinal endothelium. Gillies et al. cultured primary bovine RCE cells on coated (fibronectin, laminin and collagen IV) polycarbonate filters and compared permeability of 3H-inulin by retinal cells vs. bovine aortic endothelial cells and bovine fibroblasts.83 In another study, they used bovine RCE monolayer model to determine how glucose level influences the permeability.84 Tretiach et al. developed a co-culture model of Müller cells and bovine RCE to understand interactions between these two cell types.85 Hayato et al. designed in vitro model with Müller (TR-MUL5) cell lines and immortalized rat RCE cells (TR-iBRB2). They reported that Müller cells play role in retinal angiogenesis as they modulated various genes in TR-iBRB2 functioning, for example, anti-angiogenic (plasminogen activator inhibitor1-PAI-1) induction and downregulation of pro-angiogenic (inhibitor of DNA binding 2-Id2).86 Shen et al. investigated apparent permeability coefficient of various drugs across TR-iBRB cells maintained on membranes coated with fibronectin. They also observed expression of P-glycoprotein and glucose transporter GLUT1 on TR-iBRB2 cells.87 Apparently for deeper understanding of barrier characteristics of RCE cells, establishment of co-culture models and in vitro – in vivo correlations were needed for application of cell-based model in high-throughput screening of the drug candidates. Churm et al. established a triple culture model to elucidate the mechanism of diseases because monoculture or dual cultures lacked the true representation of microenvironment of the native tissue.88 They grew RCE cells (HRMVEC/ACBRI181) RPE cells (ARPE-19) and Müller glial cells for 5 days and observed that upregulation of PEDF and downregulation of VEGF due to increase in expression of tight junction protein (TJP1). REC cells are microvascular cells with retina barrier specific properties and preferred over macrovascular cells originating from umbilical vein and arterial tissues in assessing screening as well as permeability potential of drugs. In BRB, REC are major physical barrier but other cells such as pericytes, glial cells have significant role in maintenance of BRB hence for better understanding of BRB physiology and functions, co-culture models involving different types of retinal cells would be warranted.
C. Stem Cells Based Models
1. Stem Cell-Based Corneal Model
Pluripotent stem cells can be derived from human somatic cells, can proliferate indefinitely (while maintained in undifferentiated state), or differentiate into several cell types.89 Hence, they are considered suitable for transplantation and corneal disease modeling. According to their differentiation potential, stem cells have been categorized into embryonic stem cells (ESCs) or human induced pluripotent stem cells (hiPSCs), although both ESCs and mesenchymal stem cells (MSCs) are able to maintaini undifferentiated and differentiated states depending upon the specific conditions provided to them. However, ESC and MSC are not widely used due to various challenges (e.g., ethical debates, heterogeneity, limited source, immune rejection).90 On the other hand, hiPSCs have self-renewing and differentiation capabilities like ESC and can be differentiated from patients’ cells as well. This further gives advantage to overcome immune rejection and ethical issues.91 Various studies described generation of mini-corneal 3D organoids from hiPSCs. These 3D organoids, which ranged from 1 to 7 mm diameter in size, recapitulate the early developmental events in vitro and reproduce morphological, anatomical, and phenotypic features similar to human corneas.92 Foster et al. developed corneal organoids from hiPSCs through several differentiation rounds and observed that the organoid model had 3 distinct layers; epithelium, stroma, and endothelium, expressing characteristics markers of each layer along with extracellular matrix collagen fibrils of stromal layer (Fig. 2).93 Such corneal organoid models hold potential as tools for investigating development and pathophysiology of various corneal conditions.93 Susaimanickam et al. generated complex 3D models recapitulating eyeball-like structures in 15 week periods comprising both primordias (retinal and corneal), adnexal tissues (like ciliary margin zone), and eyelid-like outer covering.94 These organoids offered promising platform for translational research and regenerative applications. In another study Joseph et al. developed a model of keratoconus (KC) with hiPSCs differentiated from fibroblasts (obtained from both normal and KC human corneal stroma cells). They observed KC-derived hiPSC have stunted growth and proliferation. They also proposed that inhibition of survival signals could be instrumental for the decreased cell survival and apoptosis of KC-specific keratocytes.95 Aberdam et al. differentiated limbal epithelial cells from hiPSCs (LiPSCs), which could be used as an alternative for toxicity studies like limbal epithelial stem cells.96 Stem cell-based models are physiological and reproducible alternatives which could efficiently alleviate the animal use in therapeutic testing prior to clinical trials. However, they cannot completely mimic the in vivo environment in terms of interactions between immune cells and corneal cells.
FIG. 2:
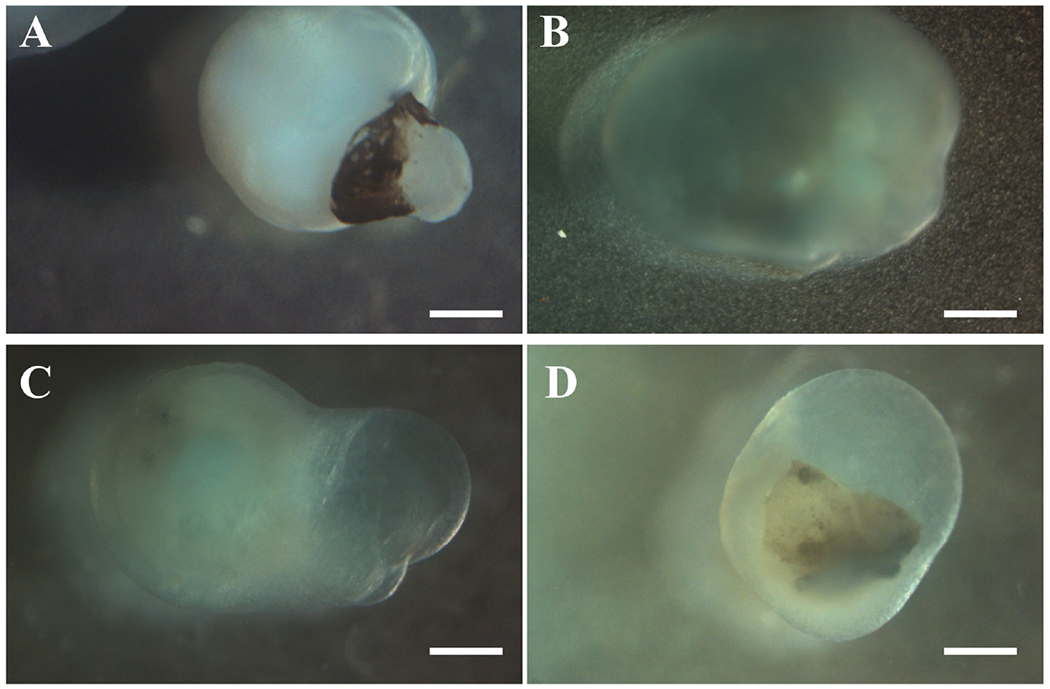
Gross morphology of corneal organoids. Bright field observations showing gross morphology of corneal organoids obtained from hiPSCs after 3 months in culture. (a) Organoids appeared translucent with a dense center. (b, d) Organoids had clear center. (c) Organoids had clear center and pigmented end. Scale bar 200 μm (Figure reproduced from Foster et al. under a Creative Commons license).
2. Stem Cell-Based Retinal Models
As there are various differences in the morphology, organization, developmental stages, and distribution and functions of proteins in retina of different species, human model systems representing retina are warranted. Stem cells of any source (endogenous or exogenous) started offering great confidence in disease modeling, translation research, and tissue replacement tissue development and drug screening potential. Retinal cells derived from hiPSC also been utilized for drug screening purposes.
Meyer et al. demonstrated protocols to differentiate retinal progenitor cells from hiPSCs and then isolated optic vesicles to study human retinal development, neuroretinal cell genesis, and analyzed pharmacologic and genetic interventions to treat a functional defect in patient (retinal degenerative disease) derived hiPSC-RPE cultures.97 In other studies, researchers used 4-hydroxytamoxifen (4-OHT) to induce photoreceptor degeneration/photoreceptor cell death in mouse retinal explants (hiPSC-derived 3D retinal organoids).98 They also measured degeneration-related attributes via a live-cell imaging system. Additionally they performed quantitative analysis of ophthalmic supplements (100 μM vitamin E, 0.2 μM astaxanthin, 20 μM anthocyanidin, 0.4 μM lutein) in attenuating light-induced photoreceptor degeneration demonstrating potential of in vitro stem cell-based model for drug screening purposes.98 The cells from the outer retina have been derived and characterized regularly from hiPSC, hence, successfully used for screening drugs for various degenerative diseases.97,99–103 Moreover, when hiPSC were derived from patients of particular disease type they possess abilities to recapitulate that disease in terms of phenotype and other morphological features. Hence many researchers performed studies on developing therapeutic approaches for various retinal diseases using patient-derived hiPSC.104–106 In AMD commonly affected cells are photoreceptors (undergoing loss) and RPE (becoming dysfunctional) cells which leads to loss of vision. Patient-derived RPE have been demonstrated to mimic various characteristic features of AMD such as flaws in response to oxidative stress, increased expression of inflammatory markers, and have been used as platform for screening potential drugs candidates for cure.107–110 Compounds such as nicotinamide was shown to alleviate RPE deterioration by downregulating drusen proteins, inflammatory factors, whereas enhancing expression of various chromatin-modifying genes, nucleosomes, and ribosome.110 Chang et al. isolated T cells from AMD patients and reprogrammed them to hiPSC cells. They then induced hiPSC to RPE cells (AMD-RPE), investigated AMD pathogenesis and used cells for in vitro drug testing. They evaluated effectiveness of curcumin in reducing ROS production. They also observed that curcumin also inhibited H2O2-induced cell death.111 Ferrar et al. differentiated hiPSCs derived RPE cells and grew them in 96- and 384-well plates. Using multiplex assays, they demonstrated expression of specific characteristics genes relevant to stem cells, hiPSC derived RPE and primary RPE cells at 2 different stages. They correlated data of high-throughput assays with RT-PCR measurements and confirmed that assays provided basis to screen several compounds targeting several retinal degenerative diseases, RPE functions, and disease pathways.112 Likewise, retinal cells (i.e., photoreceptors derived from PSCs) could exhibit characteristics retinitis pigmentosa (RP9) features by showing upregulated expression of various markers of apoptosis, oxidative stress, and lipid oxidation, and were used for testing drugs with potential to alleviate the disease progression.100,113–115 Jin et al. developed hiPSC from RP9 patients and differentiated them into retinal rod photoreceptors. They also observed that patient-derived rod cells exhibited RP9 phenotype, expressing stress markers, causing their degeneration. They observed variable responses to antioxidants (e.g. α-tocopherol exposure in patient-derived photoreceptors and normal photoreceptors). They demonstrated that patient-derived hiPSCs hold potential in personalized medicine and screening of therapeutics.116
As various differentiation protocols helped in gaining insight to phenotypes and morphology of the outer retina cells, latest refinements in differentiation strategies were also warranted to understand differentiation of cell of inner retina cells, especially retinal ganglionic cells (RGC).117–120 Degeneration of RGC results in vision loss as RGC are crucial for transmission of information from retina to the brain. Thus differentiation of RGCs from PSCs of optic neuropathies patients withhold potential in allowing drug screening and developing personalized treatments in retinal disorders.117,120 Cheng et al. obtained hiPSC from patient diagnosed with optic atrophy and differentiated them to RGC. Enhanced apoptosis and less differentiation potential were observed in the patient-derived hiPSC. They demonstrated that addition of β-estrogen or noggin promoted differentiation potential of hiPSC in the presence of neural induction medium and suggested that role of apoptosis in pathogenesis disease. Hence, they established proof of concept that hiPSC have potential to recapitulate degenerative processes associated with inner retinal diseases to some extent and can facilitate drug screening studies oriented towards the rescue of RGC deterioration.120 Although a large number of these studies were successful in establishing the capability of differentiated retinal cells (derived from hiPSC) in screening the target molecules bearing neuroprotective effects, these strategies had few drawbacks, for example, long protocols for differentiation, lack of 3D organization, absence of effects of cell–cell interaction. Retinal organoids on the other hand can differentiate in an approach that better recapitulates the functioning, structural design, and spatial arrangement and microenvironment of the retina.
Itro et al. developed 3D retinal organoids using mouse hiPSC and were successful in inducing cell death by treatment with 4-hydroxytamoxifen (4-OHT); which was comparable to degeneration observed in retinal explants from mouse. They demonstrated applicability of retinal organoids in drug screening to explain the protective mechanism by various supplements on photoreceptor degeneration induced by 4-OHT.98 There are various challenges in analysis of 3D models due to their heterogeneity, lack of specific inherent geometric arrangement, and presence of various cell types as compared to 2D arrangements which have homogeneous composition facilitating easy access to entire population during experimental data assessment. Thus, analysis of experimental variables is more challenging in 3D models due to accommodation for various depths/thickness, penetration of dyes or lasers, resolution, imaging, variable diffusion kinetics of drug molecules.121,122 Vergara et al. developed retina organoids and established a platform which involved use of a fluorescence microplate reader allowing 3D (xyz dimensions) detection as well as wavelength selection. They optimized various parameters for sensitivity, detection of signals coming from fluorescent reporter, evaluated performance and validated automated reporter quantification (ARQ) technology for practical applications in high-throughput screening.121 Most of the studies involving stem cells were able to regenerate properties of one or other type of retinal neurons or their arbitrary mixture but they lacked physiological characters such as synaptic connectivity between different neurons, layering, and functional rods and cons. The use of stem cells in the corneal and retinal research helped in gaining advancements in understanding diseases. However, the protocols for differentiation of stem cells to retinal lineages are lengthy; more improvements are needed to obtain sustainable platforms better mimicking human ocular tissues.
D. 3D In Vitro Models
1. 3D In Vitro Cornea Models
3D in vitro models have been widely explored for drug testing, permeability studies, translational research, and tissue engineering due to their superiority over conventional 2D systems in terms of recapitulating the microenvironment of native tissues; better representation of cell–cell as well cell–matrix interactions and higher stability.123 Postnikoff et al. cultured HCE on a curved Millicell-HA membrane and designed a 3-5 layered stratified, curved 3D epithelial model. They demonstrated that a quantitative model was sensitive for cytotoxicity and biocompatible for testing of contact lenses and potential in better understanding of interactions between cells and material used.124 Fligor et al. developed hiPSC-based retina ganglionic organoids and observed self-organization of RGC in the organoids and identified factors affecting neuritis outgrowths (Fig. 3).125 It was reported that RGCs derived from organoids expressed diverse phenotypes and mimicked the RGC in developmental and degeneration pathways. McLelland et al. derived retinal organoids sheets from hiPSC and successfully transplanted them to rat models of retinal degeneration. They evaluated that transplanted sheets were able to integrated into host cells, differentiated into retinal layers and improved the visual function in the rat model.126 This study demonstrated therapeutic potential of 3D retina organoids. Shokoomand et al. developed 3D co-culture model of RPE (hRPE) along with primate choroidal endothelial cells (RF-6A) in polycaprolactone (PCL)-gelatin based electrospun scaffold. They observed that porous ultrastructure of PCL-gelatin scaffolds supported attachment, differentiation, proliferation as well as migration of both types of cells. They implied that 3D co-culture model would be useful in understanding interaction between cells and pathway involved in AMD.127 Lu et al., developed a complex 3D model for the ocular surface studies.128 This model contained both conjunctival epithelial cells and lacrimal gland cell spheroids for recapitulating aqueous and mucin layers present in the tear film. They also induced the inflammation to study dry eye pathogenesis and see the response of co-culture to the therapeutics for comparison with monoculture conditions.128
FIG. 3:
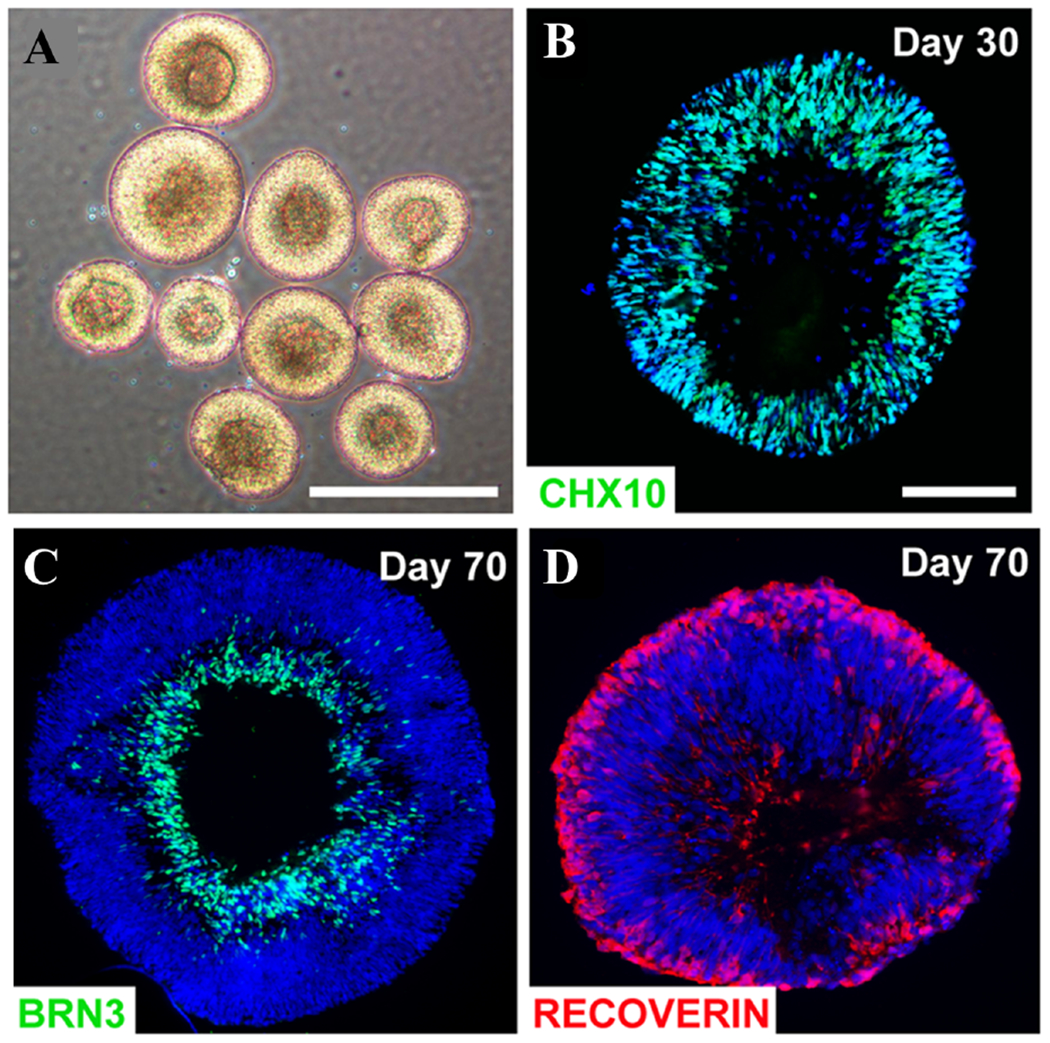
Retinal organoids with different ganglionic cell layers. (a) Brightfield images displaying stratified morphology of early retinal organoids. (b) Differentiation day 30: almost all cells within retinal organoids expressed the retinal progenitor marker CHX10. (c, d) Differentiation day 70 days, BRN3-positive cells resided in basal layers while Recoverin-positive photoreceptors occupied more apical layers. Scale bars equal 500 μm for (a) and 100 μm for (b, c, d). Scale bar in b applies to (c, d) (Figure reproduced from Fligor et al. under a Creative Commons license).
3D printing is a method that involves production of 3D objects using any material ranging from synthetic to organic including digital data. It is also known as additive manufacturing as it deals with deposition of several layers of material until the desired structure is obtained. 3D printing technology has growing applications ranging from tissue engineering,129 cancer research,130–132 pharmaceuticals,133 and medical.134 Wu et al. used extrusion-based 3D bioprinting platform to print HCE in collagen, alginate and gelatin-based hydrogel to be cross-linked using calcium chloride. They reported high viability and increased expression of cytokeratin 3 by tuning and controlling degradation of hydrogel by altering molar ratio of sodium alginate/sodium citrate. Their studies demonstrated application of 3D printing in tissue regeneration/engineering. Gibney et al. reported 3D printing using thin films of collagen formed by recombinant collagen type III (RHCIII). They printed both corneal keratocytes and MSC in RHCIII printed scaffolds and observed better proliferation and alignment in MSCs.135 A team of Spanish researchers were working on designing and printing cornea where need for transparency was supposed to be addressed by printing parallel collagen fibers by pre-determining specific distances between them.136 Isaacson et al. 3D printed an artificial cornea using FRESH method with the help of extrusion-based printer. They printed stem cells in alginate, collagen-based hydrogel and allowed them to differentiate into stromal cells. The printed corneas were designed to match patient’s corneal specifications in terms of geometry, thickness. and shape.137 A biotech company, Precisebio, reported about development and transplantation of 3D printed corneal graft in animals successfully. They claimed of using 4D technology for printing instead of 3D and referred 4th dimension as time needed for building connections among bioprinted cells and fibers (https://www.precise-bio.com/bio-tech-firm-develops-3d-printed-replacement-cornea-for-human-eyes/). Pandoram Technologies a banglore (India) based biotechnology firm mentioned about 3D bioprinting liquid corneas with potential for wound healing. The researchers were planning to conduct clinical trials in 2020 (https://www.docwirenews.com/future-of-medicine/3d-printing-cornea-tissue-to-combat-blindness/). In another study, researchers mentioned about 3D printing corneal stromal scaffolds for high throughput screening.138 This report established proof of concept for the rapid fabrication of corneal stromal equivalents. They printed transparent, smooth HCK cells bearing cornea with optimum curvature (Fig. 4). This study broadened the scope 3D printing as toll to design organized tissues on large scale for screening purposes.138 Hyeonji et al. bioengineered a transparent corneal stroma using 3D cell printing technology. They printed stromal analogues recapitulating the native corneal structure by introducing shear stress to bioink comprised of decellularized extracellular matrix (ECM). They controlled the shear stress by varying the nozzle size and thus manipulated the arrangement of printed collagen fibers exhibiting tissue like specificity and structural organization. Further, during in vivo studies they also observed that collagen fibrils remodeled along the printing path lattice pattern created by collagen fibrils after 4 weeks.139 Recently, researchers in Japan developed a 3D cornea-on-a-chip model which was claimed to substitute rabbits in ocular drug testing. This device was capable of testing 4 different samples simultaneously while maintaining same conditions. The sample section comprised of upper and one lower channel, which were separated by a clear, porous polyester membrane. Corneal cells were seeded in the upper channel (on top of the membrane). Cells take 7 days to grow and eventually form a barrier. To mimic blinking of eyelid and tears, movement of fluid was accommodated in 2 channels (https://www.drugtargetreview.com/news/58364/cornea-on-a-chip-could-allow-for-more-accurate-ophthalmic-drug-testing/). Phan et al. developed a 3D model to accommodate for tear break up, tear deposition and blinking mechanism to study the effect of these factors on drug delivery.140 In their model, the eyelids were 3D printed and PVA made flexible membrane (secured by teeth). They compared the water content and wetting ability of the materials with the bovine tissues (cornea and sclera). In the model, artificial tear solution generated with the help of microfluidic pump was delivered to eyelids. During every blink, the eyelid were made to flex and slide across the eyeball, ensuring that the 2 surfaces in contact and spreading uniform tear film. The model could be used for high-throughput screening studies by connecting about four units together (Fig. 5).140 3D printing of corneal tissues offers various advantages as compared to other tissues due to cellular homogeneity, avascular nature, and low metabolism of cornea. Also, use of stem cells in 3D printed matrix majorly comprising of collagen mimics the human cornea. However, for designing 3D printed in vitro model with drug screening applications incorporation of various factors such as epithelial barrier, thickness of the layers, curvature of the cornea, blinking, tear fluid, tear flow is desired all together in one model.
FIG. 4:
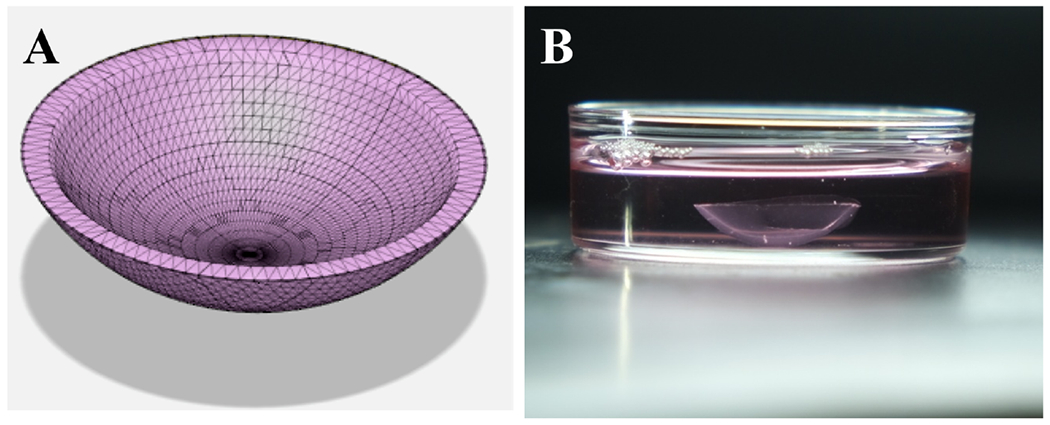
3D printed cornea model. (a) Corneal stroma model designed in Autodesk fusion 360. (b) 3D printed cornea after cross-linking placed in the media (reprinted from Kutlehria et al. with permission from John Wiley and Sons, copyright 2020).
FIG. 5:
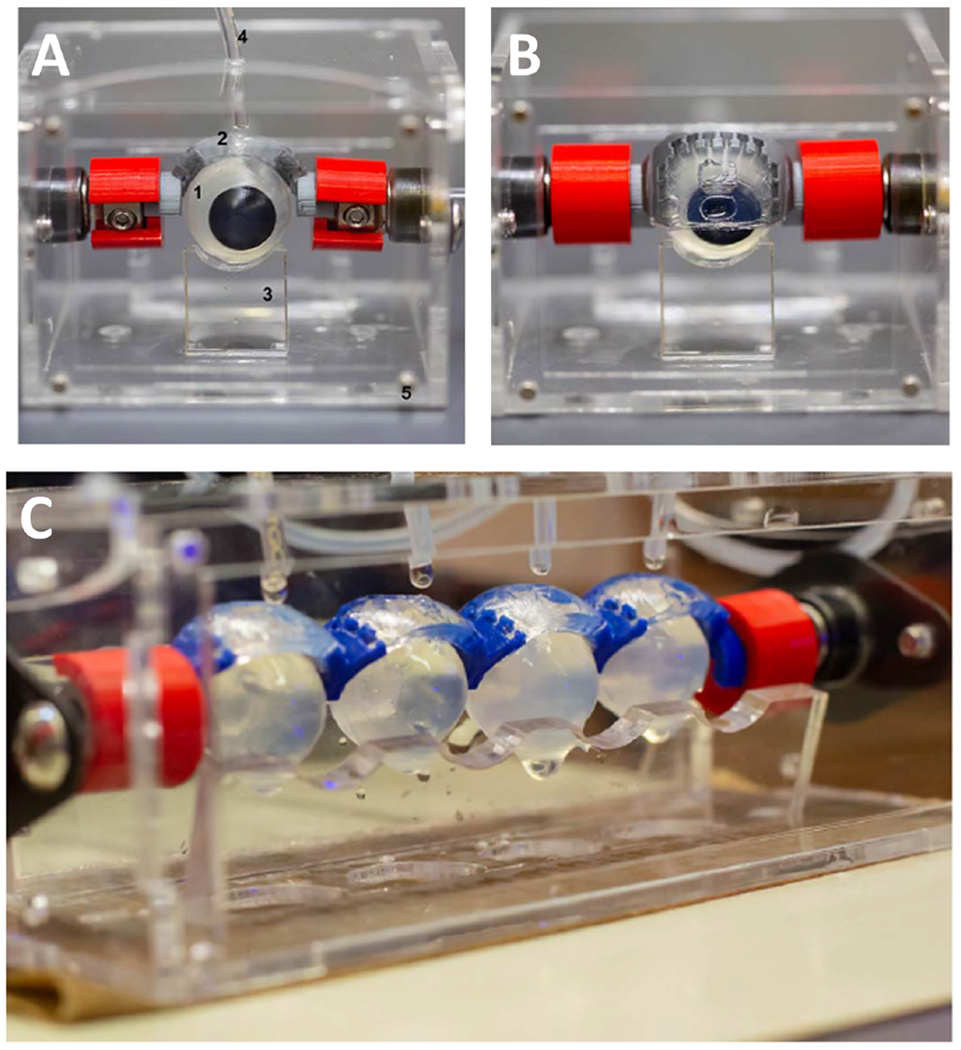
Blink model (a) Fully assembled blink model; 1. eyeball, 2. eyelids, 3. lower eyelids, 4. tubing attached to a microfluidic pump, and 5. acrylic chambers. (b) Eyelid in the closed position. (c) For high throughput testing-blink model can be connected with 4 eyelids (Figure reproduced from Phan et al. under a Creative Commons license).
2. 3D Printed Retinal Models
Human retina being complex neural tissue is comprised of more than 50 cell types, making it difficult to target specific population of cells during treatment of retinal diseases. In such scenario bio-printing of various types of retinal cells holds potential as a great tool in research, drug development, and disease modeling. A research team led by Dr Erin Lavik was awarded by National Eye Institute (NEI) for proposing a retina model. They proposed the use of a type of screen printing to print layers retinal neurons derived from adult neural progenitor for high-throughput assays (https://3dprint.com/189603/3d-printed-retinal-model/).
Shi et al. performed 3D bio-printing of RPE cells with photoreceptors (PR) using alginate, pluronic based hybrid gel to create a retinal model.141 First, they printed ARPE cells on polycaprolactone ultrathin membranes and allowed them to grow for 2 weeks. Thereafter they printed Y79 (PR equivalent cells) cells laden in gel in distinctive patterns on the ARPE cells layers (Fig. 6).141 They investigated bioprinted cells for morphology, viability, and quality, and observed high quality and viability in the printed scaffolds. Their hybrid 3D bioprinting advancement offered potential for cell–cell complex interactions to support further research. RGCs are generally lost during injuries and are incapable to regenerate. Various studies have been performed using 3D bioprintng technology to represent RGC layer of retina by depositing RGC in orderly manner. Lorber et al. attempted to print RGC along with glial cells using inkjet printing techniques.142 They observed that cells could withstand the vibration force occurring due to piezoelectric printing and did not lose viability during printing. They also demonstrated that glial cells maintained their growth promoting properties even after printing and increased growth of RGC neurons.142 Kadnor et al. demonstrated combined use of electrospinning along with thermal inkjet-based 3D printing for precise orientation of RGCs on the scaffold.
FIG. 6:
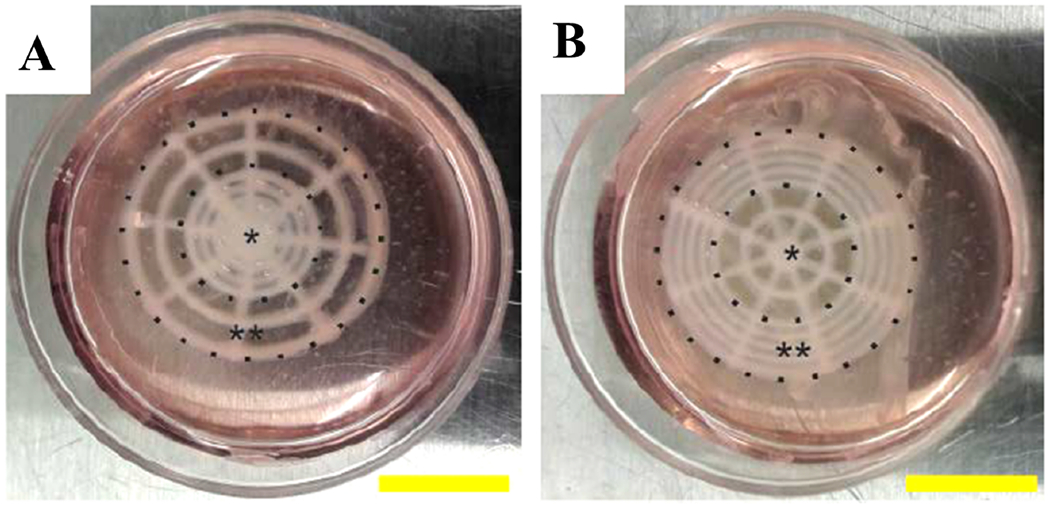
3D printed retinal equivalents with 2 distinct Y79 cell-seeding density. (a) High average cell density at the center (HC). (b) High average cell density at the periphery (HP); *central area, **periphery; scale bar: 10 mm (Figure reproduced from Shi et al. under a Creative Commons license).
Dendritic cells were aligned with radial scaffolds. They demonstrate that cell survival, electrophysiology, and growth of neuritis were maintained during polylactic acid-based electrospun-guided 3D printing approach.143 Both these studies showed successful printing of RGC cells using different 3D printing technologies. Wang et al. attempted to recapitulate the microenvironment of retina by functionally modifying hyaluronic acid (HA) in order to obtain hydrogel with compressive modulus similar to that of retina. They synthesized methacrylated HA with different degree of cross-linking. After encapsulating RPE cells and retinal progenitor cells (RPC) in the hydrogels, they showed 3D bioprinting of bilayer constructs. They observed that co-culture with RPE cells enhanced maturation and development in PRs and more than 70% viability in the cells printed in high-methacrylated hyaluronic acid (HMHA-GM) containing polymerizable hydrogel.144 Through this study they established the potential of 3D printing technology in designing co-culture systems to mimic complex environment of native retina. So far, researchers have demonstrated successful printing of ganglionic cell along with glial cells142 and RPE cells with photoreceptors144 while maintaining their viability and functions. However, investigations are needed to determine if other retinal cells e.g. mueller, horizontal, amacrine and bipolar cells can also be effectively printed avoiding functional and viability loss. Other challenge would be to mimic the precise spatial arrangement existing in this complex tissue. Besides, to recapitulate the functional retina accurate printing must be conjugated with high densities of cells would be needed.
In the past, Kadnor et al. could achieve density of 30 cell/mm2 while average density of cell in human retina is about 2100 cells/mm2.143,145 Additionally, a steadfast high-throughput printing platform, containing multi-head printing tools would be desirable while maintaining cell viability during the process of 3D printing. Besides, microfluidics systems would be needed to mimic vasculature supply nutrition and oxygen to growing cells and to facilitate their long-term survival. Glaucoma is one of the causes for blindness and involved elevated increased intraocular pressure (IOP) and degeneration of RGCs.146 More understanding was warranted to explain how elevated IOP led to degeneration of RGC. Various researchers developed glaucoma models to study the effect of IOP on the degeneration of RGC. Wu et al., studied how increase in hydrostatic pressure leads to changes to RGC axon, cell body area dendrites, and total neurite length. In their tunable platform, the hydrostatic pressure could be changed by altering the height of a liquid reservoir connected to a 3D printed adapter. The model facilitated monitoring of RGC under varying pressures. They observed that pressure variations significantly changed the neurite length, axon length, and dendritic branching.146 The model was useful in drug screening because it facilitated understanding biophysical mechanism involved in glaucoma. Torrejon et al. designed an in vitro 3D model of human trabercular network (HTM) to better understand pathology of steroid-induced glaucoma, as well as physiology of outflow. Steroids induced a fibrotic state mimicking HTM during glaucoma, and outflow was regulated. The disease model mimicked characteristics of glaucomatous HTM physiology and outflow resistance. The model also responded to intraocular pressure reducing agent (e.g., ROCK Inhibitor). The bioengineered perfusion platform for physiological and pathological studies was related to regulation of outflow and screening of new therapeutic agents.147
Undoubtedly, 3D printed retinal models offer insights to the underlying mechanisms of posterior eye diseases, together with their potential in drug screening and validations. However, the major challenge in printing retinal models is precise printing of retinal cells at high densities, proper spatial arrangement, and their connection to each other synaptically while establishing various horizontal and vertical connections that would make retina functional and capable of transmission of visual information. Lastly, 3D printed complex tissues like retina will need a stringent approval process by regulatory authorities to ensure applicability and functionality.
E. Organ on Chip Models
Microphysiological systems (MPS) are commonly called organ-on-a-chip tools as designed by researchers. MPS are in vitro platforms which are integrative, microfabricated, and are designed to simulate functional units of human organs.148 MPS have several advantages as they accommodate for flow, pressure, cell–cell interactions, and cell–tissue interplays. Puleo et al. designed the first microfluidic device which contained collagen vitrigel (CV) as substrate for microtissue patches of cornea. They demonstrated applications of microfluidic device by measuring the permeability of fluorescein across a range of corneal tissue structures while varying growth conditions, chemical exposures, and CV etching levels.149 Seo et al. modeled blinking by incorporating a hydrogel- based eyelid to mimic spontaneous eye blinking. They emphasized that their realistic system could be a platform for preclinical drug screening, demonstrated its application as a dry eye disease model and evaluated therapeutic potential of lubricin.150 Mattern et al. developed a novel system called Dynamic Micro Tissue Engineering System using HCE-T cells.151,152 They measured trans-epithelial resistance (TEER) by positioning electrodes within the arrangement and also microfluidic device to offer applicability of model in drug screening. This helped in noninvasive real-time monitoring of the system. Inert polycarbonate was used instead of polydimethysiloxane (PDMS) to ensure exposure of 2–3 layered corneal epithelium to a fluid flow. They injected BAK in the system to compare the model with static test methods.152 They observed that the incorporated flow recapitulated the in vivo kinetics better than static models. For applicability of such model in preclinical studies where small volumes are available, downsizing of the compartments would be important. Bennet et al. developed another cornea chip model by using immortalized HCE cells.153 They seeded 5 monolayers of epithelial cells to create a structure mimicking layered composition of the corneal epithelium. The model attempted to recapitulate microenvironment of the eye by accommodating for tear flow and blinking. Tear flow and blinking rate play a crucial role in influencing residence time of the drug. They compared static flow, continuous flow, and pulsating flow. Pulsating flow is an attempt to imitate the eye blinking rate. The authors analyzed drug mass transport and investigated the corneal permeation using different ocular drug formulations: suspension (Pred Forte) and liquid eye drops (Zaditor). In ophthalmic chip development, besides regeneration of corneal tissues, efforts were made to re-create retinal tissue as well. During transplantation there are various challenges ranging from migration of transplanted cells, maturation of precursor cells to final integration with the native cells.154 In such situations real-time observations are critical, hence models must be transparent and optically accessible. Such critical attributes of transparency and accessibility are not viable with animal models, creating a dire need for organ on chip models for retina. Su et al. fabricated a microfluidic chip–retinal synaptic regeneration (Retinal SR) chip to explore the mechanism of regeneration of retinal synapse.155 The Retinal SR-chip involved seeding of murine precursor cells into microchambers linked by several arrays of microchannels. They observed emergence of axons from one population of cells towards other. Their automated method helped in analysis and even enumeration of synaptic connections, monitoring electrophysiological activity of retina. To validate functionality of chip they used variable concentrations of glycine and monitored its effect on SR.
They also quantified synaptic connections and assessed their kinetics (during SR) using an image-based analytical method. This microfluidic SR chip was a device for high-throughput investigation of retinal SR and screening precursor cells during transplantation (Fig. 7).155 Mishra et al. studied retinal cell migratory functions and behaviors of both mouse, human retinas using microfluidic chip called μRetina chip. They coupled computer simulations with experimental validations and monitored cell migration using real-time imaging systems.156 Their human retina model had 3 compartments linked to each other with micro channels. The arc-shaped chamber in the center served as imitation of retinal geometry and seeding of progenitor cells, while 2 chambers at top and bottom were reservoirs. Through these chips the authors attempted to establish model which can help in monitoring migration behaviors of the cells and thus facilitate development of cell therapies. In another chip model by Dodson et al., there were 12 channels to facilitate delivery of drugs or signaling molecules. Moreover, device had some suctions channels enabling flattening of retinal explants that aided in imaging and localized analysis of slices of tissues as well.157 They also exposed explants to lipopolysacchrides (LPS) to recapitulate inflammation microenvironment and establish applicability of model towards disease modeling and drug screening. Chip systems have been developed to understand the patho-physiological changes and cellular mechanism of AMD. Kaji et al. made easy co-culture chip designs, which consisted of ARPE-19 cells (seeded on upper side) co-cultured with human umbilical vein endothelial cells (HUVECs) cells (seeded on bottom side) in 2 separate channels with a porous membrane in between. They investigated VEGF concentrations on the apical channel in addition to basal sides while maintaining low and high glucose containing medias and observed that tissue possessed properties similar to native tissues.158 Complex model platform were designed to study angiogenesis and effects of VEGF inhibitors. Chung et al. grew an ARPE-19 cell line (representing RPE monolayer) with HUVEC cells (forming blood vessel network) embedded in fibrin gel.159 They also created RPE and choroid layers gap by injecting fibrin gel (central channel) and supported angiogenesis by filling fibroblast in (in lateral channels) (Fig. 8). They observed regression of blood vessels on injecting anti-VEGF bevacizumab.159 In another study Nafian developed a glaucomaon-a-chip (GOC) model to investigate the effect of high pressure and neuroprotective agents on viability of RGCs. Based on simulations generated from physical parameters they developed a chip with 3 layers, culture wells, and interconnecting microchannels. ECM air plasma modification and membrane coatings were perfomed on the bottom surface of the wells. The authors isolated and purified RGCs isolated from postnatal Wistar rats for the studies. Further, they studied the effect of brain-derived-neurotrophic factor (BDNF) and BDNF mimetic agent RNYK on the RGCs under normal pressure and elevated pressure condition for 2 days. They observed about 2-fold reduction in the RGC death rate in presence of BDNF and RNYK.160 This model was instrumental in preliminary screening of specific pathways and cell types for studying damage involved in glaucoma These MPS/organ on chip (OoC) models show great potential in recapitulating structural complexity, functionality, and microenvironment of specific sections more accurately as compared to conventional in vitro models. They were also capable of overcoming limitations of conventional models such as lack of interactions, interspecies variability, transparency, and optical accessibility, and thus were more predictive and appropriate. Nonetheless, more advancement is needed. OoC models so far developed have narrow focus on either a particular aspect of ocular tissue or a specific part such as retina, cornea, or choroid. Thus, models with broader applicability, comprehensive coverage, and holistic recapitulation by focusing on complex integrity, multi-cellularity, vasculature within the ocular tissue are still needed.
FIG. 7:
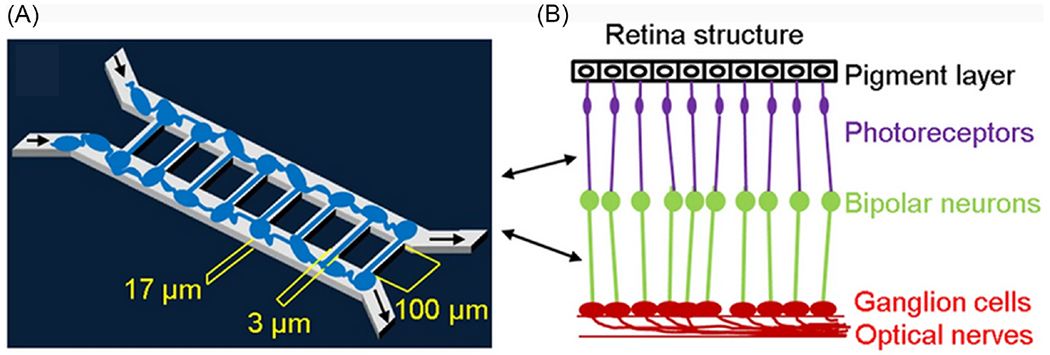
Microfluidic chip design and retinal synapse principal function. (a) Design of retinal synapse regeneration (RSR) chip to mimic retinal structure. The RSR-Chip consists of two chambers connected by 100 microchannels. Two populations of retinal cells are seeded in the two chambers and form synaptic connections in the microchannels. (b) Image of microchannels in RSR-Chip. Scale bar, 200 μm (Figure reproduced from Su et al. under a Creative Commons license).
FIG. 8:
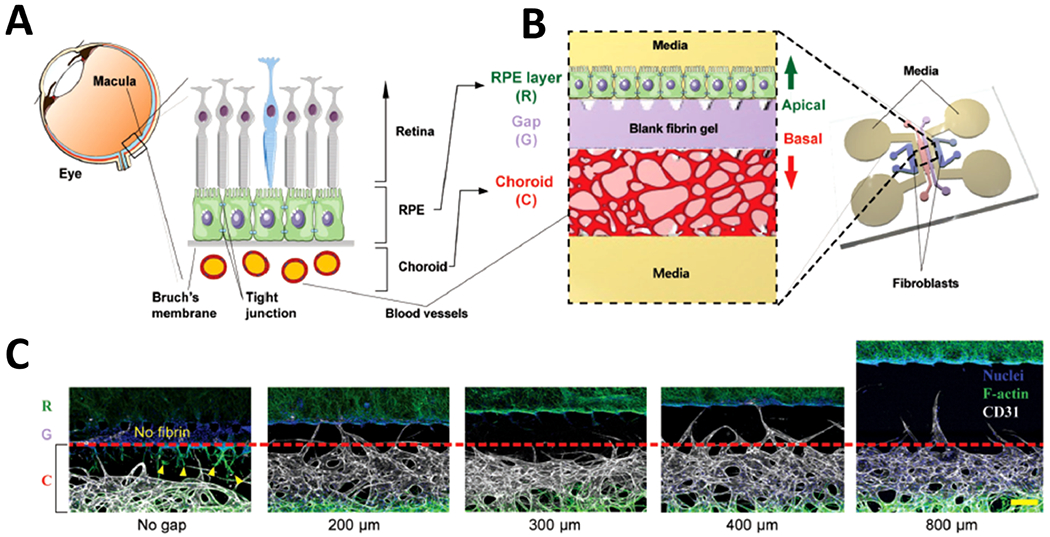
Microfluidic eye-on-a-chip model-3D RPE-choroid. (a) Schematic of RPE–choroid complex in the neural retina of the eye. (b) Design showing microfluidic device mimicking the RPE–choroid system in vitro. (c) Optimization of the device design by testing gap channels with various widths (reprinted from Chung et al. with permission from John Wiley and Sons, copyright 2017).
IV. CONCLUSION
Today researchers and pharmaceutical companies are moving towards in vitro models, not only due to legislation preferences for adherence to 3Rs (replacement, reduction, and refinement) for animal use, but also in search of more relevant, cheaper, less time consuming, and humane methods of disease studies and product testing. Progress has been made in developing in vitro models involving human cells, tissues, organoids, 3D printing, and MPS technology for disease modeling and drug development in the field of ophthalmology. Complex models involving use of stem cells, 3D printing, and OoC approaches hold potential in mimicking complexity of this organ while offering opportunity to simultaneously create diseased background (by use patient-derived cells) and optimum microenvironment for cellular growth and interactions. As a result, various physiological relevant models have been investigated for anterior (cornea, trabecular mesh) and posterior compartment (retina) of the eye. However, most of the models focus on a particular tissue and not the whole organ. In fact, many ocular diseases being complex are not restricted to just one tissue, they affect various tissues. Hence, to deal with the pressing need of better therapeutic agents and more realistic models, an integrative approach would be desired. Therefore, fusion of interdisciplinary approaches and different technologies like stem cells, microfabrication, 3D printing, biomaterial science while developing OoC models would be able to bring advancements and translations in ocular disease modeling and drug discovery.
ABBREVIATIONS:
- 3D
3-dimensional
- 4-OHT
4-hydroxytamoxifen
- AM
amniotic membrane
- ARQ
automated reporter quantification
- BAK
benzalkonium chloride
- BDNF
brain-derived-neurotrophic factor
- BM
Bruch’s membrane
- BRB
blood retinal barrier
- CV
collagen vitrigel
- ECM
extracellular matrix
- ESC
embryonic stem cells
- GOC
glaucoma-on-a-chip
- HA
hyaluronic acid
- HCE
human cultures of epithelial cells
- HCJC
human conjunctival cells
- hESC-RPE
human stem cell-derived RPE
- hiPSC
human induced pluripotent stem cells
- HMHA-GM
high-methacrylated hyaluronic acid
- HTM
human trabecular meshwork
- HUVEC
human umbilical vein endothelial cells
- KC
keratoconus
- LiPSC
limbal epithelial cells from iPSCs
- LPS
lipopolysaccharide
- MGSH40
macrogolglycerol hydroxystearate
- MPS
microphysiological systems
- NEI
National Eye Institute
- PCL
polycaprolactone
- PS80
polysorbate 80
- RCE
retinal capillary endothelium
- retinal SR
retinal synaptic regeneration
- RGC
retinal ganglionic cells
- RHCIII
recombinant collagen type III
- RPC
retinal progenitor cells
- RPE
retinal pigmental epithelium
- TEER
trans-epithelial electrical resistance
- TJP
tight junction protein
- VEGF
vascular endothelial growth factors
REFERENCES
- 1.Chuang K, Fields MA, Del Priore LV. Focus: Genome editing: Potential of gene editing and induced pluripotent stem cells (iPSCs) in treatment of retinal diseases. Yale J Biol Med. 2017. December 19;90(4):635–42. [PMC free article] [PubMed] [Google Scholar]
- 2.Cholkar K, Patel SP, Vadlapudi AD, Mitra AK. Novel strategies for anterior segment ocular drug delivery. J Ocular Pharmacol Therapeut. 2013;29(2):106–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shafaie S, Hutter V, Cook MT, Brown MB, Chau DY. In vitro cell models for ophthalmic drug development applications. Biores Open Access. 2016;5(1):94–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson SL, Ahearne M, Hopkinson A. An overview of current techniques for ocular toxicity testing. Toxicology. 2015;327:32–46. [DOI] [PubMed] [Google Scholar]
- 5.Huhtala A, Salminen L, Tähti H, Uusitalo H. Corneal models for the toxicity testing of drugs and drug releasing materials. In: Ashammakhi N, editor. Topics in multifunctional biomaterials and devices. 2008. p. 1–24. [Google Scholar]
- 6.Ubels JL, Clousing DP. In vitro alternatives to the use of animals in ocular toxicology testing. Ocul Surf. 2005;3(3):126–42. [DOI] [PubMed] [Google Scholar]
- 7.Iwata T, Tomarev S. Animal models for eye diseases and therapeutics. In: Conn PM, editor. Sourcebook of models for biomedical research. Humana Press; 2008. p. 279–87. [Google Scholar]
- 8.Kutlehria S, Vhora I, Bagde A, Chowdhury N, Behl G, Patel K, Singh M. Tacrolimus loaded PEG-cholecalciferol based micelles for treatment of ocular inflammation. Pharm Res. 2018;35(6):117. [DOI] [PubMed] [Google Scholar]
- 9.Barabino S, Dana MR. Animal models of dry eye: A critical assessment of opportunities and limitations. Invest Ophthalmol Vis Sci. 2004;45(6):1641–6. [DOI] [PubMed] [Google Scholar]
- 10.Liu C-H, Wang Z, Sun Y, Chen J. Animal models of ocular angiogenesis: From development to pathologies. FASEB J. 2017;31(11):4665–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barile FA. Validating and troubleshooting ocular in vitro toxicology tests. J Pharmacol Toxicol Methods. 2010;61(2):136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutlehria S, Bagde A, Patel N, Singh M. Whole-eye perfusion model for screening of the ocular formulations via confocal laser scanning microscopy. AAPS PharmSciTech. 2019;20(7):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eskes C, Bessou S, Bruner L, Curren R, Harbell J, Jones P, Kreiling R, Liebsch M, McNamee P, Pape W. Eye irritation. Altern Lab Anim. 2005;33(Suppl 1):47–81. [DOI] [PubMed] [Google Scholar]
- 14.Zhou EH, Paolucci M, Dryja TP, Manley T, Xiang C, Rice DS, Prasanna G, Chen A. A compact whole-eye perfusion system to evaluate pharmacologic responses of outflow facility. Invest Ophthalmol Vis Sci. 2017;58(7):2991–3003. [DOI] [PubMed] [Google Scholar]
- 15.Cakmak HB, Cagil N, Simavli H, Raza S. Corneal white-to-white distance and mesopic pupil diameter. Int J Ophthal. 2012;5(4):505–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qazi Y, Wong G, Monson B, Stringham J, Ambati BK. Corneal transparency: Genesis, maintenance and dysfunction. Brain Res Bull. 2010;81(2-3):198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norman RE, Flanagan JG, Rausch SM, Sigal IA, Tertinegg I, Eilaghi A, Portnoy S, Sled JG, Ethier CR. Dimensions of the human sclera: Thickness measurement and regional changes with axial length. Exp Eye Res. 2010;90(2):277–84. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X-R, Zhang Z-Y, Hoffman MR. Conjunctival thickness measured by optical coherence tomography. Ophthalmology. 2013;120(6):1305. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed I, Patton T. Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Invest Ophthalmol Vis Sci. 1985;26(4):584–7. [PubMed] [Google Scholar]
- 20.White CE, Olabisi RM. Scaffolds for retinal pigment epithelial cell transplantation in age-related macular degeneration. J Tissue Eng. 2017;8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Remington LA. Chapter 4 - Retina. In: Remington LA, editor. Clinical anatomy and physiology of the visual system. 3rd ed. Saint Louis: Butterworth-Heinemann; 2012. p. 61–92. [Google Scholar]
- 22.Forest DL, Johnson LV, Clegg DO. Cellular models and therapies for age-related macular degeneration. Dis Model Mech. 2015;8(5):421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell M, Humphries P. The blood-retina barrier: Tight junctions and barrier modulation. Adv Exp Med Biol. 2012;763:70–84. [PubMed] [Google Scholar]
- 24.Chowdhury N, Vhora I, Patel K, Bagde A, Kutlehria S, Singh M. Development of hot melt extruded solid dispersion of tamoxifen citrate and resveratrol for synergistic effects on breast cancer cells. AAPS PharmSciTech. 2018;19(7):3287–97. [DOI] [PubMed] [Google Scholar]
- 25.Doddapaneni R, Patel K, Chowdhury N, Singh M. Noscapine chemosensitization enhances docetaxel anticancer activity and nanocarrier uptake in triple negative breast cancer. Exper Cell Res. 2016;346(1):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel K, Chowdhury N, Doddapaneni R, Boakye CH, Godugu C, Singh M. Piperlongumine for enhancing oral bioavailability and cytotoxicity of docetaxel in triple-negative breast cancer. J Pharm Sci. 2015;104(12):4417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kutlehria S, Behl G, Patel K, Doddapaneni R, Vhora I, Chowdhury N, Bagde A, Singh M. Cholecalciferol-PEG conjugate based nanomicelles of doxorubicin for treatment of triple-negative breast cancer. AAPS PharmSciTech. 2018;19(2):792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nottingham E, Sekar V, Mondal A, Safe S, Rishi A, Singh M. The role of self nano emulsifying drug delivery systems of CDODA-Me in sensitizing Erlotinib resistant nonsmall cell lung cancer. J Pharm Sci. 2020;109(6):1867–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferdous AJ, Stembridge NY, Singh M. Role of monensin PLGA polymer nanoparticles and liposomes as potentiator of ricin A immunotoxins in vitro. J Control Release. 1998;50(1-3):71–8. [DOI] [PubMed] [Google Scholar]
- 30.Boakye CH, Patel K, Doddapaneni R, Bagde A, Marepally S, Singh M. Novel amphiphilic lipid augments the co-delivery of erlotinib and IL36 siRNA into the skin for psoriasis treatment. J Control Release. 2017;246:120–32. [DOI] [PubMed] [Google Scholar]
- 31.Boakye CH, Patel K, Patel AR, Faria HA, Zucolotto V, Safe S, Singh M. Lipid-based oral delivery systems for skin deposition of a potential chemopreventive DIM derivative: Characterization and evaluation. Drug Deliv Transl Res. 2016;6(5):526–39. [DOI] [PubMed] [Google Scholar]
- 32.Tripathi B, Tripathi R. Cytotoxic effects of benzalkonium chloride and chlorobutanol on human corneal epithelial cells in vitro. Lens Eye Toxic Res. 1989;6(3):395–403. [PubMed] [Google Scholar]
- 33.Tripathi BJ, Tripathi RC. Hydrogen peroxide damage to human corneal epithelial cells in vitro: Implications for contact lens disinfection systems. Arch Ophthalmol. 1989;107(10):1516–9. [DOI] [PubMed] [Google Scholar]
- 34.Tripathi B, Tripathi R, Millard C, Borisuth N. Cytotoxicity of hydrogen peroxide to human corneal epithelium in vitro and its clinical implications. Lens Eye Toxic Res. 1990;7(3-4):385–401. [PubMed] [Google Scholar]
- 35.Scott L, Eskes C, Hoffmann S, Adriaens E, Alepée N, Bufo M, Clothier R, Facchini D, Faller C, Guest R. A proposed eye irritation testing strategy to reduce and replace in vivo studies using bottom–up and top–down approaches. Toxicol In Vitro. 2010;24(1):1–9. [DOI] [PubMed] [Google Scholar]
- 36.Han B, Schwab IR, Madsen TK, Isseroff RR. A fibrin-based bioengineered ocular surface with human corneal epithelial stem cells. Cornea. 2002;21(5):505–10. [DOI] [PubMed] [Google Scholar]
- 37.Ramaesh K, Dhillon B. Ex vivo expansion of corneal limbal epithelial/stem cells for corneal surface reconstruction. Eur J Ophthalmol. 2003;13(6):515–24. [DOI] [PubMed] [Google Scholar]
- 38.Narvekar P, Bhatt P, Fnu G, Sutariya V. Axitinib-loaded poly (lactic-co-glycolic acid) nanoparticles for age-related macular degeneration: Formulation development and in vitro characterization. Assay Drug Dev Technol. 2019;17(4):167–77. [DOI] [PubMed] [Google Scholar]
- 39.Guo C, Zhang Y, Yang Z, Li M, Li F, Cui F, Liu T, Shi W, Wu X. Nanomicelle formulation for topical delivery of cyclosporine A into the cornea: In vitro mechanism and in vivo permeation evaluation. Sci Rep. 2015;5:12968. [Google Scholar]
- 40.Schuerer N, Stein E, Inic-Kanada A, Pucher M, Hohenadl C, Bintner N, Ghasemian E, Montanaro J, Barisani-Asenbauer T. Implications for ophthalmic formulations: Ocular buffers show varied cytotoxic impact on human corneal-limbal and human conjunctival epithelial cells. Cornea. 2017;36(6):712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rangarajan R, Ketelson HA, Do R, McCanna DJ, Suko A, Enstone D, Subbaraman LN, Dantam J, Jones LW. Effect of artificial tear formulations on the metabolic activity of human corneal epithelial cells after exposure to desiccation. J Vis Exp. 2020;(159). doi: 10.3791/60812. [DOI] [PubMed] [Google Scholar]
- 42.Bucolo C, Fidilio A, Platania CBM, Geraci F, Lazzara F, Drago F. Antioxidant and osmoprotecting activity of taurine in dry eye models. J Ocul Pharmacol Ther. 2018;34(1-2):188–94. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman HM, Choi JH, Clousing DP, Ubels JL, McCarey BE, Edelhauser HF. Corneal epithelial testing strategies for safety evaluation of ophthalmic formulations. Cutan Ocular Toxicol. 2007;26(4):311–27. [DOI] [PubMed] [Google Scholar]
- 44.Ban Y, Cooper LJ, Fullwood NJ, Nakamura T, Tsuzuki M, Koizumi N, Dota A, Mochida C, Kinoshita S. Comparison of ultrastructure, tight junction-related protein expression and barrier function of human corneal epithelial cells cultivated on amniotic membrane with and without air-lifting. Exp Eye Res. 2003;76(6):735–43. [DOI] [PubMed] [Google Scholar]
- 45.Nagai N, Nakazawa Y, Ito Y, Kanai K, Okamoto N, Shimomura Y. A nanoparticle-based ophthalmic formulation of dexamethasone enhances corneal permeability of the drug and prolongs its corneal residence time. Biol Pharm Bull. 2017;40(7):1055–62. [DOI] [PubMed] [Google Scholar]
- 46.Alaminos M, Sánchez-Quevedo MDC, Munoz-Avila JI, Serrano D, Medialdea S, Carreras I, Campos A. Construction of a complete rabbit cornea substitute using a fibrin-agarose scaffold. Invest Ophthalmol Vis Sci. 2006;47(8):3311–7. [DOI] [PubMed] [Google Scholar]
- 47.Duan X, McLaughlin C, Griffith M, Sheardown H. Biofunctionalization of collagen for improved biological response: Scaffolds for corneal tissue engineering. Biomaterials. 2007;28(1):78–88. [DOI] [PubMed] [Google Scholar]
- 48.Griffith M, Osborne R, Munger R, Xiong X, Doillon CJ, Lay cock NL, Hakim M, Song Y, Watsky MA. Functional human corneal equivalents constructed from cell lines. Science. 1999;286(5447):2169–72. [DOI] [PubMed] [Google Scholar]
- 49.Reichl S, Bednarz J, Müller-Goymann C. Human corneal equivalent as cell culture model for in vitro drug permeation studies. Br J Ophthalmol. 2004;88(4):560–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reichl S, Döhring S, Bednarz J, Müller-Goymann CC. Human cornea construct HCC—An alternative for in vitro permeation studies? A comparison with human donor corneas. Eur J Pharm Biopharm. 2005;60(2):305–8. [DOI] [PubMed] [Google Scholar]
- 51.Doillon C, Watsky MA, Hakim M, Wang J, Munger R, Laycock N, Osborne R, Griffith M. A collagen-based scaffold for a tissue engineered human cornea: Physical and physiological properties. Int J Artif Organs. 2003;26(8):764–73. [DOI] [PubMed] [Google Scholar]
- 52.Tegtmeyer S, Papantoniou I, Müller-Goymann CC. Reconstruction of an in vitro cornea and its use for drug permeation studies from different formulations containing pilocarpine hydrochloride. Eur J Pharm Biopharm. 2001;51(2):119–25. [DOI] [PubMed] [Google Scholar]
- 53.Tegtmeyer S, Reichl S, Müller-Goymann C. Cultivation and characterization of a bovine in vitro model of the cornea. Pharmazie. 2004;59(6):464–71. [PubMed] [Google Scholar]
- 54.Zieske JD, Mason VS, Wasson ME, Meunier SF, Nolte CJ, Fukai N, Olsen BR, Parenteau NL. Basement membrane assembly and differentiation of cultured corneal cells: Importance of culture environment and endothelial cell interaction. Exp Cell Res. 1994;214(2):621–33. [DOI] [PubMed] [Google Scholar]
- 55.Ju C, Gao L, Wu X, Pang K. A human corneal endothelium equivalent constructed with acellular porcine corneal matrix. Indian J Med Res. 2012;135(6):887–94. [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao B, Cooper LJ, Brahma A, MacNeil S, Rimmer S, Fullwood NJ. Development of a three-dimensional organ culture model for corneal wound healing and corneal transplantation. Invest Ophthalmol Vis Sci. 2006;47(7):2840–6. [DOI] [PubMed] [Google Scholar]
- 57.Reich S, Muller-Goymann C. Development of an organotypical cornea construct as an in vitro model for permeation studies. Ophthalmologe. 2001;98(9):853–8. [DOI] [PubMed] [Google Scholar]
- 58.Stern M, Klausner M, Alvarado R, Renskers K, Dickens M. Evaluation of the EpiOcularTM tissue model as an alternative to the Draize eye irritation test. Toxicol In Vitro. 1998;12(4):455–61. [DOI] [PubMed] [Google Scholar]
- 59.Kaluzhny Y, Kandárová H, Hayden P, Kubilus J, d’Argembeau-Thornton L, Klausner M. Development of the EpiOcular™ eye irritation test for hazard identification and labelling of eye irritating chemicals in response to the requirements of the EU cosmetics directive and REACH legislation. Altern Lab Anim. 2011;39(4):339–64. [DOI] [PubMed] [Google Scholar]
- 60.Alépée N, Leblanc V, Adriaens E, Grandidier M, Lelièvre D, Meloni M, Nardelli L, Roper C, Santirocco E, Toner F. Multi-laboratory validation of SkinEthic HCE test method for testing serious eye damage/eye irritation using liquid chemicals. Toxicol In Vitro. 2016;31:43–53. [DOI] [PubMed] [Google Scholar]
- 61.Kulkarni AA, Chang W, Shen J, Welty D. Use of Clonetics® human corneal epithelial cell model for evaluating corneal penetration and hydrolysis of ophthalmic drug candidates. Invest Ophthalmol Vis Sci. 2011;52(14):3259. [Google Scholar]
- 62.Kaluzhny Y, Kinuthia MW, Truong T, Lapointe AM, Hayden P, Klausner M. New human organotypic corneal tissue model for ophthalmic drug delivery studies. Invest Ophthalmol Vis Sci. 2018;59(7):2880–98. [DOI] [PubMed] [Google Scholar]
- 63.Defoe DM, Ahmad A, Chen W, Hughes BA. Membrane polarity of the Na+-K+ pump in primary cultures of Xenopus retinal pigment epithelium. Exp Eye Res. 1994;59(5):587–96. [DOI] [PubMed] [Google Scholar]
- 64.Chang C-W, Ye L, Defoe DM, Caldwell RB. Serum inhibits tight junction formation in cultured pigment epithelial cells. Invest Ophthalmol Vis Sci. 1997;38(6):1082–93. [PubMed] [Google Scholar]
- 65.Hartnett ME, Lappas A, Darland D, McColm JR, Lovejoy S, D’Amore PA. Retinal pigment epithelium and endothelial cell interaction causes retinal pigment epithelial barrier dysfunction via a soluble VEGF-dependent mechanism. Exp Eye Res. 2003;77(5):593–9. [DOI] [PubMed] [Google Scholar]
- 66.Peng S, Rahner C, Rizzolo LJ. Apical and basal regulation of the permeability of the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44(2):808–17. [DOI] [PubMed] [Google Scholar]
- 67.Lu SC, Sun W-M, Nagineni CN, Hooks JJ, Kannan R. Bidirectional glutathione transport by cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1995;36(12):2523–30. [PubMed] [Google Scholar]
- 68.Huang W, Prasad PD, Kekuda R, Leibach FH, Ganapathy V. Characterization of N5-methyltetrahydrofolate uptake in cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1997;38(8):1578–87. [PubMed] [Google Scholar]
- 69.Hamann S, La Cour M, Lui GM, Bundgaard M, Zeuthen T. Transport of protons and lactate in cultured human fetal retinal pigment epithelial cells. Pflugers Arch. 2000;440(1):84–92. [DOI] [PubMed] [Google Scholar]
- 70.Han Y-H, Sweet DH, Hu D-N, Pritchard JB. Characterization of a novel cationic drug transporter in human retinal pigment epithelial cells. J Pharmacol Exp Ther. 2001;296(2):450–7. [PubMed] [Google Scholar]
- 71.Dunn K, Aotaki-Keen A, Putkey F, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62(2):155–70. [DOI] [PubMed] [Google Scholar]
- 72.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu C-P, Morin GB, Harley CB, Shay JW, Lichtsteiner S, Wright WE. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279(5349):349–52. [DOI] [PubMed] [Google Scholar]
- 73.Nabi IR, Mathews AP, Cohen-Gould L, Gundersen D, Rodriguez-Boulan E. Immortalization of polarized rat retinal pigment epithelium. J Cell Sci. 1993;104(1):37–49. [DOI] [PubMed] [Google Scholar]
- 74.Hellinen L, Pirskanen L, Tengvall-Unadike U, Urtti A, Reinisalo M. Retinal pigment epithelial cell line with fast differentiation and improved barrier properties. Pharmaceutics. 2019;11(8):412. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Hellinen L, Hagström M, Knuutila H, Ruponen M, Urtti A, Reinisalo M. Characterization of artificially re-pigmented ARPE-19 retinal pigment epithelial cell model. Sci Rep. 2019;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bennis A, Jacobs J, Catsburg LA, Ten Brink JB, Koster C, Schlingemann RO, van Meurs J, Gorgels TG, Moerland PD, Heine VM. Stem cell-derived retinal pigment epithelium: The role of pigmentation as maturation marker and gene expression profile comparison with human endogenous retinal pigment epithelium. Stem Cell Rev Rep. 2017;13(5):659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhatt P, Narvekar P, Lalani R, Chougule MB, Pathak Y, Sutariya V. An in vitro assessment of thermo-reversible gel formulation containing sunitinib nanoparticles for neovascular age-related macular degeneration. AAPS PharmSciTech. 2019;20(7):281. [DOI] [PubMed] [Google Scholar]
- 78.Hellinen L, Hongisto H, Ramsay E, Kaarniranta K, Vellonen K-S, Skottman H, Ruponen M. Drug flux across RPE cell models: The hunt for an appropriate outer blood–retinal barrier model for use in early drug discovery. Pharmaceutics. 2020;12(2):176. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Spencer C, Abend S, McHugh KJ, Saint-Geniez M. Identification of a synergistic interaction between endothelial cells and retinal pigment epithelium. J Cell Mol Med. 2017;21(10):2542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cai H, Gong J, Del Priore LV, Tezel TH, Fields MA. Culturing of retinal pigment epithelial cells on an ex vivo model of aged human Bruch’s membrane. J Vis Exp. 2018;(134):e57084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Palanisamy K, Karunakaran C, Raman R, Chidambaram S. Optimization of an in vitro bilayer model for studying the functional interplay between human primary retinal pigment epithelial and choroidal endothelial cells isolated from donor eyes. BMC Res Notes. 2019;12(1):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Engelmann K, Valtink M. RPE cell cultivation. Graefes Arch Clin Exp Ophthalmol. 2004;242(1):65–7. [DOI] [PubMed] [Google Scholar]
- 83.Gillies MC, Su T, Naidoo D. Electrical resistance and macromolecular permeability of retinal capillary endothelial cells in vitro. Curr Eye Res. 1995;14(6):435–42. [DOI] [PubMed] [Google Scholar]
- 84.Gillies MC, Su T, Stayt J, Simpson JM, Naidoo D, Salonikas C. Effect of high glucose on permeability of retinal capillary endothelium in vitro. Invest Ophthalmol Vis Sci. 1997;38(3):635–42. [PubMed] [Google Scholar]
- 85.Tretiach M, Van Driel D, Gillies MC. Transendothelial electrical resistanceof bovine retinal capillary endothelial cells is influenced by cell growth patterns: An ultrastructural study. Clin Exp Ophthalmol. 2003;31(4):348–53. [DOI] [PubMed] [Google Scholar]
- 86.Abukawa H, Tomi M, Kiyokawa J, Hori S, Kondo T, Terasaki T, Hosoya K-I. Modulation of retinal capillary endothelial cells by Müller glial cell-derived factors. Mol Vis. 2009;15:451–7. [PMC free article] [PubMed] [Google Scholar]
- 87.Shen J, Cross ST, Tang-Liu DD, Welty DF. Evaluation of an immortalized retinal endothelial cell line as an in vitro model for drug transport studies across the blood-retinal barrier. Pharm Res. 2003;20(9):1357–63. [DOI] [PubMed] [Google Scholar]
- 88.Churm R, Dunseath GJ, Prior SL, Thomas RL, Banerjee S, Owens DR. Development and characterization of an in vitro system of the human retina using cultured cell lines. Clin Exp Ophthalmol. 2019;47(8):1055–62. [DOI] [PubMed] [Google Scholar]
- 89.Wu N, Doorenbos M, Chen DF. Induced pluripotent stem cells: Development in the ophthalmologic field. Stem Cells Int. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu J, Slevin M, Guo B-Q, Zhu S-R. Induced pluripotent stem cells as a potential therapeutic source for corneal epithelial stem cells. Int J Ophthalmol. 2018;11(12):2004–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schermer A, Galvin S, Sun T-T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103(1):49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bai J, Wang C. Organoids and microphysiological systems: New tools for ophthalmic drug discovery. Front Pharmacol. 2020;11:407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Foster JW, Wahlin K, Adams SM, Birk DE, Zack DJ, Chakravarti S. Cornea organoids from human induced pluripotent stem cells. Sci Rep. 2017;7(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Susaimanickam PJ, Maddileti S, Pulimamidi VK, Boyinpally SR, Naik RR, Naik MN, Reddy GB, Sangwan VS, Mariappan I. Generating minicorneal organoids from human induced pluripotent stem cells. Development. 2017;144(13):2338–51. [DOI] [PubMed] [Google Scholar]
- 95.Joseph R, Srivastava OP, Pfister RR. Modeling keratoconus using induced pluripotent stem cells. Invest Ophthalmol Vis Sci. 2016;57(8):3685–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aberdam E, Petit I, Sangari L, Aberdam D. Induced pluripotent stem cell-derived limbal epithelial cells (LiPSC) as a cellular alternative for in vitro ocular toxicity testing. PLoS One. 2017;12(6):e0179913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meyer JS, Howden SE, Wallace KA, Verhoeven AD, Wright LS, Capowski EE, Pinilla I, Martin JM, Tian S, Stewart R. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011;29(8):1206–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ito S-I, Onishi A, Takahashi M. Chemically-induced photoreceptor degeneration and protection in mouse iPSC-derived three-dimensional retinal organoids. Stem Cell Res. 2017;24:94–101. [DOI] [PubMed] [Google Scholar]
- 99.Burnight E, Wiley L, Drack A, Braun T, Anfinson K, Kaalberg E, Halder J, Affatigato L, Mullins R, Stone E. CEP290 gene transfer rescues Leber congenital amaurosis cellular phenotype. Gene Ther. 2014;21(7):662–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lukovic D, Castro AA, Delgado ABG, Bernal MdlAM, Pelaez NL, Lloret AD, Espejo RP, Kamenarova K, Sánchez LF, Cuenca N. Human iPSC derived disease model of MERTK-associated retinitis pigmentosa. Sci Rep. 2015;5:12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singh R, Kuai D, Guziewicz KE, Meyer J, Wilson M, Lu J, Smith M, Clark E, Verhoeven A, Aguirre GD. Pharmacological modulation of photoreceptor outer segment degradation in a human iPS cell model of inherited macular degeneration. Mol Ther. 2015;23(11):1700–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leach LL, Buchholz DE, Nadar VP, Lowenstein SE, Clegg DO. Canonical/β-catenin Wnt pathway activation improves retinal pigmented epithelium derivation from human embryonic stem cells. Invest Ophthalmol Vis Sci. 2015;56(2):1002–13. [DOI] [PubMed] [Google Scholar]
- 103.Schwarz N, Carr A-J, Lane A, Moeller F, Chen LL, Aguila M, Nommiste B, Muthiah MN, Kanuga N, Wolfram U. Translational read-through of the RP2 Arg120stop mutation in patient iPSC-derived retinal pigment epithelium cells. Hum Mol Genet. 2015;24(4):972–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sridhar A, Langer KB, Fligor CM, Steinhart M, Miller CA, Ho-A-Lim KT, Ohlemacher SK, Meyer JS. Human pluripotent stem cells as in vitro models for retinal development and disease. In: Ballios B, Young M, editors. Regenerative medicine and stem cell therapy for the eye. Cham, Switzerland: Springer; 2018. p. 17–49. [Google Scholar]
- 105.Wu SM, Hochedlinger K. Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nature Cell Biol. 2011;13(5):497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yvon C, Ramsden CM, Lane A, Powner MB, da Cruz L, Coffey PJ, Carr A-JF. Using stem cells to model diseases of the outer retina. Comput Struct Biotechnol J. 2015;13:382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Singh R, Shen W, Kuai D, Martin JM, Guo X, Smith MA, Perez ET, Phillips MT, Simonett JM, Wallace KA. iPS cell modeling of Best disease: Insights into the pathophysiology of an inherited macular degeneration. Hum Mol Genet. 2013;22(3):593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang J, Li Y, Chan L, Tsai Y-T, Wu W-H, Nguyen HV, Hsu C-W, Li X, Brown LM, Egli D. Validation of genome-wide association study (GWAS)-identified disease risk alleles with patient-specific stem cell lines. Hum Mol Genet. 2014;23(13):3445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kokkinaki M, Sahibzada N, Golestaneh N. Human induced pluripotent stem-derived retinal pigment epithelium (RPE) cells exhibit ion transport, membrane potential, polarized vascular endothelial growth factor secretion, and gene expression pattern similar to native RPE. Stem Cells. 2011;29(5):825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saini JS, Corneo B, Miller JD, Kiehl TR, Wang Q, Boles NC, Blenkinsop TA, Stern JH, Temple S. Nicotinamide ameliorates disease phenotypes in a human iPSC model of age-related macular degeneration. Cell Stem Cell. 2017;20(5):635–47.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chang Y-C, Chang W-C, Hung K-H, Yang D-M, Cheng Y-H, Liao Y-W, Woung L-C, Tsai C-Y, Hsu C-C, Lin T-C. The generation of induced pluripotent stem cells for macular degeneration as a drug screening platform: Identification of curcumin as a protective agent for retinal pigment epithelial cells against oxidative stress. Front Aging Neurosci. 2014;6:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ferrer M, Corneo B, Davis J, Wan Q, Miyagishima KJ, King R, Maminishkis A, Marugan J, Sharma R, Shure M. A multiplex high-throughput gene expression assay to simultaneously detect disease and functional markers in induced pluripotent stem cell-derived retinal pigment epithelium. Stem Cells Transl Med. 2014;3(8):911–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tucker BA, Mullins RF, Streb LM, Anfinson K, Eyestone ME, Kaalberg E, Riker MJ, Drack AV, Braun TA, Stone EM. Patient-specific iPSC-derived photoreceptor precursor cells as a means to investigate retinitis pigmentosa. Elite. 2013;2:e00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yoshida T, Ozawa Y, Suzuki K, Yuki K, Ohyama M, Akamatsu W, Matsuzaki Y, Shimmura S, Mitani K, Tsubota K. The use of induced pluripotent stem cells to reveal pathogenic gene mutations and explore treatments for retinitis pigmentosa. Mol Brain. 2014;7(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li Y, Wu W-H, Hsu C-W, Nguyen HV, Tsai Y-T, Chan L, Nagasaki T, Maumenee IH, Yannuzzi LA, Hoang QV. Gene therapy in patient-specific stem cell lines and a preclinical model of retinitis pigmentosa with membrane frizzled-related protein defects. Mol Ther. 2014;22(9):1688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jin Z-B, Okamoto S, Osakada F, Homma K, Assawachananont J, Hirami Y, Iwata T, Takahashi M. Modeling retinal degeneration using patient-specific induced pluripotent stem cells. PLoS One. 2011;6(2):e17084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ohlemacher SK, Sridhar A, Xiao Y, Hochstetler AE, Sarfarazi M, Cummins TR, Meyer JS. Stepwise differentiation of retinal ganglion cells from human pluripotent stem cells enables analysis of glaucomatous neurodegeneration. Stem Cells. 2016;34(6):1553–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Deng F, Chen M, Liu Y, Hu H, Xiong Y, Xu C, Liu Y, Li K, Zhuang J, Ge J. Stage-specific differentiation of iPSCs toward retinal ganglion cell lineage. Mol Vis. 2016;22:536–47. [PMC free article] [PubMed] [Google Scholar]
- 119.Teotia P, Chopra DA, Dravid SM, Van Hook MJ, Qiu F, Morrison J, Rizzino A, Ahmad I. Generation of functional human retinal ganglion cells with target specificity from pluripotent stem cells by chemically defined recapitulation of developmental mechanism. Stem Cells. 2017;35(3):572–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen J, Riazifar H, Gian M-X, Huang T. Modeling autosomal dominant optic atrophy using induced pluripotent stem cells and identifying potential therapeutic targets. Stem Cell Res Ther. 2016;7(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vergara MN, Flores-Bellver M, Aparicio-Domingo S, McNally M, Wahlin KJ, Saxena MT, Mumm JS, Canto-Soler MV. Three-dimensional automated reporter quantification (3D-ARQ) technology enables quantitative screening in retinal organoids. Development. 2017;144(20):3698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aasen DM, Vergara MN. New drug discovery paradigms for retinal diseases: A focus on retinal organoids. J Ocul Pharmacol Ther. 2020;36(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Elliott NT, Yuan F. A review of three-dimensional in vitro tissue models for drug discovery and transport studies. J Pharm Sci. 2011;100(1):59–74. [DOI] [PubMed] [Google Scholar]
- 124.Postnikoff CK, Pintwala R, Williams S, Wright AM, Hileeto D, Gorbet MB. Development of a curved, stratified, in vitro model to assess ocular biocompatibility. PLoS One. 2014;9(5):e96448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fligor CM, Langer KB, Sridhar A, Ren Y, Shields PK, Edler MC, Ohlemacher SK, Sluch VM, Zack DJ, Zhang C. Three-dimensional retinal organoids facilitate the investigation of retinal ganglion cell development, organization and neurite outgrowth from human pluripotent stem cells. Sci Rep. 2018;8(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McLelland BT, Lin B, Mathur A, Aramant RB, Thomas BB, Nistor G, Keirstead HS, Seiler MT. Transplanted hESC-derived retina organoid sheets differentiate, integrate, and improve visual function in retinal degenerate rats. Invest Ophthalmol Vis Sci. 2018;59(6):2586–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shokoohmand A, Jeon JE, Theodoropoulos C, Baldwin JG, Hutmacher DW, Feigl B. A novel 3D cultured model for studying early changes in age-related macular degeneration. Macromol Biosci. 2017;17(12):1700221. [DOI] [PubMed] [Google Scholar]
- 128.Lu Q, Yin H, Grant MP, Elisseeff JH. An in vitro model for the ocular surface and tear film system. Sci Rep. 2017;7(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu K, Shin SR, van Kempen T, Li YC,Ponraj V,Nasajpour A,Mandla S,Hu N, Liu X, Leijten J. Gold nanocomposite bioink for printing 3D cardiac constructs. Adv Funct Mater. 2017;27(12):1605352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mondal A, Gebeyehu A, Miranda M, Bahadur D, Patel N, Ramakrishnan S, Rishi AK, Singh M. Characterization and printability of sodium alginate-gelatin hydrogel for bioprinting nScLc co-culture. Sci Rep. 2019;9(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mondal A, Gebeyehu A, Subramanian R, Rishi A, Singh M. Bioprinted (3D) co-cultured spheroids with NSCLC PDX cells and cancer associated fibroblasts (CAFs) using alginate/gelatin hydrogel Cancer Res. 2018;78(13 Suppl):5018. [Google Scholar]
- 132.Wang Y, Shi W, Kuss M, Mirza S, Qi D, Krasnoslobodtsev A, Zeng J, Band H, Band V, Duan B. 3D bio-printing of breast cancer models for drug resistance study. ACS Biomater Sci Eng. 2018;4(12):4401–11. [DOI] [PubMed] [Google Scholar]
- 133.Trenfield SJ, Awad A, Goyanes A, Gaisford S, Basit AW. 3D printing pharmaceuticals: Drug development to frontline care. Trends Pharmacol Sci. 2018;39(5):440–51. [DOI] [PubMed] [Google Scholar]
- 134.Gianniopoulos AA, Mitsouras D, Yoo S-J, Liu PP, Chatzizisis YS, Rybicki FJ. Applications of 3D printing in cardiovascular diseases. Nat Rev Cardiol. 2016;13(12):701–18. [DOI] [PubMed] [Google Scholar]
- 135.Gibney R, Matthyssen S, Patterson J, Ferraris E, Zakaria N. The human cornea as a model tissue for additive biomanufacturing: A review. Procedia CIRP. 2017;65:56–63. [Google Scholar]
- 136.Sommer AC, Blumenthal EZ. Implementations of 3D printing in ophthalmology. Graefes Arch Clin Exp Ophthalmol. 2019;257(9):1–8. [DOI] [PubMed] [Google Scholar]
- 137.Isaacson A, Swioklo S, Connon CJ. 3D bioprinting of a corneal stroma equivalent. Exp Eye Res. 2018;173:188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kutlehria S, Dinh TC, Bagde A, Patel N, Gebeyehu A, Singh M. High-throughput 3D bioprinting of corneal stromal equivalents. J Biomed Mater Res B Appl Biomater. 2020;108(7):2981–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kim H, Jang J, Park J, Lee K-P, Lee S, Lee D-M, Kim KH, Kim HK, Cho D-W. Shear-induced alignment of collagen fibrils using 3D cell printing for corneal stroma tissue engineering. Biofabrication. 2019;11(3):035017. [DOI] [PubMed] [Google Scholar]
- 140.Phan C-M, Walther H, Qiao H, Shinde R, Jones L. Development of an eye model with a physiological blink mechanism. Transl Vis Sci Technol. 2019;8(5):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shi P, Yeong WY, Laude A, Tan EYS. Hybrid three-dimensional (3D) bioprinting of retina equivalent for ocular research. Int J Bioprint. 2017;3(2):138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lorber B, Hsiao W-K, Hutchings IM, Martin KR. Adult rat retinal ganglion cells and glia can be printed by piezoelectric inkjet printing. Biofabrication. 2013;6(1):015001. [DOI] [PubMed] [Google Scholar]
- 143.Kador KE, Grogan SP, Dorthé EW, Venugopalan P, Malek MF, Goldberg JL, D’lima DD. Control of retinal ganglion cell positioning and neurite growth: Combining 3D printing with radial electrospun scaffolds. Tissue Eng Part A. 2016;22(3-4):286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang P, Li X, Zhu W, Zhong Z, Moran A, Wang W, Zhang K, Chen S. 3D bioprinting of hydrogels for retina cell culturing. Bioprinting. 2018;12:e00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fenton OS, Paolini M, Andresen JL, Müller FJ, Langer R. Outlooks on three-dimensional printing for ocular biomaterials research. J Ocul Pharmacol Ther. 2020;36(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wu J, Mak HK, Chan YK, Lin C, Kong C, Leung CKS, Shum HC. An in vitro pressure model towards studying the response of primary retinal ganglion cells to elevated hydrostatic pressures. Sci Rep. 2019;9(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Torrejon KY, Papke EL, Halman JR, Stolwijk J, Dautriche CN, Bergkvist M, Danias J, Sharfstein ST, Xie Y. Bioengineered glaucomatous 3D human trabecular meshwork as an in vitro disease model. Biotechnol Bioeng. 2016;113(6):1357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011;21(12):745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Puleo CM, Ambrose WM, Takezawa T, Elisseeff J, Wang T-H. Integration and application of vitrified collagen in multilayered microfluidic devices for corneal microtissue culture. Lab Chip. 2009;9(22):3221–7. [DOI] [PubMed] [Google Scholar]
- 150.Seo J, Byun WY, Alisafaei F, Georgescu A, Yi Y-S, Massaro-Giordano M, Shenoy VB, Lee V, Bunya VY, Huh D. Multiscale reverse engineering of the human ocular surface. Nat Med. 2019;25(8):1310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mattern K, Beißner N, Reichl S, Dietzel A. DynaMiTES–A dynamic cell culture platform for in vitro drug testing PART 1–Engineering of microfluidic system and technical simulations. Eur J Pharm Biopharm. 2018;126:159–65. [DOI] [PubMed] [Google Scholar]
- 152.Beißner N, Mattern K, Dietzel A, Reichl S. DynaMiTES–A dynamic cell culture platform for in vitro drug testing PART 2–Ocular DynaMiTES for drug absorption studies of the anterior eye. Eur J Pharm Biopharm. 2018;126:166–76. [DOI] [PubMed] [Google Scholar]
- 153.Bennet D, Estlack Z, Reid T, Kim J. A microengineered human corneal epithelium-on-a-chip for eye drops mass transport evaluation. Lab Chip. 2018;18(11):1539–51. [DOI] [PubMed] [Google Scholar]
- 154.Haderspeck JC, Chuchuy J, Kustermann S, Liebau S, Loskill P. Organ-on-a-chip technologies that can transform ophthalmic drug discovery and disease modeling. Expert Opin Drug Discov. 2019;14(1):47–57. [DOI] [PubMed] [Google Scholar]
- 155.Su P-J, Liu Z, Zhang K, Han X, Saito Y, Xia X, Yokoi K, Shen H, Qin L. Retinal synaptic regeneration via microfluidic guiding channels. Sci Rep. 2015;5:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mishra S, Thakur A, Redenti S, Vazquez M. A model microfluidics-based system for the human and mouse retina. Biomed Microdevices. 2015;17(6):107. [DOI] [PubMed] [Google Scholar]
- 157.Dodson KH, Echevarria FD, Li D, Sappington RM, Edd JF. Retina-on-a-chip: A microfluidic platform for point access signaling studies. Biomed Microdevices. 2015;17(6):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kaji H, Ito S, Nagamine K, Nishizawa M, Nagai N, Abe T, editors. Characterization of retinal pigment epithelial cells and endothelial cells within a microfluidic device towards a retina on a chip. 18th International Conference on Miniaturized Systems for Chemistry and Life Sciences MicroTAS; 2014. [Google Scholar]
- 159.Chung M, Lee S, Lee BJ, Son K, Jeon NL, Kim JH. Wet-AMD on a chip: Modeling outer blood-retinal barrier in vitro. Adv Healthc Mater. 2018;7(2). [DOI] [PubMed] [Google Scholar]
- 160.Nafian F, Azad BKD, Yazdani S, Rasaee MJ, Daftarian N. A lab-on-a-chip model of glaucoma. BioRxiv. 2019:704510. [DOI] [PMC free article] [PubMed] [Google Scholar]


