Abstract
Frequent cyanobacterial blooms in eutrophic waters produce a variety of toxins such as microcystins (MCs), which are seriously harmful to waterbodies and human health. The spatiotemporal distribution characteristics of the MC-LR concentration in drinking water sources in seven river basins in China were investigated in this study. The removal rate of MC-LR in the purification process of water treatment plants and the human health risk of MC-LR in drinking water are also discussed. The results show that the detection frequency of MC-LR in source water was 55.46% and its concentration ranged from 0.06 × 10−3 to 52 × 10−3 μg L−1 (mean of 12.47 × 10−3 μg L−1), which are both below China's drinking water quality standard for algal toxins. The MC-LR concentration in lakes and reservoirs was higher than that in rivers, and exhibited an obvious spatiotemporal variation. The mean removal rate of MC-LR varied with river basin, and was also slightly higher for the advanced water treatment process (97.46%) in comparison to that of the conventional process (96.74%). The concentration of MC-LR in 8.26% of treated water samples was higher than that of raw water, thus indicating that MC-LR may be further released during the purification process. The risk index of MC-LR in treated water samples ranged from 2.29 × 10−3 to 8.40 × 10−3 (mean of 4.73 × 10−3), which corresponded to an extremely low level of risk. However, intensive monitoring should still be carried out in some high-concentration watersheds during the summer to ensure the safety of public drinking water.
Frequent cyanobacterial blooms in eutrophic waters produce a variety of toxins such as microcystins (MCs), which are seriously harmful to waterbodies and human health.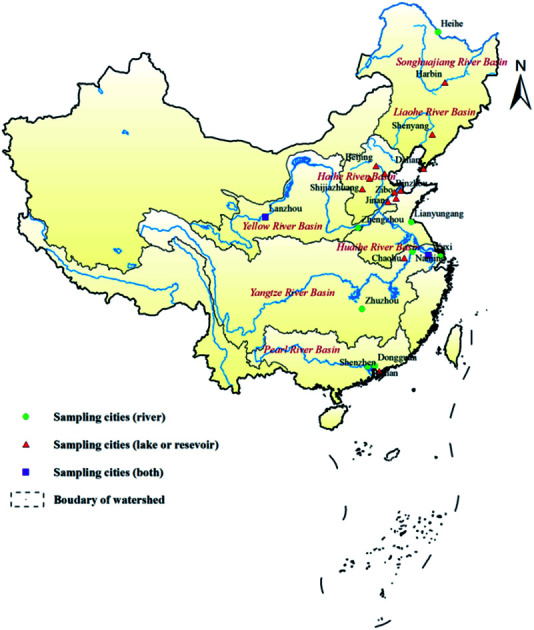
1. Introduction
In recent years, a large amount of anthropogenically produced nitrogen and phosphorus have entered waterbodies via surface runoff, sewage systems, and atmospheric dry and wet deposition processes, thus resulting in the eutrophication of waterbodies and algal blooms.1 Cyanobacterial blooms not only cause a decline in water quality, but also produce a series of highly toxic secondary metabolites, which pose a serious threat to people and the safety of drinking water.2 Microcystins (MCs) are the most frequently occurring of all algal toxins3 with a cyclic heptapeptide structure. Since the 1980s, when the structure of MCs was first identified, at least 246 variants have been found.4 Globally, among all of these MC congeners, MC-LR, MC-RR, and MC-YR are the variants most frequently detected in water samples, and in some cases, they are predominant.5–8
In Asia, many studies have detected MC-LR, MC-RR, and MC-YR, among which, MC-LR is the most commonly reported and most well-studied congener; hence, it is currently considered to be the reference compound due to its high, acute toxicity and high occurrence. According to a toxicokinetic study, MCs mediate their toxicity by uptake into hepatocytes via a carrier-mediated transport system, followed by inhibition of serine/threonine protein phosphatases.8 Ikehara et al. investigated the effect of 21 MC analogs on the activity of protein phosphatase 2A and found that MC-LR was the strongest inhibitor.9 The inhibition of protein phosphatases results in an increase in protein phosphorylation, which affects several processes, thus leading to various cellular responses such as apoptosis, reduced DNA repair, and tumor promotion.10 These responses have mainly been investigated with MC-LR.11 Long-term drinking of water contaminated with MCs will cause liver injury, induce liver cancer, intestinal cancer, and may produce genotoxicity. Epidemiological studies have found that the high incidence of primary liver cancer in some areas of southern China (e.g., Jiangsu Taixing and Haimen) is related to the contamination of drinking water by MCs.12,13 In Serbia, more than 80% of reservoirs used for water supply have experienced algae blooms over the past 80 years, with levels of MC-LR reaching up to 650 μg L−1 in the reservoir. The study by Svirčev et al. evidenced that there was a significant increase in the incidence of primary liver cancer in regions where these waters were used for human consumption in the 10 years previous to their investigation.14
The recognition that MCs represent a serious hazard to animal and human health led various countries to define specific guidelines and reference values for MC-LR and the group of MCs, thus requiring risk management measures to be put in place to protect human health. The water quality standard for algal toxins in drinking water recommended by the WHO is 1.0 μg L−1.15 Australian scholars recommend 1.0 μg L−1 as the upper limit for safe drinking water.16 The standard of algal toxins in the “Environmental Quality Standards for Surface Water” of China is 1.0 μg L−1.17
Many techniques have been used to analyze MCs, such as immunological and biological assays, UV absorbance, and mass spectrometry.18–20 Enzyme linked immunosorbent assay (ELISA) kits used to determine algal toxins are relatively inexpensive, simple to operate, and rapid, but they are less sensitive and selective for target algal toxins.18,21,22 The method, which is based on direct injection coupled with liquid chromatography with tandem mass spectrometry (LC-MS/MS), is recommended by the United States Environmental Protection Agency (US EPA) and can be used for the detection of six MC congeners (MC-LA, MC-LF, MC-LR, MC-LY, MC-RR, and MC-YR) in drinking water samples.23 However, the poor sensitivity of this method is one of its limitations. Other detection methods have been used for analyte separation, with UV absorbance commonly employed, but their limitations include low sensitivity, low specificity, and interference from complex matrices.24,25
To enhance the sensitivity and specificity of the analytical methods for the detection and quantification of MCs in water samples and complex matrices, ultra-performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS) has been developed. Combined with the sample pretreatment method of solid phase extraction (SPE), the detection limit varies with the concentration of the sample, and the lowest limit can reach 5.0 ng L−1.26
Pirsaheb et al. developed a new extraction method based on liquid-phase microextraction and the freezing of a deep eutectic solvent (LPME-FDES) for the determination of common pesticides in water samples prior to their analysis using high performance liquid chromatography-ultraviolet detection (HPLCUV) or metal in blood samples.27 The extraction methodology is simple, rapid, cheap, and green, since only small amounts of non-toxic solvents are necessary.28,29 The combination of continuous sample drop flow microextraction (CSDFME) with gas chromatography-electron-capture detection (GC-ECD) has been developed as a high preconcentration technique for the determination of chlorophenols (CPs) in environmental water samples.30 Dispersive liquid–liquid microextraction based on the solidification of floating organic drops has been applied to the simultaneous extraction and determination of contaminant traces because it is both fast and inexpensive.31–33 Solid phase extraction (SPE) coupled with ion pair based-surfactant assisted dispersive liquid–liquid microextraction based on the solidification of floating organic drop (IP-SA-DLLME-SFO) method was developed by Sadeghi. It has high sensitivity, accuracy, and credibility for the preconcentration and determination of environmental samples.34 Compared with the recent microextraction techniques mentioned above that determine organic and inorganic compounds, the solid-phase extraction method used in this study has simple operation steps, it can effectively reduce the impact of the surrounding environment on the system, and it can obtain more accurate results.
The problem of MCs pollution in the water environment, especially in drinking water sources, has therefore attracted long-term attention from scholars worldwide. Hernandez et al. (2009) concluded that although the risk of cancer from MCs in drinking water is currently less than the risk of cancer from smoking, the hazard represented by MCs should not be underestimated.35 As the eutrophication of waterbodies intensifies, cyanobacteria continue to erupt, and it seems inevitable that MCs will endanger human health to a greater extent. Hiroshi et al. determined that the highest concentration of total cellular microcystin in Lake Taihu in China was 50 μg L−1, which thus exceeds the drinking water limit (1.0 μg L−1) and may affect human health.36 Additionally, MC-LR has recently been reported as the major MC present in some reservoirs in Africa.37,38 The eutrophication and MCs pollution of waterbodies in developed countries such as Japan are also serious issues. For example, the concentration of MC-LR in a lake in Japan in June 1992 reached 1.5 μg L−1.39 In Australian freshwaters, the presence of MCs has been widely reported since 2000; for example, Cirés et al. determined that MC-LR was the most abundant MC variant.40 In southern Manitoba, Canada, MC-LR was detected in 44% of waterbodies at a concentration of between 0.1 and 0.6 μg L−1.41 According to the survey of Duong et al., the concentration of MCs in Hoan Kiem Lake in Vietnam reached 46.0 μg L−1.42 Vasconcelos et al. investigated the occurrence of MCs in lakes and reservoirs in central Mexico, and found that their concentrations ranged from 4.9 to 78.0 μg L−1.43 Generally, MCs are more commonly detected in lake waterbodies, with higher concentrations in some areas.44 In addition, there are significant regional differences in the release characteristics of MCs, and the peak time in some regions also varies.45,46
Many studies have been undertaken to date regarding the concentration of MCs in waterbodies in certain water basins, most of which have focused on the biotoxicity and environmental occurrence of MCs as well as the study of cyanobacterial blooms. However, recent research studies have typically been conducted over relatively small spatial scales and/or during the cyanobacteria outbreak season only. Such approaches cannot fully characterize the spatiotemporal distribution of MC-LR. More in-depth research is also required in order to undertake comparisons between seasons. In addition, health risks, such as non-carcinogenic risks, have seldom been reported during the evaluation of drinking water in previous studies. Concentration detection and the purification process of drinking water produced from raw water sources have rarely been accounted for in evaluation systems.
Therefore, in the present study, both raw and treated water samples were collected from 80 treatment plants across China. The spatiotemporal distribution of the MC-LR in seven major basins and its concentration in raw and treated water samples are assessed in this study. The removal rate of MC-LR in water treatment plants is also discussed, and a human health risk assessment was also undertaken. Accordingly, this study aimed to contribute to the understanding of MC-LR pollution in surface waters that are used as drinking water sources, and may provide a basis for the development of further prevention and control measures.
2. Materials and methods
2.1. Study areas and sample collection
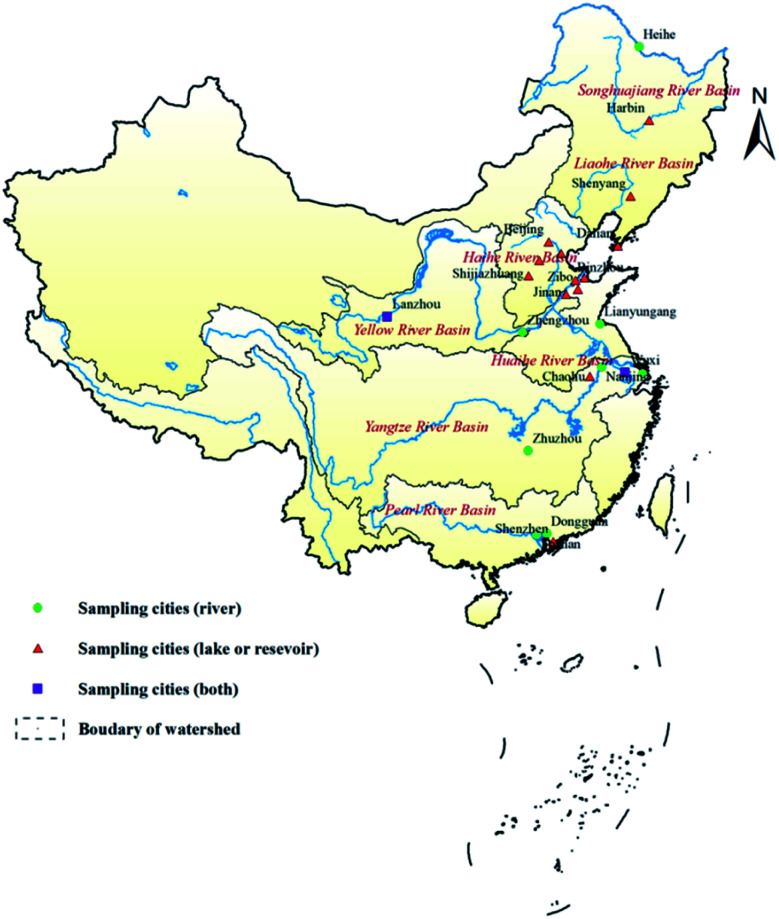
119 raw water samples (59 from lakes or reservoirs and 60 from rivers) were collected from 7 major river basins, and 121 treated water samples were collected from 80 water treatment plants in 23 cities from May 2015 to December 2017 (Table 1, Fig. 1). As the raw water samples were taken directly from waterbodies that act as sources for water treatment plants, the concentrations of MC-LR in these samples were taken as being representative of the source water concentration. In Fig. 1, the green dots indicate the cities in which water samples were collected from rivers, red triangles indicate the cities in which samples were collected from lakes or reservoirs, and purple squares indicate the cities in which samples were collected from rivers and lakes or reservoirs.
Sampling location information.
| Number | River basin | City | Number of influent samples |
|---|---|---|---|
| 1 | Yangtze river | Nanjing, Zhuzhou, Wuxi, Shanghai, Chaohu | 44 (9 from lakes or reservoirs, 35 from rivers) |
| 2 | Songhua river | Harbin, Heihe | 3 (2 from lakes or reservoirs, 1 from rivers) |
| 3 | Pearl river | Shenzhen, Dongguan, Foshan | 22 (8 from lakes or reservoirs, 14 from rivers) |
| 4 | Yellow river | Jinan, Zhengzhou, Lanzhou, Baoding, Zibo, Binzhou, Dongying | 34 (27 from lakes or reservoirs, 7 from rivers) |
| 5 | Liaohe river | Dalian, Shenyang | 6 (from lakes or reservoirs) |
| 6 | Haihe river | Shijiazhuang, Beijing, Baoding | 7 (from lakes or reservoirs) |
| 7 | Huaihe river | Lianyungang | 3 (from rivers) |
Fig. 1. Location of sampling cities in seven major river basins in China.
2.1.1. Sample processing and determination
SPE-UPLC-MS was used to determine the MC-LR concentration in the samples. Each water sample was first filtered through a 0.45 μm filter and concentrated by solid-phase extraction using a C18 cartridge, which was activated using 10 mL of methanol and 10 mL of pure water in sequence. The sample was then rinsed, eluted, and nitrogen-blown. The volume was subsequently adjusted. After filtering through a 0.2 μm disposable needle filter, all samples were injected and MCs were determined by UPLC-MS/MS.
2.1.2. Instruments and operating conditions
A SPE-UPLC-MS system (Waters®, USA) and Masslynx TM 4.1 workstation were used in this study. The chromatographic conditions involved a column ACQUITY UPLC® BEH C18 (1.7 μm, 2.1 mm × 50 mm). The MS used an electrospray ion source (ESI+), capillary voltage of 3.00 kV, ion source temperature of 110 °C, desolvent gas temperature of 380 °C, desolvent gas flow rate of 900 L h−1, and cone hole backflush gas flow rate of 50 L h−1.
2.1.3. Experimental reagents and chemicals
A MC-LR standard product obtained from Taiwan Algae Research Co., Ltd. was used. The MC-LR standard solution was made up to a 20 μg mL−1 stock solution with methanol and stored at −4 °C. The solid phase extraction column was obtained from Waters Oasis® HLB.DC HP (20 μm, 2.1 mm × 30 mm, reusable), and disposable needle filters (25 mm) were purchased from Acrodisc GHP 0.2 μm (Pull, USA).
2.2. Removal rate calculation
The removal rate of MC should be calculated using the following formula:Removal rate = (C1 − C2)/C1 × 100%,where C1 is the concentration of MC-LR in influent water and C2 is the concentration of MC-LR in effluent water.
2.3. Health risk assessment method
Humans may be exposed to MCs both directly and indirectly. Consumption of drinking water is a main direct route, and indirect exposure include the consumption of MCs-containing freshwater fish and shellfish, contaminated crops, vegetables that have been irrigation with MCs contaminated water, other food of an animal origin, and food supplements.47
Risk assessments are usually divided into carcinogenic and non-carcinogenic risks according to the carcinogenicity of the pollutant of interest. At present, most studies on the carcinogenicity of MCs are still in the exploration stage, and no carcinogenic intensity coefficient of MC-LR has yet been declared by the EPA.48 Therefore, this study only considers the oral intake of MC-LR through drinking water consumption. The hazard quotient (HQ) was used to assess the noncarcinogenic risks of MC-LR to humans. It is generally believed that the response of an organism to non-carcinogenic substances has a dose threshold, and it is considered that there is no adverse health effect if the risk is below the threshold. HQ is defined as the ratio of the long-term daily intake dose to the reference dose due to exposure. The single toxicant non-carcinogenic health risk assessment model recommended by the US EPA was used for evaluation.48 The calculation formula is as expressed by eqn (1):23
| HQ = CDI/RfD | 1 |
where CDI is the long-term daily intake (oral or inhalation) dose (of a pollutant) (μg per kg per d) and RfD is the maximum exposure reference dose (μg per kg per d), which is available from the US EPA48,49 and Integrated Risk Information System (IRIS, https://www.epa.gov/iris). When HQ is less than 1, the non-carcinogenic health risk level is considered to be acceptable; otherwise, a non-carcinogenic health risk is considered present.50
The CDI for oral and inhalation exposure can be calculated using eqn (2):
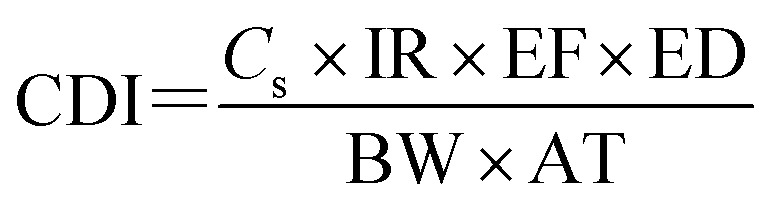 |
2 |
where Cs is the content of the contaminant of interest in a medium (e.g., water, food, or air) (μg kg−1 or μg L−1); IR is the intake of water, food, or air (mg per d or L per d); EF is the exposure frequency (365 d year−1); ED is the exposure period (non-carcinogens, 30 years; carcinogens, 70 years); BW is the average body weight (kg); AT is the average exposure time (non-carcinogens, 30 × 365 = 10 950 d; carcinogens, 70 × 365 = 25 550 d).
This article only considers the risk of MCs to human health through drinking. Therefore, the formula for calculating the non-carcinogenic risk can be simplified as eqn (3):
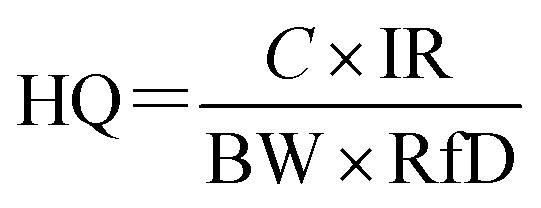 |
3 |
where C is the concentration of MCs in drinking water (μg L−1); IR is the drinking water intake (L per d); RfD is the reference dose for oral exposure of MC-LR.
2.4. Statistical analysis
Data were analyzed and plotted using Excel 2013 and Origin 2017. ArcGIS 10.5 was used for the sampling profile.
3. Results and discussion
3.1. Temporal and spatial distribution of MC-LR
Of the 59 samples of raw water from lakes/reservoirs, 36 (61.02%) contained MC-LR. The concentration ranged from 0.06 × 10−3 to 52 × 10−3 μg L−1 (mean of 11.44 × 10−3 μg L−1) and the highest concentration was found in Shijiazhuang located in the Haihe River basin. Of the 60 samples of raw water from rivers, 30 (50%) contained MC-LR. The concentration ranged from 0.22 × 10−3 to 37 × 10−3 μg L−1 (mean of 5.29 × 10−3 μg L−1) and the highest concentration was found in Zhengzhou City located in the Yellow River basin. Comparison of the mean MC-LR concentrations revealed that lakes/reservoirs had higher concentrations than rivers, which may have related to the lack of hydrodynamic conditions in the lakes/reservoirs and the eutrophication of waterbodies. However, all of the raw water samples were below the water quality standards for algae toxins in drinking water (1.0 μg L−1, China's GB5749-2006 and the WHO, see Section 1).
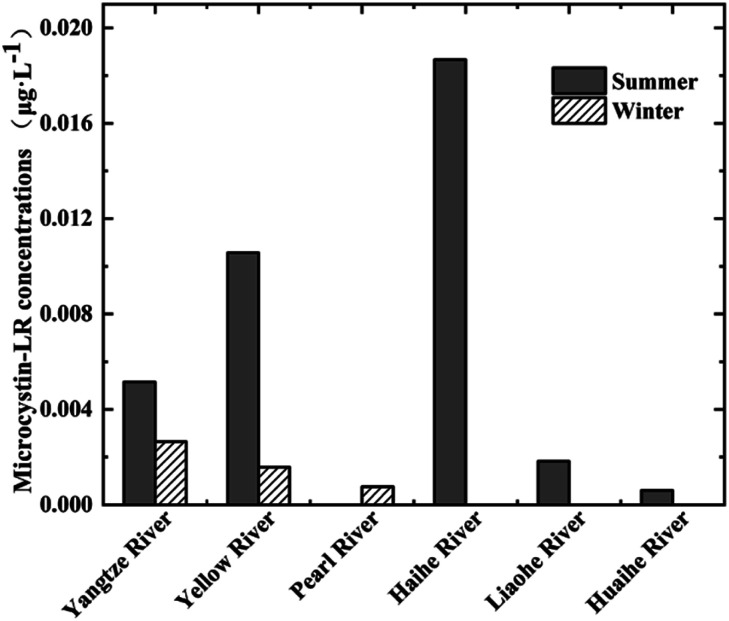
The concentration of MC-LR in the raw water samples was greatly affected by season. Of the 45 raw water samples collected from lakes/reservoirs during the summer (May to October), 29 (64.44%) contained MC-LR at a concentration ranging from 0.06 × 10−3 to 52 × 10−3 μg L−1 (mean of 7.59 × 10−3 μg L−1). Of the 14 raw water samples collected from lakes/reservoirs during the winter (November to April), 7 (50%) contained MC-LR at a concentration ranging from 0.40 × 10−3 to 3 × 10−3 μg L−1 (mean of 1.07 × 10−3 μg L−1). The seasonal data for lake/reservoir samples are shown in Fig. 2, which illustrates the seasonal variation of the MC-LR concentration in the different river basins. Summer concentrations were higher than winter concentrations, which was consistent with the seasonal trend of algal biomass. Additional data are given in Online Resource 1.
Fig. 2. Concentration of MC-LR in raw water samples collected from lakes/reservoirs in various river basins in China during the summer and winter from 2015 to 2017 (MC-LR was not detected in the water samples from Songhua River basin.).
Lezcano et al. investigated the environmental factors affecting MCs and found that the concentration of MCs exhibited a significant positive correlation with water temperature.51 Hu et al. found that MCs increased slowly in low temperature conditions, and that the low temperature adaptability of Microcystis was weaker than other algae.52 Xiao et al. also found that the concentration of MC-LR varied with the season, whereby an increased (decreased) water temperature corresponded to an increased (decreased) concentration, which is in general agreement with our results.53 This temperature relationship infers that the summer is suitable for the growth of cyanobacteria, and thus MC-LR pollution can occur easily at this time. This important seasonal variation between the concentration of MCs in water samples collected from the reservoir has also been reported for Portuguese reservoirs.54
Our findings suggest the need to control the eutrophication of surface waterbodies and to regularly monitor MC-LR concentration levels, especially during the high algae period in summer. Algae cells and MC-LR in raw water should also be removed to ensure the safety of treated water and to prevent algal toxin poisoning. Additionally, given that the concentration of MC-LR in our study was the highest in lakes and reservoirs in the Haihe River basin, this basin should be prioritized for monitoring during the summer.
3.2. Removal rate of MC-LR by the purification process
MCs are chemically stable, degrade slowly in water, and can accumulate in aquatic organisms.55 MCs must be controlled and eliminated during drinking water treatment. Of the 121 treated water samples, 19 (15.7%) contained MC-LR at a concentration ranging from 0.03 × 10−2 to 1.10 × 10−2 μg L−1 (mean of 0.31 × 10−2 μg L−1; median of 0.19 × 10−2 μg L−1). Thus, treated water sample concentrations were also lower than the drinking water standard (1 μg L−1) in China, and were clearly lower than those determined for the raw water samples. This indicates that although the water treatment procedure in the studied treatment plants did not completely eliminate the MCs, it did significantly reduce the concentration of MC-LR in the source water.
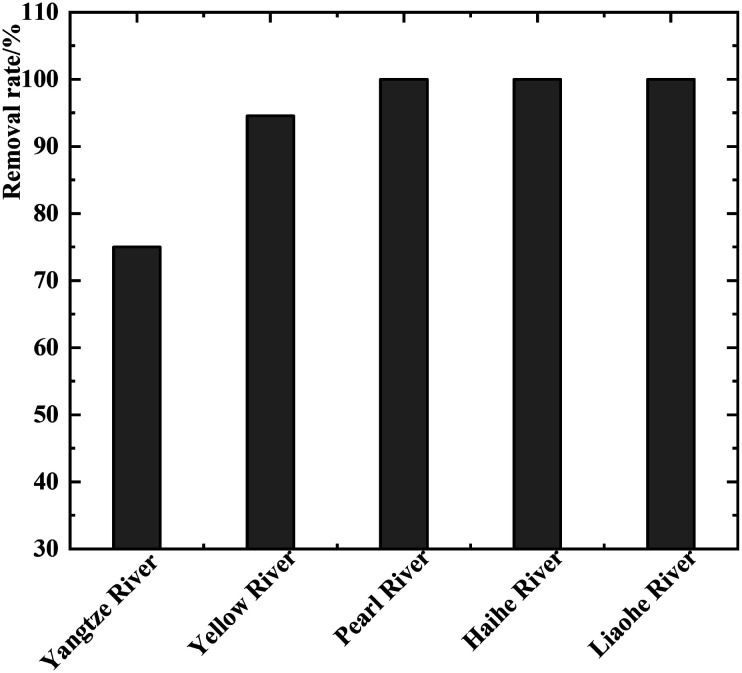
The MC-LR removal rate was calculated to range between 0% and 100% (mean of 93.91%). As shown in Fig. 3, the removal rate of MC-LR in Liaohe, Haihe, and Pearl River water treatment plants was relatively complete. The mean removal rate of MC-LR in the treatment plant in the Yellow River basin was 94.56%, which was higher than that of the treatment plant in the Yangtze River basin. MC-LR pollution thus seriously threatens drinking water safety in this area, and it is critical to improve the MC-LR removal rate.
Fig. 3. Removal rate of MC-LR in water samples collected from lakes/reservoirs in various river basins in China during the summer and winter from 2015 to 2017.
The drinking water treatment procedure can be divided into simple treatment, conventional treatment, and advanced treatment. The water treatment plants that were sampled in this study all adopt conventional or advanced treatments. The conventional treatment procedure includes coagulation, sedimentation, filtration, and disinfection to remove turbidity and the microbial content, and to reduce the colloidal organic matter content in water. Advanced treatment increases the use of ozone or activated carbon on the basis of the original treatment procedure to ensure that the drinking water quality meets the requirements of legislation.
In our study, the MC-LR removal rate in the treatment plants that use the conventional treatment procedure ranged from 25% to 100% in the source water for samples taken during the summer (mean of 96.74%). In the water treatment plants that use the advanced treatment procedure, the MC-LR removal rate ranged from 64.52% to 100% (mean of 97.46%). Hence, the rate of the advanced treatment was slightly better than that of the conventional treatment.
It was also observed that the concentration of MC-LR in 8.26% of the treated water samples was higher than that in the raw water samples. This indicates that MC-LR may be further released during the purification process in some water treatment plants using the conventional treatment procedure. Wang et al. reported that the pre-chlorination process easily destroyed the cyanobacterial cell structure and released more extracellular toxins, which led to an increase in the concentration of dissolved algal toxins in the treated water.56 According to a study by Jiang, the concentration of MCs increased after pre-chlorination and sand filtration.57 Advanced treatment can effectively remove the increased algal toxins by ozone and activated carbon, whereas the conventional treatment procedure has no effective countermeasures.
The maximum and mean MCs concentration in the treated water samples during the summer were higher than those of the raw water samples in this study. This demonstrates that there is still a risk that algal toxins are not removed during water treatment. Moreover, the results support the need to control the eutrophication of surface waters that are used as drinking water sources to reduce the occurrence of algal blooms.
3.3. Non-carcinogenic risk assessment of MC-LR in source water and effluent water
MC-LR enters the human body through three phases (the mouth, stomach, and small intestine) via the consumption of drinking water. Any part of MC-LR that is excreted from the body during the digestion stage will not affect human health. Therefore, it is suggested that in order to accurately assess the actual health risks of MC-LR through drinking water exposure, the bioavailable concentration of MC-LR (after gastrointestinal digestion) should be considered.58
Lü Chen et al. collated data on reported MC-LR concentrations in source and drinking water in China from various publicly released domestic and foreign studies.59 The HQ recommended by the US EPA was adopted to assess the non-carcinogenic risks of MC-LR posed by consuming contaminated source water and drinking water.49 The results show that in China, from April 1998 to June 2016, the concentrations of MC-LR in Chaohu Lake, Taihu Lake, Dianshan Lake, Dianchi Lake, the Yangtze River, Pearl River, and other water bodies ranged from ND to 54.90 μg L−1, and the HQ of lake water ranged from 0 to 51.00. The maximum HQ for lake (reservoir) water in China was greater than 1, indicating that the level of MC-LR has non-carcinogenic health risks.
According to the “Research Report on Environmental Exposure Behavior Patterns of Chinese Populations” issued by the Ministry of Environmental Protection in China,60 the average weight of adults in China is 60.6 kg and the amount of drinking water consumed is 1.85 L per d per person. Therefore, the IR in eqn (3) is 1.85 L per d and BW is 60.6 kg. In 1998, the WHO adopted the highest dose (NOAEL) from the MC-LR toxicity test of Fawell et al. for 13 weeks, which resulted in a TDI of 0.04 μg kg−1.61 The guideline value of MCs in drinking water is 1 μg L−1 and the TDI of MC-LR recommended by the WHO is 0.04 μg per kg per d.15 The US EPA recommends that if the RfD value is not specified, its value can be replaced by the TDI value.62 Therefore, the health risk assessment was performed using 0.04 μg per kg per d as the RfD value of MC-LR. For the health risk assessment, C in eqn (3) is the concentration of algal toxin in the source water or treated water. The risk grades of Ding et al. were used to evaluate the risk of MC-LR in waterbodies into five levels: extremely low, low, medium, high, and extremely high (Table 2).63
Relationship between the hazard quotient (HQ) value and the risk degree.
| HQ value | Risk degree |
|---|---|
| HQ ≤ 0.03 | Extremely low |
| 0.03 < HQ ≤ 0.71 | Low |
| 0.71 < HQ ≤ 1 | Medium |
| 1 < HQ ≤ 2.86 | High |
| HQ > 2.86 | Extremely high |
The results showed that among the 45 raw water samples taken during the summer that contained MC-LR, the HQ risk index for MC-LR ranged from 0.05 × 10−3 to 3.97 × 10−2 (mean of 8.57 × 10−3), i.e., generally extremely low or low risk level (Table 2). After treatment, the HQ for the 10 samples taken during the summer that contained MC-LR ranged from 2.29 × 10−3 to 8.40 × 10−3 (mean of 4.73 × 10−3), i.e., extremely low risk level.
4. Conclusions
MC-LR can be detected extensively in surface waters used as drinking water sources in important water basins in China, even during the winter. The detection rate of MC-LR in 119 surface water samples collected from 23 cities located in seven major water basins was 55.46% for the period from May 2015 to December 2017. The detection rate and mean concentration of MC-LR in water samples collected from lakes or reservoirs were 61.02% and 11.44 × 10−3 μg L−1, respectively, which were higher than those for samples collected from rivers (50% and 5.29 × 10−3 μg L−1). All of the detected concentrations of MC-LR in source water samples were below the surface water quality standards for MC-LR in China. The concentration of MC-LR in source water samples exhibited clear temporal characteristics. The detected rate and mean concentration of MC-LR in source water samples collected from lakes/reservoirs during the summer were 64.44% and 7.59 × 10−3 μg L−1, respectively, which were higher than those for samples taken during the winter (50% and 1.07 × 10−3 μg L−1). The mean concentration of MC-LR in source water samples collected from the Haihe River basin was higher than that of the other river basins. The removal rate of MC-LR in water treatment plants that use advanced treatment was slightly little higher than that for conventional treatment. However, the higher concentration of MC-LR in treated water samples was observed in several water treatment plants that use conventional treatment. This suggests that more MCs could be released into water through the destruction of the cyanobacterial cell structure during treatment. Generally, the non-carcinogenic risk associated with the concentration of MC-LR in the surface source water samples was low, even for samples taken during the summer, and the level of risk was in some cases further reduced after treatment. However, the total (original) concentration of MC-LR were used to calculate the non-carcinogenic health risk rather than the bioavailable concentration, which is a limitation of this study, and further research in this area is needed. There is still a need to control the eutrophication of surface waterbodies to ensure the safety of drinking water. Additionally, there is scope to elaborate on the need for further study regarding the release of MC-LR during water treatment.
This study assessed the temporal and spatial distribution of MC-LR in lakes, reservoirs, and rivers of seven major river basins, as well as its concentration in raw and treated water. The removal rate of MC-LR in water treatment plants and the human health risks are also discussed, which can help further understanding of the pollution of MC-LR in surface water used as a source of drinking water and provide a basis for to further improve methods of prevention and control.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by the Technology Program for Water Pollution Control and Treatment (No. 2014ZX07405001 and No. 2017ZX07401004).
References
- Pavagadhi S. Gong Z. Balasubramanian R. Environ. Toxicol. Chem. 2013;32(7):1574–1581. doi: 10.1002/etc.2203. [DOI] [PubMed] [Google Scholar]
- Apeldoorn M. E. V. Egmond H. P. V. Speijers G. J. A. Bakker G. J. I. Mol. Nutr. Food Res. 2007;51(1):7–60. doi: 10.1002/mnfr.200600185. [DOI] [PubMed] [Google Scholar]
- Palus J. Dziubaltowska E. B. Stańczyk M. g. Lewińska D. a. Mankiewicz-Boczek J. Izydorczyk K. Bonislawska A. Jurczak T. Zalewski M. Wlsowicz W. Int. J. Occup. Med. Env. 2007;20(1):48–65. doi: 10.2478/v10001-007-0008-2. [DOI] [PubMed] [Google Scholar]
- Spoof L. and Catherine A., Appendix 3: Tables of Microcystins and Nodularins, John Wiley & Sons, Ltd, 2017, pp. 526–538 [Google Scholar]
- Fetscher A. E. Howard M. D. A. Stancheva R. Kudela R. M. Stein E. D. Sutula M. A. Busse L. B. Sheath R. G. Harmful Algae. 2015;49:105–116. doi: 10.1016/j.hal.2015.09.002. [DOI] [Google Scholar]
- Greer B. Mcnamee S. Boots B. Cimarelli L. Guillebault D. Helmi K. Marcheggiani S. Panaiotov S. Breitenbach U. Akcaalan R. Harmful Algae. 2016;55:31–40. doi: 10.1016/j.hal.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Fatma G. Uzunmehmetoğlu O. Y. Diler Ö. Metcalf J. S. Sci. Total Environ. 2016;562:860–868. doi: 10.1016/j.scitotenv.2016.04.027. [DOI] [PubMed] [Google Scholar]
- Catherine A., Bernard C., Spoof L. and Bruno M., Microcystins and Nodularins, John Wiley & Sons, Ltd, 2017 [Google Scholar]
- Ikehara T. Imamura S. Sano T. Nakashima J. Kuniyoshi K. Oshiro N. Yoshimoto M. Yasumoto T. Toxicon. 2009;54:539–544. doi: 10.1016/j.toxicon.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Buratti F. M. Manganelli M. Vichi S. Stefanelli M. Funari E. Arch. Toxicol. 2017;91:1049–1130. doi: 10.1007/s00204-016-1913-6. [DOI] [PubMed] [Google Scholar]
- Cazenave J. Bistoni M. de los Á. Zwirnmann E. Wunderlin D. A. Wiegand C. Environ. Toxicol. 2006;21(1):22–32. doi: 10.1002/tox.20151. [DOI] [PubMed] [Google Scholar]
- Zhou X. F. Dong Z. H. Yu S. Z. China Cancer. 1999;8(3):350–351. [Google Scholar]
- Lu W. G. Lin W. Y. J. Transp. Med. 2001;15(5):469–470. [Google Scholar]
- Svirčev Z. Drobac D. Tokodi N. Vidovi M. Duli T. Journal of Environmental Science and Health. 2013;31:181–200. doi: 10.1080/10590501.2013.824187. [DOI] [PubMed] [Google Scholar]
- WHO, Cyanobacterial toxins: microcystin-LR, Guidelines for drinking-water quality, 1998, pp. 95–110 [Google Scholar]
- Australian National Health and Medical Research Council, Drinking Water Guidelines, NH & MRC, Canberra, Australia, 2005 [Google Scholar]
- State Environmental Protection Administration of China, GB5746–2006, 2006
- Mcelhiney J. Lawton L. A. Toxicol. Appl. Pharmacol. 2005;203:219–230. doi: 10.1016/j.taap.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Merel S. Walker D. Chicana R. Snyder S. Baures E. Thomas O. Environ. Int. 2013;59:303–327. doi: 10.1016/j.envint.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Oehrle S. A. Southwell B. Westrick J. Toxicon. 2010;55:965–972. doi: 10.1016/j.toxicon.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Stanford B. D., Adams C., Rosenfeldt E. J. and Wert E. C., Water Research, 2017 [DOI] [PubMed] [Google Scholar]
- Sangolkar L. N. Maske S. S. Chakrabarti T. Water Res. 2006;40:3485–3496. doi: 10.1016/j.watres.2006.08.010. [DOI] [PubMed] [Google Scholar]
- U.S. E.P.A., METHOD 544. Determination of microcystins and nodularin in drinking water by solid phase extraction and liquid chromatography/tandem mass spectrometry (LC/MS/MS), U.S. E.P.A, Washington DC.available at https://cfpub.epa.gov/si/si_public_file_download.cfm?p_download_id=522920&Lab=NERL, accessed May 21, 2015 [Google Scholar]
- Meriluoto J. A. O. Spoof L. E. M. Adv. Exp. Med. Biol. 2008;619:483–499. doi: 10.1007/978-0-387-75865-7_21. [DOI] [PubMed] [Google Scholar]
- Welker M. Bickel H. Fastner J. Water Res. 2002;36:4659–4663. doi: 10.1016/S0043-1354(02)00194-X. [DOI] [PubMed] [Google Scholar]
- Ortea P. M. Allis O. Healy B. M. Lehane M. Shuilleabhain A. N. Furey A. James K. J. Chemosphere. 2004;55:1395–1402. doi: 10.1016/j.chemosphere.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Pirsaheb M. Fattahi N. RSC Adv. 2018;8:11412–11418. doi: 10.1039/C8RA00912K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akramipour R. Golpayegani M. R. Gheini S. Fattahi N. Talanta. 2018;186:17–23. doi: 10.1016/j.talanta.2018.04.042. [DOI] [PubMed] [Google Scholar]
- Akramipour R. Golpayegani M. R. Ghasemi M. Noori N. Fattahi N. New J. Chem. 2019;43:6951–6958. doi: 10.1039/C9NJ00979E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimaei M. Sharafi K. Moradi M. Ghaffari H. R. Biglari H. Arfaeinia H. Fattahi N. Anal. Methods. 2017;9:2865–2872. doi: 10.1039/C7AY00530J. [DOI] [Google Scholar]
- Pirsaheb M. Fattahi N. Shamsipur M. Khodadadi T. J. Sep. Sci. 2013;36:684–689. doi: 10.1002/jssc.201200872. [DOI] [PubMed] [Google Scholar]
- Ataee M. Ahmadi-Jouibari T. Fattahi N. Int. J. Environ. Anal. Chem. 2016;96:271–283. doi: 10.1080/03067319.2016.1150464. [DOI] [Google Scholar]
- Rezaee M. Khalilian F. Mashayekhi H. A. Fattahi N. Anal. Methods. 2014;6:3456–3461. doi: 10.1039/C3AY42244E. [DOI] [Google Scholar]
- Sadeghi M. Nematifar Z. Irandoust M. Fattahi N. Hamzei P. Barati A. Ramezani M. Shamsipur M. RSC Adv. 2015;5:100511–100521. doi: 10.1039/C5RA15311E. [DOI] [Google Scholar]
- Hernandez J. M. López-Rodas V. Costas E. Med. Hypotheses. 2009;72:539–540. doi: 10.1016/j.mehy.2008.11.041. [DOI] [PubMed] [Google Scholar]
- Hiroshi S. Aimin H. Yasushi I. Song W. Takahiro K. Zhenjia Z. Hiroyuki K. Sci. World J. 2013:1–7. [Google Scholar]
- Yeshiemebet M. Demeke K. Lisa S. Jussi M. Environ. Sci. Pollut. Res. 2018;25:26861–26873. doi: 10.1007/s11356-018-2727-2. [DOI] [Google Scholar]
- Benard S. Steve O. Thomas R. Lewis S. Rainer K. Toxins. 2018;10:275. doi: 10.3390/toxins10070275. [DOI] [Google Scholar]
- Tanaka Y. Takenaka S. Matsuo H. Kitamori S. Tokiwa H. Toxicol. Environ. Chem. Rev. 1991;39(1–2):21–27. [Google Scholar]
- Cirés S. Alvarez-Roa C. Wood S. A. Puddick J. Loza V. Heimann K. Toxicon. 2014;88:62–66. doi: 10.1016/j.toxicon.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Davis J. V., Water-Resources Investigations Report, 1998, vol. 98–4164, available at https://pubs.usgs.gov/wri/wri98-4164/WRIR98-4164.pdf, accessed February 14, 2020 [Google Scholar]
- Duong T. T. Jähnichen S. Le T. P. Q. Ho C. T. Hoang T. K. Nguyen T. K. Vu T. N. Dang D. K. Environ. Earth Sci. 2013;71(5):2419–2427. doi: 10.1007/s12665-013-2642-2. [DOI] [Google Scholar]
- Vasconcelos V. Martins A. Vale M. Antunes A. Azevedo J. Welker M. Lopez O. Montejano G. Toxicon. 2010;56(3):425–431. doi: 10.1016/j.toxicon.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Reuters, some 400,000 in Ohio without drinking water, tests show lower toxin levels, http://www.indiaenvironmentportal.org.in/content/397866/some-400000-in-ohio-without-drinking-water-tests-show-lower-toxin-levels/, 2014
- Gibble C. M. Kudela R. M. Harmful Algae. 2014;39:146–153. doi: 10.1016/j.hal.2014.07.004. [DOI] [Google Scholar]
- Ji-Qing Q. I. Qiu-Hua L. I. Chen W. S. Xin-Yang M. A. Chen Q. Xiong M. J. Han M. S. Sun R. G. Chin. J. Ecol. 2019;38(09):2741–2748. [Google Scholar]
- Manganelli M. Scardala S. Stefanelli M. Palazzo F. Testai E. Ann. Ist. Super. Sanita. 2012;48(4):415–428. doi: 10.4415/ANN_12_04_09. [DOI] [PubMed] [Google Scholar]
- Emergency U. S. E. P. A. and Response R., Risk assessment guidance for superfund. Volume I: human health evaluation manual (Part A), 1989, vol. 804, pp. 636–640 [Google Scholar]
- U.S. E.P.A., The risk assessment guidelines of 1986, Office of Health and Environmental Assessment, 1986 [Google Scholar]
- Zhang L. E. Huang D. Yang J. Wei X. Qin J. Ou S. Zhang Z. Zou Y. Environ. Pollut. 2017;222:118–125. doi: 10.1016/j.envpol.2016.12.074. [DOI] [PubMed] [Google Scholar]
- Lezcano M. Á. Quesada A. El-Shehawy R. Harmful Algae. 2018;71:19–28. doi: 10.1016/j.hal.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Hu X. Zhang R. Ye J. Wu X. Zhang Y. Wu C. Environ. Sci. Pollut. Res. 2017;25(6):5921–5933. doi: 10.1007/s11356-017-0956-4. [DOI] [PubMed] [Google Scholar]
- Xiao C. C. Chen M. J. Mei F. B. Fang X. Li J. L. Huang T. R. Li Y. D. Deng W. Guangxi Med. J. 2018;40(15):1706–1709. [Google Scholar]
- Rodrigues M. A. Reis M. P. Mateus M. C. Toxicon. 2013;74:8–18. doi: 10.1016/j.toxicon.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Figueiredo D. R. D. Azeiteiro U. M. Esteves S. M. Goncalves F. J. M. Pereira M. J. Ecotoxicol. Environ. Saf. 2004;59(2):151–163. doi: 10.1016/j.ecoenv.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Wang S. F. Guo C. R. Xu X. Y. Zhu L. Chin. J. Appl. Ecol. 2016;27(5):1683–1692. doi: 10.13287/j.1001-9332.201605.026. [DOI] [PubMed] [Google Scholar]
- Jiang L. Water and Wastewater. 2017;53(09):11–15. [Google Scholar]
- Sharafi K. Nodehi R. N. Mahvi A. H. Pirsaheb M. Nazmara S. Mahmoudi B. Yunesian M. Food Chem. 2019;299:125126.125121–125126.125128. doi: 10.1016/j.foodchem.2019.125126. [DOI] [PubMed] [Google Scholar]
- Lü C. Zeng H. Wang J. Shu W. Q. J. Environ. Occup. Med. 2018;35(9):841–848. [Google Scholar]
- Ministry of Environmental Protection of the People’s Republic of China (MEPC), Research Report on Environmental Exposure Behavior Patterns of Chinese Population (Adults), 2013 [Google Scholar]
- Fawell J. K. Mitchell R. E. Everett D. J. Hill R. E. Hum. Exp. Toxicol. 1999;18(3):162–167. doi: 10.1177/096032719901800305. [DOI] [PubMed] [Google Scholar]
- United U. S. E. P. A. States Environmental Protection Agency (USEPA) Reference Dose (RfD): Description and Use in Health Risk Assessments Background Document 1A-March 15, 2016 [Google Scholar]
- Jiannan D. Shanshan Z. Xuyue W. Jingguo W. Hua Z. Ecology Environmental Sciences. 2018;27(11):2095–2101. [Google Scholar]