Abstract
Five typing methods, including biotyping (API ID32; BioMérieux, Marcy l'Etoile, France), quantitative antibiogram typing based on actual zone sizes, plasmid typing, randomly amplified polymorphic DNA (RAPD) analysis (with primer M13 and primer set ERIC-2–1026), and pulsed-field gel electrophoresis (PFGE), were compared with a previously performed method of DNA fingerprinting by AFLP (amplified fragment length polymorphism analysis) for their performance in the typing of blood isolates of Staphylococcus epidermidis. Sixteen epidemiologically unrelated strains and 11 sets of four blood culture isolates from 11 patients with septicemia were used. The stabilities and reproducibilities of the patterns, the discriminatory capacities of the methods, and the ability to apply the methods to blood culture isolates were used as performance criteria. All strains tested were typeable by each method, and the patterns were stable and reproducible. The numbers of different types within the collection of 16 epidemiologically different isolates were 5 by biotyping, 14 by antibiogram typing, 4 by plasmid typing, 9 by the RAPD assay (combination of results with primer M13 and primer set ERIC-2–1026), and 16 by PFGE. Within the 11 sets of four blood culture isolates the types found by quantitative antibiogram typing, plasmid typing, and PFGE were unique for each set, whereas by biotyping and RAPD analysis some types were observed in more than one set. The results of biotyping did not correspond with the results of the other methods or the results of AFLP. For 6 of the 11 sets, the results of all methods except those of biotyping corresponded completely. Quantitative antibiogram typing, PFGE, and AFLP proved to be the most accurate of the six typing methods tested.
Coagulase-negative staphylococci (CoNS) have increasingly been recognized as the cause of bacteremia in patients with neutropenia and indwelling prosthetic devices. To date CoNS are the most frequent species in blood cultures, and this is particularly the case for Staphylococcus epidermidis (1, 17). In immunocompromised patients, bacteremia caused by CoNS has far-reaching clinical consequences. First, the combination of neutropenia and bloodstream infections with S. epidermidis will usually require the use of antibiotics during neutropenic periods, and if present, the use of deep intravascular catheters may have to be reconsidered. Second, if septicemia caused by S. epidermidis in patients with indwelling prosthetic devices like artificial heart valves, vascular protheses, and joints is thought to be the result of infection at the site of the device, revision of the protheses will be considered. However, such removal is not without risk for the patients.
S. epidermidis is an inhabitant of the human skin, and it is often cultured from blood as a contaminant. Therefore, the clinical significance of this species in cultures of blood from an individual patient suspected of having bacteremia is often not clear, and positive blood cultures for patients with neutropenia or patients with indwelling prosthetic devices place both the clinician and the microbiologist in a dilemma. Although no strict criteria for the diagnosis of bacteremia exist, several clinical and laboratory characteristics are associated with genuine bacteremia. One of these is the repetitive culture of S. epidermidis from serial blood samples from a single patient, which is considered an indication of bacteremia rather than contamination (5, 9).
Comparative typing of isolates from multiple sequential blood cultures for a single patient can be used to assess the clinical significance of positive cultures for vulnerable patients. As a consequence, the results of comparative typing influence the decision to prescribe antibiotics or to consider the removal of an artificial device. Several methods of S. epidermidis strain identification have been described, including biochemical characterization (7), cluster analysis of antibiotic susceptibility profiles (19), plasmid profiling (11, 18), randomly amplified polymorphic DNA (RAPD) analysis (13), and large restriction fragment analysis by pulsed-field gel electrophoresis (PFGE) (10, 19). There is no generally accepted method for the typing of S. epidermidis, and there is a growing awareness of the need for a polyphasic approach by the use of different typing methods to draw final conclusions about strain identity.
Before using a particular technique for the typing of specific bacterial species, the technique must be evaluated critically. Struelens (21) proposed strict criteria for the evaluation of typing methods, including typeability, reproducibility, stability, discriminatory power, epidemiological concordance, and typing system concordance, before the typing methods can be applied to an appropriate strain collection. Recently, we have evaluated AFLP (amplified fragment length polymorphism analysis) for the typing of S. epidermidis with a predefined collection of strains (20). AFLP is a patented high-resolution DNA fingerprinting method that is based on the selective amplification of restriction fragments (25) and that is increasingly applied for microbial typing (16). In the previous study (20), AFLP had a high discriminatory capacity for S. epidermidis. In the present study we evaluated five other typing methods, using the same strain collection, and together with AFLP, these methods were compared for their performances in the typing of blood isolates of S. epidermidis by the criteria of Struelens (21).
MATERIALS AND METHODS
Test criteria.
Five methods for the epidemiological typing of S. epidermidis were compared by the use of three criteria. First, the in vitro stability and reproducibility of the typing results were tested with three strains (ATCC 14990T, LUH1024, and LUH3085). For each strain, four cultures were obtained by serial subcultivation on different media and under different incubation conditions. Details of the subculturing and selection of cultures for typing are described elsewhere (20). Second, the discriminatory capacities of the methods were determined with 16 epidemiologically unrelated isolates (see Table 1). Finally, the methods were applied to 11 sets of blood culture isolates from 11 patients with septicemia. For these sets variations in the patterns of isolates from each patient and of isolates from different patients were investigated (see Table 2).
TABLE 1.
Origin and typing results obtained by different typing methods with epidemiologically unrelated S. epidermidis strainsa
| Strain | Originb | Profile obtained by different methods
|
||||||
|---|---|---|---|---|---|---|---|---|
| Biotyping (API ID32) | Plasmid typing | RAPD typing
|
PFGE | AFLP no. | ||||
| M13 | ERIC-2–1026 | Comb.c | ||||||
| ATCC 14990T | DSMd (G) | 366030210 | 1 | 1 | 1 | 1 | 1 | 1 |
| LUH1024 | Leiden (H1)(NL) | 166032210 | 2 | 2 | 2 | 2 | 2 | 2 |
| LUH3085 | W.E. Kloos (US)e | 366032210 | 2 | 1 | 3 | 3 | 3 | 3 |
| LUH3088 | W.E. Kloos (US)f | 366032210 | 2 | 1 | 1 | 1 | 4 | 4 |
| LUH3090 | Leiderdorp (NL) | 366032210 | 3 | 1 | 1 | 1 | 5 | 1 |
| LUH3092 | Enschede (NL) | 567032210 | 2 | 3 | 4 | 4 | 6 | 5 |
| LUH3095 | Enschede (NL) | 166032210 | 3 | 4 | 3 | 5 | 7 | 6 |
| LUH3097 | Maastricht (NL) | 166032210 | 4 | 2 | 2 | 2 | 8 | 7 |
| LUH3099 | Maastricht (NL) | 166032210 | 3 | 3 | 3 | 6 | 9 | 8 |
| LUH4002 | Gouda (NL) | 166032210 | 1 | 5 | 2 | 7 | 10 | 9 |
| LUH4004 | Gouda (NL) | 366032210 | 3 | 1 | 1 | 1 | 11 | 10 |
| LUH4007 | Leiden (H1)(NL) | 366032210 | 1 | 2 | 5 | 8 | 12 | 11 |
| LUH6014 | Weert (NL) | 367032210 | 1 | 1 | 3 | 3 | 13 | 12 |
| LUH6015 | Weert (NL) | 366032210 | 3 | 2 | 5 | 8 | 14 | 13 |
| LUH6047 | Leiden (H2)(NL) | 367032210 | 3 | 1 | 5 | 9 | 15 | 14 |
| LUH9013 | The Hague (NL) | 366032210 | 3 | 2 | 5 | 8 | 16 | 15 |
Types within this set of strains are arbitrarily coded for each typing method. AFLP was performed in a previous study (20).
DSM, Deutsche Sammlung von Mikroorganismen und Zellkulturen; G, Germany; NL, The Netherlands; US, United States; H1, hospital 1; H2, hospital 2.
Comb., combination of codes obtained by RAPD analyses with primer M13 and primer set ERIC-2–1026.
Strain DSM20044.
Strain WAW815.
Strain SM225.
TABLE 2.
Epidemiological features and typing results for 44 blood culture strains of S. epidermidis from 11 patients suspected of having septicemia obtained by six methodsa
| Patient no. | Wardb | Strain | Date of isolation (yr/mo/day) | Profile obtained by different methods
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biotyping (API ID32) | Antibiogram typing | Plasmid profiling | RAPD analysis
|
PFGE | AFLP no. | ||||||
| M13 | ERIC-2–1026 | Comb.c | |||||||||
| 1 | IC surgery | LUH4007 | 92/12/20 | 366032210 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| LUH4008 | 92/12/19 | 366032210 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| LUH4010 | 92/12/22 | 366032210 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| LUH4015 | 92/12/19 | 366032210 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| 2 | Hematology | LUH4019 | 93/11/20 | 166032210 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| LUH4020 | 93/11/21 | 166032210 | 2 | 2 | 2 | 2 | 2 | 3 | 2 | ||
| LUH4021 | 93/11/21 | 166032210 | 2 | 2 | 2 | 2 | 2 | 4 | 2 | ||
| LUH4022 | 93/11/21 | 166032210 | 2 | 2 | 2 | 2 | 2 | 4 | 2 | ||
| 3 | Nephrology | LUH4034 | 93/01/28 | 367022210 | 3 | 3 | 3 | 3 | 3 | 5 | 3 |
| LUH4036 | 93/01/28 | 366022210 | 3 | 3 | 3 | 3 | 3 | 5 | 3 | ||
| LUH4038 | 93/01/28 | 366022210 | 3 | 3 | 3 | 3 | 3 | 5 | 3 | ||
| LUH4039 | 93/01/28 | 366022210 | 3 | 3 | 3 | 3 | 3 | 5 | 3 | ||
| 4 | Cardiology | LUH4041 | 93/04/14 | 367030200 | 4 | 4 | 4 | 4 | 4 | 6 | 4 |
| LUH4042 | 93/04/14 | 367030200 | 4 | 4 | 4 | 4 | 4 | 6 | 4 | ||
| LUH4043 | 93/04/14 | 367030200 | 4 | 4 | 4 | 4 | 4 | 6 | 4 | ||
| LUH4044 | 93/04/14 | 367030200 | 4 | 4 | 4 | 4 | 4 | 7 | 4 | ||
| 5 | IC cardiology | LUH4096 | 93/06/17 | 363232200 | 5 | 5 | 5 | 5 | 5 | 8 | 5 |
| LUH4097 | 93/06/17 | 163022200 | 5 | 5 | 5 | 5 | 5 | 8 | 5 | ||
| LUH4098 | 93/06/17 | 163022200 | 5 | 5 | 5 | 5 | 5 | 8 | 5 | ||
| LUH4099 | 93/06/17 | 163022210 | 5 | 5 | 5 | 5 | 5 | 8 | 5 | ||
| 6 | Cardiology | LUH6073 | 94/09/21 | 366032210 | 6 | 6 | 6 | 1 | 6 | 9 | 6 |
| LUH6074 | 94/09/21 | 366032210 | 6 | 6 | 6 | 1 | 6 | 9 | 7 | ||
| LUH6075 | 94/09/16 | 366032210 | 7 | 7 | 7 | 6 | 7 | 10 | 8 | ||
| LUH6076 | 94/09/16 | 362022210 | 7 | 6 | 6 | 6 | 8 | 11 | 9 | ||
| 7 | Pediatrics | LUH6077 | 95/02/21 | 366022210 | 8 | 8 | 8 | 1 | 9 | 12 | 10 |
| LUH6078 | 95/02/22 | 366032210 | 8 | 8 | 8 | 1 | 9 | 12 | 10 | ||
| LUH6079 | 95/02/21 | 366032210 | 8 | 8 | 8 | 1 | 9 | 12 | 10 | ||
| LUH6080 | 95/02/22 | 366032210 | 8 | 8 | 8 | 1 | 9 | 12 | 10 | ||
| 8 | Hematology | LUH6081 | 94/11/07 | 266224610 | 9 | 9 | 9 | 7 | 10 | 13 | 11 |
| LUH6082 | 94/11/06 | 266224610 | 10 | 9 | 9 | 7 | 10 | 13 | 11 | ||
| IC Int. Med. | LUH6083 | 94/11/10 | 266224610 | 9 | 9 | 9 | 7 | 10 | 13 | 11 | |
| LUH6084 | 94/11/09 | 266224610 | 9 | 9 | 9 | 7 | 10 | 13 | 11 | ||
| 9 | Hematology | LUH6085 | 94/10/05 | 166032210 | 11 | 10 | 10 | 1 | 11 | 14 | 12 |
| LUH6086 | 94/10/05 | 166032210 | 11 | 10 | 10 | 1 | 11 | 14 | 12 | ||
| LUH6087 | 94/10/06 | 166032210 | 11 | 10 | 10 | 1 | 11 | 14 | 12 | ||
| LUH6088 | 94/10/11 | 166032210 | 11 | 10 | 10 | 1 | 11 | 14 | 12 | ||
| 10 | Hematology | LUH6089 | 93/11/20 | 166032210 | 12 | 11 | 11 | 2 | 12 | 15 | 13 |
| LUH6090 | 93/11/21 | 166032210 | 12 | 11 | 11 | 2 | 12 | 15 | 13 | ||
| LUH6091 | 93/11/21 | 166032210 | 12 | 11 | 11 | 2 | 12 | 15 | 13 | ||
| LUH6092 | 93/11/21 | 166032210 | 12 | 11 | 11 | 2 | 12 | 15 | 13 | ||
| 11 | Hematology | LUH6093 | 94/01/17 | 366032210 | 13 | 12 | 1 | 2 | 13 | 16 | 14 |
| LUH6094 | 94/01/15 | 366032210 | 13 | 12 | 1 | 2 | 13 | 16 | 14 | ||
| LUH6095 | 94/01/15 | 366032210 | 14 | 13 | 5 | 2 | 14 | 17 | 15 | ||
| LUH6096 | 94/01/19 | 366032210 | 13 | 12 | 1 | 2 | 13 | 16 | 16 | ||
Types are arbitrarily coded within this set of strains for each typing method. AFLP was performed in a previous study (20).
IC, intensive care; Int. Med., Internal Medicine.
Combination of codes obtained by RAPD analysis with primer M13 and primer set ERIC-2–1026.
Strains.
Two collections with a total of 60 S. epidermidis isolates were used. Collection I comprised 16 strains, including 13 epidemiologically unrelated strains from several institutes in The Netherlands, ATCC 14990T (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmmH [DSM], Braunschweig, Germany), and LUH3088 and LUH3085, which were from W. E. Kloos, North Carolina State University, Raleigh. Collection II comprised 11 sets of four blood culture isolates from 11 patients suspected of having septicemia. Each set represented isolates from one patient, and the criteria of the Centers for Disease Control and Prevention were applied to the diagnosis of septicemia (5). Further details about the isolates in collections I and II are given in Table 1 and Table 2, respectively.
Biotyping.
The API ID32 system (BioMérieux, Marcy l'Etoile, France) was used to differentiate the strains below the species level. Patterns were read visually after an incubation period of 24 h. Each different code was considered a biotype.
Quantitative antibiogram typing.
The determination of the antibiograms was performed as described previously (19). A panel of antibiotics that comprised agents with different mechanisms of action was selected for its ability to discriminate between isolates and included chloramphenicol (30 μg), cefamandole (30 μg), ciprofloxacin (5 μg), clindamycin (10 μg), erythromycin (15 μg), fusidic acid (10 μg), gentamicin (10 μg), methicillin (10 μg), mupirocin (5 μg), penicillin (10 μg), rifampin (5 μg), streptomycin (10 μg), tetracycline (30 μg), and trimethoprim (1.25 μg). All disks except for clindamycin, methicillin, and mupirocin disks were obtained from Becton Dickinson (Cockeysville, Md.). Clindamycin, methicillin, and mupirocin disks were obtained from Oxoid (Basingstoke, United Kingdom). Isolates were comparatively typed on the basis of the similarities of their antibiotic susceptibilities. For this purpose zones were (semi)automatically read with the Biomic Video Reader (J. A. Kemme, J. H. Sloos, C. P. A. van Boven, and L. Dijkshoorn, 4th Int. Meeting Bacterial Epidemiological Markers, 1997). The unweighted values of the diameters of the inhibition zones were subjected to cluster analysis with the SPSS statistical software package (14). In the case of single colonies within the inhibition zone of methicillin, the zone was read as the smallest possible diameter (6 mm). Squared euclidean distances were calculated between all possible pairs of isolates, and clusters were generated by the method of Ward (26). Grouping of the isolates was depicted in a dendrogram.
Plasmid typing.
Plasmid DNA analysis was performed as described previously (8). Briefly, an overnight culture was lysed, and after centrifugation the supernatant was treated successively with RNase and proteinase. DNA was precipitated with ethanol, and the samples were electrophoresed in a 0.7% agarose gel with 0.5× TBE (Tris-borate-EDTA) buffer at a constant voltage of 100 V for 2.5 h. Plasmids of Escherichia coli 39RB61 (23) and E. coli V517 (12) were included as a reference for plasmid size determination. If no plasmids were observed, the isolation was done up to three times before it was concluded that an isolate was typeable or not. Each unique pattern was defined as a plasmid type.
RAPD analysis.
The S. epidermidis strains were grown on blood agar plates, and the DNAs were isolated by the method described by Boom et al. (4). RAPD profiles were obtained by PCR with core primer M13 (5′-GAG GTT GGC GGT TCT-3′) and primer set ERIC-2 and 1026 (5′-AAG TAA GTG ACT GGG GTG AGC G-3′ and 5′-TAC ATT CGA GGA CCC CTA AGT G-3′, respectively). The PCR mixture (100 μl per reaction mixture) consisted of 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 2.5 mM MgCl2, 0.01% gelatin, 0.1% (vol/vol) Triton X-100, deoxyribonucleotide triphosphates (final concentration, 0.2 mM each), 100 pmol of each primer(s), 100 ng of template DNA, and 0.5 U of super Taq DNA polymerase in reactions with primer M13 or 1.5 U of super Taq DNA polymerase in reactions with the ERIC-2–1026 primer set. For PCR with primer M13 the amplification program was as follows: 2 min of denaturation at 94°C, followed by 35 cycles of 94°C for 1 min, 25°C for 1 min, and 72°C for 4 min and a final cycle of 94°C for 1 min, 25°C for 1 min, and 72°C for 8 min. For PCR with primer set ERIC-2–1026 the amplification was initiated with 2 cycles of 94°C for 5 min, 35°C for 5 min, and 72°C for 5 min, followed by 30 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 2 min and a final cycle of 94°C for 1 min, 60°C for 1 min, and 72°C for 8 min. The amplified DNA products were separated and visualized by electrophoresis on 1.2% agarose gels containing 0.5 μg of ethidium bromide per ml. The gels were photographed under UV transillumination with Polaroid film, and the patterns were evaluated visually. Each unique pattern was defined as a RAPD type.
PFGE.
The isolation, digestion, and visualization of the DNA were performed as described previously (19). Briefly, a sample of an overnight culture of the isolate was mixed with the same amount of 2% low-melting-point agarose, and this mixture was hardened in molds. Next, the cells were lysed by overnight incubation of the samples with lysostaphin and lysozyme at a temperature of 4°C, followed by proteinase K treatment for 24 h at 55°C. After the DNA was washed in TE (Tris-EDTA) buffer, it was digested in restriction buffer containing 150 U of SmaI per ml. Restriction fragments were separated electrophoretically in a 0.7% agarose gel with the CHEF-DRII apparatus (Bio-Rad, Richmond, Calif.). Electrophoresis conditions were 200 V for 22 h at 14°C in 0.5× TE buffer, with pulse times ranging from 1 to 30 s. The DNA was visualized by staining with 0.5 μg of ethidium bromide per ml in water, and the gel was photographed. On each gel, one or more specimens of digested S. epidermidis LUH3088, i.e., a clinical isolate of our culture collection, were included as a reference. The criteria for the delineation of types were strict, with each unique pattern defining a PFGE type.
RESULTS
In vitro stabilities and reproducibilities of patterns.
Repeated testing of S. epidermidis ATCC 14990T, LUH1024, and LUH3085 resulted in a single type per method for each strain, although for plasmid typing up to three isolations were sometimes needed before plasmids were observed (data not shown). The four subcultures of each of the three strains showed the same type for each strain by each of the methods used. Thus, all strains tested were typeable by each method, and the patterns were stable and reproducible.
Discriminatory capacity.
Five different biotypes were distinguished among the 16 isolates (Table 1). The patterns of the grouping of the strains in the dendrogram that resulted from cluster analysis of the quantitative antibiogram typing assay were heterogeneous, and the maximum distance between two clusters of isolates was 4,031 (100%). The minimum distance between two epidemiologically unrelated isolates (isolates LUH4004 and LUH6014) was 152. Therefore, in this study, the delineation level of 152 was defined as the cutoff value for epidemiological relatedness, and isolates with a delineation level of 152 or less were defined as belonging to one antibiogram type. Thus, 14 antibiogram types were distinguished (data not shown).
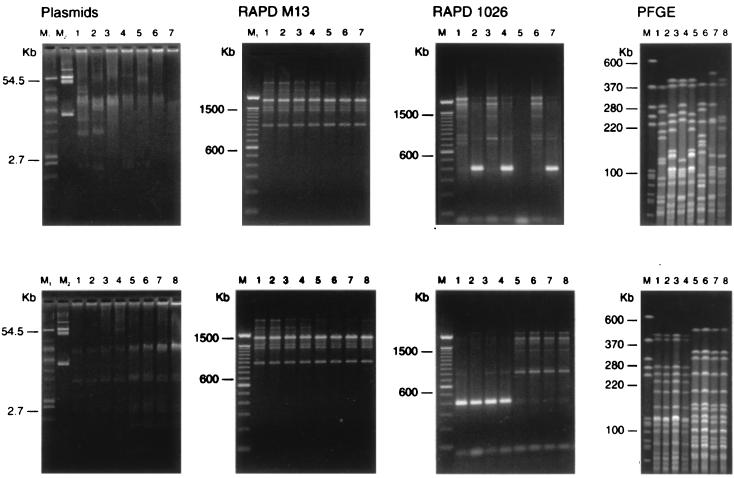
For the isolates within collection I the distinct electrophoretic profiles obtained by each of the three remaining typing methods were arbitrarily numbered (Table 1). An example of the patterns obtained by plasmid profiling, RAPD analysis, and PFGE of three isolates from collection I is shown in Fig. 1.
FIG. 1.
Example of patterns obtained by plasmid profiling RAPD analysis (with primers M13 [RAPD M13] and ERIC-2–1026 [RAPD 1026]), and PFGE. Lanes M1, E. coli 39RB61; lanes M2, E. coli V517. For PFGE LUH3088 was used as a marker. (Top panels) Patterns of seven epidemiologically unrelated S. epidermidis strains, including LUH3092, LUH1025, LUH3090, LUH3097, LUH3085, LUH6014, and LUH4007, in lanes 1 to 7, respectively. For details about the strains, see Table 1. (Bottom panels) Patterns of two sets of four blood culture isolates of S. epidermidis, including LUH4007, LUH4008, LUH4010, LUH4015, LUH6089, LUH6090, LUH6091, and LUH6092, in lanes 1 to 8, respectively. For details about the strains, see Table 2.
By the plasmid typing assay, the number of bands ranged from one to seven. Among the panel of 16 epidemiologically different isolates, four different plasmid patterns (types) were observed.
By the RAPD assay five different types were distinguished among the 16 epidemiologically different isolates with both primer M13 and primer set ERIC-2–1026. The isolates allocated to the five different types with each primer were not the same for both primers. Therefore, the combination of the results obtained with primer M13 and primer set ERIC-2–1026 gave nine different types.
Characterization of the DNAs of the isolates in collection I by PFGE resulted in 16 different patterns, which was a priori the maximum number of types to be distinguished.
Typing results for multiple sequential blood isolates.
Table 2 shows the results of the five typing methods investigated and the results of AFLP (20). Ten biotypes were obtained by biotyping (API ID32). Strains of biotype 366032210 were observed in four patients (patients 1, 6, 7, and 11), strains of biotype 166032210 were observed in three patients (patients 2, 9, and 10), and strains of biotype 366022210 were observed in two patients (patients 3 and 7). Strains of each of the remaining seven biotypes were observed in one patient each.
By using the quantitative antibiogram for typing with a delineation level of 152 as the cutoff value for epidemiological relatedness, 14 antibiogram types were distinguished. Strains of each type could be allocated to one patient only. The maximum distance between two clusters of isolates was 4,955 (100%). Patients 6, 8, and 11 each had isolates of different antibiogram types. The maximum distances between the isolates from each of these patients were 4,955, 286, and 1,970, respectively. For each of the eight remaining patients with isolates of the same antibiogram type, the maximum distances between the isolates ranged from 7 to 109 (data not shown).
By plasmid typing, 13 different types were observed, with each type being unique for isolates from each patient. Only one type was observed in nine patients, whereas in two sets of blood cultures (those for patients 6 and 11), two types were found within each set.
By RAPD analysis, 14 types were distinguished among the 44 isolates by combination of the results obtained with primer M13 and primer set ERIC-2–1026. For patients 6 and 11, three and two different types could be distinguished in each set, respectively. For the remaining nine patients only one type was found.
By PFGE 17 different types were observed among the isolates from the 11 patients. Each type was unique for each patient. Three types were observed in patients 2 and 6, and patients 4 and 11 each had isolates of two PFGE types. The remaining seven patients had isolates of only one type each. An example of the patterns obtained by plasmid typing, RAPD analysis, and PFGE of the isolates from two patients is shown in Fig. 1.
Comparison of results of the different methods including AFLP.
The results of each method used for typing of the blood culture isolates from 11 patients (Table 2) were compared with those of each of the other methods used and also with the results of AFLP (20). The biotypes obtained did not correspond to the results obtained by the other methods evaluated. For patients 1, 3, 5, 7, 9, and 10 the results of the six typing methods except those of biotyping corresponded completely. For patient 8 the results of all typing methods except those of antibiogram typing corresponded. For patients 2 and 4 the results of all methods except those of PFGE were in agreement. The correspondence between the typing results was less clear for patients 6 and 11. For patient 6 the only correspondence observed was between the results of RAPD analysis and PFGE, whereas for patient 11 the results of antibiogram typing, plasmid typing, RAPD analysis, and PFGE corresponded.
DISCUSSION
The observation of multiple blood cultures positive for S. epidermidis for patients with septicemia is usually interpreted as an indication of true bacteremia, whereas a single positive blood culture is suspected as a representation of contamination (9). It is also assumed that the isolates in a set of positive blood cultures represent the same strain. In this study, six typing methods were compared for their possible usefulness in elucidating the identities of S. epidermidis strains in repetitive cultures of blood from patients with septicemia. The criteria of Struelens (21) for the evaluation of typing methods were used in this study, although the number of isolates used to test the discriminatory capacity was less than the recommended number. The collection of strains used for this purpose was carefully composed.
Biotyping and determination of susceptibilities to antibiotics can be performed in laboratories with no advanced equipment for molecular typing techniques. Although the simplicity of biotyping with one of the API systems is attractive for practical purposes, the number of possible different profiles for S. epidermidis has been found to be limited (6, 7). In this study the discriminatory capacity of biotyping with the API ID32 system was also found to be low, with only five different types observed among 16 epidemiologically unrelated isolates (Table 1). Furthermore, it should be noted that these differences in coding were mostly based on only one test.
For antibiogram typing, the selection of antibiotics is crucial (15). In addition, it has been recommended that the actual sizes of the inhibition zones rather than qualitative results in terms of resistance or susceptibility be used (3). Biotyping and antibiogram typing are both relatively simple methods that can be performed in any diagnostic laboratory. By this combination of methods it was possible to successfully identify the strains found in blood cultures (8). In the present study biotyping had no added value over antibiogram typing. This finding underscores the fact that the determination of actual zone sizes combined with cluster analysis increases the discriminatory capacity of antibiogram typing.
Plasmid typing has been found to be useful for the determination of the relatedness of blood culture isolates of S. epidermidis (11). In the present study conflicting results were found with this method. Only four types were distinguished among the 16 unrelated strains (collection I), suggesting a limited discriminatory capacity. However, among the strains in collection II, at least one isolate from each of the 11 patients had a unique plasmid type. Because only collection I strains were used for determination of the discriminatory capacity, we concluded that the discriminatory power of plasmid typing is low. Besides, the use of plasmids for typing purposes was not without shortcomings, because each isolate must be tested three times in order to obtain clear patterns. Thus, it is concluded that this method, although simple to perform, is not suitable for the typing of bacteria in daily clinical practice.
Marquet-van der Mee et al. (13) used PCR fingerprinting for the typing of S. epidermidis isolates. Only 1 of 45 randomly designed primers had an acceptable discriminatory power, which means that the correct choice of primer is crucial. Several other experimental variables may also influence the results of RAPD analysis, including cycling conditions, RNA contamination, the concentrations of the reagents, the type of DNA polymerase used, or the visualization technique (24). In the present study, by RAPD analysis as a DNA amplification typing method, only nine different types were obtained among the collection of 16 epidemiologically different S. epidermidis isolates. Thus, RAPD analysis proved not to be suitable as a single method for the typing of S. epidermidis. The possibility that more, different patterns could have been observed by the use of another primer or primer combination cannot be excluded.
PFGE is another DNA-based method for the typing of S. epidermidis and has been used in several epidemiological studies (10, 19). It has been advocated as the “gold standard” for the typing of Staphylococcus aureus (2). A maximum of 16 types was observed among the strains in collection I, whereas among the strains in collection II, each type was unique for each patient. In patients 2 and 4 more than one type was observed, which was not the case for the other methods used. It cannot be excluded that the latter observation has been caused by small genetic alterations, which can lead to differences in up to three fragments (22). Despite the criteria used (22), the interpretation of PFGE patterns can be a source of discussion. Although it is known that mutations can lead to differences in the number of bands, the number of bands must be taken into account.
Our earlier study (20) showed the high resolution of AFLP. The number of different types obtained by AFLP was largely concordant with that obtained by PFGE in the present study. In our hands AFLP and PFGE were superior to plasmid typing and RAPD analysis for the typing of S. epidermidis strains.
A long antibiotic treatment course or removal of an invasive prothesis are possible consequences of the determination that an S. epidermidis strain is not a contaminant. Because of the clinical importance of elucidation of repetitive blood cultures as positive for S. epidermidis, a typing method must be easy to perform and results must be unequivocal. In the present study, the results of antibiogram typing, PFGE, and AFLP were highly discriminatory and correlated with the epidemiological features, demonstrating the usefulness of these methods for the typing of S. epidermidis. The discriminatory capacities of biotyping (API ID32), plasmid typing, and RAPD analysis appeared to be relatively low, and these methods are not recommended as single methods for the typing of S. epidermidis. Some of the results seem quite predictable, but it is interesting that the results of quantitative antibiogram typing seemed to be accurate in this study and that PFGE was perhaps overdiscriminatory. Antibiogram determination combined with cluster analysis is a simple method for rapid screening for strain identity, whereas PFGE or AFLP can be used as confirmatory DNA-based methods. PFGE and AFLP require high degrees of expertise and equipment, but the other methods can be performed in almost any laboratory. Numerous reference laboratories are capable of performing PFGE in a routine setting. Considering the impact of the diagnosis of septicemia caused by S. epidermidis in neutropenic patients or patients with indwelling prosthetic devices, application of these methods in order to find the ultimate explanation should not be a problem.
REFERENCES
- 1.Banerjee S N, Emori T G, Culver D H, Gaynes R P, Jarvis W R, Horan T, Edwards J R, Tolson J, Henderson T, Martone W J. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. Am J Med. 1991;91:86S–89S. doi: 10.1016/0002-9343(91)90349-3. [DOI] [PubMed] [Google Scholar]
- 2.Bannerman T L, Hannock G A, Tenover F C. Pulsed field gel electrophoresis as a replacement for bacteriophage typing of Staphylococcus aureus. J Clin Microbiol. 1995;3:551–555. doi: 10.1128/jcm.33.3.551-555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanc D S, Petignat C, Moreillon P, Wenger A, Bille J, Francioli P. Quantitative antibiogram as a typing method for the prospective epidemiological surveillance and control of MRSA: comparison with molecular methods. Infect Control Hosp Epidemiol. 1996;17:654–659. doi: 10.1086/647198. [DOI] [PubMed] [Google Scholar]
- 4.Boom R, Sol C J A, Salimans M M M, Jansen C L, Wertheim-van Dillen P M E, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garner J S, Jarvis W R, Emori T G, Horan T C, Hughes J M. CDC definitions for nosocomial infections. Am J Infect Control. 1988;16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 6.Geary C, Jordens J Z, Richardson J F, Hawcroft D M, Mitchell C J. Epidemiological typing of coagulase-negative staphylococci from nosocomial infections. J Med Microbiol. 1997;46:195–203. doi: 10.1099/00222615-46-3-195. [DOI] [PubMed] [Google Scholar]
- 7.Hébert G A, Cooksey R C, Clark N C, Hill B C, Jarvis W R, Thornsberry C. Biotyping coagulase-negative staphylococci. J Clin Microbiol. 1988;26:1950–1956. doi: 10.1128/jcm.26.10.1950-1956.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herwaldt L A, Boyken L D, Pfaller M A. Biotyping of coagulase-negative staphylococci: 108 isolates from nosocomial bloodstream infections. Diagn Microbiol Infect Dis. 1990;13:461–466. doi: 10.1016/0732-8893(90)90077-9. [DOI] [PubMed] [Google Scholar]
- 9.Herwaldt L A, Geiss M, Kao C. The positive predictive value of isolating coagulase-negative staphylococci from blood cultures. Clin Infect Dis. 1996;22:14–20. doi: 10.1093/clinids/22.1.14. [DOI] [PubMed] [Google Scholar]
- 10.Huebner J, Pier G B, Maslow J N, Muller E, Shiro H, Parent M, Kropec A, Arbeit R D, Goldmann D A. Endemic nosocomial transmission of Staphylococcus epidermidis bacteremia isolates in a neonatal intensive care unit over 10 years. J Infect Dis. 1994;169:526–531. doi: 10.1093/infdis/169.3.526. [DOI] [PubMed] [Google Scholar]
- 11.Khatib R, Riederer K M, Clarks J A, Khatib S, Brisky L E, Wilson F M. Coagulase-negative staphylococci in multiple blood cultures: strain relatedness and determinants of same-strain bacteremia. J Clin Microbiol. 1995;33:816–820. doi: 10.1128/jcm.33.4.816-820.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macrina F L, Kopecko D J, Jones K R, Ayers D J, McCowen S M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978;1:417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- 13.Marquet-van der Mee N, Mallet S, Loulerge J, Audurier A. Typing of Staphylococcus epidermidis strains by random amplification of polymorphic DNA. FEMS Microbiol Lett. 1995;128:39–44. doi: 10.1111/j.1574-6968.1995.tb07497.x. [DOI] [PubMed] [Google Scholar]
- 14.Norusis M J. SPSS for windows professional statistics, release 5. Chicago, Ill: SPSS Inc.; 1992. pp. 83–109. [Google Scholar]
- 15.Richardson J F, Marples R R. Changing resistance to antimicrobial drugs, and resistance typing in clinically significant strains of Staphylococcus epidermidis. J Med Microbiol. 1982;15:475–484. doi: 10.1099/00222615-15-4-475. [DOI] [PubMed] [Google Scholar]
- 16.Savelkoul P H M, Aarts H J M, de Haas J, Dijkshoorn L, Duim B, Otsen M, Rademakers J L W, Schouls L, Lenstra J A. Amplified-fragment length polymorphism analysis: the state of the art. J Clin Microbiol. 1999;37:3083–3091. doi: 10.1128/jcm.37.10.3083-3091.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaberg D R, Culver D H, Gaynes R P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991;91:72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 18.Sloos J H, Dijkshoorn L, Trienekens T A M, van Harsselaar B, van Dijk Y, van Boven C P A. Multiresistant Staphylococcus epidermidis in a neonatal care unit. Clin Microbiol Infect. 1996;1:44–49. doi: 10.1111/j.1469-0691.1996.tb00199.x. [DOI] [PubMed] [Google Scholar]
- 19.Sloos J H, Horrevorts A M, Dijkshoorn L, van Boven C P A. Multiresistant Staphylococcus epidermidis in neonates in a secondary care hospital. J Clin Pathol. 1998;51:62–67. doi: 10.1136/jcp.51.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sloos J H, Janssen P, van Boven C P A, Dijkshoorn L. AFLP™ typing of Staphylococcus epidermidis in multiple sequential blood cultures. Res Microbiol. 1998;149:221–228. doi: 10.1016/s0923-2508(98)80082-x. [DOI] [PubMed] [Google Scholar]
- 21.Struelens M J. Consensus guidelines for appropriate use and evaluation of microbial epidemiologic typing systems. Clin Microbiol Infect. 1996;2:2–11. doi: 10.1111/j.1469-0691.1996.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 22.Tenover F C, Arbeit R D, Goering R V, Mickelsen P A, Murray B E, Persing D H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995;9:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Threlfall E J, Frost J A. The identification, typing and fingerprinting of salmonella: laboratory aspects and epidemiological application. J Appl Bacteriol. 1990;68:5–16. doi: 10.1111/j.1365-2672.1990.tb02542.x. [DOI] [PubMed] [Google Scholar]
- 24.Tyler K D, Wang G, Tyler S D, Johnson W M. Factors affecting reliability and reproducibility of amplification based DNA fingerprinting of representative bacterial pathogens. J Clin Microbiol. 1997;35:339–346. doi: 10.1128/jcm.35.2.339-346.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vos P, Hogers R, Bleeker M, Reijans M, van der Lee T, Hornes M, Freijters A, Pot J, Peleman J, Kuiper M, Zabeau M. AFLP: a new method for DNA fingerprinting. Nucleic Acids Res. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward J H. Hierarchical grouping to optimize an objective function. J Am Stat Assoc. 1963;58:236–244. [Google Scholar]