Abstract
Regenerative endodontics has introduced numerous procedures such as pulp implantation, revascularization, and postnatal stem cell therapy. Revascularization has been successfully implemented clinically nowadays, thus providing dentists with outrageous results. Platelet-rich fibrin (PRF) used either alone or along with bone graft promotes bone growth and vascularization. This matrix promotes migration, cell attachment, and proliferation of osteoblast that leads to bone formation. PRF consists of a packed fibrin complex consisting of leukocytes, cytokines, and glycoproteins such as thrombospondin. The usage of PRF has reported high success rates in surgical cases such as sinus lift procedures, healing of extraction sockets, and management of periapical abscesses. Compared to platelet-rich plasma, PRF is more economical, easy to prepare, and feasible to use in daily clinical practices. Revascularization compromised the induction of a blood clot into the root canal space, which emerged as a clinical triumph. This further led to platelet concentrates as an autologous scaffold on which revascularization could occur. The applications of PRF in regenerative endodontics are numerous, such as an agent for repairing iatrogenic perforation of the pulpal floor and for the revascularization of immature permanent teeth with necrotic pulps. It acts as a matrix for tissue ingrowth. Evidence of progressive thickening of dentinal walls, root lengthening, regression in the periapical lesion, and apical closure was reported. Further studies are needed to clarify the precise mechanism of action of PRF for dental pulp regeneration both in vitro and in vivo. The current review aims at the present uses of PRF in regenerative endodontics dentistry and its application with future recommendations and limitations.
1. Introduction
Procedures that involve the usage of materials that encourage healing and repair of the pulp dentin complex after restoring the infectious or diseased tooth tissue are known as regenerative procedures [1]. According to the concepts of regenerative therapy in dentistry, this procedure can turn a dead tooth into a vital one. Regenerative endodontics has introduced numerous procedures such as pulp implantation, revascularization, and postnatal stem cell therapy. Revascularization has been successfully implemented clinically nowadays, thus providing dentists with outrageous results [2]. Due to the increased rate of root canal treatment failure and posttreatment complications, regenerative endodontics plays an essential role in overcoming these complications. The goal is to prevent aggressive invasive instrumentation and radiographic exposure [3]. This is performed by the reimposition of β and T lymphocytes that aid in defense against the pathogens leading to pulp damage. The canal is completely sealed, and the vitality of the tooth is sustained, leading to the prevention of tooth fracture and periapical reinfection [4].
Healing is known to be a demand in surgical procedures, and it is achieved by a series of events: cellular organization, chemical signals, and extracellular matrix for tissue repair [5]. Platelet-rich fibrin used either alone or along with bone graft promotes bone growth and vascularization. This matrix promotes migration, cell attachment, and proliferation of osteoblast that leads to bone formation [6]. Cytokines released by PRF play a significant role in blood vessel formation and immune system stimulation to fight foreign pathogens [7]. Studies claim that PRF that is prepared using low centrifugal forces leads to the effective concentration of leukocytes and growth factors related to those prepared at high centrifugal forces [8]. A study has proved the enhanced results of PRF consumption along with iliac crest bone graft in patients with cleft alveolar ridge defects; relatively, in cases where iliac crest graft alone was used, results were unsatisfactory [9].
Similarly, an orthodontic surgical case was pursued using PRF, cancellous bone allograft, bovine bone matrix, and metronidazole, leading to complete healing with no complication [10]. Results obtained from studies have shown that when PRF is combined with the biomaterials, the respective substitute's revitalization power increases and is more suitable and acceptable for the defected tissue space. PRF develops the cell-to-cell interaction; thus, proper incorporation of biomaterial is attained [11]. The current review aims at the uses of PRF in regenerative endodontics and dentistry and its applications with future application and limitations.
2. Materials and Composition
Being an unnatural biomechanical complex, platelet-rich fibrin (PRF) is synthesized by homologized plasma. This is performed by the formation of a fibrin clot which occurs during centrifugation of human-derived blood. This clot later contains a copious number of cytokines, growth factors, and platelets resulting from a polymerization reaction; apart from this, there is no foreign enzyme or anticoagulant needed for its production. Transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), insulin growth factor-1 (IGF-1), fibroblast growth factor (FGF), and epidermal growth factor (EGF) are the cytokines present in PRF which contribute in osteoblast proliferation, angiogenesis, wound healing, and collagen formation [5], Figure 1.
Figure 1.
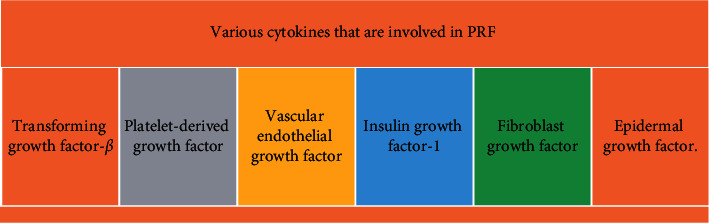
The various cytokines that are involved in PRF.
3. Classification of Plasma-Rich Fibrin
Initially, platelet concentrates in transfusion medicine were intended to treat and prevent hemorrhage due to various conditions. Several years ago, blood-derived products were used to seal wounds and promote healing using fibrin glues. These fibrin glues consisted of concentrated fibrinogen [6]. Autologous origin reduces the risk of contamination [7]. Consequently, further exploration in the field led to the replacement of fibrin glue with platelet concentrates, which was first described by Whitman et al. [8]. Consequently, platelet-rich fibrin (PRF) as a tool for tissue regeneration in medicine was introduced in 2001 [9]. The concept was extrapolated from the first-generation platelet concentrate, i.e., platelet-rich plasma (PRP). Its efficacy in various medical fields was astounding despite the setback of inhibiting the coagulation cascade because of an anticoagulant to the preparation [10–12]. With further advancement, Leukocyte PRF (L-PRF) came into being. It is named as such because of the higher leukocyte count. L-PRF acts as a three-dimensional fibrin matrix that entraps growth factors, with the added benefit of the absence of anticoagulants to the formulation [13–15]. A classification was proposed based on leukocyte and fibrin content in which platelet concentrates were categorized into four categories [16], as shown in Figure 2.
Figure 2.
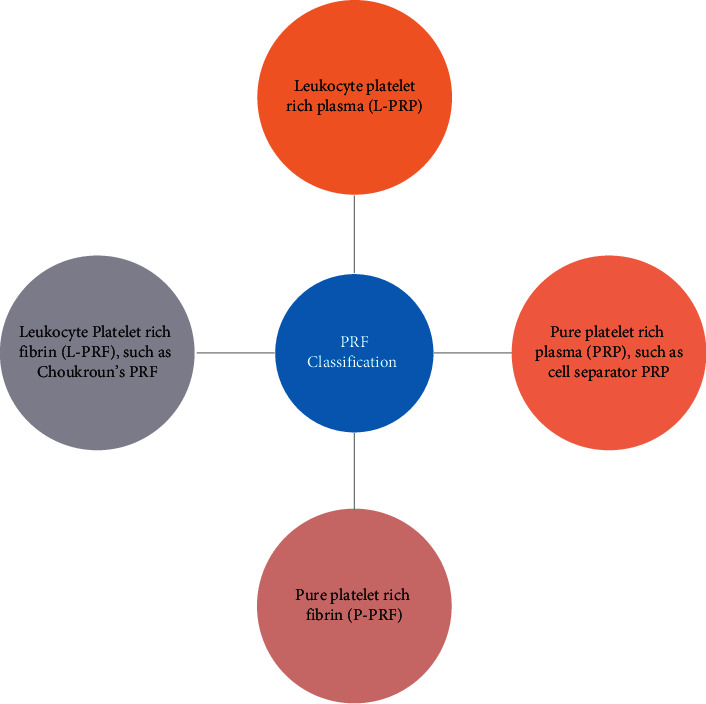
Classification based on leukocytes.
3.1. Leucocyte-Poor or Pure Platelet-Rich Plasma (P-PRP)
Pure platelet concentrates were first developed for topical use as a supplemental application to the classical platelet units, and their first clinical implementation was reported in maxillofacial surgery [8, 17].
The method of producing platelet concentrates for topical use is called plasmapheresis, which requires a cell separator that separates the blood into different components such as platelets, leukocytes, and erythrocytes, which can then be used and readministered to the patient [18].
3.2. Leucocyte- and Platelet-Rich Plasma (L-PRP)
The primary goal of developing an alternative, more convenient method was to incorporate platelet concentrates in day-to-day practice without the need for a transfusion laboratory. Initially, the product collected had high quantities of leucocytes which were hard to eliminate without the help of a cell separator. However, the alteration in collection parameters made way for pure (leucocyte free) PRP to be collected through more rigorous experimentation. The main setback of this technique is the essential requirement of expensive and complex centrifuges and preparation kits. Furthermore, like fibrin glue, the final product dissolves quickly. Consequently, L-PRP is rendered uncommon for daily use [19].
3.3. Leucocyte-Poor or Pure Platelet-Rich Fibrin (P-PRF) Concentrates
To produce pure platelet-rich fibrin, a small quantity of blood is collected into a collection tube. Trisodium citrate is added as an anticoagulant and a separator gel, and the suspension is centrifuged at high speed for six minutes. The separated buffy coat and platelet-poor plasma (PPP) are transferred into another tube containing calcium chloride and centrifuged. The stable clot is collected and being used.
The company that developed this method claimed that a “natural” platelet formulation is produced since bovine thrombin was not added. However, these claims are questionable because synthetic compounds are involved in the process (i.e., anticoagulant and separator gel) [19].
3.4. Leucocyte- and Platelet-Rich Fibrin (L-PRF) Concentrates
Choukroun et al. developed a simple technique for producing L-PRF in France [20]. Venous blood is drawn into glass tubes and centrifuged at a low speed [21]. Platelet activation and polymerization of fibrin are instantaneous due to the absence of anticoagulants. The PRF clot produced has several clinical applications in oral [22], maxillofacial surgery [23, 24], ENT [25], and plastic surgery [26]. This preparation has the advantage of gradual dissolution after application, and the three-dimensional fibrin mesh slowly remodels, corresponding to the physiological blood clot.
Moreover, the technique is simple and efficient; higher quantities are obtainable, involving only natural constituents as reactants. Thus, L-PRF is most appropriate for everyday practices, and many countries such as France, Israel, and Italy have already employed it [19].
4. Mechanism of Action of Platelet-Rich Fibrin
PRF consists of a packed fibrin complex consisting of leukocytes, cytokines, and glycoproteins such as thrombospondin. In a condensed PRF scaffold, leukocytes hold an integral position in growth factor release in addition to an immune response. Promotion of tissue regeneration and wound healing is achieved due to this concentrated suspension of platelets rich in growth factors. Transforming growth factor beta (TGF-β) accelerates reactionary dentinogenesis by stimulating odontoblastic activity [27]. Leukocytes, cytokines, and lymphocytes inhibit infection and inflammatory cascades. Vascular endothelial growth factor (VEGF) aids in angiogenesis which is pivotal in revascularization [28], Figure 3.
Figure 3.
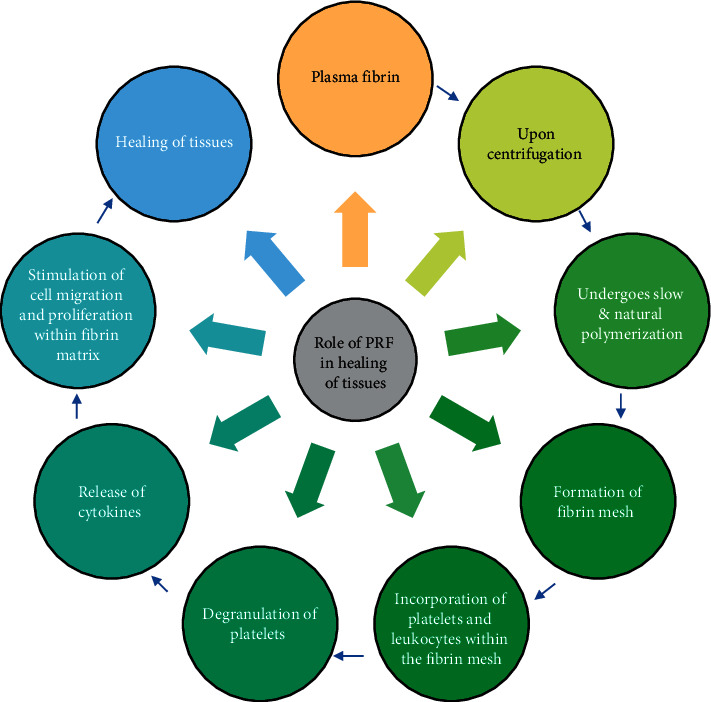
Role of PRF in healing of tissues.
4.1. Role of Fibrin in Angiogenesis
Cytokines including FGF, VEGF, angiopoietin, and PDGF get entrapped into the fibrin matrix's three-dimensional structure, resulting in a slowly progressing release, which is imperative in angiogenesis [29]. Fibrin causes the enhanced expression of αvβ3 integrin which stimulates the binding of endothelial cells to fibrin, fibronectin, and vitronectin [30].
4.2. Fibrin-Assisted Immune Response
Adhesion to endothelial cells, fibrinogen, and the transmigration of neutrophils is aided by fibrin. Fibrin acts by heightening the expression of CD11c/CD18 receptors on endothelial cells [31]. The wound colonization by macrophages is modulated by fibrin and fibronectin.
4.3. Effect of Fibrin on Mesenchymal Stem Cells
The fibrin matrix provides a scaffold for undifferentiated mesenchymal cells and promotes differentiation, imperative for tissue regeneration [22].
4.4. Effect of Fibrin on Osseous Tissue
Similarly, fibrin also acts as a scaffold for bone morphogenic protein, and its sustained release from within the fibrin matrix induces bone formation. Steady release of VEGF, FGF, and PDGF promotes angiogenesis. The circulating stem cells get entrapped into the fibrin clot resulting in hemostasis, thus enabling tissue restoration [32].
5. PRF in Dentistry
The usage of PRF has reported high success rates in surgical cases such as sinus lift procedures. Bone height and width are maintained, and wound healing is also achieved. In cases where immediate implants are to be placed, PRF has been reported to play a significant role in rapid healing of the extraction socket PRF, along with bone graft, and is known to give a synergetic effect. Intrabony defects are managed with open flap debridement and PRF to gain the clinical attachment loss [33]. According to a case report, an avulsed tooth with a periapical abscess was treated by shaping the canal, and a triple antibiotic paste was placed until the follow-up. In return, the antibiotic paste was removed, and the canal was irrigated. Revascularization was performed, and PRF was prepared and inserted in the canal, followed by biodentine and glass ionomer cement placement. After 6 months of follow-up, PRF helped in the apex's closure and repair and thickening of radicular dentin [34].
5.1. Platelet-Rich Fibrin in Regenerative Endodontics
Numerous conditions require endodontic intervention, including dental caries and pulpitis, prevalent in more than two-thirds of the world's population [35]. Additionally, dental trauma amongst children results in pulpal tissue damage, with concerns mainly in the immature tooth, as treatment options are limited in such cases of open apices [36]. However, with the advent of the treatment modality of revascularization, the tooth's survival has increased. It has positively impacted the management of symptoms, and postoperative radiographs confirm the physiological root completion [37].
Traditionally, revascularization compromised the induction of a blood clot into the root canal space [38], which emerged as a clinical triumph [39]. This further led to platelet concentrates as an autologous scaffold on which revascularization could occur [40]. The applications of platelet-rich fibrin (PRF) in regenerative endodontics are numerous. Bains et al. employed it as the agent for repairing iatrogenic perforation of the pulpal floor of the mandibular first molar when used in combination with MTA [41]. PRF is ideal for the revascularization of immature permanent teeth with necrotic pulps by providing a scaffold rich in growth factors, enhancing cellular proliferation and differentiation. It acts as a matrix for tissue ingrowth [42]. Furthermore, the gradual release of growth factors as the fibrin matrix resorbs ensures a steady healing process [43]. Evidence of progressive thickening of dentinal walls, root lengthening, regression in the periapical lesion, and apical closure was reported by Shivashankar et al., following the use of PRF on a tooth with pulpal necrosis and open apex [44].
Similarly, successful healing and apexification with the combined use of MTA as an apical barrier and autologous platelet-rich fibrin membrane as an internal matrix were reported by Rudagi K. and B. Rugadi. [45]. Additionally, PRF enhanced dental pulp cell proliferation, upregulation in alkaline phosphatase activity, and increased osteoprotegerin expression in a time-dependent fashion [46]. Pulpotomy in young permanent teeth using PRF has been reported to have affirmative results [47]. Moreover, instead of solely using biomaterials for the bone augmentation following treatment of periapical defects, the combination of PRF with a biomaterial (β-TCP) offers a better treatment alternative for swift healing [48]. It exhibits a more predictable radiographic and clinical bony regeneration [49].
Cases of traumatic immature teeth with necrotic pulp were treated with calcium hydroxide that induced a calcific barrier, but it reduces the organic support of dentin (radicular), which ceases root fracture. In order to prevent the chances of fracture, platelet-rich fibrin serves to be the best alternative that maintains tooth vitality and strength [42].
6. Clinical Implementation
6.1. PRF in Oral and Maxillofacial Surgery (OMFS)
The management of dimensional changes of the alveolar bone directly following tooth extraction has become an area of interest amongst researchers [50]. A study of 23 patients showed that the use of PRF resulted in reduced changes in dimension of the alveolar bone before implant placement in comparison to natural socket healing [51]. Furthermore, it was seen that there was nearly a ten-fold decrease in the rate of osteomyelitis infections when the third molar extraction sockets were filled using PRF [52]. Additionally, there are multiple other uses of PRF in oral and maxillofacial surgery.
PRF is routinely utilized in sinus lift procedures, particularly as a filling material during sinus lift and implant surgeries [53]. Several studies have proven the efficacy of L-PRF in new bone formation and its role in wound healing. Procedures such as alveolar ridge augmentation involve the use of PRF-based membranes [54]. Preliminary positive outcomes were observed when such membranes were used in patients on anticoagulant therapy and the prevention and treatment of patients on bisphosphonate-induced osteonecrosis of the jaw. L-PRF can be used to fill up cavities after tumor removal [55] and should be further investigated as a regenerative material after excision of malignant tumor tissue. It can be used in plastic reconstructive surgeries as an adjunct with graft material (adipocytes) [26]. Further experimentation and understanding of the appropriate surgical methodology are required in other fields of OMFS, such as orthognathic surgery (Lefort osteotomies, etc.), to benefit from L-PRF properties [56].
6.2. PRF in Periodontics
PRF is used in the regeneration of the periodontium. It is enriched with soluble growth factors and cytokines, including TGF-β1, VEGF, ILGF, PDGF, and Il-1, -4, and -6, which mainly aid tissue regeneration and accelerate wound healing [57]. There is a notable improvement in the clinical outcome and the radiographic reduction in the intrabony defect depth when either bone grafts or pharmacological agents such as metformin gel is used in combination with PRF of being used alone [57]. Similarly, investigations of periodontal regeneration of class II furcation defects using PRF were studied. According to this, there was a significant improvement in clinical attachment loss (CAL) gains with the use of PRF when compared to open flap debridement (OFP) [58–60]. In conclusion, these results signify the tissue repair potential using PRF for furcation defects.
According to a study carried out, during the coverage of gingival recession by raising a coronal advancement flap, the addition of PRF to the area showed a reduction in matrix metalloproteinase 8 and IL beta levels initially, and an increase in matrix metalloproteinase 1 level at day 10 was observed. This resulted in promoting periodontal wound healing in the earlier phase of the process [57].
6.3. Future Recommendation
To maintain bone height procedures in avulsion and cystic excision cases where implants are placed, we need to use platelet-rich fibrin. Compared to platelet-rich plasma (PRP), platelet-rich fibrin (PRF) is more economical, easy to prepare, and feasible to use in daily clinical practices. In periodontal procedures where guided tissue regeneration (GTR) is performed to overcome intrabony defects, platelet-rich fibrin (PRF) membranes are expected to be replaced. They are compatible with patients who have diabetes, who smoke, or who use anticoagulants, while using platelet-rich plasma (PRP) cannot be facilitated. Fibrin membrane acts as a natural stimulating barrier and prevents soft- and hard-tissue integration, promoting bone regeneration and filling the defect [61]. Studies are needed to clarify the precise mechanism of action of PRF for dental pulp regeneration both in vitro and in vivo.
Contributor Information
Anand Marya, Email: amarya@puthisastra.edu.kh.
Mohmed Isaqali Karobari, Email: dr.isaq@gmail.com.
Data Availability
All data used to support the findings of this study are included in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Murray P. E., Garcia-Godoy F., Hargreaves K. M. Regenerative endodontics: a review of current status and a call for action. Journal of Endodontics . 2007;33(4):377–390. doi: 10.1016/j.joen.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Johns D., Shivashankar V., Krishnamma S., Johns M. Use of photoactivated disinfection and platelet-rich fibrin in regenerative endodontics. Journal of Conservative Dentistry . 2014;17(5):p. 487. doi: 10.4103/0972-0707.139850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah N., Logani A., Bhaskar U., Aggarwal V. Efficacy of revascularization to induce apexification/apexogensis in infected, nonvital, immature teeth: a pilot clinical study. Journal of Endodontics . 2008;34(8):919–925. doi: 10.1016/j.joen.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Sedgley C. M., Messer H. H. Are endodontically treated teeth more brittle? Journal of Endodontics . 1992;18(7):332–335. doi: 10.1016/s0099-2399(06)80483-8. [DOI] [PubMed] [Google Scholar]
- 5.Khiste S. V., Naik Tari R. Platelet-rich fibrin as a biofuel for tissue regeneration. International Scholarly Research Notices . 2013;2013:6. doi: 10.5402/2013/627367.627367 [DOI] [Google Scholar]
- 6.Matras H. Die wirkungen vershiedener fibrinpraparate auf kontinuitat-strennungen der rattenhaut. Osterr Z Stomatol . 1970;67(9):338–359. [PubMed] [Google Scholar]
- 7.Gibble J., Ness P. Fibrin glue: the perfect operative sealant? Transfusion . 1990;30(8):741–747. doi: 10.1046/j.1537-2995.1990.30891020337.x. [DOI] [PubMed] [Google Scholar]
- 8.Whitman D. H., Berry R. L., Green D. M. Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. Journal of Oral and Maxillofacial Surgery . 1997;55(11):1294–1299. doi: 10.1016/s0278-2391(97)90187-7. [DOI] [PubMed] [Google Scholar]
- 9.Choukroun J., Adda F., Schoeffler C., Vervelle A. Opportunities in implant dentistry: PRF. Implantodontie . 2001;42:p. e62. [Google Scholar]
- 10.Anfossi G., Trovati M., Mularoni E., Massucco P., Calcamuggi G., Emanuelli G. Influence of propranolol on platelet aggregation and thromboxane B2 production from platelet-rich plasma and whole blood. Prostaglandins, Leukotrienes and Essential Fatty Acids . 1989;36(1):1–7. doi: 10.1016/0952-3278(89)90154-3. [DOI] [PubMed] [Google Scholar]
- 11.Fijnheer R., Pietersz R., Korte D., et al. Platelet activation during preparation of platelet concentrates: a comparison of the platelet-rich plasma and the buffy coat methods. Transfusion . 1990;30(7):634–638. doi: 10.1046/j.1537-2995.1990.30790385523.x. [DOI] [PubMed] [Google Scholar]
- 12.Marx R. E. Platelet-rich plasma: evidence to support its use. Journal of Oral and Maxillofacial Surgery . 2004;62(4):489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Toffler M. Guided bone regeneration (GBR) using cortical bone pins in combination with leukocyte- and platelet-rich fibrin (L-PRF) Compendium of Continuing Education in Dentistry (Jamesburg, N. J.: 1995) . 2014;35(3):192–198. [PubMed] [Google Scholar]
- 14.Lekovic V., Milinkovic I., Aleksic Z., et al. Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. Journal of Periodontal Research . 2012;47(4):409–417. doi: 10.1111/j.1600-0765.2011.01446.x. [DOI] [PubMed] [Google Scholar]
- 15.Shivashankar V. Y., Johns D. A., Vidyanath S., Sam G. Combination of platelet rich fibrin, hydroxyapatite and PRF membrane in the management of large inflammatory periapical lesion. Journal of Conservative Dentistry: JCD . 2013;16(3):261–4. doi: 10.4103/0972-0707.111329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Corso M., Mazor Z., Rutkowski J. L., Ehrenfest D. M. D. The use of leukocyte- and platelet-rich fibrin during immediate postextractive implantation and loading for the esthetic replacement of a fractured maxillary central incisor. Journal of Oral Implantology . 2012;38(2):181–187. doi: 10.1563/aaid-joi-d-12-cl.3802. [DOI] [PubMed] [Google Scholar]
- 17.Marx R. E., Carlson E. R., Eichstaedt R. M., Schimmele S. R., Strauss J. E., Georgeff K. R. Platelet-rich plasma. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology . 1998;85(6):638–646. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 18.Weibrich G., Kleis W. K. G., Hafner G., Hitzler W. E., Wagner W. Comparison of platelet, leukocyte, and growth factor levels in point-of-care platelet-enriched plasma, prepared using a modified curasan kit, with preparations received from a local blood bank. Clinical Oral Implants Research . 2003;14(3):357–362. doi: 10.1034/j.1600-0501.2003.00810.x. [DOI] [PubMed] [Google Scholar]
- 19.Dohan Ehrenfest D. M., Rasmusson L., Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends in Biotechnology . 2009;27(3):158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Naik B., Karunakar P., Jayadev M., Marshal V. R. Role of platelet rich fibrin in wound healing: a critical review. Journal of conservative dentistry: JCD . 2013;16(4):284–293. doi: 10.4103/0972-0707.114344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dohan D. M., Del Corso M., Charrier J. B. Cytotoxicity analyses of choukroun’s platelet-rich fibrin (PRF) on a wide range of human cells: the answer to a commercial controversy. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology . 2007;5(103):587–593. doi: 10.1016/j.tripleo.2007.03.016. [DOI] [Google Scholar]
- 22.Choukroun J., Diss A., Simonpieri A., et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. part IV: clinical effects on tissue healing. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology . 2006;101(3):e56–e60. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Choukroun J., Diss A., Simonpieri A., et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology . 2006;101(3):299–303. doi: 10.1016/j.tripleo.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 24.Diss A., Dohan D. M., Mouhyi J., Mahler P. Osteotome sinus floor elevation using choukroun’s platelet-rich fibrin as grafting material: a 1-year prospective pilot study with microthreaded implants. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology . 2008;105(5):572–579. doi: 10.1016/j.tripleo.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 25.Choukroun J. I., Braccini F., Diss A., Giordano G., Doglioli P., Dohan D. M. Influence of platelet rich fibrin (PRF) on proliferation of human preadipocytes and tympanic keratinocytes: a new opportunity in facial lipostructure (Coleman's technique) and tympanoplasty? Revue de laryngologie-otologie-rhinologie . 2007;128(1-2):27–32. [PubMed] [Google Scholar]
- 26.Braccini F., Dohan D. The relevance of choukroun’s platelet rich fibrin (PRF) during facial aesthetic lipostructure (coleman’s technique): preliminary results. Revue de Laryngologie Otologie Rhinologie . 2007;128(4):255–260. [PubMed] [Google Scholar]
- 27.Smith A. J., Murray P. E., Sloan A. J., Matthews J. B., Zhao S. Trans-dentinal stimulation of tertiary dentinogenesis. Advances in Dental Research . 2001;15(1):51–54. doi: 10.1177/08959374010150011301. [DOI] [PubMed] [Google Scholar]
- 28.Toffler M., Holtzclaw D., del Corso M., Toscano N. Introducing choukroun’s platelet rich fibrin (PRF) to the reconstructive surgery milieu. Journal of Implant and Advanced Clinical Dentistry . 2009;1(6):21–30. [Google Scholar]
- 29.Dvorak H. F., Harvey V. S., Estrella P., Brown L. F., McDonagh J., Dvorak A. M. Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing. Laboratory Investigation; a Journal of Technical Methods and Pathology . 1987;57(6):673–686. [PubMed] [Google Scholar]
- 30.Feng X., Clark R. A. F., Galanakis D., Tonnesen M. G. Fibrin and collagen differentially regulate human dermal microvascular endothelial cell integrins: stabilization of αv/β3 mRNA by fibrin1. Journal of Investigative Dermatology . 1999;113(6):913–919. doi: 10.1046/j.1523-1747.1999.00786.x. [DOI] [PubMed] [Google Scholar]
- 31.Loike J. D., Sodeik B., Cao L., et al. CD11c/CD18 on neutrophils recognizes a domain at the N terminus of the A alpha chain of fibrinogen. Proceedings of the National Academy of Sciences . 1991;88(3):1044–1048. doi: 10.1073/pnas.88.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawamura M., Urist M. R. Human fibrin is a physiologic delivery system for bone morphogenetic protein. Clinical Orthopaedics and Related Research . 1988;(235):302–310. doi: 10.1097/00003086-198810000-00031. [DOI] [PubMed] [Google Scholar]
- 33.Miron R. J., Zucchelli G., Pikos M. A., et al. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clinical Oral Investigations . 2017;21(6):1913–1927. doi: 10.1007/s00784-017-2133-z. [DOI] [PubMed] [Google Scholar]
- 34.Bakhtiar H., Esmaeili S., Fakhr Tabatabayi S., Ellini M. R., Nekoofar M. H., Dummer P. M. H. Second-generation platelet concentrate (platelet-rich fibrin) as a scaffold in regenerative endodontics: a case series. Journal of Endodontics . 2017;43(3):401–408. doi: 10.1016/j.joen.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 35.Larsen T., Fiehn N. E. Dental biofilm infections—an update. Apmis . 2017;125(4):376–384. doi: 10.1111/apm.12688. [DOI] [PubMed] [Google Scholar]
- 36.Ray H. L., Jr., Marcelino J., Braga R., Horwat R., Lisien M., Khaliq S. Long-term follow up of revascularization using platelet-rich fibrin. Dental Traumatology . 2016;32(1):80–84. doi: 10.1111/edt.12189. [DOI] [PubMed] [Google Scholar]
- 37.Jeeruphan T., Jantarat J., Yanpiset K., Suwannapan L., Khewsawai P., Hargreaves K. M. Mahidol study 1: comparison of radiographic and survival outcomes of immature teeth treated with either regenerative endodontic or apexification methods: a retrospective study. Journal of Endodontics . 2012;38(10):1330–1336. doi: 10.1016/j.joen.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 38.Ding R. Y., Cheung G. S. P., Chen J., Yin X. Z., Wang Q. Q., Zhang C. F. Pulp revascularization of immature teeth with apical periodontitis: a clinical study. Journal of Endodontics . 2009;35(5):745–749. doi: 10.1016/j.joen.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Cehreli Z. C., Isbitiren B., Sara S., Erbas G. Regenerative endodontic treatment (revascularization) of immature necrotic molars medicated with calcium hydroxide: a case series. Journal of Endodontics . 2011;37(9):1327–1330. doi: 10.1016/j.joen.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 40.Nosrat A., Seifi A., Asgary S. Regenerative endodontic treatment (revascularization) for necrotic immature permanent molars: a review and report of two cases with a new biomaterial. Journal of Endodontics . 2011;37(4):562–567. doi: 10.1016/j.joen.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Bains R., Bains V. K., Loomba K., Verma K., Nasir A. Management of pulpal floor perforation and grade II Furcation involvement using mineral trioxide aggregate and platelet rich fibrin: a clinical report. Contemporary Clinical Dentistry . 2012;3(Suppl 2):S223–S227. doi: 10.4103/0976-237X.101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keswani D., Pandey R. K. Revascularization of an immature tooth with a necrotic pulp using platelet‐rich fibrin: a case report. International Endodontic Journal . 2013;46(11):1096–1104. doi: 10.1111/iej.12107. [DOI] [PubMed] [Google Scholar]
- 43.Simonpieri A., Del Corso M., Sammartino G., Dohan Ehrenfest D. M. The relevance of choukroun’s platelet-rich fibrin and metronidazole during complex maxillary rehabilitations using bone allograft. part I: a new grafting protocol. Implant Dentistry . 2009;18(2):102–111. doi: 10.1097/id.0b013e318198cf00. [DOI] [PubMed] [Google Scholar]
- 44.Shivashankar V. Y., Johns D. A., Vidyanath S., Kumar M. R. Platelet rich fibrin in the revitalization of tooth with necrotic pulp and open apex. Journal of Conservative Dentistry: JCD . 2012;15(4):395–8. doi: 10.4103/0972-0707.101926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudagi K., Rudagi B. One-step apexification in immature tooth using grey mineral trioxide aggregate as an apical barrier and autologus platelet rich fibrin membrane as an internal matrix. Journal of Conservative Dentistry . 2012;15(2):p. 196. doi: 10.4103/0972-0707.94582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang F. M., Yang S. F., Zhao J. H., Chang Y. C. Platelet-rich fibrin increases proliferation and differentiation of human dental pulp cells. Journal of Endodontics . 2010;36(10):1628–1632. doi: 10.1016/j.joen.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Hiremath H., Saikalyan S., Kulkarni S. S., Hiremath V. Second-generation platelet concentrate (PRF) as a pulpotomy medicament in a permanent molar with pulpitis: a case report. International Endodontic Journal . 2012;45(1):105–112. doi: 10.1111/j.1365-2591.2011.01973.x. [DOI] [PubMed] [Google Scholar]
- 48.Kim B. J., Kwon T. K., Baek H. S., et al. A comparative study of the effectiveness of sinus bone grafting with recombinant human bone morphogenetic protein 2-coated tricalcium phosphate and platelet-rich fibrin-mixed tricalcium phosphate in rabbits. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology . 2012;113(5):583–592. doi: 10.1016/j.tripleo.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 49.Jayalakshmi K., Agarwal S., Singh M. P., Vishwanath B. T., Krishna A., Agarwal R. Platelet-rich fibrin with β-tricalcium phosphate—a noval approach for bone augmentation in chronic periapical lesion: a case report. Case Reports in Dentistry . 2012;2012:6. doi: 10.1155/2012/902858.902858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Risi V., Clementini M., Vittorini G., Mannocci A., De Sanctis M. Alveolar ridge preservation techniques: a systematic review and meta-analysis of histological and histomorphometrical data. Clinical Oral Implants Research . 2015;26(1):50–68. doi: 10.1111/clr.12288. [DOI] [PubMed] [Google Scholar]
- 51.Hauser F., Gaydarov N., Badoud I., Vazquez L., Bernard J.-P., Ammann P. Clinical and histological evaluation of postextraction platelet-rich fibrin socket filling. Implant Dentistry . 2013;22(3):295–303. doi: 10.1097/id.0b013e3182906eb3. [DOI] [PubMed] [Google Scholar]
- 52.Hoaglin D. R., Lines G. K. Prevention of localized osteitis in mandibular third-molar sites using platelet-rich fibrin. International Journal of Dentistry . 2013;2013:4. doi: 10.1155/2013/875380.875380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Del Corso M., Toffler M., Dohan Ehrenfest D. Use of an autologous leukocyte and platelet-rich fibrin (L-PRF) membrane in post-avulsion sites: an overview of choukroun’s PRF. Journal of Implant and Advanced Clinical Dentistry . 2010;1(9):27–35. [Google Scholar]
- 54.Tatullo M., Marrelli M., Cassetta M., et al. Platelet rich fibrin (PRF) in reconstructive surgery of atrophied maxillary bones: clinical and histological evaluations. International Journal of Medical Sciences . 2012;9(10):872–880. doi: 10.7150/ijms.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Charrier J. B., Monteil J. P., Albert S., Collon S., Bobin S., Dohan Ehrenfest D. M. Relevance of choukroun’s platelet-rich fibrin (PRF) and SMAS flap in primary reconstruction after superficial or subtotal parotidectomy in patients with focal pleiomorphic adenoma: a new technique. Revue de Laryngologie Otologie Rhinologie . 2008;129(4-5):313–318. [PubMed] [Google Scholar]
- 56.Simonpieri A. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 2: bone graft, implant and reconstructive surgery. Current Pharmaceutical Biotechnology . 2012;13(7):1231–1256. doi: 10.2174/138920112800624472. [DOI] [PubMed] [Google Scholar]
- 57.Verma U. P., Yadav R. K., Dixit M., Gupta A. Platelet-rich fibrin: a paradigm in periodontal therapy–a systematic review. Journal of International Society of Preventive & Community Dentistry . 2017;7(5):p. 227. doi: 10.4103/jispcd.JISPCD_429_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma A., Pradeep A. R. Autologous platelet-rich fibrin in the treatment of mandibular degree II furcation defects: a randomized clinical trial. Journal of Periodontology . 2011;82(10):1396–1403. doi: 10.1902/jop.2011.100731. [DOI] [PubMed] [Google Scholar]
- 59.Bajaj P., Pradeep A. R., Agarwal E., et al. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of mandibular degree II furcation defects: a randomized controlled clinical trial. Journal of Periodontal Research . 2013;48(5):573–581. doi: 10.1111/jre.12040. [DOI] [PubMed] [Google Scholar]
- 60.Pradeep A. R., Karvekar S., Nagpal K., Patnaik K., Raju A., Singh P. Rosuvastatin 1.2 mg in situ gel combined with 1 : 1 mixture of autologous platelet-rich fibrin and porous hydroxyapatite bone graft in surgical treatment of mandibular class II furcation defects: a randomized clinical control trial. Journal of Periodontology . 2016;87(1):5–13. doi: 10.1902/jop.2015.150131. [DOI] [PubMed] [Google Scholar]
- 61.Del Corso M. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 1: periodontal and dentoalveolar surgery. Current Pharmaceutical Biotechnology . 2012;13(7):1207–1230. doi: 10.2174/138920112800624391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used to support the findings of this study are included in the article.


