Abstract
目的
拟探索结合超声检查与单纯根据临床查体两种临床场景下银屑病关节炎(psoriatic arthritis,PsA)患者临床分型的差异。
方法
入选2010年1月至2020年10月就诊北京大学第一医院风湿免疫科且临床和超声结果完整的PsA患者,根据患者的临床资料对其进行表型分类,再进一步结合能量多普勒与灰阶超声所发现的附着点炎及指(趾)炎对所有患者进行再次的表型分类,应用韦恩图表示纳入超声前后PsA各临床表型分组,临床表型构成比采用χ2检验或Fisher’s精确检验,纳入超声前后临床表型差异应用Wilcoxon符号秩检验。
结果
共纳入227例PsA患者,分别存在一种或多种临床表现。临床查体发现:209(92.1%,209/227)例患者有银屑病皮损,98(43.2%,98/227)例患者有指(趾)甲病变,219(96.5%,219/227)例患者有外周关节炎表现,25(11.0%,25/227)例患者脊柱受累,80(35.2%,80/227)例患者存在指(趾)炎,18(7.9%,18/227)例患者存在附着点炎。纳入超声评估后,发现另外18例患者超声下有指(趾)炎表现,另外80例患者超声下有急性附着点炎表现,其中异常回声减低55例,肌腱增厚62例,48例可见多普勒血流信号。与单纯根据临床查体分型相比,联合超声检查后对227例患者进行分型,发现最常见的单纯外周关节炎型患者明显减少(49.8% vs. 27.8%,P < 0.001),外周关节炎合并附着点炎患者比例明显增多(4.4% vs. 18.1%,P < 0.001),外周关节炎合并附着点炎和指(趾)炎的患者也明显增多(1.8% vs. 17.6%,P < 0.001)。
结论
超声是发现附着点炎及指(趾)炎的有利工具,借助超声检查可以有效辅助风湿科医生更好地鉴别PsA的病变性质和类型,准确划分临床表型,并进一步指导治疗。
Keywords: 银屑病关节炎, 肌肉骨骼超声, 临床表型, 附着点炎, 指(趾)炎
Abstract
Objective
To explore the difference in phenotype recognition of PsA patients in two clinical scenarios, physical examination with and without ultrasound assessment.
Methods
PsA patients who visited the rheumatology and clinical immunology department of Peking University First Hospital between January 2010 and October 2020, with complete data of clinical and ultrasound assessment were enrolled. The phenotypes were first identified based on physical examination only, and then combined with enthesitis and dactylitis shown on power doppler and gray-scale ultrasound. The phenotype groupings without and with ultrasound assessment were presented with Wayne diagram. The distributions of different clinical phenotypes were compared by using χ2 test or Fisher's exact test. The differences of clinical phenotypes with and without ultrasound assessment were compared by using Wilcoxon signed rank test.
Results
A total of 227 patients with PsA were enrolled with one or more clinical domains. Physical examination revealed that psoriasis was in 209 (92.1%, 209/227) patients, nail involvement in 98 (43.2%, 98/227) patients, peripheral arthritis in 219 (96.5%, 219/227) patients, axial involvement in 25 (11.0%, 25/227) patients, dactylitis in 80 (35.2%, 80/227) patients, and enthesitis in 18 (7.9%, 18/227) patients. Besides 18 patients with clinical enthesitis, ultrasound scan revealed acute enthesitis in 80 patients, with hypoechogenicity (55 cases), tendon thickening (62 cases), and presence of Doppler signals (48 cases). Similarly, dactylitis on ultrasound was found in 18 patients besides those patients with clinical dactylitis. Compared with the phenotypes recognized based on physical examination only, the additional ultrasound assessment revealed that the most common phenotypes, peripheral arthritis was significantly less frequently recognized (49.8% vs. 27.8%, P < 0.001), however on the other hand, the proportion of the patients with peripheral arthritis and enthesitis was significantly increased (4.4% vs. 18.1%, P < 0.001). The phenotype of peripheral arthritis combined with enthesitis, and dactylitis was also dramatically increased (1.8% vs. 17.6%, P < 0.001).
Conclusion
Ultrasound is a useful tool to identify enthesitis and dactylitis. With the aid of ultrasound assessment, rheumatologists can better identify the lesions of PsA, accurately identify the phenotypes, and further guide the subsequent treatment.
Keywords: Psoriatic arthritis, Musculoskeletal ultrasound, Clinical phenotype, Enthesitis, Dactylitis
银屑病关节炎(psoriatic arthritis, PsA)是一种慢性自身免疫性关节炎性疾病[1]。全球银屑病的患病率为0.14%~1.99%[2],而在银屑病患者中,约30%的患者存在关节炎[3]。与其他关节炎不同,PsA的临床表现复杂多样,可能呈现为外周关节炎、指(趾)炎、附着点炎、脊柱炎、银屑病皮疹、指(趾)甲病变等多种表型。银屑病和PsA研究评价组(Group for Research and Assessment of Psoriasis and Psoriatic Arthritis, GRAPPA)建议根据不同的临床表型决定治疗策略,从而更好地改善PsA患者预后[4]。
指(趾)炎表现为整个手指或足趾的弥漫肿胀(腊肠指/趾),是PsA重要的特征性表现,在PsA患者中,指(趾)炎的平均发生率约为25%[5]。根据2019年欧洲抗风湿病联盟(European League Against Rheumatism,EULAR)的推荐,单关节炎或者寡关节炎的治疗应该首选非甾体抗炎药或局部注射糖皮质激素,但合并指(趾)炎的患者需要尽快应用传统合成的改善病情的抗风湿药[6]。附着点是肌腱、韧带或关节囊与骨相连支配关节活动的部位,附着点局部的炎症称为附着点炎[7]。附着点炎也同样是PsA的特征性表现,平均患病率为30%[5]。根据2015年GRAPPA和2019年EULAR的治疗推荐,如果附着点炎症对非甾体抗炎药或局部注射糖皮质激素的反应欠佳,应考虑应用生物制剂[4, 6]。
PsA不同的临床表型很大程度上影响我们的治疗决策,因此,准确的临床分型是PsA治疗的基础。现阶段主要根据临床评估对指(趾)炎和附着点炎进行判定,然而仅依靠体格检查区分外周关节炎、附着点炎或指(趾)炎往往有一定困难。近年来的一些研究发现,肌肉骨骼超声较体格检查能更敏感地发现附着点病变[8],同时也越来越关注指(趾)炎的超声下表现[9]。纳入超声评估后,PsA的临床分型是否发生变化,该领域尚无相关研究,因此, 我们拟探索结合超声评估与单纯根据临床查体两种临床场景下PsA患者临床分型的差异。
1. 资料与方法
1.1. 病例来源
2010年1月至2020年10月就诊于北京大学第一医院风湿免疫科的PsA患者,所有患者均符合《银屑病关节炎分类标准》(classification criteria for psoriatic arthritis, CASPAR)[10],且初次就诊时有完整的肌肉骨骼超声检查结果。本试验获得北京大学第一医院生物医学研究伦理委员会批准(批准号:2019研267),患者均签署知情同意书。
1.2. 临床评估
收集上述患者性别、年龄、病程、体重指数(body mass index, BMI),是否存在外周关节炎、脊柱炎、附着点炎、指(趾)炎、皮损、指(趾)甲病变等。本文中定义存在1个及1个以上外周关节肿胀或压痛为外周关节炎,脊柱炎定义为存在典型临床症状(炎性腰背痛和/或脊柱强直)和/或影像学检查(X线、电子计算机断层扫描或核磁共振成像)结果,附着点炎由临床医生基于患者症状由查体判定,就诊时由临床医生判定是否存在指(趾)炎、皮损和指(趾)甲病变等表现。
1.3. 肌肉骨骼超声检查
肌肉骨骼超声由超声检查经验丰富且不知晓病情的风湿免疫科医生完成。对所有患者均完成有症状关节的超声检查,每个部位进行纵向和横断面扫描。除有症状关节外,2018年后就诊患者常规完成8对附着点部位附着点炎检查,包括双侧肱骨内上髁(屈肌总腱附着点)、肱骨外上髁(伸肌总腱附着点)、鹰嘴粗隆(肱三头肌附着点)、髌骨上缘(股四头肌附着点)、髌骨下缘(髌腱上端附着点)、胫骨粗隆(髌腱下端附着点)、跟骨上缘(跟腱附着点)、跟骨下缘(跖筋膜附着点)。本研究采用美国GE公司E9超声设备,探头型号为ML6-15,频率为6~15 MHz。
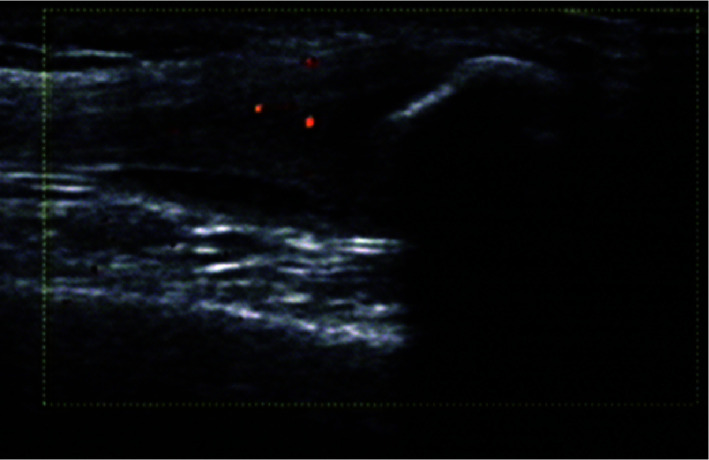
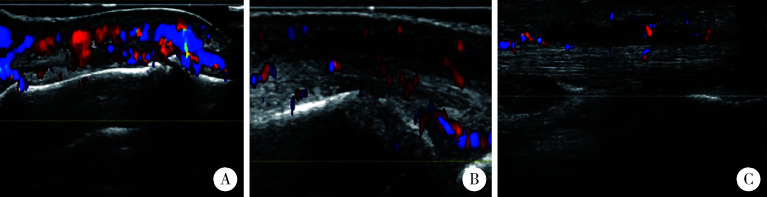
超声下滑膜炎、腱鞘炎、腱周炎[11]、附着点炎[12]、软组织水肿[13]按照风湿病预后评估组织(Outcome Measures in Rheumatology, OMERACT)统一定义。本研究中定义急性附着点炎(两个垂直切面见到肌腱/韧带附着点处的异常低回声和/或增厚,伴或不伴有多普勒信号)为超声下附着点炎(图 1)。指(趾)炎参考指(趾)炎整体超声(dactylitis global sonographic,DACTOS)评分,共包括滑膜炎、伸肌腱腱周炎、屈肌腱腱鞘炎、软组织水肿4种表现[14]。本研究中,当患者存在屈肌腱腱鞘炎1种表现或上述表现中任意2种或以上时,定义为超声下指(趾)炎(图 2)。
图 1.
超声下附着点炎表现
Enthesitis on ultrasound
图 2.
超声下指(趾)炎表现
Dactylitis on ultrasound
A, peritendon extensor inflammation; B, synovitis; C, soft tissue oedema, flexor tenosynovitis.
1.4. 统计学分析
采用SPSS 22.0软件进行统计学分析。对于分类变量,按照百分比表示。计量资料如符合正态分布采用x±s表示,如不符合正态分布,采用M (P25, P75)表示。用韦恩图[15]表示PsA各临床表型分组,临床表型构成比采用χ2检验或Fisher’s精确检验,纳入超声前后临床表型差异应用Wilcoxon符号秩检验,P < 0.05为差异有统计学意义。
2. 结果
2.1. 一般情况及临床表现
共纳入227例PsA患者,其中男性119例(52.4%),中位年龄39(31,51)岁,关节炎的中位病程3(0.7,7)年,中位BMI为24.02(22.21,26.35)kg/m2。患者可存在一种或多种临床表现,其中219(96.5%,219/227)例患者有外周关节炎表现,25(11.0%,25/227)例患者存在脊柱受累表现,80(35.2%,80/227)例患者存在指(趾)炎,18(7.9%,18/227)例患者存在附着点炎,209(92.1%,209/227)例患者有银屑病皮损,98(43.2%,98/227)例患者存在指(趾)甲病变。
2.2. 超声下附着点炎
经过超声检查,除外18例临床附着点炎患者,发现另外115(55.0%,115/209)例患者有超声下附着点病变表现,其中慢性附着点病变80例,可表现为一种或多种慢性附着点病变特点,其中钙化31例、骨赘53例、骨侵蚀34例。80(69.6%,80/115)例患者存在急性附着点病变,可表现为一种或多种急性附着点病变特点,其中异常回声减低55例,肌腱增厚62例,48例有多普勒血流信号。超声所发现的急性附着点炎最常见于手远端指间关节伸肌腱附着点处(21例),其次为跖筋膜(19例)、足趾伸肌腱附着点处(15例)、手近端指间关节伸肌腱附着点处(9例)以及伸肌总腱附着点处(9例,表 1)。
表 1.
超声下附着点炎的部位分布
Locations of enthesitis revealed by ultrasound
| Sites | n |
| Extensor tendon of distal interphalangeal joint | 21 |
| Plantar fascia | 19 |
| Extensor tendon of toe | 15 |
| Extensor tendon of proximal interphalangeal joint | 9 |
| Common extensor tendon | 9 |
| Tuberosity of tibia | 8 |
| Achilles’ tendon | 6 |
| Superior margin of patella | 6 |
| Inferior margin of patella | 2 |
| Olecranon tuberosity | 2 |
| Posterior tibial tendon | 2 |
| Common flexor tendon | 2 |
| Iliotibial tract | 2 |
| Medial collateral ligament | 2 |
2.3. 超声下指(趾)炎
经过超声检查,除外80例临床指(趾)炎患者,发现额外46(31.3%,46/147)例患者有超声下指(趾)炎相关表现,其中伸肌腱腱周炎43例、滑膜炎13例、屈肌腱腱鞘炎7例、皮下软组织水肿1例。在46例患者中,符合本研究超声下指(趾)炎定义的患者有18例,其中1例同时存在屈肌腱腱鞘炎、伸肌腱腱周炎和滑膜炎,10例同时存在腱周炎和滑膜炎,2例同时存在腱鞘炎和滑膜炎,1例同时存在腱周炎和腱鞘炎,1例同时存在腱周炎和皮下软组织水肿,3例仅存在屈肌腱腱鞘炎。
2.4. 纳入超声评估前后PsA患者临床分型的差异
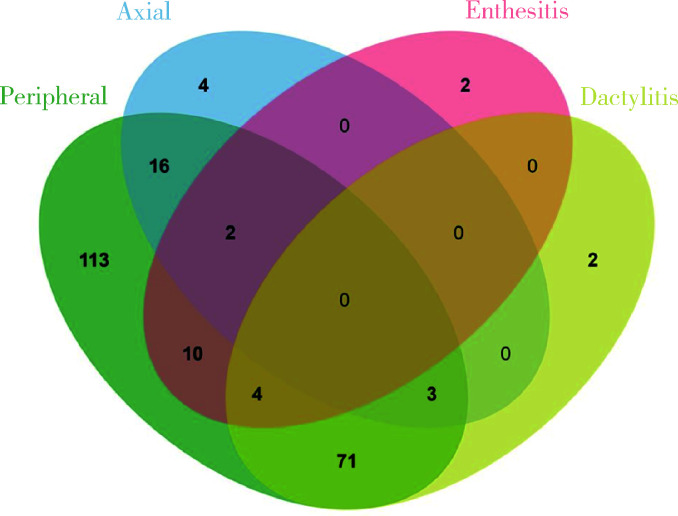
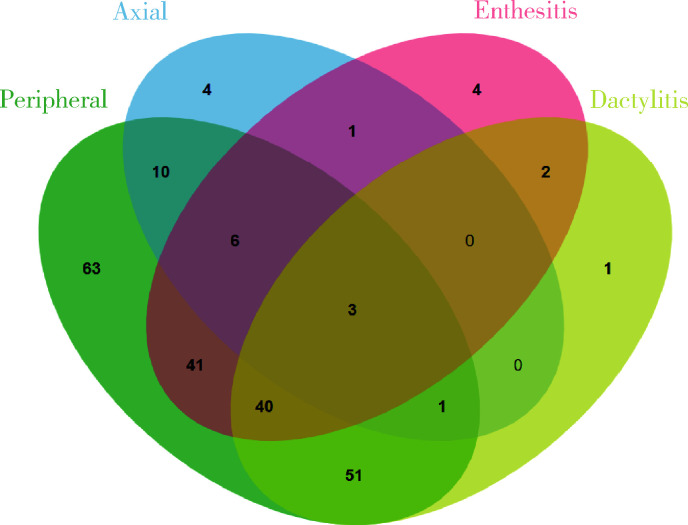
单纯根据体格检查对227例患者进行分型,发现最常见的表型是单纯外周关节炎型(113例),其次为外周关节炎合并指(趾)炎型(71例)、外周关节炎合并中轴受累(16例),其他临床表型共27例(图 3)。然而,结合超声评估后发现,最常见的临床表型仍然是单纯外周关节炎型(63例)以及外周关节炎合并指(趾)炎(51例),但是例数明显减少,而同时发现外周关节炎合并附着点炎(41例),外周关节炎合并附着点炎和指(趾)炎(40例),其他临床表型共32例(图 4)。与单纯根据临床查体分型相比,结合超声检查结果进行的分型显示(表 2):单纯外周关节炎型患者明显减少(49.8% vs. 27.8%,P < 0.001),外周关节炎合并附着点炎患者明显增多(4.4% vs. 18.1%,P < 0.001);外周关节炎合并附着点炎和指(趾)炎的患者比例也明显增加(1.8% vs. 17.6%,P < 0.001),提示结合超声评估发现更多PsA患者存在多种病变。纳入超声前后临床表型构成比差异无统计学意义(P=0.724)。
图 3.
纳入超声前PsA临床分型
Clinical phenotypes of PsA patients without ultrasound assessment
图 4.
纳入超声后PsA临床分型
Clinical phenotypes of PsA patients with ultrasound assessment
表 2.
有无超声辅助下PsA患者临床表型分类的差异比较
Comparisons of PsA phenotypes with and without ultrasound assessment
| Phenotypes | Without ultrasound, n (%) | With ultrasound, n (%) | χ2 | P |
| Peripheral arthritis only | 113 (49.8) | 63 (27.8) | 23.197 | < 0.001 |
| Peripheral arthritis + dactylitis | 71 (31.3) | 51 (22.5) | 4.484 | 0.034 |
| Peripheral arthritis + axial disease | 16 (7.0) | 10 (4.4) | 1.469 | 0.226 |
| Peripheral arthritis + enthesitis | 10 (4.4) | 41 (18.1) | 21.228 | < 0.001 |
| Peripheral arthritis + enthesitis+ dactylitis | 4 (1.8) | 40 (17.6) | 32.616 | < 0.001 |
| Axial disease only | 4 (1.8) | 4 (1.8) | > 0.999 | |
| Peripheral arthritis + axial disease+ dactylitis | 3 (1.3) | 1 (0.4) | 0.623 | |
| Peripheral arthritis + axial disease+ enthesitis | 2 (0.9) | 6 (2.6) | 0.285 | |
| Enthesitis only | 2 (0.9) | 4 (1.8) | 0.685 | |
| Dactylitis only | 2 (0.9) | 1 (0.4) | > 0.999 | |
| Peripheral arthritis + axial+ enthesitis+ dactylitis | 0 | 3 (1.3) | 0.248 | |
| Enthesitis+ dactylitis | 0 | 2 (0.9) | 0.499 | |
| Axial disease+ dactylitis | 0 | 0 | > 0.999 | |
| Axial disease+ enthesitis | 0 | 1 (0.4) | > 0.999 | |
| Axial disease+ enthesitis+ dactylitis | 0 | 0 | > 0.999 |
3. 讨论
近年来,随着对PsA认识的深入以及更多种类药物的应用,PsA患者的治疗效果有了较大的改善,而且目前也已经在PsA患者的治疗中提出了目标治疗的理念(treat to target, T2T),总体改善了患者的临床结局[16]。然而,PsA的临床治疗策略由不同的临床表型所决定,因此,对PsA临床表型的正确识别在治疗过程中非常重要。
超声是探查附着点炎的便捷工具,且明显优于临床评估[17-18]。本研究发现,临床附着点炎的发生率仅为7.9%,这一研究低于既往报道[5],可能与我们没有常规进行利兹大学附着点炎指数(Leeds enthesitis index, LEI)[19]评估相关。纳入肌肉骨骼超声检查后,在另外55.0%的患者中发现了附着点病变,其中69.6%的患者存在急性改变。目前已有研究表明,超声下附着点病变与PsA患者的影像学进展密切相关。Polachek等[20]的研究发现,马德里超声附着点炎指数(Madrid, sonography enthesitis index,MASEI)每增加10,反映手足关节损伤X线改良Steinbrocker评分(modified Steinbrocker score,mSS)增加42%,关节损毁的风险增加1.77倍,同时改良的Stoke强直性脊柱炎脊柱评分(modified Stoke ankylosing spondylitis spine score, mSASSS)也增加1.18倍,因此,早期发现超声下附着点炎,制订合适的治疗策略至关重要。
没有临床症状但是超声下发现的附着点炎称为亚临床附着点炎,这种亚临床附着点炎普遍存在。Michelsen等[21]对141例PsA患者的研究发现,超声发现跟腱附着点病变的患者中有16%的病例没有临床症状。Falsetti等[22]发现86%的代谢综合征患者存在超声下附着点炎表现,而Bakirci等[23]在健康人群中发现超声下附着点改变也普遍存在,年龄增大、BMI增高、男性、经常体育锻炼都是出现超声下附着点病变的独立预测因素,这些提示我们需要综合各方面的信息来评估超声下的附着点病变。本研究中52.4%患者为男性,中位年龄39岁,BMI值24.02 kg/m2,不存在年龄过大、明显超重等可能影响超声评估的因素,因此本研究结果有临床参考价值。此外,为尽量减少干扰因素的影响,本研究中仅纳入超声下急性附着点病变,定义更为严格。
本研究还发现80例(35.2%,80/227)患者存在临床指(趾)炎,这与既往文献报道相符[5],但是在临床无指(趾)炎的患者中,仍有46例(31.3%,46/147)患者存在超声下指(趾)炎的表现,其中18例符合本研究中对超声下指(趾)炎的定义。由于指(趾)炎的病理基础复杂,超声下可见的表现多样,目前国际上尚没有公认的超声下指(趾)炎定义。本研究采用了目前大家最为接受的DACTOS评分。Girolimetto等[24]在治疗有应答与无应答以及达到缓解和未达到缓解的临床指(趾)炎患者中,已经对DACTOS评分进行了验证,这一评分可以一定程度上反映PsA指(趾)炎的临床特征和治疗反应。在指(趾)炎的解剖中,屈肌腱和滑车是最关键的结构,在手指屈伸动作的过程中,屈肌腱在滑车内移动,屈肌腱与滑车之间产生附着点炎,炎症沿着整根手指或足趾蔓延,表现为临床上常见的腊肠指(趾)[25]。屈肌腱腱鞘炎是指(趾)炎最为常见的超声下表现,Kane等[26]发现,96%的指(趾)炎存在超声下屈肌腱腱鞘炎,而软组织水肿、滑膜炎、伸肌腱腱周炎也是指(趾)炎可见的超声下表现,但相对发生率更低[27],因此,本研究中采用超声下存在屈肌腱腱鞘炎1种表现,或屈肌腱腱鞘炎、伸肌腱腱周炎、滑膜炎、软组织水肿中任意至少2种表现定义为超声下指(趾)炎是科学合理的。
基于上述超声所见的病变,我们发现在超声辅助下PsA患者的临床表型与根据单纯临床查体的分型发生了很大变化,例如,单纯外周关节炎型由49.8% 下降至27.8%。临床上最常应用LEI进行外周附着点炎评估,但其评估部位为双侧肱骨外上髁、股骨内侧髁及跟腱附着点[19]。然而我们的研究发现,超声下附着点病变最多见于手足等小关节,提示现有的临床附着点炎评估存在局限性,更说明一部分临床查体考虑为关节炎的患者其本质是附着点炎,印证了单纯依靠临床评估来决定PsA的临床表型存在明显局限性。关节炎与附着点炎的治疗策略不同[4, 6],因此部分患者治疗效果不理想很可能是最初确定临床表型时存在偏差,导致治疗方案选择的错误。PsA的诊断延迟6个月,就会对影像学进展和功能结局产生不良影响[28],风湿科免疫医生不应仅满足于PsA的及时诊断,更需要进行正确的临床分型,超声作为简便的工具可以帮助我们更准确地进行PsA的临床分型,从而进一步改善预后。
本研究首次纳入超声技术对PsA临床分型进行探索,可以发现更多的附着点炎及指(趾)炎表现,能帮助临床医生更好地鉴别PsA的病变性质,从而进一步指导PsA临床表型的准确划分,因此有重要意义。本研究存在一定局限性:首先,是回顾性研究,时间跨度较大,超声检查部位存在不完全一致的问题,这可能对超声附着点炎好发部位的检出存在一定偏倚,同时也造成本研究中临床附着点炎的检出率较既往研究低,目前仍有学者认为临床附着点炎与超声下附着点炎存在本质上的区别,因此需要开展前瞻性研究来探讨超声下附着点炎的意义;其次,指(趾)炎目前仍缺乏统一的超声下定义,未来我们拟开展进一步研究,以探讨超声下不同病变指导指(趾)炎治疗的意义。
Funding Statement
北京大学第一医院青年临床研究专项课题科研基金(2019CR28)、北京大学第一医院交叉临床研究专项科研基金(2021CR30)
Supported by the Youth Clinical Research Project of Peking University First Hospital (2019CR28), and the Interdisciplinary Clinical Research Project of Peking University First Hospital (2021CR30)
References
- 1.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376(10):957–970. doi: 10.1056/NEJMra1505557. [DOI] [PubMed] [Google Scholar]
- 2.Parisi R, Iskandar IYK, Kontopantelis E, et al. Global psoriasis atlas. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369:m1590. doi: 10.1136/bmj.m1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogdie A, Weiss P. The epidemiology of psoriatic arthritis. Rheum Dis Clin North Am. 2015;41(4):545–568. doi: 10.1016/j.rdc.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coates LC, Kavanaugh A, Mease PJ, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68(5):1060–1071. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 5.Pittam B, Gupta S, Harrison NL, et al. Prevalence of extra-articular manifestations in psoriatic arthritis: a systematic review and meta-analysis. Rheumatology (Oxford) 2020;59(9):2199–2206. doi: 10.1093/rheumatology/keaa062. [DOI] [PubMed] [Google Scholar]
- 6.Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79(6):700–712. doi: 10.1136/annrheumdis-2020-217159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGonagle D, Benjamin M. Rheumatology. Netherlands: Elsevier; 2018. pp. 1082–1089. [Google Scholar]
- 8.Alcalde M, Acebes JC, Cruz M, et al. A sonographic enthesitic index of lower limbs is a valuable tool in the assessment of ankylosing spondylitis. Ann Rheum Dis. 2007;66(8):1015–1019. doi: 10.1136/ard.2006.062174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felbo SK, Østergaard M, Sørensen IJ, et al. Which ultrasound lesions contribute to dactylitis in psoriatic arthritis and their reliability in a clinical setting. Clin Rheumatol. 2021;40(3):1061–1067. doi: 10.1007/s10067-020-05483-9. [DOI] [PubMed] [Google Scholar]
- 10.Taylor W, Gladman D, Helliwell P, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheumatol. 2006;54(8):2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 11.Wakefield RJ, Balint PV, Szkudlarek M, et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32(12):2485–2487. [PubMed] [Google Scholar]
- 12.Balint PV, Terslev L, Aegerter P, et al. Reliability of a consensus-based ultrasound definition and scoring for enthesitis in spondyloarthritis and psoriatic arthritis: an OMERACT US initiative. Ann Rheum Dis. 2018;77(12):1730–1735. doi: 10.1136/annrheumdis-2018-213609. [DOI] [PubMed] [Google Scholar]
- 13.Tinazzi I, Idolazzi L, Zabotti A, et al. Ultrasonographic detection, definition and quantification of soft tissue oedema in psoriatic dactylitis. Med Ultrason. 2019;21(4):414–421. doi: 10.11152/mu-2258. [DOI] [PubMed] [Google Scholar]
- 14.Zabotti A, Sakellariou G, Tinazzi I, et al. Novel and reliable dactylitis global sonographic (DACTOS) score in psoriatic arthritis. Ann Rheum Dis. 2020;79(8):1037–1043. doi: 10.1136/annrheumdis-2020-217191. [DOI] [PubMed] [Google Scholar]
- 15.Bardou P, Mariette J, Escudié F, et al. Jvenn: an interactive venn diagram viewer. BMC Bioinformatics. 2014;15(1):293. doi: 10.1186/1471-2105-15-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coates LC, Moverley AR, McParland L, et al. Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open-label, randomised controlled trial. Lancet. 2015;386(10012):2489–2498. doi: 10.1016/S0140-6736(15)00347-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riente L, Carli L, Delle Sedie A. Ultrasound imaging in psoriatic arthritis and ankylosing spondylitis. Clin Exp Rheumatol. 2014;32(Suppl 80):26–33. [PubMed] [Google Scholar]
- 18.Helliwell PS. Assessment of enthesitis in psoriatic arthritis. J Rheumatol. 2019;46(8):869–870. doi: 10.3899/jrheum.181380. [DOI] [PubMed] [Google Scholar]
- 19.Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheumatol. 2008;59(5):686–691. doi: 10.1002/art.23568. [DOI] [PubMed] [Google Scholar]
- 20.Polachek A, Cook R, Chandran V, et al. The association between sonographic enthesitis and radiographic damage in psoriatic arthritis. Arthritis Res Ther. 2017;19(1):189. doi: 10.1186/s13075-017-1399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michelsen B, Diamantopoulos AP, Soldal DM, et al. Achilles enthesitis defined by ultrasound is not associated with clinical enthesitis in patients with psoriatic arthritis. RMD Open. 2017;3(2):e000486. doi: 10.1136/rmdopen-2017-000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falsetti P, Conticini E, Baldi C, et al. Diffuse peripheral enthesitis in metabolic syndrome: a retrospective clinical and power doppler ultrasound study[J/OL]. Reumatol Clin (Engl Ed), 2021, 2 (2021-02-24)[2021-06-24]. https://pubmed.ncbi.nlm.nih.gov/33640321.
- 23.Bakirci S, Solmaz D, Stephenson W, et al. Entheseal changes in response to age, body mass index, and physical activity: an ultrasound study in healthy people. J Rheumatol. 2020;47(7):968–972. doi: 10.3899/jrheum.190540. [DOI] [PubMed] [Google Scholar]
- 24.Girolimetto N, Zabotti A, Tinazzi I, et al. Sensitivity to change and clinical correlations of the novel dactylitis global sonographic (DACTOS) score in psoriatic arthritis. Rheumatology (Oxford) 2021;60(9):4103–4111. doi: 10.1093/rheumatology/keaa885. [DOI] [PubMed] [Google Scholar]
- 25.McGonagle D, Tan AL, Watad A, et al. Pathophysiology, assessment and treatment of psoriatic dactylitis. Nat Rev Rheumatol. 2019;15(2):113–122. doi: 10.1038/s41584-018-0147-9. [DOI] [PubMed] [Google Scholar]
- 26.Kane D, Greaney T, Bresnihan B, et al. Ultrasonography in the diagnosis and management of psoriatic dactylitis. J Rheumatol. 1999;26(8):1746–1751. [PubMed] [Google Scholar]
- 27.Girolimetto N, Giovannini I, Crepaldi G, et al. Psoriatic dactylitis: current perspectives and new insights in ultrasonography and magnetic resonance imaging. J Clin Med. 2021;10(12):2604. doi: 10.3390/jcm10122604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74(6):1045–1050. doi: 10.1136/annrheumdis-2013-204858. [DOI] [PubMed] [Google Scholar]