Abstract
This study demonstrates the level of heavy metal pollution in the Halda River, the only natural breeding source of carps in Bangladesh. Water was collected from 12 different sampling points along the Halda River. Water at various locations was found satisfactory in terms of the assessed physicochemical parameters (pH, electrical conductivity, and total dissolved solids). The presence of various cations and anions was also studied using ion chromatography. Atomic absorption spectroscopy was used to identify and quantify various heavy metals in the collected water samples. Among the heavy metals, Cd, Cr, Fe, Pb, Cu, and As concentration exceeded the safe limit suggested by WHO. The calculated heavy metal pollution index and metal index were found higher than the critical index value. The single-factor assessment (P i) and Nemerow's multi-factor index (P N) of heavy metals was calculated to find out the degree of pollution in the Halda River. The maximum values of P i (Cd), P i (Pb), P i (As), P i (Cu), and P i (Cr) were determined to be 26.67, 260.00, 17.00, 208.76 and 2.80 respectively. The maximum value of P N was found to be 289.04. The discharge of effluents from various large and small industries near the Halda River is considered to be the major source of the identified heavy metals. Multivariate statistical analysis such as principal component analysis, Pearson correlation matrix and cluster analysis revealed that most of the heavy metals originated from different anthropogenic sources. Multivariate analysis also showed that Co, Mn, Cu, Cr, Pb, Cd, NH4+, NO3- mainly came from artificial sources whereas Fe, Ca, As mainly originated from natural sources. Arsenic (As) also came from artificial sources with Cu.
Keywords: Heavy metal, Pollution, Halda river, Statistical analysis, Anthropogenic sources
Heavy metal; Pollution; Halda River; Statistical analysis; Anthropogenic sources.
1. Introduction
The excessive pollution of surface water by various heavy metals is a global public health concern, as heavy metals are potentially responsible for causing deleterious effects on human health through food chain. Metals having density greater than 5 g/cm3 are called heavy metals [1]. These metals include chromium (Cr), nickel (Ni), cadmium (Cd), arsenic (As), lead (Pb), zinc (Zn), copper (Cu), cobalt (Co), molybdenum (Mo), manganese (Mn), mercury (Hg), vanadium (V), iron (Fe), rare metals etc. Contamination of food items with heavy metals is a global health concern all over the world particularly in the major cities of the developing countries [1]. Food chain contamination with heavy metals is dangerous as they are persistent environmental pollutants and non-biodegradable having long biological half-lives. Heavy metals are toxic to humans even at very low concentration [2]. Heavy metals lead to numerous hazards including cardiovascular, kidney, nervous, bone diseases, carcinogenesis, mutagenesis and teratogenesis, nutrient deficiency, enzyme malfunctioning, renal tubular dysfunction accompanied by osteomalacia and other complications etc. [3, 4]. Some essential heavy metals such as Zn, Cu, Mn, Fe maintain proper metabolic activities in living organisms while other non-essential heavy metals such as Cd, Ni, Hg, Pb, Cr, As cause toxicity in human body [5, 6]. The unrestricted disposal of industrial waste, agrochemicals, transportation waste etc. leads to the severe pollution of soil, water and, air by heavy metals. Heavy metals are easily transported from this polluted environment into the food chain. Heavy metals are naturally available on earth's crust. The contamination of environment by heavy metals and human exposure stems from various anthropogenic activities such as industrial production, smelting and mining operations, agricultural and domestic use of metals and metal-containing compounds [7, 8]. The industrial sources of heavy metals are petroleum combustion, nuclear power stations, metal processing in refineries, coal burning in power plants, high tension lines, microelectronics, textiles, plastics, paper processing plants, wood preservation etc. [9, 10].
The pollution level of heavy metals in surface waters of Bangladesh is increasing rapidly due to unplanned urbanization, industrialization and uncontrolled use of agrochemicals. The insufficiently treated waste disposed from various industries into the river water makes serious problem to aquatic flora and fauna. Photosynthetic activity is inhibited in polluted water due to reduced light penetration. The elevated pollution level is a great threat to human and animals all over the country. Fish production and availability is badly reduced in polluted water [11].
Water is an essential element of the ecosystem and a vital element for all living creatures. River water is widely used for various domestic, urban, agricultural, and industrial purposes. The Halda River, in the south-east region of Bangladesh, is a natural source of fertilized carp eggs due to the inherent biochemical properties of its water [12]. Indian major carps are spawn naturally in the Halda River, which makes this river a unique heritage in Bangladesh. Generally, fisherman from the nearby areas hugely support their daily lives by collecting Indian major carps such as Rohu (Labeo rohita), Katla (Gibelion catla), and Mrigal (Cirrhinus cirrhosis) from the Halda River. Several other species are consumed by local community as potential source of proteins such as Liza parse, Otolithoides pama, Rhinomugil corsula, Apocryptes bato, Macrobrachium rosenbergii, Notropis atherinoides, and Pseudapocryptes elongates [13]. Apart from being the natural breeding ground for major carps, this river water has widespread use in agriculture, irrigation, livestock rearing, fish farming, bathing, recreation, and drinking [14]. Unfortunately, this river water is being polluted due to waste disposal into the river. The river passes through the industrial zones and is severely polluted with industrial wastes, sand excretion, and sewage discharge. Four canals are considered as the major discharge routes for polluters into the Halda River, which are Madari, Khondakia, Mondakini, and Chengkhali canals [15].
In this present study, it was aimed to investigate the heavy metal pollution in the Halda River water by collecting water samples from different locations of the river. Significant pollution level in this river by various heavy metals has been found in this study. The statistical analysis of the recorded data provides a thorough insight into the contribution of different heavy metals in overall water pollution and their probable sources. It is expected that the findings from this study will pave the way for enhancing public consciousness on the pollution level of the Halda River water and will encourages relevant authorities to take stern action for reducing the water pollution to ensure the healthy use of this river water.
2. Materials and methods
2.1. Study location
In this study, water samples were collected from Madarikhal area near Modunaghat (famous for several heavy industries) along the Halda River, which is situated between 22°25′13″-22º48′51.37″N and 91º45′00″-91°52′33″E (Figure 1). It is a 98 km long river that passes through Raozanupazilas, Fatikchhari, Chandgaonthana, and Hathazari of Chittagong before merging with the River Karnaphuli [16]. The river depth varies between 2.0-11.5 m and width ranges from 120-200 m [17]. The sampling points were recorded with the global positioning system (GPS) tracer (Germin-62s, USA). Several potential factors including human activities taking place near the riverbanks (e.g., farming, harvesting, bathing) and geographical proximity of industrial and urban discharges to this river were taken into consideration before selecting the sampling points. During the last few decades, the river ecosystem has been severely disrupted due to the discharge of household waste, industrial waste, and domestic raw sewage from the surrounding habitation directly into this river.
Figure 1.
Map of sampling points along the Halda River.
2.2. Sample collection
The water samples were collected in new polypropylene bottles (2 L) capped with double stoppers. Several steps were followed to prepare the polypropylene bottles for collecting water samples such as treating with detergent, washing with plenty of running tap water, immersing in 5% HNO3 (Merck, Germany) overnight, rinsing with deionized water, and finally drying in the air. To distinguish the collected samples, the dried bottles were labeled with special identification number. Samples were collected from 12 different points along the Halda River at a depth of 10–15 cm below the water surface. During sampling, bubble formation and suspended particles were carefully avoided. The collected samples were placed in polypropylene bottles containing 0.4% ultra-pure HNO3 (Assay: 68–70% Merck, Germany) and were stored in a refrigerator.
2.3. Sample preparation
For the determination of heavy metals, atomic absorption spectrophotometric (AAS) method was used in this study. For this purpose, 500 mL of each sample was filtered through Whatman No. 41 filter paper. The filtrate was acidified with 1 mL 65% HNO3. The acidified sample was then divided into two parts in two beakers each containing 250 mL volume. The volume was then reduced to 25 mL by heating on a hotplate. The concentrated samples were then subjected to atomic absorption spectrophotometer for trace metals analysis (Pb, Cd, Cr, Cu, Zn, Co, Mn). For As analysis, the sample was mixed with 0.5 mL potassium iodide, 0.5 mL ascorbic acid, 3 mL HCl and kept for 2 h.
2.4. Quality control
Highly pure (purity: 99.98%) standard solutions of target elements were purchased from Varian Inc, the USA. Ultra-pure HNO3 and other chemicals were purchased from E. Merck, Germany. From stock solution (1000 mg/L concentration), several working standard solutions were prepared using Milli-Q water. The quantification of the studied metals was based on the calibration curves. The accuracy of the analytical methods was assessed by analyzing the Certified Reference Materials NIST 1640 (water matrix). The recovery rate of 90%–99% was earned and considered satisfactory for the analysis. Triplicates were performed for each sample analysis.
2.5. Analytical methods
2.5.1. Physicochemical parameters
At the time of sample collection, various physicochemical parameters were measured such as pH, electrical conductivity (EC), total dissolved solids (TDS), and salinity. For the measurement of pH of the collected samples, a pH meter (Jenway, 3051, UK) was used. A conductivity meter (Hanna Instruments, HI 8033, UK) was used to determine EC, TDS, and salinity using the standard procedure (APHA, 2005). Before taking readings, the electrode was rinsed thoroughly with distilled water and wiped gently using dry tissue paper.
2.5.2. Anion and cation test
In this study, ion chromatography (IC) method was employed for the analysis of various anions (fluoride, chloride, nitrite, bromide, sulfate, nitrate, phosphate) and cations (sodium, potassium, magnesium, calcium, ammonium). Volumetric method was used to determine the concentration of HCO3-. Anion self-regenerating suppressor with conductivity detector was used for separating and detecting the ions. IC is a very effective method for determining a wide range of ions with detection limit below 1 part per billion (ppb).
2.5.3. Heavy metal analysis
A total of 11 heavy metals was analyzed in this study. Flame atomic absorption spectrophotometer (Model varian AA 240) was used for determining Pb, Cd, Cr. Co, Fe, Mn, Ca, As, Cu, and Zn. The instrument was equipped with a Hydride generator system (Model VGA-77) and controlled with software (Version 5.01). Mercury (Hg) was determined by cold vapour atomic absorption spectrophotometer (Model Varian AA 240 FS).
2.6. Pollution assessment methods of heavy metals
2.6.1. Heavy metal pollution index (HPI)
Heavy metal pollution index (HPI) is a powerful technique for the assessment of water quality based on heavy metal concentration. HPI is defined as a rating reflecting the composite influence of different dissolved heavy metals [18]. The critical pollution index value for drinking water should be less than 100 [19]. In this study, HPI was calculated using the standard procedure [20] and the values are presented in Table 1.
| HPI = ∑WiQi/ΣWi | (1) |
| Qi = Σ(Mi−Ii)/(Si−Ii) × 100 | (2) |
Where, Qi is the sub-index of the ith parameter; Wi is the unit weightage of the ith parameter; n is the number of parameters; Mi is the monitored value of heavy metal of ith parameter; Ii is the ideal value of ith parameter; Si is the standard value of the ith parameter.
Table 1.
The computational method for calculating HPI.
| Heavy Metals | Mean Value Mi(ug/L) |
Standard permissible value, Si (ug/L) | Highest desirable value, Ii (ug/L) | Unit Weightage Wi | Sub Index Qi | Wi x Qi |
|---|---|---|---|---|---|---|
| Cd | 9.03E-06 | 3E-03 | 0 | 33.33E+03 | 3.3 E-02 | 2.98E-07 |
| Cr | 5.83E-05 | 5.0E-02 | 0 | 2.0E+03 | 8.5E-03 | 4.99E-07 |
| Mn | 3.0E-04 | 5.0E-01 | 1.0E+02 | 2.0E+02 | 8.8 E-01 | 3.0 E-04 |
| Pb | 1.0 E-03 | 1.0E-02 | 0 | 10.0E+03 | 1.22 | 1.3 E-03 |
| Cu | 2.6 E-01 | 2.00 | 50.00 | 50.00 | 8.68E-01 | 2.26 E-01 |
| Zn | 2.9E-03 | 3.00 | 5.0 E+03 | 33.33 | 6.69E-01 | 1.9 E-03 |
| As | 8.3E-02 | 1.0E-02 | 0 | 10.0E+03 | 98.59 | 8.20 |
2.6.2. Metal index (MI)
The metal index (MI) value was calculated according to the following equation [21]:
| MI = ∑ Ci/(MAC)ix 100 | (3) |
Where, MI is the metal index, C is the concentration of each element in solution, MAC is the maximum allowed concentration for each element, and the subscript i is the ith sample.
2.6.3. Single factor pollution index
The single factor index method was applied to assess the pollution degree of one pollutant in the sediment samples [22]. In our present work, this method was used to assess the pollution degree of one pollutant in the river water samples. This method could find out the most significant pollutant, which contributes most to the pollution at each sampling site. The pollution index for a single pollutant was established according to:
| Pi = Ci / Si | (4) |
Where, Pi is the single factor pollution index; Ci (μg/L) is the measured concentration of heavy metals; and Si (μg/L) is the standard value of the pollutants. The value of the single factor index Pi≤ 1indicates clean lines of pollution degree, 1< Pi ≤ 2 is regarded as low pollution degree, 2 < Pi ≤ 3 is moderate, and Pi> 3 indicates high levels of pollution degree [23, 24].
2.6.4. Nemerow's pollution index
To understand the comprehensive pollution level, the Nemerow pollution index was used. .Since different heavy metals may have impacts on one site, this method could provide a reasonable interpretation of the heavy metal pollution at each site as a whole [22]. The Nemerow pollution index can be calculated by Eq.:
| PN = √ (max Pi)2 + (P̄i)2 / 2 | (5) |
Where PN is Nemerow's pollution index; max Pi is the maximum single pollution index among the pollutants, and P̄i is the average mean of single pollution indexes among all the pollutants.
The value of Nemerow's pollution index PN ≤ 1 indicates the clarity of water, 1<PN≤2.5 is low clarity, 2.5<PN≤7 is moderate, and PN>7 indicates high clarity [23, 24].
2.7. Multivariate statistical analysis
Principal component analysis (PCA) reduces the dimension of large sets of variables to a small set of variables by a linear combination of original data. Linear combination generates new variables which are orthogonal and not correlated to each other and that still contains most of the information in the large set. It extracts the eigenvalues and eigenvectors from the covariance matrix of original variables [25]. This analysis has been used to extract principal components (PC) from the sampling points as well as to evaluate possible sources and variation of heavy metals in surface water sample.
Pearson's correlation matrix is used to signify the association among the pairs of variables. The correlation coefficient matrix measures how well the variance of each constituent can be explained by relationship with each other [26]. In this study, IBM SPSS software (version 20) was used to perform statistical analysis of experimental river water data.
3. Results and discussion
3.1. Physicochemical analysis of river water
The results of physicochemical parameters of the Halda River water are presented in Table 2. The finding reveals that the pH values in the study areas ranged from 5.51 to 6.42. However, the highest pH value was recorded at the sampling point S1, whereas the lowest pH value was observed at S12. The pH values of the adjacent waterbodies are significantly affected by industrial or domestic waste [27]. The pH values are also potentially related to various human activities such as washing, bathing, and latrines along the waterbodies [28]. The pH of waterbodies is also influenced by air temperature, various chemical, and biochemical reactions [29]. Most of the fishes can tolerate pH ranging from 5.0 to 9.0 and aquatic life is not directly impacted by a smaller change in pH. However, the availability and solubility of various chemicals are influenced with pH change, which aggravates nutrient problems for the aquatic life. Here, it could be concluded that the pH values of the Halda River water have no significant impact on the aquatic animals and plants of this river.
Table 2.
Physicochemical properties of the Halda River water.
| Sample ID | EC |
pH | TDS |
|---|---|---|---|
| (μS/cm) | (mg/L) | ||
| S1 | 33.4 | 6.42 | 20.1 |
| S2 | 33.1 | 6.22 | 19.8 |
| S3 | 33.7 | 6.16 | 20.3 |
| S4 | 33.5 | 6.05 | 20.1 |
| S5 | 32.5 | 5.84 | 19.94 |
| S6 | 33.6 | 5.87 | 20.2 |
| S7 | 32.8 | 6.21 | 20.1 |
| S8 | 33.6 | 6.14 | 20.1 |
| S9 | 32.9 | 5.98 | 19.55 |
| S10 | 31.3 | 5.98 | 18.74 |
| S11 | 33.6 | 5.88 | 20.2 |
| S12 | 32.8 | 5.51 | 19.62 |
| Average | 33.06 | 6.02 | 19.89 |
| STDEV | 0.69 | 0.24 | 0.43 |
| CV% | 2.07 | 3.9 | 2.17 |
| Standard (WHO) | 800–1000 | 6.5–8.5 | 1000 |
Minor differences in the total dissolved solids (TDS) levels were found in the collected samples and the values ranged between 18.74 and 20.30 mg/L. The highest TDS value was observed at the sampling point S3, while the lowest TDS value was detected at the sampling point S10. Excessive TDS leads to the increase in water temperature, hinders photosynthesis, and reduces water clarity [30]. However, TDS values for different sampling areas (Table 2) were found far below the WHO guideline value of 1000 mg/L, which has been specified for the protection of fisheries, aquatic life, and for domestic water supply [31].
The total concentration of ionized constituents of water is usually represented by measuring electrical conductivity (EC) and a higher conductivity reflects higher water pollution [32]. In this study, the EC values of the water samples, collected from different locations, were almost identical with mean value of 33.07 μS/cm (Table 2). The EC values were also far below the WHO guideline value of 80–1000 μS/cm.
From the physicochemical parameter analysis, it could be concluded that the water of the Halda River is satisfactory in terms of EC, pH, and TDS values.
3.2. Analysis of anions and cations
To identify the presence of ions, the collected water samples were analyzed by ion chromatography. The domestic and/or agricultural use of river water is compromised due to the presence of various dissolved salts in elevated concentrations. The sodium part of NaCl salt is connected to kidney and heart diseases, while the chloride part is harmless [32]. A salty taste may be rendered by NaCl at 250 mg/L concentration. Excessive chlorides in inland water are considered as an index of pollution. The presence of various anions with their respective concentration is described in Table 3. Various anions were quantified in this study according to the following decreasing order: chloride > sulphate > nitrate > fluoride. In this study, phosphate and bromide were found below their LOD (limit of detection) values, which are 0.5 ppm and 0.2 ppm respectively. Fluoride was found in only 5 samples with highest value of 0.29 mg/L at the sampling point S4. All of these values were far below the safe limit suggested by WHO. The highest and lowest values for chloride (8.93 mg/L, 6.30 mg/L), sulphate (4.26 mg/L, 3.47 mg/L), and nitrate (9.54 mg/L, 0.29 mg/L) were within the range of standard reference values [31]. The maximum and minimum concentration of HCO3- (83.70 mg/L and 68.38 mg/L) was found in sample S9 and S4 respectively. The responsible source of HCO3- in surface water could be the rock weathering process [25].
Table 3.
Concentration of anions in the Halda River water.
| Sample ID | Fl−(mg/L) | Cl−(mg/L) | SO42-(mg/L) | NO3-(mg/L) | HCO3-(mg/L) |
|---|---|---|---|---|---|
| S1 | 0.09 ± 0.01 | 7.23 ± 0.47 | 3.92 ± 0.26 | 1.51 ± 0.38 | 70.70 ± 4.8 |
| S2 | <0.02 | 6.68 ± 1.00 | 3.55 ± 0.5 | 0.37 ± 0.04 | 68.77 ± 3.7 |
| S3 | 0.28 ± 0.03 | 7.52 ± 0.11 | 3.94 ± 0.11 | 0.73 ± 0.01 | 76.60 ± 4.9 |
| S4 | 0.29 ± 0.00 | 7.51 ± 0.4 | 3.99 ± 0.04 | 0.23 ± 0.01 | 68.38 ± 6.77 |
| S5 | <0.02 | 7.92 ± 0.1 | 4.20 ± 0.01 | 0.46 ± 0.01 | 82.48 ± 6.87 |
| S6 | <0.02 | 8.93 ± 0.2 | 4.18 ± 0.02 | 0.50 ± 0.02 | 79.47 ± 5.98 |
| S7 | <0.02 | 8.93 ± 0.1 | 4.24 ± 0.01 | 1.26 ± 0.02 | 78.58 ± 8.1 |
| S8 | <0.02 | 8.88 ± 0.8 | 4.26 ± 0.08 | 0.34 ± 0.01 | 83.70 ± 6.8 |
| S9 | 0.07 ± 0.02 | 7.60 ± 0.2 | 4.16 ± 0.02 | 9.54 ± 0.02 | 78.73 ± 7.56 |
| S10 | <0.02 | 6.30 ± 2.5 | 3.47 ± 0.36 | 0.29 ± 0.01 | 76.89 ± 5.7 |
| S11 | <0.02 | 8.03 ± 0.1 | 4.20 ± 0.04 | 0.45 ± 0.01 | 77.46 ± 5.67 |
| S12 | 0.09 ± 0.01 | 7.86 ± 0.1 | 4.23 ± 0.01 | 0.53 ± 0.01 | 78.10 ± 6.12 |
| Standard (WHO) | 1.5 | 250 | 500 | 50 | - |
The cationic test results, shown in Table 4, reveals that sodium, ammonium, potassium, magnesium, calcium level in the collected samples were much lower than their standard reference values [31]. From the anionic and cationic test results, it could be stated that the Halda River water was not significantly polluted. The hardness of a waterbody depends on the presence of Ca and Mg cations in the water. The earth's crust is rich with Ca, which potentially contributes to the presence of Ca in the waterbodies. Lower concentration of Ca (1–2 mg/L) is usually found in river water, while the Ca concentration in lime river zone could be high as 100 mg/L [32].
Table 4.
Concentration of cations in the Halda River water.
| Sample ID | Na+ (mg/L) | NH4+ (mg/L) | K+ (mg/L) | Mg2+ (mg/L) | Ca2+ (mg/L) |
|---|---|---|---|---|---|
| S1 | 8.4 ± 0.28 | 0.3 ± 0.02 | 3.35 ± 0.14 | 3.33 ± 0.12 | 6.31 ± 0.2 |
| S2 | 8.96 ± 0.18 | 0.45 ± 0.05 | 3.42 ± 0.09 | 3.53 ± 0.07 | 6.7 ± 0.07 |
| S3 | 9.56 ± 0.04 | 0.36 ± 0.05 | 3.57 ± 0.12 | 3.81 ± 0.11 | 8.31 ± 0.2 |
| S4 | 9.03 ± 0.16 | 0.47 ± 0.04 | 3.38 ± 0.13 | 3.53 ± 0.11 | 6.51 ± 0.11 |
| S5 | 10.3 ± 0.28 | 0.36 ± 0.05 | 3.78 ± 0.13 | 4.07 ± 0.07 | 9.06 ± 0.13 |
| S6 | 10.6 ± 0.40 | 0.29 ± 0.01 | 3.88 ± 0.1 | 4.02 ± 0.07 | 7.92 ± 0.35 |
| S7 | 10.8 ± 0.20 | 0.19 ± 0.01 | 3.95 ± 0.03 | 4.05 ± 0.18 | 7.69 ± 0.24 |
| S8 | 10.8 ± 0.13 | 0.45 ± 0.02 | 3.97 ± 0.13 | 4.3 ± 0.06 | 8.45 ± 0.45 |
| S9 | 10.7 ± 0.16 | 0.37 ± 0.03 | 3.85 ± 0.08 | 4.03 ± 0.11 | 7.50 ± 0.21 |
| S10 | 9.9 ± 0.04 | 0.41 ± 0.01 | 3.88 ± 0.33 | 4.05 ± 0.06 | 7.50 ± 0.30 |
| S11 | 10.63 ± 0.10 | 0.38 ± 0.03 | 4.03 ± 0.13 | 3.83 ± 0.13 | 7.37 ± 0.04 |
| S12 | 10.27 ± 0.24 | 0.36 ± 0.07 | 3.41 ± 0.05 | 4.1 ± 0.14 | 7.79 ± 0.02 |
| Standard (WHO) | 200 | Not found | 10 | 50 | 75 |
∗BDL: Below Detection Limit.
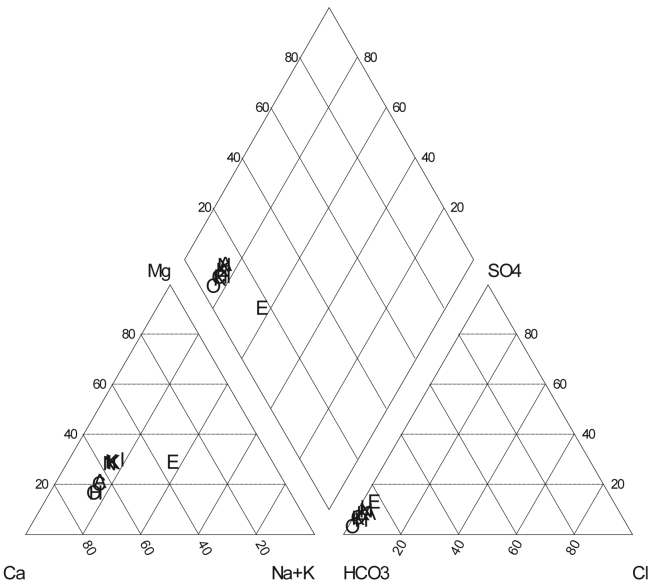
The concentration (meq/L) of major cations and anions, obtained from water sample analysis, were used to prepare piper diagram (Figure 2) to identify the hydrochemical facies and water type. The plot reveals the dominance of Ca2+, Mg2+ and HCO3- ions in the water system. Two types of hydrochemical facies of analyzed water samples have been found, they are Na–Ca–Mg–HCO3- and Mg–Na–HCO3- type (Table 5). The rock source deduction analysis of Aqua chem. Software (version 4) suggests that their possible sources may be comprised of either dolomite (CaCO3. MgCO3) or calcite (CaCO3) in the aquifer system. It should also be noted that the hardness of studied water samples was below limit value set by WHO, where the standard value of hardness for drinking water is 0–75 mg/L.
Figure 2.
Piper diagram of studied water samples collected from the Halda River.
Table 5.
The properties of water found from piper diagram.
| Sample ID | Water type | Total hardness mg/L CaCO3 | Total alkalinity mg/L CaCO3 |
|---|---|---|---|
| S1 | Mg–Na–HCO3 | 34.30 | 57.99 |
| S2 | Na–Ca–Mg–HCO3 | 31.27 | 56.40 |
| S3 | Na–Ca–Mg–HCO3 | 36.44 | 62.83 |
| S4 | Na–Ca–Mg–HCO3 | 30.79 | 56.08 |
| S5 | Ca–Na–Mg–HCO3 | 39.39 | 67.65 |
| S6 | Na–Ca–Mg–HCO3 | 36.33 | 65.18 |
| S7 | Na–Ca–Mg–HCO3 | 35.88 | 64.45 |
| S8 | Na–Ca–Mg–HCO3 | 38.81 | 68.65 |
| S9 | Na–Ca–Mg–HCO3 | 35.33 | 64.57 |
| S10 | Na–Ca–Mg–HCO3 | 35.41 | 63.06 |
| S11 | Na–Ca–Mg–HCO3 | 34.18 | 63.53 |
| S12 | Na–Ca–Mg–HCO3 | 36.34 | 64.06 |
3.3. Heavy metals contamination in the Halda River water
The concentration of heavy metals (Pb, Cr, Cd, As, Zn, Cu, Mn, Fe, Co, Ca, Hg) in the Halda River water is presented as mean ± standard deviation in Table 6. The concentration of these metals was compared with the permissible limit set by WHO and most of the metals exceeded maximum permissible limits as drinking water except Mn, Zn, and Hg [31].
Table 6.
Concentration of heavy metals in the Halda River water.
| Concentration of Heavy Metals in Water | ||||||||
|---|---|---|---|---|---|---|---|---|
| Element |
Unit |
S1 |
S2 |
S3 |
S4 |
S5 |
S6 |
S7 |
| Co | (μg/L) | <5.0 | 90.0 ± 3.19 | 20.0 ± 0.71 | 10.0 ± 0.35 | <5.0 | <5.0 | 170.0 ± 6.02 |
| Cd | (μg/L) | 10.0 ± 0.36 | 80.0 ± 2.85 | 10.0 ± 0.36 | 10.0 ± 0.36 | <1.0 | <1.0 | <1.0 |
| Cr | (μg/L) | 130.0 ± 4.72 | 50.0 ± 1.82 | 40.0 ± 1.45 | 80.0 ± 2.90 | 40.0 ± 1.45 | 50.0 ± 1.82 | 120.0 ± 4.36 |
| Fe | (μg/L) | 424.0 ± 67.59 | 311.0 ± 49.57 | 346.0 ± 55.16 | 398.0 ± 63.44 | 383.0 ± 61.05 | 457.0 ± 72.85 | 459.0 ± 73.16 |
| Mn | (μg/L) | 610.0 ± 4.81 | 440.0 ± 3.47 | 785.0 ± 61.94 | 295.0 ± 23.28 | 325.0 ± 25.64 | 385.0 ± 30.38 | 1185.0 ± 93.50 |
| Pb | (μg/L) | 2200.0 ± 78.98 | 1700.0 ± 61.03 | 900.0 ± 32.31 | 700.0 ± 25.13 | 700.0 ± 25.13 | 1400.0 ± 50.26 | 1000.0 ± 35.9 |
| Ca | (mg/L) | 3.61 ± 0.86 | 3.83 ± 0.71 | 4.32 ± 0.32 | 3.92 ± 0.62 | 3.97 ± 0.97 | 4.06 ± 0.6 | 3.99 ± 0.98 |
| Cu | (mg/L) | 25.54 ± 1.12 | 341.83 ± 15.01 | 387.70 ± 17.02 | 17.06 ± 7.49 | 204.70 ± 18.98 | 138.92 ± 6.09 | 575.39 ± 25.26 |
| Zn | (μg/L) | 940.8 ± 27.48 | 446.0 ± 60.43 | 803.0 ± 19 | 423.6 ± 37.40 | 583.6 ± 79.08 | 528.0 ± 71.54 | 575.2 ± 77.94 |
| Hg | (μg/L) | <0.3 | <0.3 | <0.3 | <0.3 | <0.3 | <0.3 | <0.3 |
| As |
(μg/L) |
12.0 ± 0.99 |
<2 |
30.0 ± 2.99 |
8.0 ± 0.77 |
4.0 ± 0.7 |
8.0 ± 0.65 |
2.0 ± 0.1 |
| Element |
Unit |
S8 |
S9 |
S10 |
S11 |
S12 |
Average |
Standard (WHO) |
| Co | (μg/L) | <5.0 | <5.0 | 60.0 ± 2.12 | <5.0 | <5.0 | 21.7 | - |
| Cd | (μg/L) | <1.0 | 30.0 ± 1.07 | <1.0 | <1.0 | 10.0 ± 0.36 | 12.5 | 3 |
| Cr | (μg/L) | 140.0 ± 5.08 | 110.0 ± 3.99 | 30.0 ± 1.09 | 60.0 ± 2.18 | <11.0 | 70.8 | 50 |
| Fe | (μg/L) | 481.0 ± 76.67 | 318.0 ± 50.69 | 380.0 ± 60.57 | 436.0 ± 69.49 | 494.0 ± 78.74 | 407.3 | 300 |
| Mn | (μg/L) | 450.0 ± 35.51 | 335.0 ± 26.43 | 175.0 ± 13.81 | 430.0 ± 33.93 | 220.0 ± 17.36 | 469.5 | 500 |
| Pb | (μg/L) | 1500.0 ± 53.85 | 2600.0 ± 93.34 | 700.0 ± 25.13 | 1000.0 ± 35.9 | 1000.0 ± 35.9 | 1283.3 | 10 |
| Ca | (mg/L) | 3.98 ± 0.21 | 3.59 ± 0.12 | 3.95 ± 0.21 | 4.63 ± 0.32 | 5.03 ± 0.42 | 4.07 | - |
| Cu | (mg/L) | 377.86 ± 16.59 | 41.75 ± 8.33 | 298.27 ± 10.09 | 352.80 ± 15.49 | 237.83 ± 10.44 | 294.076 | 2.00 |
| Zn | (μg/L) | 398.8 ± 54.08 | 389.8 ± 52.82 | 491.6 ± 66.61 | 519.2 ± 70.35 | 492.8 ± 66.77 | 549.4 | 3000 |
| Hg | (μg/L) | <0.3 | <0.3 | <0.3 | <0.3 | <0.3 | <0.3 | 1 |
| As | (μg/L) | 170.0 ± 1.7 | 126.0 ± 12 | 82.0 ± 8 | 132.0 ± 11.09 | 136.0 ± 13 | 60.0 | 10 |
The average concentration of Pb was found to be 1283.3 μg/L that was more than 25 times higher than the standard value of 50 μg/L [31]. Jolly et al. carried out a similar study on another river, Shitalakhya, in Bangladesh and reported 16 μg/L concentration of Pb [33]. Islam et al. observed the seasonal variation in Pb concentration and reported 35.0 μg/L Pb level in winter and 27.0 μg/L in summer season in River Korotoa [34]. Ahmad et al. found 65.45 μg/L of Pb level in water sample collected from River Buriganga [35]. The source of Pb can be attributed to vehicle exhaust, metal plating, wastewater discharge, fertilizer etc. [36]. The excessive concentration of Pb found in this study may be correlated to the discharge of effluents from industries located at Nandirhat near the river Halda. Lead (Pb) affects haem biosynthesis and erythropoiesis. Chronic Pb intoxication is responsible for cancers and anaemia in adults, reproductive harm in male, hormonal imbalance and IQ drop in young children [37, 38].
The average concentration of Cr was found to be 70.8 μg/L (30–140 μg/L), that also exceeded the WHO permissible limit of 50 μg/L. Islam et al. found similar data on River Korotoa. The authors reported 83 μg/L Cr during winter and 73 μg/L Cr during summer season [34]. Ahmad et al. found an extremely high concentration of Cr (587.44 μg/L) in River Buriganga [35], while Jolly et al. reported only 18 μg/L Cr in River Shitalakhya [33]. Commercially, Cr compounds are used in leather tanning, dyes and pigments, chrome plating, industrial welding, wood preservation etc. [39]. The high concentration of Cr could be associated with the tanneries as well as shipping related activities near the River Halda [40]. Chromium (Cr) must be removed from water before drinking as it is carcinogenic [41].
In this study, the Cd concentration at different points of along the Halda River were ranged from 10.0 ± 0.36 μg/L to 80.0 ± 2.85 μg/L. However, some sampling points found Cd concentration below the detection limit but the average value (12.5 μg/L) in rest of the sites exceeded their permissible limit of 3 μg/L [31]. Islam et al. found 11 μg/L Cd concentration in winter season in Korotoa River and 8 μg/L in summer season [34]. Ahmad et al. reported 9.34 μg/L Cd concentration in Buriganaga River water [35]. Recently, Jolly et al. found 3 μg/L of Cd concentration in Shitalakhya River water [33]. Metal industry, coal combustion and waste disposal are considered to be the major sources of Cd [42]. Cadmium (Cd) has carcinogenic effect, which also disturbs bone metabolism, generates various toxic effects in the body, affects kidneys and deforms reproductive and endocrine systems. Ca excretion can occur from exposure to Cd, which causes skeletal demineralization with subsequent consequence to the increased risk of bone fragility and fractures [43]. Reduced birth weight and premature death might occur due to exposure to Cd during pregnancy [44].
The present study revealed that the average value of As in the Halda River water was 60.0 μg/L (2.0 ± 0.1 μg/L to 170.0 ± 1.7 μg/L), that was 6 times higher than the WHO recommended value (10 μg/L). Conducting a similar study on Korotoa River, Islam et al. found 46 μg/L and 37 μg/L As levels during winter and summer season respectively [34]. Jolly et al. found As level within the permissible limit (10 μg/L) on water from Shitalakhya River [33]. Arsenic (As), available in the aquifer sediment with iron oxides, has been considered to be released by microbially-driven reductive dissolution in an organic-rich environment. In addition, As use for mining activities, industrial purposes, metal processing, fertilizers, and pesticides are other major source of contamination [45, 46]. Arsenic (As) has association with hypertension and impacts on the cardiovascular system and it may cause hepatic damage [47].
In this study, the average Zn concentration was recorded as 549.4 μg/L, that was below the permissible limit i.e., 3000 μg/L [31]. Conducting similar research on Shitalakya River water, Jolly et al. reported 56 μg/L of Zn concentration [33]. Zn is introduced into waterbodies through artificial pathways such as coal-fired power stations, steel production, burning of waste materials etc. [48]. Zn is an essential trace element and is relatively harmless, however, exposure to excessive concentration might lead to toxicity [49].
Among all the studied metals, Cu was in the highest concentration in all the sampling points of the Halda River. The lowest concentration of Cu detected in water samples was 17.06 ± 7.49 μg/L in sampling point S4 and the highest concentrations recorded was 575.39 ± 25.26 μg/L in sampling point S7. Copper (Cu) is largely released from agricultural fields, agrochemical industries, and urban sewage [34]. An overwhelming Cu intake could lead to several problems such as impairment of organs, vomiting, nausea, kidney failure, hemolytic jaundice, central nervous system depression etc. [50].
The average concentration of Mn was recorded as 469.5 μg/L that was below the permissible limit of 500 μg/L [31]. Lowest concentration of Mn was recorded for sampling point S10, while highest concentration was recorded for sampling point S7. Manganese (Mn) is an essential nutrient, which is toxic at high levels of exposure. It is an abundant transition metal in the earth's crust. Manganese (Mn) can exist in surface water in a broad range of oxidation states, which are controlled by microbial activity and dissolved oxygen levels [51]. Manganese (Mn) is an essential mineral in trace amounts, however, excessive exposure results in detrimental health effects and toxicity. Long-term exposure with Mn leads to Fe-deficiency anaemia as well as the impairment of the Cu-dependent metalloenzyme activity [52].
The concentration of Fe in the Halda River ranged from 311.0 ± 49.57 μg/L to 494.0 ± 78.74 μg/L that exceeded the permissible limit (300 μg/L) set by the WHO for drinking water [31]. High concentration of Fe in water could be linked to the effluent discharge from metal alloys industries. Fe is an essential element in most biological system. When an excessive amount of Fe accumulates in the body, Fe poisoning occurs. Excessive amount of Fe can irritate the stomach and the digestive tract [53]. The average concentration of Co and Ca was 21.7 μg/L and 4.07 mg/L respectively. In this study, none of the samples were detected with Hg. All forms of Hg are toxic, and the effect of Hg includes nephrotoxicity, neurotoxicity, and gastrointestinal toxicity [54].
3.4. Heavy metal pollution index
The HPI values for 12 water samples were calculated and the results were displayed in Figure 3. The HPI value is used to assess the quality of river water samples. The mean heavy metal pollution indexes of the Halda River water were calculated to be1797.21, which are found above the critical index value 100. Majority of the collected samples showed HPI value above the critical index value. Sampling points S2, S6, and S7 were exception. The high pollution index values could be attributed to the leaching of heavy metals from various industries (e.g., fertilizer, pigments, paper etc.) along the Halda River. It should be clearly stated that among various heavy metals, Cu, and As were the potential contributors to high pollution index value.
Figure 3.
Heavy metal Pollution Index (HPI) and Metal index (MI).
3.5. Mean metal index
The mean metal index (MI) value of the collected sample was calculated to be 0.038. The MI values showed that, in the Halda River, the sampling sites S1 and S3 were observed slightly affected with respect to heavy metals (Figure 3). This result could be correlated to effluent discharged from various large-scale as well as many small-scale industries located near the Halda River.
3.6. Single factor pollution index
The single pollution indexes (Pi) of Cd are greater than 3. It concludes that sampling site S1 to S4, S9, and S12 are extremely polluted with Cd. For As, sampling site S8 to S12 are highly contaminated. Single pollution indexes (Pi) of Pb and Cu also reveal that all of the sampling sites are highly contaminated with Pb and Cu. Sites S1, S7, S8, S9 are moderately contaminated by Cr. Except the site S7, rest of the sites possessed low contamination level of Mn. All the sampling sites were clean with respect to Zn. These results express that the contamination position is mainly located at the respective sampling sites or near to the sampling sites. The assessment of Nemerow's pollution index (PN) reveals that all the water samples were highly polluted. Single pollution index and Nemerow's pollution index are shown in Table 7. The distribution pattern of the contamination of different heavy metals in different sampling sites are shown in Figure 4.
Table 7.
Single Pollution Index and Nemerow's Pollution Index of samples collected from the Halda River.
| Sample | Pi(Cd) | Pi(As) | Pi(Zn) | Pi(Pb) | Pi(Cr) | Pi(Mn) | Pi(Cu) | PN |
|---|---|---|---|---|---|---|---|---|
| S1 | 3.33 | 1.2 | 0.31 | 220.00 | 2.60 | 1.22 | 127.69 | 233.84 |
| S2 | 26.67 | 0 | 0.15 | 170.00 | 1.00 | 0.88 | 170.92 | 198.74 |
| S3 | 3.33 | 3 | 0.27 | 90.00 | 0.80 | 1.57 | 193.85 | 166.71 |
| S4 | 3.33 | 0.8 | 0.14 | 70.00 | 1.60 | 0.59 | 85.29 | 87.29 |
| S5 | 0.00 | 0.4 | 0.19 | 70.00 | 0.80 | 0.65 | 102.35 | 94.83 |
| S6 | 0.00 | 0.8 | 0.19 | 140.00 | 1.00 | 0.77 | 69.46 | 146.48 |
| S7 | 0.00 | 0.2 | 0.18 | 100.00 | 2.40 | 2.37 | 287.70 | 228.21 |
| S8 | 0.00 | 17 | 0.19 | 150.00 | 2.80 | 0.9 | 188.93 | 193.46 |
| S9 | 10.00 | 12.6 | 0.13 | 260.00 | 2.20 | 0.67 | 208.76 | 289.04 |
| S10 | 0.00 | 8.2 | 0.16 | 70.00 | 0.60 | 0.35 | 149.14 | 127.21 |
| S11 | 0.00 | 13.2 | 0.17 | 100.00 | 1.20 | 0.86 | 176.40 | 159.52 |
| S12 | 3.33 | 13.6 | 0.16 | 100.00 | 0.00 | 0.44 | 118.92 | 126.96 |
Figure 4.
The distribution pattern of the contamination of heavy metals in sampling sites.
3.7. Pearson correlation matrix analysis
The Pearson correlation matrix of heavy metals are shown in Table 8. The p value indicates level of significance of correlation matrix. It also signifies the strength of correlations among heavy metals. For example, p value having 0.01 and 0.05 indicates strong and significant correlations respectively. Pearson correlation matrix suggests that the correlation in between Co and Mn (r = 0.650) is significant, and Mn possesses positive correlation with Zn (r = 0.392) and strong correlation with Cu (r = 0.704). Cu and Co (r = 0.642) also exhibit significant correlation. As is positively correlated with Fe (r = 0.303) and Ca (r = 0.397). Cr, NH4+ and Pb, NO3- are associated with significant correlation (r = 0.578) and (r = 0.718) respectively. The strong positive correlation could indicate similar source of origin of heavy metals in different water samples.
Table 8.
Pearson correlation matrix analysis for all heavy metals and selective anions found in studied water sample.
| Elements | Fe | Mn | Cr | Pb | Cd | Co | Ca | Cu | Zn | As | NH4+ | NO3- |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | 1 | |||||||||||
| Mn | .104 | 1 | ||||||||||
| Cr | .110 | .456 | 1 | |||||||||
| Pb | -.260 | .022 | .550 | 1 | ||||||||
| Cd | -.645∗ | .086 | -.073 | .443 | 1 | |||||||
| Co | .079 | .650∗ | .135 | .164 | .233 | 1 | ||||||
| Ca | .498 | .121 | -.624∗ | -.527 | .274 | .168 | 1 | |||||
| Cu | .109 | .704∗ | .440 | .184 | .110 | .652∗ | .096 | 1 | ||||
| Zn | .114 | .392 | .212 | .135 | .260 | .193 | .096 | -.053 | 1 | |||
| As | .303 | -.368 | .061 | .162 | -.196 | -.389 | .397 | .194 | .265 | 1 | ||
| NH4+ | .175 | .541 | .578∗ | .013 | .146 | .478 | -.512 | .402 | .040 | -.387 | 1 | |
| NO3- | -.427 | .042 | .345 | .718∗∗ | .217 | .125 | -.405 | .323 | -.225 | .271 | .072 | 1 |
∗. Significant correlation at the 0.05 level (2-tailed).
∗∗. Significant correlation at the 0.01 level (2-tailed).
3.8. Principal component analysis
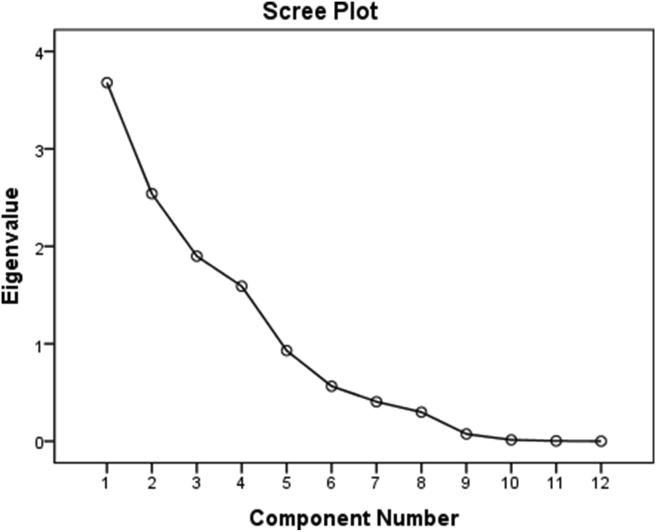
To find out reliable idea of the different sources of heavy metals contributing to the water collected from the different locations of the Halda River, Principal Component Analysis (PCA) was used to identify major elements associated with sources. The Kaiser-Meyer-Olkin (KMO) test value was 0.684 that is good for sampling adequacy. Barlett's test of sphericity reveals a high significant value of p < 0.001 which proves that correlation matrix is nonidentity matrix. To maximize the sum of variances of the factor coefficients, varimax rotation is used which better explains the possible factors that influence heavy metals. A scree plot of eigenvectors as a function of factor number is shown in Figure 5. The number of prominent principal components was considered on the basis of the Kaiser criterion having eigenvalue greater than 1 [55]. There are four factors according to this criterion, which represents 80.91% of the total variance. The scree plot is used to identify the number of PCs. The factor loadings, cumulative percentage, and percentage of variance are explained by each factor as listed in Table 9 and the component plot in rotated space of principal component analysis is shown in Figure 6.
Figure 5.
Scree plot of the characteristic roots of principal component analysis.
Table 9.
Principal component analysis (PCA) with varimax rotation for all heavy metal found in studied water sample.
| Rotated Component Matrixa | ||||
|---|---|---|---|---|
| Elements | Component |
|||
| PC1 | PC2 | PC3 | PC4 | |
| Co | .870 | -.248 | -.243 | .070 |
| Mn | .863 | .045 | .198 | -.292 |
| Cu | .831 | .292 | .057 | .337 |
| NH4+ | .700 | .152 | -.213 | -.304 |
| Pb | -.065 | .891 | -.210 | -.034 |
| NO3- | .000 | .796 | -.268 | .301 |
| Cr | .458 | .734 | .214 | -.276 |
| Ca | -.200 | -.572 | .465 | .482 |
| Fe | .011 | -.195 | .859 | .046 |
| Cd | .063 | .139 | -.821 | .134 |
| Zn | -.029 | .122 | .371 | -.733 |
| As | -.251 | .339 | .452 | .723 |
Extraction Method: Principal Component Analysis.
Rotation Method: Varimax with Kaiser Normalization.
Rotation converged in 8 iterations.
Figure 6.
Component plot in rotated space of principal component analysis.
In our study, four principal components PC1, PC2, PC3 and PC4 explain more than 30%, 21%, 16%, and 13% of variance respectively. The first principal component PC1 was found to be highly loaded with Co (0.870), Mn (0.863), Cu (0.831), NH4+ (0.700) and relatively low loadings with Cr (0.458). Co, Mn, Cu can be originated from untreated furnace oil which is released from heavy oil-based power plant located near the Halda River, probably it is the dominating source in factor 1. Generally, different metals are used as additives during refining process; metals can be absorbed from tankages and supply vessels as well as natural presence of these metals in the source rock from which the crude was extracted [56]. NH4+ and Cr show positive correlation with each other. (NH4)2Cr2O7 salt is used for leather tanning in tannery industry. Therefore, this could be a probable source of Cr and NH4+ in the Halda River.
The second principal component PC2 was loaded with Pb (0.891), NO3- (0.796), Cr (0.734). Lead (Pb) value for surface soil has been estimated to be 25 mg/kg on the global scale; levels above this suggest an anthropogenic influence [57]. Not only fugitive Pb, batteries, lead-based paints, the corrosion of lead pipes in areas of soft water and sewage sludge, Pb-bearing glass are all significant sources of Pb. Along with leather tanning, Cr normally originate from the metallurgical industry. River water can be contaminated with nitrate directly as the result of runoff of nitrate containing fertilizers. Atmosphere which carries nitrogen-containing compounds derived from automobiles and other sources, can also contributes nitrate in river water. Pb shows strong association with NO3-. Generally Pb(NO3)2 is used as pigments in lead paints, for dyeing and printing calico and other textiles. Therefore, this component reveals both natural and anthropogenic sources [58].
The third principal component PC3 have been found to be loaded with Fe (0.859), Ca (0.465), As (0.452). Iron is predominantly present in the Earth's crust and soil with a median value of 2.1% [59]. It is present mostly as Fe2+ in ferro-magnesian silicates and as Fe3+ in iron oxides and hydroxides which is the result of weathering. The anthropogenic sources of iron have been reported to include the iron and steel industry sewage [60].
Rivers generally contain 1–2 ppm Ca, but the concentration increases about 100 ppm in lime areas rivers. The average As abundance in the Earth's crust is 5 mg/kg. More than 200 mineral forms of As have been reported. Arsenopyrite, realgar, and orpiment are the major mineral hosts of As. These are the compounds of As(III) that were formed under reducing, subsurface conditions. Arsenic is also found in iron pyrite, galena, and chalcopyrite. Reductive dissolution of arsenic-rich iron oxyhydroxides also leads to As contamination [61, 62, 63, 64].
In fourth component PC4, As (0.723) is dominantly loaded along with Ca and Cu. Industrial uses of As is probable source for this factor. Generally, As is used in pharmaceutical industry, paint and dye industry, glass industry, pulp and wood industry.
3.9. Cluster analysis
Hierarchical Cluster Analysis (HCA) is an algorithm which is used to classify the objects of the system into groups based on their data similarities. In this study, HCA with centroid linkage method is further employed to explore the associations between heavy metals and some significant cation and anion (Figure 7). Two clusters are distinguished: the first cluster is primarily composed of Ca, Pb, Mn, Zn, Fe, As, Cr, Co, Cd, NO3- and NH4+. The second cluster is composed of Cu solely. This analysis reveals that Cr, Co, Cd, are mostly associated with NO3- and NH4+. Probably Zn, Fe, As are associated with each other by single source of origin. It also indicates that the major sources of discharge of Cu into river water are different from others.
Figure 7.
Dendrogram shows the clustering heavy metals and selective cations and anion.
4. Conclusion
The Halda River, in the south-east region of Bangladesh, is considered as an important river because it is the only natural breeding source of carps. This study thoroughly explores the water quality of the Halda River in terms of pH, electrical conductivity, total dissolved solids, anions, cations, and various toxic heavy metals. The river water quality at the 12 sampling points was found satisfactory in terms of pH, electrical conductivity, total dissolved solids, anions, and cations. However, the water was found to be polluted with various toxic heavy metals and 6 of the heavy metals (Cd, Cr, Fe, Pb, Cu, and As) largely exceeded their safe limit (for drinking water) suggested by world health organization (WHO). The direct discharges of effluents from the nearby industries are considered as the major source of heavy metals in the Halda River water. From multivariate statistical analysis, the probable major sources of heavy metal contamination in the Halda River water have been identified. Based on the findings from this study, it could be stated that an urgent and strict action from relevant authority is required to prevent the uncontrolled discharge of industrial effluents from the nearby industries to protect this economically significant Halda River from further deterioration of its water quality.
Declarations
Author contribution statement
Moumita Dey: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Asma Akter: Performed the experiments.
Saiful Islam & Shaikat Chandra Dey: Analyzed and interpreted the data; Wrote the paper.
Tasrina Rabia Choudhury & Konica Jannat Fatema: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Bilkis Ara Begum: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Noakhali Science and Technology University, Noakhali 3814, Bangladesh.
Data availability statement
Data associated with this study has been deposited at the Department of Food Technology and Nutrition Science, Noakhali Science and Technology University, Noakhali 3814, Bangladesh.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to the Analytical Chemistry Laboratory, Chemistry Division, Atomic Energy Centre, Dhaka 1000, Bangladesh and Atmospheric and Environmental Laboratory, Chemistry Division, Atomic Energy Centre, Dhaka 1000, Bangladesh for providing analytical support to carry out this study. The authors also thank Mr. Shishir K. Sarker, Ph.D. Candidate at the Department of Earth and Environmental Sciences, University of Kentucky, Lexington, USA for his support in drawing the Map of the sampling points along the Halda River.
Contributor Information
Moumita Dey, Email: moumita.ftns@nstu.edu.bd.
Tasrina Rabia Choudhury, Email: tsarina.rabia@gmail.com.
References
- 1.Nagajyoti P.C., Lee K.D., Sreekanth T.V.M. Heavy metals, occurrence and toxicity for plants: a review. Environ. Chem. Lett. 2010;8:199–216. [Google Scholar]
- 2.Muchuweti M., Birkett J.W., Chinyanga E., Zvauya R., Scrimshaw M.D., Lester J.N. Heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: implications for human health. Agric. Ecosyst. Environ. 2006;112:41–48. [Google Scholar]
- 3.Järup L. Hazards of heavy metal contamination. Br. Med. Bull. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 4.Itanna F. Metals in leafy vegetables grown in Addis Ababa and toxicological implications. Ethiop. J. Health Dev. 2002;16:295–302. [Google Scholar]
- 5.Jolly Y.N., Iqbal S., Rahman M.S., Kabir J., Akter S., Ahmad I. Energy dispersive X-ray fluorescence detection of heavy metals in Bangladesh cows’ milk. Heliyon. 2017;3 doi: 10.1016/j.heliyon.2017.e00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smical A.-I., Hotea V., Oros V., Juhasz J., Pop E. Studies on transfer and bioaccumulation of heavy metals from soil into lettuce. Environ. Eng. Manage. J. 2008;7:609–615. [Google Scholar]
- 7.He Z.L., Yang X.E., Stoffella P.J. Trace elements in agroecosystems and impacts on the environment. J. Trace Elem. Med. Biol. 2005;19:125–140. doi: 10.1016/j.jtemb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Shallari S., Schwartz C., Hasko A., Morel J.L. Heavy metals in soils and plants of serpentine and industrial sites of Albania. Sci. Total Environ. 1998;209:133–142. [PubMed] [Google Scholar]
- 9.Arruti A., Fernández-Olmo I., Irabien A. Evaluation of the contribution of local sources to trace metals levels in urban PM2.5 and PM10 in the Cantabria region (Northern Spain) J. Environ. Monit. 2010;12:1451–1458. doi: 10.1039/b926740a. [DOI] [PubMed] [Google Scholar]
- 10.Sträter E., Westbeld A., Klemm O. Pollution in coastal fog at Alto Patache, northern Chile. Environ. Sci. Pollut. Res. 2010;17:1563–1573. doi: 10.1007/s11356-010-0343-x. [DOI] [PubMed] [Google Scholar]
- 11.Arefin M.A., Mallik A. Sources and causes of water pollution in Bangladesh: a technical overview. Bibechana. 2018;15:97–112. [Google Scholar]
- 12.Saimon M.K., Mustafa M.G., Sarker B.S., Hossain M.B., Rahman M.M. Marketing channels of Indian carp fry collected from theHalda River and livelihood of the fry traders. Asian J. Agric. Res. 2016;10:28–37. [Google Scholar]
- 13.Ahmed A.S.S., Hossain M.B., Semme S.A., Babu S.M.O.F., Hossain K., Moniruzzaman M. Accumulation of trace elements in selected fish and shellfish species from the largest natural carp fish breeding basin in Asia: a probabilistic human health risk implication. Environ. Sci. Pollut. Res. 2020;27:37852–37865. doi: 10.1007/s11356-020-09766-1. [DOI] [PubMed] [Google Scholar]
- 14.Tsai C.-f., Islam M.N., MD R.K., Rahman K.U.M. Spawning of major carps in the lower Halda River, Bangladesh. Estuaries. 1981;4:127–138. [Google Scholar]
- 15.Bhuyan M.S., Bakar M.A. Seasonal variation of heavy metals in water and sediments in the Halda River, Chittagong, Bangladesh. Environ. Sci. Pollut. Res. 2017;24:27587–27600. doi: 10.1007/s11356-017-0204-y. [DOI] [PubMed] [Google Scholar]
- 16.Kabir H., Kibria M., Jashimuddin M., Hossain M.M. Conservation of a river for biodiversity and ecosystem services: the case of the Halda–the unique river of Chittagong, Bangladesh. Int. J. River Basin Manag. 2015;13:333–342. [Google Scholar]
- 17.Patra R.W.R., Azadi M.A. Limnology of the Halda River. J. Noami. 1985;2 [Google Scholar]
- 18.Sirajudeen J., Arulmanikandan S., Manivel V. Heavy metal pollution index of ground water of Fathima Nagar area near Uyyakondan channel Tiruchirappalli district, Tamilnadu, India. World J. Pharm. Pharmaceut. Sci. 2014;4:967–975. [Google Scholar]
- 19.Reza R., Singh G., Kumar J. Application of heavy metal pollution index for ground water quality assessment in Angul district of Orissa, India. Int. J. Res. Chem. Environ. 2011;1:118–122. [Google Scholar]
- 20.Mohan S.V., Nithila P., Reddy S.J. Estimation of heavy metals in drinking water and development of heavy metal pollution index. J. Environ. Sci. Health, Part A: Environ. Sci. Eng. Toxicol. 1996;31:283–289. [Google Scholar]
- 21.Tamasi G., Cini R. Heavy metals in drinking waters from Mount Amiata (Tuscany, Italy). Possible risks from arsenic for public health in the Province of Siena. Sci. Total Environ. 2004;327:41–51. doi: 10.1016/j.scitotenv.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Yan N., Liu W., Xie H., Gao L., Han Y., Wang M., Li H. Distribution and assessment of heavy metals in the surface sediment of Yellow River, China. J. Environ. Sci. 2016;39:45–51. doi: 10.1016/j.jes.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y., Yu H., Sun Y., Chen J. Novel assessment method of heavy metal pollution in surface water: a case study of Yangping River in Lingbao City, China. Environ. Eng. Res. 2017;22:31–39. [Google Scholar]
- 24.Zhaoyong Z., Abuduwaili J., Fengqing J. Heavy metal contamination, sources, and pollution assessment of surface water in the Tianshan Mountains of China. Environ. Monit. Assess. 2015;187:33. doi: 10.1007/s10661-014-4191-x. [DOI] [PubMed] [Google Scholar]
- 25.Chabukdhara M., Nema A.K. Assessment of heavy metal contamination in Hindon River sediments: a chemometric and geochemical approach. Chemosphere. 2012;87:945–953. doi: 10.1016/j.chemosphere.2012.01.055. [DOI] [PubMed] [Google Scholar]
- 26.Liu C.-W., Lin K.-H., Kuo Y.-M. Application of factor analysis in the assessment of groundwater quality in a Blackfoot disease area in Taiwan. Sci. Total Environ. 2003;313:77–89. doi: 10.1016/S0048-9697(02)00683-6. [DOI] [PubMed] [Google Scholar]
- 27.Campbell I.C. A biological investigation of an organically polluted urban stream in Victoria. Aust. J. Mar. Freshw. Res. 1978;29:275–291. [Google Scholar]
- 28.Effendi H., Romanto, Wardiatno Y. Water quality status of Ciambulawung river, Banten Province, based on pollution index and NSF-WQI. Procedia Environ. Sci. 2015;24:228–237. [Google Scholar]
- 29.Manjare S.A., Vhanalakar S.A., Muley D.V. Analysis of water quality using physicochemical parameters Tamdalge tank in Kolhapur district, Maharashtra. Int. J. Adv. Biotechnol. Res. 2010;1:115–119. http://www.bipublication.com/files/ijabarv1i220108.pdf [Google Scholar]
- 30.Rahman M.S., Gagnon G.A. Bench-scale evaluation of drinking water treatment parameters on iron particles and water quality. Water Res. 2014;48:137–147. doi: 10.1016/j.watres.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 31.WHO . World Health Organization; Geneva: 1993. Guidelines for Drinking-Water Quality. [Google Scholar]
- 32.Florescu D., Ionete R.E., Sandru C., Iordache A., Culea M. The influence of pollution monitoring parameters in characterizing the surface water quality from Romania southern area. Rom. J. Phys. 2011;56:1001–1010. https://rjp.nipne.ro/2011_56_7-8/1001_1010.pdf [Google Scholar]
- 33.Jolly Y., Rana S., Akter S., Rahman M.S., Rahman M.M., Sultana M.S. Appraisal of metal pollution in the aquatic environment of Shitalakhya River, Bangladesh and its ecological risk assessment. J. Nat. Sci. Sustain. Technol. 2018;12:289–313. [Google Scholar]
- 34.Islam M.S., Ahmed M.K., Raknuzzaman M., Habibullah-Al-Mamun M., Islam M.K. Heavy metal pollution in surface water and sediment: a preliminary assessment of an urban river in a developing country. Ecol. Indicat. 2015;48:282–291. [Google Scholar]
- 35.Ahmad M.K., Islam S., Rahman M.S., Haque M.R., Islam M.M. Heavy metals in water, sediment and some fishes of Buriganga River, Bangladesh. Int. J. Environ. Res. 2010;4:321–332. [Google Scholar]
- 36.Karrari P., Mehrpour O., Abdollahi M.A. Systematic review on status of lead pollution and toxicity in Iran; Guidance for preventive measures. DARU J. Pharm. Sci. 2012;20:2. doi: 10.1186/1560-8115-20-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siddiqui M.K.J., Srivastava S., Mehrotra P.K. Environmental exposure to lead as a risk for prostate cancer. Biomed. Environ. Sci. 2002;15:298–305. [PubMed] [Google Scholar]
- 38.Tandon S.K., Chatterjee M., Bhargava A., Shukla V., Bihari V. Lead poisoning in Indian silver refiners. Sci. Total Environ. 2001;281:177–182. doi: 10.1016/s0048-9697(01)00845-2. [DOI] [PubMed] [Google Scholar]
- 39.Barnhart J. Occurrences , uses, and properties of chromium. Regul. Toxicol. Pharmacol. 1997;26:S3–S7. doi: 10.1006/rtph.1997.1132. [DOI] [PubMed] [Google Scholar]
- 40.Brady J.P., Ayoko G.A., Martens W.N., Goonetilleke A. Temporal trends and bioavailability assessment of heavy metals in the sediments of Deception Bay, Queensland, Australia. Mar. Pollut. Bull. 2014;89:464–472. doi: 10.1016/j.marpolbul.2014.09.030. [DOI] [PubMed] [Google Scholar]
- 41.Cohen M.D., Kargacin B., Klein C.B., Costa M. Mechanisms of chromium carcinogenicity and toxicity. Crit. Rev. Toxicol. 1993;23:255–281. doi: 10.3109/10408449309105012. [DOI] [PubMed] [Google Scholar]
- 42.Rzętała M.A. Cadmium contamination of sediments in the water reservoirs in Silesian Upland (southern Poland) J. Soils Sediments. 2016;16:2458–2470. [Google Scholar]
- 43.Wu X., Jin T., Wang Z., Ye T., Kong Q., Nordberg G. Urinary calcium as a biomarker of renal dysfunction in a general population exposed to cadmium. J. Occup. Environ. Med. 2001;43:898–904. doi: 10.1097/00043764-200110000-00009. https://journals.lww.com/joem/Abstract/2001/10000/Urinary_Calcium_as_a_Biomarker_of_Renal.9.aspx [DOI] [PubMed] [Google Scholar]
- 44.Henson M.C., Chedrese P.J. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp. Biol. Med. 2004;229:383–392. doi: 10.1177/153537020422900506. [DOI] [PubMed] [Google Scholar]
- 45.Islam F.S., Gault A.G., Boothman C., Polya D.A., Charnock J.M., Chatterjee D., Lloyd J.R. Role of metal-reducing bacteria in arsenic release from Bengal delta sediments. Nature. 2004;430:68–71. doi: 10.1038/nature02638. [DOI] [PubMed] [Google Scholar]
- 46.Stuckey J.W., Schaefer M.V., Kocar B.D., Benner S.G., Fendorf S. Arsenic release metabolically limited to permanently water-saturated soil in Mekong Delta. Nat. Geosci. 2016;9:70–76. [Google Scholar]
- 47.Lee M.-Y., Jung B.-I., Chung S.-M., Bae O.-N., Lee J.-Y., Park J.-D., Yang J.-S., Lee H., Chung J.H. Arsenic-induced dysfunction in relaxation of blood vessels. Environ. Health Perspect. 2003;111:513–517. doi: 10.1289/ehp.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wuana R.A., Okieimen F.E. Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. Int. Scholarly Res. Not. 2011;2011:402647. [Google Scholar]
- 49.Plum L.M., Rink L., Haase H. The essential toxin: impact of zinc on human health. Int. J. Environ. Res. Publ. Health. 2010;7:1342–1365. doi: 10.3390/ijerph7041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hashem E.Y., Seleim M.M., El-Zohry A.M. Environmental method for spectrophotometric determination of copper (II) Green Chem. Lett. Rev. 2011;4:241–248. [Google Scholar]
- 51.Tobiason J.E., Bazilio A., Goodwill J., Mai Xuyen., Nguyen C. Manganese removal from drinking water sources. Curr. Pollut. Rep. 2016;2:168–177. [Google Scholar]
- 52.Crossgrove J., Zheng W. Manganese toxicity upon overexposure. NMR Biomed. 2004;17:544–553. doi: 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fraga C.G., Oteiza P.I. Iron toxicity and antioxidant nutrients. Toxicology. 2002;180:23–32. doi: 10.1016/s0300-483x(02)00379-7. [DOI] [PubMed] [Google Scholar]
- 54.Tchounwou P.B., Ayensu W.K., Ninashvili N., Sutton D. Environmental exposure to mercury and its toxicopathologic implications for public health. Environ. Toxicol. 2003;18:149–175. doi: 10.1002/tox.10116. [DOI] [PubMed] [Google Scholar]
- 55.Kaiser H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960;20:141–151. [Google Scholar]
- 56.Akpoveta O.V., Osakwe S.A. Determination of heavy metal contents in refined petroleum products. IOSR J. Appl. Chem. 2014;7:1–2. [Google Scholar]
- 57.Kabata-Pendias A. fourth ed. CRC Press/Taylor & Francis Group; Boca Raton, FL, USA: 2010. Trace Elements in Soils and Plants. [Google Scholar]
- 58.Akter S., Islam S.M.A., Rahman M.O., Mamun K.M., Kabir M.J., Rahman M.S., Begum B.A., Abedin M.J., Tushar S.I., Jolly Y.N. Toxic elements accumulation in vegetables from soil collected from the vicinity of a Fertilizer factory and possible health risk assessment. Open Access J. Biomed. Eng.Biosci. 2019;3:277–288. [Google Scholar]
- 59.Rose A.W., Hawkes H.E., Webb J.S. second ed. Vol. 17. Academic Press; London: 1981. pp. 298–299. (Geochemistry in mineral Exploration). Earth-Sci. Rev. [Google Scholar]
- 60.Reimann C., Caritat P. de. Springer; 1998. Chemical Elements in the Environment: Factsheets for the Geochemist and Environmental Scientist. (Online) [Google Scholar]
- 61.Calcium and water: reaction mechanisms, environmental impact and health effects. https://www.lenntech.com/periodic/water/calcium/calcium-and water.htm?fbclid=IwAR1rLNAzWeNz46fd5MeQ6RekU6PcvNTqiqQoBnBzE5U1tasqm2tGotwlyMQ. Accessed: 18 December 2021.
- 62.Goldschmidt V.M. Clarendon Press; Oxford: 1954. Geochemistry. [Google Scholar]
- 63.Wedepohl K.H. Springer-Verlag Berlin Heidelberg; 1969. Handbook of Geochemistry; p. 1969. Softcover, Series ISNN: 0072-9817. [Google Scholar]
- 64.Nickson R., McArthur J., Burgess W., Ahmed K.M., Ravenscroft P., Rahman M. Arsenic poisoning of Bangladesh groundwater. Nature. 1998;395:338. doi: 10.1038/26387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study has been deposited at the Department of Food Technology and Nutrition Science, Noakhali Science and Technology University, Noakhali 3814, Bangladesh.