Abstract
Based on a long-term field investigation on chigger mites in southwest China from 2001 to 2019, the present study analyzed the infestation and distribution of chigger mites on the Chevrieri's field mouse (Apodemus chevrieri) in the region. A total of 12,516 individuals of chigger mites were collected from 1981 A. chevrieri mice, and 12,281 chiggers were identified as 107 species, 11 genera and 3 subfamilies in 2 families, which revealed a high species diversity of the mites on A. chevrieri mice. Of 1981 A. chevrieri mice, 633 ones were infested with chiggers with a relatively high overall prevalence (PM = 31.95%), mean abundance (MA = 6.32) and mean intensity (MI = 19.77). Of the 107 chigger species identified from A. chevrieri mice, three ones were the most dominant and they were Leptrombidium scutellare, L. densipunctatum and L. cricethrionis, which showed aggregated distribution among different individuals of the mice. A slightly positive association existed between every two dominant chigger species, which implied that the dominant chigger species tend to co-exist on A. chevrieri. The infestations of A. chevrieri with chiggers varied in different latitudes, altitudes and landscapes and they showed some heterogeneity along different environmental gradients. The logistic regression analysis showed that the risk factors for chigger infestations on A. chevrieri were landscapes, ages and altitudes, which implied that the environmental factors and host ages could influence the infestations of the mice with the mites. A theoretical curve of the species abundance distribution of chigger mites on A. chevrieri was successfully fitted by Preston's lognormal model, suggesting that the species abundance distribution conforms to the lognormal distribution pattern. The expected total species of chigger mites on A. chevrieri was roughly estimated to be 136 species and about 29 rare chigger species were probably missed in the sampling field investigation.
Keywords: Acari, Chigger mite, Ectoparasite, Rodent, Apodemus chevrieri, Southwest China
Graphical abstract
Highlights
-
•
Infestation and distribution of chigger mites on Apodemus chevrieri in Southwest China.
-
•
Risk factors of infestations of Apodemus chevrieri with chigger mites.
-
•
It is the first time to study the chigger mites of Apodemus chevrieri in such a wide geographical region.
1. Introduction
Chigger mites (trombiculid mites) are a group of tiny arthropods and they belong to families Trombiculidae and Leeuwenhoekiidae in the subclass Acari (Li et al., 1997; Ren et al., 2014; Ding et al., 2021a). Some literatures claim that there are over 3000 species of chigger mites recorded in the world and more than 400 species in China (Li et al., 1997; Zhan et al., 2013; Lv et al., 2021). Some other literatures, however, indicate that over 3700 species of chigger mites have been documented throughout the world and more than 510 species in China (Ding et al., 2020, 2021a, 2021b). Of many chigger mite species documented in various literatures, some species are actually synonyms or invalid species, and therefore it has been a long time to obtain an exact number of chigger mite species (Nielsen et al., 2021; Li et al., 1997; Vercammen-Grandjean and Langston, 1976). A latest review on the annotated world checklist of chigger mites indicates that there are 3013 species of chigger mites (Trombiculidae and Leeuwenhoekiidae) in the world, excluding some synonyms or invalid species (Nielsen et al., 2021). However, the exact species of chigger mites recorded in China are still unknown and they may have exceeded 500 species (Li et al., 1997; Duan et al., 2009).
In the complicated life cycle of chigger mites, only the larvae (often called “chiggers”) are the ectoparasites of some other animals, especially rodents (Li et al., 1997). The larvae of chigger mites (chiggers) are the exclusive vector of scrub typhus (tsutsugamushi diseases) caused by the causative agent Orentia tsutsugamushi, Ot (Xiang and Guo, 2021). In current years, scrub typhus is widespread in the Asia-Pacific region including many parts of China (especially in the south and southwest) with around 1,000,000 cases appearing annually (Li et al., 1997; Latif et al., 2017). Besides transmitting scrub typhus, chiggers are also proved to be the potential vector of hemorrhagic fever with renal syndrome, HFRS (Peng et al., 2018; Xiang and Guo, 2021).
Rodents and some other small mammals (e.g. insectivores and tree shrews) usually harbor lots of chiggers on their body surface and chiggers are common ectoparasites of rodents (Peng et al., 2016; Ding et al., 2021a). The Chevrieri's field mouse, Apodemus chevrieri (Miline-Edwards, 1868), is a common species of rodent in China and it is widely distributed in many parts of China such as Yunnan, Guizhou, Sichuan and Chongqing, etc. (Wlison et al., 2017). As a typical wild rodent species, A. chevrieri is often found in crop fields and various farmlands, bush areas, grasslands and open woodlands in its distributed regions (Wlison et al., 2017). Besides destroying crops and other agricultural plants as an important pest in the distribution regions, A. chevrieri is also a common reservoir host and infectious source of some zoonotic diseases (zoonoses), e. g. bartonellosis, plague, HFRS and some other zoonoses (Men et al., 2007; Tsai et al., 2010; Wang et al., 2018).
From 2001 to 2019, a long-term field investigation on chigger mites was carried out in southwest China, which covered five provincial regions including Yunnan, Guizhou, Chongqing, Sichuan and the east part of Tibet (Xizang Autonomous Region). Based on the investigation data, the present paper studied the infestation and distribution of chigger mites on A. chevrieri in southwest China, which is the first time to study the chigger mites of A. chevrieri in such a wide geographical region.
2. Materials and methods
2.1. Field investigation and collection of chigger mites
All the A. chevrieri mice, together with some other rodents and small mammals (insectivores and tree shrews, etc.), were captured with mousetraps (Guixi Mousetrap Apparatus Factory, Guixi, Jiangxi, China). The mousetraps were set in different type of habitats in the evening and checked the following morning. Every trapped animal host was put into a pre-marked cloth bag separately and then brought to the field laboratory where the animal host was anesthetized with ether. Over a large white tray, chiggers were collected from each host with a special bistoury or curette (Lv et al., 2019, Lv et al., 2021). After the collection of chiggers, every host was identified to species according to its appearance (the body size, body shape and coat color, etc.) and some measurements such as the body weight, body length and the lengths of ears, tail and hind feet (Huang et al., 1995; Wlison et al., 2017). The ages and genders (sexes) of A. chevrieri mice and other small mammal hosts were determined based on the body size, the relative ratio of head to trunk, color depth of pelage, the developmental status of genitalia and the distance from urethra to anus, etc. (Zheng et al., 2012; Zhuo et al., 2016; Morris, 1972). The identified A. chevrieri mice, together with the chiggers on the mice, were selected as the target of the present study. In the laboratory, the preserved chiggers in 70% of ethanol were isolated from some other “non-mite” impurities, the scurf and debris from the mice's skin, under a stereo microscope, and then made into slide-mounted specimens with Hoyer's medium. With the help of some relevant taxonomic literatures including taxonomic monographs and identification keys, the slide-mounted chiggers were identified to species under microscopes after dehydration and transparent process (Li et al., 1997; Vercammen-Grandjean and Langston, 1976). The capture and use of animals for research were officially approved by the local wildlife affairs authority and the Animal Ethics Committee of Dali University, which followed the international standards of animal euthanasia, 2013 AVMA guidelines (Cima, 2013). The representative specimens of animal hosts and chigger mites are deposited in the specimen repository of the Institute of Pathogens and Vectors of Dali University.
2.2. General statistics of chigger mites
The constituent ratio (Cr), prevalence (PM), mean abundance (MA) and mean intensity (MI) were used to calculate the infestation of A. chevrieri with chigger mites (Ding et al., 2021a; Liu, 2020a), in which Cr represents the percentage of a certain chigger species on A. chevrieri mice, PM the percentage of the infested mice with chiggers, MA the average number of chiggers per examined mouse and MI the average number of chiggers per infested mouse. The richness index (richness, S), Shannon-Wiener's diversity index (H′), Pielou's evenness (E) and Simpson's dominance index (D) were used to describe the chigger community structure on the mice (Magurran, 1998; Ding et al., 2021a; Lv et al., 2021).
In the above formulae, Ni = the individuals of chigger mite species i on the host (A. chevrieri), N = the total individuals of all the species of chigger mites and Si = the species i in the community.
2.3. Logistic regression analysis of risk factors related to chigger infestations
The logistic regression model was used to analyze the risk factors of infestations of A. chevrieri mice with chigger mites. All the observation variables with statistical significance (P < 0.05) were taken as single factor covariates, and the infestation of A. chevrieri mice with chigger mites was taken as dependent variables. The statistics and the 95% confidence interval (CI) were made under SPSS 25.0 software, and P < 0.05 was considered to be of statistical significance. (Truett et al., 1967; Jiang, 2015; Liu, 2020).
2.4. Distribution of species abundance and estimation of total species of chigger mites
In a semi-logarithmic rectangular system, X-axis scaled with log-intervals (log3N) was marked with chigger individuals and Y-axis with arithmetic scales was marked with chigger species. Preston's lognormal model was used to fit the theoretical curve of species abundance distribution as the following formula (Preston, 1948; Peng et al., 2016). Based on the species abundance distribution, the expected total species of chigger mites (ST) on A. chevrieri were estimated by the method based on Preston's lognormal model (Ding et al., 2021a; Preston, 1948; Peng et al., 2017).
(e = 2.71828 …) (Preston's lognormal distribution model)
where stands for the theoretical species number at R-th log interval, S(R) the actual chigger mite species in R-th log interval, R the log interval R and R0 the mode log interval. And S0 represents the mite species at R0 log interval and β the spread constant of the distribution. The value of β was determined according to the best determination coefficient (R2) in statistics, n is the number of log intervals in the fitting of theoretical curve, and ST is the estimated total species of chigger mites on A. chevrieri mice (Peng et al., 2017; Ding et al., 2021a).
2.5. Measurement of spatial distribution patterns
The Cassie index (CA) and clumping index (I) were used to measure the spatial distribution pattern of the dominant mite species among the different individuals of the host, A. chevrieri mice (Kuno, 1991; Xiang et al., 2021b; Ding et al., 2021a).
In the above formulae, m and σ2 represent the mean and variance of chigger mites on the host A. chevrieri. When CA and I > 0, the spatial distribution was determined as the aggregated distribution.
2.6. Measurement of interspecific association
Based on the establishment of a contingency table (see Table 4 in “Results”), the association coefficient (V) was used to calculate the interspecific association between any two species of chigger mites (chigger species) on the mouse host, A. chevrieri mice. Chi-square test was used for testing the statistical significance of V (Guo et al., 2006; Yin et al., 2021).
Table 4.
Analysis on interspecific association between any two dominant species of chigger mites on Chevrieri's field mice (Apodemus chevrieri) in southwest China (2001–2019).
| Leptotrombidium densipunctatum | Leptotrombidium cricethrionis | ||||||
|---|---|---|---|---|---|---|---|
| + | – | total | + | – | total | ||
| Leptotrombidium scutellare | + | 15 (a) | 146 (b) | 161 (a + b) | 63 (a) | 104 (b) | 167 (a + b) |
| – | 93 (c) | 1727 (d) | 1820 (c + d) | 64 (c) | 1756 (d) | 1820 (c + d) | |
| Total | 108 (a + c) | 1873 (b + d) | 1981 (n) | 127 (a + c) | 1860 (b + d) | 1987 (n) | |
| Association coefficient | V = 0.05 | V = 0.39 | |||||
| Chi-square | χ2 = 11.203 | χ2 = 6.109 | |||||
| Significance | <0.05 | <0.05 | |||||
In the above formula, V = the association coefficient between chigger species X and Y; a = the host individuals on which chigger species X and Y simultaneously occur; b = the host individuals on which chigger species Y occurs, but chigger species X does not occur; c = the host individuals on which chigger species X occurs, but chigger species Y does not occur; and d = the host individuals on which both chigger species X and Y do not occur. When V > 0 and P < 0.05, the interspecific relationship between chigger species X and Y is determined as the positive association, and when V < 0 and p < 0.05, the negative association. And P is the significance probability in Chi-square test (χ 2).
3. Result
3.1. Collection and identification of chigger mites
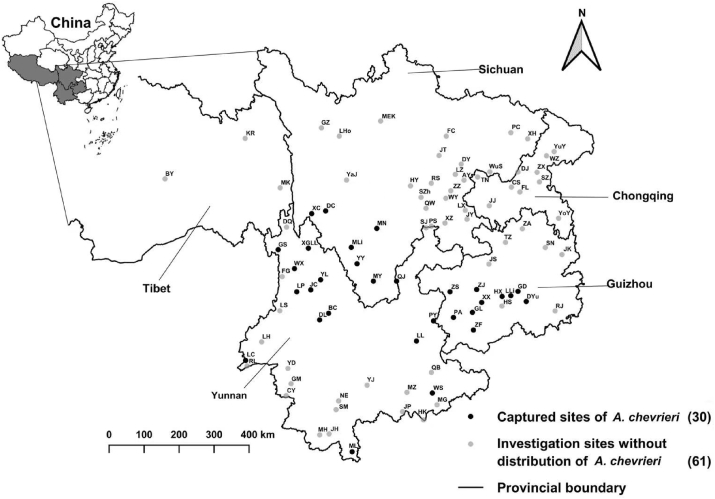
A total of 1981 A. chevrieri mice were captured from 30 sites of 91 investigated ones in southwest China. The result showed that A. chevrieri mice were not distributed in all the region of southwest China and they were mainly from Yunnan, Sichuan and Guizhou (Fig. 1, Table 1). From the body surface of 1981 A. chevrieri mice, 12516 individuals of chigger mites (chiggers) were collected. Of 12,516 collected mites, 12,281 ones were identified as 107 species, 10 genera in 3 subfamilies under 2 families (Table 2), and the rest 235 mites remained unidentified because of broken body, dirt-covered body, blurred structure or suspected new species.
Fig. 1.
The 91 investigation sites and the captured sites where Chevrieri's field mice (Apodemus chevrieri) were captured in southwest China (2001–2019).
Table 1.
The 91 investigation sites and the captured sites where Chevrieri's field mice (Apodemus chevrieri) were captured in southwest China (2001–2019).
| No. | Abbr. | Investigation sites | No. | Abbr. | Investigation sites | No. | Abbr. | Investigation sites |
|---|---|---|---|---|---|---|---|---|
| 1 | AY | Anyue | 32 | JY | Jiangyang (Luzhou city) | 63 | SM | Simao |
| 2 | BC | Binchuan* | 33 | KR | Karuo (Changdu city) | 64 | SN | Sinan |
| 3 | BY | Bayi (Linzhi city) | 34 | LC | Longchuan* | 65 | SZ | Shizhu |
| 4 | CS | Changshou | 35 | LH | Lianghe | 66 | SZh | Shizhong (Leshan city) |
| 5 | CY | Cangyuan | 36 | LHo | Luhuo | 67 | TN | Tongnan |
| 6 | DC | Daocheng* | 37 | LL | Luliang* | 68 | TZ | Tongzhi |
| 7 | DJ | Dianjiang | 38 | LLi | Longli* | 69 | WS | Wenshan* |
| 8 | DL | Dali* | 39 | LP | Lanping* | 70 | WuS | Wusheng |
| 9 | DQ | Deqin | 40 | LS | Lushui | 71 | WX | Weixi* |
| 10 | DY | Daying | 41 | LX | Luxian | 72 | WY | Weiyuan |
| 11 | DYu | Duyun* | 42 | LZ | Lezhi | 73 | WZ | Wanzhou |
| 12 | FC | Fucheng (Mianyang city) | 43 | MEK | Maerkang | 74 | XC | Xiangcheng* |
| 13 | FG | Fugong | 44 | MG | Maguan | 75 | XGLL | Xianggelila* |
| 14 | FL | Fuling | 45 | MH | Menghai | 76 | XH | Xuanhan |
| 15 | FY | Fuyuan* | 46 | MK | Mangkang | 77 | XX | Xixiu (Anshun city)* |
| 16 | GD | Guiding* | 47 | ML | Mengla* | 78 | XZ | Xuzhou(Yibin city) |
| 17 | GL | Guanling* | 48 | MLi | Muli* | 79 | YaJ | Yajiang |
| 18 | GM | Gengma | 49 | MN | Mianning* | 80 | YD | Yongde |
| 19 | GS | Gongshan* | 50 | MY | Miyi* | 81 | YJ | Yuanjiang |
| 20 | GZ | Ganzi | 51 | MZ | Mengzi | 82 | YL | Yulong* |
| 21 | HK | Hekou | 52 | NE | Ninger | 83 | YoY | Youyang |
| 22 | HS | Huishui | 53 | PA | Puan* | 84 | YuY | Yunyang |
| 23 | HX | Huaxi (Guiyang city)* | 54 | PC | Pingchang | 85 | YY | Yanyuan* |
| 24 | HY | Hongya | 55 | PS | Pingshan | 86 | ZA | Zhengan |
| 25 | JC | Jianchuan* | 56 | QB | Qiubei | 87 | ZF | Zhenfeng* |
| 26 | JH | Jinghong | 57 | QJ | Qiaojia* | 88 | ZJ | Zhijin* |
| 27 | JJ | Jiangjin | 58 | QW | Qianwei | 89 | ZS | Zhongshanzx (Liupanshui city)* |
| 28 | JK | Jiangkou | 59 | RJ | Rongjiang | 90 | ZX | Zhongxian |
| 29 | JP | Jinping | 60 | RL | Ruili | 91 | ZZ | Zizhong |
| 30 | JS | Jinsha | 61 | RS | Renshou | |||
| 31 | JT | Jintang | 62 | SJ | Suijiang |
Annotation: The investigated sites (counties) marked with “*” were the captured sites where A. chevrieri mice were captured.
Table 2.
Identified chigger mites from Chevrieri's field mice (Apodemus chevrieri) in southwest China between 2001 and 2019.
| Taxonomic taxa of chigger mites(Family, Subfamily, Genus and Species) | Individuals | Taxonomic taxa of chigger mites(Family, Subfamily, Genus and Species) | Individuals | Taxonomic taxa of chigger mites(Family, Subfamily, Genus and Species) | Individuals |
|---|---|---|---|---|---|
| 1. Family Trombiculidae | L. imphalum Vercammen-Grandjean and Langston, 1975 | 6 | N. sinica (Wang, 1964) | 4 | |
| 1.1 Subfamily Trombiculinae | L. jinmai Wen and Xiang, 1984 | 54 | N. tongtianhensis Yang et al.,1995 | 13 | |
| 1.1.1 Genus Ascoschoengastia | L. kunmingense Wen and Xiang, 1984 | 5 | 1.1.7 Genus Trombiculindus | ||
| A. leechi (Domrow, 1962) | 1 | L. laojunshanense Yu et al., 1986 | 4 | T. bambusoides Wang and Yu, 1965 | 255 |
| 1.1.2 Genus Eutrombicula | L. lianghense Yu et al., 1983 | 12 | T. chilie Wen and Xiang, 1984 | 2 | |
| E. wichmanni (Oudemans, 1905) | 1 | L. linji Wen and Sun, 1984 | 1 | T. cuneatus Traub and Evans,1951 | 3 |
| 1.1.3 Genus Helenicula | L. longchuanense Yu et al., 1981 | 5 | T. jilie Wen and Xiang, 1984 | 1 | |
| H. kohlsi (Philip and Woodward, 1946) | 4 | L. longimedium Wen and Xiang, 1984 | 277 | T. nujiange Wen and Xiang, 1984 | 136 |
| H. lanius (Radford, 1946) | 1 | L. lushanense Wang and Song, 1991 | 12 | T. yunnanus (Wang and Yu, 1965) | 125 |
| H. hsui Zhao, 1990 | 86 | L. neotebraci Xiang and Wen,1986 | 6 | 1.1.8 Genus Walchia | |
| H. simena (Hsu and Chen, 1957) | 247 | L. nyctali Wen and Sun,1984 | 2 | W. acutascuta Chen, 1980 | 1 |
| H. yunnanensis Wen and Xiang, 1984 | 12 | L. pallidum (Nagayo et al., 1919) | 5 | W. enode Gater, 1932 | 1 |
| 1.1.4 Genus Herpetacarus | L. parapalpale (Womersley et al., 1952) | 2 | W. ewingi (Fuller, 1949) | 204 | |
| H. hastoclavus Yu et al., 1979 | 393 | L. qujingense Yu et al., 1981 | 39 | W. koi (Chen and Hsu, 1957) | 59 |
| H. tenuiclavus Yu et al., 1979 | 15 | L. rufocanum Wang and Liu,1989 | 1 | W. micropelta (Traub and Evans, 1957) | 36 |
| 1.1.5 Genus Leptrombidium | L. rupestre Traub and Nadchatram, 1967 | 61 | W. xishaensis Zhao et al., 1986 | 11 | |
| L. akamushi (Brumpt, 1910) | 9 | L. robustisetum Yu et al., 1983 | 36 | 1.2 Subfamily Gahrliepiinae | |
| L. alpinum Yu and Yang, 1986 | 61 | L. saltuosum Yu et al., 1982 | 11 | 1.2.1 Genus Gahrliepia | |
| L. apodevrieri Wen and Xiang,1984 | 125 | L. scutellare (Nagayo et al., 1921) | 2312 | G. agrariusia Hus et al., 1965 | 4 |
| L. bambicola Xiang and Wen, 1984 | 78 | L. shuqui Wen and Xiang, 1984 | 88 | G. chekiangensis Chu, 1964 | 33 |
| L. baoshui Wen and Xiang, 1984 | 89 | L. shuyui Wen et al., 1984 | 254 | G. chungkingensis Jeu et al., 1963 | 1 |
| L. bayanense Yang, 1994 | 1 | L. sinicum Yu et al., 1981 | 222 | G. eurypunctata Jeu et al., 1983 | 1 |
| L. biji Wen and Xiang, 1984 | 421 | L. svnotupaium Wen and Xiang, 1984 | 5 | G. deqinensis Yu and Yang, 1982 | 9 |
| L. biluoxueshanense Yu et al., 1982 | 2 | L. spicanisetum Yu et al., 1986 | 3 | G. latiscutata Chen and Fan, 1981 | 15 |
| L. bishanense Yu, 1986 | 84 | L. suense Wen, 1984 | 19 | G. lengshui Wen and Xiang, 1984 | 1 |
| L. cangjiangense Yu et al., 1981 | 37 | L. trapezoidum Wang et al., 1981 | 19 | G. linguipelta Jeu et al., 1983 | 41 |
| L. caudatum Wen et al., 1984 | 386 | L. turdicola Vercammen-Grandjean et Langston, 1976 | 20 | G. longipedalis Yu and Yang, 1986 | 2 |
| L. cricethrionis Wen et al., 1984 | 795 | L. wangi Yu et al., 1986 | 611 | G. madun Wen and Xiang, 1984 | 6 |
| L. cuonae Wang et al., 1996 | 2 | L. wenense Wu et al., 1982 | 17 | G. megascuta Hsu et al., 1965 | 61 |
| L. deliense (Walch, 1922) | 42 | L. xiaguanense Yu et al., 1981 | 318 | G. miyi Wen and Song, 1984 | 106 |
| L. densipunctatum Yu et al., 1982 | 1791 | L. xiaowei Xiang and Wen, 1984 | 37 | G. myriosetosa (Wang, 1964) | 9 |
| L. deplanoscutum Yu et al., 1981 | 1 | L. xishani Wen and Xiang, 1984 | 24 | G. orientalis Wen and Xiang, 1984 | 10 |
| L. dianchi Wen and Xiang, 1984 | 20 | L. yongshengense Yu and Yang, 1986 | 87 | G. radiopunctata (Hsu et al., 1965) | 7 |
| L. ejingshanense Yu et al., 1982 | 11 | L. yui (Chen and Hsu, 1955) | 205 | G. silvatica Yu and Yang, 1982 | 15 |
| L. eothenomydis Yu and Yang, 1986 | 77 | L. yunlingense Yu et al., 1981 | 97 | G. yunnanensis (Hsu et al., 1965) | 204 |
| L. fujianense Liao and Wang,1983 | 718 | L. zhongdianense Yu et al., 1981 | 12 | 2. Family Leeuwenhoekiidae | |
| L. gongshanense Yu et al., 1981 | 39 | 1.1.6 Genus Neotrombicula | 2.1 Subfamily Leeuwenhoekiinae | ||
| L. gemiticulum (Traub et al.,1958) | 1 | N. aeretes Hsu and Yang,1985 | 108 | 2.1.1 Genus Chatia | |
| L. hiemale Yu et al., 1982 | 217 | N. deqinensis Yu and Wang,1981 | 1 | C. alpina Shao and Wen, 1984 | 7 |
| L. hsui Yu et al., 1986 | 19 | N. japonica (Tanaka et al., 1930) | 69 | 2.1.2 Genus Shunsennia | |
| L. huangchuanense Yang,1994 | 32 | N. longmenis Wen and Xiang, 1984 | 6 | S. scabriselosa (Huang, 1986) | 7 |
| Total | 12,281 individuals, 107 species, 11genera, 3 subfamilies, 2 families | ||||
Annotation: Of 12,516 collected chigger mites, 12,281 ones were identified to species.
3.2. Infestation, community structure and spatial distribution of dominant species of chigger mites
A total of 12,516 individuals of chigger mites were collected from 1981 A. chevrieri mice (hosts). Of 1981 A. chevrieri hosts, 633 of them were infested with chigger mites with 31.95% of overall prevalence (PM = 31.95%, 633/1981), 6.32 of overall mean abundance (MA = 6.32, 12516/1981) and 19.77 of overall mean intensity (MI = 19.77, 12516/633). Based on the identified 107 species (12,281 individuals) of chigger mites, the community structure of the mites on A. chevrieri mice was calculated with the species richness S = 107, Shannon-Wiener's diversity index H’ = 3.27, Pielou's evenness index E = 0.699 and Simpson's dominance index D = 0.075. The unidentified 235 individuals of chigger mites (12516-12281 = 235) were not included in the community calculation because their exact species were unknown.
Of 107 species of chigger mites identified from A. chevrieri mice, three of them were dominant chigger species and they all belong to the genus Leptrombidium. The three dominant chigger species were Leptrombidium scutellare with the constituent ratio Cr = 18.47%, L. densipunctatum with Cr = 14.31%, and L. cricethrionis with Cr = 6.35%. The total constituent ratio of three dominant species of chigger mites reached 39.13% (4898/12516). Leptotrombidium scutellare was the first dominant chigger species with the highest constituent ratio (Cr = 18.47%), prevalence (PM = 8.13%) and mean abundance (MA = 1.17) (P < 0.05). The mean intensity of L. densipunctatum (MI = 16.58) was higher than those of other two chigger species with statistical significance (P < 0.05).
The Cassie index (CA) and clumping index (I) of three dominant species of chigger mites and all the 107 chigger species were all higher than the border value “0”, and therefore all the chigger species were determined to be of the aggregated distribution among different individuals of A. chevrieri mice (Table 3).
Table 3.
The constituent ratios, infestation indices and dispersion coefficients of three dominant species of chigger mites on Chevrieri's field mice (Apodemus chevrieris) in southwest China (2001–2019).
| Dominant species of chigger mites | Constituent ratios of chigger mites |
Infestations of chigger mites |
Dispersion coefficient |
||||
|---|---|---|---|---|---|---|---|
| Individuals | Cr (%) | PM (%) | MA | MI | CA | I | |
| Leptotrombidium scutellare | 2312 | 18.47 | 8.13 | 1.17 | 14.36 | 106.76 | 124.60 |
| Leptotrombidium densipunctatum | 1791 | 14.31 | 5.45 | 0.90 | 16.58 | 74.31 | 67.18 |
| Leptotrombidium cricethrionis | 795 | 6.35 | 6.11 | 0.40 | 6.57 | 54.27 | 21.78 |
| Total 107 species of chigger species | 12516 | 100 | 31.95 | 6.32 | 19.77 | 15.47 | 97.77 |
3.3. Analysis on interspecific association between dominant species of chigger mites
The analysis on interspecific association between any two main species of chigger mites showed that a slightly positive association existed between the dominant chigger mite species L. densipunctatum and L. scutellare (V = 0.05, χ2 = 11.203, P < 0.05), and between L. cricethrionis and L. scutellare (V = 0.39, χ2 = 6.109, P < 0.05) on the mouse host, A. chevrieri (Table 4).
3.4. Chigger infestations in different landscapes and on different ages of hosts
The infestations of A. chevrieri mice with chigger mites varied in different landscapes and on different ages of the hosts. Apodemus chevrieri mice in the mountainous landscape harbored much more individuals of chigger mites than those in the flatland landscape. All the infestation indices of chigger mites in mountainous landscape (PM = 34.35, MA = 6.20 and MI = 20.09) were significantly higher than those in the flatland landscape (PM = 10.89, MA = 1.18 and MI = 10.86) (P < 0.001). The prevalence (PM) and mean abundance (MA) of chigger mites on the adult A. chevrieri mice (PM = 34.45 and MA = 7.29) were higher than those on the juvenile mice (PM = 2.24, MA = 2.82) with P < 0.001. The mean intensity of chigger mites on the adult A. chevrieri mice (MI = 21.17) was also higher than that on the juvenile mice (MI = 12.21) with P < 0.05 (Table 5).
Table 5.
Overall infestations of Chevrieri's field mice (Apodemus chevrieri) with chigger mites in different landscapes and on different ages of the hosts in southwest China (2001–2019).
| Different landscapes and different ages of hosts | Number of captured hosts, A. chevrieri | Infested hosts, A. chevrieri | Individuals of chigger mites |
Overall infestations of chigger mites on the host, A. chevrieri |
|||
|---|---|---|---|---|---|---|---|
| Individuals | Cr (%) | PM (%) | MA | MI | |||
| mountainous landscape | 1779 | 611 | 12277 | 98.09 | 34.35 | 6.20 | 20.09 |
| flatland landscape | 202 | 22 | 239 | 1.91 | 10.89 | 1.18 | 10.86 |
| Total | 1981 | 633 | 12516 | 100.00 | 31.95 | 6.32 | 19.77 |
| Juvenile hosts | 429 | 99 | 1209 | 9.66 | 2.24 | 2.82 | 12.21 |
| Adult hosts | 1550 | 534 | 11307 | 90.34 | 34.45 | 7.29 | 21.17 |
| Totala | 1979 | 633 | 12516 | 100 | 31.99 | 6.32 | 19.77 |
Annotation: Two individuals of A. chevrieri mice without gender record were not included in the above table.
3.5. Infestations of Apodemus chevrieri mice with chigger mites in different latitudes and altitudes
As shown in Table 6, the A. chevrieri mice in latitude 24–26°N harbored much more individuals of chigger mites than those in other latitudes. The prevalence (PM = 17.01%) and mean abundance (MA = 3.40) of chigger mites in latitude 24–26°N were also higher than those in other latitudes with P < 0.001. The mean intensity (MI) in latitude 28–30°N was higher than that in other latitudes (P < 0.001). The prevalence (PM) and mean abundance (MA) of A. chevrieri mice in 2000–3000m (PM = 36.97 and MA = 7.17) were higher than those in other altitudes (P < 0.001), and the mean intensity (MI) of A. chevrieri mice in altitude 1000–2000m was higher than that in other altitudes (P < 0.05) (Table 6).
Table 6.
Overall infestation of Chevrieri's field mice (Apodemus chevrieri) with chigger mites in different latitude gradients and altitude gradients in southwest China (2001–2019).
| Different latitude gradients and altitude gradients | Number of captured mice | Infested mice | Individuals of chigger mites |
Overall infestations of chigger mites on the mice |
|||
|---|---|---|---|---|---|---|---|
| Individuals | Cr(%) | PM (%) | MA | MI | |||
| latitude <24°N | 2 | 1 | 19 | 0.15 | 0.05 | 0.01 | 19.00 |
| latitude 24–26°N | 770 | 337 | 6740 | 53.85 | 17.01 | 3.40 | 20.00 |
| latitude 26–28°N | 1101 | 205 | 2626 | 20.98 | 10.35 | 1.33 | 12.81 |
| latitude >28°N | 108 | 90 | 3131 | 25.02 | 4.54 | 1.58 | 34.79 |
| Total | 1981 | 633 | 12516 | 100.00 | 31.95 | 6.32 | 19.77 |
| altitude <1000M | 3 | 1 | 19 | 0.15 | 0.33 | 6.33 | 19.00 |
| altitude 1000–2000M | 604 | 145 | 3662 | 29.26 | 24.01 | 6.06 | 25.26 |
| altitude 2000–3000M | 1117 | 413 | 8014 | 64.03 | 36.97 | 7.17 | 19.40 |
| altitude >3000M | 257 | 74 | 821 | 6.56 | 28.79 | 3.19 | 11.09 |
| Total | 1981 | 633 | 12516 | 100.00 | 31.95 | 6.32 | 19.77 |
3.6. Logistic regression analysis of the factors related to the infestations of chigger mites on Apodemus chevrieri mice
Based on the result of univariate factor analysis, the variables of landscapes (χ2 = 45.894 P < 0.001), altitude gradients (χ2 = 31.675 P < 0.001), latitude gradients (χ2 = 27.185 P < 0.001) and host ages (χ2 = 19.983 P < 0.001) were put into the logistic regression analysis. Of the above variables, the variables of latitude gradients (OR = 0.670 95%, CI: 0.564–0.797) were negatively correlated with the infestations of chigger mites on A. chevrieri mice, which were the protective factors. The variables of landscapes (OR = 4.652 95%, CI: 2.947–7.342), altitudes (OR = 1.243%, CI: 1.068–1.446) and host ages (OR = 1.995 95%, CI: 1.552–2.565) were the risk factors for the infestations of chigger mites on A. chevrieri mice (P < 0.001) (Table 7).
Table 7.
Logistic regression analysis of the factors related to the infestations of chigger mites on Chevrieri's field mouse (Apodemus chevrieri) in southwest China (2001–2019).
| B | S.E. | Wald | df | Sig. | OR | 95% CI |
|||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Step 4d | landscapes (1) | 1.537 | 0.233 | 43.570 | 1 | 0.000 | 4.652 | 2.947 | 7.342 |
| altitudes | 0.217 | 0.077 | 7.894 | 1 | 0.005 | 1.243 | 1.068 | 1.446 | |
| latitudes | −0.400 | 0.088 | 20.552 | 1 | 0.000 | 0.670 | 0.564 | 0.797 | |
| host ages | 0.691 | 0.128 | 29.012 | 1 | 0.000 | 1.995 | 1.552 | 2.565 | |
| constant | −2.983 | 0.426 | 48.980 | 1 | 0.000 | 0.051 | |||
3.7. Species abundance distribution and total species estimation of chigger mites on Apodemus chevrieri mice
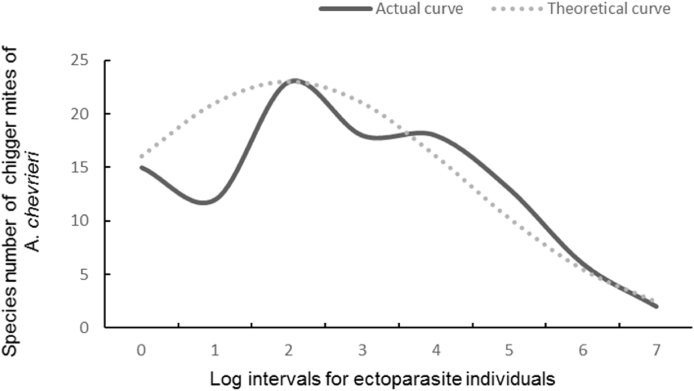
Of the 107 species and 12516 individuals of chiggers collected from A. chevrieri mice, most chigger species were rare species with 5–13 individuals (Table 8). By using Preston log-normal distribution model, the species abundance distribution of chigger mite community on A. chevrieri mice was fitted as (β = 0.30, R2 = 0.68), and the theoretical curve showed a parabolic tendency (Table 8 and Fig. 2). Based on fitting the species abundance distribution, the expected total species of chigger mites on A. chevrieri mice were estimated to be 136 species (ST = 136), 29 more than the actually collected species (S = 107).
Table 8.
Theoretical curve fitting for the species abundance distribution of chigger mite community on Chevrieri's field mice (Apodemus chevrieri) in southwest China (2001–2019).
| Log intervals | Individual ranges in each log interval | Midpoint values of each individual range | Actual chigger mite species | Theoretical chigger mite species |
|---|---|---|---|---|
| 0 | 0–1 | 1 | 15 | 16.05 |
| 1 | 2–4 | 3 | 12 | 21.02 |
| 2 | 5–13 | 9 | 23 | 23.00 |
| 3 | 14–40 | 27 | 18 | 21.02 |
| 4 | 41–121 | 81 | 18 | 16.05 |
| 5 | 122–364 | 243 | 13 | 10.23 |
| 6 | 365–1093 | 729 | 6 | 5.45 |
| 7 | 1094–3280 | 2187 | 2 | 2.42 |
Annotation: R0 = 2, S0 = 23 and β = 0.30 (R2 = 0.68) in the theoretical curve fitting for the species abundance distribution of chigger community.
Fig. 2.
Theoretical curve fitting for the species abundance distribution of chigger mite community on Chevrieri's field mice (Apodemus chevrieri) in southwest China (2001–2019).
4. Discussion
4.1. Species diversity, infestation and community structure of chigger mites on Apodemus chevrieri mice
The original data of the present study came from a long-term field investigation in 91 sites (counties) of southwest China, which covered the five provincial regions, Yunnan, Guizhou, Sichuan, Chongqing and Tibet. The present study is the first time to systematically report the infestation and distribution of chigger mites on A. chevrieri in southwest China where is a very wide geographical region with a total of 2,500,000 km2. A previous study from Yunnan province of southwest China reported that 1414 chigger mites (61 species) were collected from 1113 brown rats (Norway rat), Rattus norvegicus (Berkenhout, 1769), with overall PM = 13.39%, MA = 1.27 and MI = 9.49 (Ding et al., 2021a), which are much lower than the chigger infestations on A. chevrieri mice in the present study. Another previous study revealed that a total of 49,850 individuals of chigger mites (175 species) were identified from 2463 Eothenomys miletus (Thomas, 1914) in three provinces of southwest China, Yunnan, Guizhou and Sichuan (Peng et al., 2015, 2016). The overall infestations of chigger mites on E. miletus voles (PM = 57.69%, MA = 20.24 and MI = 35.08) are obviously higher than those on A. chevrieri mice in the present study (Peng et al., 2015, 2016). The species diversity and infestations of chigger mites on rodents (rats, mice and voles, etc.) can be influenced by a series of factors, including host species and various environmental factors. Different species of rodents with different biological characteristics are usually different in the susceptibility of infesting with chigger mites and some other ectoparasites (Ding et al., 2021a; Peng et al., 2015, 2016). The cross infestations of chigger mites among different hosts are very common because of their low host specificity. The rodents living in complicated wild habitats with diverse vegetation usually harbor more chigger mites and some other ectoparasites with higher species diversity and infestations than those living the residential areas of humans (Peng et al., 2016; Guo et al., 2013). Apodemus chevrieri mice and E. miletus voles are two species of typical wild rodents (Peng et al., 2016; Guo et al., 2013; Huang et al., 1995), and therefore the species diversity and infestations of chigger mites on A. chevrieri mice and E. miletus voles are much higher than those on R. norvegicus rats which often lives in the residential areas of humans (Ding et al., 2021a; Peng et al., 2016; Guo et al., 2013).
Because of low host specificity of chigger mites, the species diversity and infestations of chigger mites on the same species of host often vary in different geographical regions, latitudes, altitudes, landscapes and habitats (Ding et al., 2021a; Peng et al., 2017). Through cross infestation, the same host species can harbor different mite species in different environments (Ding et al., 2021a; Peng et al., 2017). Different geographical regions with different latitudes, altitudes and climates can lead to the different species richness (species diversity) and species compositions of chigger mites and some other ectoparasites on the same species of hosts (Peng et al., 2016; Ding et al., 2021a), and this may explain that the infestations of A. chevrieri mice with chigger mites varied in different latitudes and altitudes. The environmental gradients could be the important risk factors of influencing the infestations of chigger mites on A. chevrieri mice. The high species diversity of chigger mites (107 species) on A. chevrieri mouse may reflect the mouse's high potential of harboring abundant mite species. The complicated topography and diverse climate types in southwest China may also contribute to the high species of chigger mites in the region (Peng et al., 2016; Ding et al., 2021a; Yin et al., 2021). Besides, the wide investigation scope in 91 investigation sites of five provincial regions with large host samples (1981 A. chevrieri mice) is also an important factor to lead to the high species of chigger mites in the present study.
The community structure of chigger mites on A. chevrieri mice showed a high diversity with a high species richness (S = 107) and diversity index (H’ = 3.27) as well as a high evenness index (E = 0.699), but a relatively low dominance index (D = 0.075). The results may indicate that there were many chigger mite species on A. chevrieri mice, but no obviously dominant species.
4.2. Dominant chigger species on Apodemus chevrieri mice and their spatial distribution patterns and interspecific relationship
Of the 107 species of chigger mites identified from A. chevrieri, there were three dominant chigger species and they were L. scutellare (Cr = 18.47%), L. densipunctatum (Cr = 14.31%) and L. cricethrionis (Cr = 6.35%). Of the three dominant chigger species, L. scutellare was the most abundant, and L. densipunctatum and L. cricethrionis came next (Table 3). Leptotrombidiu scutellare is one of six main vectors of scrub typhus in China, and L. cricethrionis is considered a potential vector of the disease (Su et al., 2012; Wu et al., 2013). As a typical wild rodent species, A. chevrieri is often found in various wild and outdoor habitats including crop fields, various farmlands, bush areas, grasslands and open woodlands and it is closely associated with human activities (Wlison et al., 2017; Huang et al., 1995). The abundant occurrence of L. scutellare and L. cricethrionis on the body surface of A. chevrieri mice would increase the possibility of transmitting scrub typhus from the mouse to humans.
The Cassie index (CA) and clumping index (I) was adopted to measure the spatial distribution of three dominant species of chigger mites among different individuals of their mouse host, A. chevrieri mice (Table 3). As a result, all the dominant species of chigger mites was determined as aggregated distribution among different individuals of A. chevrieri mice. The aggregated distribution is a very common distribution pattern of ectoparasites including chigger mites (Ding et al., 2021a; Xiang et al., 2021). The aggregated distribution is considered to be beneficial to the survival, spread, mating, reproduction and defense of the parasites (Peng et al., 2016; Liu et al., 2020b; Ding et al., 2021a), and it may also facilitate the transmission of some zoonotic diseases by vector mites from rodents to humans.
The association coefficient (V) used in the present study is a common and applicable way to measure the interspecific relationship between any two species of chigger mites on their hosts (Liu et al., 2020b; Yin et al., 2021). The result showed that the interspecific association between any two of three dominant chigger species (L. scutellare, L. densipunctatum and L. cricethrionis) were slightly positive (V > 0, P < 0.05), which indicates that the dominant chigger species have a tendency to co-exist on the body surface of A. chevrieri mice.
4.3. Infestation variations of chigger mites in different environments and on different ages of hosts
In comparison with the A. chevrieri mice in the flatland landscape, the mice in the mountainous landscape harbored much more chigger mites with much higher infestation indices (PM, MA and MI) with statistical significance (P < 0.001) (Table 5). The result reflects the infestation variations of chigger mites in different landscapes. In southwest China, the mountainous landscapes are usually associated with complex environments, diverse vegetation and high biodiversity in comparison with the flatland landscapes (Ding et al., 2021a; Pongsiri et al., 2009; Peng et al., 2018). The complex environments, diverse vegetation and high biodiversity in the mountainous landscape may lead to the higher infestations of chigger mites on A. chevrieri mice.
The infestations of chigger mites in different latitudes and altitudes also showed some variations. The A. chevrieri mice in the N24°-26° latitude harbored more chigger mites with higher PM and MA (P < 0.001). The mean intensity (MI) in the N28°-30° latitude was higher than that in other latitudes (P < 0.001) (Table 6). Apodemus chevrieri mice in the altitude 2000–3000m harbored a large number of chigger mites with higher PM and MA (Table 6). Some previous studies also reported the infestation variations of gamsid mites and chigger mites on the same rodent species, the Asian house rat (oriental house rat) Rattus tanezumi (Temminck, 1844) and the brown rat R. norvegicus in different latitudes and altitudes (Yin et al., 2021; Lv, 2021). The infestation variations of chigger mites in different latitudes and altitudes reflect the influence of environmental factors on the mites, which may be associated with the different vegetation, temperature, humidity and rainfall in different environmental gradients (Yin et al., 2021; Lv, 2021).
The adult A. chevrieri mice harbored much more chigger mites with significantly higher infestations (PM, MA and MI) than the juvenile mice (Table 5), and this is consistent with some previous studies (Ding et al., 2021a; Peng et al., 2015). The result suggests an age-bias of the A. chevrieri mice in the infestations of chigger mites. The larger body size of adult hosts and their wider range of activities in the external environments may increase the risk of infesting ectoparasites including chigger mites (Poulin, 1997; Kataranovski et al., 2011).
The logistic regression analysis used in the present study is a common way to evaluate the risk factors of some diseases in clinical studies (Truett et al., 1967; Liu, 2020), but it was seldom used in analyzing the infestations of chigger mites on rodent hosts. The result of the present study showed that the risk factors of influencing the infestations of A. chevrieri mice with chigger mites included the landscape, host age and altitude. The infestations of rodents (rats, mice and voles, etc.) with ectoparasitic mites and some other ectoparasites can be influenced by a series of complex factors including host species, environmental factors and climatic factors (Kataranovski et al., 2011; Liu, 2020). This result of the present paper reflects the influence of the host factor (host age) and environmental factors (landscape and altitude) on chigger mites.
4.4. Species abundance distribution of chigger mites on Apodemus chevrieri mice
The species abundance distribution is to illustrate the relationship between species and individuals in a certain community (Ding et al., 2021a; McGill et al., 2007; Preston 1948). In the present study, the log-normal distribution model based on Preston’ methods was applied to describe the species abundance distribution of the chigger mite community on A. chevrieri mice. The theoretical species abundance distribution of chigger mites (chigger mite community) on A. chevrieri mice was fitted as by the lognormal distribution model with the determination coefficient R2 = 0.68 (Fig. 2), which is similar to that of chigger mites and some other ectoparasites on some other species of rodents (Ding et al., 2021a; Guo et al., 2016).
In ecological studies, it is often necessary to roughly estimate the expected total number of species in a given community. There are a series of methods to estimate the expected total species and one of them is the method based on Preston's lognormal model in the present study (Ding et al., 2021a). The total expected number of chigger species on A. chevrieri mice was roughly estimated to be 136 species (ST = 136), and the result suggests that there are about 29 rare chigger species probably missed in the actual field investigation. Some rare species are too rare to be found, and it is impossible to collect all the rare species in an actual field investigation (Ding et al., 2021a; Chao and Shen, 2003; Peng et al., 2017). In order to find more rare species of chiggers and some other ectoparasites, abundant host samples with a wide geographical scope are often recommended in the field investigations (Peng et al., 2016).
5. Conclusion
The Chevrieri's field mouse (A. chevrieri) has a great potential to harbor lots of chigger mites with high species diversity and infestation. Leptrombidium scutellare, L. densipunctatum and L. cricethrionis are the dominant chigger species on the mouse in southwest China and they are of aggregated distribution on the host A. chevrieri. The infestations of A. chevrieri with chigger mites vary with different latitudes, altitudes and landscapes, and the environmental factors and host ages often influence the infestations of the mouse with the mites. Abundant host samples with a wide geographical scope are recommended in the field investigations to find more rare chigger species and some other ectoparasites.
Supplemental statement
The capture of A. chevrieri mice was officially approved by the local authority of wildlife service in Dali Prefecture, Yunnan Province, China. The use of animals for the research was officially approved by the Animal Ethics Committee of Dali University.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to express our sincere thanks to the following people who contributed to the field investigations and laboratory work: Yun-Ji Zou, Qiao-Hua Wang, Wen-Yu Song, Yong Zhang, Cong-Hua Gao, Nan Zhao, Jian-Chang He, Guo-Li Li, Yan-Liu Li, Xue-Song He, De-Cai Ouyang, Shuang-Lin Wang, some colleagues and college students. The present study was supported by the National Natural Science Foundation of China (No. 81960380 and 82160400) to Xian-Guo Guo, and the Innovation Team of Vector Biology, Dali University (No. ZKLX2019104).
References
- Chao A., Shen T.J. Non-parametric estimation of Shannon's index of diversity when there are unseen species in sample. Environ. Ecol. Stat. 2003;10:429–443. doi: 10.1023/A:1026096204727. [DOI] [Google Scholar]
- Cima G. AVMA guidelines for the euthanasia of animal. J. Am. Vet. Med. Assoc. 2013;242:715–716. [Google Scholar]
- Ding F., Guo X.G., Song W.Y., Fan R., Zhao C.F., Mao K.Y., Zhang Z.W., Peng P.Y., Lin H., Dong W.G., Qian T.J., et al. Infestation and distribution of chigger mites on brown rat (Rattus norvegicus) in Yunnan province, southwest China. Trop. Biomed. 2021;38:111–121. doi: 10.47665/tb.38.1.020. [DOI] [PubMed] [Google Scholar]
- Ding F., Jiang W.L., Guo X.G., Fan R., Zhao C.F., Zhang Z.W., Mao K.Y., Xiang R. Infestation and related ecology of chigger mites on the Asian house rat (Rattus tanezumi) in Yunnan province, southwest China. Kor. J. Parasitol. 2021;59(4):377–392. doi: 10.3347/kjp.2021.59.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding F., Jiang W.L., Guo X.G., Fan R., Mao K.Y., Zhao C.F., Zhang Z.W., Qian T.J., Yang Z.H. A preliminary report on Walchia micropelta in Yunnan Province. Sichuan J. Zool. 2020;39:555–562. doi: 10.11984/j.issn.1000-7083.20200129. (in Chinese) [DOI] [Google Scholar]
- Duan H.S., Yang Z.Q., Xu G.Q., Zhou S.L., Wen X.M., Pan X.X., Liu Y.R. Trombiculid mite distribution in Hubei Province and zoogeographical demarcation of the province. J. Pathog Biol. 2009;4:687–688. doi: 10.13350/j.cjpb.2009.09.006. (in Chinese) [DOI] [Google Scholar]
- Guo X.G., Dong W.G., Men X.Y., Qian T.J., Wu D., Ren T.G., Qin F., Song W.Y., Yang Z.H., Fletcher Q.E. Species abundance distribution of ectoparasites on Norway rats (Rattus norvegicus) from a localized area in Southwest China. J. Arthropod-Borne. Dis. 2016;10:192–200. [PMC free article] [PubMed] [Google Scholar]
- Guo X.G., Qian T.J., Meng X.Y., Dong W.G., Shi W.X., Wu D. Preliminary analysis of chigger communities associated with house rats (Rattus flavipectus) from six counties in Yunnan, China. Syst. Appl. Acarol-UK. 2006;11:13–21. doi: 10.11158/saa.11.1.2. [DOI] [Google Scholar]
- Guo X.G., Speakman J.R., Dong W.G., Men X.Y., Qian T.J., Wu D., Qin F., Song W.Y. Ectoparasitic insects and mites on Yunnan red-backed voles (Eothenomys miletus) from a localized area in southwest China. Parasitol. Res. 2013;112:3543–3549. doi: 10.1007/s00436-013-3537-6. [DOI] [PubMed] [Google Scholar]
- Huang W.J., Chen Y.X., Wen Y.X. Fudan University Press; Shanghai: 1995. Rodents of China; pp. 1–308. (in chinese) [Google Scholar]
- Jiang J.F. Academy of Military Medieal Sciences; 2015. Eeologieal and Epidemiological Studies on Rodent Hosts of Hantavirus in Beijing. (in chinese) [Google Scholar]
- Kataranovski M., Mirkov I., Belij S., Popov A., Petrovic Z., Gaci Z., Kataranovski D. Intestinal helminths infection of rats (Ratus norvegicus) in the Belgrade area (Serbia): the effect of sex, age and habitat. Parasite. 2011;18(2):189–196. doi: 10.1051/parasite/2011182189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno E. Sampling and analysis of insect populations. Annu. Rev. Entomol. 1991;36:285–304. [Google Scholar]
- Latif A., Liu B.Y., Chen Z., Sun Y., Shi Y.L., Zong J., Li J.J., Ren C.P., Zhang X.C., Liu X.N., Yu X.J., et al. Orientia tsutsugamushi infection in rodents in Anhui Province of China. Infect. Genet. Evol. 2017;56:14–18. doi: 10.1016/j.meegid.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Li J.C., Wang D.Q., Chen X.B. Guangdong Science and Technology Publishing House; Guangzhou: 1997. Trombiculid Mites of China (Studies on Vector and Pathogen of Tsutsugamushi Disease) pp. 1–570. (in chinese) [Google Scholar]
- Liu Z. Yunnan Province, Southwest China. Dali University; 2020. Ecological research on gamasid mites on three species of domestic rodents (Rattus tanezumi, Rattus norvegicus and Mus musculus) (in chinese) [Google Scholar]
- Liu Z., Guo X.G., Fan R., Zhao C.F., Mao K.Y., Zhang Z.W., Zhao Y. Ecological analysis of gamasid mites on the body surface of Norway rats (Rattus norvegicus) in Yunnan Province, Southwest China. Biologia. 2020;75:1325–1336. doi: 10.2478/s11756-019-00383-z. [DOI] [Google Scholar]
- Lv Y. Guizhou University; 2021. Researches on the Distribution of Leptotrombidium delicense and Some Other Important Chigger Mites in Southwest China. (in chinese) [Google Scholar]
- Lv Y., Guo X.G., Jin D.C., Song W.Y., Fan R., Zhao C.F., Zhang Z.W., Mao K.Y., Peng P.Y., Lin H., Zhao Y., Qian T.J., Dong W.G. Host selection and seasonal fluctuation of Leptotrombidium deliense (Walch, 1922) (Trombidiformes: Trombiculidae) at a localized area of southern Yunnan, China. Syst. Appl. Acarol. 2019;24:2253–2271. [Google Scholar]
- Lv Y., Guo X.G., Jin D.C., Song W.Y., Peng P.Y., Lin H., Fan R., Zhao C.F., Zhang Z.W., Mao K.Y., Qian T.J., Dong W.G., Yang Z.H. Infestation and seasonal fluctuation of chigger mites on the Southeast Asian house rat (Rattus brunneusculus) in southern Yunnan Province, China. Int. J. Parasitol. Parasites. Wildl. 2021;14:141–149. doi: 10.1016/j.ijppaw.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magurran A.E. Measuring richness and evenness. Trends Ecol. Evol. 1998;13(4):165–166. doi: 10.1016/s0169-5347(97)01290-1. [DOI] [PubMed] [Google Scholar]
- McGill B.J., Etienne R.S., Gray J.S., Alonso D., Anderson M.J., Benecha H.K., Dornelas M., Enquist B.J., Green J.L., He F., Hurlbert A.H., Magurran A.E., Marquet P.A., Maurer B.A., Ostling A., Soykan C.U., Ugland K.I., White E.P. Species abundance distributions: moving beyond single prediction theories to integration within an ecological framework. Ecol. Lett. 2007;10:995–1015. doi: 10.1111/j.14610248.2007.01094.x. [DOI] [PubMed] [Google Scholar]
- Men X.Y., Guo X.G., Dong W.G., et al. Ectoparasites of chevrier's field mouse, Apodemus chevrieri, in a focus of plague in southwest China. Med. Vet. Entomol. 2007;21(3):297–300. doi: 10.1111/j.1365-2915.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- Morris P. A review of mammalian age determination methods. Mamm Rev. 1972;2(3):69–104. [Google Scholar]
- Nielsen D.H., Robbins R.G., Rueda L.M. Annotated world checklist of the Trombiculidae and Leeuwenhoekiidae (1758–2021) (Acari: Trombiculoidea), with notes on nomenclature, taxonomy, and distribution. Zootaxa. 2021;4967(1):1243. doi: 10.11646/zootaxa.4967.1.1. [DOI] [PubMed] [Google Scholar]
- Peng P.Y., Guo X.G., Jin D.C., Dong W.G., Qian T.J., Qin F., Yang Z.H., Fan R. Landscapes with different biodiversity influence distribution of small mammals and their ectoparasitic chigger mites: a comparative study from Southwest China. PLoS One. 2018;13 doi: 10.1371/journal.pone.0189987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng P.Y., Guo X.G., Jin D.C., Dong W.G., Qian T.J., Qin F., Yang Z.H. Species abundance distribution and ecological niches of chigger mites on small mammals in Yunnan Province, Southwest China. Biologia. 2017;72:1031–1040. doi: 10.1515/biolog-2017-0119. [DOI] [Google Scholar]
- Peng P.Y., Guo X.G., Song W.Y., Hou P., Zou Y.J., Fan R. Ectoparasitic chigger mites on large oriental vole (Eothenomys miletus) across Southwest, China. Parasitol. Res. 2016;115:623–632. doi: 10.1007/s00436-015-4780-9. [DOI] [PubMed] [Google Scholar]
- Peng P.Y., Guo X.G., Song W.Y., Hou P., Zou Y.J., Fan R., He X.S. Analysis of ectoparasites (chigger mites, gamasid mites, fleas and sucking lice) of the Yunnan red-backed vole (Eothenomys miletus) sampled throughout its range in Southwest China. Med. Vet. Entomol. 2015;29:403–415. doi: 10.1111/mve.12134. [DOI] [PubMed] [Google Scholar]
- Pongsiri M.J., Roman J., Ezenwa V.O., Goldberg T.L., Koren H.S., Newbold S.C., Ostfeld R.S., Pattanayak S.K., Salkeld D.J. Biodiversity loss affects global disease ecology. Bioscience. 2009;59:945–954. [Google Scholar]
- Poulin R. Species richness of parasite assemblages: evolution and patterns. Annu. Rev. Ecol. Systemat. 1997;28:341–358. doi: 10.1146/annurev.ecolsys.28.1.341. [DOI] [Google Scholar]
- Preston F.W. The commonness, and rarity, of species. Ecology. 1948;29:254–283. doi: 10.2307/1930989. [DOI] [Google Scholar]
- Ren T.G., Guo X.G., Jin D.C. Two new species of chigger mites in the Genus Gahrliepia (Acari: Trombiculidae) from China. Pakistan J. Zool. 2014;46:1657–1662. [Google Scholar]
- Su J.J., Wang Y., Zhou J., Bin Y., Yang Z.Q. Advances in research of tsutsugamushi disease epidemiology in China in recent years. Chin. J. Hyg. Insect. Equip. 2012;18:160–163. doi: 10.19821/j.1671-2781.2012.02.026. (in chinese) [DOI] [Google Scholar]
- Truett J., Cornfield J., Kannel W. A multivariate analysis of the risk of coronary heart disease in Framingham. J. Chron. Dis. 1967;20(7):511–524. doi: 10.1016/0021-9681(67)90082-3. [DOI] [PubMed] [Google Scholar]
- Tsai Y.L., Chuang S.T., Chang C.C., Kass P.H., Chomel B.B. Bartonella species in small mammals and their ectoparasites in Taiwan. Am. J. Trop. Med. Hyg. 2010;83(4):917–923. doi: 10.4269/ajtmh.2010.10-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen-Grandjean P.H., Langston R.L. George Williams Hooper Foundation, University of California; San Francisco: 1976. The chigger mites of the World (Acarina: Trombiculidae et Leeuwenhoekiidae). III. Leptotrombidium complex; p. 1061. [Google Scholar]
- Wang B., Li W., Zhou J.H., Li B., Zhang W., Yang W.H., Pan H., Wang L.X., Bock C.T., Shi Z.L., Zhang Y.Z., Yang X.L. Chevrier's field mouse (Apodemus chevrieri) and Père David's vole (Eothenomys melanogaster) in China Carry Orthohepeviruses that form two Putative novel genotypes within the species Orthohepevirus C. Virol. Sin. 2018;33(1):44–58. doi: 10.1007/s12250-018-0011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlison D.E., Lacher T.E., Mittermeier R.A. Handbook of mammals of the world. Rodents Ⅱ. Lynx. Edicions. Barceloma. 2017;7:784. [Google Scholar]
- Wu G.H., Jiang Z.K., Wang L., Ding L.Y., Mao C.Q., Ma B.Y. Accordance and identification of vector chigger mites of tsutsugamushi disease in China. Chin. J. Hyg. Insect. Equip. 2013;19:286–292. (in chinese) [Google Scholar]
- Xiang R., Guo X.G. Research advances of Leptotrombidium scutellare in China. Kor. J. Parasitol. 2021;59(1):1–8. doi: 10.3347/kjp.2021.59.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang R., Guo X.G., Zhao C.F., et al. Infestation and distribution of gamasid mites on Himalayan field rat (Rattus nitidus) in Yunnan Province of Southwest China. Biologia. 2021;76:1763–1773. doi: 10.2478/s11756-021-00679-z. [DOI] [Google Scholar]
- Yin P.W., Guo X.G., Jin D.C., Fan R., Zhao C.F., Zhang Z.W., Huang X.B., Mao K.Y. Distribution and host selection of tropical rat mite, Ornithonyssus bacoti, in Yunnan province of southwest China. Animals. 2021;11:110. doi: 10.3390/ani11010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y.Z., Guo X.G., Speakman J.R., Zuo X.H., Wu D., Wang Q.H., Yang Z.H. Abundances and host relationships of chigger mites in Yunnan Province, China. Med. Vet. Entomol. 2013;27(2):194–202. doi: 10.1111/j.1365-2915.2012.01053.x. https://doi:10.1111/j.1365-2915.2012.01053.x [DOI] [PubMed] [Google Scholar]
- Zheng Z.M., Jiang Z.K., Chen A.G. Shanghai Jiao Tong University Press; Shanghai: 2012. Rodent Zoology; p. 714. (in Chinese) [Google Scholar]
- Zhuo Y.P., Guo G.Z., Sun L.Y., Zhang Q.R., Su H.J., Liu Q.S. Studying progress of the age division standard in rodents. J. Anim. Sci. Vet. Med. 2016;35:53–57. [Google Scholar]