Abstract
Introduction
Dravet Syndrome (DS) is a rare epileptiform disorder typically presenting within the first year of life of a normally developing infant. It is characterized by several prolonged seizures that are often resistant to current anti-epileptic drug (AED) regimens. This paper outlines the history and clinical trials of the drug fenfluramine, a drug that when used in addition to AED regimens may provide hope to children affected by DS.
Body
Fenfluramine (3-trifulormethyl-N-ethylamphetamine) is an amphetamine derivative that primarily affects serotonin neurotransmitter levels. It was initially prescribed in the 1960s as an appetite suppressant marketed as a weight loss drug. However, it was removed from the markets due to its association with cardiac valvopathies. It continued to by studied in epilepsy by Gastaut in the 1980s in children with self-induced syncope and irretractable epilepsy. In 2012, Ceulemans et al. studied the use of fenfluramine in patients with DS. Following the success of that retrospective case study, Nabbout et al. and Legae et al. conducted two randomized control trials leading to the FDA approval of fenfluramine under its trade name Fintepla in 2020.
Discussion
The success of the randomized control trials suggests the addition of fenfluramine to current AED regimens may lead to better control of seizures in patients with DS. The side effects of fenfluramine prove to be manageable and the concern for valvopathies has not been reproducible with low dose fenfluramine.
Keywords: Dravet syndrome, Antiepileptic, Epilepsy, Pharmacology, Seizures
Graphical abstract
Highlights
-
•
Fenfluramine presents as a viable treatment option for seizure activity in patients with Dravet Syndrome.
-
•
Most common adverse events observed included decreased appetite, pyrexia, diarrhea, nasopharyngitis, and fatigue.
-
•
Antiepileptic cost is an impactful component of DS care and fenfluramine offers similar if not superior add-on therapy.
-
•
Concern for valvopathies has not been reproducible with low dose fenfluramine.
1. Introduction
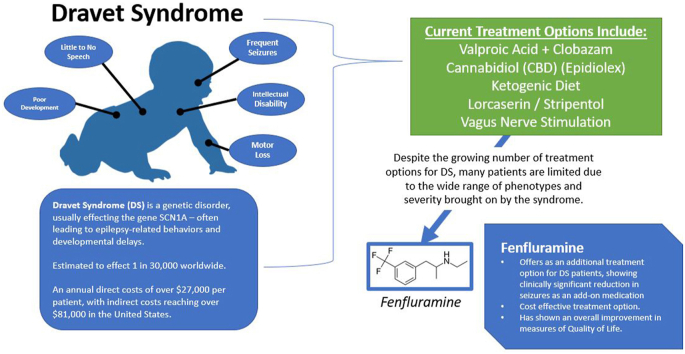
Dravet Syndrome (DS) is a life-long, rare, and devastating myoclonic seizure disorder, typically presenting in the first year of life in an otherwise healthy child. Children born with DS often reach expected neurotypical milestones for the first several months of development. However, once seizures begin, typically within the first year of life, the epileptic activity is associated with encephalopathy with severe cognitive, speech, and behavioral impairment. Often, the child's first seizure is provoked by fever. Several parents describe that their child's first seizure occurred shortly after their 4-month vaccine appointment in association with fever. Seizures in DS patients are frequent, often more than 4 times per month, tend to last longer often greater than 10 min and can result in status epilepticus or sudden unexpected death in epilepsy (SUDEP) (Anwar et al., 2019; Aras et al., 2015).
While the diagnosis of DS remains a clinical diagnosis based on specific clinical criteria, greater than 75% of children with DS exhibit an SCN1A gene mutation, which of often de novo. While the pathogenesis of DS remains poorly understood, the SCN1A gene codes for the alpha unit of the Nav1.1 voltage-gated sodium channel. Most mutations of this gene involve a truncation mutation producing no functional proteins. This sodium channel is required for generation and propagation of action potentials in the central nervous system. It is paradoxical that inhibition of an excitatory channel would lead to an epileptiform disorder. Research is ongoing to try and unravel this paradox (Chopra and Isom, 2014).
The purpose of this review is to outline the clinical use, trial results, and pharmaceutical development of fenfluramine as an anti-epileptic drug (AED) for children with DS. Current treatment for DS includes valproic acid or clobazam with stiripentol. However, DS still remains difficult to control and often refractory to therapy. Fenfluramine hopes to provide DS patients with better seizure control.
2. Fenfluramine and its mechanism of action
Fenfluramine (3-trifulormethyl-N-ethylamphetamine) is an amphetamine derivative that primarily affects serotonin neurotransmitter levels. The drug was initially developed and prescribed in the 1960s and 1970s for weight loss. The appetite-suppressant-effect was thought to be due to the fenfluramine's modulation of serotonin levels in the brain. This assumption was demonstrated by Fuller et al., in 1988 when they showed that fenfluramine affected the release of serotonin from neurotransmitter vesicles (Fuller et al., 1988).
Serotonin, which acts through 5-HT receptors in the CNS plays a role in several functions including mood, sleep, muscle contractions, endocrine functions, and appetite. It was first found to be associated with epilepsy by Bonnycastle in 1957 when his lab demonstrated an increase level of 5-HT in a rat brain after the administrated of an anti-epileptic drug (Bonnycastle et al., 1957). Since the initial discovery of fenfluramine's action on serotonin vesicles by Fuller in 1988, the drug has been shown to affect over 14 different subtypes of the 5-HT receptor. When studied in Zebrafish, the drug demonstrated anti-elliptic effects through antagonism of 5-HT1D and 5-HT2C (and possible 5-HT2A) receptors. This same study implicates fenfluramine also acts through the sigma-1 receptor, which may be involved in the pathophysiology of DS (Sourbron et al., 2017).
By the 1980s, over 60 million patients were taking fenfluramine for appetite suppression and weight loss. Fenfluramine was often combined off-label with phentermine to sustain the appetite-suppressant effects of fenfluramine leading to the creation of the Fen-Phen combination. However, in the late 1990s, fenfluramine was removed from the market in the United States due to its relationship with valvular heart disease and pulmonary hypertension (Connolly et al., 1997). Fenfluramine would continue to be studied within the scope of epilepsy and was approved by the U.S. Food and Drug Administration (FDA) for the treatment of DS in June of 2020.
3. Fenfluramine's efficacy and clinic study review
Fenfluramine was first used as a behavioral modifying drug in the treatment of epilepsy. In 1984, Gastaut published a case series which resulted in the elimination of self-induced syncope in patients with autism spectrum disorder (Gastaut, 1984). Gastaut additionally observed a reduction in seizure frequency in self-induced photosensitive epilepsy patients treated with fenfluramine (Aicardi and Gastaut, 1985). A follow-up pilot study was performed in 1987, examining the use of fenfluramine in 33 patients over an unspecified length of time with severe childhood epilepsy with the primary diagnosis of irretractable epilepsy. This study showed a greater than 50% reduction in seizure frequency in 46% of the patients when adding 0.5–1.5 mg/kg/day of fenfluramine to the patient's current AED regimen (Gastaut and Zifkin, 1987).
In 2012, fenfluramine was studied in a retrospective case series in patients with DS. This study resulted in 7 of the 12 patients enrolled becoming seizure-free, 1 patient with 75% reduction in seizure frequency, 2 patients with no significant response, 1 patient stopped due to lack of efficacy, and 1 patient stopped who was controlled with another AED (Ceulemans et al., 2012).
In 2019, Nabbout conducted a double-blind, parallel-group, placebo-controlled, phase 3 randomized clinical study involving patients with DS who were on a treatment regimen that involved stiripentol plus clobazam or valproic acid (Nabbout et al., 2020). The study included 87 pediatric patients ages 2–18 with DS who did not have any underlying cardiac or valvular insufficiencies. The patients were randomized to either receive fenfluramine 0.4 mg/kg day in addition to their treatment or regimen or a placebo medication. The patients who received fenfluramine were estimated to have a 54.0% greater reduction in MCSF (monthly convulsive seizure frequency) compared to the patient's receiving the placebo medication (P < 0.001). The treatment group also had significantly more patients experience a clinically meaningful (determined to be >/ = 50%) reduction in mean MCSF that the placebo group. 22 out of 43 patients in the treatment group compared to 2 of 44 patients in the placebo group experienced a clinically meaningful reduction in mean MCSF. The treatment group also experienced significantly longer seizure-free intervals (median, 22.0 days vs 130.0 days; P = 0.004).
Nabbout's trial was the first phase 3 clinical trial for the use of fenfluramine in the treatment of DS. It concluded that fenfluramine demonstrated both statistically significant and clinically meaningful efficacy in the treatment of DS within their patient cohort. Therefore, fenfluramine may be an effective treatment option for patients with DS whose seizures are not adequately controlled on stiripentol plus AED regimen.
In 2019, Lagae et al. conducted a similar randomized, double-blind, placebo-controlled clinical trial that also demonstrated encouraging results for the use of fenfluramine in patients with DS. The study enrolled 119 patients ages 2–18 with DS who were randomly assigned to receive either fenfluramine 0.2 mg/kg, fenfluramine 0.7 mg/kg per day or placebo. The study found a median reduction in seizure frequency of 74.9% in the fenfluramine 0.7 mg/kg group. This represents a decrease in seizures from 20.7 seizures per 28 days to 4.7 seizures per 28 days. Both doses of the fenfluramine showed statistically significant reduction in mean MCSF compared to the placebo group (Lagae et al., 2019; Lagae et al., 2019). This study reached a similar conclusion as the Nabbout study; fenfluramine provides a significantly greater reduction in convulsive seizure frequency compared with placebo and could be an effective new treatment for patients with DS. This study did not allow concomitant stiripentol use as the Nabbout trial did.
Patients who completed these two studies were enrolled in an open-label extension (OLE) study to assess long-term safety and efficacy of fenfluramine in these patients (Sullivan et al., 2020). The Nabbout and Lagae studies took place over a period of 15 and 14 weeks respectively. The open-label extension study had a median study period of 37 weeks. The study demonstrated that the efficacy of fenfluramine was sustained throughout the longer study period. The patients were all down-titrated (or underwent a false down-titration if placebo group) from 0.4 or 0.7 mg/kg day to 0.2 mg/kg per day for a period of 14 days before being titrated up based off disease control and patients’ ability to tolerate fenfluramine. Of note, this OLE was conducted by Zogenix, the makers of Fintepla (fenfluramine).
4. Side effects of fenfluramine
In both Nabbout's and Lagae's case control studies and the Fintepla OLE, the authors note that the drug was well tolerated by patients. The most common adverse events observed in the three studies included decreased appetite, pyrexia, diarrhea, nasopharyngitis, and fatigue (Table 1). In the Lagae study 5 patients (N = 79) withdrew due to adverse effects, but the study did not specify which effects led to the participants withdrawal (Lagae et al., 2019). Within the Nabbout study, 2 patients (N = 43) withdrew due to unspecified adverse events (Nabbout et al., 2020). Finally, the Sullivan et al. study had 1 participant withdraw at 71 days of treatment due to adverse effects (N = 232) (Sullivan et al., 2020). Note that Table 1 lists the documented adverse events from the Nabbout et al., Lagae et al., and the Sullivan et al. Studies (Nabbout et al., 2020; Lagae et al., 2019; Sullivan et al., 2020). ∗ Values include the adverse events from the patients in the Fenfluramine 0.2 mg/kg/day and the patients receiving Fenfluramine 0.7 mg/kg/day. ∗∗This study only reported adverse events that occurred in greater than 10% of patients.
Table 1.
A comparison of side effects experienced by participants from controlled case studies (Nabbout et al., 2020; Lagae et al., 2019) and an open label extension (Sullivan et al., 2020) of Fintepla. The most common adverse events observed in the three studies included decreased appetite, pyrexia, diarrhea, nasopharyngitis, and fatigue. Inputs labeled with (∗) represent very common effects (>1/10). Inputs labeled with (∗∗) represent common effects (≥1/100 to <1/10) in accordance with the FDA and EMA guidelines. Of note, the studies did not have adverse effects that met the criteria of rare (≥1/10,000 to <1/1,000) or very rare (<1/10,000) adverse effects. The column with a (†) contains no data points because the open-label study was a placebo-uncontrolled study and all participants received fenfluramine. Additionally, the data from the three studies could not be pooled due to the recruitment of patients from Lagae et al., and the Nabbout et al. study.
| Side Effects | % Adverse Events [rank] in those Receiving Fenfluramine (Lagae et al., 2019) (N = 79) | % Adverse Events [rank] in those Receiving Placebo (Lagae et al., 2019) (N = 40) | % Adverse Events [rank] in those Receiving Fenfluramine (Nabbout et al., 2020) (N = 43) | % Adverse Events [rank] in those Receiving Placebo (Nabbout et al., 2020) (N = 44) | % Adverse Events [rank] in those Receiving Fenfluramine (Sullivan et al., 2020) (N = 232)) | % Adverse Events [rank] in those Receiving Placebo (Sullivan et al., 2020) (N = 0)† |
|---|---|---|---|---|---|---|
| Patients with at least 1 adverse Event | 94.9 | 65.0 | 97.7 | 95.4 | 89.7 | N/A |
| Decreased Appetite | 29.1∗ | 5.0∗∗ | 44.2∗ | 11.4∗ | 15.9∗ | N/A |
| Diarrhea | 24.1∗ | 7.5∗∗ | 23.3∗ | 6.8∗∗ | 10.8∗ | N/A |
| Fall | 5.1∗∗ | 5.0∗∗ | 0.0 | 0.0 | 0.0 | N/A |
| Fatigue | 10.1∗ | 2.5∗∗ | 25.6∗ | 4.5∗∗ | 0.0 | N/A |
| Lethargy | 13.9∗ | 5.0∗∗ | 14.0∗ | 4.5∗∗ | 0.0 | N/A |
| Nasopharyngitis | 13.9∗ | 5.0∗∗ | 16.3∗ | 34.1∗ | 19.4∗ | N/A |
| Pyrexia | 11.4∗ | 7.5∗∗ | 25.6∗ | 9.1∗∗ | 21.6∗ | N/A |
| Seizure | 8.9∗∗ | 12.5∗ | 4.7∗∗ | 15.9∗ | 11.2∗ | N/A |
| Somnolence | 12.7∗ | 7.5∗∗ | 0.0 | 0.0 | 0.0 | N/A |
| Upper Respiratory Tract Infection | 10.1∗ | 12.5∗ | 0.0 | 0.0 | 10.3∗ | N/A |
| Vomiting | 8.9∗∗ | 10.0∗ | 0.0 | 0.0 | 0.0 | N/A |
| Weight Decrease | 8.9∗∗ | 0.0 | 0.0 | 0.0 | 0.0 | N/A |
| Blood Glucose Decrease | 0.0 | 0.0 | 14.0∗ | 4.5∗∗ | 0.0 | N/A |
| Bronchitis | 0.0 | 0.0 | 11.6∗ | 4.5∗∗ | 0.0 | N/A |
| Abnormal Heart Valves | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | N/A |
The black label should be addressed, when fenfluramine was combined with phentermine, Fen-Phen, it was found to have a correlation with valvular heart disease and pulmonary hypertension (Connolly et al., 1997). For this reason, the drug was discontinued in the United States. In the recent case control study and OLE, the patients were all screened for heart disease with echocardiograms. Each study notes that all patients exhibited normal valvular function with no pulmonary arterial hypertension at the end of the trials. Additionally, for the OLE study, it should be noted that the adverse events for the placebo arms were not available, which in turn was not possible to compare the frequency of adverse events between treatments and placebo arms.
5. Alternatives treatments for Dravet Syndrome
Currently, the most common therapy for DS includes the use or combination of valproic acid and clobazam which is based on the result from a retrospective study conducted by Dressler et al., 2015. It is suggested to add the other if seizures are not controlled on the first choice. Stiripentol can be added to the regimen as a second line therapy. The ketogenic diet is considered second line treatment for DS patients.
The ketogenic diet is a high-fat, low-carbohydrate diet that is considered a non-pharmacological effective treatment of DS in addition to other seizure disorders. The diet is usually recommended after the patient has failed 3 anti-epileptic drugs. However, since the diet has fewer neurotoxic side effects compared to pharmacologic agents, the diet is often initiated sooner. The diet has been studied in a retrospective study conducted by Laux and Blackford where 20 patients with DS who had the SCN1A mutation were evaluated. Results demonstrated that 13 of the 20 patients had greater than 50% reduction in seizure frequency (Laux and Blackford, 2013). Additionally, the diet was studied in a mouse model where SCN1A mutant mice were placed on the ketogenic diet for 2 weeks. The mice showed an elevated seizure threshold after the 2 weeks on the diet (Caraballo et al., 2005).
Stiripentol is currently one of the only FDA approved drugs for the treatment of DS. It was recently approved in the United States in 2018. Stiripentol is used as an adjunctive agent with clobazam as it interacts with clobazam increasing the concentration of its active metabolites. Stiripentol is also thought to exert its effect at the post synaptic GABA receptor by enhancing GABAergic transmission (Fisher, 2009).
Another medication with serotonin activity that shows promise in DS is Lorcaserin. The drug, which acts as a 5-HT 2C receptor agonist, was studied by Griffin et al., in the zebrafish DS model and found a decrease in the severity and frequency of seizures (Griffin et al., 2017). Tolete et al., published a case series (n = 35) studying the impacts of lorcaserin on patients with refractory seizures including those with Lennox Gastaut syndrome and DS (Tolete et al., 2018). The results showed a median percentage reduction of 47.7% (p < 0.01). With these promising impacts, Esai Inc will be completing a phase 3 trial that studies the efficacy of Lorcaserin compared to place in dravet patients. This study should conclude in the fall of 2021. Until then, the evidence while promising is not enough to support use in the DS population.
Epidiolex (GW Pharmaceuticals) is a new pharmaceutical formulation which uses a high concentration of the cannabinoid cannabidiol (CBD) in a sesame seed oil carrier (Devinsky et al., 2017). The extract used in Epidiolex has a purity as high as 99% and lacks psychoactive characteristics associated with the tetrahydro cannabinoids (Sekar and Pack, 2019). Epidiolex varies from the over-the-counter CBD products offered due to the purity of CBD extract juxtaposed to the openly available CBD products which sometimes have no CBD within their solution (Devinsky et al., 2016). Investigators conducted a randomized double-blinded control study in children ages 2–18 years of age with DS. Patients receiving CBD saw their average convulsive seizure frequency decrease by 38.9% from baseline compared to a decrease of 13.3% in the placebo group (p = 0.01). The most common adverse effects of cannabidiol were vomiting, fatigue, pyrexia, and somnolence (Devinsky et al., 2017). As cannabidiol has shown efficacy compared to placebo in several seizure disorders, it is suggested that CBD is a general anti-epileptic and not specific to DS.
Surgical treatments of DS are ineffective in DS given that the condition is of genetic origin and has a diffuse impact on the brain. Focal resection is one surgical approach in epilepsy patients. Even so, focal resection was unlikely to impact seizure burden in one study of DS patients with the SCN1A mutation (N = 6) (Skjei et al., 2015). Deep brain stimulation of the subthalamic or anterior thalamic nucleus was approved for use in treatment resistant seizure patients in the US in 2018; however, demonstration of its benefits in DS patients is limited. Vagus nerve stimulation has also shown to be an effective treatment for breakthrough seizures. It can be used in patients who have refractory seizures and are poor surgical candidates for DBS. It continues to be studied in DS patients, with no clear benefit yet demonstrated (Anwar et al., 2019).
6. Conclusion
Our review of the current literature involving fenfluramine's use in DS show that this therapy is a viable option for treatment. Where DS is often refractory to most treatments, the addition of fenfluramine as an option introduces one more factor to improve the quality of life in DS patients. While the valvopathies discussed in the Connolly Et al., did seem worrisome the current studies reviewed in this paper did not show any abnormalities suggestive of valvopathy (Connolly et al., 1997; Nabbout et al., 2020; Lagae et al., 2019; Sullivan et al., 2020). This may suggest a dose response for valvopathy as the maximum fenfluramine dosing used in patients with DS was far lower than that used in the weight loss regimens. The other side effects discussed above were limited and tolerable to the patients. In light of the alternative, which would be a recurrent seizure in the DS patient the side effects seemed acceptable.
Aside from the seizures and the neurodevelopmental decline in DS, one factor that makes DS a devastating disease aside from the impact on the patient is the cost of treatment and the loss to productivity by the caregivers. The Children's Hospital of Colorado administered a survey to 60 DS caregivers to assess the direct and indirect costs of DS. Their study results (response rate = 34) showed an average annual direct cost of U.S. $27,276 with a 95% confidence interval (CI) of $15,757 to U.S. $41,904. Still, even more burdensome, was the indirect cost due to factors that included loss to productivity with an average annual cost of U.S. $81,582 with a 95% CI of U.S. $57,253 – U.S. $106,378 (Whittington et al., 2018). One key area of expenses for the caregiver is the monthly cost of AEDs. In the European Journal of Pediatric Neurology, a survey of 93 caregivers uncovered that total AED cost was the third leading element in the cost of DS care with the costs of inpatient care and care grade expenses coming in first and second place, respectively. Incremental cost-effectiveness ratio (ICER) is a measure of a medications cost-effectiveness when compared to a competitor (Strzelczyk et al., 2019). Weston, Et al., performed a cost-effect analysis of fenfluramine and found a lower ICER for fenfluramine when compared to cannabidiol as an add-on therapy (Weston G et al., 2021). Altogether, AED cost is an impactful component of DS care and fenfluramine offers similar if not superior add-on therapy when cost is considered.
CRediT authorship contribution statement
Kayla Simon: Writing – original draft. Hunter Sheckley: Writing – original draft. Christopher L. Anderson: Writing – review & editing. Zhao Liu: Supervision. Paul R. Carney: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Aicardi J., Gastaut H. Treatment of self-induced photosensitive epilepsy with fenfluramine. N. Engl. J. Med. 1985;313:1419. doi: 10.1056/NEJM198511283132218. [DOI] [PubMed] [Google Scholar]
- Anwar A., Saleem S., Patel U.K., Arumaithurai K., Malik P. Dravet syndrome: an overview. Cureus. 2019;11 doi: 10.7759/cureus.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aras L.M., Isla J., Mingorance-Le Meur A. The European patient with Dravet syndrome: results from a parent-reported survey on antiepileptic drug use in the European population with Dravet syndrome. Epilepsy Behav. 2015;44:104–109. doi: 10.1016/j.yebeh.2014.12.028. [DOI] [PubMed] [Google Scholar]
- Bonnycastle D.D., Giarman N.J., Paasonen M.K. Anticonvulsant compounds and 5-hydroxytryptamine in rat brain. Br. J. Pharmacol. Chemother. 1957;12:228–231. doi: 10.1111/j.1476-5381.1957.tb00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraballo R.H., et al. Ketogenic diet in patients with Dravet syndrome. Epilepsia. 2005;46:1539–1544. doi: 10.1111/j.1528-1167.2005.05705.x. [DOI] [PubMed] [Google Scholar]
- Ceulemans B., et al. Successful use of fenfluramine as an add-on treatment for Dravet syndrome. Epilepsia. 2012;53:1131–1139. doi: 10.1111/j.1528-1167.2012.03495.x. [DOI] [PubMed] [Google Scholar]
- Chopra R., Isom L.L. Untangling the dravet syndrome seizure network: the changing face of a rare genetic epilepsy. Epilepsy Current. 2014;14:86–89. doi: 10.5698/1535-7597-14.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly H.M., et al. Valvular heart disease associated with fenfluramine–phentermine. N. Engl. J. Med. 1997;337:581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- Devinsky O., et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15:270–278. doi: 10.1016/S1474-4422(15)00379-8. [DOI] [PubMed] [Google Scholar]
- Devinsky O., et al. Trial of cannabidiol for drug-resistant seizures in the dravet syndrome. N. Engl. J. Med. 2017;376:2011–2020. doi: 10.1056/NEJMoa1611618. [DOI] [PubMed] [Google Scholar]
- Dressler A., et al. Efficacy and tolerability of the ketogenic diet in Dravet syndrome - comparison with various standard antiepileptic drug regimen. Epilepsy Res. 2015;109:81–89. doi: 10.1016/j.eplepsyres.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Fisher J.L. The anti-convulsant stiripentol acts directly on the GABA(A) receptor as a positive allosteric modulator. Neuropharmacology. 2009;56:190–197. doi: 10.1016/j.neuropharm.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R.W., Snoddy H.D., Robertson D.W. Mechanisms of effects of d-fenfluramine on brain serotonin metabolism in rats: uptake inhibition versus release. Pharmacol. Biochem. Behav. 1988;30:715–721. doi: 10.1016/0091-3057(88)90089-5. [DOI] [PubMed] [Google Scholar]
- Gastaut H. [Efficacy of fenfluramine for the treatment of compulsive behavior disorders in psychotic children] Presse Med. 1984;13:2024–2025. [PubMed] [Google Scholar]
- Gastaut H., Zifkin B.G. The risk of automobile accidents with seizures occurring while driving: relation to seizure type. Neurology. 1987;37:1613–1616. doi: 10.1212/wnl.37.10.1613. [DOI] [PubMed] [Google Scholar]
- Griffin A., et al. Clemizole and modulators of serotonin signalling suppress seizures in Dravet syndrome. Brain. 2017;140:669–683. doi: 10.1093/brain/aww342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagae L., et al. Fenfluramine hydrochloride for the treatment of seizures in Dravet syndrome: a randomised, double-blind, placebo-controlled trial. Lancet. 2019;394:2243–2254. doi: 10.1016/S0140-6736(19)32500-0. [DOI] [PubMed] [Google Scholar]
- Laux L., Blackford R. The ketogenic diet in Dravet syndrome. J. Child Neurol. 2013;28:1041–1044. doi: 10.1177/0883073813487599. [DOI] [PubMed] [Google Scholar]
- Nabbout R., et al. Fenfluramine for treatment-resistant seizures in patients with dravet syndrome receiving stiripentol-inclusive regimens: a randomized clinical trial. JAMA Neurol. 2020;77:300–308. doi: 10.1001/jamaneurol.2019.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar K., Pack A. 2019. Epidiolex as Adjunct Therapy for Treatment of Refractory Epilepsy: a Comprehensive Review with a Focus on Adverse Effects; p. F1000Res8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjei K.L., et al. Clinical and histopathological outcomes in patients with SCN1A mutations undergoing surgery for epilepsy. J. Neurosurg. Pediatr. 2015;16:668–674. doi: 10.3171/2015.5.PEDS14551. [DOI] [PubMed] [Google Scholar]
- Sourbron J., Smolders I., de Witte P., Lagae L. Pharmacological analysis of the anti-epileptic mechanisms of fenfluramine in scn1a mutant zebrafish. Front. Pharmacol. 2017;8:191. doi: 10.3389/fphar.2017.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzelczyk A., et al. Burden-of-illness and cost-driving factors in Dravet syndrome patients and carers: a prospective, multicenter study from Germany. Eur. J. Paediatr. Neurol. 2019;23:392–403. doi: 10.1016/j.ejpn.2019.02.014. [DOI] [PubMed] [Google Scholar]
- Sullivan J., et al. Fenfluramine HCl (Fintepla(®) ) provides long-term clinically meaningful reduction in seizure frequency: analysis of an ongoing open-label extension study. Epilepsia. 2020;61:2396–2404. doi: 10.1111/epi.16722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolete P., et al. Lorcaserin therapy for severe epilepsy of childhood onset: a case series. Neurology. 2018;91:837–839. doi: 10.1212/WNL.0000000000006432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston G P.A., Adams E., Linley W., Hawkins N., Schwenkglenks M., Hamlyn-Williams C., Toward T. Elsevier, ISPOR—The Professional Society for Health Economics and Outcomes Research; 2021. A Cost-Effectiveness Analysis of Fenfluramine for the Treatment of Seizures for Patients with Dravet Syndrome (DS) in the UK Setting. 24 S164-165. [Google Scholar]
- Whittington M.D., et al. The direct and indirect costs of Dravet Syndrome. Epilepsy Behav. 2018;80:109–113. doi: 10.1016/j.yebeh.2017.12.034. [DOI] [PubMed] [Google Scholar]