Abstract
Background
Implementation of adjuvant therapies in non-metastatic melanoma improved treatment outcomes in some patients; however, adjuvant therapy can be associated with significant cost and risk of toxicity. Therefore, there is an unmet need to better identify patients at high risk of recurrence.
Patients and methods
We carried out an ultrasensitive droplet digital PCR (ddPCR)-based detection of BRAFV600E-mutated circulating tumor DNA (ctDNA) from blood samples prospectively collected before surgery, 1 hour after surgery, and then serially during follow-up.
Results
In 80 patients (stages ≤III), BRAFV600E mutations were detected in 47.2% of tissue, in 37.7% of ctDNA samples collected before surgery, and in 25.9% of ctDNA samples collected 1 hour after surgery. Patients with detected ctDNA in blood collected 1 hour after surgery compared to patients without detected ctDNA had higher likelihood of melanoma recurrence (P < 0.001) and shorter median disease-free survival (P = 0.001) and overall survival (P = 0.003).
Conclusions
Ultrasensitive ddPCR can detect ctDNA in pre- and post-surgical blood samples from patients with resectable melanoma. Detection of ctDNA in post-surgical samples is associated with inferior treatment outcomes.
Key words: circulating tumor DNA, liquid biopsy, melanoma
Highlights
-
•
Ultrasensitive ddPCR can detect ctDNA in pre- and post-surgical samples.
-
•
Detection of ctDNA 1 hour after surgery is associated with inferior treatment outcomes.
-
•
There were no associations between ctDNA detection at other timepoints and clinical outcomes.
Introduction
Cutaneous melanoma is the fifth most common cancer in the United States with a median age at diagnosis of 65 years.1 Treatment of locally advanced and metastatic melanoma has been challenging because of the high recurrence rate and limited therapeutic options. Even though patients with early disease are potentially curable with surgery, 13% develop recurrent locoregional or metastatic disease within 2 years with a median overall survival (OS) of 1-2 years.2 Addition of adjuvant therapy after surgical resection improved outcomes for some patients; however, it was also associated with significant cost and increased risk of bothersome or even permanent side-effects.3, 4, 5 Therefore, there is an unmet need to identify patients at high risk of recurrence who can benefit most from post-surgical adjuvant therapy.
Approximately half of the patients with melanoma harbor BRAFV600 mutations followed by NRAS mutations and some less frequent alterations such as class II and III BRAF alterations, MEK1 mutations, KIT mutations, or other alterations.6 Mutated DNA can be detected in fragments of circulating tumor DNA (ctDNA), which are released to the circulation from dying tumor cells in patients with advanced and to less extent early-stage cancers.7 We hypothesize that the presence of ctDNA in blood from patients with early-stage melanoma is associated with unfavorable disease-free survival (DFS). Because the quantity of ctDNA decreases with less advanced disease,7 we developed and used an ultrasensitive technique for the detection of BRAFV600E-mutated ctDNA in blood from patients with early-stage melanoma and compared ctDNA detection to clinical outcomes.
Patients and methods
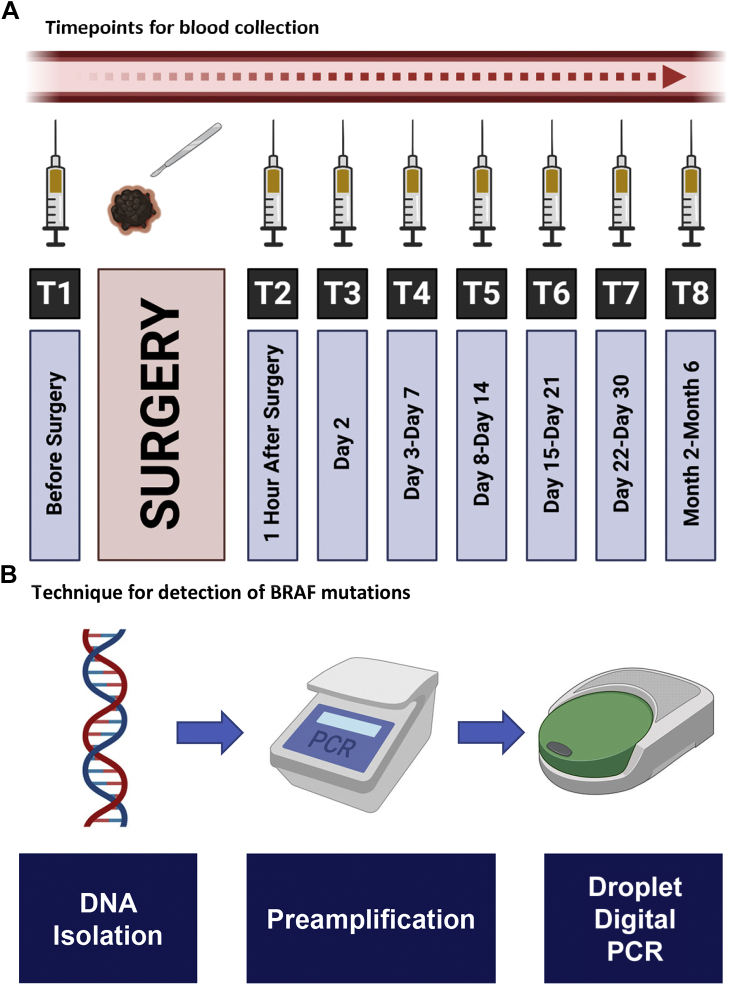
Patients with newly diagnosed early-stage melanoma who underwent definitive surgery at Charles University (Czech Republic) between January 2014 and July 2020 were invited to participate in this study. Institutional review board approval was obtained from Charles University and patients consented to collection of both archival tumor tissue and serial blood samples. Plasma samples were obtained from patients before and after surgery as well as at follow-up visits according to predefined timepoints (Figure 1). Peripheral blood samples were collected from the cubital vein using K3EDTA Vacutainer tubes (Greiner Bio-One, Kremsmünster, Austria). Plasma was separated by two-step centrifugation of 6 ml of blood [950 relative centrifugal force (RCF) for 10 min at 4°C and then 11 000 RCF for 10 min at 4°C] and then stored at −80°C until further use.
Figure 1.
Different timepoints for blood collection as well as summary of the technique.
Cell-free DNA (cfDNA) was isolated from an average of 3 ml plasma (0.5-5 ml) using QIAamp Circulating Nucleic Acid Kit (QIAGEN, Hilden, Germany) and from corresponding formalin-fixed paraffin blocks using QIAamp DNA FFPE Tissue Kit (QIAGEN, Hilden, Germany). cfDNA was quantified using Quanti-iT PicoGreen dsDNA Assay Kit (Invitrogen, MA) on SpectraMax M2 (Molecular Devices, CA) where reference standard curve assay was used with serial dilutions for accurate quantification. Unproportionate pre-amplification of mutant and wild-type (WT) copies (favoring mutant alleles) was done using Q5 High-Fidelity PCR Kit (New England BioLabs, MA). QIAquick PCR Purification kits (QIAGEN, Hilden, Germany) were used to purify DNA after pre-amplification, and droplet digital PCR (ddPCR) (BioRad, CA) was carried out on pre-amplified cfDNA for detection of BRAFV600E mutations.
Clinical and epidemiological data were obtained from patients' medical records where disease staging was defined based on American Joint Committee on Cancer (AJCC) Staging Manual, eighth edition.8 Patients with medical records showing another primary tumor were excluded from analysis. Recurrence rate was defined as the percentage of patients developing recurrent melanoma during the study. DFS was defined as the time between curative surgery and development of disease recurrence or death of any cause. OS was calculated as the difference between date of patient's death or last follow-up and date of diagnosis.
Statistical analysis was carried out using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., NY). Median and interquartile range (IQR) were used for description of continuous variables, while frequencies and percentages were used to describe categorical variables. Statistical significance was tested using Pearson’s chi-square test, Fisher’s exact test, and Mann–Whitney test, each when appropriate. Survival analysis was done using Kaplan–Meier analysis, and log-rank test was used to assess statistical significance. A P value of <0.05 was considered statistically significant.
Results
A total of 80 patients were included in the study with a median age at diagnosis of 60 years. Most patients were males (n = 43, 53.8%), had stage II disease (n = 30, 37.5%), had no nodal involvement (n = 49, 61.3%), and had no associated ulceration (n = 46, 57.5%). The median Breslow thickness in the studied cohort was 2.2 mm (IQR, 3) (Table 1). Only 17 patients (21.3%) received adjuvant interferon (n = 16) or nivolumab (n = 1).
Table 1.
Patient characteristics
| Age [median years (IQR)] | 60 (21) | |
| Gender [n (%)] | Males | 43 (53.8) |
| Females | 37 (46.3) | |
| Stage [n (%)] | Stage 0 | 4 (5) |
| Stage IA | 12 (15) | |
| Stage IB | 12 (15) | |
| Stage IIA | 11 (13.8) | |
| Stage IIB | 10 (12.5) | |
| Stage IIC | 9 (11.3) | |
| Stage IIIA | 1 (1.3) | |
| Stage IIIB | 4 (5) | |
| Stage IIIC | 14 (17.5) | |
| Stage IIID | 1 (1.3) | |
| Unknown | 2 (2.5) | |
| Nodal involvement [n (%)] | Yes | 19 (23.8) |
| No | 49 (61.3) | |
| Unknown | 12 (15) | |
| Ulceration [n (%)] | Yes | 34 (42.5) |
| No | 46 (57.5) | |
| Breslow thickness [median mm (IQR)] | 2.2 (3) | |
| Pathology [n (%)] | Malignant melanoma (NOS) | 11 (13.8) |
| In situ | 4 (5) | |
| Lentigo maligna | 3 (3.8) | |
| Acrolentigionous | 6 (7.5) | |
| Nodular melanoma | 23 (28.7) | |
| Superficial spreading | 23 (28.7) | |
| Other types | 9 (11.3) | |
| Unknown | 1 (1.3) | |
| Tissue BRAF [n (%)] | Mutated | 23 (28.7) |
| Wild type | 29 (36.3) | |
| Unavailable | 28 (35) | |
| Relapse [n (%)] | Yes | 19 (23.8) |
| No | 61 (76.3) | |
| Vital status [n (%)] | Alive | 72 (90) |
| Dead | 8 (10) | |
IQR, interquartile range; NOS, not otherwise specified.
Tumor tissue samples, adequate for BRAFV600E mutation testing, were available in 52 patients (65%) and 23 of these 52 (44.2%) patients demonstrated BRAFV600E mutation in the tumor tissue. There were no significant differences between tissue BRAFV600E-mutated samples and BRAFV600E-WT samples in terms of recurrence risk [21.7% (n = 5) for tissue-mutated cohort versus 33.3% (n = 10) for tissue-WT cohort, P = 0.314], DFS (median survival not reached in both groups, P = 0.158), or OS (median survival not reached in both groups, P = 0.713) (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2021.100357).
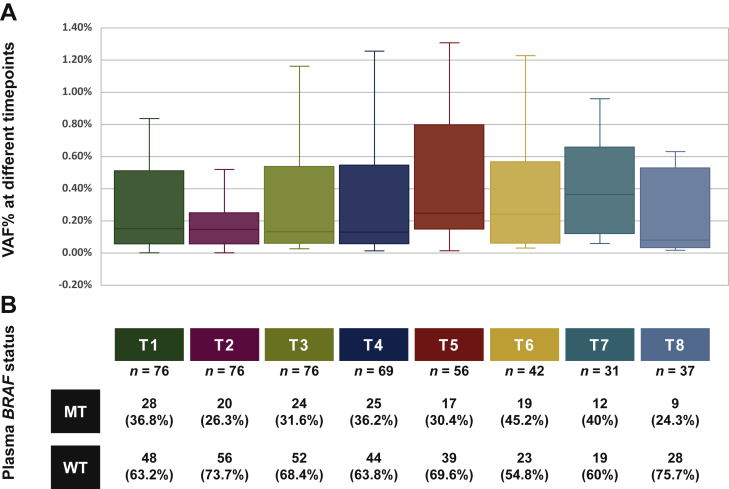
Of 80 patients included in this study, 76 (95%) had plasma samples available at baseline, at 1 hour after surgery, and at 1 day after surgery and were tested for presence of BRAFV600E mutation in ctDNA. Figure 2 shows the distribution of variant allele frequencies for BRAFV600E at different timepoints and frequencies of BRAFV600E mutations in pre- and post-operative samples.
Figure 2.
Plasma BRAFV600E variant allele frequency (VAF%) at different timepoints (A) as well as frequency of plasma BRAFV600E mutations (B).
MT, mutant; WT, wild type.
BRAFV600E-mutated ctDNA was detected in plasma samples collected before surgery in 28 patients (36.8%). We tried to explore the concordance between tissue and plasma BRAFV600E mutation status. In 49 patients, who had both tumor tissue and pre-surgical ctDNA available, we found agreement between BRAFV600E mutation status in the tumor tissue and plasma in 23 patients (46.9%). BRAFV600E mutations were present in tumor tissue only in 13 patients (26.5%) and in plasma only in 13 (26.5%) out of 49 patients (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100357).
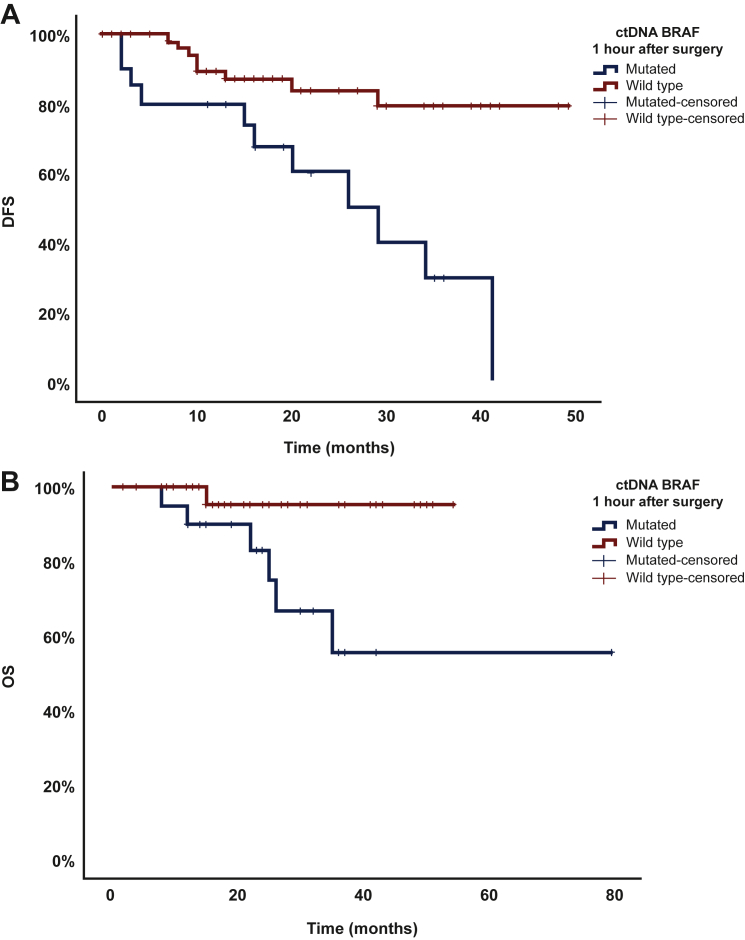
In patients with plasma collected before surgery (n = 76), presence of BRAFV600E-mutated ctDNA in pre-surgical specimens was associated with higher rate of melanoma recurrence [39.3% (n = 11) versus 16.7% (n = 8), P = 0.028] and death [21.4% (n = 6) versus 4.2% (n = 2), P = 0.046]; however, this did not translate into difference in median DFS (41 months versus not reached, P = 0.214) or median OS (not reached in both groups, P = 0.077, Table 2). At 1-hour post-surgery, BRAFV600E mutations were detected in 26.3% (n = 20) of patients. Immediate conversion from pre-operative ctDNA mutated to post-operative ctDNA wild-type BRAFV600E occurred in 20% (n = 15) of patients. Patients with BRAFV600E-mutated ctDNA at 1 hour after surgery compared to patients with BRAFV600E-WT ctDNA had higher likelihood of overall recurrence [55% (n = 11) versus 14.3% (n = 8), P < 0.001], recurrence risk at 6 months [20% (n = 4) versus 0% (n = 0), P = 0.004], and recurrence risk at 24 months [35% (n = 7) versus 12.5% (n = 7), P = 0.042]. They also had shorter DFS (29 months versus median survival not reached, P = 0.001) and OS (median survival not reached in both groups, P = 0.003; Figure 3). At the second day after surgery, BRAFV600E mutations in ctDNA were associated with higher rate of recurrence [41.7% (n = 10) versus 17.3% (n = 9), P = 0.023] but not with a difference in median DFS (41 months versus not reached, P = 0.214) or OS (not reached in both groups, P = 0.077). At all other follow-up timepoints, there was no significant difference between ctDNA-mutant and -WT groups in terms of recurrence risk, DFS, or OS (all P > 0.05) (Table 2).
Table 2.
Associations between clinical outcomes and ctDNA mutation status at different timepoints for the overall population
| Recurrence at 6 months |
Recurrence at 24 months |
Overall progression probability |
Death probability |
DFS |
OS |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | P | n (%) | P | n (%) | P | n (%) | P | Median (months) | P | Median (months) | P | ||
| T1 (n = 76) | MT (n = 28) | 2 (7.1) | 0.623 | 7 (25) | 0.258 | 11 (39.3) | 0.028 | 6 (21.4) | 0.046 | 41 | 0.214 | NR | 0.077 |
| WT (n = 48) | 2 (4.2) | 7 (14.6) | 8 (16.7) | 2 (4.2) | NR | NR | |||||||
| T2 (n = 76) | MT (n = 20) | 4 (20) | 0.004 | 7 (35) | 0.042 | 11 (55) | <0.001 | 6 (30) | 0.003 | 29 | 0.001 | NR | 0.003 |
| WT (n = 56) | 0 (0) | 7 (12.5) | 8 (14.3) | 2 (3.6) | NR | NR | |||||||
| T3 (n = 76) | MT (n = 24) | 3 (12.5) | 0.09 | 7 (29.2) | 0.120 | 10 (41.7) | 0.023 | 3 (12.5) | 0.702 | 41 | 0.129 | NR | 0.943 |
| WT (n = 52) | 1 (1.9) | 7 (13.5) | 9 (17.3) | 5 (9.6) | NR | NR | |||||||
| T4 (n = 69) | MT (n = 25) | 2 (8) | 0.617 | 4 (16) | 0.756 | 7 (28.0) | 0.785 | 3 (12) | 1 | 41 | 0.600 | NR | 0.570 |
| WT (n = 44) | 2 (4.5) | 9 (20.5) | 11 (25) | 5 (11.4) | NR | NR | |||||||
| T5 (n = 56) | MT (n = 17) | 3 (17.6) | 0.079 | 5 (29.4) | 0.739 | 7 (41.2) | 0.339 | 4 (23.5) | 0.228 | 41 | 0.746 | NR | 0.437 |
| WT (n = 39) | 1 (2.6) | 9 (23.1) | 11 (28.2) | 4 (10.3) | NR | NR | |||||||
| T6 (n = 42) | MT (n = 19) | 1 (5.3) | 0.452 | 2 (10.5) | 0.428 | 4 (21.1) | 1 | 3 (15.8) | 0.644 | NR | 0.307 | NR | 0.790 |
| WT (n = 23) | 0 (0) | 5 (21.7) | 6 (26.1) | 2 (8.7) | NR | NR | |||||||
| T7 (n = 31) | MT (n = 12) | 2 (16.7) | 0.548 | 3 (25) | 0.704 | 5 (41.7) | 0.712 | 3 (25) | 0.364 | 41 | 0.935 | NR | 0.612 |
| WT (n = 19) | 1 (5.6) | 6 (33.3) | 6 (33.3) | 2 (11.1) | NR | NR | |||||||
| T8 (n = 37) | MT (n = 9) | 1 (11.1) | 0.432 | 2 (22.2) | 1 | 2 (22.2) | 1 | 1 (11.1) | 1 | NR | 0.680 | NR | 0.469 |
| WT (n = 28) | 1 (3.6) | 5 (17.9) | 8 (28.6) | 4 (14.3) | 34 | NR | |||||||
Highlighted comparisons indicate statistical significance (P < 0.05); NR indicates that median survival was not reached.
ctDNA, circulating tumor DNA; DFS, disease-free survival; MT, mutant; OS, overall survival; WT, wild type.
Figure 3.
Difference in disease-free survival (DFS) (A) and overall survival (OS) (B) between circulating tumor DNA (ctDNA)-mutant group (MT) and ctDNA-wild-type group (WT) at 1 hour after surgery (T2).
Patients with mutant ctDNA BRAFV600E had shorter DFS (29 months versus median survival not reached, P = 0.001) and OS (median survival not reached in both groups, P = 0.003).
Finally, in order to investigate if the above reported associations with outcomes differed with respect to the tissue BRAFV600E mutation status, we carried out separate subgroup analyses for tissue BRAFV600E-mutated and tissue BRAFV600E-WT subgroups. In 23 patients with the BRAFV600E mutation in the tumor tissue, patients with detectable BRAFV600-mutated ctDNA 1 hour after surgery compared to patients with undetectable ctDNA had higher overall risk of recurrence [57.1% (n = 4) versus 6.7% (n = 1), P = 0.021], higher risk for death [57.1% (n = 4) versus 0% (n = 0), P = 0.005], shorter median DFS (26 months versus not reached, P = 0.002), and shorter median OS (not reached in both groups, P < 0.001) (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2021.100357). In tissue BRAFV600E-WT cohort (n = 29), we found an increased rate of overall recurrence risk in patients with detectable BRAFV600E-mutated ctDNA at 1 hour after surgery compared to patients without detectable ctDNA [66.7% (n = 6) versus 23.5% (n = 4), P = 0.046]. However, there was no difference between the two groups in rate of death [22.2% (n = 2) versus 5.9% (n = 1), P = 0.268], or median OS (not reached in both groups, P = 0.286) with exception of a trend toward shorter median DFS in patients with detectable ctDNA (29 months versus not reached, P = 0.06), (Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2021.100357).
Discussion
Melanoma is a common skin malignancy that can be cured in early stages if completely surgically removed.9, 10, 11, 12 Adjuvant therapy decreases recurrence rates in high-risk patients but that comes at also increased cost and risk of toxicity.3, 4, 5,13 Identification of biomarkers that can predict patient outcomes can plausibly help in better selection of patients likely to benefit from adjuvant therapy. In our study, we carried out a clinical validation of the ultrasensitive detection of BRAFV600E mutations in ctDNA from blood samples of patients with resectable melanoma. We identified BRAFV600E mutations in tumor tissue in 47.2% of patients and in blood collected before surgery in 37.7% of patients, which is comparable to previously published reports of BRAF testing in non-metastatic melanoma.6,14 Tumors at earlier stages shed less ctDNA compared to metastatic tumors with larger disease burden and it could have accounted for the difference between BRAFV600E mutation frequency in the tumor and ctDNA.15 Interestingly, detection of BRAFV600E-mutated ctDNA in blood collected 1 hour after surgery was associated with higher overall recurrence rate, higher recurrence rate at 6 and 24 months, shorter DFS, and shorter OS. Also, 20% of patients had conversion of ctDNA detection before surgery to no detection 1 hour after surgery, which can be plausibly explained by a short half-life of ctDNA spanning from 8 to 147 min.16
Most of the current data on the utility of ctDNA detection in melanoma come from studies in stage IV disease,17, 18, 19, 20, 21, 22 where detection rate of mutant BRAF can be up to 90% and is intended to be used for selection of targeted therapies or prognosis determination.17,23 In early-stage melanoma, there has been emerging evidence suggesting the utility of ctDNA detection to identify patients with stage II or III resected melanoma at high risk of recurrence. For instance, Tan et al. reported that pre-operative and post-operative detection of ctDNA in blood from patients with stage III melanoma was associated with shorter relapse-free survival and distant metastasis-free survival.14 Similarly, Lee et al. reported that detection of ctDNA in pre-operative blood samples from patients with stage III melanoma was associated with more significant nodal involvement, high lactate dehydrogenase levels, and worse melanoma-specific survival.24 In our study, other post-operative timepoints, except for the first post-surgical collection, did not demonstrate statistically significant association with outcomes and reasons behind that phenomenon remain unclear. It is plausible though that tumor manipulation during surgery could have led to release of more mutant ctDNA, which was then detected at the 1-hour post-operative timepoint.
Current melanoma treatment guidelines establish surgical resection as the standard of care for localized and locoregional disease with the possibility of adding adjuvant therapy in high-risk patients.25 Our results suggest that ctDNA detection at 1 hour after surgery can identify patients with higher risk of recurrence. While this observation needs to be confirmed, testing for ctDNA can be considered for future investigations as a selection tool for adjuvant therapy.26
We noticed that nearly half of the patients with no BRAFV600E mutation in tumor tissue had BRAFV600E-mutated ctDNA detected in at least one timepoint. This plasma-specific mutation pattern has also been described in previous reports in other tumor types.27,28 This can be possibly explained by the tumor heterogeneity that cannot be addressed during lesion biopsy mutation analysis, while shedding from different tumor clones in plasma gives a more holistic overview of the genomic profile.29 In order to investigate whether observed differences were not driven by patients with BRAFV600E mutation in the tumor tissue, we carried out a separate subgroup analysis for both the tissue BRAFV600E-mutated and tissue BRAFV600E-WT groups to make sure the benefit was not driven by the tissue-mutated cohort. Even though tissue-mutated cohort showed more pronounced associations with outcomes, the recurrence risk was also higher for patients with ctDNA detection at 1 hour after surgery in the tissue BRAFV600E-WT group.
Our study has several limitations, which include the relatively small number of patients with diverse AJCC stages. In addition, 35% of patients did not have available tumor tissue for confirmatory BRAFV600E mutation analysis. Also, not all patients had all planned timepoints collected, which also could have impacted our analysis. Nevertheless, our results provide a proof of concept that detection of BRAFV600E mutations in ctDNA isolated from blood collected after surgery using the ultrasensitive ddPCR-based approach can identify patients at higher risk of disease recurrence and shorter survival, which warrants further investigation especially with respect to indication of adjuvant therapy.
Acknowledgments
Funding
This study was supported by the LTAUSA19080 Project as part of the INTER-EXCELLENCE program (INTER-ACTION subprogram) funded by the Ministry of Education, Youth and Sports in the Czech Republic (LTAUSA19080); National Center for Advancing Translational Sciences [grant number UL1 TR000371]; National Institutes of Health through MD Anderson’s Cancer Center Support Grant [grant number P30CA016672]; Rising Tide Foundation [grant number CR-18-600]; and Andrew Sabin Family Foundation (FJ).
Disclosure
SGC is a current employee at Tempus Labs. FM-B has research support from Aileron Therapeutics, AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences, Curis, CytomX Therapeutics, Daiichi Sankyo Co., Debiopharm International, eFFECTOR Therapeutics, Genentech, Guardant Health, Klus Pharma, Takeda Pharmaceutical (formerly Millennium Pharmaceutical), Novartis, Puma Biotechnology, and Taiho Pharmaceutical; provided consulting role for AbbVie, Aduro BioTech, Alkermes, AstraZeneca, DebioPharm, eFFECTOR Therapeutics, F. Hoffman-La Roche, Genentech, IBM Watson, Infinity Pharmaceuticals, Jackson Laboratory, Kolon Life Science, OrigiMed, PACT Pharma, Parexel International, Pfizer, Samsung Bioepis, Seattle Genetics, Tyra Biosciences, and Xencor, Zymeworks; is on the advisory committee of Black Diamond, Eisai, Immunomedics, Inflection Biosciences, Karyopharm Therapeutics, Mersana Therapeutics, Puma Biotechnology, Seattle Genetics, Silverback Therapeutics, Spectrum Pharmaceuticals, and Zentalis; received honoraria from Chugai Biopharmaceuticals, Mayo Clinic, and Rutgers Cancer Institute of New Jersey; and received travel-related support from Beth Israel Deaconess Medical Center. FJ has research support from Astex, Novartis, BioMed Valley Discoveries, Fore Bio, Deciphera, Bristol-Myers Squibb, Asana, Ideaya Biosciences, Sanofi, Merck, F-star, JSI Innopharm, Bioxcel, Lilly, Bicara, PureTech Health, FujiFilm Pharmaceuticals, Sotio, Synlogic, NextCure, and Hutchinson Medipharma; is on the Scientific Advisory Boards of Ideaya Biosciences, Synlogic, Sotio, Puretech Health, Deciphera, Crown Bioscience, Asana, Fore Bio, Novartis, Bicara, and PegaOne; is a paid consultant for Mersana Therapeutics, Flame Bio, Cardiff Oncology, MedinCell, and Immunomet; has ownership interests in Cardiff Oncology and Monte Rosa Therapeutics; and has the leadership position in Monte Rosa Therapeutics. The remaining authors have declared no conflicts of interest.
Supplementary data
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Von Schuckmann L.A., Hughes M.C.B., Ghiasvand R., et al. Risk of melanoma recurrence after diagnosis of a high-risk primary tumor. JAMA Dermatol. 2019;155(6):688–693. doi: 10.1001/jamadermatol.2019.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eggermont A.M.M., Blank C.U., Mandala M., et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378(19):1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 4.Weber J., Mandala M., Del Vecchio M., et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377(19):1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 5.Long G.V., Hauschild A., Santinami M., et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377(19):1813–1823. doi: 10.1056/NEJMoa1708539. [DOI] [PubMed] [Google Scholar]
- 6.Hodis E., Watson I.R., Kryukov G.V., et al. A landscape of driver mutations in melanoma. Cell. 2012;150(2):251–263. doi: 10.1016/j.cell.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bettegowda C., Sausen M., Leary R.J., et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amin M.B., Edge S.B., Greene F.L., et al., editors. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing; New York City, NY: 2016. [Google Scholar]
- 9.Che G., Huang B., Xie Z., et al. Trends in incidence and survival in patients with melanoma, 1974-2013. Am J Cancer Res. 2019;9(7):1396–1414. [PMC free article] [PubMed] [Google Scholar]
- 10.Ossio R., Roldán-Marín R., Martínez-Said H., Adams D.J., Robles-Espinoza C.D. Melanoma: a global perspective. Nat Rev Cancer. 2017;17(7):393–394. doi: 10.1038/nrc.2017.43. [DOI] [PubMed] [Google Scholar]
- 11.Garbe C., Keim U., Eigentler T.K., et al. Time trends in incidence and mortality of cutaneous melanoma in Germany. J Eur Acad Dermatol Venereol. 2019;33(7):1272–1280. doi: 10.1111/jdv.15322. [DOI] [PubMed] [Google Scholar]
- 12.Dawson S.-J., Tsui D.W.Y., Murtaza M., et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368(13):1199–1209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 13.Hodi F.S., O'Day S.J., McDermott D.F., et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan L., Sandhu S., Lee R.J., et al. Prediction and monitoring of relapse in stage III melanoma using circulating tumor DNA. Ann Oncol. 2019;30(5):804–814. doi: 10.1093/annonc/mdz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang R.S.P., Xiao J., Pavlick D.C., et al. Circulating cell-free DNA yield and circulating-tumor DNA quantity from liquid biopsies of 12 139 cancer patients. Clin Chem. 2021;67:1554–1566. doi: 10.1093/clinchem/hvab176. [DOI] [PubMed] [Google Scholar]
- 16.Chen K., Zhao H., Shi Y., et al. Perioperative dynamic changes in circulating tumor DNA in patients with lung cancer (DYNAMIC) Clin Cancer Res. 2019;25(23):7058–7067. doi: 10.1158/1078-0432.CCR-19-1213. [DOI] [PubMed] [Google Scholar]
- 17.Xi L., Pham T.H.T., Payabyab E.C., Sherry R.M., Rosenberg S.A., Raffeld M. Circulating tumor DNA as an early indicator of response to T-cell transfer immunotherapy in metastatic melanoma. Clin Cancer Res. 2016;22(22):5480–5486. doi: 10.1158/1078-0432.CCR-16-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forthun R.B., Hovland R., Schuster C., et al. ctDNA detected by ddPCR reveals changes in tumour load in metastatic malignant melanoma treated with bevacizumab. Sci Rep. 2019;9(1):17471. doi: 10.1038/s41598-019-53917-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J.H., Long G.V., Menzies A.M., et al. Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with anti–programmed cell death 1 antibodies. JAMA Oncol. 2018;4(5):717–721. doi: 10.1001/jamaoncol.2017.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray E.S., Rizos H., Reid A.L., et al. Circulating tumor DNA to monitor treatment response and detect acquired resistance in patients with metastatic melanoma. Oncotarget. 2015;6(39):42008–42018. doi: 10.18632/oncotarget.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEvoy A.C., Warburton L., Al-Ogaili Z., et al. Correlation between circulating tumour DNA and metabolic tumour burden in metastatic melanoma patients. BMC Cancer. 2018;18(1):726. doi: 10.1186/s12885-018-4637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seremet T., Jansen Y., Planken S., et al. Undetectable circulating tumor DNA (ctDNA) levels correlate with favorable outcome in metastatic melanoma patients treated with anti-PD1 therapy. J Transl Med. 2019;17(1):303. doi: 10.1186/s12967-019-2051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santiago-Walker A., Gagnon R., Mazumdar J., et al. Correlation of BRAF mutation status in circulating-free DNA and tumor and association with clinical outcome across four BRAFi and MEKi clinical trials. Clin Cancer Res. 2016;22(3):567–574. doi: 10.1158/1078-0432.CCR-15-0321. [DOI] [PubMed] [Google Scholar]
- 24.Lee J.H., Long G.V., Boyd S., et al. Circulating tumour DNA predicts response to anti-PD1 antibodies in metastatic melanoma. Ann Oncol. 2017;28(5):1130–1136. doi: 10.1093/annonc/mdx026. [DOI] [PubMed] [Google Scholar]
- 25.Michielin O., van Akkooi A.C.J., Ascierto P.A., Dummer R., Keilholz U., ESMO Guidelines Committee Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(12):1884–1901. doi: 10.1093/annonc/mdz411. [DOI] [PubMed] [Google Scholar]
- 26.Parikh A.R., Van Seventer E.E., Siravegna G., et al. Minimal residual disease detection using a plasma-only circulating tumor DNA assay in patients with colorectal cancer. Clin Cancer Res. 2021;27(20):5586–5594. doi: 10.1158/1078-0432.CCR-21-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasi P.M., Le A.D., Barrett A. Comparative landscape of actionable somatic alterations in advanced cholangiocarcinoma from circulating tumor and tissue-based DNA profiling. J Clin Oncol. 2021;39(3_suppl):342. [Google Scholar]
- 28.Mack P., Minichielle K., Redman M., et al. LUNGMAP Master Protocol (LUNGMAP): concordance between plasma ctDNA and tissue molecular analysis. J Thorac Oncol. 2021;16(3):S163–S164. [Google Scholar]
- 29.Gerlinger M., Rowan A.J., Horswell S., et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.