Introduction
On March 15, 2021, Abbott (Lake Forest, IL) issued a safety notification for a subset of its St Jude Assurity and Endurity pacemakers. Owing to a manufacturing defect of epoxy in the device headers, there was concern for moisture ingress into the devices, leading to premature battery depletion, shortened duration between Elective Replacement Indicator and End of Service, and possible loss of pacing. Initial recommendations included remote monitoring through their Merlin.net monitoring, which provides an automated alert within 24 hours of a device reaching Elective Replacement Indicator or End of Service.1 Subsequently, the US Food and Drug Administration labeled this advisory as a Class I recall on May 13, 2021, but no additional patient management recommendations were made. Here we present 2 cases from different healthcare systems of patients with such devices who, despite remote monitoring, suffered unexpected complete loss of pacing via these devices, with symptoms requiring urgent device replacement.
Case report
Case 1
The first patient is an 81-year-old man who presented in 2017 with complete heart block. He underwent uncomplicated implantation of a St Jude Assurity PM2272 pacemaker. Additional past medical history includes persistent atrial fibrillation status post left atrial appendage closure, aortic valve disease in the setting of ischemic cardiomyopathy status post aortic valve replacement, and coronary artery bypass grafting. In September 2020, owing to recurrent noise episodes on his St Jude Tendril ventricular pacing lead with intermittent inhibition of pacing, a new right ventricular lead was placed and the old lead capped. He had no other issues with his pacemaker and was highly compliant with regular clinic and remote monitoring follow-up.
During his last in-office pacemaker interrogation in January 2021, there was ≈10 years of estimated battery life remaining (Figure 1A). He continued to be monitored via the Merlin remote monitoring system. In April 2021, he was scheduled to make a routine 3-month transmission. When this transmission was not received, he was contacted by our device team and Abbott technical services, who determined that his device was not transmitting. He was brought into our clinic, where we were unable to communicate with his device. His electrocardiogram demonstrated him to be in atrial fibrillation with a junctional escape at 40 beats/min (Figure 1B). He reported being fatigued and lightheaded but denied syncope over the preceding few weeks. He underwent generator change the same day, with prompt improvement in symptoms.
Figure 1.
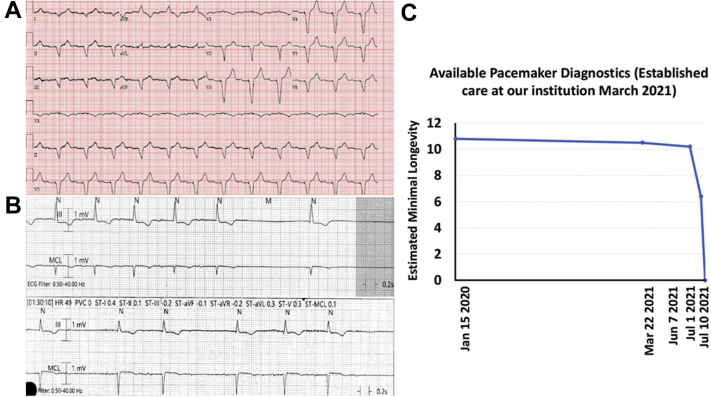
A: Device interrogation in January 2021 showing 95% predicted battery life and normal function on remote interrogation B: Electrocardiogram (ECG) on April 30, 2021. There was no communication with the device, which was at end of life. ECG showed atrial fibrillation with a junctional escape.
Case 2
The second patient is a 93-year-old who underwent uncomplicated implantation of a St. Jude Assurity PM2240 dual-chamber pacemaker in 2017 at an outside facility, in the setting of syncope, sinus node dysfunction, and intermittent second-degree heart block. Additional past cardiovascular history includes diabetes, hypertension, and hypercholesterolemia. Routine follow-up was reportedly uncomplicated until February 2021, at which point he established care with our system. The patient was seen in our clinic to transition care of his device to our system, including his remote management. At that time his electrocardiogram showed sinus rhythm with ventricular pacing (Figure 2A). In March 2021, the pacemaker’s battery life estimate was ≈10.5 years and in June specifically his battery voltage was 2.98 V with an estimated longevity of 10 years and 2 months. In June 2021, the patient was sent a letter notifying him of the advisory, recommending that all patients be enrolled in remote management, which he was, and to increase the frequency of remote transmissions.
Figure 2.
A: Baseline electrocardiogram of the patient in case 2 demonstrating sinus rhythm with right ventricular pacing. B: The presentation electrocardiogram of the patient in case 2 with pacemaker failure demonstrating sinus rhythm with first-degree atrioventricular (AV) block, sinus pauses, and intermittent second-degree AV block with failure to sense and pace the pacemaker. C: The available pacemaker diagnostics highlighting the abrupt battery failure occurring between July 1 and July 10 with lack of capture of the battery failure from July 1 to July 10 by remote monitoring.
In early July 2021 a remote transmission was urgently received owing to abrupt status change in his device, which had gone into a VVI backup mode. The patient was contacted and evaluated in clinic, at which time St. Jude technical services was contacted. No extrinsic trigger or interference was identified as a source for the backup mode. Under manufacturer recommendations, the device was reset to nominal settings and then reprogrammed to the prior settings. Underlying rhythm at this time was found to be sinus bradycardia, and lead parameters were stable and within normal limits. Of note, battery longevity at that time was significantly lower, at 5.7–6.4 years. No further Merlin remote monitoring transmissions were received from the device.
Ten days after that event, the patient presented to the emergency department with a 2-day history of lightheadedness, fatigue, and near-syncope. Electrocardiogram on presentation revealed marked sinus bradycardia with a heart rate of 46 beats/min, with intrinsic induction and no evidence of pacemaker activity. Telemetry during hospitalization revealed sinus pauses, first-degree AV block, and intermittent second-degree heart block with bradycardia (Figure 2B). On multiple attempts, the device could not be interrogated, consistent with the generator being at an end-of-life status. The patient underwent urgent pacemaker generator change, with return of normal pacing function and resolution of symptoms.
Discussion
These cases highlight several key points in the management of patients with cardiac rhythm management (CRM) devices under advisory/recall. First, they identify potential limitations in remote monitoring for the detection of CRM device–related failures. Specifically, in both cases communication between the pacemaker generator and Merlin communicator failed because of abrupt battery failure prior to loss of pacing, and as a result no alert was sent to the following physicians. Importantly, both patients had a history of compliance with remote monitoring protocols for their pacemakers. In these cases, both standard remote monitoring protocols and more frequent monitoring as suggested by the manufacturer were inadequate to identify a complete battery failure and could result in a serious adverse event and possibly death
Additionally, in the second case it appears an unexpected reversion to backup mode may have been an early warning sign to impending battery failure. Unfortunately, there were no data upon which to base this judgment at the time of patient presentation, and manufacturer recommendations at the time were followed to simply reprogram the device. Nevertheless, the battery longevity estimate was markedly diminished in a short period of time, and we believe based on this experience that such patients should undergo prompt generator change in the setting of unexpected behavior of an advisory device, particularly if there is any level of dependency on the device.
Finally, these cases emphasize potential conflicts between industry recommendations for managing CRM device advisories/recalls and patient safety. As noted above, the industry recommendation for managing this advisory/recall was reliance on remote monitoring, which, in these cases, failed. While the second case did transmit that the device was in a backup mode (∼6 years of estimated battery life), no further transmission was made with resumption of his baseline programing prior to battery failure. Furthermore, these cases and prior incidences of delayed reporting of CRM device malfunction by industry2,3 highlight the need for health systems, medical societies, and healthcare providers to provide important checks and balances in managing CRM device advisory/recall in order to keep patient safety the top priority.
Following the event described in case 1, Intermountain amended their systemwide notification response for this advisory/recall and requested that all pacemaker-dependent patients with an affected device be scheduled for an urgent in-person or remote video visit. During these visits, there was a shared-decision discussion regarding whether to perform prophylactic generator replacement or continued remote monitoring. Most pacemaker-dependent patients opted for generator replacement. Finally, at the largest hospital within the Intermountain system 46 of 169 patients that had received a pacemaker generator affected by this recall were identified as deceased. Manual chart review of these 46 patients identified 3 pacemaker-dependent patients who died from sudden cardiac death. Unfortunately, the status of the pacemaker generator in each of these 3 cases at the time of death is not known. However, given the outcomes in case 1 and 2 that were possible only because both patients were not pacemaker dependent, these sudden death episodes identified at Intermountain Medical Center are concerning and should be considered at minimum as possibly-probably associated with abrupt battery failure. It is also important to emphasize that both of our reported cases occurred within the same metropolitan area during a similar time window, and likely represent only a fraction of actual cases worldwide.
Key Teaching Points.
-
•
The US Food and Drug Administration advisory issued on March 15, 2021, and subsequently upgraded to a Class I recall affecting a subset of Abbott (Lake Forest, IL) pacemakers (models PM1152, PM1160, PM1172, PM1240, PM1272, PM2152, PM2160, PM2172, PM2240, PM2260, PM2272) with recommendations for close remote monitoring of implanted devices, may not detect all device failures.
-
•
Pacemakers affected by the recall may fail owing to a loss of radiofrequency transmitting capabilities and subsequent loss of pacing without forewarning.
-
•
Implanting physicians and practices who manage patients with these devices should be aware of this possible failure mode. Consideration should be given to close monitoring or to prophylactic generator change in appropriate patients.
Footnotes
Funding Sources: The authors have no funding sources to disclose.
Conflicts of Interest: R.F. is a member of the Medical Advisory Board of Abbott.
References
- 1.Abbott Medical Safety Notification: For a subset of AssurityTM and EndurityTM pacemakers. https://www.cardiovascular.abbott/content/dam/bss/divisionalsites/cv/pdf/reports/assurity-endurity-032021-DDL-US.pdf
- 2.Maron B.J., Harris K.M., Maron M.S. The Guidant Affair revisited...but this time with good news. Heart Rhythm. 2020;17:512–513. doi: 10.1016/j.hrthm.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 3.DOJ US St. Jude Agrees to Pay $27 Million for Allegedly Selling Defective Heart Devices. 2021. https://www.justice.gov/opa/pr/st-jude-agrees-pay-27-million-allegedly-selling-defective-heart-devices