Abstract
Objective
Neutralizing antibodies are among the factors used to measure an individual's immune status for the control of infectious diseases. We aimed to confirm the persistence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralizing antibody levels in patients who had recovered from coronavirus disease 2019 (COVID-19).
Methods
Plasma donors in South Korea who had completely recovered from SARS-CoV-2 infection had follow-up testing to determine the persistence of neutralizing antibodies using a plaque-reduction neutralization test and ELISA.
Results
Of the 111 participants—aged 20–29 years, 37/111 (33.3%); 30–39 years, 17/111 (15.3%); 40–49 years, 23/111 (20.7%); 50–59 years, 21/111 (18.9%); 60–65 years, 13/111 (11.7%); male, 43/111 (38.7%); female, 68/111 (61.3%)—66.1% still had neutralizing antibodies approximately 9 months (range 255–302 days) after confirmation of the diagnosis.
Conclusions
In this study we analysed the titre of neutralizing antibodies associated with predicting immune status in individuals with natural infection. Information about the persistence and change in levels of neutralizing antibodies against SARS-CoV-2 can be utilized to provide evidence for developing vaccination schedules for individuals with previous infection.
Keywords: COVID-19, ELISA, Neutralizing antibody, Plaque-reduction neutralizing test, SARS-CoV-2
Introduction
It is important to confirm the change in antibody levels and the persistence of neutralizing antibodies in individuals who recover from natural infections in order to determine their infection status, predict prevention of reinfection, and establish vaccination policies in the context of a pandemic [1,2]. In this study we aimed to confirm the development and maintenance of neutralizing antibodies in South Korean patients who had had coronavirus disease 2019 (COVID-19) during the early phase of the pandemic and had recovered completely.
Methods
Blood collection
Blood samples were collected from healthy individuals who had fully recovered from COVID-19 approximately 3 months (140 days), 6 months (181 days), and 9 months (271 days) after the confirmation of COVID-19 in February or March 2020. The participants were aged ≥19 years, lived in South Korea, and had agreed to become plasma donors. The participants were recruited through the plasma donation recruitment notice and consented for their plasma specimens to be used for research.
Plaque-reduction neutralization tests (PRNT) and enzyme-linked immunosorbent assay (ELISA)
PRNTs were performed as previously described [[3], [4], [5]] using severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (clade S; hCoV-19/South Korea/KCDC03/2020, EPI_ISL_407,193) obtained from the National Culture Collection for Pathogens in South Korea. PRNT titres ≥1:20 were considered positive for SARS-CoV-2 neutralizing antibodies [6]. Neutralizing antibodies (nAbs) were also tested using the SARS-CoV-2 Surrogate Virus Neutralization Test (sVNT) Kit (GeneScript), and total antibodies (IgG, IgM and IgA) were measured using the STANDARD E COVID-19 Total Ab Kit (SD Corporation). The nAb ELISA used a competitive ELISA detection method involving protein–protein interaction between human angiotensin-converting enzyme 2 (ACE2) receptors attached to the surface of the plate in competition with a SARS-CoV-2 receptor binding domain fragment conjugated with horseradish peroxidase and neutralizing antibodies in plasma samples. The recombinant COVID-19 antigens, containing nucleocapsids and spike proteins, were used to detect IgM/IgA/IgG antibodies. These tests, including the ELISA, were performed according to the manufacturer's protocol [7].
Analysis of neutralizing antibody responses pattern
The nAb response and duration patterns were analysed as previously described with minor modification [8]. Briefly, the nAb response patterns were classified into three patterns: (a) negative, cases in which nAb titres remained undetected 3 months after infection and the measurable nAb PRNT titres were <1:20 within 9 months, (b) waning, cases in which nAb titres were present 3 months after diagnosis, but decreased by more than 30% or to < 1:20 within 6–9 months, and (c) persistent, cases in which nAb titres were maintained for 9 months with minimal reduction and continued to increase after infection.
Statistical analysis
Statistical comparisons of the data were performed using the one-way analysis of variance. All analyses were performed using PRISM (GraphPad) software; p values < 0.05 were considered statistically significant.
Ethics approval
The study was approved by the Korea Disease Control and Prevention Agency Institutional Review Board (IRB No. 2021-06-01-P-A). Participants provided written informed consent.
Results
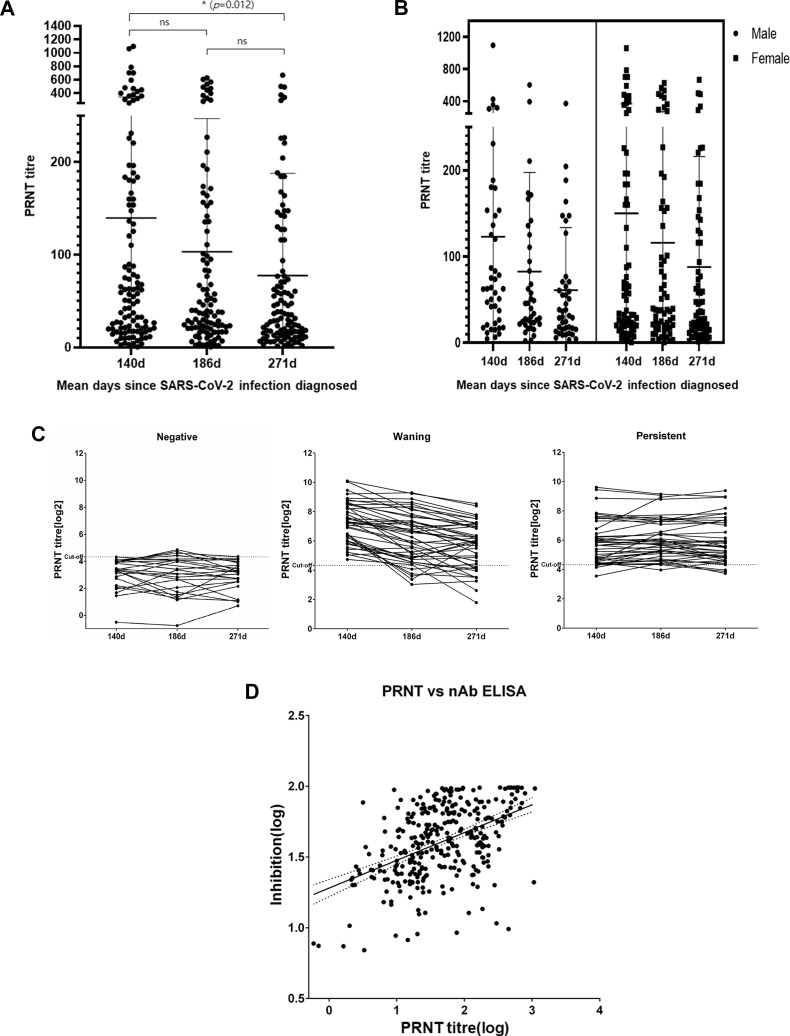
The neutralizing antibody responses of 111 participants aged 20–65 years—20–29 years, 37 (33.3%); 30–39 years, 17 (15.3%); 40–49 years, 23 (20.7%); 50–59 years, 21 (18.9%); 60–65 years, 13 (11.7%)—of whom 43 (39%) were male and 68 (61%) were female, were analysed. The mean times (range) of blood sample collection were 140 (117–161), 187 (173–211), and 271 (255–302) days after the confirmation of a COVID-19 diagnosis. According to the PRNT results, the proportion of participants who maintained neutralizing antibodies was 76.6% (85/111), 76.5% (78/102) and 66.1% (72/109) at 140 days, 187 days and 271 days, respectively, after their COVID-19 diagnosis. Neutralizing antibody levels were generally maintained for between 3 and 6 months and started to decrease significantly thereafter. Of the participants, 39.6% (44/111) had a waning pattern, 38.7% (43/111) had a persistent pattern, and 21.6% (24/111) had a negative pattern (Fig. 1 C and Supplementary Material Table S1). The nAb responses did not differ significantly according to gender (Fig. 1A and B and Table 1 ). According to the nAb ELISA results, the proportion of participants with a sustained nAb response decreased significantly with time: 73.9%, 64.7%, and 64.2%, on days 140, 187, and 271, respectively. In addition, the neutralizing antibody levels of younger (20–39 years), middle-aged (40–59 years) and older (60–65 years) participants decreased by 49.7%, 37.4%, and 37.4%, respectively, 3 and 9 months after their COVID-19 diagnosis. The rate of decrease did not differ significantly by age (Supplementary Material Fig. S1 and Table S2). The Spearman correlation (r value) of the total antibodies measured using the PRNT method and the nAb using ELISA was 0.450 (Fig. 1D). In contrast to the nAb, the total antibodies were maintained for 9 months in 93.6% (102/111) of participants (Supplementary Material Table S3). None of the participants tested positive for SARS-CoV-2 on PCR testing at the time of each plasma donation.
Fig. 1.
Neutralizing antibody responses against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). (A) Levels of neutralization antibodies in all participants, (B) antibody levels by gender, and (C) antibody levels by response pattern. (D) Correlation between the neutralizing antibody enzyme-linked immunosorbent assay (nAb ELISA) and plaque-reduction neutralization test (PRNT) results. ∗p < 0.05, statistically significant difference; ns, not significant.
Table 1.
Neutralizing antibody response (plaque-reduction neutralization test (PRNT50) and ELISA) in patients with coronavirus disease 2019 (COVID-19) according to time from diagnosis
| 140 d |
187 d |
271 da |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Male (n = 43) | Female (n = 68) | Total (n = 111) | Male (n = 39) | Female (n = 63) | Total (n = 102) | Male (n = 43) | Female (n = 66) | Total (n = 109) | |
| PRNT titre | |||||||||
| 40 | 4 (9.3%) | 16 (23.5%) | 20 (18.0%) | 12 (30.8%) | 16 (25.4%) | 28 (27.5%) | 9 (20.9%) | 13 (19.7%) | 22 (20.2%) |
| 80 | 13 (30.2%) | 9 (13.2%) | 22 (19.8%) | 8 (20.5%) | 7 (11.1%) | 15 (14.7%) | 11 (25.6%) | 11 (16.7%) | 22 (20.2%) |
| 160 | 8 (18.6%) | 4 (5.9%) | 12 (10.8%) | 6 (15.4%) | 9 (14.3%) | 15 (14.7%) | 4 (9.3%) | 9 (13.6%) | 13 (11.9%) |
| 320 | 5 (11.6%) | 11 (16.2%) | 16 (14.4%) | 4 (10.2%) | 6 (9.5%) | 10 (9.8%) | 3 (7.0%) | 7 (10.6%) | 10 (9.2%) |
| 640 | 3 (7.0%) | 7 (10.3%) | 10 (9.0%) | 2 (5.1%) | 7 (11.1%) | 9 (8.8%) | 1 (2.3%) | 3 (4.5%) | 4 (3.7%) |
| 1280 | 1 (2.3%) | 4 (5.9%) | 5 (4.5%) | 0 (0.0%) | 1 (1.6%) | 1 (0.9%) | 0 (0.0%) | 1 (1.5%) | 1 (0.9%) |
| Negative (<20) Positive |
9 (20.9%) 34 (79.1%) |
17 (25.0%) 51 (75.0%) |
26 (23.4%) 85 (76.6%) |
7 (17.9%) 32 (82.1%) |
17 (27.0%) 46 (73.0%) |
24 (23.5%) 78 (76.5%) |
15 (34.9%) 28 (65.1%) |
22 (33.3%) 44 (66.7%) |
37 (33.9%) 72 (66.1%) |
|
140 d |
187 da |
271 da |
|||||||
| nAb on ELISA | |||||||||
| Negative | 10 (23.3%) | 19 (27.9%) | 29 (26.1%) | 16 (41.0%) | 20 (31.7%) | 36 (35.3%) | 17 (39.5%) | 22 (33.3%) | 39 (35.8%) |
| Positive | 33 (76.7%) | 49 (72.1%) | 82 (73.9%) | 23 (59.0%) | 43 (68.3%) | 66 (64.7%) | 26 (60.5%) | 44 (66.7%) | 70 (64.2%) |
ELISA, enzyme-linked immunosorbent assay; nAb, neutralizing antibody.
p < 0.05 compared to mean of 140 d.
Discussion
Neutralizing antibodies are important to confirm the immunity of individuals and to establish public health policies regarding vaccination. According to the PRNT results in this study, 76.5% of individuals had neutralizing antibodies 6 months after diagnosis of SARS-CoV-2 infection, and this decreased to 66.1% after 9 months. Previous studies have found that neutralizing antibodies are maintained for 6–8 months following SARS-CoV-2 infection [9,10]. Many studies have also shown nAb measured using ELISA (which may replace the PRNT method even though it has lower sensitivity than the PRNT method). In this study, 63.4% of the blood donors had sustained nAb responses 9 months after infection detected using ELISA. The difference in the sensitivity of the ELISA and PRNT methods for detecting SARS-CoV-2 nAb may be attributable to difference in the detection system. Generally, nAb ELISA for SARS-CoV-2 targets the protein of human ACE2 receptors for binding neutralizing antibodies in plasma, whereas PRNT uses living cells with ACE receptors and other factors. Furthermore, recent studies have reported that coreceptors or cofactors may influence SARS-CoV-2 infection [[11], [12], [13]]. These factors may also have contributed to the difference in the results of nAb testing between PRNT and nAb ELISA.
It may be useful to evaluate the duration of neutralizing antibodies using nAb ELISA using a large number of samples during the pandemic period. However, comparison of nAb titres, positivity rates, and changes in titres over time after infection measured using the two methods must be interpreted carefully, considering the differences between the PRNT and nAb ELISA methods and their limitations.
Currently, COVID-19 vaccines are being rolled out in stages in many countries, including South Korea. The analysis of neutralizing antibodies against SARS-CoV-2 in this study could inform public health policies against COVID-19, including the need for COVID-19 vaccination in individuals with a history of COVID-19 due to natural SARS-CoV-2 infection.
This study has some limitations. First, the information on the clinical features of COVID-19 (including disease severity) in the participants could not be obtained from the medical institutions in which the blood was collected. Second, the main purpose of this study was to compare the results of antibodies measured using the PRNT and ELISA methods. However, a reference standard for serological methods of antibody measurement could not be defined between the two analysis methods. For more detailed information, long-term follow-up studies should be conducted to confirm the duration of nAb levels. Moreover, further research should be conducted to determine the mechanisms for the different patterns of development and maintenance of nAb levels.
Author contributions
SMS, JWK, JSY, KCK and JYL were involved in the design of this study. JWK, SJ, YJ and HMW performed the experiments. SMS, WJK, JSY and KCK assembled the data. SMS, JWK, JSY, KCK and JYL were involved in writing and all authors approved the manuscript.
Transparency declaration
The authors declare that they have no conflict of interest regarding the publication of this research note. This study was supported by intramural funds (2019-NG-044-02 and 2020-NI-039-00) from the Korea National Institute of Health.
Acknowledgements
The authors would like to thank the blood donors and Dae Seong Kim (Korean Red Cross) for organizing the collection of blood samples.
Editor: M. Cevik
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2021.12.012.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Perera R.A., Mok C.K., Tsang O.T., Lv H., Ko R.L., Wu N.C., et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). March 2020. Euro Surveill. 2020;25:2000421. doi: 10.2807/1560-7917.ES.2020.25.16.2000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeewandara C., Jayathilaka D., Gomes L., Wijewickrama A., Narangoda E., Idampitiya D., et al. SARS-CoV-2 neutralizing antibodies in patients with varying severity of acute COVID-19 illness. Sci Rep. 2021;11:2062. doi: 10.1038/s41598-021-81629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padoan A., Bonfante F., Pagliari M., Bortolami A., Negrini D., Zuin S., et al. Analytical and clinical performances of five immunoassays for the detection of SARS-CoV-2 antibodies in comparison with neutralization activity. EBiomedicine. 2020;62:103101. doi: 10.1016/j.ebiom.2020.103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okba N.M.A., Müller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., et al. Severe acute respiratory syndrome coronavirus 2-specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muruato A.E., Fontes-Garfias C.R., Ren P., Garcia-Blanco M.A., Menachery V.D., Xie X., et al. A high-throughput neutralizing antibody assay for COVID-19 diagnosis and vaccine evaluation. Nat Commun. 2020;11:1–6. doi: 10.1038/s41467-020-17892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valcourt E.J., Manguiat K., Robinson A., Chen J.C., Dimitrova K., Philipson C., et al. Evaluation of a commercially-available surrogate virus neutralization test for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) Diagn Microbiol Infect Dis. 2020;99:115294. doi: 10.1016/j.diagmicrobio.2020.115294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan C.W., Chia W.N., Qin X., Liu P., Chen M.I., Tiu C., et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotech. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 8.Chia W.N., Zhu F., Ong S.W.X., Young B.E., Fong S.W., Le Bert N., et al. Dynamics of SARS-CoV-2 neutralizing antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2:e240–e249. doi: 10.1016/S2666-5247(21)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.L’Huillier A.G., Meyer B., Andrey D.O., Arm-Vernez I., Baggio S., Didierlaurent A., et al. Antibody persistence in the first 6 months following SARS-CoV-2 infection among hospital workers: a prospective longitudinal study. Clin Microbiol Infect. 2021;27:784. doi: 10.1016/j.cmi.2021.01.005. e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choe P.G., Kim K.H., Kang C.K., Suh H.J., Kang E.K., Lee S.Y., et al. Antibody responses 8 months after asymptomatic or mild SARS-CoV-2 infection. Emerg Infect Dis. 2021;27:928. doi: 10.3201/eid2703.204543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clausen T.M., Sandoval D.R., Spliid C.B., Pihl J., Perrett H.R., Painter C.D., et al. SARS-CoV-2 infection depends on cellular heparin sulfate and ACE2. Cell. 2020;183:1043–1057. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang K., Chen W., Zhang Z., Deng Y., Lian J.Q., Du P., et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5:283. doi: 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Føns S., Krogfelt K.A. How can we interpret SARS-CoV-2 antibody test results? Pathog Dis. 2021;79 doi: 10.1093/femspd/ftaa069. ftaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.