Abstract
Current nucleoside-modified RNA lipid nanoparticle (modmRNA-LNP) technology has successfully paved the way for the highest clinical efficacy data from next-generation vaccinations against SARS-CoV-2 during the COVID-19 pandemic. However, such modmRNA-LNP technology has not been characterized in common pre-existing inflammatory or immune-challenged conditions, raising the risk of adverse clinical effects when administering modmRNA-LNPs in such cases. Herein, we induce an acute-inflammation model in mice with lipopolysaccharide (LPS) intratracheally (IT), 1 mg kg−1, or intravenously (IV), 2 mg kg−1, and then IV administer modmRNA-LNP, 0.32 mg kg−1, after 4 h, and screen for inflammatory markers, such as pro-inflammatory cytokines. ModmRNA-LNP at this dose caused no significant elevation of cytokine levels in naive mice. In contrast, shortly after LPS immune stimulation, modmRNA-LNP enhanced inflammatory cytokine responses, Interleukin-6 (IL-6) in serum and Macrophage Inflammatory Protein 2 (MIP-2) in liver significantly. Our report identifies this phenomenon as inflammation exacerbation (IE), which was proven to be specific to the LNP, acting independent of mRNA cargo, and was demonstrated to be time- and dose-dependent. Macrophage depletion as well as TLR3 −/− and TLR4−/− knockout mouse studies revealed macrophages were the immune cells involved or responsible for IE. Finally, we show that pretreatment with anti-inflammatory drugs, such as corticosteroids, can partially alleviate IE response in mice. Our findings characterize the importance of LNP-mediated IE phenomena in gram negative bacterial inflammation, however, the generalizability of modmRNA-LNP in other forms of chronic or acute inflammatory and immune contexts needs to be addressed.
Keywords: mRNA, Lipid nanoparticle, Inflammation, Toxicity, Nanoparticle, Adverse effect
Graphical abstract
1. Introduction
Lipid nanoparticles (LNPs) are considered among the most advanced systems for delivering nucleic acids, such as mRNA, siRNA, and miRNA. After decades of development, the first LNP-nucleic acid therapeutic, ONPATTRO® (Patisiran; Alnylam, Inc.), was approved in August 2018 to deliver siRNA for a rare genetic disorder [1]. Therapeutic protein and immunoglobulin encoding mRNAs have been evaluated in preclinical and clinical trials for a vast number of diseases including both inflammatory and non-inflammatory conditions [[2], [3], [4], [5]]. We developed modmRNA as a means to introduce therapeutic proteins in vivo [[6], [7], [8], [9]]. Most importantly, the modmRNA-LNP vaccine platform is the basis for the first two Emergency Use Authorization (EUA) COVID-19 vaccines [10,11].
Lipid nanoparticles are a versatile class of synthetic nanocarriers, consisting generally of a mixture of phospholipid, sterol, PEGylated lipid, and ionizable lipid that become charged in the low endosomal pH. Compared to conventional cationic liposomes, LNPs are considered as relatively safe carriers for mRNA delivery supported by comprehensive studies [12]. However, the drug carrier properties of LNP still have the risk of inducing side effects, and this topic has received less attention in the literature. Therefore, it is necessary to define their safety and therapeutic efficacy under defined physiological conditions to be able to characterize their therapeutic margin for specific clinical indications more accurately. Previously, we investigated the efficacy of modmRNA-LNP in naive animals [9]. LNP delivery requires mechanisms to enable the nucleic acid cargo to enter the cell and escape from intracellular endosomes, thus potentially eliciting innate immune pathways [13]. While such immune activation could be a desirable advantage for vaccination [14,15], in other indications it can be detrimental to treatment success and predispose patients to unexpected adverse reactions. Thus, understanding the impact of modmRNA-LNP on innate immune activation or in the setting of ongoing immune activation in diseased states can be greatly beneficial towards clinical implementation of these therapeutics.
To bridge this gap, here we studied the effects of modmRNA-LNP in healthy mice vs. mice experiencing lipopolysaccharide (LPS) induced inflammatory responses. Although fairly benign in the healthy state, LNP potentiated existing inflammation in mice that had received the bacterial cell wall component LPS intratracheally (IT) or intravenously (IV). This is the first indication of this phenomenon of inflammation-exacerbation (IE) by LNP. We investigated the IE phenomenon mechanistically, and compared LNP to other nanoparticulate delivery systems. Finally, we have characterized potential auxiliary agents attenuating this phenomenon, and protocols to minimize IE exacerbation following delivery of modmRNA-LNP. Considering the possibility of LNP being used in humans with ongoing inflammation due to a variety of conditions and diseases, this study delineates a potential risk and how to moderate it.
2. Materials and methods
2.1. Ethics statement
The investigators faithfully adhered to the Guide for the Care and Use of Laboratory Animals by the Committee on Care of Laboratory Animal Resources Commission on Life Sciences, National Research Council.
2.2. Mice
Mouse studies were conducted under protocols approved by the University of Pennsylvania (UPenn) IACUCs. The animal facilities at the University of Pennsylvania are fully accredited by the American Association for Assessment and Accreditation of Laboratory Animal Care.
C57BL/6 J mice. C57BL/6 J mice were purchased from Jackson laboratories.
B6(Cg)-Tlr4tm1.2Karp/J mice (Tlr4 KO). Tlr4 −/− (Tlr4KO) on C57BL/6 background were purchased from Jackson laboratories. Tlr4 KO mice lack exon 3 of the toll-like receptor 4 (Tlr4) gene.
B6;129S1-Tlr3tm1Flv/J mice (Tlr3 KO). Tlr3 −/− (Tlr4KO) on C57BL/6 background were purchased from Jackson laboratories. The toll-like receptor 3 (Tlr3) gene was disrupted by replacement of exon 1 with a floxed neo cassette via homologous recombination.
2.3. mRNA
mRNAs were produced using T7 RNA polymerase (Megascript, Ambion) on linearized plasmids. mRNAs were transcribed to contain 101 nucleotide-long poly(A) tails. m1Ψ-5′-triphosphate (TriLink) instead of UTP was used to generate modified nucleoside-containing mRNA. Capping of the in vitro transcribed mRNAs was performed co-transcriptionally using the trinucleotide cap1 analog, CleanCap (TriLink). mRNA was purified by cellulose purification, as described. [16] All mRNAs were analyzed by native agarose gel electrophoresis and were stored frozen at −20 °C.
2.4. Preparation of modmRNA-LNP formulations
m1ψ-containing mRNAs were encapsulated in LNP using a self-assembly process in which an aqueous solution of mRNA at pH=4 is rapidly mixed with a solution of lipids dissolved in ethanol [17]. Unless stated otherwise, LNP used in this study was similar in composition to those described previously [17,18], which contain an ionizable cationic lipid (proprietary to Acuitas), phosphatidylcholine, cholesterol, and PEG-lipid. The diameter of the nanoparticles was ~80 nm as measured by dynamic light scattering using a Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, UK) instrument. modmRNA-LNP formulations were stored at −80 °C at a concentration of mRNA of ~1 μg μL-1.
DLin-MC3-DMA-LNP and C12–200-LNP was manufactured by Genevant Sciences using a controlled mixing process (US 9,005,654) in which an aqueous solution of acetate buffer at pH=5 was combined with an ethanolic solution of lipids, containing DLin-MC3-DMA or C12–200, 1,2-distearoyl-sn-glycero-3-phosphocholine, cholesterol, and a PEG-conjugated lipid at a molar ratio of 50:10:38.5:1.5. Ethanol was removed by tangential flow ultrafiltration, followed by buffer exchange and concentration. The formulation was adjusted to 20 mg mL−1 total lipid and sterile filtered through a 0.2 μm polyethersulfone membrane prior to storage at -80 °C. The diameter of the nanoparticles was ~80 nm as measured by dynamic light scattering using a Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, UK) instrument.
LNP without ionizable lipids were prepared in-house. Cholesterol (Sigma-Aldrich), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC, Avanti, Alabaster, AL), and 1,2-dimyristoyl-snglycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (ammonium salt) (C14-PEG2000, Avanti) were combined into an ethanol phase at molar ratios of 77:20:3, respectively, in a total volume of 112.5 μL. A separate aqueous phase was prepared containing 25 μg luciferase mRNA and 10 mM citrate buffer at pH=3 in a total volume of 337.5 μL. The lipid and mRNA phases were combined using a microfluidic device and syringe pump as described previously [19]. The LNP solution was dialyzed against PBS for 2 h and then concentrated using 2 mL Amicon centrifugal filters with 50,000 MWCO (Millipore Sigma) at 800 x g for 20 min. Lastly, LNP was sterile filtered through syringe filters with 0.2 μm pores and the mRNA concentration was determined using the A260 measurement by Nanodrop. LNP was stored at 4 °C until use. All materials and reagents were RNase free and were handled using RNase-free technique throughout the formulation and characterization steps.
2.5. Monoclonal antibody-conjugated lipid nanoparticles
LNP was conjugated with mAbs specific for platelet endothelial cell adhesion molecule-1 (PECAM-1) or plasmalemma vesicle-associated protein (PLVAP). Anti-mouse PLVAP antibody was produced in-house from hybridoma secreting rat anti-mouse PLVAP IgG2a, clone MECA32, which was obtained from the Developmental Studies Hybridoma Bank (developed under the auspices of the NICHD and maintained at the University of Iowa, Department of Biology). Anti-mouse-PECAM-1/CD31 antibody (clone MEC13, BioLegend, San Diego, CA), anti-mouse PLVAP antibody, or control isotype-matched IgG were coupled to LNP via SATA–maleimide conjugation chemistry, as described previously [9]. Briefly, LNP was modified with DSPE-PEG-maleimide by a post-insertion technique. The antibody was modified with N-succinimidyl S-acetylthioacetate (SATA) (Sigma-Aldrich) to introduce sulfhydryl groups allowing conjugation to maleimide. SATA was deprotected using 0.05 M hydroxylamine followed by removal of the unreacted components by G-25 Sephadex Quick Spin Protein columns (Roche Applied Science, Indianapolis, IN). The reactive sulfhydryl group on the antibody was then conjugated to maleimide moieties using thioether conjugation chemistry. Purification was performed using Sepharose CL-4B gel filtration columns (Sigma-Aldrich). mRNA content was calculated by performing a modified Quant-iT RiboGreen RNA assay (Invitrogen).
2.6. Other nanoparticulate formulations preparation
Liposomes were prepared by thin film hydration method described previously [20]. Liposomes were composed of 58% Dipalmitoylphosphatidylcholine (DPPC), 40% Cholesterol, and 2% 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000] (DSPE-PEG (2 k)).
Nanogels (NGs) were prepared as previously reported [21]. Briefly, the solution of Rhodamine B isothiocyanate–dextran and lysozyme in water (1:1 M ratio, pH = 7) were lyophilized. The lyophilized powder was then allowed to react at 60 °C under 79% relative humidity in a desiccator containing saturated KBr solution for 18 to 24 h. The final powder was dissolved in water (5 mg mL−1, based on dextran and lysozyme together), the pH was adjusted to 10.7 using 0.1 N sodium hydroxide, and the solution was further reacted at 80 °C for 30 min.
AAV empty viral capsids were provided by Junwei Sun of University of Pennsylvania's Center for Advanced Retinal and Ocular Therapeutics (CAROT).
2.7. LPS-mediated acute systemic inflammation model in mice
Lipopolysaccharide (LPS) from E. coli O111:B4 (Cat# L2630, Sigma-Aldrich) at 0.2 or 2 mg kg−1 was injected IV (retro-orbital intravenous injection) into adult C57BL/6, Tlr4 KO, or Tlr3 KO mice, 6–8 week old (The Jackson Laboratory, Bar Harbor, ME). Four hours after the LPS challenge, either unconjugated or endothelial-targeted modmRNA-LNP (0.032 or 0.32 mg-mRNA kg−1) were injected IV. Four hours later, blood, liver, and spleen were harvested and cytokine measurement was performed on the serum and tissue homogenate.
In studies to deplete the macrophages from liver and spleen, clodronate-liposome at 50 mg kg−1 was injected IV 48 h before the modmRNA-LNP administration.
In treatment groups, Dexamethasone-21-Phosphate (0.5 mg mL−1 in PBS) was injected twice either subcutaneously (SC) or intraperitoneally (IP) as 2 mg kg−1 into the mice, one hour after LPS injection and immediately after modmRNA-LNP administration.
In the IE kinetic model, mice were first administered with either LPS or modmRNA-LNP, then respectively treated with modmRNA-LNP or LPS at t = 0 h, t = 4 h, t = 1 h, or t = 24 h.
2.8. LPS-mediated acute lung inflammation model in mice
Adult C57BL/6 mice, 6–8 week old were anesthetized with an intraperitoneal injection of ketamine (75 mg kg−1) and xylazine (1.5 mg kg−1) followed by endotracheal intubation with a 20-gauge angiocatheter. A PE-10 catheter (outer diameter 0.024″) was inserted and the Lipopolysaccharide (LPS) from E. coli O111:B4 (1 mg kg−1) was instilled [22]. Four hours after the LPS challenge, unconjugated modmRNA-LNP or endothelial-targeted modmRNA-LNP (0.32 mg-mRNA kg−1) were injected IV. Four hours later, protein and leukocyte content were analyzed in Bronchoalveolar lavage (BAL).
2.9. Oleic acid-mediated acute lung inflammation model in mice
Oleic acid (OA) intravenous injection models key aspects of acute respiratory distress syndrome (ARDS) caused by trauma, in which bone fractures release fat emboli, which lodge in the pulmonary vasculature, where they induce local inflammation and pulmonary edema [23]. OA (Sigma, Cat. 75,090–5 mL, Analytic standard, liquid form) was mixed with 50 μL of sterile 0.1% BSA in PBS, at an OA dosage of 0.3 mL kg−1 mouse body weight. Mice were anesthetized with 3% isoflurane for ~3 min and intravenously injected via the retro-orbital route. Four hours after OA injection, mice were IV-injected with modmRNA-LNPs, then sacrificed for analysis of luciferase expression and bronchoalveolar lavage at either 8 or 24 h after the initial OA IV injection.
2.10. BAL analysis
BAL samples were first centrifuged for 6 min at 350g and protein concentration was measured in the supernatant by the DC Protein Assay (Bio-Rad, Hercules, CA). The spinned cells were then washed with PBS 1× and dispersed in FACS buffer (PBS 1× containing 0.5% FBS) as single-cell suspensions. The samples were stained with anti-CD45-APC (Cat# 103111, BioLegend) to analyze the leukocyte content by flow cytometry (Accuri C6 instrument, Becton Dickinson, San Jose, CA).
2.11. TNF-α-mediated acute brain inflammation model in mice
Intrastriatal (i.s.) TNF injection was performed [24]. Briefly, mice were anesthetized using an IP administration of ketamine/xylazine and placed in a rodent stereotaxic frame (David Kopf Instruments, Tujunga, CA, USA). A total 2.5 μL of 200 μg/mL mouse-recombinant TNF (R&D systems, Minneapolis, MN, USA) in PBS was administered by i.s. injection using a 10-μL Nanofil microsyringe over a 3-min period. Control animals did not receive any surgical procedure to avoid any induced inflammation. 17 h after the injury, endothelial-targeted modmRNA-LNP (here VCAM1-targeted modmRNA-LNP, 0.32 mg-mRNA kg−1) were injected IV. Four hours later, blood, liver, and brain were harvested and cytokine measurement was performed on serum and tissue homogenates.
2.12. Cytokine measurements following intravenous administration of modmRNA-LNP in naive and inflamed mouse models
Cytokine measurements in serum and tissue homogenates were performed as previously described [9]. Briefly, at 4 h post- modmRNA-LNP treatment either in naive or inflamed mouse models, animals were euthanized and blood samples were collected in heparin and spun at 1500 ×g for 10 min at 4 °C. Collected tissues such as livers, spleens, or brains were homogenized in PBS containing protease inhibitor cocktail (1×). Lysis buffer was added to the homogenates followed by incubation at 4 °C for one hour. The cell lysate was centrifuged for 10 min at 16,000g at 4 °C. The levels of Macrophage Inflammatory Protein 2 (MIP-2) cytokine in liver or brain, and Interleukin 6 (IL-6) in plasma or brain were assessed by ELISA according to manufacturer's protocol (DuoSet ELISA kits, R and D systems, Minneapolis, MN). The LEGENDplex Mouse Inflammation Panel (Biolegend) was also used for the preliminary screening. Measurement was performed on an Accuri C6 flow cytometer (Becton Dickinson, Franklin Lakes, NJ) using LEGENDplex v.7.0 software for data analysis.
2.13. Single cell analysis upon modmRNA-LNP administration using flow cytometry
To analyze cellular distribution of modmRNA-LNP within the lungs, in wild type and LPS-treated mice, mRNA translation was tracked with single-cell resolution using flow cytometry. DIO-tagged LNP were conjugated to antibodies, as described above. Four hours after LPS instillation at 1 mg kg−1, PECAM-targeted fluorescent LNP were IV injected in mice at 0.32 mg kg−1. After 30 min, the lungs were perfused and harvested. The lungs were first digested and cell suspensions were passed through 100 μm nylon strainer. To lyse RBCs, ACK lysis buffer (QualityBiological, Gaithersburg, MD) was used. Anti-CD31, anti-CD45, and anti-F4/80 were used for staining endothelial cells, leukocytes, and macrophages/monocytes, respectively. Flow cytometry was performed on Accuri C6 instrument (Becton Dickinson, San Jose, CA).
2.14. Luciferase modmRNA translation in vivo, bioluminescence imaging
At 4 h post- modmRNA-LNP treatment either in naive or above-mentioned inflamed mice models, bioluminescence imaging was performed as described previously [9] using an IVIS Spectrum imaging system (Caliper Life Sciences, Waltham, MA). Briefly, D-luciferin was administered to mice intraperitoneally at a dose of 150 mg kg−1. After 5 min, the mice were euthanized; spleens and livers were harvested, and immediately placed on the imaging platform. Tissue luminescence was measured on the IVIS imaging system using an exposure time of 5 s or longer to ensure that the signal obtained was within operative detection range. Bioluminescence values were also quantified by measuring photon flux (photons/s) in the region of interest using LivingImage software provided by Caliper.
2.15. Real-time quantitative RT-PCR (qRT-PCR)
qRT-PCR was used to quantitate the effect of unconjugated modmRNA-LNP on the expression of MIP-2, TLR-3 and TLR-4 proteins in livers and spleens from either naive or inflamed mouse models. Total RNA was extracted from tissues using Trizol reagent (Life Technologies) according to the manufacturer's instructions. Consequently, cDNA was synthesized using the reverse transcriptase kit from Promega (Promega Inc., Madison WI) as per manufacturer's protocol. Quantitative real-time PCR was conducted on a Bio-Rad CFX 9000 qPCR instrument using RT2 SYBR Green reagent (QIAGEN, Hilden, Germany).
3. Results
3.1. modmRNA-LNP exacerbates LPS-mediated inflammation in mice
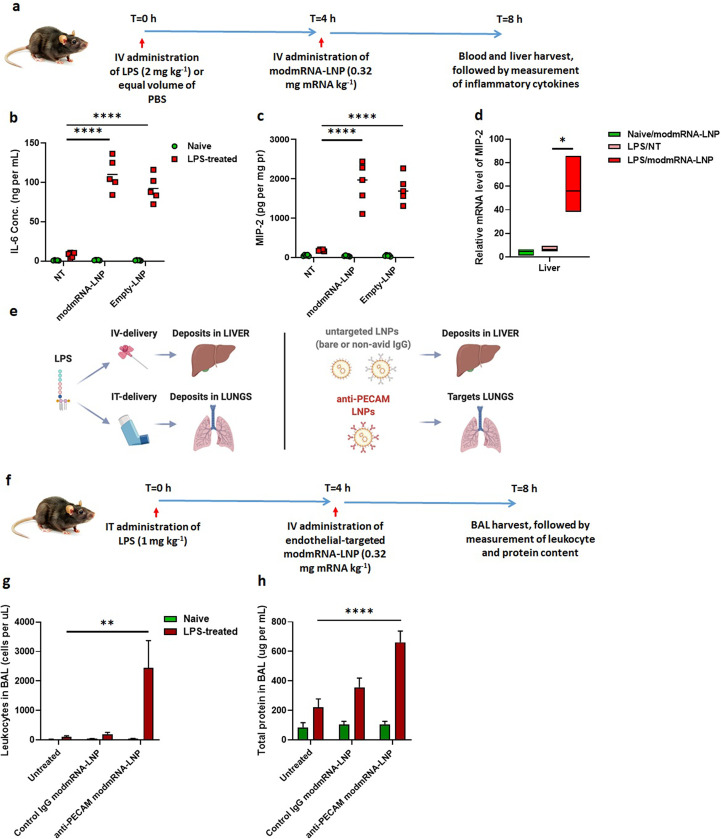
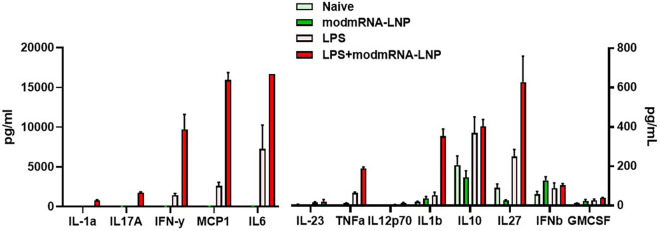
To investigate the effect of modmRNA-LNP on a pre-existing inflammatory condition, C57BL/6 J mice were first treated with either LPS (2 mg kg−1) or an equal volume of 1× PBS via retro-orbital IV injection (t = 0 h) (Fig. 1a). Four hours later (t = 4 h), the animals were IV injected (retro-orbital administration) with modmRNA-LNP (0.32 mg-mRNA kg−1) or equivalent lipid mass of empty-LNP, which contain an ionizable cationic lipid (proprietary to Acuitas), phosphatidylcholine, cholesterol, and PEG-lipid. Blood and liver were harvested four hours after modmRNA-LNP treatment (T = 8 h) and pro-inflammatory cytokines, interleukin 6 (IL-6) and macrophage inflammatory protein 2 (MIP-2), were quantified by ELISA. The dose combination of LPS and modmRNA-LNP was chosen based on the initial dose screening study (Supplementary Fig. 1). The cytokines were based on our cytokine screening study (Supplementary Fig. 2) and those already suggested in the literature to be representative pro-inflammatory cytokines [25,26]. All the modmRNA-LNP tested were endotoxin-free.
Fig. 1.
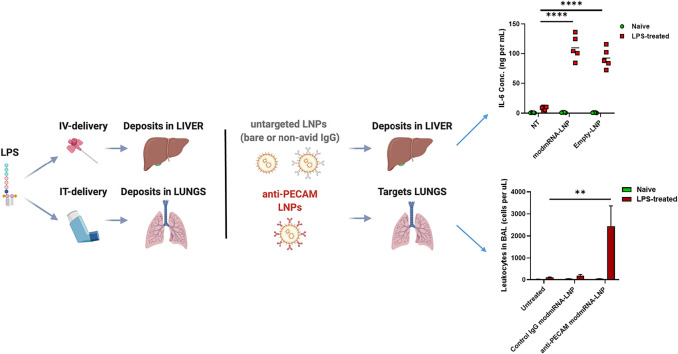
Inflammation exacerbation with sequential application of modmRNA-LNP and LPS. (a) Experimental time-line for the inflammation exacerbation (IE) study in IV-LPS model. Pro-inflammatory cytokines of interleukin-6 (IL-6) in serum (b) and macrophage inflammatory protein (MIP-2) in liver homogenate (c) upon treatment with LPS (2 mg kg−1) followed by modmRNA-LNP (0.32 mg-mRNA kg−1or equivalent lipid mass of empty-LNP) were compared to the mice treated with LPS only (2 mg kg−1) or modmRNA-LNP only. In (b) and (c), each symbol represents one animal and horizontal lines show the mean. (d) Relative expression levels of MIP-2 normalized to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were compared in liver tissue homogenates from mice treated with modmRNA-LNP, LPS only, or LPS/modmRNA-LNP by Quantitative PCR (qPCR) analysis. (e) Schematic of localization of modmRNA-LNPs vs. LPS. In experiments explained in (a - d), LPS was administered IV, leading to most of the LPS localizing to the liver, and modmRNA-LNP was untargeted, leading to deposition in the liver. To study IE in the lungs (f-h), LPS was administered intratracheally (IT), and modmRNA-LNP was targeted to the lungs by conjugation to anti-PECAM antibodies. (f) Experimental time-line for the inflammation exacerbation (IE) study in IT-LPS model. Mice were intratracheally instilled with LPS and intravenously injected with anti-PECAM modmRNA-LNP or control IgG modmRNA-LNP, followed by measurement of the bronchoalveolar lavage (BAL) levels of leukocytes (g) and protein (h), both which are measures of lung inflammation. Error bars indicate SEM. Group size is 5 animals for (b) and (c) and 3–5 animals for (d, g, and h). Statistical analysis was performed by one-way (d) or two-way (b,c,g, and h) ANOVA with Bonferroni correction, comparing modmRNA-LNP or empty-LNP to non-treated (NT) in LPS-challenged mice, (*P < 0.05, **P < 0.01, ****P < 0.0001).
Supplementary Fig. 1.
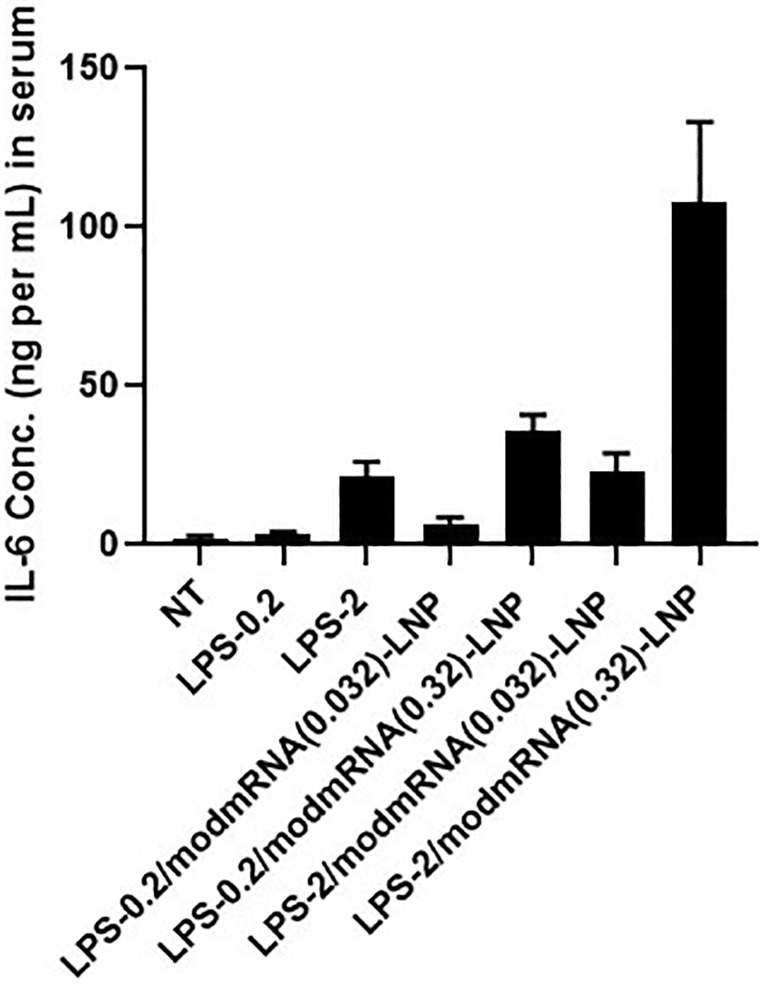
Dose-Screening for LPS and modmRNA-LNP in inflammation exacerbation model. Pro-inflammatory cytokine of interleukin-6 (IL-6) in serum was measured upon treatment with LPS (0.2 or 2 mg kg−1, injected IV) followed by modmRNA-LNP (0.032 or 0.32 mg-mRNA kg−1, injected IV).
Supplementary Fig. 2.
Systemic inflammatory cytokine release screening in vivo. Cytokine levels (LEGENDplex Mouse Inflammation Panel [Biolegend]) in plasma samples of mice treated with LPS (2 mg kg−1, injected IV) followed by modmRNA-LNP (0.32 mg-mRNA kg−1, injected IV) was compared to LPS only and modmRNA-LNP only conditions.
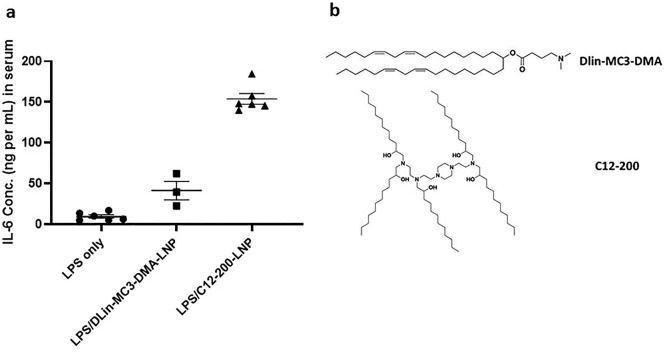
There was a 14-fold increase in IL-6 expression in serum from LPS-treated mice injected with modmRNA-LNP compared to mice treated with only LPS (Fig. 1b). Also, a 94-fold increase in IL-6 expression was detected in the serum of modmRNA-LNP, LPS-treated animals compared to modmRNA-LNP treated naïve mice. As shown in Fig. 1c, an 11-fold increase in MIP-2 expression in the liver was observed in LPS-treated mice injected with modmRNA-LNP in comparison to only LPS-treated control animals. Similarly, a 52-fold increase in MIP-2 was observed in LPS-treated modmRNA-LNP mice in comparison to naïve mice receiving modmRNA-LNP. These data demonstrate that co-treatment with LPS and modmRNA-LNP induces pro-inflammatory cytokine expression in blood. A similar pattern occurs when empty-LNP were used instead of modmRNA-LNP, suggesting the effect is LNP-dependent and not modmRNA-dependent (Fig. 1b and c). To further assess if we can generalize this phenomenon to other standard LNP formulations, we evaluated two other empty LNP formulations (DLin-MC3-DMA-LNP and C12–200-LNP) in the same IE setting, and observed a very similar IE trend (Supplementary Fig. 3a). The molecular structure of ionizable lipids used in these LNPs are listed in Supplementary Fig. 3b. As depicted in Supplementary Fig. 1, 10-fold lower doses of LPS (0.2 mg kg−1) and modmRNA-LNP (0.032 mg-mRNA kg−1) did not induce a significant IE effect. This shows that the IE phenomenon is also dose-dependent.
Supplementary Fig. 3.
Evaluation of inflammation exacerbation induced by other standard LNP formulations. Pro-inflammatory cytokine of interleukin-6 (IL-6) in serum was measured upon treatment with LPS (2 mg kg−1, injected IV) followed by empty LNP (injected IV, mass equivalent to 0.32 mg-mRNA kg−1) (a). The molecular structure of ionizable lipids related to these LNPs are listed as (b).
To further confirm the IE effect of modmRNA-LNP in LPS-mediated inflammation in mice, qPCR of MIP-2 was performed in liver tissue homogenates from either naïve or LPS-treated mice receiving modmRNA-LNP. In accordance with the MIP-2 ELISA data, the LPS/modmRNA-LNP treated animals have 11- and 8-fold increase in MIP-2 mRNA expression compared to naïve/modmRNA-LNP and LPS-only control animals, respectively (Fig. 1d). Together, these data suggest an IE effect in response to LNP introduction in the presence of LPS-mediated inflammation. In addition, IL-6 and MIP-2 are signature biomarkers modulated by this process.
To determine whether the site of inflammation is important for the IE effect, mice were treated with intra-tracheal instilled LPS (IT-LPS) at a dose of 1 mg kg−1. Lung targeted anti-PECAM [platelet endothelial cell adhesion molecule-1] modmRNA-LNP (0.32 mg-mRNA kg−1) induced an IE pattern when injected IV four hours post-IT-LPS treatment (Fig. 1 f–h). In this case, both the number of leukocytes (Fig. 1g) and protein concentration (Fig. 1h) in bronchoalveolar lavage (BAL), which are markers of inflammation, were increased [27,28] compared to naïve mice receiving lung-targeted modmRNA-LNP and no LPS.
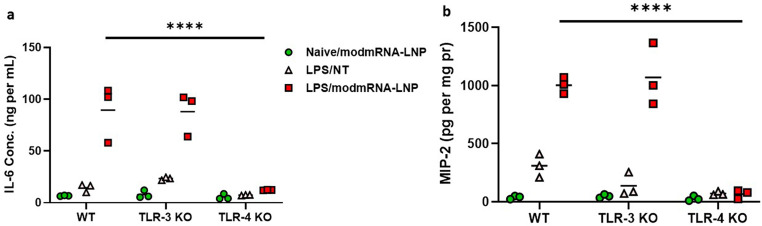
At the cellular level, LPS is known to mediate activation and signaling of Toll-like receptor-4 (TLR-4) [[29], [30], [31], [32]]. Similarly, unmodified RNA binds to a series of TLRs such as TLR-3, TLR-7, and TLR-8 resulting in the release of type I interferons and activation of interferon-inducible genes [6]. However, we demonstrated that such activation does not occur with nucleoside-modified, purified mRNA [6] (Fig. 1b, c, d, g, h), which is the type of mRNA we used in this study. To evaluate whether TLRs are immunogenic regulators of LPS/modmRNA-LNP induced IE, both TLR-3 and TLR-4 knockout (KO) mice were screened in this IE model for IL-6 and MIP-2 cytokines. TLR-3 KO mice showed no significant difference of cytokine release compared to WT C57BL/6 J mice for these two IE biomarkers in response to any treatment groups including non-treated, LPS-only, or LPS/modmRNA-LNP treated mice (Fig. 2). Similarly, we did not observe any up-regulation in TLR-3 modmRNA level in liver or spleen when comparing LPS-only and LPS/modmRNA-LNP treated wild type mice (Supplementary Fig. 4). In contrast, the LPS/modmRNA-LNP treated TLR-4 KO mice exhibited no increase in the levels of IL-6 and MIP-2 in serum compared to modmRNA-LNP only.
Fig. 2.
Inflammation-exacerbation in TLR-3 knockout but not TLR-4 knockout mice. Pro-inflammatory cytokines of interleukin-6 (IL-6) in serum (a) and macrophage inflammatory protein (MIP-2) in liver homogenate (b) of wild-type (WT), TLR-3 KO, or TLR-4 KO upon treatment with LPS (2 mg kg−1) followed by modmRNA-LNP (0.32 mg-mRNA kg−1 or equivalent lipid mass of empty-LNP) were compared to the mice treated with LPS only (2 mg kg−1) or modmRNA-LNP only. Each symbol represents one animal and horizontal lines show the mean. Error bars indicate SEM. Group size is 3 animals. Statistical analysis was performed by two-way ANOVA with Bonferroni correction, comparing LPS/modmRNA-LNP among wild-type and knockout mice (****P < 0.0001).
Supplementary Fig. 4.
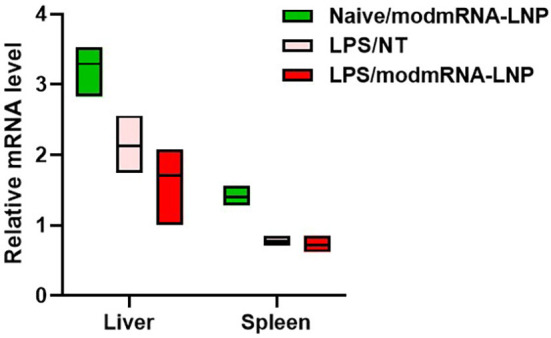
Relative expression levels of Toll-like receptor 3 (TLR-3) normalized to Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were compared in liver and spleen tissue homogenates from mice treated with modmRNA-LNP, LPS only, or LPS/modmRNA-LNP by Quantitative PCR (qPCR) analysis.
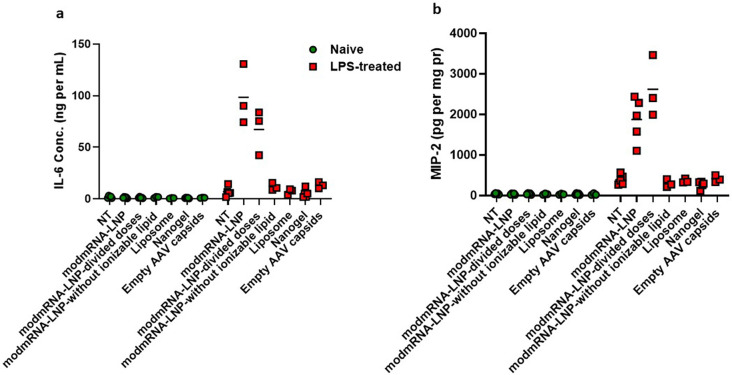
We next investigated the relationship between the LNP formulation and IE. The ionizable lipid is the most important component of LNP for both RNA encapsulation and endosomal escape [12], and we sought to evaluate a panel of nanoparticles that did not contain this component. These included LNP formulations without an ionizable lipid, other lipid particles such as liposomes, and those of a completely different nature such as polymeric nanoparticles, nanogels, and empty AAV capsids. We prioritized nanoparticles that already have or may have therapeutic applications in the future, and assessed them in our IE model. None of the mentioned nanoparticles in their recommended dose range caused IE (Supplementary Fig. 5).
Supplementary Fig. 5.
Evaluation of Inflammation exacerbation among different nanoparticles. Pro-inflammatory cytokines of interleukin-6 (IL-6) in serum (a) and macrophage inflammatory protein (MIP-2) in liver homogenate (b) of wild-type (WT), TLR-3 KO, or TLR-4 KO upon treatment with LPS (2 mg kg−1) followed by modmRNA-LNP (0.32 mg-mRNA kg−1 or equivalent lipid mass of empty-LNP) were compared to the mice treated with LPS only (2 mg kg−1) or modmRNA-LNP only. Each symbol represents one animal and horizontal lines show the mean. Error bars indicate SEM. Group size is 3–5 animals. Statistical analysis was performed by two-way ANOVA with Bonferroni correction, comparing LPS/ modmRNA-LNP among wild-type and knockout mice (****P < 0.0001).
3.2. LPS pre-treatment decreases the levels of mRNA encoded protein
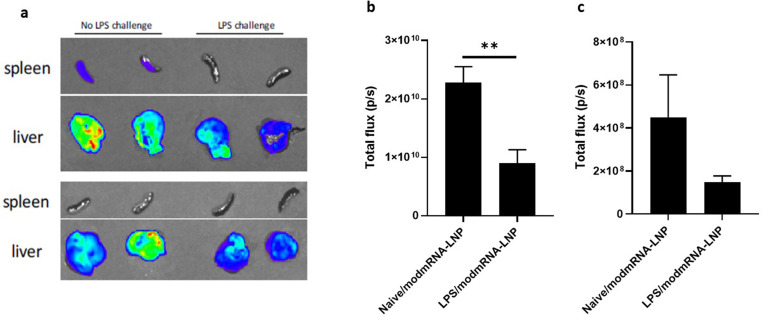
To determine whether pre-treatment with LPS has an effect on the levels of encoded protein produced by the mRNA, we utilized bioluminescence imaging to measure protein expression 4 h following injection of mRNA-LNP with encapsulated luciferase mRNA [9,33]. Results in Fig. 3 indicate that LPS-challenged mice experienced an ~4 fold reduction in luciferase expression in the liver and ~ 3.3 fold in the spleen. This data suggests that LPS pre-treatment decreases either the bio-distribution or translation of the delivered mRNA-LNP.
Fig. 3.
Effect of LPS pre-treatment on the efficiency of mRNA expression. A representative sample set of dissected spleens and livers (a) were analyzed 5 min after the administration of D-luciferin. The bioluminescent signal was quantified as total flux (p/s) in liver (b) and spleen (c), and compared between naïve and LPS-treated mice receiving modmRNA-LNP (0.32 mg-mRNA kg−1) as IV injections four hours post-IV-LPS (2 mg kg−1) treatment. Group size is 4 animals and error bars indicate SEM. Statistical analysis was performed by Student's t-test, comparing LPS/modmRNA-LNP to naïve/modmRNA-LNP (**P < 0.01).
3.3. IE is dependent on macrophages
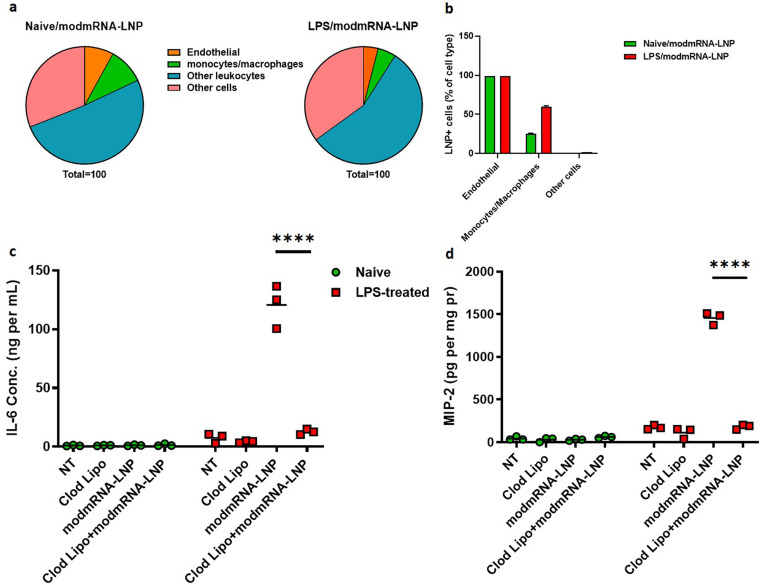
Data presented depicts the potentiation by modmRNA-LNP on the existing LPS inflammatory background. To track down any difference in LNP biodistribution in LPS vs. naive model, we performed flow cytometry on lung single cell preparations to determine cell type distribution in naive vs. LPS-treated mice. From our flow analysis, % cells positive for LNP indicated a 3-fold increase in monocyte/macrophage populations taking up LNP in LPS-treated mice compared to naive, 60% to 20% respectively (Fig. 4b). This outcome suggests a potential role for macrophages being involved or responsible for IE.
Fig. 4.
Macrophage engagement in inflammation exacerbation (IE). (a) and (b) Flow cytometric analysis of cell populations receiving PECAM-1 targeted DIO-tagged LNPs in lung tissue. Staining was performed against CD31 for endothelial cells, CD45 for leukocytes, and F4/80 for monocytes/macrophages. (a) Pie chart representative of total cell recovery from lung in naive vs. LPS-treated mice. (b) Percent of sub-cell populations positive for LNP. Pro-inflammatory cytokines of interleukin-6 (IL-6) in serum (c) and macrophage inflammatory protein (MIP-2) in liver homogenate (d) of either naïve or LPS-treated mice under different regimens (Clod lipo, modmRNA-LNP, or a combination of Clod lipo and modmRNA-LNP). LPS (2 mg kg−1) and modmRNA-LNP (0.32 mg-modmRNA kg−1) were administered via retro-orbital intravenous injections. In Clod lipo treatment, clodronate liposomes were injected via IV at a concentration of 50 mg kg−1. In Clod lipo+modmRNA-LNP regimen, clodronate liposomes were injected IV at a concentration of 50 mg kg−1 48 h prior to modmRNA-LNP administration. Each symbol represents one animal and horizontal lines show the mean. Error bars indicate SEM. Group size is 3 animals. Statistical analysis was performed by two-way ANOVA with Bonferroni correction, comparing Clod Lipo+modmRNA-LNP to modmRNA-LNP (****P < 0.0001).
Clodronate-encapsulated liposomes have previously been demonstrated to deplete macrophages and dendritic cells across numerous species including mice and pigs [[34], [35], [36]]. To confirm LPS/modmRNA-LNP induced IE is dependent on macrophages to elicit the IE response, naive or LPS-treated C57BL/6 J mice were treated with clodronate liposomes, modmRNA-LNP, or a combination of clodronate liposomes and modmRNA-LNP. Cytokines IL-6 and MIP-2 were measured in the serum and liver, respectively. Results in Fig. 4c and d indicate naïve mice had a low immunogenic response of both IL-6 and MIP-2 expression when treated with either clodronate liposomes, modmRNA-LNP, or the combination of clodronate liposomes and modmRNA-LNP. In LPS-treated mice, clodronate pre-treatment significantly reduced the modmRNA-LNP IE effect 10-fold in serum IL-6 and 8-fold in liver MIP-2 to a level equivalent to values in untreated mice. This data suggests that macrophages and/or dendritic cells are likely the primary regulators of the LPS/modmRNA-LNP IE immune response.
3.4. Dexamethasone alleviates inflammation exacerbation
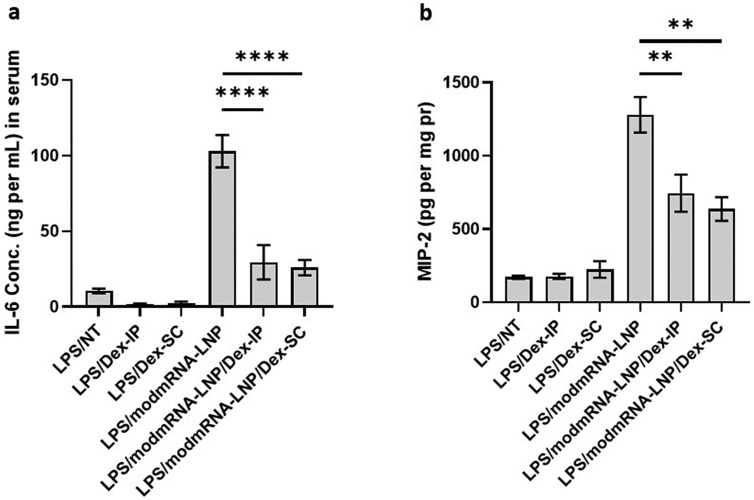
Pharmaceutical anti-inflammatory medications have long been proven to alleviate inflammation in various parts of the body. Dexamethasone is a long lasting (36–54 h) anti-inflammatory corticosteroid medication, which is widely known to decrease swelling, prevent allergic reactions, and treat autoimmune diseases [[37], [38], [39]]. We also tested Pioglitazone, an anti-diabetic drug, which has been shown to mediate its anti-inflammatory effects via attenuation of NLRP3 inflammasome activation [[40], [41], [42]]. To evaluate whether Dexamethasone or Pioglitazone alleviates the IE response in our mouse model, animals were treated as previously described in Fig. 1a. In addition, Dexamethasone-21-Phosphate (0.5 mg mL−1 in PBS) was injected twice via two different routes of administration (ROA): intraperitoneal injection (IP), and subcutaneous (SC) at 2 mg kg−1 into the mice, one hour after LPS injection and simultaneously with modmRNA-LNP administration. Pioglitazone was administered IV at the same time intervals as Dexamethasone. Results in Fig. 5a depict an alleviation of plasma IL-6 concentration by 3.5- and 4-fold in LPS/modmRNA-LNP/Dex-IP and LPS/modmRNA-LNP/Dex-SC compared to LPS/modmRNA-LNP treated mice. Fig. 5b exemplifies a similar pattern of inflammation alleviation as shown by MIP-2 expression in the liver. At a lower extent, Pioglitazone showed partial but significant improvement on cytokine secretion in the IE model (Supplementary Fig. 6).
Fig. 5.
Attenuation of inflammation-exacerbation by Dexamethasone. Pro-inflammatory cytokines of interleukin-6 (IL-6) in serum (a) and macrophage inflammatory protein (MIP-2) in liver homogenate (b) of LPS-treated mice under different treatment regimens. LPS (2 mg kg−1) and modmRNA-LNP (0.32 mg-mRNA kg−1) at 4 h post-LPS treatment were administered via retro-orbital intravenous injections. Dexamethasone-21-Phosphate (0.5 mg mL−1 in PBS) was injected twice either subcutaneously (SC) or intraperitoneally (IP) as 2 mg kg−1 into the mice, one hour after LPS injection and simultaneously with modmRNA-LNP administration. Each column represents the mean ± SEM. Group size is 3–6 animals. Statistical analysis was performed by one-way ANOVA with Bonferroni correction, comparing LPS/modmRNA-LNP/Dex-IP or LPS/modmRNA-LNP/Dex-SC to modmRNA-LNP (**P < 0.01 and ****P < 0.0001).
Supplementary Fig. 6.
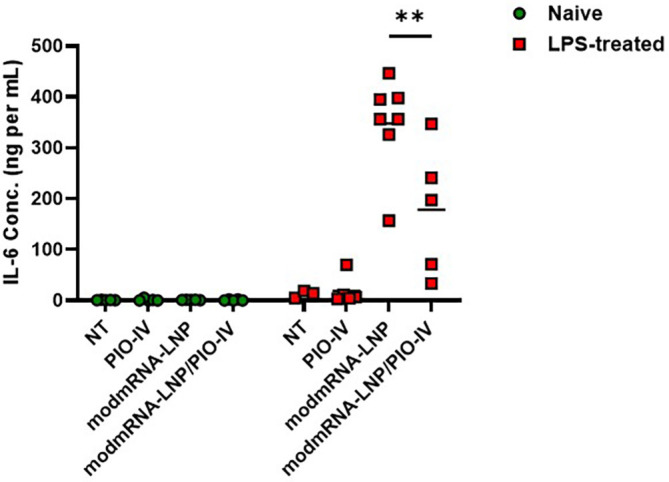
Attenuation effect of Pioglitazone on inflammation exacerbation. Pro-inflammatory cytokine of interleukin-6 (IL-6) in serum of either naïve or LPS-treated mice was measured under different treatment regimens. LPS (2 mg kg−1) and modmRNA-LNP (0.32 mg-mRNA kg−1) at 4 h post-LPS treatment were administered via retro-orbital intravenous injections. Pioglitazone (20 mg kg−1) was injected twice intraperitoneally, one hour after LPS injection and simultaneously with modmRNA-LNP administration. Each symbol represents one animal and horizontal lines show the mean. Error bars indicate SEM. Group size is 5 animals. Statistical analysis was performed by two-way ANOVA with Bonferroni correction, comparing LPS/ modmRNA-LNP/PIO-IV to LPS/ modmRNA-LNP (**P < 0.01).
3.5. Temporal or spatial effect of LPS and modmRNA-LNP administrations attenuate inflammation exacerbation
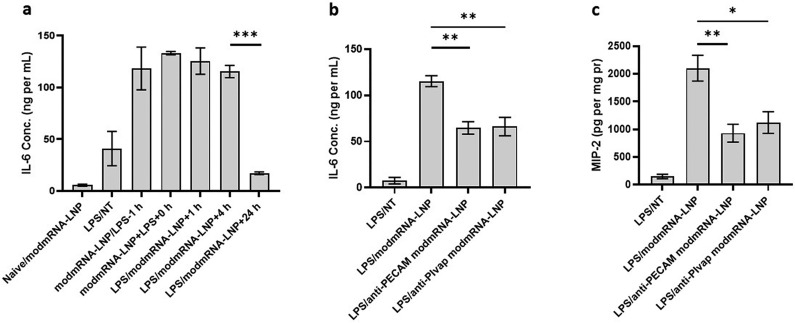
In our original mouse IE model, modmRNA-LNP was administered at the t = 4 h time-point post LPS treatment, and tissues were harvested at t = 8 h (treatment designated as LPS/modmRNA-LNP + 4 h in Fig. 6 ). We were interested in investigating the systemic relationship between LPS and modmRNA-LNP induced IE with respect to permutations of treatment inductions and time-points. To better understand the IE kinetic phenomena, mice were first treated with either LPS or modmRNA-LNP, then respectively treated with modmRNA-LNP or LPS at t = 0 h, t = 1 h, t = 4 h, or t= 24 h after the initial injection. Results in Fig. 6a indicate that when modmRNA-LNP and LPS are administered simultaneously (modmRNA-LNP + LPS + 0 h), levels of IL-6 are similar to our original IE model (LPS/modmRNA-LNP + 4 h). Additionally, there is no significant difference in IL-6 production when LPS is given 1 or 4 h before or after modmRNA-LNP. However, the IE phenotype is significantly reduced for IL-6 when mice are first challenged with LPS and then administered modmRNA-LNP at t = 24 h (Fig. 6a). These results signify a loss of the IE response from modmRNA-LNP when administered 24 h after LPS.
Fig. 6.
Attenuation of inflammation exacerbation via temporally or spatially controlled LPS and modmRNA-LNP administrations. Pro-inflammatory cytokines of interleukin-6 (IL-6) in serum (a, b) and macrophage inflammatory protein (MIP-2) in liver homogenate (c) of either naïve or LPS-treated mice under different treatment regimens. LPS at 2 mg kg−1, and modmRNA-LNP, anti-PECAM modmRNA-LNP, or anti-PlVAP modmRNA-LNP at 0.32 mg-mRNA kg−1 were administered via retro-orbital intravenous injection. Results shown in (a) are obtained from the timepoint study in which mice were first administered with either LPS or mRNA-LNP, then respectively treated with modmRNA-LNP or LPS at the same time (modmRNA-LNP + LPS + 0 h), t = 1 h (modmRNA-LNP/LPS-1 h, in which LPS was administered 1 h post-mRNA-LNP treatment, or LPS/modmRNA-LNP + 1 h, in which LPS was administered 1 h before modmRNA-LNP treatment), t= 4 h (LPS/modmRNA-LNP + 4 h, in which LPS was administered 4 h before modmRNA-LNP treatment), or t = 24 h (LPS/modmRNA-LNP + 24 h, in which LPS was administered 24 h before modmRNA-LNP treatment). Each column represents Mean ± SEM. Group size is 3 animals. Statistical analysis was performed by one-way ANOVA with Bonferroni correction, comparing LPS/modmRNA-LNP to either LPS/modmRNA-LNP + 24 h, LPS/anti-PECAM modmRNA-LNP, or LPS/anti-PLVAP modmRNA-LNP (*P < 0.05, **P < 0.01, and ***P < 0.001).
To further characterize the IE model, we studied the effect of localization of the two hits. Intravenous injection of LPS causes systemic pro-inflammatory state manifested by elevation of the markers and mediators in blood and predominantly in liver, followed by small and large intestines and kidneys among other organs [43,44]. To achieve redirection of the modmRNA-LNP from liver to lung tissue, we administered endothelial-targeted modmRNA-LNP (either anti-PECAM modmRNA-LNP or anti-PLVAP [plasmalemma vesicle-associated protein] modmRNA-LNP) instead of non-targeted modmRNA-LNP in the original model. We previously reported that anti-PECAM modmRNA-LNP was highly localized to the lungs [9]. Similarly, studies for nanoparticles targeted against PLVAP reported substantial lung accumulation for PLVAP-targeted nanoparticles [45,46]. As shown in Fig. 6b and c, both anti-PECAM modmRNA-LNP and anti-PLVAP modmRNA-LNP treatments triggered significantly lower systemic inflammatory response; IL-6 (Fig. 6b) and MIP-2 (Fig. 6c), respectively in serum and liver, when compared to modmRNA-LNP that primarily targeted the liver. We therefore demonstrated that when the two hits are locally distanced, in this case by using lung targeted modmRNA-LNP in systemic IV-LPS setting, the IE effect was attenuated.
3.6. Inflammation exacerbation does not happen with other stimulants such as TNF-α or oleic acid
In order to evaluate if inflammation exacerbation is a general phenomenon to all inflammation inducing agents, we performed the IE experiment in two other models.
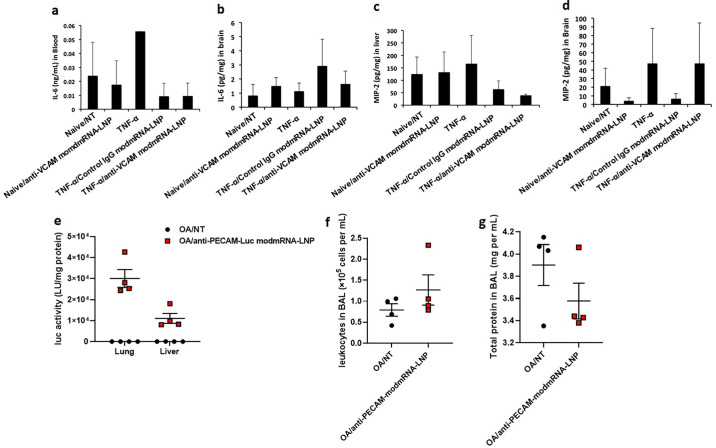
We chose Intrastriatal TNF-α model to study a physiologic model involving the brain to assess the potentiating effect from LNP treatment. Cytokines, such as tumor necrosis factor alpha (TNF-α) are important mediators of inflammatory diseases in the CNS [47]. They induce the expression of adhesion molecules on the surface of brain endothelial cells and are associated with blood-brain barrier (BBB) disruption [48]. One of the most important adhesion molecule receptors that is highly upregulated on endothelial cells upon TNF-α stimulation is vascular cell adhesion molecule 1 (VCAM-1) [49]. We therefore used intrastriatal (i.s.) TNF administration into mouse right striatum to create a localized brain inflammatory model similar to a published one [24]. In our previous studies, the IV injection of anti-VCAM-modmRNA-LNP elevated cellular uptake and translation in the ipsilateral hemisphere (inflamed zone) up to 71-fold compared to control-IgG-LNP [50]. Interestingly, we did not observe any elevation in pro-inflammatory markers in the brain in this model (Supplementary Fig. 7).
Supplementary Fig. 7.
Inflammation exacerbation is not observed in Intrastriatal (i.s.) TNF-α or oleic acid model. In Intrastriatal (i.s.) TNF-α model, pro-inflammatory cytokines of interleukin-6 (IL-6) in serum (a) and macrophage inflammatory protein (MIP-2) in brain (b) and liver (c) homogenates of wild-type (WT) upon treatment with TNF-α (0.5 μg in 2.5 μl) followed by Control IgG-modmRNA-LNP or anti-VCAM1-modmRNA-LNP (0.32 mg-mRNA kg−1) were compared to the mice treated with TNF-α only. (e) Mice were IV-injected with oleic acid (OA) to induce pulmonary inflammation. Four hours after OA, mice were injected with anti-PECAM-modmRNA-LNP (0.32 mg-mRNA kg−1), and sacrificed four hours after that. There was robust expression of luciferase (e), but no IE, as measured bronchoalveolar lavage (BAL) leukocyte count (f) or protein level (g).
Given that the brain is immune-privileged, we next wanted to check if IE was present in non-LPS inflammation in an organ in which we already observed IE: the lungs. Therefore, we used another common mouse model of acute lung injury and inflammation, intravenous injection of oleic acid (OA). IV OA mimics the acute pulmonary inflammation seen with trauma, in which bone fractures release fat emboli, which lodge in the pulmonary vasculature, where they induce local inflammation and pulmonary edema [23]. Using the same time course to examine IE as we used in intratracheal LPS (Fig. 1 g and h), we did not observe any of the common metrics of pulmonary inflammation, such as increased BAL protein or leukocyte count. (Supplementary Fig. 7).
4. Discussion
LNPs have been widely studied for delivering different types of nucleic acids, such as siRNA and mRNA [12]. Nucleic acid-LNP complexes provide optimal particle sizes (100 nm diameter or less), high encapsulation efficiencies, well-designed robust manufacturing processes, and low surface charge to minimize interactions with serum proteins [12,51,52]. There are now several nucleic acid-LNP in clinical trials that have been reviewed elsewhere [51,[53], [54], [55]]. Moderna's mRNA-1273-LNP and BioNTech/Pfizer BNT162b2 vaccines for SARS-CoV-2 have received emergency approval. Although multiple safety studies were performed before and during these clinical trials and in post-marketing analyses, evaluation of mRNA-LNP use and its application in pathological conditions are lacking.
It has been demonstrated that nucleic acid nanoparticulate carriers can greatly impact immune system activation [[57], [58], [59], [60]]. Compared to the parent cationic lipids used in early lipoplex formulations, ionizable lipids in recent versions of LNP formulations provide a safe and effective platform for delivering nucleic acids such as siRNA and mRNA in the clinic [1,56,61]. In our hands, modmRNA-LNP when administered to naïve mice and in clinical dose ranges, did not cause any acute nor chronic toxic/inflammatory effect [9,50,62]. It was reported that mRNA-LNP with diverse formulation designs and dosing regimens in a healthy, non-inflammatory context caused a transient increase in cytokines such as IL-6, macrophage inflammatory protein-1β (MIP-1β), monocyte chemotactic protein-1 (MCP-1), granulocyte-colony stimulating factor (GCS-F), or granulocyte-macrophage colony-stimulating factor (GM-CSF) [[63], [64], [65], [66]], although the mRNA used was either not nucleoside-modified or the dsRNA contaminants were not removed leaving the mRNA potentially immunogenic. Similarly, LNP used for delivering siRNA showed an immediate immune activation response [67]. This acute cytokine release is usually considered a trivial reaction, and in most cases, even at doses well above clinical range, returned to baseline level in 96 h [[63], [64], [65], [66]]. However, there is not enough evidence on how this panel looks when there is pre-existing inflammation.
In the current study, we investigated the use of modmRNA-LNP in mice in an inflammatory state. Although completely benign in the healthy state, modmRNA-LNP potentiated existing inflammation in mice that had received the bacterial cell wall component LPS either IV or IT. We refer to this phenomenon as the inflammation-exacerbation (IE) effect. We first demonstrated the IE phenomenon through measurement of pro-inflammatory cytokines in mice that received LPS (IV administration as 2 mg kg−1) with a 4-h post-LPS administration of modmRNA-LNP (injected IV as 0.32 mg-mRNA kg−1). The fact that lower doses of LPS (0.2 mg kg−1) and modmRNA-LNP (0.032 mg-mRNA kg−1) did not induce a significant IE effect suggests that the IE model is dose-dependent and it does not occur with low doses of each, i.e. LPS or modmRNA-LNP. Notably, the dose of modmRNA-LNP that led to the IE effect was lower than typically used to deliver mRNA encoded monoclonal antibodies in animal models [68], but much higher than used for SARS-CoV-2 vaccines.
We then demonstrated that ongoing inflammation reduced the modmRNA delivery efficacy, as measured by translation of the encoded protein as determined by luminescence imaging following injection with LNP encapsulating luciferase modmRNA. LPS (tested here at 2 mg kg−1) considerably decreased luminescence in mice injected with luciferase modmRNA-LNP (0.32 mg-mRNA kg−1). Similarly, Lokugamage et al. [69] reported that LPS suppresses the cellular translation process and can decrease transfection efficiency of Cre mRNA-LNP to a considerable extent in Ai14 mice. Lokugamage et al. [69] used LPS (intraperitoneal injection) at 0.1 mg kg−1, which they considered as non-toxic. After 6 h, they delivered the Cre mRNA-LNP intravenously at 0.3 mg kg−1. They reported that three days post mRNA-LNP injection, compared to mice that were not treated with LPS, the percentage of tdTomato+ cells (at three categories of Kupffer cells, liver endothelial cells, or hepatocytes) isolated from mice treated with LPS decreased to ~0%. Although the timelines of our study differs from Lokugamage et al. [69] and they only tested one low dose of LPS, their findings are in line with our luciferase modmRNA translation data upon LPS administration. For the first time, we demonstrated that a combination of pre-existing inflammation and modmRNA-LNP application can not only affect the levels of mRNA-encoded protein, but more importantly, lead to enhanced inflammatory reactions.
We further confirmed the IE phenomenon as a reaction to LNP, and not modmRNA, as empty-LNP led to the same inflammation potentiation effect as modmRNA-LNP. Compared to conventional lipid-based carriers for nucleic acid delivery, LNPs are one of the safest available carriers due, in part, to their limited interaction with biological components [12,70]. As mentioned above, we did not observe harmful effects when delivering modmRNA-LNP in naïve mice and at doses up to 1 mg kg−1 [9,50,62]. Some immunostimulatory lipids can be recognized by TLR-2 and TLR-4 on the cell surface of macrophages and other cells and trigger downstream events including induction of cytokine and chemokine genes and an inflammatory response [67,71]. Application of modmRNA-LNP in LPS-administered TLR-4 knockout mice did not result in any IE phenomenon. We also investigated the possible formulation ingredient of LNP responsible for IE. Cholesterol was found to be non-immunogenic and compatible with a large variety of cationic and procationic lipids with different packing parameters, generating LNP with proven efficiency in vitro and in vivo [72]. We hypothesized that the ionizable lipid could play a central role in inflammation-exacerbation by LNP, and showed that three different ionizable lipids could each still induce the IE effect when incorporated into LNP. We also demonstrated that modmRNA-LNP without a potent ionizable lipid does not initiate the IE effect. It is of note to mention that LNP without ionizable lipids form approximately 80 nm particles by DLS, but it is possible that LNP without ionizable lipids are not stable after in vivo delivery, and therefore its kinetics could be very different. Similarly, we demonstrated that other tested drug delivery systems (liposome, nanogel, and AAV empty capsids) with different design parameters than LNP, did not cause a similar IE outcome. The effect of formulation design on the IE model needs to be further investigated by studies focused on particle interaction with extracellular and intracellular components in vivo to be able to draw a better conclusion.
To seek possible strategies to make mRNA-LNP available for inflammatory conditions, we demonstrated that macrophage depletion via IV administration of clodronate liposomes removed the IE effect. Depletion of liver Kupffer cells, spleen macrophages, and dendritic cells prior to administration of adenoviral vectors or lipoplexes has similarly been shown to alleviate an acute inflammatory response [73,74]. Other than macrophage removal, we demonstrated that temporal or spatial control of the two stimulants (LPS and modmRNA-LNP) could resolve the effect. With a 24 h time-difference between LPS and modmRNA-LNP, a significant decrease of the enhanced innate immune response was observed. We also demonstrated that delivery of each agent to different organs triggered significantly lower systemic inflammatory responses. Therefore, when in a disease context, and by targeting the modmRNA-LNP against tissue markers, it may be possible to redirect them from liver and spleen, which are assumed to be the source of the IE initiating event with systemic inflammation.
We then investigated possible drug therapies to attenuate the IE effect. Dexamethasone administration alleviated the inflammatory response substantially. It was reported that LNP containing unmodified mRNA co-loaded with lipid-conjugated dexamethasone, enhanced the protein expression in a mouse liver by up to 6.6-fold when injected IV [75]. A mixture of immune suppressants including Dexamethasone is given before Patisiran siRNA-LNP [1], Alnylam® Pharmaceuticals' FDA-approved formulation for TTR-mediated amyloidosis. However, the indication of immune suppressants pre-Patisiran treatment are to prevent adverse infusion-related immune events [60,76], which could include LNP-related pro-inflammatory consequences. While these prior studies indicated a potential for a mild immune effect of LNP, the current study is the first to identify the phenomenon of IE, in which LNP increases inflammation by an order of magnitude when there is a concomitant background inflammatory stimulus. We believe that these studies could considerably advance the field of nanomedicine and specifically modmRNA-LNP.
Finally, we evaluated the possibility of inducing the IE phenomenon with other inflammatory agents such as TNF-α or Oleic acid. We could not observe any exacerbation effect by administering modmRNA-LNP in any of these two conditions. Therefore, the IE cannot be extrapolated to all inflammatory conditions. The fact that IE is a very powerful effect with LPS, but not two other inflammatory stimuli, provides key mechanistic insights. Additionally, this points to key clinical situations in which IE might lead to increased morbidity and mortality. Many potential applications of modmRNA-LNP include those of acute critical illnesses, such as ARDS, stroke, sepsis, and more, as these diseases seem perfectly suited to the hours-to-days time-course of expression of modmRNA-encoded therapeutic proteins. However, in all of these common illnesses, patients have a very high likelihood of a concomitant gram-negative bacterial infection and release of endogenous TLR4 agonists from damaged cells. For example, approximately one third of stroke patients develop pneumonia within a week of neurological injury. Such a high rate of bacterial infection implies these patients may be at risk of IE, and thus mitigation strategies for IE are urgently needed.
5. Conclusion
We report the discovery of inflammation exacerbation (IE) mediated through acute LPS-induced inflammation and LNP delivery. IE was first characterized with very high levels of pro-inflammatory cytokine concentrations in liver tissue and in serum. We further provide evidence that IE is both inflammatory reagent- and LNP-specific, and the phenomena is independent from mRNA cargo. Additionally, IE is a time-sensitive and dose-dependent event. We also show the effect of IE can be alleviated with corticosteroids, such as Dexamethasone. Altogether, our discovery of LPS and LNP-mediated IE will likely provide new information on the potential behavior of benign modmRNA-LNP in specific conditions and advance understanding on potential adverse effects.
The following are the supplementary data related to this article.
Author contributions
H.P., J.S.B., D.W., and V.M. conceived and designed the experiments; H.P., P.P., T.E.P., H.S., Q.L., R.S., M.Z., A.Y., O.A.M., A.N., N.P., V.V.S., R.K., J.M., T.U., R.S.R., X. H., K.L., and J.H. performed the experiments; H.P., J.S.B., and M.J.M., analyzed the data; H.P. wrote the paper. All authors read and approved the final manuscript.
Funding sources
This work was supported by the NIH under award numbers HL134839, HL155106, HL138269, HL153510, HL154662, AI124429, and funding from BioNTech.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
References
- 1.Suhr O.B., Coelho T., Buades J., Pouget J., Conceicao I., Berk J., Schmidt H., Waddington-Cruz M., Campistol J.M., Bettencourt B.R., Vaishnaw A., Gollob J., Adams D. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a phase II multi-dose study. Orphanet. J. Rare Dis. 2015;10:109. doi: 10.1186/s13023-015-0326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlsson L., Clarke J.C., Yen C., Gregoire F., Albery T., Billger M., Egnell A.C., Gan L.M., Jennbacken K., Johansson E., Linhardt G., Martinsson S., Sadiq M.W., Witman N., Wang Q.D., Chen C.H., Wang Y.P., Lin S., Ticho B., Hsieh P.C.H., Chien K.R., Fritsche-Danielson R. Biocompatible, purified VEGF-A mRNA improves cardiac function after intracardiac injection 1 week post-myocardial infarction in swine. Mol. Ther. Methods Clin. Dev. 2018;9:330–346. doi: 10.1016/j.omtm.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu X., Yin L., Theisen M., Zhuo J., Siddiqui S., Levy B., Presnyak V., Frassetto A., Milton J., Salerno T., Benenato K.E., Milano J., Lynn A., Sabnis S., Burke K., Besin G., Lukacs C.M., Guey L.T., Finn P.F., Martini P.G.V. Systemic mRNA therapy for the treatment of fabry disease: preclinical studies in wild-type mice, fabry mouse model, and wild-type non-human primates. Am. J. Hum. Genet. 2019;104:625–637. doi: 10.1016/j.ajhg.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richner J.M., Himansu S., Dowd K.A., Butler S.L., Salazar V., Fox J.M., Julander J.G., Tang W.W., Shresta S., Pierson T.C., Ciaramella G., Diamond M.S. Modified mRNA vaccines protect against zika virus infection. Cell. 2017;168:1114–1125.e1110. doi: 10.1016/j.cell.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahl K., Senn J.J., Yuzhakov O., Bulychev A., Brito L.A., Hassett K.J., Laska M.E., Smith M., Almarsson Ö., Thompson J., Ribeiro A.M., Watson M., Zaks T., Ciaramella G. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol. Ther. 2017;25:1316–1327. doi: 10.1016/j.ymthe.2017.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karikó K., Buckstein M., Ni H., Weissman D. Suppression of RNA recognition by toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Karikó K., Muramatsu H., Keller J.M., Weissman D. Increased erythropoiesis in mice injected with submicrogram quantities of pseudouridine-containing mRNA encoding erythropoietin. Mol. Therap. 2012;20:948–953. doi: 10.1038/mt.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardi N., Hogan M.J., Pelc R.S., Muramatsu H., Andersen H., DeMaso C.R., Dowd K.A., Sutherland L.L., Scearce R.M., Parks R., Wagner W., Granados A., Greenhouse J., Walker M., Willis E., Yu J.-S., McGee C.E., Sempowski G.D., Mui B.L., Tam Y.K., Huang Y.-J., Vanlandingham D., Holmes V.M., Balachandran H., Sahu S., Lifton M., Higgs S., Hensley S.E., Madden T.D., Hope M.J., Karikó K., Santra S., Graham B.S., Lewis M.G., Pierson T.C., Haynes B.F., Weissman D. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543:248–251. doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parhiz H., Shuvaev V.V., Pardi N., Khoshnejad M., Kiseleva R.Y., Brenner J.S., Uhler T., Tuyishime S., Mui B.L., Tam Y.K., Madden T.D., Hope M.J., Weissman D., Muzykantov V.R. PECAM-1 directed re-targeting of exogenous mRNA providing two orders of magnitude enhancement of vascular delivery and expression in lungs independent of apolipoprotein E-mediated uptake. J. Control. Release. 2018;291:106–115. doi: 10.1016/j.jconrel.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliver Sara E., Gargano Julia W., Marin Mona, Wallace Megan, Curran Kathryn G., Chamberland Mary, McClung Nancy, Campos-Outcalt Doug, Morgan Rebecca L., Mbaeyi Sarah, Romero José R., Talbot H. Keipp, Lee Grace M., Bell Beth P., Dooling K. 2020. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine — United States, December 2020, in, MMWR Morb Mortal Wkly Rep; pp. 1922–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver Sara E., Gargano J.W., Marin Mona, Wallace Megan, Curran Kathryn G., Chamberland Mary, McClung Nancy, Campos-Outcalt Doug, Morgan Rebecca L., Mbaeyi Sarah, Romero José R., Talbot H. Keipp, Lee Grace M., Bell Beth P., Dooling K. 2021. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Moderna COVID-19 Vaccine — United States, December 2020, in, MMWR Morb Mortal Wkly Rep; pp. 1653–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulkarni J.A., Cullis P.R., van der Meel R. Lipid nanoparticles enabling gene therapies: from concepts to clinical utility. Nucleic Acid Ther. 2018;28:146–157. doi: 10.1089/nat.2018.0721. [DOI] [PubMed] [Google Scholar]
- 13.Maugeri M., Nawaz M., Papadimitriou A., Angerfors A., Camponeschi A., Na M., Hölttä M., Skantze P., Johansson S., Sundqvist M., Lindquist J., Kjellman T., Mårtensson I.-L., Jin T., Sunnerhagen P., Östman S., Lindfors L., Valadi H. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat. Commun. 2019;10:4333. doi: 10.1038/s41467-019-12275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shirai S., Shibuya M., Kawai A., Tamiya S., Munakata L., Omata D., Suzuki R., Aoshi T., Yoshioka Y. Lipid nanoparticles potentiate CpG-Oligodeoxynucleotide-based vaccine for influenza virus. Front. Immunol. 2020;10:3018. doi: 10.3389/fimmu.2019.03018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reichmuth A.M., Oberli M.A., Jaklenec A., Langer R., Blankschtein D. mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv. 2016;7:319–334. doi: 10.4155/tde-2016-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baiersdorfer M., Boros G., Muramatsu H., Mahiny A., Vlatkovic I., Sahin U., Kariko K. A facile method for the removal of dsRNA contaminant from in vitro-transcribed mRNA. Mol. Ther. Nucleic. Acids. 2019;15:26–35. doi: 10.1016/j.omtn.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maier M.A., Jayaraman M., Matsuda S., Liu J., Barros S., Querbes W., Tam Y.K., Ansell S.M., Kumar V., Qin J., Zhang X., Wang Q., Panesar S., Hutabarat R., Carioto M., Hettinger J., Kandasamy P., Butler D., Rajeev K.G., Pang B., Charisse K., Fitzgerald K., Mui B.L., Du X., Cullis P., Madden T.D., Hope M.J., Manoharan M., Akinc A. Biodegradable lipids enabling rapidly eliminated lipid nanoparticles for systemic delivery of RNAi therapeutics. Mol. Ther. 2013;21:1570–1578. doi: 10.1038/mt.2013.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayaraman M., Ansell S.M., Mui B.L., Tam Y.K., Chen J., Du X., Butler D., Eltepu L., Matsuda S., Narayanannair J.K., Rajeev K.G., Hafez I.M., Akinc A., Maier M.A., Tracy M.A., Cullis P.R., Madden T.D., Manoharan M., Hope M.J. Maximizing the potency of siRNA lipid nanoparticles for hepatic gene silencing in vivo. Angew. Chem. Int. Ed. Eng. 2012;51:8529–8533. doi: 10.1002/anie.201203263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen D., Love K.T., Chen Y., Eltoukhy A.A., Kastrup C., Sahay G., Jeon A., Dong Y., Whitehead K.A., Anderson D.G. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. J. Am. Chem. Soc. 2012;134:6948–6951. doi: 10.1021/ja301621z. [DOI] [PubMed] [Google Scholar]
- 20.Hood E.D., Greineder C.F., Dodia C., Han J., Mesaros C., Shuvaev V.V., Blair I.A., Fisher A.B., Muzykantov V.R. Antioxidant protection by PECAM-targeted delivery of a novel NADPH-oxidase inhibitor to the endothelium in vitro and in vivo. J. Control. Release. 2012;163:161–169. doi: 10.1016/j.jconrel.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrer M.C., Shuvaev V.V., Zern B.J., Composto R.J., Muzykantov V.R., Eckmann D.M. Icam-1 targeted nanogels loaded with dexamethasone alleviate pulmonary inflammation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0102329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenner J.S., Bhamidipati K., Glassman P.M., Ramakrishnan N., Jiang D., Paris A.J., Myerson J.W., Pan D.C., Shuvaev V.V., Villa C.H., Hood E.D., Kiseleva R., Greineder C.F., Radhakrishnan R., Muzykantov V.R. Mechanisms that determine nanocarrier targeting to healthy versus inflamed lung regions. Nanomedicine. 2017;13:1495–1506. doi: 10.1016/j.nano.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matute-Bello G., Frevert C.W., Martin T.R. Animal models of acute lung injury. Am. J. Phys. Lung Cell. Mol. Phys. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montagne A., Gauberti M., Macrez R., Jullienne A., Briens A., Raynaud J.S., Louin G., Buisson A., Haelewyn B., Docagne F., Defer G., Vivien D., Maubert E. Ultra-sensitive molecular MRI of cerebrovascular cell activation enables early detection of chronic central nervous system disorders. Neuroimage. 2012;63:760–770. doi: 10.1016/j.neuroimage.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Norman J., Denham W., Denham D., Yang J., Carter G., Abouhamze A., Tannahill C.L., MacKay S.L.D., Moldawer L.L. Liposome-mediated, nonviral gene transfer induces a systemic inflammatory response which can exacerbate pre-existing inflammation. Gene Ther. 2000;7:1425–1430. doi: 10.1038/sj.gt.3301240. [DOI] [PubMed] [Google Scholar]
- 26.Di Gioacchino M., Petrarca C., Lazzarin F., Di Giampaolo L., Sabbioni E., Boscolo P., Mariani-Costantini R., Bernardini G. Immunotoxicity of nanoparticles. Int. J. Immunopathol. Pharmacol. 2011;24:65s–71s. [PubMed] [Google Scholar]
- 27.Shuvaev V.V., Christofidou-Solomidou M., Bhora F., Laude K., Cai H., Dikalov S., Arguiri E., Solomides C.C., Albelda S.M., Harrison D.G., Muzykantov V.R. Targeted detoxification of selected reactive oxygen species in the vascular endothelium. J. Pharmacol. Exp. Ther. 2009;331:404–411. doi: 10.1124/jpet.109.156877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner J.S., Pan D.C., Myerson J.W., Marcos-Contreras O.A., Villa C.H., Patel P., Hekierski H., Chatterjee S., Tao J.-Q., Parhiz H., Bhamidipati K., Uhler T.G., Hood E.D., Kiseleva R.Y., Shuvaev V.S., Shuvaeva T., Khoshnejad M., Johnston I., Gregory J.V., Lahann J., Wang T., Cantu E., Armstead W.M., Mitragotri S., Muzykantov V. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat. Commun. 2018;9:2684. doi: 10.1038/s41467-018-05079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poltorak A., He X., Smirnova I., Liu M.Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 30.Chow J.C., Young D.W., Golenbock D.T., Christ W.J., Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 31.Hoshino K., Takeuchi O., Kawai T., Sanjo H., Ogawa T., Takeda Y., Takeda K., Akira S. Cutting edge: toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 32.Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr. Opin. Immunol. 2000;12:20–26. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 33.Pardi N., Tuyishime S., Muramatsu H., Kariko K., Mui B.L., Tam Y.K., Madden T.D., Hope M.J., Weissman D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wibroe P.P., Anselmo A.C., Nilsson P.H., Sarode A., Gupta V., Urbanics R., Szebeni J., Hunter A.C., Mitragotri S., Mollnes T.E., Moghimi S.M. Bypassing adverse injection reactions to nanoparticles through shape modification and attachment to erythrocytes. Nat. Nanotechnol. 2017;12:589–594. doi: 10.1038/nnano.2017.47. [DOI] [PubMed] [Google Scholar]
- 35.Weisser S.B., van Rooijen N., Sly L.M. Depletion and reconstitution of macrophages in mice. J. Vis. Exp. 2012:4105. doi: 10.3791/4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kozicky L.K., Sly L.M. In: Mouse Models of Innate Immunity: Methods and Protocols. Allen I.C., editor. Springer; New York, New York, NY: 2019. Depletion and reconstitution of macrophages in mice; pp. 101–112. [Google Scholar]
- 37.Newton R. Molecular mechanisms of glucocorticoid action: what is important? Thorax. 2000;55:603–613. doi: 10.1136/thorax.55.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swantek J.L., Cobb M.H., Geppert T.D. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-alpha) translation: glucocorticoids inhibit TNF-alpha translation by blocking JNK/SAPK. Mol. Cell. Biol. 1997;17:6274–6282. doi: 10.1128/mcb.17.11.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han J., Thompson P., Beutler B. Dexamethasone and pentoxifylline inhibit endotoxin-induced cachectin/tumor necrosis factor synthesis at separate points in the signaling pathway. J. Exp. Med. 1990;172:391–394. doi: 10.1084/jem.172.1.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heliövaara M.K., Herz M., Teppo A.M., Leinonen E., Ebeling P. Pioglitazone has anti-inflammatory effects in patients with type 2 diabetes. J. Endocrinol. Investig. 2007;30:292–297. doi: 10.1007/BF03346296. [DOI] [PubMed] [Google Scholar]
- 41.Swanson C.R., Joers V., Bondarenko V., Brunner K., Simmons H.A., Ziegler T.E., Kemnitz J.W., Johnson J.A., Emborg M.E. The PPAR-γ agonist pioglitazone modulates inflammation and induces neuroprotection in parkinsonian monkeys. J. Neuroinflammation. 2011;8:91. doi: 10.1186/1742-2094-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y., Yu B., Wang L., Yang M., Xia Z., Wei W., Zhang F., Yuan X. Pioglitazone ameliorates glomerular NLRP3 inflammasome activation in apolipoprotein E knockout mice with diabetes mellitus. PLoS One. 2017;12 doi: 10.1371/journal.pone.0181248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ning Y., Kim J.K., Min H.K., Ren S. Cholesterol metabolites alleviate injured liver function and decrease mortality in an LPS-induced mouse model. Metabolism. 2017;71:83–93. doi: 10.1016/j.metabol.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Gong Q., He L., Wang M., Zuo S., Gao H., Feng Y., Du L., Luo Y., Li J. Comparison of the TLR4/NFκB and NLRP3 signalling pathways in major organs of the mouse after intravenous injection of lipopolysaccharide. Pharm. Biol. 2019;57:555–563. doi: 10.1080/13880209.2019.1653326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shuvaev V.V., Khoshnejad M., Pulsipher K.W., Kiseleva R.Y., Arguiri E., Cheung-Lau J.C., LeFort K.M., Christofidou-Solomidou M., Stan R.V., Dmochowski I.J., Muzykantov V.R. Spatially controlled assembly of affinity ligand and enzyme cargo enables targeting ferritin nanocarriers to caveolae. Biomaterials. 2018;185:348–359. doi: 10.1016/j.biomaterials.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Myerson J.W., Braender B., McPherson O., Glassman P.M., Kiseleva R.Y., Shuvaev V.V., Marcos-Contreras O., Grady M.E., Lee H.-S., Greineder C.F., Stan R.V., Composto R.J., Eckmann D.M., Muzykantov V.R. Flexible nanoparticles reach sterically obscured endothelial targets inaccessible to rigid nanoparticles. Adv. Mater. 2018;30:e1802373. doi: 10.1002/adma.201802373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Probert L., Selmaj K. TNF and related molecules: trends in neuroscience and clinical applications. J. Neuroimmunol. 1997;72:113–117. doi: 10.1016/s0165-5728(96)00176-2. [DOI] [PubMed] [Google Scholar]
- 48.Fabry Z., Raine C.S., Hart M.N. Nervous tissue as an immune compartment: the dialect of the immune response in the CNS. Immunol. Today. 1994;15:218–224. doi: 10.1016/0167-5699(94)90247-X. [DOI] [PubMed] [Google Scholar]
- 49.Carlos T.M., Schwartz B.R., Kovach N.L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., et al. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990;76:965–970. [PubMed] [Google Scholar]
- 50.Marcos-Contreras O.A., Greineder C.F., Kiseleva R.Y., Parhiz H., Walsh L.R., Zuluaga-Ramirez V., Myerson J.W., Hood E.D., Villa C.H., Tombacz I., Pardi N., Seliga A., Mui B.L., Tam Y.K., Glassman P.M., Shuvaev V.V., Nong J., Brenner J.S., Khoshnejad M., Madden T., Weissmann D., Persidsky Y., Muzykantov V.R. Selective targeting of nanomedicine to inflamed cerebral vasculature to enhance the blood-brain barrier. Proc. Natl. Acad. Sci. U. S. A. 2020;117:3405–3414. doi: 10.1073/pnas.1912012117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 52.Ernsting M.J., Murakami M., Roy A., Li S.D. Factors controlling the pharmacokinetics, biodistribution and intratumoral penetration of nanoparticles. J. Control. Release. 2013;172:782–794. doi: 10.1016/j.jconrel.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gómez-Aguado I., Rodríguez-Castejón J., Vicente-Pascual M., Rodríguez-Gascón A., Solinís M., Del Pozo-Rodríguez A. Nanomedicines to deliver mRNA: state of the art and future perspectives. Nanomaterials (Basel) 2020;10 doi: 10.3390/nano10020364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.BioNTech . 2020. A Trial Investigating the Safety and Effects of Four BNT162 Vaccines against COVID-2019 in Healthy Adults. [Google Scholar]
- 55.Moderna . 2020. Safety and Immunogenicity Study of 2019-nCoV Vaccine (mRNA-1273) for Prophylaxis of SARS-CoV-2 Infection (COVID-19) [Google Scholar]
- 56.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S.P., Neuzil K., Raabe V., Bailey R., Swanson K.A., Li P., Koury K., Kalina W., Cooper D., Fonter-Garfias C., Shi P.-Y., Tuereci O., Tompkins K.R., Walsh E.E., Frenck R., Falsey A.R., Dormitzer P.R., Gruber W.C., Sahin U., Jansen K.U. 2020. Phase 1/2 Study to Describe the Safety and Immunogenicity of a COVID-19 RNA Vaccine Candidate (BNT162b1) in Adults 18 to 55 Years of Age: Interim Report, medRxiv. 2020.2006.2030.20142570. [Google Scholar]
- 57.Parhiz H., Khoshnejad M., Myerson J.W., Hood E., Patel P.N., Brenner J.S., Muzykantov V.R. Unintended effects of drug carriers: big issues of small particles. Adv. Drug Deliv. Rev. 2018;130:90–112. doi: 10.1016/j.addr.2018.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakurai H., Kawabata K., Sakurai F., Nakagawa S., Mizuguchi H. Innate immune response induced by gene delivery vectors. Int. J. Pharm. 2008;354:9–15. doi: 10.1016/j.ijpharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 59.Kim J.Y., Choung S., Lee E.J., Kim Y.J., Choi Y.C. Immune activation by siRNA/liposome complexes in mice is sequence- independent: lack of a role for toll-like receptor 3 signaling. Mol. Cell. 2007;24:247–254. [PubMed] [Google Scholar]
- 60.Szebeni J., Muggia F., Gabizon A., Barenholz Y. Activation of complement by therapeutic liposomes and other lipid excipient-based therapeutic products: prediction and prevention. Adv. Drug Deliv. Rev. 2011;63:1020–1030. doi: 10.1016/j.addr.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 61.Coelho T., Adams D., Silva A., Lozeron P., Hawkins P.N., Mant T., Perez J., Chiesa J., Warrington S., Tranter E., Munisamy M., Falzone R., Harrop J., Cehelsky J., Bettencourt B.R., Geissler M., Butler J.S., Sehgal A., Meyers R.E., Chen Q., Borland T., Hutabarat R.M., Clausen V.A., Alvarez R., Fitzgerald K., Gamba-Vitalo C., Nochur S.V., Vaishnaw A.K., Sah D.W., Gollob J.A., Suhr O.B. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N. Engl. J. Med. 2013;369:819–829. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]
- 62.Pardi N., Secreto A.J., Shan X., Debonera F., Glover J., Yi Y., Muramatsu H., Ni H., Mui B.L., Tam Y.K., Shaheen F., Collman R.G., Karikó K., Danet-Desnoyers G.A., Madden T.D., Hope M.J., Weissman D. Administration of nucleoside-modified mRNA encoding broadly neutralizing antibody protects humanized mice from HIV-1 challenge. Nat. Commun. 2017;8:14630. doi: 10.1038/ncomms14630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramaswamy S., Tonnu N., Tachikawa K., Limphong P., Vega J.B., Karmali P.P., Chivukula P., Verma I.M. Systemic delivery of factor IX messenger RNA for protein replacement therapy. Proc. Natl. Acad. Sci. U. S. A. 2017;114:E1941–E1950. doi: 10.1073/pnas.1619653114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thran M., Mukherjee J., Pönisch M., Fiedler K., Thess A., Mui B.L., Hope M.J., Tam Y.K., Horscroft N., Heidenreich R., Fotin-Mleczek M., Shoemaker C.B., Schlake T. mRNA mediates passive vaccination against infectious agents, toxins, and tumors. EMBO Mol. Med. 2017;9:1434–1447. doi: 10.15252/emmm.201707678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Hoecke L., Roose K. How mRNA therapeutics are entering the monoclonal antibody field. J. Transl. Med. 2019;17:54. doi: 10.1186/s12967-019-1804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schlake T., Thess A., Thran M., Jordan I. mRNA as novel technology for passive immunotherapy. Cell. Mol. Life Sci. 2019;76:301–328. doi: 10.1007/s00018-018-2935-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abrams M.T., Koser M.L., Seitzer J., Williams S.C., DiPietro M.A., Wang W., Shaw A.W., Mao X., Jadhav V., Davide J.P., Burke P.A., Sachs A.B., Stirdivant S.M., Sepp-Lorenzino L. Evaluation of efficacy, biodistribution, and inflammation for a potent siRNA nanoparticle: effect of dexamethasone co-treatment. Mol. Therap. 2010;18:171–180. doi: 10.1038/mt.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rybakova Y., Kowalski P.S., Huang Y., Gonzalez J.T., Heartlein M.W., DeRosa F., Delcassian D., Anderson D.G. mRNA delivery for therapeutic anti-HER2 antibody expression in vivo. Mol. Ther. 2019;27:1415–1423. doi: 10.1016/j.ymthe.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lokugamage M.P., Gan Z., Zurla C., Levin J., Islam F.Z., Kalathoor S., Sato M., Sago C.D., Santangelo P.J., Dahlman J.E. Mild innate immune activation overrides efficient nanoparticle-mediated RNA delivery. Adv. Mater. 2020;32:1904905. doi: 10.1002/adma.201904905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morrissey D.V., Lockridge J.A., Shaw L., Blanchard K., Jensen K., Breen W., Hartsough K., Machemer L., Radka S., Jadhav V., Vaish N., Zinnen S., Vargeese C., Bowman K., Shaffer C.S., Jeffs L.B., Judge A., MacLachlan I., Polisky B. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat. Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- 71.Takeda K., Kaisho T., Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 72.Draghici B., Ilies M.A. Synthetic nucleic acid delivery systems: present and perspectives. J. Med. Chem. 2015;58:4091–4130. doi: 10.1021/jm500330k. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y., Chirmule N., Gao G.-P., Qian R., Croyle M., Joshi B., Tazelaar J., Wilson J.M. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]
- 74.Sakurai F., Terada T., Yasuda K., Yamashita F., Takakura Y., Hashida M. The role of tissue macrophages in the induction of proinflammatory cytokine production following intravenous injection of lipoplexes. Gene Ther. 2002;9:1120–1126. doi: 10.1038/sj.gt.3301784. [DOI] [PubMed] [Google Scholar]
- 75.Ohto T., Konishi M., Tanaka H., Onomoto K., Yoneyama M., Nakai Y., Tange K., Yoshioka H., Akita H. Inhibition of the inflammatory pathway enhances both the in vitro and in vivo transfection activity of exogenous in vitro-transcribed mRNAs delivered by lipid nanoparticles. Biol. Pharm. Bull. 2019;42:299–302. doi: 10.1248/bpb.b18-00783. [DOI] [PubMed] [Google Scholar]
- 76.Walker M., Makropoulos D., Achuthanandam R., Bugelski P.J. Recent advances in the understanding of drug-mediated infusion reactions and cytokine release syndrome. Curr. Opin. Drug. Discov. Devel. 2010;13:124–135. [PubMed] [Google Scholar]