Abstract
Increasing market pressure to reduce the use of antibiotics and the Veterinary Feed Directive of 2019 have led to expanded research on alternate antibiotic solutions. This review aimed to assess the benefits of using essential oils (EOs) and their nanoemulsions (NEs) as feed supplements for poultry and their potential use as antibiotic alternatives in organic poultry production. Antibiotics are commonly used to enhance the growth and prevent diseases in poultry animals due to their antimicrobial activities. EOs are a complex mixture of volatile compounds derived from plants and manufactured via various fermentation, extraction, and steam distillation methods. EOs are categorized into 2 groups of compounds: terpenes and phenylpropenes. Differences among various EOs depend on the source plant type, physical and chemical soil conditions, harvest time, plant maturity, drying technology used, storage conditions, and extraction time. EOs can be used for therapeutic purposes in various situations in broiler production as they possess antibacterial, antifungal, antiparasitic, and antiviral activities. Several studies have been conducted using various combinations of EOs or crude extracts of their bioactive compounds to investigate their complexity and applications in organic poultry production. NEs are carrier systems that can be used to overcome the volatile nature of EOs, which is a major factor limiting their application. NEs are being progressively used to improve the bioavailability of the volatile lipophilic components of EOs. This review discusses the use of these nonantibiotic alternatives as antibiotics for poultry feed in organic poultry production.
Key words: antibiotic alternatives, essential oils, nanoemulsions, organic poultry
Abbreviations: AGPs, antibiotic growth promoters; AI, avian influenza; BWG, body weight gain; EOs, essential oils; FCR, feed conversion ratio; GIT, gastrointestinal tract; kg, kilogram; MDA, malondialdehyde; mg, milligram; ND, Newcastle disease; NEs, nano emulsions; PCU, population correction unit; ROS, reactive oxygen species; SOD, superoxide dismutase
INTRODUCTION
Subtherapeutic levels of antibiotics have been primarily utilized in the broiler industry to improve growth performance while decreasing morbidity and mortality (Abd El-Hack et al., 2020a,b); however, antibiotics are banned by the European Union due to the resulting presence of antibiotic residues in poultry products and the dramatic increase in the emergence of bacterial antibiotic resistance (Namdeo et al., 2020). Accordingly, alternate strategies are required to enhance poultry growth performance. Phytogenic feed supplements such as essential oils (EOs) are appropriate alternatives to boost growth efficiency in the meat production industry and improve broiler production (Namdeo et al., 2020).
EOs are a composite mixture of fragrant, fluid-based compounds formed via different methods of fermentation, extraction, and steam distillation. As a mixture of volatiles, their effect is a result of these components and their interactions (Bakkali et al., 2008; El-Tarabily et al., 2021). Variations in EOs activity are primarily determined by plant genotype, physical and chemical soil conditions, harvest time, plant maturity, drying technology, storage period, and extraction processes used (Bakkali et al., 2008). These differences also depend on the form, origin, method of inclusion in poultry diet, intake level, and composition of EOs as well as basal diet digestibility, hygiene measures used, and the environmental conditions of poultry production (Brenes and Roura, 2010).
EOs show the potential for restorative manipulation under various circumstances in broiler production. EOs enhance poultry production by stimulating the activity of various digestive enzymes, decreasing the number of fermentation products, reducing the number of pathogenic microorganisms, intensifying prececal nutrient digestion, ameliorating intestinal accessibility of significant nutrients, and improving antioxidant status and immune response (Brenes and Roura, 2010). In addition, EOs show hypocholesterolemic and coccidiostatic effects (Brenes and Roura, 2010). Commonly used EOs in broiler diets include anize, oregano, cinnamon, garlic, thyme, and turmeric (Lee, 2002). EOs are mainly categorized into 2 classes: terpenes and phenylpropenes (Lee, 2002). Terpenes are monoterpenes, sesquiterpenes, and diterpene units of 2, 3, or 4 isoprene units (Gopi et al., 2014). Phenylpropenes are phenylpropanoid volatiles derived from the amino acid phenylalanine. The structural diversity of phenylpropenes is derived from variation of the substituents on the benzene ring and in the position of the double bond in the propenyl side chain (Atkinson, 2018). Phenylpropenes such as eugenol, chavicol, estragole, and anethole contribute to the flavor and aroma of a number of important herbs and spices. They have been shown to function as floral attractants for pollinators and to have antifungal and antimicrobial activities (Atkinson, 2018).
Antibiotics are commercially used to prevent diseases in poultry because of their antimicrobial activities (Landoni and Albarellos, 2015). They are also essential in the treatment of many mammalian diseases (Abdelnour et al., 2020a); some antibiotics even have nutritive value as feed additives that enhance animal growth and performance (Abdelnour et al., 2020a; Abou-Kassem et al., 2021). Furthermore, antibiotics are used to increase the efficacy of poultry production and ensure proper development, strength, and immunity in animal farming industries (Reda et al., 2021a). However, overuse of antibiotics increases the amount of antibiotic-resistant bacteria as well as animal and human resistance factors (Abdelnour et al., 2020b; Saad et al., 2021a). Thus, researchers have been motivated to study several antibiotic alternatives to be used in poultry diets to reduce these negative antibiotic effects, improve consumer health, and protect the environment (Pan and Yu, 2014; Vlaicu et al., 2017; Alagawany et al., 2021a).
According to Abdelnour et al. (2020a), antibiotic alternatives should be nontoxic for animals, entirely excreted from the body and leave no residue, nonpolluting, palatable, and easily degradable and have a stable bioavailability. Additionally, additives should have no side effects on the animals while also improving diet efficiency and animal growth. They must also increase beneficial microbial load and reduce harmful microbial loads (Abdelnour et al., 2020a; Alagawany et al., 2021a; Alagawany et al., 2021b). Finally, antibiotic alternatives should maintain public health as well as animal health and productivity and be environmentally friendly. Antibiotic alternatives include EOs, organic acids, enzymes, nanoparticles, phytoncides, phytogenic feed additives, immunomodulators, probiotics, bacteriophages, and bacteriocins (Mehdi et al., 2018; El-Saadony et al., 2021a; Yaqoob et al., 2021).
Phytobiotics are potential antibiotic alternatives in poultry production (Windisch et al., 2008). They are compounds derived from plant extracts that enhance animal growth and performance (Mehdi et al., 2018). Specifically, they comprise a mixture of organic and bioactive compounds that are capable of preventing the development of antibiotic resistance (Suresh et al., 2018; El-Saadony et al., 2021c; El-Saadony et al., 2021d). Most phytobiotics, such as polypeptides and polyphenols, are secondary metabolites (Nabavi et al., 2015); and they are antimicrobial agents produced during the plant's metabolism (Hashemi and Davoodi, 2011). Plants also produce different bioactive compounds, that play an essential role against external stressors (El-Saadony et al., 2020a; El-Saadony et al., 2021b).
The antimicrobial and immunomodulatory activities of phytobiotics are important characteristics that allow for their use as feed supplements for poultry (Yang et al., 2018). Phytobiotics are known for maintaining growth, enhancing the immune system, and reducing stress in poultry (Yang et al., 2018). Several studies found that phytobiotics can improve poultry growth performance (Ghasemi et al., 2014; Li et al., 2015) and reduce microbiota colonization in poultry (Upadhyay et al., 2017) by nearly eliminating pathogen transmission (Abd El-Hack et al., 2020c; Ashour et al., 2020). Additionally, phytobiotics improve poultry growth by enhancing intestinal microbiota (Mountzouris et al., 2011) and preventing subclinical infections, thus resulting in improved nutrient uptake (Huyghebaert et al., 2011).
The volatile compounds in EOs, a type of phytobiotic, possess antimicrobial activities (Mahmoud and Croteau, 2002; Jayasena and Jo, 2013) that make them potential antibiotic alternatives (Chaves et al., 2008); however, their low bioavailability limits their use (Mahmoud and Croteau, 2002). Nanoemulsions (NEs) provide a medium for ensuring the bioavailability of EOs. NEs are regularly used in the food industry to encapsulate, protect, and deliver bioactive components having low water-solubility (McClements, 2012). In addition, several lipophilic compounds can be encapsulated by NEs to increase their bioavailability (Acosta, 2009).
Thus, we address nonantibiotic replacements, such as plant-derived EOs and their NEs, as novel alternatives to antibiotics. This review also focuses on the biological implications and antioxidant and antimicrobial activities of EOs, intestinal morphology and immunity of poultry animals, and broiler economy.
Antibiotics in the Poultry Industry
Antibiotics are natural, semisynthetic, or synthetic compounds that are commonly applied orally, parentally, or topically in humans and other animals to prevent and treat diseases (Phillips et al., 2004; Diaz-Sanchez et al., 2015). Antimicrobial agents have been widely used since the 1950s (Mathew et al., 2007) and the discovery of antibiotics has had an evident impact on medicine (Davies and Davies, 2010).
Antibiotics encourage growth, prophylaxis, or act as therapeutic agents in livestock production (Chattopadhyay, 2014). However, antibiotic growth promoters refers to a low sub-therapeutic dose of any antibiotic used to reduce or control the bacterial population in livestock (Ronquillo and Hernandez, 2017). Clostridium, Salmonella, and Mycoplasma genera cause various diseases in poultry, such as salmonellosis and mycoplasmosis, resulting is an economic loss to poultry production. Antibiotics effectively controlled these diseases (Singer and Hofacre, 2006; Mathew et al., 2007; Abd El-Hack et al., 2021a).
Poultry was ranked second in the consumption of antimicrobials with 148 mg/population correction unit, followed by cattle with 45 mg/population correction unit; whilst, pigs ranked first with 172 mg/population correction unit. Antimicrobials are the primary weapon for increasing animal production to cover increased food demand by preventing disease and increasing growth and feed efficiency (Van Boeckel et al., 2015). The utilization of antibiotic growth promoters is increasing due to their importance to livestock companies and extensive and intensive agricultural operations (Phillips et al., 2004; Van Boeckel et al., 2015). Fortifying poultry feed with antibiotics increased the growth performance and immunity of chickens. Additionally, it reduced gut microbes that threaten the herd's immunity (Neish, 2002; Kumar et al., 2018). Therefore, antibiotic utilization can increase poultry production.
Recent studies in poultry nutrition showed that antibiotic growth promoters could promote growth (Chattopadhyay, 2014) and maintain bird health by reducing the bacterial load, thinning the mucosal layer, and inducing the immune system (Lee et al., 2012). The critical role of antibiotics in promoting productivity is through controlling the gut microbiota in poultry intestine (Singh et al., 2013) by decreasing bile salt hydrolase activity; this can be brought about by reducing the bacterial enzymes in the intestine, which in turn, negatively affects digestion and the use of fats (Lin, 2014).
Antibiotics promote growth by increasing the host's nutrient absorption capacity and the load of beneficial bacterial with nonantagonistic functions (Butaye et al., 2003; Phillips et al., 2004). The utilization of antibiotic growth promoters is intrinsically linked with the modification of intestinal microbiota and growth promotion (Lin, 2014). Many antibiotics are bacteriostatic, that is, they only inhibit the bacterial population, whereas others are bactericidal and kill the bacteria. Both types of antibiotics are available in the market (Kohanski et al., 2010); however, few of these have been thoroughly investigated for use in livestock (Butaye et al., 2003). Up to 90% of the antibiotics used in animal production are excreted to the environment in a raw format or as other metabolites (Carvalho and Santos, 2016).
The misuse and overuse of antibiotics in livestock production induced antibiotic resistance in pathogenic bacteria (Wegener, 2003; Phillips et al., 2004). Antibiotic resistance can naturally occur through random chromosome mutations that are vertically transmitted when cells divide or by horizontal gene transfer wherein other bacterial resistance genes are transferred (Diarra and Malouin, 2014; Toutain et al., 2016). Thus, resistance is a defense mechanism of microbes against antibiotics, and the emergence of antibiotic-resistant bacterial strains is a critical problem (Khameneh et al., 2016; Marquardt and Li., 2018) affecting the effectiveness of antibiotics in treating diseases (Wegener, 2003).
Eliminating antibiotics from poultry diet negatively affects the performance and growth of the animal due to the increased incidence of diseases (Huyghebaert et al., 2011). As bacteria already possess antibiotic resistance mechanisms, it is crucial to investigate alternatives for various nonantibiotic alternatives (Suresh et al., 2018). Thus, the issue of antibiotic resistance must be addressed through multifaceted and interdisciplinary research to discover alternatives to antibiotics (Allen et al., 2013). Determining substitutes for antibiotics is increasingly necessary to sustain and enhance public health globally (Goossens et al., 2005).
Biological Implications of EOs
Bioactive compounds, in general, affect digestive enzymes, amylases, and proteases (Jang et al., 2007) and improve poultry growth by enhancing absorbent cells in the gut (Jamroz et al., 2006). Important plants can be used as alternative feed additives for antibiotic growth promoters in poultry production (Windisch et al., 2008). Phytobiotics and their derivatives are safer than antibiotics, are not used for medical or veterinary purposes, and positively affect animal production (Hashemi and Davoodi, 2011). Herbs, spices, and EOs contain many bioactive compounds depending on their origin and processing (Goossens et al., 2005).
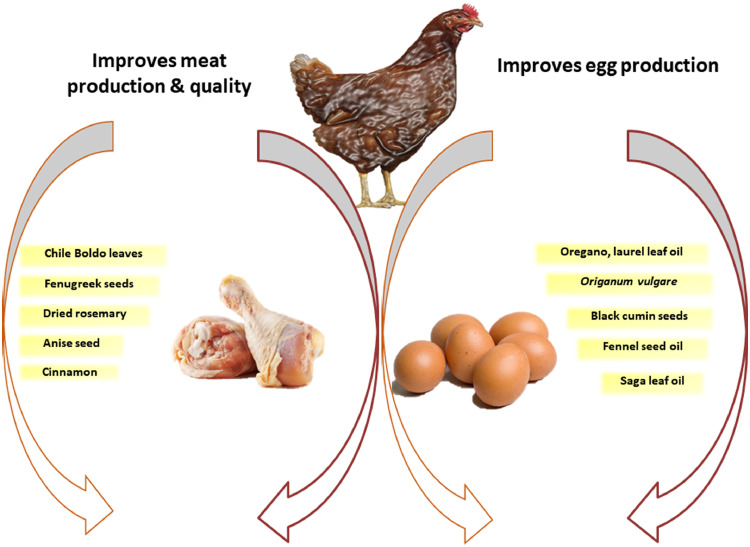
EOs are natural, volatile, aromatic oily fluids extracted from various parts of the plant (Bakkali et al., 2008). EOs are effective digestive stimulants, growth promoters, hypolipidemic agents, immunomodulators, antioxidants, antimicrobial, and antiparasitic agents. Some of the EOs that positively affect meat quality and egg production are shown in Figure 1.
Figure 1.
Some of the essential oils with a positive effect on meat quality, and egg production.
Effect of EOs on the Growth Performance of Chickens
Importance of EOs in Poultry Production
Previous studies on chickens have indicated that the inclusion of EOs in the diet improves growth performance by stimulating the secretion of digestive enzymes, which results in increased digestion and absorption of nutrients with an improved gut transit rate (Jamroz et al., 2005). In addition, EOs have been shown to have a positive effect on the activity of trypsin and amylase enzymes when included in poultry feed (Jamroz et al., 2005). Incorporating EOs theoretically reduces the incidence of intestinal diseases caused by undesirable bacteria and favors the growth of beneficial gut microbiota, thus improving growth performance (Bento et al., 2013). Factors such as temperature, viscosity, visual appearance, and feed intake are influenced by saliva production, food nutrient content, particle size, feed toxicity, and social interaction (Bölükbaşi et al., 2006).
Many experiments have demonstrated the beneficial effect of EOs on feed intake in poultry. For example, elevated feed intake was observed when broiler diets were supplemented with a mixture of EOs from clove, oregano, and anize at 200 and 400 mg/kg for 3 wk (Ertas et al., 2005). In the same study, bioactive compounds such as thymol, anethole, eugenol, and carvacrol found in the EOs were responsible for increasing appetite, which ultimately increased feed intake (Ertas et al., 2005). Similarly, improved overall feed intake was observed in broilers consuming feed supplemented with 18 mg/kg each of cinnamon, oregano, thyme, and eucalyptus EOs, which was attributed to the enhanced gut microbiota (Ulfah, 2006).
Other investigators studied the effects of oregano EOs on broiler efficiency for a period of 42 d using the following dietary treatments: basal diet, basal diet + 8 mg/kg of avilamycin (AVI); basal diet + 300 mg/kg of oregano EOs (OEO300); and basal diet + 600 mg/kg of oregano EOs (OEO600). The OEO300 and OEO600 groups showed significant increases in average daily feed consumption and average daily gain compared with the control group on d 42 (P < 0.05) (Peng et al., 2016). In addition, bodyweight gain (BWG) increased in the groups supplemented with EOs due to the antimicrobial action and stimulation of various digestive enzymes, which enhanced nutrient utilization (Hernandez et al., 2004). A BWG increase of 8 to 16% in the group supplemented with 200 ppm EOs was observed compared with the antibiotic and control groups, thus demonstrating the growth-enhancing properties of EOs in poultry (Ertas et al., 2005).
The feed conversion ratio (FCR) is a measure of how efficiently is feed mass converted to body mass over a fixed time. Cinnamon powder, which contains cinnamaldehyde, was shown to improve the FCR in broilers (Hernandez et al., 2004). A diet supplemented with cinnamaldehyde decreased harmful bacteria in the gut, leading to increased nutrient digestibility. Similarly, broiler feed supplemented with a mixture of EOs at 200 ppm significantly (P < 0.05) improved FCR compared with the antibiotic (by 6%) and control groups (by 12%; Ertas et al., 2005). Likewise, broilers fed with myrtle leaf, oregano, sage leaf, laurel leaf, citrus peel, and fennel seed EOs showed significant improvements in FCR (Namdeo et al., 2020). In another study, supplementing broiler diets with thyme extract EO increased secretion of digestive enzymes, such as amylase and chymotrypsin, and improved intestinal absorption rates and feed utilization (Wade et al., 2018).
Mechanisms of Action of EOs on the Digestive System of Poultry Animals
EOs show a beneficial influence on the avian digestive system by recovering the microbiota balance and improving nutrient absorption, primarily due to the presence of terpenoid substances that improve FCR (Mountzouris et al., 2011). These compounds also enhances the production of digestive enzymes, which further contributes to better digestion and nutrient absorption (Mountzouris et al., 2011).
EOs influence feed taste and smell, and enhance protein digestion by increasing gastric secretions and HCl concentrations (Gopi et al., 2014). Although some EOs can irritate the intestinal mucosal lining and cause inflammation, it is necessary to carefully select the EOs supplements (Gopi et al., 2014).
Effects of EOs on Feed Consumption, Increased Body Weight, and Conversion Ratios
Many EOs have been shown to increase poultry growth. For example, Tiihonen et al. (2010) reported that EOs supplementation at 5 g/t cinnamaldehyde and 15 g/t thymol in broiler diet decreased undesirable bacterial growth, thus maintaining a healthy gut microbiota and improving growth performance. A similar study showed that a mixture of caraway, basil, lemon, laurel, sage, oregano, thyme, and tea EOs enhanced broiler growth (Khattak et al., 2014). The tecnaroma herbal mixture at 100, 200, 300, 400, and 500 g/t of feed also significantly (P < 0.05) increased the average daily gain and body weight (BW) and improved FCR compared with the control. Likewise, significant increase in BW was detected in broilers supplemented with EOs blends at 300 and 600 g/kg of feed (Peng et al., 2016). Thus, feed conversion greatly improved poultry development when anize, citrus, sage, oregano, and laurel components were added to the feed by increasing nutrient availability through the modulation of the intestinal ecosystem (Çabuk et al., 2006).
EOs also increase growth, reduce disease, and improve the health of animals with subpar hygiene. For example, EOs prevented and cured necrotic enteritis in poultry (Jerzsele et al., 2012). In addition, cinnamon, pepper, and oregano EOs increased digestibility among chickens and enhanced FCR (Hernandez et al., 2003; Windisch et al., 2008). EOs, including carvacrol, cinnamaldehyde, and capsicum oleoresin, positively influence broiler productivity and nutrient use in areas with poor hygiene (Bravo et al., 2014). These effects may be mediated by gastrointestinal microbial alterations, improved local immunity against Eimeria infections, enhanced liver antioxidant status, and improved nutritional energy and nutrient usage (Karadas et al., 2014). The biological effects of EOs in poultry production are shown in Table 1.
Table 1.
Biological effects of essential oils (EOs) in poultry production.
| Essential Oils | Effect | References |
|---|---|---|
| The phytobiotic bioactive substances | Increases the activity of amylase and protease. Affects digestive enzyme development and activity. | (Jang et al., 2007) |
| EOs | Minimizes intestinal diseases caused by unwanted bacteria. Promotes good gut microbiota development. Enhances growth performance. | (Bento et al., 2013) |
| Thymol, anetole, eugenol and carvacrol | Increases feed intake. | (Ertas et al., 2005) |
| Cinnamon, oregano, thyme, and eucalyptus EOs | Balances gut microbiota. | (Ulfah, 2006) |
| Oregano EOs | Increases body weight gain in essential oil supplementation, combined with its antimicrobial activity and stimulation of various digestive enzymes that boost nutrient usage. | (Hernandez et al., 2004) |
| Cinnamon powder | Contains cinnamaldehyde that helps increases feed conversion ratio in broilers. | (Decker and Park, 2010) |
| EOs mixture | 200 ppm greatly increases feed conversion ratio by 6 and 12% compared to the antibiotic and the control groups, respectively. | (Ertas et al., 2005) |
| (Myrtle leaf oil, oregano oil, sage leaf oil, laurel leaf oil, citrus peel oil, and fennel seed oil) | Shows considerable improvement in feed conversion ratio. | (Çabuk et al., 2006) |
| Thyme extract | Increases secretion of digestive enzymes, i.e. amylase and chymotrypsin. Increases absorption rates in the intestine. Enhances feed utilization. | (Wade et al., 2018) |
| EOs consisting of 5 g/ton cinnamaldehyde and 15 g/ton thymol | Decreases the growth of undesirable bacteria and enhances beneficial intestinal microbiota growth. Improves growth performance of broilers. | (Tiihonen et al., 2010) |
| Mixture of EOs from caraway, basil, lemon, laurel, sage, oregano, thyme, and tea | Enhances growth performance. | (Khattak et al., 2014) |
| EOs blend at 300 and 600 g/kg of feed | Enhances growth performance. | (Peng et al., 2016) |
| Carvacol and thymol | Increases SOD activity. | (Hashemipour et al., 2013) |
| Ginger EOs at 150 mg/kg | Increases total SOD activity and decreases malondialdehyde concentrations in the liver, which may be attributed to the presence of many antioxidant compounds such as shogaol, gingerol, zingerone, and diarylheptanoids in ginger root. | (Habibi et al., 2014) |
| Cinnamon bark oil at 300 mg/kg diet | Enhances antioxidant status in broilers as SOD activity was significantly increased in cinnamon bark oil–complemented birds compared to the antibiotic treatment. | (Chowdhury et al., 2018) |
| Lemongrass (Cymbopogon citratus) EOs | Inhibits pathogenic bacteria, such as Salmonella typhimurium, Salmonella enterica, Escherichia coli, Staphylococcus aureus, Listeria monocytogenes, Klebsiella pneumoniae, and Candida albicans. | (Fagbemi et al., 2009; Naik et al., 2010; Singh and Ebibeni, 2016; Juniatik et al., 2017) |
| Trans-cinnamaldehyde and eugenol | Decreases Salmonella enteritidis in 20-day-old broiler poultry. | (Kollanoor-Johny et al., 2012a) |
| Curcumin, carvacrol, piperin, thymol, and eugenol | Decreases the colonization and proliferation of Clostridium perfringens in the broilers gastrointestinal tract. | (Mitsch et al., 2004) |
| Oreganum aetheroleum EOs | Improves broiler chickens immunity against Escherichia coli infections. Enhances cell-mediated and humoral immune responses. | (Abd El-Ghany and Ismail, 2014) |
| Oregano and thyme EOs | Reduces the number of a broad range of pathogenic bacteria such as Salmonella strains in the chicken gastrointestinal tract. | (Koščová et al., 2006) |
| Thyme, oregano, rosemary, clove, and cinnamon | Preserves the intestinal wall from damage due to the effects of coccidial multiplication. Promotes growth. | (Hashemi and Davoodi, 2011) |
| Anise, citrus, sage, oregano, and bay leaf | Improves nutrients availability by adjusting the intestinal ecosystem. | (Çabuk et al., 2006) |
| Inclusion of 300 and 600 mg/kg oregano essential oil (Origanum spp.) | Improves the birds’ average daily gain. | (Peng et al., 2016) |
| Trans-cinnamaldehyde at 0.75% and eugenol at 1% as an antimicrobial additive in the feed for 5 d prior to slaughter | Reduces cecal colonization of Salmonella enteritidis by 1.5 log10 CFU/g. | (Kollanoor-Johny et al., 2012b) |
| Trans-cinnamaldehyde | Reduces egg-borne transmission of Salmonella enteritidis in commercial layers. | (Abd El-Hack et al., 2021c) |
| Trans-cinnamaldehyde for 66 d at 1 and 1.5% to 40-wk and 25-wk old layer hens | Reduces Salmonella enteritidis on the egg shell and in the yolk without causing any harmful effect on the growth, egg production, and consumer acceptability of eggs. | (Galiş et al., 2013) |
| Carvacrol and thymol | Decreases Campylobacter colonization, although consistency in the antimicrobial effect across the experiments was a problem. | (Abd El-Hack et al., 2021d) |
| Thymol at 0.25 and 1%, or carvacrol at 1%, or a combination of the molecules at 0.5% | Efficient against Campylobacter colonization in broilers. | (Arsi et al., 2014) |
| Garlic and oregano EOs | Reduces population of Clostridium spp. | (Kirkpinar et al., 2011) |
| Cinnamaldehyde and thymol | Shows selective antibacterial properties and reduces yeast, and mold growth. | (Bento et al., 2013) |
| Cinnamon oil | Induces detrimental changes in Escherichia coli cell wall. | (Rahimi et al., 2011) |
| Ajwain oil (AJO), clove oil (CLO), and cinnamon oil (CNO) (400, 600, 300 mg/kg) of diet, respectively | Decreases the counts of Escherichia coli and Clostridium species in pre-cecal contents. | (Chowdhury et al., 2018) |
| Thymol supplementation | Increases intestine length, and the width and depth of the villi. Improves nutrient absorption. | (Alcicek et al., 2003) |
| Carvacol (5.4%), cinnamaldehyde (2.9%), capsicum oleoresin (2.18%) | Increases villus length and intestinal diameter. | (Awaad et al., 2014) |
| Mentha piperita leaves | Enhances histomorphology structure of mucosa of small intestine of broilers. | (Hamedi et al., 2017) |
| Cinnamon oil at 0.3 mg/g of diet | Improves height of villi in duodenum, jejunum, and ileum. | (Chowdhury et al., 2018) |
| Anise, oregano, and citrus peel | Decreases cholesterol by lower the very-low-density lipoprotein levels and increased total flavonoids. | (Hong et al., 2012) |
| Cinnamon essential oil at 300 mg/kg | Reduces cholesterol level and 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase enzyme post-transcriptionally without changing mRNA levels of the enzyme. | (Qureshi et al., 1996; Chowdhury et al., 2018) |
| Thymol, ionone, and carvacrol | Induces a presumed regulatory nonsterol product. | (Elson, 1996) |
| Garlic | Enriches assembly of gamma interferon, interleukins, tumor necrosis factor alpha. Increases phagocytosis of antigen-presenting cells and macrophages. | (Hanieh et al., 2010) |
| Eucalyptus and peppermint EOs | Shows higher hemagglutinin-inhibition antibody titers against both avian influenza (AI) and Newcastle vaccines as compared to control. Shows specific antibody response against influenza vaccine virus. | (Talazadeh and Mayahi, 2017) |
| Thymol EOs | Shows higher (p<0.05) spleen index than birds of control group. Increases the level of secretory immunoglobulin A (p<0.05) in duodenum and ileum mucosa of finisher group. | (Yang et al., 2018) |
| Allium sativum, Echinacea purpure | Effective against intestinal parasites, including the Eimeria species. | (Zhai et al., 2007) |
| Oregano EOs at 300 mg/kg in experimentally infected (Eimeria tenella) birds | Decreases number of Eimeria tenella oocysts. | (Giannenas et al., 2004) |
| Phenols | Exhibits oocysticidal activity against Eimeria tenella. | (Williams, 1997) |
| Supplementation of a natural blend of EOs (basil, lemon, caraway, oregano, laurel, sage, thyme, and tea) | Increases carcass weight, breast weight, and breast meat. | (Khattak et al., 2014)) |
| Oregano EOs supplementation at 600 mg/kg | Displays high breast muscle percent together with augmented dressing percent, eviscerated rate, and leg muscle percent. | (Peng et al., 2016) |
| Ocimum basilicum EOs | Shows antimicrobial activity against a wide range of Gram-negative and Gram-positive bacteria, yeast, and mold. | (Citarasu, 2010) |
| Sunflower oil nanoemulsion | Shows antibacterial activity against foodborne bacteria such as Listeria monocytogenes, Salmonella typhi, and Staphylococcus aureus. Shows high fungicidal and a sporicidal activity against Rhizopus nigricans, Aspergillus niger, Penicillium species, Bacillus cereus, and Bacillus circulans. | (Joe et al., 2012) |
Abbreviations: AI, avian influenza; CFU, colony forming units; FCR, feed conversion ratio; GIT, gastrointestinal tract; HI, hemagglutinin-inhibition; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A reductase; ND, Newcastle disease; SOD, super oxide dismutase; VLDL, very low-dentistry lipoprotein.
Effect of EOs on Antioxidant Activity
Lipid oxidation (lipid peroxidation) and free radical formation are natural processes that breakdown membrane structure, disrupt transportation processes, and cause cell organelle dysfunction. In cell membranes, phospholipids are significantly more susceptible to damage due to the oxidation that corresponds with the degree of fatty acid unsaturation. Polyunsaturated fatty acids enable important cell membrane properties, such as permeability and fluidity (Pisoschi and Pop, 2015).
Synthetic antioxidants (e.g., butylated hydroxyl anisole, butylated hydroxyl toluene, tert-butylhydroquinone, and propyl gallates) have been used to retard lipid peroxidation's effect of scavenging peroxyl radicals; however, their use was debated because of their carcinogenic effects. Because of the negative effects of these synthetic antioxidants, consumers have become more interested in natural antioxidant sources (Hashemipour et al., 2013). The chemical structure and high redox properties of EOs can neutralize free radicals, thus quenching singlet and triplet oxygen, and chelate transitional metals. EOs are a rich source of such phenolic, natural antioxidant compounds (Rahimi et al., 2011); however, EOs can act as pro-oxidants depending on dosage and type, by destroying cell and organelle integrity and causing additional cytotoxic effects on living cells (Bakkali et al., 2008).
EOs positively impact antioxidant expression, such as superoxide dismutase (SOD), glutathione peroxidase, and catalase, and prevent the formation of reactive oxygen species (ROS) and off-flavor polyunsaturated fatty acid oxidation (Marcinčák et al., 2008; Miguel, 2010). Thus, the dietary supplementation of EOs in poultry may enormously improve their quality of life (Decker and Park, 2010). In several studies, a significant improvement in antioxidant activity was observed upon EOs supplementation in poultry. For example, when an equal mixture of carvacrol and thymol was supplemented to broilers at 60, 100, and 200 mg/kg, it was reported that SOD activity (P < 0.05) increased in a dose-dependent manner (Hashemipour et al., 2013).
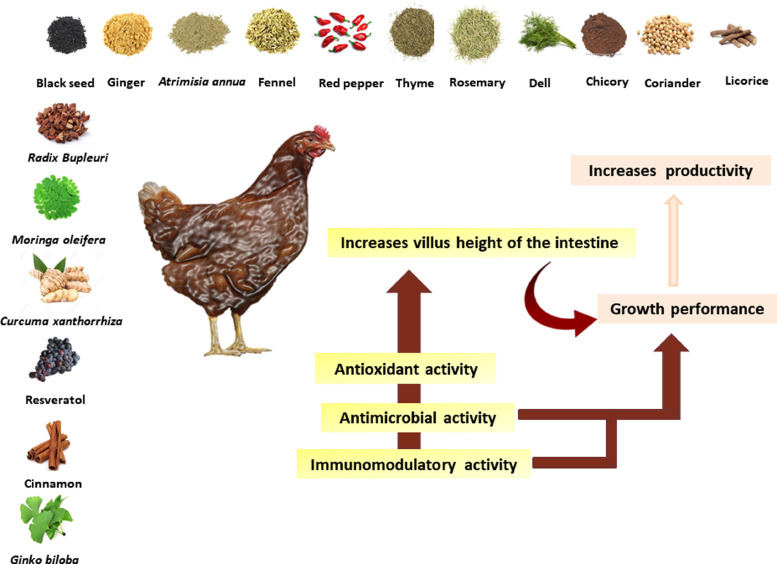
Furthermore, Habibi et al. (2014) showed that ginger EOs supplementation at 150 mg/kg increased the total SOD activity and decreased the malondialdehyde levels in the liver. Several antioxidant compounds may cause these changes in the ginger root, such as shogaol, gingerol, and zingerone (Habibi et al., 2014). Similarly, Chowdhury et al. (2018) demonstrated that cinnamon bark oil (300 mg/kg diet) substituted for antibiotics (50 mg/kg diet) resulted in improved antioxidant status in broilers. In addition, SOD increased significantly in cinnamon oil-supplemented birds as compared to the antibiotic treatment (Chowdhury et al., 2018). The effects of different EOs on the antioxidant activities in poultry are shown in Table 1. The effects of EOs on growth performance and poultry productivity due to their antioxidant, antimicrobial, and immunomodulatory effects are shown in Figure 2.
Figure 2.
Effects of essential oils on growth performance and poultry productivity due to their antioxidant, antimicrobial, and immunomodulatory effects.
Antimicrobial Activity of EOs
Major Bacterial Foodborne Pathogens in Poultry
The bacterial pathogens Salmonella and Campylobacter, which are transmitted through poultry products, are the most common infectious bacteria in human disease. On farms, poultry (including broilers, layers, and turkeys) can serve as the primary source of product contamination (Gantois et al., 2009). The primary site for Salmonella colonization in poultry is the ceca, which leads to Salmonella spp. on the carcass and eggshells (Gantois et al., 2009; Abd El-Hack et al., 2020a). Egg infection with Salmonella is caused by the injection of infected feces through the eggshell before or after oviposition (Gantois et al., 2009). Similarly, Campylobacter mainly colonizes the mucus of the ceca and the epithelial cells. Once birds become colonized, the bacteria rapidly disseminate to the entire flock via high levels of fecal shedding and fecal-oral transmission through water and feed (Lee and Newell, 2006). In an organic setting, the likelihood of birds being exposed to Salmonella and Campylobacter is relatively high due to their outdoor access. These pathogens could infect birds from the soil or water (Lee and Newell, 2006; Noormohamed and Fakhr, 2014).
Organic poultry products can provide ideal hosts for the transmission of Salmonella and Campylobacter to humans (Sato et al., 2004; Noormohamed and Fakhr, 2014). There is evidence that organic poultry harbors similar or higher pathogens levels than conventionally raised poultry. Cui et al. (2005) studied pathogen incidence in organic poultry and found that 76% and 61% of the samples were contaminated with Campylobacter or Salmonella, respectively. They also observed a similar prevalence for Campylobacter in organic and commercial chickens, but found more Salmonella in organic chicken samples (Cui et al., 2005). Noormohamed and Fakhr (2014) reported similar Campylobacter prevalence in organic and conventional chickens in a comparable study.
In Louisiana, USA, approximately 21 to 22% of Salmonella prevalence was reported in sampled organic and conventional chicken carcasses (Lestari et al., 2009). Overbeke et al. (2006) also observed a similar prevalence of Salmonella from conventional and organic chicken samples. It was concluded that >74% of the poultry carcasses sampled from both conventional and organic sources were contaminated with Campylobacter, and 27% of the US Department of Agriculture organic-certified carcasses had Salmonella contamination (Vaarst et al., 2019).
Consumers may assume that organically raised poultry carries fewer foodborne pathogens than conventionally raised birds given their lower bird density, outdoor access, pesticide, and antibiotic use (Vaarst et al., 2019). This assumption may increase the potential for contamination by mishandling of the raw product (Vaarst et al., 2019). This situation warrants effective organic-friendly antimicrobial strategies on the farm and during processing to improve poultry meat and egg quality (Vaarst et al., 2019). Unfortunately, organic poultry operations currently have limited effective interventions against these harmful pathogens.
Antimicrobial Functions of EOs against Poultry Pathogens
Broiler chicken productivity is optimal with a healthy gastrointestinal tract (GIT) (Sugiharto, 2016). A balanced population of pathogenic and beneficial microflora in the GIT plays a crucial role in the digestion and absorption of nutrients (Patterson and Burkholder, 2003; Kročko et al., 2012). Escherichia coli, Salmonella typhimurium, and Clostridium perfringens are the 3 major pathogens found in the poultry gut (La Ragione et al., 2004); however antibiotic resistance concerns caused antibiotics used as growth promotors to be banned in poultry diets, which has led to an urgent need to explore the alternative for antibiotic growth promoters (Hajati and Rezaei, 2010).
Phytobiotics, especially EOs, have positively impacted poultry production and feed consumption by enhancing the taste and flavoring of feed (Grashorn, 2010). Phytobiotics can also efficiently inhibit pathogenic bacteria, as demonstrated by lemongrass (Cymbopogon citratus) phytobiotics, which inhibited S. typhimurium (Oussalah et al., 2007), Salmonella enterica (Singh and Ebibeni, 2016), E. coli, Staphylococcus aureus, Listeria monocytogenes (Asaolu et al., 2009), and Klebsiella pneumonia (Fagbemi et al., 2009; Naik et al., 2010). Lemongrass oil and kaffir lime oil (Citrus hystrix) also showed antifungal properties against pathogenic yeasts, such as Candida albicans (Juniatik et al., 2017).
EOs are hydrophobic (Bakkali et al., 2008; Sánchez-González et al., 2011), with antibacterial activities but with low aqueous solubility (Khumpirapang et al., 2017). One efficient method for the delivery of self-nano-emulsifying drug delivery systems is to improve oral bioavailability (Nagaraju et al., 2019) and solubility (Bansal and Jamil, 2018) to control drug release (Baskar et al., 2018). The formula optimization analysis goal is to determine the variable level from which a high-quality product can be generated (Basalious et al., 2010).
EOs contain many secondary metabolites, such as isoprenoid compounds (Brewer, 2011). Studies on the antimicrobial activities of EOs of plant origin have demonstrated that these EOs can be very useful as feed supplements for poultry (Burt, 2004). EOs promote bile secretion and stimulate digestive enzyme activity and mucus, thus facilitating intestinal functions (Platel and Srinivasan, 2004). Therefore, EOs are used in poultry diets for their antioxidant and antimicrobial activities, which modify the gut microbiota and stimulate the digestion process by affecting the metabolic processes of starch, protein, and fat digestion (Lee, 2002; Hernandez et al., 2004; Goossens et al., 2005).
Several phyto-EOs have been shown to inhibit pathogen emergence in poultry (Micciche et al., 2019). For example, the fortification of poultry diets with cinnamaldehyde and eugenol reduced the Salmonella enteritidis load in broilers (Kollanoor-Johny et al., 2012a). Diets supplemented with EOs, such as curcumin, carvacrol, piperine, thymol, and eugenol, reduced C. perfringens in the chicken GIT (Abd El‐Hack et al., 2021a). EOs display potential actions against C. perfringens, E. coli (Jang et al., 2007), and Campylobacter spp. (Kelly et al., 2017), which inhabit the digestive systems of broiler chickens (Windisch et al., 2008).
When animal diets supplemented with the antibiotic ciprofloxacin and Oreganum aetheroleum EOs were compared, it was found that the EOs reduced the amount of E. coli by enhancing the immune responses and more successfully treating the infection (Abd El-Ghany et al., 2013). Furthermore, in the chicken gut, oregano and thyme EOs effectively reduced pathogenic bacteria, such as Salmonella spp. (Koščová et al., 2006). In addition, some EOs, such as rosemary, clove, thyme, oregano, and cinnamon, increased the strength of the intestinal wall by affecting coccidial multiplication. Thus, EOs can be used as growth promoters (Hashemi and Davoodi, 2011).
Supplementation of 1 g/kg thyme EOs significantly augmented the BWG of broiler chickens compared to the inclusion of 10 g/kg of whole thyme leaves, (Cross et al., 2007). EOs composed of an oregano, anize, and clove mixture increased the BWG by approximately 16% relative to the control group (Ertas et al., 2005). The addition of an EO mixture of anize, oregano, and bay leaves to chicken diets at a concentration of 200 mg/kg led to the modification of the gut microbiota and significantly increased chicken growth compared to the control group both immediately and after 5 wk of trial (Ertas et al., 2005; Çabuk et al., 2006). The addition of oregano EOs at 300 to 600 mg/kg to broiler feed improved the average daily gain of the birds (Peng et al., 2016). Several EOs have been used as a substitute for antibiotic growth promoters to improve chicken production (Khattak et al., 2014; Pirgozliev et al., 2015; Peng et al., 2016). Corresponding decreases in pathogenic bacterial populations increased nutrient availability, minimized nutrient competition, and avoided multiple GIT diseases (Yitbarek, 2015).
The hydrophobicity and the ability of EOs to penetrate the membrane of bacterial cells containing lipids and their antimicrobial activities are important characteristics that are responsible for EO effectiveness in poultry diets (Smith-Palmer et al., 2004). Exposure to EOs increases bacterial membrane permeability, which leads to cell lysis due to cell content leakage (Carson et al., 2002). The hydrophobicity of the volatile compounds in EOs enables their interaction with the lipid contents in the bacterial cell membrane, which affects the cell's properties, and leads to the death of the pathogenic bacteria. It also affects the permeability of the bacterial membranes, allowing the active compounds to penetrate the cell and bind with specific proteins as another inhibitory activity (São Pedro et al., 2013).
Chemical groups found in the active compounds of EOs have been posited to have antimicrobial activities (Carson et al., 2002; Burt, 2004; Smith-Palmer et al., 2004). EOs contain more than 100 active compounds (Bilia et al., 2014; Calo et al., 2015). Terpenic compounds (monoterpenes, sesquiterpenes, and diterpene), alcohols, acids, esters, epoxides, aldehydes, ketones, amines, and sulfides are typically the chemical contents of petrol. Hence, the use of EOs as an antimicrobial agent was hypothesized to reduce the population of harmful bacteria in the broiler gut (Smith-Palmer et al., 2004).
A limiting factor in the use of EOs as an alternative to antibiotics is its high volatility and the thermolabile, photolabile, and less stable nature of most of its constituents (Yitbarek, 2015). Due to the high volatility of many EO constituents, EOs oxidize readily when exposed directly to heat, air, light, and humidity (Bilia et al., 2014). EOs oxidize when exposed to light and heat because they include unsaturated carbon chains. The oxidation leads to the formation of terpenes and sesquiterpenes with lacons rings and terpenoids (Vigan, 2010). These compounds are characterized by being unstable and fat soluble with limited bioavailability. The low solubility of EOs in water reduces its uptake in biological fluids; therefore, a new approach is needed to overcome these limiting factors to improve EO bioavailability (Natrajan et al., 2015).
EOs have been used in medicine, food preservation, cosmetics, and perfumery for centuries (Venkitanarayanan et al., 2013). Originating from plants, extracts of volatile plant compounds and most of their active components present an array of safe alternatives that are natural, less toxic, environmentally friendly, and generally recognized by the US Food and Drug Administration as safe feed additives (Venkitanarayanan et al., 2013; Upadhyay et al., 2014). As EOs and their biological constituents contain many active chemical groups, their antimicrobial activities may be due to many different mechanisms (Burt, 2004). This unique characteristic significantly reduces the likelihood that bacteria will develop resistance to EOs. Recently, researchers have focused on utilizing EO compounds against foodborne pathogens in organic chickens (Kollanoor-Johny et al., 2010).
In vitro experimental testing of EOs and their compounds have revealed promising results. For example, trans-cinnamaldehyde is the most critical element in cinnamon bark extract, and eugenol is a natural phytophenolic mixture in the clove mixture of EOs (Kollanoor-Johny et al., 2010). Thymol and its structural carvacrol isomers are derived from many plant sources, including Thymus, Origanum, and Carum spp. (Kollanoor-Johny et al., 2010).
Kollanoor-Johny et al. (2010) evaluated the in vitro efficacy of several active EO compounds, including trans-cinnamaldehyde, eugenol, thymol, and carvacrol, against Salmonella and Campylobacter in commercial broiler and layer chickens using a modified cecal medium (Kollanoor-Johny et al., 2010). Each compound was added (concentrations of 0.1–1.2%) to chicken cecal substances inoculated with 7.0 log10 CFU/mL of S. enteritidis or 5.0 log10 CFU/mL Campylobacter (Kollanoor-Johny et al., 2010). Reduced S. enteritidis and Campylobacter spp. populations were found in the cecal content, demonstrating that these EO compounds were bactericidal against both pathogens, with trans-cinnamaldehyde and eugenol the most effective (Kollanoor-Johny et al., 2010).
In vivo trials have also shown encouraging antibacterial activities, confirming the in vitro findings. Kollanoor-Johny et al. (2010) examined the effectiveness of trans-cinnamaldehyde and eugenol against S. enteritidis in broiler chickens by supplementing the diets for 20 d with either 0.5% or 0.75% trans-cinnamaldehyde or 0.75% or 1% eugenol. The birds were then challenged with 5.0 log10 CFU/mL on d 8. At the end of the study, the S. enteritidis population in the cecum was determined. Both concentrations of trans-cinnamaldehyde and 1% eugenol decreased S. enteritidis colonization in the cecum (3.0 log10 CFU/g; Kollanoor-Johny et al., 2010). The pH in the cecum altered neither the compound nor the endogenous cecal counts of microflora.
In follow-up tests, Kollanoor-Johny et al. (2012b) studied the antibacterial efficacy of trans-cinnamaldehyde and eugenol in decreasing S. enteritidis in broiler chickens. Trans-cinnamaldehyde was applied at 0.75% and eugenol at 1% as an antimicrobial additive in the feed for 5 d before slaughter. Both EOs compounds reduced the colonization of S. enteritidis (Kollanoor-Johny et al., 2012b). In addition, the trans-cinnamaldehyde egg transmission of S. enteritidis was decreased by in-feed supplementation in commercial chickens (Kollanoor-Johny et al., 2012b). Furthermore, trans-cinnamaldehyde supplementation for 66 d decreased S. eteritidis by 1% and 1.5% at 40 and 25 wk, respectively. There were no deleterious consequences in the eggshell or yolk in regards to development, egg production, and consumer egg acceptability (Kollanoor-Johny et al., 2012b).
Additionally, Upadhyaya et al. (2013) reported the rapid inactivation capacity of EO compounds against eggshell S. enteritidis. Arsi et al. (2014) also evaluated the effectiveness of carvacrol and thymol EO compounds in broiler chickens on the populations of Campylobacter spp. Although consistent antimicrobial effects across experiments was a concern, these EOs reduced Campylobacter colonization (Arsi et al., 2014). Thymol at 0.25% and 1% was inhibitory to Campylobacter spp. colonization, while carvacrol at 1% and the combination of thymol and carvacrol at 0.5% inhibited Campylobacter spp. (Arsi et al., 2014).
The antibacterial action related to the hydrophobicity of EOs that causes damage to the bacterial cell membranes and the leakage of ions and other cellular constituents is important. In particular, trans-cinnamaldehyde negatively affects glucose absorption and ATP synthesis (Arsi et al., 2014). Another EOs mode of action is the inhibition of the main bacterial enzymes, such as amino acid decarboxylases (Arsi et al., 2014). This inhibition reduces S. enteritidis motility and its invasion of abdominal epithelial cells and oviduct epithelial cell macrophages by down-regulating essential virulence genes (Kollanoor-Johny et al., 2012a). DNA microarray studies have shown that genes involved Salmonella pathogenicity, specifically the Island 1, type 3 secretion system, surface proteins, metabolic pathway, and electron acceptors under anaerobiotic conditions, were down-regulated in S. enteritidis by these antimicrobial agents (Kollanoor-Johny, 2011).
Metal chelation by flavonoids and phenols, disruption of the membrane by phenolics and terpenoids, and genetic material damage by alkaloids and coumarin that may impede the growth of microorganisms are several examples of the antimicrobial mechanisms exhibited by EOs (Cowan, 1999). The hydrophobicity of EOs enables them to separate lipids in the bacterial cell wall, which allows them to accumulate in the lipid layer, thus disrupting cell membrane integrity and ion transportation; this results in the subsequent loss of cellular components and lysis of bacterial cells (Burt, 2004).
EOs also cause acidification inside the cell, blocking cellular energy production, collapsing the proton pump, and reducing membrane potential (Burt, 2004). In addition, EOs are active against Gram-positive bacteria (Li et al., 2015) because they are lipophilic in nature. Small molecular weight components inside EOs disrupt cell membranes by crossing the bacterial cell wall through the lipopolysaccharide layer or diffusion through the membrane proteins (Dorman and Deans, 2000).
Garlic and oregano EOs have positive impacts on the intestinal microflora of broilers. In one study, 4 diets were formulated: a basal diet; a basal diet + oregano EOs at 300 mg/kg; a basal diet + garlic EOs at 300 mg/kg; and a basal diet + oregano and garlic EOs at 150 mg/kg. The total populations of Streptococcus, Lactobacillus spp., and coliforms in the ileum were not affected by these diet treatments; however, a significant (P < 0.05) decrease in the population of Clostridium spp. was observed in birds supplemented with the garlic EOs, oregano EOs, and garlic EOs + oregano EOs compared with only basal diet supplementation (Kirkpinar et al., 2011).
EOs, such as cinnamaldehyde and thymol, have selective antibacterial properties and inhibit yeast and mold growth (Bento et al., 2013), with a beneficial effect on the GIT by managing pathogens, reducing stress induced by oxidative diseases, and stabilizing the intestinal microbiota (Bento et al., 2013). The findings by Zheng and Wang (2001) agree with the above findings. They revealed the effect of two herbs and virginiamycin on intestinal bacterial inhabitants in broilers. Four diets were formulated: basal diet + 15 ppm of virginiamycin, basal diet + 0.1% dose of garlic, thyme, or a blend of the two. Ileo-cecum digesta of the supplemented groups showed a significant decrease in the E. coli population compared to the control; however, a significant increase in the lactic acid bacteria count was observed in the thyme group compared to the other groups (Zheng and Wang, 2001).
Similarly, the supplementation of cinnamon oil revealed detrimental changes in the cell walls of treated E. coli (Rahimi et al., 2011). Chowdhury et al. (2018) studied the impact of cinnamon, ajwain, and cloves EOs at 300, 400, and 600 mg/kg of diet, respectively, as an antibiotic substitute to estimate their effect on the intestine of broiler chickens. They reported that cinnamon bark oil lowered the counts of precaecal E. coli and Clostridium spp.
Effect of EOs on Intestinal Morphology
The gastrointestinal mucosa is the first tissue that contacts dietary components. The height and depth of the villus crypt is the most critical measure of the small intestine's digestive ability. Any change in this ratio alters digestion and absorption (Pluske et al., 1997). Adding antibiotics to chicken diets decreased intestinal wall density, led to weight loss, and increased intestinal length (Hedemann et al., 2003).
Many scientists have reported increased villus height due to EO supplementation (Garcia et al., 2007; Samanta et al., 2010). The villus height caused by the EOs may correlate with its antioxidant properties (El-Baroty et al., 2010). Components present in EOs, such as cinnamaldehyde, prevent villi damage by increasing antioxidant enzyme activity, but in cinnamon EOs, the phenolic group acts as hydrogen donors (El-Baroty et al., 2010).
Thymol supplementation increased the intestinal length, width, and depth of the villi, thus creating better conditions for nutrient absorption (Alcicek et al., 2003). Similarly, a combination of carvacrol (5.4%), cinnamaldehyde (2.9%), and capsicum oleoresin (2.18%) significantly (P < 0.05) increased villus length and the intestinal diameter in the treated group compared to other birds (Awaad et al., 2014). Furthermore, studies revealed that feeding the leaves of Mentha piperita partially improved the histomorphological structure of the small intestine's mucosa in chickens (Hamedi et al., 2017). Chowdhury et al. (2018) revealed that supplementation of cinnamon EOs at 0.3 mg/g diet increased the height of villi in the duodenum, jejunum, and ileum compared to the control group (Chowdhury et al., 2018).
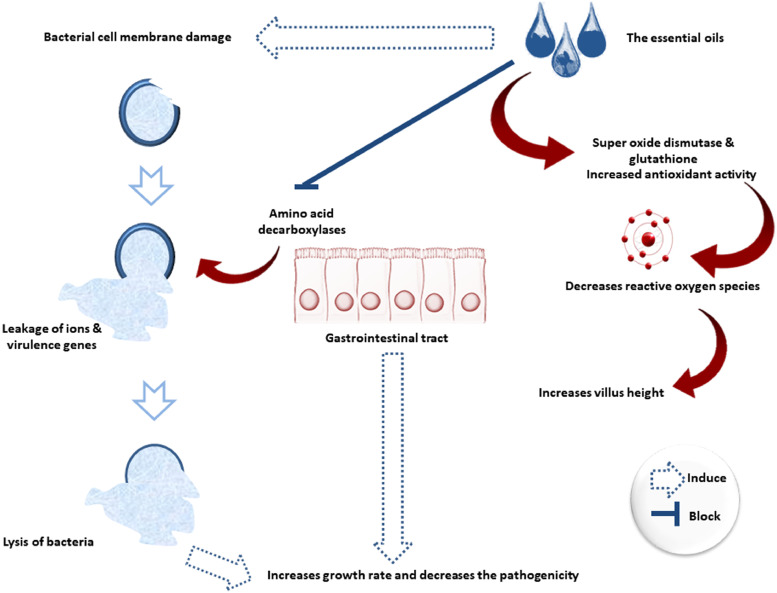
Figure 3 shows how oxygen radicals invade the surface mucus during digestion and shorten the intestine's villi length. In addition, EOs attract oxygen and quencher-free radicals via antioxidants, such as catalase, glutathione peroxidase, and SOD.
Figure 3.
Effects of essential oils on the poultry intestinal morphology and bacterial cell wall and cell membrane.
Impact of EOs on the Metabolism of Lipids
EOs supplementation can decrease cholesterol levels. Bioactive compounds present in EOs, such as cineole, borneol, citral, menthone, geraniol, menthol, fenchyl alcohol, fenchone, and ionone, are essential in inhibiting hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG-CoA) activity (Yu et al., 1994), a crucial enzyme in the synthesis of cholesterol. For example, a 2% reduction in serum cholesterol level has been observed in poultry upon the inhibition of 5% HMG-CoA reductase activity (Case et al., 1995).
A correlation exists between both low-density lipoproteins (LDL), total cholesterol and HMG-CoA reductase activity in chickens, but not with high-density lipoproteins (HDL) (Qureshi et al., 1983). In an experiment to understand EOs as potential growth or hypocholesterolemic agents, 24-day-old broilers were divided into three equal groups: antibiotic or positive control (100 ppm oxytetracycline), negative control (no antibiotic and no EOs), and EOs (125 ppm), including EOs originated from anize, oregano, and citrus peel (Hong et al., 2012). The results from this study showed reduced cholesterol serum levels in the groups treated with antibiotics or EOs; however, the EOs supplementation was found to have higher total polyphenolic compounds, lower very-low-density lipoprotein levels, and higher total flavonoids (Hong et al., 2012). Recently, a study performed by Chowdhury et al. (2018) showed that in broiler chickens, a significant reduction in serum cholesterol concentrations could be achieved by supplementing them with cinnamon EOs at 300 mg/kg. EOs down-regulate the HMG-CoA reductase enzyme post-transcriptionally without changing mRNA levels (Qureshi et al., 1996).
EOs inhibition of hepatic HMG-CoA reductase is irrespective of the enzyme's cycle and hormones, such as glucocorticoids, insulin, glucagon, and triiodothyronine (Middleton and Hui, 1982). Two regulators were required to completely inhibit cholesterol synthesis: LDL-derived cholesterol and mevalonate-derived nonsterol, which controlled the HMG-CoA reductase activity (Goldstein and Brown, 1990). Thymol, ionone, and carvacrol can induce a regulatory nonsterol product (Elson, 1996).
Immunomodulatory Activity of EOs
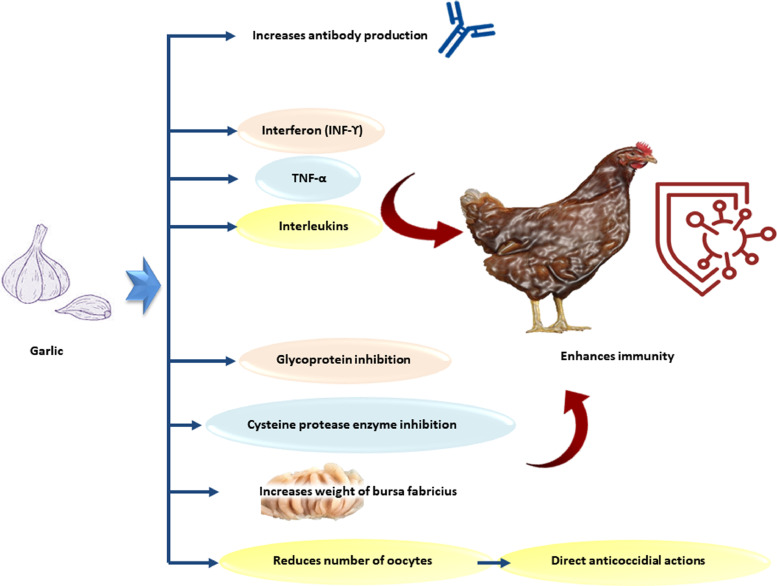
Researchers have investigated the various immunomodulatory capacities of EOs, and have found that the inclusion of EOs in chicken feed significantly increased the development of antibodies toward Pasteurella multocida, Leptospira pomona, and S. enteritidis (Szigeti et al., 1998). A study conducted by Hanieh et al. (2010) revealed that garlic enhances the assembly of interferon (INF-γ), interleukins, and tumor necrosis factor α (Figure 4, Table 1). Similarly, it increased the phagocytosis of antigen-presenting cells and macrophages. Zheng and Wang (2001) showed that the bursa of Fabricius in the garlic-supplemented diet was significantly (P < 0.05) increased in comparison to the other groups; however, no significant effect was observed on the relative weight of the spleen. Awaad et al. (2014) studied the immunostimulant impacts of eucalyptus and peppermint EOs on humoral and cell-mediated immunity in birds vaccinated against Avian Influenza (AI) and Newcastle disease (ND). The volatile oil-treated group showed higher hemagglutinin-inhibition antibody titers against AI and ND vaccines than the control group. The specific antibody response against the influenza vaccine virus was significantly increased compared to control due to supplementation with 0.2% thyme extract (Talazadeh and Mayahi, 2017).
Figure 4.
The immunomodulatory and the anticoccidial effect of garlic essential oils.
Yang et al. (2018) studied the effect of thymol EOs on broiler chicken immunity. Dietary treatments were as follows: basal diet only without any supplementation (control); basal diet with 0.15 g/kg enramycin in the growing period; basal diet with 0.30 g/kg thymol EOs in the growing period, and basal diet with 0.30 g/kg thymol EOs in the finishing period. They concluded that birds in the grower and finisher phases fed 0.30 g/kg EOs displayed a superior (P < 0.05) spleen index compared to birds of the control group. The duodenum and ileum mucosal levels of secretory immunoglobulin A were substantially (P < 0.05) improved in the finisher group compared to the other groups (Yang et al., 2018).
Anticoccidial Effect of EOs
EOs are powerful botanical products that can interfere directly or indirectly with parasitic metabolism by amplifying host immunity and antioxidant status to effectively control and eradicate parasitic invasion, particularly in coccidiosis management (Machado et al., 2010). Direct anticoccidial activities include glycoprotein inhibition (Rodrigues Goulart et al., 2004), ultrastructural changes in the mitochondrial membrane (Ueda-Nakamura et al., 2006), and cysteine protease enzyme inhibition (Ogungbe and Setzer, 2008; Figure 4).
Indirect antiparasitic activities of EOs involve improving host immunity (Machado et al., 2010) and improving antioxidant effects by scavenging ROS (Bakkali et al., 2008). Plants such as Allium cepa, Origanum vulgare (Milhau et al., 1997), Allium sativum, Echinacea purpurea (Zhai et al., 2007), Chenopodium ambrosioides, and Mentha spp. were efficacious against intestinal parasites, including the Eimeria species (Giannenas et al., 2004).
Giannenas et al. (2004) revealed that supplementing oregano EOs (300 mg/kg) in experimentally infected (Eimeria tenella) birds resulted in reduced oocyst mass per gram of excreta. The coccidial infection level was estimated from the number of Eimeria oocysts shed in the birds’ excreta (Giannenas et al., 2004). O. vulgare was widely investigated for E. tenella anticoccidial activity (Silva et al., 2009), as well as mixed Eimeria species infection (Hume et al., 2006), E. maxima (Tsinas et al., 2011), and E. acervuline (Tsinas et al., 2011).
For controlling avian coccidiosis, the supplementation of EOs and coccidial vaccinations have been reported as an effective alternative by various researchers (Küçükyilmaz et al., 2012). Furthermore, many scientists have shown the oocysticidal activity of phenols against E. tenella (Williams, 1997). Bioactive components in EOs (Küçükyilmaz et al., 2012) reduced fecal oocyst shedding and mitigated intestinal epithelium degradation in affected birds (Bozkurt et al., 2013).
Impact of EOs on Carcass Characteristics
Improvement in chicken meat production depends on increased muscle proportions, enhanced growth, and the reduction in abdominal fat in chickens (Musa et al., 2006). Alcicek et al. (2003) reported that the combination of EOs extracted from different herbs grown in Turkey at 24, 48, or 72 mg/kg diet improved BW, feed intake (except at d 42), FCR, and carcass yield at 21 and 42 d. They also showed that supplementation above 48 mg of a combination of EOs per kg had no additional beneficial effect on BW, feed intake, FCR, and carcass yield (Alcicek et al., 2003).
Khattak et al. (2014) showed an increase in carcass weight, breast weight, and breast meat with the supplementation of natural EOs (basil, lemon, caraway, oregano, laurel, sage, thyme, and tea). These findings indicate that broiler carcass characteristics were improved with dietary supplementation of EOs; however, dietary supplementation of oregano EOs at 300 mg/kg showed a nonsignificant impact on carcass characteristics (Alp et al., 2012). Sang-oh et al. (2013) demonstrated that the dressing percentage and thigh and breast muscle were significantly (P < 0.05) higher in birds fed a basal diet supplemented with cinnamon powder compared to a nonsupplemented diet.
In broiler chicks supplemented with graded levels of cinnamon powder (0, 3, 5, and 7%) to the basal diet, the highest dressing percentage and commercial cuts (breast, drumstick, and thigh) occurred at a 5% (Eltazi, 2014). Likewise, oregano EOs supplements at 600 mg/kg doses were associated with more considerable breast muscle, dressing, and leg muscle percentages than the controls (Peng et al., 2016).
Impact of EOs on Broiler Economy
Incorporating EOs as a feed supplement in organic poultry production has many variable effects on broiler economy. For instance, Wade et al. (2018) found significantly higher BWG and improved FCR in birds fed a basal diet supplemented with thyme EOs.
Potential Uses of NEs
The goal of drug formulation research in recent decades has been to identify a mechanism for delivering medications that can extend and sustain drug release after application (Maderuelo et al., 2011). This improved medication delivery system is being studied extensively because it has various advantages over traditional systems. In pharmaceutical research, colloids are extremely important. Colloidal particles can be measured in nanometers and are referred to as nanoparticles (Kamble et al., 2010).
A nanoparticle, also known as an ultrafine particle, is a small particle of matter with a dimension of 1 to 100 nm (El-Saadony et al., 2020b; Reda et al., 2020; Abd El-Hack et al., 2021b; El-Saadony et al., 2021e). Nanoparticles can transport and protect oxygen from oxidation, protect EOs from degradation, and improve their stability to enhance products and function. Nanocarriers positively affect the products and control the release of bioactive molecules (Liang et al., 2012). In addition, it facilitates EO activity by providing various diffusion properties due to its nanoscale dimensions (São Pedro et al., 2013). Recent advances in nanoscience and nanoscale technology have shown improvements in the antimicrobial activities of many antimicrobial agents using nanoparticles (El-Saadony et al., 2021f; El-Saadony et al., 2021g; Reda et al., 2021b). In addition, nanobiotechnology has gained considerable interest in recent decades, and uses biological agents, including microorganisms or plant extracts, to biosynthesize nanoparticles (Sheiha et al., 2020; El-Saadony et al., 2021h; El-Saadony et al., 2021i; Saad et al., 2021b).
Emulsions containing particles in the 10 to 100 nm range are known as NEs. They are fine emulsions of oil in water that require slight agitation when added to other aqueous media (Mason et al., 2006; Wang et al., 2009). When NEs are diluted, nano-droplets with sizes ranging 20 to 200 nm are produced, which increases the solubility and bioavailability of lipophilic materials in water. NEs are more stable and can be improved (Chakraborty et al., 2009). These nano-sized colloids are used to provide lipophilic bioactive materials in the food, pharmaceutical, and cosmetic industries (McClements and Rao, 2011; Ghosh et al., 2013).
Emulsions stabilize and improve the antimicrobial effects of oils in aqueous solutions. Since the 1980s, the focus has been on droplet emulsions in the NE range, which possess specific characteristics, such as transparency to the naked eye and a unique Tyndall effect (Salager et al., 2001). NEs typically contain oil in their exterior shell layer and a central component of health-friendly compounds inside. NEs are relatively stable for long periods without any major changes in their physical properties (Pathania et al., 2018). EO-based NEs are a more effective alternative to antibiotics in poultry nutrition than pure oil because of their increased surface area and small droplet size (Pathania et al., 2018).
The encapsulation of EOs by NEs is considered an innovative technology that can help overcome the limiting factors of EO usage. The solubility and bioavailability of EOs are enhanced when encapsulated (Calo et al., 2015) and their microbial stability is improved (Jayasena and Jo, 2013). Additionally, encapsulated EOs also protects EOs against possible thermal or photo-degradation, thus increasing their stability (São Pedro et al., 2013).
The therapeutic efficacy of encapsulated EOs can be maintained by using NEs to reduce volatility, increase stability, change solubility, and preserve therapeutic efficacy (Bilia et al., 2014). A high-energy emulsification procedure and a self-emulsification or low-energy method are both employed to make NEs. High mechanical energy inputs, such as high shearing, high-pressure homogenizers, or ultrasounds, are used in the high-energy process. Self-emulsification uses chemical energy from the mixture, using phase transitions during emulsification, resulting from the shift in the spontaneous curvature of the surfactant (Solè et al., 2010). NEs are created when oil droplets are scattered throughout the aquatic phase, while water droplets, distributed throughout the oil phase, form water-in-oil NEs (McClements and Rao, 2011).
The efficacy of EO NEs has been proven in the food industry. Sunflower oil NEs possess strong antibacterial activity against major foodborne pathogens, such as Bacillus cereus, B. circulans, Listeria monocytogenes, and S. typhi, and pathogenic fungi, including Penicillium spp., Aspergillus niger, and Rhizopus nigricans. Adding sunflower oil to foods such as milk, raw chicken, vegetables, and apple juice reduced microbial loads (Joe et al., 2012). In addition, the active components in NE remained stable after a year of storage at room temperature when zedoary turmeric oil was employed. When zedoary turmeric oil NE was added to mice diets, the active components grew faster and were more bioavailable than raw turmeric oil (Zhao et al., 2010).
According to Sood et al. (2014), brain-targeted intranasal administration of curcumin NEs exhibited no adverse side effects. During in vitro diffusion experiments, NEs achieved significantly higher dissolution rates than drug solutions. For example, higher flux and penetration into the nasal mucosa of sheep can be achieved with mucoadhesive NEs (Sood et al., 2014). NE-based edible coating containing ginger EOs can help increase the life of breast fillets (Noori et al., 2018). In the poultry industry, antimicrobial treatments are the most practical and cost effective choice for farmers (Venkitanarayanan et al., 2013).
NEs can be used to effectively deliver lipophilic active substances into the water sources of livestock (Vandamme and Anton, 2010). Since infected birds reduce feed intake while drinking, antimicrobial compounds are added to the drinking water (Landoni and Albarellos, 2015). Drugs added to drinking water are distinguished by their therapeutic efficacy, low cost, simplicity of drug application, and dose adjustment (Vermeulen et al., 2002).
CONCLUSION
EOs are natural, volatile compounds that have an intense flavor. In poultry processing, EOs can be a novel alternative to antibiotics. The antibacterial action of EOs can play an essential role in improving GIT enzyme activity by reducing pathogens and preserving gut health. EOs have a positive effect on the intestinal environment and function. EOs contain predominantly lipophilic and aromatic bioactive compounds, although they do have some limiting aspects for usage due to their low solubility and stability. NEs are one prospective approach for efficiently delivering EOs in poultry farming.
Acknowledgments
ACKNOWLEDGMENTS
Prof. Khaled A. El-Tarabily thanks library at Murdoch University, Australia for the valuable online resources and comprehensive databases.
Author contributions: All authors were equally contributed in writing this review article. All authors reviewed and approved the final version of the manuscript.
DISCLOSURES
Authors declare no conflict of interests.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2021.101584.
Appendix. Supplementary materials
References
- Abd El-Ghany W.A., Ismail M. Tackling experimental colisepticaemia in broiler chickens using phytobiotic essential oils and antibiotic alone or in combination. Iran. J. Vet. Res. 2014;15:110–115. [Google Scholar]
- Abd El-Ghany W.A., Hatem M.E., Ismail M. Evaluation of the efficacy of feed additives to counteract the toxic effects of aflatoxicosis in broiler chickens. Int. J. Anim. Vet. Adv. 2013;5:171–182. [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Swelum A.A., Arif M., Abo Ghanima M.M., Shukry M., Noreldin A., Taha A.E., El-Tarabily K.A. Curcumin, the active substance of turmeric: its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021;101:5747–5762. doi: 10.1002/jsfa.11372. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., Alaidaroos B.A., Farsi R.M., Abou-Kassem D.E., El-Saadony M.T., Saad A.M., Shafi M.E., Albaqami N.M., Taha A.E., Ashour E.A. Impacts of supplementing broiler diets with biological curcumin, Zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals. 2021;11:1878. doi: 10.3390/ani11071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shehata A.M., Arif M., Paswan V.K., Batiha G.E.-S., Khafaga A.F., Elbestawy A.R. Approaches to prevent and control Campylobacter spp. colonization in broiler chickens: a review. Environ. Sci. Pollut. Res. 2021;28:4989–5004. doi: 10.1007/s11356-020-11747-3. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Zabermawi N.M., Arif M., Batiha G.E., Khafaga A.F., Abd El-Hakim Y.M., Al-Sagheer A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: a review. Int. J. Biol. Macromol. 2020;164:2726–2744. doi: 10.1016/j.ijbiomac.2020.08.153. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Alshahrani O.A., Saghir S.A., Al-Wajeeh A.S., Al-Shargi O.Y., Taha A.E., Mesalam N.M., Abdel-Moneim A.-M.E. Prebiotics can restrict Salmonella populations in poultry: a review. Anim. Biotechnol. 2021;19:1–10. doi: 10.1080/10495398.2021.1883637. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Qattan S.Y., Batiha G.E., Khafaga A.F., Abdel-Moneim A.M.E., Alagawany M. Probiotics in poultry feed: a comprehensive review. J. Anim. Physiol. Anim. Nutr. (Berl). 2020;104:1835–1850. doi: 10.1111/jpn.13454. [DOI] [PubMed] [Google Scholar]
- Abdelnour S.A., Swelum A.A., Salama A., Al-Ghadi M.Q., Qattan S.Y.A., Abd El-Hack M.E., Khafaga A.F., Alhimaidi A.R., Almutairi B.O., Ammari A.A., El-Saadony M.T. The beneficial impacts of dietary phycocyanin supplementation on growing rabbits under high ambient temperature. Ital. J. Anim. Sci. 2020;19:1046–1056. [Google Scholar]
- Abdelnour S.A., El-Saadony M.T., Saghir S.A.M., Abd El-Hack M.E., Al-shargi O.Y.A., Al- Gabri N., Salama A. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livest. Sci. 2020;240 [Google Scholar]
- Abou-Kassem D.E., Mahrose K.M., El-Samahy R.A., Shafi M.E., El-Saadony M.T., Abd El-Hack M.E., Emam M., El-Sharnouby M., Taha A.E., Ashour E.A. Influences of dietary herbal blend and feed restriction on growth, carcass characteristics and gut microbiota of growing rabbits. Ital. J. Anim. Sci. 2021;20:896–910. [Google Scholar]
- Acosta E. Bioavailability of nanoparticles in nutrient and nutraceutical delivery. Curr. Opin. Colloid Interface Sci. 2009;14:3–15. [Google Scholar]
- Alagawany M., Madkour M., El-Saadony M.T., Reda F.M. Paenibacillus polymyxa (LM31) as a new feed additive: antioxidant and antimicrobial activity and its effects on growth, blood biochemistry, and intestinal bacterial populations of growing Japanese quail. Anim. Feed Sci. Technol. 2021;276 [Google Scholar]
- Alagawany M., El-Saadony M.T., Elnesr S.S., Farahat M., Attia G., Madkour M., Reda F. Use of lemongrass essential oil as a feed additive in quail's nutrition: its effect on growth, carcass, blood biochemistry, antioxidant and immunological indices, digestive enzymes and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcicek A., Bozkurt M., Çabuk M. The effect of an essential oil combination derived from selected herbs growing wild in Turkey on broiler performance. S. Afr. J. Anim. Sci. 2003;33:89–94. [Google Scholar]
- Allen H.K., Levine U.Y., Looft T., Bandrick M., Casey T.A. Treatment, promotion, commotion: antibiotic alternatives in food-producing animals. Trends. Microbiol. 2013;21:114–119. doi: 10.1016/j.tim.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Alp M., Midilli M., Kocabagli N., Yilmaz H., Turan N., Gargili A., Acar N. The effects of dietary oregano essential oil on live performance, carcass yield, serum immunoglobulin G level, and oocyst count in broilers. J. Appl. Poult. Res. 2012;21:630–636. [Google Scholar]
- Arsi K., Donoghue A.M., Venkitanarayanan K., Kollanoor-Johny A., Fanatico A.C., Blore P.J., Donoghue D.J. The efficacy of the natural plant extracts, thymol and carvacrol against Campylobacter colonization in broiler chickens. J. Food Saf. 2014;34:321–325. [Google Scholar]
- Asaolu M.F., Oyeyemi O.A., Olanlokun J.O. Chemical compositions, phytochemical constituents and in vitro biological activity of various extracts of Cymbopogon citratus. Pak. J. Nutr. 2009;8:1920–1922. [Google Scholar]
- Ashour E.A., Abd El-Hack M.E., Shafi M.E., Alghamdi W.Y., Taha A.E., Swelum A.A., Tufarelli V., Mulla Z.S., El-Ghareeb W.R., El-Saadony M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture. 2020;10:457. [Google Scholar]
- Atkinson R.G. Phenylpropenes: occurrence, distribution, and biosynthesis in fruit. J. Agric. Food Chem. 2018;66:2259–2272. doi: 10.1021/acs.jafc.6b04696. [DOI] [PubMed] [Google Scholar]
- Awaad M., Elmenawey M., Ahmed K.A. Effect of a specific combination of carvacrol, cinnamaldehyde, and Capsicum oleoresin on the growth performance, carcass quality and gut integrity of broiler chickens. Vet. World. 2014;7:284–290. [Google Scholar]
- Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils. a review. Food Chem. Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Bansal M., Jamil S. Micellar microparticles: a novel approach to topical drug delivery system. Int. J. Appl. Pharm. 2018;10:1–5. [Google Scholar]
- Basalious E.B., Shawky N., Badr-Eldin S.M. SNEDDS containing bioenhancers for improvement of dissolution and oral absorption of lacidipine. I: development and optimization. Int. J. Pharm. 2010;391:203–211. doi: 10.1016/j.ijpharm.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Baskar V., Salim Meeran I., Subramani A., Sruthi J.Ali, Shabeer T.K. Historic review on modern herbal nanogel formulation and delivery methods. Int. J. Pharm. Pharm. Sci. 2018;10:1–10. [Google Scholar]
- Bento M.H.L., Ouwehand A.C., Tiihonen K., Lahtinen S., Nurminen P., Saarinen M.T., Schulze H., Mygind T., Fischer J. Essential oils and their use in animal feeds for monogastric animals – effects on feed quality, gut microbiota, growth performance and food safety: a review. Vet. Med. 2013;58:449–458. [Google Scholar]
- Bilia A.R., Guccione C., Isacchi B., Righeschi C., Firenzuoli F., Bergonzi M.C. Essential oils loaded in nanosystems: a developing strategy for a successful therapeutic approach. Evid. Based Complement. Alternat. Med. 2014;2014 doi: 10.1155/2014/651593. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bölükbaşi Ş.C., Erhan M.K., Özkan A. Effect of dietary thyme oil and vitamin E on growth, lipid oxidation, meat fatty acid composition. S. Afr. J. Anim. Sci. 2006;36:189–196. [Google Scholar]
- Bozkurt M., Giannenas I., Küçükyilmaz K., Christaki E., Florou-Paneri P. An update on approaches to controlling coccidia in poultry using botanical extracts. Br. Poult. Sci. 2013;54:713–727. doi: 10.1080/00071668.2013.849795. [DOI] [PubMed] [Google Scholar]
- Bravo D., Pirgozliev V., Rose S.P. A mixture of carvacrol, cinnamaldehyde, and capsicum oleoresin improves energy utilization and growth performance of broiler chickens fed maize-based diet. J. Anim Sci. 2014;92:1531–1536. doi: 10.2527/jas.2013-6244. [DOI] [PubMed] [Google Scholar]
- Brenes A., Roura E. Essential oils in poultry nutrition: main effects and modes of action. Anim. Feed Sci. Technol. 2010;158:1–14. [Google Scholar]
- Brewer M.S. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011;10:221–247. [Google Scholar]
- Burt S. Essential oils: their antibacterial properties and potential applications in foods - a review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Butaye P., Devriese L.A., Haesebrouck F. Antimicrobial growth promoters used in animal feed: effects of less well known antibiotics on gram-positive bacteria. Clin. Microbiol. Rev. 2003;16:175–188. doi: 10.1128/CMR.16.2.175-188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çabuk M., Bozkurt M., Alcicek A., Akbaþ Y., Küçükyýlmaz K. Effect of a herbal essential oil mixture on growth and internal organ weight of broilers from young and old breeder flocks. S. Afr. J. Anim. Sci. 2006;36:135–141. [Google Scholar]
- Calo J.R., Crandall G.P., O'Bryan C.A., Ricke S.C. Essential oils as antimicrobials in food systems - a review. Food Control. 2015;54:111–119. [Google Scholar]
- Carson C.F., Mee B.J., Riley T.V. Mechanism of action of Melaleuca alternifolia (tea tree) oil on Staphylococcus aureus determined by time-kill, lysis, leakage, and salt tolerance assays and electron microscopy. Antimicrob. Agents. Chemother. 2002;46:1914–1920. doi: 10.1128/AAC.46.6.1914-1920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho I.T., Santos L. Antibiotics in the aquatic environments: a review of the European scenario. Environ. Int. 2016;94:736–757. doi: 10.1016/j.envint.2016.06.025. [DOI] [PubMed] [Google Scholar]
- Case G.L., He L., Mo H., Elson C.E. Induction of geranyl pyrophosphate pyrophosphatase activity by cholesterol-suppressive isoprenoids. Lipids. 1995;30:357–359. doi: 10.1007/BF02536045. [DOI] [PubMed] [Google Scholar]
- Chakraborty S., Shukla D., Mishra B., Singh S. Lipid-an emerging platform for oral delivery of drugs with poor bioavailability. Eur. J. Pharm. Biopharm. 2009;73:1–15. doi: 10.1016/j.ejpb.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay M.K. Use of antibiotics as feed additives: a burning question. Front. Microbiol. 2014;5:334. doi: 10.3389/fmicb.2014.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves A.V., He M.L., Yang W.Z., Hristov A.N., McAllister T.A., Benchaar C. Effects of essential oils on proteolytic, deaminative and methanogenic activities of mixed ruminal bacteria. Can. J. Anim. Sci. 2008;88:117–122. [Google Scholar]
- Chowdhury S., Mandal G.P., Patra A.K., Kumar P., Samanta I., Pradhan S., Samanta A.K. Different essential oils in diets of broiler chickens: 2. Gut microbes and morphology, immune response, and some blood profile and antioxidant enzymes. Anim. Feed Sci. Technol. 2018;236:39–47. [Google Scholar]
- Citarasu T. Herbal biomedicines: a new opportunity for aquaculture industry. Aquac. Int. 2010;18:403–414. [Google Scholar]
- Cowan M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross D.E., McDevitt R.M., Hillman K., Acamovic T. The effect of herbs and their associated essential oils on performance, dietary digestibility and gut microflora in chickens from 7 to 28 days of age. Br. Poult. Sci. 2007;48:496–506. doi: 10.1080/00071660701463221. [DOI] [PubMed] [Google Scholar]
- Cui S., Ge B., Zheng J., Meng J. Prevalence and antimicrobial resistance of Campylobacter spp. and Salmonella serovars in organic chickens from Maryland retail stores. Appl. Environ. Microbiol. 2005;71:4108–4111. doi: 10.1128/AEM.71.7.4108-4111.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Davies D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010;74:417–433. doi: 10.1128/MMBR.00016-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker E.A., Park Y. Healthier meat products as functional foods. Meat Sci. 2010;86:49–55. doi: 10.1016/j.meatsci.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Diarra M.S., Malouin F. Antibiotics in Canadian poultry productions and anticipated alternatives. Front. Microbiol. 2014;5:282. doi: 10.3389/fmicb.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Sanchez S., Moscoso S., Solis de los Santos F., Andino A., Hanning I. Antibiotic use in poultry: a driving force for organic poultry production. Food Prot. Trends. 2015;35:440–447. [Google Scholar]
- Dorman H.J., Deans S.G. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- El-Baroty G.S., Abd El-Baky H.H., Farag R.S., Saleh M.A. Characterization of antioxidant and antimicrobial compounds of cinnamon and ginger essential oils. Afr. J. Biochem. Res. 2010;4:167–174. [Google Scholar]
- El-Saadony M.T., Saad A.M., Taha T.F., Najjar A.A., Zabermawi N.M., Nader M.M., AbuQamar S.F., El-Tarabily K.A., Salama A. Selenium nanoparticles, from Lactobacillus paracasei HM1 capable of antagonizing animal pathogenic fungi, as a new source from human breast milk. Saudi J. Biol. Sci. 2021;28:6782–6794. doi: 10.1016/j.sjbs.2021.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Abd El-Hack M.E., Swelum A.A., Al-Sultan S.I., El-Ghareeb W.R., Hussein E.O., Ba-Awadh H.A., Akl B.A., Nader M.M. Enhancing quality and safety of raw buffalo meat using the bioactive peptides of pea and red kidney bean under refrigeration conditions. Ital. J. Anim. Sci. 2021;20:762–776. [Google Scholar]
- El-Saadony M.T., Alagawany M., Patra A.K., Kar I., Tiwari R., Dawood M.A., Dhama K., Abdel-Latif H.M. The functionality of probiotics in aquaculture: an overview. Fish Shellfish Immunol. 2021;117:36–52. doi: 10.1016/j.fsi.2021.07.007. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Abd El-Hack M.E., Taha A.E., Fouda M.M.G., Ajarem J.S., Maodaa N.S., Allam A.A., Elshaer N. Ecofriendly synthesis and insecticidal application of copper nanoparticles against the storage pest Tribolium castaneum. Nanomaterials. 2020;10:587. doi: 10.3390/nano10030587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Elsadek M.F., Mohamed A.S., Taha A.E., Ahmed B.M., Saad A.M. Effects of chemical and natural additives on cucumber juice's quality, shelf life, and safety. Foods. 2020;9:639. doi: 10.3390/foods9050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Zabermawi N.M., Burollus M.A., Shafi M.E., Alagawany M., Yehia N., Askar A.M., Alsafy S.A., Noreldin A.E., Khafaga A.F., Dhama K., Elnesr S.S., Elwan H.A.M., Di Cerbo A., El-Tarabily K.A., Abd El-Hack M.E. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: a review. Food Rev. Int. 2021 doi: 10.1080/87559129.2021.1944183. [DOI] [Google Scholar]
- El-Saadony M.T., Sitohy M.Z., Ramadan M.F., Saad A.M. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II) Innov. Food Sci. Emerg. Technol. 2021;69 [Google Scholar]
- El-Saadony M.T., Alkhatib F.M., Alzahrani S.O., Shafi M.E., Abdel-Hamid S.E., Taha T.F., Aboelenin S.M., Soliman M.M., Ahmed N.H. Impact of mycogenic zinc nanoparticles on performance, behavior, immune response, and microbial load in Oreochromis niloticus. Saudi J. Biol. Sci. 2021;28:4592–4604. doi: 10.1016/j.sjbs.2021.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Desoky E.M., Saad A.M., Eid R.S., Selem E., Elrys A.S. Biological silicon nanoparticles improve Phaseolus vulgaris L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. J. Environ. Sci. 2021;106:1–14. doi: 10.1016/j.jes.2021.01.012. [DOI] [PubMed] [Google Scholar]
- El-Saadony M.T., Saad A.M., Najjar A.A., Alzahrani S.O., Alkhatib F.M., Shafi M.E., Selem E., Desoky E.M., Fouda S.E.E., El-Tahan A.M., Hassan M.A.A. The use of biological selenium nanoparticles in controlling Triticum aestivum L. crown root and rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 2021;28:4461–4471. doi: 10.1016/j.sjbs.2021.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Saadony M.T., Khalil O.S., Osman A., Alshilawi M.S., Taha A.E., Aboelenin S.M., Shukry M., Saad A.M. Bioactive peptides supplemented raw buffalo milk: biological activity, shelf life and quality properties during cold preservation. Saudi J. Biol. Sci. 2021;28:4581–4591. doi: 10.1016/j.sjbs.2021.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C.E. Novel lipids and cancer: isoprenoids and other phytochemicals. Adv. Exp. Med. Biol. 1996;399:71–86. doi: 10.1007/978-1-4613-1151-5_6. [DOI] [PubMed] [Google Scholar]
- El-Tarabily K.A., El-Saadony M.T., Alagawany M., Arif M., Batiha G.E., Khafaga A.F., Elwan H.A., Elnesr S.S., Abd El-Hack M.E. Using essential oils to overcome bacterial biofilm formation and their antimicrobial resistance. Saudi J. Biol. Sci. 2021;28:5145–5156. doi: 10.1016/j.sjbs.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltazi S.M. Effect of using cinnamon powder as natural feed additive on performance and carcass quality of broiler chickens. Int. J. Innov. Agric. Bio. Res. 2014;2:1–8. [Google Scholar]
- Ertas O.N., Guler T., Çiftçi M., DalkIlIç B., Simsek U.G. The effect of an essential oil mix derived from oregano, clove and anise on broiler performance. Int. J. Poult. Sci. 2005;4:879–884. [Google Scholar]
- Fagbemi J.F., Ugoji E., Adenipekun T., Adelowotan O. Evaluation of the antimicrobial properties of unripe banana (Musa sapientum L.), lemon grass (Cymbopogon citratus S.) and turmeric (Curcuma longa L.) on pathogens. Afr. J. Biotechnol. 2009;8:1176–1182. [Google Scholar]
- Galiş A.M., Marcq C., Marlier D., Portetelle D., Van I., Beckers Y., Théwis A. Control of Salmonella contamination of shell eggs—preharvest and postharvest methods: a review. Compr. Rev. Food Sci. 2013;12:155–182. [Google Scholar]
- Gantois I., Ducatelle R., Pasmans F., Haesebrouck F., Gast R., Humphrey T.J., Van Immerseel F. Mechanisms of egg contamination by Salmonella enteritidis. FEMS Microbiol. Rev. 2009;33:718–738. doi: 10.1111/j.1574-6976.2008.00161.x. [DOI] [PubMed] [Google Scholar]
- Garcia V., Catala-Gregori P., Hernandez F., Megias M.D., Madrid J. Effect of formic acid and plant extracts on growth, nutrient digestibility, intestine mucosa morphology, and meat yield of broilers. J. Appl. Poult. Res. 2007;16:555–562. [Google Scholar]
- Ghasemi H.A., Kasani N., Taherpour K. Effects of black cumin seed (Nigella sativa L.), a probiotic, a prebiotic and a synbiotic on growth performance, immune response and blood characteristics of male broilers. Livest. Sci. 2014;164:128–134. [Google Scholar]
- Ghosh V., Saranya S., Mukherjee A., Chandrasekaran N. Cinnamon oil nanoemulsion formulation by ultrasonic emulsification: investigation of its bactericidal activity. J. Nanosci. Nanotechnol. 2013;13:114–122. doi: 10.1166/jnn.2013.6701. [DOI] [PubMed] [Google Scholar]
- Giannenas I.A., Florou-Paneri P., Papazahariadou M., Botsoglou N.A., Christaki E., Spais A. Effect of diet supplementation with ground oregano on performance of broiler chickens challenged with Eimeria tenella. Archiv. Fur. Geflugelkunde. 2004;68:247–252. [Google Scholar]
- Goldstein J.L., Brown M.S. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Goossens H., Ferech M., Vander Stichele R., Elseviers M., ESAC Group Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- Gopi M., Karthik K., Manjunathachar H.V., Tamilmahan P., Kesavan M., Dashprakash M., Balaraju B.L., Purushothaman M.R. Essential oils as a feed additive in poultry nutrition. Adv. Anim. Vet. Sci. 2014;2:1–7. [Google Scholar]
- Grashorn M.A. Use of phytobiotics in broiler nutrition-an alternative to infeed antibiotics. J. Anim. Feed. Sci. 2010;19:338–347. [Google Scholar]
- Habibi R., Sadeghi G.H., Karimi A. Effect of different concentrations of ginger root powder and its essential oil on growth performance, serum metabolites and antioxidant status in broiler chicks under heat stress. Br. Poult. Sci. 2014;55:228–237. doi: 10.1080/00071668.2014.887830. [DOI] [PubMed] [Google Scholar]
- Hajati H., Rezaei M. The application of prebiotics in poultry production. Int. J. Poult. Sci. 2010;9:298–304. [Google Scholar]
- Hamedi S., Shomali T., Ghaderi H. Effect of dietary inclusion of Mentha piperita L. on histological and histomorphometrical parameters of the small intestine in broiler chickens. Org. Agric. 2017;7:105–110. [Google Scholar]
- Hanieh H., Narabara K., Piao M., Gerile C., Abe A., Kondo Y. Modulatory effects of two levels of dietary Alliums on immune response and certain immunological variables, following immunization, in White Leghorn chickens. Anim. Sci. J. 2010;81:673–680. doi: 10.1111/j.1740-0929.2010.00798.x. [DOI] [PubMed] [Google Scholar]
- Hashemi S.R., Davoodi H. Herbal plants and their derivatives as growth and health promoters in animal nutrition. Vet. Res. Commun. 2011;35:169–180. doi: 10.1007/s11259-010-9458-2. [DOI] [PubMed] [Google Scholar]
- Hashemipour H., Kermanshahi H., Golian A., Veldkamp T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci. 2013;92:2059–2069. doi: 10.3382/ps.2012-02685. [DOI] [PubMed] [Google Scholar]
- Hedemann M.S., Højsgaard S., Jensen B.B. Small intestinal morphology and activity of intestinal peptidases in piglets around weaning. J. Anim. Physiol. Anim. Nutr. 2003;87:32–41. doi: 10.1046/j.1439-0396.2003.00405.x. [DOI] [PubMed] [Google Scholar]
- Hernandez F., Madrid J., Garcia V., Orengo J., Megias M.D. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poult. Sci. 2004;83:169–174. doi: 10.1093/ps/83.2.169. [DOI] [PubMed] [Google Scholar]
- Hernández T., Canales M., Avila J.G., Duran A., Caballero J., Romo de Vivar A., Lira R. Ethnobotany and antibacterial activity of some plants used in traditional medicine of Zapotitlán de las Salinas, Puebla (México) J. Ethnopharmacol. 2003;88:181–188. doi: 10.1016/s0378-8741(03)00213-7. [DOI] [PubMed] [Google Scholar]
- Hong J.C., Steiner T., Aufy A., Lien T.F. Effects of supplemental essential oil on growth performance, lipid metabolites and immunity, intestinal characteristics, microbiota and carcass traits in broilers. Livest. Sci. 2012;144:253–262. [Google Scholar]
- Hume M.E., Clemente-Hernández S., Oviedo-Rondón E.O. Effects of feed additives and mixed Eimeria species infection on intestinal microbial ecology of broilers. Poult. Sci. 2006;85:2106–2111. doi: 10.1093/ps/85.12.2106. [DOI] [PubMed] [Google Scholar]
- Huyghebaert G., Ducatelle R., Van Immerseel F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011;187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Jamroz D., Wiliczkiewicz A., Wertelecki T., Orda J., Skorupińska J. Use of active substances of plant origin in chicken diets based on maize and locally grown cereals. Br. Poult. Sci. 2005;46:485–493. doi: 10.1080/00071660500191056. [DOI] [PubMed] [Google Scholar]
- Jamroz D., Wertelecki T., Houszka M., Kamel C. Influence of diet type on the inclusion of plant origin active substances on morphological and histochemical characteristics of the stomach and jejunum walls in chicken. J. Anim. Physiol. Anim. Nutr. 2006;90:255–268. doi: 10.1111/j.1439-0396.2005.00603.x. [DOI] [PubMed] [Google Scholar]
- Jang S.I., Ko Y.H., Kang S.Y., Lee C.Y. Effect of a commercial essential oil on growth performance, digestive enzyme activity and intestinal microflora population in broiler chickens. Anim. Feed Sci. Technol. 2007;134:304–315. [Google Scholar]
- Jayasena D.D., Jo C. Essential oils as potential antimicrobial agents in meat and meat products: a review. Trends Food Sci. Technol. 2013;34:96–108. [Google Scholar]
- Jerzsele A., Szeker K., Csizinszky R., Gere E., Jakab C., Mallo J.J., Galfi P. Efficacy of protected sodium butyrate, a protected blend of essential oils, their combination, and Bacillus amyloliquefaciens spore suspension against artificially induced necrotic enteritis in broilers. Poult. Sci. 2012;91:837–843. doi: 10.3382/ps.2011-01853. [DOI] [PubMed] [Google Scholar]
- Joe M.M., Bradeeba K., Parthasarathi R., Sivakumaar P.K., Chauhan P.S., Tipayno S., Benson A., Sa T. Development of surfactin based nanoemulsion formulation from selected cooking oils: evaluation for antimicrobial activity against selected food associated microorganisms. J. Taiwan Inst. Chem. Eng. 2012;43:172–180. [Google Scholar]
- Juniatik M., Hida K., Wulandari F.P., Pangestuti N., Munawaroh N., Martien R., Pratiwi S.U.T. Formulation of nanoemulsion mouthwash combination of lemongrass oil (Cymbopogon citratus) and kaffir lime oil (Citrus hystrix) for anticandidiasis against Candida albicans ATCC 10231. Trad. Med. J. 2017;22:7–15. [Google Scholar]
- Kamble V.A., Jagdale D.M., Kadam V.J. Solid lipid nanoparticles as drug delivery system. Int. J. Pharma. Bio. Sci. 2010;1:1–9. [Google Scholar]
- Karadas F., Pirgozliev V., Rose S.P., Dimitrov D D., Oduguwa O., Bravo D. Dietary essential oils improve the hepatic antioxidative status of broiler chickens. Br. Poult. Sci. 2014;55:329–334. doi: 10.1080/00071668.2014.891098. [DOI] [PubMed] [Google Scholar]
- Kelly C., Gundogdu O., Pircalabioru G., Cean A., Scates P., Linton M., Pinkerton L., Magowan E., Stef L., Simiz E., Pet I., Stewart S., Stabler R., Wren B., Dorrell N., Corcionivoschi N. The in vitro and in vivo effect of carvacrol in preventing Campylobacter infection, colonization and in improving productivity of chicken broilers. Foodborne Pathog. Dis. 2017;14:341–349. doi: 10.1089/fpd.2016.2265. [DOI] [PubMed] [Google Scholar]
- Khameneh B., Diab R., Ghazvini K., Fazly Bazzaz B.S. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog. 2016;95:32–42. doi: 10.1016/j.micpath.2016.02.009. [DOI] [PubMed] [Google Scholar]
- Khattak F., Ronchi A., Castelli P., Sparks N. Effects of natural blend of essential oil on growth performance, blood biochemistry, cecal morphology, and carcass quality of broiler chickens. Poult. Sci. 2014;93:132–137. doi: 10.3382/ps.2013-03387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khumpirapang N., Pikulkaew S., Müllertz A., Rades T., Okonogi S. Self-microemulsifying drug delivery system and nanoemulsion for enhancing aqueous miscibility of Alpinia galanga oil. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpinar F., Bora Ünlü H., Özdemir G. Effects of oregano and garlic essential oils on performance, carcass, organ and blood characteristics and intestinal microflora of broilers. Livest. Sci. 2011;137:219–225. [Google Scholar]
- Kohanski M.A., Dwyer D.J., Collins J.J. How antibiotics kill bacteria: from targets to networks. Nat. Rev. Microbiol. 2010;8:423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollanoor-Johny A., Darre M.J., Donoghue A.M., Donoghue D.J., Venkitanarayanan K. Antibacterial effect of trans-cinnamaldehyde, eugenol, carvacrol, and thymol on Salmonella enteritidis and Campylobacter jejuni in chicken cecal contents in vitro. J. Appl. Poult. Res. 2010;19:237–244. [Google Scholar]
- Kollanoor-Johny A. PhD Diss. Univ. Connecticut; Connecticut: 2011. Investigating the potential of natural antimicrobial molecules for reducing Salmonella enterica serovar enteritidis colonization in chickens. [Google Scholar]
- Kollanoor-Johny A., Mattson T., Baskaran S.A., Amalaradjou M.A., Babapoor S., March B., Valipe S., Darre M., Hoagland T., Schreiber D., Khan M.I., Donoghue A., Donoghue D., Venkitanarayanan K. Reduction of Salmonella enterica serovar enteritidis colonization in 20-day-old broiler chickens by the plant-derived compounds trans-cinnamaldehyde and eugenol. Appl. Environ. Microbiol. 2012;78:2981–2987. doi: 10.1128/AEM.07643-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollanoor-Johny A., Upadhyay A., Baskaran S.A., Upadhyaya I., Mooyottu S., Mishra N., Darre M.J., Khan M.I., Donoghue A.M., Donoghue D.J., Venkitanarayanan K. Effect of therapeutic supplementation of the plant compounds trans-cinnamaldehyde and eugenol on Salmonella enterica serovar enteritidis colonization in market-age broiler chickens. J. Appl. Poult. Res. 2012;21:816–822. [Google Scholar]
- Koščová J., Nemcová R., Gancarčíková S., Jonecová Z., Sciranková L., Bomba A., Buleca V. Effect of two plant extracts and Lactobacillus fermentum on colonization of gastrointestinal tract by Salmonella enterica var. Düsseldorf in chicks. Biologia. 2006;61:775–778. [Google Scholar]
- Kročko M., Canigová M., Bezeková J., Lavová M., Haščík P., Ducková V. Effect of nutrition with propolis and bee pollen supplements on bacteria colonization pattern in gastrointestinal tract of broiler chickens. J. Anim. Sci. Biotechnol. 2012;45:63–67. [Google Scholar]
- Küçükyilmaz K., Bozkurt M., Selek N., Güven E., Eren H., Atasever A., Bintaş E., Çatlı A.U., Çınar M. Effects of vaccination against coccidiosis, with and without a specific herbal essential oil blend, on performance, oocyst excretion and serum IBD titers of broilers reared on litter. Ital. J. Anim. Sci. 2012;11:1. [Google Scholar]
- Kumar S., Chen C., Indugu N., Werlang G.O., Singh M., Kim W.K., Thippareddi H. Effect of antibiotic withdrawal in feed on chicken gut microbial dynamics, immunity, growth performance and prevalence of foodborne pathogens. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0192450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Ragione R.M., Narbad A., Gasson M.J., Woodward M.J. In vivo characterization of Lactobacillus johnsonii FI9785 for use as a defined competitive exclusion agent against bacterial pathogens in poultry. Lett. Appl. Microbiol. 2004;38:197–205. doi: 10.1111/j.1472-765x.2004.01474.x. [DOI] [PubMed] [Google Scholar]
- Landoni M.F., Albarellos G. The use of antimicrobial agents in broiler chickens. Vet. J. 2015;205:21–27. doi: 10.1016/j.tvjl.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Lee K.W., Ho Hong Y., Lee S.H., Jang S.I., Park M.S., Bautista D.A., Ritter G.D., Jeong W., Jeoung H.Y., An D.J., Lillehoj E.P., Lillehoj H.S. Effects of anticoccidial and antibiotic growth promoter programs on broiler performance and immune status. Res. Vet. Sci. 2012;93:721–728. doi: 10.1016/j.rvsc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Lee K.-W. PhD Diss. Uttercht University; Uttercht, Netherlands: 2002. Essential oils in broiler nutrition. [Google Scholar]
- Lee M.D., Newell D.G. Campylobacter in poultry: filling an ecological niche. Avian Dis. 2006;50:1–9. doi: 10.1637/7474-111605R.1. [DOI] [PubMed] [Google Scholar]
- Lestari S.I., Han F., Wang F., Ge B. Prevalence and antimicrobial resistance of Salmonella serovars in conventional and organic chickens from Louisiana retail stores. J. Food Prot. 2009;72:1165–11672. doi: 10.4315/0362-028x-72.6.1165. [DOI] [PubMed] [Google Scholar]
- Li H.L., Zhao P.Y., Lei Y., Hossain M.M., Kim I.H. Phytoncide, phytogenic feed additive as an alternative to conventional antibiotics, improved growth performance and decreased excreta gas emission without adverse effect on meat quality in broiler chickens. Livest. Sci. 2015;181:1–6. [Google Scholar]
- Liang R., Xu S., Shoemaker C.F., Li Y., Zhong F., Huang Q. Physical and antimicrobial properties of peppermint oil nano emulsions. J. Agric. Food Chem. 2012;60:7548–7555. doi: 10.1021/jf301129k. [DOI] [PubMed] [Google Scholar]
- Lin J. Antibiotic growth promoters enhance animal production by targeting intestinal bile salt hydrolase and its producers. Front. Microbiol. 2014;5:33. doi: 10.3389/fmicb.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado M., Dinis A.M., Salgueiro L., Cavaleiro C., Custódio J.B., do Céu Sousa M. Anti-Giardia activity of phenolic-rich essential oils: effects of Thymbra capitata, Origanum virens, Thymus zygis subsp. sylvestris, and Lippia graveolens on trophozoites growth, viability, adherence, and ultrastructure. Parasitol. Res. 2010;106:1205–1215. doi: 10.1007/s00436-010-1800-7. [DOI] [PubMed] [Google Scholar]
- Maderuelo C., Zarzuelo A., Lanao J.M. Critical factors in the release of drugs from sustained release hydrophilic matrices. J. Control. Release. 2011;154:2–19. doi: 10.1016/j.jconrel.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Mahmoud S.S., Croteau R.B. Strategies for transgenic manipulation of monoterpene biosynthesis in plants. Trends Plant Sci. 2002;7:366–373. doi: 10.1016/s1360-1385(02)02303-8. [DOI] [PubMed] [Google Scholar]
- Marcinčák S., Cabadaj R., Popelka P., Šoltýsová L. Antioxidative effect of oregano supplemented to broilers on oxidative stability of poultry meat. Slov. Vet. Res. 2008;45:61–66. [Google Scholar]
- Marquardt R.R., Li S. Antimicrobial resistance in livestock: advances and alternatives to antibiotics. Anim. Front. 2018;8:30–37. doi: 10.1093/af/vfy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason T.G., Wilking J.N., Meleson K., Chang C.B., Graves S.M. Nano emulsions: formation, structure, and physical properties. J. Phys. Condens. Matter. 2006;18:R635. [Google Scholar]
- Mathew A.G., Cissell R., Liamthong S. Antibiotic resistance in bacteria associated with food animals: a United States perspective of livestock production. Foodborne Pathog. Dis. 2007;4:115–133. doi: 10.1089/fpd.2006.0066. [DOI] [PubMed] [Google Scholar]
- McClements D.J., Rao J. Food-grade nano emulsions: formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011;51:285–330. doi: 10.1080/10408398.2011.559558. [DOI] [PubMed] [Google Scholar]
- McClements D.J. Nano emulsions versus microemulsions: terminology, differences, and similarities. Soft Matter. 2012;8:1719–1729. [Google Scholar]
- Mehdi Y., Létourneau-Montminy M.P., Gaucher M.L., Chorfi Y., Suresh G., Rouissi T., Brar S.K., Côté C., Ramirez A.A., Godbout S. Use of antibiotics in broiler production: global impacts and alternatives. Anim. Nutr. 2018;4:170–178. doi: 10.1016/j.aninu.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micciche A., Rothrock Jr M.J., Yang Y., Ricke S.C. Essential oils as an intervention strategy to reduce Campylobacter in poultry production: a review. Front. Microbiol. 2019;10:1058. doi: 10.3389/fmicb.2019.01058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton B., Hui K.P. Inhibition of hepatic S-3-hydroxy-3-methylglutaryl-CoA reductase and in vivo rates of lipogenesis by a mixture of pure cyclic monoterpenes. Biochem. Pharmacol. 1982;31:2897–2901. doi: 10.1016/0006-2952(82)90261-1. [DOI] [PubMed] [Google Scholar]
- Miguel M.G. Antioxidant and anti-inflammatory activities of essential oils: a short review. Molecules. 2010;15:9252–9587. doi: 10.3390/molecules15129252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhau G., Valentin A., Benoit F., Mallié M., Bastide J.-M., Pélissier Y., Bessière J.-M. In vitro antimalarial activity of eight essential oils. JEOR. 1997;9:329–333. [Google Scholar]
- Mitsch P., Zitterl-Eglseer K., Köhler B., Gabler C., Losa R., Zimpernik I. The effect of two different blends of essential oil components on the proliferation of Clostridium perfringens in the intestines of broiler chickens. Poult. Sci. 2004;83:669–675. doi: 10.1093/ps/83.4.669. [DOI] [PubMed] [Google Scholar]
- Mountzouris K.C., Paraskevas V., Tsirtsikos P., Palamidi I., Steiner T., Schatzmayr G., Fegerosa K. Assessment of a phytogenic feed additive effect on broiler growth performance, nutrient digestibility and caecal microflora composition. Anim. Feed. Sci. Technol. 2011;168:223–231. [Google Scholar]
- Musa H., Chen G.H., Cheng J.H., Li B.C., Mekki D.M. Study on carcass characteristics of chicken breeds raised under the intensive condition. Int. J. Poult. Sci. 2006;5:530–533. [Google Scholar]
- Nabavi S.F., Di Lorenzo A., Izadi M., Sobarzo-Sánchez E., Daglia M., Nabavi S.M. Antibacterial effects of cinnamon: from farm to food, cosmetic and pharmaceutical industries. Nutrients. 2015;7:7729–7748. doi: 10.3390/nu7095359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraju R., Saritha D., Bose P.S.C., Ravi G., Ravi V. A review on current status of self-emulsifying drug delivery systems. Int. J. Med. Biomed. Stud. 2019;3:232–248. [Google Scholar]
- Naik M.I., Fomda B.A., Jaykumar E., Bhat J.A. Antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selected pathogenic bacterias. Asian Pac. J. Trop. Med. 2010;3:535–538. [Google Scholar]
- Namdeo S., Baghel R., Nayak S., Khar A., Pal R.P., Chaurasiya A., Thakur S., Reddy B. Essential oils: a potential substitute to antibiotics growth promoter in broiler diet. J. Entomol. Zool. Stud. 2020;8:1643–1649. [Google Scholar]
- Natrajan D., Srinivasan S., Sundar K., Ravindran A. Formulation of essential oil-loaded chitosan-alginate nanocapsules. J. Food Drug Anal. 2015;23:560–568. doi: 10.1016/j.jfda.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neish A.S. The gut microflora and intestinal epithelial cells: a continuing dialogue. Microbes Infect. 2002;4:309–317. doi: 10.1016/s1286-4579(02)01543-5. [DOI] [PubMed] [Google Scholar]
- Noori S., Zeynali F., Almasi H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control. 2018;84:312–320. [Google Scholar]
- Noormohamed A., Fakhr M.K. Prevalence and antimicrobial susceptibility of Campylobacter spp. in Oklahoma conventional and organic retail poultry. Open Microbiol. J. 2014;8:130–137. doi: 10.2174/1874285801408010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogungbe I.V., Setzer W.N. Cruzain inhibition by terpenoids: a molecular docking analysis. Nat. Prod. Commun. 2008;3:865–868. [Google Scholar]
- Oussalah M., Caillet S., Saucier L., Lacroix M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157: H7, Salmonella typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control. 2007;18:414–420. [Google Scholar]
- Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathania R., Khan H., Kaushik R., Khan M.A. Essential oil nano emulsions and their antimicrobial and food applications. Curr. Res. Nutr. Food Sci. 2018;6:626–643. [Google Scholar]
- Patterson J.A., Burkholder K.M. Application of prebiotics and probiotics in poultry production. Poult. Sci. 2003;82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- Peng Q.Y., Li J.D., Li Z., Duan Z.Y., Wu Y.P. Effects of dietary supplementation with oregano essential oil on growth performance, carcass traits and jejunal morphology in broiler chickens. Anim. Feed. Sci. Technol. 2016;214:148–153. [Google Scholar]
- Phillips I., Casewell M., Cox T., De Groot B., Friis C., Jones R., Nightingale C., Preston R., Waddell J. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J. Antimicrob. Chemother. 2004;53:28–52. doi: 10.1093/jac/dkg483. [DOI] [PubMed] [Google Scholar]
- Pirgozliev V., Bravo D., Mirza M.W., Rose S.P. Growth performance and endogenous losses of broilers fed wheat-based diets with and without essential oils and xylanase supplementation. Poult. Sci. 2015;94:1227–1232. doi: 10.3382/ps/peu017. [DOI] [PubMed] [Google Scholar]
- Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: a review. Eur. J. Med. Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- Platel K., Srinivasan K. Digestive stimulant action of spices: a myth or reality? Indian J. Med. Res. 2004;119:167–179. [PubMed] [Google Scholar]
- Pluske J.R., Hampson J.D., Williams I.H. Factors influencing the structure and function of the small intestine in the weaned pig: a review. Livest. Prod. Sci. 1997;51:215–236. [Google Scholar]
- Qureshi A.A., Lehmann J.W., Peterson D.M. Amaranth and its oil inhibit cholesterol biosynthesis in 6-week-old female chickens. J. Nutr. 1996;126:1972–1978. doi: 10.1093/jn/126.8.1972. [DOI] [PubMed] [Google Scholar]
- Qureshi A.A., Din Z.Z., Abuirmeileh N., Burger W.C., Ahmad Y., Elson C.E. Suppression of avian hepatic lipid metabolism by solvent extracts of garlic: impact on serum lipids. J. Nutr. 1983;113:1746–1755. doi: 10.1093/jn/113.9.1746. [DOI] [PubMed] [Google Scholar]
- Rahimi S., Zadeh Z.T., Torshizi M.A.K., Omidbaigi R., Rokni H. Effect of the three herbal extracts on growth performance, immune system, blood factors and intestinal selected bacterial population in broiler chickens. J. Agric. Sci. Technol. 2011;13:527–539. [Google Scholar]
- Reda F.M., El-Saadony M.T., Elnesr S.S., Alagawany M., Tufarelli V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals. 2020;10:754. doi: 10.3390/ani10050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F., El-Saadony M.T., El-Rayes T., Farahat M., Attia G., Alagawany M. Dietary effect of licorice (Glycyrrhiza glabra) on quail performance, carcass, blood metabolites and intestinal microbiota. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F.M., El-Saadony M.T., El-Rayes T.K., Attia A.I., El-Sayed S.A., Ahmed S.Y., Madkour M., Alagawany M. Use of biological nano zinc as a feed additive in quail nutrition: biosynthesis, antimicrobial activity and its effect on growth, feed utilisation, blood metabolites and intestinal microbiota. Ital. J. Anim. Sci. 2021;20:324–335. [Google Scholar]
- Rodrigues Goulart H., Kimura E.A., Peres V.J., Couto A.S., Aquino Duarte F.A., Katzin A.M. Terpenes arrest parasite development and inhibit biosynthesis of isoprenoids in Plasmodium falciparum. Antimicrob. Agents. Chemother. 2004;48:2502–2509. doi: 10.1128/AAC.48.7.2502-2509.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquillo M.G., Hernandez J.C.A. Antibiotic and synthetic growth promoters in animal diets: review of impact and analytical methods. Food Control. 2017;72:255–267. [Google Scholar]
- Saad A.M., Mohamed A.S., El-Saadony M.T., Sitohy M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT - Food Sci. Technol. 2021;148 [Google Scholar]
- Saad A.M., El-Saadony M.T., El-Tahan A.M., Sayed S., Moustafa M.A., Taha A.E., Taha T.F., Ramadan M.M. Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi J. Biol. Sci. 2021;28:5674–5683. doi: 10.1016/j.sjbs.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salager J.L., Antón R.E., Anderez J.M., Aubry J.M. Formulation des micro- émulsions par la méthode HLD. Techniques de l'Ingénieur. 2001;157:2001. [Google Scholar]
- Samanta S., Haldar S., Ghosh T.K. Comparative efficacy of an organic acid blend and bacitracin methylene disalicylate as growth promoters in broiler chickens: effects on performance, gut histology, and small intestinal milieu. Vet. Med. Int. 2010;2010:1–8. doi: 10.4061/2010/645150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-González L., Vargas M., González-Martínez C., Chiralt A., Chafer M. Use of essential oils in bioactive edible coatings: a review. Food Eng. Rev. 2011;3:1–16. [Google Scholar]
- Sang-Oh P., Chae-Min R., Byung-Sung P., Jong H. The meat quality and growth performance in broiler chickens fed diet with cinnamon powder. J. Environ. Biol. 2013;34:127–133. [PubMed] [Google Scholar]
- São Pedro A., Espirito Santo I., Silva C.V., Detoni C., Albuquerque E., Politécnica E. In: Microbial Pathogens and Strategies for Combating Them. Méndez-Vilas A., editor. Formatex Research Center Publisher; Badajoz, Spain: 2013. The use of nanotechnology as an approach for essential oil-based formulations with antimicrobial activity; pp. 1364–1374. [Google Scholar]
- Sato K., Bartlett P.C., Kaneene J.B., Downes F.P. Comparison of prevalence and antimicrobial susceptibilities of Campylobacter spp. isolates from organic and conventional dairy herds in Wisconsin. Appl. Environ. Microbiol. 2004;70:1442–1447. doi: 10.1128/AEM.70.3.1442-1447.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiha A.M., Abdelnour S.A., Abd El-Hack M.E., Khafaga A.F., Metwally K.A., Ajarem J.S., Maodaa S.N., Allam A.A., El-Saadony M.T. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals. 2020;10:430. doi: 10.3390/ani10030430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M.A., Pessotti B.M., Zanini S.F., Colnago G.L., Rodrigues M.R., de Nunes L., Zanini M.S., Martins I.V. Intestinal mucosa structure of broiler chickens infected experimentally with Eimeria tenella and treated with essential oil of oregano. Ciênc. Rural. 2009;39:1471–1477. [Google Scholar]
- Singer R.S., Hofacre C.L. Potential impacts of antibiotic use in poultry production. Avian Dis. 2006;50:161–172. doi: 10.1637/7569-033106R.1. [DOI] [PubMed] [Google Scholar]
- Singh B.R., Ebibeni N. Aerobic microbiome of vagina of apparently healthy pregnant large black sows in Nagaland and antimicrobial resistance in isolates. Glob. J. Pathol. Microbiol. 2016;4:8–17. [Google Scholar]
- Singh P., Karimi A., Devendra K., Waldroup P.W., Cho K.K., Kwon Y.M. Influence of penicillin on microbial diversity of the cecal microbiota in broiler chickens. Poult. Sci. 2013;92:272–276. doi: 10.3382/ps.2012-02603. [DOI] [PubMed] [Google Scholar]
- Smith-Palmer A., Stewart J., Fyfe L. Influence of subinhibitory concentrations of plant essential oils on the production of enterotoxins A and B and alpha-toxin by Staphylococcus aureus. J. Med. Microbiol. 2004;53:1023–1027. doi: 10.1099/jmm.0.45567-0. [DOI] [PubMed] [Google Scholar]
- Solè I., Pey C.M., Maestro A., González C., Porras M., Solans C., Gutiérreza J.M. Nano-emulsions prepared by the phase inversion composition method: preparation variables and scale up. J. Colloid Interface Sci. 2010;344:417–423. doi: 10.1016/j.jcis.2009.11.046. [DOI] [PubMed] [Google Scholar]
- Sood S., Jain K., Gowthamarajan K. Optimization of curcumin nanoemulsion for intranasal delivery using design of experiment and its toxicity assessment. Colloids Surf. B. Biointerfaces. 2014;113:330–337. doi: 10.1016/j.colsurfb.2013.09.030. [DOI] [PubMed] [Google Scholar]
- Sugiharto S. Role of nutraceuticals in gut health and growth performance of poultry. J. Saudi Soc. Agric. Sci. 2016;15:99–111. [Google Scholar]
- Suresh G., Das R.K., Kaur Brar S., Rouissi T., Ramirez A.A., Chorfi Y., Godbout S. Alternatives to antibiotics in poultry feed: molecular perspectives. Crit. Rev. Microbiol. 2018;44:318–335. doi: 10.1080/1040841X.2017.1373062. [DOI] [PubMed] [Google Scholar]
- Szigeti G., Pálfi V., Nagy B., Edes I., Nagy G., Szmolény G., Bago G., Radvanyi S. New type of immuno-stimulant to increase antibody production in response to viral and bacterial vaccines. Magyar Allatorvosok Lapja. 1998;120:719–721. [Google Scholar]
- Talazadeh F., Mayahi M. Immune response of broiler chickens supplemented with pediatric cough syrup including thyme extract in drinking water against influenza vaccine. J. Herbmed. Pharmacol. 2017;6:33–36. [Google Scholar]
- Tiihonen K., Kettunen H., Bento M.H.L., Saarinen M., Lahtinen S., Ouwehand A.C., Schulze H., Rautonen N. The effect of feeding essential oils on broiler performance and gut microbiota. Br. Poult. Sci. 2010;51:381–392. doi: 10.1080/00071668.2010.496446. [DOI] [PubMed] [Google Scholar]
- Toutain P-.L., Ferran A.A., Bousquet-Melou A., Pelligand L., Lees P. Veterinary medicine needs new green antimicrobial drugs. Front. Microbiol. 2016;7:1196. doi: 10.3389/fmicb.2016.01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsinas A., Giannenas I., Voidarou C., Tzora A., Skoufos J. Effects of an oregano based dietary supplement on performance of broiler chickens experimentally infected with Eimeria acervulina and Eimeria maxima. Poult. Sci. 2011;48:194–200. [Google Scholar]
- Ueda-Nakamura T., Mendonça-Filho R.R., Morgado-Díaz J.A., Maza P.K., Prado Dias Filho B., Aparício Garcia Cortez D., Alviano D.S., Rosa M., Lopes A.H., Alviano C.S., Nakamura C.V. Antileishmanial activity of Eugenol-rich essential oil from Ocimum gratissimum. Parasitol. Int. 2006;55:99–105. doi: 10.1016/j.parint.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Ulfah M. Essential oils as multi-function feed additive (MFA) to improve broilers performance, metabolism, dung consistency and efficiency of production. J. Agric. Rural. Dev. Trop. Subtrop. 2006;88:50–55. [Google Scholar]
- Upadhyay A., Upadhyaya I., Kollanoor-Johny A., Venkitanarayanan K. Combating pathogenic microorganisms using plant-derived antimicrobials: a mini review of the mechanistic basis. BioMed. Res. Int. 2014;2014 doi: 10.1155/2014/761741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay A., Arsi K., Wagle B.R., Upadhyaya I., Shrestha S., Donoghue A.M., Donoghue D.J. Trans-cinnamaldehyde, carvacrol, and eugenol reduce Campylobacter jejuni colonization factors and expression of virulence genes in vitro. Front. Microbiol. 2017;8:713. doi: 10.3389/fmicb.2017.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya I., Upadhyay A., Kollanoor-Johny A.M., Baskaran S.A., Mooyottu S., Darre M.J., Venkitanarayanan K. Rapid inactivation of Salmonella enteritidis on shell eggs by plant-derived antimicrobials. Poult. Sci. 2013;92:3228–3235. doi: 10.3382/ps.2013-03126. [DOI] [PubMed] [Google Scholar]
- Vaarst, M., K. Horsted, and V. Maurer. 2019. Organic poultry farming: opportunities and challenges. Pages 307–328 in Improving Organic Animal Farming. M. Vaarst and S. Roderick, ed. 1st. Burleigh Dodds Science Publishing, Cambridge, UK.
- Van Boeckel T.P., Brower C., Gilbert M., Grenfell B.T., Levin S.A., Robinson T.P., Teillant A., Laxminarayan R. Global trends in antimicrobial use in food animals. PNAS. 2015;112:5649–5654. doi: 10.1073/pnas.1503141112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overbeke I., Duchateau L., De Zutter L., Albers G., Ducatelle R. A comparison survey of organic and conventional broiler chickens for infectious agents affecting health and food safety. Avian Dis. 2006;50:196–200. doi: 10.1637/7448-093005R.1. [DOI] [PubMed] [Google Scholar]
- Vandamme T.F., Anton N. Low-energy nanoemulsification to design veterinary controlled drug delivery devices. Int. J. Nanomed. 2010;2010:867–873. doi: 10.2147/IJN.S13273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkitanarayanan K., Kollanoor-Johny A., Darre M.J., Donoghue A.M., Donoghue D.J. Use of plant-derived antimicrobials for improving the safety of poultry products. Poult. Sci. 2013;92:493–501. doi: 10.3382/ps.2012-02764. [DOI] [PubMed] [Google Scholar]
- Vermeulen B., De Backer P., Remon J.P. Drug administration to poultry. Adv. Drug Deliv. Rev. 2002;54:795–803. doi: 10.1016/s0169-409x(02)00069-8. [DOI] [PubMed] [Google Scholar]
- Vigan M. Essential oils: renewal of interest and toxicity. Eur. J. Dermatol. 2010;20:685–692. doi: 10.1684/ejd.2010.1066. [DOI] [PubMed] [Google Scholar]
- Vlaicu P.A., Saracila M., Panaite T.D., Tabuc C., Bobe E., Criste R.D. Effect of the dietary grape seeds and rosehip oils given to broilers (14–42 days) reared at 32°C on broiler performance, relative weight of carcass cuts and internal organs and balance of gut microflora. Archi. Zootech. 2017;20:77–88. [Google Scholar]
- Wade M.R., Manwar S.J., Kuralkar S.V., Waghmare S.P., Ingle V.C., Hajare S.W. Effect of thyme essential oil on performance of broiler chicken. J. Entomol. Zool. Stud. 2018;6:25–28. [Google Scholar]
- Wang L., Dong J., Chen J., Eastoe J., Li X. Design and optimization of a new self-nanoemulsifying drug delivery system. J. Colloid. Interface Sci. 2009;330:443–448. doi: 10.1016/j.jcis.2008.10.077. [DOI] [PubMed] [Google Scholar]
- Wegener H.C. Antibiotics in animal feed and their role in resistance development. Curr. Opin. Microbiol. 2003;6:439–445. doi: 10.1016/j.mib.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Williams R.B. Laboratory tests of phenolic disinfectants as oocysticides against the chicken coccidium Eimeria tenella. Vet. Res. 1997;141:447–448. doi: 10.1136/vr.141.17.447. [DOI] [PubMed] [Google Scholar]
- Windisch W., Schedle K., Plitzner C., Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008;86:E140–E148. doi: 10.2527/jas.2007-0459. [DOI] [PubMed] [Google Scholar]
- Yang X., Xin H., Yang C., Yang X. Impact of essential oils and organic acids on the growth performance, digestive functions and immunity of broiler chickens. Anim. Nutr. 2018;4:388–393. doi: 10.1016/j.aninu.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaqoob M., Abd El-Hack M.E., Hassan F., El-Saadony M.T., Khafaga A., Batiha G., Yehia N., Elnesr S., Alagawany M., El-Tarabily K., Wang M. The potential mechanistic insights and future implications for the effect of prebiotics on poultry performance, gut microbiome, and intestinal morphology. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yitbarek M.B. Phytogenics as feed additives in poultry production: a review. Int. J. Extensive Res. 2015;3:49–60. [Google Scholar]
- Yu S.G., Abuirmeileh N.M., Qureshi A.A., Elson C.E. Dietary beta-ionone suppresses hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. J. Agric. Food Chem. 1994;42:1493–1496. [Google Scholar]
- Zhai Z., Liu Y., Wu L., Senchina D.S., Wurtele E.S., Murphy P.A., Kohut M.L., Cunnick J.E. Enhancement of innate and adaptive immune functions by multiple Echinacea species. J. Med. Food. 2007;10:423–434. doi: 10.1089/jmf.2006.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Wang C., Chow A.H., Ren K., Gong T., Zhang Z., Zheng Z. Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of Zedoary essential oil: formulation and bioavailability studies. Int. J. Pharm. 2010;383:170–177. doi: 10.1016/j.ijpharm.2009.08.035. [DOI] [PubMed] [Google Scholar]
- Zheng W., Wang S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001;49:5165–5170. doi: 10.1021/jf010697n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.