Abstract
Objective:
Preclinical and clinical evidence suggests that oxytocin administration decreases food intake and weight. The mechanisms underlying the anorexigenic effects of oxytocin in humans are unknown but critical to study to consider oxytocin as a neurohormonal weight loss treatment. Complementing ongoing research into metabolic and food motivation mechanisms of oxytocin, we hypothesized that in humans, oxytocin improves cognitive control over behavior.
Methods:
In a randomized, double-blind, placebo-controlled crossover study of 24 IU single-dose intranasal oxytocin, ten men with overweight or obesity completed a stop-signal task assessing ability and strategy to suppress behavioral impulses, in which they performed a choice-reaction task (go task) but had to withhold their response when prompted (stop task). It was hypothesized that oxytocin would improve suppression of behavioral impulses.
Results:
After receiving oxytocin, compared to placebo, participants showed increased reaction times in the go task (M=936 vs. 833 ms, p=0.012, 95% CI [29, 178]) and displayed fewer stop errors (M=36.41 vs. 41.15%, p=0.049, 95% CI [−9.43, −0.03]).
Conclusions:
Oxytocin triggers increased proactive control over behavior. Future studies need to further characterize the impact of oxytocin on cognitive control and investigate its potential role in the anorexigenic effects of oxytocin in human obesity.
Keywords: Cognitive control, Impulse control, Obesity, Overweight, Oxytocin
Introduction
Recent advances in nutrition suggest that the hypothalamic neuropeptide oxytocin acts as a critical central nervous system factor in mediating food intake and weight. Oxytocin is produced in the paraventricular and supraoptic nuclei of the hypothalamus, decreases food intake in rodent, primate, and human obesity,1–7 and induces weight loss with minimal side effects.2, 8–10 As a consequence, oxytocin is under investigation as a potential new neurohormonal treatment for obesity. In the U.S., 69% of adults have overweight, with 35% meeting criteria for obesity11, and rates of associated health risks, such as diabetes, cardiovascular disease, and premature mortality, are rising. Although complications resulting from obesity may be reversible with successful weight reduction, achieving and maintaining meaningful weight loss is difficult with available pharmacological and lifestyle interventions, and current treatment options are associated with complications12 and safety and tolerability problems.13 Thus, there is a clear need for effective, tolerable obesity therapies.
The mechanisms underlying the beneficial effects of oxytocin on food intake and weight in humans are not well understood but are important to determine the potential of oxytocin as a weight loss treatment. In humans, intranasal oxytocin reduces caloric consumption, particularly of palatable foods, without affecting subjective appetite.3, 4 In addition, when hunger- and reward-driven eating was contrasted, oxytocin affected hedonic eating (snacking in a satiety state) more than homeostatic food intake.6 These findings indicate that oxytocin might exert some of its effect on food intake in humans through altering eating behavior in response to hedonic urges to eat rather than hunger signals.
Cognitive control – the group of mechanisms that regulate impulses, habits, decision-making, and personal autonomy14 – plays a key role in hedonic food intake, and human obesity is characterized by reduced cognitive control, linked to poor clinical outcomes. Individuals with obesity, compared to healthy controls, show impaired cognitive control over impulsive actions, impaired cognitive flexibility, and more risky decision-making.15–20 Cognitive control has been shown to predict food intake,21 and improvements in cognitive control following an 8-week weight loss intervention that included cognitive and behavioral strategies to achieve goals predicted weight loss in individuals with obesity.16 In addition, behavioral and neuroimaging studies demonstrate that individuals with obesity, compared with control participants with normal weight, show an increased responsiveness to reward attainment and a stronger bias for (instantly) rewarded behaviors and choices.22–24 Increased responsiveness to food reward amplifies the potential consequences of reduced cognitive control in the context of food intake, as it makes individuals with low cognitive control more susceptible to overeating, poor dietary choices, and ultimately weight gain.25 These data highlight cognitive control (and its interplay with reward- or habit-driven behavioral impulses) as a promising target in the search for novel approaches in obesity management.
Oxytocin could alter cognitive control. Oxytocin receptors are present in cognitive control brain regions,26 and oxytocin promotes cortical control over subcortical brain structures.27, 28 In a randomized, double-blind, placebo-controlled crossover study in ten men with overweight or obesity, using an exploratory whole-brain analysis, we have shown that a single dose of 24 IU intranasal oxytocin (the same dose that reduced food intake in previous studies3, 4, 6, 7) increased functional magnetic resonance imaging (fMRI) activity in the anterior cingulate cortex and frontopolar prefrontal cortex (both of which have been associated with cognitive control29) with a simultaneous attenuation of fMRI activity in brain areas processing food reward when participants viewed images of palatable food items compared to non-food objects.30 Our findings are supported by Spetter et al. (2018)31 who showed that in 15 men with normal weight, 24 IU single-dose intranasal oxytocin altered neural activation in reward-related brain regions and the cognitive control network during a task requiring the engagement of both reward evaluation and cognitive control processes. While alterations of neural activity in the cognitive control brain network are a promising first indication that oxytocin might affect cognitive control, it remains unclear whether those alterations translate into behavioral effects. In their neuroimaging study, Spetter et al. showed that oxytocin also reduced food intake during a breakfast in the same sample by 12%. However, it is unclear whether this reduction in food intake is related to increases in cognitive control, reduced reward responsiveness, or other factors. To prove that the observed oxytocin-induced changes in neural activity within the cognitive control network translate into behavioral advantages (i.e., reduced impulsive behavior), behavioral testing of cognitive control without involvement of reward processing and other factors that can affect food intake (e.g., homeostatic drive) is required.
We aimed to provide first behavioral evidence for an effect of oxytocin on cognitive control over behavioral impulses in individuals with overweight or obesity. We asked the same group of men with overweight or obesity who underwent neuroimaging following oxytocin administration30 to also complete a well-established paradigm to test the ability and strategy to control pre-existing behavioral impulses, namely a stop-signal task.32 In this paradigm, participants perform a simple choice-reaction task (go task). Occasionally, after the stimulus for the go task is provided, a signal indicates the need to suppress the response for the go task and thus to stop an already generated behavioral impulse from being executed (stop task). Performance in this task reflects an individual’s ability and strategy to control undesired behavioral impulses. First, the duration of the process of suppressing the behavioral impulse can be estimated in this task (stop-signal reaction time, SSRT), and a shorter SSRT indicates a higher reactive ability to inhibit behavioral impulses.33, 34 Second, the stop-signal task is well suited to detect strategic shifts in cognitive control that introduce proactive tonic inhibition of behavioral impulses by increasing the response threshold for the go response.35–38 We hypothesized that in men with overweight or obesity, administration of a single dose of 24 IU intranasal oxytocin, compared to placebo, would alter cognitive processing translating into improved performance in this task. Augmented performance would be revealed by improving reactive control over behavioral impulses (indicated by a reduced SSRT; Hypothesis 1) and/or triggering an increase in meta-control capacities through upregulated proactive control by adapting a more cautious behavioral strategy (expressed as an increased response time in the go task and higher accuracy in stop trials; Hypothesis 2).
Methods
Participants
Ten men with overweight or obesity who were otherwise healthy took part in this study. Results from an fMRI paradigm that was performed during the same visits have been previously reported.30 Participants were required to be 18–45 years of age with a body mass index (BMI) between 25 and 40 kg/m2, stable weight over the preceding three months, and regular breakfast habits (≥4/week). Exclusion criteria for this study comprised any psychiatric diagnosis, psychotropic medication, a current or past eating disorder by the Structured Clinical Interview for DSM-IV-TR Axis I Disorders,39 excessive exercise (running >25 miles or exercising >10 h in any one week) over the past three months, active substance use, smoking, a history of diabetes mellitus, cardiovascular disease, or gastrointestinal tract surgery, untreated thyroid disease, a hematocrit value below the normal range, and a contraindication for MRI.
Procedures
This investigation was part of a clinical trial investigating the effects of intranasal oxytocin administration on homeostatic and hedonic food motivation (clinicaltrials.gov registration number: NCT02276677). The study was approved by the Partners HealthCare Institutional Review Board and conducted in accordance with the Declaration of Helsinki. Participants gave their written informed consent prior to participation.
Participants completed an outpatient screening (to determine eligibility30) and two main study visits at the Massachusetts General Hospital (MGH) Translational Clinical Research Center. The main study visits were completed in the morning after a 10-h fast 6 to 24 days apart. Screening visits were completed between November 2014 and May 2015; main study visits took place between December 2014 and May 2015. At 7.30 am, intranasal oxytocin (24 IU, Syntocinon®, Novartis, Basel, Switzerland, provided by Victoria Pharmacy Zürich, Switzerland) or placebo (same inactive ingredients and packaging, Victoria Pharmacy) were self-administered (three sprays per nostril) under the supervision of a nurse practitioner. For this randomized, double-blind, placebo-controlled crossover study, participants were randomized 1:1 to one of two drug orders (i.e., oxytocin – placebo or placebo – oxytocin) by the MGH research pharmacy. Participants and study staff were blinded to the randomization. Fifteen minutes after administration of oxytocin or placebo, participants started the stop-signal task.
Stop-Signal Task
Participants categorized geometrical shapes (square vs. diamond; go stimuli) but were instructed to withhold the response when a stop signal (tone) appeared.32, 40 Each trial started with a fixation cross at the screen center for 500 ms, followed by the go stimulus, which was displayed until a response was provided or for a maximum of 3,000 ms. Participants responded with the left index finger (“Z” key) to squares and right index finger (“/” key) to diamonds on a QWERTY keyboard. A blank-screen inter-trial interval (ITI) of 1,000 ms preceded the next trial, resulting in a constant response stimulus interval (RSI) of 1,500 ms. During practice trials only, a feedback was displayed for 300 ms following erroneous trials indicating the type of error (i.e., “wrong,” “too slow,” or “stop error”), while a blank screen was displayed for 300 ms following correct trials. A blank-screen ITI of 700 ms was added to maintain the 1,500 ms RSI. All visual stimuli were presented in white on a black background. In a randomly selected 25% of all trials, a sinus tone (750 Hz, 75 ms) indicated to withhold the response to the go stimulus. The time delay between the onset of the imperative stimulus and the stop signal was adjusted based on the individual responses employing a staircase tracking algorithm (one-up one-down procedure, 50-ms intervals, initial stop-signal delay: 250 ms41). Participants completed 12 practice trials and three experimental blocks with 64 trials each (total number of test trials: 192). Stimulus presentation and data recording were realized using Presentation® software (Neurobehavioral Systems, Inc., Berkeley, CA, USA) on an HP Elitebook 8560p laptop.
Data Analysis
From the responses in go and stop trials, individual mean response times (RTs) in go trials as well as error rates in both go and stop trials were calculated. For analysis of go RTs, erroneous go trials (2.47%) and correct RTs deviating more than 3 SD from the individual mean RT at each session (0.77%) were excluded. In addition, using the mathematical framework of the independent race model,33, 42 the duration of the inhibitory control process (SSRT) was estimated.
Statistical analyses were performed using SPSS® Statistics (Version 23, IBM, Armork, NY, USA). Paired t-tests or Wilcoxon signed-rank tests (for non-normally distributed data as determined by Shapiro-Wilk test) contrasting oxytocin and placebo visits were performed for RT and error rate in go trials, error rate in stop trials, and SSRT. Cohen’s d and r = Z/sqrt(N*2) are reported as effect size estimates for paired t-tests and Wilcoxon signed-rank tests, respectively.
Results
Participant Characteristics
Participants were between 23 and 43 years old (M=31.4 years, SD=5.8 years) with a BMI between 25.3 and 33.7 kg/m2 (M=28.9 kg/m2, SD=2.4 kg/m2). Additional participant characteristics for this sample have been previously reported.30
Performance in the Stop-Signal Task
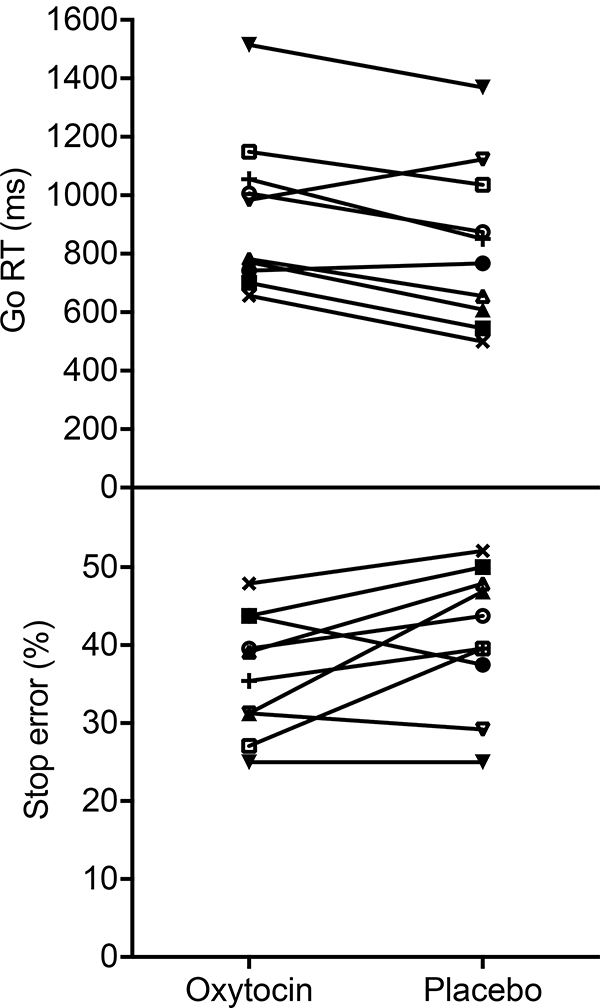
Participants displayed slower go RTs following oxytocin administration (M=936 ms, SD=263 ms) compared to placebo (M=833 ms, SD=278 ms); t(9)=3.13, p=0.012, 95% confidence interval [CI] [29, 178], d=0.99. In addition, they made fewer stop errors following oxytocin administration (M=36.41%, SD=7.67%) versus placebo (M=41.15%, SD=8.86%); t(9)=−2.28, p=0.049, 95% CI [−9.43, −0.03], d=−0.72 (Figure 1). Individual plots of differences in go RT and stop error rate between conditions are displayed in Figure 2. The error rate in go trials did not differ between oxytocin (Mdn=1.22%, IQR=0.52 – 2.43) and the placebo condition (Mdn=1.74%, IQR=0.52 – 3.47); Z=−0.53, p=0.599, r=−0.12. Similarly, the SSRT was not different between oxytocin (M=147 ms, SD=110 ms) and placebo visits (M=140 ms, SD=154 ms); t(9)=0.21, p=0.840, 95% CI [−70, 84], d=0.06.
Figure 1.
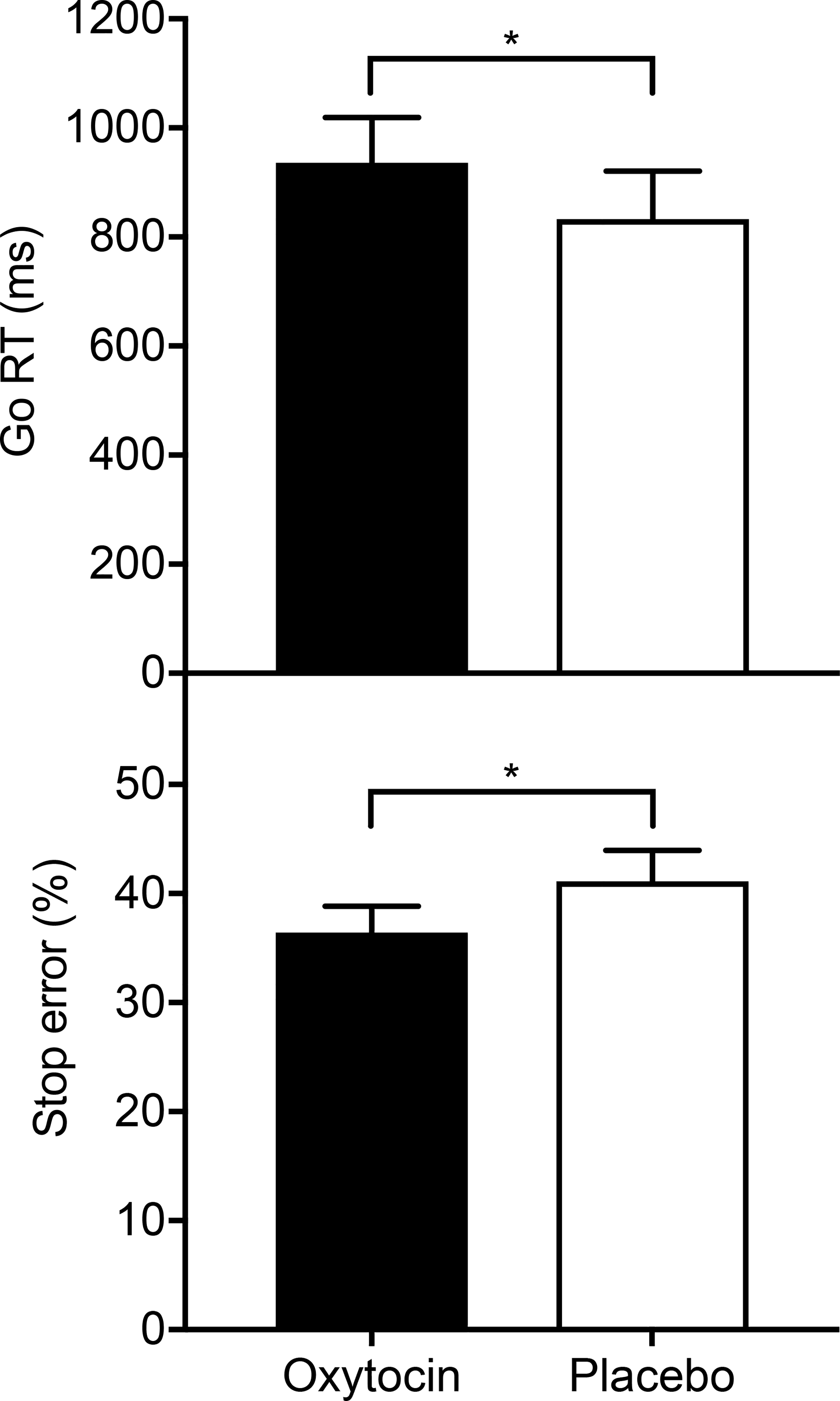
Response time in go trials (go RT) and percentage of erroneous responses in stop trials (stop error) following intranasal oxytocin and placebo administration (N=10). After receiving oxytocin, men with overweight or obesity showed increased go RTs and fewer stop errors, which is consistent with a more cautious behavioral strategy triggered by an increase in proactive control. Error bars represent SEM. *p<0.05.
Figure 2.
Response time in go trials (go RT) and percentage of erroneous responses in stop trials (stop error) following intranasal oxytocin and placebo administration for each individual (N=10). The observed overall pattern of increased go RTs combined with fewer stop errors is consistently present in eight out of ten individuals, suggesting that the reported finding reflects a general effect of oxytocin.
As this was a pilot study investigating a novel research question, no a-priori power analysis was performed. Achieved power was determined using G*Power (Version 3.1.9.6; Heinrich Heine University, Düsseldorf, Germany).43 With ten participants in this randomized, double-blind, placebo-controlled crossover study and an observed effect size of d=0.99 for the difference in response speed in the go task, we had a power of 1-β=0.80 to detect a difference at a two-tailed significance level of α=0.05.
Discussion
To our knowledge, this is the first study to investigate the effects of oxytocin on cognitive control ability and strategy in individuals with overweight or obesity. In this randomized, double-blind, placebo-controlled crossover pilot investigation in men with overweight or obesity, we demonstrate that a single dose of 24 IU intranasal oxytocin increases proactive control over behavioral impulses in a stop-signal task. These data highlight the modulation of cognitive control pathways with behavioral effects in the studied population.
After receiving oxytocin, men with overweight or obesity showed slower response times in the go task and a reduced frequency of executing pre-existing impulses when prompted not to do so. This response pattern is consistent with a more cautious behavioral strategy (Hypothesis 2). The strategic character of this increase in go response threshold is further highlighted by the simultaneous reduction of stop errors. As the stop-signal task adjusts to successful suppressions of responses in stop trials by presenting the stop signal 50 ms later at the next occasion, a lower number of stop errors can only be achieved by a continuous slowing that closely follows the automatic calibration of the stop-signal onset that is part of the task.36 This response pattern of error aversion and a strategic shift in go response threshold has been theoretically derived (goal priority hypothesis37) and repeatedly documented in the literature.35, 36, 38 It has also been linked to personality traits triggering this response pattern in a subgroup of individuals but not others in a standard stop-signal task as applied here as well as the result of explicit instructions encouraging participants to adopt a deliberate strategic control shift.38 We extend these findings by providing novel evidence that a change in endocrine state by intranasal oxytocin administration can induce a similar strategic shift. As participants could not reliably distinguish between oxytocin and placebo when prompted at the end of the study, it can be assumed that the observed behavioral change occurred without deliberation (e.g., via interoceptive signaling).
While the sample size of this pilot investigation is small, the individual results show that the observed overall pattern is consistently present in eight out of ten participants, suggesting that the reported finding reflects a general effect of oxytocin. Furthermore, an a-posteriori power analysis indicated an achieved power of 80% for the observed slowing in the go task. While replications in larger sample sizes, including studying the role of BMI and sex, represent key next steps, this behavioral evidence for a beneficial effect of oxytocin on cognitive control to regulate behavioral impulses critically extends previous findings from neuroimaging studies showing that (the same dose of) intranasal oxytocin increases fMRI activation in the cognitive control brain network,30, 31 proving that oxytocin can alter control over behavioral impulses in men with overweight or obesity.
The observed findings cannot be explained by a general oxytocin-induced cognitive slowing. In more detail, performance in stop trials is typically described by a horse race model.33 As such, general cognitive slowing would reduce speed of both go and stop process, which would result in an unaltered difference in duration between the two. As this difference in durations determines the outcome in stop trials, a general slowing would result in an unchanged stop error rate, while the current results show that the slowing in the go task under oxytocin was accompanied by a decrease in stop error rate in this condition.
The SSRT did not differ across conditions, thus, the present study provides no evidence that oxytocin alters the reactive ability to suppress impulses (Hypothesis 1). However, the observed strategic trade-off biases the estimation of the SSRT (i.e., the SSRT is estimated based on the distribution of the RTs in the go task together with the stop error probability).36 Therefore, the present findings cannot conclusively state that oxytocin does not alter reactive inhibition of behavioral impulses, and further studies that suppress strategic trade-offs (e.g., by setting stringent time-out constraints for the go task) will allow for determining the impact of oxytocin on the SSRT (with the limitation of not capturing potential strategic control shifts as detected here).
Oxytocin might trigger a proactive slowing strategy to reduce negative feedback. In the stop-signal task, failure to successfully inhibit a button press following the stop signal (tone) is experienced as frustrating by participants even when clear instructions are provided that occasional failures to suppress the response are part of the task. Avoiding the experience of committing a stop error can gain higher priority when the negative feedback of a failed response inhibition becomes more salient due to oxytocin’s effect on enhancing the salience of socially relevant stimuli (with an unwanted behavioral expression against a presented rule being a social event).44 This idea of a strategic control shift towards more cautious behavior also aligns with a recent study reporting that oxytocin reduced risk-taking in decision-making during an Iowa Gambling Task in healthy men.45
In summary, this pilot investigation provides first evidence that oxytocin increases proactive control over behavioral impulses in men with overweight or obesity. The reported link between oxytocin and cognitive control over behavioral impulses opens exciting avenues for future research, as cognitive control can reduce behavioral engagement in urges to eat. This link could thus represent a potential mechanism for the previously reported effects of oxytocin in reducing food intake and weight in humans. Future studies are required to further characterize the effects of oxytocin on cognitive control of behavioral impulses in a larger sample including both men and women and establish the predictive value and necessity of altered control over behavioral impulses for oxytocin effects on food intake, and ultimately weight, in human obesity. Furthermore, investigating whether such effects might be modulated by BMI, as observed for the inhibitory impact of oxytocin administration on caloric intake,7 or metabolic state represent key follow-up research questions.
Study Importance Questions.
What is already known?
The neurohormone oxytocin decreases food intake and induces weight loss in rodent, primate, and human obesity.
In humans, oxytocin reduces food intake without altering subjective appetite, making the mechanisms underlying its anorexigenic effect elusive; however, they are important to study to consider oxytocin as a potential neurohormonal weight loss treatment.
What does this study add?
In a first investigation of the effects of oxytocin on cognitive control ability and strategy, we show that a single-dose of 24 IU intranasal oxytocin increases proactive control in men with overweight or obesity.
How might these results change the direction of research?
Our results open a new line of investigation into the impact of oxytocin on cognitive control of behavioral impulses and its predictive value for the effects of oxytocin on food intake and weight in human obesity.
Understanding the mechanisms underlying the anorexigenic effects of oxytocin will allow us to evaluate the potential of oxytocin as a weight loss treatment as well as determine which individuals will likely benefit the most from this intervention.
Acknowledgements
We would like to thank our participants as well as the staff at the MGH Translational Clinical Research Center. Data will be made available upon request.
Funding:
This work was supported by the Boston Nutrition Obesity Research Center/NIH grant 5P30DK046200-20, Harvard Catalyst/NIH grant 1UL1TR001102, Nutrition Obesity Research Center at Harvard/NIH grant P30DK040561, and NIH grant K23MH092560. The funding sources had no involvement in the design of the study, collection, analysis, and interpretation of the data, or decision to publish this manuscript.
Footnotes
Disclosures: EAL is on the scientific advisory board and has a financial interest in OXT Therapeutics, a company developing an intranasal oxytocin and long-acting analogs of oxytocin to treat obesity and metabolic disease. EAL’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. This company was not involved in any way in this research. The other authors declare no conflicts of interests.
Clinical trial registration: clinicaltrials.gov registration number: NCT02276677
References
- 1.Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides 1989;10(1):89–93. [DOI] [PubMed] [Google Scholar]
- 2.Blevins JE, Graham JL, Morton GJ, Bales KL, Schwartz MW, Baskin DG, Havel PJ. Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. Am J Physiol Regul Integr Comp Physiol 2015;308(5):R431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burmester V, Higgs S, Terry P. Rapid-onset anorectic effects of intranasal oxytocin in young men. Appetite 2018;130:104–9. [DOI] [PubMed] [Google Scholar]
- 4.Lawson EA, Marengi DA, DeSanti RL, Holmes TM, Schoenfeld DA, Tolley CJ. Oxytocin reduces caloric intake in men. Obesity (Silver Spring) 2015;23(5):950–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olson BR, Drutarosky MD, Chow MS, Hruby VJ, Stricker EM, Verbalis JG. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides 1991;12(1):113–8. [DOI] [PubMed] [Google Scholar]
- 6.Ott V, Finlayson G, Lehnert H, Heitmann B, Heinrichs M, Born J, Hallschmid M. Oxytocin reduces reward-driven food intake in humans. Diabetes 2013;62(10):3418–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thienel M, Fritsche A, Heinrichs M, Peter A, Ewers M, Lehnert H, Born J, Hallschmid M. Oxytocin’s inhibitory effect on food intake is stronger in obese than normal-weight men. Int J Obes (Lond) 2016;40(11):1707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blevins JE, Thompson BW, Anekonda VT, Ho JM, Graham JL, Roberts ZS, Hwang BH, Ogimoto K, Wolden-Hanson T, Nelson J, Kaiyala KJ, Havel PJ, Bales KL, Morton GJ, Schwartz MW, Baskin DG. Chronic CNS oxytocin signaling preferentially induces fat loss in high-fat diet-fed rats by enhancing satiety responses and increasing lipid utilization. Am J Physiol Regul Integr Comp Physiol 2016;310(7):R640–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden-Hanson T, Baskin DG, Schwartz MW, Blevins JE. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am J Physiol Endocrinol Metab 2012;302(1):E134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Wu C, Chen Q, Chen X, Xu Z, Wu J, Cai D. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS One 2013;8(5):e61477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014;311(8):806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 2013;347:f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 2014;311(1):74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monsell S, Driver J. Banishing the control homunculus. In: Monsell S, Driver J, editors. Attention and performance XVIII: control of cognitive processes. Cambridge, MA, USA: MIT Press; 2000. p. 3–32. [Google Scholar]
- 15.Chamberlain SR, Derbyshire KL, Leppink E, Grant JE. Obesity and dissociable forms of impulsivity in young adults. CNS Spectr 2015;20(5):500–7. [DOI] [PubMed] [Google Scholar]
- 16.Kulendran M, Vlaev I, Sugden C, King D, Ashrafian H, Gately P, Darzi A. Neuropsychological assessment as a predictor of weight loss in obese adolescents. Int J Obes (Lond) 2014;38(4):507–12. [DOI] [PubMed] [Google Scholar]
- 17.Mole TB, Irvine MA, Worbe Y, Collins P, Mitchell SP, Bolton S, Harrison NA, Robbins TW, Voon V. Impulsivity in disorders of food and drug misuse. Psychol Med 2015;45(4):771–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nederkoorn C, Smulders FT, Havermans RC, Roefs A, Jansen A. Impulsivity in obese women. Appetite 2006;47(2):253–6. [DOI] [PubMed] [Google Scholar]
- 19.Perpina C, Segura M, Sanchez-Reales S. Cognitive flexibility and decision-making in eating disorders and obesity. Eat Weight Disord 2017;22(3):435–44. [DOI] [PubMed] [Google Scholar]
- 20.Sellaro R, Colzato LS. High body mass index is associated with impaired cognitive control. Appetite 2017;113:301–9. [DOI] [PubMed] [Google Scholar]
- 21.Guerrieri R, Nederkoorn C, Stankiewicz K, Alberts H, Geschwind N, Martijn C, Jansen A. The influence of trait and induced state impulsivity on food intake in normal-weight healthy women. Appetite 2007;49(1):66–73. [DOI] [PubMed] [Google Scholar]
- 22.Opel N, Redlich R, Grotegerd D, Dohm K, Haupenthal C, Heindel W, Kugel H, Arolt V, Dannlowski U. Enhanced neural responsiveness to reward associated with obesity in the absence of food-related stimuli. Hum Brain Mapp 2015;36(6):2330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanderBroek-Stice L, Stojek MK, Beach SR, vanDellen MR, MacKillop J. Multidimensional assessment of impulsivity in relation to obesity and food addiction. Appetite 2017;112:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weller RE, Cook EW, 3rd, Avsar KB, Cox JE. Obese women show greater delay discounting than healthy-weight women. Appetite 2008;51(3):563–9. [DOI] [PubMed] [Google Scholar]
- 25.Appelhans BM. Neurobehavioral inhibition of reward-driven feeding: implications for dieting and obesity. Obesity (Silver Spring) 2009;17(4):640–7. [DOI] [PubMed] [Google Scholar]
- 26.Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn Sci 2009;13(1):27–35. [DOI] [PubMed] [Google Scholar]
- 27.Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry 2007;62(10):1187–90. [DOI] [PubMed] [Google Scholar]
- 28.Riem MM, van IMH, Tops M, Boksem MA, Rombouts SA, Bakermans-Kranenburg MJ. No laughing matter: intranasal oxytocin administration changes functional brain connectivity during exposure to infant laughter. Neuropsychopharmacology 2012;37(5):1257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niendam TA, Laird AR, Ray KL, Dean YM, Glahn DC, Carter CS. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci 2012;12(2):241–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plessow F, Marengi DA, Perry SK, Felicione JM, Franklin R, Holmes TM, Holsen LM, Makris N, Deckersbach T, Lawson EA. Effects of intranasal oxytocin on the blood oxygenation level-dependent signal in food motivation and cognitive control pathways in overweight and obese men. Neuropsychopharmacology 2018;43(3):638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spetter MS, Feld GB, Thienel M, Preissl H, Hege MA, Hallschmid M. Oxytocin curbs calorie intake via food-specific increases in the activity of brain areas that process reward and establish cognitive control. Sci Rep 2018;8(1):2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Logan GD. On the ability to inhibit thought and action: a user’s guide to the stop signal paradigm. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory, and language. San Diego, CA, USA: Academic Press; 1994. p. 189–239. [Google Scholar]
- 33.Logan GD, Cowan WB. On the ability to inhibit thought and action: a theory and an act of control. Psychol Rev 1984;91(3):295–327. [DOI] [PubMed] [Google Scholar]
- 34.Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends Cogn Sci 2008;12(11):418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bissett PG, Logan GD. Balancing cognitive demands: control adjustments in the stop-signal paradigm. J Exp Psychol Learn Mem Cogn 2011;37(2):392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leotti LA, Wager TD. Motivational influences on response inhibition measures. J Exp Psychol Hum Percept Perform 2010;36(2):430–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liddle EB, Scerif G, Hollis CP, Batty MJ, Groom MJ, Liotti M, Liddle PF. Looking before you leap: a theory of motivated control of action. Cognition 2009;112(1):141–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sella F, Bonato M, Cutini S, Umilta C. Living on the edge: strategic and instructed slowing in the stop signal task. Psychol Res 2013;77(2):204–10. [DOI] [PubMed] [Google Scholar]
- 39.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP). New York, NY, USA: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 40.Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform 1984;10(2):276–91. [DOI] [PubMed] [Google Scholar]
- 41.Logan GD, Schachar RJ, Tannock R. Impulsivity and inhibitory control. Psychol Sci 1997;8(1):60–4. [Google Scholar]
- 42.Logan GD, Van Zandt T, Verbruggen F, Wagenmakers EJ. On the ability to inhibit thought and action: general and special theories of an act of control. Psychol Rev 2014;121(1):66–95. [DOI] [PubMed] [Google Scholar]
- 43.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39(2):175–91. [DOI] [PubMed] [Google Scholar]
- 44.Shamay-Tsoory SG, Abu-Akel A. The social salience hypothesis of oxytocin. Biol Psychiatry 2016;79(3):194–202. [DOI] [PubMed] [Google Scholar]
- 45.Bozorgmehr A, Alizadeh F, Sadeghi B, Shahbazi A, Ofogh SN, Joghataei MT, Razian S, Heydari F, Ghadirivasfi M. Oxytocin moderates risky decision-making during the Iowa Gambling Task: a new insight based on the role of oxytocin receptor gene polymorphisms and interventional cognitive study. Neurosci Lett 2019:134328. [DOI] [PubMed] [Google Scholar]