Abstract
Alterations in the expression of integrin receptors for extracellular matrix (ECM) proteins are strongly associated with the acquisition of invasive and/or metastatic properties by human cancer cells. Despite this, comparatively little is known of the biochemical mechanisms that regulate the expression of integrin genes in cells. Here we demonstrate that the Ras-activated Raf–MEK–extracellular signal-regulated kinase (ERK) signaling pathway can specifically control the expression of individual integrin subunits in a variety of human and mouse cell lines. Pharmacological inhibition of MEK1 in a number of human melanoma and pancreatic carcinoma cell lines led to reduced cell surface expression of α6- and β3-integrin. Consistent with this, conditional activation of the Raf-MEK-ERK pathway in NIH 3T3 cells led to a 5 to 20-fold induction of cell surface α6- and β3-integrin expression. Induced β3-integrin was expressed on the cell surface as a heterodimer with αv-integrin; however, the overall level of αv-integrin expression was not altered by Ras or Raf. Raf-induced β3-integrin was observed in primary and established mouse fibroblast lines and in mouse and human endothelial cells. Consistent with previous reports of the ability of the Raf-MEK-ERK signaling pathway to induce β3-integrin gene transcription in human K-562 erythroleukemia cells, Raf activation in NIH 3T3 cells led to elevated β3-integrin mRNA. However, unlike immediate-early Raf targets such as heparin binding epidermal growth factor and Mdm2, β3-integrin mRNA was induced by Raf in a manner that was cycloheximide sensitive. Surprisingly, activation of the Raf-MEK-ERK signaling pathway by growth factors and mitogens had little or no effect on β3-integrin expression, suggesting that the expression of this gene requires sustained activation of this signaling pathway. In addition, despite the robust induction of cell surface αvβ3-integrin expression by Raf in NIH 3T3 cells, such cells display decreased spreading and adhesion, with a loss of focal adhesions and actin stress fibers. These data suggest that oncogene-induced alterations in integrin gene expression may participate in the changes in cell adhesion and migration that accompany the process of oncogenic transformation.
Adhesion of cells to extracellular matrix (ECM) is mediated by a family of transmembrane proteins known as integrins that are expressed on the cell surface as α/β-heterodimers (21, 30, 40). Different combinations of α and β subunits give rise to a multiplicity of ECM receptors, the expression of which shows considerable cell type specificity (40). Moreover, the intracellular regions of integrin subunits are believed to mediate the assembly of components of the focal adhesion complex, which in turn participate in marshalling the actin cytoskeleton (14, 21, 72). Regulation of integrin function is believed to be essential in promoting stable cell adhesion as well as being required for cell migration.
In addition to their role in cell adhesion and migration, engagement and clustering of integrins elicits a series of signal transduction events that participate in the control of cell cycle progression and apoptosis in a process known as “outside-in” signaling. For example, integrin engagement can elicit activation of members of the Src and FAK family of protein tyrosine kinases (26, 49). These initial signaling events promote the activation of Ras and Rho family GTPases that in turn influence the activation of a number of intracellular signaling pathways (16, 47). A second mode of integrin regulation known as “inside-out” signaling has also been described. For example, the Ras-activated Raf–MEK–extracellular signal-regulated kinase (ERK) signaling pathway can influence the activation state of the αIIbβ3 (also known as gpIIb/IIIa) integrin as measured by the binding of a monoclonal antibody that recognizes the activated form of this integrin. Interestingly, the mechanism of alteration of the αIIbβ3 integrin affinity state is independent of de novo RNA and protein synthesis and may be due to the direct modification of preexisting integrin subunits on the surface of the cell (39).
In addition to their role in normal cell physiology, there is an extensive body of literature indicating that alterations in the expression of specific integrin subunits on the surface of cancer cells contributes to the invasive and metastatic properties of the cells (43, 67, 79). For example, reduced expression of α5β1-integrin in K-562, CHO, and HT-29 cells and of α2β1 in breast cancer cells correlates with increased tumorigenicity. Moreover, in certain circumstances, elevated expression of α6-, α3- or β3-integrins appears to be closely associated with oncogenic transformation and tumor progression. Indeed, there is strong evidence that the expression of αvβ3-integrin is tightly correlated with the acquisition of invasive and/or metastatic behavior by melanoma and glioblastoma cells (1, 29, 32, 59, 61, 71, 78). Moreover, ectopic expression of αvβ3-integrin in a benign human melanoma cell line can promote invasion and metastasis when tested in a mouse xenograft assay (50).
In addition to a direct role in promoting tumor cell invasion and migration, αvβ3-integrin is implicated in the angiogenesis and neovascularization of tumors by normal endothelial cells. The induced expression of this integrin heterodimer on the surface of sprouting endothelial cells is believed to be essential for endothelial cell migration, proliferation, and tubulogenesis (10, 41, 79, 80). Indeed, direct binding of the MMP-2 matrix metalloprotease to αvβ3-integrins may be important in promoting localized ECM degradation and cell invasion (12). For these reasons, a number of αvβ3-integrin antagonists are being clinically tested for their efficacy in treating cancer (11, 36, 57).
Despite the extensive literature on the role of integrins in cancer, little is known about the intracellular signaling pathways that regulate the expression of integrin genes in cancer cells (9, 83, 85). Here we demonstrate that pharmacological inhibition of MEK1 activity led to decreased expression of α6- and β3-integrins in a number of human melanoma and pancreatic carcinoma cell lines, consistent with a role for the Raf-MEK-ERK pathway in regulating integrin gene expression in certain human tumors. Moreover, selective activation of the Raf-MEK-ERK pathway in NIH 3T3 cells led to increased expression of a number of integrins, of which β3- and α6-integrin were most prominent. Raf-induced β3-integrin expression was observed in a variety of mouse fibroblasts and in mouse and human endothelial cells. In NIH 3T3 cells, induced expression of β3-integrin was preceded by increased β3-integrin mRNA, consistent with the previously described ability of the Raf-MEK-ERK signaling pathway to transactivate the β3-integrin gene (44, 81, 83, 85). Surprisingly, β3-integrin was induced in response to sustained activation of the Raf-MEK-ERK signaling pathway and not in response to the transient activation of this pathway elicited by growth factors and mitogens. However, despite the induced expression of αvβ3-integrins on the surface of NIH 3T3 cells, Raf-transformed cells displayed profound alterations in intracellular architecture and cell morphology, leading to decreased cell adhesion and increased cell motility. These data indicate that signaling pathways downstream of Ras can influence the expression of integrin genes associated with invasion and metastasis of human tumor cells.
MATERIALS AND METHODS
Retrovirus expression vectors.
Vectors for the expression of ΔRaf:ER* and ΔMEK1:ER* proteins were generated by fusing DNA sequences encoding activated forms of A-Raf, Raf-1, B-Raf, or MEK1 to a modified form of the hormone binding domain of the mouse estrogen receptor (ERTM) that responds to 4-hydroxytamoxifen (4-HT) and ICI compounds but not to 17-β-estradiol or phenol red as described previously (51). DNA sequences encoding ΔRaf:ER* and ΔMEK1:ER* were inserted into the replication-defective retrovirus vector pBabepuro3 (pBP3) for expression in mammalian cells (60). Retrovirus constructs expressing EGFPΔRaf-1:ER, c-Myc:ERTM, and Akt:ER* have been described previously (48, 51, 88). Retrovirus vectors (pZAS4) encoding v-Myc or v-Ha-Ras and resistance to mycophenolic acid were provided by J. Kaplan (45). Additional details of retrovirus expression vectors are available on request.
Cell culture, virus production, and virus infection.
Cells were cultured at 37°C in a humidified atmosphere containing 6% (vol/vol) CO2, in phenol red-free Dulbecco's modified Eagle's medium supplemented with 10% (vol/vol) fetal calf serum (FCS), penicillin, streptomycin and gentamicin. 4-HT (Sigma) was stored at −20°C as a 1 mM stock in ethanol and diluted directly into the cell culture medium. Ecotropic or amphotropic retrovirus stocks were obtained by transient transfection of retroviral vector DNAs into either BOSC 23, Phoenix-E, or Phoenix-A packaging cells and used to infect target cells as described previously (63, 88). NIH 3T3 cells expressing the TrkA/nerve growth factor (NGF) receptor were provided by Kevin Pumiglia and Stu Decker and cultured as described previously (65).
Cell staining and flow cytometry.
Cells were cultured as described above and stimulated as described in the text. They were harvested by trypsinization and washed with Flow medium (Ca2+- and Mg2+-free phosphate-buffered saline containing 1% [wt/vol] bovine serum albumin and 1 mM sodium azide) prior to staining. Phycoerythrin (PE) or biotin-coupled anti-integrin antisera used for murine cell staining were purchased from Pharmingen: anti-β1-biotin (CD29, Ha2/5), anti-β3-PE (CD61, 2C9.G2), anti-β4 (CD104, 346-11A), anti-αv-biotin (CD51, H9.2B8), anti-α1 (CD49a, Ha31/8), anti-α2 (CD49b, HMa2), anti-α4-PE (CD49d, R1-2), anti-α5-PE (CD49e, 5H10-27), and anti-α6-PE (CD49f, GoH3). Avidin-PE, avidin-fluorescein isothiocyanate (FITC), goat F(ab′)2 fragments, and anti-rat PE secondary reagents were obtained from Caltag or Vector Laboratories. Anti-hamster immunoglobulin G-biotin (secondary reagent for anti-α1- and anti-α2 integrin in conjunction with avidin-PE) was obtained from Pharmingen. Human β3-integrin was detected using a PE-coupled mouse monoclonal antibody (MAb) (VI-PL2) with a similarly coupled MAb (MOPC-21) as an isotype control (Pharmingen). Human α6-integrin was detected using an FITC-coupled rat MAb (GoH3) with a rat immunoglobulin G2a (R35-95) as an isotype control (Pharmingen). Cells were stained in 100 μl of Flow buffer, analyzed using a FACScalibur flow cytometer and quantitated using CellQuest software (Becton Dickinson).
Biotinylation and Western blotting.
Cells were biotinylated using membrane-impermeant NHS-LC-biotin (Pierce Chemicals) dissolved at 0.5 mg/ml in Tris-buffered saline (TBS) and incubated at room temperature for 30 min. The cells were washed with phosphate-buffered saline and harvested into Gold lysis buffer (GLB) containing 1% (vol/vol) Triton X-100 (33, 70). β3-Integrin was immunoprecipitated using an antiserum raised against the intracellular region of the protein, kindly provided by Mark Ginsberg. Immune complexes were collected using protein A-agarose and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blots were prepared. The Western blots were probed with streptavidin coupled to horseradish peroxidase (HRP) (Amersham) and visualized using the enhanced chemiluminescence technique (Amersham). The same antiserum was used to detect β3-integrin in GLB extracts of whole cells by standard Western blotting techniques. Phospho-specific anti-ERK1 and anti-ERK2 antisera and antisera against ERK1 and ERK2 were from New England Biolabs and Santa Cruz Biotechnology, respectively, and were used as previously described (4).
Fluorescence microscopy.
NIH 3T3 cells in the absence or presence of activated Raf were fixed in 4% (wt/vol) paraformaldehyde, permeabilized with 0.1% (vol/vol) Triton X-100, and then stained with phalloidin-FITC (Molecular Probes), as specified by the manufacturer, to visualize polymerized actin. To detect focal adhesions, fixed and permeabilized cells were costained with an anti-vinculin MAb (VIN-11-5 [Sigma], 1:100 dilution) and visualized using a Texas red-coupled anti-mouse antiserum. Dual fluorescence of the FITC and Texas red fluorophores was visualized using a Nikon microscope equipped with a 100× oil immersion lens and the appropriate fluorescence filter sets. Cells were photographed using a Nikon camera and 400ASA Kodak color film.
Quantitation of cellular mRNAs.
The expression of cellular mRNAs was quantitated using a simultaneous RNase protection assay (RPA) as described previously (53, 55). RPA probes for mouse heparin binding epidermal growth factor (HB-EGF) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNAs have been described previously (53, 55). The RPA probe for mouse β3-integrin was prepared by in vitro transcription of a 600-bp coding-sequence fragment, which was linearized with EcoRI and transcribed using T7 RNA polymerase.
RESULTS
Regulation of integrin subunit expression by the Raf-MEK-ERK signaling pathway in human cancer cells.
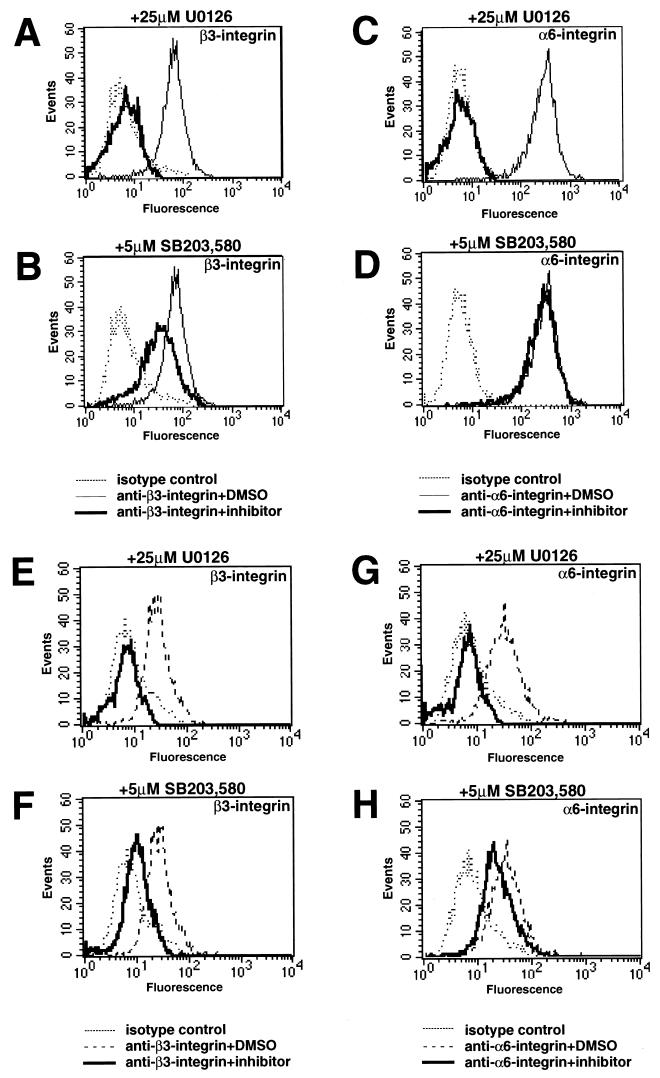
Expression of α6- or β3-integrin in certain human cancer cells is associated with progression to a more invasive and/or metastatic state (79). To determine if the Ras-regulated Raf-MEK-ERK pathway may play a role in the regulation of these integrin subunits, we assessed the expression of β3- and α6-integrin in human CFPAC pancreatic cancer cells and WM793 melanoma cells. CFPAC cells express activated K-Ras, but the activation status of the Raf-MEK-ERK pathway in WM793 cells is unknown (31). The cells were treated with either UO126, a pharmacological inhibitor of MEK1, SB203,580, a pharmacological inhibitor of p38 mitogen-activated protein (MAP) kinase, or dimethyl sulfoxide (DMSO) as a solvent control, and the expression of cell surface β3- and α6-integrins was assessed by cell staining and flow cytometry (Fig. 1). In both CFPAC (Fig. 1A to D) and WM793 (Fig. 1E to H) cells, inhibition of MEK1 by UO126 completely abolished the cell surface expression of both β3-integrin (Fig. 1A and C) and α6-integrin (Fig. 1E and G). SB203,580 led to a modest decrease in β3-integrin expression in both CFPAC and WM793 cells, whereas it had little or no effect on the expression of α6-integrin in these cells. Similar experiments conducted with a panel of pancreatic cancer and melanoma cell lines revealed that inhibition of MEK1 by UO126 led to decreased β3-integrin expression in four of seven pancreatic cancer cell lines (CFPAC, BxPC3, AsPC1, and Capan-2) and four of four melanoma cell lines (WM793, WM9, WM164, and WM1205) tested. These data indicate that cell surface α6- and β3-integrin expression is promoted by the Raf-MEK-ERK signaling pathway in certain human cancer cell lines.
FIG. 1.
Expression of β3- and α6-integrins in human cancer cell lines. CFPAC pancreatic cancer cells (A to D) or WM793 melanoma cells (E to H) were treated with either UO126, SB203,580, or DMSO as a solvent control for 48 h as indicated and then stained for the cell surface expression of β3- or α6-integrins by flow cytometry as described in Materials and Methods.
Cell surface expression of integrins following Raf activation in NIH 3T3 cells.
We have previously described the utility of conditionally active forms of Raf protein kinases (Raf:ER) in exploring the role of the Raf-MEK-ERK pathway in a variety of physiological processes (70). Addition of 4-HT to NIH 3T3 cells expressing ΔRaf-1:ER elicits immediate activation of MEK1, ERK1, and ERK2, leading to rapid changes in gene expression (54, 55). At 16 to 24 h after ΔRaf-1:ER activation, NIH 3T3 cells show dramatic alterations in cell morphology (70) (see Fig. 8).
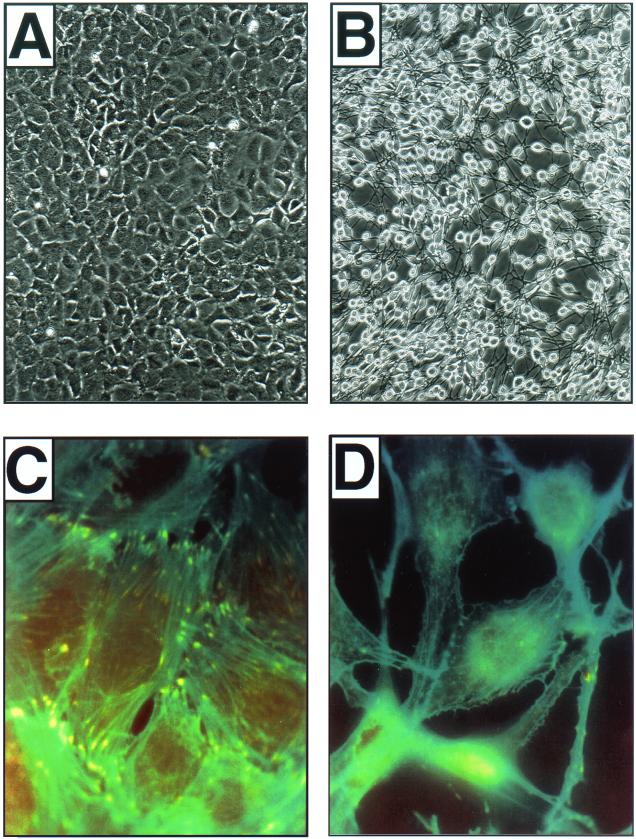
FIG. 8.
Raf-transformed fibroblasts display alterations in intracellular architecture and cell morphology. (A and B) NIH 3T3 cells expressing ΔB-Raf:ER* were either left untreated (A) or treated with 1 μM 4-HT for 24 h (B), at which time they were photographed using a Polaroid charge-coupled device camera. Total magnification, ca. ×80. (C and D) NIH 3T3 cells expressing ΔB-Raf:ER* were plated on vitronectin-coated glass microscope slides and either left untreated (C) or treated with 1 μM 4-HT for 24 h (D). At this time, the cells were fixed and costained with phalloidin-FITC to visualize polymerized actin (green) and antivinculin antisera to visualize focal adhesions (red). Areas of colocalization are false colored in yellow. Total magnification, ca. ×400.
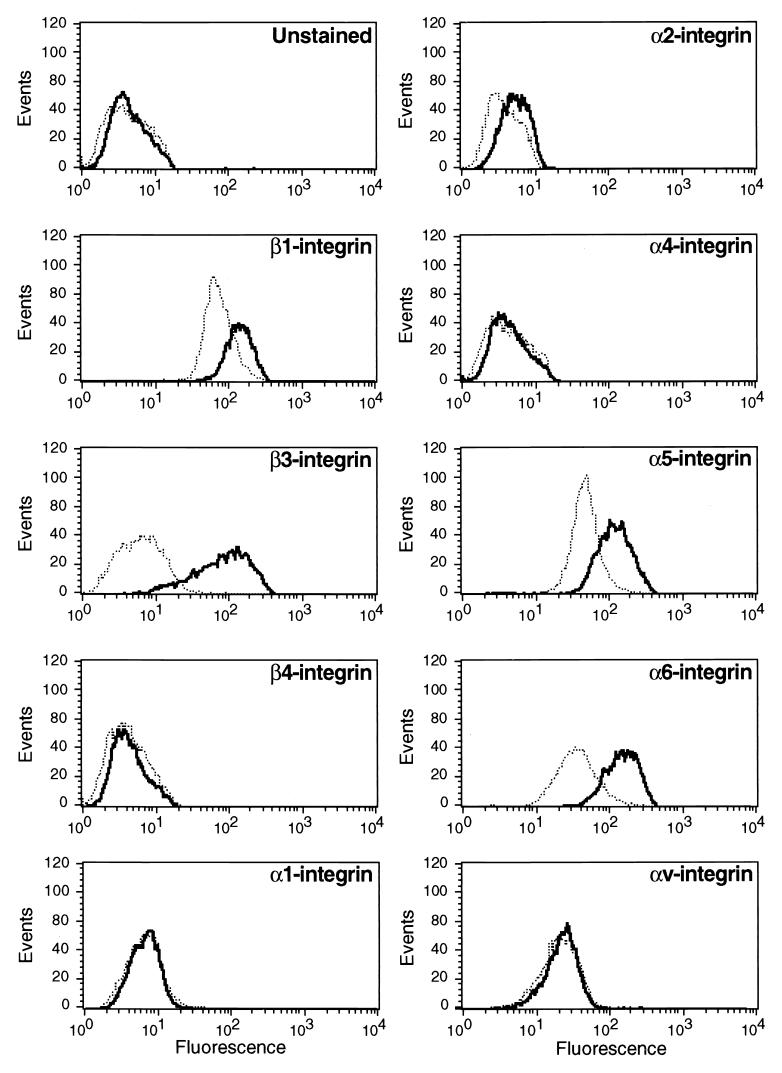
To determine if activation of the Raf-MEK-ERK pathway can lead to alterations in integrin gene expression, NIH 3T3 cells expressing ΔB-Raf:ER* (88) were either untreated or treated with 4-HT for 24 h, at which time cell surface integrin expression was assessed by flow cytometry (FACScan) of cells stained with a panel of anti-integrin MAbs as described in Materials and Methods. As controls, the cells were stained with the appropriate antibody isotypes in the absence or presence of activated Raf (data not shown). Raf activation had little or no effect on the expression of α1-, α2-, α4-, αv-, or β4-integrin subunits (Fig. 2). However, Raf activation led to increased expression of α5-, α6-, β1-, and, most strikingly, β3-integrin. A 10- to 30-fold induction of β3-integrin expression was observed in multiple iterations of this experiment using both clonal and pooled populations of NIH 3T3 cells expressing ΔB-Raf:ER*. The ability of Raf to induce β3-integrin expression was unaffected by the absence or presence of FCS. Furthermore, induction of β3-integrin was observed at both low and high levels of Raf activation, which promote or inhibit NIH 3T3 cell cycle progression, respectively (88). No alterations in integrin subunit expression were detected in parental NIH 3T3 cells or in cells expressing a kinase-inactive ΔRaf-1:ER fusion protein in the absence or presence of 4-HT (data not shown). Furthermore, no alterations in β3-integrin expression were observed following addition of 4-HT to NIH 3T3 cells expressing conditionally active Akt (Akt:ER*) or c-Myc (c-Myc:ERTM) (data not shown) (24, 48, 51, 58). Finally, as observed in the human cancer cell lines in Fig. 1, induction of β3-integrin by ΔB-Raf:ER* was inhibited by the selective MEK1 inhibitors PD098059 and UO126 but not by LY294002, an inhibitor of phosphatidylinositol 3′-kinase (23, 25). Taken together, these data suggest that the Raf-MEK-ERK pathway can influence the expression of a specific subset of integrin subunits on the surface of NIH 3T3 cells. Moreover, in NIH 3T3 cells, as in human cancer cells, Raf-induced β3-integrin expression requires the downstream activation of MEK.
FIG. 2.
Expression of integrin subunits following Raf activation in NIH 3T3 cells. NIH 3T3 cells expressing ΔB-Raf:ER* were either untreated (dotted line) or treated with 100 nM 4-HT for 24 h (solid line) at which time the cells were harvested and either left unstained or stained for the cell surface expression of particular α- and β-integrin subunits as indicated. The expression of integrin subunits was detected by flow cytometry as described in Materials and Methods.
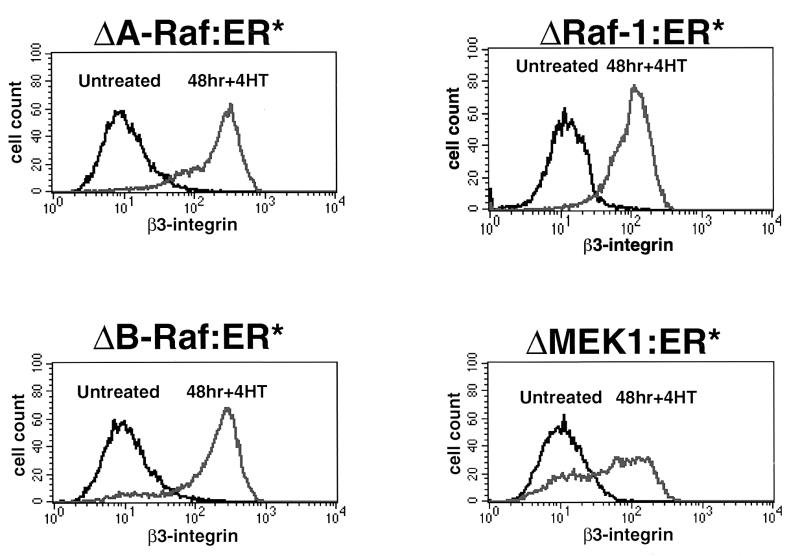
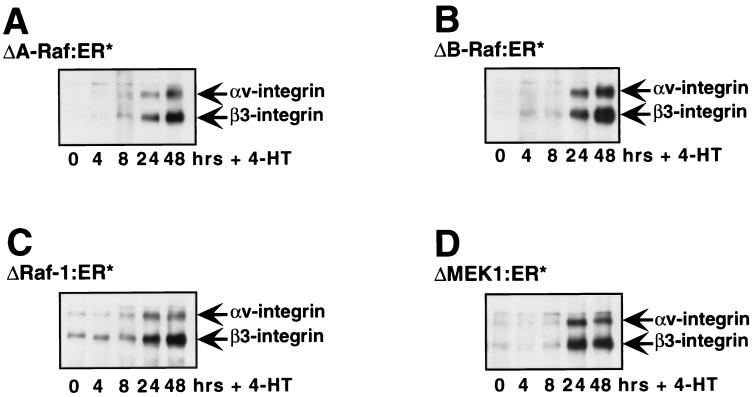
To confirm these data, we assessed the ability of conditionally active forms of A-Raf, Raf-1, and MEK1 to induce the expression of β3-integrin (4, 8, 64). Pooled populations of NIH 3T3 cells expressing ΔA-Raf:ER*, ΔRaf-1:ER*, ΔB-Raf:ER*, or ΔMEK1:ER* were derived by retrovirus infection as described in Materials and Methods. As expected, addition of 4-HT to cells expressing ΔA-Raf:ER*, ΔRaf-1:ER*, ΔB-Raf:ER*, or ΔMEK1:ER* led to induced β3-integrin expression (Fig. 3). In general, the kinetics of β3-integrin induction were more rapid in response to conditional activation of B-Raf and Raf-1 activity (data not shown), consistent with the fact that these forms of Raf are more potent activators of the ERK MAP kinase pathway than is A-Raf or MEK1 (4, 8, 64, 88).
FIG. 3.
Induction of β3-integrin by Raf and MEK1. NIH 3T3 cells expressing ΔA-Raf:ER*, ΔRaf-1:ER*, ΔB-Raf:ER*, or ΔMEK1:ER* were either left untreated or treated with 100 nM 4-HT for 48 h, at which time the cells were harvested and stained for the cell surface expression of β3-integrin as described in Materials and Methods. The expression of β3-integrin subunits was detected by flow cytometry.
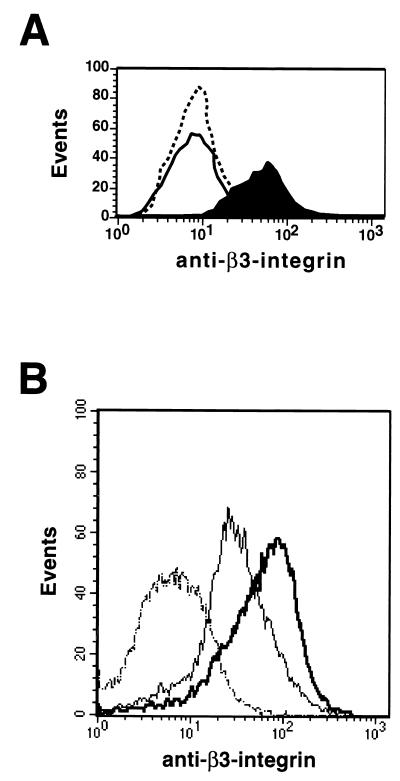
Finally, infection of NIH 3T3 cells with a retrovirus encoding oncogenic v-Ha-Ras, but not a similar virus encoding v-Myc, led to induced β3-integrin expression (Fig. 4A). To define which pathways downstream of Ras were required for β3-integrin induction, we utilized NIH 3T3 cells expressing various effector domain mutants of Ras (82, 84). A form of activated human H-Ras that is capable of activating the Raf-MEK-ERK pathway (T35S) induced β3-integrin expression in NIH 3T3 cells. However, forms of H-Ras that activate phosphatidylinositol 3′-kinase (Y40C) or Ral.GDS (E37G), but fail to activate the Raf-MEK-ERK pathway also failed to induce β3-integrin expression in NIH 3T3 cells (data not shown). Taken together, these data are consistent with a model in which the Ras-activated Raf-MEK-ERK pathway can regulate cell surface β3-integrin expression in NIH 3T3 cells.
FIG. 4.
Induction of β3-integrin by Ras. (A) NIH 3T3 cells were infected with retroviruses encoding either v-Myc or v-Ha-Ras, and virus-infected cells were selected over 14 days in mycophenolic acid as described in Materials and Methods. Parental (dotted line), v-Myc-expressing (solid line), and v-Ha-Ras-expressing (filled curve), cells were stained for the cell surface expression of β3-integrin. (B) Human microvascular endothelial cells immortalized by the expression of the catalytic subunit of telomerase and expressing EGFPΔRaf-1:ER were derived by the use of the appropriate amphotropic retroviruses. Cells were either left untreated (thin line) or treated with 1 μM 4-HT (thick line), at which time they were stained either with an isotype control (dotted line) or with an antiserum that recognizes human β3-integrin.
To test whether the ability of Raf to induce β3-integrin was a property solely of NIH 3T3 cells, we derived populations of primary mouse embryo fibroblasts, BALB/c cells, and Swiss 3T3 cells expressing EGFPΔRaf-1:ER, a green fluorescent protein-tagged form of conditional Raf-1 (88, 90). Activation of EGFPΔRaf-1:ER in all of these cells led to induced β3-integrin expression (data not shown). Moreover, since β3-integrin plays an important role in the invasion and migration of endothelial cells, we expressed EGFPΔRaf-1:ER in the mouse endothelioid cell lines MS-1 and SVEC. Although the basal levels of β3-integrin expression were considerably higher in these cells than in mouse fibroblasts, Raf activation led to elevated cell surface β3-integrin expression (data not shown). Finally, using the catalytic subunit of telomerase (hTERT), we have derived telomerase immortalized human microvascular endothelial (TIME) cells that retain the endothelial characteristics of the primary cells from which they were derived (E. Venetsanakos and M. McMahon, unpublished data). By subsequent retrovirus infection, we derived TIME cells expressing EGFPΔRaf-1:ER. As with the mouse endothelioid cell lines, TIME cells express a high basal level of β3-integrin on the surface of the cell; however, subsequent activation of EGFPΔRaf-1:ER led to increased expression of β3-integrin (Fig. 4B). These data suggest that the Raf-MEK-ERK pathway has the capacity to regulate the expression of β3-integrin in a number of human and mouse cell types.
Induction of cell surface αv β3-integrin heterodimers by Raf and MEK.
To confirm the results obtained by flow cytometry, proteins on the surface of NIH 3T3 cells were biotinylated at different times after the activation of ΔRaf:ER* or ΔMEK-1:ER*. Cell extracts were prepared, and β3-integrin (and associated proteins) was immunoprecipitated under nonreducing conditions using an antiserum against the carboxy terminus of the protein (gift of Mark Ginsberg, Scripps Institute). Western blots were prepared, and biotinylated proteins in the immunoprecipitates were detected using streptavidin coupled to horseradish peroxidase (Fig. 5). As expected from the flow cytometry experiments, activation of ΔRaf:ER* or ΔMEK1:ER* proteins led to induced expression of the 97-kDa β3-integrin subunit that was detected between 8 and 24 h after 4-HT addition. In each cell line a protein of approximately 125 kDa was observed to coimmunoprecipitate with the induced β3-integrin. β3-Integrin forms heterodimers with only two different α-integrin subunits: the 125-kDa αv subunit and the 114-kDa gpIIb subunit. Since gpIIb expression is restricted to megakaryocytes and platelets and is not expressed in fibroblasts, it seemed highly likely that the coprecipitating protein corresponded to αv-integrin. This was confirmed by reciprocal immunoprecipitations of these cell lysates with antisera against αv-integrin, which coprecipitated Raf-induced β3-integrin (data not shown). Hence, prior to Raf activation, NIH 3T3 cells express αv-integrin that is readily detected by flow cytometry, presumably as a heterodimer with a variety of other β-integrin subunits (40, 73) (Fig. 2). Experiments presented here suggest that following Raf activation all of the cells express β3-integrin as a heterodimer with αv-integrin without any change in the overall level of αv-integrin expression (Fig. 2). Consequently, these data suggest that Raf-induced β3-integrin expression most likely leads to a reassortment of the pattern of integrin heterodimers found on the surface of the cell.
FIG. 5.
Elevated αvβ3-integrin expression on the surface of Raf-transformed cells. NIH 3T3 cells expressing ΔA-Raf:ER* (A), ΔB-Raf:ER* (B), ΔRaf-1:ER* (C), or ΔMEK1:ER* (D) were either left untreated (0 h) or treated with 100nM 4-HT for 4, 8, 24, or 48 h as indicated. Cells were then subjected to biotinylation using the membrane-impermeant reagent NHS-LC-biotin. Cell extracts were prepared, and β3-integrin and its associated proteins were immunoprecipitated using a polyclonal anti-β3-integrin antiserum. Immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blots were prepared. Biotinylated proteins in the immunoprecipitates were detected by probing the Western blots with streptavidin-horseradish peroxidase.
Raf-induced β3-integrin mRNA.
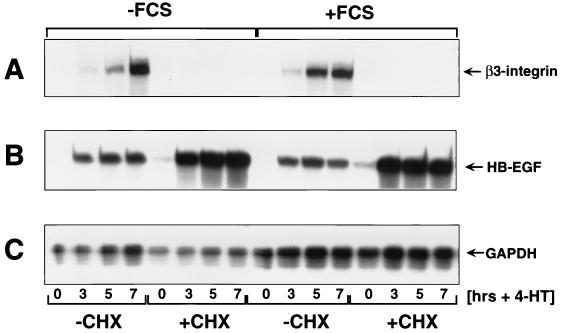
To determine the mechanism by which Raf induced β3-integrin expression, RNA samples were isolated from NIH 3T3 cells at different times after the activation of ΔB-Raf:ER*. This experiment was conducted in the absence or presence of FCS or cycloheximide, as indicated in Fig. 6. These conditions allowed us to determine whether changes in mRNA expression are immediate-early, as defined by cycloheximide sensitivity, or if they require the presence of serum factors. The expression of β3-integrin, HB-EGF, and GAPDH mRNAs was assessed using an RPA (Fig. 6A to C, respectively). HB-EGF is a previously characterized, AP-1/Ets regulated, immediate-early Raf-responsive gene, and GAPDH mRNA is unaffected by Raf activation (54, 55).
FIG. 6.
Delayed-early induction of β3-integrin mRNA by Raf. NIH 3T3 cells expressing ΔB-Raf:ER* in the absence or presence of FCS were either left untreated (−CHX) or treated with 10 μg of cycloheximide per ml (+CHX) for 1 h. At this time, the cells were either left untreated (0 h) or treated with 1 μM 4-HT for 3, 5, or 7 h as indicated. At this time, total cellular RNA was prepared and probed for the expression of β3-integrin (A), HB-EGF (B), and GAPDH (C) mRNAs using a simultaneous RPA as described in Materials and Methods.
Activation of ΔB-Raf:ER* led to induced expression of both β3-integrin and HB-EGF mRNAs. However, the induction of β3-integrin mRNA lagged behind that of HB-EGF, which was maximally induced after 3 h of Raf activity. As demonstrated previously, the induction of HB-EGF mRNA by Raf was resistant to cycloheximide and was unaffected by the absence of FCS (Fig. 6B). Indeed, HB-EGF mRNA was superinduced by Raf in the presence of cycloheximide (55). By contrast, although the induction of β3-integrin mRNA was unaffected by the absence of FCS, it was abrogated by pretreatment of cells with cycloheximide (Fig. 6A). The level of GAPDH mRNA was unaffected by ΔB-Raf:ER* activation. These data indicate that the induced expression of cell surface β3-integrin is probably mediated by elevated expression of its cognate mRNA. Moreover, like cyclin D1, β3-integrin is a delayed-early target of the Raf-MEK-ERK signaling pathway in NIH 3T3 cells (4). These data are consistent with previous observations that phorbol esters can induce the de novo transcription of the β3-integrin gene in K-562 cells through the Raf-MEK-ERK pathway (44, 81, 83, 85).
Induction of β3-integrin by sustained activation of the ERK MAP kinase pathway.
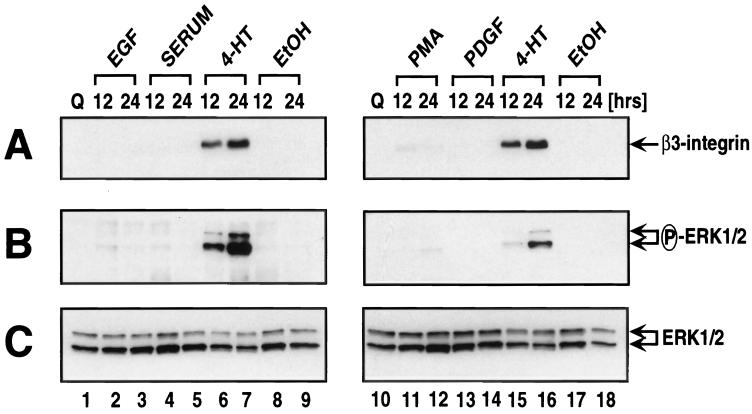
A large number of Ras- and Raf-responsive genes such as those encoding Mdm2, HB-EGF, transforming growth factor β1, c-Myc, Fra-1, JunB, cyclin D1, and p21Cip1 have been identified by many groups (4, 6, 19, 46, 55, 66, 76, 88). Invariably such genes are also induced by mitogens such as FCS, lysophosphatidic acid, phorbol esters, or specific polypeptide growth factors that activate the Raf-MEK-ERK signaling pathway such as platelet-derived growth factor (PDGF) or EGF. On the assumption that β3-integrin might also be mitogen induced, serum-deprived NIH 3T3 cells expressing EGFPΔRaf-1:ER were treated for 12 or 24 h with concentrations of EGF, PDGF, FCS, or phorbol esters sufficient to activate the Raf-MEK-ERK pathway leading to cell cycle progression. As a control, cells were treated with 4-HT to activate Raf. Cell extracts were prepared, and β3-integrin expression was assessed by Western blotting (Fig. 7A). As expected, activation of Raf led to robust induction of β3-integrin expression, but, to our surprise, β3-integrin expression was not induced by the various mitogens used in this experiment. It appeared that the induction of β3-integrin in this experiment correlated with the sustained activation of ERK1 and ERK2 that is observed in Ras- and Raf-transformed cells (Fig. 7B). To confirm this observation, this experiment was repeated using parental NIH 3T3 cells as well as other clonal and pooled populations of ΔRaf:ER-expressing cells. Under no circumstances did we observe β3-integrin induction by growth factor or mitogen treatment of NIH 3T3 cells. In addition, this experiment was repeated to include a larger number of earlier and later time points spanning a time course from 6 to 72 h following mitogen addition to ensure that we had not simply failed to detect a transient induction of β3-integrin by the choice of time points in the initial experiments. In these latter experiments, β3-integrin expression was detected by cell staining with anti-β3-integrin antisera and flow cytometry, a more sensitive measure of expression. Again, β3-integrin was induced by Raf activation but was not observed at any time in response to the mitogens or growth factors listed above (data not shown). The failure of these agents to induce β3-integrin expression was not a reflection of their inability to induce cell cycle progression since we and others have demonstrated that all of these agents are potent inducers of DNA synthesis in quiescent NIH 3T3 cells (64, 69). In addition, induction of β3-integrin by sustained activation of the Raf-MEK-ERK pathway was not a property of all delayed-early genes since cyclin D1 was induced efficiently in NIH 3T3 cells by all of the agents tested in this experiment (data not shown).
FIG. 7.
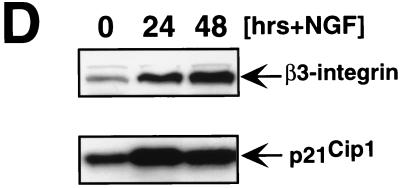
Induction of β3-integrin by sustained ERK MAP kinase activation. (A to C) NIH 3T3 cells expressing EGFPΔRaf-1:ER were cultured in serum-free medium for 36 h prior to the addition of 10 ng of EGF per ml, 20% (vol/vol) FCS, 1 μM 4-HT, 0.1% (vol/vol) ethanol (EtOH), 50 ng of phorbol esters per ml (PMA), or 10 ng of PDGF per ml for 12 or 24 h. Cell extracts were prepared and probed for the expression of β3-integrin (A), the activation of the ERK MAP kinases (B), and the overall expression of the ERK MAP kinases as a loading control (C) using the appropriate antisera as described in Materials and Methods. (D) NIH 3T3 cells expressing the NGF receptor (65) were either left untreated or treated with 10 ng of NGF per ml for 24 or 48 h, at which time the expression of β3-integrin and p21Cip1 was assessed by Western blotting with the appropriate antisera.
A major difference between the activation of the ERK MAP kinases by Raf and that mediated by mitogens or polypeptide growth factors is that Raf elicits sustained ERK activation whereas the other agents activate ERKs with transient kinetics. Others have reported that NGF treatment of NIH 3T3 cells expressing the NGF receptor leads to sustained ERK activation and induction of p21Cip1-mediated cell cycle arrest in a manner identical to that elicited by Raf (65, 88). When we treated these cells with NGF, we observed induced expression of β3-integrin, concomitant with p21Cip1 induction, consistent with the hypothesis that β3-integrin expression requires sustained ERK MAP kinase activation (Fig. 7D). Furthermore, these data suggest that the induction of β3-integrin is not specific to Ras or Raf per se but requires sustained activation of the ERK MAP kinase pathway that may be elicited by a number of means.
Effects of Raf activation on intracellular architecture and cell morphology.
The αvβ3-integrin complex is an important receptor for vitronectin in mouse fibroblasts but is also capable of promoting adhesion to a variety of other ECM proteins (38). Consequently, the induced expression of αvβ3-integrin on the surface of NIH 3T3 cells might be expected to promote cell adhesion and spreading as well as to promote the formation of focal adhesions and assembly of actin stress fibers. To address this, NIH 3T3 cells expressing ΔB-Raf:ER* were plated on vitronectin-coated coverslips in the presence of FCS and either left untreated or treated with 4-HT for 24 h (Fig. 8C and D). These cells were stained to detect polymerized and bundled actin (green) or vinculin (red) and to detect assembled focal adhesions at the tips of actin stress fibers (yellow) as described in Materials and Methods. For comparison, phase-contrast photomicrographs (at a lower magnification) of similarly treated cells are also presented (Fig. 8A and B).
Prior to Raf activation, NIH 3T3 cells display a flat, nonrefractile cell morphology and display contact inhibition (Fig. 8A). Such cells have abundant actin stress fibers and focal adhesions and display little detectable cortical actin (Fig. 8C). Furthermore, these cells require trypsinization to remove them from the dish and, when observed by time-lapse cinemicroscopy, are relatively nonmotile. By contrast, cells expressing activated ΔRaf-1:ER display a highly rounded, refractile morphology with numerous cell extensions and pile up on one another as a consequence of the loss of contact inhibition (Fig. 8B). Moreover, these cells display an almost complete loss of focal adhesions, have significantly reduced numbers of actin stress fibers, and display elevated levels of cortical actin, which is often associated with membrane ruffling (Fig. 8D). Importantly, the overall expression of vinculin, paxillin, and FAK is unchanged in Raf-transformed cells; therefore the loss of detectable focal adhesions is not due to reduced expression of these crucial components. In addition, Raf-transformed cells are readily detached from the dish without the use of trypsin. Finally, when observed by time-lapse cinemicroscopy, Raf-transformed cells display a high degree of cell motility (A. Bhat, unpublished observations). These data indicate that, although Raf promotes the expression of cell surface αvβ3-integrin, the effects of Raf on intracellular architecture, cell morphology, and adhesion run counter to the simple expectation that cell-ECM attachment might be increased in these cells.
DISCUSSION
Although alterations in the expression and activity of integrins have been reported to play an important role in the acquisition of migratory, invasive, or metastatic properties by human tumor cells, the signal transduction pathways that elicit these changes remain poorly characterized (43, 67, 79). Here we demonstrate that the Raf-MEK-ERK signaling pathway can promote the expression of α6- and β3-integrin. These integrin subunits have previously been associated with increased invasion and metastasis of a number of human tumor cells. Although the mechanisms of α6-integrin induction by the Raf-MEK-ERK pathway remain to be elucidated, induced cell surface β3-integrin expression is accompanied by elevated β3-integrin mRNA levels. The Raf-MEK-ERK pathway promoted the expression of β3-integrin in a number of nontransformed fibroblastic and endothelial cells as well as in a number of human cancer cell lines. However, β3-integrin induction is not a universal marker for the sustained activation of the ERK MAP kinase pathway in mammalian cells. Indeed, Raf activation in RIE-1 rat colonic epithelial cells, DKO-4 human colon cancer-derived cells, and IMR-90 human fibroblasts had no effects on β3-integrin expression (references 5, 66, 74, and 90 and data not shown). These data are consistent with the fact that β3-integrin is associated with the invasion and metastasis of specific types of human cancers (79). However, it is possible that Raf activation may lead to alterations in the expression or activity of other integrin subunits in different cell types.
There is at least one other situation where the expression of β3-integrin is under the control of the Raf-MEK-ERK pathway. Phorbol ester treatment or expression of activated MEK1 in human K-562 erthyroleukemia cells leads to coordinate induction of both β3-integrin and its heterodimerization partner in megakaryocytes and platelets, αIIb-integrin (44, 83). Under these circumstances, the Raf-MEK-ERK pathway promotes the transcriptional activation of the β3-integrin gene. Although the transcription factors required for β3-integrin induction are unknown, the gene promoter has potential binding sites for MZF-1, Sp1, GATA, Myb, Ets, and E2F transcription factors (81). Although there is precedent for ERK-mediated regulation of Ets and Sp1 transcription factors, further analysis of the promoter is required to confirm a role for these transcription factors in the control of β3-integrin expression in both NIH 3T3 and K-562 cells (52, 54, 56, 84, 89). Moreover, it is not clear if β3-integrin induction by the Raf-MEK-ERK pathway in NIH 3T3 cells relies on the same biochemical mechanisms observed in K-562 cells. Hence, a comparative analysis of these two cell types is currently under way using NIH 3T3 and K-562 cells expressing conditionally active forms of Raf and MEK1.
An unanticipated observation in this study was the apparent inability of mitogens and growth factors to induce β3-integrin expression in NIH 3T3 cells. To our knowledge, the β3-integrin gene is the first gene induced as a consequence of the sustained activation of the ERK MAP kinase pathway elicited by activated Ras, Raf, and MEK but not by growth factors and mitogens that elicit transient ERK MAP kinase activation. This raises the possibility that the β3-integrin gene may be a member of a group of genes that display similar properties. The advent of cDNA microarrays and high-throughput gene expression analysis will allow us to search for such genes in a systematic fashion (22, 42). Having identified the transcription factors that mediate Raf induction of β3-integrin, it will be interesting to determine why sustained, but not transient, Raf activation elicits β3-integrin expression and if there are additional signals specific to transformed cells that are required for β3-integrin expression (2, 68, 75).
It is interesting that, in principle, the induced expression of even a single integrin subunit could have significant effects on the global pattern of integrin heterodimers expressed on the surface of the cell. In this case the induction of β3-integrin was not accompanied by a concomitant increase in the expression of its heterodimerization partner αv-integrin. Since β3-integrin must appropriate a certain amount of αv-integrin for its cell surface expression, it seems reasonable to surmise that there must be a reassortment of the dimerization of β-integrin subunits on the surface of the cell or down-regulation of the expression of β-integrin subunits that form heterodimers with αv such as β5-, β6- and β8-integrins. Unfortunately, cell-staining reagents for flow cytometry were not available to assess the expression of these integrins in mouse cells. Such observations are compounded by the fact that Raf also induced the expression of other integrin subunits such as α6. In addition to effects on global patterns of integrin expression, it is clear that the Raf-MEK-ERK pathway can influence the activation state of integrins by posttranslational mechanisms (39). These observations serve to illustrate that the activation of a single signaling pathway can have profound effects on the expression and/or activity of these key cell adhesion molecules.
There is ample evidence that the expression of αvβ3-integrin on the surface of melanoma cells can promote increased cell migration, invasion, and metastasis (79). These effects may be a consequence of the ability of integrins to activate a variety of cell-signaling pathways leading to inhibition of apoptosis (71, 79). Indeed, the ability of integrins to influence the activity of Rho family GTPases, one of which, RhoC, has been reported to confer metastatic potential on melanoma cells, may be important in this regard (17, 18). Despite this, it is unclear if β3-integrin expression plays a role in the ability of Ras and Raf to transform NIH 3T3 cells. It has previously been shown that the capacity of human melanoma cells to form metastatic lung tumors in an experimental system is dependent on the expression of αvβ3-integrin (50). Consequently, it is provocative that forms of Ras that activate the Raf-MEK-ERK pathway in NIH 3T3 cells and thereby induce β3-integrin expression can elicit metastatic lung tumors when injected into the tail vein of a nude mouse. By contrast, forms of Ras that do not activate the Raf-MEK-ERK pathway fail to elicit metastatic lung tumors, although they retain the capacity to elicit local subcutaneous tumors (82). The recent description of mice with compromised β3-integrin expression or function will permit us to study the role of β3-integrin in Ras-induced oncogenic transformation and metastasis (38, 49).
Despite the reported role of αvβ3-integrin in the metastatic behavior of human melanoma cells, it is unclear whether the Raf-MEK-ERK signaling pathway influences β3-integrin expression in these cells. Although Ras mutations have been found in approximately 30% of metastatic melanomas, there is no apparent correlation reported between Ras activation, progression to a metastatic phenotype, and β3-integrin expression (3, 7). Although Ras mutations are rarely detected in invasive glioblastoma cells, the frequent alterations or overexpression of the receptors for EGF, PDGF, and fibroblast growth factor may contribute to β3-integrin expression in a manner similar to that described above in NIH 3T3 cells overexpressing the NGF receptor (15, 28, 65). The use of pharmacological inhibitors of signaling pathways should allow us to address the role of signal pathways in the control of β3-integrin expression in a wider variety of human cancer cell lines (20, 23, 25).
The expression of αvβ3-integrin on the surface of endothelial cells is important in the process of angiogenesis and the neovascularization of tumors. Although a role for the Raf-MEK-ERK pathway in β3-integrin expression in endothelial cells has not previously been described, we demonstrate that Raf activation leads to elevated β3-integrin in TIME cells. Further evidence that the Raf-MEK-ERK pathway plays an important role in angiogenesis is suggested by the fact that disruption of the B-Raf gene leads to a failure of endothelial cell differentiation accompanied by increased apoptosis. Consequently, B-Raf-nullizygous embryos die of vascular hemorrhage (86, 87). Although these data indicate an important role for B-Raf in endothelial cell development, it is unlikely that these effects are due solely to effects on β3-integrin expression, since β3-integrin-nullizygous mice display normal vasculogenesis (38).
Although Raf activation in NIH 3T3 cells elicited increased cell surface αvβ3-integrin expression, the overall consequences of Raf transformation are a loss of focal adhesions and actin stress fibers leading to decreased attachment to extracellular matrix (70, 77). This somewhat paradoxical observation indicates that the effects of Raf on cell adhesion are complex. On the one hand, the induction of integrin expression might be predicted to increase cell adhesion, a prediction borne out by the fact that ectopic expression of human β3-integrin in NIH 3T3 promotes cell adhesion and spreading (data not shown). However, presumably because of effects of Ras and Raf on other components of the cell adhesion machinery, the phenotype of Raf-transformed cells is a loss of focal adhesions and cell rounding. Indeed, this combination of biochemical events seems more likely to promote cell migration as opposed to stable ECM attachment, a hypothesis that would be consistent with the role of β3-integrin in melanoma cell migration (50).
Although the effects of Ras on cell morphology are thought to be mediated by Rho family GTPases (13, 91), it is clear that the effects of Raf on the actin cytoskeleton and focal adhesions in NIH 3T3 cells occur in the absence of any decrease in the GTP loading of Rho, Rac, or cdc42 protein (M. Woodrow and M. McMahon, unpublished observations). Moreover, the characteristic morphology of Ras-transformed cells can be reverted by pharmacological inhibition of MEK (35; M. McMahon, unpublished observations). Taken together, these data argue for an important role for the Raf-MEK-ERK pathway in Ras-induced alterations in intracellular architecture. Indeed, in MDCK cells the effects of Raf on the actin cytoskeleton and cell migration occur in the absence of decreased GTP loading of Rho family GTPases and appear to be associated with the induced expression of Rnd3, an endogenous inhibitor of Rho signaling (27, 34, 37, 62). Regardless of the mechanisms involved, it is clear that activation of the ERK MAP kinase pathway has pleiotropic effects on cell adhesion and migration that may influence the invasion and metastasis of transformed cells. It will be of considerable interest to reveal the full spectrum of molecular mechanisms underlying these observations and to determine the extent to which they participate in the aberrant behavior of human cancer cells.
ACKNOWLEDGMENTS
We are most grateful to Boris Bastian, David Cheresh, Stu Decker, Mark Ginsberg, Meenhard Herlyn, Josh Kaplan, Chandra Kumar, Lewis Lanier, Kevin Pumiglia, Craig Webb, and George Vande Woude for critical advice, materials, and reagents for this study. We also thank all the members of the McMahon laboratory and Emma Lees, David Parry, and Dan Mahony for discussion and advice. We thank Steve Robbins, Steen Hansen, and David Dankort for critical review of the manuscript.
M.M. acknowledges Schering Plough Corp. and the UCSF Cancer Center for funding to support this project. In addition, D.W. was supported by the award of a Senior Postdoctoral Fellowship from the California Division of the American Cancer Society and S.G. was supported by a fellowship from the Novartis Foundation and the Swiss National Science Foundation.
REFERENCES
- 1.Albelda S M, Mette S A, Elder D E, Stewart R, Damjanovich L, Herlyn M, Buck C A. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990;50:6757–6764. [PubMed] [Google Scholar]
- 2.Alberts A S, Geneste O, Treisman R. Activation of SRF-regulated chromosomal templates by Rho-family GTPases requires a signal that also induces H4 hyperacetylation. Cell. 1998;92:475–487. doi: 10.1016/s0092-8674(00)80941-1. [DOI] [PubMed] [Google Scholar]
- 3.Albino A P, Shea C R, McNutt N S. Oncogenes in melanomas. J Dermatol. 1992;19:853–867. doi: 10.1111/j.1346-8138.1992.tb03796.x. [DOI] [PubMed] [Google Scholar]
- 4.Aziz N, Cherwinski H, McMahon M. Complementation of defective colony-stimulating factor 1 receptor signaling and mitogenesis by Raf and v-Src. Mol Cell Biol. 1999;19:1101–1115. doi: 10.1128/mcb.19.2.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnard J A, Graves-Deal R, Pittelkow M R, DuBois R, Cook P, Ramsey G W, Bishop P R, Damstrup L, Coffey R J. Auto- and cross-induction within the mammalian epidermal growth factor-related peptide family. J Biol Chem. 1994;269:22817–22822. [PubMed] [Google Scholar]
- 6.Bortner D M, Langer S J, Ostrowski M C. Non-nuclear oncogenes and the regulation of gene expression in transformed cells. Crit Rev Oncog. 1993;4:137–160. [PubMed] [Google Scholar]
- 7.Bos J L. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 8.Bosch E, Cherwinski H, Peterson D, McMahon M. Mutations of critical amino acids affect the biological and biochemical properties of oncogenic A-Raf and Raf-1. Oncogene. 1997;15:1021–1033. doi: 10.1038/sj.onc.1201270. [DOI] [PubMed] [Google Scholar]
- 9.Boudreau N, Andrews C, Srebrow A, Ravanpay A, Cheresh D A. Induction of the angiogenic phenotype by Hox D3. J Cell Biol. 1997;139:257–264. doi: 10.1083/jcb.139.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks P C, Clark R A, Cheresh D A. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 11.Brooks P C, Stromblad S, Klemke R, Visscher D, Sarkar F H, Cheresh D A. Anti-integrin alpha v beta 3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Investig. 1995;96:1815–1822. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks P C, Stromblad S, Sanders L C, von Schalscha T L, Aimes R T, Stetler-Stevenson W G, Quigley J P, Cheresh D A. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- 13.Campbell S L, Khosravi-Far R, Rossman K L, Clark G J, Der C J. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- 14.Cary L A, Han D C, Guan J L. Integrin-mediated signal transduction pathways. Histol Histopathol. 1999;14:1001–1009. doi: 10.14670/HH-14.1001. [DOI] [PubMed] [Google Scholar]
- 15.Cavenee W K, Scrable H J, James C D. Molecular genetics of human cancer predisposition and progression. Mutat Res. 1991;247:199–202. doi: 10.1016/0027-5107(91)90015-g. [DOI] [PubMed] [Google Scholar]
- 16.Chaudhary A, King W G, Mattaliano M D, Frost J A, Diaz B, Morrison D K, Cobb M H, Marshall M S, Brugge J S. Phosphatidylinositol 3-kinase regulates raf1 through pak phosphorylation of serine 338. Curr Biol. 2000;10:551–554. doi: 10.1016/s0960-9822(00)00475-9. [DOI] [PubMed] [Google Scholar]
- 17.Clark E A, Golub T R, Lander E S, Hynes R O. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- 18.Clark E A, King W G, Brugge J S, Symons M, Hynes R O. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook S J, Aziz N, McMahon M. The repertoire of fos and jun proteins expressed during the G1 phase of the cell cycle is determined by the duration of mitogen-activated protein kinase activation. Mol Cell Biol. 1999;19:330–341. doi: 10.1128/mcb.19.1.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 21.Dedhar S. Integrins and signal transduction. Curr Opin Hematol. 1999;6:37–43. doi: 10.1097/00062752-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 22.DeRisi J L, Iyer V R. Genomics and array technology. Curr Opin Oncol. 1999;11:76–79. doi: 10.1097/00001622-199901000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eilers M, Picard D, Yamamoto K R, Bishop J M. Chimaeras of myc oncoprotein and steroid receptors cause hormone-dependent transformation of cells. Nature. 1989;340:66–68. doi: 10.1038/340066a0. [DOI] [PubMed] [Google Scholar]
- 25.Favata M F, Horiuchi K Y, Manos E J, Daulerio A J, Stradley D A, Feeser W S, Van Dyk D E, Pitts W J, Earl R A, Hobbs F, Copeland R A, Magolda R L, Scherle P A, Trzaskos J M. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 26.Filardo E J, Brooks P C, Deming S L, Damsky C, Cheresh D A. Requirement of the NPXY motif in the integrin beta 3 subunit cytoplasmic tail for melanoma cell migration in vitro and in vivo. J Cell Biol. 1995;130:441–450. doi: 10.1083/jcb.130.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foster R, Hu K Q, Lu Y, Nolan K M, Thissen J, Settleman J. Identification of a novel human Rho protein with unusual properties: GTPase deficiency and in vivo farnesylation. Mol Cell Biol. 1996;16:2689–2699. doi: 10.1128/mcb.16.6.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Furnari F B, Huang H J, Cavenee W K. Genetics and malignant progression of human brain tumours. Cancer Surv. 1995;25:233–275. [PubMed] [Google Scholar]
- 29.Gehlsen K R, Davis G E, Sriramarao P. Integrin expression in human melanoma cells with differing invasive and metastatic properties. Clin Exp Metastasis. 1992;10:111–120. doi: 10.1007/BF00114587. [DOI] [PubMed] [Google Scholar]
- 30.Giancotti F G, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 31.Giannini C D, Roth W K, Piiper A, Zeuzem S. Enzymatic and antisense effects of a specific anti-Ki-ras ribozyme in vitro and in cell culture. Nucleic Acids Res. 1999;27:2737–2744. doi: 10.1093/nar/27.13.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gladson C L, Cheresh D A. Glioblastoma expression of vitronectin and the alpha v beta 3 integrin. Adhesion mechanism for transformed glial cells. J Clin Investig. 1991;88:1924–1932. doi: 10.1172/JCI115516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gold M R, Law D A, DeFranco A L. Stimulation of protein tyrosine phosphorylation by the B-lymphocyte antigen receptor. Nature. 1990;345:810–813. doi: 10.1038/345810a0. [DOI] [PubMed] [Google Scholar]
- 34.Guasch R M, Scambler P, Jones G E, Ridley A J. RhoE regulates actin cytoskeleton organization and cell migration. Mol Cell Biol. 1998;18:4761–4771. doi: 10.1128/mcb.18.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta S, Plattner R, Der C J, Stanbridge E J. Dissection of ras-dependent signaling pathways controlling aggressive tumor growth of human fibrosarcoma cells: evidence for a potential novel pathway. Mol Cell Biol. 2000;20:9294–9306. doi: 10.1128/mcb.20.24.9294-9306.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutheil J C, Campbell T N, Pierce P R, Watkins J D, Huse W D, Bodkin D J, Cheresh D A. Targeted antiangiogenic therapy for cancer using Vitaxin: a humanized monoclonal antibody to the integrin alphavbeta3. Clin Cancer Res. 2000;6:3056–3061. [PubMed] [Google Scholar]
- 37.Hansen S H, Zegers M M, Woodrow M, Rodriguez-Viciana P, Chardin P, Mostov K E, McMahon M. Induced expression of Rnd3 is associated with transformation of polarized epithelial cells by the Raf-MEK-extracellular signal-regulated kinase pathway. Mol Cell Biol. 2000;20:9364–9375. doi: 10.1128/mcb.20.24.9364-9375.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodivala-Dilke K M, McHugh K P, Tsakiris D A, Rayburn H, Crowley D, Ullman-Cullere M, Ross F P, Coller B S, Teitelbaum S, Hynes R O. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Investig. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes P E, Renshaw M W, Pfaff M, Forsyth J, Keivens V M, Schwartz M A, Ginsberg M H. Suppression of integrin activation: a novel function of a Ras/Raf- initiated MAP kinase pathway. Cell. 1997;88:521–530. doi: 10.1016/s0092-8674(00)81892-9. [DOI] [PubMed] [Google Scholar]
- 40.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 41.Hynes R O, Wagner D D. Genetic manipulation of vascular adhesion molecules in mice. J Clin Investig. 1996;98:2193–2195. doi: 10.1172/JCI119027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iyer V R, Eisen M B, Ross D T, Schuler G, Moore T, Lee J C F, Trent J M, Staudt L M, Hudson J, Jr, Boguski M S, Lashkari D, Shalon D, Botstein D, Brown P O. The transcriptional program in the response of human fibroblasts to serum. Science. 1999;283:83–87. doi: 10.1126/science.283.5398.83. [DOI] [PubMed] [Google Scholar]
- 43.Juliano R L, Varner J A. Adhesion molecules in cancer: the role of integrins. Curr Opin Cell Biol. 1993;5:812–818. doi: 10.1016/0955-0674(93)90030-t. [DOI] [PubMed] [Google Scholar]
- 44.Kang C D, Do I R, Kim K W, Ahn B K, Kim S H, Chung B S, Jhun B H, Yoo M A. Role of Ras/ERK-dependent pathway in the erythroid differentiation of K562 cells. Exp Mol Med. 1999;31:76–82. doi: 10.1038/emm.1999.13. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan J M, Mardon G, Bishop J M, Varmus H E. The first seven amino acids encoded by the v-src oncogene act as a myristylation signal: lysine 7 is a critical determinant. Mol Cell Biol. 1988;8:2435–2441. doi: 10.1128/mcb.8.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kerkhoff E, Houben R, Loffler S, Troppmair J, Lee J E, Rapp U R. Regulation of c-myc expression by Ras/Raf signalling. Oncogene. 1998;16:211–216. doi: 10.1038/sj.onc.1201520. [DOI] [PubMed] [Google Scholar]
- 47.King W G, Mattaliano M D, Chan T O, Tsichlis P N, Brugge J S. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohn A D, Barthel A, Kovacina K S, Boge A, Wallach B, Summers S A, Birnbaum M J, Scott P H, Lawrence J C, Jr, Roth R A. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J Biol Chem. 1998;273:11937–11943. doi: 10.1074/jbc.273.19.11937. [DOI] [PubMed] [Google Scholar]
- 49.Law D A, DeGuzman F R, Heiser P, Ministri-Madrid K, Killeen N, Phillips D R. Integrin cytoplasmic tyrosine motif is required for outside-in alphaIIbbeta3 signalling and platelet function. Nature. 1999;401:808–811. doi: 10.1038/44599. [DOI] [PubMed] [Google Scholar]
- 50.Li X, Chen B, Blystone S D, McHugh K P, Ross F P, Ramos D M. Differential expression of alphav integrins in K1735 melanoma cells. Invasion Metastasis. 1998;18:1–14. doi: 10.1159/000024494. [DOI] [PubMed] [Google Scholar]
- 51.Littlewood T D, Hancock D C, Danielian P S, Parker M G, Evan G I. A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res. 1995;23:1686–1690. doi: 10.1093/nar/23.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 53.McCarthy S A, Aziz N, McMahon M. Identification of immediate-early gene targets of the Raf-1 serine/threonine protein kinase using an estradiol-dependent fusion protein, delta Raf-1:ER. Methods Mol Biol. 1997;85:137–151. doi: 10.1385/0-89603-489-5:137. [DOI] [PubMed] [Google Scholar]
- 54.McCarthy S A, Chen D, Yang B S, Garcia Ramirez J J, Cherwinski H, Chen X R, Klagsbrun M, Hauser C A, Ostrowski M C, McMahon M. Rapid phosphorylation of Ets-2 accompanies mitogen-activated protein kinase activation and the induction of heparin-binding epidermal growth factor gene expression by oncogenic Raf-1. Mol Cell Biol. 1997;17:2401–2412. doi: 10.1128/mcb.17.5.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCarthy S A, Samuels M L, Pritchard C A, Abraham J A, McMahon M. Rapid induction of heparin-binding epidermal growth factor/diphtheria toxin receptor expression by Raf and Ras oncogenes. Genes Dev. 1995;9:1953–1964. doi: 10.1101/gad.9.16.1953. [DOI] [PubMed] [Google Scholar]
- 56.Merchant J L, Du M, Todisco A. Sp1 phosphorylation by Erk 2 stimulates DNA binding. Biochem Biophys Res Commun. 1999;254:454–461. doi: 10.1006/bbrc.1998.9964. [DOI] [PubMed] [Google Scholar]
- 57.Miller W H, Keenan R M, Willette R N, Lark M W. Identification and in vivo efficacy of small-molecule antagonists of integrin alphavbeta3 (the vitronectin receptor) Drug Discov Today. 2000;5:397–408. doi: 10.1016/s1359-6446(00)01545-2. [DOI] [PubMed] [Google Scholar]
- 58.Mirza A M, Kohn A D, Roth R A, McMahon M. Oncogenic transformation of cells by a conditionally active form of the protein kinase Akt/PKB. Cell Growth Differ. 2000;11:279–292. [PubMed] [Google Scholar]
- 59.Montgomery A M, Reisfeld R A, Cheresh D A. Integrin alpha v beta 3 rescues melanoma cells from apoptosis in three- dimensional dermal collagen. Proc Natl Acad Sci USA. 1994;91:8856–8860. doi: 10.1073/pnas.91.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nip J, Shibata H, Loskutoff D J, Cheresh D A, Brodt P. Human melanoma cells derived from lymphatic metastases use integrin alpha v beta 3 to adhere to lymph node vitronectin. J Clin Investig. 1992;90:1406–1413. doi: 10.1172/JCI116007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nobes C D, Lauritzen I, Mattei M G, Paris S, Hall A, Chardin P. A new member of the Rho family, Rnd1, promotes disassembly of actin filament structures and loss of cell adhesion. J Cell Biol. 1998;141:187–197. doi: 10.1083/jcb.141.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pritchard C A, Samuels M L, Bosch E, McMahon M. Conditionally oncogenic forms of the A-Raf and B-Raf protein kinases display different biological and biochemical properties in NIH 3T3 cells. Mol Cell Biol. 1995;15:6430–6442. doi: 10.1128/mcb.15.11.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pumiglia K M, Decker S J. Cell cycle arrest mediated by the MEK/mitogen-activated protein kinase pathway. Proc Natl Acad Sci USA. 1997;94:448–452. doi: 10.1073/pnas.94.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ries S, Biederer C, Woods D, Shifman O, Shirasawa S, Sasazuki T, Oren M, McMahon M, McCormick F. Opposing effects of Ras on p53: transcriptional activation of mdm2 and induction of p19ARF. Cell. 2000;103:321–330. doi: 10.1016/s0092-8674(00)00123-9. [DOI] [PubMed] [Google Scholar]
- 67.Ruoslahti E. Fibronectin and its integrin receptors in cancer. Adv Cancer Res. 1999;76:1–20. doi: 10.1016/s0065-230x(08)60772-1. [DOI] [PubMed] [Google Scholar]
- 68.Sahai E, Alberts A S, Treisman R. RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. EMBO J. 1998;17:1350–1361. doi: 10.1093/emboj/17.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Samuels M L, McMahon M. Inhibition of platelet-derived growth factor-and epidermal growth factor-mediated mitogenesis and signaling in 3T3 cells expressing ΔRaf-1:ER, an estradiol-regulated form of Raf-1. Mol Cell Biol. 1994;14:7855–7866. doi: 10.1128/mcb.14.12.7855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Samuels M L, Weber M J, Bishop J M, McMahon M. Conditional transformation of cells and rapid activation of the mitogen- activated protein kinase cascade by an estradiol-dependent human raf-1 protein kinase. Mol Cell Biol. 1993;13:6241–6252. doi: 10.1128/mcb.13.10.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sanders L C, Felding-Habermann B, Mueller B M, Cheresh D A. Role of alpha V integrins and vitronectin in human melanoma cell growth. Cold Spring Harbor Symp Quant Biol. 1992;57:233–240. doi: 10.1101/sqb.1992.057.01.028. [DOI] [PubMed] [Google Scholar]
- 72.Sattler M, Pisick E, Morrison P T, Salgia R. Role of the cytoskeletal protein paxillin in oncogenesis. Crit Rev Oncog. 2000;11:63–76. [PubMed] [Google Scholar]
- 73.Sheppard D. In vivo functions of integrins: lessons from null mutations in mice. Matrix Biol. 2000;19:203–209. doi: 10.1016/s0945-053x(00)00065-2. [DOI] [PubMed] [Google Scholar]
- 74.Shirasawa S, Furuse M, Yokoyama N, Sasazuki T. Altered growth of human colon cancer cell lines disrupted at activated Ki-ras. Science. 1993;260:85–88. doi: 10.1126/science.8465203. [DOI] [PubMed] [Google Scholar]
- 75.Sotiropoulos A, Gineitis D, Copeland J, Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell. 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 76.Stacey K J, Fowles L F, Colman M S, Ostrowski M C, Hume D A. Regulation of urokinase-type plasminogen activator gene transcription by macrophage colony-stimulating factor. Mol Cell Biol. 1995;15:3430–3441. doi: 10.1128/mcb.15.6.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stanton V P, Jr, Cooper G M. Activation of human raf transforming genes by deletion of normal amino- terminal coding sequences. Mol Cell Biol. 1987;7:1171–1179. doi: 10.1128/mcb.7.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Uhm J H, Gladson C L, Rao J S. The role of integrins in the malignant phenotype of gliomas. Front Biosci. 1999;4:D188–D199. doi: 10.2741/uhm. [DOI] [PubMed] [Google Scholar]
- 79.Varner J A, Cheresh D A. Integrins and cancer. Curr Opin Cell Biol. 1996;8:724–730. doi: 10.1016/s0955-0674(96)80115-3. [DOI] [PubMed] [Google Scholar]
- 80.Varner J A, Cheresh D A. Tumor angiogenesis and the role of vascular cell integrin alphavbeta3. Important Adv Oncol. 1996;1996:69–87. [PubMed] [Google Scholar]
- 81.Villa-Garcia M, Li L, Riely G, Bray P F. Isolation and characterization of a TATA-less promoter for the human beta 3 integrin gene. Blood. 1994;83:668–676. [PubMed] [Google Scholar]
- 82.Webb C P, Van Aelst L, Wigler M H, Woude G F. Signaling pathways in Ras-mediated tumorigenicity and metastasis. Proc Natl Acad Sci USA. 1998;95:8773–8778. doi: 10.1073/pnas.95.15.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Whalen A M, Galasinski S C, Shapiro P S, Nahreini T S, Ahn N G. Megakaryocytic differentiation induced by constitutive activation of mitogen-activated protein kinase kinase. Mol Cell Biol. 1997;17:1947–1958. doi: 10.1128/mcb.17.4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.White M A, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, Wigler M H. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- 85.Wilhide C C, Jin Y, Guo Q, Li L, Li S X, Rubin E, Bray P F. The human integrin beta3 gene is 63 kb and contains a 5′-UTR sequence regulating expression. Blood. 1997;90:3951–3961. [PubMed] [Google Scholar]
- 86.Wojnowski L, Stancato L F, Larner A C, Rapp U R, Zimmer A. Overlapping and specific functions of braf and craf-1 proto-oncogenes during mouse embryogenesis. Mech Dev. 2000;91:97–104. doi: 10.1016/s0925-4773(99)00276-2. [DOI] [PubMed] [Google Scholar]
- 87.Wojnowski L, Zimmer A M, Beck T W, Hahn H, Bernal R, Rapp U R, Zimmer A. Endothelial apoptosis in Braf-deficient mice. Nat Genet. 1997;16:293–297. doi: 10.1038/ng0797-293. [DOI] [PubMed] [Google Scholar]
- 88.Woods D, Parry D, Cherwinski H, Bosch E, Lees E, McMahon M. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol Cell Biol. 1997;17:5598–5611. doi: 10.1128/mcb.17.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang B S, Hauser C A, Henkel G, Colman M S, Van Beveren C, Stacey K J, Hume D A, Maki R A, Ostrowski M C. Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Mol Cell Biol. 1996;16:538–547. doi: 10.1128/mcb.16.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhu J, Woods D, McMahon M, Bishop J M. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 1998;12:2997–3007. doi: 10.1101/gad.12.19.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zohn I M, Campbell S L, Khosravi-Far R, Rossman K L, Der C J. Rho family proteins and Ras transformation: the RHOad less traveled gets congested. Oncogene. 1998;17:1415–1438. doi: 10.1038/sj.onc.1202181. [DOI] [PubMed] [Google Scholar]