Abstract
In this work, four new dry water fire extinguishing agents (FEAs) were prepared by hydrophobic SiO2 and aqueous solution under certain conditions. The dry water FEAs were developed and we conducted two types of fire extinguishing experiments (i.e., class A solid fire and class B liquid fire test). Thermocouples and a color video camera were used to measure burning temperature and record the fire extinguishing process. Results indicate that the new dry water FEAs have the ability to extinguish A, B and C fires, and have a better cooling effect than dry powder FEA. It is noted, compared with traditional FEAs, that dry water FEAs have the advantages of high efficiency and high speed, and have a potential application prospect.
Four new dry water fire extinguishing agents (FEAs) were developed by hydrophobic SiO2 and aqueous solution. Among them, dry water FEA with ammonium dihydrogen phosphate has the best extinguishing effect via temperature changes by thermocouples.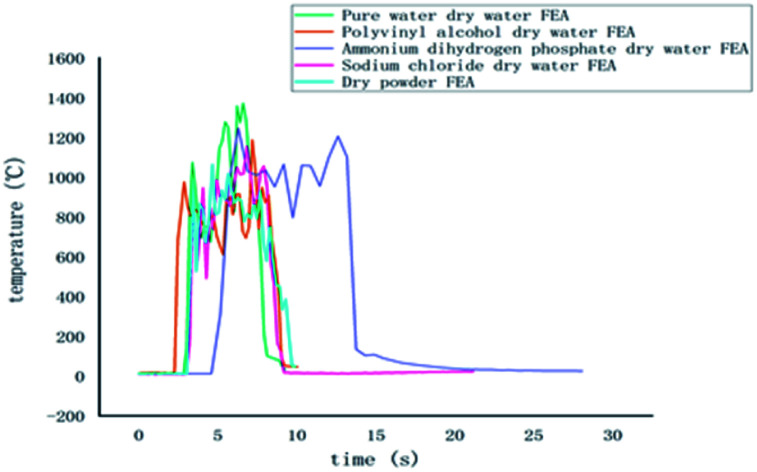
1. Introduction
Due to the rapid economy development, the number of buildings and their height increases with each passing day. The frequent occurrence of complex environment fires such as high-rise building fires poses a great threat to the safety of human life and property. In order to reduce the economic loss and casualties and improve the efficiency of fire extinguishing agents (FEAs), it is particularly important to develop a new type of FEA.
In the mid-20th century, a halon gas FEA was first studied and used,1 but halon halogenated hydrocarbons will decompose O3, which will damage the ozone layer structure for a long time. Currently, FEAs are divided into inert gas fire extinguishing agents, halogenated hydrocarbon fire extinguishing agents and aerosol fire extinguishing agents.2–4 Their common advantages are: no harm to people and the environment, high fire extinguishing efficiency and smaller equipment.5 However, there are also many disadvantages:6,7 inert gas extinguishing agents will expand and discharge in the storage and use process, halogenated hydrocarbon extinguishing agent emission has a long duration. The production process of aerosol extinguishing agents is complex, and the raw material cost is high. Therefore, a new type of extinguishing agent with high efficiency, environmental protection, and low cost should be developed.
Dry water material is a kind of material which takes liquid as the main component and disperses into droplet particles with extremely small size at high speed, and hydrophobic powder is uniformly coated on the outer surface of the droplets.8,9 It is found that the water content is more than 90%, and is widely used in the storage of methane, carbon dioxide, cosmetics, pharmaceuticals, and other fields.10–15 Because of the large amount of aqueous solution covered by dry water materials, it can be used in the field of fire extinguishing agent. The mechanism of dry water extinguishing agent is different from that of traditional dry powder extinguishing agent and fine water mists extinguishing agent, it combines the advantages of both, and has the combined mechanisms of physical cooling and chemical inhibition. In this paper, chemical additives such as ammonium dihydrogen phosphate are added to distilled water to modify dry water materials and investigate the fire-extinguishing effect of different components of dry water materials.
In the paper, four types of dry water extinguishing agents were developed by using hydrophobic SiO2 and an aqueous solution containing additives as raw materials utilizing the high-speed dispersion method. Fire extinguishing experiments were carried out on class A and class B fire extinguishing conditions (class A refers to the fire of solid substances, class B refers to liquid and fusible solid fire, class C refers to gas fire in GB4968-2008 in China).16 Meanwhile, ABC dry powder extinguishing agents were used as a reference to evaluate the extinguishing performance of dry water extinguishing agents, and select the dry water extinguishing agents with the best extinguishing effect. Meanwhile, ABC dry powder FEAs were taken as a reference to evaluate the extinguishing performance of dry water FEAs and select the dry water FEAs with the best extinguishing effect.
2. Experimental design
2.1. Experimental materials and instruments
Hydrophobic SiO2 was produced from Shanghai Yuanjiang Chemical Co., Ltd, (Shanghai, P. R. China), ammonium dihydrogen phosphate was produced from Guangzhou Linguo Chemical Fertilizer Co., Ltd, (Guangdong, P. R. China), sodium chloride was provided by Hunan Mingde Chemical Co., Ltd, (Hunan, P. R. China), and polyvinyl alcohol was purchased from Beijing Chenqi Chemical Technology Co., Ltd, (Beijing, P. R. China). The FS400-ST round tube high-speed dispersing machine and GB-2008 electronic analytical balance were purchased from Shanghai Lichen Bangxi Technology Co., Ltd, (Shanghai, P. R. China). SG50-TB61M optical microscope was produced from Suzhou Shenying Optical Co., Ltd, (Jiangsu, P. R. China). The K-type armored thermocouples and R-type platinum–rhodium thermocouples were purchased from Hebi Ruipu Instrument Co., Ltd, (Henan, P. R. China).
In the experiments, hydrophobic silica, distilled water, ammonium dihydrogen phosphate, sodium chloride and polyvinyl alcohol were used to prepare ammonium dihydrogen phosphate solution, sodium chloride solution and polyvinyl alcohol solution. K-type armored thermocouples are selected to detect the temperature of the class A fire. It is composed of thermocouples wire, high-temperature resistant metal tube shell, and insulating material. It can measure the temperature range from 0 to 1600 °C. It is often used with a temperature recorder and temperature conversion module. In this experiment, R-type platinum–rhodium thermocouples are selected to detect the temperature of class B fire. It is made of metal platinum wire (0.05 mm) and rhodium wire (0.05 mm). The positive electrode is the platinum–rhodium alloy, and the negative electrode is pure platinum. The temperature range is 0–1600 °C. And In this experiments, ABC dry powder FEA was used for the comparative test.
2.2. Preparation of dry water FEAs
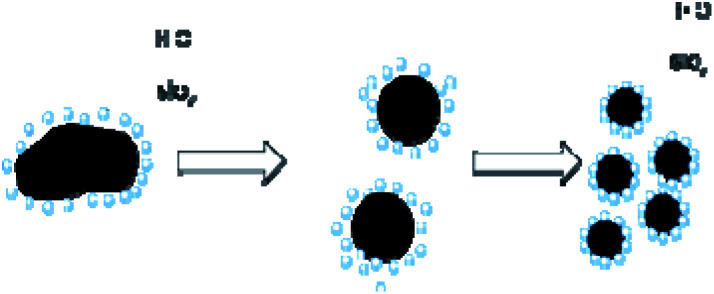
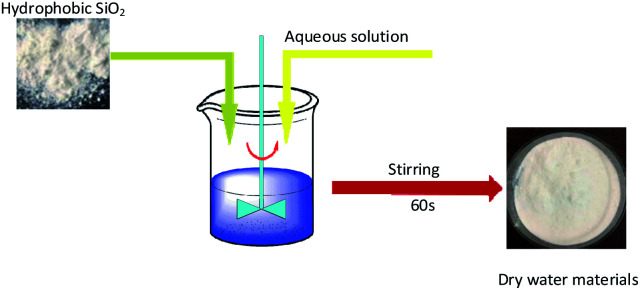
In experiments, 10% ammonium dihydrogen phosphate solution, 10% sodium chloride solution, and 0.5% polyvinyl alcohol solution were prepared with hydrophobic silica, distilled water, ammonium dihydrogen phosphate, sodium chloride, and polyvinyl alcohol respectively. Fig. 1 shows the preparation mechanism of dry powder FEAs. The preparation processes of the dry water FEA are shown in Fig. 2. Hydrophobic silica is mixed with an aqueous solution at a specific mass ratio and uniformly mixed by a high-speed dispersing machine to form a dry water FEA.
Fig. 1. Preparation mechanism of dry water FEA.
Fig. 2. Preparation process of dry water FEA.
The mass ratio of the hydrophobic SiO2 and the above solutions were set as 1 : 10 respectively, the speed of the high-speed dispersing mixer was set as 4000 rpm and the stirring time was 60 s, and the pure water dry water FEA, ammonium dihydrogen phosphate dry water FEA, sodium chloride dry water extinguishing agent and polyvinyl alcohol dry water FEA were obtained. Table 1 shows the preparation parameters of dry water FEA.
Preparation parameters of dry water fire FEA.
| Types of FEAs | Stirring speed (rpm) | Mixing time (s) | M SiO2 : Maqueous solution | Additive |
|---|---|---|---|---|
| Pure water dry water FEA | 4000 | 60 | 1 : 10 | 0 |
| Ammonium dihydrogen phosphate dry water FEA | 4000 | 60 | 1 : 10 | 10% ammonium dihydrogen phosphate |
| Sodium chloride dry water FEA | 4000 | 60 | 1 : 10 | 10% sodium chloride |
| PVA dry water FEA | 4000 | 60 | 1 : 10 | 0.5% polyvinyl alcohol |
2.3. Fire extinguishing mechanism of dry water FEAs
The fire extinguishing mechanism of dry water FEAs mainly includes physical and chemical suppression. The physical mechanism is that when a large amount of water enters the fire in the particulate matter, it evaporates quickly to absorb heat, and then the extinguishing components will decompose and absorb heat. When the remaining heat after absorption cannot maintain the combustion chain reaction, flame propagation is suppressed. At the same time, the hydrophobic silica in the dry water extinguishing agent will adhere to the surface of the burning material to prevent the heat exchange between the surface of the burning material and the flame. At the same time, the large amount of nitrogen that drives the dry water extinguishing agent prevents outside air from entering the fire site and interrupts the supply of oxidant needed to maintain the combustion reaction. The chemical suppression mechanism is that the gas products decomposed from the extinguishing components in the dry water extinguishing agent can capture the free radicals in the flame. When the concentration of free radicals in the fire decreases, the reaction speed between free radicals and fuel molecules will slow down, and when the heat released by the reaction decreases, the fuel evaporation speed will slow down. For example, ammonium dihydrogen phosphate decomposes ammonia gas, water vapor, and phosphorus pentoxide solid at high temperature.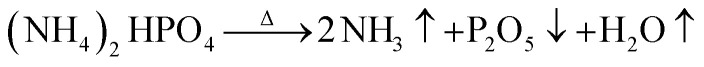
The free radicals produced by the reaction can cut off the combustion reaction chain. At the same time, ammonia and water vapor can isolate the contact between oxygen and combustibles. Phosphorus pentoxide solids can cover the surface of combustibles to prevent combustion. Sodium chloride has the effect of extinguishing flames, which can destroy the center of the chain reaction during combustion and promote the interruption of the reaction chain.
2.4. Experiments design for class A and class B fire extinguishing
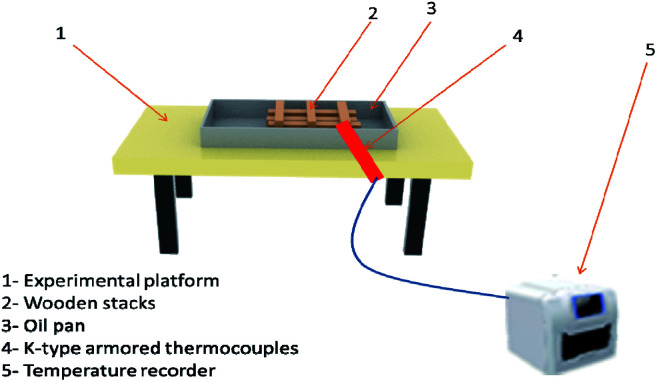
Class A fire conditions are shown in Fig. 3, including metal platforms, oil tray, and wood stacks. The wood strips were pretreated so that their moisture content was between 10% and 14%. When the moisture content is 12%, the density should be 0.5 ± 0.05 g cm−3. The wooden stack was made of 200 mm in length and 15 mm × 15 mm in cross-section. There were 3 layers in total. Each layer of wood was at right angles to each other and the spacing was the same. The size of the oil tray is 400 mm × 300 mm × 40 mm. The platform was used to hold the square oil tray, and the wood stacks were ignited by the flame of the oil tray below it.
Fig. 3. Class A fire simulation.
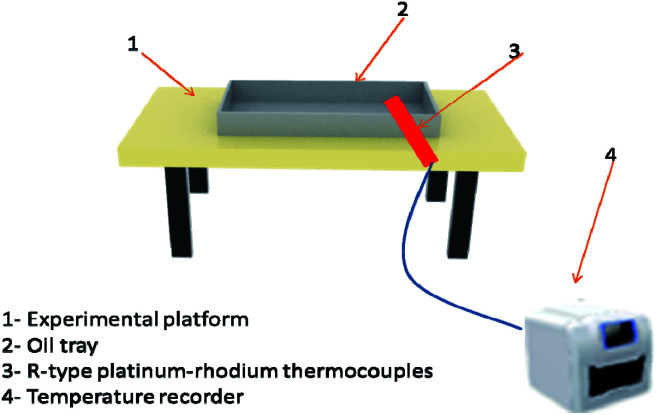
Class B fire simulation system was composed of an experimental platform, oil tray, R-type platinum–rhodium thermocouples, and a temperature recorder, as shown in Fig. 4. The size of the oil tray is 400 mm × 300 mm × 40 mm. Water at a depth of 20 mm was injected first, followed by 200 ml of no. 90 gasoline. The oil tray was placed on the metal platform. According to the evaluation method of dry powder fire-FEA in GB4066-2017 (China),17 if the FEA can extinguish class B fire, it can be considered to have the fire-extinguishing ability of class C fire. Therefore, the fire-extinguishing situation of class C fire can be measured according to the fire-extinguishing result of class B fire.
Fig. 4. Class B fire simulation.
2.5. Fire extinguishing experiment processes
(1) Pack the prepared dry water FEA into empty fire extinguisher cylinders (2 kg level). Record the weight of the fire extinguisher W1, fill it with nitrogen, keep the pressure at 1.2 ± 0.1 MPa, install the nozzle and pressure valve.
(2) Build fire models of class A and B, then fix the thermocouples and temperature recorders to test the fire extinguishing temperature.
(3) Turn on the temperature measurement system, start the fire, and time it. While the fuel burns to 53–57% of the original content, use the dry water extinguisher to spray out the dry water.
(4) During the spraying process, hold the handle to maintain the maximum spraying state, the nozzle should be in front of the fire target when spraying.
(5) Timing ends when the flame goes out. When the temperature drops to room temperature, record the data and weigh the weight of the extinguisher W2.
Repeat the experiments 2 times. If two experiments continuously succeed or fail, the third experiment can be not necessary.
3. Experimental results and discussion
3.1. Morphology characterization
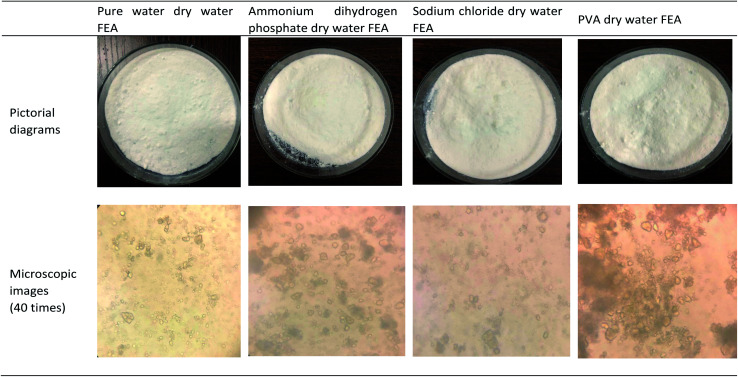
The direct diagram and microscopic observation appearance of the developed dry water extinguishing agent are shown in Fig. 5. It was found that the direct morphology and microstructure of dry water fire extinguishing agent added with ammonium dihydrogen phosphate and sodium chloride do not change. The particle size of dry water FEA added with polyvinyl alcohol increases (Fig. 5).
Fig. 5. Four pictorial diagrams and microscopic images of dry water FEAs.
3.2. Class A fire results
As shown in Table 2, it can be found that the fire-extinguishing time of class A is within 2 s, among which the dry water of ammonium dihydrogen phosphate has the shortest fire-extinguishing time of 1.50 s, followed by dry powder, and the longest time is pure dry water FEA, because its internal solution only contains distilled water, and the fire-extinguishing method is relatively simple. The least amount of FEAs consumption is ammonium dihydrogen phosphate dry water, i.e. 30.37 g. While, the most is the pure water dry water, i.e. 36.85 g, and the two experiments of each group of fire extinguishers were successful. Compared with the application of ABC dry powder FEAs, it is found that, under the same fire type, the use amount and time of several groups of dry water FEAs are close to that of ABC dry powder, and the agents' consumption and the time of dry water FEAs added with ammonium dihydrogen phosphate are less than that of ABC dry powder, which shows that the prepared dry water material has the ability to extinguish class A fire and has the same extinguishing effect as the existing FEAs.
Performances of all kinds of FEA to extinguish class A fire.
| Type of FEAs | Fire extinguishing time (s) | Average fire extinguishing time (s) | Consumption of FEAs (g) | Average consumption of FEAs (g) | Fire situation |
|---|---|---|---|---|---|
| Pure water dry water FEA | 1.78, 1.76 | 1.77 | 36.03, 37.67 | 36.85 | Out, out |
| PVA dry water FEA | 1.56, 1.59 | 1.58 | 34.52, 35.62 | 35.07 | Out, out |
| Ammonium dihydrogen phosphate dry water FEA | 1.42, 1.48 | 1.50 | 29.32, 31.42 | 30.37 | Out, out |
| Sodium chloride dry water FEA | 1.65, 1.60 | 1.63 | 33.12, 32.56 | 32.84 | Out, out |
| Dry powder FEA | 1.53, 1.55 | 1.54 | 31.26, 32.54 | 31.90 | Out, out |
3.3. Analysis of dry water extinguishing agents extinguishing class A fire
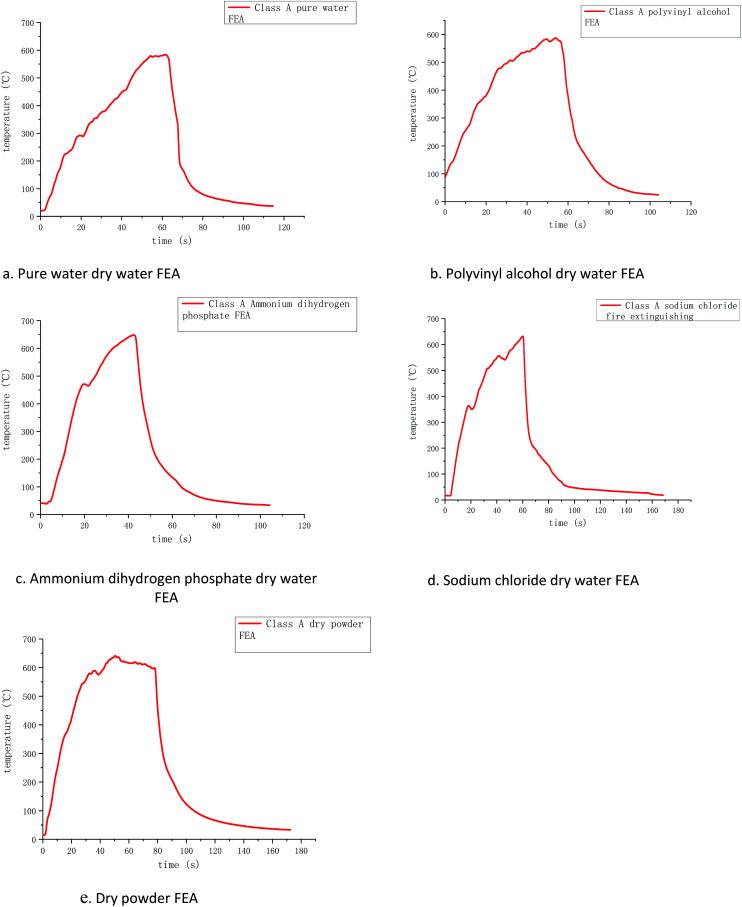
The temperature and time curves of several FEAs measured by the k-type thermocouples are shown in Fig. 6, where the horizontal coordinate is the time after ignition and the vertical coordinate is the temperature change of the whole fire extinguishing process. Because K-type armored thermocouples can resist impacting and work under high temperature for a long time, they were used to measure the temperature changes of class A fire.
Fig. 6. Variation of class A fire temperature in the tests with five samples as suppressants.
Through the temperature change curves, it can be found that the fire-extinguishing process of class A fire is divided into the pre-combustion stage, free burning stage, and fire-extinguishing stage. The time of the pre-combustion stage and free burning stage in several groups of simulation experiments were all around 60 s, and their maximum temperatures were around 600 °C. This indicates that the maximum burning temperature of selected wood was about 600 °C, so the fire extinguishing experiments were conducted at this time to ensure that other conditions were basically the same.
In the pre-combustion stage, the gasoline was fully burned, and then the flame ignited the wood stacks above. It can be found that the initial temperature in Fig. 6 was higher because the thermocouples detected the hot air flow caused by the ignition of the gasoline under it, increasing in the initial temperature. However, since the initial temperature is relatively low and within a controllable range, it can be ignored.
When the gasoline burns out, the energy released by the gasoline combustion makes the wood stacks start to enter the free burning stage. At this time, the measured temperature is completely the flame temperature of the wood stacks burning. After 60 s, several groups of wood stacks reach the highest value of free combustion temperature, and at this time, the firefighting begins. Due to the rate and flame fluctuation of different groups of wood stacks, the maximum temperature reached will be different, but basically remain within a certain range.
During the fire extinguishing stage, the dry water FEA contacts with the flame, and the dry water structure is rapidly ruptured under the influence of high temperature, and a large number of solutions flow out to give full play to the fire extinguishing effect. The temperature of each thermocouple quickly dropped to about 200 °C, which can be found that ammonium dihydrogen phosphate dry water fire when the temperature drops fastest, the highest temperature to 200 °C almost perpendicular to the axis of the curve. The fire extinguishing image of polyvinyl alcohol is similar to that of pure water dry water FEA, and it decreases rapidly at the highest temperature of 200 °C. This is because the addition of PVA dry water only changes the coating strength, but has no effect on the extinguishing performance of the aqueous solution. Adding sodium chloride dry water FEA can extinguish the flame quickly, making the temperature in the oil tray drop rapidly, slightly steeper than the cooling curve of pure water, indicating that sodium chloride has a promoting effect on extinguishing the fire.
Compared with ABC dry powder FEA, it is found that the cooling curve of several groups of dry water FEA are roughly the same as that of ABC dry powder FEA, both of which can drop to 200 °C within 10 s, but the cooling curve of ammonium dihydrogen phosphate dry water is steeper than that of ABC dry powder, indicating that ammonium dihydrogen phosphate dry water has a better cooling effect from high temperature to low temperature. The temperature of the fire extinguisher decreases from 200 °C to 50 °C. The dry water of pure water, polyvinyl alcohol, and ammonium dihydrogen phosphate are used for about 35 s, the dry water of sodium chloride is used for about 50 s, and the dry powder of ABC is used for about 80 s. It shows that the cooling effect of the dry water extinguisher is more remarkable than that of the dry powder of ABC at about 200 °C, which is due to the large amount of water contained in the dry water extinguish. It can continuously absorb heat and evaporation, which has the effect of cooling combustible materials rapidly.
3.4. Mechanism of dry water FEA to extinguish class A fire
Fig. 7 is a screenshot of the burning process of class A fire and the shooting of dry water FEA. When the dry water material is sprayed to the burning wood stack, the top flame is instantly suppressed, because the wood stack is built layer by layer, there are still a few flames and sparks below, but with the continuous spraying of dry water FEA, the bottom flame is completely extinguished, and there are no obvious sparks and no renewed phenomenon in 10 minutes. There are two kinds of burning conditions in class A wood stack fire. One is the tiny combustible material decomposed by the wood under the high temperature, which produces flame together with oxygen under the high temperature. The other is that there is no obvious flame on the surface of the wood stack after burning, but it is still red and hot to form scorching charcoal. At this time, if it can continue to burn after encountering combustible substances, this situation becomes smoldering,18 and most of the re-ignition is caused by this kind of smoldering.
Fig. 7. Ammonium dihydrogen phosphate dry water FEA extinguishes class A fire.
Because of the two burning conditions of type A wood stacking fire, the extinguishing process of dry water FEA can be divided into two aspects. For open fire, the dry water FEA driven by pressure first reaches the top of the flame, and the solution inside the heated cracked dry water flows out, contacting with the flame to absorb heat by evaporation. The high-temperature water vapor formed rapidly rises and drives away the air above the flame, thus reducing the oxygen content above the flame. At the same time, due to the low density of dry water FEA, part of the dry water will fly into the air during the process of ejecting, which also plays a role in reducing the oxygen content. Dry water FEA with a modifier such as ammonium dihydrogen phosphate dry water, ammonium dihydrogen phosphate when heated will quickly decompose ammonia gas and phosphoric acid, the production of free radicals can block the combustion process, these can effectively extinguish the open fire.
3.5. Class B fire results
As shown in Table 3, the average extinguishing time of category B FEA is about 3 s, and the longest is pure water dry water FEA 3.02 s, because its internal solution only contains distilled water, so the extinguishing method is relatively simple. The minimum extinguishing time of dry powder and ammonium dihydrogen phosphate is 2.48 s and 2.65 s respectively. The minimum amount of fire 112.19 g of ammonium dihydrogen phosphate and the maximum amount of FEA is 134.01 g of pure dry water. Two experiments of each group of FEA were successful in extinguishing the fire. By comparing the use of ABC dry FEA, it is found that in the same fire type, only ammonium dihydrogen phosphate dry water usage is slightly less than dry powder, other dry water FEA usage is more than dry powder. Ammonium dihydrogen phosphate dry water fire extinguisher takes longer to extinguish class B fire than class A fire extinguisher, and the amount of fire extinguisher is more than that of class A fire extinguisher, because of the high temperature of oil fire and the large burning area.
Performances of all kinds of fire FEAs to extinguish class B fire.
| Type of FEA | Fire extinguishing time (s) | Mean time to extinguish (s) | Consumption of fire FEA (g) | Average FEA consumption (g) | Fire situation |
|---|---|---|---|---|---|
| Pure water dry water FEA | 2.94, 3.12 | 3.02 | 132.23, 135.78 | 134.01 | Out, out |
| PVA dry water FEA | 2.88, 2.96 | 2.92 | 129.58, 134.42 | 132.00 | Out, out |
| Ammonium dihydrogen phosphate dry water FEA | 2.62, 2.68 | 2.65 | 108.74, 115.63 | 112.19 | Out, out |
| Sodium chloride dry water FEA | 2.84, 2.72 | 2.78 | 121.58, 127.93 | 124.76 | Out, out |
| Dry powder dry water FEA | 2.44, 2.51 | 2.48 | 117.52, 121.69 | 119.61 | Out, out |
3.6. Analysis of dry water FEA extinguishing class B fire
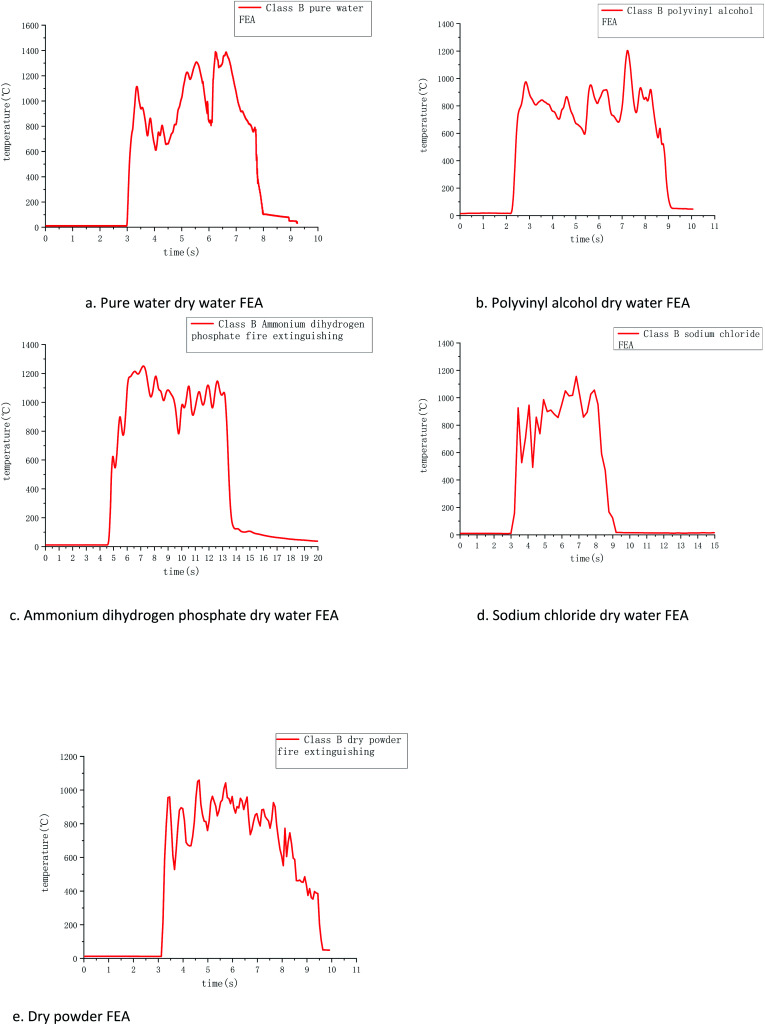
Temperature and time curves of several dry water extinguishers extinguishing type B fires measured by R-type thermocouples are shown in Fig. 8. The combustion process of category B fire can be divided into the rapid heating stage, combustion fluctuation stage, and extinguishing stage from the temperature change chart measured by thermocouples, it can be found that the temperature of type B fire rises very quickly, and the temperature rises within 0.5 s. This is because flammable liquids have certain volatility, and the gasoline chosen in the experiment is more volatile, because the carbon chain is smaller, it can be burned more fully in a short time, releasing enough energy to make the temperature rise rapidly, and this stage is obviously different from class A fire.
Fig. 8. Variation of class B fire temperature in the tests with four samples as suppressants.
During the combustion fluctuation period, the flame temperature fluctuates up and down with time, this is because the gasoline volatilization accompanies the gasoline combustion process, and the instantaneous ignition of the volatile gasoline gas when it reaches the top of the flame makes the temperature rise, while the gasoline combustion range in different areas of the oil tray is not consistent, resulting in the process of competing for the oxygen above the gasoline makes the oxygen supply in some areas too little, at this time the temperature will temporarily drop. In order to ensure the consistency of the experiment, the fire extinguishing is carried out when the temperature measured by the thermocouples is above 1000 °C.
Comparing several groups of temperature change images in the cooling intervals, it is found that the FEA prepared by dry water can make the temperature drop within 2 s. Among them, ammonium dihydrogen phosphate dry water FEA has the best cooling effect, sodium chloride is second, and pure water and polyvinyl alcohol are slower. Ammonium dihydrogen phosphate can break down the ammonia and phosphoric acid, produce free radicals that can cut off the combustion reaction chains, sodium chloride has the effect of restraining flame. The decrease of PVA is slower than that of pure water, which is due to the large particle size dry water FEA formed by PVA, which makes the content of FEAs scattered to various areas in the process of extinguishing not dense enough. As a result, the oil fire that is cooled is quickly heated by the oil fire that is not cooled, making the cooling process floating.
Compared with dry powder FEA, dry powder FEA time is the shortest, but the slowest cooling process, it is because the dry powder FEA contains a lot of ammonium salt compounds, these substances can have the effect of effective extinguishing, but when the flame was extinguished, the oil temperature is still high. However, the cooling effect of dry powder FEA is not as good as that of water solution in dry water FEAs, and the temperature dropping process of dry powder FEA appears to rise again and again. It takes about 5 seconds to drop from the highest temperature to 50 °C, but the cooling after 50 °C is slower, and it is not recorded by the temperature recorder.
3.7. Mechanism of dry water FEAs to extinguish class B fire
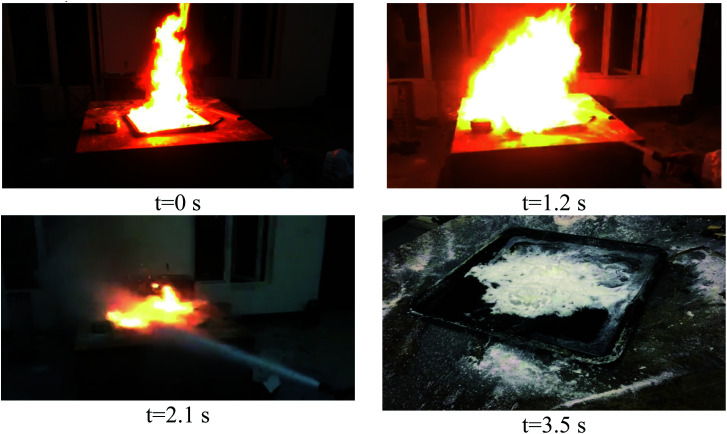
Fig. 9 is a photographic screenshot of the combustion process and dry water extinguishing agent fire extinguishing in class B fire. The fire grew instantly larger as the dry water FEA spewed out and the flame was pushed forward to the edge of the plate. This is because the pressure of FEA is high when it is ejected, the flame in the area opposite to FEA is suppressed, and even the oil layer is peeled off. But the surrounding area cannot be suppressed due to insufficient air pressure, while oxygen squeezed away by the central area is in contact with the flames around the oil tray, causing the fire to grow in an instant. With the continuous spraying of dry water FEA, the surrounding flame is suppressed, the flame height is controlled, and finally the flame is suppressed to nearly equal height with the flame oil tray until it extinguished.
Fig. 9. Ammonium dihydrogen phosphate dry water FEA extinguishes class B fire.
The biggest difference between class B fire and class A fire is that the combustible liquids in class B fires are volatile. During the combustion process, they continuously volatilize into the air to form very small droplets, which can burn more fully after contacting with the air. In the initial stage of fire extinguisher spraying, the airflow carried by fire extinguisher will reach the ignition area before the extinguisher, so that part of the combustible liquid will be blown into the air, at the same time, it will accelerate the airflow above, speed up the fresh air supply, and make the starting flame bigger. The principle of extinguishing the fire lies in the balance of the cooling of dry water FEA and the rate of combustion and heat release. If the cooling rate is fast, the flame is extinguished, it can be seen that polyvinyl alcohol and sodium chloride dry water cooling process curve jitter, indicating that the process of dry water FEA cooling effect and gasoline combustion heating at the same time, so that the temperature rise and fall. Ammonium phosphate dry water can also be faster to extinguish the flame, and the cooling effect is obvious, indicating that in the destruction of B-type fires, the chemicals produced by the decomposition of ammonium dihydro phosphate in the chemical effect (such as ammonium, phosphoric acid, etc.), while water plays a role in rapid heat absorption cooling flammable liquid. However, pure dry water has no additives in it, but it can also extinguish class B fires, indicating that the hydrophobic SiO2 coated on the outside after the structure is broken plays a good role in isolating oxygen. This also shows that the modifier of dry water FEA has a great influence on the extinguishing effect, and in the process of extinguishing class B fire, both physical and chemical extinguishing principles are used.
4. Conclusions
In this paper, four groups of dry water FEA were prepared. The method of filling and spraying fire extinguisher was used to compare with ABC dry powder for extinguishing. The fire model of class A and class B was simulated, the fire extinguishing process and temperature change were recorded, and the following conclusions were obtained.
(1) All four groups of dry water FEAs can effectively extinguish class A fire and class B fire, and the extinguishing effect is similar to that of ABC dry powder FEA, so it is considered that dry water FEA has the ability to extinguish class A fire, class B fire, and class C fire.
(2) Dry water FEA with ammonium dihydrogen phosphate added in four groups of dry water FEA has the best extinguishing effect, and the cooling effect of dry water FEA is better than that of dry powder in the extinguishing process of category B fire.
(3) The extinguishing mechanism of dry water FEA combines physical cooling and chemical inhibition, which can not only use the free radicals generated to cut off the combustion reaction chain but also use aqueous solution heat absorption to cool down.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This study was supported by National Natural Science Foundation of China (No. 11872002, No. 11502001), the authors expressed their sincere gratitude here.
References
- Wang W. Zhai H. Li Y. et al., Selection of Gas Fire Extinguishing System Based on Halon Substitutes. Fire Sci. Technol. 2002;21(6):32–33. [Google Scholar]
- Chen X. Fan A. Yuan B. et al., Renewable biomass gel reinforced core-shell dry water material as novel fire extinguishing agent. J. Loss Prev. Process Ind. 2019;59:14–22. doi: 10.1016/j.jlp.2019.02.008. [DOI] [Google Scholar]
- Wei J.-long. Jia Xu-hong. Wei Z.-xu. et al., Research progress of haloalkane alternative airborne portable fire extinguisher. Fire Sci. Technol. 2017;36(07):983–986. [Google Scholar]
- Jin J. An F. Yi S. et al., Simulation on Release Characteristics of the Gas Extinguishing Agent in Fire Extinguisher Vessel with Different Filling Conditions Based on AMESim. Procedia Eng. 2018;211:315–324. doi: 10.1016/j.proeng.2017.12.018. [DOI] [Google Scholar]
- Chen M. Xie Y. Wu H. et al., Modeling solubility of nitrogen in clean fire extinguishing agent by Peng-Robinson equation of state and a correlation of Henry’s law constants. Appl. Therm. Eng. 2017;110:457–468. doi: 10.1016/j.applthermaleng.2016.08.179. [DOI] [Google Scholar]
- Wang T. Hu Y. Zhang P. Pan R. Study on thermal decomposition properties and its decomposition mechanism of pentafluoroethane (HFC-125) fire extinguishing agent. J. Fluorine Chem. 2016;190:48–55. doi: 10.1016/j.jfluchem.2016.08.006. [DOI] [Google Scholar]
- Wang Q. Shao G. Duan Q. et al., The Efficiency of Heptafluoropropane Fire Extinguishing Agent on Suppressing the Lithium Titanate Battery Fire. Fire Technol. 2016;52(2):387–396. doi: 10.1007/s10694-015-0531-9. [DOI] [Google Scholar]
- Dieter S., Franz-Theo S. and Helmut B., et al., Predominantly aqueous compositions in a fluffy powdery form approximating powdered solids behavior and process for forming same, US pat., 3393155, 1968
- Binks B. P. Tyowua A. T. Particle-Stabilized powdered water-in-oil emulsions. Langmuir. 2016;32(13):3110–3115. doi: 10.1021/acs.langmuir.6b00140. [DOI] [PubMed] [Google Scholar]
- Carter B. O. Adams D. J. Cooper A. I. Pausing a stir: heterogeneous catalysis in “dry water”. Green Chem. 2010;12(5):783–785. doi: 10.1039/B922508K. [DOI] [Google Scholar]
- Carter B. O. Wang W. Adams D. J. Cooper A. I. Gas storage in “dry water” and “dry gel” clathrates. Langmuir. 2010;26(5):3186–3193. doi: 10.1021/la903120p. [DOI] [PubMed] [Google Scholar]
- Saleh K. Forny L. Guigon P. Pezron I. Dry water: from physico-chemical aspects to process-related parameters. Chem. Eng. Res. Des. 2011;89(5A):537–544. doi: 10.1016/j.cherd.2010.06.005. [DOI] [Google Scholar]
- Wang W. Bray C. L. Adams D. J. et al., Methane storage in dry water gas hydrates. J. Am. Chem. Soc. 2008;130:11608–11609. doi: 10.1021/ja8048173. [DOI] [PubMed] [Google Scholar]
- Forny L. Pezron I. Saleh K. et al., Storing water in powder form by self-assembling hydrophobic silica nanoparticles. Powder Technol. 2007;171:15–24. doi: 10.1016/j.powtec.2006.09.006. [DOI] [Google Scholar]
- Qiu C. Study on Methane Storage Using Hydrate Based on Dry Gel. Guangdong Chem. Ind. 2012;12(39):77–79. [Google Scholar]
- Yao S. and Zheng W., National standard of China, General Administration of quality supervision, inspection and Quarantine of the people's Republic of China, Tianjin Fire Research Institute and Ministry of public security of China, Fire classification, GB/T4968-2008, National Standardization Administration of China, 2008 [Google Scholar]
- Dai D., Li S., Liu Y., et al., National standard of China, General Administration of quality supervision, inspection and Quarantine of the people's Republic of China, Tianjin Fire Research Institute and Ministry of public security of China, Dry Powder fire extinguishing agent, GB4066-2017, National Standardization Administration of China, 2008 [Google Scholar]
- Chen C., Combustion Science, Beijing, China Machine Press, 2012 [Google Scholar]