To the editor:
Seroconversion rates in kidney transplant recipients (KTRs) after 2-dose BNT162b2 (Pfizer–BioNTech) mRNA vaccination are in the range of 3%–59% and thus are significantly lower compared with the >90% achieved in healthy controls.1 In convalescent coronavirus disease 2019 (COVID-19) patients, antibody levels decline only slightly after 6–8 months, whereas vaccine-induced immunity appears to decrease more rapidly.2, 3, 4 Recent data suggest that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern (VoCs) partially escape humoral immune responses induced by natural infection with a nonescaping variant or vaccination.5 However, little is known about protection against VoCs in KTRs who recovered from COVID-19 or were immunized with 2 doses of BNT162b2.
We compared humoral immunity in 18 KTRs hospitalized for COVID-19 infection with immunity in 25 KTRs with seroconversion after 2-dose BNT162b2 vaccination. Nucleocapsid antibodies were measured after the second vaccination in vaccinated patients or at hospitalization in COVID-19–infected patients to exclude prior SARS-CoV-2 infection. Baseline characteristics, including immunosuppressive regimens, are given in Supplementary Table S1. COVID-19 disease severity ranged from moderate to critical, with 2 COVID-19–related deaths (Supplementary Table S2). Immunosuppressive antimetabolite medication was stopped in all COVID-19 patients, and 9 of 18 (50%) patients received corticosteroids only (Supplementary Table S3). Eight patients had infection with the original SARS-CoV-2 strain, 8 patients with the VoC B.1.1.7 (alpha), and 2 patients with B.1.351 (beta). Serum was collected at a median (interquartile range [IQR]) of 72 (67–77) days after hospitalization, or 62 (54–64) days after prime vaccination for COVID-19–infected or vaccinated KTRs, respectively. We determined anti–wild-type SARS-CoV-2 spike S1 IgG, neutralizing surrogate antibodies, and performed a bead-based multiplex analysis of various SARS-CoV-2 target epitopes in 16 convalescent KTRs available for follow-up and in all 25 vaccinated KTRs. In addition, neutralizing antibodies to wild-type, B.1.1.7 (alpha), B.1.351 (beta), and B.1.617.2 (delta) were measured using a full virus assay (Supplementary Methods).
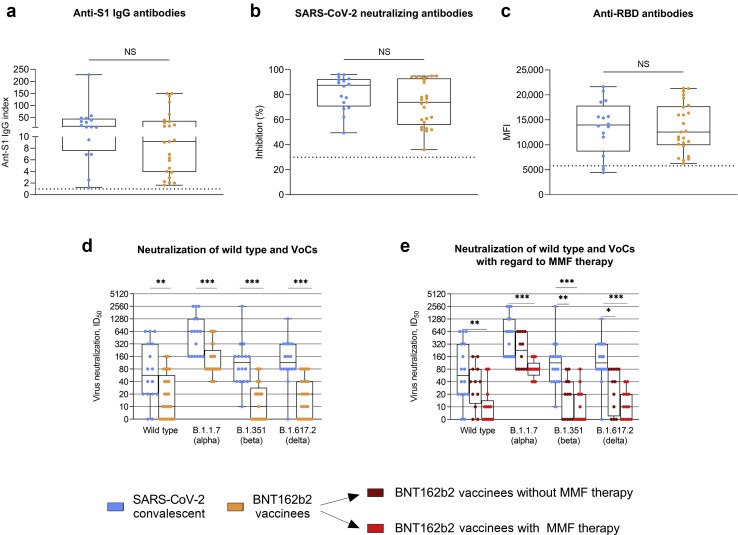
Our data show that there is no significant difference between convalescent or vaccinated KTRs for commercially available tests, such as anti-S1 IgG, neutralizing antibodies determined by a surrogate virus neutralization assay, or anti–receptor-binding domain antibodies (Figure 1 a–c). In a bead-based analysis of antibodies against different SARS-CoV-2 target epitopes, convalescent KTRs showed a broader reactivity against various SARS-CoV-2 target epitopes with significantly higher anti-S2 and anti-nucleocapsid antibody levels compared with vaccinated KTRs (for both, P < 0.001; Supplementary Figure S1A). No significant differences in antibody levels to the S1 proteins of the 4 common cold coronaviruses were detected in convalescent compared with vaccinated KTRs (Supplementary Figure S1B).
Figure 1.
Neutralization of wild type, B.1.1.7, B.1.351, and B.1.617.2 in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) convalescent and 2-dose BNT162b2 mRNA vaccinated kidney transplant recipients. (a) SARS-CoV-2 IgG antibodies in 16 SARS-CoV-2 convalescent and 25 seroconverted BNT162b2 vaccinated kidney transplant recipients 2 to 3 months after infection or prime vaccination, respectively. The dashed black line represents the cutoff for detection. A semiquantitative index ≥1 defined positivity. (b) SARS-CoV-2 neutralizing antibodies, as determined by a surrogate virus neutralization test in SARS-CoV-2 convalescent and BNT162b2 vaccinated transplant recipients. The dashed black line represents the cutoff for detection. Binding inhibition ≥30% defined positivity. (c) Antibodies against the SARS-CoV-2 receptor-binding domain (RBD) protein, as determined by a bead-based assay, in SARS-CoV-2 convalescent and BNT162b2 vaccinated kidney transplant recipients. The mean fluorescence intensity (MFI) value of the reactivity is given on the y axis, with the dashed black line indicating the cutoff for detection. (d) Titers of neutralizing antibodies against wild type, B.1.1.7, B.1.351, and B.1.617.2 variants of concern (VoCs) in SARS-CoV-2 convalescent and BNT162b2 vaccinated kidney transplant recipients, as determined by serial 2-fold serum dilutions using VeroE6 target cells. The ID50 equals the serum dilution that inhibits 50% of the infectivity. (e) Titers of neutralizing antibodies against wild type, B.1.1.7, B.1.351, and B.1.617.2 VoCs in 16 SARS-CoV-2 convalescent kidney transplant recipients 2 to 3 months after hospitalization compared with 12 and 13 2-dose BNT162b2 vaccinated kidney transplant recipients with and without mycophenolate mofetil (MMF) maintenance therapy, respectively. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. NS, nonsignificant.
In a full virus assay, convalescent KTRs had significantly higher activity of neutralizing antibodies against wild-type and all tested VoCs with a median (IQR) serum dilution that inhibits 50% of the infectivity (ID50) of 60 (20–320) for neutralization against the wild-type, 640 (160–1280) for B.1.1.7, 120 (40–160) for B.1.351, and 120 (80–320) for B.1.617.2 compared with 2-dose vaccinated KTRs with a median (IQR) ID50 of 10 (0–60), 80 (80–240), 10 (0–30), and 20 (0–40), respectively (P < 0.01 for wild type and P < 0.001 for B.1.1.7, B.1.351, and B.1.617.2; Figure 1d). Higher neutralizing activity against the wild type and all tested VoCs in COVID-19 convalescent KTRs seemed to be independent of the initially causative SARS-CoV-2 strain (Supplementary Figure S2). In addition, KTRs infected with the wild-type strain (N = 8) were analyzed separately and showed significantly higher neutralizing activity against the wild type and all tested VoCs compared with 2-dose vaccinated KTRs (Supplementary Figure S3). Neutralization against B.1.1.7 was higher compared with neutralization against B.1.351 or B.1.617.2 in both convalescent and vaccinated KTRs, which has also recently been demonstrated for BNT162b2 vaccinated and COVID-19 convalescent healthy cohorts.6 , 7
Kantauskaite et al. showed that seroconversion in SARS-CoV-2–vaccinated KTRs is impaired in patients on mycophenolate mofetil (MMF) maintenance therapy.8 As no patient remained on MMF therapy during COVID-19 infection, it was not possible to differentiate the effect of infection from cessation of immunosuppression in mounting a broad humoral response in our cohort. However, cessation of MMF does not exclusively explain the higher neutralization titers in COVID-19–infected KTRs as seroconverted KTRs without antimetabolite therapy (12 of 25, 48%) still showed lower neutralization titers against B.1.351 and B.1.617.2 compared with COVID-19 convalescent KTRs (Figure 1e).
Despite detectable seroconversion in commercially available assays, 8 of 25 (32%), 12 of 25 (48%), and 8 of 25 (32%) 2-dose vaccinated KTRs did not show neutralization against wild type, B.1.351, or B.1.617.2, respectively. In contrast, only 2 of 16 (13%) and 1 of 16 (6%) COVID-19 convalescent KTRs did not show detectable neutralizing activity against wild type and B.1.617.2, respectively. Until now, it was not possible to define humoral or cellular cutoff values that confer protective immunity. To address this question, Khoury et al. modeled SARS-CoV-2 immune protection across different convalescent and vaccine studies. They estimated a “50% protective neutralization level” at an in vitro neutralization titer (ID50) between 1:10 and 1:30, which best predicted protection against severe COVID-19.9 However, these estimates are derived from the general population and may not be applicable to immunosuppressed patients.
Our data suggest that 60 to 80 days after SARS-CoV-2 infection, most convalescent KTRs showed strong neutralization against all tested VoCs. In contrast, at least one-third of vaccinated KTRs showed insufficient neutralization against the VoCs B.1.351 or B.1.617.2, even though antibodies were detectable in commercially available tests. Therefore, a third booster dose seems reasonable even in vaccinated KTRs with seroconversion to protect against breakthrough infections caused by VoCs.
Disclosure
All the authors declared no competing interests.
Data Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Acknowledgments
Funding for this study has been received by the Dietmar Hopp Stiftung (grant number 1DH2111111). LB is funded by the Rahel Goitein-Straus Program of the Heidelberg Faculty of Medicine. RB is supported by the program for surveillance and control of SARS-CoV-2 mutations of the state of Baden-Württemberg, the German Federal Research Network Applied Surveillance and Testing within the Network University Medicine, the DKFZ@fightCOVID initiative, and the Helmholtz Association's Initiative and Networking Fund Project “Virological and immunological determinants of COVID-19 pathogenesis – lessons to get prepared for future pandemics (KA1-Co-02 “COVIPA”).” CS is funded by the Physician Scientist Program of the Heidelberg Faculty of Medicine.
We thank Iris Arnold and Sabine Bönisch at the Department of Nephrology, Heeyoung Kim at the Department of Infectious Diseases, Molecular Virology, and Verena Backendorf at the Department of Immunology (all at Heidelberg University Hospital, Heidelberg, Germany) for their technical support.
Footnotes
Supplementary Study Design.
Supplementary Methods.
Table S1. Baseline characteristics of kidney transplant recipients at the time of COVID-19 hospitalization or prime vaccination.
Table S2. Clinical courses of COVID-19 in infected kidney transplant recipients.
Table S3. Management of immunosuppression in COVID-19–infected kidney transplant recipients.
Figure S1. IgG antibodies against different SARS-CoV-2 target epitopes and against the spike S1 protein of common cold coronaviruses in SARS-CoV-2 convalescent and 2-dose BNT162b2 vaccinated kidney transplant recipients.
Figure S2. Neutralization against wild type, B.1.1.7, B.1.351, and B.1.617.2 variants of concern in SARS-CoV-2 convalescent kidney transplant recipients stratified for causative SARS-CoV-2 strain and in 2-dose BNT162b2 vaccinated kidney transplant recipients.
Figure S3. Neutralization of wild type, B.1.1.7, B.1.351, and B.1.617.2 in wild-type SARS-CoV-2 convalescent and 2-dose BNT162b2 mRNA vaccinated kidney transplant recipients.
Supplementary References.
Supplementary Material
References
- 1.Carr E.J., Kronbichler A., Graham-Brown M., et al. Review of early immune response to SARS-CoV-2 vaccination among patients with CKD. Kidney Int Rep. 2021;6:2292–2304. doi: 10.1016/j.ekir.2021.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer B. Waning antibodies to SARS-CoV-2 – don’t panic. Lancet Reg Health Eur. 2021;4:100115. doi: 10.1016/j.lanepe.2021.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wajnberg A., Amanat F., Firpo A., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.L’Huillier A.G., Meyer B., Andrey D.O., et al. Antibody persistence in the first 6 months following SARS-CoV-2 infection among hospital workers: a prospective longitudinal study. Clin Microbiol Infect. 2021;27:784.e1–784.e8. doi: 10.1016/j.cmi.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geers D., Shamier M.C., Bogers S., et al. SARS-CoV-2 variants of concern partially escape humoral but not T cell responses in COVID-19 convalescent donors and vaccine recipients. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates T.A., Leier H.C., Lyski Z.L., et al. Neutralization of SARS-CoV-2 variants by convalescent and BNT162b2 vaccinated serum. Nat Commun. 2021;12:5135. doi: 10.1038/s41467-021-25479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C., Ginn H.M., Dejnirattisai W., et al. Reduced neutralization of SARS-CoV-2 B.1.617 by vaccine and convalescent serum. Cell. 2021;184:4220–4236.e13. doi: 10.1016/j.cell.2021.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kantauskaite M, Müller L, Kolb T, et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am J Transplant. 2022;22:634–639. [DOI] [PMC free article] [PubMed]
- 9.Khoury D.S., Cromer D., Reynaldi A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.