Abstract
Background:
Despite their popularity, the efficacy of interventions targeting gut microbiota to improve depressive symptoms is unknown. Our objective is to summarize the effect of microbiome-targeting interventions on depressive symptoms.
Methods:
We conducted a systematic review and meta-analysis. We searched MEDLINE, Embase, PsycINFO, Database of Abstracts of Reviews of Effects, Cochrane Database of Systematic Reviews and the Cochrane Controlled Register of Trials from inception to Mar. 5, 2021. We included studies that evaluated probiotic, prebiotic, synbiotic, paraprobiotic or fecal microbiota transplant interventions in an adult population (age ≥ 18 yr) with an inactive or placebo comparator (defined by the absence of active intervention). Studies must have measured depressive symptoms with a validated scale, and used a randomized controlled trial study design. We conducted a random effects meta-analysis of change scores, using standardized mean difference as the measure of effect.
Results:
Sixty-two studies formed the final data set, with 50 included in the meta-analysis. Probiotic, prebiotic, and synbiotic interventions on depressive symptoms showed statistically significant benefits. In the single studies evaluating each of fecal microbiota transplant and paraprobiotic interventions, neither showed a statistically significant benefit.
Interpretation:
Despite promising findings of benefit of probiotic, prebiotic and synbiotic interventions for depressive symptoms in study populations, there is not yet strong enough evidence to favour inclusion of these interventions in treatment guidelines for depression. Critical questions about species administered, dosage and timing relative to other antidepressant medications remain to be answered.
Study registration:
PROSPERO no. 143178
Mounting evidence supports the concept of a microbiota–gut–brain axis and suggests that this axis is perturbed in neuropsychiatric disorders. The gut microbiota regulates host exposure to its products by modulating gut epithelial and blood–brain permeability,1,2 both of which are altered in patients with major depressive disorder.3–6 In addition, patients with major depressive disorder have shown substantial shifts in both the relative abundance of taxa and the neuroactive metabolic potential of the gut microbiota, compared with healthy controls.7–12
Because of this compelling preclinical data,1–12 interventions affecting the microbiota–gut–brain axis are a potential treatment modality for depressive symptoms. Multiple systematic reviews have been conducted to assess the effect of microbiota-targeting interventions on depressive symptoms, but they include diverse populations and different study designs, include different subsets of the interventions targeting the gut microbiota and, not surprisingly, report conflicting findings.13–15 The objective of this study is to summarize the effect of microbiome-targeting interventions on depressive symptoms.
Methods
Design
We conducted a systematic review and meta-analysis, following Cochrane recommendations for best practice, and the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) for reporting.16,17 We registered the protocol with PROSPERO (ID: 143178). As domain knowledge was refined, so too was the protocol and analysis strategy, in consultation with domain experts; deviations from the registered protocol are outlined in Appendix 1, Section 1, available at www.cmajopen.ca/content/9/4/E1195/suppl/DC1. Briefly, we decided to focus on depressive symptoms, rather than all mental health outcomes, to enhance interpretability.
Search strategy
On July 3, 2019, we searched MEDLINE, Embase, PsycINFO, the Database of Abstracts of Reviews of Effects, Cochrane Database of Systematic Reviews and the Cochrane Controlled Register of Trials from inception; we updated our search on Mar. 5, 2021. We used search terms for gut microbiota-targeting interventions and depression, such as “probiotics” and “depression.” Search terms were intentionally broad, to avoid excluding relevant interventions or outcomes at this stage. We searched Medical Subject Headings (MeSH), text words and keywords (Appendix 1, Section 2). A research librarian developed the search strategy, which underwent Peer Review of Electronic Search Strategies (PRESS) review.18
We filtered search results to exclude studies published in a language other than English or French, those using animal models, and commentaries, editorials, letters and case reports. One author hand-searched reference lists of identified systematic reviews.
Study selection
Eight authors (M.H., J.L., L.E.D., B.F., L.M., O.E., R.D., N.C.A.C.) screened titles and abstracts independently and in duplicate. During title and abstract screening, we refined inclusion criteria in consultation with domain experts. To ensure that all abstract reviewers shared an understanding of the review objective, reviewers and domain experts calibrated with batches of 100 abstracts until 100% agreement was reached, before proceeding to review all remaining abstracts independently and in duplicate. We used the same procedure at full-text assessment, with batches of 10 full-texts assessed by the same 8 authors (M.H., J.L., L.E.D., B.F., L.M., O.E., R.D., N.C.A.C.). Any citation included by either reviewer proceeded to full-text review, which was also conducted independently and in duplicate. Reviewers discussed disagreements until consensus was reached.
We included randomized controlled trials that evaluated microbiome-targeting interventions (i.e., probiotic, prebiotic, synbiotic, paraprobiotic or fecal microbiota transplant) in adults aged 18 and older, that measured depressive symptoms with a validated scale and used a placebo or control comparator in which the active substance in the intervention was not administered (Table 1). We considered any study population for inclusion. To be considered a validated outcome, we required there to be a publication describing validity of each tool in any population (Appendix 1, Section 3).
Table 1:
Study inclusion criteria
| Criterion | Description |
|---|---|
| Population | Human, aged 18 years or older |
| Intervention | Probiotic (consumption of live microorganisms) Prebiotic (compound[s] to induce growth or activity of gut microbiota) Synbiotic (combination of probiotic and prebiotic) Paraprobiotic (sterilized or inactivated bacteria) Fecal microbiota transplant |
| Comparator | Placebo or control, defined by the absence of intervention |
| Outcome | Depressive symptoms, measured with a validated tool |
| Design | Randomized controlled trials |
Data extraction and quality assessment
In addition to assessing study quality, 8 authors (M.H., J.L., L.E.D., B.F., L.M., O.E., R.D., N.C.A.C.) used standardized forms to extract author, year, study design, population inclusion and exclusion criteria, follow-up, sample size, intervention(s), dose, additional supplements, depressive symptom outcome(s), independently and in duplicate. They also assessed study quality with the Cochrane Risk of Bias 2.0 tool.19 We used a hierarchy developed by an expert psychiatrist a priori to select an outcome from each study for inclusion in meta-analysis20 when the same mental health outcome was measured with more than 1 validated tool, whereby we prioritized observer-rated tools above self-rated tools, commonly used tools over less commonly used tools, and tools measuring specific symptoms over those measuring mixed symptoms.
Statistical analysis
We used random effects models with methods described by DerSimonian and Laird,21 as specified for meta-analysis a priori. We summarized effect size with the standardized mean difference of change scores after treatment, which expresses difference in effects between interventions in units of standard deviations. In accordance with the Cochrane Collaboration’s recommendations for best practice, we used Hedges’ g to correct for bias, often encountered in studies of small sample size.22 Where only pre- and post-treatment scores were provided, we used the conservative correlation coefficient of 0.5 to estimate change scores.23 We summarized heterogeneity quantitatively with I2.
We conducted the meta-analysis and generated forest plots with the “metafor” package for R statistical software, and generated figures with the “ggplot2” package. We considered participant populations with a diagnosis of depression at baseline separately from participant populations where the presence of depression at baseline was not specified. In a sensitivity analysis, we removed studies deemed high risk of bias from estimates of effect. We visually inspected funnel plots for publication bias, and supplemented with trim and fill analysis.24
Ethics approval
Because this analysis uses only previously published data, ethics approval was not required.
Results
We identified 33 757 unique records. After abstract review, we assessed 231 full texts for eligibility, including 17 records identified through hand-searching. Of the full texts, we excluded 169 for the following reasons: not adult population (n = 7), intervention or comparator not of interest (n = 11), outcome not of interest (n = 76), study design not of interest (n = 53), abstract only or conference proceeding (n = 10), duplicate (n = 11) and not available in English or French (n = 1) (Figure 1). Reasons for full text exclusion are in Appendix 1, Section 4. The final data set included 62 studies with 5059 patients.
Figure 1:
Flow diagram, based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.16
Study characteristics
Characteristics of included studies can be found in Appendix 1, Section 5. The most common intervention type was probiotics (n = 51),25–75 followed by synbiotics (n = 7),64,72,76–80 prebiotics (n = 7),31,64,73,81–84 paraprobiotics (n = 1)85 and fecal microbiota transplant (n = 1 study).86 Four studies included more than 1 active intervention, with each intervention included separately in our meta-analysis.31,64,72,73 Sixteen distinct tools were used to evaluate depressive symptoms. The most used tools were the Hospital Anxiety and Depression Scale — Depression score (n = 18) and the Beck Depression Inventory (n = 16).
We excluded 2 studies from the meta-analysis, given the lack of studies with the same intervention or population with which to pool effect sizes.76,86 Of the 50 studies included in the meta-analysis, the intervention was a probiotic in 44 studies (n = 9 in populations with depression, n = 35 in populations without depression), a prebiotic in 5 studies (n = 3 in populations with depression, n = 2 in populations without depression) and a synbiotic in 6 studies (all in populations without depression).
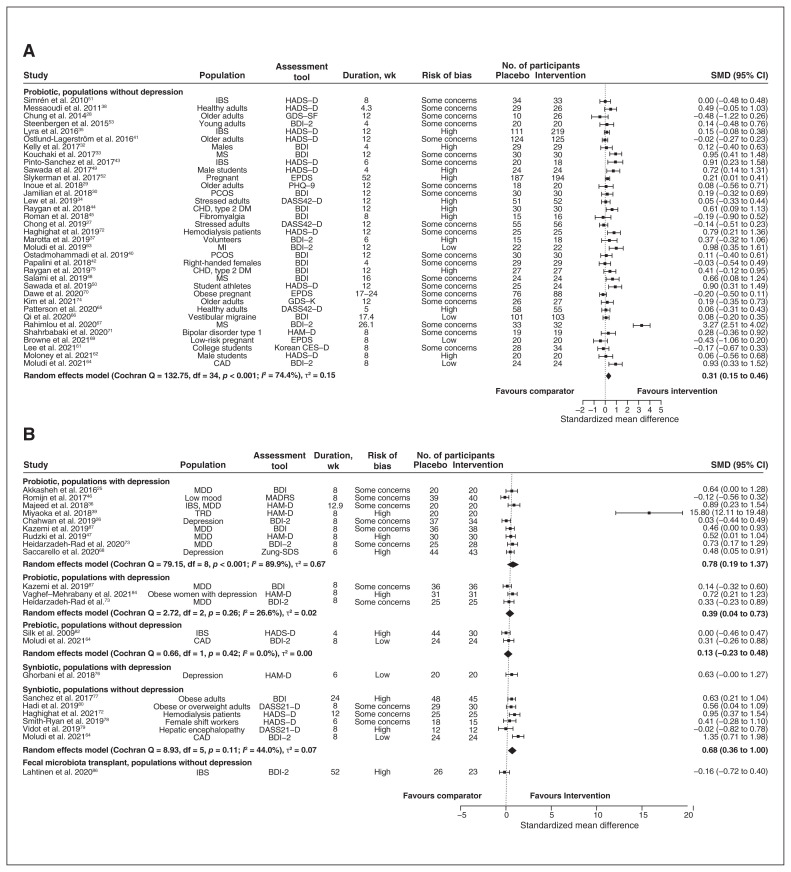
One study evaluated synbiotics in a population with depression,76 and another evaluated fecal microbiota transplant in a population without depression;86 neither of these had other studies with which to pool effect estimates. These 2 studies presented sufficient information for meta-analysis and are therefore included in Figure 2.
Figure 2:
Forest plot of (A) probiotic interventions in populations without depression, (B) probiotic interventions in populations with depression, prebiotic interventions in populations with and without depression, synbiotic interventions in populations with and without depression, and fecal microbiota transplant interventions in populations without depression. Note: BDI = Beck Depression Inventory, CAD = coronary artery disease, CES-D = Centre for Epidemiological Studies Depression Scale, CHD = coronary heart disease, CI = confidence interval, DASS21-D = Depression Anxiety and Stress Scales – 21 Items, Depression Scale, DASS42-D = Depression Anxiety Stress Scales – 42 Items, Depression Scale, DM = diabetes mellitus, EPDS = Edinburgh Postnatal Depression Scale, GDS-K = Geriatric Depression Scale – Korean Version, GDS-SF = Geriatric Depression Scale – Short Form, HADS-D = Hospital Anxiety and Depression Scale — Depression score, HAM-D = Hamilton Depression Rating Scale, IBS = irritable bowel syndrome, MADRS = Montgomery–Åsberg Depression Rating Scale, MDD = major depressive disorder, MI = myocardial infarction, MS = multiple sclerosis, PCOS = polycystic ovary syndrome, PHQ-9 = Patient Health Questionnaire – 9, SMD = standardized mean difference, TRD = treatment-resistant depression, Zung-SDS = Zung Self-Rating Depression Scale.
The remaining 10 studies failed to present necessary information for inclusion in meta-analysis. Of these 10 studies, 7 studies evaluated a probiotic, 2 studies evaluated a prebiotic and 1 study evaluated a paraprobiotic (Appendix 1, Section 6). None of the studies that had insufficient information for meta-analysis reported statistically significant differences from interventions.
Probiotic interventions
Among studies with probiotic interventions, defined as consumption of live microorganisms, the most common genera of bacteria administered were Lactobacillus (n = 41) and Bifidobacterium (n = 29). Other genera administered were Bacillus, Clostridium, Lactococcus, Streptococcus, Weisella and Lacticaseibacillus. Twenty-four studies administered probiotics from more than 1 genus. Among 9 studies with participants with depression, probiotic interventions offered statistically significant benefits (Hedges’ g 0.78, 95% confidence interval [CI] 0.19 to 1.37, τ2 = 0.67, I2 = 89.9%) (Figure 2).
One study, a visual outlier in Figure 2, was unique in the administration of Clostridium.39 This study by Miyaoka and colleagues39 was also unique in the requirement that participants with treatment-resistant depression be on a stable dose of selective serotonin reuptake inhibitor or serotonin–noradrenalin reuptake inhibitor for at least 1 month before enrolment. Exclusion of the visual outlier resulted in an effect size of 0.41 (95% CI 0.17 to 0.65, τ2 = 0.05, I2 = 42.9%), with markedly reduced heterogeneity and between-study variance.
In 35 studies that enrolled participants without depression, probiotics also offered statistically significant benefits (Hedges’ g 0.31, 95% CI 0.15 to 0.46, τ2 = 0.15, I2 = 74.4%) (Figure 2).
Prebiotic interventions
We identified 7 studies evaluating the effect of prebiotic interventions, or compounds in food that induce growth or activity of gut microbiota.64,73,81–84,87 Three studies with prebiotic interventions enrolled participants with depression, with statistically significant benefits (Hedges’ g 0.39, 95% CI 0.04 to 0.73, τ2 = 0.02, I2 = 26.6%) (Figure 2). Among 2 studies enrolling participants without depression, we did not observe any statistically significant effects (Hedges’ g 0.13, 95% CI −0.23 to 0.48, τ2 = 0.00, I2 = 00.0%) (Figure 2).
Synbiotic interventions
Seven studies evaluated the effects of synbiotics, or combinations of prebiotics and probiotics.64,72,76–80 In the meta-analysis of 6 study populations without depression, synbiotic interventions offered statistically significant benefit (Hedges’ g 0.68, 95% CI 0.36 to 1.00, τ2 = 0.07, I2 = 44.0%). The seventh study,76 conducted in participants with depression, did not find a significant effect (standardized mean difference 0.63, 95% CI −0.002 to 1.27) (Figure 2).
Paraprobiotics
One trial evaluated the effect of paraprobiotics, or sterilized bacteria, and reported no statistically significant effect of intervention when measured with the Hospital Anxiety and Depression Scale — Depression score.85
Fecal microbiota transplant
We identified 1 trial that evaluated the effect of fecal microbiota transplant.86 In this study, patients with irritable bowel syndrome were randomized to autologous or allogenic fecal microbiota transplant via colonoscopy.86 There were no differences in depressive symptoms, as measured with the Beck Depression Inventory, when compared with baseline or between groups at any time point (Figure 2).86
Risk of bias
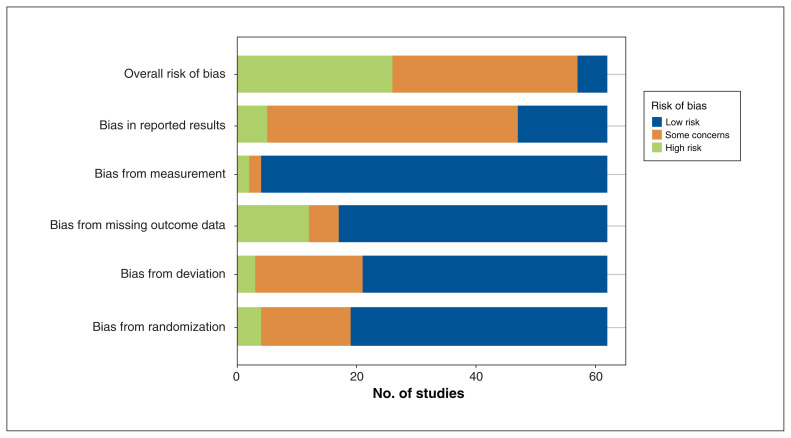
Although many studies were deemed low risk of bias in multiple domains, only 5 trials were deemed low risk of bias overall (Figure 3) (Appendix 1, Section 7).63,64,66,69,76 Most studies were low risk of bias for their approach to measurement, but the study by Miyaoka and colleagues39 was deemed high risk of bias in this domain given the lack of blinding.
Figure 3:
Risk of bias for included studies, assessed with the Cochrane Risk of Bias tool, version 2.0.19
Sensitivity analysis
After removing studies deemed “high” risk of bias from meta-analysis, estimates of effect for probiotics in populations without depression and for prebiotics in populations with depression were similar to base case estimates. The magnitude of effect for probiotics in populations with depression was markedly smaller, with reduced between-study variance and heterogeneity. The magnitude of effect for synbiotics in populations without depression was larger than base case estimates, with reduced between-study variance and heterogeneity. Notably, we observed a statistically significant benefit in all analyses involving participants with depression (Table 2).
Table 2:
Summary of analyses
| Variable | Base case estimates | Estimates, excluding Miyaoka et al.39 | Estimates, excluding studies deemed high risk of bias | Trim and fill analysis | Trim and fill analysis, excluding Miyaoka et al.39 |
|---|---|---|---|---|---|
| Probiotic interventions | |||||
| Participants with depression |
9 studies Hedges’ g 0.78 (95% CI 0.19 to 1.37) τ2 = 0.67 I2 = 89.9% |
8 studies Hedges’ g 0.41 (95% CI 0.17 to 0.65) τ2 = 0.05 I2 = 42.9% |
6 studies Hedges’ g 0.39 (95% CI 0.07 to 0.72) τ2 = 0.09 I2 = 57.0% |
9 studies; 0 missing Hedges’ g 0.39 (95% CI 0.19 to 1.37) τ2 = 0.67 I2 = 89.9% |
9 studies; 2 missing Hedges’ g 0.31 (95% CI 0.08 to 0.55) τ2 = 0.07 I2 = 50.4% |
| Participants without depression |
35 studies Hedges’ g 0.31 (95% CI 0.15 to 0.46) τ2 = 0.15 I2 = 74.4% |
NA |
24 studies Hedges’ g 0.36 (95% CI 0.13 to 0.59) τ2 = 0.26 I2 = 81.4% |
35 studies; 0 missing Hedges’ g 0.31 (95% CI 0.15 to 0.46) τ2 = 0.15 I2 = 74.4% |
NA |
| Prebiotic interventions | |||||
| Participants with depression |
3 studies Hedges’ g 0.39 (95% CI 0.04 to 0.73) τ2 = 0.02 I2 = 26.6% |
NA |
2 studies Hedges’ g 0.41 (95% CI 0.17 to 0.65) τ2 = 0.05 I2 = 42.9% |
NA | NA |
| Participants without depression |
2 studies Hedges’ g 0.78 (95% CI 0.19 to 1.37) τ2 = 0.67 I2 = 89.9% |
NA | NA | NA | NA |
| Synbiotic interventions | |||||
| Participants without depression |
6 studies Hedges’ g 0.68 (95% CI 0.36 to 1.00) τ2 = 0.67 I2 = 89.9% |
NA |
4 studies Hedges’ g 0.82 (95% CI 0.42 to 1.21) τ2 = 0.07 I2 = 41.7% |
6 studies; 1 missing Hedges’ g 0.77 (95% CI 0.43 to 1.11) τ2 = 0.11 I2 = 54.1% |
NA |
Note: CI = confidence interval, NA = not applicable.
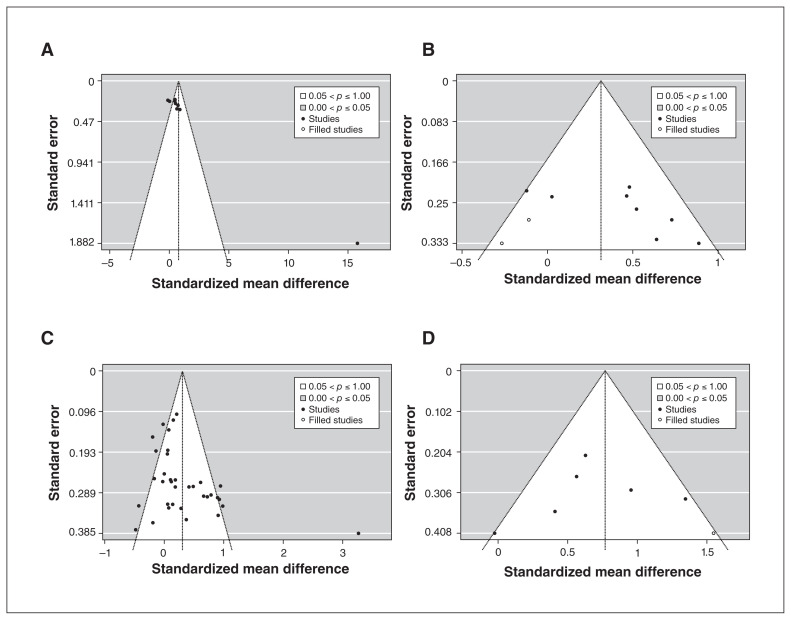
All 4 funnel plots show that few studies found intervention benefits with small standard error, suggesting the presence of publication bias (Figure 4). In our trim and fill analysis, excluding the study by Miyaoka and colleagues,39 2 missing studies are estimated on the left side of the funnel plot, with an effect estimate of 0.31 (95% CI 0.08–0.55, τ2 = 0.07, I2 = 50.4%) in participants with depression (Figure 4B). In our trim and fill analysis of synbiotic interventions in populations without depression, 1 missing study is estimated on the right side of the funnel plot, with an effect estimate of 0.77 (95% CI 0.43 to 1.11, τ2 = 0.11, I2 = 54.1%). For other meta-analyses, there were insufficient studies to generate meaningful funnel plots.
Figure 4:
Funnel plots from trim and fill analysis of probiotic interventions in populations with depression, with (A) and without (B) study by Miyaoka et al.,39 and of (C) probiotic and (D) synbiotic interventions in populations without depression.
Interpretation
This meta-analysis suggests a statistically significant benefit of probiotic, prebiotic and synbiotic interventions for depressive symptoms in study populations, both with and without depression. None of the studies excluded from the meta-analysis for lack of required information showed statistically significant evidence of benefit. In the single studies evaluating fecal microbiota transplant and paraprobiotic interventions, the interventions did not show statistically significant benefits.85,86 The body of evidence included in this systematic review is hindered by heterogeneous study quality and the likely presence of publication bias.
The lack of statistically significant evidence of benefit in many single studies may be from the measurement of depressive symptoms as a secondary outcome. Studies are rarely powered for measurement of secondary outcomes and, in the case of a small-to-medium effect size, they are underpowered to detect differences. If this is the case for studies examining paraprobiotic interventions or fecal microbiota transplants, further study and additional meta-analysis will be useful to improve precision in estimates of effect.
Effect sizes for synbiotic interventions were larger than for prebiotic or probiotic interventions, suggesting that the combination of interventions holds greater promise than solely prebiotic or probiotic interventions. Although complicated by risk of bias in included studies and the likely presence of publication bias, the magnitude of effect for synbiotic interventions in participants without depression is nearly the sum of prebiotic and probiotic interventions. Unfortunately, too few studies existed for meta-analysis of effects in participants with depression.
The effect of the probiotic intervention reported by Miyaoka and colleagues39 was an outlier. This was the only study administering adjunctive Clostridium to patients already being treated with antidepressant medications, for which change in depressive symptoms was a primary outcome. When this study was excluded, estimated effect sizes between groups with or without depression were of similar magnitude, with confidence intervals that overlap almost entirely. Bifidobacterium- and Lactobacillus-containing probiotics are produced commercially, are widely available and were used as the probiotic interventions in most included studies. The effect size estimated when excluding the study by Miyaoka and colleagues39 may better reflect those achievable with commercially available products.
Although many studies evaluated effect sizes for similar species of bacteria, the 1 study that used Clostridium showed a far greater effect,39 raising questions about why the body of literature is fixated on the same bacteria. Rather than focusing on interventions with limited potential for patient benefit, this would suggest broadening the scope of study to first identify the types of interventions most likely to produce positive effects. Caution is warranted in interpreting the magnitude of effect estimates, given their susceptibility to bias in individual studies and the likely presence of publication bias in the body of literature as a whole.
Limitations
The primary limitation of this work is likely the high-level evidence synthesis. The standardized mean difference assumes that the same outcome is measured in each study. Many of the tools used to evaluate depressive symptoms assess slightly different facets of the same phenomenon, with substantial overlap. Definitive estimates of efficacy are hindered by heterogeneity of treatment, dosage, study populations and risk of bias. However, a strength of this review is that the tools used to measure outcomes were not part of inclusion criteria; therefore, we captured all validated tools measuring depressive symptoms.
We limited searches to English and French to reduce the number of records screened. Although this strategy may have removed relevant articles in other languages, evidence suggests that language bias does not systematically affect meta-analysis findings beyond reduced precision.88 Because our objective was to summarize evidence, we elected to stay within the confines of published literature. Therefore, we did not contact authors for studies not presenting sufficient information for inclusion in meta-analysis.
Conclusion
Our objective was to summarize the effects of interventions targeting gut microbiota on depressive symptoms. This body of evidence is hindered by heterogeneous study quality and the likely presence of publication bias. Although findings are promising, there is not yet strong enough evidence to favour inclusion of these interventions in treatment guidelines for depression. Critical questions about species administered, dosage and timing relative to other antidepressant medications remain to be answered.
Supplementary Material
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Mark Hofmeister, Fiona Clement, Scott Patten, Laura Dowsett and Valerie Taylor contributed to the conception and design of the work. Mark Hofmeister, Fiona Clement, Joyce Li, Laura Dowsett, Brenlea Farkas, Liza Mastikhina, Oluwaseun Egunsola, Ruth Diaz and Noah Cooke contributed to the acquisition, analysis and interpretation of data. All of the authors drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This work was supported by an Alberta Addiction and Mental Health Research Hub’s Depression Knowledge Translation Research Priority Grant. Scott Patten is supported by the Cuthbertson and Fisher Chair in Pediatric Mental Health. Funders were not involved in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data sharing: The present study used aggregate, published data that are publicly available. No additional data are available for sharing.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/9/4/E1195/suppl/DC1.
References
- 1.Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powell N, Walker MM, Talley NJ. The mucosal immune system: master regulator of bidirectional gut-brain communications. Nat Rev Gastroenterol Hepatol. 2017;14:143–59. doi: 10.1038/nrgastro.2016.191. [DOI] [PubMed] [Google Scholar]
- 3.Najjar S, Pearlman DM, Devinsky O, et al. Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: a review of clinical and experimental evidence. J Neuroinflammation. 2013;10:142. doi: 10.1186/1742-2094-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Najjar S, Pearlman DM, Hirsch S, et al. Brain biopsy findings link major depressive disorder to neuroinflammation, oxidative stress, and neurovascular dysfunction: a case report. Biol Psychiatry. 2014;75:e23–6. doi: 10.1016/j.biopsych.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 5.Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuroendocrinol Lett. 2008;29:117–24. [PubMed] [Google Scholar]
- 6.Maes M, Kubera M, Leunis J-C, et al. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J Affect Disord. 2012;141:55–62. doi: 10.1016/j.jad.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–94. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Naseribafrouei A, Hestad K, Avershina E, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155–62. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- 9.Valles-Colomer M, Falony G, Darzi Y, et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat Microbiol. 2019;4:623–32. doi: 10.1038/s41564-018-0337-x. [DOI] [PubMed] [Google Scholar]
- 10.Lin P, Ding B, Feng C, et al. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J Affect Disord. 2017;207:300–4. doi: 10.1016/j.jad.2016.09.051. [DOI] [PubMed] [Google Scholar]
- 11.Madan A, Thompson D, Fowler JC, et al. The gut microbiota is associated with psychiatric symptom severity and treatment outcome among individuals with serious mental illness. J Affect Disord. 2020;264:98–106. doi: 10.1016/j.jad.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Stevens BR, Roesch L, Thiago P, et al. Depression phenotype identified by using single nucleotide exact amplicon sequence variants of the human gut microbiome. Mol Psychiatry. 2021;26:4277–87. doi: 10.1038/s41380-020-0652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace CJK, Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry. 2017;16:14. doi: 10.1186/s12991-017-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikolova V, Zaidi SY, Young AH, et al. Gut feeling: randomized controlled trials of probiotics for the treatment of clinical depression: systematic review and meta-analysis. Ther Adv Psychopharmacol. 2019;9:2045125319859963. doi: 10.1177/2045125319859963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13–23. doi: 10.1016/j.neubiorev.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J, Thomas J, Chandler J, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. London (UK): Cochrane; updated February 2021. [Google Scholar]
- 18.McGowan J, Sampson M, Salzwedel DM, et al. PRESS peer review of electronic search strategies: 2015 Guideline explanation and elaboration (PRESS E&E) Ottawa: Canadian Agency for Drugs and Technologies in Health; 2016. [accessed 2019 July 30]. Available: https://www.cadth.ca/sites/default/files/pdf/CP0015_PRESS_Update_Report_2016.pdf. [Google Scholar]
- 19.Higgins JPT, Savović J, Page MJ, et al., editors. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0) London (UK): Cochrane; 2016. [accessed 2019 Dec 31]. Available: https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/archive-rob-2-0-2016. [Google Scholar]
- 20.Watt JA, Goodarzi Z, Veroniki AA, et al. Comparative efficacy of interventions for reducing symptoms of depression in people with dementia: systematic review and network meta-analysis. BMJ. 2021:372. doi: 10.1136/bmj.n532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Cochrane Handbook for Systematic Reviews of Interventions Version 510. London (UK): Cochrane; 2011. [accessed 2020 Jan. 6]. The standardized mean difference. Available: https://handbook-5-1.cochrane.org/chapter_9/9_2_3_2_the_standardized_mean_difference.htm. [Google Scholar]
- 23.Follmann D, Elliot P, Suh I, et al. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol. 1992;45:769–73. doi: 10.1016/0895-4356(92)90054-q. [DOI] [PubMed] [Google Scholar]
- 24.Cochrane Handbook for Systematic Reviews of Interventions Version 510. London (UK): Cochrane; 2011. [accessed 2020 May 8]. Trim and fill. Available: https://handbook-5-1.cochrane.org/chapter_10/10_4_4_2_trim_and_fill.htm. [Google Scholar]
- 25.Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition. 2016;32:315–20. doi: 10.1016/j.nut.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Chahwan B, Kwan S, Isik A, et al. Gut feelings: a randomised, triple-blind, placebo-controlled trial of probiotics for depressive symptoms. J Affect Disord. 2019;253:317–26. doi: 10.1016/j.jad.2019.04.097. [DOI] [PubMed] [Google Scholar]
- 27.Chong HX, Yusoff’l NAA, Hor Y-Y, et al. Lactobacillus plantarum DR7 alleviates stress and anxiety in adults: a randomised, double-blind, placebo-controlled study. Benef Microbes. 2019;10:355–73. doi: 10.3920/BM2018.0135. [DOI] [PubMed] [Google Scholar]
- 28.Chung Y-C, Jin H-M, Cui Y, et al. Fermented milk of Lactobacillus helveticus IDCC3801 improves cognitive functioning during cognitive fatigue tests in healthy older adults. J Funct Foods. 2014;10:465–74. [Google Scholar]
- 29.Inoue T, Kobayashi Y, Mori N, et al. Effect of combined bifidobacteria supplementation and resistance training on cognitive function, body composition and bowel habits of healthy elderly subjects. Benef Microbes. 2018;9:843–53. doi: 10.3920/BM2017.0193. [DOI] [PubMed] [Google Scholar]
- 30.Jamilian M, Mansury S, Bahmani F, et al. The effects of probiotic and selenium co-supplementation on parameters of mental health, hormonal profiles, and biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome. J Ovarian Res. 2018;11:80. doi: 10.1186/s13048-018-0457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kazemi A, Noorbala AA, Azam K, et al. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: a randomized clinical trial. Clin Nutr. 2019;38:522–8. doi: 10.1016/j.clnu.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Kelly JR, Allen AP, Temko A, et al. Lost in translation? The potential psychobiotic Lactobacillus rhamnosus (JB-1) fails to modulate stress or cognitive performance in healthy male subjects. Brain Behav Immun. 2017;61:50–9. doi: 10.1016/j.bbi.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 33.Kouchaki E, Tamtaji OR, Salami M, et al. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2017;36:1245–9. doi: 10.1016/j.clnu.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Lew L-C, Hor Y-Y, Yusoff NAA, et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: a randomised, double-blind, placebo-controlled study. Clin Nutr. 2019;38:2053–64. doi: 10.1016/j.clnu.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Lyra A, Hillilä M, Huttunen T, et al. Irritable bowel syndrome symptom severity improves equally with probiotic and placebo. World J Gastroenterol. 2016;22:10631–42. doi: 10.3748/wjg.v22.i48.10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majeed M, Nagabhushanam K, Arumugam S, et al. Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: a randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr Res. 2018;62 doi: 10.29219/fnr.v62.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marotta A, Sarno E, Del Casale A, et al. Effects of probiotics on cognitive reactivity, mood, and sleep quality. Front Psychiatry. 2019;10:164. doi: 10.3389/fpsyt.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Messaoudi M, Lalonde R, Violle N, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105:755–64. doi: 10.1017/S0007114510004319. [DOI] [PubMed] [Google Scholar]
- 39.Miyaoka T, Kanayama M, Wake R, et al. Clostridium butyricum MIYAIRI 588 as adjunctive therapy for treatment-resistant major depressive disorder: a prospective open-label trial. Clin Neuropharmacol. 2018;41:151–5. doi: 10.1097/WNF.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 40.Ostadmohammadi V, Jamilian M, Bahmani F, et al. Vitamin D and probiotic co-supplementation affects mental health, hormonal, inflammatory and oxidative stress parameters in women with polycystic ovary syndrome. J Ovarian Res. 2019;12:5. doi: 10.1186/s13048-019-0480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Östlund-Lagerström L, Kihlgren A, Repsilber D, et al. Probiotic administration among free-living older adults: a double blinded, randomized, placebo-controlled clinical trial. Nutr J. 2016;15:80. doi: 10.1186/s12937-016-0198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papalini S, Michels F, Kohn N, et al. Stress matters: randomized controlled trial on the effect of probiotics on neurocognition. Neurobiol Stress. 2018;10:100141. doi: 10.1016/j.ynstr.2018.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinto-Sanchez MI, Hall GB, Ghajar K, et al. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153:448–59e8. doi: 10.1053/j.gastro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Raygan F, Ostadmohammadi V, Bahmani F, et al. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2018;84:50–5. doi: 10.1016/j.pnpbp.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Roman P, Estévez AF, Miras A, et al. A pilot randomized controlled trial to explore cognitive and emotional effects of probiotics in fibromyalgia. Sci Rep. 2018;8:10965. doi: 10.1038/s41598-018-29388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romijn AR, Rucklidge JJ, Kuijer RG, et al. A double-blind, randomized, placebo-controlled trial of Lactobacillus helveticus and Bifidobacterium longum for the symptoms of depression. Aust N Z J Psychiatry. 2017;51:810–21. doi: 10.1177/0004867416686694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rudzki L, Ostrowska L, Pawlak D, et al. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology. 2019;100:213–22. doi: 10.1016/j.psyneuen.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 48.Salami M, Kouchaki E, Asemi Z, et al. How probiotic bacteria influence the motor and mental behaviors as well as immunological and oxidative biomarkers in multiple sclerosis? a double blind clinical trial. J Funct Foods. 2019;52:8–13. [Google Scholar]
- 49.Sawada D, Kawai T, Nishida K, et al. Daily intake of Lactobacillus gasseri CP2305 improves mental, physical, and sleep quality among Japanese medical students enrolled in a cadaver dissection course. J Funct Foods. 2017;31:188–97. [Google Scholar]
- 50.Sawada D, Kuwano Y, Tanaka H, et al. Daily intake of Lactobacillus gasseri CP2305 relieves fatigue and stress-related symptoms in male university Ekiden runners: a double-blind, randomized, and placebo-controlled clinical trial. J Funct Foods. 2019;57:465–76. [Google Scholar]
- 51.Simrén M, Ohman L, Olsson J, et al. Clinical trial: the effect of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome (IBS)L a randomized, double-blind, controlled study. Aliment Pharmacol Ther. 2010;31:218–27. doi: 10.1111/j.1365-2036.2009.04183.x. [DOI] [PubMed] [Google Scholar]
- 52.Slykerman RF, Hood F, Wickens K, et al. Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine. 2017;24:159–65. doi: 10.1016/j.ebiom.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steenbergen L, Sellaro R, van Hemert S, et al. A randomized controlled trial to test the effect of multispecies probiotics on cognitive reactivity to sad mood. Brain Behav Immun. 2015;48:258–64. doi: 10.1016/j.bbi.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Cremon C, Guglielmetti S, Gargari G, et al. Effect of Lactobacillus paracasei CNCM I-1572 on symptoms, gut microbiota, short chain fatty acids, and immune activation in patients with irritable bowel syndrome: a pilot randomized clinical trial. United European Gastroenterol J. 2018;6:604–13. doi: 10.1177/2050640617736478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dickerson F, Adamos M, Katsafanas E, et al. Adjunctive probiotic microorganisms to prevent rehospitalization in patients with acute mania: a randomized controlled trial. Bipolar Disord. 2018;20:614–21. doi: 10.1111/bdi.12652. [DOI] [PubMed] [Google Scholar]
- 56.Rao AV, Bested AC, Beaulne TM, et al. A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome. Gut Pathog. 2009;1:6. doi: 10.1186/1757-4749-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–401. 1401.e1–4. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:1581–90. doi: 10.1111/j.1572-0241.2006.00734.x. [DOI] [PubMed] [Google Scholar]
- 59.Wong RK, Yang C, Song G-H, et al. Melatonin regulation as a possible mechanism for probiotic (VSL#3) in irritable bowel syndrome: a randomized double-blinded placebo study. Dig Dis Sci. 2015;60:186–94. doi: 10.1007/s10620-014-3299-8. [DOI] [PubMed] [Google Scholar]
- 60.Kato-Kataoka A, Nishida K, Takada M, et al. Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Benef Microbes. 2016;7:153–6. doi: 10.3920/BM2015.0100. [DOI] [PubMed] [Google Scholar]
- 61.Lee D-S, Kim M, Nam S-H, et al. Effects of oral probiotics on subjective halitosis, oral health, and psychosocial health of college students: a randomized, double-blind, placebo-controlled study. Int J Environ Res Public Health. 2021;18:1143. doi: 10.3390/ijerph18031143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moloney GM, Long-Smith CM, Murphy A, et al. Improvements in sleep indices during exam stress due to consumption of a Bifidobacterium longum. Brain Behav Immun Health. 2021;10:100174. doi: 10.1016/j.bbih.2020.100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moludi J, Alizadeh M, Mohammadzad MHS, et al. The effect of probiotic supplementation on depressive symptoms and quality of life in patients after myocardial infarction: results of a preliminary double-blind clinical trial. Psychosom Med. 2019;81:770–7. doi: 10.1097/PSY.0000000000000749. [DOI] [PubMed] [Google Scholar]
- 64.Moludi J, Khedmatgozar H, Nachvak SM, et al. The effects of co-administration of probiotics and prebiotics on chronic inflammation, and depression symptoms in patients with coronary artery diseases: a randomized clinical trial. Nutr Neurosci. 2021 Feb 28; doi: 10.1080/1028415X.2021.1889451. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 65.Patterson E, Griffin SM, Ibarra A, et al. Lacticaseibacillus paracasei Lpc-37 R improves psychological and physiological markers of stress and anxiety in healthy adults: a randomized, double-blind, placebo-controlled and parallel clinical trial (the Sisu study) Neurobiol Stress. 2020;13:100277. doi: 10.1016/j.ynstr.2020.100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qi X, Fan G, Jia H. The probiotic Lactobacillus casei Shirota attenuates symptoms of vestibular migraine: a randomised placebo-controlled double-blind clinical trial. Benef Microbes. 2020;11:469–76. doi: 10.3920/BM2020.0058. [DOI] [PubMed] [Google Scholar]
- 67.Rahimlou M, Hosseini SA, Majdinasab N, et al. Effects of long-term administration of Multi-Strain Probiotic on circulating levels of BDNF, NGF, IL-6 and mental health in patients with multiple sclerosis: a randomized, doubleblind, placebo-controlled trial. Nutr Neurosci. 2020 June 5; doi: 10.1080/1028415X.2020.1758887. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 68.Saccarello A, Montarsolo P, Massardo I, et al. Oral administration of S-Adenosylmethionine (SAMe) and Lactobacillus plantarum HEAL9 improves the mild-to-moderate symptoms of depression: a randomized, double-blind, placebo-controlled study. Prim Care Companion CNS Disord. 2020:22. doi: 10.4088/PCC.19m02578. [DOI] [PubMed] [Google Scholar]
- 69.Browne PD, Bolte AC, Besseling-van der Vaart I, et al. Probiotics as a treatment for prenatal maternal anxiety and depression: a double-blind randomized pilot trial. Sci Rep. 2021;11:3051. doi: 10.1038/s41598-021-81204-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dawe JP, McCowan LME, Wilson J, et al. Probiotics and maternal mental health: a randomised controlled trial among pregnant women with obesity. Sci Rep. 2020;10:1291. doi: 10.1038/s41598-020-58129-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shahrbabaki ME, Sabouri S, Sabahi A, et al. The efficacy of probiotics for treatment of bipolar disorder-Type 1: a randomized, double-blind, placebo controlled trial. Iran J Psychiatry. 2020;15:10–6. [PMC free article] [PubMed] [Google Scholar]
- 72.Haghighat N, Rajabi S, Mohammadshahi M. Effect of synbiotic and probiotic supplementation on serum brain-derived neurotrophic factor level, depression and anxiety symptoms in hemodialysis patients: a randomized, double-blinded, clinical trial. Nutr Neurosci. 2021;24:490–9. doi: 10.1080/1028415X.2019.1646975. [DOI] [PubMed] [Google Scholar]
- 73.Heidarzadeh-Rad N, Gökmen-Özel H, Kazemi A, et al. Effects of a psychobiotic supplement on serum brain-derived neurotrophic factor levels in depressive patients: a post hoc analysis of a randomized clinical trial. J Neurogastroenterol Motil. 2020;26:486–95. doi: 10.5056/jnm20079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim C-S, Cha L, Sim M, et al. Probiotic supplementation improves cognitive function and mood with changes in gut microbiota in community-dwelling older adults: a randomized, double-blind, placebo-controlled, multicenter trial. J Gerontol A Biol Sci Med Sci. 2021;76:32–40. doi: 10.1093/gerona/glaa090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Raygan F, Ostadmohammadi V, Asemi Z. The effects of probiotic and selenium co-supplementation on mental health parameters and metabolic profiles in type 2 diabetic patients with coronary heart disease: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38:1594–8. doi: 10.1016/j.clnu.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 76.Ghorbani Z, Nazari S, Etesam F, et al. The effect of synbiotic as an adjuvant therapy to fluoxetine in moderate depression: a randomized multicenter trial. Arch Neurosci. 2018;5:e60507. [Google Scholar]
- 77.Sanchez M, Darimont C, Panahi S, et al. Effects of a diet-based weight-reducing program with probiotic supplementation on satiety efficiency, eating behaviour traits, and psychosocial behaviours in obese individuals. Nutrients. 2017;9:284. doi: 10.3390/nu9030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith-Ryan AE, Mock MG, Trexler ET, et al. Influence of a multistrain probiotic on body composition and mood in female occupational shift workers. Appl Physiol Nutr Metab. 2019;44:765–73. doi: 10.1139/apnm-2018-0645. [DOI] [PubMed] [Google Scholar]
- 79.Vidot H, Cvejic E, Finegan LJ, et al. Supplementation with synbiotics and/or branched chain amino acids in hepatic encephalopathy: a pilot randomised placebo-controlled clinical study. Nutrients. 2019;11:1810. doi: 10.3390/nu11081810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hadi A, Sepandi M, Marx W, et al. Clinical and psychological responses to synbiotic supplementation in obese or overweight adults: a randomized clinical trial. Complement Ther Med. 2019;47:102216. doi: 10.1016/j.ctim.2019.102216. [DOI] [PubMed] [Google Scholar]
- 81.Azpiroz F, Dubray C, Bernalier-Donadille A, et al. Effects of scFOS on the composition of fecal microbiota and anxiety in patients with irritable bowel syndrome: a randomized, double blind, placebo controlled study. Neurogastroenterol Motil. 2017;29 doi: 10.1111/nmo.12911. [DOI] [PubMed] [Google Scholar]
- 82.Silk DBA, Davis A, Vulevic J, et al. Clinical trial: the effects of a transgalactooligosaccharide prebiotic on faecal microbiota and symptoms in irritable bowel syndrome. Aliment Pharmacol Ther. 2009;29:508–18. doi: 10.1111/j.1365-2036.2008.03911.x. [DOI] [PubMed] [Google Scholar]
- 83.Smith AP. The concept of well-being: relevance to nutrition research. Br J Nutr. 2005;93:S1–5. doi: 10.1079/bjn20041351. [DOI] [PubMed] [Google Scholar]
- 84.Vaghef-Mehrabany E, Ranjbar F, Asghari-Jafarabadi M, et al. Calorie restriction in combination with prebiotic supplementation in obese women with depression: effects on metabolic and clinical response. Nutr Neurosci. 2021;24:339–53. doi: 10.1080/1028415X.2019.1630985. [DOI] [PubMed] [Google Scholar]
- 85.Nishida K, Sawada D, Kawai T, et al. Para-psychobiotic Lactobacillus gasseri CP 2305 ameliorates stress-related symptoms and sleep quality. J Appl Microbiol. 2017;123:1561–70. doi: 10.1111/jam.13594. [DOI] [PubMed] [Google Scholar]
- 86.Lahtinen P, Jalanka J, Hartikainen A, et al. Randomised clinical trial: faecal microbiota transplantation versus autologous placebo administered via colonoscopy in irritable bowel syndrome. Aliment Pharmacol Ther. 2020;51:1321–31. doi: 10.1111/apt.15740. [DOI] [PubMed] [Google Scholar]
- 87.Kazemi A, Noorbala AA, Azam K, et al. Effect of prebiotic and probiotic supplementation on circulating pro-inflammatory cytokines and urinary cortisol levels in patients with major depressive disorder: a double-blind, placebo-controlled randomized clinical trial. J Funct Foods. 2019;52:596–602. [Google Scholar]
- 88.Morrison A, Polisena J, Husereau D, et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28:138–44. doi: 10.1017/S0266462312000086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.