Abstract
Staphylococcus aureus can be a harmless coloniser, but it can also cause severe infections in humans, livestock and wildlife. Regarding the latter, only few studies have been performed and knowledge on virulence factors is insufficient. The aim of the present study was to study S. aureus isolates from deceased wild beavers (Castor fiber). Seventeen isolates from eleven beavers, found in Germany and Austria, were investigated. Antimicrobial and biocide susceptibility tests were performed. Isolates were characterised using S. aureus-specific DNA microarrays, spa typing and whole-genome sequencing. From two isolates, prophages were induced by mitomycin C and studied by transmission electron microscopy. Four isolates belonged to clonal complex (CC) 8, CC12, and CC398. Twelve isolates belonged to CC1956 and one isolate was CC49. The CC49 and CC1956 isolates carried distinct lukF/S genes related to the Panton-Valentine leukocidin (PVL) from human isolates of S. aureus. These genes were located on related, but not identical, Siphovirus prophages. The beavers, from which those isolates originated, suffered from abscesses, purulent organ lesions and necrotising pneumonia, i.e., clinical manifestations resembling symptoms of severe PVL-associated disease in humans. It might thus be assumed that the “Beaver Leukocidin (BVL, lukF/S-BV)”-positive strains are beaver-specific pathogens, and further studies on their clinical role as well as on a possible transmissibility to other species, including humans, are warranted.
Subject terms: Ecology, Microbiology, Molecular biology, Zoology
Introduction
Staphylococcus aureus is a widespread and extremely versatile bacterium that colonises about 20–30% of any human population. Beyond asymptomatic, usually nasal, colonisation, it is also able to cause clinical infections ranging from superficial skin and soft tissue infections (SSTI) to life-threatening conditions such as necrotizing fasciitis, septicaemia, endocarditis or pneumonia. An important public health issue is the presence of antimicrobial resistance genes on mobile genetic elements spreading across different Staphylococcus species including S. aureus. The most notable examples are mec genes on horizontally transmissible mobile genetic elements, so-called Staphylococcal Cassette Chromosome mec (SCCmec) elements, that gave rise to genetically diverse methicillin-resistant S. aureus (MRSA).
S. aureus carriage, or infection, is not restricted to humans. S. aureus has been found in a wide variety of wildlife and domestic animals (for details and references, see Supplemental File 1). In general, domestic animals or animals in zoos or wildlife rehabilitation centres might have acquired S. aureus directly from humans or can be involved into chains of transmissions of very specific clones such as livestock-associated MRSA. Wild animals usually carry S. aureus belonging to poorly known lineages that are usually only identified in conspicuous outbreaks1,2 or if they cause concern related to human health and antimicrobial resistance, such as in the case of mecC. Few systematic studies have yet been performed in wildlife so evidence is anecdotal and knowledge on wildlife lineages of S. aureus is still sparse. It can be assumed that S. aureus causes similar conditions in animals as in humans. Many animals might be asymptomatically colonised by S. aureus while in some cases, purulent SSTI such as abscesses as well as pneumonia or sepsis might be observed. This raises the question whether the multitude of virulence factors known from S. aureus might be related to a host specificity of the strains they reside in, i.e., if an apparent redundancy of virulence factors serves as adaption to diverse host organisms.
One major class of S. aureus virulence factors are bicomponent leukocidins that consist of two components aggregating to polymeric pores in host leukocyte membranes leading to cell death. They are encoded by pairs of co-localised and co-expressed genes3,4. Some can be essentially found in every S. aureus isolate, regardless of host specificity and clonal complex (CC) affiliation. These include lukA/B (with lukG/H and lukX/Y being synonyms5–8) and lukF/S from the haemolysin gamma gene locus which could be regarded as species markers, similarly as lukF/S-int in Staphylococcus intermedius/pseudintermedius.
Moreover, there are lukD/E genes which are located on a genomic island that is absent from some S. aureus lineages (CC9, CC22, CC30, CC45, CC59, CC93, CC182, CC398, CC509 and CC772) as well as from most of the CCs recently re-categorised as Staphylococcus argenteus. Generally, there is no variation between isolates and strains belonging to the same lineage although few isolates might show random deletions of lukD/E and neighbouring genes.
Finally, there are different phage-borne genes lukF/S-PV, lukM/lukF-P83 and lukP/Q distributed across different lineages. The presence of Panton-Valentine leukocidin (lukF/S-PV, PVL) is strongly associated with SSTI in humans, such as abscesses and carbuncles, but it can also be identified in rapidly progressing, life-threatening necrotising pneumonia. It has first been described nearly a century ago, and was associated with worldwide outbreaks in the 1940s and 1960s as well as with a recent global emergence of virulent, community-associated MRSA9–14. LukM/lukF-P83-positive isolates originate from cattle, small ruminants and, rarely, from pigs or rodents1,11,15–18 and lukP/Q was recently observed in horse isolates19. The molecular epidemiology of these leukocidins strongly suggests host specificity, which was also supported by results of an experimental study indicating that PVL is effective in killing neutrophils of humans and rabbits, but not of macaques and mice20. As genes of these bicomponent leukocidins are located on prophages, one might speculate that the infection of S. aureus by bacteriophages predetermines both, host specificity of a given S. aureus strain as well as clinical manifestation in the vertebrate host of origin.
In this study, we present additional evidence for host specificity by describing a novel phage-borne bicomponent leukocidin related to PVL from diseased Eurasian (or European) beavers (Castor fiber).
Results
Isolates and typing
The isolates characterised as well as strain affiliations, geographic origins and clinical presentations are summarised in Table 1. Autopsy images showing typical aspects of putrid infections in some animals are shown in Fig. 1. The complete microarray hybridisation patterns are provided as Supplemental file 2 and some relevant features will be discussed in the descriptions of the respective strains. While all German isolates yielded hybridisation signals for lukF/S-PV, frequently only weak positive or ambiguous results for the lukS-PV probe were observed. This prompted further investigations, including the detection of PVL by lateral flow assay21 (Table 1) and whole genome sequencing (see below).
Table 1.
Details of animals, isolates and strains.
| Animal | Geographic origin | Isolate | Pathology | MLST | spa | Strain ID according to array | PVL by lateral flow |
|---|---|---|---|---|---|---|---|
| A | Berlin | WT19 | Multifocal moderate to severe suppurative necrotising pneumonia and suppurative pyelonephritis. Found dead. | ST4614a | t3058 | CC1956-MSSA (lukF-PV + /lukS-PV?)b | Positive |
| B | Berlin | WT63 | Severe suppurative necrotising pneumonia and multiple small abscesses in spleen, kidney, caecum, mesenteric lymph nodes and intercostal muscles. Found dead. | ST4614 | t3058 | CC1956-MSSA (lukF-PV + /lukS-PV?) | Positive |
| WT64 | ST4614 | t3058 | CC1956-MSSA (lukF-PV + /lukS-PV?) | Positive | |||
| C (“Tiffy”) | Bavaria | WT65 | Severe abscessing mandibular lymphadenitis, severe suppurative cystitis. Died in a wildlife rehabilitation centre. | ST49 | t208 | CC49-MSSA (lukF-PV + /lukS-PV?) | Positive |
| D | Berlin | WT66 | Severe fibrinous-purulent myocarditis, suppurative pyelonephritis (right kidney only) and prostatitis. Found dead. | ST4614 | t3058 | CC1956-MSSA (lukF-PV + /lukS-PV?) | Positive |
| WT67a | ST4614 | t3058 | CC1956-MSSA (lukF-PV + /lukS-PV?) | Positive | |||
| WT67b | ST4614 | t3058 | CC1956-MSSA (lukF-PV + /lukS-PV?) | Positive | |||
| WT68 | ST4614 | t3058 | CC1956-MSSA (lukF-PV + /lukS-PV?) | Positive | |||
| WT69 | ST4614 | t3058 | CC1956-MSSA (lukF-PV + /lukS-PV?) | Positive | |||
| E | Berlin | WT70 | Suppurative necrotising pneumonia, suppurative pyelonephritis and lymphadenitis (popliteal lymph nodes), multiple abscesses in caecum wall. Found dead. | ST4614 | t3058 | CC1956-MSSA (lukF-PV + /lukS-PV?) | Positive |
| F | Berlin | WT71 | Abscesses subcutaneous (chest wall/axilla) and in popliteal lymph nodes. Euthanised due to poor physical condition. | ST4614 | t3058 | CC1956-MSSA (lukF-PV + /lukS-PV?) | Positive |
| G | Berlin | WT110 | Suppurative necrotising pneumonia, enlarged thyroid gland containing small yellowish abscesses, suppurative pyelonephritis, splenomegaly, multiple abscesses in caecum and colon walls as well as in skin. Cachexia. Found dead. | ST4614 | t3058 | CC1956-MSSA (lukF-PV + /lukS-PV?) c | Positive |
| WT111 | ST4614 | t3058 | CC1956-MSSA (lukF-PV + /lukS-PV?) c | Negative | |||
| H | Marchfeld Channel, Austria | B1 | Nasal swab. Good physical condition except for gunshot traumata including parts of a bullet in the right hindleg with surrounding acute necrotizing inflammation. All other organs unremarkable. | N/A | t085 | CC398-MSSA | N/A |
| I | Lower Austria | B2 | Abscessing lymph node, knee. Shot dead. | N/A | t008 | CC8-MSSA | N/A |
| J | Eisenstadt region, Austria | B3 | Pneumonia (Streptococcus pyogenes, Morganella morganii). Splenomegaly. S. aureus originated from enrichment culture of lung tissue. Found dead. | N/A | t394 | CC8-MSSA (sed/j/r +) | N/A |
| K | Lower Austria | B4 | Nasal Swab. Healthy animal shot dead. | N/A | t156 | CC12-MSSA (sep +) | N/A |
aST4614 is a single locus variant of ST1956; arcC-6, aroE-291, glpF-6, gmk-2, pta-7, tpi-225, yqiL-585.
bClearly positive for lukF-PV, weekly positive or ambiguous signal for lukS-PV.
cWT110 and WT111 differed in haemolysis on Columbia blood agar and were thus handled separately although array analysis eventually revealed identical strain affiliations.
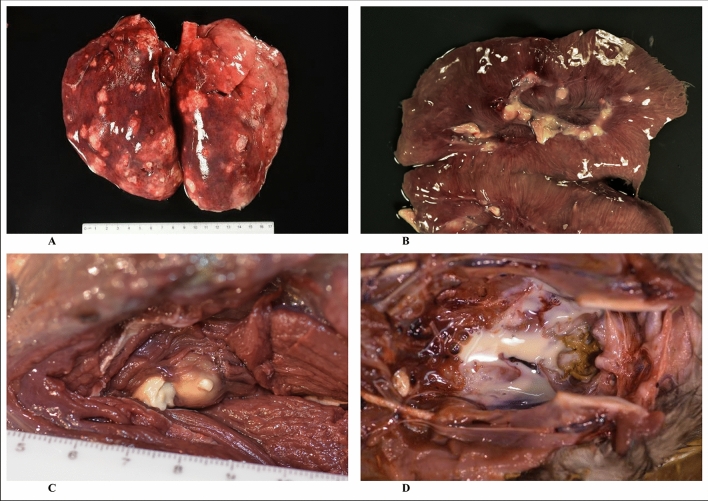
Figure 1.
Pathological lesions of Eurasian beavers (C. fiber) infected with BVL-positive S. aureus. (A) Severe suppurative necrotizing pneumonia (animal B); (B) severe suppurative pyelonephritis (animal G); (C) caseous lymphadenitis, popliteal lymph node (animal E); (D) urinary bladder with pyuria (animal C).
Phenotypic and genotypic resistance properties of the S. aureus isolates
Antimicrobial susceptibility testing revealed that all beaver isolates from Germany were susceptible to all antimicrobial agents tested. The distribution of minimal inhibitory concentration (MIC) values and test ranges are displayed in Supplemental File 3a. The phenotypic data corresponded well with microarray data, since none of the corresponding resistance genes was identified. In contrast, two of the Austrian isolates showed macrolide resistance with one of them also being lincosamide resistant. One isolate also exhibited tetracycline resistance. These phenotypes corresponded with the detection of genes erm(A), erm(C) and tet(M), respectively (Supplemental file 2 and 3b).
The chromosomal variant of the metallothiol transferase gene fosB was present in all CC1956 isolates. Sequence analysis revealed a frame shift at position 108 creating a stop codon at positions (pos.) 146.0.148 compared to the reference sequence (N315, GenBank BA000018.3 [2,389,328.0.2,389,747]). This resulted in a truncated protein of 48 amino acids (aa) rather than 139 aa as for the original fosB gene product. The mutation was present in all available sequences (i.e., Oxford Nanopore and Illumina of WT19 as well as Illumina of WT63, WT64, WT66, WT67a, WT67b, WT68, WT69, WT70, WT71, WT110 and WT111). While fosB was originally implicated in fosfomycin resistance, it appears to be linked to certain CCs. Indeed, it was also present in the CC8 and CC12 beaver isolates (B2, B3, B4) as well as in the reference sequences of the respective CCs (Supplemental File 2). The fosB gene was absent from the CC49 isolate WT65 and from the CC49 reference sequence of Tager 104, GenBank CP012409.1, as well as from the CC398 isolate B1. Moreover, all sequenced isolates (from animals A to G) harboured a gene designated tet(38), encoding a major facilitator superfamily permease. While this gene was implicated in low-level tetracycline resistance when overexpressed22, its mere presence certainly is not associated with phenotypic tetracycline resistance as it can be found in virtually every S. aureus genome.
Biocide susceptibility testing of the CC49/1956 isolates revealed unimodal MIC distributions (Supplemental File 3b), with ranges encompassing not more than three to four dilution steps for each of the biocides (benzalkonium chloride, 0.00003–0.00025%; polyhexanide, 0.000125–0.0005%; chlorhexidine, 0.00006–0.00025% and octenidine, 0.00006–0.00025% with percentages given as mass per volume). The four remaining isolates showed MIC values of 0.0000125–0.00025% for benzalkonium chloride, 0.0005–0.001% for polyhexanide, 0.00006–0.000125% for chlorhexidine, and 0.000125–0.00025% for octenidine.
The chromosomal heavy metal resistance markers arsB/R and czrB were detected by hybridisation in all four CC1956 isolates tested as well as in the CC49 isolate. This was confirmed by sequencing. There was no evidence for plasmid- or SCC-borne heavy metal resistance markers.
The sequence of the phage-borne leukocidin genes in WT19 and WT65
As mentioned above, CC49/CC1956 beaver isolates yielded occasionally ambiguous hybridisation intensities for lukS-PV probes prompting further investigation assuming that the specifically designed oligonucleotides were not able to bind optimally at the target due to mismatches, i.e., allelic variants. Sequencing revealed the presence of distinct alleles of phage-borne leukocidin genes (Figs. 2a/b and 3a/b). The sequences from the two sequenced beaver isolates were identical to each other despite their origin from different prophages in different CCs. In general, the beaver alleles, hitherto referred to as “Beaver Leukocidin” or BVL, lukF/S-BV, appeared to be closer related to the PVL genes from human strains of S. aureus than to those from ruminants and horses (see Figs. 2a/b and 3a/b and the percentages of homologies as provided in Supplemental File 4). There was no evidence for recombination/chimerism in lukF-BV and lukS-BV as mismatches compared to other sequences were evenly distributed across the entire sequences. Sequences of lukF-BV and lukS-BV were also related but clearly distinct from core genomic lukF/S-int of S. intermedius/pseudintermedius.
Figure 2.
(a) Alignment of the lukF-BV sequences, of other phage-borne leukocidin F component sequences from S. aureus and of lukF-int from S. intermedius/pseudintermedius. (b) Alignment of the amino acid sequences of the corresponding lukF gene products.
Figure 3.
(a) Alignment of the lukS-BV sequences, of other phage-borne leukocidin S component sequences from S. aureus and of lukS-int from S. intermedius/pseudintermedius. (b) Alignment of the amino acid sequences of the corresponding lukS gene products.
lukF/S-BV and the agr locus
Two isolates from one animal, WT110 and WT111 (Table 1), differed in hemolysis on Columbia blood agar and were thus handled separately although array analysis eventually revealed the same strain affiliations. They also differed in BVL production as shown by lateral flow tests. Sequencing using both, Illumina and Oxford nanopore technologies, revealed a substitution from A to T in position 706 of the agrA gene that results in a premature stop codon at position 236 of the agrA gene product (Supplemental File 5) suggesting that agr played a role in the observed phenotype and the regulation of BVL.
Core genome and genomic islands of the CC1956 isolate WT19
As revealed by array experiments (Supplemental File 1) and confirmed by genome sequencing of WT19, CC1956 isolates presented with agr IV alleles and capsule type 5. They were positive for cna, but they lacked seh and egc enterotoxin genes, ORF CM14 as well as sasG. Leukocidin genes lukX/Y, lukD/E and lukF/S-hlg were present. This is also in accordance with previously sequenced BVL-negative CC1959 isolates (SAMEA3251370, SAMEA3251372, SAMEA3251377, SAMEA3251376, SAMEA3251380; Supplemental File 2).
The WT19 genome (Supplemental Files 6a and 6b) harboured two uncharacterised enterotoxin genes (pos. 1,940,148..1,940,900 and pos. 1,939,378..1,940,121). Both were also found in DAR4145 (CC772) where they also formed a genomic island at approximately the same position within the genome (GenBank CP010526.1: RU53_RS09775, pos. 1,968,336..1,969,061 and RU53_RS09780, pos. 1,969,088..1,969,840). One of these two genes (“seu2” = RU53_RS09780) was covered by the second array-based assay23 and it was found in all four isolates tested with this array.
Mobile genetic elements in the CC1956 isolate WT19
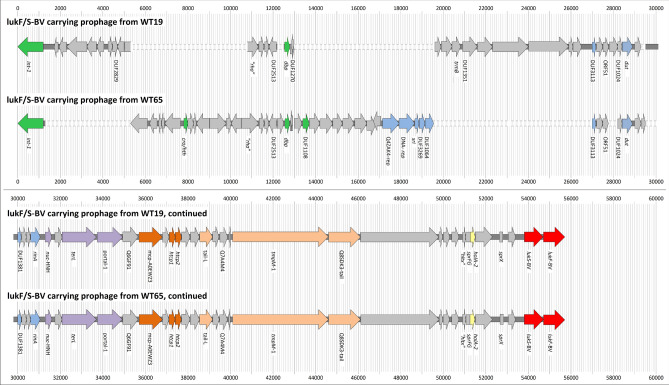
The lukF/S-BV prophage was integrated into the lipase 2 gene (lip2, “geh”, “sal3”, “salip35”, GenBank CP000253.1 [314,326..316,398]), and spanned pos. 322,629 to 365,636. Besides leukocidin genes, it also included genes associated with the different modules of a typical Siphoviridae genome (lysogeny, DNA metabolism, packaging and capsid morphogenesis, tail morphogenesis, host cell lysis24,25; see Supplemental File 7/Fig. 4).
Figure 4.
Schematic representation of the aligned sequences of the lukF/S-BV prophages from WT19 and WT65.
Furthermore, there was a small pathogenicity island at pos. 869,706 to 884,748 that included pif encoding a phage interference protein, a gene for a small terminase subunit, genes for “putative proteins” as well as a gene (scn2) coding for a paralog of a complement inhibitor SCIN family protein and a gene for a variant of the von Willebrand factor binding protein Vwb (vwb3). Thus, it is considered a staphylococcal pathogenicity island (SaPI) related to the one in S0385, GenBank AM990992.1.
Another prophage integrated between rpmF and isdB, pos. 1,107,447 to 1,146,132. A third prophage was located between a truncated nikB and Q5HG37, pos. 1,425,279 to 1,481,870. Finally, there was a forth prophage between Q5HDU4 and sarV (actually interrupting an MFS transporter between those genes), pos. 2,340,832 to 2,386,591. This prophage sequence corresponded to the phage that was detected by nanopore sequencing after induction by Mitomycin C (see below and Supplemental File 8).
Phage morphology and sequencing of phages from the CC1956 isolate WT19
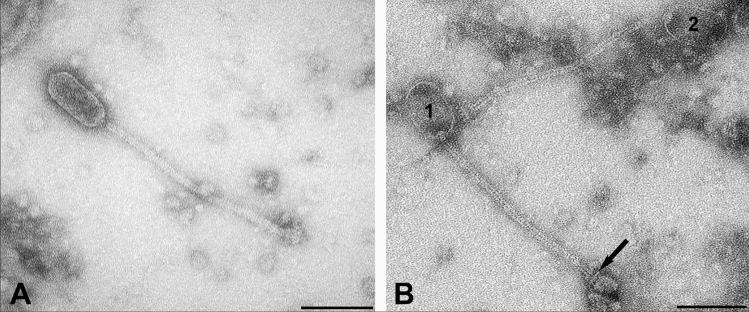
In three separate preparations, large numbers of phages were observed that were well contrasted with uranyl acetate and with phosphotungstic acid. Phages had elongated capsids. The non-contractile thin tails were straight or slightly curved and ended in a bulb-shaped base plate. Based on these characteristics, they were assigned to the order Caudovirales, family Siphoviridae.
Capsids were measured in 40 phages, tails in 34 and base plates in 33 phages. Based on these measurements, two distinct populations could be differentiated (Fig. 5). In one (Fig. 5A), the prolate, distinctly pentagonal capsids averaged 39 ± 5 nm (range 32–46 nm) in diameter and 92 ± 8 nm (range 80–104 nm) in length. Tails were 276 ± 20 nm (range 243–310 nm) long, had a diameter of 11 ± 1 nm (range 10–12 nm) and had a stacked discs appearance. Their baseplates were 16 nm (range 16–31 nm) by 27 nm (range 19–33 nm). The other population (Fig. 5B) had elongated oval capsids with a maximal diameter of 55 ± 2 nm (range 51–60 nm) diameter and 93 ± 5 nm (range 85–100 nm) length. Their tails measured 287 ± 12 nm (range 275–313 nm) in length and 9 ± 1 nm (8–10) in diameter and had a rail-road-track morphology. Dimensions of baseplates were 25 nm (range 21–30 nm) by 29 nm (range 23–39 nm).
Figure 5.
Transmission electron micrograph of two distinct prolate phages resulting from Mitomycin C treatment of S. aureus CC1956 isolate WT19. A, Phage particle with pentagonal 38 nm in diameter capsid and a 12 nm thick tail with stacked disc appearance; B, Two phage particles (1, 2) with oval capsids of 55 nm in diameter and 9 nm thick tails with rail-road-track morphology. The base plate is separated from the tail by a transversal disc (arrow). Negative contrast preparation with uranyl acetate. Bars = 100 nm.
Oxford Nanopore sequencing of one of these phage preparations (Supplemental File 8) yielded just one circular contig with a coverage of 724. Its sequence was identical to that of the forth prophage, between Q5HDU4 and sarV, except for a loss of a single triplet out of a total length of 46,387 nt.
Core genome and genomic islands of the CC49 isolate WT65
The CC49 isolate carried agr group II alleles and capsule type 5. It was positive for sasG, but lacked seh and egc enterotoxin genes, ORF CM14 and the collagen adhesion gene cna. A truncated copy of the enterotoxin S gene (GenBank CP000046, pos. 2,203,972.0.2,204,196) was found as well as leukocidin genes lukG/H = lukX/Y, lukD/E and lukF/S-hlg. With regard to presence and alleles of chromosomal markers such as MSCRAMM or ssl genes, the genome of WT65 (Supplemental Files 7a and 7b) is closely related to the CC49 reference sequences such as Tager 104, GenBank CP012409.1 (Supplemental File 2).
Mobile genetic elements in the CC49 isolate WT65
One prophage was integrated into the lip2 gene spanning pos. 311,401 to 354,724. The prophage included the lukF/S-BV genes as well as genes associated with the different modules of a typical Siphoviridae genome (Supplemental File 7/Fig. 4). Sequences corresponding to the lysogeny and replication modules were clearly different compared to the lukF/S-BV-prophage in the CC1956 isolate WT19 while approximately the second half of the two respective prophage sequences (the lower part of the alignment in Fig. 4) were virtually identical in gene content, order and orientation.
Other mobile genetic elements (Supplemental File 9a/b) included a small pathogenicity island, pos. 402,133 to 416,237 (between rpsR encoding 30S ribosomal protein S18 and its terminator), that included hypothetical proteins, a gene of a terminase small subunit, vwb3 (encoding a “von Willebrand factor” binding protein) and the scn2 gene (putative paralog of complement inhibitor). Between the genes ktrB and groL, pos. 2,029,208 to 2,042,866, another SaPI was identified that contained additional, slightly different copies of vwb3 and scn2 genes as well as terminase small subunit, integrase and excisionase (xis-AIO21657) genes. Finally, five genes between pos.1,334,169 and 1,339,503 were annotated as phage capsid genes although no other phage-related genes were found in this region.
Phage morphology and sequencing of phages from the CC49 isolate WT65
Four separate phage preparations were examined. In one of them, few phage-like structures were detected. These findings could not be confirmed in the following preparations. Thus, they were interpreted as artefacts, also given that it was not possible to induce a sufficient amount of phages for Oxford Nanopore sequencing.
Discussion
There are only few reports on S. aureus in beavers. One paper, published in 1969, described the presence of S. aureus in several mammal species, including beavers, but results obtained by serological typing methods utilised then cannot be translated into CC affiliations26. A recent paper described a rare presence of S. aureus in minced beaver meat without providing further details27. While we are not aware of published S. aureus typing data from beavers (except for one MLST profile, see below), we observed CC8, CC12, CC49, CC398 and CC1956 in beavers from Germany and Austria. Isolates from two CCs, CC49 and CC1956, were conspicuous in yielding evidence for the presence of a distinct, phage-borne bicomponent leukotoxin.
CC49 is known to be associated with rodents, such as European red squirrels (Sciurus vulgaris)1,2, brown rats (Rattus norvegicus, see below), yellow-necked mice (Apodemus flavicollis) and bank voles (Myodes glareolus)28, but also with other mammals such as Australian wallabies29 and European wildcats (Felis silvestris)30. It has been reported from humans, causing orthopaedic infections31 or SSTI32. A livestock-associated CC49 MRSA strain with a SCCmec VT element has been reported from domestic pigs in Switzerland33 as well as mecC-MRSA from humans34 and from brown rats35 in Belgium. While lukM/lukF-P83 was reported in CC49 isolates being implicated in an outbreak of exudative dermatitis in red squirrels1, to the best of our knowledge no PVL genes or variants thereof have been reported from CC49 so far.
CC1956 is another rodent-associated lineage. It has been found in field voles (Microtus agrestis), common voles (Microtus arvalis) and bank voles from Germany28,30 as well as in common voles and wood mice (Apodemus sylvaticus) from Spain36. The MLST database37 provides an example of ST1956 from an English red squirrel in respiratory distress (https://pubmlst.org/bigsdb?page=info&db=pubmlst_saureus_isolates&id=3900). A single locus variant, ST1959, was observed in a beaver (C. fiber) from Iowa (https://pubmlst.org/bigsdb?page=info&db=pubmlst_saureus_isolates&id=3903) Other variants came from a human from Poland (ST1960, https://pubmlst.org/bigsdb?page=info&db=pubmlst_saureus_isolates&id=3904) and from an unnamed animal from Spain (ST2766, https://pubmlst.org/bigsdb?page=info&db=pubmlst_saureus_isolates&id=5333). In addition, there are a couple of sequences in the NCBI BioSample database/Sequence Read Archive (SRA; SAMEA3251370, SAMEA3251372, SAMEA3251377, SAMEA3251376, SAMEA3251380) but since they contain no additional host or geographic metadata beside a description of a “small mammal” origin, this information is essentially useless. The German vole isolates and the SRA sequences did not contain lukF/S-PV or lukM/F-P83. The ST1959 isolate from Iowa, however, was described as PVL-positive, suggesting it to be an unrecognised specimen of BVL-positive CC1956.
As both, CC49 and CC1956, are rodent-associated lineages, it can be speculated that the S. aureus strains belong to the native microbiome of beavers, or alternatively originate from other wild mammals not yet sufficiently studied. However, none of the yet described rodent strains harboured lukF/S-BV, whereas they carried either no phage-borne leukocidins, or lukM/F-P83. This raises the question of the origin or source of S. aureus infections in beavers. One might speculate that beavers were infected with human or livestock-associated strains of S. aureus through contact to human or domestic animal´s offal, hospital or agricultural wastewater or sewage. This could have been the case for the BVL-negative Austrian CC8, CC12 and CC398 isolates as these lineages are common, widespread and promiscuous with regard to host species30,31,38–44. For the CC49 and CC1956 isolates, however, the distinct and unique sequence of lukF/S-BV as well as the presence of identical lukF/S-BV alleles in different strains and on different prophages indicate a beaver-specific origin rather than accidental or interspecies infections.
All animals involved were subjected to pathological and bacteriological diagnostics and those with BVL-positive S. aureus presented with severe/fatal disease (Fig. 1). These observations suggest that lukF/S-BV in beaver strains of S. aureus induces essentially the same pathology as lukF/S-PV in human strains and that it might be associated with mortality. In the BVL-negative cases from Austria, S. aureus could be regarded as coloniser (B1, B3, B4) or as cause of a localised, non-fatal infection (B2). One might pointedly say that animals with BVL-negative strains died with S. aureus while those with BVL-positive strains died from S. aureus infection, but case numbers are too low yet for a definite assessment. There are no systematic data neither on S. aureus morbidity and mortality in beavers, nor on a possible asymptomatic carriage. Further investigations on host specificity and clinical significance would require animal experiments which are clearly beyond the scope of the present study. In the absence of such experiments, further diagnostic studies on sick or dead beavers are warranted. Investigations of asymptomatic carriage of S. aureus in beavers might target captive beavers as well as road-killed or shot animals which could serve as surrogate for a healthy control group, presenting at least with a non-staphylococcal cause of death as this was the case for the Austrian beavers described herein.
The geographic distribution of lukF/S-BV-positive S. aureus still needs to be studied. It was interesting that all Berlin isolates were assigned to CC1956 and the one from Bavaria to CC49 while the Austrian ones belonged to other CCs, but the number of animals studied does not yet allow to draw conclusions on the geographic range of these strains and their phages. Similarly, the prevalence of BVL-positive strains among beavers and their possible impact on wild beaver populations that just recover after a century at the brink of extinction warrant further investigation. A possible occurrence among related rodent species, such as North American beaver (Castor canadensis) or among those that co-exist in the same habitat, such as coypus (Myocastor coypus), muskrat (Ondatra zibethicus) or water vole (Arvicola spec.), as well as in other CCs of S. aureus also still need to be studied.
Our observations also emphasise the need for different technologies for toxin or toxin gene detection. Assays that rely on short primers and probes, such as array hybridisation or PCR, might fail to recognise deviant alleles of target genes. Protein-/antibody-based assays might be more forgiving (as it was here the case when using the PVL assay), but are, of course, less easily available. Genome sequencing is an “open” approach that can be employed to find unknown alleles or unknown genes. However, the costs in terms of both, labour and expenses, are still too high for routine applications. This restricts the use of sequencing technologies, especially if costs are an issue. Unfortunately, this is the case in veterinary settings, but also in medical settings in most parts of the world.
Finally, the question of phylogeny of leukocidin genes remains. The beaver alleles are more closely related to the PVL sequences known from human isolates of S. aureus and their prophages than to the lukM/F-P83 leukocidin sequences from ungulates or to lukF/S-int from canines (Figs. 2 and 3). It is tempting to assume that this might indicate a spill-over to humans from beavers hunted for and consumed as food, as recently speculated for Yersinia species45. Indeed, beavers have been hunted close to extinction, for fur as well as for meat that was allowed to be consumed during lent. Manipulation of dead animals or preparation of meat might have facilitated a transmission of S. aureus, and/or staphylococcal phages, but this of course cannot be proven. A possible counter argument might be the high prevalence and diversity of PVL-positive S. aureus in humans outside the natural range of beavers, i.e., in Africa, the Indian subcontinent and the Middle East. A related issue is a potential infection risk for humans, given that human and beaver leukocidin genes are similar. Handling of dead or live beavers, for research or food27, should thus be performed under at least basic biosafety precautions, and the prevalence of BVL phages in beavers and related rodents should be investigated as well as possible SSTI in humans who handled beavers or beaver carcasses.
In summary, all CC49 and CC1956 isolates from fatally diseased beavers harboured the novel genes, lukF-BV and lukS-BV, which appeared to be closely related to PVL genes. Autopsy findings of affected beavers suggested significant virulence and resembled the pathology associated with PVL-positive S. aureus in humans. Further studies are needed to investigate the prevalence and distribution of lukF-BV and lukS-BV and its association with clinical symptoms.
Material and methods
Animals and isolates
Thirteen S. aureus isolates from seven wild beavers from Germany were investigated (Table 1). This included six adult beavers, found dead (n = 5) or moribund (n = 1) in Berlin, and one juvenile that died in captivity in Bavaria. Furthermore, four S. aureus isolates originated from beavers shot (n = 3) or found dead (n = 1) in Austria. Animals were subjected to post-mortem and bacteriological examinations. S. aureus isolates were identified by coagulase testing (BD BBL Rabbit Coagulase Plasma, Becton Dickinson, New Jersey, USA), hyaluronidase production using the Streptococcus equi decapsulation test46 and MALDI-TOF MS. Of three beavers, more than one isolate was investigated. These two (animals B, G) or five isolates (animal D) originated from different organs and/or displayed different phenotypes during primary culture, but were found to be indistinguishable upon genotyping.
Susceptibility testing
Antimicrobial susceptibility testing was performed for 28 antimicrobial agents (Supplemental File 3a) and three combinations of antimicrobial agents by broth microdilution according to Clinical and Laboratory Standards Institute (CLSI) standards47,48. Biocide susceptibility testing was performed for benzalkonium chloride, chlorhexidine, octenidine, and polyhexanide using commercial microtitre plates (sifin diagnostics GmbH, Berlin, Germany) and the protocol from Schug et al.49 modified as follows. The inoculum was prepared by adding 30 µL bacterial suspension of a density of 0.5 McFarland to 12 mL single concentrated tryptic soy broth (TSB) and the microtiter plates were inoculated with 100 µL per well according to the manufacturer’s recommendation.
Spa and multi-locus sequence typing
Multi-locus sequence typing (MLST), which is based on sequencing of fragments of arcC, aroE, glpF, gmk, pta, tpi and yqiL50, was performed by deducing MLST sequences and types from the Illumina sequences of all samples of animals A-G (see below). The sequences of the MLST alleles were analysed using the S. aureus pubMLST website37 (https://pubmlst.org/bigsdb?db=pubmlst_saureus_seqdef&page=sequenceQuery). Spa typing was performed according to previously published protocols51, confirming results using the Illumina sequences and using the nomenclature on the Ridom website (http://spa.ridom.de/).
Microarray-based typing
Isolates were characterised using the DNA microarray-based Interarray S. aureus kit (fzmb GmbH, Bad Langensalza, Germany). Primer and probe sequences have been previously described in detail7,8. Protocols and procedures were in accordance with manufacturer’s instructions. The array covers 336 different targets related to approximately 170 different genes and their allelic variants allowing detection of virulence and resistance factors as well as CC and strain identification based on automated comparison to a database of reference experiments. Briefly, S. aureus was cultured on Colombia blood agar. DNA extraction was performed after enzymatic lysis7,8. A multiplexed linear amplification was performed using one specific primer per target. During this step, biotin-16-dUTP was randomly incorporated into the amplicons. After incubation and washing, hybridisation was performed to probes immobilised on the array. Hybridisation was detected by streptavidin horseradish peroxidase, generating a localised precipitation of a dye resulting in formation of visible spots. Microarrays were then photographed and analysed with a designated reader (Alere Technologies/Abbott, Jena, Germany) and software. A second microarray (Alere Technologies/Abbott, Jena, Germany23) was used on four CC1956 isolates, (WT19, WT63, WT67b, WT68) and one CC49 isolate (WT65) in order to detect additional markers (Supplemental File 2).
PVL lateral flow test
The expression of PVL was determined by a PVL lateral flow device21 (Senova, Weimar, Germany) according to the manufacturer’s instructions.
Phage induction
Phage preparation was performed as described previously52. In summary, cultures were grown in 2×TY medium (prepared using NaCl by Roth, Heidelberg, Germany, as well as tryptone and yeast extract by Becton Dickinson, Le Pont de Claix, France) in an incubator at 30 °C for 18 h overnight. 100 mL of fresh medium was inoculated with 1 mL of the overnight culture and cultured at 37 °C until the middle of the exponential growth phase. mitomycin C (Roche, Basel, Switzerland) was added at a final concentration of 0.5 μg/mL, and cultivation was continued at 30 °C until the OD at 600 nm decreased for the first time (by 0.4 for WT19 and 1.5 for WT65). After centrifugation at 4 °C for 12 min at 3,000 × g, 0.1 N NaOH was added to the supernatant until pH = 7.0 was reached. The neutralized supernatant was filtered using a 0.20 µm cellulose acetate (CA) membrane filter (Sartorius, Göttingen, Germany) and the filtrate was stored at 4 °C.
Phage detection by transmission electron microscopy (TEM)
Suspensions from phage preparations were vortexed briefly. Then, drops of 30 µL were placed on a plate of dental wax. 300 mesh copper grids filmed with formvar, coated with carbon, and hydrophilized by glow discharge (immediately before use) were floated on the drops for 30 min. Then the grids were briefly rinsed in 3 drops of distilled water and the excess liquid was drained on wet filter paper. Finally, one grid of each preparation was contrasted on one drop of 1% phosphotungstic acid and one drop of 1% uranyl acetate for 1 min. The excess contrast medium was drained on wet filter paper. After air drying, the grids were examined by transmission electron microscopy (Tecnai 12, FEI Deutschland GmbH, Dreieich, Germany) at 80 kV. Representative micrographs were taken with a digital camera (TEMCAM FX416, TVIPS, Gauting, Germany).
Phage DNA Isolation
To sequence pure phage DNA (pDNA), DNA extraction was performed according to the method described previously53. The phage filtrate was first treated with 10 µg/mL DNase I (Sigma Aldrich, Steinheim, Germany) and 10 µg/mL RNAse (Qiagen, Hilden, Germany) for 1 h at 37 °C, followed by treatment with 20 mM EDTA, 50 µg/mL proteinase K, and 0.2% SDS and another incubation for 1 h at 65 °C and 300 rpm. A phenol chloroform extraction was performed as described previously53. For better separation of the phases, phase lock gel light tubes (Quantabio, Beverly, USA) were used in each step. DNA was concentrated in a SpeedVac vacuum concentrator (Eppendorf, Hamburg, Germany; 25 min at room temperature and 1400 rpm), and the final concentration was measured using the Qubit 4 fluorometer (ThermoFisher Scientific, Waltham, USA).
Whole-genome sequencing (WGS) by Illumina
All isolates from animals A to G were subjected to whole genome sequencing (WGS) with the Illumina MiSeq platform (Illumina, Inc., San Diego, USA). The DNA was extracted using the QIAamp® DNA Mini Kit (QIAGEN, Hilden, Germany) with adaptations for staphylococci as described previously54.
The libraries for WGS were prepared using the Nextera XT DNA Library Preparation Kit (Illumina, Inc., San Diego, USA) according to the manufacturer’s recommendations. The 2 × 300 bp paired-end sequencing in 40-fold multiplexes was performed on the Illumina MiSeq platform (Illumina, Inc., San Diego, USA).
Oxford Nanopore sequencing
Oxford Nanopore Technology (ONT) sequencing of the isolates WT19, WT65, WT110 and WT111 as well as of the phage DNA prepared from isolate WT19 was performed using two different MinION flow cells (FLO-MIN106D for the isolated bacteria, and two FLO-FLG001 for phage DNA, all containing an R9.4.1 pore). Library preparations were done using the 1D genomic DNA by ligation kit (SQK-LSK109, ONT, Oxford, UK), and the native barcoding expansion kit (EXP-NBD104 and EXP-NBD114, ONT) for the isolated bacteria following manufacturer’s instructions with minor adaptations. In short, prior to the library preparation, an AMPure bead (Agencourt AMPure XP, Beckman Coulter, Krefeld, Germany) clean up step was performed. Potential nicks in DNA and DNA ends were repaired in a combined step using NEBNext FFPE DNA Repair Mix and NEBNext Ultra II End repair/dA-tailing Module (NewEngland Biolabs, Ipswich, USA) by tripling the incubation time. A subsequent second AMPure bead purification was followed by the ligation of sequencing adapters onto prepared ends followed by a clean-up step with AMPure beads. For bacterial DNA, an additional barcoding and clean-up step was performed prior to adapter ligation. Sequencing buffer and loading beads were added to the library. An initial quality check of the flow cells (FLO-MIN106D; ID FAL13739 and FAP37324 as well as FLO-FLG001; ID: AES237) showed around 1289, 509 and 18 active pores, respectively, at the start of sequencing. DNA samples from WT19, WT65, WT110 and WT111 as well as from the WT19 phage preparation were used for loading that comprised a total amount of around 120 ng as measured by Qubit 4. The sequencing ran for 48 h using the MinKNOW software version 20.06.5.
Sequence assembly and polishing
The 300 bp paired-end reads of all eleven isolates generated by Illumina MiSeq sequencer were de novo assembled into contigs with a minimum size of 200 bp using Unicycler v0.4.755 at default settings.
For all nanopore data sets (bacterial isolates as well as phage DNA from isolate WT19), the guppy basecaller (v4.2.2, ONT) translated and trimmed the MinION raw data (fast5) into quality tagged sequence reads (4,000 reads per fastq-file). To get a smaller and better subset of reads Filtlong (v0.2.0) was used only for bacteria DNA with a median read quality of 14 and a minimum read length of 1,000 bp. The reads of the phage WT19 sequence run were not filtered by Filtlong. The median read quality of 12.5 and a N50 read length of 340 bp was highly suitable for assembly. Flye (v2.8.3) was used to assemble the reads to high quality contigs. Then, a racon-medaka (4-times racon v1.4.3; 1-time medaka v1.2.0) pipeline was applied for polishing. Besides that, sequences were additionally polished by pilon (v1.23) using Illumina sequence data. The NCBI Prokaryotic Genome Annotation Pipeline (PGAP version 2021–01-11.build 5132) was used for annotation of all assembled contigs for the staphylococcal isolates in combination with an in-house database of published staphylococcal sequences.
Ethics
No animal experiments were performed, and no animal was purposefully killed for this study. Isolates originated from samples which have been submitted to the Leibniz Institute for Zoo and Wildlife Research, Berlin, or to the University of Veterinary Medicine, Vienna, for routine diagnostics and wildlife surveillance.
Supplementary Information
Acknowledgements
The authors thank Nadine Jahn, Zoltan Mezö, Claudia Szentiks (Leibniz Institute for Zoo and Wildlife Research, Berlin), Kerstin Müller (Small Animal Clinic, Department of Veterinary Medicine at the Freie Universität Berlin), Berit Arendt (beaver management, BUND Nature Conservation Bavaria e.V.), Petra Krienke and Inga Eichhorn (Institute of Microbiology and Epizootics, Freie Universität Berlin) as well as Lisa Wolf and Jana Jagiela (Friedrich-Loeffler-Institut, Jena) for support and assistance.
Author contributions
S.M. and A.T.F. designed the study, analysed data and wrote the manuscript; S.B.V. performed experiments, analysed data and wrote the manuscript; H.K., K.M., G.W., I.L. obtained samples and performed experiments (sampling, bacteriological work); E.L.T. performed experiments (TEM); M.R., S.D.B., D.H., C.D. performed experiments and analysed data (sequencing); E.M. performed experiments (sequencing, arrays, PVL tests); S.S. and R.E. designed the study, supervised experiments and revised the manuscript. All authors revised and corrected the various drafts and approved the final version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The Jena group acknowledges support by the German Federal Ministry of Education and Research, within the ADA project (13GW0456C) aiming on the development of rapid tests for the detection of resistance genes and virulence factors in human and zoonotic isolates of S. aureus/MRSA. The FU Berlin group acknowledges funding by the German Federal Ministry of Education and Research under project number 01KI1727D as part of the Research Network Zoonotic Infectious Diseases.
Data availability
Accession numbers are FU-Berlin_WT19, CP084892 and FU-Berlin_WT65, CP084107. The BioProject accession number for the strains sequenced within this project is PRJNA763345. All other data are provided within the manuscript, or as Supplemental Files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Stefan Monecke, Andrea T. Feßler, Sindy Burgold-Voigt, Stefan Schwarz and Ralf Ehricht.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-03823-6.
References
- 1.Simpson VR, et al. Association of a lukM-positive clone of Staphylococcus aureus with fatal exudative dermatitis in red squirrels (Sciurus vulgaris) Vet. Microbiol. 2013;162:987–991. doi: 10.1016/j.vetmic.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 2.Simpson VR, et al. Mortality in red squirrels (Sciurus vulgaris) associated with exudative dermatitis. Vet. Record. 2010;167:59–62. doi: 10.1136/vr.b4887. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko J, Kamio Y. Bacterial two-component and hetero-heptameric pore-forming cytolytic toxins: Structures, pore-forming mechanism, and organization of the genes. Biosci. Biotechnol. Biochem. 2004;68:981–1003. doi: 10.1271/bbb.68.981. [DOI] [PubMed] [Google Scholar]
- 4.Szmigielski S, Prevost G, Monteil H, Colin DA, Jeljaszewicz J. Leukocidal toxins of staphylococci. Zentralbl Bakteriol. 1999;289:185–201. doi: 10.1016/s0934-8840(99)80105-4. [DOI] [PubMed] [Google Scholar]
- 5.DuMont AL, et al. Identification of a crucial residue required for Staphylococcus aureus LukAB cytotoxicity and receptor recognition. Infect. Immun. 2014;82:1268–1276. doi: 10.1128/iai.01444-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ventura CL, et al. Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS ONE. 2010;5:e11634. doi: 10.1371/journal.pone.0011634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monecke S, et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant Staphylococcus aureus. PLoS ONE. 2011;6:e17936. doi: 10.1371/journal.pone.0017936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monecke S, Slickers P, Ehricht R. Assignment of Staphylococcus aureus isolates to clonal complexes based on microarray analysis and pattern recognition. FEMS Immunol. Med. Microbiol. 2008;53:237–251. doi: 10.1111/j.1574-695X.2008.00426.x. [DOI] [PubMed] [Google Scholar]
- 9.Panton P, Valentine F. Staphylococcal toxin. Lancet. 1932;1:506–508. doi: 10.1016/S0140-6736(01)24468-7. [DOI] [Google Scholar]
- 10.Kaneko J, Kimura T, Narita S, Tomita T, Kamio Y. Complete nucleotide sequence and molecular characterization of the temperate staphylococcal bacteriophage phiPVL carrying Panton-Valentine leukocidin genes. Gene. 1998;215:57–67. doi: 10.1016/s0378-1119(98)00278-9. [DOI] [PubMed] [Google Scholar]
- 11.Zou D, Kaneko J, Narita S, Kamio Y. Prophage, phiPV83-pro, carrying panton-valentine leukocidin genes, on the Staphylococcus aureus P83 chromosome: comparative analysis of the genome structures of phiPV83-pro, phiPVL, phi11, and other phages. Biosci. Biotechnol. Biochem. 2000;64:2631–2643. doi: 10.1271/bbb.64.2631. [DOI] [PubMed] [Google Scholar]
- 12.Vandenesch F, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 2003;9:978–984. doi: 10.3201/eid0908.030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan MS. Diagnosis and treatment of Panton-Valentine leukocidin (PVL)-associated staphylococcal pneumonia. Int. J. Antimicrob. Agents. 2007;30:289–296. doi: 10.1016/j.ijantimicag.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Boakes E, et al. Distinct bacteriophages encoding Panton-Valentine leukocidin (PVL) among international methicillin-resistant Staphylococcus aureus clones harboring PVL. J. Clin. Microbiol. 2011;49:684–692. doi: 10.1128/JCM.01917-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaneko J, Muramoto K, Kamio Y. Gene of LukF-PV-like component of Panton-Valentine leukocidin in Staphylococcus aureus P83 is linked with lukM. Biosci. Biotechnol. Biochem. 1997;61:541–544. doi: 10.1271/bbb.61.541. [DOI] [PubMed] [Google Scholar]
- 16.Lahuerta-Marin A, et al. First report of lukM-positive livestock-associated methicillin-resistant Staphylococcus aureus CC30 from fattening pigs in Northern Ireland. Vet. Microbiol. 2016;182:131–134. doi: 10.1016/j.vetmic.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Schlotter K, et al. Leukocidin genes lukf-P83 and lukM are associated with Staphylococcus aureus clonal complexes 151, 479 and 133 isolated from bovine udder infections in Thuringia, Germany. Vet. Res. 2012;43:42. doi: 10.1186/1297-9716-43-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada T, et al. Leukotoxin family genes in Staphylococcus aureus isolated from domestic animals and prevalence of lukM-lukF-PV genes by bacteriophages in bovine isolates. Vet. Microbiol. 2005;110:97–103. doi: 10.1016/j.vetmic.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Koop G, et al. Identification of LukPQ, a novel, equid-adapted leukocidin of Staphylococcus aureus. Sci. Rep. 2017;7:40660. doi: 10.1038/srep40660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Löffler B, et al. Staphylococcus aureus panton-valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010;6:e1000715. doi: 10.1371/journal.ppat.1000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senok A, et al. Lateral Flow Immunoassay for the Detection of Panton-Valentine Leukocidin in Staphylococcus aureus from skin and soft tissue infections in the United Arab Emirates. Front. Microbiol. 2021;2:2. doi: 10.3389/fcimb.2021.754523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truong-Bolduc QC, Dunman PM, Strahilevitz J, Projan SJ, Hooper DC. MgrA is a multiple regulator of two new efflux pumps in Staphylococcus aureus. J. Bacteriol. 2005;187:2395–2405. doi: 10.1128/jb.187.7.2395-2405.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monecke S, et al. Diversity of SCCmec elements in Staphylococcus aureus as observed in South-Eastern Germany. PLoS ONE. 2016;11:e0162654. doi: 10.1371/journal.pone.0162654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deghorain M, Van Melderen L. The Staphylococci phages family: an overview. Viruses. 2012;4:3316–3335. doi: 10.3390/v4123316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia G, Wolz C. Phages of Staphylococcus aureus and their impact on host evolution. Infect. Genet. Evol. 2014;21:593–601. doi: 10.1016/j.meegid.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 26.Plommet MG, Wilson JB. Serological typing of Staphylococcus aureus from wild animals. J. Comp. Pathol. 1969;79:425–433. doi: 10.1016/0021-9975(69)90062-0. [DOI] [PubMed] [Google Scholar]
- 27.Ziomek M, et al. Microbiological changes in meat and minced meat from beavers (Castor fiber L.) during refrigerated and frozen storage. Foods. 2021 doi: 10.3390/foods10061270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mrochen DM, et al. Wild rodents and shrews are natural hosts of Staphylococcus aureus. Int. J. Med. Microbiol. 2018;308:590–597. doi: 10.1016/j.ijmm.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 29.Chen MMS, Monecke S, Brown MH. Clonal diversity of methicillin-sensitive Staphylococcus aureus from South Australian wallabies. One Health. 2016 doi: 10.1016/j.onehlt.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Monecke S, et al. Diversity of Staphylococcus aureus Isolates in European Wildlife. PLoS ONE. 2016;11:e0168433. doi: 10.1371/journal.pone.0168433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luedicke C, Slickers P, Ehricht R, Monecke S. Molecular fingerprinting of Staphylococcus aureus from bone and joint infections. Eur. J. Clin. Microbiol. Infect. Dis. 2010;29:457–463. doi: 10.1007/s10096-010-0884-4. [DOI] [PubMed] [Google Scholar]
- 32.Davis R, Hossain MJ, Liles MR, Panizzi P. Complete Genome Sequence of Staphylococcus aureus Tager 104, a Sequence Type 49 Ancestor. Genome Announc. 2013 doi: 10.1128/genomeA.00706-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Overesch G, Buttner S, Rossano A, Perreten V. The increase of methicillin-resistant Staphylococcus aureus (MRSA) and the presence of an unusual sequence type ST49 in slaughter pigs in Switzerland. BMC Vet. Res. 2011;7:30. doi: 10.1186/1746-6148-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deplano A, Vandendriessche S, Nonhoff C, Denis O. Genetic diversity among methicillin-resistant Staphylococcus aureus isolates carrying the mecC gene in Belgium. J. Antimicrob. Chemother. 2014;69:1457–1460. doi: 10.1093/jac/dku020. [DOI] [PubMed] [Google Scholar]
- 35.Paterson GK, et al. The newly described mecA homologue, mecALGA251, is present in methicillin-resistant Staphylococcus aureus isolates from a diverse range of host species. J. Antimicrob. Chemother. 2012;67:2809–2813. doi: 10.1093/jac/dks329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gómez P, et al. Detection of methicillin-resistant Staphylococcus aureus (MRSA) carrying the mecC gene in wild small mammals in Spain. J. Antimicrob. Chemother. 2014;69:2061–2064. doi: 10.1093/jac/dku100. [DOI] [PubMed] [Google Scholar]
- 37.Jolley KA, Bray JE, Maiden MCJ. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018;3:124. doi: 10.12688/wellcomeopenres.14826.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Argudín MA, et al. Clonal complexes and diversity of exotoxin gene profiles in methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolates from patients in a Spanish hospital. J. Clin. Microbiol. 2009;47:2097–2105. doi: 10.1128/jcm.01486-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donker GA, et al. The population structure of Staphylococcus aureus among general practice patients from The Netherlands. Clin. Microbiol. Infect. 2009;15:137–143. doi: 10.1111/j.1469-0691.2008.02662.x. [DOI] [PubMed] [Google Scholar]
- 40.Monecke S, Luedicke C, Slickers P, Ehricht R. Molecular epidemiology of Staphylococcus aureus in asymptomatic carriers. Eur. J. Clin. Microbiol. Infect. Dis. 2009;28:1159–1165. doi: 10.1007/s10096-009-0752-2. [DOI] [PubMed] [Google Scholar]
- 41.Chroboczek T, et al. Clonal complex 398 methicillin susceptible Staphylococcus aureus: A frequent unspecialized human pathogen with specific phenotypic and genotypic characteristics. PLoS ONE. 2013;8:e68462. doi: 10.1371/journal.pone.0068462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomez-Sanz E, Torres C, Ceballos S, Lozano C, Zarazaga M. Clonal dynamics of nasal Staphylococcus aureus and Staphylococcus pseudintermedius in dog-owning household members. Detection of MSSA ST(398) PLoS ONE. 2013;8:e69337. doi: 10.1371/journal.pone.0069337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monecke S, et al. Genotyping of Staphylococcus aureus isolates from diseased poultry. Vet. Microbiol. 2013;162:806–812. doi: 10.1016/j.vetmic.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 44.da Gonçalves Silva A, et al. A phylogenomic framework for assessing the global emergence and evolution of clonal complex 398 methicillin-resistant Staphylococcus aureus. Microb. Genom. 2017;3:e000105. doi: 10.1099/mgen.0.000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Susat J, et al. A 5,000-year-old hunter-gatherer already plagued by Yersinia pestis. Cell Rep. 2021;35:109278. doi: 10.1016/j.celrep.2021.109278. [DOI] [PubMed] [Google Scholar]
- 46.Murray RG, Pearce RH. The detection and assay of hyaluronidase by means of mucoid streptococci. Can. J. Res. E Med. Sci. 1949;27:254–264. doi: 10.1139/cjr49e-035. [DOI] [PubMed] [Google Scholar]
- 47.CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals. CLSI Supplement VET01S. 5th ed. (Clinical and Laboratory Standards Institute, 2021).
- 48.CLSI. Performance Standards for Antimicrobial Susceptibility Testing, CLSI Supplement M100. 31th ed., (Clinical and Laboratory Standards Institute, 2021).
- 49.Schug AR, et al. Biocide susceptibility testing of bacteria: Development of a broth microdilution method. Vet. Microbiol. 2020;248:108791. doi: 10.1016/j.vetmic.2020.108791. [DOI] [PubMed] [Google Scholar]
- 50.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin .Microbiol. 2000;38:1008–1015. doi: 10.1128/JCM.38.3.1008-1015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harmsen D, et al. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 2003;41:5442–5448. doi: 10.1128/JCM.41.12.5442-5448.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raya RR, Hbert E, M. Isolation of phage via induction of lysogens. Methods Mol. Biol. 2009;501:23–32. doi: 10.1007/978-1-60327-164-6_3. [DOI] [PubMed] [Google Scholar]
- 53.Hagemann, R. in Gentechnologische Arbeitsmethoden—ein Handbuch (ed R. Hagemann) 51 (Akademie-Verlag Berlin 1990).
- 54.Scholtzek AD, et al. Molecular Characterization of Equine Staphylococcus aureus Isolates Exhibiting Reduced Oxacillin Susceptibility. Toxins. 2019 doi: 10.3390/toxins11090535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017;13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Accession numbers are FU-Berlin_WT19, CP084892 and FU-Berlin_WT65, CP084107. The BioProject accession number for the strains sequenced within this project is PRJNA763345. All other data are provided within the manuscript, or as Supplemental Files.