Abstract
Cardiovascular disease is the leading cause of human death worldwide. Drug thrombolysis, percutaneous coronary intervention, coronary artery bypass grafting and other methods are used to restore blood perfusion for coronary artery stenosis and blockage. The treatments listed prolong lifespan, however, rate of mortality ultimately remains the same. This is due to the irreversible damage sustained by myocardium, in which millions of heart cells are lost during myocardial infarction. The lack of pragmatic methods of myocardial restoration remains the greatest challenge for effective treatment. Exosomes are small extracellular vesicles (EVs) actively secreted by all cell types that act as effective transmitters of biological signals which contribute to both reparative and pathological processes within the heart. Exosomes have become the focus of many researchers as a novel drug delivery system due to the advantages of low toxicity, little immunogenicity and good permeability. In this review, we discuss the progress and challenges of EVs in myocardial repair, and review the recent development of extracellular vesicle-loading systems based on their unique nanostructures and physiological functions, as well as the application of engineering modifications in the diagnosis and treatment of myocardial repair.
Keywords: extracellular vesicles, myocardial repair, diagnosis and treatment, drug delivery system, engineering strategy
Introduction
About 16.5 million people die of cardiovascular disease every year and is still the leading cause of death according to the Global Burden of Disease (GBD) study (1). The case fatality rate from cardiovascular diseases is expected to rise further due to unhealthy lifestyles and aging population. Ischemic heart disease can be divided into coronary artery disease and myocardial disease. Coronary artery occlusion results in blood flow restriction, leading to myocardial hypoxia and subsequent tissue death (2). The damage dealt to the ischemic myocardium becomes main contributor of deteriorating heart failure and eventual mortality. The adult human left ventricle contains about 2 to 4 billion cardiomyocytes which are terminally differentiated cells lacking the ability to re-enter the cell cycle and proliferate (3). Large numbers of cardiac muscle cells die when ischemia occurs and are eventually replaced with non-contractile scar tissue. Therefore, it is of utmost importance to explore novel and clinically pragmatic strategies for myocardial repair.
Extracellular vesicles (EVs) are a group of membranous vesicles released by all cell types. These vesicles can range in diameter from 30 to 1,000 nm (4, 5). Exosomes are a subset of extracellular vesicle about 30–150 nm in size, with characteristic transmembrane proteins, such as CD63. Microvesicles are another common EVs with a particle size of 100–1,000 nm. Different from exosomes, which are secreted by cells, microvesicles are formed by cell membrane bubbling. Due to the limitation of the separation method, generally obtained exosomes refer to a mixed population of small EVs (sEVs). Since most published data cannot accurately determine whether the function of exosomes is generic EV activity or exosome-specific activity, we thus chose here to use the generic term EVs to represent the types of vesicles isolated non-specifically. These can be isolated from amniotic fluid, urine, cerebrospinal fluid, lymph and other body fluids (6–9). As an important carrier of intercellular information exchange, EVs are widely involved in the processes of myocardial angiogenesis, myocardial fibrosis and immune inflammatory response, and are expected to become a new target for clinical treatment of cardiovascular diseases (10–13). EVs contain a large number of endogenous proteins with different functions, including: tetraspanins proteins, heat shock proteins, endogenous cellular proteins, and lipid-related proteins such as phospholipase. In addition, EVs contain a variety of different types of RNA molecules such as mRNAs, circRNAs, miRNAs, snoRNAs, lincRNAs, and rRNA (14–16). The biological effect of EVs is conferred upon delivery of these proteins and RNA molecules to the recipient cells. EVs are widely studied, can be targeted, biocompatible, and immunogenic, which provide a potential therapeutic tool for clinical cardiovascular diseases.
EVs play an important role in intercellular communication, providing a new acellular therapy (16–19). Although many advances have been made in basic research on EVs repairing damaged myocardium, there are still challenges in clinical use and some key questions remain to be answered. In view of the great potential of EVs for myocardial repair, with the continuous exploration and solution of key problems, it will have a very broad clinical prospect. In this review, we discuss the advances and challenges of EVs in cardiovascular studies based on the structure and physiological function, development, advantages, engineering modification and application of EVs drug loading systems in myocardial repair and diagnosis.
Mechanism of EVS Repairing Damaged Myocardium
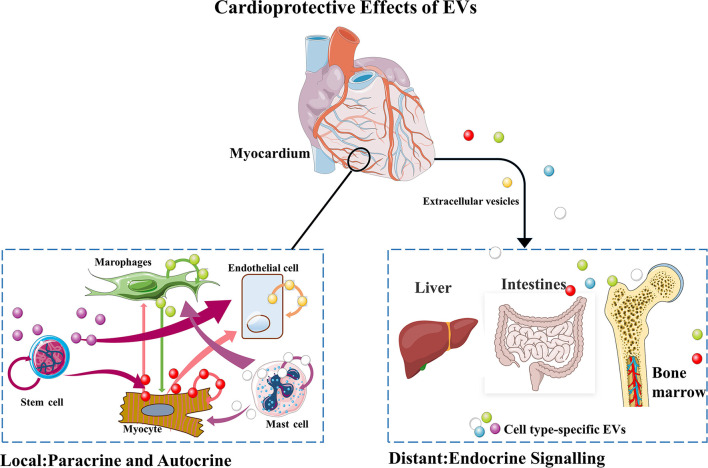
Since the discovery of EVs, basic research on EVs have emerged especially in the field of cardiovascular disease. The role of EVs in cardiovascular disease varies due to the diversity of their origin, production pathway and content. As shown in Figure 1, EVs that have therapeutic effects on ischemic myocardium include local source (for example, paracrine and autocrine) and distant source (for example, mesenchymal stem cells derived from bone marrow). Recent studies have found that some clinical drugs can play a cardioprotective role by regulating EVs production. For example, Lionetti has demonstrated that conventional cardiovascular drugs increase release of EVs promoting proliferation of cardiac progenitor cells (20). More interestingly, changes in the in vivo distribution of EVs may also play a role in cardiac protection (21). These studies further illustrate the important roles of EVs in myocardial repair. In this section, we will summarize the mechanisms of EVs of different origins in myocardial repair.
Figure 1.
Mechanisms of EVs-mediated local and distal communications of the heart.
Role of Cardiomyocyte-Derived EVs in Myocardial Repair
EVs are involved in the process of cardiovascular pathology, the communication between cardiomyocytes, fibroblasts, smooth muscle cells and endothelial cells, and the regulation of cardiac regeneration, ventricular remodeling and angiogenesis (22–24). Cardiomyocytes are the main cells of the heart and their EVs play different roles in myocardial repair (25). Myocardial ischemia and I/R injury are the main causes of myocardial injury. And in this part, we mainly describe the effect of cardiomyocyte-derived EVs on injured myocardium.
Myocardial infarction is a common manifestation of ischemic heart disease/coronary artery disease (26). It is characterized by sudden interruption of coronary blood flow and necrosis of supplying cardiomyocytes. Although primary coronary angioplasty and drug therapy repair the impaired cardiac function to some extent, the mortality rate remains high. More and more evidences have shown that paracrine factors play an important role in the process of myocardial infarction, the changes of microRNAs (miRs) in circulation can accurately reflect the myocardial injury in vivo (4), and the nearby living myocardium can protect myocardial cells from hypertrophy by capturing EVs. Circulating miRs released by the damaged myocardium after acute myocardial infarction (AMI) can also be transferred to distal organs through circulation via exosomes and affect the biological activity of recipient cells functionally. Yang et al. (27) found that miR-30 was highly enriched in exosomes isolated from serum of AMI patients or hypoxic cardiomyocyte culture medium, and miR-30a mediated up-regulation of core autophagy regulators Beclin-1, Atg12 and LC3II/LC3I. Regulating autophagy through exosomes is also a promising strategy for the treatment of ischemic heart disease. It has been found that rat cardiomyocytes can promote survival and inhibit apoptosis by releasing a variety of paracrine factors, including insulin-like growth factor-1, vascular endothelial growth factor (VEGF) and transforming growth factor-β, under co-culture conditions (28, 29). Cardiac Progenitor Cells participate in cardiac function recovery by secreting EVs (CPC EVs) through a paracrine mechanism. CPC EVs can reduce scar formation, alleviate undesirable remodeling, and improve cardiac function in acute and chronic myocardial infarction models (28). These findings are supported by other preclinical models of myocardial infarction (26).
The process of myocardial ischemia/reperfusion (I/R) involves injury of endothelial cell function and structure in a number of ways, including mass release of reactive oxygen species (ROS), direct injury of white blood cells, calcium overload, and reduced secretion of NO. EVs derived from cardiomyocytes were initially discovered under hypoxia and re-oxygenation conditions (6–8). Cardiomyocytes secreted exosomes containing circHIPK3 under hypoxic conditions to protect injured cardiomyocytes. In addition, the inflammatory response is involved in all aspects of I/R injury (25, 30). Chen et al. (31) found that eNOS activation in cardiac microvascular endothelial cells (CMECs) required a crosstalk between cardiomyocytes (CMs) and CMECs through the uptake of CM-derived sEVs. Tongxinluo induced CM-sEVs contain increased levels of long Intergenic non-protein coding RNA, regulator of reprogramming (Linc-ROR). Upon uptake into CMECs, linc-ROR downregulates its target miR-145-5p leading to activation of the eNOS pathway by facilitating the expression of p70s6k1 in these cells. The activation of CMEC-derived eNOS works to increase survival in both the CMECs and the CMs themselves.
Role of Immune EVs in Myocardial Repair
Immune cells play an important regulatory role in the development of myocardial ischemia, I/R injury, septic cardiomyopathy and chemotherapy-related cardiomyopathy (32–34). EVs derived from immune cells show pleiotropism in pathological states. EVs have therapeutic potential of anti-apoptosis and anti-fibrosis, promoting angiogenesis, inhibiting ventricular remodeling, improving cardiac function and inhibiting local inflammatory response (35).
In the process of myocardial injury, macrophages are recruited to the damaged area to initiate release EVs into the peripheral tissue. Macrophage-derived EVs aggravate myocardial injury, inflammation, and promote myocardial fibrosis. Studies demonstrate that in the process of myocardial ischemia, macrophage derived exosomes deliver miR-155 to cardiac fibroblasts, which inhibit proliferation and promoted inflammation, suggesting that macrophage derived EVs containing miR-155 aggravate myocardial injury (36). Macrophages themselves are also receptors for miR-155, and endothelial cell derived EVs containing miR-155 can promote macrophage polarization (37).
Antigen presenting T cells have the ability to release specific EVs. Treg cells can improve the healing and remodeling after myocardial infarction (38, 39) and delays the progression of atherosclerosis (40). However, compared with cardiovascular diseases, Treg-derived exosomes are more recognized in organ transplantation and have a greater application prospect (41–44). The regulatory effect of DC-based EVs on the heart may depend on Treg activation, but it is still difficult to determine whether Treg exosomes have a cardioprotective effect. Studies have shown that Treg inhibits the effect of other T cells (43, 44), such as Th1 cells dependent on the transfer of exosomal miRNAs to receptor cells (45). Neutrophils are the most abundant white blood cells in human peripheral blood, accounting for about 50 to 70% of the total number of white blood cells. They play an important role in the innate immune system and are the first line of defense for the body to respond to the invasion of pathogens. They can resist the invasion of external pathogens through various ways such as phagocytosis, degranulation and production of reactive oxygen species (46). In the early stage of myocardial infarction, neutrophils and monocytes rapidly infiltrate the infarct region (47), releasing inflammatory EVs and triggering an inflammatory cascade (48). Neutrophil-derived and mast cell-derived EVs play an important role in initiating injury-related molecular patterns (DAMPs) of the innate immune response.
Stem Cell-Derived EVs in Myocardial Repair
Different types of stem cell (SC) derived EVs can convey different biological information. EVs with cardioprotective function may come from marrow mesenchymal stem cell (MSC), embryonic stem cells, and hematopoietic stem cells. Various strategies have been tried for the treatment of cardiovascular disease with SC transplantation therapy. Studies have found that the paracrine factors of transplanted cells, not the transplanted cells themselves, play a major role in repairing damaged tissue.
Cardioprotective Effects of Embryonic Stem Cells (ESC), Induced Pluripotent Stem Cells (iPSC), and Their Derrivatives
Khan et al. (49) found that ESC exosomes enhanced angiogenesis, cardiac progenitor cells (CPC) survival, proliferation and cardiac repair after myocardial infarction, and also participated in anti-inflammatory effects, enhanced cardiac function and reduced fibrosis. Wang et al. reported that iPSC exosomes protected cardiomyocytes from H2O2-induced oxidative stress by inhibiting caspase 3/7 activation, and alleviated IR injury in mouse myocardium by delivering cardiac protective miRs such as miR-21 and miR-210 (50). iPSC-derived cardiomyocytes from human placental amniotic mesenchymal stem cells were successfully implanted in mice to improve myocardial activity and cardiac function after myocardial infarction (51). Transplantation of ESC and iPSC derived cardiomyocytes has been employed, however problems such as arrythmia and poor retention of transplanted cells over time limit practical clinical applications (52, 53). iPSC/ESC cardiomyocyte EV therapy improves heart function without the risk of poor engraftment and induction of arrythmia, while allowing for the generation of patient specific EVs.
Properties of Multipotent CPC and MSC Derived EVs
Studies on CPCs and their derived exosomes therapeutic potential have demonstrated improvements in cardiac function (54). Physoxic conditions (5% O2) in cultured CPCs increase the number of EVs released while maintaining basal cell gene expression and cell morphology as opposed to hypoxic conditions (55), demonstrating changes in the microenvironment of EV donor cells modulate dosage of EV release in specific contexts. Therefore, regulation of CPC secretion can affect the paracrine potential of their EVs.
MSC-EVs has been shown to have similar or even better therapeutic activity than parent MSCs in inhibiting inflammation, oxidative damage and the proliferation of fibrosis in damaged tissues (56, 57). In ischemic cardiovascular disease, MSC-EV therapy reduces cardiomyocyte apoptosis, thereby reducing the extent of infarction and improving functional recovery and new vessel formation. Bone marrow derived MSCs have immunosuppressive properties, and the use of MSC-derived EVs alone can avoid the occurrence of immune rejection and enhance the repair of damaged cardiac muscle (58). Ju et al. (59) demonstrate that intramyocardial injection of MSC-derived exosomes can promote myocardial cell proliferation and angiogenesis, while reducing infarct size in mice with myocardial infarction. These results suggest that MSC-derived exosomes can play a protective role in the early stage of myocardial infarction.
Engineering Strategies of EVS in the Diagnosis And Treatment Of Cardiovascular Diseases
To improve the therapeutic effect of chemical and biomolecular drugs, researchers have used nanoparticles of various scales as drug carriers (60). However, the clinical transformation of vectors faces two major problems: Safety of materials and vectors and rapid clearance of reticuloendothelial system (RES) or mononuclear Phagocyte system (MPS) (61). Compared with nano-carriers constructed from artificial materials, endogenous nano-carriers have the advantage of biocompatibility in vivo and have broad prospects in improving drug delivery and therapeutic effect. EVs, as nanocarriers, have the advantages of being similar to cell membranes, small in size, negatively charge, less recognized by immune cells, and penetration of deep tissues (62). Therefore, EVs may serve as an ideal natural nanomaterial for the delivery of myocardial repair drugs (Table 1).
Table 1.
Summary of application of extracellular vesicles (EVs) as carriers in myocardial repair.
| Origin | Isolation strategy | Type and size | Cargo loading | Type of disease | clinical outcomes | References |
|---|---|---|---|---|---|---|
| Mesenchymal stem cell | Centrifugation Total Exosome Isolation reagent (Invitrogen) | Exosomes 135 nm | Lamp2b+IMTP transfection | AMI | IMTP-exosomes exert enhanced therapeutic effects | (63) |
| Induced pluripotent stem cell–derived cardiomyocytes (iCMs) | Differential ultracentrifugation method | EVs 98–677 nm | Mitochondrion iCMs self-contain | Myocardial infarction | M-EVs improve mitochondrial function and prevent post-MI LV remodeling | (64) |
| Mesenchymal stem cell | Ultracentrifugation | EVs | Chronic myocardial ischemia | Mesenchymal cell–derived EVs induct capillary and arteriolar growth resulting in increased cardiac output and stroke volume | (65) | |
| Genetically modified MSCs overexpressing CD47 | Ultracentrifugation | EVs 90–350 nm | miR-21 Electroporation | I/R injury | miR21-loaded CD47-Evs exert anti-apoptosis effects, alleviate cardiac inflammation, improve cardiac morphology and the functional recovery of the I/R myocardium | (66) |
| Mesenchymal stem cell Raw 264.7 | Exosome isolation kit (Beyotime, China) LiposoFast extruder apparatus (Avestin, Canada) | Hybrid EVs 109.76 nm | RAW 264.7 membrane fusion-extrusion | I/R injury | Mon-Exos were shown to promote endothelial maturation during angiogenesis and modulate macrophage subpopulations after MI/RI offering additional techniques to help clinicians better manage regenerative therapeutics for ischemic heart diseases | (58) |
| Ultrafiltration by centrifugation (UFC) | Chimeric EVs 30–150 nm | DPS/ischemic homing peptide/incubation | I/R injury | IschCDC-EVs greatly enhances localization to injured myocardium as a potential targeting carrier of CVD | (67) | |
| HEK293 cells expressing CTP-tagged FLAG-LAMP2b | Sartorius 10-kDa (5 L) poly- ether sulfone membranes | Chimeric EVs <150 nm | siRAGE-loaded C-sEVs | Myocarditis | C-sEVs may be a useful drug delivery vehicle for the treatment of heart disease | (68) |
Myocardial Repair Strategies Using EVs as Carriers
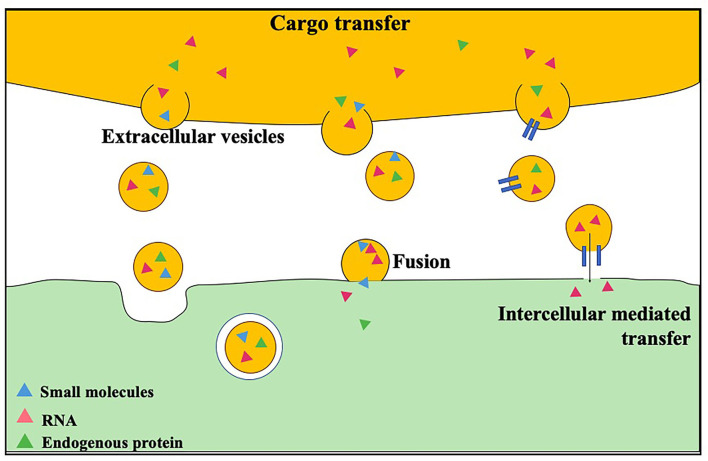
The research on EVs delivery of drugs is increasing gradually (69, 70). Some small molecule chemical drugs and gene drugs have been successfully loaded into EVs, showing great potential in the treatment of tumors, cardiovascular diseases and neurological diseases. EVs can accumulate drugs in treated cells, improve the stability and blood circulation time of small molecule drugs, and improve the efficacy of small molecule drugs (Figure 2). Sun et al. (71) showed that curcumin-loaded exosomes can increase the concentration of curcumin in vivo, increase the stability of the drug, and improve its anti-inflammatory and antioxidant effects.
Figure 2.
Loading and transshipment of EVs.
RNA is an unstable macromolecular material making it difficult to achieve effective delivery. Existing deliveries include use of cationic liposomes, dendritic macromolecules, cationic polymer particles, but the carrier is still in the process of clinical application. This technology faces problems with security, stability, and tissue targeting (72). Overexpression of specific microRNAs via transfection in donor cells enables their packaging into EVs. For example, overexpression of miR-125b targeting SIRT7 in bone marrow MSC-derived exosomes down-regulated the levels of Bax, caspase-3 apoptotic proteins and IL-1β, IL-6, TNF-α inflammatory factors, and up-regulated the expression of Bcl-2, in order to repair the myocardial injury of I/R-rats (73). In addition, miR-181a delivered by exosomes of MSCs may inhibit the inflammatory response through c-Fos and promote the polarization of Treg cells to protect myocardial injury caused by miRs (74–77). These results suggest that donor cell engineering allows for directed cargo loading of small nucleotides into EVs, providing an effective therapeutic strategy for ischemia-reperfusion injury.
EVs can also be used as carriers of proteins for myocardial repair. Proteins can be genetically engineered from donor cells or encapsulated directly into EVs. In the former, transfected donor cells with a plasmid carrying the target gene are used to synthesize the protein encoded by the inserted gene. These proteins are loaded into exosomes. The supernatant of the cell culture is collected for isolation and purification of exosomes. In addition, proteins can be directly encapsulated in exosomes. Studies have shown that Yim et al. (78) encapsulated proteins in exosomes through an optically reversible protein-protein interaction, which significantly increased the level of target proteins in the receptor cells in vivo and in vitro, further proving the possibility of EV cargo design for not only nucleotides, but also protein.
Design and Modification Strategies for Tissue Specific Targeting of EVs
EVs are considered as ideal natural drug carriers due to their good histocompatibility and low immunogenicity, but drug loading and targeted delivery are the problems that must be solved when EVs are used for cardiac repair. To complete drug loading and targeted delivery, researchers have paved the way for engineering EVs. For example, gene engineering technology can be used to express targeted proteins or peptides to the surface of EVs, which can improve the targeting effect of the drug and enhance the efficacy. However, the complexity of the genetic modification process has greatly hindered its widespread application. Recently, modification methods based on physical and chemical properties have been developed. In this section, we will summarize the design and modification strategies of EVs as therapeutic platforms. As shown in Table 2, we summarize strategies for the engineering of EVs.
Table 2.
Strategies for cargo loading into EVs.
| Strategiesa | Methods | Advantages | Disadvantages | Prominent examples | References |
|---|---|---|---|---|---|
| Cargo loading into donor cells | Co-incubation | Simple and feasible; No damage to membrane integrity | Poor specificity; Low loading efficiency | Delivery of DHA and S1P | (79) |
| Transfection | Simple and feasible; No damage to membrane integrity | Induce donor cell apoptosis; Impair biological responses; Inefficient packaging | Delivery of miRNA-181a, Lamp2b, IMTP and MiR21 | (63, 74, 80) | |
| Direct loading into EVs | Electroporation | Simple and quick; Higher loading efficiency than transfection | EVs aggregation; siRNA precipitation; Not suitable for some RNAs with special structures | Delivery of MiR21 | (66) |
| Extrusion | Efficient packaging | Cause potential damages to biofunctional contents | Targeted delivery of MSC-exosomes | (58) | |
| Freeze and thaw cycles | Higher loading efficiency | EVs aggregation; Lower drug loading capacity than extrusion | Delivery of curcumin and miR-144-3p | (81) | |
| General modification of EVs membrane | Chimeric EVs | Cell membrane targeting ability | Cost of presenting chimeric peptides | Targeted delivery of MSCs and CDC-XOs | (82, 83) |
| New engineered EVs-based platforms | Hybrid EVs | Easy preparation and scalability; Adjustable physical parameters | May lose biological functions of integral EVs; Low homogeneity | Delivery of HELIOS | (84) |
| New engineered EVs-based platforms EVs membrane camouflaged NVs | Maintain complex EVs membrane structure; Specific targeting ability; High therapy efficacy | Low scalability; Increase the difficulty of fabrication; Time-consuming | Delivery of MiR-21 mimics | (85) |
CDC-XOs, cardiosphere-derived cell exosomes; CREKA, cysteine-arginine-glutamic acid-lysine-alanine; DHA, docosahexaenoic acid; HELIOS, highly efficient life-support intracellular opto-driven system; IMTP, ischemic myocardium-targeting peptide CSTSMLKAC; LFA1, lymphocyte function-associated antigen1 or αLβ2 integrin; Mac1, macrophage receptor 1 or integrin αMβ2; MSC, mesenchymal stem cell; NVs, nanovesicles; S1P, sphingosine-1 phosphate.
Tian et al. (86) conjugated functional ligand RGDyk cyclic peptide to the surface of EVs through biological orthogonal reaction between the EVs with surface modification of cyclostyle and the azide polypeptide. They encapsulated them with curcumin to target the cerebral lesion area in a cerebral artery occlusion mouse model. This effectively inhibited inflammatory response and apoptosis in the focus region, and developed a novel exon-based targeted drug delivery vector for ischemic brain injury. Ligand fragments or homing peptides discovered by phage display and in vivo biopanning methods fused to the enriched molecules on the external part of exosomes have been exploited to improve the ability of exosomes to target specific tissues or organs carrying cognate receptors.
The homing peptide of 9AA (CSTSMLKAC, 9AA) can specifically target the myocardial cells of MIRI (87). Xu Wang used technology of molecular cloning and lentivirus packaging to engineer exosomal enriched membrane protein (Lamp2b) fused with ischemic myocardium-targeting peptide 9AA. The result found that exosomes engineered by IMTP can specially target ischemic myocardium, and mesenchymal stem cell-derived IMTP-exosomes exert enhanced therapeutic effects on AMI.
In addition to peptides, binding targeted proteins such as nanosomic and signal regulatory protein α to exosome surfaces has also become an effective targeting strategy (88). For cardiovascular diseases, gene modification can also be used to overexpress bioactive substances with cardio protective effects in exosomes, such as miRNA or proteins to enhance exosome mediated cardio protective effects and reverse the effect of the pathological environmental secretome. Ni et al. (89) found that tissue matrix metalloproteinase inhibitor 2 (TIMP2) modified MSCs derived exosomes from human umbilical cord blood activated the Akt/Sfrp2 pathway, inhibiting cardiomyocyte apoptosis and extracellular matrix remodeling while promoting angiogenesis, improving heart function following myocardial ischemia. Compared with normal HUC-EXO, HUC-ExotimP2 increased the number of CD31+ and lectin immune-active cells in myocardial infarction rats and promoted angiogenic activity in the infarct area.
Although exosomes have shown promising in the field of drug delivery, it is not easy to achieve specific targeting of exosomes through surface modification, and the reaction conditions need to be strictly controlled to avoid the destruction and aggregation of exosomes caused by improper temperature, pressure and osmotic pressure. At present, the efficiency of exosome targeted drug delivery is not ideal as modifications result in clearing by the immune system.
Problems in the Preparation of EVs
EVs have favorable biocompatibility, non-immunogenicity and non-tumorigenesis. They are physiologically more stable than graphted cells, circulate throughout the body, and can cross the blood-brain barrier, and are more resistant to freezing and thawing than cells, have the advantage of long-term storage (90), and have natural properties for therapeutic use. In addition, EVs prove suitable for modification to deliver drugs to target cells (91). However, there is still a lack of standardized methods for the collection, separation and purification of EVs, and different separation methods lead to large differences in the purity, size and concentration of EVs (92), hindering the introduction of EVs into clinical practice.
The most common EVs separation technique is differential centrifugation, however, EVs obtained by this technique often contain aggregates of cell culture medium, cell proteins and particles (93). In addition, production of EVs is difficult to scale up due to time-consuming isolation process, low yield, and need for specialized equipment. Another common separation technique is based on monoclonal antibodies to isolate EVs-associated antigens (94). However, the disadvantage of this technique is low specificity: non-EVs materials or cells carrying the antigen can easily bind with the antibody, which greatly reduces the purity of the extracted EVs (95, 96). High performance liquid chromatography can provide highly purified EVs, but this technique also requires expensive equipment and low yields, which limits its widespread use (93). At present, there is still a lack of a consensus, standardized high specificity, high yield EVs isolation and purification method.
The lack of appropriate preservation methods is another major problem that limits the clinical use of EVs. Generally, EVs are stored at −80°C, become unstable under long term storage (97). Studies have shown that the surface and morphological characteristics of EVs change and the protein degrades after 4 days of storage at −80°C (98). The size of EVs decreased when stored at 4 or 37°C, indicating structural change or degradation. These studies show that the storage conditions of EVs are relatively harsh, which limits its clinical use (99).
Before clinical use of EVs in the treatment of cardiovascular diseases, there are still many difficulties to be solved: the criteria for isolation and identification, high yield and more economical protocols are still to be developed. Current storage conditions are harsh, and the in vivo dynamics of EVs have not been studied in detail. EVs have irreplaceable advantages in the treatment of cardiovascular diseases, including those diseases that lack effective drug therapy (100).
Application of Engineered EVS in Myocardial Repair
Natural EVs are an ideal drug delivery system, but there are still some problems to be solved, such as separation, identification criteria, and high yield. The emergence of engineered EVs can overcome the challenges of mass production, identification criteria, and isolation and purification to advance the clinical application of exosomes. At the same time, the emergence of block copolymers provides the possibility for the personalized customization of engineered extracellular vesicle, improves the targeting of drugs, and enables the drugs to be specifically concentrated at the injured myocardium, thus increasing the intensity of action while reducing the dose of drugs.
Preparation of Engineered EVs
Engineered EVs are nano-vesicles made of natural EVs or cell membranes by special methods. Engineered EVs can be divided into multilayer vesicles 50–1,000 nm and single-layer vesicles, in which single-layer vesicles are further divided into small vesicles (SUV) 20–100 nm, large vesicles (LUV) 100–1,000 nm and large vesicle (GUV) 1–200 μm (5, 101). Engineered EVs also need to be given targeted, stimulus-responsive properties. To target cardiomyocytes, EVs need to be modified by genetic engineering or chemistry.
The exosomal donor cells are genetically modified to express the targeted peptide, effectively expressing the targeted peptide on secreted exosomes (63). Kim et al. (102) used lentiviral vectors fused with lysosomal associated membrane glycoprotein 2B and ischemic myocardial targeting peptide to genetically modify bone marrow MSCs to express ischemic myocardial targeting peptide. Fluorescence microscopy imaging showed that the number of exosomes expressing ischemic myocardial targeting peptide was greater than that of natural exosomes. Li et al. (103) reported a programmed exosome that would provide human antigen R with extremely high affinity to RNA. HuR and exosome transmembrane protein CD9 sequence were fused to modify donor cells, and the modified donor cells expressed the fusion protein HuR-CD9. These generated exosomes actively increased RNA loading. Ohno et al. (104) transfected pDisplay vector with GE11 peptide specifically binding to expression of EGFR into HEK293 cells to successfully express G11 peptide on the isolated exosome membrane. In subsequent experiments, they demonstrated the inhibitory effect of EGF-specific exosomes delivered Let-7a miRNA to EGFR-expressing xenograft breast cancer tissue in RAG2 −/− mice. Gene modification in exosome donor cells can greatly improve the stability of functional exosomes, but it is expensive and time-consuming to perform gene manipulation in donor cells.
At present, there are two chemical modification methods for artificial vesicles: wet chemistry method and microfluidic technology (101). The wet chemical methods grouped with the thin-film hydration method is the most classic (105). This method can be used in the synthesis of multilayer vesicles (106). Hammons et al. (107) used the film hydration method to dissolve dioleyl-phosphatidylcholine and poly-oxyethylene in chloroform, which was dried by rotary evaporation and then vacuum drying. Next, aqueous solution containing carbon nanotube pore protein was used for hydration. Ultrasound was used to remove the film on the inner wall of the container and the sample was extruded through the 200 nm polycarbonate film with a micro extruder to prepare hybrid bionic vesicles (108). Based on the film hydration method, a team replaced the “skeleton” required for self-assembly with block copolymers instead of pure phospholipids which broadened the application scope of the original method (109). In 2018, inkjet printing technology began being used to prepare emulsions (110). Inkjet technology can print amphiphilic molecules directly onto the receiving medium to form vesicles (111), which can quickly encapsulate fragile biomolecules such as proteins or other biological complexes, maintaining some degree of structural integrity of these molecules (112).
Engineered EVs for the Diagnosis and Treatment of Myocardial Repair
Currently, the reassembly of phospholipid membranes with different functions has become a new direction of engineered EVs, which makes it possible for EVs to have specificity, enabling repair and anti-inflammatory functions at the same time. However, there are few related studies, and they are mainly used in cardiac ischemia and ischemia reperfusion injury in cardiovascular diseases.
Use of Engineered EVs in the Repair of Injured Myocardium
MSC-derived exosomes have attracted attention as paracrine components that mediate cardiac stem cell repair (63, 113, 114). Zhang et al. (58) obtained monocyte cell membrane isolated from RAW264.7 monocyte macrophages and EVs derived from MSCs through the fusion and extrusion of 0.2 μm polycarbonate membrane to obtain a monocyte simulation MSC-EVs. And the analog can imitate monocyte recruitment characteristics, enhancing myocardial recovery after ischemia-reperfusion injury by increased targeting efficiency, reducing collagen volume, reversing left ventricular anterior wall hypertrophy, reducing inflammation reaction to protect heart function, and promoting the role of angiogenesis. It has previously been reported that MSC-derived exosomes can repair damaged tissues (56), but how to precisely deliver exosomes into recipient cells in vivo remains a problem. As early as 2002, Lestini et al. (113) showed that polypeptides can direct liposomes to receptors expressed on pathologically stimulated vascular cells. Based on this, in recent years, a peptide known as a homing peptide has been used in the treatment of myocardial ischemia. Wang et al. (63) fused an exosome enriched membrane protein (Lamp2b) with the ischemic myocardial homing peptide CSTSMLKAC (IMTP) using molecular cloning and lentiviral packaging techniques. The results showed that MSC-derived IMTP exosomes had enhanced therapeutic effect on AMI. Antes et al. (67) designed a modular extracellular vesicle-anchoring platform DPS composed of a combination of 1, 2-BIS (Dimethylphosphino)ethane, polyethylene glycol and Streptavidin. CDC-EVs loaded with ischemic myocardial homing peptide coupled with DPS have proven to enhance the localization of injured myocardium and play a better role in myocardial repair.
Scaffolds for Controled Release and Local Targetting of EVs
To achieve precise drug delivery, engineered EVs need to add stimulus-response modules. Drug release can be achieved by temperature, light, ultrasound, magnetic field or electric field stimulation. This greatly improves the controllability of drug delivery, thus increasing drug targeting and reducing drug side effects to achieve more accurate and controllable treatment of diseases.
MSC-EVs have high therapeutic potential for tissue repair. There is evidence of functional engineered EVs isolated from human bone marrow MSCs by introducing lentiviral particles containing BMP2-expressing plasmids. By enhancing the BMP2 signaling cascade in target cells and tissues, the repair of tissues such as myocardium and bone are promoted (115). However, this does not meet all treatment needs. Previous studies on EVs administration have explored the mechanisms of local application (116), systemic administration (117), intrathecal administration (118), vitreous injection (119), and nasal administration (120), but problems such as high demand, low efficiency and strong ectopic effect of EVs have limited its application. Huang et al.'s team encapsulated it in alginate saline gel to promote angiogenesis and regeneration of tissues such as skin, bone, and heart muscle, achieving location or site specificity. These physical wrapping methods resulted in EVs being released within hours of being placed in the desired location (121). Recent studies have used EVs encapsulated in alginate hydrogels for regenerative medicine applications in the treatment of myocardial ischemia or myocardial infarction (122).
EVs for Individualized Therapy and Diagnosis of Disease
Personalized treatment, also known as personalized medicine or precision medicine, refers to a treatment mode that takes the genetic information and specific disease conditions of patients as guidance and makes targeted treatment plans to improve the cure rate and reduce side effects of patients. Since the “Precision Medicine” plan was put forward in 2015, various countries around the world have launched personalized medicine research projects with huge investment. As a treatment mode of precision medicine, personalized drug screening and drug delivery are required according to patients' physical conditions and disease development. Tailored to the individual situation of each cardiovascular patient, the best treatment plan is designed to achieve a specific medical model that maximizes treatment effect and minimizes adverse reactions (123). Compared with the simple surgical resection for most cardiovascular patients in the past, this treatment method emphasizes patient specific therapies.
Given the limited regenerative capacity of the human heart, stem cell-derived cardiomyocytes are a promising source of alternative cell therapy. iPSC-derived cardiomyocytes (iCMs) have shown potential to attenuate ischemic injury and restore cardiac function in preclinical myocardial infarction models. At present, cell transplantation poses a variety of risks to the recipient. However, the collection and administration of EV's from patient autologous cells could become an effective personalized treatment method.
Use of Endogenous Circulating EVs in the Diagnosis of Injured Myocardium
The conventional diagnostic methods of heart failure include echocardiography, MRI imaging and identification of myocardial necrosis markers. Exosomes show great diagnostic potential as biomarkers of AMI, and we focus on the diagnostic value of miRNAs and proteins carried by exosomes (124, 125).
miRNAs found in serum and plasma are characterized by stability, time, and tissue specificity. Exosomes, as effective carriers of miRNAs, may provide a new possibility for the diagnosis of AMI. Beg et al. studied the plasma exosome miRNA in patients with heart failure and normal controls with average LVEF of (22.2 ± 7.2) %, and found that the ratio of miR-146a/miR-16 in peripheral blood of patients with heart failure was significantly higher (126). Studies have shown that exosome miR-146a has a cardiomyocyte protective function and has a protective effect on oxidative stress (114). The exosomes isolated from the serum of patients with myocardial infarction contain miRNA-183. Studies have confirmed that the level of miRNA-183 is positively correlated with the degree of myocardial ischemia injury, and classified miRNA-183 as a new biomarker for the diagnosis of myocardial infarction. miRNA-183 is highly enriched in exosomes in patient serum with AMI (127–129). It is worth noting that exosome derived miRNAs can also be detected in urine. In AMI rats, exosome associated miR-1 specifically increased in both blood and urine (91, 130), suggesting that urine derived exosomes may be a new diagnostic method for AMI. The non-invasive extraction of urine may greatly improve the diagnostic rate of myocardial infarction and has a good clinical application prospect. Though miRs is the leading diagnostic marker in the context of exosomes, the potential value of exosomal specific carrier proteins should not be overlooked in the diagnostic evaluation of myocardial infarction.
Summary and Outlook
EVs contain a variety of proteins, nucleic acids, and lipids, as carriers of intercellular material and information, they can play an important role in influencing the course of disease by regulating interactions between heterogeneous cell microenvironments. EV cargo is effected by disease states, which give circulating EVs the potential as a diagnostic biomarker, which earlier and more accurately reflect the clinical progress of certain diseases, treatment response and prognosis judgement. The study of EVs provides an option for acellular therapy for regeneration and improvement of cardiac function. A large number of experiments have shown that EVs have great therapeutic potential in the treatment of myocardial injury and are also ideal drug delivery tools.
Although many advances have been made in basic research on extracellular vesicle myocardial repair, many challenges remain and some key questions need to be answered in depth before clinical transformation can occur. For example, which cell-derived EVs are most effective at repairing the heart muscle; What are the exact mechanisms of selection and packaging of extracellular vesicle contents; How to regulate the process of extracellular vesicle production; How to efficiently prepare and purify exosomes. How to maximize the retention of EVs in the body so that they can function properly. In addition, allogeneic cells are often used in current studies because of the convenience of obtaining them, but their immunogenicity, individual rejection and in vivo safety remain to be confirmed. In vivo pharmacokinetic properties, mode of administration, safety, and industrialization must be fully evaluated before clinical transformation can be achieved. The current studies on exosomes as biomarkers in cardiovascular diseases are mostly based on small samples of patients. Moreover, the therapeutic effect of exosomes has only been confirmed in animal experiments and multiple heart-related cells, and no systematic human experiments have been conducted. Despite the above challenges, with the continuous development of cell drug delivery systems, clinical transformation of nano-biomimetic drug delivery systems, the field demonstrates a promising future.
Author Contributions
CL and NB were responsible for writing the main body of the article. TD and HC retrieved the literature. JT and XL modified the format. DH and YD made charts. PY, JL, ZF, and LW provided ideas and guidance for the writing of the article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (82104495, 82174161, 81804132, 81803019, and 82002253), Macao Youth Scholars Program (AM2021023), Scientific research projects of Guangdong Bureau of traditional Chinese Medicine (No. 20200513093851), Guangdong Basic and Applied Basic Research Foundation (2021A1515012573 and 2019A1515111108), Science and Technology Foundation of Guangzhou City (202102010257), State Key Laboratory of Dampness Syndrome of Chinese Medicine Research Foundation (SZ2021ZZ21), the TCM Research Fund of Guangdong Provincial Hospital of Chinese Medicine (YN2019MJ15), and the Fund of Science and Technology Innovation Strategy of Guangdong Province (191900105).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019 update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76:2982–3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taggart C, Wereski R, Mills NL, Chapman AR. Diagnosis, investigation and management of patients with acute and chronic myocardial injury. J Clin Med. (2021) 10:2331. 10.3390/jcm10112331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller P, Lemcke H, David R. Stem cell therapy in heart diseases - cell types, mechanisms and improvement strategies. Cell Physiol Biochem. (2018) 48:2607–55. 10.1159/000492704 [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Hu YW, Zheng L, Wang Q. Characteristics and roles of exosomes in cardiovascular disease. DNA Cell Biol. (2017) 36:202–11. 10.1089/dna.2016.3496 [DOI] [PubMed] [Google Scholar]
- 5.Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. (2018) 7:1535750. 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Rooij E, Purcell AL, Levin AA. Developing MicroRNA therapeutics. Circ Res. (2012) 110:496–507. 10.1161/CIRCRESAHA.111.247916 [DOI] [PubMed] [Google Scholar]
- 7.Charoenviriyakul C, Takahashi Y, Morishita M, Matsumoto A, Nishikawa M, et al. Cell type-specific and common characteristics of exosomes derived from mouse cell lines: yield, physicochemical properties, and pharmacokinetics. Eur J Pharm Sci. (2017) 96:316–22. 10.1016/j.ejps.2016.10.009 [DOI] [PubMed] [Google Scholar]
- 8.Gnecchi M, Zhang ZP, Ni AG, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. (2008) 103:1204–19. 10.1161/CIRCRESAHA.108.176826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang HZ, Wan KJ, Zhou Y, He XX, He DG, Cheng H, et al. A three-dimensional multipedal DNA walker for the ultrasensitive detection of tumor exosomes. Chem Commun. (2020) 56:12949–52. 10.1039/D0CC04360E [DOI] [PubMed] [Google Scholar]
- 10.Zaldivia MTK, McFadyen JD, Lim B, Wang XW, Peter K. Platelet-derived microvesicles in cardiovascular diseases. Front Cardiovasc Med. (2017) 4:74. 10.3389/fcvm.2017.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu SH, Zhang YJ, Li YL, Luo LM, Zhao YL, Yao Y. Extracellular vesicles in cardiovascular diseases. Cell Death Discov. (2020) 6:68. 10.1038/s41420-020-00305-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleury A, Martinez MC, Le Lay S. Extracellular vesicles as therapeutic tools in cardiovascular diseases. Front Immunol. (2014) 5:370. 10.3389/fimmu.2014.00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Abreu RC, Fernandes H, Martins PAD, Sahoo S, Emanueli C, Ferreira L. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat Rev Cardiol. (2020) 17:685–97. 10.1038/s41569-020-0389-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang C, Hopfner F, Katsikoudi A, Hein R, Catli C, Evetts S, et al. Serum neuronal exosomes predict and differentiate Parkinson's disease from atypical parkinsonism. J Neurol Neurosur Ps. (2020) 91:720–9. 10.1136/jnnp-2019-322588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang HZ, He DG, Wan KJ, Sheng XW, Cheng H, Huang J, et al. In situ multiplex detection of serum exosomal microRNAs using an all-in-one biosensor for breast cancer diagnosis. Analyst. (2020) 145:3289–96. 10.1039/D0AN00393J [DOI] [PubMed] [Google Scholar]
- 16.Luo RH, Liu MM, Yang Q, Cheng HJ, Yang HM, Li MH, et al. Emerging diagnostic potential of tumor-derived exosomes. J Cancer. (2021) 12:5035–45. 10.7150/jca.59391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu PP, Zhang B, Ocansey DK, Wenrong W, Qian H. Extracellular vesicles: a bright star of nanomedicine. Biomaterials. (2021) 269:120467. 10.1016/j.biomaterials.2020.120467 [DOI] [PubMed] [Google Scholar]
- 18.Chinnappan M, Srivastava A, Amreddy N, Razaq M, Pareek V, Ahmed R, et al. Exosomes as drug delivery vehicle and contributor of resistance to anticancer drugs. Cancer Lett. (2020) 486:18–28. 10.1016/j.canlet.2020.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu CP, Chen ZD, Ye ZY, He DY, Dang Y, Li ZW, et al. Therapeutic applications of functional nanomaterials for prostatitis. Front Pharmacol. (2021) 12:685465. 10.3389/fphar.2021.685465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casieri V, Matteucci M, Pasanisi EM, Papa A, Barile L, Fritsche-Danielson, et al. Ticagrelor enhances release of anti-hypoxic cardiac progenitor cell-derived exosomes through increasing cell proliferation in vitro. Sci Rep. (2020) 10:2494. 10.1038/s41598-020-59225-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S, Chen X, Bao L, Liu T, Yuan P, Yang X, et al. Treatment of infarcted heart tissue via the capture and local delivery of circulating exosomes through antibody-conjugated magnetic nanoparticles. Nat Biomed Eng. (2020) 4:1063–75. 10.1038/s41551-020-00637-1 [DOI] [PubMed] [Google Scholar]
- 22.Alfi E, Thairi C, Femmino S, Alloatti G, Moccia F, Brizzi MF, et al. Extracellular vesicles (EVs) in ischemic conditioning and angiogenesis: focus on endothelial derived EVs. Vasc Pharmacol. (2021) 140:106873. 10.1016/j.vph.2021.106873 [DOI] [PubMed] [Google Scholar]
- 23.Yuan HX, Chen CY, Li YQ, Ning DS, Li Y, Chen YT, et al. Circulating extracellular vesicles from patients with valvular heart disease induce neutrophil chemotaxis via FOXO3a and the inhibiting role of dexmedetomidine. Am J Physiol-Endoc M. (2020) 319:E217–31. 10.1152/ajpendo.00062.2020 [DOI] [PubMed] [Google Scholar]
- 24.Chen YT, Yuan HX, Ou ZJ, Ou JS. Microparticles (exosomes) and atherosclerosis. Curr Atheroscler Rep. (2020) 22:23. 10.1007/s11883-020-00841-z [DOI] [PubMed] [Google Scholar]
- 25.Yu H, Wang Z. Cardiomyocyte-derived exosomes: biological functions and potential therapeutic implications. Front Physiol. (2019) 10:1049. 10.3389/fphys.2019.01049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balbi C, Costa A, Barile L, Bollini S. Message in a bottle: upgrading cardiac repair into rejuvenation. Cells. (2020) 9:724. 10.3390/cells9030724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, Li Y, Chen X, Cheng X, Liao Y, Yu X. Exosomal transfer of miR-30a between cardiomyocytes regulates autophagy after hypoxia. J Mol Med. (2016) 94:711–24. 10.1007/s00109-016-1387-2 [DOI] [PubMed] [Google Scholar]
- 28.Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res. (2014) 103:530–41. 10.1093/cvr/cvu167 [DOI] [PubMed] [Google Scholar]
- 29.Barile L, Cervio E, Lionetti V, Milano G, Ciullo A, Biemmi V, et al. Cardioprotection by cardiac progenitor cell-secreted exosomes: role of pregnancy-associated plasma protein-A. Cardiovasc Res. (2018) 114:992–1005. 10.1093/cvr/cvy055 [DOI] [PubMed] [Google Scholar]
- 30.Lu J, Xu LW, Zeng ZF, Xue CQ, Li JL, Chen X, et al. Normothermic ex vivo heart perfusion combined with melatonin enhances myocardial protection in rat donation after circulatory death hearts via inhibiting NLRP3 inflammasome-mediated pyroptosis. Front Cell Dev Biol. (2021) 9:733183. 10.3389/fcell.2021.733183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen G, Xu C, Gillette TG, Huang T, Huang P, Li Q, et al. Cardiomyocyte-derived small extracellular vesicles can signal eNOS activation in cardiac microvascular endothelial cells to protect against Ischemia/Reperfusion injury. Theranostics. (2020) 10:11754–74. 10.7150/thno.43163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AKZ, Schwanekamp JA, et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature. (2020) 577:405. 10.1038/s41586-019-1802-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boag SE, Andreano E, Spyridopoulos I. Lymphocyte communication in myocardial ischemia/reperfusion injury. Antioxid Redox Sign. (2017) 26:660–675. 10.1089/ars.2016.6940 [DOI] [PubMed] [Google Scholar]
- 34.Lin H, Wang WT, Lee MD, Meng QH, Ren HS. Current status of septic cardiomyopathy: basic science and clinical progress. Front Pharmacol. (2020) 11:210. 10.3389/fphar.2020.00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang WY, Wang M. New insights into the immunomodulatory role of exosomes in cardiovascular disease. Rev Cardiovasc Med. (2019) 20:153–60. 10.31083/j.rcm.2019.03.528 [DOI] [PubMed] [Google Scholar]
- 36.Wang CX, Zhang CC, Liu LX, Xi A, Chen BY, Li YL, et al. Macrophage-derived mir-155-containing exosomes suppress fibroblast proliferation and promote fibroblast inflammation during cardiac injury. Mol Ther. (2017) 25:192–204. 10.1016/j.ymthe.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He S, Wu C, Xiao J, Li D, Sun Z, Li M. Endothelial extracellular vesicles modulate the macrophage phenotype: potential implications in atherosclerosis. Scand J Immunol. (2018) 87:e12648. 10.1111/sji.12648 [DOI] [PubMed] [Google Scholar]
- 38.Hofmann U, Beyersdorf N, Weirather J, Podolskaya A, Bauersachs J, Ertl G, et al. Activation of CD4(+) T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. (2012) 125:1652–U46. 10.1161/CIRCULATIONAHA.111.044164 [DOI] [PubMed] [Google Scholar]
- 39.Weirather J, Hofmann UDW, Beyersdorf N, Ramos GC, Vogel B, Frey A, et al. Foxp(3+) CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. (2014) 115:55–67. 10.1161/CIRCRESAHA.115.303895 [DOI] [PubMed] [Google Scholar]
- 40.Sharma M, Schlegel MP, Afonso MS, Brown EJ, Rahman K, Weinstock A, et al. Regulatory T cells license macrophage pro-resolving functions during atherosclerosis regression. Circ Res. (2020) 127:335–53. 10.1161/CIRCRESAHA.119.316461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agarwal A, Fanelli G, Letizia M, Tung SL, Boardman D, Lechler R, et al. Regulatory T cell-derived exosomes: possible therapeutic and diagnostic tools in transplantation. Front Immunol. (2014) 5:555. 10.3389/fimmu.2014.00555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song JP, Huang J, Chen X, Teng X, Song ZZ, Xing Y, et al. Donor-derived exosomes induce specific regulatory T cells to suppress immune inflammation in the allograft heart. Sci Rep. (2016) 6:20077. 10.1038/srep20077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aiello S, Rocchetta F, Longaretti L, Faravelli S, Todeschini M, Cassis L, et al. Extracellular vesicles derived from T regulatory cells suppress T cell proliferation and prolong allograft survival. Sci Rep. (2017) 7:11518. 10.1038/s41598-017-08617-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu XS, Huang CB, Song B, Xiao Y, Fang MQ, Feng JY, et al. CD4(+)CD25(+) regulatory T cells-derived exosomes prolonged kidney allograft survival in a rat model. Cell Immunol. (2013) 285:62–8. 10.1016/j.cellimm.2013.06.010 [DOI] [PubMed] [Google Scholar]
- 45.Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, et al. MicroRNA-containing T-regulatory-cell-derived exosomes suppress pathogenic T helper 1 cells. Immunity. (2014) 41:89–103. 10.1016/j.immuni.2014.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A. Neutrophil function: from mechanisms to disease. Annu Rev Immunol. (2012) 30:459–89. 10.1146/annurev-immunol-020711-074942 [DOI] [PubMed] [Google Scholar]
- 47.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. (2014) 11:255–65. 10.1038/nrcardio.2014.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frangogiannis NG, Lindsey ML, Michael LH, Youker KA, Bressler RB, Mendoza LH, et al. Resident cardiac mast cells degranulate and release preformed TNF-alpha, initiating the cytokine cascade in experimental canine myocardial ischemia/reperfusion. Circulation. (1998) 98:699–710. 10.1161/01.CIR.98.7.699 [DOI] [PubMed] [Google Scholar]
- 49.Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. (2015) 117:52–64. 10.1161/CIRCRESAHA.117.305990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol. (2015) 192:61–9. 10.1016/j.ijcard.2015.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fang YH, Wang SPH, Gao ZH, Wu SN, Chang HY, Yang PJ, et al. Efficient cardiac differentiation of human amniotic fluid-derived stem cells into induced pluripotent stem cells and their potential immune privilege. Int J Mol Sci. (2020) 21:2359. 10.3390/ijms21072359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tu C, Zoldan J. Moving iPSC-derived cardiomyocytes forward to treat myocardial infarction. Cell Stem Cell. (2018) 23:322–3. 10.1016/j.stem.2018.08.011 [DOI] [PubMed] [Google Scholar]
- 53.Yu Y, Qin N, Lu XA, Li J, Han XA, Lei W, et al. Human embryonic stem cell-derived cardiomyocyte therapy in mouse permanent ischemia and ischemia-reperfusion models. Stem Cell Res Ther. (2019) 10:167. 10.1186/s13287-019-1271-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barile L, Milano G, Vassalli G. Beneficial effects of exosomes secreted by cardiac-derived progenitor cells and other cell types in myocardial ischemia. Stem Cell Investig. (2017) 4:93. 10.21037/sci.2017.11.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dougherty JA, Patel N, Kumar N, Rao SG, Angelos MG, Singh H, et al. Human cardiac progenitor cells enhance exosome release and promote angiogenesis under physoxia. Front Cell Dev Biol. (2020) 8:130. 10.3389/fcell.2020.00130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao T, Sun F, Liu J, Ding T, She J, Mao F, et al. Emerging role of mesenchymal stem cell-derived exosomes in regenerative medicine. Curr Stem Cell Res Ther. (2019) 14:482–94. 10.2174/1574888X14666190228103230 [DOI] [PubMed] [Google Scholar]
- 57.Fujita Y, Kadota T, Araya J, Ochiya T, Kuwano K. Clinical application of mesenchymal stem cell-derived extracellular vesicle-based therapeutics for inflammatory lung diseases. J Clin Med. (2018) 7:355. 10.3390/jcm7100355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang N, Song YA, Huang ZY, Chen J, Tan HP, Yang HB, et al. Monocyte mimics improve mesenchymal stem cell-derived extracellular vesicle homing in a mouse MI/RI model. Biomaterials. (2020) 255:120168. 10.1016/j.biomaterials.2020.120168 [DOI] [PubMed] [Google Scholar]
- 59.Ju CW, Shen Y, Ma GS, Liu YT, Cai JW, Kim IM, et al. Transplantation of cardiac mesenchymal stem cell-derived exosomes promotes repair in ischemic myocardium. J Cardiovasc Transl. (2018) 11:420–8. 10.1007/s12265-018-9822-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu D, Yang F, Xiong F, Gu N. The smart drug delivery system and its clinical potential. Theranostics. (2016) 6:1306–23. 10.7150/thno.14858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haque S, Whittaker MR, McIntosh MP, Pouton CW, Kaminskas LM. Disposition and safety of inhaled biodegradable nanomedicines: opportunities and challenges. Nanomed Nanotechnol. (2016) 12:1703–24. 10.1016/j.nano.2016.03.002 [DOI] [PubMed] [Google Scholar]
- 62.Vader P, Mol EA, Pasterkamp G, Schiffelers RM. Extracellular vesicles for drug delivery. Adv Drug Deliver Rev. (2016) 106:148–56. 10.1016/j.addr.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 63.Wang X, Chen YH, Zhao ZN, Meng QY, Yu Y, Sun JC, et al. Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. J Am Heart Assoc. (2018) 7:e008737. 10.1161/JAHA.118.008737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ikeda G, Santoso MR, Tada Y, Li AM, Vaskova E, Jung JH, et al. Mitochondria-rich extracellular vesicles from autologous stem cell-derived cardiomyocytes restore energetics of ischemic myocardiums. J Am Coll Cardiol. (2021) 77:1073–88. 10.1016/j.jacc.2020.12.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Potz BA, Scrimgeour LA, Pavlov VI, Sodha NR, Abid MR, Sellke FW. Extracellular vesicle injection improves myocardial function and increases angiogenesis in a swine model of chronic ischemia. J Am Heart Assoc. (2018) 7:e008344. 10.1161/JAHA.117.008344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peng Z, Chang Y, Fan J, Ji W, Su C. Phospholipase A2 superfamily in cancer. Cancer Lett. (2021) 497:165–77. 10.1016/j.canlet.2020.10.021 [DOI] [PubMed] [Google Scholar]
- 67.Antes TJ, Middleton RC, Luther KM, Ijichi T, Peck KA, Liu WJ, et al. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J Nanobiotechnol. (2018) 16:61. 10.1186/s12951-018-0388-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim H, Mun D, Kang JY, Lee SH, Yun N. Improved cardiac-specific delivery of RAGE siRNA within small extracellular vesicles engineered to express intense cardiac targeting peptide attenuates myocarditis. Mol Ther-Nucl Acids. (2021) 24:1024–32. 10.1016/j.omtn.2021.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pi YN, Xia BR, Jin MZ, Jin WL, Lou G. Exosomes: powerful weapon for cancer nano-immunoengineering. Biochem Pharmacol. (2021) 186:114487. 10.1016/j.bcp.2021.114487 [DOI] [PubMed] [Google Scholar]
- 70.Fu P, Zhang J, Li H, Mak M, Xu W, Tao Z. Extracellular vesicles as delivery systems at nano-/micro-scale. Adv Drug Deliver Rev. (2021). 10.1016/j.addr.2021.113910. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun DM, Zhuang XY, Xiang XY, Liu YL, Zhang SY, Liu CR, et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther. (2010) 18:1606–14. 10.1038/mt.2010.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patil ML, Zhang M, Taratula O, Garbuzenko OB, He HX, Minko T. Internally cationic polyamidoamine PAMAM-OH dendrimers for sirna delivery: effect of the degree of quaternization and cancer targeting. Biomacromolecules. (2009) 10:258–66. 10.1021/bm8009973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen Q, Liu Y, Ding XY, Li QF, Qiu FY, Wang MH, et al. Bone marrow mesenchymal stem cell-secreted exosomes carrying microRNA-125b protect against myocardial ischemia reperfusion injury via targeting SIRT7. Mol Cell Biochem. (2020) 465:103–14. 10.1007/s11010-019-03671-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wei ZL, Qiao SH, Zhao JX, Liu YH, Li QL, Wei ZH, et al. miRNA-181a over-expression in mesenchymal stem cell-derived exosomes influenced inflammatory response after myocardial ischemia-reperfusion injury. Life Sci. (2019) 232:116632. 10.1016/j.lfs.2019.116632 [DOI] [PubMed] [Google Scholar]
- 75.Wang XY, Guan XH, Yu ZP, Wu J, Huang QM, Deng KY, et al. Human amniotic stem cells-derived exosmal miR-181a-5p and miR-199a inhibit melanogenesis and promote melanosome degradation in skin hyperpigmentation, respectively. Stem Cell Res Ther. (2021) 12:501. 10.1186/s13287-021-02570-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu J, Zhu MY, Tang QL. Human umbilical cord mesenchymal stem cells-derived exosomal microRNA-181a retards nasopharyngeal carcinoma development by mediating KDM5C. J Cancer Res Clin. (2021) 147:2867–77. 10.1007/s00432-021-03684-6 [DOI] [PubMed] [Google Scholar]
- 77.Gu L, Ren F, Fang XR, Yuan LW, Liu GL, Wang SL. Exosomal MicroRNA-181a derived from mesenchymal stem cells improves gut microbiota composition, barrier function, and inflammatory status in an experimental colitis model. Front Med. (2021) 8:660614. 10.3389/fmed.2021.660614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yim N, Ryu SW, Choi K, Lee KR, Lee S, Choi H, et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein-protein interaction module. Nat Commun. (2016) 7:12277. 10.1038/ncomms12277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maldonado C, Nguyen MD, Bauer P, Nakamura S, Khundmiri SJ, Perez-Abadia G, et al. Rapid lipid modification of endothelial cell membranes in cardiac ischemia/reperfusion injury: a novel therapeutic strategy to reduce infarct size. Cardiovasc Drug Ther. (2021) 35:113–23. 10.1007/s10557-020-07101-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song Y, Zhang C, Zhang JX, Jiao ZY, Dong NG, Wang GB, et al. Localized injection of miRNA-21-enriched extracellular vesicles effectively restores cardiac function after myocardial infarction. Theranostics. (2019) 9:2346–60. 10.7150/thno.29945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kang JY, Kim H, Mun D, Yun N, Joung B. Co-delivery of curcumin and miRNA-144-3p using heart-targeted extracellular vesicles enhances the therapeutic efficacy for myocardial infarction. J Control Release. (2021) 331:62–73. 10.1016/j.jconrel.2021.01.018 [DOI] [PubMed] [Google Scholar]
- 82.Chen J, Song YN, Huang ZY, Zhang N, Xie XX, Liu X, et al. Modification with CREKA improves cell retention in a rat model of myocardial lschemia reperfusion. Stem Cells. (2019) 37:663–76. 10.1002/stem.2983 [DOI] [PubMed] [Google Scholar]
- 83.Vandergriff A, Huang K, Shen DL, Hu SQ, Hensley MT, Caranasos TG, et al. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics. (2018) 8:1869–78. 10.7150/thno.20524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng DW, Xu L, Li CX, Dong X, Pan P, Zhang QL, et al. Photo-powered artificial organelles for ATP generation and life-sustainment. Adv Mater. (2018) 30:e1805038. 10.1002/adma.201805038 [DOI] [PubMed] [Google Scholar]
- 85.Tan HP, Song YN, Chen J, Zhang N, Wang QZ, Li QY, et al. Platelet-like fusogenic liposome-mediated targeting delivery of miR-21 improves myocardial remodeling by reprogramming macrophages post myocardial ischemia-reperfusion injury. Adv Sci. (2021) 8:2100787. 10.1002/advs.202100787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. (2018) 150:137–49. 10.1016/j.biomaterials.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 87.Kanki S, Jaalouk DE, Lee S, Yu AYC, Gannon J, Lee RT. Identification of targeting peptides for ischemic myocardium by in vivo phage display. J Mol Cell Cardiol. (2011) 50:841–8. 10.1016/j.yjmcc.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kooijmans SAA, Aleza CG, Roffler SR, van Solinge WW, Vader P, Schiffelers RM. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J Extracell Vesicles. (2016) 5:31053. 10.3402/jev.v5.31053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ni J, Liu XJ, Yin YH, Zhang PY, Xu YW, Liu Z. Exosomes derived from TIMP2-modified human umbilical cord mesenchymal stem cells enhance the repair effect in rat model with myocardial infarction possibly by the Akt/Sfrp2 pathway. Oxid Med Cell Longev. (2019) 2019:1958941. 10.1155/2019/1958941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ha D, Yang NN, Nadithe V. Exosomes as therapeutic drug carriers and delivery vehicles across biological membranes: current perspectives and future challenges. Acta Pharm Sin B. (2016) 6:287–96. 10.1016/j.apsb.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cheow ESH, Cheng WC, Lee CN, de Kleijn D, Sorokin V, Sze SK. Plasma-derived extracellular vesicles contain predictive biomarkers and potential therapeutic targets for Myocardial Ischemic (MI) injury. Mol Cell Proteomics. (2016) 15:2628–40. 10.1074/mcp.M115.055731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tang YT, Huang YY, Zheng L, Qin SH, Xu XP, An TX, et al. Comparison of isolation methods of exosomes and exosomal RNA from cell culture medium and serum. Int J Mol Med. (2017) 40:834–44. 10.3892/ijmm.2017.3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu R, Greening DW, Zhu HJ, Takahashi N, Simpson RJ. Extracellular vesicle isolation and characterization: toward clinical application. J Clin Invest. (2016) 126:1152–62. 10.1172/JCI81129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lai RC, Yeo RWY, Tan KH, Lim SK. Exosomes for drug delivery - a novel application for the mesenchymal stem cell. Biotechnol Adv. (2013) 31:543–51. 10.1016/j.biotechadv.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 95.Xue F, Chen Y, Wen Y, Abhange K, Zhang W, Cheng G, et al. Isolation of extracellular vesicles with multivalent aptamers. Analyst. (2021) 146:253–61. 10.1039/D0AN01420F [DOI] [PubMed] [Google Scholar]
- 96.Liangsupree T, Multia E, Riekkola ML. Modern isolation and separation techniques for extracellular vesicles. J Chromatogr A. (2021) 1636:461773. 10.1016/j.chroma.2020.461773 [DOI] [PubMed] [Google Scholar]
- 97.Jeyaram A, Jay SM. Preservation and storage stability of extracellular vesicles for therapeutic applications. Aaps J. (2018) 20:1. 10.1208/s12248-017-0160-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maroto R, Zhao YX, Jamaluddin M, Popov VL, Wang HW, Kalubowilage M, et al. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J Extracell Vesicles. (2017) 6:1359478. 10.1080/20013078.2017.1359478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sokolova V, Ludwig AK, Hornung S, Rotan O, Horn PA, Epple, et al. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloid Surface B. (2011) 87:146–50. 10.1016/j.colsurfb.2011.05.013 [DOI] [PubMed] [Google Scholar]
- 100.Batrakova EV, Kim MS. Using exosomes, naturally-equipped nanocarriers, for drug delivery. J Control Release. (2015) 219:396–405. 10.1016/j.jconrel.2015.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Leggio L, Arrabito G, Ferrara V, Vivarelli S, Paterno G, Marchetti B, et al. Mastering the tools: natural versus artificial vesicles in nanomedicine. Adv Healthc Mater. (2020) 9:2000731. 10.1002/adhm.202000731 [DOI] [PubMed] [Google Scholar]
- 102.Kim H, Yun N, Mun D, Kang JY, Lee SH, Park H, et al. Cardiac-specific delivery by cardiac tissue-targeting peptide-expressing exosomes. Biochem Bioph Res Co. (2018) 499:803–8. 10.1016/j.bbrc.2018.03.227 [DOI] [PubMed] [Google Scholar]
- 103.Li ZL, Zhou XY, Wei MY, Gao XT, Zhao LB, Shi RJ, et al. In vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Lett. (2019) 19:19–28. 10.1021/acs.nanolett.8b02689 [DOI] [PubMed] [Google Scholar]
- 104.Ohno S, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor MicroRNA to breast cancer cells. Mol Ther. (2013) 21:185–91. 10.1038/mt.2012.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ai YJ, Xie RX, Xiong JL, Liang QL. Microfluidics for biosynthesizing: from droplets and vesicles to artificial cells. Small. (2020) 16:1903940. 10.1002/smll.201903940 [DOI] [PubMed] [Google Scholar]
- 106.Jesorka A, Orwar O. Liposomes: technologies and analytical applications. Annu Rev Anal Chem. (2008) 1:801–32. 10.1146/annurev.anchem.1.031207.112747 [DOI] [PubMed] [Google Scholar]
- 107.Hammons JA, Ingolfsson HI, Lee JRI, Carpenter TS, Sanborn J, Tunuguntla T, et al. Decoupling copolymer, lipid and carbon nanotube interactions in hybrid, biomimetic vesicles. Nanoscale. (2020) 12:6545–55. 10.1039/C9NR09973E [DOI] [PubMed] [Google Scholar]
- 108.Shah VM, Nguyen DX, Patel P, Cote B, Al-Fatease A, Pham YG, et al. Liposomes produced by microfluidics and extrusion: a comparison for scale-up purposes. Nanomed Nanotechnol. (2019) 18:146–56. 10.1016/j.nano.2019.02.019 [DOI] [PubMed] [Google Scholar]
- 109.Kotoucek J, Hubatka F, Masek J, Kulich P, Velinska K, Bezdekova J, et al. Preparation of nanoliposomes by microfluidic mixing in herring-bone channel and the role of membrane fluidity in liposomes formation. Sci Rep. (2020) 10:5595. 10.1038/s41598-020-62500-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Deng R, Yang L, Bain CD. Combining inkjet printing with emulsion solvent evaporation to pattern polymeric particles. ACS Appl Mater Interfaces. (2018) 10:12317–22. 10.1021/acsami.8b02017 [DOI] [PubMed] [Google Scholar]
- 111.Hedegaard CL, Collin EC, Redondo-Gómez C, Nguyen LTH, Ng KW, Castrejón-Pita AA, et al. Hydrodynamically guided hierarchical self-assembly of peptide–protein bioinks. Adv Funct Mater. (2018) 28:1703716. 10.1002/adfm.20170371625855820 [DOI] [Google Scholar]
- 112.Wang X, Zhong X, Gong F, Chao Y, Cheng L. Newly developed strategies for improving sonodynamic therapy. Materials Horizons. (2020) 7:2028–2046. 10.1039/D0MH00613K [DOI] [Google Scholar]
- 113.Lestini BJ, Sagnella SM, Xu Z, Shive MS, Richter NJ, Jayaseharan JM, et al. Surface modification of liposomes for selective cell targeting in cardiovascular drug delivery. J Control Release. (2002) 78:235–47. 10.1016/S0168-3659(01)00505-3 [DOI] [PubMed] [Google Scholar]
- 114.Ibrahim AGE, Cheng K, Marban E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. (2014) 2:606–19. 10.1016/j.stemcr.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang CC, Kang MY, Lu Y, Shirazi S, Diaz JI, Cooper LF, et al. Functionally engineered extracellular vesicles improve bone regeneration. Acta Biomater. (2020) 109:182–94. 10.1016/j.actbio.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ma YB, Ge SH, Zhang JH, Zhou D, Li L, Wang XF, et al. Mesenchymal stem cell-derived extracellular vesicles promote nerve regeneration after sciatic nerve crush injury in rats. Int J Clin Exp Patho. (2017) 10:10032–9. [PMC free article] [PubMed] [Google Scholar]
- 117.Hu Y, Zhang Y, Ni CY, Chen CY, Rao SS, Yin H, et al. Human umbilical cord mesenchymal stromal cells-derived extracellular vesicles exert potent bone protective effects by CLEC11A-mediated regulation of bone metabolism. Theranostics. (2020) 10:2293–308. 10.7150/thno.39238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Noori L, Arabzadeh S, Mohamadi Y, Mojaverrostami S, Mokhtari T, Akbari, et al. Intrathecal administration of the extracellular vesicles derived from human Wharton's jelly stem cells inhibit inflammation and attenuate the activity of inflammasome complexes after spinal cord injury in rats. Neurosci Res. (2021) 170:87–98. 10.1016/j.neures.2020.07.011 [DOI] [PubMed] [Google Scholar]
- 119.Mathew B, Ravindran S, Liu XR, Torres L, Chennakesavalu M, Huang CC, et al. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials. (2019) 197:146–60. 10.1016/j.biomaterials.2019.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pusic KM, Kraig RP, Pusic AD. IFN gamma-stimulated dendritic cell extracellular vesicles can be nasally administered to the brain and enter oligodendrocytes. PLoS ONE. (2021) 16:e0255778. 10.1371/journal.pone.0255778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang CC, Kang MY, Shirazi S, Lu Y, Cooper LF, Gajendrareddy P, et al. 3D Encapsulation and tethering of functionally engineered extracellular vesicles to hydrogels. Acta Biomater. (2021) 126:199–210. 10.1016/j.actbio.2021.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lv KQ, Li QJ, Zhang L, Wang YC, Zhong ZW, Zhao J, et al. Incorporation of small extracellular vesicles in sodium alginate hydrogel as a novel therapeutic strategy for myocardial infarction. Theranostics. (2019) 9:7403–16. 10.7150/thno.32637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Agrahari V, Agrahari V, Burnouf PA, Chew CH, Burnouf T. Extracellular microvesicles as new industrial therapeutic frontiers. Trends Biotechnol. (2019) 37:707–29. 10.1016/j.tibtech.2018.11.012 [DOI] [PubMed] [Google Scholar]
- 124.Ma T, Chen YQ, Chen YH, Meng QY, Sun JC, Shao LB, et al. JMicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells Int. (2018) 2018:3290372. 10.1155/2018/3290372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Femmino S, Penna C, Margarita S, Comita S, Brizzi MF, Pagliaro P. Extracellular vesicles and cardiovascular system: biomarkers and cardioprotective effectors. Vasc Pharmacol. (2020) 135:106790. 10.1016/j.vph.2020.106790 [DOI] [PubMed] [Google Scholar]
- 126.Beg F, Wang R, Saeed Z, Devaraj S, Masoor K. Inflammation-associated microRNA changes in circulating exosomes of heart failure patients. BMC Res Notes. (2017) 10:751. 10.1186/s13104-017-3090-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhao XX, Jia YP, Chen HZ, Yao HM, Guo WL. Plasma-derived exosomal miR-183 associates with protein kinase activity and may serve as a novel predictive biomarker of myocardial ischemic injury. Exp Ther Med. (2019) 18:179–87. 10.3892/etm.2019.7555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tong KL, Mahmood Zuhdi AS, Wan Ahmad WA, Vanhoutte PM, de Magalhaes JP, Mustafa MR, et al. Circulating MicroRNAs in young patients with acute coronary syndrome. Int J Mol Sci. (2018) 19:1467. 10.3390/ijms19051467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xing J, Xie T, Tan W, Li R, Yu C, Han X. microRNA-183 improve myocardial damager via NF-kb pathway: in vitro and in vivo study. J Cell Biochem. (2019) 120:10145–54. 10.1002/jcb.28298 [DOI] [PubMed] [Google Scholar]
- 130.Cheng YH, Wang XB, Yang J, Duan XX, Yao Y, Shi XL, et al. A translational study of urine miRNAs in acute myocardial infarction. J Mol Cell Cardiol. (2012) 53:668–76. 10.1016/j.yjmcc.2012.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]