Abstract
Background:
Lower socioeconomic status (SES) has consistently been associated with poorer outcomes in individuals with cystic fibrosis (CF). Previous studies have compared outcomes for children with and without private insurance coverage, however the potential role of changes in insurance status on early health outcomes in children with CF remains unknown.
Objectives:
To describe the variability in insurance status in early childhood and to evaluate whether insurance variability was associated with poorer outcomes at age 6.
Methods:
Retrospective observational study using the Cystic Fibrosis Foundation Patient Registry. Insurance status was defined as: always private (including Tricare), exclusively public, or intermittent private insurance (private insurance and exclusively public insurance in separate years) during the first 6 years of life. Outcomes at age 6 included body mass index (BMI) and FEV1 percent predicted (maxFEV1pp).
Results:
From a 2000–2011 birth cohort (n=8,109), 42.3% always had private insurance, 30.0% had exclusively public insurance, and 27.6% had intermittent private insurance. BMI percentiles did not differ between groups; however, children with intermittent private insurance and exclusively public insurance had a 3.3% and 6.6% lower maxFEV1pp at age 6, respectively, compared to those with always private insurance.
Conclusions:
A substantial proportion of young children in a modern CF cohort have public or intermittent private insurance coverage. While public insurance has been associated with poorer health outcomes in CF, variability in health insurance coverage may also be associated with an intermediate risk of disparities in lung function as early as age 6.
Keywords: cystic fibrosis (CF), registry, health insurance, early childhood, BMI, FEV1pp
1. Introduction
Cystic fibrosis (CF) is a multisystem genetic disorder, characterized by growth deficits and a decline in pulmonary status over time. Earlier diagnosis through prenatal testing and newborn screening (NBS) has allowed increased attention to optimizing CF outcomes, specifically interventions to improve early nutritional status. These efforts, in addition to aggressive treatment of chronic respiratory infections and management of co-morbidities, demonstrate that interventions in childhood can impact long-term health outcomes and survival.1–3 Despite these advances, variations in disease severity and progression persist, irrespective of genotype and associated co-morbidities.4,5 One proposed explanation for the heterogeneity in CF health outcomes is socioeconomic status (SES), as lower SES has consistently been associated with poorer clinical outcomes in individuals with CF.6
SES is a multi-dimensional construct used to describe the social standing or class of an individual.7–9 Insurance status is one component of SES that is commonly used as an individual-level SES measure to approximate an individual’s financial resources. While public insurance status has not been associated with differences in health care access or utilization in CF,10 comparing individuals with non-private insurance (Medicaid or no insurance) to those with private insurance has demonstrated that non-private insurance is associated with worse outcomes on key measures of health among children and adolescents with CF, including weight and lung function.11,12 However, it is recognized that many individuals in the US, even those with chronic diseases,13,14 have variability in insurance coverage over time, often associated with changes in employment and income. Understanding the association between variability in insurance coverage and key measures of health is important for a better understanding of how SES impacts health outcomes in CF.
Tumin et al. recently reported findings from a U.S.-based adolescent and young adult CF cohort and demonstrated a statistically significant difference in lung function among individuals with continuous private insurance compared to individuals with continuous public insurance as well as those with intermittent public insurance.15 These findings suggest that among older children and young adults with CF, variability in insurance status may be associated with health outcomes in between individuals who were always covered by public insurance compared to those who were never eligible for public insurance. Variability in insurance coverage during a critical period of development such as early childhood is shown to place individuals, especially those at lower income levels, at particular risk for worse health outcomes,16–18 however it is unknown if this association exists for children younger than 6 years of age with CF.
Given early lung function and nutritional status predict long-term morbidity and mortality in CF,19–21 it is important to identify if SES factors such as insurance variability in the preschool age place individuals at higher risk for poor outcomes on key health measures as early as age 6 years in order to address early disparities, which may persist throughout the lifespan. The purpose of this study was to describe the variability in health insurance coverage in early childhood and to determine whether exclusively public and intermittent private insurance coverage was associated with poorer clinical outcomes compared to continuous private insurance coverage. We hypothesized that exclusively public and intermittent private insurance coverage would be associated with worse nutritional and respiratory outcomes at 6 years compared to continuous private insurance coverage.
2. Methods
2.1. Study population
We performed a retrospective cohort study utilizing data from the Cystic Fibrosis Foundation Patient Registry (CFFPR), a longitudinal, encounter-based registry of individuals with CF treated at CFF accredited centers in the United States.22 The study population included children born between January 1, 2000 and December 31, 2011, enrolled in the CFFPR between 2000–2017, and diagnosed with CF before age 2. Subjects were excluded if they had fewer than 3 annualized insurance entries prior to 6 years of age, were exclusively uninsured (viewed as a distinct group from those with exclusive public insurance), received a lung transplantation or passed away prior to age 6 or did not have an encounter between 6–6.99 years. This study was approved by The Johns Hopkins University Institutional Review Board (IRB00175552).
2.2. Primary Exposure
Participant’s type of insurance was collected annually in the CFFPR and early life insurance status was categorized for this study as always private, exclusively public or intermittent private using all available insurance data prior to 6 years. Individuals who had commercial or private insurance (i.e., health insurance through their parent’s employers, including TriCare or other military health plans) at every annualized encounter were categorized as always private. Individuals with exclusively taxpayer-funded or public health insurance (Medicare, Medicaid, State Special Needs Programs, and Indian Health Service) at each annualized encounter were categorized as exclusively public. Individuals who had both private and public insurance in an annualized encounter were defined as having private insurance for that year, as many individuals receive public insurance due to medical status, special health care needs or medical expenditures.23 Individuals who had private insurance in at least one annual encounter and exclusively public insurance in at least one annual encounter were categorized as intermittent private.
2.3. Primary Outcomes
Primary outcome data were recorded at clinical encounters in the CFFPR. The primary outcomes of interest were: (1) best forced expiratory volume in 1 second % predicted (maxFEV1pp) based on the Global Lung Initiative reference equations24 between 6–6.99 years (when spirometry can reliably be performed) and (2) highest body mass index (BMI) percentile based on the Center for Disease Control growth charts between 6–6.99 years.
2.4. Secondary Outcomes
Secondary outcome data were recorded at clinical encounters in the CFFPR, except for exacerbations, which were recorded annually. Secondary outcomes included mean BMI percentile between 6–6.99 years, mean FEV1pp between 6–6.99 years, as well as variability of FEV1pp, defined as the difference between the highest and lowest FEV1pp values between 6–6.99 years of age for individuals with more than one FEV1pp measurement in that timeframe.
2.5. Statistical Analysis
Demographic and clinical characteristics of children were compared between insurance groups (always private, exclusively public and intermittent private) using ANOVA and χ2 tests for continuous and categorical variables, respectively. Comparisons of lung function and BMI measures between 6–6.99 years were compared between groups. Missing data were not imputed.
Unadjusted and multivariable linear regressions were used to evaluate the association between insurance status and all FEV1pp and BMI outcomes at age 6–6.99 years. Two separate multivariable analyses were conducted. Initially, we included covariates identified a priori that have been previously reported to be associated with nutritional status or lung function in CF including sex, race (white vs. non-white), ethnicity (Hispanic vs. non-Hispanic), age at diagnosis, year of birth, CFTR genotype (0,1, or 2 F508del variants), exocrine pancreatic status defined using (1) the Clinical and Functional Translation of CFTR project (www.cftr2.org), (2) fecal elastase or (30) pancreatic enzyme supplementation, surgically placed feeding tube (gastrostomy or jejunostomy) status and rates of methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa (Pa) chronic endobronchial infection, defined as having at least one positive culture in at least two separate years between 4–6.99 years (likely chronic infection with MRSA or Pa). As a sensitivity analysis, a second multivariable model included other SES covariates including log of residential median household income (ascertained from residential zip code closest to time of birth and 2010 U.S. census data (www.census.gov), and a dummy categorical variable of the highest level of maternal education at or prior to 6 years of age (as recorded in CFFPR). Children with private coverage throughout the study period were the reference group. A p<0.05 was considered statistically significant for all comparisons. Statistical analyses were performed using Stata version 15.0.
3. Results
A total of 10,826 children enrolled in the CFFPR were born between January 1, 2000 and December 31, 2011. Of these, 1,951 (18.0%) individuals were diagnosed with CF after 2 years of age, 707 (6.5%) had <3 annualized insurance entries prior to 6 years, 12 (0.11%) were exclusively uninsured, and 59 (0.5%) died or received a lung transplantation prior to 6 years, resulting in 8,109 children who met inclusion criteria and comprised the analytic population. Of these, 3,433 (42.3%) were classified as always having private insurance coverage, 2,439 (30.0%) as having exclusively public insurance coverage, and 2,237 (27.6%) had intermittent private insurance coverage (Figure 1).
Figure 1.
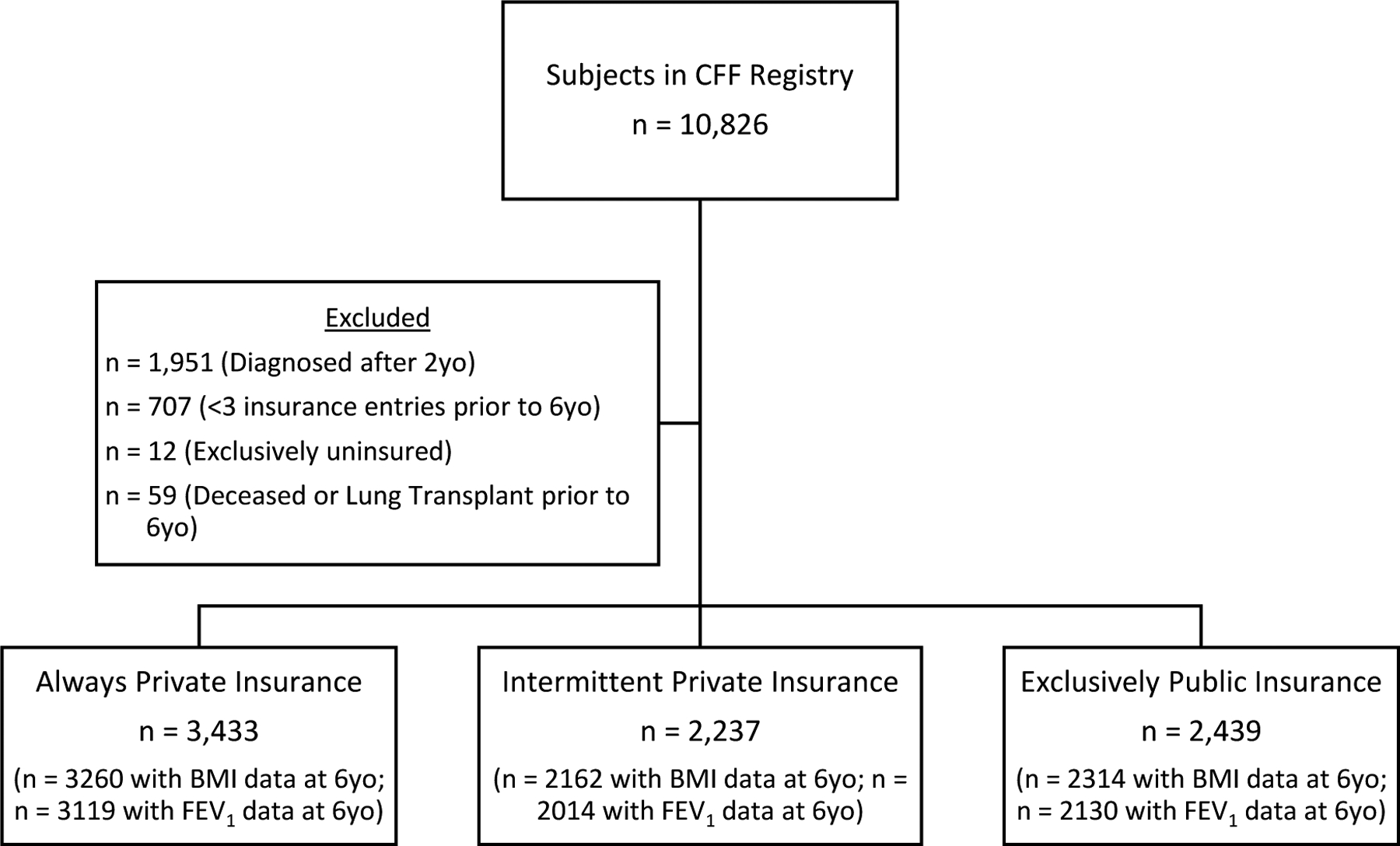
Study Population (n = 8,109)
Demographic and clinical characteristics of children, by early life insurance status, are presented in Table 1. Almost 11% of children with intermittent private insurance were non-white, while almost 16% of children with exclusively public insurance were non-white, while approximately 11% of children with intermittent private insurance were Hispanic and almost 20% of children with exclusively public insurance were Hispanic. This contrasts with children with private insurance, where only 4% of children were non-white and 5% were Hispanic. Maternal education and median household income by zip code were highest in children with private insurance, followed by those with intermittent private and those children with exclusively public insurance. The highest proportion of children with no copies of F508del variants were in the exclusively public insurance group. Of note, feeding tube placement by 6 years differed by insurance status with tube placement reported in 15.3% of those with always private insurance, 22.6% of those with intermittent private insurance, and 24.6% of those with exclusively public insurance. Number of outpatient visits between 6–6.99 years of age did not differ by insurance status, however those with public and intermittent private insurance spent more days hospitalized between 6–6.99 years than those with private insurance.
Table 1.
Study Population
| Mean ± S.D. | Entire Study Population (n = 8,109) |
Always Private (n = 3,433) |
Intermittent Private (n = 2,237) |
Exclusively Public (n = 2,439) |
P Value | |
|---|---|---|---|---|---|---|
| Sex (% female) | 49.8% | 50.1% | 47.5% | 51.3% | 0.028 | |
| Race (% non-white) | 9.8% | 4.4% | 10.6% | 16.7% | <0.001 | |
| Hispanic (% yes) | 10.9% | 5.3% | 10.5% | 19.2% | <0.001 | |
| Age at Diagnosis (years) | 0.23 ± 0.39 | 0.22 ± 0.40 | 0.23 ± 0.38 | 0.24 ± 0.38 | 0.07 | |
| CFTR Genotype (% reported) | 2 F508del variants | 48.7% | 49.5% | 51.0% | 45.6% | <0.001 |
| 1 F508del variants | 38.6% | 39.7% | 38.4% | 37.3% | ||
| 0 F508del variants | 12.7% | 10.8% | 10.6% | 17.2% | ||
| Diagnosed by Newborn Screen (% yes) | 43.6% | 42.7% | 43.8% | 44.7% | 0.34 | |
| Pancreatic Status (% Insufficient) | 86.7% | 84.3% | 89.5% | 87.4% | <0.001 | |
| Feeding tube placement by 6yo (% yes) | 20.1% | 15.3% | 22.6% | 24.6% | <0.001 | |
| Number of Outpatient Visits at 6yo (n=7,722) | 4.5 ± 1.9 | 4.5 ± 1.9 | 4.5 ± 2.0 | 4.5 ± 2.0 | 0.47 | |
| Number of Hospital Days at 6yo (n=7,776) | 3.2 ± 9.2 | 1.8 ± 5.9 | 3.3 ± 9.9 | 5.0 ± 11.6 | <0.001 | |
| Median household income at birth ($ ‘000s; n=8,004) | 58.0 ± 22.1 | 67.2 ± 24.7 | 54.1 ± 18.2 | 48.5 ± 15.7 | <0.001 | |
| Highest level of maternal education at 6yo (% yes; n=6,648) | Less than high school | 5.4% | 0.4% | 4.6% | 13.5% | <0.001 |
| High school graduate | 25.1% | 10.3% | 29.5% | 42.9% | ||
| Some College | 21.4% | 14.9% | 27.2% | 25.6% | ||
| College graduate | 40.4% | 60.7% | 34.3% | 16.0% | ||
| Masters/doctoral degree | 7.7% | 13.7% | 4.4% | 2.1% | ||
| Any MRSA positive culture (by 6yo) | 37.1% (n=8,105) |
29.6% (n=3,430) |
38.9% (n=2,236) |
45.8% (n=2,439) |
<0.001 | |
| Any Pa positive culture (by 6yo) | 71.4% (n=8,105) |
68.7% (n=3,430) |
72.1% (n=2,236) |
74.7% (n=2,439) |
<0.001 | |
| Likely MRSA Chronic Endobronchial Infection (by 6yo) | 18.5% (n=7,381) |
13.9% (n=3,131) |
19.3% (n=2,049) |
24.3% (n=2,201) |
<0.001 | |
| Likely Pa Chronic Endobronchial Infection (by 6yo) | 18.3% (n=7,381) |
15.7% (n=3,131) |
18.8% (n=2,049) |
21.6% (n=2,201) |
<0.001 | |
| Number of Pulmonary Exacerbations (between 4–6.99yo) | 0.8 ± 1.7 (n=7,578) |
0.5 ± 1.1 (n=3,203) |
0.8 ± 1.8 (n=2,109) |
1.1 ± 2.1 (n=2,266) |
<0.001 | |
The proportion of children with positive cultures for common respiratory pathogens differed between insurance status groups (Table 1). Children with exclusively public insurance had the highest rate of at least one respiratory culture positive for MRSA (45.8%), followed by those with intermittent private insurance (38.9%) and those with always private insurance (29.6%), with similar findings for Pa (74.7%, 72.1%, and 68.7%, respectively). A greater proportion of individuals with exclusively public insurance and intermittent private insurance had chronic infection with MRSA (24.3% and 19.3% respectively) than among children with always private insurance (13.9%); similar findings were seen with Pa chronic infection (21.6%, 18.8%, 15.7%, respectively). Finally, PEx between 4–6 years also differed by insurance group with the highest mean number of exacerbations, 1.1 (SD=2.1) occurring in children with exclusively public insurance followed by 0.8 (SD=1.8) in children with intermittent private insurance and 0.5 (SD=1.1) in children with always private insurance.
Differences between the included and excluded populations are detailed in Supplemental Table 1. Notably those excluded were more likely to be non-White or Hispanic and have no F508del variants. Those excluded had lower maximum BMI percentile at age 6, but were less likely to ever culture MRSA or Pa and did not differ on FEV1pp or pulmonary exacerbations at age 6.
3.1. Lung Function
The average maxFEV1pp between 6–6.99 years for the study population was 101.9±17.1% (Table 2). In crude analyses, children with intermittent private insurance had a 3.3% (95% CI: −4.3, −2.2) lower maxFEV1pp at 6–6.99 years and children with exclusively public insurance had a 6.6% (95% CI: −7.7, −5.6) lower maxFEV1pp at 6–6.99 years compared to those with always private insurance (Table 3). For children with intermittent private insurance, these results persisted after adjustment for demographic and clinical factors (2.6% lower; 95% CI: −3.6, −1.6), and after accounting for other SES covariates (1.9% lower; 95%CI: −3.0, −0.8). Similarly, children with exclusively public insurance had significantly lower maxFEV1pp compared to children with private coverage in both adjusted models (β =−5.8; 95%CI: −6.9, −4.8 and β =−4.4; 95%CI: −5.6, −3.2). Results were consistent when comparing the mean FEV1pp at 6–6.99 years between insurance categories while variability in FEV1pp did not differ by insurance status in the adjusted models.
Table 2.
Respiratory and Nutritional Outcomes at 6 Years of Age
| Mean ± S.D. | Entire Study Population (n = 8,109) |
Always Private (n = 3,433) |
Intermittent Private (n = 2,237) |
Exclusively Public (n = 2,439) |
P Value |
|---|---|---|---|---|---|
| FEV 1 pp Maximum 6yo | 101.9 ± 17.1 (n=7,263) |
104.9 ± 15.6 (n=3,119) |
101.5 ± 16.8 (n=2,014) |
98.1 ± 18.8 (n=2,130) |
<0.001 |
| FEV 1 pp Mean 6yo | 93.7 ± 17.5 (n=7,263) |
97.1 ± 15.9 (n=3,119) |
93.0 ± 17.1 (n=2,014) |
89.3 ± 18.9 (n=2,130) |
<0.001 |
| FEV1pp variability (Maximum 6yo – minimum 6yo) | 19.4 ± 13.2 (n=6,643) |
18.2 ± 12.5 (n=2,886) |
19.9 ± 13.5 (n=1,832) |
20.6 ± 13.7 (n=1,925) |
<0.001 |
| BMI Percentile Maximum 6yo | 65.5 ± 23.1 (n=7,736) |
65.3 ± 23.1 (n=3,260) |
65.5 ± 22.9 (n=2,162) |
65.8 ± 23.4 (n=2,314) |
0.75 |
| BMI Percentile Mean 6yo | 56.0 ± 24.3 (n=7,736) |
56.6 ± 24.3 (n=3,260) |
55.7 ± 23.9 (n=2,162) |
55.7 ± 24.6 (n=2,314) |
0.27 |
Table 3.
Adjusted and Unadjusted Models for Lung Function and BMI at 6 Years of Age by Insurance Status
| Unadjusted Model* | Adjusted Model 1** | Adjusted Model 2*** | |||||
|---|---|---|---|---|---|---|---|
| β value (95% C.I.) |
P value | β value (95% C.I.) |
P value | β value (95% C.I.) |
P value | ||
|
FEV1pp (Max. 6yo; n=5,961) |
Always Private | 0.0 | - | 0.0 | - | 0.0 | - |
| Intermittent Private | −3.3 (−4.3, −2.2) |
<0.001 | −2.6 (−3.6, −1.6) |
<0.001 | −1.9 (−3.0, −0.8) |
0.001 | |
| Exclusively Public | −6.6 (−7.7, −5.6) |
<0.001 | −5.8 (−6.9, −4.8) |
<0.001 | −4.4 (−5.6, −3.2) |
<0.001 | |
|
FEV1pp (Mean 6yo; n=5,961) |
Always Private | 0.0 | - | 0.0 | - | 0.0 | - |
| Intermittent Private | −3.9 (−4.9, −2.9) |
<0.001 | −2.9 (−4.0, −1.9) |
<0.001 | −2.1 (−3.2, −1.0) |
<0.001 | |
| Exclusively Public | −7.6 (−8.7, −6.6) |
<0.001 | −6.4 (−7.4, −5.3) |
<0.001 | −4.6 (−5.8, −3.4) |
<0.001 | |
|
FEV1pp variability (Max. 6yo – min. 6yo; n=5,480) |
Always Private | 0.0 | - | 0.0 | - | 0.0 | - |
| Intermittent Private | 1.7 (0.8, 2.5) |
<0.001 | 1.1 (0.3, 2.0) |
0.010 | 0.8 (−0.1, 1.7) |
0.10 | |
| Exclusively Public | 2.3 (1.5, 3.1) |
<0.001 | 1.5 (0.6, 2.4) |
0.001 | 0.8 (−0.2, 1.8) |
0.13 | |
|
Maximum BMI (Percentile; 6,342) |
Always Private | 0.0 | - | 0.0 | - | 0.0 | - |
| Intermittent Private | 0.0 (−1.4, 1.4) |
0.99 | 0.0 (−1.4, 1.4) |
0.98 | 0.2 (−1.3, 1.6) |
0.84 | |
| Exclusively Public | 0.7 (−0.7, 2.0) |
0.32 | 0.1 (−1.3, 1.5) |
0.87 | 0.5 (−1.2, 2.1) |
0.58 | |
|
Mean BMI (Percentile; 6,342) |
Always Private | 0.0 | - | 0.0 | - | 0.0 | - |
| Intermittent Private | −1.0 (−2.5, 0.4) |
0.16 | −0.6 (−2.0, 0.9) |
0.43 | −0.5 (−2.1, 1.0) |
0.49 | |
| Exclusively Public | −0.7 (−2.2, 0.7) |
0.31 | −0.7 (−2.2, 0.7) |
0.32 | −0.5 (−2.2, 1.2) |
0.56 | |
Unadjusted model includes FEV1pp measure or BMI percentile as the dependent variable and insurance status as a dummy variable with the “always private insurance” set as the reference group.
Adjusted model 1 adds demographic and clinical covariates of cohort (year of birth), sex, age at diagnosis, race, ethnicity, number of F508del CFTR variants (dummy variable), exocrine pancreatic status, presence of feeding tube by 6 years of age, any MRSA, and any Pa to the unadjusted model.
Adjusted model 2 adds other SES covariates of log of estimated median household income at birth and highest level of maternal education (dummy variable) at 6yo to adjusted model 1.
3.2. Nutritional Status
The average maximum BMI percentile between 6–6.99 years for the study population was 65.5 ± 23.1 (Table 2). In contrast to lung function, neither maximum nor mean BMI percentile between 6–6.99 years differed by insurance category in unadjusted models or adjusted models including demographic and clinical characteristics (Table 3). Moreover, there were no group differences in the proportion of children with underweight BMI percentiles (<10th percentile or <50th percentile) or overweight BMI percentiles (>85th percentile or >95th percentile) (Supplemental Table 2 and 3). To examine whether the higher rate of feeding tube placement in those with public or intermittent private insurance may have contributed to any observed differences in BMI outcomes, we evaluated BMI percentile in those who did not have a feeding tube placed. For individuals without feeding tubes, no difference was seen in maximum or mean BMI percentiles between insurance categories (ANOVA p=0.33 and p=0.86, respectively).
4. Discussion
This study evaluated the association between early life health insurance status, as a proxy for SES, and respiratory and nutritional outcomes among children with CF at 6 years. While a plurality of individuals in our study always had private insurance coverage, a large segment of the pediatric population (27.6%) had intermittent private coverage (receiving private and exclusively public insurance in different years) prior to age 6. Consistent with studies in adolescents and adults, very young children with exclusively public insurance had the lowest maxFEV1pp at age 6. Importantly our results suggest that individuals who intermittently have private coverage during early childhood also have lung function at 6 years that is between peers who consistently have private insurance coverage and peers with exclusively public coverage, suggesting a stepwise association with this SES proxy.
These results support the importance of carefully addressing early social determinants of health given evidence that these early disparities may be amplified as children enter adolescence and adulthood. The recent study by Tumin and colleagues, which also used CFFPR data, reported 30–40% of individuals aged 12–23 years had intermittent public coverage and found a more rapid decline in lung function associated with public insurance compared to private insurance within this older cohort.15 Similar to our findings with FEV1pp, they also observed a more rapid decline with intermittent public insurance coverage compared to private insurance, although not as rapid as that seen with public insurance. Taken together, these results provide strong evidence that the combination of societal factors that prevent individuals from having continuous private insurance coverage (i.e., educational attainment, annual household income and occupational status) are associated with disease severity and pulmonary decline over time.
In addition to identifying non-genetic factors such as SES (including insurance variability) that contribute to the heterogeneity in CF disease, these findings also highlight the need for improved detection of early lung function changes given the growing evidence that CF disparities in lung function begin in early childhood but may not become clinically apparent until the first year that pulmonary function testing is regularly assessed. While FEV1pp remains a strong predictor of mortality in CF, young patients often cannot perform the maneuvers required for valid spirometry testing making it less useful in detecting early lung disease in CF. As such, there continues to be a need to expand the clinical use of pulmonary function testing techniques such as multiple breath washout25 and forced oscillation techniques26 to detect the evolution of inflammation and obstruction in otherwise asymptomatic children and inform interventions to prevent pulmonary function decline among higher risk groups.
While CFTR modulator therapies may improve respiratory symptoms and grant extensions on lifespan for individuals with qualifying mutations, our results indicate that individuals with exclusively public and intermittently private insurance are less likely to have an F508del variant and more likely to identify as Hispanic, which is associated with lower FEV1 and higher rates of mortality.27 Given the relative proportion of Hispanic patients diagnosed with CF has been rising over time, reflecting national population trends, we have concerns that some of these disparities may increase; thus further focus is needed to understand the intersection between race/ethnicity and SES on health outcomes in CF. Although we identified intermittent private insurance coverage as being associated with poorer outcomes compared to private insurance, it is unclear how changes in health care delivery through legislation may change outcomes as well; the Patient Protection and Affordable Care Act (ACA; 2010) has not appeared to increase private coverage or use of routine care among those with CF who were eligible.28
Contrary to our hypothesis, we did not observe any differences in BMI percentiles between insurance coverage groups, as was similarly noted in Tumin et al.’s older cohort. This may be secondary to the creation of CFF nutritional guidelines, an increased focus on early nutritional surveillance, as well as effective interventions for malnutrition, benefiting all individuals with CF regardless of SES. Additionally, despite the known association between lower childhood SES and higher BMI noted in the general population, we did not see an association between insurance status and the proportion of BMI percentiles that would be classified as overweight or obese. Of note, we did observe that those with public insurance, as well as those with intermittent private insurance were more likely to have feeding tubes placed by age 6. Although BMI percentiles are similar across insurance coverage groups in subjects without feeding tubes, we cannot exclude the possibility that the higher rate of feeding tube placement in the public and intermittent private insurance groups may contribute to similar BMI percentiles across the groups. Thus, it is possible nutritional outcomes are being achieved due to additional procedures such as surgically placed feeding tubes. Irrespective of BMI percentile, those with lower SES as measured by insurance status demonstrated lower lung function starting at age 6, indicating that optimizing nutritional status, while imperative, may not overcome the deleterious effects of lower SES on lung health.
Understanding how SES impacts early health outcomes in CF remains critically important for modifying outcomes. Prior research by Schechter and colleagues demonstrated no significant differences in age at diagnosis or disparities in access to routine CF care (use of chronic pulmonary medications, clinic visits or rates of treatment of PEx) to explain worse health outcomes among individuals with public insurance compared to those with private insurance coverage.10,29,30 Other possible explanations for these differences in outcomes could include environmental exposures, including second-hand smoke11,12 and ambient air pollution,32,33 which contribute to CF lung disease34 and are associated with lower SES. Additionally, lower SES is associated with higher rates of chronic endobronchial infection with MRSA, thought to be related to overcrowding and nutritional status.35 Indeed, individuals with exclusively public and intermittent private insurance in our study were more likely to have chronic infection with MRSA and Pa, which are associated with air pollution exposure36,37 and severity of lung disease.38,39 Similarly, the association between SES and health outcomes in CF has been shown to be influenced by nonadherence40 and mental health co-morbidities, including anxiety and depression.41 Together, our findings in conjunction with the existing literature suggest that to improve lung outcomes for children with CF and public or intermittent private insurance, we may need to more aggressively identify and treat PEx and address factors associated with poverty such as reducing exposure to air pollution, addressing housing overcrowding, incorporating strategies to improve treatment adherence, and treating mental health diagnoses in addition to the aggressive nutritional interventions already provided.
There are several limitations to the present investigation. First, insurance status was used as a proxy for SES. As noted, SES is multi-factorial and there is no measure of SES considered to be the gold standard; as can be seen in our results the effect size associated with insurance status decreases when other markers of SES are added to the model (maternal education and estimated household income). Insurance status, which is often linked to household income, is one component of SES, however the use of broad categories (i.e., private, public, and intermittent private) may not capture the subtle nuances of insurance coverage, such as variation in U.S. state eligibility criteria for public insurance, the extent of coverage for specific therapies with private and/or public insurance, and the impact of disease severity and medical expenditures on eligibility for public insurance coverage. Additionally, insurance coverage is only one financial barrier to access to healthcare, the CFFPR does not capture insurance copays and other out-of-pocket healthcare expenses in the registry, and the median health care cost for CF based on private insurance claims is at least $131,000 per year in 2016.42 Secondly, the single annual measure of insurance status may not reflect variability within a given year or over time. Given the timespan of the cohort used in this study it is possible that cohort effects may exist in terms of care practices, income, Medicaid eligibility, etc. Another limitation is the generalizability of our results to the entire CF population, as our excluded population may have overall milder disease based on pancreatic sufficiency and is more likely to be non-White or Hispanic. Finally, there may be bias due to residual confounding resulting from other unmeasured confounders such as environmental exposures including secondhand smoke exposure and neighborhood effects such as pollution, which may impact exacerbations as well as early health outcomes.
In conclusion, our findings suggest that insurance status, as a proxy for income and other social determinants of health, influences health outcomes in CF, specifically the factors that result in individuals not having private insurance coverage during early life, is associated with lower lung function at age 6, albeit less so for those with intermittent private insurance coverage. Given that Tumin et al. found that these disparities associated with health insurance may persist into adulthood, it is critically important to address the unique barriers that individuals with CF and lower SES face in achieving their health goals and to determine how and why these disparities arise so early in childhood. CF teams are uniquely positioned to be able to provide treatment and services to delay the onset of lung function decline in these higher-risk children and to support advocacy at the local and national level for solutions to address non-medical factors that affect CF management and health outcomes.
Supplementary Material
Highlights:
Children with CF often switch between public and private insurance coverage
Public insurance and variable insurance are associated with lower FEV1 and more pulmonary exacerbations at age 6
Lung function disparities begin in early childhood and may be amplified throughout the lifespan
Identifying risk factors for early lung function decline is essential for addressing health disparities
Acknowledgment:
The authors would like to thank the Cystic Fibrosis Foundation for the use of CF Foundation Patient Registry data to conduct this study. Additionally, we would like to thank the patients, care providers, and clinic coordinators at CF Centers throughout the United States for their contributions to the CF Foundation Patient Registry.
Funding:
This work was supported by the Cystic Fibrosis Foundation [DICKIN19B0] and the National Heart, Lung and Blood Institute [R01 HL128475].
Conflict of Interest Statement:
All authors disclose that they have no financial interests in the subject of this manuscript. The funding sources have no role in the analysis or drafting of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton H. Evidence-Based Practice Recommendations for Nutrition-Related Management of Children and Adults with Cystic Fibrosis and Pancreatic Insufficiency: Results of a Systematic Review. Journal of the American Dietetic Association. 2008;108(5):832–839. doi: 10.1016/j.jada.2008.02.020 [DOI] [PubMed] [Google Scholar]
- 2.Yen EH, Quinton H, Borowitz D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J Pediatr. 2013;162(3):530–535.e1. doi: 10.1016/j.jpeds.2012.08.040 [DOI] [PubMed] [Google Scholar]
- 3.Konstan MW, Butler SM, Wohl MEB, et al. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr. 2003;142(6):624–630. doi: 10.1067/mpd.2003.152 [DOI] [PubMed] [Google Scholar]
- 4.Cystic Fibrosis Foundation Patient Registry. 2019. Annual Data Report. Bethesda, Maryland. [Google Scholar]
- 5.Lester LA, Kraut J, Lloyd-Still J, et al. ΔF508 Genotype Does Not Predict Disease Severity in an Ethnically Diverse Cystic Fibrosis Population. Pediatrics. 1994;93(1):114–118. [PubMed] [Google Scholar]
- 6.Schechter MS, Shelton BJ, Margolis PA, Fitzsimmons SC. The association of socioeconomic status with outcomes in cystic fibrosis patients in the United States. Am J Respir Crit Care Med. 2001;163(6):1331–1337. doi: 10.1164/ajrccm.163.6.9912100 [DOI] [PubMed] [Google Scholar]
- 7.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic Status in Health Research: One Size Does Not Fit All. JAMA. 2005;294(22):2879. doi: 10.1001/jama.294.22.2879 [DOI] [PubMed] [Google Scholar]
- 8.Cheng TL, Goodman E, THE COMMITTEE ON PEDIATRIC RESEARCH. Race, Ethnicity, and Socioeconomic Status in Research on Child Health. Pediatrics. 2015;135(1):e225–e237. doi: 10.1542/peds.2014-3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daly MC, Duncan GJ, McDonough P, Williams DR. Optimal indicators of socioeconomic status for health research. Am J Public Health. 2002;92(7):1151–1157. doi: 10.2105/ajph.92.7.1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schechter MS, McColley SA, Silva S, Haselkorn T, Konstan MW, Wagener JS. Association of Socioeconomic Status with the Use of Chronic Therapies and Healthcare Utilization in Children with Cystic Fibrosis. J Pediatr. 2009;155(5):634–9.e1-4. doi: 10.1016/j.jpeds.2009.04.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ong T, Schechter M, Yang J, et al. Socioeconomic Status, Smoke Exposure, and Health Outcomes in Young Children With Cystic Fibrosis. Pediatrics. 2017;139(2):e20162730. doi: 10.1542/peds.2016-2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oates GR, Baker E, Rowe SM, et al. Tobacco smoke exposure and socioeconomic factors are independent predictors of pulmonary decline in pediatric cystic fibrosis. J Cyst Fibros. 2020;19(5):783–790. doi: 10.1016/j.jcf.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Short PF, Graefe DR. Battery-Powered Health Insurance? Stability In Coverage Of The Uninsured. Health Affairs. 2003;22(6):244–255. doi: 10.1377/hlthaff.22.6.244 [DOI] [PubMed] [Google Scholar]
- 14.Sommers BD. Loss of Health Insurance Among Non-elderly Adults in Medicaid. J Gen Intern Med. 2009;24(1):1–7. doi: 10.1007/s11606-008-0792-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tumin D, Crowley EM, Li SS, Wooten W, Ren CL, Hayes D. Patterns of Health Insurance Coverage and Lung Disease Progression in Adolescents and Young Adults with Cystic Fibrosis. Annals of the American Thoracic Society. Published online September 4, 2020. doi: 10.1513/AnnalsATS.201911-839OC [DOI] [PubMed] [Google Scholar]
- 16.Buchmueller T, Orzol SM, Shore-Sheppard L. Stability of children’s insurance coverage and implications for access to care: evidence from the Survey of Income and Program Participation. Int J Health Care Finance Econ. 2014;14(2):109–126. doi: 10.1007/s10754-014-9141-1 [DOI] [PubMed] [Google Scholar]
- 17.Aiken KD, Freed GL, Davis MM. When Insurance Status Is Not Static: Insurance Transitions of Low-Income Children and Implications for Health and Health Care. Ambulatory Pediatrics. 2004;4(3):237–243. doi: 10.1367/A03-103R.1 [DOI] [PubMed] [Google Scholar]
- 18.Tumin D, Miller R, Raman VT, Uffman JC, Tobias JD. Patterns of Health Insurance Discontinuity and Children’s Access to Health Care. Maternal and Child Health Journal. 2019;23(5):667–677. doi: 10.1007/s10995-018-2681-0 [DOI] [PubMed] [Google Scholar]
- 19.Konstan MW, Morgan WJ, Butler SM, et al. Risk Factors For Rate of Decline in Forced Expiratory Volume in One Second in Children and Adolescents with Cystic Fibrosis. The Journal of Pediatrics. 2007;151(2):134–139.e1. doi: 10.1016/j.jpeds.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 20.McColley SA, Schechter MS, Morgan WJ, Pasta DJ, Craib ML, Konstan MW. Risk factors for mortality before age 18 years in cystic fibrosis. Pediatr Pulmonol. 2017;52(7):909–915. doi: 10.1002/ppul.23715 [DOI] [PubMed] [Google Scholar]
- 21.Sanders DB, Zhang Z, Farrell PM, Lai HJ. Early life growth patterns persist for 12 years and impact pulmonary outcomes in cystic fibrosis. Journal of Cystic Fibrosis. 2018;17(4):528–535. doi: 10.1016/j.jcf.2018.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knapp EA, Fink AK, Goss CH, et al. The Cystic Fibrosis Foundation Patient Registry. Design and Methods of a National Observational Disease Registry. Annals ATS. 2016;13(7):1173–1179. doi: 10.1513/AnnalsATS.201511-781OC [DOI] [PubMed] [Google Scholar]
- 23.Musumeci M, Chidambaram P. Medicaid’s Role for Children with Special Health Care Needs: A Look at Eligibility, Services, and Spending. :11. [Google Scholar]
- 24.Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. European Respiratory Journal. 2012;40(6):1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subbarao P, Milla C, Aurora P, et al. Multiple-Breath Washout as a Lung Function Test in Cystic Fibrosis. A Cystic Fibrosis Foundation Workshop Report. Ann Am Thorac Soc. 2015;12(6):932–939. doi: 10.1513/AnnalsATS.201501-021FR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brennan S Correlation of forced oscillation technique in preschool children with cystic fibrosis with pulmonary inflammation. Thorax. 2005;60(2):159–163. doi: 10.1136/thx.2004.026419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assessing Differences in Mortality Rates and Risk Factors Between Hispanic and Non-Hispanic Patients With Cystic Fibrosis in California | Elsevier Enhanced Reader. doi: 10.1378/chest.14-2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tumin D, Li SS, Kopp BT, et al. The effect of the affordable care act dependent coverage provision on patients with cystic fibrosis. Pediatric Pulmonology. 2017;52(4):458–466. doi: 10.1002/ppul.23648 [DOI] [PubMed] [Google Scholar]
- 29.Schechter MS, Margolis PA. Relationship between socioeconomic status and disease severity in cystic fibrosis. J Pediatr. 1998;132(2):260–264. [DOI] [PubMed] [Google Scholar]
- 30.Schechter MS, McColley SA, Regelmann W, et al. Socioeconomic Status and the Likelihood of Antibiotic Treatment for Signs and Symptoms of Pulmonary Exacerbation in Children with Cystic Fibrosis. The Journal of Pediatrics. 2011;159(5):819–824.e1. doi: 10.1016/j.jpeds.2011.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanders DB, Bittner RCL, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med. 2010;182(5):627–632. doi: 10.1164/rccm.200909-1421OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goss CH, Newsom SA, Schildcrout JS, Sheppard L, Kaufman JD. Effect of Ambient Air Pollution on Pulmonary Exacerbations and Lung Function in Cystic Fibrosis. Am J Respir Crit Care Med. 2004;169(7):816–821. doi: 10.1164/rccm.200306-779OC [DOI] [PubMed] [Google Scholar]
- 33.Goeminne PC, Kiciński M, Vermeulen F, et al. Impact of air pollution on cystic fibrosis pulmonary exacerbations: a case-crossover analysis. Chest. 2013;143(4):946–954. doi: 10.1378/chest.12-1005 [DOI] [PubMed] [Google Scholar]
- 34.Szczesniak R, Rice JL, Brokamp C, et al. Influences of environmental exposures on individuals living with cystic fibrosis. Expert Review of Respiratory Medicine. 2020;14(7):737–748. doi: 10.1080/17476348.2020.1753507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oates GR, Harris WT, Rowe SM, et al. Area Deprivation as a Risk Factor for Methicillin-resistant Staphylococcus aureus Infection in Pediatric Cystic Fibrosis. Pediatr Infect Dis J. 2019;38(11):e285–e289. doi: 10.1097/INF.0000000000002419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Psoter KJ, De Roos AJ, Mayer JD, Kaufman JD, Wakefield J, Rosenfeld M. Fine Particulate Matter Exposure and Initial Pseudomonas aeruginosa Acquisition in Cystic Fibrosis. Annals of the American Thoracic Society. 2015;12(3):385–391. doi: 10.1513/AnnalsATS.201408-400OC [DOI] [PubMed] [Google Scholar]
- 37.Psoter KJ, De Roos AJ, Wakefield J, Mayer JD, Rosenfeld M. Air pollution exposure is associated with MRSA acquisition in young U.S. children with cystic fibrosis. BMC Pulm Med. 2017;17. doi: 10.1186/s12890-017-0449-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pittman JE, Calloway EH, Kiser M, et al. Age of Pseudomonas aeruginosa Acquisition and Subsequent Severity of Cystic Fibrosis Lung Disease. Pediatr Pulmonol. 2011;46(5):497–504. doi: 10.1002/ppul.21397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dasenbrook EC, Merlo CA, Diener-West M, Lechtzin N, Boyle MP. Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. Am J Respir Crit Care Med. 2008;178(8):814–821. doi: 10.1164/rccm.200802-327OC [DOI] [PubMed] [Google Scholar]
- 40.Oates GR, Stepanikova I, Gamble S, Gutierrez HH, Harris WT. Adherence to airway clearance therapy in pediatric cystic fibrosis: Socioeconomic factors and respiratory outcomes. Pediatric Pulmonology. 2015;50(12):1244–1252. doi: 10.1002/ppul.23317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quittner AL, Goldbeck L, Abbott J, et al. Prevalence of depression and anxiety in patients with cystic fibrosis and parent caregivers: results of The International Depression Epidemiological Study across nine countries. Thorax. 2014;69(12):1090–1097. doi: 10.1136/thoraxjnl-2014-205983 [DOI] [PubMed] [Google Scholar]
- 42.Grosse SD, Do TQN, Vu M, Feng LB, Berry JG, Sawicki GS. Healthcare expenditures for privately insured US patients with cystic fibrosis, 2010–2016. Pediatric Pulmonology. 2018;53(12):1611–1618. doi: 10.1002/ppul.24178 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


