Abstract
The neurobiology of schizophrenia involves multiple facets of pathophysiology, ranging from its genetic basis over changes in neurochemistry and neurophysiology, to the systemic level of neural circuits. Although the precise mechanisms associated with the neuropathophysiology remain elusive, one essential aspect is the aberrant maturation and connectivity of the prefrontal cortex that leads to complex symptoms in various stages of the disease. Here, we focus on how early developmental dysfunction, especially N-methyl-D-aspartate receptor (NMDAR) development and hypofunction, may lead to the dysfunction of both local circuitry within the prefrontal cortex and its long-range connectivity. More specifically, we will focus on an “all roads lead to Rome” hypothesis, i.e., how NMDAR hypofunction during development acts as a convergence point and leads to local gamma-aminobutyric acid (GABA) deficits and input-output dysconnectivity in the prefrontal cortex, which eventually induce cognitive and social deficits. Many outstanding questions and hypothetical mechanisms are listed for future investigations of this intriguing hypothesis that may lead to a better understanding of the aberrant maturation and connectivity associated with the prefrontal cortex.
Keywords: prefrontal cortex, NMDA receptor hypofunction, development, GABAergic interneurons, aberrant network, schizophrenia
I. Introduction
Schizophrenia (SZ) is a neurodevelopmental disorder with both cognitive and social deficits that are resistant to current therapies. To improve the treatment of these impairments, it is essential to clarify the pathophysiological processes underlying the progression of SZ. A large body of evidence indicates a dysfunctional local circuitry, particularly gamma-aminobutyric acid (GABA)ergic deficits within the prefrontal cortex (PFC) 1 and its connections with other brain regions, especially those related to the limbic systems 2. Over the last two decades, numerous neurophysiological (EEG, electroencephalogram) and neuroimaging (fMRI, functional magnetic resonance imaging) studies of patients with SZ have provided strong evidence for both aberrant local prefrontal network activity and brain-wide dysconnectivity, i.e., abnormal functional integration of information processes in the PFC and the brain 3–10. While the evidence for dysconnectivity in SZ is strong 9, 11–13, its etiology is complicated, and its mechanisms and significance for clinical symptoms remain subject to debate 9, 14–16. Nevertheless, in the past two decades, the framework of the dysconnectivity hypothesis has led to significant progress in the field. This dysconnectivity theory hypothesizes that the core neuropathology in SZ is aberrant N-methyl-D-aspartate receptor (NMDAR)-mediated synaptic plasticity and abnormal neuromodulation of NMDAR expression and function by disrupted neuromodulatory transmitters such as dopamine, serotonin, and acetylcholine 9, 14, 17, 18. Undeniably, we agree that NMDAR hypofunction is a critical player and a convergence point of the neuropathophysiology of SZ, especially for cognitive and social deficits 19.
However, here we argue against the causal role of neuromodulatory transmitters (such as dopamine, etc.) in the induction of NMDAR hypofunction. On the contrary, based on recent evidence, we hypothesize that NMDAR hypofunction occurs first 20, which in turn causes dysfunctional GABAergic circuitry locally within the PFC and disruptions to the incoming and outgoing connections with other brain regions, including neuromodulatory systems 21. We agree that this claim remains in debate as substantial literature showing that dopamine and/or other monoamine systems play essential roles in the prefrontal function and synaptic plasticity in both animals and humans 22, 23. However, evidence from clinical 22, 23 and pre-clinical 15, 16, 24–28 studies supports that the hypothesis NMDA hypofunction occurs earlier during or before juvenile period 19, 29 compared to the dopamine dysfunction a later stage of adolescence. This raises questions about the sequential changes or logical causal role of NMDAR vs. dopamine hypothesis. More studies on the causal roles of dopamine dysfunction in the disconnected network during earlier periods are needed.
The resulting GABA deficiency and subsequent dopamine/serotonin dysfunction lead to progressive symptoms, including cognitive and social function impairments and psychosis. Indeed, current antipsychotic medications that target the dopaminergic system demonstrate minimal therapeutic efficacy in treating cognitive and social deficits in SZ 30. In contrast, targeting NMDAR dysfunction during early disease stages appears to be a promising avenue for prevention and therapeutic intervention for cognitive and social deficits in various animal models, including the NMDAR antagonist MK-801 model, for SZ 31–33.
Mechanistically, how these two major hypotheses, NMDAR hypofunction and local/long-range dysconnectivity, are initiated and reconciled remains unexplored. A large body of evidence from both animal models and human studies implicates dysfunction of NMDARs in disease development and symptom manifestation of SZ 34–44. Recent studies, including ours, have suggested that NMDAR hypofunction is likely a convergence point that occurs during the early stage of the disease. This may consequentially initiate GABA deficits 19, 20, 41, 45, leading to aberrant local circuitry and long-range disconnections among different brain regions, including dopamine systems 22, 46, thereby accounting for key clinical features of SZ.
The PFC must precisely filter essential information from the numerous signals it receives from cortical and subcortical brain regions to perform the executive function. Local GABAergic interneurons (INs) are critical for gating incoming information to excitatory neurons 47. Additionally, long-range inputs drive inhibitory neurons to maintain the E/I balance within the prefrontal circuits 48, 49. The currently limited evidence suggests that distinct afferents may have unique NMDAR subunit compositions with differential functional roles for connectivity during development 50, 51. It is therefore vital to understand how PFC pyramidal neurons and different IN subtypes are modulated in unique ways across development by distinct afferent inputs from major upstream regulatory centers, including the mediodorsal thalamus (MD) and other thalamic nuclei, as well as other inputs from the limbic systems such as the ventral hippocampus (vHipp) and basolateral amygdala.
In this review, we focus on recent literature, especially the progress made within the past ten years, to present 1) clinical evidence of dysconnectivity; 2) the role of NMDAR hypofunction in aberrant local PFC circuitry and long-range dysconnectivity; 3) MD-PFC dysconnectivity and interneuron subtype specificity in SZ; 4) reconciling NMDAR hypofunction with local circuit and global dysconnectivity; and 5) outstanding questions and future perspectives.
II. Clinical evidence of brain-wide dysconnectivity
Since the dysconnection hypothesis for SZ was introduced more than 20 years ago 11, 12, 52, neuroscience has witnessed tremendous advances. The evidence for a systemic dysfunctional integration and connectivity is overwhelming, and now it is widely accepted that SZ is characterized by large-scale cortical (e.g., thalamocortical and corticocortical) dysconnectivity compared with healthy individuals 5, 9, 14. These findings involve the PFC and key subcortical and associative cortical regions mostly related to the limbic systems 3–5, 7, 9, 14, 53–55. Combined with the clinical findings, recent modeling of intrinsic and extrinsic connectivity also suggests a specific failure of intrinsic gain regulation within the PFC or modulation of synaptic efficacy in hierarchically subordinate structures 4, 7, 56, which supports the hypothesis of failures in self-monitoring or homeostatic plasticity proposed by Friton and colleagues 9, 14. Based on these clinical measurements and modeling, Yang et al. proposed that functional cortical hierarchy between the association and sensory regions underlies preferential network connectivity disturbances in associative vs. primary sensory (e.g., visual and auditory) cortices 56, and even widespread early-stage prefrontal hyperconnectivity 55 in SZ. The neuroimaging correlations of dysconnection appear to be stable over time, enabling the differentiation between patients and control subjects 57. It has even been proposed that these systemic measures of dysconnection may serve as neurobiological indices for defining SZ patients 9. However, this raises an intriguing question about the underlying biological trait abnormalities that implicate a failure in the modulation of synaptic efficacy relative to the potential compensatory homeostatic state of abnormalities that produce symptoms and signs 9.
Measures of functional connectivity with EEG or fMRI have also been linked to a genetic basis. A meta-analysis 58 reported that putative SZ risk variants reduced functional connectivity, which is supported by recent studies 59, providing more evidence for the dysconnection hypothesis 9, 52. Interestingly, dysconnectivity is mostly implicated in the PFC and other association areas, raising the question of how such differential impairment can be explained if biological abnormalities are common across the neocortex. This question has motivated a study of brain dysconnectivity in SZ that combined fMRI with a large-scale cortical network model of the human cortex 56, 60, and an intriguing hypothesis of macroscopic gradients of synaptic excitation and inhibition (E/I) balance across the neocortex that warrants further exploration 7.
III. The role of NMDA hypofunction in the aberrant local circuit and long-range dysconnectivity of the mPFC
Although there is strong evidence for global functional network dysconnectivity revealed by neuroimaging and neurophysiology, SZ is also clearly linked to disruption of the local prefrontal circuit, particularly imbalanced E/I ratio based on clinical 6, 10, 61, computation 56, and animal 62, 63 studies. Clinical literature from cross-diagnostic analysis indicates a convergent neural mechanism governing E/I balance in patients with SZ 61. There is also evidence supporting a link between glutamate-mediated cortical disinhibition, effective-connectivity deficits, and computational performance in psychosis 10. The question is how these two frameworks - global versus localized neural dysfunction – work together to display behavioral phenotypes, particularly cognitive and social impairments in SZ, that are dependent on proper PFC function. To answer this question, we elucidate the basic prefrontal circuitry that functions to execute normal cognition and social behavior, the distinct and prolonged developmental trajectory of the PFC, its unique connections with other brain regions, how NMDAR development and function are intertwined with the development of PFC circuitry, as well as new questions raised and hypothetical mechanisms learned from studies in other cortices for further investigations.
NMDAR in the development of prefrontal GABAergic interneurons
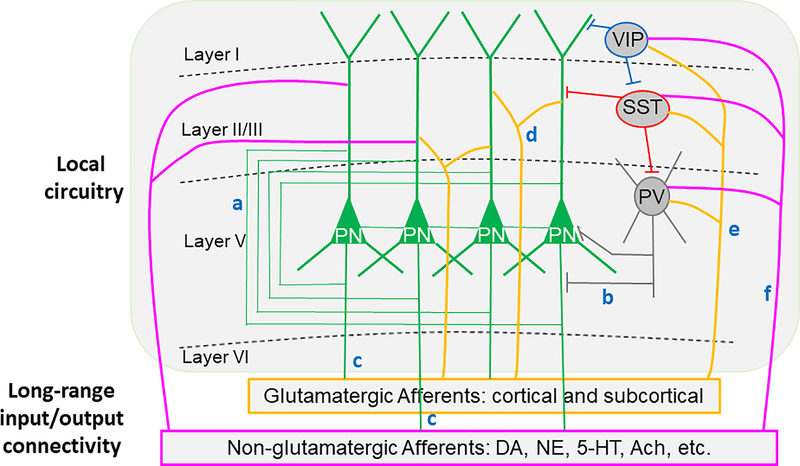
The PFC exhibits laminar and neuronal connections within the local circuitry. As shown in Figure 1, the excitatory glutamatergic pyramidal cells connect each other to form recurrent excitation and innervate GABAergic interneurons to initiate feedback inhibition. The pyramidal neurons project to other cortical regions to establish corticocortical connections and subcortical regions as descending innervations. Within the PFC local circuitry, a diverse array of GABAergic IN subtypes regulates cortical activity 64. The three most abundant INs include fast-spiking (FS) parvalbumin (PV)-INs (which target the perisomatic region of pyramidal neurons), somatostatin-expressing (SST)-INs (which target pyramidal neuron dendrites, gate input-specific information processing, and inhibit PV-INs 65) and vasointestinal peptide-expressing (VIP) cells (which inhibit SST-INs to induce disinhibition of pyramidal neurons 47, 66, 67. In addition, the local prefrontal circuitry receives both glutamatergic afferents and non-glutamatergic neuromodulatory afferents from the cortical and subcortical regions to enable feedforward excitation and inhibition 48, 68, while the pyramidal neurons in the PFC project to the limbic systems and other cortical and subcortical structures (Figure 1) that are different from other cortical regions such as primary sensory, auditory, and visual cortices.
Figure 1.
Illustration showing the simplified local prefrontal circuitry and long-range input/output connectivity. The excitatory glutamatergic pyramidal cells connect to each other to form recurrent excitation (a) and innervate GABAergic interneurons to form feedback inhibition (b). The pyramidal neurons project to other cortical and subcortical regions to form corticocortical connections and subcortical regions as descending innervations (c). In turn, local prefrontal circuitry, including both pyramidal neurons and GABAergic cells are also innervated by cortical and subcortical excitatory glutamatergic inputs (green) to form forward excitation (d) and feedforward inhibition (e). Both pyramidal neurons and GABAergic interneurons are also regulated by subcortical non-glutamatergic neuromodulatory afferents (red) from the dopamine (DA), norepinephrine (NE), serotonin (5-HT), and acetylcholine (Ach) cells. Despite these seemingly clear connections, exactly how they interact to affect PFC-associated functions remains to be determined. There are also many unknowns among these connections, and their roles in behavioral deficits associated with SZ symptoms.
Despite these seemingly clear connections, exactly how they interact to affect PFC-associated functions remains to be determined, especially those associated with different subtypes of GABAergic interneurons, such as VIP and SST, even in the normal brain. There are many unknowns among these connections. For example, how does NMDAR hypofunction induce an abnormal neurodevelopment in the local circuitry of different PFC neuronal subtypes and their long-range connections with other brain regions, including both glutamatergic and non-glutamatergic inputs and outputs? Consequently, it remains unclear how these changes may induce behavioral deficits associated with SZ symptoms.
SZ is a neurodevelopmental disorder, and most, if not all, of the functional and structural changes occur during the early stages of postnatal development, especially during juvenile and adolescent periods, as presented in both human 69–71 and animal 63, 72–76 studies. The delayed maturation of the PFC and, in particular, the delayed maturation of GABAergic INs in this region through adolescence critically contribute to susceptibility to cognitive and social deficits 15, 16, 77–85. Therefore, it is essential to elucidate when and how the local prefrontal circuitry forms and matures across development and the role of different cell types in the PFC in regulating cognitive and behaviors. It is also essential to understand how various excitatory afferents from cortical and subcortical regions (e.g., thalamus vs. hippocampus vs. amygdala, etc.) regulates the development of synaptic function in PV-, SST-, and VIP-INs vs. pyramidal neurons within the PFC, as illustrated in Figure 1.
The activity/experience-dependence of synaptic and circuit formation during development, which relies on NMDAR-mediated plasticity, has been extensively studied in somatosensory, auditory, and visual cortices 86–88. These studies suggest that GluN2B-containing NMDARs are the pillar in the maintenance of critical-period plasticity. In the PFC, the developmental GluN2B-to-GluN2A switch occurs later than in other brain regions and marks the transition from juvenile to adult neural processing and maturation of associative learning abilities 89. This may leave open a long critical period window for plasticity and may underlie the delayed maturation of the PFC 90. In addition, existing evidence assists us in speculating that the time course of NMDAR maturation is likely cell-type-specific 91, 92.
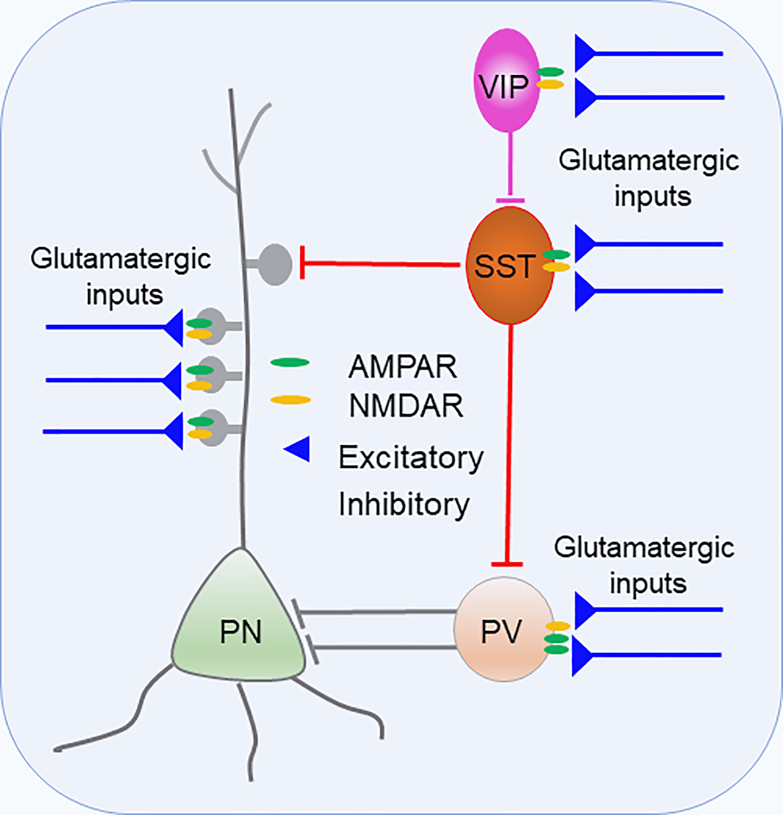
For example, the NMDAR hypofunction hypothesis has largely focused on NMDAR expression and function in PV cells 19, 20, 41, 42, 93. But paradoxically, PV-INs express relatively low levels of NMDARs 51, 94–98 (Figure 2), and they are enriched with GluN2A subunit 99–101. In contrast, SST- and VIP-INs express relatively more NMDARs and are enriched with GluN2B subunits (Figure 3), which have significantly slower decays than NMDARs expressing the GluN2A subunit 102–104. Previous studies reported that NMDAR subunit composition controls synaptogenesis and synapse stabilization 105. The distinct composition of NMDAR subunits endows the three types of interneurons with different magnitudes of plasticity in network activity during cognitive performance. All three types of interneurons in the PFC are crucially involved in cognitive function, social behavior, and SZ 103, 106–109. The limited current evidence suggests different IN subtypes show distinct changes in NMDAR function across neurodevelopment. For example, Koppensteinier et al. 92 showed that NMDA-mediated ESPC amplitude in SST-IN increases from adolescence to adulthood. In contrast, the amplitude of NMDA-mediated ESPCs in PV-INs decreases from the juvenile stage to adolescence, consistent with our previous report in rat PFC (Wang 2009). However, more research is needed to fully understand NMDAR expression and function in the different IN subtypes across neurodevelopment and how these may go awry in SZ.
Figure 2.
Prefrontal local circuit. Glutamatergic afferents innervate both excitatory pyramidal neurons (PN) and inhibitory GABAergic IN subtypes 248. These inputs are capable to generate diverse feedforward control on the prefrontal local network through PV-, SST, or VIP-INs. PV-INs express relatively low levels of NMDARs 51, 94–98, and they are enriched with GluN2A subunit 99–101. In contrast, SST- and VIP-INs express relatively more NMDARs and are enriched with GluN2B subunits 102–104.
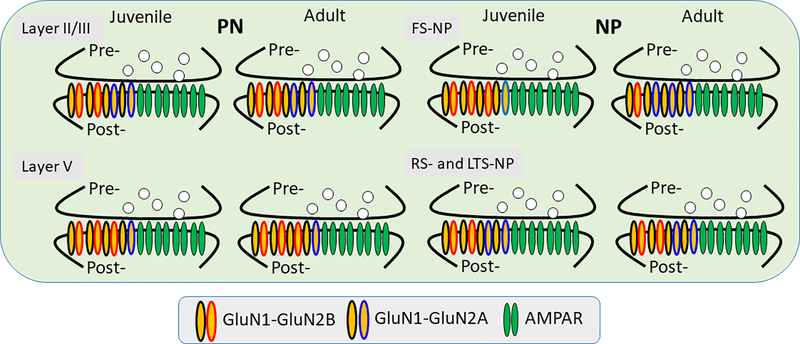
Figure 3.
Schematic model showing the development of NMDAR subunits in different types of PFC neurons. Layer II/III pyramidal neurons (PN) exhibit equal amount of GluN2A and GluN2B subunits, whereas layer V pyramidal neurons expresses more GluN2B subunits during the juvenile to adulthood development. In contrast, in the GABAergic interneurons (non-pyramidal neurons (NP), fast-spiking (FS) interneurons exhibits clear GluN2B-to-GluN2A subunit switch whereas regular spiking (RS) and low-threshold spiking (LTS) interneurons exhibit similar NMDAR subunit expression to the layer II/III pyramidal neurons. In summary, except a subset of FS-NPs, which show a sharp increase of GluN2A and decrease of GluN2B, all other cells exhibit no GluN2B-to-GluN2A switch during postnatal development, differing from other brain regions 95, 155, 185, 249.
Nevertheless, abundant evidence has shown that NMDAR blocker ketamine decreases resting-state functional network connectivity 110 and modulates hippocampal neurochemistry and functional connectivity in health subjects 111. These effects have not only emphasized the importance of NMDARs in neural connectivity but also neurons in other cortical regions (e.g., ventral tegmental area, VTA) and other cell types (e.g., SST-INs 106 in NMDAR hypofunction for SZ 42. Several recent studies have also provided interesting yet inconsistent reports regarding the relationship between NMDAR function and proper connectivity in the PFC. For example, Flores-Barrera et al. reported a late adolescent expression of GluN2B in the apical (but not basal) dendrites in mPFC layer 5 pyramidal neurons, with ventral hippocampal (vHipp) inputs requiring postsynaptic PKA and D1 signaling for GluN2B maturation 112. In contrast, by deleting GluN2B in PFC pyramidal neurons, Miller et al. showed a selective enhancement of mediodorsal thalamus (MD)-PFC but not a vHipp-PFC pathway in layer 2/3 pyramidal neurons, and that activation of MD-PFC pathway induces anti-depressive behaviors in young adult mice 113. This enhanced connectivity is consistent with a clinical report of increased connectivity during the early stage of the disease 55. It provides causal evidence that NMDAR dysfunction can lead to aberrant functional connectivity.
Notably, the mPFC is a convergent target of multiple long-range inputs, including those from vHipp and MD 114, 115. Given that these long-range inputs innervate pyramidal neurons 116 and INs 48, 68, 117 in both layers 2/3 and 5 (Figure 1), these findings raise important questions regarding how NMDAR hypofunction affects the excitatory activity from the MD and other long-range inputs, which in turn, shape the local inhibitory circuitry formed by different subtypes of INs within the PFC, and whether altered MD activity influences the inhibitory circuit formation among INs.
Recent studies have suggested an afferent-specific role of NMDARs for the circuit integration of INs 118 and input-specific NMDAR-dependent potentiation of dendritic (i.e., SST-INs) GABAergic inhibition 119. Tonic GABAergic activity also facilitates dendritic calcium signaling and short-term plasticity 120. Dendritic NMDARs in PV-INs even underlie supralunar integration of feedback excitation from local pyramidal neurons and thus enable strong and stable neuronal assemblies 121. Consequently, NMDAR ablation in corticolimbic PV-INs induces hippocampus-PFC functional hypoconnectivity after adolescence in a mouse model for SZ 63. Therefore, we expect an input- and cell type-specific, as well as age-dependent, modulation, as we and others have reported that cortical INs are differentially modulated by their inputs, including glutamatergic and monoaminergic fibers 21, 50, 122–126 (see Figure 1).
IV. MD-mPFC dysconnectivity and interneuron subtype specificity in SZ
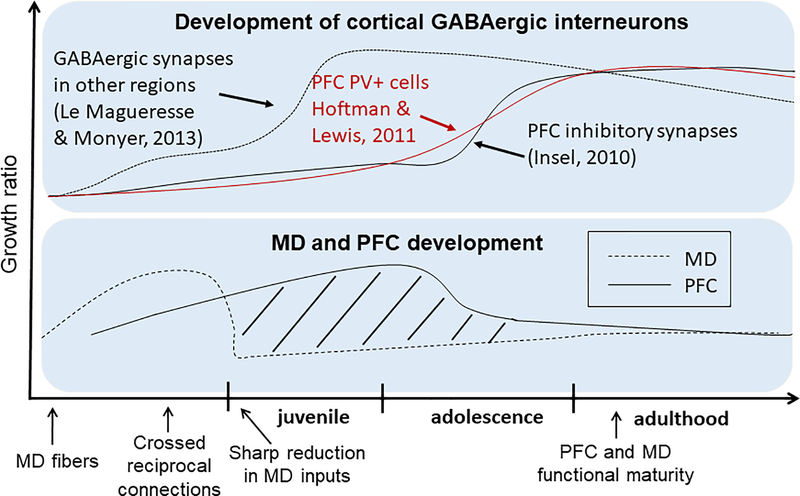
The MD is a major upstream regulatory center to the PFC 127–130. Excitatory afferents from the MD reach PFC cortical layers far earlier during development than the maturation of GABAergic INs and synapses 117 (Figure 4). Additionally, the MD and PFC share extensive reciprocal connections 114, 131 with SZ patients demonstrating reduced connectivity between these two brain regions 5, which is associated with cognitive and social deficits 49. Recent studies indicate that the MD plays distinct roles in sophisticated cognitive processes 116, 130, 132–134 and social dominance 135. In particular, MD inhibition in mice disrupts thalamofrontal connectivity and working memory 49, 131, 136, and goal-directed behavior 129, 137. In adulthood, we recently reported that acute chemogenetic inhibition of MD activity results in a significant reduction in GABAergic signaling and increased E/I balance within the mPFC, as well as abnormalities in cognitive and social behaviors. Furthermore, by selectively activating PV-INs in the mPFC with a chemogenetic tool, E/I balance was restored, and working memory and social deficits were ameliorated. These findings highlight the importance of thalamocortical activation of PV-INs in PFC-dependent behaviors 68.
Figure 4.
Developmental trajectory of cortical GABAergic interneurons and maturation of MD and PFC. Upper panel: Distinct developmental trajectories of GABAergic inhibitory synapses and PV+ cells in the PFC (red and black lines) vs other brain regions (dashed line) 75, 250, 251. Lower panel: MD afferent density (dashed line) and volume of the PFC (solid line). Dashed lines represent the different developmental window between MD activity and PFC function. Lower panel is modified from 117, and copyright is permitted for reuse of the figure.
Thus far, our study, as well as work from others, has mainly focused on PV cells in normal adult animals 42, leaving the role of SST and VIP cells in MD-mediated PFC function untested. In fact, we do not even know how MD inputs directly regulate SST and VIP-INs despite the dense distribution of both SST and VIP cells 138–141, as well as MD fibers 114, 142, 143 in superficial layers of the PFC. SST and VIP mRNA and peptide levels are both decreased in the PFC of patients with SZ 107, 144. Additionally, both cell types are critically involved in cognition 67, 145–147. It is thus essential to understand how MD and other inputs’ activity regulates different PFC INs during development and to determine whether MD inputs are essential for the reduction of SST and VIP in animal models for SZ. It is conceivable that the MD, as a major source of glutamatergic inputs, plays a critical role in the development of NMDARs in both pyramidal neurons and different subtypes of INs in the PFC (Figure 4).
V. Reconciling NMDAR hypofunction with local and global dysconnectivity
A basic question that has haunted the field is what causes GABA deficits and abnormal connectivity in neurodevelopmental disorders such as SZ? It is known that in response to normal and abnormal stimulation, synapses mediating local and long-range connections between different cell types exhibit sequential alterations through different mechanisms during development. More importantly, during adolescent development, GABAergic cell function becomes dominant compared to pyramidal neurons because they are receiving more glutamatergic innervations 78. For example, PV cells are enriched with excitatory inputs during adolescent development through regulation of excitatory synapse pruning on PV cells via ErbB4 splicing 148–151. This effect is both activity- and NMDAR-dependent, and disruption of any of these processes produces SZ-like cognitive and social deficits 20, 78, 149, 152, 153.
NMDARs significantly contribute to information transfer and integration at synapses during repetitive activity and to the generation of persistent activity of neural assemblies for working memory function 90, 154–156. The mechanisms for changes in NMDAR function, number, and subunit composition at a given synapse and cell type, as well as the identity and function of auxiliary subunits of NMDARs in the developing rodent PFC 51, 95, 96, 155, 157, remain understudied compared to other cortical regions 158–160.
NMDAR hypofunction and dendritic spine plasticity
Given the unique biophysical properties and development of NMDARs in the PFC, especially the high level of GluN2B 7, 90, the plasticity of NMDAR-mediated synaptic transmission is crucial to the circuit formation and maturation of the PFC. A growing body of evidence suggests the existence of synaptic pathology in SZ 15. NMDAR hypofunction in pyramidal neurons appears to be responsible for spine density alteration of cortical pyramidal cells as SZ-related spine density reduction is induced by GluN1 knockdown in the pyramidal neurons 20, 161, 162. Pharmacologically, subchronic PCP treatment decreases the number of dendritic synapses in the rat PFC 163, 164, but the underlying mechanisms remain to be determined. However, it has been demonstrated that mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonist ketamine 165. A remarkable recent rodent study provides additional support with in vivo dendritic spine monitoring in mPFC 166. Specifically, prolonged exposure to chronic unpredictable stress increases “depressive-like behaviors” in the mice and causes a retraction of dendritic spines in the mPFC. Interestingly, these changes are effectively normalized by systemic administration of ketamine 166, highlighting the importance of NMDAR in regulating dendritic spine plasticity in the PFC.
PFC dysconnectivity and NMDAR hypofunction in non-human primate and human studies
Prior neural recording studies in monkey PFC also characterized how blocking NMDAR alters physiological signals in prefrontal neurons related to working memory and executive control. Specifically, blocking NMDAR weakens delay period activity associated with working memory 154, reduces the strength and task selectivity of neural signals reflecting executive control in rule-based tasks 167, 168, and modifies prefrontal oscillations reflecting trial outcome 169. In particular, blockade of GluN2B in the dlPFC markedly reduces the persistent firing of the delay cells needed for neuronal representations of visual space 154, 170. However, studies probing how NMDAR hypofunction affects prefrontal function in non-human primates remain limited 171. Two different NMDAR antagonists, PCP and MK-801, have been often used in rodent behavioral studies, while ketamine has been often used in non-human primates to induce cognitive disturbances 171–176. Moreover, non-human studies tend to use acute and bolus injection of NMDAR antagonists equivalent to those achieved in human subjects, in which ketamine produced resting brain hyperconnectivity and SZ-like cognitive deficits 177, 178. Systemic administration of ketamine also reduces the persistent firing of delay cells in primates. This effect may explain why this drug can mimic or worsen SZ’s cognitive symptoms 170.
In a recent study, Zick et al. further reported that blocking NMDAR-mediated synaptic transmission by PCP in primate disrupts spike timing in prefrontal circuits that lead to activity-dependent synaptic disconnection of these circuits over time, potentially linking synchrony and connectivity deficits in SZ 179. Administration of ketamine also yields a circuit-level mechanism that links NMDAR hypofunction to synaptic connections to behaviors, including decision-making biases 180. Based on these observations, although the causal biology underlying SZ remains not well understood, it is predicted to involve a malfunction in how neurons adjust synaptic connections in response to patterns of activity in networks 181.
Interestingly, a recent study reported that the effects of NMDAR antagonists on prefrontal cortical connectivity model in early rather than chronic SZ 4 [also see 110, 111, 182, 183], emphasizing their critical roles in neurodevelopment. However, despite the progress, compared with rodent studies, how NMDA hypofunction affects the development of prefrontal circuitry and synaptic connectivity in non-human primates remains to be determined. In the primate dlPFC, the density of excitatory synapses decreases by 40–50% during adolescence 184, but whether this significant change is associated with NMDAR-mediated transmission is unknown. Gonzalez-Burgos et al. reported that during early postnatal development (3-month-old monkey), excitatory inputs to layer 3 pyramidal neurons exhibited immature properties, including higher release probability, lower AMPA/NMDA ratio, and higher expression of GrinN2B subunits compared to preadolescent (15 months old) and adult (42 or 84 months old) monkeys. These findings indicate that functionally immature synapses’ contribution to prefrontal cortical development significantly decreases before adolescence begins, at least for monkey PFC layer 3 pyramidal neurons 184. These findings are slightly different from those reported in the rodent mPFC, as shown in Figure 3 90, 95, 155, 185. Whether there exists a laminar difference of NMDAR subunit development in non-human primate PFC remains to be determined.
NMDAR hypofunction, prefrontal E/I balance, and global dysconnectivity
Despite extensive information regarding the regulation of NMDAR function and trafficking in cultured neurons and expression systems 186, 187, much remains to be explored about the molecular basis of activity-dependent NMDAR plasticity in vivo, especially during different stages of development 188. A significant challenge is determining the precise contribution of NMDAR in a cell-type- and afferent/pathway-specific manner, as recently reported in the prefrontal neurons 151, 189. The new technologies described in the future directions would be vital in addressing the challenge and the outstanding questions enlisted below. A recent review study has provided strong evidence of cell-type actions for NMDAR hypofunction 42. Specifically, NMDAR hypofunction can produce SZ-related effects through action on various circuits and cell types, including not only prefrontal pyramidal neurons and PV-INs, but also VTA dopamine neurons and other brain regions 42, as illustrated in Figure 1.
Still, all of these assumptions depend on how NMDAR hypofunction leads to global E/I imbalance, local circuit dysfunction, and regional dysconnectivity that underlie SZ 6. Postmortem work indicates IN dysfunction involves the altered interplay of GABA and glutamate across the brain 190. NMDARs on GABAergic INs, especially PV-INs, form the cornerstone of the NMDA hypofunction model of SZ 10, 19, 42, 191, and are critical for the regulation of E/I balance in neural microcircuits 192.
An important recent discovery is the macroscopic gradients of synaptic E/I in the neocortex 7. Areas of the neocortex differ not only due to their input-output patterns but also their physiological properties. Specifically, when cortical regions were plotted by rank order of SST cell to PV cell ratio values, the ratio of SST- to PV-INs is surprisingly low in primary sensory and motor areas and high in association areas, including frontal areas, revealing a macroscopic gradient of synaptic inhibition in the mouse cortex. Notably, PV cells are twice as abundant as SST cells in V1, but SST cells are 4-fold more numerous than PV cells in frontal areas per this ratio calculation 7. Importantly, there is an increasing gradient of synaptic excitation along the cortical hierarchy, including the expression level of GluN2B that plays a crucial role in the NMDAR dependent recurrent excitation that supports cognition 90, 154–156. However, a gradient of NMDAR-dependent excitation in a multiregional cortex, especially those in SST cells, remains elucidated in future research 7. The notion of macroscopic gradients has begun to be applied to studies of mental disorders. For instance, SZ is characterized by large-scale cortical dysconnectivity 14.
Interestingly, dysconnectivity is primarily implicated in the PFC and other association areas, raising the question of how such differential impairment can be explained if biological trait abnormalities are common across the neocortex 56. Still, the relationship between macroscopic gradients and NMDAR hypofunction leading to global E/I balance and local circuit dysfunction remains to be determined. Given the selective role of GABAergic interneurons in NMDAR hypofunction hypothesis 19, 192, and the gradient GluN2B ratio 193 and regional difference of NMDAR-mediated currents between PFC and V1 155, we predict that an NMDAR hypofunction condition will likely produce a regional difference in E/I balance, contributing to the various symptoms of SZ. Indeed, recent theoretical accounts have proposed E/I imbalance as a possible mechanistic, network-level hypothesis underlying neural and behavioral dysfunction across neurodevelopmental disorders, particularly SZ 61.
Investigation of the link between NMDAR homeostatic plasticity and the molecular mechanisms governing NMDAR trafficking 194, 195 may serve to bridge the gap in our understanding of how these processes integrate activity over multiple timescales to support cognitive functions. While emerging evidence suggests that activity-dependent regulation of NMDARs plays an essential role in behaviors, further studies are needed to test the role of NMDAR plasticity in neuropsychiatric conditions, such as the NMDAR dysregulation implicated in SZ 186.
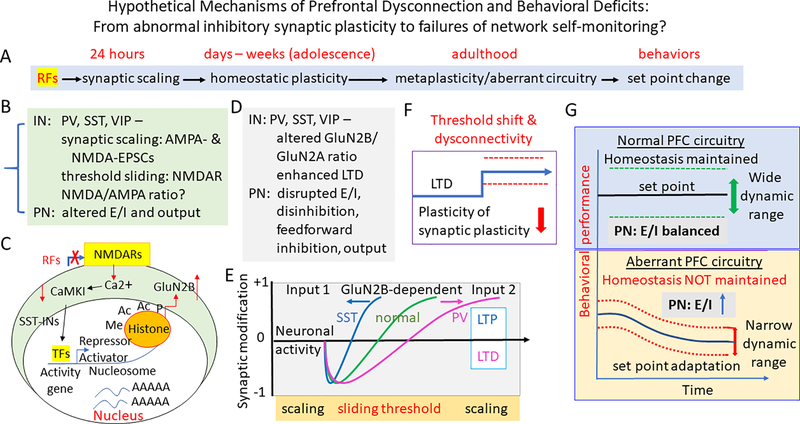
Box 1. How NMDAR hypofunction may contribute to the abnormal inhibition, disrupted synaptic plasticity, and dysconnection - Lessons learned from the studies in other brain regions and the hypothetical mechanisms (Figure 5).
Despite the progress reviewed above, the mechanisms underlying how NMDAR hypofunction may contribute to abnormal inhibition, disrupted plasticity, disconnection, and eventual behavioral deficits remains elusive. However, significant progress has been made in other cortices, and we propose to test these hypothetical mechanisms in the PFC circuitry, as illustrated in Figure 5. Beyond their well-established role as Hebbian signals for long-term potentiation and depression (LTP and LTD) of fast synaptic transmission 158, 159, 196–201, NMDARs themselves are also dynamically regulated by an activity-dependent LTP 188, 202. Specifically, the unique functional properties of the NMDAR, including high Ca2+ permeability, negative slope conductance that enables signal amplification, and slow NMDAR-EPSP kinetics, make NMDAR plasticity a powerful mechanism for the fine-tuning of information encoding and storage 7, 156. Since Ca2+ is a secondary messenger, NMDAR plasticity has far-reaching implications beyond amplitude changes of NMDAR-mediated synaptic responses 188.
The immediate responses to risk factors involve both translational and posttranslational modifications such as receptor trafficking related to epigenetic regulation (e.g., epigenetic histone acetylation, methylation, phosphorylation via activator or repressor) (Figure 5A–C) 88, 203. These changes will likely result in similar synaptic scaling of both AMPA- and NMDA-EPSCs in prefrontal neurons, as previously reported in visual cortical neurons 204, 205. Importantly, activity-dependent NMDAR plasticity appears to be a universal mechanism; it occurs in not only excitatory but also inhibitory synapses, including effects on NMDA/AMPA ratio and E/I balance in different cell types and connections 206, 207 (Figure 5B, D). Further, NMDAR-dependent effect is also observed on behavioral representation, as pioneered in the study of primary visual cortex 160, 208–210.
While most studies support the postsynaptic locus of NMDARs, the existence and functional role of NMDARs localized to the presynaptic site has received significant attention in both PFC 211 and other cortical regions 212–215. There is evidence from different brain regions that presynaptic NMDARs act as coincidence-detectors and play an essential role in some forms of spike-timing-dependent plasticity 212, 216–218. The subunit composition of presynaptic NMDARs can also be developmentally regulated, thereby modulating the inducibility of spike-timing-dependent plasticity 219. A recent study illustrated the distinct roles for pre- and post-synaptic NMDARs in visual circuit development and revealed extensive transsynaptic regulation of form and function 220. Our research indicated that, in response to NMDAR blockade during adolescence, these presynaptic receptors themselves underwent plastic changes differentially in the fast-spiking INs vs. pyramidal cells in the PFC 221. Still, it remains unclear whether presynaptic NMDARs from distinct long-range afferents or cell types within the PFC have different identities and functions.
Although we have focused primarily on the mechanisms and implications of rapid, synapse-specific NMDAR plasticity, mounting evidence mainly from the sensory cortices indicates that NMDARs can participate in homeostatic plasticity 202, This process acts over relatively longer timescales and can underlie metaplasticity 197, 208, 222–226, as illustrated hypothetically in the PFC in Figure 5. Specifically, visual experience-dependent homeostatic plasticity of excitatory synapses observed in superficial layers of the visual cortex is dependent on NMDAR function. Interestingly, both strengthening of synapses induced by visual deprivation and weakening by the reinstatement of visual experience were blocked in the absence of functional NMDARs. This finging suggests that sensory experience-dependent homeostatic adaptation depends on NMDARs, supporting the sliding threshold model of plasticity and input-specific homeostatic control observed in vivo 208. Especially during adolescent development, NMDAR subunit-dependent homeostatic plasticity in inhibitory neurons occurs in cell-type and input-specific manner through a sliding threshold mechanism 197, 198, such as differential LTP induction in SST vs. LTD in PV cells, respectively (Figure 5D, E). Similarly, homeostatic plasticity of GABAergic neurons and their synaptic connections with excitatory neurons also occur, although the molecular mechanisms remain to be determined 119, 227, 228. NMDAR hypofunction would weaken or disrupt both normal Hebbian plasticity between prefrontal interneurons and long-range afferents, meanwhile, reshape the homeostatic plasticity in the mPFC and its connections with downstream targets. These homeostatic changes in plasticity, in turn, induce threshold shift and/or disconnectivity, resulting in a reduced metaplasticity 222, 229 (Figure 5F). Consequently, an aberrant local circuit and dysfunctional connections are formed, a new set point with a narrower dynamic range for information is adopted, and abnormal behavioral changes follow under an aberrant connectivity condition 230, 231 (Figure 5G).
Box 1_Figure 5.
A summary illustration of the perspective mechanisms associated with dysconnectivity and behavioral deficits – from abnormal synaptic function and plasticity to failures of self-monitoring. A, Timeline of synaptic- and circuit-specific alterations and behavioral changes during developmental dysconnectivity. B-G, In response to normal and abnormal stimulation, synapses mediating local and long-range connections between different cell types exhibit sequential alterations through different mechanisms during development. B & C, The immediate responses (within 24 hours) to risk factors (RFs) are associated with both translational and posttranslational modifications such as receptor trafficking (B) related to epigenetic regulation (e.g., epigenetic histone acetylation, methylation, phosphorylation via activator or repressor) (C) 88, 203, resulting in changes in excitatory synaptic strength/synaptic scaling of both AMPA- & NMDA-EPSCs 204, 205 in prefrontal neurons which consequently disrupts E/I balance in the local prefrontal circuit. D & E, Later on, especially during adolescent development, NMDAR subunit-dependent homeostatic plasticity in inhibitory neurons occurs in cell-type and input-specific manner through a sliding threshold mechanism 197, 198, such as differential LTP induction in SST vs. LTD PV cells, respectively. NMDAR hypofunction would weaken or disrupt both normal Hebbian plasticity between prefrontal interneurons and long-range afferents, meanwhile reshape the homeostatic plasticity in the mPFC and its connections with downstream targets. F, These homeostatic changes in plasticity (as described in D & E), in turn, induce threshold shift and/or disconnectivity, resulting in a reduced metaplasticity 222, 229. G, Consequently, an aberrant local circuit and dysfunctional connections are formed, a new set point with a narrower dynamic range for information is adopted, and abnormal behavioral changes follow under an aberrant connectivity condition 230, 231. IN: interneuron; PN: pyramidal neuron; PPF: paired-pulse facilitation; PPD: paired-pulse depression; RFs: regulatory factors; TFs: transcription factors.
In summary, mechanistically, pyramidal cells and IN subtypes can be regulated in response to activity levels via various mechanisms (Figure 5). Therefore, the maturation of different inputs to the PFC during juvenile and adolescent development can stimulate the neurons and neuronal network, leading to distinct changes in protein expression levels depending on the identity of the postsynaptic cell. One such family of proteins is the NMDAR subunits, which are known to vary among IN subtypes and pyramidal neurons. NMDAR subunit composition is important for plasticity and regulation of cortical development during critical juvenile and adolescent periods. Therefore, alteration of input and output activity during development is expected to disrupt GABAergic circuitry and neuronal connectivity with other brain regions, with cell-type- and input/output-specific effects on synaptic plasticity and underlying molecular changes. Eventually, these homeostatic changes could lead to a shift of threshold for plasticity, i.e., metaplasticity, and consequently, a set-point adaption.
VI. Conclusion and future directions
Aberrant neural network connectivity has been widely recognized as constituting major functional and structural changes in the neuropathophysiology of SZ. However, what an aberrant circuitry actually looks like remains an enigma. Brain cells are highly plastic, allowing an organism to learn and adapt to its environment, including response to mutant genes and risk factors. This ongoing plasticity is essentially unstable during development, leading to aberrant circuit activity. Homeostatic plasticity provides a compensatory mechanism in controlling neuronal network activity and stability. Many of these homeostatic modifications may occur not only within the subcellular neuronal compartments but also in different levels of connections from synaptic inputs at both excitatory and inhibitory cells to modulation of neuronal outputs, as proposed in a recent review 232. Here we have summarized the current progress, and we outline the outstanding questions in Box 2 and future directions.
Outstanding questions.
When and how is local prefrontal circuitry formed and matured during development, especially during juvenile and adolescent periods? What are the roles of different subtypes of INs in the regulation of cognitive and social functions during adolescent development? What are the roles of NMDARs in the development of local prefrontal circuitry among the different subtypes of INs?
Past studies focused on the roles of DA, GABA, NMDARs in genetic and neurodevelopmental models for SZ. Many aspects of the circuit- and cell-type-specific connections, as well as input/output specificity, remain unclear or unexplored, especially those related to homeostatic plasticity and metaplasticity. For example, the pre- and post-synaptic NMDARs in different subtypes of INs, particularly SST and VIP cells, and their roles in development, remain untested.
For the PFC, given the most important factor is prolonged and delayed maturation during adolescence, all findings reported in other brain regions, especially those from the sensory, auditory, and visual cortices, usually do not completely apply to the PFC. Therefore, the key question is to elucidate the unique properties among each brain region, given the potential macroscopic gradient differences.
One critical issue is the monoamine systems in the mPFC, which exhibit prominent delayed maturation, and macroscopic gradient distribution compared with other regions. How these neuromodulatory systems are affected by NMDAR hypofunction during development is barely explored. Are the NMDAR hypofunction and neuromodulation mutually dependent on each other or mutually exclusive in the dysfunctional status? We propose that during the early (e.g., juvenile and adolescent) stages of development, NMDAR hypofunction dominates the process of dysfunction, which in turn results in differential impairments in a system-specific manner, and in adulthood, both NMDAR hypofunction and DA dysfunction co-exist and impact each other.
Given the large number of genetic mutations associated with symptoms of SZ, it is unlikely that a single locus for pathology exists at the cellular or synaptic level. How a complex substrate induces a distinct behavioral phenotype that represents a genuinely ‘brain-wide’ aberrant function and disconnection, deserves to be explored.
New technologies for SZ studies
Technology has opened new frontiers in the study of the pathophysiology of mental disorders, including SZ. Specifically, recent technical advances have enabled us to map not only local synaptic connections among different cell subtypes 233, but also their up- and down-stream connectivity 234, 235, as well as their molecular phenotypes and neuronal activity in animal models. These techniques include the transcriptomic approach RNAseq 236 for molecular phenotype, neurological tracing methods such as Tracing the Relationship of Inputs and Outputs (TRIO) and cell-type-specific tracing of the relationship between input and outputs (cTRIO) 237, 238, iTango for neuromodulatory circuits 239, optogenetics 240 and chemogenetics 241, as well as calcium imaging and various fluorescent sensors 242, 243. These technologies will provide novel insights into the understanding of what an aberrant circuit and dysfunctional connection look like under different etiological conditions and whether the alterations due to different etiology share similar or different mechanisms in a global scale of input-output level connectivity and macroscopic gradient of whole brain activity.
However, although these cutting-edge tools are useful in model organisms, primarily rodents, and even non-human primates, critical advances in human brain disorders are seriously hindered by our lack of ability to monitor and manipulate circuitry in safe and minimally invasive ways. Clinical intervention with novel cell- and circuit-specific tools are required to focus on research designed to elucidate the aberrant circuit and connectivity of PFC in patients with SZ 10. The ambitious ‘Research Domain Criteria’ (RDoC) project initiated by The National Institutes of Mental Health aims to develop new tools in assessing behavior and neurobiological measurements encompassing positive and negative valence systems, cognitive systems, social systems, and arousal/regulatory systems. These comprehensive analyses of these systems at the cellular, brain network, physiological, and behavioral levels will likely yield the direct information associated with the aberrant connectivity of SZ 244. Other clinical tools such as proton magnetic resonance spectroscopy (1H-MRS for tissue glutamate and GABA), PET/SPECT, Mismatch negativity/P300/gamma-band oscillations 245, 246, post-mortem neurochemical/biochemical findings remain useful in elucidating the pathological alterations in neural circuit and connectivity 44. All these measures offer noninvasive endophenotypes or biomarkers that may find a powerful application in SZ research 247.
Concluding remarks
It is increasingly clear that synaptic plasticity can occur at various levels of connections during both learning and homeostatic adaptation, including different cell types in the PFC and their input and output connectivity. It is clear that excitatory and inhibitory neurons undergo complementary forms of functional and structural plasticity in order to maintain an optimal E/I balance and ultimately regulate network stability. Yet, many outstanding questions remain unanswered. It is essential to understand whether excitatory and inhibitory neurons display different forms of homeostatic plasticity by using different sensors and effectors of neuronal or network activity, and how different forms of homeostatic plasticity, at either the pre- or post-synaptic sites, are merged to form a newly adapted set-point for behavioral performance. It is likely that each cell type will alter its input-output relation within the network in a different way, and thus the type of stimulus or the level of network activity will be altered towards specific forms of functional and structural homeostasis and meta-plasticity, especially during the development. We conclude that the aberrant prefrontal cortical circuitry and dysconnection in SZ are attributed to the “all roads lead to Rome” NMDAR-mediated plastic changes during development, ranging from abnormal synaptic plasticity to an adapted set point with dysfunctional self-monitoring. Targeting this process during the early stages is likely more effective in preventing or treating the disease.
Acknowledgments
This study was supported by NIH R21MH110678, the NIH R01MH085666, NARSAD Independent Award 2015, and Pennsylvania Commonwealth 4100072545 (CURE 2016) to W. J. Gao.
Abbreviations:
- AMPAR
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- BC
basket cell
- ChC
chandelier cell
- EEG
electroencephalogram
- E/I
excitation/inhibition
- fMRI
functional magnetic resonance imaging
- FS
fast spiking
- INs
interneurons
- LTD
long term depression
- LTP
long term potentiation
- MC
Martinotti cell
- MD
mediodorsal thalamus
- mPFC
medial prefrontal cortex
- NMDAR
N-methyl-D-aspartate receptor
- PN
pyramidal neuron
- PPD
paired-pulse depression
- PPF
paired-pulse facilitation
- PV
parvalbumin
- sEPSC
spontaneous excitatory postsynaptic current
- SST
somatostatin
- SZ
Schizophrenia
- TRIO
tracing the relationship between input and output
- vHipp
ventral hippocampus
- VIP
vasoactive intestinal peptide
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
References
- 1.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature Rev Neurosci 2005; 6(4): 312–324. [DOI] [PubMed] [Google Scholar]
- 2.Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN et al. Limbic System Abnormalities Identified in Schizophrenia Using Positron Emission Tomography With Fluorodeoxyglucose and Neocortical Alterations With Deficit Syndrome. Archives of General Psychiatry 1992; 49(7): 522–530. [DOI] [PubMed] [Google Scholar]
- 3.Anticevic A, Cole MW, Murray JD, Corlett PR, Wang X-J, Krystal JH. The role of default network deactivation in cognition and disease. Trends in cognitive sciences 2012; 16(12): 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anticevic A, Corlett PR, Cole MW, Savic A, Gancsos M, Tang Y et al. N-Methyl-D-Aspartate receptor antagonist effects on prefrontal cortical connectivity better model early than chronic schizophrenia. Biological psychiatry 2014; 77(6): 569–580. [DOI] [PubMed] [Google Scholar]
- 5.Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C et al. Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA psychiatry 2015; 72(9): 882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anticevic A, Lisman J. How Can Global Alteration of Excitation/Inhibition Balance Lead to the Local Dysfunctions That Underlie Schizophrenia? Biological psychiatry 2017; 81(10): 818–820. [DOI] [PubMed] [Google Scholar]
- 7.Wang X-J. Macroscopic gradients of synaptic excitation and inhibition in the neocortex. Nature Reviews Neuroscience 2020; 21(3): 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hunt MJ, Kopell NJ, Traub RD, Whittington MA. Aberrant Network Activity in Schizophrenia. Trends in Neurosciences 2017; 40(6): 371–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friston K, Brown HR, Siemerkus J, Stephan KE. The dysconnection hypothesis (2016). Schizophr Res 2016; 176(2–3): 83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Limongi R, Jeon P, Mackinley M, Das T, Dempster K, Théberge J et al. Glutamate and Dysconnection in the Salience Network: Neurochemical, Effective Connectivity, and Computational Evidence in Schizophrenia. Biological psychiatry 2020; 88(3): 273–281. [DOI] [PubMed] [Google Scholar]
- 11.Weinberger DR. A connectionist approach to the prefrontal cortex. The Journal of neuropsychiatry and clinical neurosciences 1993; 5(3): 241–253. [DOI] [PubMed] [Google Scholar]
- 12.Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci 1995; 3(2): 89–97. [PubMed] [Google Scholar]
- 13.Krajcovic B, Fajnerova I, Horacek J, Kelemen E, Kubik S, Svoboda J et al. Neural and neuronal discoordination in schizophrenia: From ensembles through networks to symptoms. Acta Physiologica 2019; 226(4): e13282. [DOI] [PubMed] [Google Scholar]
- 14.Stephan KE, Friston KJ, Frith CD. Dysconnection in Schizophrenia: From Abnormal Synaptic Plasticity to Failures of Self-monitoring. Schizophr Bull 2009; 35(3): 509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selemon LD. A role for synaptic plasticity in the adolescent development of executive function. Transl Psychiatry 2013; 3: e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selemon LD, Zecevic N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl Psychiatry 2015; 5: e623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friston KJ. Schizophrenia and the disconnection hypothesis. Acta psychiatrica Scandinavica Supplementum 1999; 395: 68–79. [DOI] [PubMed] [Google Scholar]
- 18.Meltzer HY, Rajagopal L, Huang M, Oyamada Y, Kwon S, Horiguchi M. Translating the N-methyl-D-aspartate receptor antagonist model of schizophrenia to treatments for cognitive impairment in schizophrenia. Int J Neuropsychopharmacol 2013; 16(10): 2181–2194. [DOI] [PubMed] [Google Scholar]
- 19.Snyder MA, Gao W-J. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Frontiers in Cellular Neuroscience 2013; 7: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakazawa K, Jeevakumar V, Nakao K. Spatial and temporal boundaries of NMDA receptor hypofunction leading to schizophrenia. NPJ schizophrenia 2017; 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avery MC, Krichmar JL. Neuromodulatory Systems and Their Interactions: A Review of Models, Theories, and Experiments. Front Neural Circuits 2017; 11: 108–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophr Bull 2009; 35(3): 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chini M, Hanganu-Opatz IL. Prefrontal Cortex Development in Health and Disease: Lessons from Rodents and Humans. Trends in Neurosciences 2021; 44(3): 227–240. [DOI] [PubMed] [Google Scholar]
- 24.Tagliabue E, Pouvreau T, Eybrard S, Meyer F, Louilot A. Dopaminergic responses in the core part of the nucleus accumbens to subcutaneous MK801 administration are increased following postnatal transient blockade of the prefrontal cortex. Behavioural brain research 2017; 335: 191–198. [DOI] [PubMed] [Google Scholar]
- 25.Jia J-M, Zhao J, Hu Z, Lindberg D, Li Z. Age-dependent regulation of synaptic connections by dopamine D2 receptors. Nat Neurosci 2013; 16(11): 1627–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kellendonk C, Simpson EH, Kandel ER. Modeling cognitive endophenotypes of schizophrenia in mice. Trends in Neurosciences 2009; 32(6): 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron 2006; 49(4): 603–615. [DOI] [PubMed] [Google Scholar]
- 28.Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron 2010; 65(4): 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakajima M, Halassa MM. Thalamic control of functional cortical connectivity. Current opinion in neurobiology 2017; 44: 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim YK, Choi J, Park SC. A Novel Bio-Psychosocial-Behavioral Treatment Model in Schizophrenia. International journal of molecular sciences 2017; 18(4): 734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li ML, Gulchina Y, Monaco SA, Xing B, Ferguson BR, Li YC et al. Juvenile Treatment with a Novel mGluR2 Agonist/mGluR3 Antagonist Compound, LY395756, Reverses Learning Deficits and Cognitive Flexibility Impairments in Adults in A Neurodevelopmental Model of Schizophrenia. Neurobiology of learning and memory 2017: 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xing B, Han G, Wang M-J, Snyder MA, Gao W-J. Juvenile treatment with mGluR2/3 agonist prevents schizophrenia-like phenotypes in adult by acting through GSK3β. Neuropharmacology 2018; 137: 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snyder MA, Gao WJ. NMDA receptor hypofunction for schizophrenia revisited: Perspectives from epigenetic mechanisms. Schizophr Res 2020; 217: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cellular and Molecular Neurobiology 2006; 26(4–6): 365–384. [DOI] [PubMed] [Google Scholar]
- 35.Moghaddam B Bringing order to the glutamate chaos in schizophrenia. Neuron 2003; 40(5): 881–884. [DOI] [PubMed] [Google Scholar]
- 36.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends in Neurosci 2008; 31(5): 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marek GJ, Behl B, Bespalov AY, Gross G, Lee Y, Schoemaker H. Glutamatergic (N-methyl-D-aspartate receptor) hypofrontality in schizophrenia: too little juice or a miswired brain? Molecular pharmacology 2010; 77(3): 317–326. [DOI] [PubMed] [Google Scholar]
- 38.Vinson PN, Conn PJ. Metabotropic glutamate receptors as therapeutic targets for schizophrenia. Neuropharmacology 2012; 62(3): 1461–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weickert CS, Fung SJ, Catts VS, Schofield PR, Allen KM, Moore LT et al. Molecular evidence of N-methyl-D-aspartate receptor hypofunction in schizophrenia. Molecular psychiatry 2013; 18(11): 1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamoto H, Hagino Y, Kasai S, Ikeda K. Specific Roles of NMDA Receptor Subunits in Mental Disorders. Current molecular medicine 2015; 15(3): 193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cohen SM, Tsien RW, Goff DC, Halassa MM. The impact of NMDA receptor hypofunction on GABAergic interneurons in the pathophysiology of schizophrenia. Schizophrenia research 2015; 167(0): 98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bygrave AM, Kilonzo K, Kullmann DM, Bannerman DM, Kätzel D. Can N-Methyl-D-Aspartate Receptor Hypofunction in Schizophrenia Be Localized to an Individual Cell Type? Frontiers in Psychiatry 2019; 10(835). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balu DT. The NMDA Receptor and Schizophrenia: From Pathophysiology to Treatment. In: Robert S (ed). Advances in Pharmacology, vol. Volume 76. Academic Press; 2016, pp 351–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uno Y, Coyle JT. Glutamate hypothesis in schizophrenia. Psychiatry and Clinical Neurosciences 2019; 73(5): 204–215. [DOI] [PubMed] [Google Scholar]
- 45.Datta D, Arnsten AFT. Unique Molecular Regulation of Higher-Order Prefrontal Cortical Circuits: Insights into the Neurobiology of Schizophrenia. ACS Chem Neurosci 2018; 9(9): 2127–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grace AA. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nature reviews Neuroscience 2016; 17(8): 524–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang GR, Murray JD, Wang XJ. A dendritic disinhibitory circuit mechanism for pathway-specific gating. Nat Commun 2016; 7: 12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferguson BR, Gao W-J. PV Interneurons: Critical Regulators of E/I Balance for Prefrontal Cortex-Dependent Behavior and Psychiatric Disorders. Front Neural Circuits 2018; 12: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kupferschmidt DA, Gordon JA. The dynamics of disordered dialogue: Prefrontal, hippocampal and thalamic miscommunication underlying working memory deficits in schizophrenia. Brain and neuroscience advances 2018; 2: 2398212818771821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar SS, Huguenard JR. Pathway-specific differences in subunit composition of synaptic NMDA receptors on pyramidal neurons in neocortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 2003; 23(31): 10074–10083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bogart LJ, O’Donnell P. Multiple long-range inputs evoke NMDA currents in prefrontal cortex fast-spiking interneurons. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2018; 43(10): 2101–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friston KJ. The disconnection hypothesis. Schizophr Res 1998; 30(2): 115–125. [DOI] [PubMed] [Google Scholar]
- 53.Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biological psychiatry 2006; 59(10): 929–939. [DOI] [PubMed] [Google Scholar]
- 54.Anticevic A, Gancsos M, Murray JD, Repovs G, Driesen NR, Ennis DJ et al. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci U S A 2012; 109(41): 16720–16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anticevic A, Hu X, Xiao Y, Hu J, Li F, Bi F et al. Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. The Journal of Neuroscience 2015; 35(1): 267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang GJ, Murray JD, Wang XJ, Glahn DC, Pearlson GD, Repovs G et al. Functional hierarchy underlies preferential connectivity disturbances in schizophrenia. Proc Natl Acad Sci U S A 2016; 113(2): E219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khadka S, Meda SA, Stevens MC, Glahn DC, Calhoun VD, Sweeney JA et al. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biological psychiatry 2013; 74(6): 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mothersill O, Kelly S, Rose EJ, Donohoe G. The effects of psychosis risk variants on brain connectivity: a review. Front Psychiatry 2012; 3: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schleifer C, Lin A, Kushan L, Ji JL, Yang G, Bearden CE et al. Dissociable Disruptions in Thalamic and Hippocampal Resting-State Functional Connectivity in Youth with 22q11.2 Deletions. The Journal of neuroscience : the official journal of the Society for Neuroscience 2019; 39(7): 1301–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmitt A, Hasan A, Gruber O, Falkai P. Schizophrenia as a disorder of disconnectivity. Eur Arch Psychiatry Clin Neurosci 2011; 261 Suppl 2(Suppl 2): S150–S154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foss-Feig JH, Adkinson BD, Ji JL, Yang G, Srihari VH, McPartland JC et al. Searching for Cross-Diagnostic Convergence: Neural Mechanisms Governing Excitation and Inhibition Balance in Schizophrenia and Autism Spectrum Disorders. Biological psychiatry 2017; 81(10): 848–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bicks LK, Koike H, Akbarian S, Morishita H. Prefrontal cortex and social cognition in mouse and man. Frontiers in Psychology 2015; 6: 1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alvarez RJ, Pafundo DE, Zold CL, Belforte JE. Interneuron NMDA Receptor Ablation Induces Hippocampus-Prefrontal Cortex Functional Hypoconnectivity after Adolescence in a Mouse Model of Schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience 2020; 40(16): 3304–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tremblay R, Lee S, Rudy B. GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits. Neuron 2016; 91(2): 260–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silberberg G, Markram H. Disynaptic inhibition between neocortical pyramidal cells mediated by Martinotti cells. Neuron 2007; 53(5): 735–746. [DOI] [PubMed] [Google Scholar]
- 66.Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci 2013; 16(8): 1068–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Batista-Brito R, Vinck M, Ferguson KA, Chang JT, Laubender D, Lur G et al. Developmental Dysfunction of VIP Interneurons Impairs Cortical Circuits. Neuron 2017; 95(4): 884–895.e889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferguson BR, Gao WJ. Thalamic Control of Cognition and Social Behavior Via Regulation of Gamma-Aminobutyric Acidergic Signaling and Excitation/Inhibition Balance in the Medial Prefrontal Cortex. Biological psychiatry 2018; 83: 657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Konradi C, Heckers S. Molecular aspects of glutamate dysregulation: implications for schizophrenia and its treatment. Pharmacol Ther 2003; 97(2): 153–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lieberman JA, Perkins D, Belger A, Chakos M, Jarskog F, Boteva K et al. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology, and therapeutic approaches. Biological psychiatry 2001; 50(11): 884–897. [DOI] [PubMed] [Google Scholar]
- 71.Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci 2002; 25: 409–432. [DOI] [PubMed] [Google Scholar]
- 72.Vincent SL, Pabreza L, Benes FM. Postnatal maturation of GABA-immunoreactive neurons of rat medial prefrontal cortex. The Journal of comparative neurology 1995; 355(1): 81–92. [DOI] [PubMed] [Google Scholar]
- 73.Erickson SL, Lewis DA. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. The Journal of comparative neurology 2002; 448(2): 186–202. [DOI] [PubMed] [Google Scholar]
- 74.Hashimoto T, Nguyen QL, Rotaru D, Keenan T, Arion D, Beneyto M et al. Protracted developmental trajectories of GABAA receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biological psychiatry 2009; 65(12): 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoftman GD, Lewis DA. Postnatal developmental trajectories of neural circuits in the primate prefrontal cortex: identifying sensitive periods for vulnerability to schizophrenia. Schizophr Bull 2011; 37(3): 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marin O Developmental timing and critical windows for the treatment of psychiatric disorders. Nature medicine 2016; 22(11): 1229–1238. [DOI] [PubMed] [Google Scholar]
- 77.Caballero A, Tseng KY. GABAergic Function as a Limiting Factor for Prefrontal Maturation during Adolescence. Trends in Neurosciences 2016; 39(7): 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Caballero A, Granberg R, Tseng KY. Mechanisms contributing to prefrontal cortex maturation during adolescence. Neuroscience and biobehavioral reviews 2016; 70: 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lewis DA. Development of the prefrontal cortex during adolescence: insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacol 1997; 16(6): 385–398. [DOI] [PubMed] [Google Scholar]
- 80.Dow-Edwards D, MacMaster FP, Peterson BS, Niesink R, Andersen S, Braams BR. Experience during adolescence shapes brain development: From synapses and networks to normal and pathological behavior. Neurotoxicology and teratology 2019; 76: 106834. [DOI] [PubMed] [Google Scholar]
- 81.Jadhav KS, Boutrel B. Prefrontal cortex development and emergence of self-regulatory competence: the two cardinal features of adolescence disrupted in context of alcohol abuse. The European journal of neuroscience 2019; 50(3): 2274–2281. [DOI] [PubMed] [Google Scholar]
- 82.Larsen B, Luna B. Adolescence as a neurobiological critical period for the development of higher-order cognition. Neuroscience and biobehavioral reviews 2018; 94: 179–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Andersen SL. Commentary on the special issue on the adolescent brain: Adolescence, trajectories, and the importance of prevention. Neuroscience and biobehavioral reviews 2016; 70: 329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fuhrmann D, Knoll LJ, Blakemore SJ. Adolescence as a Sensitive Period of Brain Development . Trends in cognitive sciences 2015; 19(10): 558–566. [DOI] [PubMed] [Google Scholar]
- 85.Delevich K, Thomas AW, Wilbrecht L. Adolescence and “Late Blooming” Synapses of the Prefrontal Cortex. Cold Spring Harbor symposia on quantitative biology 2018; 83: 37–43. [DOI] [PubMed] [Google Scholar]
- 86.Mardinly AR, Spiegel I, Patrizi A, Centofante E, Bazinet JE, Tzeng CP et al. Sensory experience regulates cortical inhibition by inducing IGF1 in VIP neurons. Nature 2016; 531(7594): 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spiegel I, Mardinly Alan R, Gabel Harrison W, Bazinet Jeremy E, Couch Cameron H, Tzeng Christopher P et al. Npas4 regulates excitatory-inhibitory balance within neural circuits through cell-type-specific gene programs. Cell 2014; 157(5): 1216–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wamsley B, Fishell G. Genetic and activity-dependent mechanisms underlying interneuron diversity. Nature reviews Neuroscience 2017; 18(5): 299–309. [DOI] [PubMed] [Google Scholar]
- 89.Dumas TC. Developmental regulation of cognitive abilities: modified composition of a molecular switch turns on associative learning. Progress in neurobiology 2005; 76(3): 189–211. [DOI] [PubMed] [Google Scholar]
- 90.Monaco SA, Gulchina Y, Gao W-J. NR2B subunit in the prefrontal cortex: A double-edged sword for working memory function and psychiatric disorders. Neuroscience & Biobehavioral Reviews 2015; 56(0): 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mierau SB, Patrizi A, Hensch TK, Fagiolini M. Cell-Specific Regulation of N-Methyl-D-Aspartate Receptor Maturation by Mecp2 in Cortical Circuits . Biological psychiatry 2016; 79(9): 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koppensteiner P, Von Itter R, Melani R, Galvin C, Lee FS, Ninan I. Diminished Fear Extinction in Adolescents Is Associated With an Altered Somatostatin Interneuron-Mediated Inhibition in the Infralimbic Cortex. Biological psychiatry 2019; 86(9): 682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S et al. GABAergic interneuron origin of schizophrenia pathophysiology. Neuropharmacology 2012; 62(3): 1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Akgul G, McBain CJ. Diverse roles for ionotropic glutamate receptors on inhibitory interneurons in developing and adult brain. J Physiol 2016; 594(19): 5471–5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang HX, Gao WJ. Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharmacol 2009; 34(8): 2028–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rotaru DC, Yoshino H, Lewis DA, Ermentrout GB, Gonzalez-Burgos G. Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J Neurosci 2011; 31(1): 142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Le Magueresse C, Monyer H. GABAergic Interneurons Shape the Functional Maturation of the Cortex. Neuron 2013; 77(3): 388–405. [DOI] [PubMed] [Google Scholar]
- 98.Matta JA, Pelkey KA, Craig MT, Chittajallu R, Jeffries BW, McBain CJ. Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity. Nat Neurosci 2013; 16(8): 1032–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. Journal of Neuroscience 2006; 26(5): 1604–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science 2007; 318(5856): 1645–1647. [DOI] [PubMed] [Google Scholar]
- 101.Xi D, Keeler B, Zhang W, Houle JD, Gao WJ. NMDA receptor subunit expression in GABAergic interneurons in the prefrontal cortex: application of laser micro dissection technique. J Neurosci Meth 2009; 176: 172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moreau AW, Kullmann DM. NMDA receptor-dependent function and plasticity in inhibitory circuits. Neuropharmacology 2013; 74: 23–31. [DOI] [PubMed] [Google Scholar]
- 103.Krystal JH, Anticevic A, Yang GJ, Dragoi G, Driesen NR, Wang X-J et al. Impaired Tuning of Neural Ensembles and the Pathophysiology of Schizophrenia: A Translational and Computational Neuroscience Perspective. Biological psychiatry 2017; 81(10): 874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Paul A, Crow M, Raudales R, He M, Gillis J, Huang ZJ. Transcriptional architecture of synaptic communication delineates GABAergic neuron identity. Cell 2017; 171(3): 522–539.e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gambrill AC, Barria A. NMDA receptor subunit composition controls synaptogenesis and synapse stabilization. Proceedings of the National Academy of Sciences 2011; 108(14): 5855–5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alherz F, Alherz M, Almusawi H. NMDAR hypofunction and somatostatin-expressing GABAergic interneurons and receptors: A newly identified correlation and its effects in schizophrenia. Schizophrenia research Cognition 2017; 8: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fung SJ, Fillman SG, Webster MJ, Shannon Weickert C. Schizophrenia and bipolar disorder show both common and distinct changes in cortical interneuron markers. Schizophr Res 2014; 155(1): 26–30. [DOI] [PubMed] [Google Scholar]
- 108.Koukouli F, Rooy M, Tziotis D, Sailor KA, O’Neill HC, Levenga J et al. Nicotine reverses hypofrontality in animal models of addiction and schizophrenia. Nature medicine 2017; 23: 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cummings KA, Clem RL. Prefrontal somatostatin interneurons encode fear memory. Nature Neuroscience 2020; 23: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scheidegger M, Walter M, Lehmann M, Metzger C, Grimm S, Boeker H et al. Ketamine Decreases Resting State Functional Network Connectivity in Healthy Subjects: Implications for Antidepressant Drug Action. PLOS ONE 2012; 7(9): e44799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kraguljac NV, Frölich MA, Tran S, White DM, Nichols N, Barton-McArdle A et al. Ketamine modulates hippocampal neurochemistry and functional connectivity: a combined magnetic resonance spectroscopy and resting-state fMRI study in healthy volunteers. Molecular psychiatry 2017; 22(4): 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Flores-Barrera E, Thomases DR, Heng L-J, Cass DK, Caballero A, Tseng KY. Late adolescent expression of gluN2B transmission in the prefrontal cortex is input-specific and requires postsynaptic protein kinase A and D1 dopamine receptor signaling. Biological psychiatry 2014; 75(6): 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miller OH, Bruns A, Ben Ammar I, Mueggler T, Hall BJ. Synaptic regulation of a thalamocortical circuit controls depression-related behavior. Cell Rep 2017; 20(8): 1867–1880. [DOI] [PubMed] [Google Scholar]
- 114.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain structure & function 2007; 212(2): 149–179. [DOI] [PubMed] [Google Scholar]
- 115.Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. Journal of Comparative Neurology 1996; 371(2): 179–207. [DOI] [PubMed] [Google Scholar]
- 116.Collins DP, Anastasiades PG, Marlin JJ, Carter AG. Reciprocal circuits linking the prefrontal cortex with dorsal and ventral thalamic nuclei. Neuron 2018; 98(2): 366–379.e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ferguson BR, Gao W-J. Development of thalamocortical connections between the mediodorsal thalamus and the prefrontal cortex and its implication in cognition. Front Hum Neurosci 2015; 8: 1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chittajallu R, Wester JC, Craig MT, Barksdale E, Yuan XQ, Akgul G et al. Afferent specific role of NMDA receptors for the circuit integration of hippocampal neurogliaform cells. Nat Commun 2017; 8(1): 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chiu CQ, Martenson JS, Yamazaki M, Natsume R, Sakimura K, Tomita S et al. Input-specific NMDAR-dependent potentiation of dendritic GABAergic inhibition. Neuron 2018; 97(2): 368–377.e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chiu C, Morse T, Nani F, Knoflach F, Hernandez M-C, Jadi M et al. Tonic GABAergic activity facilitates dendritic calcium signaling and short-term plasticity. bioRxiv 2020: 2020.2004.2022.055137. [Google Scholar]
- 121.Cornford JH, Mercier MS, Leite M, Magloire V, Häusser M, Kullmann DM. Dendritic NMDA receptors in parvalbumin neurons enable strong and stable neuronal assemblies. eLife 2019; 8: e49872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Anastasiades PG, Collins DP, Carter AG. Mediodorsal and ventromedial thalamus engage distinct L1 circuits in the prefrontal cortex. Neuron 2021; 109(2): 314–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Toth K, McBain CJ. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat Neurosci 1998; 1(7): 572–578. [DOI] [PubMed] [Google Scholar]
- 124.Toth K, McBain CJ. Target-specific expression of pre- and postsynaptic mechanisms. J Physiol 2000; 525 Pt 1: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gao WJ, Wang Y, Goldman-Rakic PS. Dopamine modulation of perisomatic and peridendritic inhibition in prefrontal cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 2003; 23(5): 1622–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bacci A, Huguenard JR, Prince DA. Modulation of neocortical interneurons: extrinsic influences and exercises in self-control. Trends in Neurosci 2005; 28(11): 602–610. [DOI] [PubMed] [Google Scholar]
- 127.Groenewegen HJ. Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience 1988; 24(2): 379–431. [DOI] [PubMed] [Google Scholar]
- 128.Leonard CM. The prefrontal cortex of the rat. I. Cortical projection of the mediodorsal nucleus. II. Efferent connections. Brain research 1969; 12(2): 321–343. [DOI] [PubMed] [Google Scholar]
- 129.Parnaudeau S, Bolkan SS, Kellendonk C. The mediodorsal thalamus: an essential partner of the prefrontal cortex for cognition. Biological psychiatry 2018; 83(8): 648–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mitchell AS, Chakraborty S. What does the mediodorsal thalamus do? Front Syst Neurosci 2013; 7: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bolkan SS, Stujenske JM, Parnaudeau S, Spellman TJ, Rauffenbart C, Abbas AI et al. Thalamic projections sustain prefrontal activity during working memory maintenance. Nat Neurosci 2017; 20(7): 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Funahashi S Thalamic mediodorsal nucleus and its participation in spatial working memory processes: comparison with the prefrontal cortex. Front Syst Neurosci 2013; 7: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Watanabe Y, Funahashi S. Thalamic mediodorsal nucleus and working memory. Neuroscience and biobehavioral reviews 2012; 36(1): 134–142. [DOI] [PubMed] [Google Scholar]
- 134.Baxter MG. Mediodorsal thalamus and cognition in non-human primates. Frontiers in systems neuroscience 2013; 7: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou T, Zhu H, Fan Z, Wang F, Chen Y, Liang H et al. History of winning remodels thalamo-PFC circuit to reinforce social dominance. Science 2017; 357(6347): 162–168. [DOI] [PubMed] [Google Scholar]
- 136.Parnaudeau S, O’Neill P-K, Bolkan Scott S, Ward Ryan D, Abbas Atheir I, Roth Bryan L et al. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron 2013; 77(6): 1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Parnaudeau S, Taylor K, Bolkan SS, Ward RD, Balsam PD, Kellendonk C. Mediodorsal thalamus hypofunction impairs flexible goal-directed behavior. Biological psychiatry 2015; 77(5): 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim D, Jeong H, Lee J, Ghim J-W, Her Eun S, Lee S-H et al. Distinct Roles of Parvalbumin- and Somatostatin-Expressing Interneurons in Working Memory. Neuron 2016; 92(4): 902–915. [DOI] [PubMed] [Google Scholar]
- 139.Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol 2002; 31(3–5): 277–287. [DOI] [PubMed] [Google Scholar]
- 140.Gal E, London M, Globerson A, Ramaswamy S, Reimann MW, Muller E et al. Rich cell-type-specific network topology in neocortical microcircuitry. Nat Neurosci 2017; 20(7): 1004–1013. [DOI] [PubMed] [Google Scholar]
- 141.Kim Y, Yang GR, Pradhan K, Venkataraju KU, Bota M, Garcia Del Molino LC et al. Brain-wide Maps Reveal Stereotyped Cell-Type-Based Cortical Architecture and Subcortical Sexual Dimorphism. Cell 2017; 171(2): 456–469.e422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rotaru DC, Barrionuevo G, Sesack SR. Mediodorsal thalamic afferents to layer III of the rat prefrontal cortex: Synaptic relationships to subclasses of interneurons. J Comp Neurol 2005; 490(3): 220–238. [DOI] [PubMed] [Google Scholar]
- 143.Kuroda M, Murakami K, Kishi K, Price JL. Thalamocortical synapses between axons from the mediodorsal thalamic nucleus and pyramidal cells in the prelimbic cortex of the rat. The Journal of comparative neurology 1995; 356(1): 143–151. [DOI] [PubMed] [Google Scholar]
- 144.Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cerebral cortex (New York, NY : 1991) 2008; 18(7): 1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pi H-J, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A. Cortical interneurons that specialize in disinhibitory control. Nature 2013; 503(7477): 521–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chevy Q, Kepecs A. When Acetylcholine Unlocks Feedback Inhibition in Cortex. Neuron 2018; 97(3): 481–484. [DOI] [PubMed] [Google Scholar]
- 147.Urban-Ciecko J, Jouhanneau J-S, Myal SE, Poulet JFA, Barth AL. Precisely timed nicotinic activation drives SST inhibition in neocortical circuits. Neuron 2018; 97(3): 611–625.e615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chung DW, Wills ZP, Fish KN, Lewis DA. Developmental pruning of excitatory synaptic inputs to parvalbumin interneurons in monkey prefrontal cortex. Proc Natl Acad Sci U S A 2017; 114(4): E629–e637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wen L, Lu Y-S, Zhu X-H, Li X-M, Woo R-S, Chen Y-J et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. PNAS, vol. 1072010, pp 1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yin D-M, Sun X-D, Bean JC, Lin TW, Sathyamurthy A, Xiong W-C et al. Regulation of Spine Formation by ErbB4 in PV-Positive Interneurons. The Journal of Neuroscience 2013; 33(49): 19295–19303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bean JC, Lin TW, Sathyamurthy A, Liu F, Yin D-M, Xiong W-C et al. Genetic Labeling Reveals Novel Cellular Targets of Schizophrenia Susceptibility Gene: Distribution of GABA and Non-GABA ErbB4-Positive Cells in Adult Mouse Brain. The Journal of Neuroscience 2014; 34(40): 13549–13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chung DW, Fish KN, Lewis DA. Pathological Basis for Deficient Excitatory Drive to Cortical Parvalbumin Interneurons in Schizophrenia. The American journal of psychiatry 2016; 173(11): 1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li B, Woo R-S, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron 2007; 54(4): 583–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang M, Yang Y, Wang C-J, Gamo Nao J, Jin Lu E, Mazer James A et al. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron 2013; 77(4): 736–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang HX, Stradtman GGr, Wang XJ, Gao WJ. A specialized NMDA receptor function in layer 5 recurrent microcircuitry of the adult rat prefrontal cortex. Proc Nat Acad Sci U S A 2008; 105(43): 16791–16796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang X-J. Synaptic basis of cortical persistent activity: the importance of NMDA receptors to working memory. The Journal of neuroscience : the official journal of the Society for Neuroscience 1999; 19(21): 9587–9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rotaru DC, Lewis DA, Gonzalez-Burgos G. The role of glutamatergic inputs onto parvalbumin-positive interneurons: relevance for schizophrenia. Rev Neurosci 2012; 23(1): 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fernandes D, Carvalho AL. Mechanisms of homeostatic plasticity in the excitatory synapse. Journal of neurochemistry 2016; 139(6): 973–996. [DOI] [PubMed] [Google Scholar]
- 159.Barnes Samuel J, Sammons Rosanna P, Jacobsen RI, Mackie J, Keller Georg B, Keck T. Subnetwork-Specific Homeostatic Plasticity in Mouse Visual Cortex In Vivo. Neuron 2015; 86(5): 1290–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.van Versendaal D, Levelt CN. Inhibitory interneurons in visual cortical plasticity. Cellular and Molecular Life Sciences 2016; 73(19): 3677–3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ultanir SK, Kim J-E, Hall BJ, Deerinck T, Ellisman M, Ghosh A. Regulation of spine morphology and spine density by NMDA receptor signaling in vivo. Proceedings of the National Academy of Sciences 2007; 104(49): 19553–19558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Alvarez VA, Ridenour DA, Sabatini BL. Distinct structural and ionotropic roles of NMDA receptors in controlling spine and synapse stability. J Neurosci 2007; 27(28): 7365–7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Flores C, Wen X, Labelle-Dumais C, Kolb B. Chronic phencyclidine treatment increases dendritic spine density in prefrontal cortex and nucleus accumbens neurons. Synapse 2007; 61(12): 978–984. [DOI] [PubMed] [Google Scholar]
- 164.Hajszan T, Leranth C, Roth RH. Subchronic phencyclidine treatment decreases the number of dendritic spine synapses in the rat prefrontal cortex. Biological psychiatry 2006; 60(6): 639–644. [DOI] [PubMed] [Google Scholar]
- 165.Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329(5994): 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 2019; 364(6436): eaat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ma L, Skoblenick K, Seamans JK, Everling S. Ketamine-Induced Changes in the Signal and Noise of Rule Representation in Working Memory by Lateral Prefrontal Neurons. The Journal of Neuroscience 2015; 35(33): 11612–11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Skoblenick K, Everling S. NMDA antagonist ketamine reduces task selectivity in macaque dorsolateral prefrontal neurons and impairs performance of randomly interleaved prosaccades and antisaccades. The Journal of neuroscience : the official journal of the Society for Neuroscience 2012; 32(35): 12018–12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Škovierová H, Vidomanová E, Mahmood S, Sopková J, Drgová A, Červeňová T et al. The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health. International journal of molecular sciences 2016; 17(10): 1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang M, Arnsten AF. Contribution of NMDA receptors to dorsolateral prefrontal cortical networks in primates. Neuroscience bulletin 2015; 31(2): 191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gilmour G, Dix S, Fellini L, Gastambide F, Plath N, Steckler T et al. NMDA receptors, cognition and schizophrenia--testing the validity of the NMDA receptor hypofunction hypothesis. Neuropharmacology 2012; 62(3): 1401–1412. [DOI] [PubMed] [Google Scholar]
- 172.Roberts BM, Holden DE, Shaffer CL, Seymour PA, Menniti FS, Schmidt CJ et al. Prevention of ketamine-induced working memory impairments by AMPA potentiators in a nonhuman primate model of cognitive dysfunction. Behavioural brain research 2010. [DOI] [PubMed] [Google Scholar]
- 173.Stoet G, Snyder LH. Effects of the NMDA antagonist ketamine on task-switching performance: evidence for specific impairments of executive control. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2006; 31(8): 1675–1681. [DOI] [PubMed] [Google Scholar]
- 174.Condy C, Wattiez N, Rivaud-Péchoux S, Gaymard B. Ketamine-induced distractibility: An oculomotor study in monkeys. Biological psychiatry 2005; 57(4): 366–372. [DOI] [PubMed] [Google Scholar]
- 175.Buccafusco JJ, Terry AV Jr. A reversible model of the cognitive impairment associated with schizophrenia in monkeys: potential therapeutic effects of two nicotinic acetylcholine receptor agonists. Biochemical pharmacology 2009; 78(7): 852–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Taffe MA, Davis SA, Gutierrez T, Gold LH. Ketamine impairs multiple cognitive domains in rhesus monkeys. Drug and alcohol dependence 2002; 68(2): 175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D’Souza DC et al. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Molecular psychiatry 2013; 18(11): 1199–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Driesen NR, McCarthy G, Bhagwagar Z, Bloch MH, Calhoun VD, D’Souza DC et al. The Impact of NMDA Receptor Blockade on Human Working Memory-Related Prefrontal Function and Connectivity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2013; 38: 2613–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zick JL, Blackman RK, Crowe DA, Amirikian B, DeNicola AL, Netoff TI et al. Blocking NMDAR Disrupts Spike Timing and Decouples Monkey Prefrontal Circuits: Implications for Activity-Dependent Disconnection in Schizophrenia. Neuron 2018; 98(6): 1243–1255.e1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cavanagh SE, Lam NH, Murray JD, Hunt LT, Kennerley SW. A circuit mechanism for decision-making biases and NMDA receptor hypofunction. Elife 2020; 9: e53664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kummerfeld E, Ma S, Blackman RK, DeNicola AL, Redish AD, Vinogradov S et al. Cognitive Control Errors in Nonhuman Primates Resembling Those in Schizophrenia Reflect Opposing Effects of NMDA Receptor Blockade on Causal Interactions Between Cells and Circuits in Prefrontal and Parietal Cortices. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 2020; 5(7): 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Duncan NW, Wiebking C, Tiret B, Marjańska M, Hayes DJ, Lyttleton O et al. Glutamate Concentration in the Medial Prefrontal Cortex Predicts Resting-State Cortical-Subcortical Functional Connectivity in Humans. PLOS ONE 2013; 8(4): e60312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Braun U, Schäfer A, Bassett DS, Rausch F, Schweiger JI, Bilek E et al. Dynamic brain network reconfiguration as a potential schizophrenia genetic risk mechanism modulated by NMDA receptor function. Proceedings of the National Academy of Sciences 2016; 113(44): 12568–12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gonzalez-Burgos G, Kroener S, Zaitsev AV, Povysheva NV, Krimer LS, Barrionuevo G et al. Functional maturation of excitatory synapses in layer 3 pyramidal neurons during postnatal development of the primate prefrontal cortex. Cerebral cortex (New York, NY : 1991) 2008; 18: 626–637. [DOI] [PubMed] [Google Scholar]
- 185.Wang HX, Gao WJ. Development of calcium-permeable AMPA receptors and their correlation with NMDA receptors in fast-spiking interneurons of rat prefrontal cortex. J Physiol 2010; 588: 2823–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nature reviews Neuroscience 2007; 8(6): 413–426. [DOI] [PubMed] [Google Scholar]
- 187.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nature reviews Neuroscience 2013; 14(6): 383–400. [DOI] [PubMed] [Google Scholar]
- 188.Hunt DL, Castillo PE. Synaptic plasticity of NMDA receptors: mechanisms and functional implications. Current opinion in neurobiology 2012; 22(3): 496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li W, Pozzo-Miller L. Differences in GluN2B-Containing NMDA Receptors Result in Distinct Long-Term Plasticity at Ipsilateral versus Contralateral Cortico-Striatal Synapses. eNeuro 2019; 6(6): ENEURO.0118–0119.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends in Neurosciences 2012; 35(1): 57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Krystal JH, D’Souza DC, Mathalon D, Perry E, Belger A, Hoffman R. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: toward a paradigm shift in medication development. Psychopharmacology 2003; 169(3–4): 215–233. [DOI] [PubMed] [Google Scholar]
- 192.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. Journal of Neuroscience 2007; 27(43): 11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Burt JB, Demirtaş M, Eckner WJ, Navejar NM, Ji JL, Martin WJ et al. Hierarchy of transcriptomic specialization across human cortex captured by structural neuroimaging topography. Nat Neurosci 2018; 21(9): 1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Chiu AM, Wang J, Fiske MP, Hubalkova P, Barse L, Gray JA et al. NMDAR-Activated PP1 Dephosphorylates GluN2B to Modulate NMDAR Synaptic Content. Cell Reports 2019; (e5): 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kyrke-Smith M, Williams JM. Bridging Synaptic and Epigenetic Maintenance Mechanisms of the Engram. Front Mol Neurosci 2018; 11: 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Galanis C, Vlachos A. Hebbian and Homeostatic Synaptic Plasticity—Do Alterations of One Reflect Enhancement of the Other? Frontiers in Cellular Neuroscience 2020; 14: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lee H-K, Kirkwood A. Mechanisms of Homeostatic Synaptic Plasticity in vivo. Frontiers in Cellular Neuroscience 2019; 13: 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Keck T, Hübener M, Bonhoeffer T. Interactions between synaptic homeostatic mechanisms: an attempt to reconcile BCM theory, synaptic scaling, and changing excitation/inhibition balance. Current opinion in neurobiology 2017; 43: 87–93. [DOI] [PubMed] [Google Scholar]
- 199.Keck T, Toyoizumi T, Chen L, Doiron B, Feldman DE, Fox K et al. Integrating Hebbian and homeostatic plasticity: the current state of the field and future research directions. Philos Trans R Soc Lond B Biol Sci 2017; 372(1715): 20160158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nature reviews Neuroscience 2004; 5(2): 97–107. [DOI] [PubMed] [Google Scholar]
- 201.Turrigiano G Too Many Cooks? Intrinsic and Synaptic Homeostatic Mechanisms in Cortical Circuit Refinement. Annual review of neuroscience 2011; 34(1): 89–103. [DOI] [PubMed] [Google Scholar]
- 202.Perez-Otano I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends in neurosciences 2005; 28(5): 229–238. [DOI] [PubMed] [Google Scholar]
- 203.Schaukowitch K, Reese AL, Kim S-K, Kilaru G, Joo J-Y, Kavalali ET et al. An Intrinsic Transcriptional Program Underlying Synaptic Scaling during Activity Suppression. Cell Reports 2017; 18(6): 1512–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Watt AJ, Sjostrom PJ, Hausser M, Nelson SB, Turrigiano GG. A proportional but slower NMDA potentiation follows AMPA potentiation in LTP. Nat Neurosci 2004; 7(5): 518–524. [DOI] [PubMed] [Google Scholar]
- 205.Watt AJ, van Rossum MC, MacLeod KM, Nelson SB, Turrigiano GG. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron 2000; 26(3): 659–670. [DOI] [PubMed] [Google Scholar]
- 206.Castillo PE, Chiu CQ, Carroll RC. Long-term plasticity at inhibitory synapses. Current opinion in neurobiology 2011; 21(2): 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rebola N, Srikumar BN, Mulle C. Activity-dependent synaptic plasticity of NMDA receptors. The Journal of physiology 2010; 588(Pt 1): 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rodriguez G, Mesik L, Gao M, Parkins S, Saha R, Lee HK. Disruption of NMDAR Function Prevents Normal Experience-Dependent Homeostatic Synaptic Plasticity in Mouse Primary Visual Cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience 2019; 39(39): 7664–7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Puścian A, Benisty H, Higley MJ. NMDAR-Dependent Emergence of Behavioral Representation in Primary Visual Cortex. Cell Reports 2020; 32(4): 107970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Forsyth JK, Bachman P, Mathalon DH, Roach BJ, Asarnow RF. Augmenting NMDA receptor signaling boosts experience-dependent neuroplasticity in the adult human brain. Proceedings of the National Academy of Sciences 2015; 112(50): 15331–15336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mathew SS, Hablitz JJ. Presynaptic NMDA receptors mediate IPSC potentiation at GABAergic synapses in developing rat neocortex. PLoS ONE 2011; 6(2): e17311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bouvier G, Larsen RS, Rodríguez-Moreno A, Paulsen O, Sjöström PJ. Towards resolving the presynaptic NMDA receptor debate. Current opinion in neurobiology 2018; 51: 1–7. [DOI] [PubMed] [Google Scholar]
- 213.Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron 2003; 39(4): 641–654. [DOI] [PubMed] [Google Scholar]
- 214.Corlew R, Brasier DJ, Feldman DE, Philpot BD. Presynaptic NMDA receptors: newly appreciated roles in cortical synaptic function and plasticity. Neuroscientist 2008; 14(6): 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Banerjee A, Larsen RS, Philpot BD, Paulsen O. Roles of Presynaptic NMDA Receptors in Neurotransmission and Plasticity. Trends in Neurosciences 2016; 39(1): 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Rodriguez-Moreno A, Paulsen O. Spike timing-dependent long-term depression requires presynaptic NMDA receptors. Nat Neurosci 2008; 11(7): 744–745. [DOI] [PubMed] [Google Scholar]
- 217.McGuinness L, Taylor C, Taylor RDT, Yau C, Langenhan T, Hart ML et al. Presynaptic NMDARs in the Hippocampus Facilitate Transmitter Release at Theta Frequency. Neuron 2010; 68(6): 1109–1127. [DOI] [PubMed] [Google Scholar]
- 218.Park H, Popescu A, Poo M-m. Essential role of presynaptic NMDA receptors in activity-dependent BDNF secretion and corticostriatal LTP. Neuron 2014; 84(5): 1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Larsen RS, Corlew RJ, Henson MA, Roberts AC, Mishina M, Watanabe M et al. NR3A-containing NMDARs promote neurotransmitter release and spike timing-dependent plasticity. Nat Neurosci 2011; 14(3): 338–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kesner P, Schohl A, Warren EC, Ma F, Ruthazer ES. Postsynaptic and Presynaptic NMDARs Have Distinct Roles in Visual Circuit Development . Cell Reports 2020; 32(4): 107955. [DOI] [PubMed] [Google Scholar]
- 221.Wang HX, Gao WJ. Prolonged exposure to NMDAR antagonist induces cell-type specific changes of glutamatergic receptors in rat prefrontal cortex. Neuropharmacology 2012; 62(4): 1808–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rebola N, Carta M, Lanore F, Blanchet C, Mulle C. NMDA receptor-dependent metaplasticity at hippocampal mossy fiber synapses. Nat Neurosci 2011; 14(6): 691–693. [DOI] [PubMed] [Google Scholar]
- 223.Lee M-C, Yasuda R, Ehlers MD. Metaplasticity at Single Glutamatergic Synapses. Neuron 2010; 66(6): 859–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Keck T, Keller Georg B, Jacobsen RI, Eysel Ulf T, Bonhoeffer T, Hübener M. Synaptic Scaling and Homeostatic Plasticity in the Mouse Visual Cortex In Vivo. Neuron 2013; 80(2): 327–334. [DOI] [PubMed] [Google Scholar]
- 225.Soares C, Lee KFH, Béïque J-C. Metaplasticity at CA1 Synapses by Homeostatic Control of Presynaptic Release Dynamics. Cell Reports 2017; 21(5): 1293–1303. [DOI] [PubMed] [Google Scholar]
- 226.Abraham WC. Metaplasticity: tuning synapses and networks for plasticity. Nature reviews Neuroscience 2008; 9(5): 387. [DOI] [PubMed] [Google Scholar]
- 227.Wenner P Mechanisms of GABAergic homeostatic plasticity. Neural plasticity 2011: 489470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Udakis M, Pedrosa V, Chamberlain SEL, Clopath C, Mellor JR. Interneuron-specific plasticity at parvalbumin and somatostatin inhibitory synapses onto CA1 pyramidal neurons shapes hippocampal output. Nat Commun 2020; 11(1): 4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends in neurosciences 1996; 19(4): 126–130. [DOI] [PubMed] [Google Scholar]
- 230.Abraham WC, Richter-Levin G. From Synaptic Metaplasticity to Behavioral Metaplasticity. Neurobiology of learning and memory 2018; 154: 1–4. [DOI] [PubMed] [Google Scholar]
- 231.Matthews GA, Tye KM. Neural mechanisms of social homeostasis. Annals of the New York Academy of Sciences 2019; 1457(1): 5–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wefelmeyer W, Puhl CJ, Burrone J. Homeostatic Plasticity of Subcellular Neuronal Structures: From Inputs to Outputs. Trends in neurosciences 2016; 39(10): 656–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Vormstein-Schneider D, Lin JD, Pelkey KA, Chittajallu R, Guo B, Arias-Garcia MA et al. Viral manipulation of functionally distinct interneurons in mice, non-human primates and humans. Nature Neuroscience 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sun Q, Li X, Ren M, Zhao M, Zhong Q, Ren Y et al. A whole-brain map of long-range inputs to GABAergic interneurons in the mouse medial prefrontal cortex. Nature Neuroscience 2019; 22(8): 1357–1370. [DOI] [PubMed] [Google Scholar]
- 235.Ahrlund-Richter S, Xuan Y, van Lunteren JA, Kim H, Ortiz C, Pollak Dorocic I et al. A whole-brain atlas of monosynaptic input targeting four different cell types in the medial prefrontal cortex of the mouse. Nat Neurosci 2019; 22(4): 657–668. [DOI] [PubMed] [Google Scholar]
- 236.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 2009; 10(1): 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE et al. Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 2015; 524: 88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Beier Kevin T, Steinberg Elizabeth E, DeLoach Katherine E, Xie S, Miyamichi K, Schwarz L et al. Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell 2015; 162(3): 622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Lee D, Creed M, Jung K, Stefanelli T, Wendler DJ, Oh WC et al. Temporally precise labeling and control of neuromodulatory circuits in the mammalian brain. Nature methods 2017; 14(5): 495–503. [DOI] [PubMed] [Google Scholar]
- 240.Deisseroth K, Hegemann P. The form and function of channelrhodopsin. Science 2017; 357(6356): eaan5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Roth Bryan L DREADDs for Neuroscientists. Neuron 2016; 89(4): 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Dana H, Sun Y, Mohar B, Hulse BK, Kerlin AM, Hasseman JP et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nature methods 2019; 16(7): 649–657. [DOI] [PubMed] [Google Scholar]
- 243.Sun F, Zeng J, Jing M, Zhou J, Feng J, Owen SF et al. A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell 2018; 174(2): 481–496.e419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Dedoncker J, Baeken C, De Raedt R, Vanderhasselt MA. Combined transcranial direct current stimulation and psychological interventions: State of the art and promising perspectives for clinical psychology Biological psychology 2020: 107991. [DOI] [PubMed] [Google Scholar]
- 245.Uhlhaas PJ, Singer W. Oscillations and neuronal dynamics in schizophrenia: the search for basic symptoms and translational opportunities. Biological psychiatry 2015; 77(12): 1001–1009. [DOI] [PubMed] [Google Scholar]
- 246.Hong LE, Summerfelt A, Buchanan RW, O’Donnell P, Thaker GK, Weiler MA et al. Gamma and delta neural oscillations and association with clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2010; 35(3): 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Shaw AD, Knight L, Freeman TCA, Williams GM, Moran RJ, Friston KJ et al. Oscillatory, Computational, and Behavioral Evidence for Impaired GABAergic Inhibition in Schizophrenia. Schizophr Bull 2020; 46(2): 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kuroda MYJ, Oda S, Price JL. Synaptic relationships between axon terminals from the mediodorsal thalamic nucleus and gamma-aminobutyric acidergic cortical cells in the prelimbic cortex of the rat. J Comp Neurol 2004; 477(2): 220–234. [DOI] [PubMed] [Google Scholar]
- 249.Xi D, Zhang W, Wang HX, Stradtman GG, Gao WJ. Dizocilpine (MK-801) induces distinct changes of N-methyl-d-aspartic acid receptor subunits in parvalbumin-containing interneurons in young adult rat prefrontal cortex. Int J Neuropsychopharmacol 2009; 12(10): 1395–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Insel TR. Rethinking schizophrenia. Nature 2010; 468(7321): 187–193. [DOI] [PubMed] [Google Scholar]
- 251.Le Magueresse C, Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron 2013; 77(3): 388–405. [DOI] [PubMed] [Google Scholar]