Abstract
Sepsis is resulted from a systemic inflammatory response to bacterial, viral, or fungal agents. The induced inflammatory response by these microorganisms can lead to multiple organ system failure with devastating consequences. Recent studies have shown altered expressions of several non-coding RNAs such as long non-coding RNAs (lncRNAs), microRNAs (miRNAs) and circular RNAs (circRNAs) during sepsis. These transcripts have also been found to participate in the pathogenesis of multiple organ system failure through different mechanisms. NEAT1, MALAT1, THRIL, XIST, MIAT and TUG1 are among lncRNAs that participate in the pathoetiology of sepsis-related complications. miR-21, miR-155, miR-15a-5p, miR-494-3p, miR-218, miR-122, miR-208a-5p, miR-328 and miR-218 are examples of miRNAs participating in these complications. Finally, tens of circRNAs such as circC3P1, hsa_circRNA_104484, hsa_circRNA_104670 and circVMA21 and circ-PRKCI have been found to affect pathogenesis of sepsis. In the current review, we describe the role of these three classes of noncoding RNAs in the pathoetiology of sepsis-related complications.
Keywords: lncRNA, miRNA, sepsis, expression, biomarker
Introduction
Sepsis is a systemic inflammatory response to different infections, namely bacterial, viral, or fungal agents. This condition is the principal source of mortality in intensive care units (1). These infectious microorganisms can stimulate inflammatory reactions through induction of cytokines release. These reactions lead to multiple organ system failure. Other factors that contribute in this devastating condition during sepsis are systemic hypotension and abnormal perfusion of the microcirculatory system (2). No specific treatment modality has been suggested for prevention of multiple organ system failure during sepsis (2). Thus, identification of sepsis-related changes at cellular and biochemical levels is important. Currently, there is no effective pharmacological therapy for sepsis. Thus, early diagnosis, resuscitation and instant administration of suitable antibiotics are essential steps in decreasing the burden of this condition {Thompson, 2019 #562}.
Lipopolysaccharide (LPS) as the main constituent of the cell wall of Gram-negative bacteria has been found to stimulate apoptotic pathways in tubular epithelial cells of kidney (3). Moreover, it can prompt acute inflammatory responses through activation of NF-κB during the course of acute kidney injury (4). This molecular pathway is an important axis in mediation of immune-related organ damage.
Recent studies have shown altered expressions of several non-coding RNAs such as long non-coding RNAs (lncRNAs), microRNAs (miRNAs) and circular RNAs (circRNAs) during sepsis. These transcripts have also been found to participate in the pathogenesis of multiple organ system failure through different mechanisms. In the current review, we describe the role of these three classes of noncoding RNAs in the pathoetiology of sepsis-related complications.
LncRNAs and Sepsis
LncRNAs are transcripts with sizes larger than 200 nucleotides. These transcripts regulate gene expression through modulation of chromatin configuration, regulation of splicing events, serving as decoys for other transcripts and making structures for recruitment of regulatory proteins (5). These transcripts participate in the regulation of immune reactions and pathoetiology of several immune-related disorders (6).
Experiments in animal model of acute lung injury have shown down-regulation of TUG1 and induction of apoptosis and inflammation. Up-regulation of TUG1 in these animals could ameliorate sepsis-associated lung injury, apoptosis and inflammatory reactions. TUG1 could also protect lung microvascular endothelial cells from deteriorative effects of LPS. In fact, TUG1 inhibits cell apoptosis and inflammatory reactions in LPS-stimulated microvascular endothelial cells through sponging miR-34b-5p and releasing GAB1 from its inhibitory effects. Cumulatively, TUG1 ameliorates sepsis-associated inflammation and apoptosis through miR-34b-5p/GAB1 axis (7). Another study has demonstrated down-regulation of TUG1 while up-regulation of miR-223 in the plasma samples of sepsis patients. They have also reported a negative correlation between expressions of TUG1 and miR-223 in sepsis patients. Besides, expression levels of TUG1 have been negatively correlated with respiratory infection, serum creatinine, white blood cell, C-reactive protein, APACHE II score, and SOFA score. Based on these results, TUG1 has been suggested as a biomarker for prediction of course and prognosis of sepsis (8). TUG1 has also been shown to interact with miR-27a. Over-expression of TUG1 has resulted in down-regulation of TNF-α, while up-regulation of miR-27a has enhanced expression of TNF-α in cardiomyocytes. TNF-α and miR-27a up-regulation could enhance LPS-induced apoptosis of cardiomyocytes. On the other hand, TUG1 up-regulation has exerted opposite effects (9).
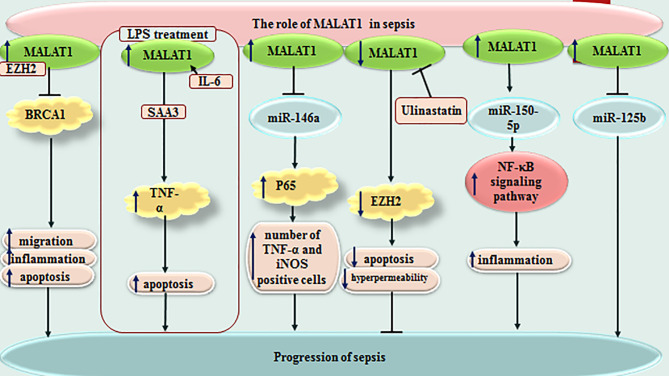
MALAT1 is another lncRNA that affects immune responses of rats with LPS-induced sepsis through influencing the miR-146a/NF-κB P65 axis (10). Moreover, MALAT1 could increase apoptosis skeletal muscle cells and sepsis-associated immune responses through down-regulating BRCA1 levels via recruitment of EZH2 (11). The miR-150-5p/NF-κB axis is another axis that mediates the effects of MALAT1 in sepsis-associated cardiac inflammation (12). In addition, the protective effects of Ulinastatin against LPS-associated dysfunction of heart microvascular endothelial cells have been shown to be exerted through down-regulation of MALAT1 (13). Most notably, MALAT1/miR-125a axis has been shown to discriminate sepsis patients based on their severity of diseases, organ damage, levels of inflammatory responses and mortality (14). Figure 1 depicts function of MALAT1 in sepsis-related events.
Figure 1.
Function of MALAT1 in sepsis-related events.
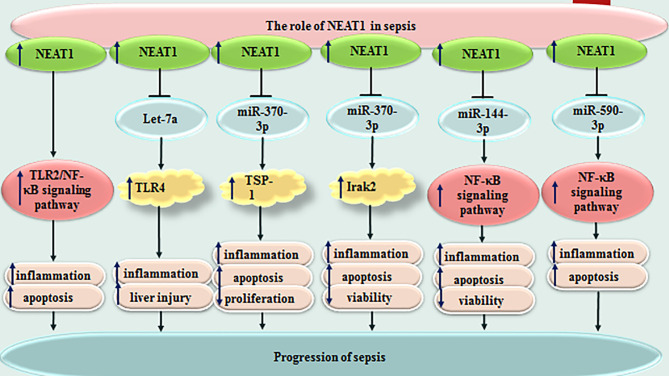
NEAT1 is another lncRNA whose participation in the pathophysiology of sepsis has been vastly investigated. This lncRNA could promote inflammatory responses and aggravate sepsis-associated hepatic damage through the Let-7a/TLR4 axis (15). Moreover, NEAT1 can accelerate progression of sepsis via miR-370-3p/TSP-1 axis (16). This lncRNA could also promote LPS-induced inflammatory responses in macrophages through regulation of miR-17-5p/TLR4 axis (17). NEAT1 silencing could suppress immune responses during sepsis through miR‐125/MCEMP1 axis (18). Figure 2 shows the function of NEAT1 in sepsis-related events. Several other lncRNAs have also been found to influence course of sepsis through modulation of immune responses ( Table 1 ).
Figure 2.
Function of NEAT1 in sepsis-related events. Several other lncRNAs have also been found to influence course of sepsis through modulation of immune responses ( Table 1 ).
Table 1.
LncRNAs and Sepsis.
| lncRNA | Expression Pattern |
Clinical Samples/ Animal Model | Assessed Cell Lines | Targets / Regulators | Signaling Pathways | Description | Reference |
|---|---|---|---|---|---|---|---|
| TUG1 | ↓ | 35 ARDS patients and 68 HCs, male C57BL/6 mice | PMVECs | ↑ miR-34b-5p, GAB1 ↓ | _ | TUG1 reduces sepsis-induced pulmonary injury, apoptosis and inflammation in ALI. | (7) |
| TUG1 | ↓ | 122 patients with sepsis and 122 HCs | _ | ↑ miR-223 | _ | Low levels of TUG1 was correlated with respiratory infection. TUG1 expression was negatively associated with Scr, WBC, SOFA score, and CRP levels and 28‐day deaths, but positively associated with albumin levels. | (8) |
| TUG1 | ↓ | _ | HUVECs | ↑ miR-27a-3p, ↓ SLIT2 | _ | Up-regulation of TUG1 reduced apoptosis, autophagy, and inflammatory response. | (19) |
| TUG1 | ↓ | 70 patients with sepsis and 70 HCs | AC16 | miR-27a, ↑ TNF-α | _ | Up-regulation of TUG1 reduced apoptosis. | (9) |
| MALAT1 | ↑ | rats with and without LPS-induced sepsis | U937 | ↓ miR-146a, ↑ P65 | ↑ NF-κB signaling pathway | Downregulation of MALAT1 decreased the number of TNF-α and iNOS positive cells. | (10) |
| MALAT1 | ↑ | BALB/c male mice | HSMKMC 3500 | ↓ BRCA1, EZH2 | _ | Downregulation of MALAT1 reduced inflammatory responses, neutrophil migration, skeletal muscle cell apoptosis, and AKT-1 phosphorylation. | (11) |
| MALAT1 | ↑ | _ | H9c2 | ↓ miR-150-5p, | ↑ NF-κB signaling pathway | Downregulation of MALAT1 reduced inflammatory response and downregulated NF-κB signaling pathway. | (12) |
| MALAT1 | ↑ | male SD rats | CMVECs | ↑ EZH2 | _ | MALAT1 significantly inhibited levels of EZH2 target genes, DAB2IP and Brachyury. Up-regulation of CRNDE increased permeability and apoptosis. Ulinastatin suppressed levels of MALAT1 and EZH2. |
(13) |
| MALAT1 | ↑ | 196 patients with sepsis and 196 HCs, | _ | ↓ miR‐125a | _ | MALAT1 expression was positively correlated with APACHE II score, SOFA score, serum creatinine, CRP, TNF‐α, IL‐1β, IL‐6, 28‐day deaths, and negatively with albumin. | (14) |
| MALAT1 | ↑ | sepsis mice | _ | ↓ miR-23a, ↑ MCEMP1 | _ | Downregulation of MALAT1 suppressed expression of MPO, IL-6, IL-10, TNF-α, and IL-1β, and reduced inflammation. | (20) |
| MALAT1 | ↑ | male C57 mice | ↑ p38 | ↑ p38 MAPK/p65 NF-κB signaling pathway | Downregulation of MALAT1 reduced MPO and inflammatory responses. | (21) | |
| MALAT1 | ↑ | _ | a lung injury inflammatory cell model | ↓ miR-149, ↑ MyD88 | ↑ NF-κB pathway | Downregulation of MALAT1 reduced the levels of MyD88, TNF-α, IL-1β, and IL-6, and prevented the NF-κB pathway. | (22) |
| MALAT1 | ↑ | CLP-induced septic mice | HUVECs, PAECs | ↓ miR-150 | ↑ NF-κB pathway | Downregulation of MALAT1 reduced apoptosis, ER stress and inflammation. | (23) |
| MALAT1 | ↑ in ARDS group | 152 patients with sepsis (41 ARDS and 111 Non-ARDS patients) | _ | _ | _ | MALAT1 expression was association with APACHE II score, SOFA score, inflammatory factors levels, and high mortality. | (24) |
| MALAT1 | ↑ | GEO dataset (GSE3140), male C57B6/L mice | HL-1 | ↑ IL-6, ↑ ↑ TNF-α, SAA3 | _ | Downregulation of MALAT1 Protected Cardiomyocytes from LPS-induced Apoptosis. | (25) |
| MALAT1 | ↑ | 190 patients with sepsis and 190 HCs | _ | ↓ miR‐125b | _ | MALAT1 expression was associated with Scr, WBC, CRP, PCT, TNF‐α, IL‐8, IL‐17, APACHE II score, SOFA score, and 28‐day deaths. | (26) |
| MALAT1 | ↑ | 120 patients with sepsis and 60 HCs | _ | _ | _ | Expression of MALAT1 was found to be an independent risk factor for sepsis, poor prognosis and septic shock. | (27) |
| MALAT1 | ↑ | female C57BL/6 mice | THP-1 | ↓ miR-214, ↑ TLR5 | _ | Downregulation of MALAT1 attenuated the burn injury and post-burn sepsis-induced inflammatory reaction. | (28) |
| KCNQ1OT1 | ↓ | male SD rats | H9c2 | ↑ miR-192-5p, ↓ XIAP | _ | Up-regulation of KCNQ1OT1 ameliorated proliferation and impeded apoptosis in sepsis-induced myocardial injury. | (29) |
| CYTOR | ↓ | male SD rats | H9c2 | ↑ miR-24, ↓ XIAP | _ | Up-regulation of CYTOR ameliorated viability and inhibited apoptosis in sepsis-induced myocardial injury. | (30) |
| lncRNA-5657 | ↑ | 15 patients with sepsis-induced ARDS and 15 non-septic and non-ARDS patients, SD rats | NR8383 | ↑ Spns2 | _ | Downregulation of lncRNA-5657 7 prevented sepsis-induced lung injury and LPS-induced inflammation. | (31) |
| RMRP | ↓ | male C57BL/6 mice | HL-1 | ↑ miR-1-5p, ↓ HSPA4 | ↑ NF-κB Pathway | Up-regulation of RMRP reduced LPS-induced damage, apoptosis and mitochondrial damage and LPS-induced sepsis. | (32) |
| NEAT1 | ↑ | 15 patients with sepsis-induced liver injury and 15 HCs | Kupffer, Raw264.7 | ↓ Let-7a, ↑ TLR4 | _ | Downregulation of NEAT1 reduced expression of inflammatory factors in sepsis-induced liver injury. | (15) |
| NEAT1 | ↑ | 25 Sepsis patients and 25 HCs | RAW 264.7 | ↓ miR-370-3p, ↑ TSP-1 | _ | Downregulation of NEAT1 prevented LPS-mediated inflammation and apoptosis and ameliorated proliferation. | (16) |
| NEAT1 | ↑ | male pathogen-free C57BL/6 mice | _ | ↓ miR-125, ↑ MCEMP1 | _ | Downregulation of NEAT1 suppressed inflammation and T lymphocyte apoptosis. | (18) |
| NEAT1 | ↑ | 68 patients with sepsis and 32 HCs | THP-1 macrophages | ↓ miR-17-5p, ↑ TLR4 | _ | Downregulation of NEAT1 prevented LPS-induced inflammatory responses in macrophages. | (17) |
| NEAT1 | ↑ | mouse with sepsis-induced lung injury | _ | ↓ miR-16-5p, ↑ BRD4 | _ | Downregulation of NEAT1 inhibited inflammation, apoptosis, pulmonary edema, MPO activity, pathological changes, promoted viability. | (33) |
| NEAT1 | ↑ | male C57 mice | _ | _ | ↑ TLR2/NF-κB signaling pathway | Downregulation of NEAT1 reduced LPS-induced myocardial pathological injury, apoptosis, oxidative stress, inflammatory responses. | (34) |
| NEAT1 | ↑ | male C57BL/6 mice | A549 | _ | ↑ HMGB1/RAGE signaling | Downregulation of NEAT1 increased viability attenuated LPS-induced apoptosis and suppressed inflammation. | (35) |
| NEAT1 | ↑ | 30 patients with sepsis and 30 HCs | HK-2 | ↓ let-7b-5p, TRAF6 | _ | Downregulation of NEAT1 increased proliferation and inhibited apoptosis and inflammation. | (36) |
| NEAT1 | ↑ | _ | RAW264.7 | ↓ miR-125a-5p, ↑ TRAF6, ↑ P-TAK1 | _ | Downregulation of NEAT1 decreased inflammation by promoting macrophage M2 polarization. | (37) |
| NEAT1 | ↑ | _patients with sepsis | HK2 | ↓ miR-93-5p, ↑ TXNIP | _ | Downregulation of NEAT1 inhibited apoptosis, inflammation and oxidative stress. | (38) |
| NEAT1 | ↑ | _ sepsis tissues and ANCTs |
AW 264.7 and HL-1 | ↓ miR-370-3p, ↑ Irak2 | _ | Downregulation of NEAT1 ameliorated viability, prevented apoptosis and the expression of inflammatory cytokines. | (39) |
| NEAT1 | ↑ | _ | HL-1 | ↓ miR-144-3p | NF-κB signaling pathway | Downregulation of NEAT1 ameliorated viability, prevented apoptosis and inflammatory response in LPS-induced myocardial cell injury. | (40) |
| NEAT1 | ↑ | 152 patients with sepsis and 150 | _ | _ | _ | Up-regulation of NEAT1 was positively associated with Acute Physiology and Chronic Health Evaluation II score, inflammatory responses, while negatively associated with IL-10. | (41) |
| NEAT1 | ↑ | C57BL/6 mice | WI-38 | ↓ miR-944, ↑ TRIM37 | _ | Downregulation of NEAT1 inhibited inflammatory responses and apoptosis. Overexpression of TRIM37 rescued influence of downregulation of NEAT1 on cell s. | (42) |
| NEAT1 | ↑ | 59 patients with sepsis, 52 patients with noninfectious SIRS, and 56 HCs | PBMCs | _ | _ | Levels of NEAT1 could be considered as a good predictor for the diagnosis of sepsis. | (43) |
| NEAT1 | ↑ | 127 patients with sepsis and 50 HCs | _ | ↑ Th1, ↑ Th17 | _ | Overexpression of NEAT1 was associated with chronic health evaluation II score, CRP level, acute physiology, and SOFA score. | (44) |
| NEAT1 | ↑ | male C57BL/6 mice | RAW264.7 | ↓ miR495-3p, ↑STAT3, ↓ miR-211 | ↑ PI3K/AKT signaling | Overexpression of NEAT1 was associated with inflammatory responses. | (45) |
| NEAT1 | ↑ | 102 patients with sepsis and 100 HCs | _ | ↓ miR‐125a | _ | High levels of NEAT1 was associated with SOFA score, APACHE II score, 28‐day deaths, and high ARDS risk. | (46) |
| NEAT1 | ↑ | Septic Mice | _ | ↑ NF-κB | _ | Downregulation of NEAT1 increased activity of nerve cells and reduced apoptosis. | (47) |
| NEAT1 | ↑ | 82 patients with sepsis and 82 HCs | _ | ↓ miR-124 | _ | NEAT1 showed a good predictive value for increased sepsis risk. NEAT1 expression was positively associated with disease severity, CRP, PCT, TNF-α, and IL-1β, 28-day deaths. |
(48) |
| NEAT1 | ↑ | 18 patients with sepsis-induced AKI and 18 HCs | HK-2 | ↓ miR-22-3p | ↑ NF-κB pathway | Downregulation of NEAT1 reduced levels of autophagy factors and inflammatory responses. | (49) |
| NEAT1 | ↑ | _ | RAW264.7 | ↓ miR-31-5p, ↑ POU2F1 | _ | Downregulation of NEAT1 reduced inflammatory response and apoptosis, and increased proliferation. | (50) |
| NEAT1 | ↑ | 22 patients with sepsis and 22 HCs, | H9c2 | ↓ miR-590-3p | NF-κB signaling pathway | Downregulation of NEAT1 reduced apoptosis and inflammatory responses in LPS-induced sepsis. | (51) |
| H19 | ↓ | 69 patients with sepsis and HCs, male BALB/c mice | _ | ↑ miR-874, ↓ AQP1 | _ | Downregulation of H19 contributed to inflammatory responses. Up-regulation of H19 ameliorated the impairment of sepsis companied myocardial dysfunction. | (52) |
| H19 | ↓ | _ | H9C2 | ↑ miR-93-5p, ↓ SORBS2 | _ | Up-regulation of H19 suppressed inflammatory responses in sepsis-induced myocardial injury. | (53) |
| H19 | ↓ | 104 patients with sepsis, and 92 HCs | _ | _ | _ | Expression of H19 was negatively associated with 28-day deaths and inflammatory response markers. | (54) |
| CASC9 | ↓ | rats | HSAECs | ↑ miR-195-5p, ↓ PDK4 | _ | Up-regulation of CASC9 promoted viability in sepsis-induced ALI. |
(55) |
| LUADT1 | ↓ | 60 patients with sepsis and 60 HCs | HCAECs | miR-195, ↓ Pim-1 | _ | Up-regulation of LUADT1 reduced apoptosis. | (56) |
| MIAT | ↑ | male SD rats | NRK-52E | ↓ miR-29a | _ | Up-regulation of MIAT promoted apoptosis in sepsis-related kidney injury. | (57) |
| MIAT | ↑ | male BALB/c mice | HL-1 | ↓ miR-330-5p, ↑ TRAF6 | ↑ NF-κB signaling | Downregulation of MIAT restrained inflammation and oxidative stress in Sepsis-Induced Cardiac Injury. | (58) |
| THRIL | ↑ | 66 patients with sepsis and 66 HCs | HBEpCs | ↓ miR-19a, ↑ TNF-α | _ | Up-regulation of THRIL promoted apoptosis. | (59) |
| THRIL | ↑ | C57BL/6 mice | MPVECs | ↓ miR-424, ↑ ROCK2 | _ | Downregulation of THRIL prevented inflammatory responses, and apoptosis in septic-induced acute lung injury. | (60) |
| THRIL | ↑ in ARDS group | 32 sepsis patients with ARDS and 77 without ARDS | _ | _ | _ | THRIL independently predicted increased risk of ARDS. THRIL was positively associated with APACHE II score, SOFA score, CRP, PCT, TNF-α, and IL-1β levels, and mortality rates. |
(61) |
| XIST | ↓ | male SD rats | HSAECs, HEK-293T | miR-16-5p | _ | Up-regulation of XIST increased viability and inhibited inflammatory response and apoptosis in sepsis-induced ALI. | (62) |
| XIST | ↓ | CLP-induced AKI mice | HK-2, TCMK-1 | ↑ miR-155-5p, ↓ WWC1 | _ | Up-regulation of XIST decreased sepsis-induced AKI. | (63) |
| XIST | ↑ | 30 patients and 10 HCs, male SD rats | Kupffer | ↑ BRD4 | _ | Downregulation of XIST reduced inflammation, oxidative stress, and apoptosis in sepsis-induced acute liver injury. | (64) |
| XIST | ↑ | GEO database: GSE94717 ( 6 patients with sepsis-induced AKI and 6 HCs) | MPC5 | ↓ miR-15a-5p, ↑ CUL3 | _ | Up-regulation of XIST enhanced apoptosis in sepsis-induced AKI. | (65) |
| xist | ↑ | _ | MCM | ↓ PGC-1α, ↓ Tfam |
_ | Downregulation of xist inhibited apoptosis and induced proliferation. | (66) |
| GAS5 | ↓ | 60 patients with sepsis and 60 HCs | AC16 | ↓ miR-214 | _ | Downregulation of GAS5 restrained apoptosis of cardiomyocytes induced by LPS. GAS5 could regulate miR-214 through methylation pathway. | (67) |
| CRNDE | ↓ | male specific-pathogen-free Wistar rats | _ | ↑ miR-29a, ↓ SIRT1 |
↑ NF-κB/PARP1 signaling | Up-regulation of CRNDE reduced apoptosis, oxidative stress and inflammatory response. | (68) |
| CRNDE | ↑ | 136 patients with sepsis and 151 HCs | THP-1 | ↓ miR-181a-5p, ↑ TLR4 | _ | Up-regulation of CRNDE was correlated with poorer OS and was a significant predictor in patients with sepsis. Downregulation of CRNDE reduced sepsis-related inflammatory pathogenesis. | (69) |
| CRNDE | ↑ | male C57 mice | _ | ↑ p65 | ↑ TLR3/NF-κB pathway | Downregulation of CRNDE reduced edema, necrosis and apoptosis in sepsis-induced AKI. | (70) |
| CRNDE | ↑ | _ | HK-2 | ↓ miR-146a | ↑ TLR4/NF-κB signaling pathway | Up-regulation of CRNDE enhanced cell injuries, inflammatory responses and apoptosis in sepsis-induced AKI. | (71) |
| CRNDE | ↓ | rats | HK-2, HEK293 | ↑ miR-181a-5p, ↓ PPARα | _ | Downregulation of CRNDE increased the urea nitrogen and serum creatinine, and reduced proliferation and promoted apoptosis. | (72) |
| CRNDE | ↓ | male SD rats | L02 | ↑ miR-126-5p, ↓ BCL2L2 | _ | Up-regulation of CRNDE increased viability and repressed apoptosis in sepsis-induced liver injury. |
(73) |
| HOTAIR | ↓ | male SD rats | HK-2 | ↑ miR-34a, ↓ Bcl-2 | _ | Up-regulation of HOTAIR reduced apoptosis in sepsis-induced AKI. | (74) |
| HULC | ↑ | 110 patients with sepsis and 100 HCs | HMEC-1, CRL-3243 | ↓ miR-128-3p, ↑ RAC1 | _ | Downregulation of HULC restrained apoptosis and inflammation, and protected HMEC-1 cells from LPS-induced injury. | (75) |
| HULC | ↑ | 174 patients with sepsis and 100 HCs | _ | _ | _ | Expression of HULC was correlated with APACHE II, SOFA score, and 28‐day deaths. It was also positively associated with Scr, WBC, and CRP, but negatively correlated with albumin. | (76) |
| HULC | ↑ | 56 patients with sepsis and 56 HCs | HUVECs | ↓ miR-204-5p, ↑ TRPM7 | _ | Downregulation of HULC promoted viability and reduced apoptosis, inflammatory responses and oxidative stress. | (77) |
| HULC | ↑ | C57BL/6 mice | HMECs | ↑ IL6, ↑ ICAM1, ↑ VCAM1 | _ | Downregulation of HULC reduced levels of pro-inflammatory factors. | (78) |
| TapSAKI | ↑ | SD rats | HK-2 | ↓ miR-22 | ↑ TLR4/NF-κB pathway | Downregulation of TapSAKI decreased inflammatory factors and renal function indicators, so decreased kidney injury. | (79) |
| ITSN1‐2 | ↑ | 309 patients with intensive care unit (ICU)‐treated sepsis and 300 HCs | _ | _ | _ | High levels of ITSN1‐2 were correlated with elevated disease severity, inflammation, and poor prognosis in sepsis patients. | (80) |
| LincRNA-p21 | ↑ | sepsis-induced ALI rat model | BEAS-2B c | _ | _ | Downregulation of LincRNA-p21 restrained apoptosis, inflammatory responses and oxidative stress in sepsis-induced ALI. | (81) |
| TCONS_ 00016233 |
↑ | 15 patients with septic AKI and non-AKI, and 15 HCs, C57BL/6J mice |
HK-2 | miR-22-3p, ↑ AIFM1 | TLR4/p38MAPK axis. | Downregulation of TCONS_00016233 restrained LPS-induced apoptosis. Up-regulation of TCONS_00016233 induced LPS-induced apoptosis and inflammatory responses. |
(82) |
| UCA1 | ↑ | C57BL/6 mice | HMECs | ↑ IL6, ↑ ICAM1, ↑ VCAM1 | _ | Downregulation of UCA1 reduced inflammatory responses. | (78) |
| NR024118 | ↓ | 82 patients with sepsis without MD, 35 patients with sepsis and MD and 82 HCs | AC16 | ↑ IL-6 | NF-κB signaling pathway | Up-regulation of NR024118 reduced the secretion of IL-6 and apoptosis, and improved LPS-induced myocardial APD duration and cell injury. | (83) |
| MIR155HG | ↑ | 28 patients with sepsis and 28 without sepsis | HL-1, RAW 264.7 | ↓ miR-194-5p, ↑ MEF2A | _ | Downregulation of MIR155HG increased viability and decreased apoptosis and inflammatory responses. | (84) |
| LUCAT1 | ↑ | GEO dataset: GSE101639 | H9C2 | ↓ miR-642a, ↑ ROCK1 | _ | Downregulation of LUCAT1 decreased inflammatory responses. | (85) |
| SOX2OT | ↑ | male C57B6/L mice | H9c2 | ↑ SOX2 | _ | Downregulation of SOX2OT reduced mitochondrial dysfunction in septic cardiomyopathy. Overexpression of SOX2OT aggravated mitochondrial dysfunction in septic cardiomyopathy |
(86) |
| MEG3 | ↑ | male C57BL/6 mice | TECs | ↓ miR-18a-3P | _ | Downregulation of MEG3 reduced number of pyroptotic cells, secretion of LDH, IL-1β, and IL-18, and expression of GSDMD in LPS-induced AKI. | (87) |
| MEG3 | ↑ | 82 patients with sepsis and 54 HCs | Human primary renal mixed epithelial cells , AC16 | _ | _ | Patients with high levels of MEG3 showed higher mortality rate, and downregulation of it inhibited apoptosis induced by LPS. | (88) |
| MEG3 | ↑ | 112 patients with sepsis and 100 HCs | _ | _ | _ | High levels of MEG3 were associated with 28‐day deaths and it was found to be a predictor of higher ARDS risk. | (89) |
| MEG3 | ↑ | 219 patients with sepsis and 219 HCs, male C57BL/6 J mice | _ | ↓ miR‐21 | _ | Lnc‐MEG3 expression was positively correlated with cardiomyopathy, APACHE II score, SOFA score, Scr, TNF‐α, IL‐1β, IL‐6, and IL‐17, 28‐day deaths, while negatively correlated with albumin. | (90) |
| MEG3 | ↓ | male C57/BL mice | Caco2 | ↑ miR-129-5p, ↓ SP-D | _ | Overexpression of MEG3 reduced villus length and apoptosis, inhibited intestinal injury and enhanced proliferation. | (91) |
| GAS5 | ↓ | _ | conditional immortalized podocyte line | ↓ PTEN | ↑ PI3K/AKT pathway | Downregulation of GAS5 elevated the Podocyte Injury. | (92) |
| LINC00472 | ↑ | male SD rats | THLE-3 | ↓ miR-373-3p, ↑ TRIM8 | _ | Downregulation of LINC00472 enhanced viability and suppressed apoptosis. | (93) |
| HOTAIR | ↑ | male e C57B6/L mice | HL-1 | ↑ p-p65, ↑ NF-κB | NF-κB pathway | Downregulation of HOTAIR restrained LPS-induced myocardial dysfunction in septic mic. HOTAIR was involved in p65 phosphorylation and NF-κB activation, leading to 15 TNF-α production. | (94) |
| HOTAIR | ↑ | male SD rats | HK-2 | ↓ miR-22, ↑ HMGB1 | _ | Downregulation of HOTAIR reduced renal function indicators (blood urea nitrogen and serum creatinine). | (95) |
| Hotairm1 | ↑ | male C57BL/6 mice | MDSCs | ↑ S100A9 localization | _ | Downregulation of Hotairm1 restrained the suppressive functions of late sepsis Gr1+CD11b+ MDSCs. Hotairm1 Was involved in shuttling S100A9 protein to the nucleus. | (96) |
| NKILA | ↑ | _ | HK2 | ↓ miR-140-5p, ↑ CLDN2 | _ | Downregulation of NKILA restrained apoptosis, autophagy and inflammation and promoted viability in sepsis-induced AKI. | (97) |
| HOXA‐AS2 | ↓ | 44 patients with sepsis and 44 HCs, adults clean Kunming mice | HK‐2 | ↑ miR‐106b‐5p | ↑ Wnt/β‐catenin and NF‐κB pathways | Up-regulation of HOXA‐AS2 increased viability and repressed apoptosis and protect cells to resist LPS‐induced damage in sepsis-induced AKI. | (98) |
| SNHG14 | ↑ | _ | HK-2 | miR-93, ↑IL-6R, ↑IRAK4 | TLR4/NF-κB pathway, ↑ NF-κB and STAT3 signaling |
Up-regulation of SNHG14 promoted oxidative stress, inflammation, and apoptosis. TLR4/NF-κB pathway induced upregulation of SNHG14. |
(99) |
| lncRNA-CCL2 | ↑ | male C57BL/6 mice | _ | ↓ SIRT1 | _ | Expression of lncRNA-CCL2 was inhibited by SIRT1 through maintaining a more repressive chromatin state in lncRNA-CCL2 locus. Downregulation of SIRT1 induced inflammatory response. |
(100) |
| DLX6-AS1 | ↑ | patients with septic AKI | HK-2 | ↓ miR-223-3p, ↑ NLRP3 | _ | Downregulation of DLX6-AS1 suppressed LPS-induced cytotoxicity and pyroptosis. Expression of DLX6-AS1 was positively correlated with levels of creatinine in the serum of patients. |
(101) |
| CASC2 | ↓ | _ patients with sepsis and HCs | HK-2 | ↑ miR-155 | ↑ NF-κB signaling pathway | The levels of CASC2 were negatively correlated with the severity of AKI. CASC2 expression induced cell viability and inhibited inflammatory response, apoptosis and oxidative stress. |
(102) |
| CASC2 | ↓ | patients with sepsis and HCs | HPAEpiC | ↑ miR-152-3p, ↓ PDK4 | _ | Up-regulation of CASC2 increased viability and restrained apoptosis, inflammatory and oxidative damages. | (103) |
| ZFAS1 | ↓ | 202 patients with sepsis and 200 HCs | _ | _ | _ | Expression of ZFAS1 was negatively associated with APACHE II, level of CRP, TNF-α, IL-6 and positively with IL-10. |
(104) |
| ZFAS1 | ↓ | male SD rats | H9C2 | ↑ miR-34b-5p, ↓ SIRT1 | _ | Up-regulation of ZFAS1 decreased inflammatory responses and apoptosis. | (105) |
| ZFAS1 | ↑ | male C57BL/6 mice | _ | ↓ miR-590-3p, SP1 | AMPK/mTOR signaling | Downregulation of ZFAS1 reduced LPS-induced pyroptosis and enhanced LPS-suppressed autophagy in sepsis-induced cardiac dysfunction. | (106) |
| ZFAS1 | ↓ | 22 patients with SIMI and 24 HCs, rats treated by LPS | H9C2 | ↑ miR-138–5p, ↓ SESN2 | _ | Up-regulation of ZFAS1 attenuated myocardial injury and inflammatory response. | (107) |
| Mirt2 | ↓ | male SD rats | _ | ↑ MiR-101 | ↓ PI3K/AKT Signaling Pathway | Up-regulation of Mirt2 inhibited inflammatory responses and improved cardiac function. | (108) |
| Mirt2 | ↓ | 40 patients with sepsis, 40 patients with sepsis‐ALI, 40 HCs | HBEpCs | ↓ miR‐1246 | _ | Up-regulation of Mirt2 inhibited LPS‐induced inflammatory response, apoptosis, and promoted miR‐1246 expression but reduced its gene methylation. | (109) |
| TCONS_00016406 | ↓ | male C57BL/6 mice | PTEC | ↑ miR-687, ↓ PTEN | _ | Up-regulation of lncRNA 6406 inhibited inflammatory responses, apoptosis and oxidative stress in LPS-induced AKI. | (110) |
| NORAD | ↑ in NS patients | 88 patients with late-onset NS and 86 patients with pneumonia neonates | RAW264.7 | ↓ miR-410-3p | _ | Expression of NORAD was closely correlated with WBC, PCT, IL-6, IL-8, and TNF-α. | (111) |
| GAS5 | ↑ | _ | THP-1 | ↓ miR-23a-3p, ↑ TLR4 | _ | Downregulation of GAS5 inhibited inflammation and apoptosis. | (112) |
| lnc‐ANRIL | ↑ | 126 patients with sepsis and 125 HCs | _ | ↓ miR‐125a | _ | lnc‐ANRIL showed good predictive values for sepsis risk. lnc‐ANRIL was positively associated with CRP and PCT levels, disease severity scale scores, and pro‐inflammatory cytokine levels, 28‐day deaths in sepsis patients, |
(113) |
| PVT1 | ↑ | 109 patients with sepsis and 100 HCs | _ | _ | _ | PVT1 was found to be an independent risk factor for sepsis ARDS. And PVT1 expression positively associated with disease severity and 28-day deaths. | (114) |
| PVT1 | ↑ | _ | THP-1 | _ | ↑ p38 MAPK signaling pathway | Downregulation of PVT1 reduced levels of IL-1β and TNF-α mRNA and inhibited the p38 MAPK signaling pathway, | (115) |
| PVT1 | ↑ | sepsis model mice | HK-2 | ↓ miR-20a-5p, ↑ NLRP3 | _ | Downregulation of PVT1 inhibited pyroptosis in septic AKI. | (116) |
| PVT1 | ↑ | Mice model with sepsis | _ | ↓ miR-29a, ↑ HMGB1 | _ | Downregulation of PVT1 reduced LPS-induced myocardial injury and alleviated M1 macrophage polarization. | (117) |
| HOTAIR | ↑ | C57BL/6 mice | Monocytes | ↓ miR-211 | _ | Overexpression of HOTAIR suppressed proliferation and promoted apoptosis. | (118) |
| HOTAIR | ↑ | LPS-induced septic cardiomyopathy mice | H9C2 | ↑ PDCD4, Lin28 | _ | Downregulation of HOTAIR inhibited inflammatory responses and apoptosis. | (119) |
| DILC | ↓ | 18 patients with sepsis and 18 HCs | PBMCs, THP-1 | ↑ IL-6 | _ | DILC suppressed the transcription of IL-6, DILC decreased levels of STAT3, p-STAT3, TLR4, TNF-α, CCL5, E-selectin and CXCR1. |
(120) |
| RMRP | ↑ | C57BL/6 mice | HK-2 | ↓ miR-206, ↑ DDX5 | _ | Downregulation of RMRP inhibited inflammatory response and apoptosis in sepsis-induced AKI. | (121) |
| GAS5 | ↑ | C57BL/6 mice | _ | ↓ miR-449b, ↑ HMGB1 | ↑ HMGB1/NF-κB pathway | Downregulation of GAS5 inhibited pro-inflammatory reaction and alleviated myocardial injury. |
(122) |
| TapSAKI | ↑ | _ | HK-2 | ↓ miR-205, ↑ IRF3 | _ | Downregulation of TapSAKI alleviated LPS-induced damage. | (123) |
| SNHG16 | ↑ | male SD rats | BEAS-2B | ↓ miR-128-3p, ↑ HMGB3 | _ | Downregulation of SNHG16 reduced the apoptosis and inflammation in sepsis-induced ALI. | (124) |
| DANCR | ↓ | 20 patients with sepsis-induced AKI and 20 HCs | HK-2 | ↑ miR-214, ↑ KLF6 | _ | Up-regulation of DANCR promoted viability and suppressed cell apoptosis and inflammatory responses. | (125) |
| CASC2 | ↓ | _ | HK2, HEK293 | ↑ miR-545-3p to regulate, ↓ PPARA | _ | Up-regulation of CASC2 increased viability and inhibited apoptosis, migration, epithelial-mesenchymal transition and oxidative stress. | (126) |
| SNHG1 | ↓ | _ | H9c2 | ↑ miR-181a-5p, ↓ XIAP | _ | Up-regulation of SNHG1 increased viability and inhibited inflammatory responses and oxidative stress. | (127) |
| SNHG14 | ↑ | _patients with sepsis | HK-2 | ↓ miR-495-3p, ↑ HIPK1 | _ | SNHG14 is upregulated in patients. SNHG14 prevented proliferation and autophagy and boosted apoptosis and inflammatory responses. | (128) |
| Linc-KIAA1737–2 | ↑ | _ | HK-2 | ↓ MiR-27a-3p | _ | Downregulation of Linc-KIAA1737–2 reduced apoptosis. | (129) |
| PlncRNA-1 | ↓ | 6 patients with septic AKI and 6 HCs | NRK-52E | ↓ BCL2 | _ | Up-regulation of PlncRNA-1 meliorated proliferation and prevented apoptosis and autophagy. | (130) |
| CDKN2B-AS1 | ↑ | sepsis patients 47 and 55 HCs | BEAS-2B | ↓ miR-140-5p , ↑ TGFBR2 | ↑ TGFBR2/smad3 pathway | Downregulation of CDKN2B-AS1 promoted viability reduced apoptosis and inflammation. | (131) |
ARDS, acute respiratory distress syndrome; HCs, healthy controls; ALI, acute lung injury; LPS, lipopolysaccharide; SD, Sprague–Dawley; AKI, acute kidney injury; SOFA, sequential organ failure assessment; Scr, serum creatinine; WBC, white blood cell; CRP, C-reactive protein; PBMCs, peripheral blood mononuclear cells; PCT, procalcitonin; APACHE, physiology and chronic health evaluation; MPO, Myeloperoxidase; NS, Neonatal sepsis; SIMI, sepsis-induced myocardial injury.
miRNAs and Sepsis
miRNAs have sizes about 22 nucleotides and regulate expression of genes through binding with different regions of target mRNAs, particularly their 3’ UTR. They can either degrade target mRNA or suppress its translation. Several miRNAs have been found to influence course of sepsis. Altered expression of these small-sized transcripts has been reported in sepsis by numerous research groups. For instance, plasma levels of miR-494-3p have been shown to be decreased in sepsis patients compared with healthy controls in correlation with up-regulation of TLR6. Expression level of miR-494-3p has been decreased in LPS-induced RAW264.7 cells, parallel with up-regulation of TLR6 and TNF-α. Forced over-expression of miR-494-3p in RAW264.7 cells could reduce TNF-α level and suppress translocation of NF-κB p65 to the nucleus. TLR6 has been shown to be targeted by miR-494-3p. Taken together, miR-494-3p could attenuate sepsis-associated inflammatory responses through influencing expression of TLR6 (132). miR-218 is another miRNA which participates in the pathoetiology of sepsis. This miRNA could reduce inflammatory responses in the sepsis through decreasing expression of VOPP1 via JAK/STAT axis (133).
miR-122 is another important miRNA in the sepsis which has superior diagnostic power compared with CRP and total leucocytes count for distinguishing sepsis from wound infection. miR-122 has also been found to be a prognostic marker for sepsis, albeit with poor specificity and accuracy values (134).
In the mice model of sepsis, decreased levels of miR-208a-5p and increased levels of SOCS2 has been associated with enhanced activity of SOD, while reduction in LDH and MDA activities. Moreover, down-regulation of miR-208a-5p has been associated with low levels TNF-α, IL-6, NF-κB p65 and HIF-1α in this animal model. miR-208a-5p silencing could decrease the extent of mitochondria swelling, and inhibit apoptosis of cardiomyocytes in animal model of sepsis. Taken together, miR-208a-5p suppression has been suggested as a modality to attenuate sepsis-related myocardial damage. This function is mediated through NF-κB/HIF-1α axis (135).
miR-21 is another miRNA whose role in sepsis has been investigated by several groups. Down-regulation of miR-21 has been shown to inhibit inflammasome activation, ASC pyroptosome, LPS-induced pyroptosis and septic shock in one study (136). On the other hand, another study in animal models of sepsis has shown that up-regulation of miR-21 reduced inflammation and apoptosis (137). Similarly, βMSCs-derived exosomes have been shown to reduce symptoms in septic mice and improve their survival rate through up-regulation of miR-21 (138).
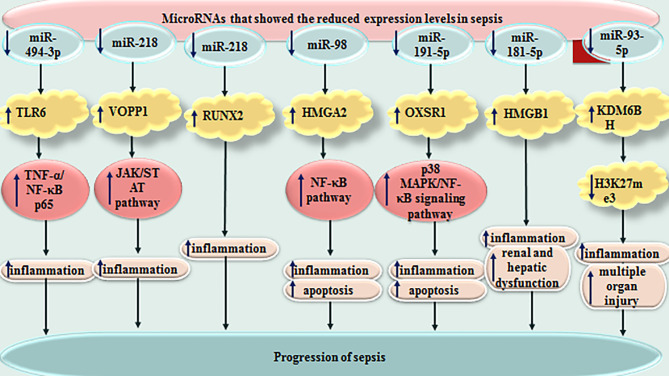
miR-328 is another miRNA which is dysregulated in sepsis patients as well as animal models of sepsis. Serum levels of this miRNA could properly differentiate sepsis from normal conditions. Thus, miR-328 has been suggested as a diagnostic biomarker for sepsis. Moreover, down-regulation of miR-328 could amend sepsis-related heart dysfunction and inflammatory responses in this tissue (139). miR-452 is another miRNA with diagnostic applications in sepsis. Notably, serum and urinary levels of this miRNA have been suggested as possible markers for early diagnosis of sepsis-associated acute kidney injury, since expression of this miRNA has been higher in sepsis patients with acute kidney injury compared with those without this condition (140) ( Table 2 ). Figure 3 depicts miRNAs that are down-regulated in sepsis.
Table 2.
Lists the function of miRNAs in the course of sepsis.
| miRNA | Pattern of Expression | Clinical Samples/Animal Model | Assessed Cell Lines | Targets / Regulators | Signaling Pathways | Description | Reference |
|---|---|---|---|---|---|---|---|
| miR-15a-5p | ↑ | GEO database: GSE94717 (6 patients with sepsis-induced AKI and 6 HCs) | MPC5 | ↓ XIST, ↓ CUL3 | _ | Downregulation of miR-15a-5p reduced apoptosis in sepsis-induced AKI. | (65) |
| miR-494-3p | ↓ | _Patients with sepsis and HCs | RAW264.7 | ↑ TLR6 | _ | Upregulation of microRNA-494-3p reduced inflammation, TNF-α level, and prevented nuclear translocation of NF-κB p65. | (132) |
| miR-218 | ↓ | 53 Patients with sepsis and 20 HCs, septic mouse model | PBMCs | ↑ VOPP1 | ↑ JAK/STAT pathway | Upregulation of microRNA-494-3p reduced inflammation. | (133) |
| miR-218 | ↓ | male S SD rats | RAW264.7 | ↑ RUNX2 | Up-regulation of miR-218 inhibited inflammatory response. | (141) | |
| miR-122 | ↑ | 25 patients with sepsis and 25 patients with local wound infections as a control group | _ | _ | _ | miR-122 showed higher AUC in comparison with CRP and TLC which had 66.6% sensitivity, 50% specificity, and 56.0% accuracy as a prognostic biomarker for sepsis. | (134) |
| miR-208a-5p | ↑ | septic mouse model | _ | ↓ SOCS2 | ↑NF-κB/HIF-1α pathway | Downregulation of miR-208a-5p decreased reduced degree of mitochondria swelling, and inhibited apoptosis. | (135) |
| miR-328 | ↑ | 110 Patients with sepsis and 89 HCs, male SD rats | _ | _ | _ | miR-328 expression was positively associated with Scr, WBC, CRP, PTC, APACHE II score, and SOFA score. miR-328 was found to be a good diagnostic value for sepsis. Downregulation of miR-328 reduced inflammatory response. | (139) |
| miR-452 | ↑ | 47 sepsis patients with AKI, 50 patients without AKI, and 10 HCs | BUMPT | NF-KB | _ | Serum and urinary miR-452 could be a potential biomarker for early detection of septic AKI. It was upregulated in sepsis patients with AKI compared with without AKI. miR-452 had high diagnostic value for AKI. | (140) |
| miR‐21 | ↓ | 219 Patients with sepsis and 219 HCs | _ | _ | _ | miR‐21 was found to be a good value in predicting sepsis risk. miR‐21 expression was negatively correlated with APACHE II, SOFA score, and 28‐day mortality risk. | (142) |
| miR‐126 | ↑ | 208 Patients with sepsis and 210 HCs | _ | _ | _ | miR‐126 expression was positively correlated with APACHE II, serum creatinine, CRP, TNF‐α, IL‐6, IL‐8, mortality rate, but negatively with IL‐10. | (143) |
| mir-103 | ↓ | 196 Patients with sepsis and 196 HCs | _ | _ | _ | mir-103 predicted high ARDS risk. Mir-103 and was negatively associated with APACHE II score, SOFA score, serum creatinine, CRP, TNF, IL- 1β, IL-6, IL-8, 28-day deaths, but positively correlated with albumin. | (144) |
| mir-107 | ↓ | 196 Patients with sepsis and 196 HCs | _ | _ | _ | mir-107 predicted high ARDS risk. mir-107 and was negatively associated with APACHE II score, SOFA score, serum creatinine, CRP, TNF, IL- 1β, IL-6, IL-8, 28-day deaths, but positively correlated with albumin | |
| miR-92a | ↑ in sepsis-induced ARDS | 53 sepsis patients (36 patients with sepsis-induced ARDS) | HPMEC, A549 | _ | ↓ Akt/mTOR signaling pathway | Downregulation of mir-92a reduced apoptosis and inflammatory response, and enhanced migration | (145) |
| miR-98 | ↓ | male C57BL/6 mice | _ | ↑ HMGA2 | ↑ NF-κB pathway | Upregulation of miR-98 prevented HMGA2, NF-κB, TNF-α, IL-6, Bcl-2 and augmented IL-10, Cleaved caspase-3 and Bax expression, it reduced LVEDP, CTn-I, BNP, ALT, AST, TBIL, LDH, and PaCO2 but elevated +dp/dt max, -dp/dt max, pH and PaO2. | (146) |
| miR‐125a | ↑ | 150 Patients with sepsis and 150 HCs | _ | _ | _ | miR‐125a expression was positively associated with Scr, APACHE II score, SOFA score. | (147) |
| miR‐125b | ↑ | 150 Patients with sepsis and 150 HCs | _ | _ | _ | miR‐125b was correlated with Scr, CRP, APACHE II score, SOFA score, and chronic obstructive pulmonary disease , and 28-day deaths. | |
| miR-199a | ↑ | male C57BL/6 mice | _ | ↓ SIRT1 | _ | Downregulation of miR-199a reduced apoptosis and inflammatory response. | (148) |
| miR-495 | ↓ | 105 Patients with sepsis and 100 HCs, rats | _ | _ | _ | miR-495 was negatively correlated with Scr, WBC, CRP, PCT, APACHE II score and SOFA score. CLP rats showed worse LVSP, LVEDP, ± dp/dtmax, and exhibited an increase in serum CTn-I, CK-MB, TNF-α, IL-6 and IL-1β. | (149) |
| miR-106a | ↑ | 50 patients with sepsis and 30 HCs, clean Kunming mice | TCMK-1 | ↓ THBS2 | _ | Downregulation of miR-106a reduced apoptosis and inflammatory response. | (150) |
| miR‐146a | _ | male C57BL/6 mice | MSCs | IL‐1β | – | IL‐1β stimulation resulted in packaging miR‐146a into exosomes. The exosomal miR‐146a was transferred to macrophages, yielded to M2 polarization, and finally led to high survival in septic mice. | (151) |
| miR-574 | ↓ | CLP-treated mice | HBE | ↑ C3 | _ | Upregulation of mir-574 increased viability, inhibited apoptosis, and reduced sepsis-induced ERS. | (152) |
| miR-195 | _ | wistar rats with sepsis | _ | TGF-β1/Smads signaling pathway, | MicroRNA-195 could promote cardiac remodeling by up-regulating the nanoantibiotics signaling pathway in sepsis rats. | (153) | |
| miR-133a | ↑ | septic mouse model | RAW264.7 | ↓ SIRT1 | _ | Downregulation of miR-133a prevented inflammatory response, sepsis-induced lung, liver and kidney injuries. | (154) |
| miR-191-5p | ↓ | female Wistar rats | _ | ↑ OXSR1 | ↑ p38 MAPK/NF-κB signaling pathway | Upregulation of miR-191-5p prevented inflammatory response and apoptosis in | (155) |
| miR-146a | ↑ | 180 patients with sepsis and 180 HCs | _ | _ | _ | MiR-146a was of good value in predicting high sepsis risk and 28-day mortality risk. MiR-146a was positively associated with biochemical indices, inflammatory cytokines, overall disease severity. | (156) |
| miR-146b | ↑ | 180 patients with sepsis and 180 HCs | _ | _ | _ | miR-146b was of good value in predicting high sepsis risk and 28-day mortality risk. MiR-146a was positively associated with biochemical indices, inflammatory cytokines, and overall disease severity. . |
|
| miR-126 | ↓ | 20 patients with sepsis and 30 patients with general infection | _ | _ | _ | miR-126 was negatively associated with the levels of caspase-3, APACHE II score, and positively with 28-day cumulative survival rate. AUC for predicting the prognosis by miR-126 was 0.823. | (157) |
| miR-223 | _ | C57BL/6 mice | RAW264.7 | _ | _ | Upregulation of mir-223 impelled M2 macrophage through lower activity of glycolysis Pathway. the Implementation of miR-223 over-expressed macrophages with IL-4 pre-conditioning alleviated sepsis severity. |
(158) |
| miR-146b | ↓ | septic mouse model | HK-2 | ↑ IRAK1 | ↑ NF-κB pathway | Treatment with hucMSC-Ex improved survival in mice with sepsis by reducing levels of IRAK1, increasing of miR-146b level, and inhibition of NF-κB activity. | (159) |
| miR-1-3p | ↑ | male SD rats | HUVECs | ↓ SERP1 | _ | miR-1-3p decreased proliferation, and increased apoptosis, and permeability and HUVECs membrane injury. | (160) |
| miR-25 | ↓ | 70 patients with sepsis and 30 patients with SIRS | _ | _ | _ | Levels of miR-25 was negatively associated with the severity of sepsis, SOFA score, CRP and PCT level, 28-day deaths, and levels of oxidative stress indicators. | (161) |
| miR-370-3p | ↑ in SAE | 12 patients with sepsis without encephalopathy, 17 patients with SAE, 20 patients with severe uremia and 12 HCs , male C57BL/6 mice | _ | _ | _ | miR-370-3p was associated with TNF-α and increased brain apoptosis in SAE mice. | (162) |
| miR-21 | ↑ | GEO database: GSE26440 (88 children with septic shock and 26 HCs), C57BL/6 mice | _ | ↓ A20, ↑ NLRP3 | ↑ NF-κB pathway | Downregulation of miR-21 inhibited inflammasome activation, ASC pyroptosome, LPS-induced pyroptosis and septic shock. | (136) |
| miR-21 | ↓ | CLP mouse model | _ | ↑ PDCD4, ↑ PTEN | PDCD4/NF-κB and PTEN/AKT pathways | rIPC protected kidneys from injury by miR-21. miR-21 was transported from ischemic limbs to the kidneys by exosomes. | (163) |
| miR-21 | ↓ | septic mouse model | MTEC | ↑ PDCD4 | ↑ NF-κB pathway | Upregulation of miR-21 reduced inflammation and apoptosis. | (137) |
| miR-21 | _ | septic mice | _ | _ | _ | Hyperoside decreased miR-21 levels so reduced inflammatory responses and increased viability. | (164) |
| miR-21 | ↓ | _ | MSCs | ↑ PDCD4 | _ | βMSCs-derived exosomes reduced symptoms in septic mice and improved their survival rate through miR-21 upregulation. | (138) |
| miR-21 | ↑ | septic C57BL/6J mice | _ | ↓ PGE2, ↓ IL-10 | _ | Downregulation of miR-21 reduced bacterial growth, systemic inflammation, organ damage, macrophage glycolysis, and increased animal survival. | (165) |
| miR-21-3p | ↑ | SD rats | TECs | ↓ AKT, ↓ CDK2, ↑ FOXO1 | – | miR-21-3p regulated lipid metabolism and increased cell cycle arrest and apoptosis. | (166) |
| miR-34 | ↑ | male C57BL/6 mice (15 control group and 15 sepsis model group) | _ | ↓ KLF4 | _ | Plasma miR-34a was positively associated with SCr and BUN. | (167) |
| miR-483-5p | ↑ | CLP-treated mice | PMVECs | ↓ PIAS1 | _ | Downregulation of miR-483-5p reduced inflammation and apoptosis and improved lung injury in mice with sepsis-induced ALI. | (168) |
| miR-181-5p | ↓ | CLP- treated mice | _ | ↑ HMGB1 | _ | Upregulation of miR-181-5p reduced inflammatory response, and sepsis-induced renal and hepatic dysfunction. | (169) |
| miR-20a | _ | SD rats | _ | _ | _ | miR-20a could deteriorated AKI via activating autophagy in sepsis rats. | (170) |
| hsa-miR-92a-3p | ↓ in sepsis-induced coagulopathy group | 116 patients with sepsis | _ | _ | _ | AUC of hsa-mir-92a-3p was 0.660. Levels of plasma hsa-mir-92a-3p were related to plasma lipocalin-2 level, activated partial thromboplastin time, and prothrombin activity. | (171) |
| miR-93-5p | ↓ | septic mouse model | HK2 | ↑ KDM6B, ↓ H3K27me3 | _ | Extracellular vesicles containing miR-93-5p reduced inflammation, apoptosis, multiple organ injury, and vascular leakage in septic mice. | (172) |
| miR-223 | ↓ | 143 patients with sepsis and 44 HCs | _ | _ | _ | Expression of miR-223 was negatively correlated with SOFA scores and positively with survival rate. Upregulation of miR-223 decreased apoptosis and increased proliferation and G1/S transition. | (173) |
| miR-34a | ↑ | male C57BL/6 mice | _ | ↓ SIRT1, ↓ ATG4B | _ | Downregulation of miR-34a reduced inflammatory response and pyroptosis, apoptosis and enhanced autophagy. | (174) |
| miR-30a | ↑ | septic rats | _ | ↓ SOCS-1 | ↑ JAK/STAT signaling pathway | Upregulation of miR-30a promoted apoptosis and inhibited proliferation. | (175) |
| miR-150-5p | ↓ | rat septic shock model | H9C2 | ↑ Akt2 | _ | Upregulation of miR-150-5p inhibited apoptosis. | (176) |
| miR-140 | ↓ | SPF male BALB/c mice | _ | _ | ↑ WNT signaling pathway | Upregulation of miR-140 inhibited apoptosis and inflammation, skeletal muscle glycolysis and atrophy. |
(177) |
| miR-22-3p | ↓ | male SD rats | HK-2 | ↑ HMGB1, ↑ PTEN | _ | Upregulation of miR-22-3p inhibited apoptosis and inflammatory response | (178) |
| miR-205-5b | ↑ | BALB/c mice | RAW264.7 | HMGB1 | _ | Down regulation of miR-205-5b increased HMGB1 expression in LPS-induced sepsis. | (179) |
| miR-526b | ↓ | BALB/c mice | HK2 | ↑ ATG7 | _ | Upregulation of miR-526b increased viability by inhibiting autophagy. | (180) |
| miR-145a | ↓ | septic mouse model | _ | ↑ Fli-1 | ↑ NF-κB signaling | Upregulation of miR-526b reduced levels of proinflammatory cytokines. | (181) |
| miR‐125a | ↑ | 150 patients with sepsis and 150 HCs | _ | _ | _ | AUC of miR‐125a: 0.749 miR‐125a was positively correlated with APACHE II score and SOFA score. |
(182) |
| miR‐125b | ↑ | 150 patients with sepsis and 150 HCs | _ | _ | _ | AUC of miR‐125b: 0.839 miR‐125b was positively correlated with APACHE II score, SOFA score CRP, TNF‐α, IL‐6, IL‐17, IL‐23, and 28‐day mortality risk. |
|
| miR-122 | ↑ | 108 patients with sepsis and 20 patients with infections without sepsis as controls | _ | _ | _ | AUC of miR-122: 0.760 miR-122 was found as independent prognostic factor for 30-day mortality. |
(183) |
| miR-135a | ↑ | _patients with sepsis and HCs, BALB/c mice | _ | _ | ↑ p38 MAPK/NF-κB pathway | Upregulation of miR-135a exacerbated inflammation and myocardial dysfunction. |
(184) |
| miR-133a | ↓ | _ | TCMK-1 | ↑ BNIP3L | ↑ NF-κB pathway | Upregulation of miR-133a reduced inflammation and apoptosis. | (185) |
| miR-223 | _ | male C57BL/6 mice | _ | _ | _ | In multiple models of experimental sepsis, miR-223 showed the complex role in the pathogenesis of septic kidney injury. | (186) |
| miR-155 | ↑ | 44 patients with severe sepsis, 102 patients with sepsis, and 19 HCs | ↑ | ↑ | ↑ | AUC of miR-155: 0.782 (for predicting 30-day mortality in ALI) | (187) |
| miR-146a | ↑ | 44 patients with severe sepsis, 102 patients with sepsis, and 19 HCs | ↑ | ↑ | ↑ | AUC of miR-146a: 0.733 (for predicting 30-day mortality in ALI), CC genotype of rs2910164 in miR-146a was correlated with worse treatment result. |
|
| miR-194 | ↑ | _ | H9c2 | ↓ Slc7a5 | ↑ Wnt/β-catenin pathway | Upregulation of miR-194 increased apoptosis. |
(188) |
| miR-30a | ↑ | male C57BL/6 mice | RAW 264.7 | ↓ ADAR1, ↓ SOCS3 | _ | Upregulation of ADAR1 (a target of miR-30a) reduced inflammation and organ damage. |
(189) |
| miR-27b | ↓ | male C57BL/6 mice | BMMSCs | ↑ JMJD3 | ↑ NF-κB signaling pathway | Upregulation of miR-27b MSC-derived exosomes reduced pro-inflammatory cytokines. | (190) |
| miR-155 | ↑ | BALB/c mice | _ | ↓ SOCS1 | ↑ JAK/STAT signaling | Downregulation of miR-155 alleviated LPS-induced mortality and liver injury | (191) |
| miR-155 | ↓ | C57BL/6 mice | _ | ↑ Arrb2 | ↑ JNK signaling pathway | Upregulation of miR-155 ameliorated late sepsis survival and its cardiac dysfunction, and reduced pro-inflammatory responses. | (192) |
| miR-155 | ↑ | _patients with sepsis and HCs, mouse septic shock model | _ | ↓ CD47 | _ | Downregulation of microRNA-155 reduced sepsis-associated cardiovascular dysfunction and mortality. | (193) |
| miR-155 | ↑ | 60 patients with sepsis and 20 HCs | _ | ↑ Foxp3 | _ | Expression of miR-155 was correlated with APACHEII score, it was significantly higher in non-survival group. | (194) |
| miR-155 | ↑ in sepsis and ALI/ARDS than sepsis but no ALI/ARDS | 156 patients with sepsis (41 with ALI and 32 with ARDS) | _ | _ | _ | AUC of miR-155: 0.87, miR-155 was positively associated with IL-1β, TNF-α levels, and ALI/ARDS score, but negatively with PaO2/FiO2. |
(195) |
| miR-29c-3p | ↑ | 86 patients with sepsis and 85 HCs, male SD rats | _ | _ | _ | AUC of miR-29c-3p: 0.872 miR-29c-3p expression was positively correlated with APACHE II score, SOFA score, levels of CRP and PCT. miR-29c-3p was found to be an independent factor in the occurrence of cardiac dysfunction. |
(196) |
| miR-125b | ↓ | 40 patients with sepsis and HCs, female and male C57BL/6 mice | _ | ↓ PTEN, ↑ MyD88 | _ | PTEN increased miR125 production through associating with the nuclear localization of Drosha-Dgcr8. Downregulation of PTEN resulted in cytokine production, MyD88 abundance and mortality. |
(197) |
| miR-203b | ↓ | 40 patients with sepsis and HCs, female and male C57BL/6 mice | _ | ↓ PTEN, ↑ MyD88 | _ | PTEN increased miR203b production through associating with the nuclear localization of Drosha-Dgcr8. Downregulation of PTEN resulted in cytokine production, MyD88 abundance and mortality. |
|
| miR-146 | ↓ | _ | EA. hy926 | _ | ↑ NF-κB signaling pathway | Upregulation of reduced levels inflammatory cytokines. | (198) |
| miR-140-5p | ↓ | male SPF rats | MLE-12 | ↑ TLR4, ↑ MyD88 | ↑ NF-κB signaling pathway | Shikonin could alleviated sepsis- induced ALI by increasing the levels of miRA-140-5p and decreasing the levels of TLR4. | (199) |
| miR-125b | ↓ | male C57BL/6 mice | HUVECs | ↑ ICAM-1, ↑ VCAM-1, ↑ TRAF6 | ↑ NF-κB signaling pathway | Upregulation of miR-125b alleviated sepsis-induced cardiac dysfunction and ameliorated survival. |
(200) |
| miR-494 | ↑ | ARDS rat models | _ | _ | ↓ Nrf2 signaling pathway | Upregulation of miR-494 increased inflammatory response, oxidative stress and ALI. | (201) |
| miR-146a | ↓ | male C57BL/6 mice | H9C2, J774 | ↑ IRAK, ↑ TRAF6 |
↑ NF-κB signaling pathway | Upregulation of miR-146 reduced levels of inflammatory cytokines and sepsis-induced cardiac dysfunction | (202) |
| miR-223 | _ | 221 patients with sepsis and 75 HCs, male C57Bl/6 mice | _ | _ | _ | Levels of serum miR-223 did not differ between critically ill patients and HCs, but ICU patients with APACHE-II score had moderately decreased circulating miR-223. | (203) |
| miR-300 | ↓ | septic mouse model | _ | ↑ NAMPT | ↓ AMPK/mTOR signaling pathway | Upregulation of miR-300 increased autophagy, cell cycle entry and reduced apoptosis and inflammatory response. | (204) |
| miR-126 | ↓ | male C57BL/6 mice | _ | ↓ HSPA12B | _ | Upregulation of HSPA12B increased levels of miR-126, upregulation of miR-126 reduced levels of dhesion molecules and improved sepsis–induced cardiac dysfunction. | (205) |
| miR-10a | ↓ | 62 patients with sepsis and 20 HCs | _ | ↑ MAP3K7 | ↑ NF-κB pathway | miR-10a expression was negatively association with disease severity scores, levels of c-reactive protein, procalcitonin, and 28-day death. | (206) |
| miR-146a | ↓ | mice | _ | ↑ Notch1 | ↑ NF-κB signaling | Upregulation of miR-146a reduced inflammatory responses of macrophages and protected mice from organ damage | (207) |
| miR-19a | ↓ | CLP mice | RAW 264.7 | ↑ Fn14 | _ | Upregulation of miR-19a reduced LPS-Induced Tubular Damage, it was found to protected mice from sepsis-induced AKI. | (208) |
| miR-214 | _ | male Kunming mice | _ | _ | _ | Upregulation of miR-214 reduced apoptosis, inflammatory response, myocardial injury, and improved cardiac function in SIMI. | (209) |
| miR-539-5p | ↓ | male C57BL/6 mice | MPVECs | ↑ ROCK1 | _ | Upregulation of miR-539-5p reduced apoptosis, inflammatory response, sepsis-induced pulmonary injury. | (210) |
| miR-155 | ↑ | 60 patients with sepsis and 30 HCs | _ | _ | _ | miR-155 was positively correlated with a higher SOFA score and a greater severity. AUC of miR-155 for 28-day survival was 0.763. miR-155 derived immunosuppression through CD39(+) Tregs. | (211) |
| miR-146a | ↑ in sepsis group compared to shame group | male BALB/C mice | _ | _ | _ | Up-regulation of miR-146a reduced levels of inflammatory cytokine TNF-α and mitigated inflammatory reaction and lung tissue injury in sepsis-induced ALI. | (212) |
| miR-7110-5p | ↑ | 52 patients with pneumonia, 44 patients with sepsis and 21 HCs | _ | _ | _ | The sensitivity and specificity of miR-7110-5p were 84.2 and 90.5% respectively. (sepsis vs HCs) | (213) |
| miR-223-3p | ↑ | 52 patients with pneumonia, 44 patients with sepsis and 21 HCs | _ | _ | _ | The sensitivity and specificity of miR-223-3p were 82.9 and 100% respectively. (sepsis vs HCs) | |
| miR-19a | ↑ | patients with sepsis | B cells from patients with sepsis | CD22 | _ | Expression of CD22 initially increased but subsequently reduced. Upregulation of miR-19a resulted in an increased BCR signaling, while overexpression of CD22 reduced the effect of miR-19a and promoted its expression. | (214) |
| miR-206 | ↑ | 63 patients with sepsis, 30 patients with septic shock and HCs | _ | _ | _ | miR-206 was positively associated with SOFA sore and APACHE-II score. It was observed an activated partial thromboplastin time and notably longer prothrombin time. | (215) |
| miR-146a | ↓ | male C57BL/6 mice | RAW264.7 | _ | ↑ NF-κB signaling | Up-regulation of miR-146a reduced apoptosis, inflammatory response, and weakened organ injury in splenic macrophages. | (216) |
| miR-19b-3p | ↓ | 103 patients with sepsis and 98 HCs | HUVECs | _ | _ | Up-regulation of miR-19b-3p reduced inflammatory response. miR-19b-3p was found to be an independent prognostic factor for 28-day survival. | (217) |
| miR-129-5p | ↓ | CLP mice | MLE-12 | ↑ HMGB1 | _ | Up-regulation of miR-129-5p reduced apoptosis, inflammatory response, , lung wet/dry weight ratio, and myeloperoxidase activity. | (218) |
| miR-23b | ↓ | 30 patients with sepsis and 30 HCs | THP-1 | ↑ ADAM10 | _ | Up-regulation of miR-23b reduced apoptosis and inflammatory response. | (219) |
| miR-150 | ↓ | 140 patients multiple trauma and 10 HCs | MDSCs | ↑ ARG1 | _ | Up-regulation of miR-150 reduced IL-6, TGF-β and IL-10. | (220) |
| miR-375 | ↓ | _ patients with sepsis, septic mice | MDSCs | ↑ miR-21 | ↑ JAK2/STAT3 pathway | Up-regulation of miR-375 reduced the number of sepsis Gr1+CD11b+ MDSCs in mice. | (221) |
| miR-31 | ↑ | male SD rats | CACO-2 | ↓ HMOX1 | ↑ NF-κB/HIF-1α pathway | Downregulation of miR-31 reduced intestinal barrier function, intestinal mucosal permeability, oxidative damage and inflammation level. | (222) |
| miR-21 and miR-181b | ↑ (in early sepsis) sustained (in late sepsis) | male BALB/c mice | MDSCs | ↑ NFI-A | _ | Down regulation of miR-21 and miR-181b decreased, immunosuppression, reprograming myeloid cells, late-sepsis mortality, and improved bacterial clearance. | (223) |
| miR-150 | ↓ slightly | 223 critically ill patients (including 138 fulfilled sepsis criteria) and 76 HCs | _ | _ | _ | serum levels of miR-150 were associated with hepatic or renal dysfunction. Low levels were correlated with an unfavorable prognosis of patients. serum levels of miR-150 were not suitable for predicting of sepsis. | (224) |
| miR-10a | ↑ | SD rats | _ | _ | ↑ TGF-β1/Smad pathway | Up-regulation of miR-10a increased ROS, TNF-α, IL-6, and MPO, and downregulation reduced sepsis-induced liver injury. | (225) |
| miR-145 | ↓ | septic mice | HUVECs | ↑ TGFBR2, ↑ SMAD2, ↑ DNMT1 | _ | Up-regulation of miR-145 reduced LPS-induced sepsis and improved the overall survival of septic mice. | (226) |
| miR-150 | ↓ | 17 patients with sepsis and 32 HCs | _ | _ | _ | Levels of miR-150 were negatively correlated with the level of disease severity, TNF-α, IL-10, and IL-18. | (227) |
| miR‐103a‐3p | ↑ | 30 patients with sepsis and 30 HCs, male C57 BL/6 mice | AML12, LO2 | ↓ FBXW7 | _ | Downregulation of miR‐103a‐3p reduced apoptosis, and inflammatory response. | (228) |
| miR-143 | ↑ | 103 patients with sepsis, 95 patients with SIRS and 16 HCs | _ | _ | _ | miR-143 was positively correlated with SOFA score and APACHE II score in patients with sepsis. For distinguishing between sepsis and SIRS, miR-143 showed a sensitivity of 78.6% and specificity of 91.6%. | (229) |
| miR-145 | ↓ | 33 patients with sepsis and 22 HCs, septic mice | BEAS-2B | ↑ TGFBR2 | _ | Up-regulation of miR-145 reduced inflammatory response and improved the overall survival of septic mice. | (230) |
| miR-150 | ↓ | C57Blk/6J mice | HPAECs | ↑ Ang2 | _ | Downregulation of miR-150 damaged adherens junctions reannealing after injury, which caused an irreversible increase in vascular permeability. Up-regulation of miR-150 reduced vascular injury and mortality. | (231) |
| miR-34b-3p | ↓ | CLP mice | RMCs | ↑ UBL4A | ↑ NF-κB signaling | Up-regulation of MiR-34b-3p reduced inflammatory response and AKI in sepsis mice | (232) |
| miR-21-3p | ↑ | _patients with sepsis, C57BL/6 mice | _ | ↓ SORBS2 | _ | Downregulation of miR-21-3p induced mitochondria ultrastructural damage and autophagy in LPS-treated mice. Levels of miR-21-3p increased in patients with cardiac dysfunction than without cardiac dysfunction. | (233) |
| miR-199a-5p | ↑ | C57BL/6 mice | HEK-293T | ↓ SP-D | ↑ NF-κB signaling | Down regulation of miR-199a-5p reduced D-lactic acid, DAO, FD-40, oxidative damage and inflammation. | (234) |
| miR-17 | ↓ | mice | BMSCs, RAW264.7 | ↑ BDR4, ↑ EZH2, ↑ TRAIL | _ | MiR-17 carried by BMSC-EVs reduced inflammation and apoptosis. | (235) |
| miR-125b | ↑ | 120 patients with sepsis and 120 HCs | _ | _ | _ | AUC of miR-125b: 0.658 MiR-125b was positively associated with APACHE II score, SOFA score, Scr, CRP, PCT, TNF-α, and IL-6 levels. miR-125b Was found to be an independent risk factor for mortality risk. |
(236) |
| miR-30e | ↓ | septic rats | _ | ↑ FOSL2 | ↑ JAK/STAT signaling | Up-regulation of miR-30e increased proliferation and reduced apoptosis. | (237) |
| miR-20b-5p | ↑ | SD rats | HEK-293T | ↓ circDMNT3B | _ | Downregulation of miR-20b-5p reduced level of d-lactic acid, FD-40, MDA, diamine oxidase, IL-10, IL-6, oxidative damage and inflammatory factors level. | (238) |
| miR-146b | ↓ | CLP mice | _ | ↑ Notch1 | _ | Up-regulation of miR-146b reduced apoptosis and inflammatory response. | (239) |
| miR-25 | ↓ | SD rats | H9C2 | ↑ PTEN, ↑ TLR4 | ↑ NF-κB signaling | Up-regulation of miR-25 reduced apoptosis and enhanced survival rate. | (240) |
| miR-21 and miR-181b |
↑ | septic mice | MDSCs, Gr1+CD11b + cells |
↑ C/EBPβ, ↑ Stat3 | _ | Stat3 and C/EBPβ increased miR-21 and miR-181b expression by binding to their promoters during sepsis. | (241) |
| miR-17-5p | ↓ | septic mice | LPS-induced macrophages | ↑ TLR4 | _ | Sch B increased miR-17-5p expression and reduced inflammation. | (242) |
| miR-200a-3p | ↑ | male C57BL/6J mice | HBMECs | ↑ NLRP3, ↓ Keap1, ↓ Nrf2, ↓ HO-1 |
_ | Up-regulation of miR-200a-3p induced inflammatory response in sepsis-induced brain injury. | (243) |
| miR-26b | ↓ | 14 patients with sepsis and 7 patients with septic shock and 21 HCs | MEG-01 | ↑ SELP, ↓ Dicer1 | _ | Low levels of miR-26b was correlated with the severity and mortality of sepsis. | (244) |
| miR-96-5p | ↓ | _ | RAW264.7 | ↑ NAMPT | ↑ NF-κB pathway | Up-regulation of miR-96-5p reduced inflammatory response. | (245) |
| miR-27a | ↑ | septic mice | _ | _ | ↑ NF-κB pathway | Downregulation of miR-27a reduced inflammatory response and promoted survival of septic mice. | (246) |
| miR-21a-3p | ↑ | specific pathogen-free SD rats | NRK52E | ↑ Ago2, ↑ Nrp-1 | _ | miR-21a-3p was found to be internalized by TECs via Nrp-1 and Ago2. | (247) |
| miR-574-5p | ↑ | 118 patients with sepsis | _ | _ | _ | miR-574-5p was associated with the death of sepsis patients. | (248) |
| miR-181b | ↓ | 26 patients with sepsis, 36 patients with sepsis plus sepsis/ARDS and 16 HCs, male C57BL/6 mice | THP-1, HUVECs | ↑ importin-α3 | ↑ NF-κB signaling pathway | Up-regulation of miR-181b reduced mortality rate, inflammation response, LPS-induced EC activation, leukocyte accumulation. | (249) |
| miR-182-5p | ↑ | pneumonia mice models | _ | _ | _ | Downregulation of miR-182-5p reduced apoptosis, inflammation response and promoted viability and proliferation. | (250) |
| miR-195 | ↑ | C57BL/6 mice | endothelial cells | ↓ BCL-2, ↓ Sirt1, ↓ Pim-1 | _ | Downregulation of miR-182-5p reduced apoptosis, and improved survival. | (251) |
| miR-205 | ↓ | male SD rats | _ | _ | ↑ HMGB1-PTEN signaling pathway | Up-regulation of miR-205 reduced apoptosis and renal injury. | (252) |
| miR-21-3p | ↑ in AKI group | 49 patients with sepsis-induced AKI and 93 sepsis patients with non-AKI | _ | ↑ Scr, ↑ Cys-C, ↑ KIM-1 |
_ | Levels of miR-21-3p was positively associated with Scr, Cys-C, and KIM-1 in the AKI group. | (253) |
| miR-181a-2-3p | ↓ | GSE46955 data set, CLP mouse model | TCMK-1 | ↑ GJB2 | _ | Up-regulation of miR-181a-2-3p reduced apoptosis and inflammatory response. | (254) |
| miR-21 | ↓ | female Wistar rats | HK-2 | ↑ PTEN, ↓ PI3K, ↓ AKT | _ | Up-regulation of miR-21 suppressed apoptosis and kidney injury. | (255) |
| miR-146a | ↓ | female ICR mice | Raw264.7 | ↑ JMJD3, NF-κB p65 | _ | GSKJ4 reduced inflammatory response by increasing miR-146a levels. Transcription of miR-146a was negatively regulated by JMJD3 through epigenetic mechanism. |
(256) |
| miR-294 | _ | _ | RAW264.7 | TREM-1 | _ | miR-294 reduced TNF-α and IL-6 secretion. | (257) |
| miR-128-3p | ↑ | CLP mouse model | TCMK-1 | ↓ NRP1 | _ | Up-regulation of miR-128-3p promoted apoptosis and inflammatory response and reduced viability. | (258) |
| miR-146a | ↓ | _ | H9C2 | ↓ ErbB4, ↑ TRAF6, ↑ IRAK1 |
_ | Up-regulation of miR-146a reduced apoptosis and inflammatory response and promoted viability. | (259) |
| miR-511 | ↑ in S mice | C57BL/6J (B) mice, SPRET/Ei (S) mice, | _ | Low protein expression of TNFR1 in S mice | _ | miR-511 was induced by glucocorticoids. miR-511 inhibited endotoxemia and experimental hepatitis. | (260) |
| miR-376b | ↓ in sepsis with AKI group | 20 Patients with sepsis with AKI, 20 patients with sepsis without AKI and 10 HCs, male C57BL/6 mice | BUMPT | NF-κB, NFKBIZ | _ | miR-376b inhibited NF-κB inhibitor ζ (NFKBIZ) expression and NF-κB inhibited miR-376b expression so they created a negative feedback loop. | (261) |
| miR-155 | ↑ | female BALB/c mice | _ | _ | _ | DXM treatment suppressed the expression of miRNA-155. |
(262) |
| miR-133a | ↑ | 223 patients with sepsis and 76 HCs, C57Bl/6 mice | _ | _ | _ | High levels of miR-133a was correlated with disease severity, inflammatory response, bacterial infection, and organ failure and predicted an unfavorable outcome of patients. | (263) |
| miR-203 | ↓ | clean grade Kunming mice | HEK-293T | ↑ VNN1 | ↓ AKT signaling pathway | Up-regulation of miR-203 reduced apoptosis, inflammatory response, MDA, ALT, and AST in lung tissues, PMN and PAM levels in BALF and increased SOD activity. | (264) |
| miR-223 | ↑ | 187 patients with sepsis and 186 HCs | _ | _ | _ | AUC for miR-223: 0.754, Plasma miR-223 was associated with disease severity and inflammatory factor levels. miR-223 was found to predict sepsis risk independently. |
(265) |
| miR-146a | ↓ | patients with sepsis and HCs | Human primary T cells | ↑ PRKCϵ | _ | Reduced levels of miR-146a contributes to the pathogenesis of sepsis. | (266) |
| miR-146-a | ↓ | 55 patients with sepsis and 60 HCs | _ | _ | _ | AUC for miR-146-a: 0.803 Serum levels of miR-146-a was negatively correlated with C-reactive protein, pro-calcitonin, IL-6 and TNF-α. |
(267) |
| miR-34a | ↑ | CLP-induced suckling rats | U937 | _ | ↑ STAT3 pathway | Up-regulation of miR-34a promoted iNOS secretion from pulmonary macrophages. | (268) |
| hsa-miR-346 | ↓ | _ | RAW264.7 | ↑ lncRNA MALAT1, ↑ SMAD3 | _ | Up-regulation of hsa-miR-346 promoted proliferation. | (269) |
| miR-214 | ↓ | male Kunming mice | _ | ↑ PTEN | ↓ AKT/mTOR pathway | Up-regulation of miR-214 reduced oxidative stress and autophagy, so ameliorated CLP-induced AKI. | (270) |
| miR-27a | ↑ | LPS induced sepsis mice model | H9C2 | ↓ rhTNFR:Fc, ↓ Nrf2 | _ | rhTNFR:Fc elevated viability and reduced apoptosis by increasing Nrf2 levels and reducing miR-27a levels. | (271) |
| miR-150 | ↓ in non-survival group | 48 patients with septic shock (23 survival patients and 25 non-survival patients) | _ | _ | _ | MiR-150 level was positively associated with cardiac index and negatively with EVLWI and PVPI. | (272) |
| miR-148a-3p | ↑ | male adult wild-type mice and myeloid-specific RBP-J-deficient mice | RAW264.7 | _ | Notch signaling and NF-κB pathway | Up-regulation of miR-148a-3p increased proinflammatory cytokines and decreased protective effect of EVs in LPS induced sepsis. | (273) |
| miR-218-5p | ↑ | male ICR mice | GMCs | ↓ HO-1 | _ | miR-218-5p was reduced in honokiol-treated septic mice, so the survival rate was increased. | (274) |
| miR-425-5p | ↓ | C57BL/6 mice | hepatocytes | ↑ RIP1 | _ | Up-regulation of miR-425-5p reduced inflammatory response and sepsis-related liver damage. | (275) |
| miR-122 | ↑ in CA group | 168 patients with sepsis (CA group and CN group) | _ | _ | _ | Serum levels of miR-122 were associated with APTT ratios, FIB and antithrombin III levels. | (275) |
| miR-101-3p | ↑ | 27 patients with SIC and 15 HCs, male SD rats | H9C2 | ↓ DUSP1 | ↑ MAPK p38 and NF-κB pathways. | Downregulation of reduced apoptosis and inflammatory response. | (276) |
| miR-124 | ↓ | mouse model of ALI | _ | ↑ MAPK14 | ↑ MAPK signaling pathway | Up-regulation of miR-124 reduced apoptosis and inflammatory response and promoted proliferation. | (277) |
| miR-942-5p | ↓ | _ | HK-2 | ↑ FOXO3 | _ | Up-regulation of miR-942-5p reduced apoptosis and inflammatory response and promoted viability. | (278) |
| miR-23a-5p | ↑ | SD rats | NR8383 | _ | _ | _ | (279) |
| miR-1298-5p | ↑ | _ | BEAS-2B | ↓ SOCS6, ↑ STAT3 |
_ | Up-regulation of miR-1298-5p induced cell permeability and inflammatory response and reduced proliferation. | (280) |
| miR-290-5p | ↓ | male C57BL/6J mice | MPC5 | ↑ CCL-2 | _ | Propofol increased levels of miR-290-5p and decreased CCL-2 and inflammatory response. | (281) |
| miR-146a | ↓ | C57BL/6 mice | BMDMs | _ | _ | Rg6 increased IL-10 and miR-146a levels so inhibited inflammatory responses. | (282) |
| miR-223 | _ | C57BL/6 mice | MSCs | Sema3A, Stat3 | _ | WT-exosomes encased high miR-223 levels induced cardio-protection in sepsis. | (283) |
| miR-608 | _ | _ | U937, HEK293T | ELANE | _ | miR-608 played an important role in posttranscriptional regulation of ELANE expression and upregulation of miR-608 reduced inflammation. | (284) |
| miR-124 | ↓ | BALB/c and C57BL/6 mice | RAW264.7 | ↓ α7nAChR, ↑ STAT3 | _ | miR-124 was found to be a critical mediator for the cholinergic anti-inflammatory effect. | (285) |
| miR-26b | ↑ in AKI group | 155 patients with sepsis (68 AKI and 87 non-AKI ) and 57 patients with non-infectious SIRS | _ | _ | _ | Urinary miR-26b levels showed an elevated mortality rate and was correlated with the severity of the disease. | (286) |
| miR-146a | _ | Rat model of SAKI | _ | _ | _ | DEX pretreatment could increase the expression level of miR-146a and reduce oxidative stress and inflammatory responses. | (287) |
| miR-29a | ↑ in AKI group | 74 patients with AKI and 41 without AKI | _ | _ | _ | AUC for miR-29a: 0.82 miR-29a was found to be an independent risk factor for mortality in the septic patients. |
(288) |
| miR-10a-5p | ↑ in AKI group | 74 patients with AKI and 41 without AKI | _ | _ | _ | AUC for miR-10a-5p: 0.75 miR-10a-5p was found to be an independent risk factor for mortality in the septic patients. |
|
| miR-155 | ↑ | septic mice | NCM460 | _ | ↑ NF-κB signaling | Up-regulation of miR-155 increased hyperpermeability to FITC-dextran, TNF-α and IL-6 levels, and decreased ZO-1 and Occludin expression. | (289) |
| miR-155 | ↑ | male C57BL/6 mice | Raw264.7, THP-1 | _ | ↑ PI3K/AKT signalling pathways | Curcumin inhibited inflammatory responses and miR-155 expression. | (290) |
| miR-497 | ↑ in myocardial injury group | 148 patients with sepsis (58 myocardial injury group and 90 non-myocardial injury group) | _ | _ | _ | Plasma miRNA-497 was correlated with cTnI in patients with myocardial injury. | (291) |
| miR-497-5p | ↑ | GEO database, male C57BL/6 mice | BEAS-2B | ↓ IL2RB | _ | Downregulation of miR-497-5p reduced apoptosis and inflammatory responses. | (292) |
| miR-30a | ↓ | _ | monocytes | ↑ STAT1, ↑ MD-2 | _ | miR-30a could inhibit STAT1-MD-2 in monocytes of sepsis. | (293) |
| miR-150 | ↓ | C57BL/6 mice | HUVECs | ↑ NF-κB1 | _ | miR-150 increased survival in patients and inhibited apoptosis and inflammatory responses. | (294) |
| miR-146a | _ | _ | THP-1 | RBM4, Ago2, p38 | _ | Up-regulation of miR-146a inhibited p38 activation and increased Ago2-RBM4 protein interaction, so reduced inflammatory responses. | (295) |
| miR-146a | _ | C57BL/6 mice | HEK293TN, J774.1 | _ | _ | Up-regulation of miR-146a reduced morphine mediated hyper-inflammation. | (296) |
| miR-27a | ↓ | septic mice | _ | ↑ TAB3 | ↑ NF-κB signaling pathway | Paclitaxel pretreatment increased miR-27a levels, so decreased inflammatory responses. | (297) |
| miR-146a | ↓ in septic patients than SIRS and HCs groups | 50 patients with sepsis, 30 patients with SIRS and 20 HCs | _ | _ | _ | AUC for miR-146a: 0.858 | (298) |
| miR-223 | ↓ in septic patients than SIRS and HCs groups | 50 patients with sepsis, 30 patients with SIRS and 20 HCs | _ | _ | _ | AUC for miR-223: 0.804 | |
| miR-339-5p | ↓ | septic mice | RAW264.7 | ↑ HMGB1, ↑ IKK-β | _ | Paeonol could reduce inflammatory responses by upregulating miR-339-5p expression. | (299) |
| miR-99b | ↑ | male C57BL/6 J mice | RAW264.7 | ↓ MFG-E8 | _ | Spherical nucleic acid increased migration by inhibiting miR-99b. | (300) |
| miR-215-5p | ↓ | _ | H9c2 | ↑ LRRFIP1, ↑ ILF3 | _ | miR-215-5p reduced inflammatory responses. | (301) |
| miR-15a | ↑ in sepsis and SIRS than HCs | 166 patients with sepsis, 32 patients with SIRS, and 24 HCs | _ | _ | _ | miR-15a could distinguish sepsis/SIRS from HCs. | (302) |
| miR-16 | ↑ in sepsis and SIRS than HCs | 166 patients with sepsis, 32 patients with SIRS, and 24 HCs | _ | _ | _ | miR-16 could distinguish sepsis/SIRS from HCs. |
miRNAs and Sepsis. AKI, Acute kidney injury; HCs, healthy controls; AUC, significant higher area under curve; CRP, C-reactive protein; TLC, total leucocytes count; SD, Sprague-Dawley; SOFA, sequential organ failure assessment; Scr, serum creatinine; WBC, white blood cell; PCT, procalcitonin; APACHE, physiology and chronic health evaluation; CLP, cecal ligation and puncture; ERS, endoplasmic reticulum stress; AUC, area under the ROC curve; SAE, sepsis-associated encephalopathy; BUN, blood urine nitrogen; rIPC, remote ischemic preconditioning; SPF, specific pathogen-free; GEO, Gene Expression Omnibus; SIMI, sepsis-induced myocardial injury; Tregs, regulatory T-cells; Sch B, Schisandrin B; DXM, dexamethasone; MDA, malondialdehyde; ALT, aminotransferase; AST, aspartate aminotransferase; PAM; pulmonary alveolar macrophages; PMN, polymorphonuclear neutrophils; BALF, bronchoalveolar lavage fluid; SOD, superoxide dismutase; CA, coagulation abnormal; CN, coagulation normal; APTT, serum activated partial thromboplastin time; FIB, fibrinogen; SIC, sepsis-induced cardiomyopathy; SIRS, systemic inflammatory response syndrome; DEX, dexmedetomidine; SAKI, sepsis-induced acute kidney injury).
Figure 3.
Down-regulated miRNAs in sepsis.
CircRNAs and sepsis
CircRNAs are a recently appreciated group of non-coding RNAs with enclosed circular configuration formed by covalent bonds between two ends of linear transcripts. However, some of these transcripts have been shown to produce proteins. They mostly exert regulatory functions in the transcriptome. Impact of circRNAs in the sepsis has been assessed by several groups (303). For instance, circC3P1 has been shown to attenuate production of inflammatory cytokines and decrease cell apoptosis in sepsis-associated acute lung injury via influencing expression of miR‐21 (304).
A microarray-based has shown differential expression of 132 circRNAs between sepsis patients and healthy controls among them have been hsa_circRNA_104484 and hsa_circRNA_104670 whose up-regulation in sepsis serum exosomes has been verified been RT-PCR. Expression levels of these two circRNAs have been suggested as diagnostic biomarkers for sepsis (305).
CircVMA21 is another circRNA that has been shown to ameliorate sepsis‐related acute kidney injury through modulation of oxidative stress and inflammatory responses via miR‐9‐3p/SMG1 axis (306). Circ_0114428/miR-495-3p/CRBN axis is another molecular axis which is involved in the pathoetiology of sepsis‐related acute kidney injury (307). Moreover, expression levels of circPRKCI have been correlated with sepsis risk, severity of sepsis and mortality during a period of 28 days (308). Table 3 summarizes the role of circRNAs in sepsis.
Table 3.
CircRNAs and Sepsis.
| circRNA | Pattern of Expression | Clinical Samples/Animal Model | Assessed Cell Lines | Targets / Regulators | Signaling Pathways | Description | Reference |
|---|---|---|---|---|---|---|---|
| circC3P1 | ↓ | male C57BL/6 mice | MPVECs | ↑ miR-21 | _ | Upregulation of circC3P1 reduced pulmonary injury, inflammatory responses and apoptosis. | (304) |
| hsa_circRNA_104484 | ↑ | 25 patients with sepsis and 22 HCs | _ | _ | _ | Hsa_circRNA_104484 showed the potential to be used as diagnostic marker for sepsis. | (305) |
| hsa_circRNA_104670 | ↑ | 25 patients with sepsis and 22 HCs | _ | _ | _ | Hsa_circRNA_104670 showed the potential to be used as diagnostic marker for sepsis. | |
| circVMA21 | ↓ | CLP rats | HK-2, WI-38 | ↑ miR-9-39, ↓ SMG1 | – | CircVMA21 reduced apoptosis, inflammatory responses and oxidative stress. | (306) |
| circ-PRKCI | ↓ | 121 patients with sepsis and 60 HCs | _ | ↑ miR-545 | _ | Low levels of circ-PRKCI were correlated with sepsis risk, clinical disease severity and 28-day mortality risk. | (308) |
| circDNMT3B | ↓ | male SD rats | Caco2 | ↑ miR-20b-5p, ↓ SOD | _ | Downregulation of circDNMT3B decreased cell survival and increased apoptosis, inflammatory responses and oxidative damage. | (238) |
| circ_0114428 | ↑ | _ | HK2 | ↓ miR-495-3p, ↑ CRBN | _ | Downregulation of circ_0114428 decreased apoptosis, inflammatory responses, oxidative stress, and ER stress. | (307) |
| circ_0001105 | ↓ | septic rats | _ | ↑ YAP1 | _ | Up-regulation of circ_0001105 decreased apoptosis, inflammatory responses and oxidative damage . | (309) |
| circ_Ttc3 | ↓ | CLP rats | _ | ↑ miR-148a, ↓ Rcan2 | _ | Up-regulation of circ_Ttc3 decreased inflammatory responses and oxidative stress in AKI rats. | (310) |
| circPRKCI | ↓ | patients with sepsis and HCs | HK2 | ↑ miR-545, ↓ ZEB2 | NF-kB pathway | Up-regulation of circPRKCI reduced LPS-induced cell injury and inflammatory responses. | (311) |
| circ_0003420 | ↑ | _patients with sepsis and HCs | Kupffer cells | ↓ NPAS4 | _ | Up-regulation of circ_0003420 increased apoptosis, inflammatory responses and decreased proliferation. | (312) |
| circ-Fryl | ↑ in ADSC exosomes | septic mouse model | ADSCs, LPS-induced AEC damage model | miR-490-3p, ↑ SIRT3 in ADSC exosomes | SIRT3/AMPK signaling | Up-regulation of circ-Fryl increased autophagy and decreased apoptosis and inflammatory responses. | (313) |
| circ_0091702 | ↓ | _ | HK2 | ↑ miR-182, ↓ PDE7A | _ | Up-regulation of circ_0091702 reduced LPS-induced cell injury. | (314) |
| circVMA21 | ↓ | _ | THP-1 | ↑ miR-199a-5p, ↓ NRP1 | _ | Up-regulation of circVMA21 reduced apoptosis, inflammatory responses and oxidative stress. | (315) |
| circTLK1 | ↑ | wistar rats | HK-2, 293T | ↓ miR-106a-5p, ↑ HMGB1 | _ | Downregulation of circTLK1 reduced apoptosis, inflammatory responses and oxidative stress. | (316) |
| circFADS2 | ↑ | 50 patients with sepsis and 50 HCs | HBEpCs | ↓ mature miR-15a-5p | _ | Up-regulation of circFADS2 reduced miR-15a-5p overexpression-induced apoptosis. | (317) |
| circ_0091702 | ↓ | _ | HK2 | ↑ miR-545-3p, ↓ THBS2. | _ | Up-regulation of circ_0091702 reduced LPS-induced HK2 cell injury. | (318) |
| hsa_circ_0068,888 | ↓ | _ | HK-2 | ↑ miR-21-5p | _ | Up-regulation of hsa_circ_0068,888 reduced inflammatory response and oxidative stress and increased viability. | (319) |
| circPTK2 | ↑ | C57BL/6 mice | BV2 microglia | ↓ miR-181c-5p, ↑ HMGB1 | _ | Downregulation of circPTK2 reduced apoptosis, inflammatory responses. | (320) |
| circ-FANCA | ↑ | 19 patients with sepsis and 19 HCs | HK2 | ↓ miR-93-5p, ↑ OXSR1 | _ | Downregulation of circ-FANCA reduced apoptosis, inflammatory responses and oxidative stress and increased proliferation. | (321) |
| circANKRD36 | ↑ | 60 patients with sepsis-induced ARDS | RAW264.7 | ↓ miR-330, ↑ ROCK1 | _ | Downregulation of circANKRD36 reduced viability and migration and alleviated inflammatory responses. | (322) |
| circPRKCI | ↓ | _ | HK2 | ↑ miR-106b-5p, ↓ GAB1 | _ | Up-regulation of circPRKCI reduced apoptosis, inflammatory responses and oxidative stress and increased viability. | (323) |
HCs, healthy controls; AKI, acute kidney injury; ARDS, acute respiratory distress syndrome.
Discussion
A vast body of literature points to the involvement of lncRNAs, miRNAs and circRNAs in the pathoetiology of sepsis-related complications. NEAT1, MALAT1, MEG3, THRIL, XIST, CRNDE, ZFAS1, HULC, MIAT and TUG1 are among lncRNAs with the strongest evidence for their participation in this process. NEAT1 as the mostly assessed lncRNA in this regard has been shown to act as a molecular sponge for let-7a, let-7b-5p, miR-370-3p, miR-124, miR-125, miR-17-5p, miR-16-5p, miR-93-5p, miR-370-3p, miR-144-3p, miR-944, miR495-3p, miR-22-3p, miR-31-5p and miR-590-3p. Through sequestering these miRNAs, NEAT1 can affect several molecular pathways in the course of sepsis. It can enhance immune responses and the related injury in target organs, thus participating in sepsis-related multiple organ damage.
Similar to lncRNAs, circRNAs influence course of sepsis mainly through acting as molecular sponges for miRNAs. circC3P1/miR-21, circVMA21/miR-9, circVMA21/miR-199a-5p, circ-PRKCI/miR-545, circPRKCI/miR-106b-5p, circDNMT3B/miR-20b-5p, circ_0114428/miR-495-3p, circ_Ttc3/miR-148a, circPRKCI/miR-454, circ-Fryl/miR-490-3p, circ_0091702/miR-182, circTLK1/miR-106a-5p, circFADS2/miR-15a-5p, circ_0091702/miR-545-3p, hsa_circ_0068,888/miR-21-5p, circPTK2/miR-181c-5p, circ-FANCA/miR-93-5p and circANKRD36/miR-330 are among circRNA/miRNA axes which are involved in the pathophysiology of sepsis-related conditions.
NF‐κB, PI3K/AKT, JAK/STAT and Wnt/β‐catenin pathways are the most important pathways being regulated by lncRNAs, circRNAs and miRNAs in the context of sepsis. These transcripts, particularly miRNAs can be used as diagnostic or prognostic markers in sepsis. Expression levels of these regulatory transcripts might be used for diagnosis of organ specific damages during the course of sepsis.
In general, the pathophysiology of sepsis is considered as an initial hyperinflammatory phase (“cytokine storm”) followed by a protracted immunosuppressive phase. Since no data is available about the differential expression of non-coding RNAs during these two distinct phases, future studies are needed to evaluate expression patterns of non-coding RNAs in these two phases. It is possible that some of the non-coding RNAs that suppress the immune response could be used as biomarkers to indicate the immunoparalysis in sepsis.
From a therapeutic point of view, several in vitro and in vivo studies have shown that up-regulation/silencing of circRNAs, lncRNAs and miRNAs can ameliorate the pathologic events in the target organs, particularly heart and kidney during sepsis. Yet, this field is still in its infancy needing verification in additional animal models and cell lines. Moreover, since sepsis is an emergency situation, any therapeutic option should be verified in terms of bioavailability, efficiency and instant amelioration of pathological events.
Since the pathoetiology of sepsis-related complications is not completely understood, high throughput sequencing strategies focusing on different classes of non-coding as well coding RNAs are necessary to find the complicated networks between these transcripts in the context of sepsis.
Author Contributions
SG-F wrote the draft and revised it. MT designed and supervised the study. NA, BH, and TK collected the data and designed the figures and tables. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Zarjou A, Agarwal A. Sepsis and Acute Kidney Injury. J Am Soc Nephrol (2011) 22(6):999–1006. doi: 10.1681/ASN.2010050484 [DOI] [PubMed] [Google Scholar]
- 2. Rossaint J, Zarbock A. Pathogenesis of Multiple Organ Failure in Sepsis. Crit Rev Immunol (2015) 35(4):277–91. doi: 10.1615/CritRevImmunol.2015015461 [DOI] [PubMed] [Google Scholar]
- 3. Li C, Wu J, Li Y, Xing G. Cytoprotective Effect of Heat Shock Protein 27 Against Lipopolysaccharide-Induced Apoptosis of Renal Epithelial HK-2 Cells. Cell Physiol Biochem (2017) 41(6):2211–20. doi: 10.1159/000475636 [DOI] [PubMed] [Google Scholar]
- 4. Ye H-Y, Jin J, Jin L-W, Chen Y, Zhou Z-H, Li Z-Y. Chlorogenic Acid Attenuates Lipopolysaccharide-Induced Acute Kidney Injury by Inhibiting TLR4/NF-κb Signal Pathway. Inflammation (2017) 40(2):523–9. doi: 10.1007/s10753-016-0498-9 [DOI] [PubMed] [Google Scholar]
- 5. Zhang X, Wang W, Zhu W, Dong J, Cheng Y, Yin Z, et al. Mechanisms and Functions of Long Non-Coding Rnas at Multiple Regulatory Levels. Int J Mol Sci (2019) 20(22):5573. doi: 10.3390/ijms20225573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flores-Concha M, Oñate N. Long Non-Coding Rnas in the Regulation of the Immune Response and Trained Immunity. Front Genet (2020) 11:718. doi: 10.3389/fgene.2020.00718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qiu N, Xu X, He Y. Lncrna TUG1 Alleviates Sepsis-Induced Acute Lung Injury by Targeting Mir-34b-5p/GAB1. BMC Pulmonary Med (2020) 20(1):1–12. doi: 10.1186/s12890-020-1084-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li N, Wu S, Yu L. The Associations of Long Non-Coding RNA Taurine Upregulated Gene 1 and Microrna-223 With General Disease Severity and Mortality Risk in Sepsis Patients. Med (2020) 99(50). doi: 10.1097/MD.0000000000023444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ge X, Liu W, Zhao W, Feng S, Duan A, Ji C, et al. Exosomal Transfer of LCP1 Promotes Osteosarcoma Cell Tumorigenesis and Metastasis by Activating the JAK2/STAT3 Signaling Pathway. Mol Ther Nucleic Acids (2020) 21:900–15. doi: 10.1016/j.omtn.2020.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou Y-L, Yang S-H, Zhang C, Zhang B, Yang X-J. Lncrna MALAT1 Modulates the Immunoreaction of Rats With Lipopolysaccharide-Induced Sepsis by Targeting the Mir-146a/NF-κb P65 Pathway. Sichuan da xue xue bao Yi xue ban= J Sichuan Univ Med Sci Ed (2018) 49(6):865–70. [PubMed] [Google Scholar]
- 11. Li Z, Liu S, Li X, Zhao W, Li J, Xu Y. Circular RNA in Schizophrenia and Depression. Front Psychiatry (2020) 11. doi: 10.3389/fpsyt.2020.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wei S, Liu Q. Long Noncoding RNA MALAT1 Modulates Sepsis-Induced Cardiac Inflammation Through the Mir-150-5p/NF-κb Axis. Int J Clin Exp Pathol (2019) 12(9):3311. [PMC free article] [PubMed] [Google Scholar]
- 13. Yu Z, Rayile A, Zhang X, Li Y, Zhao Q. Ulinastatin Protects Against Lipopolysaccharide-Induced Cardiac Microvascular Endothelial Cell Dysfunction via Downregulation of Lncrna MALAT1 and EZH2 in Sepsis. Int J Mol Med (2017) 39(5):1269–76. doi: 10.3892/ijmm.2017.2920 [DOI] [PubMed] [Google Scholar]
- 14. Liu W, Geng F, Yu L. Long Non-Coding RNA MALAT1/Microrna 125a Axis Presents Excellent Value in Discriminating Sepsis Patients and Exhibits Positive Association With General Disease Severity, Organ Injury, Inflammation Level, and Mortality in Sepsis Patients. J Clin Lab Anal (2020) 34(6):e23222. doi: 10.1002/jcla.23222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang C-C, Niu F. Lncrna NEAT1 Promotes Inflammatory Response in Sepsis-Induced Liver Injury via the Let-7a/TLR4 Axis. Int Immunopharmacol (2019) 75:105731. doi: 10.1016/j.intimp.2019.105731 [DOI] [PubMed] [Google Scholar]
- 16. Wu X, Fang Y, Zheng F, Zhang Y, Li Q. Lncrna NEAT1 Facilitates the Progression of Sepsis Through Up-Regulating TSP-1 via Sponging Mir-370-3p. Eur Rev Med Pharmacol Sci (2020) 24(1):333–44. doi: 10.26355/eurrev_202001_19931 [DOI] [PubMed] [Google Scholar]
- 17. Li Y, Guo W, Cai Y. NEAT1 Promotes LPS-Induced Inflammatory Injury in Macrophages by Regulating Mir-17-5p/TLR4. Open Med (2020) 15(1):38–49. doi: 10.1515/med-2020-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen JX, Xu X, Zhang S. Silence of Long Noncoding RNA NEAT1 Exerts Suppressive Effects on Immunity During Sepsis by Promoting Microrna-125-Dependent MCEMP1 Downregulation. IUBMB Life (2019) 71(7):956–68. doi: 10.1002/iub.2033 [DOI] [PubMed] [Google Scholar]
- 19. Dong Y, Fan G, Li Y, Zhou Q. TUG1 Represses Apoptosis, Autophagy, and Inflammatory Response by Regulating Mir-27a-3p/SLIT2 in LPS-Treated Vascular Endothelial Cells. J Surg Res (2020) 256:345–54. doi: 10.1016/j.jss.2020.05.102 [DOI] [PubMed] [Google Scholar]
- 20. Xie W, Chen L, Chen L, Kou Q. Silencing of Long Non-Coding RNA MALAT1 Suppresses Inflammation in Septic Mice: Role of Microrna-23a in the Down-Regulation of MCEMP1 Expression. Inflamm Res (2020) 69(2):179–90. doi: 10.1007/s00011-019-01306-z [DOI] [PubMed] [Google Scholar]
- 21. Lin L, Niu G, Zhang X. Influence of Lncrna MALAT1 on Septic Lung Injury in Mice Through P38 MAPK/P65 NF-κb Pathway. Eur Rev Med Pharmacol Sci (2019) 23(3):1296–304. doi: 10.26355/eurrev_201902_17025 [DOI] [PubMed] [Google Scholar]
- 22. Liang WJ, Zeng XY, Jiang SL, Tan HY, Yan MY, Yang HZ. Long Non-Coding RNA MALAT1 Sponges Mir-149 to Promote Inflammatory Responses of LPS-Induced Acute Lung Injury by Targeting Myd88. Cell Biol Int (2020) 44(1):317–26. doi: 10.1002/cbin.11235 [DOI] [PubMed] [Google Scholar]
- 23. Liu L, Yan L-N, Sui Z. Microrna-150 Affects Endoplasmic Reticulum Stress via MALAT1-Mir-150 Axis-Mediated NF-κb Pathway in LPS-Challenged Huvecs and Septic Mice. Life Sci (2021) 265:118744. doi: 10.1016/j.lfs.2020.118744 [DOI] [PubMed] [Google Scholar]
- 24. Huang X, Zhao M. High Expression of Long Non-Coding RNA MALAT1 Correlates With Raised Acute Respiratory Distress Syndrome Risk, Disease Severity, and Increased Mortality in Sepstic Patients. Int J Clin Exp Pathol (2019) 12(5):1877. [PMC free article] [PubMed] [Google Scholar]
- 25. Zhuang Y, Xu D, Wang G, Sun J, Huang Y, Wang S. IL-6 Induced Lncrna MALAT1 Enhances TNF-α Expression in LPS-Induced Septic Cardiomyocytes via Activation of SAA3. Eur Rev Med Pharmacol Sci (2017) 21(2):302–9. [PubMed] [Google Scholar]
- 26. Geng F, Liu W, Yu L. Potential Role of Circulating Long Noncoding RNA MALAT1 in Predicting Disease Risk, Severity, and Patients' Survival in Sepsis. J Clin Lab Anal (2019) 33(8):e22968. doi: 10.1002/jcla.22968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen J, He Y, Zhou L, Deng Y, Si L. Long Non−Coding RNA MALAT1 Serves as an Independent Predictive Biomarker for the Diagnosis, Severity and Prognosis of Patients With Sepsis. Mol Med Rep (2020) 21(3):1365–73. doi: 10.3892/mmr.2020.10923 [DOI] [PubMed] [Google Scholar]
- 28. Gao F, Chen R, Xi Y, Zhao Q, Gao H. Long Noncoding RNA MALAT1 Regulates Sepsis in Patients With Burns by Modulating Mir−214 With TLR5. Mol Med Rep (2019) 19(5):3756–66. doi: 10.3892/mmr.2019.10028 [DOI] [PubMed] [Google Scholar]
- 29. Sun F, Yuan W, Wu H, Chen G, Sun Y, Yuan L, et al. Lncrna KCNQ1OT1 Attenuates Sepsis-Induced Myocardial Injury via Regulating Mir-192-5p/XIAP Axis. Exp Biol Med (2020) 245(7):620–30. doi: 10.1177/1535370220908041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen T, Zhu C, Ye C. Lncrna CYTOR Attenuates Sepsis-Induced Myocardial Injury via Regulating Mir-24/XIAP. Cell Biochem Funct (2020) 38(7):976–85. doi: 10.1002/cbf.3524 [DOI] [PubMed] [Google Scholar]
- 31. Liu F, Hu S, Zhao N, Shao Q, Li Y, Jiang R, et al. Lncrna-5657 Silencing Alleviates Sepsis-Induced Lung Injury by Suppressing the Expression of Spinster Homology Protein 2. Int Immunopharmacol (2020) 88:106875. doi: 10.1016/j.intimp.2020.106875 [DOI] [PubMed] [Google Scholar]
- 32. Han Y, Cai Y, Lai X, Wang Z, Wei S, Tan K, et al. Lncrna RMRP Prevents Mitochondrial Dysfunction and Cardiomyocyte Apoptosis via the Mir-1-5p/Hsp70 Axis in LPS-Induced Sepsis Mice. Inflammation (2020) 43(2):605–18. doi: 10.1007/s10753-019-01141-8 [DOI] [PubMed] [Google Scholar]
- 33. Yin J, Han B, Shen Y. Lncrna NEAT1 Inhibition Upregulates Mir-16-5p to Restrain the Progression of Sepsis-Induced Lung Injury via Suppressing BRD4 in a Mouse Model. Int Immunopharmacol (2021) 97:107691. doi: 10.1016/j.intimp.2021.107691 [DOI] [PubMed] [Google Scholar]
- 34. Wang S, Liu G, Xian H, Si J, Qi S, Yu Y. Lncrna NEAT1 Alleviates Sepsis-Induced Myocardial Injury by Regulating the TLR2/NF-κb Signaling Pathway. Eur Rev Med Pharmacol Sci (2019) 23(11):4898–907. doi: 10.26355/eurrev_201906_18078 [DOI] [PubMed] [Google Scholar]
- 35. Zhou H, Wang X, Zhang B. Depression of Lncrna NEAT1 Antagonizes LPS-Evoked Acute Injury and Inflammatory Response in Alveolar Epithelial Cells via HMGB1-RAGE Signaling. Mediators Inflamm (2020) 2020. doi: 10.1155/2020/8019467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gao C, Zou X, Chen H, Shang R, Wang B. Long Non-Coding RNA Nuclear Paraspeckle Assembly Transcript 1 (NEAT1) Relieves Sepsis-Induced Kidney Injury and Lipopolysaccharide (LPS)-Induced Inflammation in HK-2 Cells. Med Sci Monit: Int Med J Exp Clin Res (2020) 26:e921906–1. doi: 10.12659/MSM.921906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang W, Guo Z-H. Downregulation of Lncrna NEAT1 Ameliorates LPS-Induced Inflammatory Responses by Promoting Macrophage M2 Polarization via Mir-125a-5p/TRAF6/TAK1 Axis. Inflammation (2020) 43(4):1548–60. doi: 10.1007/s10753-020-01231-y [DOI] [PubMed] [Google Scholar]
- 38. Yang J, Wu L, Liu S, Hu X, Wang Q, Fang L. Long Non-Coding RNA NEAT1 Promotes Lipopolysaccharide-Induced Injury in Human Tubule Epithelial Cells by Regulating Mir-93-5p/TXNIP Axis. Med Microbiol Immunol (2021) 210(2):121–32. doi: 10.1007/s00430-021-00705-6 [DOI] [PubMed] [Google Scholar]
- 39. Xiao T, Sun C, Xiao Y, Li Y. Lncrna NEAT1 Mediates Sepsis Progression by Regulating Irak2 via Sponging Mir-370-3p. Biol Open (2020) 9(6):bio049353. doi: 10.1242/bio.049353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wei J, Wu C, Chen J, Shang F, Guo S, Zhang X, et al. Lncrna NEAT1 Promotes the Progression of Sepsis-Induced Myocardial Cell Injury by Sponging Mir-144-3p. Eur Rev Med Pharmacol Sci (2020) 24(2):851–61. doi: 10.26355/eurrev_202001_20069 [DOI] [PubMed] [Google Scholar]
- 41. Huang Q, Huang C, Luo Y, He F, Zhang R. Circulating Lncrna NEAT1 Correlates With Increased Risk, Elevated Severity and Unfavorable Prognosis in Sepsis Patients. Am J Emergency Med (2018) 36(9):1659–63. doi: 10.1016/j.ajem.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 42. Chen C, Zhang H, Ge M, Ye J, Li R, Wang D. Lncrna NEAT1 Acts as a Key Regulator of Cell Apoptosis and Inflammatory Response by the Mir-944/TRIM37 Axis in Acute Lung Injury. J Pharmacol Sci (2021) 145(2):202–12. doi: 10.1016/j.jphs.2020.11.009 [DOI] [PubMed] [Google Scholar]
- 43. Huang S, Qian K, Zhu Y, Huang Z, Luo Q, Qing C. Diagnostic Value of the Lncrna NEAT1 in Peripheral Blood Mononuclear Cells of Patients With Sepsis. Dis Markers (2017) 2017. doi: 10.1155/2017/7962836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu B, Ren H, Chen J. Lncrna NEAT1 Correlates With Th1 and Th17 and Could Serve as an Assistant Biomarker in Sepsis. Biomarkers Med (2021) 1177–86. doi: 10.2217/bmm-2020-0906 [DOI] [PubMed] [Google Scholar]
- 45. Xia D, Yao R, Zhou P, Wang C, Xia Y, Xu S. Lncrna NEAT1 Reversed the Hindering Effects of Mir-495-3p/STAT3 Axis and Mir-211/PI3K/AKT Axis on Sepsis-Relevant Inflammation. Mol Immunol (2020) 117:168–79. doi: 10.1016/j.molimm.2019.10.009 [DOI] [PubMed] [Google Scholar]
- 46. Yang Y, Yang L, Liu Z, Wang Y, Yang J. Long Noncoding RNA NEAT 1 and its Target Microrna-125a in Sepsis: Correlation With Acute Respiratory Distress Syndrome Risk, Biochemical Indexes, Disease Severity, and 28-Day Mortality. J Clin Lab Anal (2020) 34(12):e23509. doi: 10.1002/jcla.23509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu W, Wang Y, Zheng Y, Chen X. Effects of Long Non-Coding RNA NEAT1 on Sepsis-Induced Brain Injury in Mice via NF-κb. Eur Rev Med Pharmacol Sci (2019) 23(9):3933–9. doi: 10.26355/eurrev_201905_17822 [DOI] [PubMed] [Google Scholar]
- 48. He F, Zhang C, Huang Q. Long Noncoding RNA Nuclear Enriched Abundant Transcript 1/Mirna-124 Axis Correlates With Increased Disease Risk, Elevated Inflammation, Deteriorative Disease Condition, and Predicts Decreased Survival of Sepsis. Med (2019) 98(32). doi: 10.1097/MD.0000000000016470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Feng Y, Liu J, Wu R, Yang P, Ye Z, Song F. NEAT1 Aggravates Sepsis-Induced Acute Kidney Injury by Sponging Mir-22-3p. Open Med (2020) 15(1):333–42. doi: 10.1515/med-2020-0401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang Y, Xue J, Qin L, Zhang J, Liu J, Yu J. Lncrna NEAT1 Promotes Inflammatory Response in Sepsis via the Mir-31-5p/POU2F1 Axis. Inflammation (2021) 44(4):1–11. doi: 10.1007/s10753-021-01436-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu L, Liu F, Sun Z, Peng Z, You T, Yu Z. Lncrna NEAT1 Promotes Apoptosis and Inflammation in LPS−Induced Sepsis Models by Targeting Mir−590−3p. Exp Ther Med (2020) 20(4):3290–300. doi: 10.3892/etm.2020.9079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fang Y, Hu J, Wang Z, Zong H, Zhang L, Zhang R, et al. Lncrna H19 Functions as an Aquaporin 1 Competitive Endogenous RNA to Regulate Microrna-874 Expression in LPS Sepsis. Biomed Pharmacother (2018) 105:1183–91. doi: 10.1016/j.biopha.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 53. Shan B, Li J-Y, Liu Y-J, Tang X-B, Zhou Z, Luo L-X. Lncrna H19 Inhibits the Progression of Sepsis-Induced Myocardial Injury via Regulation of the Mir-93-5p/SORBS2 Axis. Inflammation (2021) 44(1):344–57. doi: 10.1007/s10753-020-01340-8 [DOI] [PubMed] [Google Scholar]
- 54. Yu B, Cui R, Lan Y, Zhang J, Liu B. Long Non-Coding RNA H19 as a Diagnostic Marker in Peripheral Blood of Patients With Sepsis. Am J Trans Res (2021) 13(4):2923. [PMC free article] [PubMed] [Google Scholar]
- 55. Wang H-R, Guo X-Y, Liu X-Y, Song X. Down-Regulation of Lncrna CASC9 Aggravates Sepsis-Induced Acute Lung Injury by Regulating Mir-195-5p/PDK4 Axis. Inflammation Res (2020) 69(6):559–68. doi: 10.1007/s00011-020-01316-2 [DOI] [PubMed] [Google Scholar]
- 56. Zhang Z, Lv M, Wang X, Zhao Z, Jiang D, Wang L. Lncrna LUADT1 Sponges Mir-195 to Prevent Cardiac Endothelial Cell Apoptosis in Sepsis. Mol Med (2020) 26(1):1–8. doi: 10.1186/s10020-020-00228-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang Y, Zhang Y, Xia F, Yang A, Qian J, Zhao H, et al. Effect of Lncrna-MIAT on Kidney Injury in Sepsis Rats via Regulating Mir-29a Expression. Eur Rev Med Pharmacol Sci (2019) 23:10942–9. doi: 10.26355/eurrev_201912_19797 [DOI] [PubMed] [Google Scholar]
- 58. Xing P-C, An P, Hu G-Y, Wang D-L, Zhou M-J. Lncrna MIAT Promotes Inflammation and Oxidative Stress in Sepsis-Induced Cardiac Injury by Targeting Mir-330-5p/TRAF6/NF-κb Axis. Biochem Genet (2020) 58(5):783–800. doi: 10.1007/s10528-020-09976-9 [DOI] [PubMed] [Google Scholar]
- 59. Liu T, Liu J, Tian C, Wang H, Wen M, Yan M. Lncrna THRIL Is Upregulated in Sepsis and Sponges Mir-19a to Upregulate TNF-α in Human Bronchial Epithelial Cells. J Inflammation (2020) 17(1):1–7. doi: 10.1186/s12950-020-00259-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen H, Hu X, Li R, Liu B, Zheng X, Fang Z, et al. Lncrna THRIL Aggravates Sepsis-Induced Acute Lung Injury by Regulating Mir-424/ROCK2 Axis. Mol Immunol (2020) 126:111–9. doi: 10.1016/j.molimm.2020.07.021 [DOI] [PubMed] [Google Scholar]
- 61. Wang Y, Fu X, Yu B, Ai F. Long Non-Coding RNA THRIL Predicts Increased Acute Respiratory Distress Syndrome Risk and Positively Correlates With Disease Severity, Inflammation, and Mortality in Sepsis Patients. J Clin Lab Anal (2019) 33(6):e22882. doi: 10.1002/jcla.22882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Song X, Li L, Zhao Y, Song Y. Down-Regulation of Long Non-Coding RNA XIST Aggravates Sepsis-Induced Lung Injury by Regulating Mir-16-5p. Hum Cell (2021) 34(5):1–11. doi: 10.1007/s13577-021-00542-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang L, Cao QM. Long Non-Coding RNA XIST Alleviates Sepsis-Induced Acute Kidney Injury Through Inhibiting Inflammation and Cell Apoptosis via Regulating Mir-155-5p/WWC1 Axis. Kaohsiung J Med Sci (2021). doi: 10.1002/kjm2.12442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shen C, Li J. Lncrna XIST Silencing Protects Against Sepsis-Induced Acute Liver Injury via Inhibition of BRD4 Expression. Inflammation (2021) 44(1):194–205. doi: 10.1007/s10753-020-01321-x [DOI] [PubMed] [Google Scholar]
- 65. Xu G, Mo L, Wu C, Shen X, Dong H, Yu L, et al. The Mir-15a-5p-XIST-CUL3 Regulatory Axis Is Important for Sepsis-Induced Acute Kidney Injury. Ren Fail (2019) 41(1):955–66. doi: 10.1080/0886022X.2019.1669460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liang D, Jin Y, Lin M, Xia X, Chen X, Huang A. Down-Regulation of Xist and Mir-7a-5p Improves LPS-Induced Myocardial Injury. Int J Med Sci (2020) 17(16):2570. doi: 10.7150/ijms.45408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li M, Zhang Z, Liu B, Chen L, Wang M. Lncrna GAS5 Upregulates Mir-214 Through Methylation to Participate in Cell Apoptosis of Sepsis. Arch Physiol Biochem (2020) 1–6. doi: 10.1080/13813455.2020.1764051 [DOI] [PubMed] [Google Scholar]
- 68. Zhu Y, Sun A, Meng T, Li H. Protective Role of Long Noncoding RNA CRNDE in Myocardial Tissues From Injury Caused by Sepsis Through the Microrna-29a/SIRT1 Axis. Life Sci (2020) 255:117849. doi: 10.1016/j.lfs.2020.117849 [DOI] [PubMed] [Google Scholar]
- 69. Wang Y, Xu Z, Yue D, Zeng Z, Yuan W, Xu K. Linkage of Lncrna CRNDE Sponging Mir-181a-5p With Aggravated Inflammation Underlying Sepsis. Innate Immun (2020) 26(2):152–61. doi: 10.1177/1753425919880946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sun B, Sui Y, Huang H, Zou X, Chen S, Yu Z. Effect of Lncrna CRNDE on Sepsis-Related Kidney Injury Through the TLR3/NF-κb Pathway. Eur Rev Med Pharmacol Sci (2019) 23(23):10489–97. doi: 10.26355/eurrev_201912_19688 [DOI] [PubMed] [Google Scholar]
- 71. Wu S, Qiu H, Wang Q, Cao Z, Wang J. Effects and Mechanism of Lncrna CRNDE on Sepsis-Induced Acute Kidney Injury. Anal Cell Pathol (2020) 2020. doi: 10.1155/2020/8576234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang J, Song J, Li Y, Shao J, Xie Z, Sun K. Down-Regulation of Lncrna CRNDE Aggravates Kidney Injury via Increasing Mir-181a-5p in Sepsis. Int Immunopharmacol (2020) 79:105933. doi: 10.1016/j.intimp.2019.105933 [DOI] [PubMed] [Google Scholar]
- 73. Li Y, Song J, Xie Z, Liu M, Sun K. Long Noncoding RNA Colorectal Neoplasia Differentially Expressed Alleviates Sepsis-Induced Liver Injury via Regulating Mir-126-5p. IUBMB Life (2020) 72(3):440–51. doi: 10.1002/iub.2230 [DOI] [PubMed] [Google Scholar]
- 74. Jiang Z, Zhang M, Fan Z, Sun W, Tang Y. Influence of Lncrna HOTAIR on Acute Kidney Injury in Sepsis Rats Through Regulating Mir-34a/Bcl-2 Pathway. Eur Rev Med Pharmacol Sci (2019) 23(8):3512–9. doi: 10.26355/eurrev_201904_17717 [DOI] [PubMed] [Google Scholar]
- 75. Yang W, Luo X, Liu Y, Xiong J, Xia H, Liu Y. Potential Role of Lncrna HULC/Mir−128−3p/RAC1 Axis in the Inflammatory Response During LPS−Induced Sepsis in HMEC−1 Cells. Mol Med Rep (2020) 22(6):5095–104. doi: 10.3892/mmr.2020.11601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wang H, Feng Q, Wu Y, Feng L, Yuan H, Hou L, et al. Association of Circulating Long Non-Coding RNA HULC Expression With Disease Risk, Inflammatory Cytokines, Biochemical Index Levels, Severity-Assessed Scores, and Mortality of Sepsis. J Clin Lab Anal (2021) 35(3):e23656. doi: 10.1002/jcla.23656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen X, Song D. LPS Promotes the Progression of Sepsis by Activation of Lncrna HULC/Mir-204-5p/TRPM7 Network in Huvecs. Biosci Rep (2020) 40(6):BSR20200740. doi: 10.1042/BSR20200740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chen Y, Fu Y, Song Y-F, Li N. Increased Expression of Lncrna UCA1 and HULC Is Required for Pro-Inflammatory Response During LPS Induced Sepsis in Endothelial Cells. Front Physiol (2019) 10:608. doi: 10.3389/fphys.2019.00608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shen J, Liu L, Zhang F, Gu J, Pan G. Lncrna Tapsaki Promotes Inflammation Injury in HK-2 Cells and Urine Derived Sepsis-Induced Kidney Injury. J Pharm Pharmacol (2019) 71(5):839–48. doi: 10.1111/jphp.13049 [DOI] [PubMed] [Google Scholar]
- 80. Zeng Q, Wu J, Yang S. Circulating Lncrna ITSN1-2 Is Upregulated, and its High Expression Correlates With Increased Disease Severity, Elevated Inflammation, and Poor Survival in Sepsis Patients. J Clin Lab Anal (2019) 33(4):e22836. doi: 10.1002/jcla.22836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang W, Li Y, Zhi S, Li J, Miao J, Ding Z, et al. Lncrna-ROR/Microrna-185-3p/YAP1 Axis Exerts Function in Biological Characteristics of Osteosarcoma Cells. Genomics (2020) 113(1 Pt 2):450–61. doi: 10.1016/j.ygeno.2020.09.009 [DOI] [PubMed] [Google Scholar]
- 82. Zhang P, Yi L, Qu S, Dai J, Li X, Liu B, et al. The Biomarker TCONS_00016233 Drives Septic AKI by Targeting the Mir-22-3p/AIFM1 Signaling Axis. Mol Therapy-Nucleic Acids (2020) 19:1027–42. doi: 10.1016/j.omtn.2019.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Qin G, Wei L, Jiang F, Li J, Zhang B, Pan D, et al. Lncrna NR024118 Is Downregulated in Sepsis and Inhibits LPS−Induced Apoptosis of Cardiomyocytes. Mol Med Rep (2021) 23(6):1–7. doi: 10.3892/mmr.2021.12073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhang C, Li J, Li H, Wang G, Wang Q, Zhang X, et al. Lncrna MIR155HG Accelerates the Progression of Sepsis via Upregulating MEF2A by Sponging Mir-194-5p. DNA Cell Biol (2021) 40(6):811–20. doi: 10.1089/dna.2021.0038 [DOI] [PubMed] [Google Scholar]
- 85. Wang J, Xin S, Yang R, Jiang J, Qiao Y. Knockdown of Lncrna LUCAT1 Attenuates Sepsis−Induced Myocardial Cell Injury by Sponging Mir-642a. Mamm Genome (2021) 32(6):1–9. doi: 10.1007/s00335-021-09890-4 [DOI] [PubMed] [Google Scholar]
- 86. Chen M, Guan Y, Li A, Zhao Y-Z, Zhang L, Zhang L, et al. Lncrna SOX2OT Mediates Mitochondrial Dysfunction in Septic Cardiomyopathy. DNA Cell Biol (2019) 38(11):1197–206. doi: 10.1089/dna.2019.4839 [DOI] [PubMed] [Google Scholar]
- 87. Deng J, Tan W, Luo Q, Lin L, Zheng L, Yang J. Long Non-Coding RNA MEG3 Promotes Renal Tubular Epithelial Cell Pyroptosis by Regulating the Mir-18a-3p/GSDMD Pathway in Lipopolysaccharide-Induced Acute Kidney Injury. Front Physiol (2021) 12. doi: 10.3389/fphys.2021.663216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chen K, Shi X, Jin Y, Wang F, Shen Q, Xu W. High Lncrna MEG3 Expression Is Associated With High Mortality Rates in Patients With Sepsis and Increased Lipopolysaccharide−Induced Renal Epithelial Cell and Cardiomyocyte Apoptosis. Exp Ther Med (2019) 18(5):3943–7. doi: 10.3892/etm.2019.8049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wu X, Chen D, Yu L. The Value of Circulating Long Non-Coding RNA Maternally Expressed Gene 3 as a Predictor of Higher Acute Respiratory Distress Syndrome Risk and 28-Day Mortality in Sepsis Patients. J Clin Lab Anal (2020) 34(11):e23488. doi: 10.1002/jcla.23488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Na L, Ding H, Xing E, Gao J, Liu B, Wang H, et al. Lnc-MEG3 Acts as a Potential Biomarker for Predicting Increased Disease Risk, Systemic Inflammation, Disease Severity, and Poor Prognosis of Sepsis via Interacting With Mir-21. J Clin Lab Anal (2020) 34(4):e23123. doi: 10.1002/jcla.23123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Du X, Tian D, Wei J, Yan C, Hu P, Wu X, et al. MEG3 Alleviated LPS-Induced Intestinal Injury in Sepsis by Modulating Mir-129-5p and Surfactant Protein D. Mediators Inflamm (2020) 2020. doi: 10.1155/2020/8232734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Fang Y, Hu J, Wang Z, Zhang S, Zhang R, Sun L, et al. GAS5 Promotes Podocyte Injury in Sepsis by Inhibiting PTEN Expression. Eur Rev Med Pharmacol Sci (2018) 22(23):8423–30. doi: 10.26355/eurrev_201812_16541 [DOI] [PubMed] [Google Scholar]
- 93. Li L, He Y, He X-J, Bi M-R, Qi Y-H, Zhu W-W. Down-Regulation of Long Noncoding RNA LINC00472 Alleviates Sepsis-Induced Acute Hepatic Injury by Regulating Mir-373-3p/TRIM8 Axis. Exp Mol Pathol (2020) 117:104562. doi: 10.1016/j.yexmp.2020.104562 [DOI] [PubMed] [Google Scholar]
- 94. Wu H, Liu J, Li W, Liu G, Li Z. Lncrna-HOTAIR Promotes TNF-α Production in Cardiomyocytes of LPS-Induced Sepsis Mice by Activating NF-κb Pathway. Biochem Biophys Res Commun (2016) 471(1):240–6. doi: 10.1016/j.bbrc.2016.01.117 [DOI] [PubMed] [Google Scholar]
- 95. Shen J, Zhang J, Jiang X, Wang H, Pan G. Lncrna HOX Transcript Antisense RNA Accelerated Kidney Injury Induced by Urine-Derived Sepsis Through the Mir-22/High Mobility Group Box 1 Pathway. Life Sci (2018) 210:185–91. doi: 10.1016/j.lfs.2018.08.041 [DOI] [PubMed] [Google Scholar]
- 96. Alkhateeb T, Bah I, Kumbhare A, Youssef D, Yao ZQ, McCall CE, et al. Long Non-Coding RNA Hotairm1 Promotes S100A9 Support of MDSC Expansion During Sepsis. J Clin Cell Immunol (2020) 11(6). [PMC free article] [PubMed] [Google Scholar]
- 97. Han D, Fang R, Shi R, Jin Y, Wang Q. Lncrna NKILA Knockdown Promotes Cell Viability and Represses Cell Apoptosis, Autophagy and Inflammation in Lipopolysaccharide-Induced Sepsis Model by Regulating Mir-140-5p/CLDN2 Axis. Biochem Biophys Res Commun (2021) 559:8–14. doi: 10.1016/j.bbrc.2021.04.074 [DOI] [PubMed] [Google Scholar]
- 98. Wu H, Wang J, Ma Z. Long Noncoding RNA HOXA-AS2 Mediates Microrna-106b-5p to Repress Sepsis-Engendered Acute Kidney Injury. J Biochem Mol Toxicol (2020) 34(4):e22453. doi: 10.1002/jbt.22453 [DOI] [PubMed] [Google Scholar]
- 99. Shi C, Zhao Y, Li Q, Li J. Lncrna SNHG14 Plays a Role in Sepsis-Induced Acute Kidney Injury by Regulating Mir-93. Mediators Inflamm (2021) 2021. doi: 10.1155/2021/5318369 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 100. Jia Y, Li Z, Cai W, Xiao D, Han S, Han F, et al. SIRT1 Regulates Inflammation Response of Macrophages in Sepsis Mediated by Long Noncoding RNA. Biochim Biophys Acta (BBA)-Molecular Basis Dis (2018) 1864(3):784–92. doi: 10.1016/j.bbadis.2017.12.029 [DOI] [PubMed] [Google Scholar]
- 101. Tan J, Fan J, He J, Zhao L, Tang H. Knockdown of Lncrna DLX6-AS1 Inhibits HK-2 Cell Pyroptosis via Regulating Mir-223-3p/NLRP3 Pathway in Lipopolysaccharide-Induced Acute Kidney Injury. J Bioenerg Biomembr (2020) 52(5):367–76. doi: 10.1007/s10863-020-09845-5 [DOI] [PubMed] [Google Scholar]
- 102. Wang M, Wei J, Shang F, Zang K, Ji T. Long Non−Coding RNA CASC2 Ameliorates Sepsis−Induced Acute Kidney Injury by Regulating the Mir−155 and NF−κb Pathway. Int J Mol Med (2020) 45(5):1554–62. doi: 10.3892/ijmm.2020.4518 [DOI] [PubMed] [Google Scholar]
- 103. Zhu L, Shi D, Cao J, Song L. Lncrna CASC2 Alleviates Sepsis-Induced Acute Lung Injury by Regulating the Mir-152-3p/PDK4 Axis. Immunol Invest (2021) 1–15. doi: 10.1080/08820139.2021.1928693 [DOI] [PubMed] [Google Scholar]
- 104. Xu Y, Shao B. Circulating Long Noncoding RNA ZNFX1 Antisense RNA Negatively Correlates With Disease Risk, Severity, Inflammatory Markers, and Predicts Poor Prognosis in Sepsis Patients. Med (2019) 98(9). doi: 10.1097/MD.0000000000014558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chen D-D, Wang H-W, Cai X-J. Long Non-Coding RNA ZFAS1 Alleviates Sepsis-Induced Myocardial Injury via Target Mir-34b-5p/SIRT1. Innate Immun (2021) 27(5):377–87. doi: 10.1177/17534259211034221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Liu J-J, Li Y, Yang M-S, Chen R, Cen C-Q. SP1-Induced ZFAS1 Aggravates Sepsis-Induced Cardiac Dysfunction via Mir-590–3p/NLRP3-Mediated Autophagy and Pyroptosis. Arch Biochem Biophys (2020) 695:108611. doi: 10.1016/j.abb.2020.108611 [DOI] [PubMed] [Google Scholar]
- 107. An L, Yang T, Zhong Y, Yin Y, Li W, Gao H. Molecular Pathways in Sepsis-Induced Cardiomyocyte Pyroptosis: Novel Finding on Long Non-Coding RNA ZFAS1/Mir-138–5p/SESN2 Axis. Immunol Lett (2021) 238:47–56. doi: 10.1016/j.imlet.2021.07.003 [DOI] [PubMed] [Google Scholar]
- 108. Zhang Z, Yu T, Geng W. Long Non-Coding RNA CCHE1 Participates in Postoperative Distant Recurrence But Not Local Recurrence of Osteosarcoma Possibly by Interacting With ROCK1. BMC Musculoskeletal Disord (2020) 21(1):462. doi: 10.1186/s12891-020-3184-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Xu X, Xu Y, Tao X, Liang G. Lncrna Mirt2 Upregulates Mir-1246 Through Methylation to Suppress LPS-Induced Lung Cell Apoptosis. Immun Inflamm Dis (2021) 9(3):695–701. doi: 10.1002/iid3.422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Liu X, Zhu N, Zhang B, Xu SB. Long Noncoding RNA TCONS_00016406 Attenuates Lipopolysaccharide-Induced Acute Kidney Injury by Regulating the Mir-687/PTEN Pathway. Front Physiol (2020) 11:622. doi: 10.3389/fphys.2020.00622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zhang H, Li L, Xu L, Zheng Y. Clinical Significance of the Serum Lncrna NORAD Expression in Patients With Neonatal Sepsis and its Association With Mir-410-3p. J Inflammation Res (2021) 14:4181. doi: 10.2147/JIR.S315985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Gao Z, Huang D. Lncrna GAS5−Mediated Mir−23a−3p Promotes Inflammation and Cell Apoptosis by Targeting TLR4 in a Cell Model of Sepsis. Mol Med Rep (2021) 24(1):1–9. doi: 10.3892/mmr.2021.12149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gui F, Peng H, Liu Y. Elevated Circulating lnc-ANRIL/Mir-125a Axis Level Predicts Higher Risk, More Severe Disease Condition, and Worse Prognosis of Sepsis. J Clin Lab Anal (2019) 33(6):e22917. doi: 10.1002/jcla.22917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Liu Y, Peng H, Gui F. Long Noncoding Plasmacytoma Variant Translocation 1 Facilitates the Surveillance of Acute Respiratory Distress Syndrome and Mortality Prediction in Sepsis. Biomarkers Med (2021) 15(6):401–12. doi: 10.2217/bmm-2020-0506 [DOI] [PubMed] [Google Scholar]
- 115. Zheng S, Li W, Liao W, Huang C, Zhou M, Zheng Y, et al. Silencing of Lncrna-PVT1 Ameliorates Lipopolysaccharide-Induced Inflammation in THP-1-Derived Macrophages via Inhibition of the P38 MAPK Signaling Pathway. Ann palliative Med (2021) 10(6):6410–8. doi: 10.21037/apm-21-1078 [DOI] [PubMed] [Google Scholar]
- 116. Deng L-T, Wang Q-L, Yu C, Gao M. Lncrna PVT1 Modulates NLRP3−Mediated Pyroptosis in Septic Acute Kidney Injury by Targeting Mir−20a−5p. Mol Med Rep (2021) 23(4):1–. doi: 10.3892/mmr.2021.11910 [DOI] [PubMed] [Google Scholar]
- 117. Luo Y-Y, Yang Z-Q, Lin X-F, Zhao F-L, Tu H-T, Wang L-J, et al. Knockdown of Lncrna PVT1 Attenuated Macrophage M1 Polarization and Relieved Sepsis Induced Myocardial Injury via Mir-29a/HMGB1 Axis. Cytokine (2021) 143:155509. doi: 10.1016/j.cyto.2021.155509 [DOI] [PubMed] [Google Scholar]
- 118. Chen J, Gu X, Zhou L, Wang S, Zhu L, Huang Y, et al. Long Non−Coding RNA−HOTAIR Promotes the Progression of Sepsis by Acting as a Sponge of Mir−211 to Induce IL−6R Expression. Exp Ther Med (2019) 18(5):3959–67. doi: 10.3892/etm.2019.8063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ni S-Y, Xu W-T, Liao G-Y, Wang Y-L, Li J. Lncrna HOTAIR Promotes LPS-Induced Inflammation and Apoptosis of Cardiomyocytes via Lin28-Mediated PDCD4 Stability. Inflammation (2021) 44(4):1–12. doi: 10.1007/s10753-021-01431-0 [DOI] [PubMed] [Google Scholar]
- 120. Huang J, Liu Y, Xie Q, Liang G, Kong H, Liu M, et al. Expression Profiling of Long Noncoding RNA and Messenger RNA in a Cecal Ligation and Puncture-Induced Colon Injury Mouse Model. Mediators Inflamm (2020) 2020. doi: 10.1155/2020/8925973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhang X, Huang Z, Wang Y, Wang T, Li J, Xi P. Long Non-Coding RNA RMRP Contributes to Sepsis-Induced Acute Kidney Injury. Yonsei Med J (2021) 62(3):262. doi: 10.3349/ymj.2021.62.3.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gao H, Ma H, Gao M, Chen A, Zha S, Yan J. Long Non-Coding RNA GAS5 Aggravates Myocardial Depression in Mice With Sepsis via the Microrna-449b/HMGB1 Axis and the NF-κb Signaling Pathway. Biosci Rep (2021) 41(4):BSR20201738. doi: 10.1042/BSR20201738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Han X, Yuan Z, Jing Y, Zhou W, Sun Y, Xing J. Knockdown of Lncrna Tapsaki Alleviates LPS-Induced Injury in HK-2 Cells Through the Mir-205/IRF3 Pathway. Open Med (2021) 16(1):581–90. doi: 10.1515/med-2021-0204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sun J, Xin K, Leng C, Ge J. Down-Regulation of SNHG16 Alleviates the Acute Lung Injury in Sepsis Rats Through Mir-128-3p/HMGB3 Axis. BMC Pulmonary Med (2021) 21(1):1–14. doi: 10.1186/s12890-021-01552-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhao H, Chen B, Li Z, Wang B, Li L. Long Noncoding RNA DANCR Suppressed Lipopolysaccharide-Induced Septic Acute Kidney Injury by Regulating Mir-214 in HK-2 Cells. Med Sci monitor: Int Med J Exp Clin Res (2020) 26:e921822–1. doi: 10.12659/MSM.921822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Hu Q, Zen W, Zhang M, Wang Z, Cui W, Liu Y, et al. Long Non-Coding RNA CASC2 Overexpression Ameliorates Sepsis-Associated Acute Kidney Injury by Regulating Mir-545-3p/PPARA Axis. J Surg Res (2021) 265:223–32. doi: 10.1016/j.jss.2021.03.047 [DOI] [PubMed] [Google Scholar]
- 127. Luo S, Huang X, Liu S, Zhang L, Cai X, Chen B. Long Non-Coding RNA Small Nucleolar RNA Host Gene 1 Alleviates Sepsis-Associated Myocardial Injury by Modulating the Mir-181a-5p/XIAP Axis In Vitro. Ann Clin Lab Sci (2021) 51(2):231–40. [PubMed] [Google Scholar]
- 128. Yang N, Wang H, Zhang L, Lv J, Niu Z, Liu J, et al. Long Non-Coding RNA SNHG14 Aggravates LPS-Induced Acute Kidney Injury Through Regulating Mir-495-3p/HIPK1. Acta Biochim Biophys Sin (2021) 53(6):719–28. doi: 10.1093/abbs/gmab034 [DOI] [PubMed] [Google Scholar]
- 129. Hu M, Wei J, Yang L, Xu J, He Z, Li H, et al. Linc-KIAA1737–2 Promoted LPS-Induced HK-2 Cell Apoptosis by Regulating Mir-27a-3p/TLR4/NF-κb Axis. J Bioenerg Biomembr (2021) 53(4):1–11. doi: 10.1007/s10863-021-09897-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Fu D, Zhou K, Liu J, Zheng P, Li P, Cheng W, et al. Long Non-Coding RNA Plncrna-1 Regulates Cell Proliferation, Apoptosis, and Autophagy in Septic Acute Kidney Injury by Regulating BCL2. Int J Clin Exp Pathol (2018) 11(1):314. [PMC free article] [PubMed] [Google Scholar]
- 131. Wang B, Sun Q, Ye W, Li L, Jin P. Long Non-Coding RNA CDKN2B-AS1 Enhances LPS-Induced Apoptotic and Inflammatory Damages in Human Lung Epithelial Cells via Regulating the Mir-140-5p/TGFBR2/Smad3 Signal Network. BMC pulmonary Med (2021) 21(1):1–12. doi: 10.1186/s12890-021-01561-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wang H, Li Y, Wang Y, Li H, Dou L. Microrna-494-3p Alleviates Inflammatory Response in Sepsis by Targeting TLR6. Eur Rev Med Pharmacol Sci (2019) 23(7):2971–7. doi: 10.26355/eurrev_201904_17578 [DOI] [PubMed] [Google Scholar]
- 133. Li J, Zhang H, Zuo Y. Microrna-218 Alleviates Sepsis Inflammation by Negatively Regulating VOPP1 via JAK/STAT Pathway. Eur Rev Med Pharmacol Sci (2018) 22(17):5620–6. doi: 10.26355/eurrev_201809_15827 [DOI] [PubMed] [Google Scholar]
- 134. Abou El-Khier NT, Zaki ME, Alkasaby NM. Study of Microrna-122 as a Diagnostic Biomarker of Sepsis. Egypt J Immunol (2019) 26(2):105–16. [PubMed] [Google Scholar]
- 135. Ouyang H, Tan Y, Li Q, Xia F, Xiao X, Zheng S, et al. Microrna-208-5p Regulates Myocardial Injury of Sepsis Mice via Targeting SOCS2-Mediated NF-κb/Hif-1α Pathway. Int Immunopharmacol (2020) 81:106204. doi: 10.1016/j.intimp.2020.106204 [DOI] [PubMed] [Google Scholar]
- 136. Xue Z, Xi Q, Liu H, Guo X, Zhang J, Zhang Z, et al. Mir-21 Promotes NLRP3 Inflammasome Activation to Mediate Pyroptosis and Endotoxic Shock. Cell Death Dis (2019) 10(6):1–13. doi: 10.1038/s41419-019-1713-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Jia P, Wu X, Dai Y, Teng J, Fang Y, Hu J, et al. Microrna-21 Is Required for Local and Remote Ischemic Preconditioning in Multiple Organ Protection Against Sepsis. Crit Care Med (2017) 45(7):e703–e10. doi: 10.1097/CCM.0000000000002363 [DOI] [PubMed] [Google Scholar]
- 138. Yao M, Cui B, Zhang W, Ma W, Zhao G, Xing L. Exosomal Mir-21 Secreted by IL-1β-Primed-Mesenchymal Stem Cells Induces Macrophage M2 Polarization and Ameliorates Sepsis. Life Sci (2021) 264:118658. doi: 10.1016/j.lfs.2020.118658 [DOI] [PubMed] [Google Scholar]
- 139. Sun B, Luan C, Guo L, Zhang B, Liu Y. Low Expression of Microrna-328 can Predict Sepsis and Alleviate Sepsis-Induced Cardiac Dysfunction and Inflammatory Response. Braz J Med Biol Res (2020) 53. doi: 10.1590/1414-431x20209501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Liu Z, Yang D, Gao J, Xiang X, Hu X, Li S, et al. Discovery and Validation of Mir-452 as an Effective Biomarker for Acute Kidney Injury in Sepsis. Theranostics (2020) 10(26):11963. doi: 10.7150/thno.50093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhou M, Zhang L, Song M, Sun W. Microrna-218 Prevents Lung Injury in Sepsis by Inhibiting RUNX2. Eur Rev Med Pharmacol Sci (2018) 22(23):8438–46. doi: 10.26355/eurrev_201812_16543 [DOI] [PubMed] [Google Scholar]
- 142. Na L, Ding H, Xing E, Zhang Y, Gao J, Liu B, et al. The Predictive Value of Microrna-21 for Sepsis Risk and its Correlation With Disease Severity, Systemic Inflammation, and 28-Day Mortality in Sepsis Patients. J Clin Lab Anal (2020) 34(3):e23103. doi: 10.1002/jcla.23103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lin R, Hu H, Li L, Chen G, Luo L, Rao P. The Potential of Microrna-126 in Predicting Disease Risk, Mortality of Sepsis, and its Correlation With Inflammation and Sepsis Severity. J Clin Lab Anal (2020) 34(9):e23408. doi: 10.1002/jcla.23408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Wang Q, Feng Q, Zhang Y, Zhou S, Chen H. Decreased Microrna 103 and Microrna 107 Predict Increased Risks of Acute Respiratory Distress Syndrome and 28-Day Mortality in Sepsis Patients. Med (2020) 99(25). doi: 10.1097/MD.0000000000020729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Gao Y, Zhang N, Lv C, Li N, Li X, Li W. Lncrna SNHG1 Knockdown Alleviates Amyloid-β-Induced Neuronal Injury by Regulating ZNF217 via Sponging Mir-361-3p in Alzheimer’s Disease. J Alzheimer's Dis (2020) Preprint):1–14. doi: 10.3233/JAD-191303 [DOI] [PubMed] [Google Scholar]
- 146. Zhu J, Lin X, Yan C, Yang S, Zhu Z. Microrna-98 Protects Sepsis Mice From Cardiac Dysfunction, Liver and Lung Injury by Negatively Regulating HMGA2 Through Inhibiting NF-κb Signaling Pathway. Cell Cycle (2019) 18(16):1948–64. doi: 10.1080/15384101.2019.1635869 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 147. Li S, Zhao D, Cui J, Wang L, Ma X, Li Y. Correlation of Microrna-125a/B With Acute Respiratory Distress Syndrome Risk and Prognosis in Sepsis Patients. J Clin Lab Anal (2020) 34(3):e23098. doi: 10.1002/jcla.23098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Liu Y, Guan H, Zhang J-L, Zheng Z, Wang H-T, Tao K, et al. Acute Downregulation of Mir-199a Attenuates Sepsis-Induced Acute Lung Injury by Targeting SIRT1. Am J Physiol-Cell Physiol (2018) 314(4):C449–C55. doi: 10.1152/ajpcell.00173.2017 [DOI] [PubMed] [Google Scholar]
- 149. Guo H, Tang L, Xu J, Lin C, Ling X, Lu C, et al. Microrna-495 Serves as a Diagnostic Biomarker in Patients With Sepsis and Regulates Sepsis-Induced Inflammation and Cardiac Dysfunction. Eur J Med Res (2019) 24(1):1–9. doi: 10.1186/s40001-019-0396-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Shen Y, Yu J, Jing Y, Zhang J. Mir-106a Aggravates Sepsis-Induced Acute Kidney Injury by Targeting THBS2 in Mice Model1. Acta cirurgica Bras (2019) 34. doi: 10.1590/s0102-865020190060000002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Song Y, Dou H, Li X, Zhao X, Li Y, Hou Y. Exosomal Mir-146a Contributes to the En-Hanced Therapeutic Efficacy of IL-1β-Primed Mesenchymal Stem Cells Against Sepsis. Stem Cells (2017) 35(5):1208–21. doi: 10.1002/stem.2564 [DOI] [PubMed] [Google Scholar]
- 152. Sun W, Li H, Gu J. Up-Regulation of Microrna-574 Attenuates Lipopolysaccharide-or Cecal Ligation and Puncture-Induced Sepsis Associated With Acute Lung Injury. Cell Biochem Funct (2020) 38(7):847–58. doi: 10.1002/cbf.3496 [DOI] [PubMed] [Google Scholar]
- 153. Zheng P, Feng X, Deng Q, Guo R, Li W, Hayakumo S, et al. Study on the Efficacy of Nanoantibiotics in Rats With Sepsis Based on Microrna-195 and TGF-β1/Smads Signaling Pathway. J Nanosci Nanotechnol (2021) 21(2):1357–64. doi: 10.1166/jnn.2021.18646 [DOI] [PubMed] [Google Scholar]
- 154. Chen L, Xie W, Wang L, Zhang X, Liu E, Kou Q. Mirna-133a Aggravates Inflammatory Responses in Sepsis by Targeting SIRT1. Int Immunopharmacol (2020) 88:106848. doi: 10.1016/j.intimp.2020.106848 [DOI] [PubMed] [Google Scholar]
- 155. Qin Y, Wang G, Peng Z. Microrna-191-5p Diminished Sepsis-Induced Acute Kidney Injury Through Targeting Oxidative Stress Responsive 1 in Rat Models. Biosci Rep (2019) 39(8):BSR20190548. doi: 10.1042/BSR20190548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Chen L, Yu L, Zhang R, Zhu L, Shen W. Correlation of Microrna-146a/B With Disease Risk, Biochemical Indices, Inflammatory Cytokines, Overall Disease Severity, and Prognosis of Sepsis. Medicine (2020) 99(22):e19754. doi: 10.1097/MD.0000000000019754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zou Q, Zhao S, Wu Q, Wang H, He X, Liu C. Correlation Analysis of Microrna-126 Expression in Peripheral Blood Lymphocytes With Apoptosis and Prognosis in Patients With Sepsis. Zhonghua wei Zhong Bing ji jiu yi xue (2020) 32(8):938–42. doi: 10.3760/cma.j.cn121430-20200213-00181 [DOI] [PubMed] [Google Scholar]
- 158. Dang CP, Leelahavanichkul A. Over-Expression of Mir-223 Induces M2 Macrophage Through Glycolysis Alteration and Attenuates LPS-Induced Sepsis Mouse Model, the Cell-Based Therapy in Sepsis. PloS One (2020) 15(7):e0236038. doi: 10.1371/journal.pone.0236038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Zhang N, Gao Y, Yu S, Sun X, Shen K. Berberine Attenuates Aβ42-Induced Neuronal Damage Through Regulating Circhdac9/Mir-142-5p Axis in Human Neuronal Cells. Life Sci (2020) 117637. doi: 10.1016/j.lfs.2020.117637 [DOI] [PubMed] [Google Scholar]
- 160. Gao M, Yu T, Liu D, Shi Y, Yang P, Zhang J, et al. Sepsis Plasma-Derived Exosomal Mir-1-3p Induces Endothelial Cell Dysfunction by Targeting SERP1. Clin Sci (2021) 135(2):347–65. doi: 10.1042/CS20200573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Yao L, Liu Z, Zhu J, Li B, Chai C, Tian Y. Clinical Evaluation of Circulating Microrna-25 Level Change in Sepsis and its Potential Relationship With Oxidative Stress. Int J Clin Exp Pathol (2015) 8(7):7675. [PMC free article] [PubMed] [Google Scholar]
- 162. Visitchanakun P, Tangtanatakul P, Trithiphen O, Soonthornchai W, Wongphoom J, Tachaboon S, et al. Plasma Mir-370-3p as a Biomarker of Sepsis-Associated Encephalopathy, the Transcriptomic Profiling Analysis of Microrna-Arrays From Mouse Brains. Shock (2020) 54(3):347–57. doi: 10.1097/SHK.0000000000001473 [DOI] [PubMed] [Google Scholar]
- 163. Pan T, Jia P, Chen N, Fang Y, Liang Y, Guo M, et al. Delayed Remote Ischemic Preconditioning Confersrenoprotection Against Septic Acute Kidney Injury via Exosomal Mir-21. Theranostics (2019) 9(2):405. doi: 10.7150/thno.29832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Zhang J, Liu Y, Liu L. Hyperoside Prevents Sepsis-Associated Cardiac Dysfunction Through Regulating Cardiomyocyte Viability and Inflammation via Inhibiting Mir-21. Biomed Pharmacother (2021) 138:111524. doi: 10.1016/j.biopha.2021.111524 [DOI] [PubMed] [Google Scholar]
- 165. De Melo P, Alvarez ARP, Ye X, Blackman A, Alves-Filho JC, Medeiros AI, et al. Macrophage-Derived Microrna-21 Drives Overwhelming Glycolytic and Inflammatory Response During Sepsis via Repression of the PGE2/IL-10 Axis. J Immunol (2021) 207(3):902–12. doi: 10.4049/jimmunol.2001251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Lin Z, Liu Z, Wang X, Qiu C, Zheng S. Mir-21-3p Plays a Crucial Role in Metabolism Alteration of Renal Tubular Epithelial Cells During Sepsis Associated Acute Kidney Injury via AKT/CDK2-FOXO1 Pathway. BioMed Res Int (2019) 2019. doi: 10.1155/2019/2821731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Jiang Q, Wu C, Zhang Q. Microrna-34a Participates in Lipopolysaccharide Mediated Sepsis Related Renal Function Impairment via Kruppel-Like Factor 4. Zhonghua wei zhong bing ji jiu yi xue (2018) 30(4):351–4. doi: 10.3760/cma.j.issn.2095-4352.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 168. Leng C, Sun J, Xin K, Ge J, Liu P, Feng X. High Expression of Mir-483-5p Aggravates Sepsis-Induced Acute Lung Injury. J toxicol Sci (2020) 45(2):77–86. doi: 10.2131/jts.45.77 [DOI] [PubMed] [Google Scholar]
- 169. Ma X, Qin J, Guo X. Mir-181-5p Protects Mice From Sepsis via Repressing HMGB1 in an Experimental Model. Eur Rev Med Pharmacol Sci (2020) 24(18):9712–20. doi: 10.26355/eurrev_202009_23063 [DOI] [PubMed] [Google Scholar]
- 170. Wang J, Tao Y, Wang Z, Mao Q. Mir-20a Promotes Kidney Injury in Sepsis Rats Through Autophagy. J Biol Regulators Homeostatic Agents (2020) 34(4):1277–83. doi: 10.23812/20-174-A [DOI] [PubMed] [Google Scholar]
- 171. Wang Y, Wang H, Zhang C, Zhang C, Yang H, Gao R, et al. Plasma Hsa-Mir-92a-3p in Correlation With Lipocalin-2 is Associated With Sepsis-Induced Coagulopathy. BMC Infect Dis (2020) 20(1):1–9. doi: 10.1186/s12879-020-4853-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. He Z, Wang H, Yue L. Endothelial Progenitor Cells-Secreted Extracellular Vesicles Containing Microrna-93-5p Confer Protection Against Sepsis-Induced Acute Kidney Injury via the KDM6B/H3k27me3/TNF-α Axis. Exp Cell Res (2020) 395(2):112173. doi: 10.1016/j.yexcr.2020.112173 [DOI] [PubMed] [Google Scholar]
- 173. Liu D, Wang Z, Wang H, Ren F, Li Y, Zou S, et al. The Protective Role of Mir-223 in Sepsis-Induced Mortality. Sci Rep (2020) 10(1):1–10. doi: 10.1038/s41598-020-74965-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Chen S, Ding R, Hu Z, Yin X, Xiao F, Zhang W, et al. Microrna-34a Inhibition Alleviates Lung Injury in Cecal Ligation and Puncture Induced Septic Mice. Front Immunol (2020) 11:1829. doi: 10.3389/fimmu.2020.01829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Yuan FH, Chen YL, Zhao Y, Liu ZM, Nan CC, Zheng BL, et al. Microrna-30a Inhibits the Liver Cell Proliferation and Promotes Cell Apoptosis Through the JAK/STAT Signaling Pathway by Targeting SOCS-1 in Rats With Sepsis. J Cell Physiol (2019) 234(10):17839–53. doi: 10.1002/jcp.28410 [DOI] [PubMed] [Google Scholar]
- 176. Zhu XG, Zhang TN, Wen R, Liu CF. Overexpression of Mir-150-5p Alleviates Apoptosis in Sepsis-Induced Myocardial Depression. BioMed Res Int (2020) 2020. doi: 10.1155/2020/3023186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Liu L, Li T-M, Liu X-R, Bai Y-P, Li J, Tang N, et al. Microrna-140 Inhibits Skeletal Muscle Glycolysis and Atrophy in Endotoxin-Induced Sepsis in Mice via the WNT Signaling Pathway. Am J Physiol-Cell Physiol (2019) 317(2):C189–C99. doi: 10.1152/ajpcell.00419.2018 [DOI] [PubMed] [Google Scholar]
- 178. Wang X, Wang Y, Kong M, Yang J. Mir-22-3p Suppresses Sepsis-Induced Acute Kidney Injury by Targeting PTEN. Biosci Rep (2020) 40(6):BSR20200527. doi: 10.1042/BSR20200527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Zhou W, Wang J, Li Z, Li J, Sang M. Microrna-205−5b Inhibits HMGB1 Expression in LPS-Induced Sepsis. Int J Mol Med (2016) 38(1):312–8. doi: 10.3892/ijmm.2016.2613 [DOI] [PubMed] [Google Scholar]
- 180. Liu Y, Xiao J, Sun J, Chen W, Wang S, Fu R, et al. ATG7 Promotes Autophagy in Sepsis−Induced Acute Kidney Injury and Is Inhibited by Mir−526b. Mol Med Rep (2020) 21(5):2193–201. doi: 10.3892/mmr.2020.11001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Wu Y, Li P, Goodwin AJ, Cook JA, Halushka PV, Zingarelli B, et al. Mir-145a Regulation of Pericyte Dysfunction in a Murine Model of Sepsis. J Infect Dis (2020) 222(6):1037–45. doi: 10.1093/infdis/jiaa184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Zhao D, Li S, Cui J, Wang L, Ma X, Li Y. Plasma Mir-125a and Mir-125b in Sepsis: Correlation With Disease Risk, Inflammation, Severity, and Prognosis. J Clin Lab Anal (2020) 34(2):e23036. doi: 10.1002/jcla.23036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Rahmel T, Schäfer ST, Frey UH, Adamzik M, Peters J. Increased Circulating Microrna-122 Is a Biomarker for Discrimination and Risk Stratification in Patients Defined by Sepsis-3 Criteria. PloS One (2018) 13(5):e0197637. doi: 10.1371/journal.pone.0197637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Zheng G, Pan M, Jin W, Jin G, Huang Y. Microrna-135a Is Up-Regulated and Aggravates Myocardial Depression in Sepsis via Regulating P38 MAPK/NF-κb Pathway. Int Immunopharmacol (2017) 45:6–12. doi: 10.1016/j.intimp.2017.01.029 [DOI] [PubMed] [Google Scholar]
- 185. Qin L, Wang M, Zhang H. Mir-133a Alleviates Renal Injury Caused by Sepsis by Targeting BNIP3L. Eur Rev Med Pharmacol Sci (2020) 24(5):2632–9. doi: 10.26355/eurrev_202003_20532 [DOI] [PubMed] [Google Scholar]
- 186. Colbert JF, Ford JA, Haeger SM, Yang Y, Dailey KL, Allison KC, et al. A Model-Specific Role of Microrna-223 as a Mediator of Kidney Injury During Experimental Sepsis. Am J Physiol-Renal Physiol (2017) 313(2):F553–F9. doi: 10.1152/ajprenal.00493.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Han Y, Li Y, Jiang Y. The Prognostic Value of Plasma Microrna-155 and Microrna-146a Level in Severe Sepsis and Sepsis-Induced Acute Lung Injury Patients. Clin Lab (2016) 62(12):2355–60. doi: 10.7754/Clin.Lab.2016.160511 [DOI] [PubMed] [Google Scholar]
- 188. Zhai Y, Ding N. Microrna-194 Participates in Endotoxemia Induced Myocardial Injury via Promoting Apoptosis. Eur Rev Med Pharmacol Sci (2018) 22(7):2077–83. doi: 10.26355/eurrev_201804_14739 [DOI] [PubMed] [Google Scholar]
- 189. Shangxun Z, Junjie L, Wei Z, Yutong W, Wenyuan J, Shanshou L, et al. ADAR1 Alleviates Inflammation in a Murine Sepsis Model via the ADAR1-Mir-30a-SOCS3 Axis. Mediators Inflamm (2020) 2020. doi: 10.1155/2020/9607535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Sun J, Sun X, Chen J, Liao X, He Y, Wang J, et al. Microrna-27b Shuttled by Mesenchymal Stem Cell-Derived Exosomes Prevents Sepsis by Targeting JMJD3 and Downregulating NF-κb Signaling Pathway. Stem Cell Res Ther (2021) 12(1):1–15. doi: 10.1186/s13287-020-02068-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Lv X, Zhang Y, Cui Y, Ren Y, Li R, Rong Q. Inhibition of Microrna−155 Relieves Sepsis−Induced Liver Injury Through Inactivating the JAK/STAT Pathway. Mol Med Rep (2015) 12(4):6013–8. doi: 10.3892/mmr.2015.4188 [DOI] [PubMed] [Google Scholar]
- 192. Zhou Y, Song Y, Shaikh Z, Li H, Zhang H, Caudle Y, et al. Microrna-155 Attenuates Late Sepsis-Induced Cardiac Dysfunction Through JNK and β-Arrestin 2. Oncotarget (2017) 8(29):47317. doi: 10.18632/oncotarget.17636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Vasques-Nóvoa F, Laundos TL, Cerqueira RJ, Quina-Rodrigues C, Soares-dos-Reis R, Baganha F, et al. Microrna-155 Amplifies Nitric Oxide/Cgmp Signaling and Impairs Vascular Angiotensin II Reactivity in Septic Shock. Crit Care Med (2018) 46(9):e945–54. doi: 10.1097/CCM.0000000000003296 [DOI] [PubMed] [Google Scholar]
- 194. Wang Q, Zhao C, Cai Q, Zhu H. Expression of Microrna-155 and Regulative T Cell in Sepsis Patients and Their Relationship. Zhonghua wei zhong bing ji jiu yi xue (2014) 26(3):179–83. doi: 10.3760/cma.j.issn.2095-4352.2014.03.011 [DOI] [PubMed] [Google Scholar]
- 195. Wang Z-F, Yang Y-M, Fan H. Diagnostic Value of Mir-155 for Acute Lung Injury/Acute Respiratory Distress Syndrome in Patients With Sepsis. J Int Med Res (2020) 48(7):0300060520943070. doi: 10.1177/0300060520943070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Zhang B, Yu L, Sheng Y. Clinical Value and Role of Microrna-29c-3p in Sepsis-Induced Inflammation and Cardiac Dysfunction. Eur J Med Res (2021) 26(1):1–7. doi: 10.1186/s40001-021-00566-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Sisti F, Wang S, Brandt SL, Glosson-Byers N, Mayo LD, Son YM, et al. Nuclear PTEN Enhances the Maturation of a Microrna Regulon to Limit Myd88-Dependent Susceptibility to Sepsis. Sci Signaling (2018) 11(528):eaai9085. doi: 10.1126/scisignal.aai9085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Gao N, Dong L. Microrna-146 Regulates the Inflammatory Cytokines Expression in Vascular Endothelial Cells During Sepsis. Die Pharmazie-An Int J Pharm Sci (2017) 72(11):700–4. doi: 10.1691/ph.2017.7600 [DOI] [PubMed] [Google Scholar]
- 199. Zhang YY, Liu X, Zhang X, Zhang J. Shikonin Improve Sepsis-Induced Lung Injury via Regulation of Mirna-140-5p/TLR4—a Vitro and Vivo Study. J Cell Biochem (2020) 121(3):2103–17. doi: 10.1002/jcb.28199 [DOI] [PubMed] [Google Scholar]
- 200. Ma H, Wang X, Ha T, Gao M, Liu L, Wang R, et al. Microrna-125b Prevents Cardiac Dysfunction in Polymicrobial Sepsis by Targeting TRAF6-Mediated Nuclear Factor κb Activation and P53-Mediated Apoptotic Signaling. J Infect Dis (2016) 214(11):1773–83. doi: 10.1093/infdis/jiw449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Ling Y, Li Z-Z, Zhang J-F, Zheng X-W, Lei Z-Q, Chen R-Y, et al. Microrna-494 Inhibition Alleviates Acute Lung Injury Through Nrf2 Signaling Pathway via NQO1 in Sepsis-Associated Acute Respiratory Distress Syndrome. Life Sci (2018) 210:1–8. doi: 10.1016/j.lfs.2018.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 202. Gao M, Wang X, Zhang X, Ha T, Ma H, Liu L, et al. Attenuation of Cardiac Dysfunction in Polymicrobial Sepsis by Microrna-146a Is Mediated via Targeting of IRAK1 and TRAF6 Expression. J Immunol (2015) 195(2):672–82. doi: 10.4049/jimmunol.1403155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Benz F, Tacke F, Luedde M, Trautwein C, Luedde T, Koch A, et al. Circulating Microrna-223 Serum Levels do Not Predict Sepsis or Survival in Patients With Critical Illness. Dis Markers (2015) 2015. doi: 10.1155/2015/384208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Li Y, Ke J, Peng C, Wu F, Song Y. Microrna-300/NAMPT Regulates Inflammatory Responses Through Activation of AMPK/Mtor Signaling Pathway in Neonatal Sepsis. Biomed Pharmacother (2018) 108:271–9. doi: 10.1016/j.biopha.2018.08.064 [DOI] [PubMed] [Google Scholar]
- 205. Zhang X, Wang X, Fan M, Tu F, Yang K, Ha T, et al. Endothelial HSPA12B Exerts Protection Against Sepsis-Induced Severe Cardiomyopathy via Suppression of Adhesion Molecule Expression by Mir-126. Front Immunol (2020) 11:566. doi: 10.3389/fimmu.2020.00566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Zheng G, Qiu G, Ge M, Meng J, Zhang G, Wang J, et al. Mir-10a in Peripheral Blood Mononuclear Cells Is a Biomarker for Sepsis and has Anti-Inflammatory Function. Mediators Inflamm (2020) 2020. doi: 10.1155/2020/4370983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Bai X, Zhang J, Cao M, Han S, Liu Y, Wang K, et al. Microrna-146a Protects Against LPS-Induced Organ Damage by Inhibiting Notch1 in Macrophage. Int Immunopharmacol (2018) 63:220–6. doi: 10.1016/j.intimp.2018.07.040 [DOI] [PubMed] [Google Scholar]
- 208. Hong J, Hu B-C, Xu L, Zheng Y, Shao Z-Q, Zhang R, et al. Microrna-19a Targets Fibroblast Growth Factor-Inducible Molecule 14 and Prevents Tubular Damage in Septic AKI. Anal Cell Pathol (2020) 2020. doi: 10.1155/2020/2894650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Ge C, Liu J, Dong S. Mirna-214 Protects Sepsis-Induced Myocardial Injury. Shock (2018) 50(1):112–8. doi: 10.1097/SHK.0000000000000978 [DOI] [PubMed] [Google Scholar]
- 210. Meng L, Cao H, Wan C, Jiang L. Mir-539-5p Alleviates Sepsis-Induced Acute Lung Injury by Targeting ROCK1. Folia histochem cytobiologica (2019) 57(4):168–78. doi: 10.5603/FHC.a2019.0019 [DOI] [PubMed] [Google Scholar]
- 211. Liu J, Shi K, Chen M, Xu L, Hong J, Hu B, et al. Elevated Mir-155 Expression Induces Immunosuppression via CD39+ Regulatory T-Cells in Sepsis Patient. Int J Infect Dis (2015) 40:135–41. doi: 10.1016/j.ijid.2015.09.016 [DOI] [PubMed] [Google Scholar]
- 212. Zhang J, Ding C, Shao Q, Liu F, Zeng Z, Nie C, et al. The Protective Effects of Transfected Microrna-146a on Mice With Sepsis-Induced Acute Lung Injury In Vivo. Zhonghua wei zhong bing ji jiu yi xue (2015) 27(7):591–4. doi: 10.3760/cma.j.issn.2095-4352.2015.07.010 [DOI] [PubMed] [Google Scholar]
- 213. Zhang W, Jia J, Liu Z, Si D, Ma L, Zhang G. Circulating Micrornas as Biomarkers for Sepsis Secondary to Pneumonia Diagnosed via Sepsis 3.0. BMC pulmonary Med (2019) 19(1):1–8. doi: 10.1186/s12890-019-0836-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Jiang Y, Zhou H, Ma D, Chen ZK, Cai X. Microrna-19a and CD22 Comprise a Feedback Loop for B Cell Response in Sepsis. Med Sci monitor: Int Med J Exp Clin Res (2015) 21:1548. doi: 10.12659/MSM.894321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Liang G, Wu Y, Guan Y, Dong Y, Jiang L, Mao G, et al. The Correlations Between the Serum Expression of Mir-206 and the Severity and Prognosis of Sepsis. Ann Palliative Med (2020) 9(5):3222–34. doi: 10.21037/apm-20-1391 [DOI] [PubMed] [Google Scholar]
- 216. Funahashi Y, Kato N, Masuda T, Nishio F, Kitai H, Ishimoto T, et al. Mir-146a Targeted to Splenic Macrophages Prevents Sepsis-Induced Multiple Organ Injury. Lab Invest (2019) 99(8):1130–42. doi: 10.1038/s41374-019-0190-4 [DOI] [PubMed] [Google Scholar]
- 217. Xu H, Liu X, Ni H. Clinical Significance of Mir-19b-3p in Patients With Sepsis and its Regulatory Role in the LPS-Induced Inflammatory Response. Eur J Med Res (2020) 25(1):1–7. doi: 10.1186/s40001-020-00408-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Yang P, Xiong W, Chen X, Liu J, Ye Z. Overexpression of Mir-129-5p Mitigates Sepsis-Induced Acute Lung Injury by Targeting High Mobility Group Box 1. J Surg Res (2020) 256:23–30. doi: 10.1016/j.jss.2020.05.101 [DOI] [PubMed] [Google Scholar]
- 219. Zhang W, Lu F, Xie Y, Lin Y, Zhao T, Tao S, et al. Mir-23b Negatively Regulates Sepsis-Induced Inflammatory Responses by Targeting ADAM10 in Human THP-1 Monocytes. Mediators Inflamm (2019) 2019. doi: 10.1155/2019/5306541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Liu Q, Wang Y, Zheng Q, Dong X, Xie Z, Panayi A, et al. Microrna-150 Inhibits Myeloid-Derived Suppressor Cells Proliferation and Function Through Negative Regulation of ARG-1 in Sepsis. Life Sci (2021) 278:119626. doi: 10.1016/j.lfs.2021.119626 [DOI] [PubMed] [Google Scholar]
- 221. Sheng B, Zhao L, Zang X, Zhen J, Chen W. Mir-375 Ameliorates Sepsis by Downregulating Mir-21 Level via Inhibiting JAK2-STAT3 Signaling. Biomed Pharmacother (2017) 86:254–61. doi: 10.1016/j.biopha.2016.11.147 [DOI] [PubMed] [Google Scholar]
- 222. Zhan C-Y, Chen D, Luo J-L, Shi Y-H, Zhang Y-P. Protective Role of Down-Regulated Microrna-31 on Intestinal Barrier Dysfunction Through Inhibition of NF-κb/Hif-1α Pathway by Binding to HMOX1 in Rats With Sepsis. Mol Med (2018) 24(1):1–14. doi: 10.1186/s10020-018-0053-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. McClure C, Brudecki L, Ferguson DA, Yao ZQ, Moorman JP, McCall CE, et al. Microrna 21 (Mir-21) and Mir-181b Couple With NFI-a to Generate Myeloid-Derived Suppressor Cells and Promote Immunosuppression in Late Sepsis. Infect Immun (2014) 82(9):3816–25. doi: 10.1128/IAI.01495-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Roderburg C, Luedde M, Vargas Cardenas D, Vucur M, Scholten D, Frey N, et al. Circulating Microrna-150 Serum Levels Predict Survival in Patients With Critical Illness and Sepsis. PloS One (2013) 8(1):e54612. doi: 10.1371/journal.pone.0054612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Zhou Y, Han W, Song D, Li Z, Ding H, Zhou T, et al. Effect of Mir-10a on Sepsis-Induced Liver Injury in Rats Through TGF-β1/Smad Signaling Pathway. Eur Rev Med Pharmacol Sci (2020) 24(2):862–9. doi: 10.26355/eurrev_202001_20070 [DOI] [PubMed] [Google Scholar]
- 226. Ma F, Li Z, Cao J, Kong X, Gong G. A TGFBR2/SMAD2/DNMT1/Mir-145 Negative Regulatory Loop Is Responsible for LPS-Induced Sepsis. Biomed Pharmacother (2019) 112:108626. doi: 10.1016/j.biopha.2019.108626 [DOI] [PubMed] [Google Scholar]
- 227. Vasilescu C, Rossi S, Shimizu M, Tudor S, Veronese A, Ferracin M, et al. Microrna Fingerprints Identify Mir-150 as a Plasma Prognostic Marker in Patients With Sepsis. PloS One (2009) 4(10):e7405. doi: 10.1371/journal.pone.0007405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Zhou YP, Xia Q. Inhibition of Mir-103a-3p Suppresses Lipopolysaccharide-Induced Sepsis and Liver Injury by Regulating FBXW7 Expression. Cell Biol Int (2020) 44(9):1798–810. doi: 10.1002/cbin.11372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Han Y, Dai Q-C, Shen H-L, Zhang X-W. Diagnostic Value of Elevated Serum Mirna-143 Levels in Sepsis. J Int Med Res (2016) 44(4):875–81. doi: 10.1177/0300060516645003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Cao X, Zhang C, Zhang X, Chen Y, Zhang H. Mir-145 Negatively Regulates TGFBR2 Signaling Responsible for Sepsis-Induced Acute Lung Injury. Biomed Pharmacother (2019) 111:852–8. doi: 10.1016/j.biopha.2018.12.138 [DOI] [PubMed] [Google Scholar]
- 231. Rajput C, Tauseef M, Farazuddin M, Yazbeck P, Amin M-R, Avin BRV, et al. Microrna-150 Suppression of Angiopoetin-2 Generation and Signaling Is Crucial for Resolving Vascular Injury. Arteriosclerosis thrombosis Vasc Biol (2016) 36(2):380–8. doi: 10.1161/ATVBAHA.115.306997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. He SY, Wang G, Pei YH, Zhu HP. Mir-34b-3p Protects Against Acute Kidney Injury in Sepsis Mice via Targeting Ubiquitin-Like Protein 4A. Kaohsiung J Med Sci (2020) 36(10):817–24. doi: 10.1002/kjm2.12255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Wang H, Bei Y, Shen S, Huang P, Shi J, Zhang J, et al. Mir-21-3p Controls Sepsis-Associated Cardiac Dysfunction via Regulating SORBS2. J Mol Cell Cardiol (2016) 94:43–53. doi: 10.1016/j.yjmcc.2016.03.014 [DOI] [PubMed] [Google Scholar]
- 234. Du X, Tian D, Wei J, Yan C, Hu P, Wu X, et al. Mir-199a-5p Exacerbated Intestinal Barrier Dysfunction Through Inhibiting Surfactant Protein D and Activating NF-κb Pathway in Sepsis. Mediators Inflamm (2020) 2020. doi: 10.1155/2020/8275026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Su Y, Song X, Teng J, Zhou X, Dong Z, Li P, et al. Mesenchymal Stem Cells-Derived Extracellular Vesicles Carrying Microrna-17 Inhibits Macrophage Apoptosis in Lipopolysaccharide-Induced Sepsis. Int Immunopharmacol (2021) 95:107408. doi: 10.1016/j.intimp.2021.107408 [DOI] [PubMed] [Google Scholar]
- 236. Zhu X. Mir-125b But Not Mir-125a Is Upregulated and Exhibits a Trend to Correlate With Enhanced Disease Severity, Inflammation, and Increased Mortality in Sepsis Patients. J Clin Lab Anal (2020) 34(3):e23094. doi: 10.1002/jcla.23094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Ling L, Zhang S-H, Zhi L-D, Li H, Wen Q-K, Li G, et al. Microrna-30e Promotes Hepatocyte Proliferation and Inhibits Apoptosis in Cecal Ligation and Puncture-Induced Sepsis Through the JAK/STAT Signaling Pathway by Binding to FOSL2. Biomed Pharmacother (2018) 104:411–9. doi: 10.1016/j.biopha.2018.05.042 [DOI] [PubMed] [Google Scholar]
- 238. Liu J, Liu Y, Zhang L, Chen Y, Du H, Wen Z, et al. Down-Regulation of Circdmnt3b Is Conducive to Intestinal Mucosal Permeability Dysfunction of Rats With Sepsis via Sponging Mir-20b-5p. J Cell Mol Med (2020) 24(12):6731–40. doi: 10.1111/jcmm.15324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Wang X, Yu Y. Mir-146b Protect Against Sepsis Induced Mice Myocardial Injury Through Inhibition of Notch1. J Mol Histol (2018) 49(4):411–7. doi: 10.1007/s10735-018-9781-4 [DOI] [PubMed] [Google Scholar]
- 240. Yao Y, Sun F, Lei M. Mir-25 Inhibits Sepsis-Induced Cardiomyocyte Apoptosis by Targetting PTEN. Biosci Rep (2018) 38(2). doi: 10.1042/BSR20171511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. McClure C, McPeak MB, Youssef D, Yao ZQ, McCall CE, El Gazzar M. Stat3 and C/Ebpβ Synergize to Induce Mir-21 and Mir-181b Expression During Sepsis. Immunol Cell Biol (2017) 95(1):42–55. doi: 10.1038/icb.2016.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Ji Z-R, Xue W-L, Zhang L. Schisandrin B Attenuates Inflammation in LPS-Induced Sepsis Through Mir-17-5p Downregulating TLR4. Inflammation (2019) 42(2):731–9. doi: 10.1007/s10753-018-0931-3 [DOI] [PubMed] [Google Scholar]
- 243. Yu J, Chen J, Yang H, Chen S, Wang Z. Overexpression of Mir−200a−3p Promoted Inflammation in Sepsis−Induced Brain Injury Through ROS−Induced NLRP3. Int J Mol Med (2019) 44(5):1811–23. doi: 10.3892/ijmm.2019.4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Szilágyi B, Fejes Z, Póliska S, Pócsi M, Czimmerer Z, Patsalos A, et al. Reduced Mir-26b Expression in Megakaryocytes and Platelets Contributes to Elevated Level of Platelet Activation Status in Sepsis. Int J Mol Sci (2020) 21(3):866. doi: 10.3390/ijms21030866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Chen X, Chen Y, Dai L, Wang N. Mir-96-5p Alleviates Inflammatory Responses by Targeting NAMPT and Regulating the NF-κb Pathway in Neonatal Sepsis. Biosci Rep (2020) 40(7):BSR20201267. doi: 10.1042/BSR20201267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Wang Z, Ruan Z, Mao Y, Dong W, Zhang Y, Yin N, et al. Mir-27a Is Up Regulated and Promotes Inflammatory Response in Sepsis. Cell Immunol (2014) 290(2):190–5. doi: 10.1016/j.cellimm.2014.06.006 [DOI] [PubMed] [Google Scholar]
- 247. Zou Z, Lin Q, Yang H, Liu Z, Zheng S. Nrp-1 Mediated Plasmatic Ago2 Binding Mir-21a-3p Internalization: A Novel Mechanism for Mir-21a-3p Accumulation in Renal Tubular Epithelial Cells During Sepsis. BioMed Res Int (2020) 2020. doi: 10.1155/2020/2370253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Wang H, Meng K, jun Chen W, Feng D, Jia Y, Xie L. Serum Mir-574-5p: A Prognostic Predictor of Sepsis Patients. Shock (2012) 37(3):263–7. doi: 10.1097/SHK.0b013e318241baf8 [DOI] [PubMed] [Google Scholar]
- 249. Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, et al. Microrna-181b Regulates NF-κb–Mediated Vascular Inflammation. J Clin Invest (2012) 122(6):1973–90. doi: 10.1172/JCI61495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Du X, Wei J, Tian D, Wu M, Yan C, Hu P, et al. Mir-182-5p Contributes to Intestinal Injury in a Murine Model of Staphylococcus Aureus Pneumonia-Induced Sepsis via Targeting Surfactant Protein D. J Cell Physiol (2020) 235(1):563–72. doi: 10.1002/jcp.28995 [DOI] [PubMed] [Google Scholar]
- 251. Zheng D, Yu Y, Li M, Wang G, Chen R, Fan G-C, et al. Inhibition of Microrna 195 Prevents Apoptosis and Multiple-Organ Injury in Mouse Models of Sepsis. J Infect Dis (2016) 213(10):1661–70. doi: 10.1093/infdis/jiv760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Zhang Y, Xia F, Wu J, Yang A, Zhang Y, Zhao H, et al. Mir-205 Influences Renal Injury in Sepsis Rats Through HMGB1-PTEN Signaling Pathway. Eur Rev Med Pharmacol Sci (2019) 23:10950–6. doi: 10.26355/eurrev_201912_19798 [DOI] [PubMed] [Google Scholar]
- 253. Wu S-Y, Zhang H, Wu W, Wu Y-Y. Value of Serum Mir-21-3p in Predicting Acute Kidney Injury in Children With Sepsis. Zhongguo Dang dai er ke za zhi= Chin J Contemp Pediatr (2020) 22(3):269–73. doi: 10.7499/j.issn.1008-8830.2020.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Yi H-X, Jiang S-Y, Yu L-H, Chen K, Yang Z-X, Wu Q. Mir-181a-2-3p Alleviates the Apoptosis of Renal Tubular Epithelial Cell via Targeting GJB2 in Sepsis-Induced Acute Kidney Injury. Mol Cell Biol (2021) 41(7):MCB. 00016–21. doi: 10.1128/MCB.00016-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Fu D, Dong J, Li P, Tang C, Cheng W, Xu Z, et al. Mirna-21 has Effects to Protect Kidney Injury Induced by Sepsis. Biomed Pharmacother (2017) 94:1138–44. doi: 10.1016/j.biopha.2017.07.098 [DOI] [PubMed] [Google Scholar]
- 256. Pan Y, Wang J, Xue Y, Zhao J, Li D, Zhang S, et al. GSKJ4 Protects Mice Against Early Sepsis via Reducing Proinflammatory Factors and Up-Regulating Mir-146a. Front Immunol (2018) 9:2272. doi: 10.3389/fimmu.2018.02272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Liu Y, Cao D, Mo G, Zhang L. Effects of Microrna-294 on Inflammatory Factor of Sepsis by Targeting Triggering Receptor Expressed on Myeloid Cells-1. Zhonghua wei zhong bing ji jiu yi xue (2014) 26(9):661–5. doi: 10.3760/cma.j.issn.2095-4352.2014.09.01 [DOI] [PubMed] [Google Scholar]
- 258. Wang L, Wang K, Tian Z. Mir-128-3p Inhibits NRP1 Expression and Promotes Inflammatory Response to Acute Kidney Injury in Sepsis. Inflammation (2020) 43:1772–9. doi: 10.1007/s10753-020-01251-8 [DOI] [PubMed] [Google Scholar]
- 259. An R, Feng J, Xi C, Xu J, Sun L. Mir-146a Attenuates Sepsis-Induced Myocardial Dysfunction by Suppressing IRAK1 and TRAF6 via Targeting Erbb4 Expression. Oxid Med Cell longevity (2018) 2018. doi: 10.1155/2018/7163057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Puimège L, Van Hauwermeiren F, Steeland S, Van Ryckeghem S, Vandewalle J, Lodens S, et al. Glucocorticoid-Induced Microrna-511 Protects Against TNF by Down-Regulating TNFR 1. EMBO Mol Med (2015) 7(8):1004–17. doi: 10.15252/emmm.201405010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Liu Z, Tang C, He L, Yang D, Cai J, Zhu J, et al. The Negative Feedback Loop of NF-κb/Mir-376b/NFKBIZ in Septic Acute Kidney Injury. JCI Insight (2020) 5(24). doi: 10.1172/jci.insight.142272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Wang Z-H, Liang Y-B, Tang H, Chen Z-B, Li Z-Y, Hu X-C, et al. Dexamethasone Down-Regulates the Expression of Microrna-155 in the Livers of Septic Mice. PloS One (2013) 8(11):e80547. doi: 10.1371/journal.pone.0080547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Tacke F, Roderburg C, Benz F, Cardenas DV, Luedde M, Hippe H-J, et al. Levels of Circulating Mir-133a Are Elevated in Sepsis and Predict Mortality in Critically Ill Patients. Crit Care Med (2014) 42(5):1096–104. doi: 10.1097/CCM.0000000000000131 [DOI] [PubMed] [Google Scholar]
- 264. Ling L, Lu H-T, Wang H-F, Shen M-J, Zhang H-B. Microrna-203 Acts as a Potent Suppressor in Septic Shock by Alleviating Lung Injury via Inhibition of VNN1. Kidney Blood Pressure Res (2019) 44(4):565–82. doi: 10.1159/000500484 [DOI] [PubMed] [Google Scholar]
- 265. Wu X, Yang J, Yu L, Long D. Plasma Mirna-223 Correlates With Risk, Inflammatory Markers as Well as Prognosis in Sepsis Patients. Med (2018) 97(27). doi: 10.1097/MD.0000000000011352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Möhnle P, Schütz SV, van der Heide V, Hübner M, Luchting B, Sedlbauer J, et al. Microrna-146a Controls Th1-Cell Differentiation of Human CD4+ T Lymphocytes by Targeting Prkcϵ. Eur J Immunol (2015) 45(1):260–72. doi: 10.1002/eji.201444667 [DOI] [PubMed] [Google Scholar]
- 267. Karam RA, Zidan HE, Karam NA, Abdel Rahman DM, El-Seifi OS. Diagnostic and Prognostic Significance of Serum Mirna-146-a Expression in Egyptian Children With Sepsis in a Pediatric Intensive Care Unit. J Gene Med (2019) 21(11):e3128. doi: 10.1002/jgm.3128 [DOI] [PubMed] [Google Scholar]
- 268. Cheng D-L, Fang H-X, Liang Y, Zhao Y, Shi C-S. Microrna-34a Promotes Inos Secretion From Pulmonary Macrophages in Septic Suckling Rats Through Activating STAT3 Pathway. Biomed Pharmacother (2018) 105:1276–82. doi: 10.1016/j.biopha.2018.06.063 [DOI] [PubMed] [Google Scholar]
- 269. Yang Q, Cao K, Jin G, Zhang J. Hsa-Mir-346 Plays a Role in the Development of Sepsis by Downregulating SMAD3 Expression and Is Negatively Regulated by Lncrna MALAT1. Mol Cell Probes (2019) 47:101444. doi: 10.1016/j.mcp.2019.101444 [DOI] [PubMed] [Google Scholar]
- 270. Sang Z, Dong S, Zhang P, Wei Y. Mir−214 Ameliorates Sepsis−Induced Acute Kidney Injury via PTEN/AKT/Mtor−Regulated Autophagy. Mol Med Rep (2021) 24(4):1–11. doi: 10.3892/mmr.2021.12322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Xue W-L, Bai X, Zhang L. Rhtnfr: Fc Increases Nrf2 Expression via Mir-27a Mediation to Protect Myocardium Against Sepsis Injury. Biochem Biophys Res Commun (2015) 464(3):855–61. doi: 10.1016/j.bbrc.2015.07.051 [DOI] [PubMed] [Google Scholar]
- 272. Yang W, Wu H, Zhang H, Liu H, Wei Y, Shi B. Prognostic Value of Picco Monitoring Combined With Plasma Microrna-150 Detection in Septic Shock Patients. Zhejiang da xue xue bao Yi xue ban= J Zhejiang Univ Med Sci (2015) 44(6):659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Bai X, Li J, Li L, Liu M, Liu Y, Cao M, et al. Extracellular Vesicles From Adipose Tissue-Derived Stem Cells Affect Notch-Mir148a-3p Axis to Regulate Polarization of Macrophages and Alleviate Sepsis in Mice. Front Immunol (2020) 11:1391. doi: 10.3389/fimmu.2020.01391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Zhang T, Xiang L. Honokiol Alleviates Sepsis-Induced Acute Kidney Injury in Mice by Targeting the Mir-218-5p/Heme Oxygenase-1 Signaling Pathway. Cell Mol Biol Lett (2019) 24(1):1–15. doi: 10.1186/s11658-019-0142-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Wang H-J, Deng J, Wang J-Y, Zhang P-J, Xin Z, Xiao K, et al. Serum Mir-122 Levels Are Related to Coagulation Disorders in Sepsis Patients. Clin Chem Lab Med (CCLM) (2014) 52(6):927–33. doi: 10.1515/cclm-2013-0899 [DOI] [PubMed] [Google Scholar]
- 276. Xin Y, Tang L, Chen J, Chen D, Wen W, Han F. Inhibition of Mir−101−3p Protects Against Sepsis−Induced Myocardial Injury by Inhibiting MAPK and NF−κb Pathway Activation via the Upregulation of DUSP1. Int J Mol Med (2021) 47(3):1–. doi: 10.3892/ijmm.2021.4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Pan W, Wei N, Xu W, Wang G, Gong F, Li N. Microrna-124 Alleviates the Lung Injury in Mice With Septic Shock Through Inhibiting the Activation of the MAPK Signaling Pathway by Downregulating MAPK14. Int Immunopharmacol (2019) 76:105835. doi: 10.1016/j.intimp.2019.105835 [DOI] [PubMed] [Google Scholar]
- 278. Luo N, Gao H, Wang Y, Li H, Li Y. Mir-942-5p Alleviates Septic Acute Kidney Injury by Targeting FOXO3. Eur Rev Med Pharmacol Sci (2020) 24(11):6237–44. doi: 10.26355/eurrev_202006_21521 [DOI] [PubMed] [Google Scholar]
- 279. Liu S, Liu C, Wang Z, Huang J, Zeng Q. Microrna-23a-5p Acts as a Potential Biomarker for Sepsis-Induced Acute Respiratory Distress Syndrome in Early Stage. Cell Mol Biol (2016) 62(2):31–7. [PubMed] [Google Scholar]
- 280. Ma J, Xu L-Y, Sun Q-H, Wan X-Y. Inhibition of Mir-1298-5p Attenuates Sepsis Lung Injury by Targeting SOCS6. Mol Cell Biochem (2021) 476(10):1–12. doi: 10.1007/s11010-021-04170-w [DOI] [PubMed] [Google Scholar]
- 281. Zheng G, Qu H, Li F, Ma W, Yang H. Propofol Attenuates Sepsis-Induced Acute Kidney Injury by Regulating Mir-290-5p/CCL-2 Signaling Pathway. Braz J Med Biol Res (2018) 51. doi: 10.1590/1414-431x20187655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Paik S, Choe JH, Choi G-E, Kim J-E, Kim J-M, Song GY, et al. Rg6, a Rare Ginsenoside, Inhibits Systemic Inflammation Through the Induction of Interleukin-10 and Microrna-146a. Sci Rep (2019) 9(1):1–15. doi: 10.1038/s41598-019-40690-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Wang X, Gu H, Qin D, Yang L, Huang W, Essandoh K, et al. Exosomal Mir-223 Contributes to Mesenchymal Stem Cell-Elicited Cardioprotection in Polymicrobial Sepsis. Sci Rep (2015) 5(1):1–16. doi: 10.1038/srep13721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Gu W, Wen D, Lu H, Zhang A, Wang H, Du J, et al. Mir-608 Exerts Anti-Inflammatory Effects by Targeting ELANE in Monocytes. J Clin Immunol (2020) 40(1):147–57. doi: 10.1007/s10875-019-00702-8 [DOI] [PubMed] [Google Scholar]
- 285. Sun Y, Li Q, Gui H, Xu D-P, Yang Y-L, Su D-F, et al. Microrna-124 Mediates the Cholinergic Anti-Inflammatory Action Through Inhibiting the Production of Pro-Inflammatory Cytokines. Cell Res (2013) 23(11):1270–83. doi: 10.1038/cr.2013.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Zhang J, Wang C, Tang X, Wei Y. Urinary Mir-26b as a Potential Biomarker for Patients With Sepsis-Associated Acute Kidney Injury: A Chinese Population-Based Study. Eur Rev Med Pharmacol Sci (2018) 22(14):4604–10. doi: 10.26355/eurrev_201807_15518 [DOI] [PubMed] [Google Scholar]
- 287. Ni J, He J, Kang L, Zhong Z, Wang L, Yin S. Effects of Dexmedetomidine Pretreatment on Rats With Sepsis-Induced Acute Kidney Injury and Mir-146a Expression. Cell Mol Biol (2020) 66(2):93–8. doi: 10.14715/cmb/2020.66.2.15 [DOI] [PubMed] [Google Scholar]
- 288. Huo R, Dai M, Fan Y, Zhou J-Z, Li L, Zu J. Predictive Value of Mirna-29a and Mirna-10a-5p for 28-Day Mortality in Patients With Sepsis-Induced Acute Kidney Injury. Nan fang yi ke da xue xue bao= J South Med Univ (2017) 37(5):646–51. doi: 10.3969/j.issn.1673-4254.2017.05.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Cao Y-Y, Wang Z, Wang Z-H, Jiang X-G, Lu W-H. Inhibition of Mir-155 Alleviates Sepsis-Induced Inflammation and Intestinal Barrier Dysfunction by Inactivating NF-κb Signaling. Int Immunopharmacol (2021) 90:107218. doi: 10.1016/j.intimp.2020.107218 [DOI] [PubMed] [Google Scholar]
- 290. Ma F, Liu F, Ding L, You M, Yue H, Zhou Y, et al. Anti-Inflammatory Effects of Curcumin Are Associated With Down Regulating Microrna-155 in LPS-Treated Macrophages and Mice. Pharm Biol (2017) 55(1):1263–73. doi: 10.1080/13880209.2017.1297838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Wu Z-J, Chen Y-F, Wang H-D, Gao F-H. Expression of Plasma Mirna-497 in Children With Sepsis-Induced Myocardial Injury and its Clinical Significance. Zhongguo dang dai er ke za zhi= Chin J Contemp Pediatr (2018) 20(1):32–6. doi: 10.7499/j.issn.1008-8830.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Lou W, Yan J, Wang W. Downregulation of Mir-497-5p Improves Sepsis-Induced Acute Lung Injury by Targeting IL2RB. BioMed Res Int (2021) 2021. doi: 10.1155/2021/6624702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Wang Y, Li T, Wu B, Liu H, Luo J, Feng D, et al. STAT1 Regulates MD-2 Expression in Monocytes of Sepsis via Mir-30a. Inflammation (2014) 37(6):1903–11. doi: 10.1007/s10753-014-9922-1 [DOI] [PubMed] [Google Scholar]
- 294. Ma Y, Liu Y, Hou H, Yao Y, Meng H. Mir-150 Predicts Survival in Patients With Sepsis and Inhibits LPS-Induced Inflammatory Factors and Apoptosis by Targeting NF-κb1 in Human Umbilical Vein Endothelial Cells. Biochem Biophys Res Commun (2018) 500(3):828–37. doi: 10.1016/j.bbrc.2018.04.168 [DOI] [PubMed] [Google Scholar]
- 295. Brudecki L, Ferguson DA, McCall CE, El Gazzar M. Microrna-146a and RBM4 Form a Negative Feed-Forward Loop That Disrupts Cytokine Mrna Translation Following TLR4 Responses in Human THP-1 Monocytes. Immunol Cell Biol (2013) 91(8):532–40. doi: 10.1038/icb.2013.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Banerjee S, Meng J, Das S, Krishnan A, Haworth J, Charboneau R, et al. Morphine Induced Exacerbation of Sepsis Is Mediated by Tempering Endotoxin Tolerance Through Modulation of Mir-146a. Sci Rep (2013) 3(1):1–12. doi: 10.1038/srep01977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Yang Q, Zhang D, Li Y, Li Y, Li Y. Paclitaxel Alleviated Liver Injury of Septic Mice by Alleviating Inflammatory Response via Microrna-27a/TAB3/NF-κb Signaling Pathway. Biomed Pharmacother (2018) 97:1424–33. doi: 10.1016/j.biopha.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 298. Wang J-F, Yu M-L, Yu G, Bian J-J, Deng X-M, Wan X-J, et al. Serum Mir-146a and Mir-223 as Potential New Biomarkers for Sepsis. Biochem Biophys Res Commun (2010) 394(1):184–8. doi: 10.1016/j.bbrc.2010.02.145 [DOI] [PubMed] [Google Scholar]
- 299. Mei L, He M, Zhang C, Miao J, Wen Q, Liu X, et al. Paeonol Attenuates Inflammation by Targeting HMGB1 Through Upregulating Mir-339-5p. Sci Rep (2019) 9(1):1–15. doi: 10.1038/s41598-019-55980-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Wang X, Hao L, Bu H-F, Scott AW, Tian K, Liu F, et al. Spherical Nucleic Acid Targeting Microrna-99b Enhances Intestinal MFG-E8 Gene Expression and Restores Enterocyte Migration in Lipopolysaccharide-Induced Septic Mice. Sci Rep (2016) 6(1):1–13. doi: 10.1038/srep31687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Yao Y, Xu K, Sun Y, Tian T, Shen W, Sun F, et al. Mir-215-5p Inhibits the Inflammation Injury in Septic H9c2 by Regulating ILF3 and LRRFIP1. Int Immunopharmacol (2020) 78:106000. doi: 10.1016/j.intimp.2019.106000 [DOI] [PubMed] [Google Scholar]
- 302. Wang H, Zhang P, Chen W, Feng D, Jia Y, Xie L-X. Evidence for Serum Mir-15a and Mir-16 Levels as Biomarkers That Distinguish Sepsis From Systemic Inflammatory Response Syndrome in Human Subjects. Clin Chem Lab Med (2012) 50(8):1423–8. doi: 10.1515/cclm-2011-0826 [DOI] [PubMed] [Google Scholar]
- 303. Geng X, Jia Y, Zhang Y, Shi L, Li Q, Zang A, et al. Circular RNA: Biogenesis, Degradation, Functions and Potential Roles in Mediating Resistance to Anticarcinogens. Epigenomics (2020) 12(3):267–83. doi: 10.2217/epi-2019-0295 [DOI] [PubMed] [Google Scholar]
- 304. Jiang WY, Ren J, Zhang XH, Lu ZL, Feng HJ, Yao XL, et al. Circc3p1 Attenuated Pro-Inflammatory Cytokine Production and Cell Apoptosis in Acute Lung Injury Induced by Sepsis Through Modulating Mir-21. J Cell Mol Med (2020) 24(19):11221–9. doi: 10.1111/jcmm.15685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Tian C, Liu J, Di X, Cong S, Zhao M, Wang K. Exosomal Hsa_Circrna_104484 and Hsa_Circrna_104670 may Serve as Potential Novel Biomarkers and Therapeutic Targets for Sepsis. Sci Rep (2021) 11(1):1–18. doi: 10.1038/s41598-021-93246-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Shi Y, Sun CF, Ge WH, Du YP, Hu NB. Circular RNA VMA21 Ameliorates Sepsis-Associated Acute Kidney Injury by Regulating Mir-9-3p/SMG1/Inflammation Axis and Oxidative Stress. J Cell Mol Med (2020) 24(19):11397–408. doi: 10.1111/jcmm.15741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. He Y, Sun Y, Peng J. Circ_0114428 Regulates Sepsis-Induced Kidney Injury by Targeting the Mir-495-3p/CRBN Axis. Inflammation (2021) 44(4):1–14. doi: 10.1007/s10753-021-01432-z [DOI] [PubMed] [Google Scholar]
- 308. Wei B, Yu L. Circular RNA PRKCI and Microrna-545 Relate to Sepsis Risk, Disease Severity and 28-Day Mortality. Scand J Clin Lab Invest (2020) 80(8):659–66. doi: 10.1080/00365513.2020.1827291 [DOI] [PubMed] [Google Scholar]
- 309. Liu S, Zhang D, Liu Y, Zhou D, Yang H, Zhang K, et al. Circular RNA Circ_0001105 Protects the Intestinal Barrier of Septic Rats by Inhibiting Inflammation and Oxidative Damage and YAP1 Expression. Gene (2020) 755:144897. doi: 10.1016/j.gene.2020.144897 [DOI] [PubMed] [Google Scholar]
- 310. Ma X, Zhu G, Jiao T, Shao F. Effects of Circular RNA Ttc3/Mir-148a/Rcan2 Axis on Inflammation and Oxidative Stress in Rats With Acute Kidney Injury Induced by Sepsis. Life Sci (2021) 272:119233. doi: 10.1016/j.lfs.2021.119233 [DOI] [PubMed] [Google Scholar]
- 311. Shi X, Ma W, Li Y, Wang H, Pan S, Pan Y, et al. Circprkci Relieves Lipopolysaccharide-Induced HK2 Cell Injury by Upregulating the Expression of Mir-545 Target Gene ZEB2. BioFactors (2020) 46(3):475–86. doi: 10.1002/biof.1620 [DOI] [PubMed] [Google Scholar]
- 312. Xiong H, Wang H, Yu Q. Circular RNA Circ_0003420 Mediates Inflammation in Sepsis-Induced Liver Damage by Downregulating Neuronal PAS Domain Protein 4. Immunopharmacol Immunotoxicology (2021) 43(3):271–82. doi: 10.1080/08923973.2021.1887212 [DOI] [PubMed] [Google Scholar]
- 313. Shen W, Zhao X, Li S. Exosomes Derived From Adscs Attenuate Sepsis-Induced Lung Injury by Delivery of Circ-Fryl and Regulation of the Mir-490-3p/SIRT3 Pathway. Inflammation (2021) 1–12. doi: 10.1007/s10753-021-01548-2 [DOI] [PubMed] [Google Scholar]
- 314. Zhang X, Dong S. Circ_0091702 Relieves Lipopolysaccharide (LPS)-Induced Cell Injury by Regulating the Mir-182/PDE7A Axis in Sepsis. Biosci Biotechnol Biochem (2021) 85(9):1962–70. doi: 10.1093/bbb/zbab100 [DOI] [PubMed] [Google Scholar]
- 315. Li X, Li R, Gong Q, Shi D, Song L, Song Y. Circular RNA Circvma21 Ameliorates Lipopolysaccharide (LPS)-Induced Acute Kidney Injury by Targeting the Mir-199a-5p/NRP1 Axis in Sepsis. Biochem Biophys Res Commun (2021) 548:174–81. doi: 10.1016/j.bbrc.2021.02.028 [DOI] [PubMed] [Google Scholar]
- 316. Xu H-P, Ma X-Y, Yang C. Circular RNA TLK1 Promotes Sepsis-Associated Acute Kidney Injury by Regulating Inflammation and Oxidative Stress Through Mir-106a-5p/HMGB1 Axis. Front Mol Biosci (2021) 8. doi: 10.3389/fmolb.2021.660269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Hong X, Li S, Wang J, Zhao Z, Feng Z. Circular RNA Circfads2 Is Overexpressed in Sepsis and Suppresses LPS-Induced Lung Cell Apoptosis by Inhibiting the Maturation of Mir-15a-5p. BMC Immunol (2021) 22(1):1–7. doi: 10.1186/s12865-021-00419-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Tan M, Bei R. Circ_0091702 Serves as a Sponge of Mir-545-3p to Attenuate Sepsis-Related Acute Kidney Injury by Upregulating THBS2. J Mol Histol (2021) 52(4):1–12. doi: 10.1007/s10735-021-09991-z [DOI] [PubMed] [Google Scholar]
- 319. Wei W, Yao Y, Bi H, Xu W, Gao Y. Circular RNA Circ_0068, 888 Protects Against Lipopolysaccharide-Induced HK-2 Cell Injury via Sponging Microrna-21–5p. Biochem Biophys Res Commun (2021) 540:1–7. doi: 10.1016/j.bbrc.2020.12.018 [DOI] [PubMed] [Google Scholar]
- 320. Li M, Hu J, Peng Y, Li J, Ren R. Circptk2-Mir-181c-5p-HMGB1: A New Regulatory Pathway for Microglia Activation and Hippocampal Neuronal Apoptosis Induced by Sepsis. Mol Med (2021) 27(1):1–15. doi: 10.1186/s10020-021-00305-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Li H, Zhang X, Wang P, Zhou X, Liang H, Li C. Knockdown of Circ-FANCA Alleviates LPS-Induced HK2 Cell Injury via Targeting Mir-93-5p/OXSR1 Axis in Septic Acute Kidney Injury. Diabetol Metab Syndrome (2021) 13(1):1–14. doi: 10.1186/s13098-021-00625-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Lin Q, Liang Q, Qin C, Li Y. Circankrd36 Knockdown Suppressed Cell Viability and Migration of LPS-Stimulated RAW264. 7 Cells by Sponging Mir-330. Inflammation (2021) 44(5):1–10. doi: 10.1007/s10753-021-01480-5 [DOI] [PubMed] [Google Scholar]
- 323. Xiong Y, Wang Y, Tian H, Li Y, Xu Q, He Z. CircPRKCI alleviates lipopolysaccharide (LPS)-induced HK-2 cell injury by regulating miR-106b-5p/GAB1 axis. J Cardiovasc Pharmacol (2021) 78(4):523–33. doi: 10.1097/FJC.0000000000001031 [DOI] [PubMed] [Google Scholar]