Staring at the nighttime sky can evoke the most basic existential questions: Where do we come from? What else is out there? The field of astronomy emerged from generations of scientists who were inspired by these questions. Each struggled with the tools of their time—gathering, as best they could, the light that trickled down from the stars—and from these scarce and distant data, they tried to make sense of our place in the universe.
The early 1990s offered a watershed moment for the field: the launching of the Hubble Space Telescope. Hubble offered some of the clearest images of stars and galaxies ever obtained and, in the process, transformed the scope of research. Questions previously in the realm of science fiction could now be answered.
While some scientists have looked outward, others have turned inward to wrestle with parallel existential dilemmas: How do we understand ourselves and our consciousness? What makes us human? These internal questions have often been most pointed in trying to understand the enigmatic nature of psychiatric illness—conditions that may seem to alter the fabric of who we are. As with astronomy, for most of human history we lacked the requisite tools to do this work—in this case, the ability to rigorously study brain function in living people.
Across the 20th century, new technologies enabled foundational work toward characterizing neuropsychiatric illnesses. Electroencephalogram (EEG) was instrumental in characterizing epilepsy and is still used in modern research programs. Computed tomography (CT) allowed for rapid, high-resolution structural imaging of the brain, including the identification of mass lesions. Positron emission tomography (PET) can now be used in diagnosing neurodegenerative disorders, in addition to other scientific applications. But each method offers only a partial view of the brain: EEG offers exceptional temporal signal but poor spatial resolution; CT offers superb structural information but no functional data; PET, which relies on measuring the activity of radioactively labeled molecules, offers higher spatial resolution of functional activity but exposes patients to radiation and has limited temporal resolution. Hence, the question remained: How can we obtain information about brain function with high spatial and temporal resolution?
A seminal insight came from Seiji Ogawa, at AT&T Bell Laboratories. In three papers published in 1990, Ogawa described an extraordinary application of another newly developed technology, magnetic resonance imaging (MRI) (1). Ogawa’s idea was to visualize, as in PET, which areas of the brain are functionally active. And, as in PET, the underlying concept depends on metabolism: when brain cells fire, they have a higher energy demand; this causes the vasculature to respond with an influx of fresh, oxygen-rich blood. Rather than relying on the uptake of radioactive isotopes, Ogawa recognized that local changes in blood oxygenation could be detected with MRI. This effect is now known as the blood oxygen level–dependent (BOLD) signal—and has created an entire field of research around functional MRI (fMRI).
Thus, the modern era of brain mapping was born. Within 2 years of Ogawa’s reports, three groups demonstrated BOLD could be used to identify task-induced brain activity in the motor and visual cortices (1). Additional papers followed, confirming that brain areas were functionally active in the ways they were already understood to be [e.g., Broca’s area in language production (2)]. Clinicians scrambled to adopt the new technology—for example, to determine a patient’s language-dominant hemisphere prior to neurosurgery (3). The dream of using functional imaging to help psychiatric patients seemed within reach, and multicolored images became a mainstay of media coverage.
As exciting as this was, it appears to have been the peak of a hype cycle. Early fMRI studies were foundational, but it is now clear that some ideas were overly reductionistic—the brain is vastly more complex than a few activated regions in an fMRI map might suggest. For instance, the smallest piece of data collected, a voxel, is generally a few millimeters in volume. Within this space, there are hundreds of thousands of neurons, including both excitatory and inhibitory cells, and currently there is no way to tease apart their activity. A further difficulty relates to temporal dynamics. While neural signals may cascade across thousands of synapses in a span of milliseconds, it takes 4 to 6 seconds to see a peak in the blood flow response to a stimulus. Taken together, this renders the BOLD signal, at best, a fuzzy and indirect measure of brain activity.
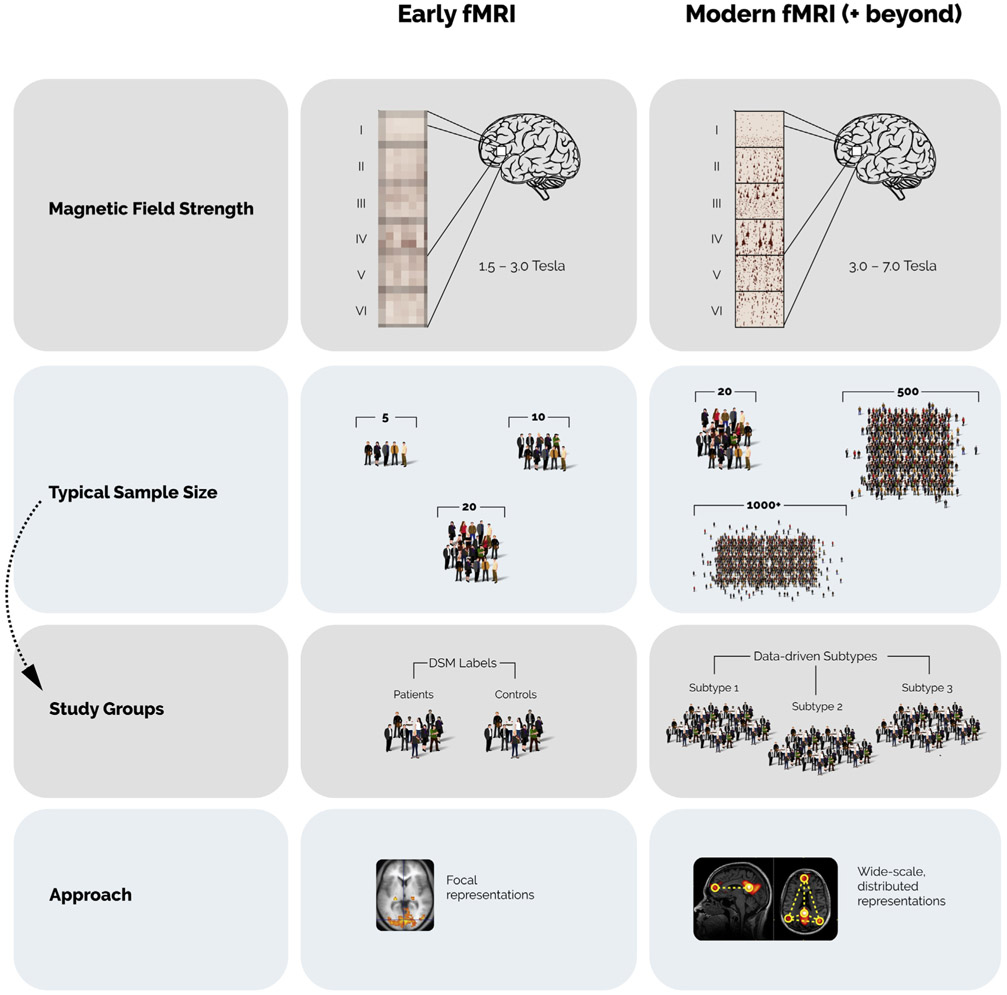
Beyond the technique itself, another historical confounding issue lay in study design: how can we navigate the enormous complexity of psychiatric phenomenology? Most early studies defined groups based on the Diagnostic and Statistical Manual (Figure 1). While the DSM offers many strengths, it was created at a time when minimal data were available regarding the pathophysiology of psychiatric illnesses and so was explicitly designed to be symptom-focused and agnostic to causality.
Figure 1.
A comparison of early and modern approaches to functional magnetic resonance imaging (fMRI) for studying psychiatric illness. Increased magnetic field strength, along with other improved imaging parameters, has increased resolution of fMRI data—in some cases, allowing for layer-specific results. Sample sizes continue to increase, with many modern datasets of >1000 individuals, allowing for the definition of data-driven subtypes of psychiatric illness, as opposed to grouping patients into DSM disease categories. As the field has advanced, there has been a shift from localizing activity in discrete regions (“focal representations”), to studying complex brain connectivity patterns (“wide-scale, distributed representations”). (Brain image at bottom left adapted from https://commons.wikimedia.org/wiki/File:1206_FMRI.jpg; brain image at bottom right adapted from https://commons.wikimedia.org/wiki/File:Default_mode_network-WRNMMC.jpg).
Over the past 20 years, it has become clear that some DSM diagnoses may be better conceptualized as syndromes rather than diseases. This was problematic for early fMRI research in which sample sizes were small: if the study groups were heterogeneous, individual studies would be unlikely to observe significant differences, and, when they did, these findings might be unlikely to replicate.
Given these issues, it is easy to feel discouraged—perhaps a natural fall from the peak of the hype cycle. So the question remains: How might we use fMRI to better understand brain function and, ultimately, to help people with psychiatric illness? Here, numerous developments offer reason for hope.
One exciting avenue is the acquisition of higher-resolution data. Whereas early research tended to report activity in whole cortical areas (Figure 1), newer studies using higher magnetic fields allow brain activity to be resolved with much greater precision. In one example, Finn et al. (4) used a 7T imaging protocol to localize activity from specific cognitive processes to distinct cortical layers in the prefrontal cortex. Such work highlights new frontiers in functional imaging and holds promise for translational research.
A second crucial development has been the ability to leverage larger, more complex datasets. With higher-resolution imaging, the amount of data per participant and study has exploded. Only with the advent of sophisticated computational tools has it been possible to meaningfully interrogate these samples. Such analytical methods are important as sample sizes increase—in some cases, up to 100,000 individuals (5). Such datasets afford the statistical power to identify real, but small, differences that would otherwise remain invisible.
Another advance has been a shift from studies focused on localizing activity within discrete regions toward whole-brain imaging incorporating complex interactions between brain areas (i.e., studying the “functional connectome”). In one fascinating study, Drysdale et al. (6) grouped patients with depression into four distinct subtypes based on functional connections and clinical symptoms. Critically, they found that these subtypes predicted differences in responsiveness to repetitive transcranial magnetic stimulation. In another example, Lake et al. (7) used a modeling approach to identify brain interactions most predictive of autism symptoms. They then showed that this model could be used to determine symptoms of inattention in a separate group of patients—supporting the notion that fMRI can identify biological signatures that transcend diagnostic categories. In both cases, it remains to be seen whether and how these findings may translate into clinical practice [particularly because of statistical and generalizability issues (8)]. Nonetheless, studies linking brain data to clinical phenotyping, and then to treatment interventions, offer a window into the potential future of precision psychiatry.
Ongoing research will further illuminate the biological basis of psychiatric illness and will likely inform new or revised diagnostic schema. This work may also pave the way for new treatments (e.g., targeted interventional approaches). Another fascinating area of study—though fraught with practical and ethical issues—is whether fMRI can be used to predict the course of illness at the individual level and in an actionable way. While media dialogue often focuses on extreme versions of this question (e.g., predicting recidivism among prisoners), there may be obvious and less controversial applications. For example, opioid use disorder represents one of the most significant public health crises in the U.S. today. One of the most vexing aspects of care is that even among patients treated with evidence-based medications, rates of those returning to use remain high (9), particularly early in treatment. A clinical risk stratification tool, as currently being tested (10), could be invaluable for enabling health care systems to focus resources on the most vulnerable patients (e.g., by ensuring optimized pharmacological management, psychotherapy, and psychosocial services).
Since its launch in 1990, Hubble has transformed our understanding of the universe, offering answers to questions that could not possibly be addressed with previous technologies. Of course, this has only paved the way to newer and bigger questions. Similarly, fMRI—despite some of its limitations—has established new foundations of knowledge and expanded the framework for future research. As technology continues to advance, we can expect to see an increasingly complete picture of the neurobiology of psychiatric illness, develop more precise interventions, and better understand the universe inside our minds.
Acknowledgments and Disclosures
Clinical Commentaries are produced in collaboration with the National Neuroscience Curriculum Initiative (NNCI). David A. Ross, in his dual roles as co-chair of the NNCI and as Education Editor of Biological Psychiatry, manages the development of these commentaries but plays no role in the decision to publish each commentary. The NNCI is funded in part by the Deeda Blair Research Initiative Fund for Disorders of the Brain through support to the Foundation for the National Institutes of Health and by National Institutes of Health Grant Nos. R25 MH08646607S1 and R44 MH115546-01.
DAR is supported by R25 MH071584-11 and by the National Center for PTSD, Clinical Neuroscience Division. CH was supported by National Institutes of Health/National Institute of General Medical Sciences Medical Scientist Training Program Grant No. T32GM007205.
The authors report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Corey Horien, Interdepartmental Neuroscience Program, Yale University School of Medicine, Yale University, New Haven, Connecticut..
R. Todd Constable, Interdepartmental Neuroscience Program, Department of Radiology and Biomedical Imaging, Department of Neurosurgery, Department of Biomedical Engineering, Yale University, New Haven, Connecticut..
David A. Ross, Department of Psychiatry, Yale University, New Haven, Connecticut.
References
- 1.Logothetis NK (2003): The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci 23:3963–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinke RM, Hu X, Stillman AE, Kim SG, Merkle H, Salmi R, Ugurbil K (1993): Functional magnetic resonance imaging of Broca’s area during internal speech. Neuroreport 4:675–678. [DOI] [PubMed] [Google Scholar]
- 3.Binder JR, Swanson SJ, Hammeke TA, Morris GL, Mueller WM, Fischer M, et al. (1996): Determination of language dominance using functional MRI: A comparison with the Wada test. Neurology 46:978–984. [DOI] [PubMed] [Google Scholar]
- 4.Finn ES, Huber L, Jangraw DC, Molfese PJ, Bandettini PA (2019): Layer-dependent activity in human prefrontal cortex during working memory. Nat Neurosci 22:1687–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller KL, Alfaro-Almagro F, Bangerter NK, Thomas DL, Yacoub E, Xu JQ, et al. (2016): Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci 19:1523–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. (2017): Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 23:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lake EMR, Finn ES, Noble SM, Vanderwal T, Shen XL, Rosenberg MD, et al. (2019): The functional brain organization of an individual allows prediction of measures of social abilities transdiagnostically in autism and attention-deficit/hyperactivity disorder. Biol Psychiatry 86:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinga R, Schmaal L, Penninx BWJH, van Tol MJ, Veltman DJ, van Velzen L, et al. (2019): Evaluating the evidence for biotypes of depression: Methodological replication and extension of Drysdale et al. (2017). Neuroimage Clin 22:101796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connery HS (2015): Medication-assisted treatment of opioid use disorder: Review of the evidence and future directions. Harvard Rev Psychiatry 23:63–75. [DOI] [PubMed] [Google Scholar]
- 10.Lichenstein SD, Scheinost D, Potenza MN, Carroll KM, Yip SW (2019): Dissociable neural substrates of opioid and cocaine use identified via connectome-based modelling [published online ahead of print Nov 12]. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]