Abstract
Whitening cosmetics have a large market scale and broad development prospects, while whitening products of traditional Chinese medicine have always been a research hotspot. In this study, the whitening active extract of Platycodon grandiflorum (PGE) was isolated and purified for the first time, and the whitening activity mechanism and chemical composition of PGE were elucidated. A total of 45 components were identified via high-performance liquid chromatography-mass spectrometry (HPLC-MS) analysis, including arbutin, syringin, chlorogenic acid, platycoside E, platycodin D3, baicalin, platycodin D, and luteolin. The scavenging rates of PGE toward DPPH and ABTS free radicals were 98.03% and 84.30%, respectively. The inhibition rate of PGE toward tyrosinase was up to 97.71%. The PGE had significant anti-inflammatory effects on RAW264.7 macrophages stimulated by lipopolysaccharide (LPS) and had significant inhibition effects on tyrosinase and melanin generation of B16F10 cells stimulated by α-MSH. The results showed that the PGE achieved a synergistic whitening effect by inhibiting the activation of oxygen free radicals on tyrosinase, antioxidation, anti-inflammatory effect, enzyme activity, and melanin generation. As a whitening agent extracted from natural plants, PGE has great potential in the research and development of plant whitening cosmetics, which lays a foundation for the further development and utilization of Platycodon grandiflorum resources and also provides a theoretical basis for the development of green and organic whitening cosmetics.
Whitening cosmetics have a large market scale and broad development prospects, while whitening products of traditional Chinese medicine have always been a research hotspot.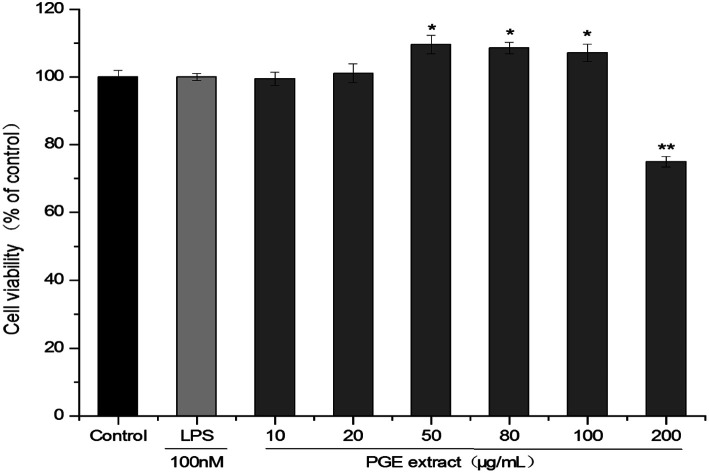
1. Introduction
In the global beauty industry, there are numerous kinds of whitening functional cosmetics, and the market share is expected to further expand. To meet the increasing demand for skin whitening agents in whitening cosmetic formulations and achieve the skin whitening effect, the cosmetic industry has introduced several chemical additives, including hydroquinone, cysteine, glutathione, vitamin A, and glucocorticoid. These compounds have good whitening effects; however, some of them have toxic side effects; for example, “rhododendrol” causes white spots in the user's skin,1 “hydroquinone” has cytotoxic and mutagenic properties,2 and “glucocorticoid” can cause hormone-dependent dermatitis.3 At present, the addition of these ingredients (rhododendrol, hydroquinone, glucocorticoid) to cosmetics is not allowed. The effect of the product is not the only standard that the consumer considers when buying cosmetics. In recent years, there has been a continuous burst of cosmetic product safety problems in the pursuit of health. Researchers have found that whitening active substances extracted from natural herbs are less toxic and have far fewer side effects. An increasing number of consumers value product safety. Furthermore, green, environmentally friendly, and organic products have become more popular, as reflected by the promotion of many direct selling brands. Therefore, the research and discovery of safe and healthy whitening skin-care ingredients have become a trend in modern development. The application of Chinese herbal extracts from natural plants as raw materials in cosmetics has gradually become a research hotspot in the development of whitening and skin-care products.4
Platycodon grandiflorum, a traditional Chinese medicinal material, has been used in China for more than 2000 years. The earliest record can be traced back to “Shennong herbal classic”.5 The traditional pharmacological action of Platycodon grandiflorum is to reduce cough and expectorate. Platycodon grandiflorum contains saponins, flavones, phenolic acids, sterols, and polysaccharides.6–8 The complexity and diversity of chemical constituents determine the diversity of their biological activities.9 Modern pharmacological research has shown that Platycodon grandiflorum has anti-inflammatory, bacteriostatic, anti-tumor, anti-oxidation, immune-regulation, and other pharmacological effects.10,11 In Korea and northern China, Platycodon grandiflorum is widely used as a raw material in food and cosmetics. Cosmetics made from Platycodon grandiflorum as the raw material, such as whitening essence, whitening milk, and whitening shower gel, are favored by consumers in Korea, Japan, and other Asia countries.12 The extract of Platycodon grandiflorum is also listed in the international cosmetic raw material catalog.13 However, the whitening active ingredients and action mechanism of Platycodon grandiflorum are still unclear, and only few preliminary studies have been conducted on its whitening activity. Gong X. J. et al. compared the tyrosinase inhibitory activities of various Chinese herbs and found that Platycodon grandiflorum had the strongest inhibitory effect.14 On this basis, Xu B. J. et al. evaluated the whitening effect of the effective constituent of Platycodon grandiflorum via a tyrosinase inhibition experiment and found that the effective constituent of Platycodon grandiflorum was probably the total saponin content.15 In this study, to further clarify the active whitening substances and whitening mechanism of Platycodon grandiflorum, we used the whitening active extract of Platycodon grandiflorum obtained through pharmacodynamic tracking method as the research object. By studying the activity and components, we clarify the composition of the whitening active substances of Platycodon grandiflorum and explore the whitening mechanism of the whitening active substances of Platycodon grandiflorum. The study will provide a theoretical basis for the better utilization of Platycodon grandiflorum as a raw material for whitening cosmetics.
Skin whitening effects involve the reduction of melanin production in the skin. Melanin is the main pigment to control the colors of skin and hair. It is a high-molecular compound widely distributed in animals and plants. When excessive melanin is produced, melanin will accumulate in the skin, forming spots and freckles, which can lead to severe skin cancer.16 Therefore, to prevent excessive skin pigmentation, it is necessary to inhibit melanin production. Melanocytes are located in the epidermal basal layer and are controlled by tyrosinase. Reactive oxygen species activate tyrosinase activity, and tyrosinase catalyzes 3,4-dihydroxyphenylalanine (DOPA) to produce DOPA quinone and then catalyzes DOPA quinone to produce melanin through autooxidation and enzyme reaction. Therefore, the inhibition of tyrosinase and melanocyte proliferation can significantly inhibit melanin production.17 Moreover, good antioxidative and anti-inflammatory effects can reduce the damage caused by oxidation and inflammation of the skin, delay skin aging, and keep skin elastic and smooth and thus exert a synergistic whitening effect.18,19
Therefore, to find a natural active extract from Platycodon grandiflorum with whitening, antioxidative, and anti-inflammatory properties, this study mainly focused on the effects of Platycodon grandiflorum extract (PGE) on tyrosinase inhibition, intracellular tyrosinase inhibition, and the inhibitory activity of cell melanin generation. The study aimed to analyze the inhibitory effect of PGE on tyrosinase activity and melanin generation and comprehensively evaluate the whitening and skin-care effect of PGE and its antioxidant and anti-inflammatory effects. The chemical composition of PGE was identified and analyzed via high-performance liquid chromatography (HPLC) and liquid chromatography (LC)/mass spectrometry (MS), and the specific whitening active material of the PGE was clarified. It is vital to develop new Platycodon grandiflorum products and promote the development of the Platycodon grandiflorum industry.
2. Materials and methods
2.1. Materials
Formic acid, methanol, 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), acetylacetone, NaOH, K2S2O8, and Ehrlich reagent were obtained from Beijing Chemical Plant. Platycodin D, arbutin, platycodin D3, platycoside E, syringin, chlorogenic acid, baicalin, and luteolin reference products were obtained from China Institute of Pharmaceutical Biological Products Identification. The chromatographically pure acetonitrile and methanol were obtained from J.T. Baker Co., USA. Tyrosinase, l-tyrosine, sodium hyaluronate, and hyaluronate were obtained from Beijing Solebo Technology Co., Ltd. The original 264.7 cells and B16F10 cells were purchased from the Cell Resource Center of the Shanghai Institute of Biological Sciences, China and stored in the laboratory of a medical college affiliated with the Changchun University of Chinese Medicine. Dulbecco's Modified Eagle's Medium (DMEM), fetal bovine serum, and antibiotics (penicillin and streptomycin) were obtained from Gibco USA. Triton X-100, α-MSH, ELISA kit (NO, IL-6, TNF-α), and cell counting kit-8 (CCK-8) kit were obtained from Changchun Baijin Biotechnology Co., Ltd. Pure water was obtained from Wahaha Food Co. Ltd.
2.2. Preparation of Platycodon grandiflorum whitening active extract
Platycodon grandiflorum was supplied by Hebei Renxin Pharmaceutical Co. Ltd. Platycodon grandiflorum was crushed into fine powder, heated, and refluxed with 95% ethanol solution three times, 1 h for each time. Then, the product was filtered, and the filtrate was recovered and merged with the filtrate obtained from three extractions. The filtrate was dried and mixed with distilled water for resolution, and n-butanol (1 : 1 volume) saturated with water was extracted five times. The n-butanol layer was combined, the n-butanol solution was removed, and the PGE was obtained. The extraction rate of PGE from Platycodon grandiflorum was 8.9%.
2.3. Cell culture
B16F10 mouse melanoma cells and RAW264.7 cells were cultured in DMEM supplemented with 10% fetal bovine serum, 100 U mL−1 penicillin, and 100 g mL−1 streptomycin in humidified air of 5% CO2 at 37 °C. When the cells were grown to the fusion state, they were digested by trypsin and the cells were passed every two days. All experiments were performed three times and repeated three times to ensure repeatability.
2.4. Cell viability assay
To evaluate the safety of PGE, the effects of PGE on B16F10 and RAW264.7 cells were determined through the CCK-8 method according to the manufacturer's instructions.20 First, B16F10 cells and RAW264.7 cells were cultured in 96-well plates at concentrations of 5 × 103 cells per well and 1 × 104 cells per well for 24 h and then treated with PGE or DMEM at different concentrations for 72 h. After treatment, 10 μL CCK-8 reagent was added to each well, and the cell was further cultured for 2 h. The absorbance at 450 nm was measured using a microplate reader.
2.5. Antioxidant activity
The antioxidant activity of PGE was determined via DPPH and ABTS free-radical scavenging tests, and ascorbic acid was used as the positive control.21,22 The results were expressed as the percentage inhibition rate.
Furthermore, PGE solution, ascorbic acid, and platycodin D with different concentrations (0.2, 0.4, 0.6, 0.8, 1.0, and 1.2 mg mL−1) were added to a clog test tube. Then, 2 mL DPPH solution was added into each test tube; the tubes were vortexed, the mixture was mixed and allowed to react in a dark chamber for 30 min, and the absorbance A1 at 520 nm was determined. Then, 2 mL methanol was used as the control group instead of DPPH solution to determine the absorbance value A2. The absorbance value A0 was determined by replacing the sample solution with 2 mL methanol as the blank control group. Adjusted the absorbance value to 0 with methanol. The DPPH radical scavenging rate was calculated according to the following formula:
| DPPH radical scavenging rate (%) = (A0 − A1 + A2)/A0 × 100. | 1 |
To prepare ABTS+· mother liquor, 35.2 mg ABTS and 6.139 mg potassium persulfate were mixed to a constant volume of 10 mL. The liquor was diluted with methanol after 12–16 h of reaction at room temperature until the absorption value of the solution at 734 nm was about 7.0 ± 0.2. Then, 30 μL PGE solution, ascorbic acid, and platycodin D with different concentrations (0.2, 0.4, 0.6, 0.8, 1.0, 1.2 mg mL−1) were mixed with 220 μL ABTS+˙ solution in a 96-well enzyme-labeled plate, and the absorbance at 734 nm was measured after reaction in the dark for 6 min.
| ABTS free-radical scavenging rate (%) = (A0 − AS)/A0 × 100, | 2 |
where A0 is the absorbance of the blank sample, and AS is the absorbance of the sample to be measured.
2.6. Lipopolysaccharide-induced anti-inflammatory activity of RAW264.7 macrophages
RAW264.7 macrophages in 1 mL medium were seeded into a 24-well plate, with 1 × 104 cells per well, and cultured overnight so that the cells adhered to the wall. The cells were cultured for 24 h after exposure to lipopolysaccharide (LPS) and PGE extracts at different concentrations (10, 50, 100 μg mL−1). The concentrations of NO, IL-6, and TNF-α in the cell supernatant were determined using an ELISA kit, as directed by the manufacturer.23
2.7. Tyrosinase activity
Using a method reported in the literature, with phosphate buffer (25 mmol, pH = 6.8) as solvent, l-tyrosine substrate solution (0.5 mg mL−1), tyrosine enzyme solution (50 U mL−1), and other solutions in 96-well plates of a 240 μL total reaction system, along with 120 μL substrate solution, 40 μL phosphate buffer, 40 μL sample solution, and blender. Then, 40 μL tyrosine enzyme solution was added to the above system. The response of constant temperature of a 37 °C water bath for 20 min was noted, and the absorbance was measured at 475 nm.24
| Tyrosinase inhibition (%) = [(A − B)/(C − D)]/(A − B) × 100, | 3 |
where A is the absorbance of the sample solution replaced by the equivalent buffer solution, B is the absorbance of the sample solution and tyrosinase solution replaced by the equivalent buffer solution, C is the absorbance of the sample solution, and D is the absorbance of the equivalent buffer solution instead of the tyrosinase solution (Table 1).
Tyrosinase catalyzed reaction system.
| Reagent | A | B | C | D |
|---|---|---|---|---|
| Phosphate buffer with pH = 6.8 | 80 | 120 | 40 | 80 |
| 0.5 mg mL−1l-tyrosine | 120 | 120 | 120 | 120 |
| PGE sample solution | — | — | 40 | 40 |
| 50 U mL−1 tyrosinase solution | 40 | — | 40 | — |
2.8. Inhibitory activity of melanin formation
B16F10 melanoma cells in 1 mL medium were seeded into a 24-well culture plate, with 1 × 104 cells per well, and cultured overnight for the cells to adhere to the wall. The cells were cultured with α-melanocyte-stimulating hormone (100 nM α-MSH) for 48 h and cultured with different PGE and arbutin concentrations (100, 150, 200 mg mL−1) for 24, 48, and 72 h. After the administration time, the cells were washed twice with PBS (pH = 7.2) and mixed with PBS 200 μL containing 1% Triton X-100. The cells were lysed by freezing and thawing. After the system was centrifuged at 12 000 rpm for 30 min, the supernatant was removed, and 1 M NaOH containing 10% dimethyl sulfoxide (300 μL) was added into the cell granules; then reacted at 80 °C for 2 h to lyse melanin in the cells.25 The absorbance at 450 nm was determined.
| Melanin production inhibition (%) = (administration group A450 − blank group A450)/(control group A450 − blank group A450) × 100. | 4 |
2.9. Cell tyrosinase activity
B16F10 melanoma cells in 1 mL medium were seeded into a 24-well culture plate, with 1 × 104 cells per well, and cultured overnight for the cells to adhere to the wall. The cells were cultured with α-melanocyte-stimulating hormone (100 nM α-MSH) for 48 h and cultured with different concentrations (100, 150, 200 mg mL−1) of PGE and arbutin for 24, 48, and 72 h. After the administration time, the cells were washed twice with PBS (pH = 7.2) and mixed with PBS 200 μL containing 1% Triton X-100. The cells were lysed by freezing and thawing. After centrifugation at 12 000 rpm for 30 min, the supernatant was deposited into a 96-well plate, 100 μL per well, and mixed with 100 μL 0.1% l-DOPA solution. After incubation at 37 °C for 2 h, the absorbance at 475 nm was immediately determined.26
| Cell tyrosinase inhibition (%) = (administration group A475 − blank group A475)/(control group A475 − blank group A475) × 100. | 5 |
2.10. Chemical composition analysis
2.10.1. HPLC analysis
The PGE sample solution was analyzed using a Shimadzu LC-2010AHT HPLC system with an ELSD6000 evaporative light scattering detector (Alltech CHROM).27 The chromatographic separation was performed on a Sepax Bio-C18 column (4.6 × 250 mm, 5 μm, from Sepax Technologies, Delaware, USA). The mobile phase system was composed of mobile phase A (acetonitrile) and mobile phase B (0.1% formic acid water). The solvent gradient is presented in Table 2. The chromatographic conditions were as follows: the flow rate was 0.8 mL min−1, the initial evaporative light scattering detection (ELSD) temperature was 105 °C, the gas flow rate was 2.8 L min−1, and the injection quantity was 20 L. Twenty batches of PGE were prepared.
Gradient elution procedures.
| Time (min) | A: acetonitrile (%) | B: 0.1% formic acid water (%) |
|---|---|---|
| 0–12 | 5.0 | 95.0 |
| 12–15 | 5.0–15.0 | 95.0–85.0 |
| 15–45 | 15.0–35.0 | 85.0–65.0 |
| 45–50 | 35.0–50.0 | 65.0–50.0 |
| 50–60 | 50.0 | 50.0 |
2.10.2. Analysis of PGE chemical composition via HPLC combined with mass spectrometry
The Q-Orbitrap high-resolution LC/MS technology was used in combination with the Q Exactive high-resolution mass spectrometer on the Ultimate 3000 RS chromatography system to analyze and identify the chemical composition of the PGE sample solution via MS.28 The MS conditions are as follows: ion source: electrospray ionization source; scan mode: positive and negative ions scanning switch; detection method: full mass/dd-MS2; resolution: 70 000 (full mass), 17 500 (dd-MS2); scanning range: 150.0–2000.0 m/z; electrospray voltage: 3.8 kV (positive); capillary temperature: 300 °C; collision gas: high-purity argon (purity ≥ 99.999%); sheath gas: nitrogen (purity ≥ 99.999%, 40 arb); auxiliary gas: nitrogen (purity ≥ 99.999%, 350 °C); data acquisition time: 30.0 min. Chromatographic conditions: chromatographic column: RP-C18 (150 × 2.1 mm, 1.8 μm, Welch); flow rate: 0.30 mL min−1; aqueous phase: 0.1% formic acid aqueous solution; organic phase: 0.1% formic acid acetonitrile; needle washing liquid: methanol; column temperature: 35 °C; injection volume: 5.00 μL. The solvent gradient is shown in Table 3.
Gradient elution procedures.
| Time (min) | A: acetonitrile (%) | B: 0.1% formic acid water (%) |
|---|---|---|
| 0–1 | 2.0 | 98.0 |
| 1–5 | 2.0–20.0 | 98.0–80.0 |
| 5–10 | 20.0–50.0 | 80.0–50.0 |
| 10–15 | 50.0–80.0 | 50.0–20.0 |
| 15–20 | 80.0–95.0 | 20.0–5.0 |
| 20–25 | 95.0 | 5.0 |
| 25–26 | 95.0–5.0 | 5.0–95.0 |
| 26–30 | 5.0 | 95.0 |
2.11. Data statistics
All experiments were conducted at least three times. The data were reported as mean value ± standard deviation. Statistical analyses were conducted using SPSS 21.0 and Origin 9.0. For multiple comparisons, data were subjected to one-way ANOVA (Turkey's post hoc) and paired t-test to determine statistical significance. p < 0.05 was considered statistically significant differences between the groups.
3. Results
3.1. The effects of PGE on the viability of B16F10 cells and 264.7 cells
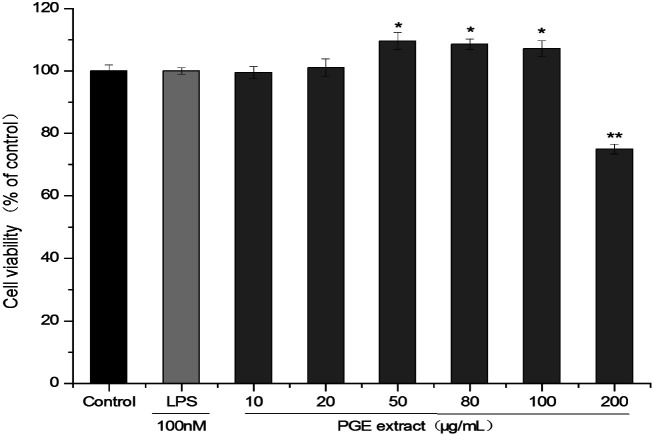
The effects of PGE on B16F10 melanoma cells and RAW264.7 macrophage were detected via the CCK-8 method. The cells were treated with different PGE concentrations for 24 h and then detected using the CCK-8 method. The results are expressed as a percentage of the survival relative to the control group. Under PGE concentrations of 10–100 μg mL−1 (cell viability: 99.42 ± 1.951–107.17 ± 2.601%), the PGE exhibited no cytotoxicity on the 264.7 cells (Fig. 1). However, a significant decrease in cell activity occurred at the PGE concentration of 200 μg mL−1 (cell viability: 74.91 ± 1.574%). Therefore, a dosage range of 10–100 μg mL−1 (The extraction rate of PGE in Platycodon Grandiflorum was 8.9%. A concentration of 10–100 μg mL−1 of PGE is equivalent to 0.11–1.12 mg mL−1 of Platycodon Grandiflorum. Adding 1 mL of drug-containing medium per well is equivalent to adding 0.11–0.12 mg of Platycodon Grandiflorum.) should be selected. Here, 10 μg mL−1, 50 μg mL−1, and 100 μg mL−1 (concentration of plant medicinal materials: 0.11 mg mL−1, 0.56 mg mL−1, 1.12 mg mL−1, respectively) were selected as low, medium, and high concentrations.
Fig. 1. Effect of PGE concentration on the activity of 264.7 cells. 264.7 Cells were treated with different PGE concentrations for 24 h, and the cell viability was determined via the CCK-8 method. The absorbance at 450 nm was measured using a microplate reader. The value represents the average of the three experiments. Data were analyzed by paired t-test. There was a significant difference between the treatment group and the control group: *p < 0.05, **p < 0.001. The error line represents the SD.
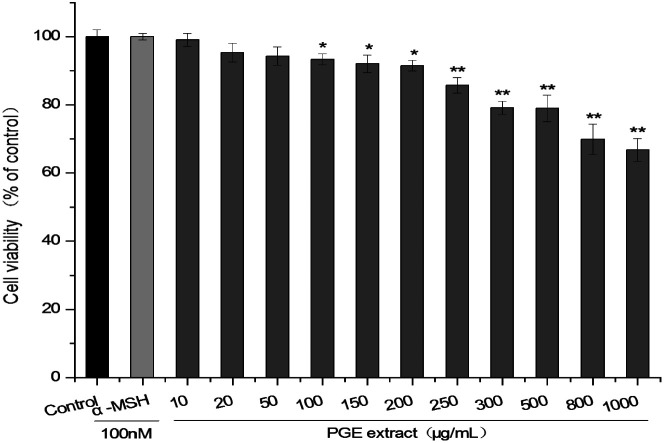
The activity of B16F10 cells was slightly different from that of 264.7 cells. When the PGE concentration was 10–200 μg mL−1 (cell viability: 99.03 ± 1.965%–91.48 ± 1.382%), the activity of B16F10 cells was not significantly different from that of the control group. However, when the concentration was 250 μg mL−1 (cell viability: 85.69 ± 2.284%), the activity of B16F10 cells began to decline, and the cell activity under PGE concentration of 1000 μg mL−1 (cell viability: 70.75 ± 3.337%) was the lowest. Therefore, a dosage range of 10–200 μg mL−1 (The extraction rate of PGE in Platycodon Grandiflorum was 8.9%. A concentration of 100–200 μg mL−1 of PGE is equivalent to 1.12–2.24 mg mL−1 of Platycodon Grandiflorum. Adding 1 mL of drug-containing medium per well is equivalent to adding 1.12–2.24 mg of Platycodon Grandiflorum.) should be selected. Here, 100 μg mL−1, 150 μg mL−1, and 200 μg mL−1 (concentration of plant medicinal materials: 1.12 mg mL−1, 1.68 mg mL−1, 2.24 mg mL−1, respectively) were selected as low, medium, and high concentrations, respectively (Fig. 2).
Fig. 2. Effect of PGE concentration on the activity of B16F10 cells. B16F10 cells were treated with different PGE concentrations for 24 h, and the cell viability was determined via the CCK-8 method. The absorbance was measured at 450 nm with a microplate reader. The value represents the average of the three experiments. Data were analyzed by paired t-test. There was a significant difference between the treatment group and the control group: *p < 0.05, **p < 0.001. The error line represents the SD.
3.2. Antioxidant capacity of PGE
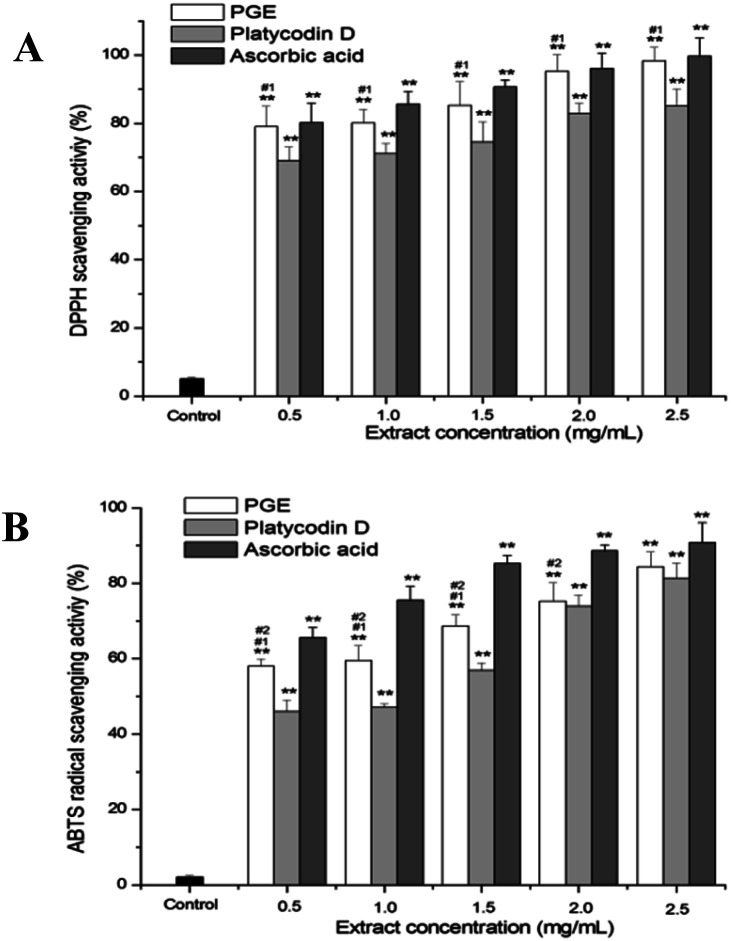
DPPH and ABTS free-radical scavenging tests were used to measure the antioxidant activity of PGE. The scavenging activities of PGE toward DPPH and ABTS radicals were determined under different PGE concentrations (0.5, 1.0, 1.5, 2.0, 2.5 mg mL−1). Platycodin D and ascorbic acid were used as the positive control. As shown in Fig. 3, the scavenging activity of PGE toward DPPH and ABTS free radicals increased in a dose-dependent manner, similar to the results of the positive control group. When the PGE concentration was 6.25 mg mL−1, the scavenging activity toward the DPPH radical was 98.03 ± 0.60%, almost the same as the scavenging activity of ascorbic acid. Moreover, PGE also showed strong scavenging activity toward ABTS radical (84.30 ± 0.53%), which was higher than that of the platycodin D control group.
Fig. 3. Antioxidant activity of PGE: (A) scavenging effect of PGE on DPPH free radical. (B) ABTS free-radical scavenging activity of PGE. PGE, platycodin D, and ascorbic acid (0.5, 1.0, 1.5, 2.0, 2.5 mg mL−1) were separately incubated with DPPH and ABTS solutions. The results are expressed as a percentage of the controls, and the data represent average of the three independent trials. Data were analyzed by one-way ANOVA (Turkey's post hoc). There was a significant difference between the treatment group and the control group: *p < 0.05, **p < 0.001. There was a significant difference between the administration group and the platycodin D group: #1p < 0.05, ##1p < 0.001. There was a significant difference between the administration group and the ascorbic acid group: #2p < 0.05, ##2p < 0.001. The error line represents the SD.
3.3. PGE reduction effect on LPS-stimulated inflammatory response of RAW264.7 macrophages
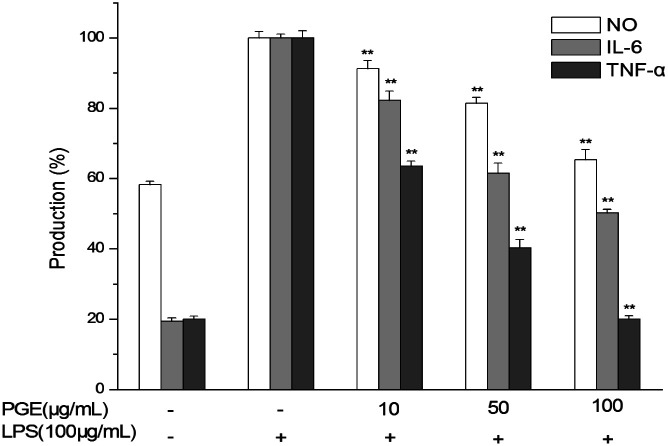
According to the results of the effect of PGE on the RAW264.7 macrophage activity in Section 3.1, PGE concentrations of 10, 50, and 100 μg mL−1 were selected as low, medium, and high doses, respectively, to test the effect of PGE on LPS-stimulated RAW264.7 macrophage inflammation. As shown in Fig. 4, the PGE dose-dependently reduced the NO production in LPS-stimulated RAW264.7 macrophages and dose-dependently reduced the levels of IL-6 and TNF-α inflammatory factors.
Fig. 4. Anti-inflammatory effects of PGE on LPS-stimulated RAW264.7 macrophage inflammatory response. The RAW264.7 macrophages were pretreated with different PGE concentrations and 100 μg mL−1 LPS. After 24 h, the cells and the culture medium were treated to determine the levels of NO, IL-6, and TNF-α. The data represent the average of the three independent experiments. Data were analyzed by paired t-test. There was a significant difference between the treatment group and the control group: *p < 0.05, **p < 0.001. The error line represents the SD.
3.4. Effects of PGE on mushroom tyrosinase activity, melanin content in B16F10 melanocytes, and intracellular tyrosinase activity
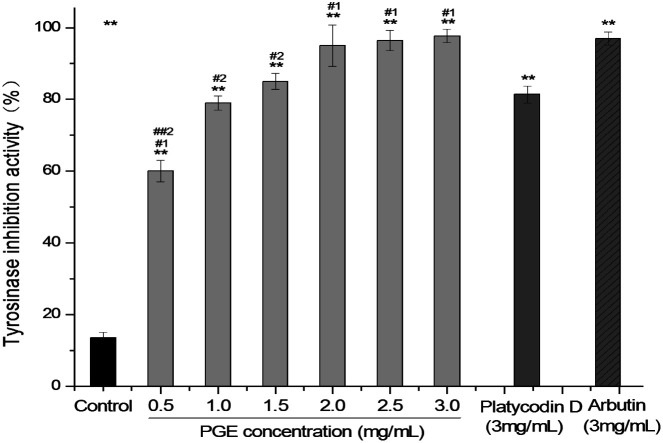
As shown in Fig. 5, the tyrosinase inhibitory activity of PGE increased significantly with the increase in the extract concentration. The higher the PGE concentration, the stronger the inhibitory activity of tyrosinase. The tyrosinase inhibitory activities of PGE at 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mg mL−1 were 60.29%, 79.32%, 85.14%, 95.23%, 96.43%, and 97.71%, respectively. However, when the PGE concentration was 2.5–3.0 mg mL−1, the tyrosinase inhibitory activity did not significantly increase; thus, it was not necessary to conduct experiments with higher concentrations. Under the same conditions, the inhibition rate of 3.0 mg mL−1 PGE (concentration of plant medicinal materials: 33.71 mg mL−1) was similar to that of arbutin (3 mg mL−1, 96.97 ± 1.849%) and higher than that of platycodin D (3 mg mL−1, 81.67 ± 2.331%).
Fig. 5. Tyrosinase inhibitory activity of PGE under different concentrations (0.5, 1.0, 1.5, 2.0, 2.5, 3.0 mg mL−1). Data represent the average of three independent experiments. Data were analyzed by one-way ANOVA (Turkey's post hoc). There was a significant difference between the treatment group and the control group: *p < 0.05, **p < 0.001. There was a significant difference between the administration group and the platycodin D group: #1p < 0.05, ##1p < 0.001. There was a significant difference between the administration group and the arbutin group: #2p < 0.05, ##2p < 0.001. The error line represents the SD.
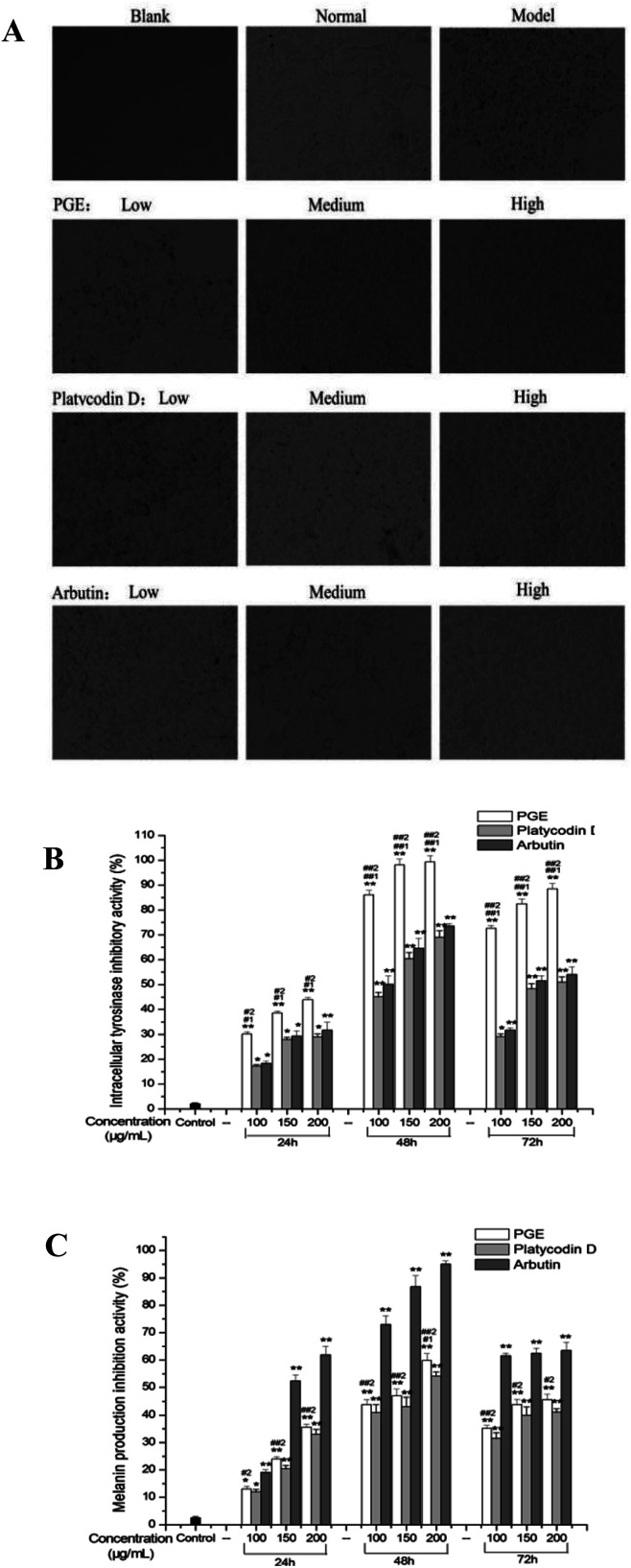
According to the effect of PGE on the activity of B16F10 melanocytes in Section 3.1, PGE concentrations of 100, 150, and 200 μg mL−1 PGE were selected as low, medium, and high doses to test the effect of PGE on the melanin content and tyrosinase activity of B16F10 melanocytes stimulated by α-MSH. As shown in Fig. 6, the tyrosinase activity of B16F10 cells stimulated by 100 nM α-MSH (control group) was significantly higher than that of non-stimulated cells. With the increase in the PGE concentration, the inhibitory activities of tyrosinase and melanin production on B16F10 cells significantly increased in a dose-dependent manner. With the increase in the administration time, the inhibitory activity of PGE on B16F10 cells tyrosinase and melanin production increased gradually.
Fig. 6. Effects of PGE on tyrosinase inhibition and melanin formation inhibition in B16F10 melanocytes. (A) Cell morphology of B16F10 in each group; (B) tyrosinase inhibition rate of α-MSH-stimulated B16F10 melanocytes by PGE; (C) inhibition of melanin production by PGE on α-MSH-stimulated B16F10 melanocytes. After stimulation with α-MSH, the cells were exposed to different PGE concentrations for 24, 48, and 72 h, and the inhibition rate was determined according to the test method. The experiment was in triplicate, and the results were expressed as the mean value. Data were analyzed by one-way ANOVA (Turkey's post hoc). There was a significant difference between the treatment group and the control group: *p < 0.05, **p < 0.001. There was a significant difference between the administration group and the platycodin D group: #1p < 0.05, ##1p < 0.001. There was a significant difference between the administration group and the arbutin group: #2p < 0.05, ##2p < 0.001. The error line represents SD.
3.5. Chemical composition of PGE
3.5.1. HPLC analysis
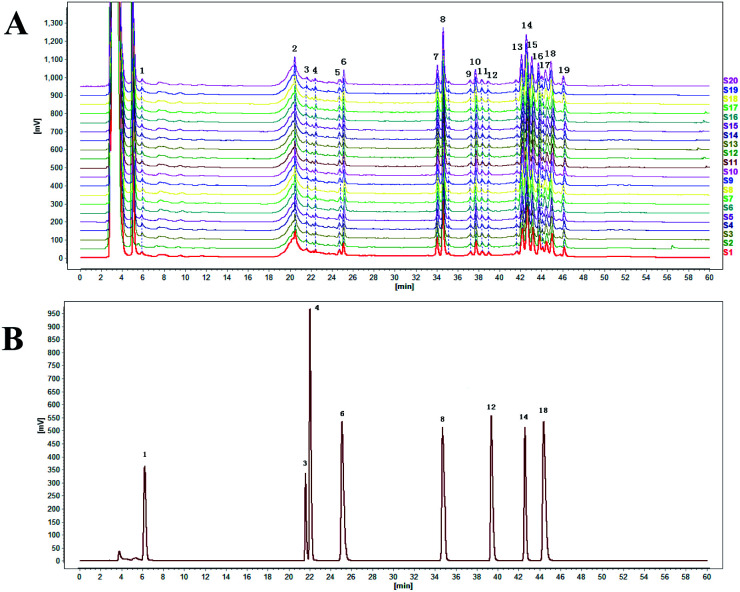
Twenty typical PGE chromatograms with good quality-control lots, obtained through HPLC analysis, are shown in Fig. 7A. Nineteen PGE peaks were identified from the HPLC fingerprint. Eight peaks of PGE, including arbutin (no. 1), syringin (no. 3), chlorogenic acid (no. 4), platycoside E (no. 6), platycodin D3 (no. 8), baicalin (no. 12), platycodin D (no. 14), and luteolin (no. 18), were identified through comparison with corresponding reference standards (Fig. 7). The relative contents of individual components in the eight peaks of PGE are presented in Table 4.
Fig. 7. (A) HPLC diagram of 20 batches of PGE samples. (B) Chromatogram of the mixed control substance.
Identification and determination of compounds in PGE by HPLC.
| Number | Retention time of control substance (min) | Retention time of PGE sample (min) | Compounds | Contents (%) |
|---|---|---|---|---|
| 1 | 5.917 ± 0.041 | 5.872 ± 0.052 | Arbutin | 0.192 ± 0.003 |
| 3 | 20.519 ± 0.124 | 20.462 ± 0.104 | Syringin | 0.022 ± 0.001 |
| 4 | 22.492 ± 0.097 | 22.421 ± 0.119 | Chlorogenic acid | 0.007 ± 0.001 |
| 6 | 25.115 ± 0.070 | 25.099 ± 0.040 | Platycoside E | 0.153 ± 0.012 |
| 8 | 34.656 ± 0.029 | 34.595 ± 0.058 | Platycodin D3 | 0.174 ± 0.032 |
| 12 | 38.922 ± 0.204 | 38.898 ± 0.194 | Baicalin | 0.019 ± 0.005 |
| 14 | 42.623 ± 0.198 | 42.583 ± 0.065 | Platycodin D | 0.184 ± 0.040 |
| 18 | 44.998 ± 0.090 | 44.914 ± 0.030 | Luteolin | 0.04 ± 0.003 |
3.5.2. HPLC-MS analysis
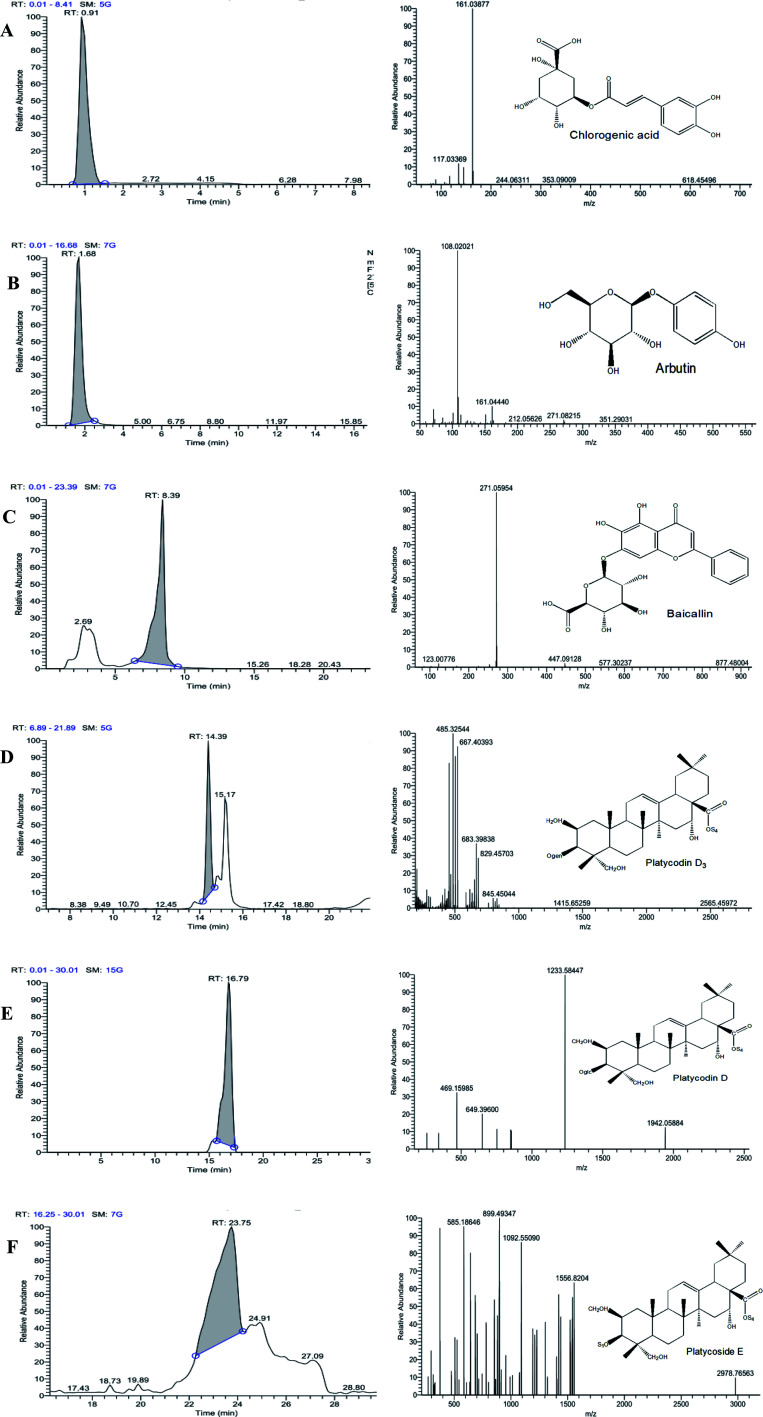
The chemical composition of PGE was analyzed via HPLC-MS (Fig. 8). The molecular ion peaks [M − H]− and [M + H]+ of each chromatographic peak in the positive- and negative-ion modes were detected via ultrahigh-performance liquid chromatography coupled with electrospray ionization-quadrupole time-of-flight-mass spectrometry, and the possible molecular weight was determined through the molecular ion peak. The different components were determined based on their specific molecular ions and fragment ions and their chromatographic peak retention time. Moreover, 45 compounds in PGE were preliminarily identified (see Table 5), mainly including chlorogenic acid, arbutin, baicalin, platycodin D3, platycodin D, and platycoside E, with peak times of 0.91, 1.68, 8.39, 14.39, 16.79, and 23.75 min, respectively.
Fig. 8. (A) Chlorogenic acid in PGE, (B) arbutin in PGE, (C) baicalin in PGE, (D) platycodin D3 in PGE, (E) platycodin D in PGE, (F) platycoside E in PGE.
List of PGE compounds identified by EHC-ELECTROspray ionization time-of-flight mass spectrometry.
| Component name | Formula | Molecular weight | t r/min | Area | [M + H]+1 | [M − H]−1 | |
|---|---|---|---|---|---|---|---|
| 1 | Choline | C5H13NO | 103.10 | 0.79 | 28 363 072.71 | 104.11 | — |
| 2 | N-α-l-Acetyl-arginine | C8H16N4O3 | 216.12 | 1.46 | 316 694.05 | 217.13 | — |
| 3 | Nicotinic acid | C6H5NO2 | 123.03 | 1.66 | 2 002 200.34 | 124.04 | — |
| 4 | Arbutin | C12H16O7 | 272.25 | 1.68 | 111 740 107.67 | — | 271.08 |
| 5 | Adenosine | C10H13N5O4 | 267.10 | 4.30 | 2 188 523.44 | 268.10 | — |
| 6 | Guanine | C5H5N5O | 151.05 | 4.70 | 775 154.87 | 152.06 | — |
| 7 | 5-Hydroxymethyl-2-furaldehyde | C6H6O3 | 126.03 | 5.36 | 23 311 959.24 | 127.04 | — |
| 8 | Caprolactam | C6H11NO | 113.08 | 7.67 | 1 106 687.15 | 114.09 | — |
| 9 | 2,3,4,9-Tetrahydro-1H-β-carboline-3-carboxylic acid | C12H12N2O2 | 216.09 | 8.26 | 22 924 490.45 | 217.10 | — |
| 10 | PEG n6 | C12H26O7 | 282.17 | 8.54 | 16 613 381.99 | 283.18 | — |
| 11 | Chlorogenic acid | C16H18O9 | 354.09 | 9.54 | 16 013 808.49 | — | 353.09 |
| 12 | PEG n8 | C16H34O9 | 370.22 | 9.77 | 27 674 930.56 | 371.23 | — |
| 13 | Baicalin | C21H18O11 | 446.08 | 13.17 | 186 540 877.8 | 447.09 | — |
| 14 | Daidzein | C15H10O4 | 254.06 | 13.34 | 2 629 710.14 | 255.07 | — |
| 15 | Citroflex 2 | C12H20O7 | 276.12 | 13.48 | 481 741.67 | 277.13 | — |
| 16 | Quercetin | C15H10O7 | 302.04 | 13.72 | 521 788.10 | 303.05 | — |
| 17 | Luteolin | C15H10O6 | 286.05 | 14.14 | 385 143 668.2 | 287.05 | — |
| 18 | Platycodin D3 | C63H102O33 | 1386.62 | 14.39 | 64 554 026.73 | 1387.64 | — |
| 19 | Diosmetin | C16H12O6 | 300.06 | 14.97 | 2 013 085.79 | 301.07 | — |
| 20 | 5,7,8-Trihydroxyflavone | C15H10O5 | 270.05 | 15.03 | 1 991 413.92 | 271.06 | — |
| 21 | N,N′-Dicyclohexylurea | C13H24N2O | 224.19 | 15.90 | 1 942 882.25 | 225.20 | — |
| 22 | 9-Oxo-ODE | C18H30O3 | 294.22 | 15.90 | 6 091 276.23 | 295.23 | — |
| 23 | Bis(4-ethylbenzylidene)sorbitol | C24H30O6 | 414.20 | 16.78 | 2 685 174.67 | 415.21 | — |
| 24 | Platycodin D | C57H92O28 | 1225.33 | 16.79 | 177 505.78 | — | 1233.57 |
| 25 | 3,5-Di-tert-butyl-4-hydroxybenzaldehyde | C15H22O2 | 234.16 | 17.65 | 3 285 930.76 | 235.17 | — |
| 26 | (±)12(13)-DiHOME | C18H34O4 | 314.25 | 17.91 | 22 222 761.43 | — | 313.24 |
| 27 | Eleutheroside B | C17H24O9 | 372.14 | 18.14 | 44 768 081.16 | 373.15 | — |
| 28 | Tributyl phosphate | C12H27O4P | 266.16 | 18.22 | 6 624 600.81 | 267.17 | — |
| 29 | α-Eleostearic acid | C18H30O2 | 278.22 | 19.02 | 13 808 673.32 | 279.23 | — |
| 30 | 9-Oxo-10(E),12(E)-octadecadienoic acid | C18H30O3 | 294.22 | 19.03 | 97 846 270.22 | 295.23 | — |
| 31 | Oleamide | C18H35NO | 281.27 | 19.28 | 829 137.62 | 282.28 | — |
| 32 | 16-Hydroxyhexadecanoic acid | C16H32O3 | 272.23 | 21.08 | 2 215 104.92 | — | 271.23 |
| 33 | Palmitoyl ethanolamide | C18H37NO2 | 299.28 | 21.11 | 1 242 465.64 | 300.29 | — |
| 34 | 1-Linoleoyl glycerol | C21H38O4 | 354.28 | 21.14 | 13 048 620.46 | 355.28 | — |
| 35 | Hexadecanamide | C16H33NO | 255.26 | 21.22 | 13 320 000.96 | 256.26 | — |
| 36 | 9S,13R-12-Oxophytodienoic acid | C18H28O3 | 292.20 | 21.53 | 649 742.30 | 293.21 | — |
| 37 | Stearamide | C18H37NO | 283.29 | 22.44 | 38 840 877.19 | 284.29 | — |
| 38 | α-Eleostearic acid | C18H30O2 | 278.22 | 22.45 | 20 545 039.37 | 279.23 | — |
| 39 | 9(Z),11(E),13(E)-Octadecatrienoic acid methyl ester | C19H32O2 | 292.24 | 22.62 | 1 997 174.74 | 293.25 | — |
| 40 | γ-Linolenic acid ethyl ester | C20H34O2 | 306.26 | 23.20 | 26 726 604.03 | 307.26 | — |
| 41 | Bis(2-ethylhexyl) phthalate | C24H38O4 | 390.28 | 23.21 | 27 051 586.57 | 391.28 | — |
| 42 | Ricinoleic acid methyl ester | C19H36O3 | 294.26 | 23.48 | 1 328 788.14 | 295.26 | — |
| 43 | Platycoside E | C69H112O38 | 1548.68 | 23.75 | 47 216 662.52 | — | 1557.68 |
| 44 | Erucamide | C22H43NO | 320.31 | 24.17 | 126 743 674.5 | 321.31 | — |
| 45 | Docosanamide | C22H45NO | 339.35 | 25.92 | 1 216 264.13 | 340.36 | — |
4. Discussion
Reactive oxygen species are a major cause of harmful skin conditions and can lead to irregular pigmentation, connective tissue degeneration, inflammatory reactions, and, in extreme cases, mutations.29 Through research confirmation, the oxygen free radical that ultraviolet ray induces can promote tyrosinase expression, causing an increase in the melanin generation.30 The increase in the number of free radicals in the skin is one of the important causes of skin aging; this increase not only damages the biological membrane but can also lead to the release of some hydrolytic enzymes from cells, the production of skin collagen fibers, the crosslinking of elastic fibers, brittleness, degeneration, elasticity loss, the thickening of the skin cutin layer, skin roughness, relaxation, and wrinkle formation.31 Therefore, the natural antioxidant substances contained in traditional Chinese medicine can capture and neutralize oxygen free radicals, thus preventing the damage caused by oxygen free radicals to the human body; delay skin aging; maintain skin elasticity and smoothness; improve skin condition and skin tone; and induce skin whitening.32 This study proves that when the PGE concentration was 6.25 mg mL−1, the scavenging rate of DPPH free radicals was 98.03 ± 0.60%, and the activity of ascorbic acid was almost the same; moreover, the ABTS radical showed strong scavenging activity (84.30 ± 0.53%), which was higher than that of the platycodin D control group. The results show that PGE had strong antioxidant activity, could reduce the generation of oxygen free radicals and oxidation reaction, weaken the tyrosinase activity, reduce melanin generation, delay skin aging, and whiten and protect the skin.
Inflammatory response is a biological process mediated by a complex cellular signaling system, and it is an important part of the body's defense mechanism against harmful stimuli (bacteria, irritants, and cell mediators). Excessive inflammatory response can lead to local blood circulation disorder, fever, parenchymal cell degeneration, necrosis, and organ dysfunction. In addition, individual components in cosmetics may promote the inflammatory response of sensitive skin; therefore, an anti-inflammatory function is the main requirement for cosmeceuticals to combat inflammatory response.33 The key mediators of inflammation include NO, TNF-α, and IL-6. Evaluating the ability of samples to reduce NO, TNF-α, and IL-6 production in RAW264.7 macrophage is a key experimental method to measure the anti-inflammatory activity of substances.34 The results showed that PGE could reduce the NO level in primitive cells in a dose-dependent manner and down-regulate the TNF-α, IL-6, and other pro-inflammatory factors of RAW264.7 macrophage stimulated by LPS. This experiment proves that PGE has a good anti-inflammatory effect, which can reduce the damage caused by inflammation, delay skin aging, keep skin elastic and smooth, and effectively play the role of cooperative whitening.
Skin pigmentation is mainly related to melanin production, and tyrosinase is the key enzyme to catalyze melanin biosynthesis.35 Under the action of tyrosinase, the tyrosine in melanocytes is oxidized into dopa, dopa quinone, dopa pigment, 5,6-dihydroxyindole, indole-5, and 6-quinone, and finally converted into melanin.15 Therefore, when the tyrosinase activity is limited, the melanin production is also limited, thus making the skin whiter or reducing hyperpigmentation.36 In this study, the melanin generation inhibition potential of PGE was first studied to determine whether the PGE could directly inhibit the tyrosinase activity in a cell-free assay system using mushroom tyrosinase as the enzyme source.37 The results show that the PGE had a strong inhibitory effect on tyrosinase, and the tyrosinase inhibitory activity of PGE increased significantly with the increase in the PGE concentration. The inhibitory rate (97.71 ± 1.886%) was higher than that of the positive control group (arbutin and platycodin D) under the same concentration when the extract concentration was 3 mg mL−1. Next, we evaluated whether PGE could exert a skin whitening effect by inhibiting the tyrosinase activity and melanin production in B16F10 melanoma cells. We used α-MSH (α-MSH binds MC1R and activates the signaling protein adenylate cyclase to increase the production of cyclic adenylate and promote tyrosinase expression) to induce B16F10 cells to activate intracellular tyrosinase activity and produce a large amount of melanin. Then arbutin and platycodin D were used as positive controls to evaluate the inhibitory effect of PGE on intracellular tyrosinase and cellular melanin generation. The results showed that with the increase in the PGE concentration, the inhibitory activities of tyrosinase and melanin production on B16F10 cells significantly increased in a dose-dependent manner. With the increase in the administration time, the inhibitory activity of PGE on B16F10 cells tyrosinase and melanin production gradually increased. Moreover, PGE showed the strongest intracellular tyrosinase inhibitory activity (inhibition rate: 106.33 ± 3.145%) and melanin generation inhibitory activity (inhibition rate: 59.80 ± 1.095%) after 48 h of administration. When the administration time was 72 h, the activity was lower than that under 48 h, indicating that the optimal PGE administration time was 48 h. The inhibitory activity of intracellular tyrosinase of PGE was significantly higher than those of arbutin and platycodin D control groups, demonstrating the excellent skin whitening activity of PGE.
The experimental results showed that the extraction rate of PGE in Platycodon grandiflorum was 8.9%, and the morphology was characterized as milky amorphous powder with good solubility. Our research group has prepared whitening cream with PGE as an active ingredient, in which the content of PGE is 2%. At the same time, we conducted human pre-experiment to study the whitening effect of whitening creams. 1–2 mL whitening cream (containing 0.02–0.04 g PGE, containing 0.22–0.45 g Platycodon grandiflorum) was applied to the forearm every day. We used PRIMOS system, Lab colorimetric system and spectrophotometer® CM-2500d (Konica Minolta, Inc., Osaka, Japan) to analyze the skin color of the forearm of the subjects. The pre-experiment results showed that the whitening cream containing 2% PGE had good whitening effect and had no skin irritation.
In this experiment, 19 common peaks were determined via HPLC combined with similarity analysis. By comparing the retention times of chromatographic charts of mixed standard substances, eight chromatographic peaks were identified and quantitatively analyzed. HPLC combined with mass spectrometry was used to identify and analyze specific chemical components in PGE, and 45 compounds in PGE were identified. By comparing the results with the experimental results of HPLC, we identified a variety of chemical components, which had different degrees of antioxidant property, anti-inflammatory property, anti-cancer property, enzyme inhibition, and immune regulation. The chemical components mainly included arbutin, syringin, chlorogenic acid, platycoside E, platycodin D3, baicalin, platycodin D, and luteolin. Moreover, arbutin can increase the activity of superoxide dismutase (SOD) enzyme in local skin tissue, reduce the tyrosine and MDA contents, and exert a good therapeutic effect on chloasma model mice. As a new whitening ingredient, arbutin has been favored by cosmetics manufacturers and consumers.38–40 Syringin has a strong antioxidant capacity and shows a strong application potential in DPPH and DMPD free-radical inhibitory activity, ferric ion reducing antioxidant power (FRAP), and metal chelating ability.41 Chlorogenic acid, the main active component of Onosma, has strong antioxidant and inhibitory activities against tyrosinase and α-amylase.42 Platycodin D has strong tyrosinase inhibitory activity and can be used as whitening and skin-care components; thus, it is worth developing and utilizing.43 Baicalin is widely used in the preparation of whitening creams and sunscreens owing to its inhibitory effects on melanin production and antibacterial activity.44–46 Luteolin extracted from rose has strong antioxidant activity and tyrosinase inhibition and antibacterial effects, making it useful as natural raw materials for skin care.47 Our research group isolated PGE via preparative HPLC and column chromatographic fractionation to obtain 14 compounds. The structures of all compounds were elucidated through spectroscopic methods. The compounds are saponins: platycodin D, platycodin D3, and platycoside E; glycosides: arbutin and syringin; acids: chlorogenic acid and nicotinic acid; flavonoids: baicalin, luteolin, daidzein, quercetin, and diosmetin; amides: erucamide and docosanamide. The results of the tyrosinase experiment showed that saponins and glycosides had strong tyrosinase inhibitory activities, among which arbutin (inhibition rate: 96.54 ± 1.585%) and platycodin D (inhibition rate: 80.96 ± 1.978%) had the strongest inhibitory activities. Acids and flavonoids had the second-strongest tyrosinase inhibitory activity, among which chlorogenic acid (inhibition rate: 75.64 ± 0.978%) and luteolin (inhibition rate: 70.91 ± 2.054%) had the strongest activity. Amides had no tyrosinase inhibitory activity. Compared with the above compounds, PGE had a stronger tyrosinase inhibitory activity (inhibition rate: 97.82 ± 2.058%). Therefore, the skin whitening activity of PGE comprises the multi-component and multi-target effect of traditional Chinese medicine, and the biological whitening activity of PGE should be the result of the combined action of multiple components.
5. Conclusions
Good antioxidant and anti-inflammatory effects can inhibit the activation of oxygen free radicals on tyrosinase and reduce melanin production. Moreover, they can reduce the damage caused by oxidation and inflammation of the skin, delay skin aging, keep the skin elastic and smooth, and effectively exert a synergistic whitening effect. The good inhibition of tyrosinase and melanin production can reduce melanin production and pigmentation in the skin and result in skin whitening. In this study, the whitening activity mechanism and pharmacodynamic material basis of PGE were determined through molecular biology and cell biology. Moreover, PGE could effectively inhibit tyrosinase activity, reducing the excessive melanin in the skin and relieving skin damage caused by oxidation and inflammation; further, the whitening active extract could significantly inhibit tyrosinase activity and the activity of B16F10 cells without potential toxic effects. Given the results, PGE has wide application potential for manufacturing whitening skin-care cosmetics. Based on the literature, cosmetics prepared from Platycodon grandiflorum and its extract have a good whitening effect on the human body. Therefore, as a whitening active substance extracted from natural Chinese herbal medicine, PGE can be used as raw materials for whitening and skin care.
Author contributions
M. M. Y. and G. Z. L conceived and designed the experiments. X. T. M., R. R. Z., and M. W performed the experiments. X. T. M., Z. H. Y., and F. Q. X analyzed the data. X. T. M. and S. S wrote the paper. All of the authors have read and agreed to the final version of the manuscript.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Key Research and Development Program: Research on Key Technologies of Ginseng Industry and Development of Massive Health Products (2017YFC1702100); the Science and Technology Development Plan Project of Jilin Province (20180623041TC); and the Changchun Science and Technology Bureau Project: Key Technologies and Product Development of Traditional Chinese Medicine Health Food Based on Jingfang and Yanfang (17YJ007).
Notes and references
- Beijing Morning Post, More than 8000 victims of Japanese Kanebo cosmetics show white spots after use, Guangxi Quality Supervision Guide, 2013, vol. 09, p. 10 [Google Scholar]
- Li C. L. Gao W. D. Jiang S. Chen Y. H. Rapid detection of hydroquinone and phenol illegally added in whitening and freckle removing cosmetics. Light. Res. Technol. 2017;33:20–21. [Google Scholar]
- Zhang G. C. Mao X. Q. Study on glucocorticoids in quick acting cosmetics. Flavour Fragrance Cosmet. 2015;04:53–55. [Google Scholar]
- Hughes K. Ho R. Butaud J. F. Filaire E. Ranouille E. Berthon J. Y. Raharivelomanana P. A selection of eleven plants used as traditional Polynesian cosmetics and their development potential as anti-aging ingredients, hair growth promoters and whitening products. J. Ethnopharmacol. 2019;245:112159. doi: 10.1016/j.jep.2019.112159. [DOI] [PubMed] [Google Scholar]
- Ji M. Y. Bo A. G. Yang M. Xu J. F. Jiang L. L. Zhou B. C. Li M. H. The Pharmacological Effects and Health Benefits of Platycodon grandiflorum-A Medicine Food Homology Species. Foods. 2020;9:142. doi: 10.3390/foods9020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. Liu Z. B. Zhang Y. W. Li W. Sun Y. J. Wang Y. F. Wang Y. H. Sun Y. S. Application of high-speed counter-current chromatography and HPLC to separate and purify of three polyacetylenes from Platycodon grandiflorum. J. Sep. Sci. 2018;41:789–796. doi: 10.1002/jssc.201700767. [DOI] [PubMed] [Google Scholar]
- Jeon D. Kim S. W. Kim H. S. Platycodin D, a bioactive component of Platycodon grandiflorum, induces cancer cell death associated with extreme vacuolation. Anim. Cells Syst. 2019;23:118–127. doi: 10.1080/19768354.2019.1588163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. Y. Shin J. H. Boo H. O. Gorinstein S. Ahn Y. G. Discrimination of Platycodon grandiflorum and Codonopsis lanceolata using gas chromatography-mass spectrometry-based metabolomics approach. Talanta. 2019;192:486–491. doi: 10.1016/j.talanta.2018.09.051. [DOI] [PubMed] [Google Scholar]
- Kim Y. J. Choi J. Y. Ryu R. Lee J. Cho S. J. Kwon E. Y. Lee M. K. Liu K. H. Rina Y. Sung M. K. Choi M. S. Platycodon grandiflorum Root Extract Attenuates Body Fat Mass, Hepatic Steatosis and Insulin Resistance through the Interplay between the Liver and Adipose Tissue. Nutrients. 2016;8:532. doi: 10.3390/nu8090532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang D. J. Huang C. Chen M. L. Chen Y. L. Fu Y. P. Paulsen B. S. Rise F. Zhang B. Z. Chen Z. L. Jia R. Y. Li L. X. Song X. Feng B. Ni X. Q. Yin Z. Q. Zou Y. F. Characterization of Inulin-Type Fructan from Platycodon grandiflorum and Study on Its Prebiotic and Immunomodulating Activity. Molecules. 2019;24:1199. doi: 10.3390/molecules24071199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boo H. O. Park J. H. Hyun K. H. Jeong K. S. Woo S. H. Evaluation of Physiological Functionalities and Anti-inflammatory Activity on in vitro Cultured Adventitious Root of Platycodon grandiflorum. J. Crop. Sci. Biotechnol. 2018;21:183–191. doi: 10.1007/s12892-018-0101-0. [DOI] [Google Scholar]
- Zhang L. Wang Y. L. Yang D. W. Zhang C. H. Zhang N. Li M. H. Liu Y. Z. Platycodon grandiflorum-An Ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2015;164:147–161. doi: 10.1016/j.jep.2015.01.052. [DOI] [PubMed] [Google Scholar]
- State Food and Drug Administration of China, Chinese name list of international cosmetic raw material standards, 2007 [Google Scholar]
- Gong X. J. Chen X. J. Sun Y. S. Xu B. J. Zheng Y. N. Inhibition of tyrosinase activity in Platycodon grandiflorum. J. Tradit. Chin. Med. 2004;04:257–259. [Google Scholar]
- Xu B. J. Zheng Y. N. Wang Y. Q. Study on tyrosinase inhibitory activity of Platycodon grandiflorum. J. Pharm. Pract. 2000;05:356. [Google Scholar]
- Zhu P. Y. Yin W. H. Wang M. R. Dang Y. Y. Ye X. Y. Andrographolide suppresses melanin synthesis through Akt/GSK3 beta/beta-catenin signal pathway. J. Dermatol. Sci. 2015;79:74–83. doi: 10.1016/j.jdermsci.2015.03.013. [DOI] [PubMed] [Google Scholar]
- Cha S. H. Ko S. C. Kim D. Jeon Y. J. Screening of marine algae for potential tyrosinase inhibitor: Those inhibitors reduced tyrosinase activity and melanin synthesis in zebrafish. J. Dermatol. 2011;38:343–352. doi: 10.1111/j.1346-8138.2010.00983.x. [DOI] [PubMed] [Google Scholar]
- Sarikurkcu C. Sahinler S. S. Ceylan O. Tepe B. Onosma pulchra: Phytochemical composition, antioxidant, skin-whitening and anti-diabetic activity. Ind. Crops Prod. 2020;154:112632. doi: 10.1016/j.indcrop.2020.112632. [DOI] [Google Scholar]
- Lee S. W. Lim J. M. Mohan H. Seralathan K. K. Park Y. J. Lee J. H. Oh B. T. Enhanced bioactivity of Zanthoxylum schinifolium fermented extract: anti-inflammatory, anti-bacterial, and anti-melanogenic activity. J. Biosci. Bioeng. 2020;129:638–645. doi: 10.1016/j.jbiosc.2019.12.003. [DOI] [PubMed] [Google Scholar]
- Liu S. Kuang M. T. Zhu H. W. Lin Y. Inhibitory effect of Cranberry on oxidative damage and apoptosis of HaCaT cells induced by UVB. Food Res. Dev. 2015;36:5–10. [Google Scholar]
- Ye X. Y. Zhu P. Y. Research progress on synthesis of melanin and whitening products. J. East China Norm. Univ., Nat. Sci. 2016;02:1–8. [Google Scholar]
- Liu G. N. Chen B. Research progress on the mechanism of elastic tissue degeneration in aging skin. J. Clin. Dermatol. 2013;42:325–328. [Google Scholar]
- Jin S. Hyun T. K. Ectopic Expression of Production of Anthocyanin Pigment 1 (PAP1) Improves the Antioxidant and Anti-Melanogenic Properties of Ginseng (Panax ginseng C.A. Meyer) Hairy Roots. Antioxidants. 2020;9:922. doi: 10.3390/antiox9100922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda C. S. Silva-Veiga F. Martins F. F. Rachid T. L. Mandarim-De-Lacerda C. A. Souza-Mello V. PPAR-alpha activation counters brown adipose tissue whitening: a comparative study between high-fat- and high-fructose-fed mice. Nutrition. 2020;78:110791. doi: 10.1016/j.nut.2020.110791. [DOI] [PubMed] [Google Scholar]
- Jiao B. Xu C. T. Li Q. Qin J. K. Luo Y. W. Yang W. G. Chemical constituents of flavonoids from Camellia oleifera and their anti-inflammatory activity in vitro. Chin. Tradit. Pat. Med. 2019;41:327–333. [Google Scholar]
- Li S. Ding J. S. Research progress of melanin biosynthesis and tyrosinase inhibitors. Cent. South Pharm. 2013;11:278–282. [Google Scholar]
- Zhao M. J. Hu J. J. Ni H. Jiang Z. D. Wang L. Research progress of melanin generation signaling pathway. J. Bioeng. 2019;35:1633–1642. doi: 10.13345/j.cjb.190084. [DOI] [PubMed] [Google Scholar]
- Zhou X. W. Jiang Z. S. Research progress of plant derived tyrosinase inhibitors. Chin. Herb. Med. 2004;06:107–110. [Google Scholar]
- Song T. Y. Chen C. H. Yang N. C. Fu C. S. Chang Y. T. Chen C. L. The Correlation of in vitro Mushroom Tyrosinase Activity with Cellular Tyrosinase Activity and Melanin Formation in Melanoma Cells A2058. J. Food Drug Anal. 2009;17:156–162. [Google Scholar]
- He D. Wu F. L. Xu X. F. Liao Y. Y. Feng H. Study on the therapeutic effect and mechanism of arbutin on chloasma rat model. Chinese Journal of Modern Medicine. 2018;28:6–10. [Google Scholar]
- Zhang F. L. Wu J. Wang G. L. Xing S. X. Research progress on the whitening mechanism and safety evaluation of α-arbutin and deoxyarbutin. J. Environ. Health. 2018;3504:370–375. [Google Scholar]
- Büşra Y. D. Sabriye S. Sezgin B. Accurate and Sensitive Reverse Phase High-Performance Liquid Chromatographic Determination of Arbutin in Blueberries and Characterization of Its Stability in Simulated Gastric Fluid and under Ultraviolet Irradiation. Anal. Lett. 2020;53:1504–1511. doi: 10.1080/00032719.2019.1710523. [DOI] [Google Scholar]
- Didem D. O. Fatma S. S. Sanem H. Ilkay E. O. Assessment of cholinesterase and tyrosinase inhibitory and antioxidant properties of Viscum album L. samples collected from different host plants and its two principal substances. Ind. Crops Prod. 2014;62:341–349. doi: 10.1016/j.indcrop.2014.08.044. [DOI] [Google Scholar]
- Cengiz S. Saliha S. S. Olcay C. Bektas T. Onosma pulchra: Phytochemical composition, antioxidant, skin-whitening and anti-diabetic activity. Ind. Crops Prod. 2020;154:112632. doi: 10.1016/j.indcrop.2020.112632. [DOI] [Google Scholar]
- Li X. N., Study on the efficacy and safety of The Traditional Chinese medicine Scutellaria baicalensis Geologist in cosmetics. Guangdong Pharmaceutical University. 2016 [Google Scholar]
- Gong S. Z., Jie Y. K. and Yuan S. M., The application of Baicalin in functional cosmetics, Daily Chemical Industry, 2003, vol. 03, pp. 200–203 [Google Scholar]
- Jeong H. S. Gu G. E. Jo A. R. Bang J. S. Yun H. Y. Baek K. J. Kwon N. S. Park K. C. Kim D. S. Baicalin-induced Akt activation decreases melanogenesis through downregulation of microphthalmia-associated transcription factor and tyrosinase. Eur. J. Pharmacol. 2015;761:19–27. doi: 10.1016/j.ejphar.2015.04.028. [DOI] [PubMed] [Google Scholar]
- Ren G. X. Xue P. Sun X. Y. Zhao G. Determination of the volatile and polyphenol constituents and the antimicrobial, antioxidant, and tyrosinase inhibitory activities of the bioactive compounds from the by-product of Rosa rugosa Thunb. var. plena Regal tea. BMC Complementary Med. Ther. 2018;18:307. doi: 10.1186/s12906-018-2374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R. X. Sun Z. H. Ren Q. Li L. Yin L. Li F. Su X. Gadd45alpha affects retinal ganglion cell injury in chronic ocular hypertension rats by regulating p38MAPK pathway. Gene. 2020;763:145030. doi: 10.1016/j.gene.2020.145030. [DOI] [PubMed] [Google Scholar]
- Chen Q. R. Kou L. Y. Wang F. W. Wang Y. Size-dependent whitening activity of enzyme-degraded fucoidan from Laminaria japonica. Carbohydr. Polym. 2019;225:115211. doi: 10.1016/j.carbpol.2019.115211. [DOI] [PubMed] [Google Scholar]
- Tepe A. S. Ozaslan M. Anti-Alzheimer, anti-diabetic, skin-whitening, and antioxidant activities of the essential oil of Cinnamomum zeylanicum. Ind. Crops Prod. 2020;145:112069. doi: 10.1016/j.indcrop.2019.112069. [DOI] [Google Scholar]
- Fernando I. P. S. Sanjeewa K. K. A. Samarakoon K. W. Kim H. S. Gunasekara U. K. D. S. S. Park Y. J. Abeytunga D. T. U. Lee W. W. Jeon Y. J. The potential of fucoidans from Chnoospora minima and Sargassum polycystum in cosmetics: antioxidant, anti-inflammatory, skin-whitening, and antiwrinkle activities. J. Appl. Phycol. 2018;30:3223–3232. doi: 10.1007/s10811-018-1415-4. [DOI] [Google Scholar]
- Jesumani V. Du H. Pei P. B. Zheng C. Q. Cheong K. L. Huang N. Unravelling property of polysaccharides from Sargassum sp. as an anti-wrinkle and skin whitening property. Int. J. Biol. Macromol. 2019;140:216–224. doi: 10.1016/j.ijbiomac.2019.08.027. [DOI] [PubMed] [Google Scholar]
- Kang S. H. Jeon Y. D. Cha J. Y. Hwang S. W. Lee H. Y. Park M. Lee B. R. Shin M. K. Kim S. J. Shin S. M. Kim D. K. Jin J. S. Lee Y. M. Antioxidant and skin-whitening effects of aerial part of Euphorbia supina Raf. Extract. BMC Complementary Altern. Med. 2018;18:256. doi: 10.1186/s12906-018-2323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe S. Y. Hong J. H. Gu Y. R. Kim I. D. Dhungana S. K. Moon K. D. Hot water extract of Glehnia littoralis leaf showed skin-whitening and anti-wrinkle properties. S. Afr. J. Bot. 2019;127:104–109. doi: 10.1016/j.sajb.2019.05.027. [DOI] [Google Scholar]
- Taddeo V. A. Epifano F. Preziuso F. Fiorito S. Caron N. Rives A. de Medina P. Poirot M. Silvente-Poirot S. Genovese S. HPLC Analysis and Skin Whitening Effects of Umbelliprenin-containing Extracts of Anethum graveolens, Pimpinella anisum, and Ferulago campestris. Molecules. 2019;24:501. doi: 10.3390/molecules24030501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R. C. Hu X. Q. Zhang B. Wang Z. Hao C. X. Xin J. Guo Q. M. Whitening Activity of Constituents Isolated from the Trichosanthes pulp. J. Evidence-Based Complementary Altern. Med. 2020;2020:2582579. doi: 10.1155/2020/2582579. [DOI] [PMC free article] [PubMed] [Google Scholar]