Abstract
Morbid obese people are more likely to contract SARS-CoV-2 infection and its most severe complications, as need for mechanical ventilation. Ketogenic Diet (KD) is able to induce a fast weight loss preserving lean mass and is particularly interesting as a preventive measure in obese patients. Moreover, KD has anti-inflammatory and immune-modulating properties, which may help in preventing the cytokine storm in infected patients. Respiratory failure is actually considered a contraindication for VLCKD, a very-low calorie form of KD, but in the literature there are some data reporting beneficial effects on respiratory parameters from ketogenic and low-carbohydrate high-fat diets. KD may be helpful in reducing ventilatory requirements in respiratory patients, so it should be considered in specifically addressed clinical trials as an adjuvant therapy for obese patients infected with SARS-CoV-2.
Keywords: SARS-CoV-2, COVID-19, ketogenic diet, low-carbohydrate high-fat diet, obesity, VLCKD, respiratory disease, respiratory failure
Introduction
Obesity and COVID-19: An Emergency in the Emergency
The pandemic of SARS-CoV-2 infection has been challenging the world for over a year. Many risk factors for the development of Coronavirus 2019 disease (COVID-19) have been identified, and among these, metabolic diseases play a major role. Severe obesity is associated with a greater risk of severe COVID-19 (1, 2), ICU admission (1) and need for invasive mechanical ventilation (2, 3). Moreover, obesity may be a risk factor for developing COVID-19 at a younger age (4).
The current pandemic, with lockdown imposed to reduce the spread of the virus, is being leading to social distancing and long times at homes. The reduced possibility of exercising outdoor and the reduced spontaneous outdoor activity, the closure of the gyms and the swimming pools, together with the increase of stress-related disorders, may lead lots of people to a worsening of the weight excess and related comorbidities.
In the real-world setting, health professionals face many practical difficulties in treating obese patients. The excess of adipose tissue hinders the diagnosis with pulmonary ultrasound, delaying the intervention in advanced stages, with consequent higher mortality (5). In addition, the facilities for severely obese patients in the wards and intensive care units are lacking, so that interventional procedures as intubation may be slow and exert a negative impact on the prognosis of the patients (5). Moreover, obese people develop a reduced response to vaccinations (6) and central obesity has been recently associated with lower antibody titres in response to COVID-19 mRNA vaccine (7). Therefore, strategies aimed to reduce weight excess are mandatory.
Ketogenic Diet
Ketogenic diet is a dietary approach characterized by the consumption of a very low amount of carbohydrates, <50 g/day, with consequent development of ketosis. Fat is used as a primary source of energy, through the beta-oxidation of fatty acids. There are different kinds of ketogenic diets, defined on the basis of the macronutrient composition.
The low-carbohydrate high-fat ketogenic diet (LCHF) is characterized by the absence of a limit for calorie intake and fat intake, which is around 80–90% of total day energy. The low-calorie diet (LCD) provides among 800–1,200 Kcal/day and the very low-calorie diet (VLCD) is characterized by an even more strict calorie restriction (<800 Kcal/day), but they do not necessary lead to ketosis. The very-low calorie ketogenic diet (VLCKD) is characterized by a similar caloric restriction, but is always associated to ketosis, and is particularly interesting for the treatment of obesity and its comorbidities. In 2019 the Italian Society of Endocrinology released a consensus statement on the administration of VLCKD for the management of metabolic diseases (8), and respiratory failure was counted among the absolute contraindications. Anyhow, the use of KDs is recently spreading in new proposed fields of application, and some pathological features which are currently considered contraindications may benefit from its tailored use on the single patient, by experienced physicians (9).
The prescription of KD is currently under consideration in other pathologies than obesity, as headache (10), polycystic ovary syndrome (11), cancer (12), and neurodegenerative diseases (13, 14).
Ketogenic Diet and COVID-19
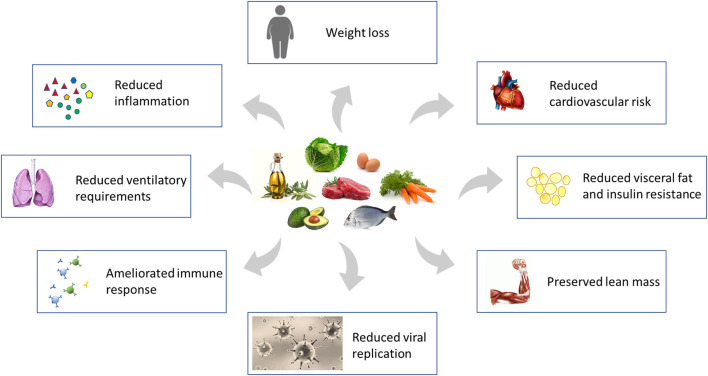
KD may be helpful in fighting COVID-19 through many mechanisms (see Figure 1). Severe SARS-CoV-2 infection determines a large innate immune response and ineffective adaptive immune response, that in some patients are associated with a cytokine storm and acute respiratory distress syndrome (15, 16). In fact, a virus infection with cytokine storm leads to an increase of reactive oxygen species and nitrogen species, which downregulate or inactivate many metabolic enzymes. Therefore, B and T cell proliferation is impaired, and cytokine production and cell death increase. These features of severe infections are related to reduced energy metabolism, altered redox state, oxidative damage and cell death, and may be at least partially dammed by KD. Ketone bodies have anti-inflammatory properties, since they are able to inhibit the NLRP3 inflammasome (17–19) and histone deacetylases (20), and consequently KD may reduce the risk of developing the cytokine storm, which is counted among the worst pathological features of COVID-19 (16, 21).
Figure 1.
Mechanisms through which Ketogenic Diet directly, and its consequent weight loss indirectly, may reduce the susceptibility to severe SARS-CoV-2 infection and stem the damage induced by the virus. Modified from Gangitano et al. (22).
KD may be able to reduce viral replication and assembly (23), because many viruses, as varicella-zoster virus (24), hepatitis C (25) and cytomegalovirus (26), are dependent on fatty acid metabolism pathway for their replication cycle, and fatty acid synthesis is usually reduced in KD, thanks to the metabolic switch induced by the diet. The metabolic switch in the liver consists in passing from the glucogenic/glycolytic pathway (fed state) to the ketogenic pathway (fast state) (23).
Thanks to these antiviral and anti-inflammatory properties, Soliman et al. proposed the use of KD and intermittent fasting as a prophylactic and adjuvant therapy for SARS-CoV-2 infection (23). KD administration has also been hypothesized as a preventive measure in obese patients, in order to achieve a fast weight loss preserving lean mass, and a supportive care for obese COVID-19 patients (22, 27).
A recent animal model study with aged mice infected with a natural murine beta coronavirus observed that the ketogenesis led to an expansion of tissue protective γδ T cells, deactivation of NLRP3 inflammasome and remodeling of inflammatory monocytes in the lungs (28).
KD administration during lockdown may rise some concerns regarding the monitoring of patients. Anyway, KD has been administered to young patients and the studies showed good results in terms of safety and maintenance of ketosis. HFKD or modified Atkins diet (MAD; macronutrient ratio fat/carbohydrates+proteins usually of 1:1, and carbohydrates limited to 10–20 g/day) have been administered to children and young patients with uncontrolled seizures, and the authors nor the members of the International Ketogenic Diet Study Group, pediatric consensus group, reported any issues (34). Patients were followed with a mixed approach of in person meetings and telemedicine visits. Similarly, another study reported no major issues using teleassistance for the maintenance of patients on KD (34). Most parents of drug-resistant epilepsy pediatric patients were satisfied of the telemedicine approach (35, 36) and would recommend it regardless of the pandemic (36).
The administration of KD in infected patients is under evaluation in ongoing clinical trials, and the effects on respiratory function have not been clearly evaluated yet. A retrospective study showed a possible beneficial effect of an eucaloric ketogenic diet (carbohydrates <30 g/day) in COVID-19 patients admitted to hospital, studying a sample of 34 patients compared with 68 patients receiving an isocaloric standard diet, but the authors did not assess respiratory function, except for the need for CPAP (Continuous Positive Airway Pressure), which did not differ among groups (37).
The aim of our study is to review the literature on ketogenic diets in respiratory patients, to consider the possibility of their safe use, from a respiratory point of view, in COVID-19 obese patients.
Low-Carbohydrate High-Fat Diets and Respiratory Function in Healthy Subjects
On a pathophysiological basis, fat oxidation produces less carbon dioxide (CO2) per amount of oxygen consumed compared to carbohydrates (CHO) (38), resulting in reduced ventilatory requirements. Therefore, ketogenic diet decreases metabolic carbon dioxide production for a given oxygen consumption, and may theoretically lead to a reduction in arterial carbon dioxide partial pressure (PETCO2), pulmonary ventilation, and CO2 body stores (29, 30).
Carbohydrates loads have been reported to precipitate respiratory failure in patients with lung compromise (39–42) since they may worse respiratory acidosis in patients unable to improve ventilation as a compensatory mechanism to excrete more CO2 (39, 43).
Over time, some authors investigated the effects of low-carbohydrate high-fat diets or supplements on respiratory parameters in healthy subjects, and most of them reported beneficial effects (see Table 1). In the majority of studies we report below, patients were administered an amount of CHO that could possibly lead to ketosis, but the development of ketosis wasn't verified.
Table 1.
Summary table of the interventional studies on the effects of low-carbohydrate dietary interventions (minimum 5 days of intervention) on ventilatory parameters and pulmonary function in spontaneously breathing patients.
| References | Patients | Diet composition | Length of the dietary intervention | Ketosis | Effect on ventilatory and pulmonary function parameters |
|---|---|---|---|---|---|
| Rubini et al. (29) | 32 healthy subjects | ketogenic diet (<30 g CHO/day, 848 Kcal) with phytoextracts, followed by low-carbohydrate no- ketogenic diet (80 g CHO/day, 938 Kcal) with phytoextracts, followed by Mediterranean diet (210 g CHO/day, 1,400 Kcal) | 20 days of ketogenic diet, 20 days of low-carbohydrate non-ketogenic diet, 2 months of Mediterranean diet | Yes | - Reduced carbon dioxide end-tidal partial pressure - No significant change in oxygen uptake, carbon dioxide production, nor expired total ventilation |
| Kwan et al. (30) | 6 healthy female subjects | low-carbohydrate diet (<50 g CHO/day), isoenergetic with the usual diet of each subject | 1 week | Yes | - Reduced pressure of expired carbon dioxide; trend for reduction in carbon dioxide production - Peak expiratory flow rate and functional residual capacity increased respect to the baseline - No change in resting ventilation and breathing frequency |
| Angelillo et al. (31) | 14 patients with COPD and chronic hypercapnia | liquid diets; low-carbohydrate high-fat (28% calories from CHO, 55% from fat), moderate-carbohydrate moderate-fat (53% calories from CHO and 30% from fat) and high-carbohydrate low-fat (74% calories from CHO, 9.4% from fat); caloric intake tailored on each patient's requirement | 5 days for each diet, sequence of diets assigned randomly | No | - Lower CO2 production and lower arterial pCO2 with the low-carbohydrate diet - No differences in oxygen consumption - Amelioration of forced vital capacity and forced expiratory volume in 1 second (FEV1) observed at the end of the administration of all diets - No change in respiratory frequency; trend for reduction in minute ventilation with the low-CHO diet |
| Tirlapur et al. (32) | 8 clinically stable COPD patients with chronic hypercapnic respiratory failure; six obese and twi non-obese | Low-calorie low-carbohydrate diet (30 g CHO/day, 600 Kcal/day) | 2–8 weeks | Not specifically evaluated | - Increased arterial oxygen tension and oxygen saturation - Reduced arterial carbon dioxide tension - Increased one-second forced expiratory volume and forced vital capacity - Reduction of nocturnal hypoxemic dips and apnoeic episodes |
| Kwan et al. (33) | 8 clinically stable COPD patients with chronic hypercapnic respiratory failure; non-obese | 2 diets isocaloric to the patients' usual diet (about 2,100 Kcal/day), and each containing 200 or 50 g of CHO/day; control diet with CHO intake around 280 g/day | 1 week for each diet | No | - Both diets increased arterial oxygen tension and decreased arterial carbon dioxide tension respect to the control diet - The 50 g CHO diet compared to the 200 g CHO diet further reduced the arterial carbon dioxide tension - Carbon dioxide production and oxygen consumption did not change - No significant changes in pulmonary function tests - Amelioration of dyspnoea during the 50 g CHO diet |
KD, Ketogenic Diet; CHO, carbohydrates; COPD, Chronic Obstructive Pulmonary Disease; CO2, carbon dioxide.
Rubini et al. (29) observed that a ketogenic diet (CHO < 30 g/day) administered for 20 days reduced PETCO2 in 32 healthy subjects, without modifications in oxygen uptake, carbon dioxide production nor expired total ventilation, which may be related to a reduction in CO2 body stores. Therefore, it may be beneficial for patients with high carbon dioxide arterial partial pressure due to respiratory insufficiency, because it lowers CO2 levels without increasing respiratory muscle fatigue, with the consequent risk of respiratory failure on mechanical basis. Interestingly, the reduction of PETCO2 was maintained even after the end of the diets, suggesting a long-term effect.
Similarly, Kwan et al. (30) observed that a ketogenic diet (50 g CHO/day) administered for 1 week to 6 healthy female subjects reduced arterial carbon dioxide tension, while resting ventilation and breathing frequency remained unchanged. Interestingly, pulmonary function tests showed an increase in peak expiratory flow rate and functional residual capacity respect to the baseline, respectively, by 5 and 10%.
Sue et al. (44) studied the effects of altering the composition of dietary fat and carbohydrate content on gas exchange, at rest and during exercise, on 8 healthy volunteers. At rest, the mean oxygen uptake did not differ, as did not the minute ventilation, while mean CO2 output was significantly less on the low-carbohydrate high-fat diet (10% of calories from CHO), compared to the high-carbohydrate diet (70% of calories from CHO). This differences was smaller during exercise, probably because of the preferential use of glycogen stores from the muscle (44).
On the other hand, one study reported negative effects of a ketogenic diet. The administration of a low-carbohydrate high-fat ketogenic diet, providing 2,400 Kcal/day for 4 weeks in 17 healthy women (<25 g of CHO/day), was associated with an earlier muscle fatigue at higher intensities and during daily life activities, probably because of the reduced glycogen stores in the muscle (45). However, a study on 7 well-trained male cyclists consuming a low-carbohydrate high-fat diet (15–82 g of CHO/day), for a long-time (at least the previous 8 months), showed that these athletes had a similar gluconeogenesis rate and reduced glycogenolysis respect to the 7 athletes fed with a mixed diet (46). These findings suggest that after a long-term adaptation to a low-carbohydrate high-fat diet, liver glycogen contributes to endogenous glucose production during exercise, and that glucose may be preferentially obtained from glycerol derived from lipolysis of intramuscular triglycerides (47), configuring an hypothetical difference among about subjects accustomed to a low-carbohydrate high-fat diet and “newbies.”
Low-Carbohydrate High-Fat Diets and Respiratory Function in Chronic Obstructive Pulmonary Disease Patients
Many Chronic Obstructive Pulmonary Disease (COPD) patients experience hypercapnia and hypoxemia, therefore a nutritional approach that decreases carbon dioxide production, and consequently respiratory muscles work, is extremely interesting.
Some authors observed an improvement of ventilatory measurements in COPD hypercapnic patients after a low-carbohydrate high-fat diet. In a small sample of 14 patients with chronic hypercapnia and COPD the administration of a low-carbohydrate diet (28% CHO) for 5 days, determined a trend of lower CO2 production, lower arterial pCO2, respect to moderate-carbohydrate moderate-fat diet and high-carbohydrate low-fat diet. An amelioration of forced vital capacity and forced expiratory volume in 1 s (FEV1) were observed for all diets, which may reflect an increase in muscle strength (31). However, some recent papers observed that a KD did not improve muscle strength in healthy women (45) and in trained athletes (48, 49), so we may hypothesize that the amelioration in FEV1, which was observed for all diets, was to be ascribed to the beneficial effects of the nutritional support rather than its composition. Also, we may speculate that the effect of the diet on muscle strength would be more evident in non-trained patients than in trained athletes.
Some authors studied the short-term alterations following the administration of a particular meal or supplement. Akrabawi et al. (38) enrolled 36 outpatients with COPD and administered a high-fat meal (55% calories from fat, 25 g of CHO) or a moderate fat meal (41% calories from fat, 35 g of CHO) and observed a higher CO2 production and O2 consumption in patients fed the moderate-fat meal in the early post-prandial time, probably reflecting the earlier absorption of the meal, and no difference for pulmonary function. A recent study on 60 low-body weight patients with COPD and elevated arterial carbon dioxide tension observed that the group administered a low-carbohydrate high-fat evening supplement with 50–75 g of CHO daily for 3 weeks had an overall improvement in ventilatory status, with decrease in VCO2, PaCO2, VO2 and minute ventilation, and an increase of PaO2 and FEV1, respect to the group administered a traditional high-carbohydrate diet (60–70% CHO) (50). This confirms the importance of nutritional therapy in malnourished COPD patients, since it has a strong impact on respiratory muscle weakness, and the importance of its macronutrient composition.
Many studies on the effects of carbohydrate loads on respiratory parameters and physical performance in chronic obstructive lung disease patients report a detrimental effect of CHO. High-carbohydrate diets may result in increased CO2 production and O2 consumption in clinically stable COPD patients (39). Kuo et al. (39) studied 12 clinically stable COPD patients and 12 healthy controls after administering an isocaloric high-fat (55.2% fat and 28.1% CHO) or high-carbohydrate (31.5% fat and 54.5% CHO) liquid meal, and found that the high-fat diet had a small effect on gas exchange parameters and ventilation, while the high-carbohydrates diet resulted in a great increase of CO2 production, O2 consumption and minute ventilation in COPD patients. Efthimiou et al. (40) studied a small sample of 10 clinically stable patients with the 6 min walking test. They observed that the group administered CHO-rich drink experienced a reduced physical performance correlated to the increased CO2 production and a perceived effort to breathe. Similar detrimental effects of CHO on walking and exercise performance were obtained in 18 patients with chronic airflow obstruction (41, 42). Increased CO2 production and increased minute ventilation after a high-carbohydrate formula, administered for nighttime enteral feeding, were observed in 10 young adult patients with cystic fibrosis with moderate to advanced lung disease (51). On the contrary, a small study with 13 patients affected by stable airways disease fed a high-carbohydrate meal (480 ml of grape juice and three-fourths cup of sucrose) showed that 7 patients who retained carbon dioxide had an increase in PaO2, probably reflecting the increased alveolar ventilation, and had not a significant increase in PaCO2, therefore the authors conclude that most patients with chronic airways disease are able to tolerate the increased endogenous carbon dioxide load resulting from a meal high in carbohydrates (52).
Low-Carbohydrate High-Fat Diets, Respiratory Failure and Mechanical Ventilation
Most patients with COPD and acute respiratory failure have marked reduction of body protein stores (53) and lower muscle concentrations of adenosine triphosphate and creatinine phosphate, and these factors may be an important determinant of respiratory failure (54). Moreover, critically ill patients are more responsive to changes in dietary composition than less critical ones (55).
Some authors suggest that a low-carbohydrate diet may be an effective tool to ameliorate respiratory failure (30, 32, 33).
A study on 8 clinically stable chronic hypercapnic respiratory failure patients with congestive heart failure showed that a very low-calorie ketogenic diet (600 Kcal/day, 30 g of CHO/day), administered for on average 1 month, was effective in ameliorating arterial oxygen tension and oxygen saturation, reducing arterial carbon dioxide tension, and ameliorating electrocardiographic abnormalities associated with hypoxiemia (32). These results were probably to be ascribed to the combined effect of diet composition and weight loss, and even two non-obese patients had beneficial effects from the diet.
Another study on 8 chronic hypercapnic respiratory failure patients administered 200 or 50 g of CHO daily for a week within an isocaloric diet, observed that the reduction in the CHO intake on both diets improved the general well-being of the patients, increased arterial oxygen tension and decreased arterial carbon dioxide tension. The 50 g CHO diet compared to the 200 g CHO diet further reduced the arterial carbon dioxide tension, suggesting that such a diet may be used in patients with intractable respiratory failure (33).
Regarding mechanically ventilated patients, in the literature there are some evidences of a beneficial effect of a low-carbohydrate high-fat diet (55–57). A study on 20 clinically stable ventilated patients showed that low-carbohydrate high-fat enteral feeding (55.2% fat, 28.1% carbohydrate) is able to reduce PaCO2 levels and the time of mechanical ventilation (55). A recent study on 100 patients with type II respiratory failure, secondary to pulmonary disease requiring mechanical ventilation, showed a great improvement in arterial carbon dioxide tension and minute volume at weaning with the low-carbohydrate high-fat feeding (55.2% fat, 28.1% carbohydrate) (56). Interestingly, similarly to Al-Saady (55), this was highly significantly associated with less time spent on mechanical ventilation (56). On the other hand, a study on 32 patients requiring mechanical ventilation reported no beneficial effects of a similar low-carbohydrate high-fat enteral nutrition (55.2% fat, 28.1% carbohydrate) in PaCO2 levels during weaning from the ventilator, respect to a standard enteral nutrition (30% fat, 53.3% carbohydrate) (58). Another recent study on 51 critically ill ventilated children with pulmonary disease observed that the low-carbohydrate high-fat diet (30% carbohydrate, 50% fat) was effective in reducing carbon dioxide tension but did not reduce the duration or level of ventilatory support (59).
Similarly to healthy subjects and respiratory patients in outpatients conditions, the acute carbohydrate loading has detrimental effects on patients with acute respiratory distress (60, 61) and acute respiratory failure (43, 62, 63), acting as a precipitating factor.
Acute respiratory failure was reported in 3 patients under ventilatory support within hours after the beginning of total parenteral nutrition, probably due its high carbohydrate content. The substantial increase of the carbon dioxide production in these patients unable to increase their ventilatory response, led to development of hypercapnia and respiratory acidosis (43).
Conclusion and Future Perspective
Ketogenic diet should be considered in severely obese people as a preventive measure for SARS-CoV-2 infection, in order to achieve a fast weight loss preserving lean mass. In addition, KD use may be hypothesized as an adjuvant therapy in obese infected patients.
Data obtained in respiratory patients, mainly lean, are indicative of the safety of low-carbohydrate high-fat diets in respiratory compromised patients, and some beneficial effects on respiratory parameters were recorded. KD administration may be helpful for obese patients with chronic hypercapnia, thanks to the reduced CO2 production induced by the diet. Many respiratory patients are malnourished, and obese patients themselves are frequently sarcopenic, therefore an adequate protein supplementation is mandatory, since malnutrition may worsen the general prognosis. Many supplements for malnourished patients are rich in carbohydrates, and this is detrimental for their respiratory function, as extensively seen above. KD may be useful for obtaining an adequate protein intake, reducing the ventilatory requirements, the dyspnea and the risk of muscle fatigue. Anyhow, the studies are pretty old, diet administration quite short, samples relatively small, and ketosis not always addressed. In addition, we focused on chronic respiratory diseases, and not on acute infective respiratory illnesses, which may present other issues. Also active infections, in fact, are considered among the contraindications for VLCKD.
Anyway, KD has anti-inflammatory effects and may reduce the risk of cytokine storm, thanks to the anti-inflammatory effects of ketone bodies, and may have a direct anti-viral effect. Therefore, ketogenic diet may be an effective adjuvant therapy in obese non-critically ill COVID-19 patients, and may even be considered in respiratory lean patients. New focused clinical trials with adequate sample sizes, led by a multidisciplinary experienced team of pneumologists, endocrinologists and nutritionists, are needed to confirm the safety and the beneficial effects on ventilatory parameters of such approach in respiratory patients.
Author Contributions
EG, LG, and CL: conceptualization. EG and RT: writing—original draft preparation. SM, AL, LG, and CL: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. (2020) 28:1195–9. 10.1002/oby.22831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caussy C, Wallet F, Laville M, Disse E. Obesity is associated with severe forms of COVID-19. Obesity. (2020) 00:2020. 10.1002/oby.22842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalligeros M, Shehadeh F, Mylona EK, Benitez G, Beckwith CG, Chan PA, et al. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity. (2020) 28:1200–4. 10.1002/oby.22859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kass DA, Duggal P, Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet. (2020) 395:1544–5. 10.1016/S0140-6736(20)31024-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muscogiuri G, Pugliese G, Barrea L, Savastano S, Colao A. Commentary: obesity: the “Achilles heel” for COVID-19? Metab Clin Exp. (2020) 108:1–3. 10.1016/j.metabol.2020.154251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honce R, Schultz-cherry S, Schultz-cherry S. Impact of obesity on influenza A virus pathogenesis, immune response, and evolution. Front Immunol. (2019) 10:1–15. 10.3389/fimmu.2019.01071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe M, Balena A, Tuccinardi D, Tozzi R, Risi R, Masi D, et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID - 19 mRNA vaccine. Diabetes Metab Res Rev. (2021) 1−10. 10.1002/dmrr.3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caprio M, Infante M, Moriconi E, Armani A, Fabbri A, Mantovani G, et al. Very-low-calorie ketogenic diet (VLCKD) in the management of metabolic diseases: systematic review and consensus statement from the Italian Society of Endocrinology (SIE). J Endocrinol Invest. (2019) 42:1365–86. 10.1007/s40618-019-01061-2 [DOI] [PubMed] [Google Scholar]
- 9.Watanabe M, Tuccinardi D, Ernesti I, Basciani S, Mariani S, Genco A, et al. Scientific evidence underlying contraindications to the ketogenic diet: an update. Obes Rev. (2020) 21:1–11. 10.1111/obr.13053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lorenzo C Di, Ballerini G, Barbanti P, Bernardini A, Arrigo GD, Egeo G, et al. Applications of ketogenic diets in patients with headache: clinical recommendations. Nutrients. (2021) 13:1–26. 10.3390/nu13072307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paoli A, Mancin L, Giacona MC, Bianco A, Caprio M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J Transl Med. (2020) 18:1–11. 10.1186/s12967-020-02277-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber DD, Aminzadeh-gohari S, Tulipan J, Catalano L, Feichtinger RG. Ketogenic diet in the treatment of cancer - where do we stand? Mol Metab. (2020) 33:102–21. 10.1016/j.molmet.2019.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wlodarek D. Role of ketogenic diets in neurodegenerative diseases (Alzheimer's disease and Parkinson's Disease). Nutrients. (2019) 11:1–11. 10.3390/nu11010169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broom GM, Shaw IC, Rucklidge JJ. The ketogenic diet as a potential treatment and prevention strategy for Alzheimer 's disease. Nutrition. (2019) 60:118–21. 10.1016/j.nut.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 15.Levy M, Thaiss CA, Elinav E. Taming the inflammasome. Nat Med. (2015) 21:213–5. 10.1038/nm.3808 [DOI] [PubMed] [Google Scholar]
- 16.Bradshaw PC, Seeds WA, Miller AC, Mahajan VR, Curtis WM. COVID-19: proposing a ketone-based metabolic therapy as a treatment to blunt the cytokine storm. Oxid Med Cell Longev. (2020) 1–33. 10.1155/2020/6401341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kornberg MD. The immunologic Warburg effect: Evidence and therapeutic opportunities in autoimmunity. Wiley Interdiscip Rev Syst Biol Med. (2020) 12:1–17. 10.1002/wsbm.1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamanashi T, Iwata M, Kamiya N, Tsunetom K, Kajitani N, Wada N, et al. Beta-hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress-induced behavioral and inflammatory responses. Sci Rep. (2017) 7:1–11. 10.1038/s41598-017-08055-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youm Y, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite β -hydroxybutyrate blocks NLRP3 inflammasome– mediated inflammatory disease. Nat Med. (2015) 21:263–71. 10.1038/nm.3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang P V, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. PNAS. (2014) 111:2247–52. 10.1073/pnas.1322269111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sukkar SG, Bassetti M. Induction of ketosis as a potential therapeutic option to limit hyperglycemia and prevent cytokine storm in COVID-19. Nutrition. (2020) 79–80:1–7. 10.1016/j.nut.2020.110967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gangitano E, Tozzi R, Gandini O, Watanabe M, Basciani S, Mariani S, et al. Ketogenic diet as a preventive and supportive care for COVID-19 patients. Nutrients. (2021) 13:1–10. 10.3390/nu13031004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soliman S, Faris MEAIE, Ratemi Z, Halwani R. Switching host metabolism as an approach to Dampen SARS-CoV-2 infection. Ann Nutr Metab. (2020) 27272. 10.1159/000510508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Namazue J, Kato T, Okuno T, Shiraki K, Yamanishi K. Evidence for attachment of fatty acid to Varicella-Zoster virus glycoproteins and effect of cerulenin on the maturation of varicella-zoster virus glycoproteins. Intervirology. (1989) 30:268–77. 10.1159/000150102 [DOI] [PubMed] [Google Scholar]
- 25.Herker E, Ott M. Unique ties between hepatitis C virus replication and intracellular lipids. Trends Endocrinol Metab. (2011) 22:241–8. 10.1016/j.tem.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koyuncu E, Purdy JG, Rabinowitz JD, Shenk T. Saturated very long chain fatty acids are required for the production of infectious human cytomegalovirus progeny. PLOS Pathog. (2013) 9:1–15. 10.1371/journal.ppat.1003333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paoli A, Gorini S, Caprio M. The dark side of the spoon- glucose, ketones and COVID-19: a possible role for ketogenic diet? J Transl Med. (2020) 18:1–9. 10.1186/s12967-020-02600-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryu S, Shchukina I, Youm YH, Qing H, Hilliard B, Dlugos T, et al. Ketogenic diet restrains aging-induced exacerbation of coronavirus infection in mice. Elife. (2021) 10:1–25. 10.7554/eLife.66522.sa2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubini A, Bosco G, Lodi A, Cenci L, Parmagnani A, Grimaldi K, et al. Effects of twenty days of the ketogenic diet on metabolic and respiratory parameters in healthy subjects. Lung. (2015) 193:939–45. 10.1007/s00408-015-9806-7 [DOI] [PubMed] [Google Scholar]
- 30.Kwan RM, Thomas S, Mir MA. Effects of a low carbohydrate isoenergetic diet on sleep behavior and pulmonary functions in healthy female adult humans. J Nutr. (1986) 116:2393–402. 10.1093/jn/116.12.2393 [DOI] [PubMed] [Google Scholar]
- 31.Angelillo AV, Sukhdarshan B, Durfee D, Dahl J, Patterson AJ, O'Donohue WJ. Effects of low and high carbohydrate feedings in ambulatory patients with chronic obstructive pulmonary disease and chronic hypercapnia. Ann Intern Med. (1985) 103:883–5. 10.7326/0003-4819-103-6-883 [DOI] [PubMed] [Google Scholar]
- 32.Tirlapur VG, Mir MA. Effect of low calorie intake on abnormal pulmonary physiology in patients with chronic hypercapneic respiratory failure. Am J Med. (1984) 77:987–94. 10.1016/0002-9343(84)90177-3 [DOI] [PubMed] [Google Scholar]
- 33.Kwan R, Mir MA. Beneficial effects of dietary carbohydrate restriction in chronic cor pulmonale. Am J Med. (1987) 82:751–8. 10.1016/0002-9343(87)90011-8 [DOI] [PubMed] [Google Scholar]
- 34.Ferraris C, Pasca L, Guglielmetti M, Marazzi C, Trentani C, Varesio C, et al. Ketogenic diet therapy provision in the COVID-19 pandemic: dual-center experience and recommendations. Epilepsy Behav. (2020) 111:1–6. 10.1016/j.yebeh.2020.107399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costa AM, Marchiò M, Bruni G, Bernabei SM, Cavalieri S, Bondi M, et al. Evaluation of e-health applications for paediatric patients with refractory epilepsy and maintained on ketogenic diet. Nutrients. (2021) 13:1–13. 10.3390/nu13041240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Semprino M, Fasulo L, Fortini S, Martorell Molina CI, González L, Ramos PA, et al. Telemedicine, drug-resistant epilepsy, and ketogenic dietary therapies: a patient survey of a pediatric remote-care program during the COVID-19 pandemic. Epilepsy Behav. (2020) 112:1–6. 10.1016/j.yebeh.2020.107493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sukkar S, Cogorno L, Pisciotta L, Pasta A, Vena A, Gradaschi R, et al. Clinical efficacy of eucaloric ketogenic nutrition in the COVID-19 cytokine storm (CSS): a retrospective analysis of mortality and Intensive Care Unit admission. Nutrition. (2021) 89:1–7. 10.1016/j.nut.2021.111236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akrabawi SS, Mobarhan S, Stoltz R, Ferguson PW. Gastric emptying, pulmonary function, gas exchange, and respiratory quotient after feeding a moderate versus high fat enteral formula meal in chronic obstructive pulmonary disease patients. Nutrition. (1996) 12:260–5. 10.1016/S0899-9007(96)90853-9 [DOI] [PubMed] [Google Scholar]
- 39.Kuo C-DD, Shiao G-MM, Lee J-DD. The effects of high-fat and high-carbohydrate diet loads on gas exchange and ventilation in COPD patients and normal subjects. Chest. (1993) 104:189–96. 10.1378/chest.104.1.189 [DOI] [PubMed] [Google Scholar]
- 40.Efthimiou J, Mounsey PJ, Benson DN, Madgwick R, Coles SJ, Benson MK. Effect of carbohydrate rich versus fat rich loads on gas exchange and walking performance in patients with chronic obstructive lung disease. Thorax. (1992) 47:451–6. 10.1136/thx.47.6.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown SE, Nagendran RC, McHugh JW, Stansbury DW, Fischer CE, Light RW. Effects of a large carbohydrate load on walking performance in chronic air-flow obstruction. Am Rev Respir Dis. (1985) 132:960–2. [DOI] [PubMed] [Google Scholar]
- 42.Brown SE, Wiener S, Brown RA, Marcarelli PA, Light RW. Exercise performance following a carbohydrate load in chronic airflow obstruction. J Appl Physiol. (1985) 58:1340–6. 10.1152/jappl.1985.58.4.1340 [DOI] [PubMed] [Google Scholar]
- 43.Covelli HD, Black JW, Olsen MS, Beekman JF. Respiratory failure precipitated by high carbohydrate loads. Ann Intern Med. (1981) 95:579–81. 10.7326/0003-4819-95-5-579 [DOI] [PubMed] [Google Scholar]
- 44.Sue YD, Chung MM, Grosvenor M, Wasserman K. Effect of altering the proportion of dietary fat and carbohydrate on excercise gas exchange in normal subjects. Am Rev Respir Dis. (1989) 139:1430–4. 10.1164/ajrccm/139.6.1430 [DOI] [PubMed] [Google Scholar]
- 45.Sjödin A, Hellström F, Sehlstedt E, Svensson M, Burén J. Effects of a ketogenic diet on muscle fatigue in healthy, young, normal-weight women: a randomized controlled feeding trial. Nutrients. (2020) 12:1–15. 10.3390/nu12040955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webster CC, Noakes TD, Chacko SK, Swart J, Kohn TA, Smith JAH. Gluconeogenesis during endurance exercise in cyclists habituated to a long-term low carbohydrate high-fat diet. J Physiol. (2016) 594:4389–405. 10.1113/JP271934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zderic TW, Davidson CJ, Schenk S, Byerley LO, Coyle EF. High-fat diet elevates resting intramuscular triglyceride concentration and whole body lipolysis during exercise. Am J Physiol Endocrinol Metab. (2004) 286:217–25. 10.1152/ajpendo.00159.2003 [DOI] [PubMed] [Google Scholar]
- 48.Paoli A, Cenci L, Pompei P, Sahin N, Bianco A, Neri M, et al. Effects of two months of very low carbohydrate ketogenic diet on body composition, muscle strength, muscle area, and blood parameters in competitive natural body builders. Nutrients. (2021) 13:1–14. 10.3390/nu13020374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greene DA, Varley BJ, Hartwig TB, Chapman P, Rigney M. A low-carbohydrate ketogenic diet reduces body mass without compromising performance in powerlifting and olympic weightlifting athletes. J Strength Cond Res. (2018) 32:3373–82. 10.1519/JSC.0000000000002904 [DOI] [PubMed] [Google Scholar]
- 50.Cai B, Zhu Y, Ma Y, Xu Z, Zao Y, Wang J, et al. Effect of supplementing a high-fat, low-carbohydrate enteral formula in COPD patients. Nutrition. (2003) 19:229–32. 10.1016/S0899-9007(02)01064-X [DOI] [PubMed] [Google Scholar]
- 51.Kane RE, Hobbs PJ, Black PG. Comparison of low, medium, and high carbohydrate formulas for nighttime enteral feedings in cystic fibrosis patients. J Parenter Enter Nutr. (1990) 14:47–52. 10.1177/014860719001400147 [DOI] [PubMed] [Google Scholar]
- 52.Gieseke T, Gurushanthaiah G, Glauser FL. Effects of carbohydrates on carbon dioxide excretion in patients with airway disease. Chest. (1977) 71:55–8. 10.1378/chest.71.1.55 [DOI] [PubMed] [Google Scholar]
- 53.Driver AG, Mcalevy MT, Smith JL. Nutritional assessment of patients with chronic obstructive pulmonary disease and acute respiratory failure. Chest. (1982) 82:568–71. 10.1378/chest.82.5.568 [DOI] [PubMed] [Google Scholar]
- 54.Gertz I, Hedenstierna G, Hellers G, Wahren J. Muscle metabolism in patients with chronic obstructive lung disease and acute respiratory failure. Clin Sci Mol Med. (1977) 52:396–403. 10.1042/cs0520395 [DOI] [PubMed] [Google Scholar]
- 55.Al-Saady NM, Blackmore CM, Bennett ED. High fat, low carbohydrate, enteral feeding lowers PaCO2 and reduces the period of ventilation in artificially ventilated patients. Intens Care Med. (1989) 15:290–5. 10.1007/BF00263863 [DOI] [PubMed] [Google Scholar]
- 56.Faramawy MA El, Allah AA, Batrawy S El, Amer H. Impact of high fat low carbohydrate enteral feeding on weaning from mechanical ventilation. Egypt J Chest Dis Tuberc. (2014) 63:931–8. 10.1016/j.ejcdt.2014.07.004 [DOI] [Google Scholar]
- 57.Cook D, Meade M, Guyatt G, Butler R, Aldawood A, Epstein S. Trials of miscellaneous interventions to wean from mechanical ventilation. Chest. (2001) 120:438S−44S. 10.1016/S0012-3692(15)50001-9 [DOI] [PubMed] [Google Scholar]
- 58.Van Den Berg B, Bogaard JM, Hop WCJ. High fat, low carbohydrate, enteral feeding in patients weaning from the ventilator. Intens Care Med. (1994) 20:470–5. 10.1007/BF01711897 [DOI] [PubMed] [Google Scholar]
- 59.El Koofy NM, Rady HI, Abdallah SM, Bazaraa HM, Rabie WA, El-ayadi AA. The effect of high fat dietary modification and nutritional status on the outcome of critically ill ventilated children: single-center study. Korean J Pediatr. (2019) 62:344–52. 10.3345/kjp.2018.06835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jih KS, Wang MF, Chow JH, Yen CC. Hypercapnic respiratory acidosis precipitated by hypercaloric carbohydrate infusion in resolving septic acute respiratory distress syndrome: a case report. Case Rep. (1996) 58:359–65. [PubMed] [Google Scholar]
- 61.Askanazi J, Elwyn DH, Silverberg PA, Rosenbaum SH, Kinney JM. Respiratory distress secondary to a high carbohydrate load: a case report. Surgery. (1980) 87:596–8. [PubMed] [Google Scholar]
- 62.Askanazi J, Rosenbaum SH, Hyman AI, Silverberg PA, Milic-Emili J, Kinney JM. Respiratory changes induced by the large glucose loads of total parenteral nutrition. JAMA. (1980) 243:1444–7. 10.1001/jama.1980.03300400028023 [DOI] [PubMed] [Google Scholar]
- 63.Askanazi J, Nordesreom J, Rosenbaum SH, Elwyn DH, Hyman AI, Carpentier YA, et al. Nutrition for the patient with respiratory failure: glucose vs. fat. Anesthesiology. (1981) 54:373–7. 10.1097/00000542-198105000-00005 [DOI] [PubMed] [Google Scholar]