Abstract
Current serologic Lyme disease tests use whole borrelia cells as the source of antigen. These assays are difficult to standardize and to optimize for sensitivity and specificity. To help solve these problems, we constructed a library of recombinant chimeric proteins composed of portions of key antigens of Borrelia burgdorferi. These proteins were then used to develop an enzyme-linked immunosorbent assay. We compared our assay with the most sensitive of three whole-cell borrelia assays. We found that the recombinant assay could detect antibodies significantly better from early Lyme disease sera (P < 0.05), and had the same sensitivity for late Lyme disease sera, as the most sensitive whole-cell borrelia assay. On potentially cross-reactive sera, the recombinant assay was more specific, but not significantly so, than the best whole-cell borrelia assay. Optimization of the recombinant assay offers the potential for a significant improvement in both sensitivity and specificity.
Lyme disease is the most common vector-borne disease in North America and Europe (26) and is an emerging problem in northern Asia (16, 18). The infectious agent in Lyme disease is the spirochete Borrelia burgdorferi, which is transmitted to humans primarily via ticks of the genus Ixodes.
B. burgdorferi infection produces a progressive or episodic disease with a wide array of clinical manifestations. Chronic disseminated infection can cause permanent damage to the nervous and musculoskeletal systems (26). The only specific sign of B. burgdorferi infection is erythema migrans (EM), a transient local response that occurs early in the course of infection in 70 to 80% of patients. None of the clinical manifestations of late Lyme disease are pathognomonic. In fact, all are characteristic of numerous other illnesses, and testing for B. burgdorferi infection is frequently an early step in the differential diagnosis of patients with rheumatologic or neurologic symptoms. Except for patients with EM, B. burgdorferi is infrequently observed in clinical samples, and direct diagnosis via microbiological techniques is not currently feasible. In the absence of EM, the laboratory diagnosis of B. burgdorferi infection is primarily dependent on the detection of a humoral immune response to the organism (2, 3, 8, 21, 24, 25). Accurate, reliable diagnostic assays are essential both to ensure early treatment of infected individuals and to exclude the large majority of uninfected patients with “Lyme-like” symptoms. Also, early treatment of Lyme disease is important to limit or prevent serious damage to the nervous and musculoskeletal systems.
Most, but not all, commercial seroassays use whole-cell B. burgdorferi preparations. Preserved B. burgdorferi cells are used as the antigen substrate for immunofluorescence assays, and crude fractions of sonicated organisms are used for most enzyme-linked immunosorbent assays (ELISAs). The use of whole cells of Borrelia spp. as the source of antigen has posed problems in optimizing the sensitivity, specificity, and reproducibility of serological tests. We developed recombinant protein-based assays to attempt to overcome these problems. We engineered recombinant chimeras, each containing portions of key antigenic proteins of B. burgdorferi, the outer surface proteins OspA, OspB, OspC, flagellin (Fla or p41), and a protein of 93 kDa (p93). By eliminating other bacterial proteins (structural and metabolic) and by creating truncated forms of the relevant antigenic proteins of B. burgdorferi, we expected to remove cross-reactive epitopes of these antigens and present only the portions that induce a strong specific immune response to B. burgdorferi. We believed that this was likely to increase both the sensitivity and specificity of the assay.
In this paper we describe the cloning, expression, purification, and serologic characterization of a recombinant chimera-based diagnostic assay for Lyme disease on an ELISA format. Our data indicate that the recombinant chimeric assay presents superior sensitivity in detecting antibodies against B. burgdorferi for the early stages of the disease, and equivalent sensitivity for the late stages of the disease, to the best whole-cell assay tested.
MATERIALS AND METHODS
Cloning of chimeric B. burgdorferi genes; protein expression and immunoblot characterization. (i) Cloning of the recombinant chimeric borrelia proteins (RCBPs).
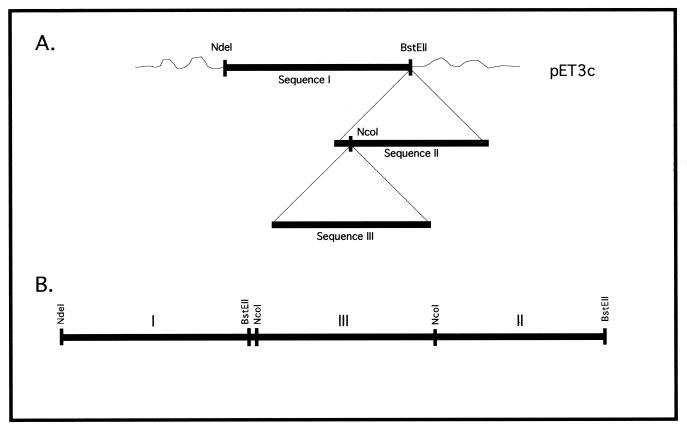
A library of chimeric proteins was generated using B. burgdorferi sequences of OspA, OspB, OspC, flagellin (p41) and p93. B. burgdorferi strain B31 was mainly used. Some chimeras utilized portions of Borrelia afzelii strain Pko or Borrelia garinii strain K48. Several versions of the chimeras were generated with the expression vector pET3c. Portions of the open reading frames of the outer surface protein (Osp) cDNAs were cloned in tandem in order to produce recombinant fusion proteins. The first group of chimeras, the OspB series, comprised the series OspB-Fla and OspB-OspC-Fla. The sequence encoding the OspB truncated fragment and the internal segment of the flagellin gene (encoding Fla or p41) were cloned sequentially into the vector on the NdeI-BamHI sites, using linkers between the sequences, creating the B-Fla chimeras. The OspC truncated fragment was then cloned between both sequences generating the chimeras B-C-Fla. The second group, the p93 series, comprised the chimeras OspA-p93, OspC-p93, and OspA-OspC-p93. The OspA or, alternatively, the OspC truncated sequences and a truncated segment of p93 were cloned sequentially using linkers, generating the chimers A-93 and C-93, respectively. The OspC truncated fragment was then cloned between the sequences of A-93, generating the chimer A-C-93. A representation of the sequential cloning of the library of the chimeras generated is shown in Fig. 1. Escherichia coli (strain DH5α) cells were transformed with the plasmid containing the chimeras, the antibiotic-resistant colonies were isolated, and the purified DNA was characterized via restriction pattern analysis.
FIG. 1.
Strategy used to clone the RCBPs. (A) General representation. (B) Sequential representation of the cloned genes.
(ii) Protein expression and immunoblot characterization.
E. coli [strain BL21 (DE3) pLysS or strain B834 (DE3)] cells were transformed with the plasmid containing the coding sequence for RCBP and grown in 10 ml of Luria-Bertani medium (5 g of NaCl, 10 g of tryptone, 5 g of yeast extract, 25 mg of chloramphenicol, 50 mg of ampicillin/ml) at 37°C with shaking. When the optical density at 600 nm reached 0.3 to 0.4, recombinant protein expression was induced by adding IPTG (isopropyl-β-d-thiogalactopyranoside) to a final concentration of 0.5 mM and cells were grown for an additional 3 h. The cultures were harvested by centrifugation at 3,800 × g for 5 min, the cells were resuspended in 20 mM NaPO4, pH 7.7, and the crude extracts were stored at −20°C overnight. Once thawed, the RCBP crude extracts were incubated with DNase (2 μg/ml) in the presence of 2.5 mM MgCl2 at room temperature for 30 min and spun at 14,000 rpm (Eppendorf 5417C) for 5 min and 5 μl of the protein sample was loaded on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel, which was either stained in Coomassie blue or used for immunoblotting. The primary antibody used for the immunoblotting was either a monoclonal antibody (MAb) or EM Lyme disease human serum. The secondary antibody used was, respectively, alkaline phosphatase-labeled anti-mouse immunoglobulin G (IgG) or anti-human IgA plus IgG plus IgM.
Protein purification.
Crude extracts were prepared according to the method of Studier et al. (27), and the resulting pooled soluble fraction was applied to an anion-exchange column (Q Sepharose Fast Flow; Pharmacia) equilibrated with either 20 mM Tris, pH 7.5, or 20 mM diethanolamine, pH 9.1, depending on the protein being purified. The bound protein was eluted using an increasing concentration of NaCl. The fractions containing the chimeric proteins were pooled and concentrated by ultrafiltration using a stirred flow cell (Amicon) with a 30- or 50-kDa cutoff membrane (depending on the RCBP purified). The protein was dialyzed against 20 mM NaPO4, pH 7.8, containing 250 mM NaCl. The protein solution was loaded onto a Ni2+ metal affinity column (chelating Sepharose Fast Flow; Pharmacia) equilibrated with 20 mM NaPO4, pH 7.8, containing 250 mM NaCl. The bound protein was eluted using an increasing concentration of imidazole. The fractions containing the chimeric protein were concentrated by ultrafiltration (Amicon). The protein concentration was then determined by the measurement of the absorbance shift when Coomassie brilliant blue G-250 reacted with protein (Bio-Rad), and the protein was stored at −70°C. Protein purity was checked by SDS-PAGE, and the reactivity was checked by immunoblotting. The yield of purified protein depends on the chimera being purified and ranges between 15 and 60 mg/liter of induced culture.
ELISA. (i) Immobilization of RCBPs onto ELISA plates.
Solutions of purified RCBPs in 140 mM sodium carbonate, pH 9.0, were used to coat commercial microwell plates (MaxiSorp; Nunc). The coating procedure was as follows. One hundred microliters of a solution containing 0.5 μg of antigen/ml was added to each well, and the microwell plate was incubated for either 1 h at room temperature or at 4°C overnight. The antigen solution was removed from the wells, the plate was washed three times with 140 mM sodium carbonate, pH 9.0, and 200 μl of blocking solution was added (2% bovine serum albumin fraction V [Sigma Chemical Co., St. Louis, Mo.] in 140 mM sodium carbonate, pH 9.0). Following a 30-min incubation at 37°C the plates were washed three times with sodium carbonate, pH 9.0, wrapped in plastic, and stored at 4°C until used (up to a week).
(ii) ELISA.
The standard procedure for the ELISAs was as follows. Serum samples were diluted 1:100 in filter-sterilized specimen diluent (10% fetal bovine serum [Gibco] in phosphate-buffered saline [PBS]–1 mM KH2PO4–10 mM Na2HPO4–137 mM NaCl–2.7 mM KCl, pH 7.4 [Boehringer Mannheim], and 100 μl of each sample was added to ELISA plate microwells. Following incubation for 1 h at 37°C, the samples were removed and the plates were washed three times in PBS-T (PBS–0.05% Tween 20). Goat anti-human IgM (Fc) and IgG (Fab) antibodies conjugated to alkaline phosphatase (Jackson ImmunoResearch Laboratories) were used as secondary antibodies. They were diluted 1:1,000 in PBS, pH 7.4, and 100 μl of the solution was added to each well. Following incubation for 30 min at 37°C, the plates were washed three times with PBS-T and 100 μl of substrate solution (5 mg of p-nitrophenylphosphate tablets dissolved in 1× diethanolamine substrate buffer to yield a 2-mg/ml solution [Kirkegaard Perry Laboratory]) was added to each well. The plates were incubated for 30 min at 37°C, and 100 μl of stop solution (5% EDTA) was added to each well. The absorbance at 410 nm was read on a microplate reader (Dynatech). As negative controls we used 10 serum samples from healthy individuals. The same negative controls were included in each plate. A sample was considered positive if it produced an average absorbance greater than the mean of the negative controls plus three standard deviations. Values between two and three standard deviations were considered equivocal and counted as positive in the final evaluations.
(iii) Commercial ELISAs.
Lyme disease ELISA kits were purchased from MarDx Diagnostics, Inc. (Carlsbad, Calif.) Sigma Chemical Co., and Wampole Laboratories, a Division of Carter Wallace, Inc. (Palatine, Ill.), and were used for comparative purposes. The tests are polyvalent and were performed according to the manufacturer instructions. As with the recombinant test, values between two and three standard deviations were considered equivocal and counted as positive.
Serum panels and MAbs.
We have access to a large bank of sera from patients who participated in multicenter clinical trials conducted by the Lyme Disease Center at the State University of New York at Stony Brook. The serum samples included in all panels were obtained singly from different subjects. All serum samples were obtained from physician-characterized patients. They were obtained at different times, so the samples used to do the screening of the chimeras were not necessarily used later in the comparison experiments. When using serum panels, some of the serum samples always get depleted and therefore are no longer available for further testing.
The panels used to do the preliminary screening of the RCBPs (see Table 2) included a total of 139 sera from patients with early Lyme disease (EM at presentation), 69 sera from patients with late Lyme disease (Lyme arthritis or neuroborreliosis), 56 sera from patients with syphilis and 50 sera from patients with immunopathological disorders (rheumatoid arthritis and systemic lupus erythematosus), 30 sera from healthy donors from an area of endemicity, and 28 normal sera from areas where the disease is not endemic. The use of sera from healthy individuals without a known history of Lyme disease from an area of endemicity is important because many people in these areas have been infected with B. burgdorferi without recognizing any specific clinical signs or symptoms. Neither the patient nor the normal control sera used to evaluate the recombinant ELISAs were prescreened; they were run blind.
TABLE 2.
Study I: serological screening of the RCBPsa
| RCBP (size [kDa]) | No. of positive results/no. of samples tested (%) for indicated serab
|
|||||
|---|---|---|---|---|---|---|
| Late Lyme | Early Lyme | Gross-reactive autoimmune disease | Syphilis | Normal, not from an area of endemicity | Normal, from an area of endemicity | |
| A-93 (62) | 9/16 (56) | 2/20 (10) | NDf | |||
| A-93 (97) | 47/53 (89)c | 49/99 (50)d | 1/40 (3) | 6/27 (22) | 0/20 (0) | 3/20 (15) |
| B-Fla (43) | 10/21 (48) | 11/47 (23) | ND | |||
| B-C-Fla (64) | 60/69 (87)e | 86/139 (62)e | 4/50 (8) | 4/56 (7) | 0/28 (0) | 5/30 (16) |
| A-93 (97) + B-C-Fla (64) | 49/51 (96) | 64/111 (58) | 2/25 (8) | 2/37 (5) | 0/20 (0) | 2/30 (7) |
Variously sized samples were chosen randomly from the panels, and the individual RCBPs were tested. Goat anti-human IgM (Fc) and IgG (Fab) antibodies conjugated to alkaline phosphatase (Jackson ImmunoResearch Laboratories) were used as the secondary antibodies. Normal sera from an area of endemicity might show some reactivity because individuals might be infected with B. burgdorferi without recognizing any clinical symptoms.
A chi-square test with correction for continuity was used to determine statistical significance between the large and small versions of the same chimeras. Differences with probabilities that were <0.05 were considered statistically significant.
P = 0.01, compared to 62-kDa protein.
P < 0.005, compared to 62-kDa protein.
P < 0.001, compared to 43-kDa protein.
ND, not determined.
The panels used to do the comparison study (see Table 3) were obtained from strictly characterized patients and were clinically classified as early Lyme (panel 1A, consisting of 41 sera from patients with EM who were culture positive, and panel 1B, consisting of 50 sera from patients with acute disseminated symptoms, multiple EM, or EM plus objective evidence of neurologic or cardiac involvement), late Lyme (panel 2A, consisting of serum from 26 patients with previous clinical history of EM and presenting signs and symptoms of late Lyme disease [arthritis or neurologic system involvement], and panel 2B, consisting of 29 sera from patients with definite organ involvement, either arthritis or neurologic, that had previously tested positive for B. burgdorferi antibodies by both ELISA and Western blotting [these sera were not prescreened before they were tested]), and potentially cross-reactive (panel 3A, consisting of 33 sera from syphilis patients, consisting of panel 3B, 27 sera from rheumatoid arthritis and systemic lupus erythematosus patients, and panel 3C, consisting of 10 normal sera from individuals from an area where the disease was not endemic). One of the panels that had been used in the screening studies was used in the comparative experiments after being further characterized (serum from patients with EM and with B. burgdorferi isolated from skin biopsies from the EM). These sera as well as the rest of the panels were run blind. MAbs directed against B. burgdorferi outer surface protein A (OspA), outer surface protein B (OspB), outer surface protein C (OspC), flagellin, and p93 were obtained as described previously (12, 22, 29).
TABLE 3.
Study II: comparison of the RCBP and WCB ELISAs for sensitivity and specificity in detecting antibodies against Lyme disease
| Serum and panela | Sensitivity (%) (no. of sera positive/no. tested) for:
|
Specificity (%) (no. of sera positive/no. tested) for:
|
Pb | ||
|---|---|---|---|---|---|
| WCB | A-93 (97 kDa) + B-C-Fla (RCBP) | WCB | A-93 (97 kDa) + B-C-Fla (RCBP) | ||
| Early Lyme | 0.0312c | ||||
| 1A | 39 (16/41) | 44 (18/41) | |||
| 1B | 54 (27/50) | 62 (31/50) | |||
| Late Lyme | NCd | ||||
| 2A | 81 (21/26) | 81 (21/26) | |||
| 2B | 100 (29/29) | 100 (29/29) | |||
| Cross-reactive | 0.125c | ||||
| 3A | 79 (7/33) | 85 (5/33) | |||
| 3B | 93 (2/27) | 100 (0/27) | |||
| 3C | 100 (0/10) | 100 (0/10) | |||
Panels are as described in Materials and Methods.
By McNemar's test. Differences with P values of <0.05 were considered statistically significant.
Combined value.
NC, not calculable.
Statistical analysis.
The statistical comparison between the small and large proteins was done using a chi-square test with correction for continuity (reference 30, section 22.3). The test used to compare the individual proteins with the combined proteins is a comparison of multiple proportions (reference 30, section 23.13). The comparison between the whole-cell borrelia (WCB) and the combined RCBPs used McNemar's exact test for correlated proportions (reference 20, section 10.5.2) because the same sera were used for both tests, giving a paired-sample design. McNemar's test evaluates whether discordant results (i.e., positive in RCBP assay and negative by WCB assay or vice versa) are evenly distributed (null hypothesis) or biased one way or the other. P values of <0.05 are considered statistically significant.
RESULTS
Selection of RCBPs.
The library of chimeras was screened with MAbs to demonstrate that their respective epitopes were present and accessible in the chimeric proteins (Table 1 and Fig. 2). The sequences that happened to have been cloned out of reading frame turned up as positive clones by restriction pattern analysis but were negative when protein expression was induced. The negative results obtained with the MAbs for induced proteins are explained by proposing that the tertiary folding of the proteins is different and that this makes the epitopes inaccessible to the MAbs. The most reactive chimeras on immunoblotting were chosen for further serological testing: OspB-Fla (43 kDa), OspB-OspC-Fla (64 kDa), OspA-p93 (62 kDa), and OspA-p93 (97 kDa).
TABLE 1.
Structural and immunochemical characterization of RCBPs
| Series and RCBP (size [kDa])a | Strain | Immunoblot result on induced positive clones
|
||||
|---|---|---|---|---|---|---|
| Anti-OspB MAb H68 | Anti-p41 MAb H9724 | Anti-OspC | Anti-OspA MAbs 184 and 105 | Anti-p93 MAb 181 | ||
| OspB | ||||||
| OspB-Fla (43) | B31 | Positive | Positive | NAb | ||
| OspB-Fla (50) | B31 | Positive | Positive | NA | ||
| OspB-Fla (41) | B31 | Positive | Positive | NA | ||
| OspB-Fla (48) | B31 | Positive | Positive | NA | ||
| OspB-OspC-Fla (64) | B31 | Positive | Positive | Positive | ||
| OspB-OspC-Fla (62) | B31 | Negative | Negative | NDc | ||
| OspB-OspC-Fla (66) | K48 | NA | NA | NA | ||
| OspB-OspC-Fla (63) | BPkoBd | NA | NA | NA | ||
| OspB-OspC-Fla (61) | BPkoB | Negative | Negative | ND | ||
| p93 | ||||||
| OspA-p93 (62) | B31 | NA | Positive | Positive | ||
| OspA-p93 (88) | B31 | NA | Negative | Positive | ||
| OspA-p93 (53) | B31 | NA | Positive | Negative | ||
| OspA-p93 (97) | B31 | NA | ND/Pos.e | Positive | ||
| OspA-OspC-p93 (83) | BPkoB | NA | NA | NA | ||
| OspC-p93 (55) | B31 | Positive | NA | Positive | ||
| OspC-p93 (83) | B31 | Positive | NA | Positive | ||
| OspC2-p93 (82) | C2-B31 | Positive | NA | Positive | ||
RCBPs chosen for serological testing are in boldface.
NA, not applicable.
ND, not determined.
BPkoB, B31-Pko-B31.
ND/Pos., MAb 184, ND/MAb 105, positive.
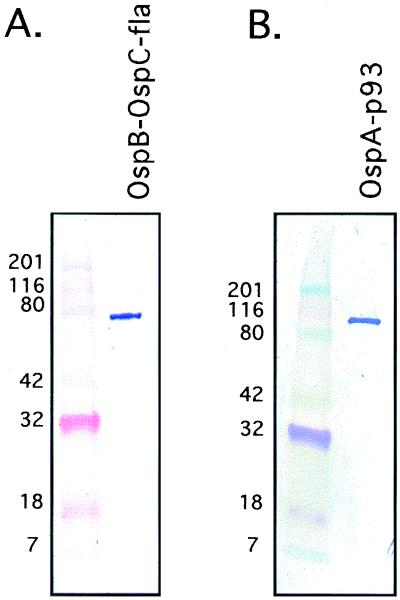
FIG. 2.
Specific reactivities of the purified RCBPs on immunoblotting using MAbs. The secondary antibody used was alkaline phosphatase-labeled anti-mouse IgG. OspB-OspC-Fla (64 kDa) was identified with MAb anti-OspC (A), and OspA-p93 (97 kDa) was identified with MAb anti-OspA LA2.2 (B). The mobilities of size standards are indicated at the left.
Development of ELISA formats with RCBPs.
Extensive tests on the titration of antigen and conjugate, the dilution of normal negative control sera, reaction temperature, and incubation times were done (data not shown). The results of these experiments indicated that an antigen concentration of 0.5 μg/ml in the coating buffer was optimal for each of the RCBPs tested. It is not necessary that the chimeric proteins be immobilized in a specific molar ratio to one another, only that enough of each protein be bound so that epitopes in that RCBP will not become a limiting factor in subsequent ELISAs of patient serum. For practical purposes these conditions were met when the anti-OspA or anti-OspC MAb capture assay mixture (used to measure the binding of the individual RCBPs) diluted at 1:100 reached an absorbance of about 1.5 or greater for each RCBP-MAb immunocomplex reaction. Serial dilutions of normal control sera indicated that the target negative baseline value of ≤0.2 absorbance units was met by the 1:100 serum dilution. The secondary antibody (conjugate) optimal dilution was 1:1,000.
Study I: serological screening of the RCBPs. (i) Sensitivity of the RCBPs.
Lyme disease serum panels composed of 139 samples of early Lyme disease sera and 69 samples of late Lyme disease sera were used to do the preliminary screening of the RCBPs for their sensitivity to detect anti-B. burgdorferi antibodies. Samples were chosen randomly, and the individual RCBPs were tested. The results are given in Table 2. A chi-square test with correction for continuity was used to test for statistical significance. A-93 with a molecular mass of 97 kDa was significantly more sensitive than A-93 with a mass of 62 kDa for both late Lyme (χ2 = 6.46, P = 0.01) and early Lyme (χ2 = 9.05, P < 0.005). B-C-Fla was significantly more sensitive than B-Fla for late Lyme (χ2 = 12.2, P < 0.001) and for early Lyme (χ2 = 19.3, P < 0.001). Thus, RCBPs A-93 (62 kDa) and B-Fla were dropped from further testing.
A mixture of A-93 (97 kDa) and B-C-Fla was formulated and tested against samples from the same blinded panels of sera. This mixture seemed to give better results than the individual chimeras. However, when the proportions were statistically tested, neither the data from late Lyme (P = ∼0.25) nor the data from early Lyme (P = ∼0.20) could reject the null hypothesis that all RCBPs were equal. This is also true when all the data were combined (P = ∼0.30). Thus, A-93 (97 kDa) and B-C-Fla individually and the two in combination had sensitivities that were not statistically distinguishable.
(ii) Specificity of the RCBPs.
To further characterize the chosen chimers in the screening studies, we tested sera from patients with diseases associated with serological responses that are known to produce cross-reactivity in currently used tests (Table 2). We used a total of 56 samples of syphilis, 50 samples of autoimmune diseases (rheumatoid arthritis and systemic lupus erythematosus), 28 normal sera from an area where the disease is not endemic, and 30 normal sera from healthy donors from an area of endemicity. Variously sized samples were chosen randomly. For all four types of sera, there was no significant difference in the specificity of A-93 (97 kDa) and B-C-Fla individually or combined. To simplify further testing, a RCBP assay mixture containing A-93 (97 kDa) plus B-C-Fla at a 1:1 molar ratio was used in the comparison study.
Study II: comparison of RCBP-based tests with commercial test kits.
Three different commercial WCB ELISAs were compared in blinded tests with a panel of 20 sera from culture-confirmed early Lyme disease patients with EM at presentation. The sensitivities of the tests were as follows: test A detected five positive sera (25%), test B detected three positive sera (15%), and test C detected one positive serum (5%). Because test A was the most sensitive, it was then compared to our RCBP assay with A-93 (97 kDa) plus B-C-Fla. The serum panels used were obtained from strictly characterized patients, were clinically classified, and were run blinded and in parallel (paired comparison) on both tests (Table 3). For the early Lyme patients, the following results were obtained: for panel 1A, with 41 EM- and culture-positive sera, 16 were positive in the WCB assay (39%) and 18 were positive in the RCBP assay (44%); for panel 1B, with 50 acute disseminated sera, 27 were positive in the WCB assay (54%) and 31 were positive in the RCBP assay (62%). The overall sensitivity of detection of antibodies to B. burgdorferi in early Lyme disease patients was 39 to 54% for the WCB assay and 44 to 62% for the RCBP assay. The difference in sensitivity between the RCBP assay and the WCB assay in the early stages of the disease was statistically significant by McNemar's exact test for correlated proportions (P = 0.0312, P < 0.05), since all the discordant results were positive in the RCBP assay and negative in the WCB assay. For the late Lyme patients, the following results were obtained: for panel 2A, with 26 sera from patients with previous clinical history of EM and presenting signs and symptoms of late Lyme disease (arthritis or neurologic system involvement), 21 were positive in both the WCB and the RCBP assays (81%); for panel 2B, with 29 not-prescreened sera from patients with definite organ system involvement that had previously tested positive for B. burgdorferi antibodies, 29 were positive in both the WCB and the RCBP assays (100%). There was no difference on the sensitivity of detection of antibodies to B. burgdorferi in the late stages of the disease for either test. For the potentially cross-reactive patients, the following results were obtained: for panel 3A, with sera from 33 syphilis patients, 7 were positive in the WCB assay (21%) and 5 were positive in the RCBP assay (15%); for panel 3B, with sera from 27 autoimmune disease patients, 2 were positive in the WCB assay (7%) and none was positive in the RCBP assay (0%). The overall specificity was 79 to 100% for the WCB assay and 85 to 100% for the RCBP assay. The difference in specificity between the RCBP and the WCB assays was not statistically significant (P = 0.125, P > 0.05). However, all discordant results were positive in the WCB assay and negative in the RCBP assay. This suggests that with a larger sample size, the RCBP assay will likely be significantly more specific.
DISCUSSION
Whole B. burgdorferi assay mixtures contain many proteins with epitopes that cross-react with antibodies against other common infectious agents. Bruckbauer et al. demonstrated extensive cross-reactivity between B. burgdorferi and the bacterial pathogens Borrelia hermsii, Treponema pallidum, Treponema phagedenis, Leptospira interrogans, Neisseria meningitidis, Haemophilus influenzae, Yersinia enterocolitica, Campylobacter jejuni, Listeria monocytogenes, Pseudomonas aeruginosa, E. coli, Salmonella enterica serovar Typhimurium, Shigella flexneri, and Legionella micdadei (1). Many healthy adults with no history of B. burgdorferi infection have detectable levels of anti-41-kDa IgG, and many individuals also have antibodies directed against other spirochetal antigens. The Borrelia proteins in the 60- to 75-kDa range, p33 and two proteins with a mass of about 20 kDa, are among the most highly cross-reactive (1, 9, 13). The immunodominant 41-kDa flagellin antigen is not cross-reactive with nonflagellated bacteria but it is highly cross-reactive with similar proteins from other spirochetes. In addition, the accuracy of indirect assays for B. burgdorferi infection can be compromised by infectious and immunopathological conditions such as viral infections, subacute bacterial endocarditis, rheumatoid arthritis, and systemic lupus erythematosus.
Recombinant protein-based assays are a natural evolutionary advance from the crude whole bacterial preparations now used, offering improvements in sensitivity, specificity, and reproducibility over whole spirochetal tests. By creating truncated forms of the relevant antigenic proteins of B. burgdorferi, we expected to remove some cross-reactive epitopes, and unlike whole-borrelia assay mixtures, which generally contain only one genospecies, multiple genospecies and variants can easily be included in these assays. These two aspects alone can be used to produce a more sensitive, specific seroassay. The two-tiered system recommended by the Centers for Disease Control and Prevention (4, 14) has been adopted because of the poor positive predictive value of current tests. Recombinant protein-based assays, by being more sensitive in the clinically crucial early stages of the disease and by limiting cross-reactive proteins, can potentially reduce the numbers of false positives and could eventually eliminate the need for the two-tiered system.
Several attempts to generate a diagnostic test with a single antigenic protein have been made (6, 10, 17), but the resulting tests proved to be not sensitive enough. We generated recombinant chimeras containing key sequences from a number of B. burgdorferi proteins, including OspA, OspB, OspC, Fla, and p93. Flagellin (Fla or p41) and p93 are two good candidate antigens for serodiagnosis of Lyme disease (14, 15). Full-length p41 is sensitive but lacks specificity. The variable middle region of p41 is a better candidate antigen for more specific serodiagnosis of the disease (5, 11, 19). This region of p41, used alone, is not very sensitive for detection of Lyme disease. As a component of chimeric constructs, however, it adds significantly to the diagnostic performance of the chimeric antigens. Therefore, this lack of sensitivity of the internal fragment of p41 was not a problem in the recombinant chimeras we selected for our serologic assays.
Recently, a significant degree of genetic diversity has been demonstrated for B. burgdorferi OspC genes (7, 28). A study of a local Long Island population showed that most of the major OspC groups found worldwide (as listed in GenBank) are found within a single population. This pattern indicated that the geographical distribution of major OspC groups is relatively homogeneous (28). Seinost et al. identified four clones of B. burgdorferi sensu stricto defined by their OspC group that cause invasive infection in humans (23). The combination of multiple OspC types in a second-generation assay could in part account for an increase in the sensitivity of this test.
Preliminary screening of the chimeric proteins indicated that different chimeric constructs vary significantly in their immunoreactivities, presumably due to secondary structural characteristics that affect the presentation of the epitopes. While individual recombinant proteins are not ideal for use as single antigens in diagnostic assays (6, 10, 17), a combination of several recombinant Borrelia antigens proved to be highly effective. Unlike tests employing WCB preparations, where improvements in sensitivity usually reduce the specificity and vice versa, the improvements in sensitivity of the ELISA using recombinant chimeric borrelia proteins was accompanied by at least an equivalent specificity for the WCB assay as was shown by the statistical analysis. These protein chimeras were constructed without knowledge of their potentiality. With further study of the genetic variability of the important epitopes, even better chimeras can be created. The construction of recombinant chimeras containing genes from several genospecies allows us to generate one protein that confers antigenicity to multiple strains, revealing the great potential and adaptability of this technique. Furthermore, the use of pure protein preparations as antigens offers greater flexibility in adapting the test to different assay formats.
ACKNOWLEDGMENTS
This study was supported by grants from NIH, SBIR, grants AI43786-01 (J.D.G.), AI38724-03 (J.D.G.), NIH ALAR 37256-01 (B.J.L. and R.J.D.), and NIH AI32454-02 (B.J.L.). It was also supported by a CDC grant, number 4313957, and by the New York State legislative initiative in Lyme disease, grant number 860042 (R.J.D.).
We thank Gina Gorgone, Laura Hannafey, Yi Ling, and Diana T. Lombardo for excellent technical assistance.
REFERENCES
- 1.Bruckbauer H R, Preac-Mursic V, Fuchs R, Wilske B. Cross-reactive proteins of Borrelia burgdorferi. Eur J Clin Microbiol Infect Dis. 1992;11:224–232. doi: 10.1007/BF02098084. [DOI] [PubMed] [Google Scholar]
- 2.Dattwyler R J, Volkman D J, Luft B J. Immunologic aspects of Lyme borreliosis. Rev Infect Dis. 1989;11(Suppl. 6):S1494–S1498. doi: 10.1093/clinids/11.supplement_6.s1494. [DOI] [PubMed] [Google Scholar]
- 3.Dattwyler R J, Volkman D J, Luft B J, Halperin J J, Thomas J, Golightly M G. Seronegative Lyme disease. Dissociation of specific T- and B-lymphocyte responses to Borrelia burgdorferi. N Engl J Med. 1988;319:1441–1446. doi: 10.1056/NEJM198812013192203. [DOI] [PubMed] [Google Scholar]
- 4.Dressler F, Whalen J A, Reinhardt B N, Steere A C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 5.Gassmann G S, Jacobs E, Deutzmann R, Gobel U B. Analysis of the Borrelia burgdorferi GeHo fla gene and antigenic characterization of its gene product. J Bacteriol. 1991;173:1452–1459. doi: 10.1128/jb.173.4.1452-1459.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerber M A, Shapiro E D, Bell G L, Sampieri A, Padula S J. Recombinant outer surface protein C ELISA for the diagnosis of early Lyme disease. J Infect Dis. 1995;171:724–727. doi: 10.1093/infdis/171.3.724. [DOI] [PubMed] [Google Scholar]
- 7.Guttman D S, Wang P, Wang L-N, Bosler E, Luft B, Dykhuisen D E. Multiple infections of Ixodes scapularis ticks by Borrelia burgdorferi as revealed by single strand conformation polymorphism. J Clin Microbiol. 1996;34:652–656. doi: 10.1128/jcm.34.3.652-656.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen K, Asbrink E. Serodiagnosis of erythema migrans and acrodermatitis chronica atrophicans by the Borrelia burgdorferi flagellum enzyme-linked immunosorbent assay. J Clin Microbiol. 1989;27:545–551. doi: 10.1128/jcm.27.3.545-551.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen K, Bangsborg J M, Fjordvang H, Pedersen N S, Hindersson P. Immunochemical characterization of and isolation of the gene for a Borrelia burgdorferi immunodominant 60-kilodalton antigen common to a wide range of bacteria. Infect Immun. 1988;56:2047–2053. doi: 10.1128/iai.56.8.2047-2053.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen K, Hindersson P, Pedersen N S. Measurement of antibodies to the Borrelia burgdorferi flagellum improves serodiagnosis in Lyme disease. J Clin Microbiol. 1988;26:338–346. doi: 10.1128/jcm.26.2.338-346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jauris-Heipke S, Fuchs R, Motz M, Preac-Mursic V, Schwab E, Soutschek E, Will G, Wilske B. Genetic heterogeneity of the genes coding for the outer surface protein C (OspC) and the flagellin of Borrelia burgdorferi. Med Microbiol Immunol. 1993;182:37–50. doi: 10.1007/BF00195949. [DOI] [PubMed] [Google Scholar]
- 12.Jiang W, Luft B J, Munoz P, Dattwyler R J, Gorevic P D. Cross-antigenicity between the major surface proteins (ospA and ospB) and other proteins of Borrelia burgdorferi. J Immunol. 1990;144:284–289. [PubMed] [Google Scholar]
- 13.Jiang W, Luft B J, Schubach W, Dattwyler R J, Gorevic P D. Mapping the major antigenic domains of the native flagellar antigen of Borrelia burgdorferi. J Clin Microbiol. 1992;30:1535–1540. doi: 10.1128/jcm.30.6.1535-1540.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson B J, Robbins K E, Bailey R E, Cao B L, Sviat S L, Craven R B, Mayer L W, Dennis D T. Serodiagnosis of Lyme disease: accuracy of a two-step approach using a flagella-based ELISA and immunoblotting. J Infect Dis. 1996;174:346–353. doi: 10.1093/infdis/174.2.346. [DOI] [PubMed] [Google Scholar]
- 15.Luft B J, Mudri S, Jiang W, Dattwyler R J, Gorevic P D, Fischer T, Munoz P, Dunn J J, Schubach W H. The 93-kilodalton protein of Borrelia burgdorferi: an immunodominant protoplasmic cylinder antigen. Infect Immun. 1992;60:4309–4321. doi: 10.1128/iai.60.10.4309-4321.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masuzawa T, Wilske B, Komikado T, Suzuki H, Kawabata H, Sato N, Muramatsu K, Isogai E, Isogai H, Johnson R C, Yanagihara Y. Comparison of OspA serotypes for Borrelia burgdorferi sensu lato from Japan, Europe and North America. Microbiol Immunol. 1996;40:539–545. doi: 10.1111/j.1348-0421.1996.tb01106.x. [DOI] [PubMed] [Google Scholar]
- 17.Mathiesen M J, Christiansen M, Hansen K, Holm A, Asbrink E, Theisen M. Peptide-based OspC enzyme-linked immunosorbent assay for serodiagnosis of Lyme borreliosis. J Clin Microbiol. 1998;36:3474–3479. doi: 10.1128/jcm.36.12.3474-3479.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikolaeva N. The review of studies in vector ecology in Russia. Bull Inst Marit Trop Med Gdynia. 1996;47:73–83. [PubMed] [Google Scholar]
- 19.Robinson J M, Pilot-Matias T J, Pratt S D, Patel C B, Bevirt T S, Hunt J C. Analysis of the humoral response to the flagellin protein of Borrelia burgdorferi: cloning of regions capable of differentiating Lyme disease from syphilis. J Clin Microbiol. 1993;31:629–635. doi: 10.1128/jcm.31.3.629-635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosner B. Fundamentals of biostatistics. 3rd ed. Boston, Mass: PWS-Kent Publishing Company; 1990. [Google Scholar]
- 21.Satz N. Immunology and diagnostic test results in Lyme borreliosis. Schweiz Med Wochenschr. 1992;122:1779–1791. [PubMed] [Google Scholar]
- 22.Schubach W H, Mudri S, Dattwyler R J, Luft B J. Mapping antibody-binding domains of the major outer surface membrane protein (OspA) of Borrelia burgdorferi. Infect Immun. 1991;59:1911–1915. doi: 10.1128/iai.59.6.1911-1915.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seinost G, Dykhuizen D E, Dattwyler R J, Golde W T, Dunn J J, Wang I N, Wormser G P, Schriefer M E, Luft B J. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect Immun. 1999;67:3518–3524. doi: 10.1128/iai.67.7.3518-3524.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sigal L H. Immunology of Lyme disease. N J Med. 1990;87:567–571. [PubMed] [Google Scholar]
- 25.Stanek G. Laboratory diagnosis and seroepidemiology of Lyme borreliosis. Infection. 1991;19:263–267. doi: 10.1007/BF01644964. [DOI] [PubMed] [Google Scholar]
- 26.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 27.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 28.Wang I N, Dykhuizen D E, Qui W, Dunn J J, Bosler E M, Luft B J. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics. 1999;151:15–30. doi: 10.1093/genetics/151.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993;61:2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zar J H. Biostatistical analysis. 3rd ed. London, United Kingdom: Prentice-Hall International, Inc.; 1996. [Google Scholar]