Abstract
Over the past few years, immune checkpoint inhibitors (ICIs) have greatly improved the survival for patients with non‐small cell lung cancer (NSCLC) without driver mutations. Compared with wild‐type tumors, tumors with epidermal growth factor receptor (EGFR) mutations show more heterogeneity in the expression level of programmed cell death‐ligand 1 (PD‐L1), tumor mutational burden (TMB), and other immune microenvironment characteristics. Whether ICIs are suitable for NSCLC patients with EGFR mutations is still worth exploring. In previous studies, no significantly improved benefits were observed with immunotherapy monotherapy in NSCLC patients with EGFR mutation. Here, we summarized and analyzed data from the clinical trials of ICIs or combined therapy in NSCLC patients with EGFR mutations. We also focused on the mechanisms affecting the efficacy of ICIs in NSCLC patients with EGFR mutations, the characteristics of potential responders, and provided insights into areas worth further investigations in future studies.
Keywords: efficacy, EGFR mutation, immune checkpoint inhibitor, non‐small cell lung cancer, tumor microenvironment
An overview of the efficacy of PD‐1/PD‐L1 inhibitors in advanced NSCLC patients with EGFR mutation. We also focused on the mechanisms that affect the efficacy of ICIs in patients with EGFR mutation and further investigation worth to be done in future.
Abbreviations
- A2AR
A2A adenosine receptor
- ABCP
atezolizumab plus bevacizumab plus carboplatin plus paclitaxel
- ACP
atezolizumab plus carboplatin plus paclitaxel
- ADO
adenosine
- ADORA1
adenosine A1 Receptor
- AE
adverse event
- AKT
protein kinase B
- ASCO
American Society of Clinical Oncology
- ATP
adenosine triphosphate
- BCP
bevacizumab plus carboplatin plus paclitaxel
- CCL2
C‐C chemokine ligand 2
- CCL22
C‐C class chemokines
- CI
confidence interval
- CTLA‐4
cytotoxic‐T‐lymphocyte‐antigen‐4
- CXCL10
CXC‐chemokine ligand 10
- DOR
duration of response
- DRR
disease control rate
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal‐regulated kinase
- FDA
Food and Drug Administration
- Foxp3
forkhead box protein 3
- GSK‐3
glycogen synthase kinase 3
- HR
hazard ratio
- ICI
immune checkpoint inhibitor
- IL‐10
interleukin‐10
- IRF1
interferon regulatory factor‐1
- JAK
Janus kinase
- mAb
monoclonal antibody
- MAPK
mitogen‐activated protein kinase
- MDSC
myeloid‐derived suppressor cell
- MHC
major histocompatibility complex
- NK
natural killer
- NSCLC
non‐small cell lung cancer
- NT5E
ecto‐5'‐nucleotidase
- ORR
overall response rate
- OS
overall survival
- PD‐1
programmed cell death protein 1
- PD‐L1
programmed death‐ligand 1
- PFS
progression‐free survival
- PI3K
phosphatidylinositol 3‑kinase
- PR
partial response
- STAT3
signal transducer and activator of transcription 3
- TAM
tumor‐associated macrophage
- TCGA
the Cancer Genome Atlas
- TGF‐β
transforming growth factor‐β
- TIL
tumor‐infiltrating lymphocyte
- TKI
tyrosine kinase inhibitor
- TMB
tumor mutational burden
- TME
tumor microenvironment
- Treg
regulatory T cell
- VEGF
vascular endothelial growth factor
- YAP
Yes‐associated protein
1. BACKGROUND
Non‐small cell lung cancer (NSCLC) is one of the most common malignant tumors in the world [1]. Over the past decade, there have been significant breakthroughs in the research of immune checkpoint inhibitors (ICIs). Programmed cell death protein 1/programmed death‐ligand 1 (PD‐1/PD‐L1) antibodies (atezolizumab, nivolumab, pembrolizumab, and durvalumab) have been approved for the treatment of NSCLC patients. Compared with traditional cytotoxic chemotherapy, ICIs offer more benefits in monotherapy or combined therapy for patients without driver mutations [2].
Epidermal growth factor receptor (EGFR) mutations are common in patients with NSCLC, accounting for nearly 20% of patients [3]. Lung cancer with EGFR mutations is more frequent in non‐smokers than in ever‐smokers. In patients with NSCLC, EGFR mutations occur in approximately 40%‐60% of non‐smokers and approximately 10%‐20% of ever‐smokers [4]. Older patients are more likely to harbor EGFR mutations than younger patients [5]. However, NSCLC patients with EGFR mutations have not been found to benefit from ICIs in several trials. A meta‐analysis based on CheckMate 057, KEYNOTE‐010, and POPLAR showed that ICI monotherapy did not prolong the overall survival (OS) of patients with EGFR mutations compared with docetaxel [6]. One study reported that EGFR mutations were associated with hyper‐progression [7]. Currently, EGFR tyrosine kinase inhibitors (TKIs) are still the first‐line choice for patients with EGFR mutations, but resistance is inevitable [8]. Treatment options remain limited for patients who are resistant to EGFR‐TKIs. Therefore, whether ICIs can benefit TKI‐treated patients with EGFR mutations should be investigated.
Here, we reviewed the clinical trials of ICIs in NSCLC patients with EGFR mutations to determine the potential reasons for poor efficacy and the potential beneficiaries, and discussed the relevant challenges and future directions. In brief, we conducted a systematic search in PubMed using terms such as “NSCLC”, “EGFR”, “ICIs”, “immunotherapy”, “TME”, “TMB”, “PD‐L1”, “PD‐1”, and references from relevant articles. We included articles in English, and there were no time limits for publication dates. We also searched conference abstracts from unpublished studies in the American Society of Clinical Oncology (ASCO) and the European Society of Medical Oncology for data analysis.
2. CLINICAL EFFICACY Of ICIs IN EGFR‐MUTANT TUMORS
To date, for patients with EGFR mutations, the majority of the results were obtained from subgroup analyses. Here, we summarized the clinical efficacy of ICIs on EGFR‐mutant tumors (Table 1).
TABLE 1.
Clinical trials of immune checkpoint inhibitors in NSCLC patients with EGFR mutations
| Clinical trial | Phase | Treatment | Subgroup | Number | Outcome |
|---|---|---|---|---|---|
| Monotherapy | |||||
| KEYNOTE‐001 | II | Pembrolizumab | EGFR (+) | 10 | ORR = 0 |
| EGFR (+/−) | 495 | ORR = 19.4%; mDOR = 12.5 months; mOS = 12 months | |||
| CheckMate 012 | I | Nivolumab | EGFR (+) | 7 | ORR = 14%; mPFS = 1.8 months; DCR = 29% |
| EGFR (−) | 30 | ORR = 30%; mPFS = 6.6 months; DCR = 50% | |||
| KEYNOTE‐010 | III | Pembrolizumab | EGFR (+), PD‐L1 ≥1% | 86 |
OS: HR = 0.88, 95% CI = 0.45‐1.70); PFS: HR, 1.79 (0.94–3.42) |
| EGFR (−), PD‐L1 ≥1% | 875 |
OS: HR = 0.66, 95% CI = 0.55‐0.80; PFS: HR = 0.83, 95% CI = 0.71‐0.98 |
|||
| CheckMate 057 | III | Nivolumab vs. docetaxel | EGFR (+) | 44 |
ORR = 11%; OS: HR‐ = 1.18 (favors docetaxel) |
| EGFR (−) | 340 | OS: HR = 0.66 | |||
| OAK | III | Atezolizumab vs. docetaxel | EGFR (+), TKI‐pretreated | 85 |
ORR = 5%; OS: HR‐ = 1.24, 95% CI = 0.71‐2.18 |
| EGFR (−) | 628 | OS: HR = 0.69, 95% CI = 0.57‐0.83 | |||
| BIRCH | II | Atezolizumab | EGFR (+), PD‐L1 (TC2/3 or IC2/3, PD‐L1‐expressing cells) | 13 |
ORR = 31%; mOS = 26 months |
| EGFR (−) | 104 | ORR = 22%; mOS = 20.1 months | |||
| ATLANTIC | II | Durvalumab | EGFR+/ALK+, TKI‐pretreated, PD‐L1<25% | 30 | mPFS = 1.9 months; mOS = 9.9 months |
| EGFR‐/ALK‐, PD‐L1<25% | 94 | mPFS = 1.9 months; mOS = 9.3 months; | |||
| EGFR+, TKI‐pretreated, PD‐L1≥25% | 66 | mOS = 16.1 months | |||
| EGFR (−), PD‐L1≥25% | 149 | mOS = 10.9 months | |||
| Combined with EGFR‐TKIs | |||||
| KEYNOTE‐021 | II | Pembrolizumab + Erlotinib | EGFR (+), TKI‐pretreated | 12 | ORR = 41.7%; mOS = NR; mPFS = 19.5 months |
| Pembrolizumab + Gefitinib | 7 | ORR = 14.3%; mOS = 13 months; mPFS = 1.4 months | |||
| CheckMate 012 | I | Nivolumab + Elotinib | EGFR (+) | 21 | ORR = 19% |
| EGFR (+), TKI‐pretreated | 20 | ORR = 15%; mPFS = 16.6 months | |||
| TATTON | I | Durvalumab + Osimertinib | EGFR (+), TKI‐pretreated | 23 | ORR = 43% |
| EGFR (+), TKI‐naive | 11 | ORR = 70% | |||
| Double immunotherapy | |||||
| CheckMate 012 | I | Nivolumab + Ipilimumab | EGFR (+) | 8 | ORR = 50% |
| EGFR (−) | 54 | ORR = 41% | |||
| Combined with chemotherapy | |||||
| IMpower 130 | III | Atezolizumab + Chemotherapy vs. Chemotherapy | EGFR+/ALK+; TKI‐pretreated | NA |
OS: 14.4 vs. 10 months, HR = 0.98; PFS: 7.0 vs. 6.0 months, HR = 0.75 |
| EGFR‐/ALK‐ | 679 |
OS: 18.6 vs. 13.9 months, HR = 0.79; PFS: 7 vs. 5.5 months, HR = 0.64 |
|||
| CT18 | II | Toripalimab + Chemotherapy | TKI‐pretreated, without T790M | 40 |
ORR = 50%; DCR = 87.5%; mPFS = 7 months |
| Others | |||||
| IMpower 150 | III | ABCP | EGFR (+), TKI‐pretreated were included | 34 |
ORR = 71%; mPFS = 10.2 months; mOS = 26.1 months |
| ABCP | EGFR (−) | 359 | mOS = 19.5 months | ||
| ACP |
EGFR (+) TKI‐pretreated were included |
45 |
ORR = 36%; mPFS = 6.9 months; mOS = 21.4 months |
||
| ACP | EGFR (−) | 350 | mOS = 19.0 months; | ||
| BCP | EGFR (+), TKI‐pretreated were included | 44 | ORR = 42%; mPFS = 7.1 months; mOS = 20.3 months | ||
| BCP | EGFR (−) | 338 | mOS = 14.7 months | ||
| ABCP vs. BCP | TKI‐pretreated with sensitive EGFR mutation | 26 vs. 32 |
mOS, 29.4 vs. 18.1 months; HR = 0.60, 95% CI = 0.31‐1.34 |
||
Abbreviations: NSCLC, non‐small cell lung cancer; EGFR, epidermal growth factor receptor; ORR, overall response rate; mDOR, median duration of response; mOS, median overall survival; mPFS, median progression‐free survival; DCR, disease control rate; PD‐L1, programmed death‐ligand 1; OS, overall survival; PFS, progression‐free survival; HR, hazard ratio; CI, confidence interval; TKI, tyrosine kinase inhibitor; ABCP, Atezolizumab + Carboplatinc + Paclitaxeld + Bevacizumab; ACP, Atezolizumab + Carboplatinc + Paclitaxeld; BCP, Bevacizumab + Carboplatinc + Paclitaxeld; wk, week; AE, adverse event; NA, not applicable.
2.1. ICI monotherapy
In the KEYNOTE‐001 phase I trial, the median progression‐free survival (PFS; 157.5 vs. 56 days) and OS (559 vs. 120 days) after pembrolizumab treatment were longer in treatment‐naïve EGFR‐mutant patients (n = 4) than in patients previously treated with EGFR‐TKIs (n = 26) [9]. However, the phase II trial of KEYNOTE‐001 did not achieve similar results as expected. Twenty‐five treatment‐naïve NSCLC patients with EGFR mutation were recruited for treatment, 11 of whom received treatment with pembrolizumab but the trial was later terminated because of lack of efficacy [10]. In the CheckMate 012 study, nivolumab also did not show superiority in the EGFR‐mutant subgroup as first‐line treatment (overall response rate [ORR] = 14%; median PFS = 1.8 months) [11]. The results of these trials demonstrated that pembrolizumab monotherapy was not an applicable first‐line treatment for TKI‐naïve NSCLC patients with EGFR mutations.
In the trial of KEYNOTE‐010 (NCT01905657), the subgroup analysis of OS indicated that for patients with PD‐L1 expression, the clinical benefits of pembrolizumab in EGFR‐mutant patients were far less than those in the EGFR‐wild population [12]. Coincidentally, the efficacy of nivolumab was not superior to that of docetaxel in the EGFR‐mutant subgroup in the CheckMate 057 trial [13]. The effectiveness of atezolizumab was evaluated in the OAK trial [14]. Eighty‐five NSCLC patients with EGFR mutations after EGFR‐TKI treatment were enrolled, and the median OS in the atezolizumab group was found to be significantly shorter than the docetaxel group (10.5 months vs. 16.2 months) [14].
2.2. ICIs combined with EGFR‐TKIs enhanced toxicity
As first‐line therapy, 19 previously untreated, EGFR‐mutant NSCLC patients were treated with a combination of erlotinib or gefitinib with pembrolizumab in the KEYNOTE‐021 trial [15]. Twelve patients received erlotinib plus pembrolizumab, and the objective response rate and median PFS were 41.7% and 19.5 months. The adverse events (AEs) of pembrolizumab plus erlotinib were similar to those of those who received monotherapy but treatment for patients with pembrolizumab plus gefitinib was discontinued because five of the patients (71.4%) had grade 3/4 liver toxicity.
As the second‐line therapy, CheckMate 012 investigated the efficacy of nivolumab plus erlotinib [16]. In the EGFR‐mutant subgroup, there was no significant increase in the rate of AEs and no improvement in clinical benefit [16]. For patients with disease progression after EGFR‐TKI treatment, a multi‐arm and phase I trial (TATTON) was established to evaluate the efficacy of osimertinib combined with durvalumab [17]. Among them, five of 23 patients (22%) developed interstitial lung disease, and 11 patients (48%) experienced AEs that were no less than grade 3. Therefore, this study was also discontinued because of the development of serious AEs. Similarly, recruitment was terminated for another phase III trial (CAURAL) that assessed the efficacy of osimertinib plus durvalumab because of AEs [18].
Serious AEs of ICIs plus EGFR‐TKIs were observed in both the first‐line and other lines of treatments. The combination therapy did not further improve efficacy but posed more safety risks to patients. Therefore, it is necessary to perform large cohort studies and safety analyses to verify efficacy and evaluate toxicity.
2.3. Efficacy of ICIs combined with chemotherapy
IMpower130 assessed the efficacy of atezolizumab plus chemotherapy in NSCLC patients who received EGFR‐TKI treatment [19]. Compared with chemotherapy, combination therapy did not lead to significant benefits in 44 patients with EGFR/ALK mutation (OS, 14.4 vs. 10 months, hazard ratio [HR] = 0.98; PFS, 7.0 vs. 6.0 months, HR = 0.75). CT18 is a phase II study that assessed the combination of toripalimab and chemotherapy for patients with EGFR mutations who were resistant to EGFR‐TKIs without T790M mutation [20, 21]. According to the data presented in the ASCO meeting in 2020 [22], the ORR, disease control rate (DRR), and median PFS were 50%, 87.5%, and 7 months, respectively. The ORR for patients with PD‐L1‐positive (PD‐L1+; TPS ≥ 1%), PD‐L1 negative (PD‐L1−), TP53 co‐mutation and TP53 wild‐type were 60%, 39%, 62%, and 14%, respectively. Notably, the ORR of patients with TP53 co‐mutation was significantly higher than TP53 wild‐type (P = 0.04). Despite the small sample size, the combination of chemotherapy and ICIs is worth further exploration. Several clinical trials evaluating chemotherapy plus ICIs in patients with EGFR mutations are underway, such as the KEYNOTE‐789 and CheckMate‐722 studies (Table 2).
TABLE 2.
Potential mechanisms affecting the efficacy of ICIs on EGFR‐mutant tumors
| EGFR/EGFR‐TKIs | Key indicators | Methods | Main mechanism and result | Reference |
|---|---|---|---|---|
| EGFR | PD‐L1 expression ↓ | Experimental data | EGFR signaling inhibits PD‐L1 expression regulated by IFN‐γ via IRF1 in vitro experiments using human cell lines | [41] |
| Clinical studies | Via the analyses of TCGA and GCLI and IHC | [43, 44, 45] | ||
| PD‐L1+/TIL+ ↓ | Clinical studies | NA | [45] | |
| PD‐L1+ T cells in the blood ↓ | Clinical studies | NA | [50] | |
| PD‐L1 expression ↑ | Experimental data | Via the downstream signaling pathway of EGFR, such as MAPK/ERK/c‐Jun, Hippo/YAP, or JAK/STAT3 | [37, 38, 39] | |
| TMB ↓ | Clinical studies | NA | [55, 105, 106] | |
| CD8+ T cells ↓ | Experimental data | Downregulation of CXCL10 inhibits effector CD8+ T cell recruitment mediated by the PI3K‐AKT pathway | [45, 61] | |
| Tregs ↑ | Experimental data | EGFR signaling upregulates Treg‐associated genes | [65] | |
| Experimental data | Upregulate CCL22 via the JNK‐c‐Jun pathway | [41] | ||
| Experimental data | Mediate the function of Treg through amphiregulin | [66, 67] | ||
| Experimental data | Facilitate the conversion of CD3+CD4+CD25− T cells to Tregs via IDO | [68] | ||
| MHC class I | Experimental data | MHC class I ↓; via IFN‐γ signaling pathways and MEK/ERK signaling pathways | [69, 70, 71] | |
| TAMs | Experimental data | Activate the EGFR signaling via EGF; Recruiting more Treg cells by producing chemokines | [72] | |
| CD73 | Experimental data | Upregulate CD73 expression via the Ras‐RAF‐ERK pathway | [35, 78, 79] | |
| Clinical studies | Tregs ↑, CD4+ TIL ↓, CD8+ TIL ↓ | [78] | ||
| Experimental data | CD73 blockade significantly inhibited tumor progression in the immune‐competent mouse model | [35] | ||
| EGFR‐TKIs | PD‐L1 expression | Experimental data | PD‐L1 expression↓ | [46] |
| Clinical studies | PD‐L1 expression↓ | [47, 48, 49] | ||
| Immunological enhancement (early stage) | Experimental data | CD8+ TIL ↑, DCs ↑, M1‐like TAMs ↑, Treg ↓ | [46] | |
| Immunosuppressive (later stage) | Experimental data | IL‐10 ↑, CCL2 ↑, MDSCs ↑ | [46] |
Abbreviations: ICI, immune checkpoint inhibitor; EGFR, epidermal growth factor receptor; TIL, tumor‐infiltrating lymphocyte; PD‐L1, programmed death‐ligand 1; TIL, tumor‐infiltrating lymphocyte; TMB, tumor mutational burden; Treg, regulatory T cell; MHC, major histocompatibility complex; TAM, tumor‐associated macrophage; IFN‐γ, interferon‐gamma; IRF1, interferon regulatory factor 1; TCGA, The Cancer Genome Atlas; IHC, immunohistochemistry; NA, not applicable; CXCL10, CXC‐chemokine ligand 10; CCL2, C‐C chemokine ligand 2; MDSC, myeloid‐derived suppressor cell; IL‐10, interleukin‐10; IDO, indoleamine 2, 3‐ dioxygenase.
2.4. ICIs combined with chemotherapy and anti‐angiogenic drugs show great benefits
Vascular endothelial growth factor (VEGF) and EGFR are critical factors in tumor progression and metastasis, and share common downstream signaling pathways [23, 24]. Previous studies have shown that the EGFR signaling pathway can induce VEGF expression to modulate angiogenesis [25]. VEGF can regulate the infiltration of immune cells (such as antigen‐presenting cells, T cytotoxic cells, and regulatory T (Treg) cells and promote the migration of myeloid‐derived inhibitory cells into tumors, thus, promoting the tumor immunosuppressive environment [26, 27]. A spectrum of preclinical and clinical studies demonstrated that the anti‐VEGF antibody can not only promote the normalization of tumor blood vessels but also relieve inhibition of the immune microenvironment [27]. The anti‐PD‐1/PD‐L1 antibody can normalize lymphocyte function and prevent immune escape [28]. These different mechanisms provide the theoretical basis for the combination therapy of the anti‐VEGF antibody and anti‐PD‐1/PD‐L1 antibody. The combination of antiangiogenic with anti‐PD‐L1 treatment significantly improved CD8+ T cell infiltration compared with antiangiogenic or anti‐PD‐L1 monotherapy (P = 0.002) [29].
Bevacizumab, an anti‐VEGF antibody, has been shown to significantly improve OS when combined with chemotherapy [30]. Additionally, researchers evaluated the efficacy of atezolizumab plus bevacizumab and chemotherapy in the IMpower150 clinical trial [31]. Patients with no prior chemotherapy were randomly assigned to atezolizumab plus bevacizumab with carboplatin and paclitaxel (ABCP), atezolizumab plus carboplatin and paclitaxel (ACP), or bevacizumab plus carboplatin and paclitaxel (BCP) groups [31]. For patients with EGFR mutations (n = 123), the median OS of ABCP, ACP, and BCP groups was 26.1, 21.4, and 20.3 months, respectively. Compared with BCP, ABCP significantly improved the median PFS (10.2 months vs. 7.1 months; HR, 0.56; 95% CI, 0.34‐0.91) of the patients. The ORR and duration of response (DOR) in the ABCP group were also higher than those in the BCP group (ORR, 73.5% vs. 40.9%; median DOR, 11.1 vs. 4.7 months). For patients with sensitive EGFR mutation, ABCP showed significant improvements in terms of PFS (10.3 vs 6.1 months, HR, 0.38; 95% CI, 0.21–0.68) compared with BCP. The four‐drug combination of IMpower150 trial offers a new option for posterior line therapy in patients with EGFR mutations. Considering the improved benefits of the IMpower150 trial, the efficacy of immunotherapy combined with antiangiogenic drugs is being evaluated in several clinical trials, which could provide more evidence for future applications (Table 2).
2.5. Double immunotherapy
PD‐1 and cytotoxic T‐lymphocyte antigen‐4 (CTLA‐4) regulate anti‐tumor immune responses in a different but complementary manner. The combination of nivolumab and ipilimumab with two cycles of platinum‐doublet chemotherapy for stage IV or recurrent NSCLC patients without EGFR/ALK mutation has been approved by the US Food and Drug Administration (FDA) [32]. A subgroup analysis of the CheckMate 012 study evaluated whether nivolumab plus ipilimumab could be used as the first‐line treatment for advanced NSCLC [33]. In the EGFR‐mutant group (n = 8), 50% of the patients achieved an objective response [33]. In another recent study (IND226) [34], 5 patients receiving EGFR‐TKI treatment received durvalumab and tremelimumab plus platinum‐doublet chemotherapy and achieved partial response (PR). These trials indicated encouraging efficacy of double ICIs, which need further confirmation.
Immunotherapy is not suitable as a first‐line treatment for NSCLC patients with EGFR mutations. For patients who failed EGFR‐TKI treatment, immunotherapy monotherapy did not show improved survival benefits compared with chemotherapy. The combination of EGFR‐TKIs and ICIs did not improve efficacy but increased toxicity. Nevertheless, the combination of immunotherapy and chemotherapy primarily showed efficacy. ICIs combined with chemotherapy and anti‐angiogenic drugs have shown promising survival benefits in the IMpower150 [31]. Overall, combined therapy may be more suitable for EGFR‐mutant patients. Currently, several clinical trials of immunotherapy combined with other treatments in NSCLC patients with EGFR mutation are ongoing (Table 2).
3. POTENTIAL MECHANISMS UNDERLYING THE LOW EFFICACY OF ICIS ON EGFR‐MUTANT NSCLC
NSCLC tumors with EGFR mutations are characterized by an immune‐inert phenotype, with low PD‐L1 expression, low TMB level, and low infiltration of cytotoxic T cells [35]. Furthermore, single‐cell analysis showed that the expression of CD73 is upregulated in the tumor cells of NSCLC with EGFR mutation, both in EGFR‐TKI naïve and TKI‐resistant tumors [35]. The EGFR signaling pathway and EGFR‐TKIs affect many aspects of immune efficacy (Table 3).
TABLE 3.
Ongoing clinical trials of PD‐1/PD‐L1 inhibitors in NSCLC patients with EGFR mutation
| NCT number | Phase | Status | Drug | Treatment | Population | Primary endpoint |
|---|---|---|---|---|---|---|
| NCT02364609 | I | Active, not recruiting | Pembrolizumab | Pembrolizumab + Afatinib | EGFR mutation with Erlotinib treatment failure | ORR, PFS |
| NCT04013542 | I | Recruiting | Nivolumab | Ipilimumab + Nivolumab + Radiation Therapy | EGFR mutation are eligible | AEs, PFS, OS, ORR, DOR |
| NCT04517526 | II | Not yet recruiting | Durvalumab |
Platinum‐based Chemotherapy + Bevacizumab + Durvalumab + Salvage SBRT |
EGFR mutation with EGFR‐TKI treatment failure | PFS, OS, ORR, DOR |
| NCT04426825 | II | Recruiting | Atezolizumab | Atezolizumab + Bevacizumab | EGFR mutation with EGFR‐TKI treatment failure | PFS, OS, ORR, DOR, AEs |
| NCT04405674 | II | Not yet recruiting | Tislelizumab | Tislelizumab + Chemotherapy | EGFR mutation with EGFR‐TKI treatment failure | PFS, OS, ORR, DOR, DCR |
| NCT04245085 (ABC‐lung) | II | Recruiting | Atezolizumab | Atezolizumab + Bevacizumab + Chemotherapy | EGFR mutation with EGFR‐TKI treatment failure | PFS, AEs, OS, ORR |
| NCT04120454 | II | Recruiting | Pembrolizumab | Ramucirumab + Pembrolizumab | EGFR mutation with EGFR‐TKI treatment failure | ORR, AEs, DCR, PFS, OS |
| NCT04147351 | II | Recruiting | Atezolizumab | Atezolizumab + Bevacizumab + Carboplatin/Cisplatin+Pemetrexed | EGFR mutation with EGFR‐TKI treatment failure | ORR, PFS |
| NCT04099836 | II | Recruiting | Atezolizumab | Atezolizumab + Bevacizumab | EGFR mutation with Osimertinib treatment failure | ORR, PFS, OS, AEs |
| NCT04042558 (GFPC 06‐2018) | II | Recruiting | Atezolizumab | Atezolizumab ± Bevacizumab + Platinum + Pemetrexed | EGFR mutations, ALK rearrangement or ROS1 fusion with targeted therapies failure | ORR, PFS, OS, DOR |
| NCT03994393 (ILLUMINATE) | II | Recruiting | Durvalumab + Tremelimumab | Durvalumab + Tremelimumab | EGFR mutation with EGFR‐TKI treatment failure | ORR, DCR, PFS, OS |
| NCT03513666 (JS001) | II | Active, not recruiting | Toripalimab | Toripalimab + Pemetrexed + Carboplatin | EGFR mutation with EGFR‐TKI treatment failure | ORR, PFS, OS, DOR |
| NCT02947386 | I/II | Recruiting | Nivolumab | Nivolumab + Nimotuzumab | EGFR mutation are eligible | ORR, irAEs |
| NCT03786692 | II | Recruiting | Atezolizumab | Carboplatin + Pemetrexed + Bevacizumab ± Atezolizumab | EGFR mutation in exon 19 or exon 21 | PFS, ORR, DOR |
| NCT03802240 | III | Recruiting | Sintilimab | Sintilimab ± IBI305 + Chemotherapy | EGFR mutation with EGFR‐TKI treatment failure | PFS, OS, ORR |
| NCT03515837 (KEYNOTE‐789) | III | Active, not recruiting | Pembrolizumab | Pemetrexed + Platinum ± Pembrolizumab | EGFR mutation with EGFR‐TKI treatment failure | PFS, OS, ORR, DOR |
| NCT03991403 | III | Recruiting | Atezolizumab | Atezolizumab + Combination Carboplatin + Paclitaxel + Bevacizumab | EGFR or ALK mutation | PFS, OS, ORR, DOR |
| NCT02864251 (CheckMate722) | III | Active, not recruiting | Nivolumab | Nivolumab + Chemotherapy vs. Nivolumab + Ipilimumab vs. Chemotherapy | EGFR mutation with EGFR‐TKI treatment failure | PFS, OS, ORR, DOR |
| NCT02454933 (CAURAL) | III | Active, not recruiting | Durvalumab | Durvalumab + Osimertinib vs. Osimertinib | EGFR mutation and T790M mutation with EGFR‐TKI treatment failure | AEs |
Abbreviations: NSCLC, non‐small cell lung cancer; EGFR, epidermal growth factor receptor; ORR, overall response rate; DOR, duration of response; OS, overall survival; PFS, progression‐free survival; DCR, disease control rate; PD‐L1, programmed death‐ligand 1; TKI, tyrosine kinase inhibitor; AE, adverse event; PD‐1, programmed cell death protein 1; irAE, immune‐related adverse event.
3.1. Effect of EGFR mutation and EGFR‐TKIs on PD‐L1 expression
Previous clinical studies have shown that NSCLC patients with high PD‐L1 expression can obtain more benefits from ICIs as compared to traditional chemotherapy [33, 36]. PD‐L1, as an immune checkpoint protein, is expressed in tumor cells and tumor‐infiltrating immune cells [37]. The expression of PD‐L1 is affected by two different mechanisms: intrinsic expression and acquired expression. EGFR mutation can upregulate PD‐L1 expression in NSCLC cells via the downstream signaling pathway of EGFR, such as mitogen‐activated protein kinase/extracellular signal‐regulated kinases/c‐Jun (MAPK/ERK/c‐Jun), Hippo/Yes‐associated protein (Hippo/YAP), and Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3) signaling pathway [38, 39, 40]. In contrast, in vitro studies have demonstrated that EGFR signaling inhibited acquired PD‐L1 expression by inhibiting IFN‐γ stimulation, which is regulated by interferon regulatory factor‐1 (IRF1) signaling [41]. In preclinical studies, the regulation of PD‐L1 expression by EGFR signaling remains contradictory. In addition, some retrospective studies and analyses of the Cancer Genome Atlas (TCGA) and the Guangdong Lung Cancer Institute indicated that PD‐L1 expression was significantly upregulated in EGFR wild‐type tumors than in EGFR‐mutant tumors [42, 43, 44, 45].
In vitro cell line experiments showed that EGFR‐TKIs downregulated PD‐L1 expression by inhibiting EGFR signaling [46]. Nevertheless, some clinical analyses demonstrated that PD‐L1 expression showed an upward trend after treatment with EGFR‐TKIs [47, 48]. After EGFR‐TKI treatment (n = 128), the proportion of patients with high PD‐L1 expression (stain intensity of tumor cells ≥ 50%) increased from 14% to 28% (P = 0.001) [48]. Gainor et al. [49] also demonstrated that 21% of patients (n = 12) had increased PD‐L1 expression in their tumor tissues after resistance to EGFR‐TKIs. PD‐L1+ T cells in the blood were also significantly increased after one week of EGFR‐TKI treatment [50]. Beyond that, most patients who had primary resistance to EGFR‐TKI showed had PD‐L1 expression and PD‐L1+CD8+ T cell infiltration [51, 52]. This may be explained by the association between EGFR‐TKI resistance and PD‐L1 upregulation. A retrospective study [48] analyzed PD‐L1 expression in 138 patients with EGFR‐mutated NSCLC who underwent re‐biopsy after progression during EGFR‐TKI treatment [51, 52]. After EGFR‐TKI treatment, patients with high PD‐L1 expression had longer OS than patients with low expression from PD‐1 inhibitor (7.1 vs. 1.7 months, P = 0.0033) [48].
3.2. Tumor mutational burden (TMB)
TMB is defined as the total number of somatic mutations in the entire tumor genome, which is an emerging biomarker for predicting the prognosis after ICI treatment. Compared with EGFR wild‐type patients, patients with EGFR mutations had a lower level of TMB. The median TMB in patients with EGFR mutation was 3.8 non‐synonymous mutations/Mb, much lower than that of wild‐type patients (7.4 non‐synonymous mutations/Mb) [53]. In particular, sensitive EGFR types had significantly lower TMB levels and immunogenicity [45].
The reduced TMB of NSCLC tumors without EGFR mutation resulted in poor efficacy of ICIs [54, 55]. TMB may be one potential explanation for the poor efficacy of ICIs in EGFR‐mutant tumors. However, there is no consistent standard for the detection, calculation method and cut‐off value of TMB. Further determining the system standard of TMB as a biomarker might help to select the appropriate population.
3.3. Tumor microenvironment (TME)
EGFR‐mutant tumors have unique TME characteristics (Figure 1). TME is the internal environment for tumor growth and development, and it is crucial for the immune regulatory network, which includes myeloid cells, T lymphocytes, cytokines, and exosomes. Treg cells, myeloid‐derived suppressor cells (MDSCs), and some cytokines often show immunosuppressive effects. Based on the difference in TME, tumors can be divided into “cold tumor” and “hot tumor”.
FIGURE 1.
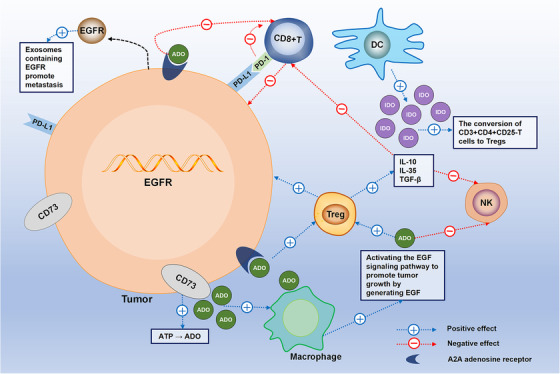
The immune characteristics of tumors with EGFR mutation. EGFR‐mutant tumors have low infiltration of CD8+ T cells and high expression of Treg and CD73. Treg cells can secrete IL‐10, IL‐35, and TGF‐β to reduce anti‐tumor immune responses mediated by NK cells and CD8+ T cells. DCs can secrete IDO, which promotes the conversion of CD3+CD4+ CD25‐T cells to Tregs. CD73 promotes ATP decomposition into ADO. A2A is an ADO receptor that is widely expressed in lung cancer. The CD73‐ADO axis promotes the efficacy of Tregs and MDSCs. ADO combined with A2AR also inhibits T cell signal transduction, thus impairing anti‐tumor immunity. Moreover, EGFR‐mutant tumors secrete exosomes containing EGFR mutations to promote distant metastasis. Abbreviations: EGFR, epidermal growth factor receptor; PD‐L1, programmed death‐ligand 1; ADO, adenosine; PD‐1, programmed cell death protein 1; DC, dendritic cell; IDO, indoleamine 2, 3‐ dioxygenase; Treg, regulatory T cell; NK, natural killer; A2AR, adenosine A2A receptor; MDSC, myeloid‐derived suppressor cell
High CD8+ T infiltration is believed to be associated with a good prognosis of NSCLC, as evidenced by several studies [49, 56, 57]. CXC‐chemokine ligand 10 (CXCL10) can recruit effector CD8+ T cells via the phosphatidylinositol 3‑kinase (PI3K)/protein kinase B (AKT) signaling pathway [58, 59]. EGFR signaling downregulates CXCL10, thus, inhibiting the recruitment of effector CD8+ T cells [60]. Nevertheless, tumors with EGFR mutations often showed lower infiltration of CD8+ tumor‐infiltrating lymphocytes (TILs) [45, 61] which can lead to immune deficiency and poor prognosis [62]. Furthermore, EGFR‐mutant tumors showed a higher ratio of PD‐L1−/TIL− but a lower ratio of PD‐L1+/TIL+ in comparison to EGFR‐wild tumors, which can lead to low responses to ICIs [45].
Tregs are highly infiltrated in tumors with EGFR mutations [63] and can attenuate the anti‐tumor immune response mediated by natural killer (NK) cells, CD4+ T cells, and CD8+ T cells by secreting interleukin‐10 (IL‐10), IL‐35, and transforming growth factor‐β (TGF‐β) [64]. The activation of EGFR mutation can upregulate Treg‐associated gene expression and recruit Treg cells by upregulating the C‐C class chemokines (CCL22) via the JNK‐c‐Jun pathway, as observed in a preclinical model [60, 65]. Preclinical studies have also shown showed that the EGFR/glycogen synthase kinase 3 (GSK‐3)/forkhead box protein 3 (Foxp3) axis mediated the inhibitory immune function of Treg through amphiregulin and promoted tumor progression [66, 67]. In addition, exosomes containing EGFR promoted the production of indoleamine 2,3‐dioxygenase secreted by DCs, which facilitated the conversion of CD3+CD4+CD25+ T cells to Tregs [68].
Other factors can also affect the TME. The major histocompatibility complex (MHC) plays an important role in antigen presentation. Previous studies demonstrated that IFN‐γ signaling pathways and MEK/ERK signaling pathways could downregulate the expression of MHC‐I and MHC‐II [69, 70]. Compared with wild‐type tumors, EGFR‐mutant tumors showed lower expression of human leukocyte antigen‐B [71]. Tumor‐associated macrophages (TAMs) can produce EGF which activates the EGFR signaling pathway and promotes tumor growth [72].
EGFR‐TKIs can relieve the inhibition of EGFR on T cells, weaken the function of Treg cells, enhance the production of IFN‐γ, and potentiate the expression of MHC‐I and MHC‐II [39, 63, 73]. However, the effect of EGFR‐TKIs on the TME may be dynamic. In a murine model, Jia et al. [46] demonstrated the dynamic effect of EGFR‐TKIs. They observed that the effect of EGFR‐TKIs on TME was beneficial in the early stage but immunosuppressive in the late stage. At the early stage, the numbers of CD8+ T cells, DCs, and M1‐like TAMs showed an increasing trend while Treg infiltration decreased. In the later stage of EGFR‐TKI treatment, the increased secretion of IL‐10 and C‐C chemokine ligand 2 (CCL2) promoted the migration and activation of MDSCs, thus suppressing immunity and promoting angiogenesis and metastasis [46, 64]. Short‐term low‐dose exposure to erlotinib led to immune‐mediated cytotoxicity in EGFR‐mutant tumors and tumor lysis of NK cells and antigen‐specific T cells. However, this enhanced immune‐mediated cytotoxicity disappeared after long‐term treatment with erlotinib [74]. The above study provides the rationale for the treatment of ICIs and erlotinib before EGFR‐TKI resistance. In addition, it is worth investigating whether the toxicity will disappear as the beneficial effects of combination therapy diminish.
CD73, which promotes immune escape by participating in the decomposition of adenosine triphosphate (ATP) into adenosine (ADO), is highly expressed in various tumors and associated with poor prognosis [75, 76, 77]. The expression of CD73 is upregulated in tumors with EGFR mutations [35, 78]. Likewise, in tumors with EGFR mutations, the top upregulated genes, such as ecto‐5'‐nucleotidase (NT5E) and adenosine A1 receptor (ADORA1), belong to the CD73‐ADO pathway [35]. In the downstream signaling pathway of EGFR, the Ras‐RAF‐ERK pathway directly regulated CD73 expression through ERK1/2 [79]. The CD73‐ADO axis promoted the efficacy of Tregs and MDSCs, thus, impairing antigen recognition and tumor‐killing functions [64]. Patients with a high expression of CD73 showed lower CD4+ TIL and CD8+ TIL than those with low expression [78]. Le et al. [35] demonstrated that CD73 blockade significantly inhibited tumor progression in an immune‐competent mouse model of EGFR‐mutant lung cancer. High expression of CD73 could predict the efficacy of ICIs in patients with EGFR mutations whereas CD73 expression had no significant effect on efficacy in patients without EGFR mutations [80]. A2A is a G‐protein‐coupled ADO receptor that is widely expressed in lung cancer. In preclinical models, A2AR (A2A adenosine receptor) blockade combined with ICIs could increase the infiltration of CD8+TILs to enhance the secretion of IFN‐γ and granzyme‐B [81]. A2AR inhibitors are not only involved in preventing negative signal transduction of T cells but also inhibit tumor cells directly [82].
In conclusion, the lower expression of PD‐L1, the lower level of TMB, and the upregulation of the immunosuppressive environment are the reasons for the disadvantage of EGFR‐mutant patients with ICIs. The effect of EGFR‐TKIs on the TME may be dynamic. Timely monitoring the dynamic changes and selecting appropriate timing windows may expand the population suitable for immunotherapy. Targeting immunoregulatory factors in the TME can also improve the efficacy of immunotherapy.
4. FUTURE DIRECTIONS AND CHALLENGES
4.1. Identify the patients who can benefit from immunotherapy
The efficacy of ICIs in NSCLC patients with EGFR mutations is associated with its heterogeneous immune characteristics. In addition, the characteristics of the immune microenvironment are influenced by many factors. Although the overall benefits of immunotherapy in EGFR‐mutant patients are poor, some patients still show superiority. It is vital to identify the patients who can benefit from immunotherapy.
4.1.1. EGFR subtypes have different responses to ICIs
Distinct EGFR mutation types have different clinical outcomes compared to ICIs. EGFR exon 19 deletions and EGFR L858R are the two most common EGFR mutations. Other EGFR mutations are called rare mutations and account for 10%‐20% of all EGFR mutations such as G719X and exon 20 insertions [83]. Patients with EGFR L858R‐mutant tumors had similar response rates (22% vs. 16%, P = 0.42) and OS (HR = 0.917, 95% confidence intervals [CI] = 0.597‐1.409, P = 0.69) as those with wild‐type [53]. Nevertheless, EGFR exon 19 deletions exhibited significantly reduced benefits than wild type (ORR, 7% vs. 22%; OS, HR = 0.69, P = 0.03) [53]. The efficacy of ICIs varies depending on the level of TMB and T cell infiltration. Similarly, clinical analysis indicated that EGFR L858R‐mutant tumors had higher TMB levels (P < 0.001) and CD8+PD‐1+ T cell infiltration than EGFR exon 19 deletions [84]. The level of TMB was reported to be positively correlated with age [85]. Furthermore, EGFR L858R‐mutant is common in the elderly and may account for higher TMB [53, 60].
A retrospective study involving 27 patients indicated that patients with uncommon EGFR mutations (such as G719X and exon 20 insertion) could obtain more clinical benefits from ICIs than those with common mutations. Patients with the uncommon subtype had higher ORR, DCR, and median PFS (ORR, 71% vs. 35.7%, P = 0.14; DCR, 57% vs. 7%, P < 0.01; mPFS, 256 vs. 50 days, HR = 0.288) [86]. For patients with EGFR G719X mutations, the ORR and PFS of ICIs and afatinib (ORR = 77.8%; median PFS = 13.8 months) were numerically similar [87].Tumors with EGFR G719X mutations had higher TMB than EGFR exon 19 deletions [84]. High PD‐L1 expression and CD8+ TILs had also been observed [88]. EGFR exon 20 insertion mutations are common in non‐smokers and women [89]. The TMB was similar to common sensitizing EGFR mutations (mean, 4.3; range, 0‐40.3 mutations/Mb), and the positive expression rate of PD‐L1 was 37%‐80% in NSCLC with EGFR exon 20 insertion [90]. Compared to classic EGFR mutants, EGFR exon 20 insertion demonstrated significantly longer PFS (HR = 0.45, P = 0.002) and OS (HR = 0.2, P < 0.001) [91].
Based on the above studies, EGFR L858R, G719X and exon 20 insertions showed potential to benefit from ICIs. However, current clinical and preclinical evidence for each mutation type is insufficient. In particular, the understanding of the biology of rare mutations is inadequate. For instance, EGFR exon 20 insertions are diverse and often associated with co‐mutations [83]. Currently, the benefits of EGFR‐TKIs and immunotherapy are limited to patients with EGFR exon 20 insertions. Although the IMpower130 and IMpower150 phase III clinical trials included some patients with exon 20 insertions, the number was too limited to draw conclusions.
Patients receiving EGFR‐TKIs often develop acquired resistance after 9‐14 months of treatment, and about 50%‐60% of this resistance is due to T790M mutations. Patients with T790M‐positive tumors could benefit from osimertinib treatment. Compared with T790M‐positive patients, patients without T790M mutation had higher PD‐L1 levels, higher infiltration of CD8+ TILs, and lower infiltration of FOXP3+ TILs [62]. In patients who received EGFR‐TKI treatment, PFS and ORR of patients without T790M mutation were higher than T790M‐positive patients (P = 0.03; P = 0.21, respectively) [86]. Haratani et al. [62] also indicated that T790M‐negative patients with EGFR‐TKI treatment failure tended to acquire more clinical benefits from nivolumab. The median PFS, ORR and DOR in patients without T790M mutation was longer or higher numerically than those with T790M mutation (2.1 months vs. 1.3 months, HR = 0.48, 95% CI: 0.20‐1.24; 24% vs. 13%, P = 1.000; 47% vs. 13%, P = 0.182) [62].
The efficacy of ICIs varies depending on the heterogeneity of the immune microenvironment of distinct EGFR mutations. At present, there are few studies on the immune characteristics and TME between different mutations. To understand the molecular mechanisms that affect the efficacy of ICIs among different types of EGFR mutations, a comparison of immunological analyses between the various types is necessary.
4.1.2. Patients with high PD‐L1 expression might benefit from ICIs
PD‐L1 expression was generally downregulated in patients with EGFR mutations. Moreover, it is extremely rare for PD‐L1 ≥ 50% to coexist with driver mutations [92]. However, some studies have shown that a small number of patients with high PD‐L1 expression benefited from immunotherapy. The ATLANTIC trial assessed the efficacy of durvalumab in EGFR‐mutant patients who received EGFR‐TKIs [93]. For EGFR‐mutant patients with at least 25% of tumor cells expressing PD‐L1 (n = 66), durvalumab was not associated with a prolongation of the PFS and ORR (median PFS, 1.9 vs. 3.3 months; ORR, 14.1% vs. 16.4%) but prolonged the OS (16.1 vs. 10.9 months) of the EGFR‐mutant patients compared to EGFR−/ALK− patients [94]. The BIRCH trial showed that in patients with PD‐L1 expression of at least 5% on tumor cells or immune cells and treated with atezolizumab as first‐line therapy, ORR was 31% (4/13) in the EGFR‐mutant group and 22% (23/104) in the wild‐type group [95]. Although the two studies mentioned above treatments, the detection and evaluation of PD‐L1 expression were inconsistent. They showed that EGFR‐mutant patients with high PD‐L1 expression might benefit from ICIs. Detecting the expression of PD‐L1 can still predict the efficacy of ICIs for patients with EGFR/ALK mutations.
4.2. Combination treatment
To date, no clinical trials have shown the appropriate efficacy and safety of EGFR‐TKIs combined with immunotherapy. A retrospective study reported that patients who received EGFR‐TKIs and progressed within 6 months had more survival benefits from subsequent immunotherapy [96]. Similarly, a case report demonstrated that two patients with EGFR‐TKI resistance had notable responses with EGFR‐TKI re‐challenge immediately after nivolumab [97]. Experimental data showed that EGFR‐TKIs could not only reduce the infiltration of immunosuppressive cells but also promote the formation of an immunosuppressive environment. This dynamic effect of EGFR‐TKIs may be a key factor in combination therapy outcomes.
Targeting the CD73‐ADO axis includes the use of small‐molecule inhibitors or human monoclonal antibodies to inhibit ADO production or neutralize ADO. A2AR blockade combined with PD‐1/PD‐L1 or CTLA‐4 inhibitors can increase infiltration of CD8+ TILs to enhance anti‐tumor response [81, 82]. Therefore, the combination of A2AR blockade combined with ICIs may contribute to the transition from “cold tumors” into “hot tumors”. Clinical trials evaluating the efficacy of several A2AR inhibitors and anti‐CD73 monoclonal antibodies are in progress for NSCLC patients with EGFR mutations, such as the NCT02503774 (AZD4635), NCT02403193 (PBF‐509), and NCT02503774 (MEDI9447, oleclumab) trials.
Radiotherapy plays an important role in anti‐tumor immunity by participating in various immunomodulatory effects [98]. Radiotherapy can activate immune pathways by producing the abscopal effect and promoting the release of cytokines to turn “cold” tumors into “hot” tumors [99]. Immunotherapy can also boost the abscopal effect to produce a powerful anti‐tumor response to radiotherapy combined with immunotherapy [100]. NCT04517526 and NCT04013542 are ongoing clinical studies evaluating the efficacy of immunotherapy combined with radiotherapy in patients with EGFR mutations (Table 2 ).
VEGF inhibitors are also an option for combination therapy. The IMpower 150 trial [31] showed that patients with EGFR mutations had improved treatment efficacy with ICIs in combination with anti‐VEGF monoclonal antibody (mAb). The four‐drug combination in the IMpower150 trial offers a new option for other lines of treatments in patients with EGFR mutations. These results strongly suggest that bevacizumab has important value for improving the immune microenvironment and promoting the efficacy of ICIs. The reduction in Treg cells and MDSCs through VEGF inhibition may lead to immunological sensitization [101]. In addition, chemotherapy can reduce tumor load, promote the release of tumor antigens, and suppress immunosuppressive cells, thereby, regulating the immune microenvironment. However, the precise mechanisms underlying these effects remain unclear. Therefore, it is necessary to explore the mechanisms in preclinical models. Several clinical trials of immunotherapy in combination with anti‐VEGF and chemotherapy are underway (Table 2) and could provide clearer evidence.
Cetuximab, an anti‐EGFR mAb, combined with ICIs has been reported to enhance immune responses in other solid tumors expressing EGFR [102]. The combinations of multikinase inhibitors and ICIs have also shown promising outcomes in gastric cancer [103]. These results may provide ideas for exploring new applications of immunotherapy to improve antitumor efficacy in NSCLC with EGFR mutations.
The blocking of abnormal increases in the EGFR signaling pathway caused by genetic changes may transform immunosuppressive tumors into an inflammatory microenvironment, providing the basis for combination immunotherapies. However, the selection of appropriate drugs, timing and treatment schemes still need further discussion. One EGFR‐mutant patient who progressed after 2 months of EGFR‐TKI treatment received four cycles of pembrolizumab combined with chemoradiotherapy and subsequently achieved complete response [104]. Future research may focus on ICIs combined with other treatment approaches, such as EGFR‐TKIs, chemotherapy, radiotherapy, and other targeted therapies.
4.3. Others
In addition, to maximize the role of immunotherapy, researching potential predictive markers is imperative. A single biomarker, such as PD‐L1 or TMB, may be insufficient to provide prognostic value. The integration of biomarkers or the selection of different biomarkers for different patients will be a new potential option in the future.
5. CONCLUSION
Based on the current studies, NSCLC patients with EGFR mutations obtain little benefit from ICIs. EGFR‐TKIs remain the first‐line treatment choice for patients with EGFR mutations. The EGFR signaling pathway not only directly regulates tumor cells but also affects the tumor microenvironment, thus, establishing an immunosuppressive microenvironment to achieve tumor avoidance. Therefore, it is very important to reverse the immunosuppressive microenvironment to improve the sensitivity of ICIs. Current studies have mainly focused on EGFR‐TKIs, anti‐CD73 mAbs, and VEGF inhibitors. Of these, combination therapy with VEGF inhibitors seems the most promising approach.
To the best of our knowledge, the current analyses of immunotherapy for NSCLC patients with EGFR mutations are limited. Most analyses were based on subgroup analysis, randomized controlled trials, or observational studies. More clinical evidence and validation are needed to determine the efficacy of immunotherapy and identify who can benefit from ICIs. Currently, several clinical studies are underway in patients with EGFR mutations, involving multiline therapy and combination therapies. Exploring the appropriate combination and application of ICIs is critical not only for EGFR‐mutated NSCLC patients but also for immunotherapy in other solid tumors containing driver mutations.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
COMPETING INTERESTS
The authors declare that they have no competing interests.
FUNDING
The study was funded by the National Natural Science Foundation of China (81972796 and 81972863), Radiation Oncology Innovate Unit, Chinese Academy of Medical Sciences (2019RU071), the Academic Promotion Program of Shandong First Medical University (2019ZL002), and the Natural Science Foundation of Shandong (ZR2019MH010 and ZR2020MH289).
AUTHORS' CONTRIBUTIONS
XJM and JMY conceived of the review and edited the manuscript. BWD, ZQH, and BW contributed to the data collection. LM analyzed the data and drafted the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
Not applicable.
Ma L, Diao B, Huang Z, Wang B, Yu J, Meng X. The efficacy and possible mechanisms of immune checkpoint inhibitors in treating non‐small cell lung cancer patients with epidermal growth factor receptor mutation. Cancer Commun. 2021;41:1314–1330. 10.1002/cac2.12229
Contributor Information
Jinming Yu, Email: sdyujinming@163.com.
Xiangjiao Meng, Email: mengxiangjiao@126.com.
REFERENCES
- 1. Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends–An Update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16‐27. [DOI] [PubMed] [Google Scholar]
- 2. Kimura H, Araya T, Yoneda T, Shirasaki H, Kurokawa K, Sakai T, et al. Long‐lasting responses after discontinuation of nivolumab treatment for reasons other than tumor progression in patients with previously treated, advanced non‐small cell lung cancer. Cancer Commun (Lond). 2019;39(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Toyooka S, Matsuo K, Shigematsu H, Kosaka T, Tokumo M, Yatabe Y, et al. The impact of sex and smoking status on the mutational spectrum of epidermal growth factor receptor gene in non small cell lung cancer. Clin Cancer Res. 2007;13(19):5763‐8. [DOI] [PubMed] [Google Scholar]
- 5. Choi YH, Lee JK, Kang HJ, Lee TS, Kim HR, Kim CH, et al. Association between age at diagnosis and the presence of EGFR mutations in female patients with resected non‐small cell lung cancer. J Thorac Oncol. 2010;5(12):1949‐52. [DOI] [PubMed] [Google Scholar]
- 6. Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint Inhibitors in Metastatic EGFR‐Mutated Non‐Small Cell Lung Cancer‐A Meta‐Analysis. J Thorac Oncol. 2017;12(2):403‐7. [DOI] [PubMed] [Google Scholar]
- 7. Kato S, Goodman A, Walavalkar V, Barkauskas DA, Sharabi A, Kurzrock R. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clinical Cancer Research. 2017;23(15):4242‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang F, Diao XY, Zhang X, Shao Q, Feng YF, An X, et al. Identification of genetic alterations associated with primary resistance to EGFR‐TKIs in advanced non‐small‐cell lung cancer patients with EGFR sensitive mutations. Cancer Commun (Lond). 2019;39(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med. 2015;372(21):2018‐28. [DOI] [PubMed] [Google Scholar]
- 10. Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T, et al. A Phase II Study of Pembrolizumab in EGFR‐Mutant, PD‐L1+, Tyrosine Kinase Inhibitor Naive Patients With Advanced NSCLC. J Thorac Oncol. 2018;13(8):1138‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, et al. Nivolumab Monotherapy for First‐Line Treatment of Advanced Non‐Small‐Cell Lung Cancer. J Clin Oncol. 2016;34(25):2980‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herbst RS, Baas P, Kim D‐W, Felip E, Pérez‐Gracia JL, Han J‐Y, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. The Lancet. 2016;387(10027):1540‐50. [DOI] [PubMed] [Google Scholar]
- 13. Borghaei H, Paz‐Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non‐Small‐Cell Lung Cancer. N Engl J Med. 2015;373(17):1627‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): a phase 3, open‐label, multicentre randomised controlled trial. The Lancet. 2017;389(10066):255‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang JC, Gadgeel SM, Sequist LV, Wu CL, Papadimitrakopoulou VA, Su WC, et al. Pembrolizumab in Combination With Erlotinib or Gefitinib as First‐Line Therapy for Advanced NSCLC With Sensitizing EGFR Mutation. J Thorac Oncol. 2019;14(3):553‐9. [DOI] [PubMed] [Google Scholar]
- 16. Gettinger S, Hellmann MD, Chow LQM, Borghaei H, Antonia S, Brahmer JR, et al. Nivolumab Plus Erlotinib in Patients With EGFR‐Mutant Advanced NSCLC. J Thorac Oncol. 2018;13(9):1363‐72. [DOI] [PubMed] [Google Scholar]
- 17. Oxnard GR, Yang JC, Yu H, Kim SW, Saka H, Horn L, et al. TATTON: a multi‐arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR‐mutant lung cancer. Ann Oncol. 2020;31(4):507‐16. [DOI] [PubMed] [Google Scholar]
- 18. Yang JC, Shepherd FA, Kim DW, Lee GW, Lee JS, Chang GC, et al. Osimertinib Plus Durvalumab versus Osimertinib Monotherapy in EGFR T790M‐Positive NSCLC following Previous EGFR TKI Therapy: CAURAL Brief Report. J Thorac Oncol. 2019;14(5):933‐9. [DOI] [PubMed] [Google Scholar]
- 19. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab‐paclitaxel chemotherapy compared with chemotherapy alone as first‐line treatment for metastatic non‐squamous non‐small‐cell lung cancer (IMpower130): a multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol. 2019;20(7):924‐37. [DOI] [PubMed] [Google Scholar]
- 20. Ren S, Zhang J, Zhao Y, Mu X, Zhou J, Bao Z, et al. A multi‐center phase II study of toripalimab with chemotherapy in patients with EGFR mutant advanced NSCLC patients resistant to EGFR TKIs: Efficacy and biomarker analysis. Journal of Clinical Oncology. 2020;38(15_suppl):e21618‐e. [Google Scholar]
- 21. Zhang J, Zhou C, Zhao Y, Mu X, Zhou J, Bao Z, et al. MA11.06 A PII Study of Toripalimab, a PD‐1 mAb, in Combination with Chemotherapy in EGFR+ Advanced NSCLC Patients Failed to Prior EGFR TKI Therapies. Journal of Thoracic Oncology. 2019;14(10). [Google Scholar]
- 22. Ren S, Zhang J, Zhao Y, Mu X, Zhou J, Bao Z, et al. A multi‐center phase II study of toripalimab with chemotherapy in patients with EGFR mutant advanced NSCLC patients resistant to EGFR TKIs: Efficacy and biomarker analysis. Journal of Clinical Oncology. 2020;38:e21618‐e. [Google Scholar]
- 23. Herbst RS, Johnson DH, Mininberg E, Carbone DP, Henderson T, Kim ES, et al. Phase I/II trial evaluating the anti‐vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER‐1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non‐small‐cell lung cancer. J Clin Oncol. 2005;23(11):2544‐55. [DOI] [PubMed] [Google Scholar]
- 24. Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, et al. Constitutive Stat3 activity up‐regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21(13):2000‐8. [DOI] [PubMed] [Google Scholar]
- 25. Kim SJ, Uehara H, Karashima T, Shepherd DL, Killion JJ, Fidler IJ. Blockade of epidermal growth factor receptor signaling in tumor cells and tumor‐associated endothelial cells for therapy of androgen‐independent human prostate cancer growing in the bone of nude mice. Clin Cancer Res. 2003;9(3):1200‐10. [PubMed] [Google Scholar]
- 26. Heine A, Held SA, Bringmann A, Holderried TA, Brossart P. Immunomodulatory effects of anti‐angiogenic drugs. Leukemia. 2011;25(6):899‐905. [DOI] [PubMed] [Google Scholar]
- 27. Hu C, Jiang X. The effect of anti‐angiogenic drugs on regulatory T cells in the tumor microenvironment. Biomed Pharmacother. 2017;88:134‐7. [DOI] [PubMed] [Google Scholar]
- 28. Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD‐1 and PD‐L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front Pharmacol. 2017;8:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Allen E, Jabouille A, Rivera LB, Lodewijckx I, Missiaen R, Steri V, et al. Combined antiangiogenic and anti‐PD‐L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med. 2017;9(385). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti‐VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol. 2018;52(Pt 2):117‐24. [DOI] [PubMed] [Google Scholar]
- 31. Reck M, Mok T, Socinski MA, Jotte RM, Lim DWT, Cappuzzo F, et al. 1293P IMpower150: Updated efficacy analysis in patients with EGFR mutations. Annals of Oncology. 2020;31:S837‐S8. [Google Scholar]
- 32. Vellanki PJ, Mulkey F, Jaigirdar AA, Rodriguez L, Wang Y, Xu Y, et al. FDA Approval Summary: Nivolumab with Ipilimumab and Chemotherapy for Metastatic Non‐Small Cell Lung Cancer, a Collaborative Project Orbis Review. Clin Cancer Res. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, et al. Nivolumab plus ipilimumab as first‐line treatment for advanced non‐small‐cell lung cancer (CheckMate 012): results of an open‐label, phase 1, multicohort study. The Lancet Oncology. 2017;18(1):31‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Juergens RA, Hao D, Ellis PM, Tu D, Mates M, Kollmannsberger C, et al. A phase IB study of durvalumab with or without tremelimumab and platinum‐doublet chemotherapy in advanced solid tumours: Canadian Cancer Trials Group Study IND226. Lung Cancer. 2020;143:1‐11. [DOI] [PubMed] [Google Scholar]
- 35. Le X. Characterization of the Immune Landscape of EGFR‐Mutant NSCLC Identifies CD73/Adenosine Pathway as a Potential Therapeutic Target. Journal of thoracic oncology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bodor JN, Boumber Y, Borghaei H. Biomarkers for immune checkpoint inhibition in non–small cell lung cancer (NSCLC). Cancer. 2020;126(2):260‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka‐Akita H, Nishimura M. B7‐H1 expression on non‐small cell lung cancer cells and its relationship with tumor‐infiltrating lymphocytes and their PD‐1 expression. Clinical Cancer Research. 2004;10(15):5094‐100. [DOI] [PubMed] [Google Scholar]
- 38. D'Incecco A, Andreozzi M, Ludovini V, Rossi E, Capodanno A, Landi L, et al. PD‐1 and PD‐L1 expression in molecularly selected non‐small‐cell lung cancer patients. Br J Cancer. 2015;112(1):95‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen N, Fang W, Zhan J, Hong S, Tang Y, Kang S, et al. Upregulation of PD‐L1 by EGFR Activation Mediates the Immune Escape in EGFR‐Driven NSCLC: Implication for Optional Immune Targeted Therapy for NSCLC Patients with EGFR Mutation. J Thorac Oncol. 2015;10(6):910‐23. [DOI] [PubMed] [Google Scholar]
- 40. Jin R, Zhao J, Xia L, Li Q, Li W, Peng L, et al. Application of immune checkpoint inhibitors in EGFR‐mutant non‐small‐cell lung cancer: from bed to bench. Ther Adv Med Oncol. 2020;12:1758835920930333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sugiyama E, Togashi Y, Takeuchi Y, Shinya S, Tada Y, Kataoka K, et al. Blockade of EGFR improves responsiveness to PD‐1 blockade in EGFR‐mutated non‐small cell lung cancer. Sci Immunol. 2020;5(43). [DOI] [PubMed] [Google Scholar]
- 42. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, et al. PD‐1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heigener DF, Reck M. Impact of PD‐L1 Expression in EGFR‐Positive NSCLC? The Answer Remains the Same…. J Thorac Oncol. 2018;13(8):1060‐1. [DOI] [PubMed] [Google Scholar]
- 44. Takada K, Toyokawa G, Tagawa T, Kohashi K, Shimokawa M, Akamine T, et al. PD‐L1 expression according to the EGFR status in primary lung adenocarcinoma. Lung Cancer. 2018;116:1‐6. [DOI] [PubMed] [Google Scholar]
- 45. Dong ZY, Zhang JT, Liu SY, Su J, Zhang C, Xie Z, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD‐1 blockade in non‐small cell lung cancer. Oncoimmunology. 2017;6(11):e1356145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jia Y, Li X, Jiang T, Zhao S, Zhao C, Zhang L, et al. EGFR‐targeted therapy alters the tumor microenvironment in EGFR‐driven lung tumors: Implications for combination therapies. Int J Cancer. 2019;145(5):1432‐44. [DOI] [PubMed] [Google Scholar]
- 47. Peng S, Wang R, Zhang X, Ma Y, Zhong L, Li K, et al. EGFR‐TKI resistance promotes immune escape in lung cancer via increased PD‐L1 expression. Mol Cancer. 2019;18(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Isomoto K, Haratani K, Hayashi H, Shimizu S, Tomida S, Niwa T, et al. Impact of EGFR‐TKI Treatment on the Tumor Immune Microenvironment in EGFR Mutation‐Positive Non‐Small Cell Lung Cancer. Clin Cancer Res. 2020;26(8):2037‐46. [DOI] [PubMed] [Google Scholar]
- 49. Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD‐1 Pathway Blockade in Non‐Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res. 2016;22(18):4585‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meniawy TM, Lake RA, McDonnell AM, Millward MJ, Nowak AK. PD‐L1 on peripheral blood T lymphocytes is prognostic in patients with non‐small cell lung cancer (NSCLC) treated with EGFR inhibitors. Lung Cancer. 2016;93:9‐16. [DOI] [PubMed] [Google Scholar]
- 51. Hsu KH, Huang YH, Tseng JS, Chen KC, Ku WH, Su KY, et al. High PD‐L1 expression correlates with primary resistance to EGFR‐TKIs in treatment naïve advanced EGFR‐mutant lung adenocarcinoma patients. Lung Cancer. 2019;127:37‐43. [DOI] [PubMed] [Google Scholar]
- 52. Su S, Dong ZY, Xie Z, Yan LX, Li YF, Su J, et al. Strong Programmed Death Ligand 1 Expression Predicts Poor Response and De Novo Resistance to EGFR Tyrosine Kinase Inhibitors Among NSCLC Patients With EGFR Mutation. J Thorac Oncol. 2018;13(11):1668‐75. [DOI] [PubMed] [Google Scholar]
- 53. Hastings K, Yu HA, Wei W, Sanchez‐Vega F, DeVeaux M, Choi J, et al. EGFR mutation subtypes and response to immune checkpoint blockade treatment in non‐small‐cell lung cancer. Ann Oncol. 2019;30(8):1311‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fang W, Ma Y, Yin JC, Hong S, Zhou H, Wang A, et al. Comprehensive Genomic Profiling Identifies Novel Genetic Predictors of Response to Anti‐PD‐(L)1 Therapies in Non‐Small Cell Lung Cancer. Clin Cancer Res. 2019;25(16):5015‐26. [DOI] [PubMed] [Google Scholar]
- 55. Rizvi H, Sanchez‐Vega F, La K, Chatila W, Jonsson P, Halpenny D, et al. Molecular Determinants of Response to Anti‐Programmed Cell Death (PD)‐1 and Anti‐Programmed Death‐Ligand 1 (PD‐L1) Blockade in Patients With Non‐Small‐Cell Lung Cancer Profiled With Targeted Next‐Generation Sequencing. J Clin Oncol. 2018;36(7):633‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brambilla E, Le Teuff G, Marguet S, Lantuejoul S, Dunant A, Graziano S, et al. Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non‐Small‐Cell Lung Cancer. J Clin Oncol. 2016;34(11):1223‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557(7706):575‐9. [DOI] [PubMed] [Google Scholar]
- 58. Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, et al. Epigenetic silencing of TH1‐type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527(7577):249‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Van Raemdonck K, Van den Steen PE, Liekens S, Van Damme J, Struyf S. CXCR3 ligands in disease and therapy. Cytokine Growth Factor Rev. 2015;26(3):311‐27. [DOI] [PubMed] [Google Scholar]
- 60. Kumagai S, Koyama S, Nishikawa H. Antitumour immunity regulated by aberrant ERBB family signalling. Nat Rev Cancer. 2021;21(3):181‐97. [DOI] [PubMed] [Google Scholar]
- 61. Mazzaschi G, Madeddu D, Falco A, Bocchialini G, Goldoni M, Sogni F, et al. Low PD‐1 Expression in Cytotoxic CD8(+) Tumor‐Infiltrating Lymphocytes Confers an Immune‐Privileged Tissue Microenvironment in NSCLC with a Prognostic and Predictive Value. Clin Cancer Res. 2018;24(2):407‐19. [DOI] [PubMed] [Google Scholar]
- 62. Haratani K, Hayashi H, Tanaka T, Kaneda H, Togashi Y, Sakai K, et al. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation‐positive non‐small‐cell lung cancer based on T790M status after disease progression during EGFR‐TKI treatment. Annals of Oncology. 2017;28(7):1532‐9. [DOI] [PubMed] [Google Scholar]
- 63. Mascia F, Schloemann DT, Cataisson C, McKinnon KM, Krymskaya L, Wolcott KM, et al. Cell autonomous or systemic EGFR blockade alters the immune‐environment in squamous cell carcinomas. Int J Cancer. 2016;139(11):2593‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lin A, Wei T, Meng H, Luo P, Zhang J. Role of the dynamic tumor microenvironment in controversies regarding immune checkpoint inhibitors for the treatment of non‐small cell lung cancer (NSCLC) with EGFR mutations. Mol Cancer. 2019;18(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD‐1 pathway contributes to immune escape in EGFR‐driven lung tumors. Cancer Discov. 2013;3(12):1355‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang S, Zhang Y, Wang Y, Ye P, Li J, Li H, et al. Amphiregulin Confers Regulatory T Cell Suppressive Function and Tumor Invasion via the EGFR/GSK‐3β/Foxp3 Axis. J Biol Chem. 2016;291(40):21085‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chang MH AH, Lee J, Jung CK, Choi YL, Park YH, Ahn JS, Park K, Ahn, MJ . Clinical impact of amphiregulin expression in patients with epidermal growth factor receptor (EGFR) wild‐type nonsmall cell lung cancer treated with EGFR‐tyrosine kinase inhibitors. Cancer 2011;117:143‐51. 2011. [DOI] [PubMed] [Google Scholar]
- 68. Huang SH, Li Y, Zhang J, Rong J, Ye S. Epidermal growth factor receptor‐containing exosomes induce tumor‐specific regulatory T cells. Cancer Invest. 2013;31(5):330‐5. [DOI] [PubMed] [Google Scholar]
- 69. Yamaki M, Sugiura K, Muro Y, Shimoyama Y, Tomita Y. Epidermal growth factor receptor tyrosine kinase inhibitors induce CCL2 and CCL5 via reduction in IL‐1R2 in keratinocytes. Exp Dermatol. 2010;19(8):730‐5. [DOI] [PubMed] [Google Scholar]
- 70. Kumai T, Matsuda Y, Oikawa K, Aoki N, Kimura S, Harabuchi Y, et al. EGFR inhibitors augment antitumour helper T‐cell responses of HER family‐specific immunotherapy. Br J Cancer. 2013;109(8):2155‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Watanabe S, Hayashi H, Haratani K, Shimizu S, Tanizaki J, Sakai K, et al. Mutational activation of the epidermal growth factor receptor down‐regulates major histocompatibility complex class I expression via the extracellular signal‐regulated kinase in non‐small cell lung cancer. Cancer Sci. 2019;110(1):52‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wyckoff J, Wang W, Lin EY, Wang Y, Pixley F, Stanley ER, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64(19):7022‐9. [DOI] [PubMed] [Google Scholar]
- 73. Brea EJ, Oh CY, Manchado E, Budhu S, Gejman RS, Mo G, et al. Kinase Regulation of Human MHC Class I Molecule Expression on Cancer Cells. Cancer Immunol Res. 2016;4(11):936‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dominguez C, Tsang KY, Palena C. Short‐term EGFR blockade enhances immune‐mediated cytotoxicity of EGFR mutant lung cancer cells: rationale for combination therapies. Cell Death Dis. 2016;7(9):e2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor‐mediated signaling in adenosine‐mediated inhibition of T‐cell activation and expansion. Blood. 1997;90(4):1600‐10. [PubMed] [Google Scholar]
- 76. Jin D, Fan J, Wang L, Thompson LF, Liu A, Daniel BJ, et al. CD73 on tumor cells impairs antitumor T‐cell responses: a novel mechanism of tumor‐induced immune suppression. Cancer Res. 2010;70(6):2245‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Antonioli L, Blandizzi C, Malavasi F, Ferrari D, Haskó G. Anti‐CD73 immunotherapy: A viable way to reprogram the tumor microenvironment. Oncoimmunology. 2016;5(9):e1216292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Park LC, Rhee K, Kim WB, Cho A, Song J, Anker JF, et al. Immunologic and clinical implications of CD73 expression in non‐small cell lung cancer (NSCLC). J Clin Oncol. 2018;36(15_suppl):12050‐. [Google Scholar]
- 79. Griesing S, Liao BC, Yang JC. CD73 Is Regulated by the EGFR‐ERK Signaling Pathway in Non‐small Cell Lung Cancer. Anticancer Res. 2021;41(3):1231‐42. [DOI] [PubMed] [Google Scholar]
- 80. Ishii H, Azuma K, Kawahara A, Kinoshita T, Matsuo N, Naito Y, et al. Predictive value of CD73 expression for the efficacy of immune checkpoint inhibitors in NSCLC. Thorac Cancer. 2020;11(4):950‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Beavis PA, Milenkovski N, Henderson MA, John LB, Allard B, Loi S, et al. Adenosine Receptor 2A Blockade Increases the Efficacy of Anti‐PD‐1 through Enhanced Antitumor T‐cell Responses. Cancer Immunol Res. 2015;3(5):506‐17. [DOI] [PubMed] [Google Scholar]
- 82. Mediavilla‐Varela M, Luddy K, Noyes D, Khalil FK, Neuger AM, Soliman H, et al. Antagonism of adenosine A2A receptor expressed by lung adenocarcinoma tumor cells and cancer associated fibroblasts inhibits their growth. Cancer Biol Ther. 2013;14(9):860‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang T, Wan B, Zhao Y, Li C, Liu H, Lv T, et al. Treatment of uncommon EGFR mutations in non‐small cell lung cancer: new evidence and treatment. Transl Lung Cancer Res. 2019;8(3):302‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhou J. Epidermal growth factor receptor tyrosine kinase inhibitor remodels tumor microenvironment by upregulating LAG‐3 in advanced non‐small‐cell lung cancer. Lung Cancer (Amsterdam, Netherlands). 2021;153:143‐9. [DOI] [PubMed] [Google Scholar]
- 85. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Yamada T, Hirai S, Katayama Y, Yoshimura A, Shiotsu S, Watanabe S, et al. Retrospective efficacy analysis of immune checkpoint inhibitors in patients with EGFR‐mutated non‐small cell lung cancer. Cancer Med. 2019;8(4):1521‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wu YL, Hirsh V, Sequist LV, Hu CP, Feng J, Lu S, et al. Does EGFR Mutation Type Influence Patient‐Reported Outcomes in Patients with Advanced EGFR Mutation‐Positive Non‐Small‐Cell Lung Cancer? Analysis of Two Large, Phase III Studies Comparing Afatinib with Chemotherapy (LUX‐Lung 3 and LUX‐Lung 6). Patient. 2018;11(1):131‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chen K, Cheng G, Zhang F, Zhu G, Xu Y, Yu X, et al. PD‐L1 expression and T cells infiltration in patients with uncommon EGFR‐mutant non‐small cell lung cancer and the response to immunotherapy. Lung Cancer. 2020;142:98‐105. [DOI] [PubMed] [Google Scholar]
- 89. Oxnard GR, Lo PC, Nishino M, Dahlberg SE, Lindeman NI, Butaney M, et al. Natural history and molecular characteristics of lung cancers harboring EGFR exon 20 insertions. J Thorac Oncol. 2013;8(2):179‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Remon J, Hendriks LEL, Cardona AF, Besse B. EGFR exon 20 insertions in advanced non‐small cell lung cancer: A new history begins. Cancer Treat Rev. 2020;90:102105. [DOI] [PubMed] [Google Scholar]
- 91. Negrao MV, Reuben A, Robichaux JP, Le X, Nilsson MB, Wu C‐j, et al. Association of EGFR and HER‐2 exon 20 mutations with distinct patterns of response to immune checkpoint blockade in non‐small cell lung cancer. Journal of Clinical Oncology. 2018;36(15_suppl):9052‐. [Google Scholar]
- 92. Rangachari D, VanderLaan PA, Shea M, Le X, Huberman MS, Kobayashi SS, et al. Correlation between Classic Driver Oncogene Mutations in EGFR, ALK, or ROS1 and 22C3‐PD‐L1 ≥50% Expression in Lung Adenocarcinoma. J Thorac Oncol. 2017;12(5):878‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Garassino MC, Cho B‐C, Kim J‐H, Mazières J, Vansteenkiste J, Lena H, et al. Durvalumab as third‐line or later treatment for advanced non‐small‐cell lung cancer (ATLANTIC): an open‐label, single‐arm, phase 2 study. The Lancet Oncology. 2018;19(4):521‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Garassino MC, Cho BC, Kim JH, Mazières J, Vansteenkiste J, Lena H, et al. Final overall survival and safety update for durvalumab in third‐ or later‐line advanced NSCLC: The phase II ATLANTIC study. Lung Cancer. 2020;147:137‐42. [DOI] [PubMed] [Google Scholar]
- 95. Peters S, Carcereny Costa E, Garassino MC, Christoph D, Kurata T, Chaft J, et al. Atezolizumab as first‐line (1L) therapy for advanced non‐small cell lung cancer (NSCLC) in PD‐L1–selected patients: Efficacy data from the BIRCH trial. Annals of Oncology. 2017;28:ii29‐ii30. [Google Scholar]
- 96. Ichihara E, Harada D, Inoue K, Shibayama T, Hosokawa S, Kishino D, et al. Characteristics of patients with EGFR‐mutant non‐small‐cell lung cancer who benefited from immune checkpoint inhibitors. Cancer Immunol Immunother. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kaira K, Kagamu H. Drastic Response of Re‐challenge of EGFR‐TKIs Immediately After Nivolumab Therapy in EGFR‐TKI‐Resistant Patients. J Thorac Oncol. 2019;14(6):e135‐e6. [DOI] [PubMed] [Google Scholar]
- 98. Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16(13):e498‐509. [DOI] [PubMed] [Google Scholar]
- 99. Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015;41(6):503‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ngwa W, Irabor OC, Schoenfeld JD, Hesser J, Demaria S, Formenti SC. Using immunotherapy to boost the abscopal effect. Nat Rev Cancer. 2018;18(5):313‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Li SJ, Chen JX, Sun ZJ. Improving antitumor immunity using antiangiogenic agents: Mechanistic insights, current progress, and clinical challenges. Cancer Commun (Lond). 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Concha‐Benavente F, Kansy B, Moskovitz J, Moy J, Chandran U, Ferris RL. PD‐L1 Mediates Dysfunction in Activated PD‐1(+) NK Cells in Head and Neck Cancer Patients. Cancer Immunol Res. 2018;6(12):1548‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kawazoe A, Fukuoka S, Nakamura Y, Kuboki Y, Wakabayashi M, Nomura S, et al. Lenvatinib plus pembrolizumab in patients with advanced gastric cancer in the first‐line or second‐line setting (EPOC1706): an open‐label, single‐arm, phase 2 trial. Lancet Oncol. 2020;21(8):1057‐65. [DOI] [PubMed] [Google Scholar]
- 104. Pizarro G, Pinto MP, Muñoz‐Medel M, Cordova‐Delgado M, Bravo ML, Nervi B, et al. Complete Response to Immunotherapy Plus Chemotherapy After an Unusual Clinical Response to Afatinib and Stereotactic Radiosurgery in a Patient With Metastatic EGFR‐Mutant Non‐Small‐Cell Lung Cancer. Clin Lung Cancer. 2020;21(4):e250‐e4. [DOI] [PubMed] [Google Scholar]
- 105. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier‐Valette C, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med. 2018;378(22):2093‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ready N, Hellmann MD, Awad MM, Otterson GA, Gutierrez M, Gainor JF, et al. First‐Line Nivolumab Plus Ipilimumab in Advanced Non‐Small‐Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J Clin Oncol. 2019;37(12):992‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


