Abbreviations
- FDR
false discovery rate
- GENIE
Genomics, Evidence, Neoplasia, Information, Exchange
- GISTIC
Genomic Identification of Significant Targets in Cancer
- MT
metastatic tumors
- NSCLC
non‐small cell lung cancer
- PT
primary tumors
- PT‐MT
primary tumors‐metastatic tumors
- SCIMET
spatial computational inference of metastatic timing
- SNV
single nucleotide mutations
- TCGA
The Cancer Genome Atlas
- TKI
tyrosine kinase inhibitor
INTRODUCTION
Lung cancer is one of the cancers with the highest morbidity and mortality worldwide. Over the two past decades, although the treatment of non‐small cell lung cancer (NSCLC) has been revolutionized by the use of tyrosine kinase inhibitor (TKI) and immune treatments, metastatic disease remains largely incurable and presents a 5‐year survival rate of approximately 10% [1].
Metastasis is an evolutionary process that involves multiple stages, such as the spread of cancer cells from the primary tumor and then their intravasation into the blood or lymphatic system, survival in the bloodstream and/or lymphatic system, embedding into a distant organ, survival in a new environment, and formation of a new metastatic tumor [2]. A previous study demonstrated that this process could be intrinsically inefficient because the majority of cancer cells either die during circulation, become stuck in capillaries, or undergo apoptosis in the blood within 24 hours. After successful colonization of distal organs, only a subset of cancer cells will develop into macrometastatic tumors [3]. However, once metastases have been established, the tumor growth follows a ‘big bang’ model [4], and current treatments frequently may fail to provide durable responses.
Each metastatic cell represents an evolutionary branch of its parental primary tumor and shares key driver changes. These cells are not under positive selection to metastasize. Rather, a set of key adaptations or hallmarks may be selected to obtain metastatic potential [5].
Herein, we focused on lung cancer metastasis from a genomic perspective, explored the differences in genomic events between metastatic and primary tumor samples, explored the evolutionary pattern and timing of lung cancer metastasis, and explored whether there are differences in evolutionary trajectories between different metastatic organs.
Genomic heterogeneity between primary and metastatic tumors in lung cancer
During the process of metastasis, each step depends on the specific phenotypic characteristics of tumor cells and their interactions with the host microenvironment and immune system. Genetic alterations obtained from primary and metastatic tumor cells may explain these phenotypic and host interactions [2].
Targeted next‐generation sequencing of 30 tumor specimens from 15 patients was performed in a previous study and demonstrated that recurrent genomic alterations between the primary tumor and matched metastasis presented a concordance rate of 94% whereas the likely passenger genomic alterations presented a concordance rate of 63% [6]. Furthermore, a 71.6% concordance rate in clonal somatic mutations was exhibited between the primary tumor and metastatic lymph nodes from 6 patients[7]. In 61 patients with brain metastases, mutations of major drivers, including EGFR, KRAS, TP53, and ALK, were highly concordant between the primary NSCLC and matched brain metastatic tumors (>80%) [8] (Figure 1). These results suggested that classic genomic drivers, such as EGFR and ALK, may be early clonal genomic events during the carcinogenesis of lung adenocarcinoma. For total genetic alterations, Tang et al. [9] revealed that the overall concordance between primary tumors (PT) and metastatic tumors (MT) was 45.6%, and it ranged from 7.3% to 80.6% per patient. Furthermore, metastatic site‐specific differences were also observed. Among lymph node, bone, brain, and adrenal gland metastases, lymph node metastases exhibited the lowest proportion of primary tumors‐metastatic tumors (PT‐MT) shared alterations while adrenal gland metastases shared the highest proportion of genomic alterations with primary tumors (Figure 1).
FIGURE 1.
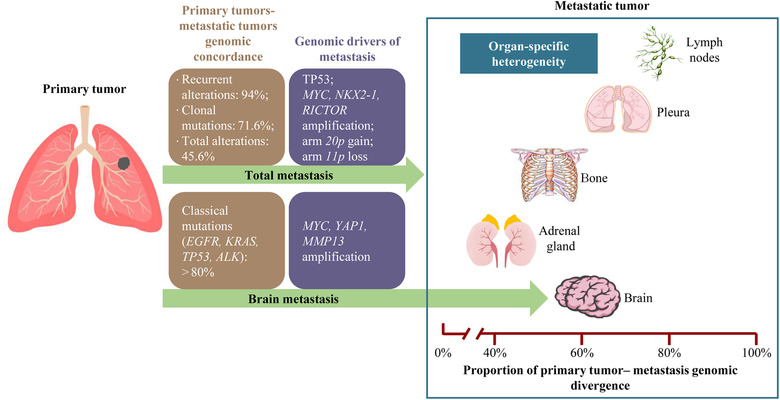
Inter‐tumor genomic heterogeneity and driver events of lung cancer metastasis. The information summarized in the figure are from previously published articles [6, 7, 8, 9, 10, 11].
Therefore, inter‐tumor genomic heterogeneity indicates the importance of comprehensively understanding the lung cancer gene landscape in clinical practice and could explain the heterogeneity of antitumor therapy efficacy.
Genomic drivers of lung cancer metastasis
From the Genomics, Evidence, Neoplasia, Information, Exchange (GENIE) cohort [10], 1897 primary and 1133 unpaired metastatic samples were directly compared. The results showed a large degree of overlap in the frequency of commonly mutated genes, with only TP53 remaining significant after adjusting for false discovery rate (FDR). Furthermore, Shih et al. [11] performed whole‐exome sequencing on 73 brain metastatic samples of lung cancer and compared them with 503 primary lung adenocarcinoma samples from The Cancer Genome Atlas (TCGA) database. By screening of Genomic Identification of Significant Targets in Cancer (GISTIC) score and validation of animal models, the authors found MYC, YAP1, and MMP13 amplification represented potential drivers of brain metastasis in lung adenocarcinoma (Figure 1). However, the above previous studies exploring the driver events of lung cancer were mainly based on nonpaired primary‐metastatic specimens, possibly due to specimen limitations.
Paired analysis between primary and metastatic tumors can reveal whether specific events are enriched at the metastatic sites within individual tumors, thus, distinguishing events that occurred before or after metastatic dissemination [5]. Tang et al. [9] performed single‐region or multiple‐region whole‐exome sequencing on 54 paired primary‐metastatic samples and found that the potential drivers of lung cancer metastasis were MYC amplification, NKX2‐1 amplification, RICTOR amplification, arm 20p gain, and arm 11p loss (Figure 1). The above somatic and molecular events can break through the evolutionary bottleneck in the process of tumor evolution and continue to be maintained in metastatic tumors, therefore, they exhibited elevated mutational frequencies in metastases. These results may help to explain the mechanism of lung cancer metastasis and promote to development of new targeted drugs.
Evolution models and timing of lung cancer metastasis
At present, two main evolution models are available for describing the process of tumor dissemination: linear evolutionary model and parallel evolutionary model [2]. Both assume that the primary tumor and its metastasis are clonally related and the differentiation is based on the time when the metastatic cells began to spread at the primary site and the genomic heterogeneity (the number of independent single‐nucleotide mutations (SNV) between the primary tumor and the metastatic tumor after the occurrence of a common ancestor) between the primary tumor and the metastatic tumor. Linear evolutionary model: metastatic cells with metastatic cloning appear in the late stage of tumorigenesis and begin to spread as soon as the primary lesion can be detected in clinical practice. Since metastasis is seeded by the most advanced primary clone or subclone, the heterogeneity of the PT‐MT gene is small. Parallel evolution model: metastatic clones or subclones appear at the early stage of the development of the primary tumor, and the clones of the primary tumor and the metastatic tumor continue to evolve parallel under different pressures, resulting in significant genetic differences between the primary lesion and the metastatic lesion (Figure 2).
FIGURE 2.
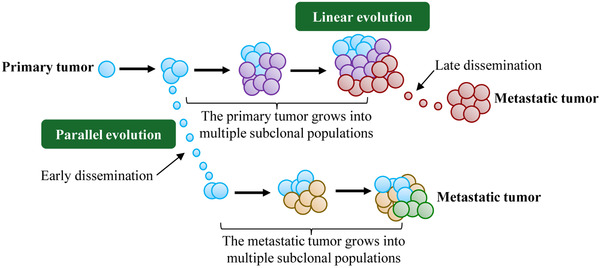
Linear evolutionary model and parallel evolutionary model of tumor metastasis. Different colored circles represent different subclones.
Different types of cancer exhibit different evolutionary patterns. Breast cancer cells [12] have the ability to metastasize at a later stage of tumorigenesis. In contrast, Hu et al. [13] reported that in colorectal cancer, most metastatic cells usually appear in the early stage of the primary tumor. To quantitatively analyze the timing of dissemination for lung cancer metastasis, Tang et al.[9] applied a published tool, spatial computational inference of metastatic timing (SCIMET)[13] to estimate the cell population of the PT at dissemination (Nd) and the mutation rate (u) using a spatial tumor growth model. It indicated that most lung cancer metastases (33/54 cases; 61.1%) preferred late dissemination. However, lung cancer had site‐specific metastatic timing, with lymph nodes showing susceptibility to early dissemination, while metastasis to the pleura, bone, adrenal gland and brain tended to occur during the late dissemination stage. Furthermore, before the primary tumor diagnosis, the time taken for tumor cells to migrate to lymph nodes was at an average of 4.26±0.74 years. However, for pleura metastases and distant metastases, the average dissemination time was only approximately 2.11±0.33 years before the detection of the PT. The results are consistent with those of a previous pan‐cancer study including breast, colorectal and lung cancer, which indicated a seeding age of 3.6 years (interquartile range = 2.8‐3.7) for overall lung cancer metastasis[14].
Overall, these results indicate the importance of early screening. Lymph node metastases likely occur earlier than distant metastases, and they needed earlier and more frequent follow‐up.
Spread trajectories of lung cancer dissemination
In colorectal cancer, Naxerova et al. [15] found that lymphatic and distant metastases shared common subclonal origin in only 35% of 17 cases, with most cases arising from independent subclones in the primary tumor. By mapping the routes of dispersal of lymphatic metastases in human colorectal cancer, Zhang et al. [16] identified three models of spreading, namely interlayer sequential spread, interlayer skip spread and intralayer spread, with 44.3% of the cases presenting interlayer sequential spread.
For lung cancer metastasis, understanding the evolutionary relationship between lymph nodes and distant metastasis is of great clinical significance for preventing distant metastasis although limited currently available relevant data. A multicancer analysis of clonality with 457 paired primary tumor and metastatic samples from 136 patients with breast, colorectal and lung cancer [14] found that polyclonal seeding was common in untreated lymph node metastases and distant metastases but less frequent in treated distant metastases, suggesting that treatment exerts strong selection pressure during tumor evolution. Recently, by reconstructing tumor phylogenies, Tang et al. [9] found that most metastases (7/8) may be directly seeded by the primary tumor but not sequentially spread from lymph nodes.
Tumor metastasis can spread through multiple routes and in different directions. However, larger sample sizes on lymph nodes and distant metastatic tumors as well as multiple distant metastatic tumors are needed to explore the evolutionary trajectories of lung cancer metastasis.
Perspectives
To our knowledge, lung cancer genomic evolution is primarily considered to be limited to early or locally advanced lung cancer [17] and the evolutionary trajectories of lung cancer metastasis are poorly understood. Tumor metastasis is a highly non‐random process [18], and lung cancer also exhibits organ‐specific morbidity and overall survival [19]. However, the mechanisms underlying the organ‐specific pattern of lung cancer metastasis remain unclear. Previous studies have found some potential metastasis drivers but the underlying mechanisms need to be further explored. A study on pan‐cancer evolution [14] suggested that clonal seeding also exhibits organ specificity. However, due to the small lung cancer sample size and the restriction of metastatic sites to the lymph nodes and brain, clonal seeding models of lung cancer metastasis have not been clarified. Herein, a more comprehensive multiple omics landscape of lung cancer metastasis should be developed to better understand the biology and characteristics of lung cancer metastasis, to prevent lung cancer metastasis and improve the overall survival of these patients.
CONCLUSIONS
Collectively, lung cancer metastasis mostly presents late dissemination and features moderate genomic heterogeneity and specific genomic drivers of metastasis. In addition, selective pressure for treatment could alter the clonal seeding patterns of lymph nodes and distant metastases. Finally, lung cancer metastasis exhibited organ‐specific patterns, including genomic heterogeneity and timing of dissemination.
DECLARATIONS
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
All authors consent to publish the paper.
FUNDING
This work was supported by the Zhongshan City People's Hospital Major Project (Top Youth Program) (Grant No. B2021003). Project of National Natural Science Foundation (Grant No. 81872510), High‐Level Hospital Construction Project (Grant No. DFJH201801), Guangdong Provincial People's Hospital Young Talent Project (Grant No. GDPPHYTP201902), GDPH Scientific Research Funds for Leading Medical Talents and Distinguished Young Scholars in Guangdong Province (Grant No. KJ012019449), and Guangdong Basic and Applied Basic Research Foundation (No. 2019B1515130002).
CONFLICT OF INTEREST
All authors indicated no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
All authors contributed to the conception and design of the study. WFT and RF drafted the manuscript. YL, JSL, ZBQ, YLW, and WZZ provided critical revisions of the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
Not applicable.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Goldstraw P, Chansky K, Crowley J, Rami‐Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11(1):39‐51. [DOI] [PubMed] [Google Scholar]
- 2. Turajlic S, Swanton C. Metastasis as an evolutionary process. Science. 2016;352(6282):169‐75. [DOI] [PubMed] [Google Scholar]
- 3. Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529(7586):298‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sottoriva A, Kang H, Ma Z, Graham TA, Salomon MP, Zhao J, et al. A Big Bang model of human colorectal tumor growth. Nat Genet. 2015;47(3):209‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Birkbak NJ, McGranahan N. Cancer Genome Evolutionary Trajectories in Metastasis. Cancer cell. 2020;37(1):8‐19. [DOI] [PubMed] [Google Scholar]
- 6. Vignot S, Frampton GM, Soria J‐C, Yelensky R, Commo F, Brambilla C, et al. Next‐Generation Sequencing Reveals High Concordance of Recurrent Somatic Alterations Between Primary Tumor and Metastases From Patients With Non‐Small‐Cell Lung Cancer. Journal of Clinical Oncology. 2013;31(17):2167‐72. [DOI] [PubMed] [Google Scholar]
- 7. Um SW, Joung J‐G, Lee H, Kim H, Kim K‐T, Park J, et al. Molecular evolution patterns in metastatic lymph nodes reflect the differential treatment response of advanced primary lung cancer. Cancer Research. 2016:0008‐5472.CAN‐16‐0873. [DOI] [PubMed] [Google Scholar]
- 8. Wang H, Ou Q, Li D, Qin T, Bao H, Hou X, et al. Genes associated with increased brain metastasis risk in non‐small cell lung cancer: Comprehensive genomic profiling of 61 resected brain metastases versus primary non‐small cell lung cancer (Guangdong Association Study of Thoracic Oncology 1036). Cancer. 2019;125(20):3535‐44. [DOI] [PubMed] [Google Scholar]
- 9. Tang WF, Wu M, Bao H, Xu Y, Lin JS, Liang Y, et al. Timing and Origins of Local and Distant Metastases in Lung Cancer. J Thorac Oncol. 2021;16(7):1136‐48. [DOI] [PubMed] [Google Scholar]
- 10. Consortium APG . AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017;7(8):818‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shih DJH, Nayyar N, Bihun I, Dagogo‐Jack I, Gill CM, Aquilanti E, et al. Genomic characterization of human brain metastases identifies drivers of metastatic lung adenocarcinoma. Nat Genet. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yates LR, Knappskog S, Wedge D, Farmery JHR, Gonzalez S, Martincorena I, et al. Genomic Evolution of Breast Cancer Metastasis and Relapse. Cancer Cell. 2017;32(2):169‐84.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu Z, Ding J, Ma Z, Sun R, Seoane JA, Scott Shaffer J, et al. Quantitative evidence for early metastatic seeding in colorectal cancer. Nat Genet. 2019;51(7):1113‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hu Z, Li Z, Ma Z, Curtis C. Multi‐cancer analysis of clonality and the timing of systemic spread in paired primary tumors and metastases. Nat Genet. 2020;52(7):701‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naxerova K, Reiter JG, Brachtel E, Lennerz JK, van de Wetering M, Rowan A, et al. Origins of lymphatic and distant metastases in human colorectal cancer. Science. 2017;357(6346):55‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang C, Zhang L, Xu T, Xue R, Yu L, Zhu Y, et al. Mapping the spreading routes of lymphatic metastases in human colorectal cancer. Nat Commun. 2020;11(1):1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jamal‐Hanjani M, Wilson GA, McGranahan N, Birkbak NJ, Watkins TBK, Veeriah S, et al. Tracking the Evolution of Non–Small‐Cell Lung Cancer. New England Journal of Medicine. 2017;376(22):2109‐21. [DOI] [PubMed] [Google Scholar]
- 18. Paget S. The distribution of secondary growths in cancer of the breast. Cancer metastasis reviews. 1889;8(2):98‐101. [PubMed] [Google Scholar]
- 19. Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, et al. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86(1):78‐84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


