Abstract
Pancreatic cancer is a highly malignant digestive system tumor with a poor prognosis. Most pancreatic cancer patients are diagnosed at an advanced stage or even metastasis due to its highly aggressive characteristics and lack of typical early symptoms. Thus, an early diagnosis of pancreatic cancer is crucial for improving its prognosis. Currently, screening is often applied in high‐risk individuals to achieve the early diagnosis of pancreatic cancer. Fully understanding the risk factors of pancreatic cancer and pathogenesis could help us identify the high‐risk population and achieve early diagnosis and timely treatment of pancreatic cancer. Notably, accumulating studies have been undertaken to improve the detection rate of different imaging methods and the diagnostic accuracy of endoscopic ultrasound‐guided fine‐needle aspiration (EUS‐FNA) which is the golden standard for pancreatic cancer diagnosis. In addition, there are currently no biomarkers with sufficient sensitivity and specificity for the diagnosis of pancreatic cancer to be applied in the clinic. As the only serum biomarker approved by the United States Food and Drug Administration, carbohydrate antigen 19‐9 (CA19‐9) is not recommended to be used in the early screening of pancreatic cancer because of its limited specificity. Recently, increasing numbers of studies focused on the discovering of novel serum biomarkers and exploring their combination with CA19‐9 in the detection of pancreatic cancer. Besides, the application of liquid biopsy involving circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), microRNAs (miRNAs), and exosomes in blood and biomarkers in urine, and saliva in pancreatic cancer diagnosis are drawing more and more attention. Furthermore, many innovative technologies such as artificial intelligence, computer‐aided diagnosis system, metabolomics technology, ion mobility spectrometry (IMS) associated technologies, and novel nanomaterials have been tested for the early diagnosis of pancreatic cancer and have shown promising prospects. Hence, this review aims to summarize the recent progress in the development of early screening and diagnostic methods, including imaging, pathological examination, serological examination, liquid biopsy, as well as other potential diagnostic strategies for pancreatic cancer.
Keywords: biomarker, diagnosis, early screening, imaging, liquid biopsy, pancreatic cancer, pathological examination, risk factor, serological examination
Pancreatic cancer is a highly malignant digestive system tumor with poor prognosis. Most pancreatic cancer patients are diagnosed at advanced stages or even metastasis due to its highly aggressive characteristics and the lack of early typical symptoms. Thus, the early diagnosis of pancreatic cancer is crucial for improving its prognosis
Abbreviations
- U.S.
United States
- PDAC
pancreatic ductal adenocarcinomas
- NCCN
National Comprehensive Cancer Network
- EUS
endoscopic ultrasonography
- USPSTF
United States Prevention Services Task Force
- NODM
new‐onset diabetes mellitus
- CT
computed tomography
- MRI/MRCP
magnetic resonance imaging/cholangiopancreatography
- FNA
fine‐needle aspiration
- ERCP
endoscopic retrograde cholangiopancreatography
- CA19‐9
carbohydrate antigen 19‐9
- FDA
Food and Drug Administration
- CEA
carcinoembryonic antigen
- CA125
carbohydrate antigen 125
- CA242
carbohydrate antigen 242
- MIC‐1
macrophage inhibitory cytokine
- MUC5AC
mucin 5AC
- ctDNA
circulating tumor DNA
- CTC
circulating tumor cell
- miRNA
microRNA
- IPMN
intraductal papillary mucinous neoplasm
- TGF‐β
transforming growth factor‐β
- JAK
Janus kinase
- STAT
signal transducer and activator of transcription
- MAPK
mitogen‐activated protein kinase
- NF‐κB
nuclear factor‐κB
- TLR
Toll‐like receptor
- BMI
body mass index
- COX2
cyclooxygenase‐2
- PanIN
pancreatic intraepithelial neoplasia
- HbA1c
hemoglobin A1c
- ENDPAC
Enriching New‐Onset Diabetes for Pancreatic Cancer
- AUC
area under the curve
- NMR
nuclear magnetic resonance
- MS
mass spectrometry
- PUMCH
Peking Union Medical College Hospital
- TAUS
transabdominal ultrasound
- CEH EUS
contrast‐enhanced endoscopic ultrasonography
- RTE
Real‐time elastography
- SL‐QPM
system‐spatial‐domain low‐coherence quantitative phase microscopy
- FNB
fine‐needle biopsy
- ELISA
enzyme‐linked immunosorbent assay
- AIP
autoimmune pancreatitis
- EpCAM
epithelial cell marker epithelial cell adhesion molecule
- GPC‐1
glypican‐1
- cfDNA
cell‐free DNA
- NGS
next‐generation sequencing
- MOB
methylation on beads
- ADAMTS1
methylation of A disintegrin and metalloproteinase with thrombospondin motifs 1
- BNC1
basonuclin‐1
- RT‐qPCR
reverse transcription‐quantitative PCR
- EV
extracellular vehicle
- crExo
circulating exosome
- APC
adenomatous polyposis coli
- HRH2
histamine receptor H2
- VOC
volatile organic compound
- IMS
ion mobility spectrometry
- GC‐IMS
gas chromatography‐ion mobility spectrometry
- GC‐TOF‐MS
gas chromatography time‐of‐flight mass spectrometry
- CI
confidence interval
- HR
hazard ratio
- OR
odds ratio
- ROC
receiver operating characteristic
- VDAC‐1
voltage‐dependent anion channel‐1
- VDAC‐2
voltage‐dependent anion channel‐2
- CHCHD3
coiled‐coil helix coiled‐coil helix domain‐containing protein 3
- SLP‐2
stomatin‐like protein 2
- TOM
translocase of the mitochondrial outer membrane
1. INTRODUCTION
Pancreatic cancer is the seventh leading cause of cancer‐related death in both males and females worldwide because of its poor prognosis; causing almost as many deaths (n = 466,003) as the number of diagnosed cases (n = 495,773) [1]. It is expected to be the second most common cause of cancer‐related death in the United States (U.S.) by 2030 [2]. Unlike with many other cancer entities, over the past several decades, its 5‐year overall survival has marginally improved but still remains no more than 9% [3, 4]. Due to the lack of typical early symptoms and its highly aggressive biological characteristics, most pancreatic cancer is diagnosed at an advanced stage and are not eligible for curative surgery; leading to dismal clinical outcomes. However, the 5‐year survival rate of patients with tumors limited to the duct epithelium can reach 100% when the tumors are smaller than 1 cm [5]. Thus, screening and early diagnosis of pancreatic cancer are crucial for improving its prognosis. It is well known that different forms of pancreatic cancer exhibit major differences in both pathology and patient outcomes. Most pancreatic cancers are characterized as pancreatic ductal adenocarcinomas (PDACs) which account for more than 85% of all malignancies of the exocrine pancreas [6]. Hence, the definition of “pancreatic cancer” we used in the study refers to PDAC.
Direct screening of pancreatic cancer patients from the general population is difficult and not cost‐effective due to the lack of high specificity tests and the low incidence of pancreatic cancer. The National Comprehensive Cancer Network (NCCN) recommends the application of endoscopic ultrasonography (EUS) for genetic/familial high‐risk individuals [7]. However, applying screening strategies for sporadic pancreatic cancer in individuals with one or more risk factors could enhance the performance of a putative screening test [8]. According to the recommendations of the U.S. Prevention Services Task Force (USPSTF) on screening for pancreatic cancer, the risk factors, except for certain inherited genetic syndrome or familial history, mainly include new‐onset diabetes mellitus (NODM), preexisting diabetes mellitus, older age, cigarette smoking, obesity, and a history of chronic pancreatitis [9]. For instance, Sharma et al. [10] developed a prediction model involving three factors including change in weight, change in blood glucose, and age at onset of diabetes to determine the risk level of pancreatic cancer in patients with NODM.
When there is clinical suspicion or evidence of dilated pancreatic and/or bile duct, the NCCN guidelines suggest that pancreatic protocol computed tomography (CT) should first be utilized for diagnosis [7]. Additionally, magnetic resonance imaging/cholangiopancreatography (MRI/MRCP), EUS, EUS‐guided fine‐needle aspiration (EUS‐FNA), and endoscopic retrograde cholangiopancreatography (ERCP) also play important roles in diagnosing pancreatic cancer. Many technological innovations of imaging or endoscopy are being applied to improve the diagnostic accuracy of pancreatic tumors [11, 12, 13]. Serological tests are also important auxiliary diagnostic methods for pancreatic cancer. The carbohydrate antigen 19‐9 (CA19‐9) is the only serum biomarker approved by the U.S. Food and Drug Administration (FDA), but cannot meet the need for a clinical diagnosis in practice due to its low sensitivity (80%, 95% confidence interval [CI] = 72%–86%) and low specificity (75%, 95% CI = 68%–80%) [14]. For this reason, some studies have focused on the combined detection of CA19‐9 together with other tumor markers such as carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), carbohydrate antigen 242 (CA242) [15], and on novel serum biomarkers, such as macrophage inhibitory cytokine‐1 (MIC‐1) and mucin 5AC (MUC5AC) [16, 17]. More recently, liquid biopsies have been utilized as a novel diagnostic approach by detecting tumor‐associated biomarkers, mainly circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), exosomes, and microRNAs (miRNAs) in a variety of extractable body fluids [18]. Diagnostic signatures consisting of serum metabolites established by using metabolomics techniques are also now attracting attention [19]. However, before these can be established as standard clinical tools, more interventional clinical trials as well as the development of an algorithm to combine the appropriate circulating markers are needed [20].
To date, still no biomarkers or panels of markers with sufficient diagnostic accuracy have been approved for the early diagnosis of pancreatic cancer. Hence, the aim of this review is to discuss current screening and diagnostic strategies and future prospects in terms of risk factors, imaging approaches, pathological examination, serological tests, liquid biopsies, and other novel early diagnostic methods of pancreatic cancer.
2. HIGH‐RISK GROUPS AND RISK FACTORS
2.1. High‐risk individuals with specific hereditary backgrounds
With insidious onset and high degree of malignancy, pancreatic cancer brings the patients an enormous burden because of the little therapeutic benefit. Therefore, the screening of pancreatic cancer in common population appears to be important and urgent for this disease entity. Routine screening for pancreatic cancer is generally not recommended for asymptomatic individuals, except for those with certain inherited genetic syndromes such as Peutz‐Jeghers syndrome or familial history of pancreatic cancer [21]. In those cases, non‐invasive imaging or highly sensitive serological tests should be performed. As mentioned above, due to its low sensitivity of 80% and specificity of 75%, CA19‐9 possesses limited diagnostic utility [14].
The NCCN guidelines suggest that EUS has a promising role in screening these high‐risk individuals [7]. In a prospective study including 78 high‐risk individuals and 149 controls with follow‐up by EUS and CT, 8 patients with pancreatic cancer, 6 patients with intraductal papillary mucinous neoplasms (IPMNs), and 3 patients with extra‐pancreatic neoplasms were identified and diagnosed [22]. A multicenter prospective cohort study from the U.S. including 216 asymptomatic high‐risk individuals reported detection of pancreatic abnormality by CT, MRI, and EUS in 11.0%, 33.3%, and 42.6%, respectively [23].
Regarding consideration of age at first screening and frequency thereafter, some investigators recommend that first screening should take place at 40‐50 years of age, or 10–15 years earlier than the onset age of pancreatic cancer patients in their family in the case of familial disease. Screening is recommended every 3 years or every 3–6 months if the first screening shows abnormalities [24]. In 2018, the International Cancer of the Pancreas Screening Consortium updated its recommendations for the management of patients with increased risk of familial pancreatic cancer. For such individuals, it was agreed that surveillance should begin at age 50 or later, otherwise 10 years earlier than the youngest relative with pancreatic cancer and that the preferred surveillance tests should be EUS and MRI/MRCP [25]. Notably, improvement of imaging‐based methods and exploration of novel biomarkers is still required to improve early pancreatic cancer diagnostic efficiency.
2.2. Risk factors for sporadic pancreatic cancer
Many patients with sporadic pancreatic cancer would inevitably be overlooked under the screening principles for genetic/familial high‐risk individuals. Thus, it is necessary to explore a high‐risk population in more detail to improve the detection rate and survival outcomes of patients with pancreatic cancer. This requires comprehensive research on the risk factors of sporadic pancreatic cancer, regarding the identification of objective factors, and taking into account that the incidence rate is higher in males than in females, and increases gradually after age 45 until it reaches a peak at the age 80 [26]. Additionally, there are also environmental factors known to contribute to the development of pancreatic cancer including lifestyle, dietary habits, and related diseases.
Unhealthy living habits promote the development of pancreatic cancer. Smokers were reported to have an approximately 2–3‐fold risk of pancreatic cancer relative to non‐smokers, but this risk decreases with increasing time since quitting smoking [27]. It was suggested that one mechanism of this cigarette smoke‐induced effect was via miR‐25‐3p and m6A modification resulting in the activation of oncogenic AKT‐p70S6K signaling, which provokes the malignant transformation of pancreatic duct epithelial cells [28]. Heavy alcohol drinking (defined as ≥420 g/week), another independent risk factor for pancreatic cancer, was associated with significant excess risk (hazard ratio [HR] = 1.69, 95% CI = 1.21–2.37) in men, but not women [29, 30]. In addition, the consumption of certain foodstuffs also increased risk, such as red meat (HR = 1.16, 95% CI = 1.01–1.33) [29]. Thus, developing and maintaining good living habits is an important proactive measure in the primary prevention of pancreatic cancer.
Individuals with certain chronic diseases, including chronic pancreatitis, diabetes, and obesity, have a higher risk of suffering from pancreatic cancer. To evaluate the association between different forms of pancreatitis and pancreatic cancer, Karlson et al. [31] conducted a large cohort study with a follow‐up duration of up to 25 years. They found that chronic pancreatitis patients had the highest standardized incidence ratio of 22.2 (95% CI = 16.2–29.6) for the development of pancreatic cancer within 4 years, and 7.6 (95% CI = 6.0–9.7) within 24 years. A meta‐analysis of 13 eligible studies revealed that the patients diagnosed with pancreatic cancer within two years of chronic pancreatitis had a pooled effect estimate of 16.16 (95% CI = 12.59–20.73) [32]. However, the risk decreased when the interim period was increased to 5 years. The mechanism by which chronic pancreatitis develops into pancreatic cancer is still unclear. Abnormally activated signaling pathways, such as transforming growth factor‐β (TGF‐β), Janus kinase (JAK)/signal transducer and activator of transcription (STAT), mitogen‐activated protein kinase (MAPK), nuclear factor‐κB (NF‐κB), and Toll‐like receptors (TLRs), may contribute to malignant transformation of pancreatic cells during long‐term pancreatitis [33]. Numerous clinical studies have demonstrated that diabetes and hyperglycemia are risk factors for pancreatic cancer. The result of a retrospective study from the U.S. showed that patients with type 2 diabetes have a doubled risk (HR = 2.17, 95% CI = 1.70–2.77) of developing pancreatic cancer compared with the general population [34]. In a large prospective clinical study in China, the risk of pancreatic cancer in diabetic patients was also approximately twice than that of the general population (HR = 1.87, 95% CI = 1.48–2.37), and increased with the duration of diabetes [35]. In addition, blood glucose levels are also closely associated with the prevalence of pancreatic cancer [35, 36]. The mechanisms of diabetes promotion of pancreatic carcinogenesis are at least partly via the high levels of insulin and insulin‐like growth factors which act on the exocrine pancreas to promote mitosis, induce cell differentiation, or impede autophagy [37, 38, 39]. As a widespread global health problem, obesity is also considered a risk factor for pancreatic cancer. A case‐control study from the United Kingdom indicated a robust causal association of increasing body mass index (BMI) with pancreatic cancer risk (odds ratio [OR] = 1.34, 95% CI = 1.09–1.65, for each standard deviation increase in BMI [4.6 kg/m2]) [38]. In a retrospective study from China, the case group included a higher proportion of overweight and obese individuals (OR = 2.48 and 95% CI = 1.98–3.11 for overweight individuals; OR = 3.5, 95% CI = 2.67–5.41 for obese individuals) emphasizing these independent risk factors for pancreatic cancer [40]. A recent study reported that obesity accelerated oncogenic Kras‐driven pancreatic ductal tumorigenesis in mice by inducing aberrant pancreatic islet cholecystokinin expression [41]. Genetic or dietary weight loss impeded pancreatic cancer progression. Additionally, a high‐fat diet was found to promote the development of pancreatic cancer, usually synergistically with obesity. Recent studies revealed that high‐fat diets could activate oncogenic Kras via cyclooxygenase‐2 (COX2), leading to pancreatic inflammation and fibrosis, and the development of pancreatic intraepithelial neoplasias (PanINs) and PDAC [42]. Conversely, acinar cells with Kras mutations had significantly reduced expression of fibroblast growth factor 21, which was found to suppress the effect of a high‐fat diet in promoting pancreatic adenocarcinoma development [43]. Additional to the above, Pang et al. [44] identified multiple risk factors for pancreatic cancer involving chronic cholecystitis (OR = 1.81, 95% CI = 1.34–2.44), cholecystotomy (OR = 16, 95% CI = 8.16–31.5) and low levels of high‐density lipoprotein cholesterol C (OR = 3.12, 95% CI = 2.52–3.86).
2.3. High‐risk population predictive model
Integrating multiple risk factors for pancreatic cancer to establish a risk prediction model could contribute to its early detection. Patients with NODM have more than twice the risk of pancreatic cancer relative to those with long‐term diabetes [45]. To identify individuals at high‐risk for PDAC among patients with NODM, Boursi et al. [46] developed and validated a predictive model including age, BMI, change in BMI, smoking, use of proton pump inhibitors and anti‐diabetic medications, as well as levels of hemoglobin A1c (HbA1c), cholesterol, hemoglobin, creatinine, and alkaline phosphatase. Based on this, only 6.19% of the NODM population would need to undergo definitive screening when setting the predicted risk threshold at 1%, but nevertheless, this was with limited sensitivity. Notably, Chari's team developed a model denoted “Enriching New‐Onset Diabetes for Pancreatic Cancer” (ENDPAC) that weighted scores for three factors including change in weight, change in blood glucose, and age at onset of diabetes [10]. An ENDPAC score of at least 3 identified patients who developed pancreatic cancer within three years of diabetes onset with an area under the curve (AUC) of 0.87 with 80% sensitivity and specificity. This simple and effective model possesses potential value for screening for pancreatic cancer.
The role of serum metabolites in the detection of pancreatic cancer has attracted a great deal of attention with the development of metabolomics technologies involving nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS) [47]. For instance, metabolite biomarkers in plasma may also contribute to the diagnosis of pancreatic cancer. Michálková et al. [48] investigated the changes of metabolites in plasma samples via NMR and developed a model comprising 12 metabolites (3‐hydroxybutyrate, lactate, glutamine, alanine, valine, lysine, citrate, histidine, isoleucine, glutamate, acetone, and dimethylamine) which had an accuracy of 94%, 100% sensitivity, and 90% specificity in distinguishing pancreatic cancer patients from healthy individuals. In addition, Mayerle et al. [19] identified a biomarker signature comprising nine metabolites together with CA19‐9 for the discrimination of PDAC from chronic pancreatitis with an AUC of 0.96 (95% CI = 0.93–0.98), a marked improvement compared to CA19‐9 alone. To establish a screening strategy for pancreatic cancer based on NODM, the metabolomic profiles of serum samples from patients with NODM and those with PDAC and NODM have been compared [49]. This identified 62 different metabolites and found that a panel including N‐succinyl‐L‐diaminopimelic and PE (18:2) had high sensitivity (93.3%) and specificity (93.1%). Currently, the studies focusing on the serum metabolomics for pancreatic cancer screening based on the NODM population are rare and further studies need to be performed.
Researchers have also established a high‐risk population predictive model through the analysis of clinical symptoms and risk factors for pancreatic cancer. A study from Peking Union Medical College Hospital (PUMCH) developed a high‐risk scoring model for pancreatic cancer based on 10 risk factors (male, age >60, alcohol, smoking, diabetes history, meat diet, family history of pancreatic cancer, pancreatitis, cholelithiasis history, and cholecystitis history) and 4 symptoms (anorexia, upper abdominal pain, weight loss, and jaundice), which exhibited an AUC of 0.981 [50]. In addition, a retrospective cohort study from Saudi Arabia noted nine factors that contributed significantly to the risk of pancreatic cancer, namely, older age, male gender, weight loss, abdominal pain, blood clots, pancreatitis, jaundice, persistent fatigue, and abnormal imaging tests [51]. This risk assessment model yielded excellent predictive utility (receiver operating characteristic [ROC] = 96.3%, 95% CI = 94.1%–98.6%) and high sensitivity (94%), which was useful for improving screening performance. Hence, exploring risk factors for pancreatic cancer comprehensively, identifying the high‐risk population effectively, and applying highly sensitive imaging‐based or serological examinations are currently the main screening strategies to improve the early diagnosis, treatment, and prognosis of pancreatic cancer.
3. TYPES OF EXAMINATION
3.1. Imaging
Medical imaging has important roles in pancreatic cancer screening and early detection, preoperative evaluation and staging, differential diagnosis, follow‐up, and treatment evaluation [52]. Nonetheless, at present, there is no standard imaging screening procedure. The USPSTF indicates that imaging‐based methods such as CT, MRI, and EUS have been tested as screening strategies in trials of high‐risk populations with inherited genetic syndromes or family history of pancreatic cancer [9]. As mentioned above, EUS performs best for screening high‐risk individuals [23].
Different imaging methods have different capabilities for the detection of early pancreatic cancer. A multicenter retrospective study from Japan was conducted to clarify the imaging features of 200 cases in stage 0 and I PDAC, 20% of which were symptomatic [11]. The diagnostic accuracy of transabdominal ultrasound (TAUS), CT, MRI, and EUS was 67.5%, 98.0%, 86.5%, and 86.5%, respectively. Moreover, the main manifestation of early pancreatic cancer in CT, EUS, and MRCP is a dilation or irregular stenosis of the main pancreatic duct. However, both CT and TAUS have limited value in the diagnosis of early PDAC because only indirect signs, such as dilation of pancreatic duct or change of pancreas contour, can be seen using these techniques. Notably, CT also detected local fatty changes of the pancreatic parenchyma in 42% stage 0 and 41.8% stage I cases, which implies the importance of detecting a local fatty change in the pancreas for identifying early‐stage pancreatic cancer [11].
EUS can detect smaller solid lesions, and has the additional advantages of not employing ionizing radiation and not requiring contrast agents, and, moreover, can acquire cytopathological results sequentially [52], unlike the low sensitivity of thin‐section triple‐phase helical CT for PDAC with a diameter <2 cm [53]. Maguchi et al. [54] compared different imaging techniques for pancreatic cancer with a diameter <2 cm and found that TAUS, CT, and EUS had a sensitivity of 52.4%, 42.8%, and 95.2% respectively. However, conventional EUS cannot distinguish very well between carcinoma and other etiologies without the use of an invasive cytological examination (EUS‐FNA) because the majority of pancreatic tumors, even those with benign etiologies, have a hypoechogenic appearance. Thus, the development of non‐invasive alternatives or methods to EUS‐FNA is necessary. A retrospective study from China revealed that the size of the neoplasm and the regularity of the margin in EUS could significantly differentiate malignant from benign pancreatic tumors, and combined diagnosis showed a sensitivity of 73.68% and specificity of 90% [55]. However, the EUS features of age, sex, location, echo pattern, and dilation of the main pancreatic duct were not informative. Recently, contrast‐enhanced endoscopic ultrasonography (CEH EUS) has been utilized as a useful minimally invasive diagnostic method, and was reported to facilitate the imaging of parenchymal perfusion and microvessels in pancreatobiliary disease. A prospective single‐center study found that the CEH EUS had higher sensitivity (94.5% vs. 83.1%) and accuracy (84.1% vs. 78.6%) than conventional EUS [13]. The results of a meta‐analysis also showed excellent pooled estimates of sensitivity and specificity of CEH EUS for pancreatic cancer diagnosis, at 93% (95% CI = 0.91–0.95) and 80% (95% CI = 0.75–0.85) respectively [56]. We believe that this modality can contribute to refining diagnostic techniques for pancreatic cancer in clinical practice.
It should be pointed out that operating EUS or CEH EUS operation is relatively challenging and accuracy is often affected by the subjectivity of endosonographers. EUS examination is often used as a part of EUS‐FNA in patients with suspected pancreatic cancer, and CEH EUS is required to confirm the diagnosis if EUS‐FNA shows a negative result due to the smaller lesions [57]. In addition, there are contraindications for MRI, including metal implants and patient claustrophobia, but this approach has yielded higher detection rates than CT for pancreatic cancer or small solid pancreatic tumors [11, 23, 58]. Nonetheless, CT is still currently used as the first choice for pancreatic cancer diagnosis in clinical practice.
3.2. Pathological examination
Over the last few years, EUS‐FNA has been considered the most advanced and accurate diagnostic technique in patients with suspected pancreatic cancer. Among those with pancreatic lesions detected by CT, the sensitivity, specificity, and accuracy of EUS‐FNA were 87.6%, 91.2%, and 88.8%, respectively [13]. A prospective single‐center study from South China reported that EUS‐FNA had a sensitivity, specificity, and accuracy of 77.8%, 100%, and 84% for pancreatic cancer diagnosis [59]. Multivariate analysis showed that abdominal pain, lesion properties, lesion metastasis, and lesion size were independent factors predicting PDAC. To increase the accuracy of early diagnosis of EUS‐FNA, investigators have made many improvements to methods and techniques. Real‐time elastography (RTE), a new technique for the assessment of tissue elasticity using ultrasound, has been used in multiple tumors to differentiate malignant from benign lesions. The combination of RTE and EUS‐FNA was reported to have higher diagnostic accuracy, sensitivity, and specificity with 94.4%, 93.4%, and 100%, respectively [60]. In addition, Bournet et al. [61] reported that pathological assessment combined with KRAS‐mutation analysis using allelic discrimination showed higher accuracy (88% vs. 73%) and sensitivity (93% vs. 85%) for PDAC diagnosis than cytopathology alone. Furthermore, novel optical system‐spatial‐domain low‐coherence quantitative phase microscopy (SL‐QPM) was demonstrated to improve the accuracy of EUS‐FNA cytological diagnosis and increase the sensitivity of cytology for identifying pancreatic cancer from 72% to 94%, even when traditional cytopathology failed to allow an accurate diagnosis [62]. For differentiating between inflammatory masses and malignancies, it was found that endoscopic ultrasound‐guided fine‐needle biopsy (EUS‐FNB) possessed higher diagnostic accuracy and sensitivity than EUS‐FNA (93.0% and 86.6% vs. 83.6% and 69.5%), and it should be considered as the preferred technique for diagnosing cancer in the setting of chronic pancreatitis [63]. Moreover, a prospective study compared the diagnostic accuracy of different techniques to obtain EUS‐FNA samples with slow‐pull and standard suction techniques [64]. There were no significant differences, but the slow‐pull technique decreased the number of slides and caused less bleeding.
ERCP plays an important role in the diagnosis and treatment of cholangio‐pancreatic diseases. Cytology using endoscopic nasopancreatic drainage has potential value for the early detection of pancreatic cancer in situ. Compared to EUS‐FNA, cytology during ERCP was more commonly applied for preoperative pathologic diagnosis in Japan, which has a detection rate of 72.2% compared to only 16.7% for EUS‐FNA in patients with stage 0 pancreatic cancer [11]. However, pancreatic juice cytology could be affected by the position and size of the catheter, and in addition, patients frequently suffer from complications such as post‐ERCP‐related pancreatitis [65]. At the PUMCH, the number of patients where ERCP is used for diagnosis has decreased in the last decade, and ERCP is now more frequently used for treatment [66]. Thus, we should adopt the appropriate diagnostic procedure for different stages of pancreatic cancer and fully consider the patient's condition.
3.3. Serological examination
There are no effective serum tumor markers for the early diagnosis of pancreatic cancer at present. Although CA19‐9 is approved by the FDA as the only serum biomarker for PDAC, it has limitations such as giving false negatives in patients with Lewis blood type‐negative phenotype and false positives in patients with obstructive jaundice. Also, the level of CA19‐9 can increase under some circumstances, such as in the presence of benign tumors, inflammatory masses, and diabetes [67]. These factors reduce the usefulness of CA19‐9, which lacks sufficient sensitivity and specificity for the early diagnosis of pancreatic cancer. However, it is commonly used as a significant prognostic factor for the evaluation of therapeutic effects, surveillance of metastasis, and survival prediction in patients with advanced pancreatic cancer [68, 69, 70]. In addition to CA19‐9, other tumor markers, such as CEA, CA125, and CA242, are also used together to diagnose pancreatic cancer. CA19‐9 seems to be associated with the highest sensitivity around 80% but has no advantages regarding specificity, which appears to be the highest for CA242, at approximately 90% [14, 15]. Notably, the sensitivity and specificity of detecting serum CA19‐9, CEA, CA125, and CA242 together were 90.4% and 93.8%, clearly higher than any single marker [15]. Consequently, patients with suspected pancreatic cancer still need to be tested for at least these four tumor markers. In addition, a team from the Shanghai Cancer Center of Fudan University divided the participants into three genotypes (Lewis‐negative, Mixed, Secretor‐negative) by Sanger sequencing and determined the best cut‐off value for each group, then applied these cut‐offs to the diagnosis of pancreatic cancer [71]. The sensitivity of CA19‐9 for the detection of stage I, II pancreatic cancer increased from 76.1% to 87.2%.
Numerous potential novel markers for pancreatic cancer diagnosis have been identified using high‐throughput screening of proteins in serum of PDAC patients, and bioinformatics analysis of available cancer genome datasets. A number of studies reported the diagnostic value of novel serum protein markers in detecting pancreatic cancer via enzyme‐linked immunosorbent assay (ELISA), and combining these with CA19‐9 could effectively improve the diagnostic accuracy for differentiating pancreatic cancer from healthy controls, benign tumors, or chronic pancreatitis (Table 1). As a special type of chronic pancreatitis, autoimmune pancreatitis (AIP) is usually misdiagnosed as pancreatic cancer. Immunoglobulin G4 (IgG4) is a commonly used diagnostic indicator of AIP but has a low sensitivity of only 72% (95% CI = 0.68–0.76) for differentiating between AIP and pancreatic cancer [72]. In a study aiming to identify a serological pattern to differentiate AIP from PDAC using routinely performed tests, four serum biomarkers including CA19‐9, globulin, eosinophil, and hemoglobin were suggested to act as independent markers, and combinations thereof identified AIP patients with 92% sensitivity and 79% specificity [73]. Moreover, researchers found that the levels of hybrid κ/λ antibodies in AIP patients were significantly higher than in pancreatic cancer patients, and the combined detection of serum hybrid κ/λ antibodies and IgG4 increased the sensitivity compared to IgG4 alone [74]. In the past, we identified several immunogenic membrane antigens (voltage‐dependent anion channel‐1 [VDAC‐1], voltage‐dependent anion channel‐2 [VDAC‐2], coiled‐coil helix coiled‐coil helix domain‐containing protein 3 [CHCHD3], stomatin‐like protein 2 [SLP‐2], and translocase of the mitochondrial outer membrane [TOM]) that bound serum IgG from pancreatic cancer patients; the levels of these specific antibodies in patients’ peripheral blood were high [75]. Therefore, the detection of specific autoantibodies in the plasma may also contribute to the early diagnosis of pancreatic cancer and needs to be validated by further clinical studies.
TABLE 1.
The diagnostic value of novel serum biomarkers and combination with CA19‐9 for pancreatic cancer diagnosis
| Study | Pancreatic cancer group | Control group | Markers | AUC (95% CI) | SEN (%) | SPE (%) | AUC (+ CA19‐9) (95% CI) | SEN (+ CA19‐9) (%) | SPE (+ CA19‐9) (%) |
|---|---|---|---|---|---|---|---|---|---|
| Kaur et al. [16] | RPC | HC+BC+CP | MUC5AC | 0.88 (0.83‐0.93) | 89.0 | 70.0 | 0.91 (0.86–0.95) | 83.00 | 83.00 |
| Mohamed et al. [17] | PC | HC | MIC | 0.92 (NA) | 94.0 | 45.8 | 0.90* (NA) | 82.00* | 66.70* |
| Mohamed et al. [17] | PC | HC | ADH | 0.82 (NA) | 62.0 | 83.3 | 0.90* (NA) | 82.00* | 66.70* |
| Lu et al. [127] | RPC | CP | IL‐6 | 0.79 (0.61‐0.91) | 61.0 | 91.0 | 0.89 (NA) | 87.30 | 90.30 |
| Martinez‐Bosch et al. [128] | PC | HC | Gal‐1 | 0.88 (NA) | 77.4 | 100 | ND | 96.00 | 100 |
| Liu et al. [129] | PC | HC | APOE+ITIH3+APOA1+APOL1 | 0.94 (NA) | 85.0 | 94.1 | 0.99 (NA) | 95.00 | 94.10 |
| Han et al. [130] | PC | HC+BPT+CP | DKK‐1 | 0.92 (NA) | 89.3 | 79.4 | ND | 96.43 | 64.13 |
| Hogendorf et al. [131] | RPC | CP | GDF‐15 | 0.83 (0.72‐0.93) | 73.8 | 76.2 | 0.89 (0.80–0.97) | 80.00 | 80.95 |
| Ferri et al. [132] | PC | CP | IGF‐1+ albumin | 0.86* (NA) | 80.9* | 95.0* | 0.96 (NA) | 93.60 | 95.00 |
| Furukawa et al. [133] | PC | CP | LRG‐1 | 0.69 (NA) | 67.7 | 54.3 | 0.94 (NA) | 75.60 | 100 |
| Honda et al. [134] | PC | HC | ApoAII‐ ATQ/AT | 0.94 (NA) | ND | ND | 0.90* (NA) | 95.40 | 98.30 |
| Jahan et al. [135] | PC | BC | TFF1+TFF2+TFF3 | 0.66 (NA) | 0.7 | 0.6 | 0.94 (NA) | 0.85 | 0.92 |
| Balasenthil et al. [136] | EPC | HC | TFPI+TNC‐FN III‐C | 0.83 (0.69‐0.97) | 0.9 | 0.8 | 0.92 (0.82–1.00) | 0.95 | 0.85 |
| Papapanagiotou et al. [137] | PC | HC | Osteonectin | 0.86 (0.70‐0.95) | 84.6 | 87.5 | ND | ND | ND |
The AUC, sensitivity and specificity of CA19‐9 alone in the detection of pancreatic cancer.
Abbreviations: RPC: Resectable pancreatic cancer; NPC: Non‐pancreatic cancer; EPC: Early stage pancreatic cancer; HC: Healthy controls; BC: Benign controls; BPT: Benign pancreatic tumors; CP: Chronic pancreatitis; IL‐6: Interleukin‐6; MUC5AC: Mucin 5AC; MIC: Macrophage inhibitory cytokine; ADH: Alcohol dehydrogenase; Gal‐1: Galectin‐1; APOE: apolipoprotein E; ITIH3: inter‐alpha‐trypsin inhibitor heavy chain H3; APOA1: apolipoprotein A‐I; APOL1: apolipoprotein L1; DKK‐1: Dickkopf‐1; GDF‐15: Growth differentiation factor‐15; IGF‐1: Insulin growth factor‐1; LRG‐1: Leucine‐rich α2‐glycoprotein‐1; ApoAII‐ATQ/AT: Apolipoprotein AII isoforms ATQ/AT; TFF: Trefoil factors; TFPI: Tissue factor pathway inhibitor; TNC‐FN III‐C: Tenascin C; ND: not described; NA: not applicable.
More recently, glycoproteomics has emerged as a subfield of proteomics, and tumor‐specific variations in protein glycosylation may also contribute to the early diagnosis of pancreatic cancer. Aronsson et al. [76] identified ten glycoprotein biomarker candidates through mapping the glycosylation profile of 1000 proteins, and subsequently verified these in serum samples from patients with pancreatic cancer. The panel including CA19‐9, IL.17E, B7.1, and DR6 showed an AUC of 0.988 at 100% sensitivity and 90% specificity for discriminating stage I pancreatic cancer from healthy controls, which was more effective than CA19‐9. In addition to compositional glycan profiling, structure‐specific glycan profiling is considered potentially able to provide biomarkers with high specificity. Through the separation, identification, and relative quantification of isomeric glycans, Liu et al. [77] discovered 25 specific‐isomeric biomarkers which were significantly altered in pancreatic cancer. A combination of all the potential biomarkers showed a higher AUC of 0.976 with 93.5% sensitivity and 90.6% specificity for discriminating between patients with pancreatic cancer and healthy controls. These results suggest that the development of innovative proteomic technology will make this technique more conducive for discovering more potential tumor‐specific biomarkers. With the rapid increase in the number of novel different types of tumor‐specific serum biomarkers, it is expected that a mature and simple algorithm will become available, combining different data. This will ensure that diagnostically efficient markers will enter clinical practice as soon as possible.
4. LIQUID BIOPSY
Over the last few decades, the use of liquid biopsy for analyzing tumor biomarkers circulating in fluids such as the blood, urine, and saliva, has received a great deal of attention. Unlike traditional tissue biopsy, liquid biopsy allows the evaluation of comprehensive cancer profiles in a non‐invasive and real‐time manner (Figure 1). Huge progress has been made in the development of devices that may contribute to the clinical application of liquid biopsy [20]. Accumulating evidence acquired using liquid biopsy involving CTCs, ctDNA, miRNAs, and exosomes have been robustly exploited by the cancer research community.
FIGURE 1.
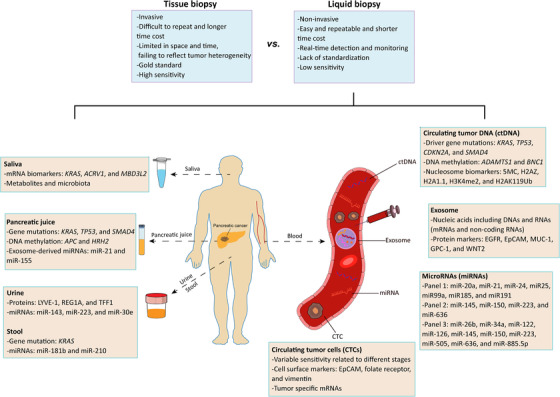
Liquid biopsy in early screening and diagnosis of pancreatic cancer. Compared to traditional tissue biopsy, liquid biopsy has more advantages (blue panel). Liquid biopsy involving circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), microRNAs (miRNAs) and exosomes represents a non‐invasive and real‐time manner to evaluate tumor heterogeneity. Beyond blood, other fluids including saliva, pancreatic juice, urine and stool also play important roles in early screening and diagnosis of pancreatic cancer (orange panel)
4.1. CTCs and ctDNA
CTCs are defined as cancer cells from solid tumors found in the peripheral blood, formed by tumor‐induced angiogenesis, and circulating through normal vessels and capillaries [78]. Therefore, the presence of CTCs usually represents the invasion and metastasis of the primary tumor. CTCs have important prognostic value for pancreatic cancer patients. Those with CTCs had worse overall survival than patients without them (HR = 1.558, 95% CI = 1.238–1.961) [79]. However, the diagnostic value of CTCs is doubted by many scientists due to their limited and variable sensitivity (varying from 25% to 100%) related to the different stages of pancreatic cancer at the time of diagnosis [79, 80]. Utilizing the microfluidic NanoVelcro CTC chip, Ankeny et al. [81] evaluated samples from patients with PDAC for the presence and number of CTCs. The presence of CTCs was confirmed as a diagnostic criterion for PDAC with 75% sensitivity and 96.4% specificity (AUC = 0.867, 95% CI = 0.798‐0.935, P < 0.001). Furthermore, CTCs appeared to be an effective biomarker for discriminating between local/regional and metastatic disease (AUC = 0.885, 95% CI = 0.80–0.969, P < 0.001) when the cut‐off value was ≥3 CTCs in 4 mL venous blood [81]. In addition to identifying CTCs by capturing them based on the expression of the epithelial cell marker epithelial cell adhesion molecule (EpCAM), emerging studies also explored other cell surface markers such as the folate receptor and the mesenchymal cell marker vimentin. These have shown encouraging diagnostic efficiency in distinguishing PDAC from benign pancreatic diseases and healthy individuals. Combined folate receptor+ or vimentin+ CTCs and CA19‐9 further improved diagnostic potency, with an AUC of 0.944 and 0.968, respectively [82, 83]. Liquid biopsy combining CTCs and other biomarkers is expected to provide a reliable and non‐invasive diagnostic method with adequate sensitivity. Notably, a combination of CTCs and glypican‐1 (GPC‐1)‐positive exosome detection displayed the highest sensitivity of 100% for the diagnosis of resectable pancreatic cancer [84]. However, CTC detection is subject to technical variability in the pre‐analytical and analytical steps [20]. The detection of CTCs proceeds first by their enrichment by density gradient centrifugation or immunological capture techniques, and immunochemical detection by antibodies against epithelial‐specific proteins or PCR‐based detection of tumor‐specific mRNAs or epithelial‐specific mRNAs [79]. Due to the rarity of CTCs in the blood, new techniques and devices are being developed and produced to improve the diagnostic accuracy of CTCs for cancer. Thus, improved technical and methodological approaches must be clarified to demonstrate the routine applicability of this methodology.
The term cell‐free DNA (cfDNA) refers to fragments of DNA found in the noncellular component of the blood. They are approximately 150–200 base pairs in length with a half‐life of an hour or less [85]. cfDNA released from apoptotic or necrotic tumor cells is often referred to as ctDNA, which is the main direction of tumor liquid biopsy work currently. Detection of cancer driver genes, such as Kras mutations in the circulation is often used to detect pancreatic cancer, whereby every mutation found in the plasma could be also identified in the primary tumor [86, 87]. Cohen et al. [86] found that Kras mutation mainly at codon 12 was detected only in 30% of patients and was more frequent in stage II than stage I patients. However, the combination of KRAS mutation and four protein markers (CA19‐9, CEA, hepatocyte growth factor, and osteopontin) increased the sensitivity to 64% with a specificity of 99.5%. In addition, by using the single‐strand library preparation and hybrid‐capture‐based cfDNA sequencing approach that could rescue short or damaged cfDNA fragments, Liu et al. [87] found that detection of Kras mutations served as an efficient marker to discriminate PDAC from healthy controls (AUC = 0.863, 95% CI = 0.830–0.898), with a higher detection rate than when using PCR‐ or next‐generation sequencing (NGS)‐based methods. Furthermore, the combination of the Kras, TP53, CDKN2A, and SMAD4 genes yielded higher diagnostic accuracy for PDAC (AUC = 0.921, 95% CI = 0.890–0.956) with 80% sensitivity and 100% specificity. Moreover, it was found that the small mutated fragments are prevalent in pre‐cancerous IPMN and early‐stage patients (stage I/II) with a dominant peak of 75–85 bp in length of KRASG12D compared to an advanced stage with a dominant peak of 150 bp. This contributed to proposing a detection method for early pancreatic cancer based on fragment size. However, the level of ctDNA varies greatly in overall cfDNA from less than 0.1% to > 90% in different patients with cancer [88]. Besides, ctDNA has a >75% detection rate in patients with advanced pancreatic cancer, but a low detection rate of only 48% in localized pancreatic cancer [89]. In addition, it is difficult to identify the source of ctDNA and to predict the potential cancer type.
Notably, epigenetic reprogramming such as DNA methylation occurs at the earliest stages of tumorigenesis. This shows different patterns in different tissue and plays an important role in cancer development and progression [90]. Thus, the detection of DNA methylation of ctDNA is considered a promising breakthrough in the early screening of tumors. Using a newly developed highly reliable technique called methylation on beads (MOB), Eissa et al. [91] found that methylation of A disintegrin and metalloproteinase with thrombospondin motifs 1 (ADAMTS1), zinc finger protein basonuclin‐1 (BNC1), and the combination of both had a sensitivity of 87.2%, 64.1%, and 97.4%, respectively, for pancreatic cancer diagnosis. For stage I/II pancreatic cancer, the two‐gene panel exhibited 94.8% sensitivity and 91.6% specificity. Furthermore, although the combination of the two genes and CA19‐9 did not result in increased sensitivity, it exhibited a higher AUC of 0.95 (95% CI = 0.9–0.98) than CA19‐9 alone (57.1%). In addition, nucleosomes can be released into the circulation; hence, intact nucleosome levels in serum or plasma might serve as potential diagnostic biomarkers for pancreatic cancer. Bauden et al. [92] identified a panel of five circulating nucleosome biomarkers comprising 5MC, H2AZ, H2A1.1, H3K4me2, and H2AK119Ub by using Nucleosomic, a novel ELISA method. This panel gave an AUC of 0.95 for the discrimination of pancreatic cancer from healthy controls, thus better than CA19‐9 alone. However, combining CA19‐9 with a panel of these four nucleosome biomarkers (5MC, H2AZ, H2A1.1, and H3K4me2) showed the highest AUC of 0.98 with 92% sensitivity and 90% specificity.
4.2. Circulating miRNAs
miRNAs are small non‐coding RNA molecules of about 18‐22 nucleotides that regulate gene expression post‐transcriptionally and play important roles in oncogenesis and tumor metastasis [93]. The difference in expression of miRNAs between pancreatic cancer and normal tissues or between different stages of pancreatic cancer is clearly a prerequisite for diagnostic biomarkers of pancreatic cancer. It was reported that the overexpression of miR‐103 and miR‐107 and low expression of miR‐155 could distinguish pancreatic cancer from normal controls [94]. Compared to non‐neoplastic ductal epithelium, miR‐155 was significantly overexpressed in both PanIN‐2 (2.6‐fold) and PanIN‐3 (7.4‐fold) [95], and miR‐196b was only expressed in PanIN‐3 tissue [96]. However, as mentioned above, tissue biopsy is an invasive methodology and has low positive detection rate for smaller lesions, leading to limited application as a routine examination.
Recently, increasing numbers of studies have reported the potential value of blood miRNAs as biomarkers for pancreatic cancer diagnosis. miRNAs are very stable in blood and can be detected by various assays such as reverse transcription‐quantitative PCR (RT‐qPCR), in situ hybridization, next‐generation sequencing, and miRNA microarray [80, 97]. By using Illumina SBS technology and hydrolysis probe‐based RT‐qPCR assays, a case‐control study from China identified a profile of miRNAs (miR‐20a, miR‐21, miR‐24, miR25, miR99a, miR185, and miR191) which were significantly differentially expressed in pancreatic cancer patients compared with controls. The application of this 7 miRNA‐based biomarker panel in pancreatic cancer diagnosis resulted in an accuracy of 83.6% that was higher than CA19‐9 (56.4%) [97]. Another study from Chinese researchers identified six significantly upregulated miRNAs in the serum of pancreatic cancer compared to normal controls, namely let‐7b‐5p, miR‐192‐5p, miR‐19a‐3p, miR‐19b‐3p, miR‐223‐3p, and miR‐25‐3p. This six‐miRNA panel yielded a high AUC of 0.978 (95% CI = 0.966‐0.998) with 93.3% sensitivity and 96.0% specificity in a validation cohort [98]. Through the detection of whole blood miRNAs profiles, a study published in JAMA reported 38 miRNAs that were significantly dysregulated in patients with pancreatic cancer compared with controls [99]. These researchers constructed 2 diagnostic panels, index I (miR‐145, miR‐150, miR‐223, and miR‐636) with an AUC of 0.86 (95% CI = 0.82–0.9), 85% sensitivity, 64% specificity, and index II (miR‐26b, miR‐34a, miR‐122, miR‐126, miR‐145, miR‐150, miR‐223, miR‐505, miR‐636, and miR‐885.5p) with an AUC of 0.93 (95% CI = 0.90–0.96), 85% sensitivity, 85% specificity. Furthermore, the combination of index I and CA19‐9 generated a higher AUC of 0.94 (95% CI = 0.90–0.98, P = 0.1) compared with CA19‐9 alone with an AUC of 0.90 (95% CI = 0.87–0.94) in the validation cohort [99].
Additionally, as reported recently, many other miRNAs possess diagnostic value for pancreatic cancer, including miR‐16, miR‐196a, miR‐1290, miR‐1246, miR‐223, miR‐5100, miR‐8073, miR‐642b‐3p, miR‐663a, miR‐21‐5p, miR‐133a, and others. Combination of certain miRNA with CA19‐9 exhibit higher efficiency for pancreatic cancer or early‐stage pancreatic cancer diagnosis (Table 2). Moreover, combining different types of biomarkers in a panel, such as miR‐21/miR‐25, CA19‐9, and MIC‐1, could improve diagnosis, compared with using a single marker [100]. Based on these studies, miRNAs could become one of the most widespread and promising non‐invasive biomarkers, and more studies in larger cohorts to validate the definitive miRNA panels are required for routine clinical applications in the future.
TABLE 2.
The diagnostic value of other miRNAs for pancreatic cancer diagnosis
| miRNA | Pancreatic cancer group vs. control group | AUC (95% CI) | SEN (%) | SPE (%) | AUC (+ CA19‐9) (95% CI) | SEN (+ CA19‐9) (%) | SPE (+ CA19‐9) (%) |
|---|---|---|---|---|---|---|---|
| miR‐16+miR‐196a [138] | PC vs. HC | 0.90 (0.85–0.94) | 87.0 | 73.5 | 0.98 (0.96–1.00) | 92.0 | 95.6 |
| miR‐1290 [139] | PC vs. HC | 0.96 (0.91–1.00) | 88.0 | 84.0 | 0.86 (NA)* | 71.0* | 90.0* |
| miR‐1290 [140] | PC vs. HC | 0.93 (0.89–0.97) | 75.0 | 97.5 | 0.99 (0.97–1.00) | 94.2 | 97.5 |
| miR‐1246 [140] | PC vs. HC | 0.85 (0.79–0.91) | 62.5 | 92.5 | 0.96 (0.93–0.99) | 89.2 | 97.5 |
| miR‐1290+ miR‐1246 [140] | PC vs. HC | ND | ND | ND | 0.99 (0.98–1.00) | 96.7 | 97.5 |
| miR‐223 [141] | PC vs. HC | 0.83 (NA) | 62.0 | 94.1 | ND | ND | ND |
| miR‐5100+ miR‐8073+ miR‐642b‐3p+ miR‐663a [142] | PC vs. HC | 0.98 (NA) | 98.6 | 87.5 | ND | ND | ND |
| miR‐21‐5p [143] | PC vs. HC | 0.78 (0.66‐0.90) | 77.0 | 80.0 | ND | ND | ND |
| miR‐133a [144] | PC vs. HC | 0.89 (NA) | 87.2 | 90.6 | ND | ND | ND |
The AUC, sensitivity and specificity of CA19‐9 alone in the detection of pancreatic cancer.
Abbreviations: PC: Pancreatic cancer; HC: Healthy controls; ND: not described; NA: not applicable.
4.3. Circulating exosomes
Exosomes are lipid bilayer‐enclosed extracellular vehicles (EVs) approximately 30–150 nm in size, consist of a lipid bilayer interspersed with various membrane proteins, and which contain a variety of nucleic acids, proteins, and lipids [101]. Exosomes are secreted by all cells including tumor cells and circulate in the blood. Recently, increasing numbers of studies have highlighted the potential diagnostic role of EVs or circulating exosomes (crExos) for the early detection of pancreatic cancer. By using multiple plasmonic assays to analyze circulating tumor‐derived EVs, Yang et al. [102] identified an EV‐based protein marker profile including EGFR, EpCAM, MUC‐1, GPC‐1, and WNT2, which showed an accuracy of 84% with 86% sensitivity and 81% specificity for the detection of PDAC. Among these biomarkers, GPC‐1, a cell surface proteoglycan, had the best diagnostic performance, indicating its potential value for pancreatic cancer detection. In another study, using mass spectrometry analyses, Melo et al. [103] determined that GPC‐1 was specifically enriched on cancer‐cell‐derived exosomes. They found that crExos from all patients with pancreatic cancer expressed significantly higher levels of GPC‐1 than healthy controls with 100% sensitivity and 100% specificity. Furthermore, mutant Kras transcripts were detected exclusively in the GPC‐1+ crExos, supporting the cancer cell origin of them [103]. To overcome the difficulty of capture and analysis of exosomes with their proteins, Lewis et al. [104] adopted a method integrating capture and analysis of crExos onto an AC electrokinetic microarray chip without using capture antibodies. They reported that GPC‐1 and CD63 possessed effective diagnostic attributes, with 99% sensitivity and 82% specificity. In addition, it was found that combining CTC and GPC‐1+ exosome detection resulted in 100% sensitivity and 80% specificity, indicating the potential value of liquid biopsy combining different biomarkers [84].
Although a number of studies reported the diagnostic value of crExos in pancreatic cancer, the isolation of cancer‐derived exosomes and separating them from non‐cancer derived exosomes remain difficult due to the lack of specific markers or identification methods. Melo et al. [103] found that the average size of PDAC crExos was significantly smaller than all other crExos, and that mutant Kras transcripts could be only detected in the GPC+ crExos. Thus, it might be promising to combine the sizing of exosomes and GPC‐1 detection to recognize the cancer‐derived exosomes and subsequently proceed with the detection of other biomarkers.
4.4. Other liquid biopsy methods
Pancreatic juice contains DNA and RNA shed from cells lining the pancreatic ducts. Genetic analysis of pancreatic juice of the patients with pancreatic cancer was able to detect mutant DNA, such as Kras, TP53, and SMAD4, for the discrimination of pancreatic cancer from the healthy controls or pre‐cancerous lesions [105, 106]. Additionally, promoters of adenomatous polyposis coli (APC) and histamine receptor H2 (HRH2) genes were found to be frequently methylated in pancreatic cancer juice, serving as potential diagnostic biomarkers [107]. Through the detection of exosome‐derived miRNAs from pancreatic juice, Japanese researchers found that miR‐21 and miR‐155 levels could discriminate PDAC patients from chronic pancreatitis patients with an AUC of 0.90 and 0.89, respectively, and that a combination with cytology displayed even higher accuracy [108]. Moreover, Kras mutation was found in the stool of 88% of pancreatic cancer patients and 19.6% of normal individuals [109]. The detection of miR‐181b and miR‐210 in the stool could distinguish patients with pancreatic cancer from normal individuals [110]. However, peripheral blood for pancreatic cancer detection is undoubtedly more convenient than pancreatic juice and fecal samples.
As an important biological fluid in the human oral cavity, saliva is convenient to collect without any invasive manipulations. Investigators have identified many salivary mRNA biomarkers such as Kras, ACRV1, and MBD3L2 to distinguish pancreatic cancer patients from healthy controls and achieved a higher accuracy by combining these biomarkers even in discriminating patients with pancreatitis [111, 112]. Additionally, salivary metabolites and microbiota have also been studied for their diagnostic value in pancreatic cancer patients and serve as potential biomarkers [113, 114]. In addition, Radon et al. [115] identified a panel of three proteins in urine samples (LYVE‐1, REG1A, and TFF1) for pancreatic cancer diagnosis through an in‐depth proteomics analysis using mass spectrometry. This panel exhibited an AUC of 0.90 (95% CI = 0.84–0.96) when comparing PDAC stage I‐II to healthy urine and showed a higher AUC of 0.97 (95% CI = 0.94–0.99) when matching plasma CA19‐9 levels. It was reported that urinary miRNAs (miR‐143, miR‐223, and miR‐30e) were significantly over‐expressed in patients with PDAC stage I comparing with age‐matched healthy individuals, and possessed potential diagnostic value in distinguishing patients with early pancreatic cancer from healthy individuals or patients with chronic pancreatitis [116].
There have been many studies that focused on liquid biopsies aiming to improve the early diagnosis of pancreatic cancer. However, the challenges emerge when it comes to their actual clinical application. This technique is not yet a standard tool. As Catherine Alix‐Panabières, a cancer cell biologist at the University Medical Centre of Montpellier, said, more interventional clinical trials are still needed, as well as the development of an algorithm to integrate multiple appropriate liquid biomarkers, where policymakers and industry must step in as well [20].
5. OTHER POTENTIAL DIAGNOSTIC STRATEGIES
Imaging‐based investigations such as CT, MRI, and EUS are widely used methods to diagnose patients with pancreatic cancer currently. However, it should be pointed out that the attention and experience of the operators are crucial. Artificial intelligence techniques and computer‐aided diagnosis systems are emerging and should provide prospective benefits. For instance, Ozkan et al. [117] developed a high‐performance computer‐aided diagnosis system with imaging processing and pattern recognition by using EUS imaging of patients with pancreatic cancer and non‐cancer patients. They identified the twenty most appropriate features which exhibited different sensitivity in three age groups (<40, 40–60, and >60 years old) of 87.5%, 85.7%, and 93.3%, respectively.
Tumors can secrete specific volatile organic compounds (VOCs) which can be detected in the odors that emanate from urine, breath, and feces, and can be sensed by canines [118, 119]. Based on this, researchers have tried to detect prostate cancer by exploiting the olfactory system of highly trained dogs [120], but few studies with dogs on pancreatic cancer have been published. Field asymmetric waveform ion mobility spectrometry (IMS) is a sensitive technique used for the detection of VOCs. Nissinen et al. [121] demonstrated that field asymmetric waveform IMS discriminated between urine samples from patients with pancreatic cancer or pre‐malignant pancreatic lesion and healthy individuals with 85% sensitivity and 75% specificity, but insufficient accuracy. In addition, Covington's group also detected urinary VOCs from participants by using IMS, and developed an algorithm distinguishing PDAC samples from healthy controls with an AUC of 0.92, 91% sensitivity, and 83% specificity [122]. Recently, the same group reported that both gas chromatography‐ion mobility spectrometry (GC‐IMS) and GC time‐of‐flight mass spectrometry (GC‐TOF‐MS) were able to differentiate PDAC from healthy controls with high confidence and an AUC in excess of 0.85. Chemical identification further suggested that 2,6‐dimethyl‐octane, nonanal, 4‐ethyl‐1,2‐dimethyl‐benzene, and 2‐pentanone play important roles in discriminating these groups [123]. Moreover, researchers also analyzed the VOCs in alveolar air from the end‐tidal breath of subjects suffering from pancreatic cancer. By using ion‐molecule reaction MS technology, Princivalle et al. [124] identified ten VOCs which significantly distinguished pancreatic cancer from controls with a high AUC of 0.99, high sensitivity of 100%, and specificity of 84%. Thus, it is a promising strategy to detect pancreatic cancer through the VOCs in urine samples or alveolar air, with the advantages of non‐invasive operation and easy collection of biological samples.
Recently, increasing studies are focusing on new nanomaterials. The biocompatibility of nanomaterials such as fluorescent nanoparticles allows their use for labeling and detecting biological molecules, thereby serving as potential early diagnostic tools for cancer [125]. For example, researchers discovered ultra pH‐sensitive fluorescent nanoprobes which were silent in the circulation but could be activated in response to the low extracellular pH in tumors, showing broad tumor imaging specificity and efficacy in tumor models including pancreatic cancer [126]. These results indicate that the integration of medicine with other disciplines, such as computer science and chemistry, is conducive for improving the detection of early pancreatic cancer.
6. CONCLUSIONS AND FUTURE PERSPECTIVES
As a highly malignant tumor with a poor prognosis, pancreatic cancer attracts constant attention from scientists worldwide who have undertaken many studies to improve its diagnostic accuracy and early detection rate. Enhancing science education and expanding the early screening population is crucial to significantly improve the prognosis of patients with pancreatic cancer. More importantly, a multidisciplinary collaboration involving both the diagnostic departments (laboratory medicine, radiology, and ultrasound) and therapeutic departments (surgery, gastroenterology, and oncology) is indispensable throughout the entire process of pancreatic cancer diagnosis and treatment as suggested in various guidelines. Thus, it is necessary to deepen the interdisciplinary integration and strengthen the cooperation among different disciplines to accelerate the scientific research production and clinical transformation.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
AVAILABILITY OF DATA AND MATERIALS
Not applicable.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS' CONTRIBUTIONS
All authors contributed substantially to the conception and design of the study. JY and RX drafted the manuscript. CW, JQ, BR, and LY provided critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
This study was supported by Non‐profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2018PT32014) and Chinese Academy of Medical Science Innovation Fund for Medical Science (2017‐I2M‐1‐001).
Yang J, Xu R, Wang C, Qiu J, Ren Bo, You L. Early screening and diagnosis strategies of pancreatic cancer: a comprehensive review. Cancer Commun. 2021;41:1257–1274. 10.1002/cac2.12204
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021. [DOI] [PubMed] [Google Scholar]
- 2. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Research. 2014;74(14):2913‐21. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7‐33. [DOI] [PubMed] [Google Scholar]
- 4. Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond). 2019;39(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ariyama J. Detection and prognosis of small pancreatic ductal adenocarcinoma. Nihon Geka Gakkai Zasshi. 1997;98(7):592‐6. [PubMed] [Google Scholar]
- 6. Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395(10242):2008‐20. [DOI] [PubMed] [Google Scholar]
- 7. Network NCC. The NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): pancreatic adenocarcinoma (version 1.2020) 2019 [cited 2020 August 15]. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx.
- 8. Hasan S, Jacob R, Manne U, Paluri R. Advances in pancreatic cancer biomarkers. Oncol Rev. 2019;13(1):410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, et al. Screening for pancreatic cancer: US preventive services task force reaffirmation recommendation statement. Jama. 2019;322(5):438‐44. [DOI] [PubMed] [Google Scholar]
- 10. Sharma A, Kandlakunta H, Nagpal SJS, Feng Z, Hoos W, Petersen GM, et al. Model to determine risk of pancreatic cancer in patients with new‐onset diabetes. Gastroenterology. 2018;155(3):730‐9.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kanno A, Masamune A, Hanada K, Maguchi H, Shimizu Y, Ueki T, et al. Multicenter study of early pancreatic cancer in Japan. Pancreatology. 2018;18(1):61‐7. [DOI] [PubMed] [Google Scholar]
- 12. Luo B, Peng F, Hong M, Su S, Fang C, Yang X, et al. ERCP combined with tumor markers in differential diagnosis of pancreatic cancer and pseudotumor‐like pancreatitis. J Buon. 2019;24(4):1568‐73. [PubMed] [Google Scholar]
- 13. Bunganič B, Laclav M, Dvořáková T, Bradáč O, Traboulsi E, Suchánek Š, et al. Accuracy of EUS and CEH EUS for the diagnosis of pancreatic tumours. Scand J Gastroenterol. 2018;53(10‐11):1411‐7. [DOI] [PubMed] [Google Scholar]
- 14. Xing H, Wang J, Wang Y, Tong M, Hu H, Huang C, et al. Diagnostic value of CA 19‐9 and carcinoembryonic antigen for pancreatic cancer: a meta‐analysis. Gastroenterol Res Pract. 2018;2018:8704751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gu YL, Lan C, Pei H, Yang SN, Liu YF, Xiao LL. Applicative value of serum CA19‐9, CEA, CA125 and CA242 in diagnosis and prognosis for patients with pancreatic cancer treated by concurrent chemoradiotherapy. Asian Pac J Cancer Prev. 2015;16(15):6569‐73. [DOI] [PubMed] [Google Scholar]
- 16. Kaur S, Smith LM, Patel A, Menning M, Watley DC, Malik SS, et al. A combination of MUC5AC and CA19‐9 improves the diagnosis of pancreatic cancer: a multicenter study. Am J Gastroenterol. 2017;112(1):172‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohamed AA, Soliman H, Ismail M, Ziada D, Farid TM, Aref AM, et al. Evaluation of circulating ADH and MIC‐1 as diagnostic markers in Egyptian patients with pancreatic cancer. Pancreatology. 2015;15(1):34‐9. [DOI] [PubMed] [Google Scholar]
- 18. Samandari M, Julia MG, Rice A, Chronopoulos A, Del Rio Hernandez AE. Liquid biopsies for management of pancreatic cancer. Transl Res. 2018;201:98‐127. [DOI] [PubMed] [Google Scholar]
- 19. Mayerle J, Kalthoff H, Reszka R, Kamlage B, Peter E, Schniewind B, et al. Metabolic biomarker signature to differentiate pancreatic ductal adenocarcinoma from chronic pancreatitis. Gut. 2018;67(1):128‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alix‐Panabières C. The future of liquid biopsy. Nature. 2020;579(7800):S9. [DOI] [PubMed] [Google Scholar]
- 21. Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, et al. Frequent detection of pancreatic lesions in asymptomatic high‐risk individuals. Gastroenterology. 2012;142(4):796‐804; quiz e14‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Canto MI, Goggins M, Hruban RH, Petersen GM, Giardiello FM, Yeo C, et al. Screening for early pancreatic neoplasia in high‐risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4(6):766‐81; quiz 665. [DOI] [PubMed] [Google Scholar]
- 23. Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, et al. Frequent detection of pancreatic lesions in asymptomatic high‐risk individuals. Gastroenterology. 2012;142(4):796‐804; quiz e14‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lami G, Biagini MR, Galli A. Endoscopic ultrasonography for surveillance of individuals at high risk for pancreatic cancer. World J Gastrointest Endosc. 2014;6(7):272‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goggins M, Overbeek KA, Brand R, Syngal S, Del Chiaro M, Bartsch DK, et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut. 2020;69(1):7‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang J, Li H, Zheng R, Zhang S, Zeng H, Sun K, et al. Incidence and mortality of pancreatic cancer in China, 2014. China Cancer. 2018;27(6):420‐5. [Google Scholar]
- 27. Bosetti C, Lucenteforte E, Silverman DT, Petersen G, Bracci PM, Ji BT, et al. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case‐Control Consortium (Panc4). Ann Oncol. 2012;23(7):1880‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang J, Bai R, Li M, Ye H, Wu C, Wang C, et al. Excessive miR‐25‐3p maturation via N(6)‐methyladenosine stimulated by cigarette smoke promotes pancreatic cancer progression. Nat Commun. 2019;10(1):1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pang Y, Holmes MV, Guo Y, Yang L, Bian Z, Chen Y, et al. Smoking, alcohol, and diet in relation to risk of pancreatic cancer in China: a prospective study of 0.5 million people. Cancer Med. 2018;7(1):229‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Naudin S, Li K, Jaouen T, Assi N, Kyrø C, Tjønneland A, et al. Lifetime and baseline alcohol intakes and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition study. Int J Cancer. 2018;143(4):801‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karlson BM, Ekbom A, Josefsson S, McLaughlin JK, Fraumeni JF, Jr , Nyrén O. The risk of pancreatic cancer following pancreatitis: an association due to confounding? Gastroenterology. 1997;113(2):587‐92. [DOI] [PubMed] [Google Scholar]
- 32. Kirkegård J, Mortensen FV, Cronin‐Fenton D. Chronic pancreatitis and pancreatic cancer risk: a systematic review and meta‐analysis. Am J Gastroenterol. 2017;112(9):1366‐72. [DOI] [PubMed] [Google Scholar]
- 33. Kolodecik T, Shugrue C, Ashat M, Thrower EC. Risk factors for pancreatic cancer: underlying mechanisms and potential targets. Front Physiol. 2013;4:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Makhoul I, Yacoub A, Siegel E. Type 2 diabetes mellitus is associated with increased risk of pancreatic cancer: a veteran administration registry study. SAGE Open Medicine. 2016;4(11):205031211668225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pang Y, Kartsonaki C, Guo Y, Bragg F, Yang L, Bian Z, et al. Diabetes, plasma glucose and incidence of pancreatic cancer: a prospective study of 0.5 million Chinese adults and a meta‐analysis of 22 cohort studies. International Journal of Cancer. 2017;140(8):1781‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. Jama. 2000;283(19):2552. [DOI] [PubMed] [Google Scholar]
- 37. Mössner J, Logsdon CD, Goldfine ID, Williams JA. Do insulin and the insulin like growth factors (IGFs) stimulate growth of the exocrine pancreas? Gut. 1987;28:51‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carrerastorres R, Johansson M, Gaborieau V, Haycock PC, Wade KH, Relton CL, et al. The role of obesity, type 2 diabetes, and metabolic factors in pancreatic cancer: a mendelian randomization study. J Natl Cancer Inst. 2017;109(9):495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hua F, Li K, Yu JJ, Lv XX, Yan J, Zhang XW, et al. TRB3 links insulin/IGF to tumour promotion by interacting with p62 and impeding autophagic/proteasomal degradations. Nat Commun. 2015;6:7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang A, Shen W. Analysis on risk factors and clinical significance in patients with pancreatic cancer. Chongqing Medicine. 2017;47(8):1064‐7. [Google Scholar]
- 41. Chung KM, Singh J, Lawres L, Dorans KJ, Garcia C, Burkhardt DB, et al. Endocrine‐exocrine signaling drives obesity‐associated pancreatic ductal adenocarcinoma. Cell. 2020;181(4):832‐47 e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Philip B, Roland CL, Daniluk J, Liu Y, Chatterjee D, Gomez SB, et al. A high‐fat diet activates oncogenic Kras and COX2 to induce development of pancreatic ductal adenocarcinoma in mice. Gastroenterology. 2013;145(6):1449‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luo Y, Yang Y, Liu M, Wang D, Wang F, Bi Y, et al. Oncogenic KRAS reduces expression of FGF21 in acinar cells to promote pancreatic tumorigenesis in mice on a high‐fat diet. Gastroenterology. 2019;157(5):1413‐28.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pang TC, Lin T, Ding G, Liu D, Jiang G. Multiple factor analysis of pancreatic cancer‐associated risk factors. Chin J Pract Surg. 2014;34(10):962‐6. [Google Scholar]
- 45. Zhang JJ, Jia JP, Shao Q, Wang YK. Diabetes mellitus and risk of pancreatic cancer in China: a meta‐analysis based on 26 case‐control studies. Prim Care Diabetes. 2019;13(3):276‐82. [DOI] [PubMed] [Google Scholar]
- 46. Boursi B, Finkelman B, Giantonio BJ, Haynes K, Rustgi AK, Rhim AD, et al. A clinical prediction model to assess risk for pancreatic cancer among patients with new‐onset diabetes. Gastroenterology. 2017;152(4):840‐50.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schmidt DR, Patel R, Kirsch DG, Lewis CA, Vander Heiden MG, Locasale JW. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J Clin. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Michálková L, Horník Š, Sýkora J, Habartová L, Setnička V. Diagnosis of pancreatic cancer via(1)H NMR metabolomics of human plasma. Analyst. 2018;143(24):5974‐8. [DOI] [PubMed] [Google Scholar]
- 49. He X, Zhong J, Wang S, Zhou Y, Wang L, Zhang Y, et al. Serum metabolomics differentiating pancreatic cancer from new‐onset diabetes. Oncotarget. 2017;8(17):29116‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang L, Li H, Qian J, Deng R, Zhou L. Establishment of a risk model based on study of risk factors for pancreatic cancer. Chin J Dig. 2005;9:515‐20. [Google Scholar]
- 51. Ahmed AE, Alzahrani FS, Gharawi AM, Alammary SA, Almijmaj FH, Alhusayni FM, et al. Improving risk prediction for pancreatic cancer in symptomatic patients: a Saudi Arabian study. Cancer Manag Res. 2018;10:4981‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. He M, Xue H, Jin Z, Zhao Y. State‐of‐the art imaging in the diagnosis and treatment of pancreatic ductal carcinoma: current role and value. Medical Journal of Peking Union Medical College Hospital. 2019;10(1):11‐8. [Google Scholar]
- 53. Bronstein YL, Loyer EM, Kaur H, Choi H, David C, DuBrow RA, et al. Detection of small pancreatic tumors with multiphasic helical CT. AJR Am J Roentgenol. 2004;182(3):619‐23. [DOI] [PubMed] [Google Scholar]
- 54. Maguchi H, Takahashi K, Osanai M, Katanuma A. Small pancreatic lesions: is there need for EUS‐FNA preoperatively? What to do with the incidental lesions? Endoscopy. 2006;38(1):S53‐6. [DOI] [PubMed] [Google Scholar]
- 55. Cui B, Fang W, Khan S, Li S, Chang Y, Wang B, et al. Endoscopic ultrasound imaging for differential diagnosis of pancreatic neoplasms: a 7‐year study in a Chinese population. Med Sci Monit. 2018;24:3653‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yamashita Y, Shimokawa T, Napoléon B, Fusaroli P, Gincul R, Kudo M, et al. Value of contrast‐enhanced harmonic endoscopic ultrasonography with enhancement pattern for diagnosis of pancreatic cancer: a meta‐analysis. Dig Endosc. 2019;31(2):125‐33. [DOI] [PubMed] [Google Scholar]
- 57. Yamashita Y, Kitano M, Ashida R. Value of endoscopy for early diagnosis of pancreatic carcinoma. Dig Endosc. 2020;32(1):27‐36. [DOI] [PubMed] [Google Scholar]
- 58. Park HJ, Jang KM, Song KD, Kim SH, Kim YK, Cha MJ, et al. Value of unenhanced MRI with diffusion‐weighted imaging for detection of primary small (≤20 mm) solid pancreatic tumours and prediction of pancreatic ductal adenocarcinoma. Clin Radiol. 2017;72(12):1076‐84. [DOI] [PubMed] [Google Scholar]
- 59. Wu L, Guo W, Li Y, Cheng T, Yao Y, Zhang Y, et al. Value of endoscopic ultrasound‐guided fine needle aspiration in pretest prediction and diagnosis of pancreatic ductal adenocarcinoma. Nan Fang Yi Ke Da Xue Xue Bao. 2018;38(10):1171‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Facciorusso A, Martina M, Buccino RV, Nacchiero MC, Muscatiello N. Diagnostic accuracy of fine‐needle aspiration of solid pancreatic lesions guided by endoscopic ultrasound elastography. Ann Gastroenterol. 2018;31(4):513‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bournet B, Selves J, Grand D, Danjoux M, Hanoun N, Cordelier P, et al. Endoscopic ultrasound‐guided fine‐needle aspiration biopsy coupled with a KRAS mutation assay using allelic discrimination improves the diagnosis of pancreatic cancer. J Clin Gastroenterol. 2015;49(1):50‐6. [DOI] [PubMed] [Google Scholar]
- 62. Ma H, Wang P, Shang D, Liu Y. Spatial‐domain low‐coherence quantitative phase microscopy to improve the cytological diagnosis of pancreatic cancer. J Investig Med. 2020;68(1):60‐7. [DOI] [PubMed] [Google Scholar]
- 63. Grassia R, Imperatore N, Capone P, Cereatti F, Forti E, Antonini F, et al. EUS‐guided tissue acquisition in chronic pancreatitis: differential diagnosis between pancreatic cancer and pseudotumoral masses using EUS‐FNA or core biopsy. Endosc Ultrasound. 2020;9(2):122‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bor R, Vasas B, Fabian A, Balint A, Farkas K, Milassin A, et al. Prospective comparison of slow‐pull and standard suction techniques of endoscopic ultrasound‐guided fine needle aspiration in the diagnosis of solid pancreatic cancer. BMC Gastroenterol. 2019;19(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Matsumoto K, Takeda Y, Onoyama T, Kawata S, Kurumi H, Ueki M, et al. Role of the preoperative usefulness of the pathological diagnosis of pancreatic diseases. World J Gastrointest Oncol. 2016;8(9):656‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yang H, Qian J, Yang A, Guo T, Mai C, Lu X, et al. Different imaging techniques in the diagnosis of pancreatic carcinoma. Medical Journal of Peking Union Medical College Hospital. 2010;1(2):137‐40. [Google Scholar]
- 67. Luo G, Jin K, Deng S, Cheng H, Fan Z, Gong Y, et al. Roles of CA19‐9 in pancreatic cancer: Biomarker, predictor and promoter. Biochim Biophys Acta Rev Cancer. 2021;1875(2):188409. [DOI] [PubMed] [Google Scholar]
- 68. Deng G, Yan H, Guo Z, Dai G. Relationship between CA199 and prognosis of patients with advanced pancreatic cancer. Academic Journal of Chinese PLA Medical School. 2019;40(1):33‐6. [Google Scholar]
- 69. Yang L, Yan H, Shi Y, Dai G. Relationship between serum CA125, CA199 levels and liver metastasis in patients with advanced pancreatic cancer. Chinese Journal of Laboratory Diagnosis. 2017;21(5):770‐3. [Google Scholar]
- 70. Usón Junior PLS, Callegaro‐Filho D, Bugano DDG, Moura F, Maluf FC. Predictive value of serum carbohydrate antigen 19‐9 (CA19‐9) for early mortality in advanced pancreatic cancer. J Gastrointest Cancer. 2018;49(4):481‐6. [DOI] [PubMed] [Google Scholar]
- 71. Luo G, Guo M, Jin K, Liu Z, Liu C, Cheng H, et al. Optimize CA19‐9 in detecting pancreatic cancer by Lewis and Secretor genotyping. Pancreatology. 2016;16(6):1057‐62. [DOI] [PubMed] [Google Scholar]
- 72. Dai C, Cao Q, Jiang M, Sun MJ. Serum immunoglobulin G4 in discriminating autoimmune pancreatitis from pancreatic cancer: a diagnostic meta‐analysis. Pancreas. 2018;47(3):280‐4. [DOI] [PubMed] [Google Scholar]
- 73. Yan T, Ke Y, Chen Y, Xu C, Yu C, Li Y. Serological characteristics of autoimmune pancreatitis and its differential diagnosis from pancreatic cancer by using a combination of carbohydrate antigen 19‐9, globulin, eosinophils and hemoglobin. PLoS One. 2017;12(4):e0174735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hao M, Li W, Yi L, Yu S, Fan G, Lu T, et al. Hybrid kappa∖lambda antibody is a new serological marker to diagnose autoimmune pancreatitis and differentiate it from pancreatic cancer. Sci Rep. 2016;6:27415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ning L, Pan B, Zhao YP, Liao Q, Zhang TP, Chen G, et al. Immuno‐proteomic screening of human pancreatic cancer associated membrane antigens for early diagnosis. Zhonghua Wai Ke Za Zhi. 2007;45(1):34‐8. [PubMed] [Google Scholar]
- 76. Aronsson L, Andersson R, Bauden M, Andersson B, Bygott T, Ansari D. High‐density and targeted glycoproteomic profiling of serum proteins in pancreatic cancer and intraductal papillary mucinous neoplasm. Scand J Gastroenterol. 2018;53(12):1597‐603. [DOI] [PubMed] [Google Scholar]
- 77. Liu Y, Wang C, Wang R, Wu Y, Zhang L, Liu BF, et al. Isomer‐specific profiling of N‐glycans derived from human serum for potential biomarker discovery in pancreatic cancer. J Proteomics. 2018;181:160‐9. [DOI] [PubMed] [Google Scholar]
- 78. Onuigbo WI. An index of the fate of circulating cancer cells. Lancet. 1963;2(7312):828‐31. [DOI] [PubMed] [Google Scholar]
- 79. Xie ZB, Yao L, Jin C, Fu DL. Circulating tumor cells in pancreatic cancer patients: efficacy in diagnosis and value in prognosis. Discov Med. 2016;22(120):121‐8. [PubMed] [Google Scholar]
- 80. Herreros‐Villanueva M, Bujanda L. Non‐invasive biomarkers in pancreatic cancer diagnosis: what we need versus what we have. Ann Transl Med. 2016;4(7):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ankeny JS, Court CM, Hou S, Li Q, Song M, Wu D, et al. Circulating tumour cells as a biomarker for diagnosis and staging in pancreatic cancer. Br J Cancer. 2016;114(12):1367‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Cheng H, He W, Yang J, Ye Q, Cheng L, Pan Y, et al. Ligand‐targeted polymerase chain reaction for the detection of folate receptor‐positive circulating tumour cells as a potential diagnostic biomarker for pancreatic cancer. Cell Prolif. 2020;53(9):e12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wei T, Zhang X, Zhang Q, Yang J, Chen Q, Wang J, et al. Vimentin‐positive circulating tumor cells as a biomarker for diagnosis and treatment monitoring in patients with pancreatic cancer. Cancer Lett. 2019;452:237‐43. [DOI] [PubMed] [Google Scholar]
- 84. Buscail E, Alix‐Panabières C, Quincy P, Cauvin T, Chauvet A, Degrandi O, et al. High clinical value of liquid biopsy to detect circulating tumor cells and tumor exosomes in pancreatic ductal adenocarcinoma patients eligible for up‐front surgery. Cancers (Basel). 2019;11(11):1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jaworski JJ, Morgan RD, Sivakumar S. Circulating cell‐free tumour DNA for early detection of pancreatic cancer. Cancers (Basel). 2020;12(12):3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cohen JD, Javed AA, Thoburn C, Wong F, Tie J, Gibbs P, et al. Combined circulating tumor DNA and protein biomarker‐based liquid biopsy for the earlier detection of pancreatic cancers. Proc Natl Acad Sci U S A. 2017;114(38):10202‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu X, Liu L, Ji Y, Li C, Wei T, Yang X, et al. Enrichment of short mutant cell‐free DNA fragments enhanced detection of pancreatic cancer. EBioMedicine. 2019;41:345‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Corcoran RB, Chabner BA. Application of cell‐free DNA analysis to cancer treatment. N Engl J Med. 2018;379(18):1754‐65. [DOI] [PubMed] [Google Scholar]
- 89. Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, et al. Detection of circulating tumor DNA in early‐ and late‐stage human malignancies. Sci Transl Med. 2014;6(224):224ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Klutstein M, Nejman D, Greenfield R, Cedar H. DNA methylation in cancer and aging. Cancer Res. 2016;76(12):3446‐50. [DOI] [PubMed] [Google Scholar]
- 91. Eissa MAL, Lerner L, Abdelfatah E, Shankar N, Canner JK, Hasan NM, et al. Promoter methylation of ADAMTS1 and BNC1 as potential biomarkers for early detection of pancreatic cancer in blood. Clin Epigenetics. 2019;11(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bauden M, Pamart D, Ansari D, Herzog M, Eccleston M, Micallef J, et al. Circulating nucleosomes as epigenetic biomarkers in pancreatic cancer. Clin Epigenetics. 2015;7:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 2016;1:15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S, et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006;24(29):4677‐84. [DOI] [PubMed] [Google Scholar]
- 95. Ryu JK, Hong SM, Karikari CA, Hruban RH, Goggins MG, Maitra A. Aberrant MicroRNA‐155 expression is an early event in the multistep progression of pancreatic adenocarcinoma. Pancreatology. 2010;10(1):66‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yu J, Li A, Hong SM, Hruban RH, Goggins M. MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin Cancer Res. 2012;18(4):981‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Liu R, Chen X, Du Y, Yao W, Shen L, Wang C, et al. Serum microRNA expression profile as a biomarker in the diagnosis and prognosis of pancreatic cancer. Clin Chem. 2012;58(3):610‐8. [DOI] [PubMed] [Google Scholar]
- 98. Zou X, Wei J, Huang Z, Zhou X, Lu Z, Zhu W, et al. Identification of a six‐miRNA panel in serum benefiting pancreatic cancer diagnosis. Cancer Med. 2019;8(6):2810‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Schultz NA, Dehlendorff C, Jensen BV, Bjerregaard JK, Nielsen KR, Bojesen SE, et al. MicroRNA biomarkers in whole blood for detection of pancreatic cancer. Jama. 2014;311(4):392‐404. [DOI] [PubMed] [Google Scholar]
- 100. Yuan W, Tang W, Xie Y, Wang S, Chen Y, Qi J, et al. New combined microRNA and protein plasmatic biomarker panel for pancreatic cancer. Oncotarget. 2016;7(48):80033‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Lane JS, Hoff DV, Cridebring D, Goel A. Extracellular vesicles in diagnosis and treatment of pancreatic cancer: current state and future perspectives. Cancers. 2020;12(6):1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yang KS, Im H, Hong S, Pergolini I, Del Castillo AF, Wang R, et al. Multiparametric plasma EV profiling facilitates diagnosis of pancreatic malignancy. Sci Transl Med. 2017;9(391):eaal3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican‐1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523(7559):177‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Lewis JM, Vyas AD, Qiu Y, Messer KS, White R, Heller MJ. Integrated analysis of exosomal protein biomarkers on alternating current electrokinetic chips enables rapid detection of pancreatic cancer in patient blood. ACS Nano. 2018;12(4):3311‐20. [DOI] [PubMed] [Google Scholar]
- 105. Yu J, Sadakari Y, Shindo K, Suenaga M, Brant A, Almario JAN, et al. Digital next‐generation sequencing identifies low‐abundance mutations in pancreatic juice samples collected from the duodenum of patients with pancreatic cancer and intraductal papillary mucinous neoplasms. Gut. 2017;66(9):1677‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yang J, Li S, Li J, Wang F, Chen K, Zheng Y, et al. A meta‐analysis of the diagnostic value of detecting K‐ras mutation in pancreatic juice as a molecular marker for pancreatic cancer. Pancreatology. 2016;16(4):605‐14. [DOI] [PubMed] [Google Scholar]
- 107. Ginesta MM, Diaz‐Riascos ZV, Busquets J, Pelaez N, Serrano T, Peinado M, et al. APC promoter is frequently methylated in pancreatic juice of patients with pancreatic carcinomas or periampullary tumors. Oncol Lett. 2016;12(3):2210‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nakamura S, Sadakari Y, Ohtsuka T, Okayama T, Nakashima Y, Gotoh Y, et al. Pancreatic juice exosomal MicroRNAs as biomarkers for detection of pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2019;26(7):2104‐11. [DOI] [PubMed] [Google Scholar]
- 109. Lu X, Xu T, Qian J, Wen X, Wu D. Detecting K‐ras and p53 gene mutation from stool and pancreatic juice for diagnosis of early pancreatic cancer. Chin Med J (Engl). 2002;115(11):1632‐6. [PubMed] [Google Scholar]
- 110. Ren Y, Gao J, Liu JQ, Wang XW, Gu JJ, Huang HJ, et al. Differential signature of fecal microRNAs in patients with pancreatic cancer. Mol Med Rep. 2012;6(1):201‐9. [DOI] [PubMed] [Google Scholar]
- 111. Liu HJ, Guo YY, Li DJ. Predicting novel salivary biomarkers for the detection of pancreatic cancer using biological feature‐based classification. Pathol Res Pract. 2017;213(4):394‐9. [DOI] [PubMed] [Google Scholar]
- 112. Zhang L, Farrell JJ, Zhou H, Elashoff D, Akin D, Park NH, et al. Salivary transcriptomic biomarkers for detection of resectable pancreatic cancer. Gastroenterology. 2010;138(3):949‐57.e1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sugimoto M, Wong DT, Hirayama A, Soga T, Tomita M. Capillary electrophoresis mass spectrometry‐based saliva metabolomics identified oral, breast and pancreatic cancer‐specific profiles. Metabolomics. 2010;6(1):78‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Farrell JJ, Zhang L, Zhou H, Chia D, Elashoff D, Akin D, et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut. 2012;61(4):582‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Radon TP, Massat NJ, Jones R, Alrawashdeh W, Dumartin L, Ennis D, et al. Identification of a three‐biomarker panel in urine for early detection of pancreatic adenocarcinoma. Clin Cancer Res. 2015;21(15):3512‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Debernardi S, Massat NJ, Radon TP, Sangaralingam A, Banissi A, Ennis DP, et al. Noninvasive urinary miRNA biomarkers for early detection of pancreatic adenocarcinoma. Am J Cancer Res. 2015;5(11):3455‐66. [PMC free article] [PubMed] [Google Scholar]
- 117. Ozkan M, Cakiroglu M, Kocaman O, Kurt M, Yilmaz B, Can G, et al. Age‐based computer‐aided diagnosis approach for pancreatic cancer on endoscopic ultrasound images. Endosc Ultrasound. 2016;5(2):101‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Sun X, Shao K, Wang T. Detection of volatile organic compounds (VOCs) from exhaled breath as noninvasive methods for cancer diagnosis. Anal Bioanal Chem. 2016;408(11):2759‐80. [DOI] [PubMed] [Google Scholar]
- 119. Boots AW, van Berkel JJ, Dallinga JW, Smolinska A, Wouters EF, van Schooten FJ. The versatile use of exhaled volatile organic compounds in human health and disease. J Breath Res. 2012;6(2):027108. [DOI] [PubMed] [Google Scholar]
- 120. Taverna G, Tidu L, Grizzi F, Torri V, Mandressi A, Sardella P, et al. Olfactory system of highly trained dogs detects prostate cancer in urine samples. J Urol. 2015;193(4):1382‐7. [DOI] [PubMed] [Google Scholar]
- 121. Nissinen SI, Roine A, Hokkinen L, Karjalainen M, Venäläinen M, Helminen H, et al. Detection of pancreatic cancer by urine volatile organic compound analysis. Anticancer Res. 2019;39(1):73‐9. [DOI] [PubMed] [Google Scholar]
- 122. Arasaradnam RP, Wicaksono A, O'Brien H, Kocher HM, Covington JA, Crnogorac‐Jurcevic T. Noninvasive diagnosis of pancreatic cancer through detection of volatile organic compounds in urine. Gastroenterology. 2018;154(3):485‐7.e1. [DOI] [PubMed] [Google Scholar]
- 123. Daulton E, Wicaksono AN, Tiele A, Kocher HM, Debernardi S, Crnogorac‐Jurcevic T, et al. Volatile organic compounds (VOCs) for the non‐invasive detection of pancreatic cancer from urine. Talanta. 2021;221:121604. [DOI] [PubMed] [Google Scholar]
- 124. Princivalle A, Monasta L, Butturini G, Bassi C, Perbellini L. Pancreatic ductal adenocarcinoma can be detected by analysis of volatile organic compounds (VOCs) in alveolar air. BMC Cancer. 2018;18(1):529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wang J, He ZW, Jiang JX. Nanomaterials: applications in the diagnosis and treatment of pancreatic cancer. World J Gastrointest Pharmacol Ther. 2020;11(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wang Y, Zhou K, Huang G, Hensley C, Huang X, Ma X, et al. A nanoparticle‐based strategy for the imaging of a broad range of tumours by nonlinear amplification of microenvironment signals. Nat Mater. 2014;13(2):204‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lu C, Zhang S, Zhang Y, Wei B. The expressions and the clinical significance of CEA, CA199, IL‐6 and MCP‐1 in the serum of patients with pancreatic cancer. Sci Technol Rev. 2012;30(14):38‐41. [Google Scholar]
- 128. Martinez‐Bosch N, Barranco LE, Orozco CA, Moreno M, Visa L, Iglesias M, et al. Increased plasma levels of galectin‐1 in pancreatic cancer: potential use as biomarker. Oncotarget. 2018;9(68):32984‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Liu X, Zheng W, Wang W, Shen H, Liu L, Lou W, et al. A new panel of pancreatic cancer biomarkers discovered using a mass spectrometry‐based pipeline. Br J Cancer. 2017;117(12):1846‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Han SX, Zhou X, Sui X, He CC, Cai MJ, Ma JL, et al. Serum dickkopf‐1 is a novel serological biomarker for the diagnosis and prognosis of pancreatic cancer. Oncotarget. 2015;6(23):19907‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hogendorf P, Durczyński A, Skulimowski A, Kumor A, Poznańska G, Strzelczyk J. Growth differentiation factor (GDF‐15) concentration combined with Ca125 levels in serum is superior to commonly used cancer biomarkers in differentiation of pancreatic mass. Cancer Biomark. 2018;21(3):505‐11. [DOI] [PubMed] [Google Scholar]
- 132. Ferri MJ, Saez M, Figueras J, Fort E, Sabat M, López‐Ben S, et al. Improved pancreatic adenocarcinoma diagnosis in jaundiced and non‐jaundiced pancreatic adenocarcinoma patients through the combination of routine clinical markers associated to pancreatic adenocarcinoma pathophysiology. PLoS One. 2016;11(1):e0147214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Furukawa K, Kawamoto K, Eguchi H, Tanemura M, Tanida T, Tomimaru Y, et al. Clinicopathological significance of leucine‐rich α2‐glycoprotein‐1 in sera of patients with pancreatic cancer. Pancreas. 2015;44(1):93‐8. [DOI] [PubMed] [Google Scholar]
- 134. Honda K, Kobayashi M, Okusaka T, Rinaudo JA, Huang Y, Marsh T, et al. Plasma biomarker for detection of early stage pancreatic cancer and risk factors for pancreatic malignancy using antibodies for apolipoprotein‐AII isoforms. Sci Rep. 2015;5:15921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Jahan R, Ganguly K, Smith LM, Atri P, Carmicheal J, Sheinin Y, et al. Trefoil factor(s) and CA19.9: a promising panel for early detection of pancreatic cancer. EBioMedicine. 2019;42:375‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Balasenthil S, Huang Y, Liu S, Marsh T, Chen J, Stass SA, et al. A plasma biomarker panel to identify surgically resectable early‐stage pancreatic cancer. J Natl Cancer Inst. 2017;109(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Papapanagiotou A, Sgourakis G, Karkoulias K, Raptis D, Parkin E, Brotzakis P, et al. Osteonectin as a screening marker for pancreatic cancer: a prospective study. J Int Med Res. 2018;46(7):2769‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Liu J, Gao J, Du Y, Li Z, Ren Y, Gu J, et al. Combination of plasma microRNAs with serum CA19‐9 for early detection of pancreatic cancer. Int J Cancer. 2012;131(3):683‐91. [DOI] [PubMed] [Google Scholar]
- 139. Li A, Yu J, Kim H, Wolfgang CL, Canto MI, Hruban RH, et al. MicroRNA array analysis finds elevated serum miR‐1290 accurately distinguishes patients with low‐stage pancreatic cancer from healthy and disease controls. Clin Cancer Res. 2013;19(13):3600‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wei J, Yang L, Wu YN, Xu J. Serum miR‐1290 and miR‐1246 as potential diagnostic biomarkers of human pancreatic cancer. J Cancer. 2020;11(6):1325‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Komatsu S, Ichikawa D, Miyamae M, Kawaguchi T, Morimura R, Hirajima S, et al. Malignant potential in pancreatic neoplasm; new insights provided by circulating miR‐223 in plasma. Expert Opin Biol Ther. 2015;15(6):773‐85. [DOI] [PubMed] [Google Scholar]
- 142. Shams R, Saberi S, Zali M, Sadeghi A, Ghafouri‐Fard S, Aghdaei HA. Identification of potential microRNA panels for pancreatic cancer diagnosis using microarray datasets and bioinformatics methods. Sci Rep. 2020;10(1):7559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Qu K, Zhang X, Lin T, Liu T, Wang Z, Liu S, et al. Circulating miRNA‐21‐5p as a diagnostic biomarker for pancreatic cancer: evidence from comprehensive miRNA expression profiling analysis and clinical validation. Sci Rep. 2017;7(1):1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Wang Z. Diagnostic performance for declined microRNA‐133a in pancreatic cancer. J Cell Biochem. 2019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


