Abstract
Context.
Targeted therapy has revolutionized lung cancer treatment and markedly increased survival, though data are lacking on patient-reported and end-of-life (EOL) outcomes among patients receiving targeted therapy.
Objectives.
This study aimed to compare quality of life (QOL), symptoms, prognostic communication, and EOL care between patients receiving targeted therapy and patients with lung cancer without targetable mutations.
Methods.
In this secondary analysis of a randomized trial of early palliative care in advanced lung cancer (n=154), we compared change in QOL and symptoms (per the Functional Assessment of Cancer Treatment [FACT]-Lung scale) over 24 weeks among patients with lung cancer receiving targeted therapy versus those without targetable mutations using linear mixed effects models, adjusted for receipt of palliative care, age and gender. We also compared prognostic communication and decedents’ EOL health care utilization using logistic regression, adjusted for palliative care.
Results.
Compared to individuals without targetable mutations, patients receiving targeted therapy (n=35) reported greater improvements in QOL (FACT-General B=0.46; 95% CI=0.19, 0.73) and symptoms (FACT-Lung Cancer Subscale B=0.12; 95% CI=0.03, 0.20) over time, independent of palliative care. Patients receiving targeted therapy were also more likely to report they rarely discussed prognosis with their clinicians (OR=2.59, 95% CI=1.01, 6.63) and were more likely to receive cancer-directed therapy in their last 14 days of life (OR=14.98, 95% CI=4.08, 54.96).
Conclusions.
Relative to patients without targetable mutations, patients with lung cancer who receive targeted therapy experience improved QOL and symptoms, are less likely to discuss prognosis early in their illness course, and more likely to continue treatment until death and die in the hospital.
Keywords: Lung cancer, targeted therapy, precision medicine, supportive oncology, oncogene-driven lung cancer, palliative care, end-of-life care, patient-reported outcomes
Background
Lung cancer is a heterogeneous disease comprised of distinct phenotypes with variable clinical behavior. In particular, lung cancer that is driven by targetable mutations in oncogenes such as epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), or c-ros oncogene-1 (ROS1), occurs more often in patients who are younger, female, Asian, and never or minimal smokers.1 These patients can receive oncogene-directed targeted therapies, which produce a more rapid response and higher response rate, have fewer side effects, and prolong progression-free survival relative to chemotherapy in patients with mutations in these genes.2 The experience of well-being among patients with lung cancer receiving targeted therapy is thus likely to be different from that of patients with lung cancer without driver mutations, yet direct comparisons of these populations are limited. In addition, how patients receiving targeted therapy communicate about their prognosis with their clinicians and their experiences of end-of-life (EOL) care have received limited attention.3
Patient-reported outcomes (PROs) provide an important window into patients’ experience of illness and treatment.4 Clinical trials of targeted therapy for patients with mutations in EGFR or ALK demonstrated improved quality of life (QOL) relative to chemotherapy.5,6 However, comparisons of QOL and symptom burden among patients with lung cancer who receive targeted therapy compared to patients without targetable mutations are lacking. Similarly, there are no comparisons of the EOL experience among patients with different lung cancer subtypes. A single-site retrospective cohort study demonstrated high rates of chemotherapy and hospitalization in the last month of life among patients with lung cancer with EGFR mutations, findings consistent with intensive EOL care.3
As treatment paradigms for lung cancer become ever more specific to molecular genetics, awareness of the corresponding variation in patient well-being and care experiences is essential to guide clinical decisions about how to provide optimal supportive care. Several randomized clinical trials have demonstrated that early palliative care integrated into oncology care from diagnosis improves QOL for patients with advanced cancer.7–10 Palliative care also improves patients’ understanding of their prognosis and decreases chemotherapy use at the end of life.11,12 Based on these findings, the American Society of Clinical Oncology recommends the early integration of palliative care for all patients with an advanced lung cancer diagnosis.13,14 Yet, these guidelines provide the same general guidance of early palliative care integration for all patients regardless of lung cancer subtype and treatment regimen. The palliative care needs of patients with lung cancer receiving targeted therapy, who live longer with fewer side effects and less treatment toxicity, have not yet been studied.
In this secondary analysis of a clinical trial of early integrated palliative and oncology care among patients with advanced lung cancer, we compared the QOL, symptom burden, illness understanding, and EOL health care utilization among patients who received targeted therapy compared to patients without targetable mutations. We also described the content of palliative care visits in this trial. We aimed to understand the differences between the experiences of illness and health care delivery of these patient subtypes to inform future development of tailored, patient-centered palliative care interventions.
Patients and Methods
Study Design
We conducted a secondary analysis of patients with advanced lung cancer enrolled in a randomized trial of early integrated palliative and oncology care at the Massachusetts General Hospital (MGH), the primary results of which have been previously reported (clinicaltrials.gov registration: NCT01401907).8 Patients were enrolled within eight weeks of diagnosis. Enrollment took place between May 2, 2011 and July 20, 2015.
Upon providing written informed consent, patients were randomized to receive palliative care integrated with oncology care or usual care. Patients in the intervention arm met with a palliative care clinician within four weeks of enrollment and monthly thereafter, with contact via telephone when in-person visits were not possible. Patients in both arms completed questionnaires prior to randomization, at 12 weeks, and at 24 weeks, or within three weeks of those time points. Palliative care clinicians reported the focus of clinic visits using a web-based data capture tool after each visit. They indicated which topics were discussed among a list of palliative care domains, such as symptom management and illness understanding.
Patient Selection
The trial included patients with advanced non-colorectal gastrointestinal cancer and lung cancer; this analysis includes only the subset of patients with nonsmall cell lung cancer (NSCLC). Patients were ≥ 18 years of age, were receiving their care at MGH, had no prior therapy, and had an Eastern Cooperative Oncology Group performance status of 0–2. Patients were excluded if they: had already been referred to palliative care or needed an immediate palliative care or hospice referral; could not read or understand English; or had significant psychiatric or cognitive impairment that impeded their ability to provide consent or participate in study activities.
Study Measures
We measured QOL and symptoms with the Functional Assessment of Cancer Treatment (FACT) – Lung scale, a measure that includes the 27-item FACT-General (FACT-G; assessment of physical, functional, emotional, and social well-being over past week; higher scores indicate better quality of life) quality of life measure and a 7-item Lung Cancer Subscale (LCS; assessment of common lung cancer symptoms over the past week; higher scores indicate lower symptom burden).15 Patients also completed the 14-item Hospital Anxiety and Depression Scale (HADS; includes anxiety and depression subscales HADS-Anxiety and HADS-Depression measuring mood symptoms over the past week; higher scores indicate greater anxiety or depression symptoms).16
To measure patients’ understanding of their prognosis and communication about prognosis with their clinicians, we used the Prognosis and Treatment Perceptions Questionnaire (PTPQ).17 For this analysis, we specifically focused on questions about prognostic communication and perception of treatment intent. Patients were asked, “How often have you had a conversation with your oncologist about the likely outcome of your cancer over time (i.e., your prognosis)?” We dichotomized responses into “never or rarely” and “sometimes, often or very often.” Patients were also asked, “If you had to choose one, what would you say is your primary goal of your current cancer treatment?” We reported whether patients chose the response “to cure my cancer” as a dichotomous variable, consistent with prior investigations.17,18 For each of these items, we included the last recorded assessment for each patient (from either 12 or 24 weeks), to minimize missing data due to progression-related loss to follow up.
Clinical Data
We extracted the following clinical data from the electronic health record (EHR): date of diagnosis, age, comorbid conditions (reported using the Charlson Comorbidity Index),19 performance status at study enrollment as documented by the treating oncologist, presence of brain metastases, and NSCLC mutation status. We also collected EOL health care utilization outcomes from the EHR, including hospitalizations and cancer-directed therapy use in the last month of life as well as hospice utilization and length of stay. We confirmed the date and location of death using the Social Security Death Index and EHR.
Statistical Analysis
We used descriptive statistics to summarize baseline clinical characteristics of the study cohort. We dichotomized the sample into two groups: patients whose tumors harbored driver mutations in EGFR, ALK, or ROS1 and received targeted therapy as first- or second-line treatment (“patients receiving targeted therapy”), versus patients whose tumors lacked these three mutations (“without targetable mutations”). We used Kaplan-Meier survival analysis to compare survival across groups.
To compare the change over time from baseline to 24 weeks in patient-reported outcomes (i.e., QOL, symptom burden, and anxiety and depression symptoms) across groups, we used linear mixed effect models that utilize maximum likelihood to impute missing data and incorporated random intercepts and random slopes. The models were adjusted for randomization to early palliative care, age, and gender; analyses included limited variables due to small sample size. We used logistic regression to compare patient report of treatment goal and frequency of prognostic conversations with clinicians, adjusted for receipt of palliative care. Additional covariates such as age and gender were not included in logistic models due to limited sample size.
For those patients who received the palliative care intervention, we described the proportion of visits that focused on each palliative care domain among the first six or fewer visits per patient. Clinicians could report more than one domain and more than one symptom per visit. Domains were selected based on National Consensus Project Clinical Practice Guidelines for Quality Palliative Care and a qualitative description of how these domains were interpreted by clinicians in this study has been previously reported.13,20 We limited the palliative care visit content description to the first six visits to correspond to the 24-week follow-up period for the self-report measures. For those visits in which clinicians reported addressing symptoms, we summarized the proportion of visits that focused on each symptom and used t-tests to compare across groups.
We used logistic regression to analyze the likelihood of hospice utilization, hospitalization and cancer-directed therapy in last 30 days of life, and death in the hospital among patients who died during the study or follow-up period, adjusted for receipt of palliative care. We also compared hospice length of stay using a nonparametric equality-of-medians test.
Results
Patient Characteristics and Clinical Course
The trial included 154 patients with NSCLC, of whom 35 (22.7%) had mutations in EGFR, ALK, or ROS1 and received targeted therapy. Baseline characteristics of these subgroups are detailed in Table 1. Patients receiving targeted therapy were younger and more likely to be female and have minimal or no smoking history than patients without targetable mutations. The most common mutation among the patients who received targeted therapy was in EGFR (74.3%). A small number of patients without targetable mutations received targeted therapy, reflecting historical use of targeted therapy among patients who were not chemotherapy candidates.21 The median overall survival from the time of diagnosis among patients receiving targeted therapy was 38.4 months compared to 12.2 months among patients without targetable mutations (p = 0.001).
Table 1.
Baseline Characteristics of Patients With Lung Cancer
| Receiving Targeted Therapy N = 35 | Without Targetable Mutations N= 119 | P | |
|---|---|---|---|
| Age in years, median (range) | 60.6 (31.4–83.6) | 65.5 (43.9–84.0) | 0.004 |
| Female gender, n (%) | 23 (65.7) | 58 (48.7) | 0.08 |
| Race/ethnicity, n (%) | |||
| White | 30 (85.7) | 107 (89.9) | 0.21 |
| American Indian/Alaskan Native | 0 | 2 (1.7) | |
| Asian | 3 (7.7) | 1 | |
| Black | 1 (2.9) | 4 (3.4) | |
| Hispanic | 0 | 1 (0.8) | |
| Other | 1 (2.9) | 4 (3.4) | |
| Smoking, n (%) | |||
| Never smoker/ <10 pack years | 27 (77.1) | 19 (16.0) | <0.001 |
| > 10 pack-years | 7 (20.0) | 98 (82.4) | |
| Comorbidity (CCI), mean (SD) | 6.6 (1.1) | 7.1 (1.2) | 0.05 |
| Performance status at enrollment, n (%) | |||
| 0 | 10 (28.6) | 25 (21.0) | 0.63 |
| 1 | 23 (65.7) | 85 (71.4) | |
| 2 | 2 (5.7) | 9 (7.6) | |
| Brain metastases at diagnosis, n (%) | 16 (45.7) | 35 (29.4) | 0.07 |
| Mutation, n (%) | |||
| EGFR | 26 (74.3) | - | |
| ALK | 7 (20.0) | - | |
| ROS1 | 2 (5.7) | ||
| Treatment regimen Chemotherapy | |||
| First line | 10 (28.6) | 111 (93.3) | |
| Second line | 11 (31.4) | 49 (41.2) | |
| Tyrosine kinase inhibitor (TKI) | |||
| First line | 24 (68.6) | 3 (2.5) | |
| Second line | 26 (74.3) | 2 (1.7) | |
| Lines of therapy, mean (SD) | 3.6 (2.1) | 2.2 (1.4) | 0.0001 |
| Randomized to receive palliative care | 23 (65.7) | 57 (47.9) | 0.06 |
Patient-Reported Outcomes and Understanding of Treatment Goals
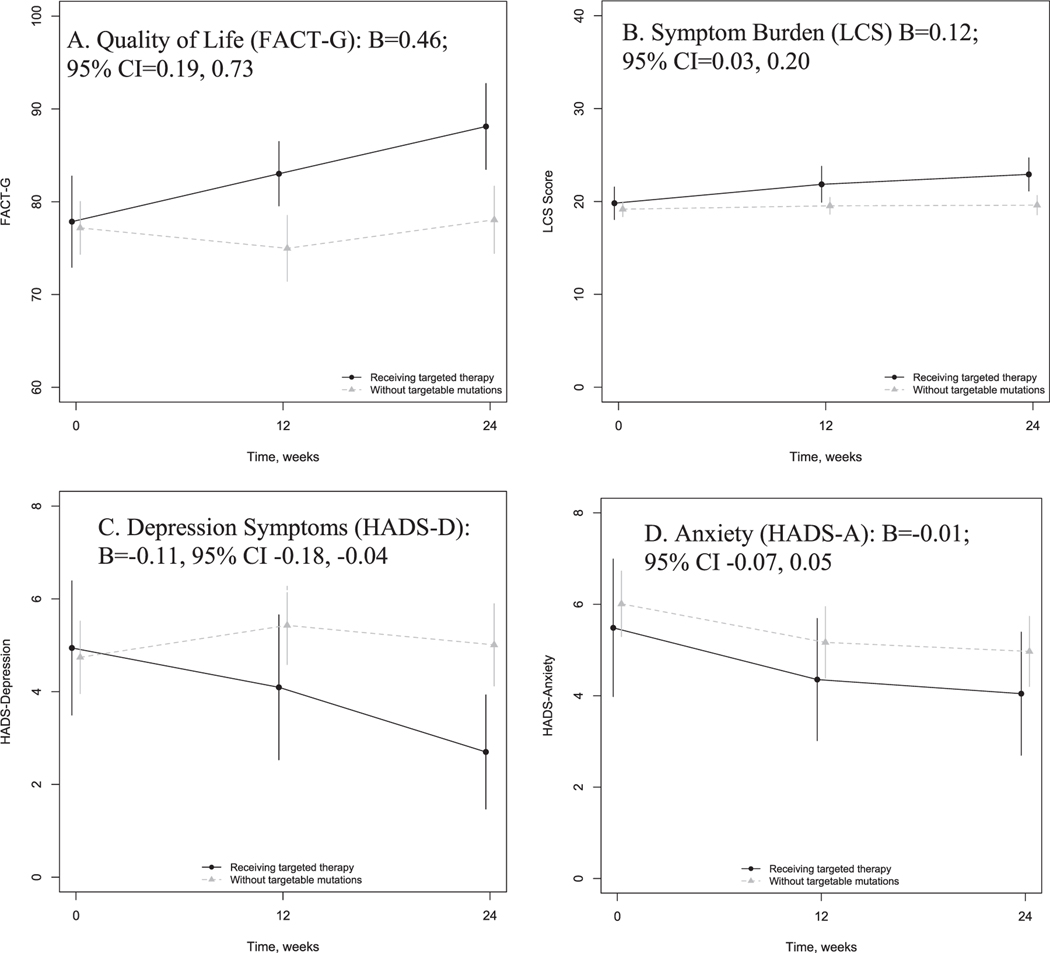
Patient-reported outcome measures at each time point are provided in Table 2 and depicted in Fig. 1. There was no difference in baseline scores and approximately a 10-point difference in FACT-G scores and 2.3-point difference in HADS-D scores across the groups at 24 weeks (Table 2). In linear mixed effects models using maximum likelihood to impute missing data, patients receiving targeted therapy reported significant improvements in QOL (FACT-G; B=0.46; 95% CI=0.19, 0.73), symptom burden (LCS; B=0.12; 95% CI=0.03, 0.20), and depressive symptoms (HADS-D; B=−0.11, 95% CI −0.18, −0.04) over time compared to patients without targetable mutations. Patient-reported anxiety symptoms did not differ significantly over time across groups (HADS-A; B=−0.01; 95% CI −0.07, 0.05).
Table 2.
Effect of Receiving Targeted Therapy on Patient-Reported Outcomes Among Patients With Lung Cancer
| Receiving Targeted Therapy N = 35 Mean, SE | Without Targetable Mutations N = 119 Mean, SE | P-value of Difference in Baseline Scores | |
|---|---|---|---|
| Quality of life –FACT-G | |||
| Baseline | 77.85, 2.42 | 77.18, 1.44 | 0.821 |
| 12 weeksa | 83.01, 1.70 | 74.95, 1.79 | |
| 24 weeksb | 88.11, 2.26 | 78.05, 1.82 | |
| Symptom burden –LCS | |||
| Baseline | 19.17, 0.41 | 19.81, 0.86 | 0.465 |
| 12 weeks | 21.85, 0.95 | 19.53, 0.45 | |
| 24 weeks | 22.92, 0.87 | 19.60, 0.52 | |
| Depression–HADS-D | |||
| Baseline | 4.94, 0.71 | 4.74, 0.39 | 0.806 |
| 12 weeks | 4.09, 0.77 | 5.43, 0.43 | |
| 24 weeks | 2.70, 0.60 | 5.01, 0.45 | |
| Anxiety –HADS-A | |||
| Baseline | 5.49, 0.74 | 6.01, 0.36 | 0.503 |
| 12 weeks | 4.35, 0.66 | 5.17, 0.39 | |
| 24 weeks | 4.04, 0.66 | 4.97, 0.39 |
At 12 weeks, n=32 in patients with NSCLC receiving targeted therapy; n=98 in patients with NSCLC without targetable mutations.
At 24 weeks, n=30 in patients with NSCLC receiving targeted therapy; n=75 in patients with NSCLC without targetable mutations.
Fig. 1.
Patient-reported outcome change over time among patients receiving targeted therapy and those with lung cancer without targetable mutations. Shown are unadjusted means and 95% confidence intervals at baseline, 12, and 24 weeks for (A) Quality of life, measured with the Functional Assessment of Cancer Therapy-General (FACT-G); (B) Symptom burden, measured with the Lung Cancer Subscale (LCS) of the FACT-Lung; (C) Depression, measured by the Hospital Anxiety and Depression Scale (HADS)-Depression subscale; and (D) Anxiety, measured by the HADS-Anxiety subscale. The unstandardized coefficient of the group-time interaction from linear mixed effects models for each patient-reported outcome are also shown, with 95% confidence intervals.
Patients receiving targeted therapy were more likely to report that they “never or rarely” discussed their prognosis with their oncologist than patients without targetable mutations (31.3% versus 15.6%; odds ratio [OR]=2.59, 95% confidence interval [CI] 1.01, 6.63). A greater proportion of patients receiving targeted therapy also reported that the intent of their treatment was curative compared to those without targetable mutations (40.6% versus 21.7%; OR 2.58, 95% CI 1.08, 6.02). For these outcomes, the last recorded assessment was at 24 weeks for 91% of patients receiving targeted therapy and 74% of patients without targetable mutations; for the remainder in each group the last available assessment was at 12 weeks.
Description of Palliative Care
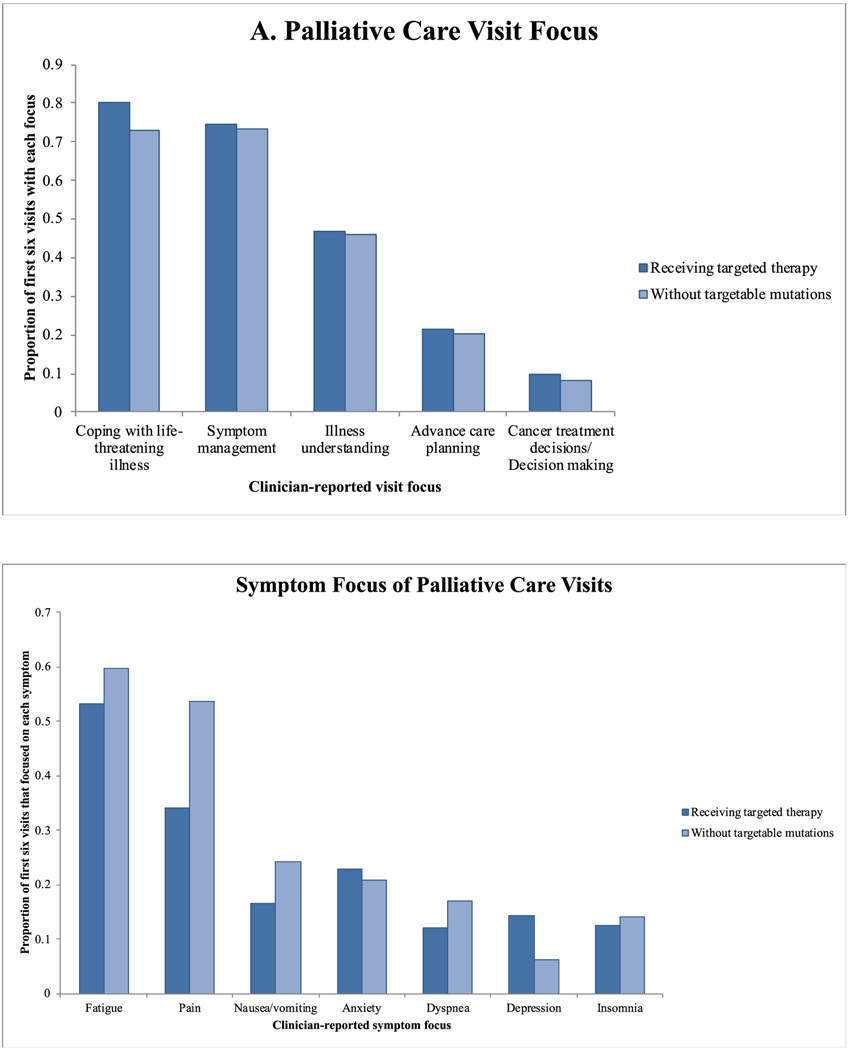
Overall, 80 patients (52.0%) were randomized to receive palliative care, including 23 patients receiving targeted therapy (14.9%). The focus of the first six palliative care visits among patients receiving targeted therapy was similar to that of visits among patients without targetable mutations, with the greatest emphasis on coping support and no differences between the groups (Fig. 2a). In addition, palliative care clinicians reported addressing symptoms of pain, nausea and vomiting, anxiety, dyspnea, depression, and insomnia for both groups of patients (Fig. 2b), with a greater proportion of visits focused on pain among the group without targetable mutations (53.6% versus 34.1%; p = 0.002) and more visits focused on depression among patients receiving targeted therapy (14.3% versus 6.2%; p = 0.02).
Fig. 2.
Overall (A) and symptom-specific (B) visit focus for palliative care visits among patients randomized to receive the palliative care intervention. The bars represent the proportion of the first six visits for each patient that focused on each topic. The focus of the visit was reported by clinicians after the visit. Clinicians could report more than one topic per visit.
End-of-Life Outcomes
Comparison of EOL outcomes among decedents (n = 127, including 24 patients receiving targeted therapy and 103 patients without targetable mutations) are detailed in Table 3. The overall proportion of patients who accessed hospice services was high (94/116 = 81.0%) and the likelihood of hospice use was similar across groups. Hospice length of stay (median 17 days in patients receiving targeted therapy vs. 19 days among patients without targetable mutations; p=0.69), and the likelihood of hospitalization in last month of life also did not differ between groups (Table 3). Patients receiving targeted therapy were significantly more likely to receive cancer-directed therapy in the last 14 days of life (OR 14.98, 95% CI 4.08, 54.96) and were more likely to die in the hospital (OR 4.36, 95% CI 1.25, 15.21) than patients without targetable mutations.
Table 3.
Decedents End-of-life Outcomes
| Receiving Targeted Therapy N (%) | Without Targetable Mutations N (%) | OR | 95% CI | P | |
|---|---|---|---|---|---|
| Patient received hospice services | 15/21 (71.4) | 79/95 (83.2) | 0.43 | 0.14, 1.36 | 0.152 |
| Hospitalization in last month of life | 12/20 (60.0) | 47/84 (56.0) | 1.55 | 0.54, 4.43 | 0.414 |
| Any cancer-directed therapy in last 14 days | 10/22 (45.5) | 5/94 (5.3) | 14.98 | 4.08, 54.96 | <0.001 |
| Cytotoxic chemotherapy in last 14 days | 3/22 (13.6) | 4/94 (4.3) | 3.01 | 0.59, 15.46 | 0.186 |
| Death in the hospital | 6/21 (28.6) | 10/94 (10.6) | 4.36 | 1.25, 15.21 | 0.021 |
Discussion
In this study, we demonstrate that patients with advanced lung cancer receiving targeted therapy experience improved QOL, symptom burden and depressive symptoms in the first 24 weeks after diagnosis relative to patients without targetable mutations, independent of the important contributions of palliative care to these outcomes. These findings are expected given the QOL improvements seen in efficacy trials of targeted therapy, yet the contrast to patients without targetable mutations is notable since these groups are effectively grouped together in recommendations for the provision of palliative care.5,6,14 Patients receiving targeted therapy also reported discussing their prognosis less often with their oncology team and were more likely to report that their treatment was curative than patients without targetable mutations in the first 24 weeks after diagnosis. Notably, patients receiving targeted therapy reported similar levels of anxiety over time as compared to patients without targetable mutations even as their QOL and symptoms improved. Finally, patients receiving targeted therapy were more likely to receive cancer-directed therapy in the last two weeks of life and die in the hospital compared to those without targetable mutations. Taken together, these findings demonstrate that patients receiving targeted therapy differ in their experience of illness and EOL health care utilization from patients without targetable mutations.
Achieving disease control to improve symptoms and QOL is a major goal of all palliative therapy for lung cancer in the metastatic setting. However, historically, the benefit of cytotoxic chemotherapy has been limited with significant toxicity for patients with advanced NSCLC.22–25 By contrast, targeted therapy with tyrosine kinase inhibitors is not only more effective than chemotherapy at achieving disease control for patients with targetable mutations, but also has fewer side effects that negatively impact patients’ QOL.5,26,27 Thus, patients receiving targeted therapy may not have the same palliative care needs as patients without targetable mutations in terms of symptom management, though this trial was not designed to answer that question and further research is needed.7
However, palliative care clinicians focus on other topics besides symptom management, including improving patients’ ability to cope with illness, helping them understand the expected outcome of their condition and preparing them for the EOL.28–30 Such support for coping and enhancing prognostic awareness affects patients’ QOL and impacts the type of EOL care they receive.12,29,31 Indeed, palliative care clinicians in this study reported that they focused on these topics for all patients, though additional research is needed to determine whether the nature and content of those conversations vary based on the treatment regimen patients receive. Further research to describe prognostic communication among patients receiving targeted therapy and their oncology clinicians is also warranted to understand opportunities to tailor palliative care integration and support.
In addition, we found that patients receiving targeted therapy had similar levels of anxiety symptoms as patients without targetable mutations, despite their improved quality of life, longer survival and perception of curative-intent treatment. This finding suggests that these factors may not be the only drivers of anxiety among patients with advanced cancer, and that other factors, such as coping with uncertainty, loss of control and ongoing reminders of cancer, may similarly affect patients regardless of whether they have effective therapy options.32 As treatment paradigms for lung cancer continue to evolve to include both targeted therapy and now immunotherapy, identifying the appropriate timing of prognostic communication and referral to palliative care, as well as the most beneficial areas of palliative care focus, will be important future research objectives.
Our group has previously demonstrated that patients who maintain inaccurate perceptions of prognosis within the first 24 weeks after diagnosis are more likely to receive chemotherapy near death.11 We observed that patients receiving targeted therapy did not discuss their prognosis as often as patients without targetable mutations with the first 24 weeks after their diagnosis. Of note, we lack information about later discussions, which is relevant given the markedly different survival across these groups. Patients with NSCLC who initially received targeted therapy also received cancer-directed therapy at the EOL more often than patients without targetable mutations. Chemotherapy in the last weeks of life is associated with increased physical and psychological distress as well as higher EOL health care utilization and thus is a National Quality Forum quality indicator of poor-quality care.23,31,33 Whether all cancer-directed therapy has the same effect on EOL outcomes as chemotherapy has not yet been extensively studied, but even less toxic treatments may prevent enrollment in hospice or increase death in the hospital when continued until the EOL.34 The findings from this study further underscore the need for clear communication about prognosis and treatment expectations early in the course of illness, as well as the elicitation of goals and values among patients receiving targeted therapy. For patients with effective treatment options like targeted therapy, it is appropriate to share in patients’ hope to gain good quality time from treatment, yet also important to offer them the opportunity to learn about how things will change when their cancer develops resistance to targeted therapy.35
This study is limited by small sample size and its design as a secondary analysis of a clinical trial to evaluate early integrated palliative care, rather than a prospective study to specifically compare outcomes and palliative care delivery among patients receiving targeted therapy versus without targetable mutations. Because of the difference in survival across groups, there was greater loss to follow-up due to death among patients without targetable mutations. Consequently, it is possible that this analysis underestimated true differences in patient-reported outcomes such as QOL and symptoms between these groups due to survival bias, since patients without targetable mutations who died during follow-up likely had worsening QOL and symptoms prior to death. The survival difference may have also affected patient reports of prognostic communication and treatment goal, since assessments occurred closer to death for patients without targetable mutations. In addition, this trial was conducted prior to approvals of immune checkpoint inhibitors, which are now part of first-line therapy for most patients with lung cancer without targetable mutations. Patients receiving these therapies likely have yet a different experience than patients receiving chemotherapy alone or targeted therapy, and thus their patient-reported outcomes are an avenue for future research. Finally, the trial was conducted at a single site where several specialists with expertise in lung cancer targeted therapy practice. Accordingly, the proportion of patients receiving targeted therapy in this study was higher than would be expected in community oncology care settings and may include patients who were more motivated to seek treatment at a tertiary care center, a possible explanation for the higher rate of cancer-directed therapy utilization at EOL.
Conclusion
Targeted therapy has transformed the experience of the subset of patients with advanced lung cancer and is a model of successful precision oncology. In this study, we demonstrated that patients receiving targeted therapy experience improved QOL, symptom burden, and depression symptoms in the first 24 weeks after diagnosis relative to patients without targetable mutations, independent of palliative care involvement. Yet, patients receiving targeted therapy were also less likely to report discussing their prognosis early in their cancer course and ultimately more likely to receive cancer-directed therapy at the EOL compared to patients without targetable mutations. These findings identify a potential role for palliative care to cultivate prognostic awareness among patients receiving highly effective therapy for life-limiting cancer in order to help them identify their goals and values and guide future treatment decisions.
Disclosures and Acknowledgments
The authors gratefully acknowledge the patients and palliative care clinicians who participated in the original clinical trial on which this study is based. The authors also acknowledge the support of an American Lung Association Clinical Patient Care Research Grant and an American Cancer Society Institutional Research Grant for this work.
This research received no specific funding/grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors declare no conflicts of interest.
Footnotes
Editorial Note: David Casarett, MD: This nice study offers a novel look at the impact on quality of life of increasingly common targeted cancer therapies.
Contributor Information
Laura A. Petrillo, Division of Palliative Care and Geriatrics, Boston, Massachusetts, USA; Massachusetts General Hospital, Boston, Massachusetts, USA.
Areej El-Jawahri, Division of Hematology and Oncology, Boston, Massachusetts, USA; Massachusetts General Hospital, Boston, Massachusetts, USA.
Emily R. Gallagher, Division of Hematology and Oncology, Boston, Massachusetts, USA; Massachusetts General Hospital, Boston, Massachusetts, USA.
Vicki A. Jackson, Division of Palliative Care and Geriatrics, Boston, Massachusetts, USA; Massachusetts General Hospital, Boston, Massachusetts, USA.
Jennifer S. Temel, Division of Hematology and Oncology, Boston, Massachusetts, USA; Massachusetts General Hospital, Boston, Massachusetts, USA.
Joseph A. Greer, Department of Psychiatry, Boston, Massachusetts, USA; Massachusetts General Hospital, Boston, Massachusetts, USA.
References
- 1.Sacher AG, Dahlberg SE, Heng J, et al. Association between younger age and targetable genomic alterations and prognosis in non-small-cell lung cancer. JAMA Oncol 2016;2:313–320. 10.1001/jamaoncol.2015.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim C, Liu SV. First-line EGFR TKI therapy in non-small-cell lung cancer: looking back before leaping forward. Ann Oncol 2019;30:1852–1855. 10.1093/annonc/mdz415. [DOI] [PubMed] [Google Scholar]
- 3.Bauman JR, Piotrowska Z, Muzikansky A, et al. End-of-life care in patients with metastatic lung cancer harboring epidermal growth factor receptor mutations. J Palliat Med 2016;19:1316–1319. 10.1089/jpm.2016.0180. [DOI] [PubMed] [Google Scholar]
- 4.Basch E The rationale for collecting patient-reported symptoms during routine chemotherapy. Am Soc Clin Oncol Educ Book 2014(34):161–165. 10.14694/EdBook_AM.2014.34.161. [DOI] [PubMed] [Google Scholar]
- 5.Chen G, Feng J, Zhou C, et al. Quality of life (QoL) analyses from OPTIMAL (CTONG-0802), a phase III, randomised, open-label study of first-line erlotinib versus chemotherapy in patients with advanced EGFR mutation-positive non-small-cell lung cancer (NSCLC). Ann Oncol 2013;24:1615–1622. 10.1093/annonc/mdt012. [DOI] [PubMed] [Google Scholar]
- 6.Shaw AT, Kim D-W, Nakagawa K, et al. Crizotinib versus Chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385–2394. 10.1056/NEJ-Moa1214886. [DOI] [PubMed] [Google Scholar]
- 7.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med 2010;363:733–742. 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 8.Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol 2016;35:834–841. 10.1200/JCO.2016.70.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of inpatient palliative care on quality of life 2 weeks after hematopoietic stem cell transplantation: a randomized clinical trial. JAMA 2016;316:2094–2103. 10.1001/jama.2016.16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakitas MA, Tosteson TD, Li Z, et al. Early versus delayed initiation of concurrent palliative oncology care: patient outcomes in the ENABLE III randomized controlled trial. J Clin Oncol 2015;33:1438–1445. 10.1200/JCO.2014.58.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Temel JS, Greer JA, Admane S, et al. Longitudinal perceptions of prognosis and goals of therapy in patients with metastatic non–small-cell lung cancer: results of a randomized study of early palliative care. J Clin Oncol 2011;29:2319–2326. 10.1200/JCO.2010.32.4459. [DOI] [PubMed] [Google Scholar]
- 12.Greer JA, Pirl WF, Jackson VA, et al. Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non–small-cell lung cancer. J Clin Oncol 2012;30:394–400. 10.1200/JCO.2011.35.7996. [DOI] [PubMed] [Google Scholar]
- 13.Ferrell BR, Temel JS, Temin S, et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2016. 10.1200/JCO.2016.70.1474. [DOI] [Google Scholar]
- 14.Hanna N, Johnson D, Temin S, et al. Systemic therapy forstage IV non–small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:3484–3515. 10.1200/JCO.2017.74.6065. [DOI] [PubMed] [Google Scholar]
- 15.Cella DF, Bonomi AE, Lloyd SR, et al. Reliability and validity of the functional assessment of cancer therapy—lung (FACT-L) quality of life instrument. Lung Cancer 1995;12:199–220. 10.1016/0169-5002(95)00450-F. [DOI] [PubMed] [Google Scholar]
- 16.Kroenke K, Spitzer RL, Williams JBW. The PHQ-9. J Gen Intern Med 2001;16:606–613. 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Jawahri A, Traeger L, Park ER, et al. Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer 2014;120:278–285. 10.1002/cncr.28369. [DOI] [PubMed] [Google Scholar]
- 18.Nipp RD, Greer JA, El-Jawahri A, et al. Coping and prognostic awareness in patients with advanced cancer. J Clin Oncol 2017;35:2551–2557. 10.1200/JCO.2016.71.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–1251. 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 20.Thomas TH, Jackson VA, Carlson H, et al. Communication differences between oncologists and palliative care clinicians: a qualitative analysis of early, integrated palliative care in patients with advanced cancer. J Palliat Med 2018;22:41–49. 10.1089/jpm.2018.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goss G, Ferry D, Wierzbicki R, et al. Randomized phase II study of gefitinib compared with placebo in chemotherapy-naive patients with advanced non-small-cell lung cancer and poor performance status. J Clin Oncol 2009;27:2253–2260. 10.1200/JCO.2008.18.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002;346:92–98. 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 23.Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol 2015;1:778–784. 10.1001/jamaoncol.2015.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Archer VR, Billingham LJ, Cullen MH. Palliative chemotherapy: no longer a contradiction in terms. Oncologist 1999;4:470–477. 10.1634/theoncologist.4-6-470. [DOI] [PubMed] [Google Scholar]
- 25.von Plessen C, Bergman B, Andresen O, et al. Palliative chemotherapy beyond three courses conveys no survival or consistent quality-of-life benefits in advanced non-small-cell lung cancer. Br J Cancer 2006;95:966–973. 10.1038/sj.bjc.6603383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sequist LV, Yang JC-H, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–3334. 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 27.Mok TS, Wu Y-L, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–957. 10.1056/NEJ-Moa0810699. [DOI] [PubMed] [Google Scholar]
- 28.Jacobsen J, Jackson V, Dahlin C, et al. Components of early outpatient palliative care consultation in patients with metastatic nonsmall cell lung cancer. J Palliat Med 2011;14:459–464. 10.1089/jpm.2010.0382. [DOI] [PubMed] [Google Scholar]
- 29.Jackson VA, Jacobsen J, Greer JA, et al. The cultivation of prognostic awareness through the provision of early palliative care in the ambulatory setting: a communication guide. J Palliat Med 2013;16:894–900. 10.1089/jpm.2012.0547. [DOI] [PubMed] [Google Scholar]
- 30.Greer JA, Jacobs JM, El-Jawahri A, et al. Role of patient coping strategies in understanding the effects of early palliative care on quality of life and mood. J Clin Oncol 2018;36:53–60. 10.1200/JCO.2017.73.7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright AA, Zhang B, Keating NL, et al. Associations between palliative chemotherapy and adult cancer patients’ end of life care and place of death: prospective cohort study. BMJ 2014;348. 10.1136/bmj.g1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Curran L, Sharpe L, Butow P. Anxiety in the context of cancer: a systematic review and development of an integrated model. Clin Psychol Rev 2017;56:40–54. 10.1016/j.cpr.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 33.National Quality Forum. Palliative and End-of-Life Care 2015–2016. NQF;2016. [Google Scholar]
- 34.Petrillo LA, El-Jawahri A, Nipp RD, et al. Performance status, survival, and end-of-life care in adults with non-small cell lung cancer (NSCLC) treated with immunotherapy. J Clin Oncol 2019;37(31_suppl):49. 10.1200/JCO.2019.37.31_suppl.49. [DOI] [Google Scholar]
- 35.Paladino J, Lakin JR, Sanders JJ. Communication strategies for sharing prognostic information with patients: beyond survival statistics. JAMA 2019;322:1345–1346. 10.1001/jama.2019.11533. [DOI] [PubMed] [Google Scholar]