Abstract
Mutations in the gene encoding hepatic nuclear factor 1-α (HNF1-α) cause a subtype of human diabetes resulting from selective pancreatic β-cell dysfunction. We have analyzed mice lacking HNF1-α to study how this protein controls β-cell-specific transcription in vivo. We show that HNF1-α is essential for the expression of glut2 glucose transporter and L-type pyruvate kinase (pklr) genes in pancreatic insulin-producing cells, whereas in liver, kidney, or duodenum tissue, glut2 and pklr expression is maintained in the absence of HNF1-α. HNF1-α nevertheless occupies the endogenous glut2 and pklr promoters in both pancreatic islet and liver cells. However, it is indispensable for hyperacetylation of histones in glut2 and pklr promoter nucleosomes in pancreatic islets but not in liver cells, where glut2 and pklr chromatin remains hyperacetylated in the absence of HNF1-α. In contrast, the phenylalanine hydroxylase promoter requires HNF1-α for transcriptional activity and localized histone hyperacetylation only in liver tissue. Thus, different HNF1-α target genes have distinct requirements for HNF1-α in either pancreatic β-cells or liver cells. The results indicate that HNF1-α occupies target gene promoters in diverse tissues but plays an obligate role in transcriptional activation only in cellular- and promoter-specific contexts in which it is required to recruit histone acetylase activity. These findings provide genetic evidence based on a live mammalian system to establish that a single activator can be essential to direct nucleosomal hyperacetylation to transcriptional targets.
Hepatic nuclear factor 1-α (HNF1-α) is an atypical homeodomain-containing protein which was first identified based on its ability to bind to critical regulatory cis elements present in the 5′-flanking region of liver-specific genes, such as the albumin, β-fibrinogen, and α1-antitripsin genes (9, 12, 18). It was initially regarded as a hepatic-specific transcriptional regulator but was later found to be expressed in other tissues, such as kidney, gut, and pancreas (4). More recently, heterozygous mutations in the gene encoding HNF1-α were identified as the most common cause of monogenic diabetes mellitus in humans (44). The discovery resulted from a positional cloning strategy with no clear a priori evidence for a role of HNF1-α in regulating glucose homeostasis (39). Interestingly, although HNF1-α is expressed in multiple tissues, the sole phenotypic abnormality identified to date in humans with mutations in the gene encoding HNF1-α is pancreatic β-cell dysfunction (4, 8). This suggests that HNF1-α must perform an essential cell-restricted role in regulating transcription of key genes in pancreatic insulin-producing cells.
Targeted disruption of the hnf1-α gene has been attained by two laboratories (23, 28). Although young heterozygous mutant mice do not have a distinct phenotype, hnf1-α homozygous null animals are small and have abnormal liver function, developing phenylketonuria due to the lack of hepatic transcription of phenylalanine hydroxylase (28, 29). These mice display glycosuria secondary to renal tubular dysfunction, and like humans with heterozygous mutations in the gene encoding HNF1-α, they develop overt diabetes (23, 30). Also in analogy to the human phenotype, nullizygous mice have severely blunted glucose-induced insulin secretion, resulting at least in part from abnormal aerobic glucose metabolism in pancreatic β-cells (14, 30). Nevertheless, the molecular defects which underlie this β-cell glucose sensing abnormality are not known. In fact, to date no distinct gene has been reported to be abnormally regulated in β-cells from mice lacking HNF1-α. However, in studies carried out with a clonal β-cell line, overexpression of HNF1 dominant-negative mutants (41, 42) has been shown to lead to a marked decrease in the mRNAs encoding insulin, the type 2 glucose transporter isoform, liver-type pyruvate kinase, aldolase B, 3-hydroxymethylglutaryl coenzyme A reductase, and mitochondrial 2-oxoglutarate. These genes represent strong candidates to explain β-cell dysfunction in mice and humans with mutations in the gene encoding HNF1-α. It is reasonable to assume that understanding the intimate molecular mechanisms involved in the transcriptional control of β-cell genes by HNF1-α could provide a rational basis to manipulate β-cell function in humans with mutations in the gene encoding HNF1-α or other forms of diabetes.
The molecular mechanisms involved in how HNF1-α controls transcription are still poorly understood. However, there is evidence which suggests that HNF1-α may be responsible for regulating the chromatin dynamics of its target genes (29, 31, 32). In hnf1-α−/− mice transcription of the phenylalanine hydroxylase gene (pah) is abolished in liver tissue, and this is associated with an abnormal methylation pattern of CpG islands and disappearance of nuclease hypersensitivity sites in the pah gene 5′-flanking region in hepatic cells (29). Furthermore, HNF1-α is necessary to elicit chromatin opening and transcription of the α-antitrypsin gene cluster in a hepatoma cell line in vitro (31). Although the molecular basis for these HNF1-α-dependent phenomena is still not understood, more recent work has shown that HNF1-α can interact with coactivator proteins which possess histone acetyltransferase (HAT) activity, such as CREB binding protein and P/CAF, and that this HAT activity is important for the potentiation of the effects of HNF1-α on a reporter minigene containing multimerized HNF1-α binding sites (32). An emerging notion derived from these studies is that HNF1-α could bind its cognate DNA element in target genes and then physically interact with HAT coactivator proteins and hence induce local conformational changes which ultimately lead to gene activation in keeping with the prevailing view, whereby histone acetylation is likely to be instrumental in transcriptional activity (for reviews, see references 34 and 43). It should be pointed out nevertheless that evidence that HNF1-α does, in fact, play a role in the acetylation of chromosomal histones is currently lacking.
In this study we have investigated the mechanisms involved in the control of β-cell-specific transcription by HNF1-α. This was done by using mice lacking HNF1-α (23) rather than by relying on cultured cell systems based on overexpression of transcription factors and episomal reporter plasmids. We first identified genetic targets which are dependent on HNF1-α exclusively in pancreatic β-cells, indicating that HNF1-α plays a β-cell-specific transcriptional regulatory role in vivo. Using chromatin immunoprecipitations, transcription factor occupancy and histone acetylation of the promoters of these β-cell genes and a known hepatic HNF1-α target were analyzed in the different tissues. The results revealed that promoter occupancy by HNF1-α in islets and liver tissue does not correlate with its requirement for gene expression. However, a close linkage was observed between tissue-specific HNF1-α-dependent gene activity and localized histone hyperacetylation. These findings provide for the first time evidence based on a live mammalian system to support the physiological relevance of the notion that a single activator can be essential to target HAT activity to promoters as a mechanism to induce transcription. Furthermore, they provide in vivo evidence to indicate that gene-specific regulation by HNF1-α is likely mediated by its ability to acetylate nucleosomal histone tails.
MATERIALS AND METHODS
Animal breeding and genotyping.
A colony of mice segregating a null hnf1-α allele on a C57BL/6J background was established locally. hnf1-α null mice were generated in the laboratory of Frank Gonzalez (National Institutes of Health, Bethesda, Md.) by Cre-loxP recombination and deletion of the first exon and have been previously described (23). Animals were kept in a controlled environment and genotyped by PCR coamplification of mouse β-globin and hnf1-α exon 1 sequences.
Pancreatic islet and hepatocyte isolation.
Four-week-old hnf1-α−/− mice and their age-matched heterozygote (+/−) and wild-type (+/+) siblings were anesthetized with urethane (15% solution, 1 ml/kg) and decapitated. Pancreatic islets were isolated by a modification of procedures previously described for rats (17). Briefly, a cannula was inserted in the main pancreatic duct and the pancreas was inflated with Hanks' balanced salt solution (HBSS) buffer containing 3 U of collagenase P (Roche) per ml. The pancreas was then removed and digested for 11 min at 37°C in a water bath with continuous agitation. The cell suspension was washed several times in cold HBSS–0.5% bovine serum albumin (BSA), and islets were purified by using a discontinuous gradient in which the lower phase was formed by a Histopaque mixture containing a 7:3 ratio of Histopaque 1077 and Histopaque 1119 (Sigma), and the upper phase was HBSS–0.5% BSA. The islet-enriched fraction was aspirated from the interface, washed three times in HBSS–0.5% BSA, and further purified by handpicking under a stereomicroscope.
Mouse hepatocytes were isolated by in situ collagenase digestion of the liver (2). A cannula was inserted in the portal vein and the liver was perfused sequentially with phosphate-buffered saline (PBS) supplemented with 0.5 mM EGTA, HBSS–0.5% BSA, and at least 20 ml of HBSS buffer containing 5 mM CaCl2 and 0.25 U of collagenase A (Roche) per ml at 37°C. The liver was then excised and transferred to a petri dish cooled on ice. Digestion was stopped by the addition of ice-cold HBSS–1% BSA. The liver was minced and filtered through a 100-μm-pore-size cell strainer (Falcon). The cell suspension was centrifuged at 50 × g for 1 min, and the supernatant was discarded. The pellet was resuspended in complete RPMI medium, and centrifugation was repeated two more times. The final pellet was resuspended in fresh RPMI and transferred to a petri dish.
RNA extraction and reverse transcription (RT)-PCR analysis.
Total RNA was extracted from 80 to 100 mg of liver, kidney, or duodenum tissue, or 25 to 100 isolated islets of individual wild-type and knockout mice, using TRIzol (Life Technologies, Inc.) according to the instructions of the manufacturer. Total RNA (120 ng for liver, kidney, and duodenum, 24 ng for islets) was heated at 70°C for 10 min in the presence of 10 ng of random primers (Promega) per μl and reverse-transcribed into cDNA in a 20-μl solution containing 3 mM MgCl2, 1 mM deoxynucleoside triphosphates, 10 mM dithiothreitol, and 200 U of SuperScript II reverse transcriptase (Life Technologies, Inc.). Reaction mixtures were incubated for 10 min at 25°C, 1 h at 42°C, and 10 min at 97°C. PCR was carried out with oligonucleotides designed to span an intron to detect genomic DNA contamination using Primerselect 4.0 (DNASTAR) software. Sequences and specific PCR amplification conditions are available upon request. Gene products of interest were coamplified with an internal control gene (β-actin or tbp). The cycling and reaction conditions were adjusted by performing test reactions at multiple cycles to ensure that the two products were in the exponential phase of amplification and showed similar amplification efficiencies. Amplification products were resolved on ethidium bromide-stained acrylamide gels, and low-exposure images were analyzed using ScionImage (Scion) software. To coamplify insulin I and II cDNAs, oligonucleotide primers with full homology to both mouse genes were designed, and the two amplification products were discriminated by selective restriction of the insulin II gene using BstEII. All reactions included a blank control (without cDNA) in addition to controls lacking reverse transcriptase.
Immunohistochemistry.
Mouse tissues were dissected, fixed in fresh 4% paraformaldehyde overnight, and then embedded in paraffin. Three-micrometer sections of paraffin-embedded tissue were deparaffinized in xylene, rehydrated through a serially diluted ethanol sequence, and heated in a microwave for 5 min in 0.01 M citrate buffer (this step was omitted when using glut2 antiserum). Sections were incubated for 30 min at room temperature in antibody diluent (DAKO Corporation) with 3% normal serum from the same species as the secondary antibody. The primary antibody (dilutions: insulin, 1:1,000; glucagon, 1:200; glut2, 1:200; and pdx1/idx1, 1:1,000) was added in DAKO antibody diluent and incubated overnight at 4°C. Slides were washed in PBS and incubated with the fluorescent-conjugated secondary antibodies (Alexa Fluor 488 and 546 from Molecular Probes and Cy3 from Jackson Immunoresearch) at a final dilution of 1:500 in DAKO antibody diluent for 45 min at room temperature. After being washed in PBS, the sections were mounted and coverslipped using ProLong Antifade mounting media (Molecular Probes). Images were collected by using a Leica TCS 4D confocal microscope and processed using Adobe Photoshop 5.0 (Adobe Systems). Guinea pig anti-insulin, rabbit anti-glut2, and rabbit anti-idx1 were kind gifts from Chris Van Schravendijk (Vrije Universiteit Brussel, Brussels, Belgium), Bernard Thorens (University of Lausanne, Lausanne, Switzerland), and Dr. Joel Habener (Howard Hughes Medical Institute, Massachusetts General Hospital, Boston, Mass.), respectively. Rabbit anti-glucagon was obtained from DAKO.
Formaldehyde cross-linking and immunoprecipitation of chromatin.
The formaldehyde cross-linking and immunoprecipitation of chromatin procedure was adapted from existing protocols for use in small amounts of cells isolated from mammalian tissues (5, 21, 26). Formaldehyde (37% HCHO–10% methanol stock solution; Merck) was diluted to 11% in a buffer containing 0.1 M NaCl, 1 mM EDTA, 0.5 mM EGTA, and 50 mM HEPES (pH 8.0) and added directly to freshly isolated cells maintained in culture medium (150 to 500 pancreatic islets or 2 × 106 hepatocytes), providing a final concentration of 1%. Fixation was allowed to proceed for 6 min at 22°C for acetylated histone immunoprecipitations or 10 min at 22°C followed by 20 min at 4°C for HNF1-α immunoprecipitations. Fixation was stopped by the addition of glycine to a final concentration of 0.125 M. Cells were collected by centrifugation, washed once in 1 ml of ice-cold PBS supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF) and protease inhibitors (Complete protease inhibitor cocktail; Roche), and swelled in 0.5 ml of 5 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) (pH 8.0), 85 mM KCl, 0.5% NP-40, 1 mM PMSF, and protease inhibitors for 15 min on ice. Approximately one volume of 400- to 625-μm glass beads (Sigma) was then added, and samples were incubated for 10 min on a vortexer at 4°C. Samples were separated from the glass beads by covering the upper end of the tube with a nylon mesh and centrifuging it inverted inside a 15-ml tube at 1,000 × g for 2 min. Pellets were resuspended in the same supernatant, transferred to a fresh 1.5-ml centrifuge tube, and pelleted again by microcentrifugation at 20,000 × g. Pellets were resuspended in 150 to 400 μl of sonication buffer (1% sodium dodecyl sulfate [SDS], 10 mM EDTA, 50 mM Tris-HCl [pH 8.1], 1 mM PMSF, and protease inhibitors) and incubated on ice for 15 min. Samples were then sonicated to an average length of 1,000 to 2,000 bp with a Bandelin sonifier for six 30-s pulses at microtip maximum output and microcentrifuged at 20,000 × g for 1 h. The efficiency of the fragmentation was always verified by purification of DNA and analysis on a 1% agarose gel stained with ethidium bromide, which typically yielded fragments spanning 500 to 2,000 bp (see Fig. 4A). The resulting supernatants were diluted 10 times in IP buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 167 mM NaCl, 16.7 mM Tris-HCl [pH 8.1], 1 mM PMSF, and protease inhibitors). This chromatin solution was precleared with 50% protein A Sepharose (Amersham Pharmacia Biotech) containing 0.1% BSA and 200 μg of sonicated salmon sperm DNA per ml for 30 min at 4°C. Precleared chromatin (500 μl, corresponding to 75 to 150 pancreatic islets or 300,000 hepatocytes) was incubated with 2 μg of affinity-purified rabbit polyclonal anti-acetylated histone H3 or H4 antibodies (Upstate Biotechnology, Inc.), 1 μg of mouse monoclonal anti-HNF1-α antibody (Transduction Laboratories), or 2 μl of preimmune rabbit serum (Jackson Immunoresearch) and was rotated at 4°C for 16 h. Two micrograms of rabbit anti-mouse immunoglobulin G (Jackson Immunoresearch) was added to the samples incubated with the anti-HNF1-α monoclonal antibody at least 1 h prior to ending the incubation. Samples were microcentrifuged at 20,000 × g for 5 min and transferred to fresh tubes prior to addition of 25 μl of protein A Sepharose containing BSA and sonicated salmon sperm DNA. Incubation was allowed to proceed for 2 h at 4°C, and the samples were then washed sequentially in low-salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl [pH 8.1], 150 mM NaCl), high-salt buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl [pH 8.1], 500 mM NaCl), LiCl buffer (0.25 M LiCl, 1% NP-40, 1% deoxycholate, 1 mM EDTA, 10 mM Tris-HCl [pH 8.1]), and three times in TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA). SDS was omitted in the wash buffers for the HNF1-α immunoprecipitated samples. Immune complexes were eluted by adding 400 μl of elution buffer (0.1 M NaHCO3, 1% SDS) and being rotated for 15 min at room temperature. Cross-links were reversed by the addition of NaCl to a final concentration of 200 mM, followed by incubation at 65°C for at least 4 h. Samples were then treated with 1 μg of proteinase K for 1 h at 45°C, extracted with phenol-chloroform, and ethanol-precipitated at −20°C overnight. The final pellets were resuspended in 25 μl of TE buffer (10 mM Tris-HCl [pH 7.5], 0.1 mM EDTA) and analyzed by PCR. Precipitated samples were left undiluted (islets) or diluted 1:3 (hepatocytes). Total input samples were diluted 1:20, 1:100, and 1:300 before PCR.
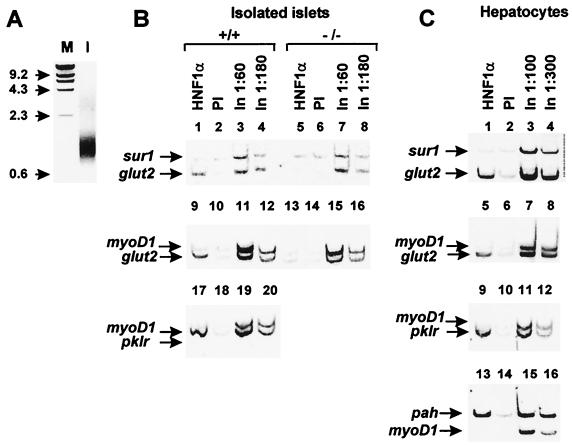
FIG. 4.
HNF1-α contacts the endogenous glut2 and pklr promoters in both pancreatic islets and hepatocytes in vivo. (A) Ethidium bromide-stained agarose gel indicating a representative example of sonicated DNA. M, molecular weight markers; I, sonicated islet DNA. (B and C) One hundred freshly isolated wild-type pancreatic islets or 3 × 105 hepatocytes were fixed with formaldehyde and immunoprecipitated using either anti-HNF1-α antibody or nonimmune serum. The precipitated samples were analyzed by duplex PCR using primers for the glut2, pklr, or pah promoter together with either the sur1 or myoD1 promoter as negative control. The HNF1-α immunoprecipitated sample is selectively enriched in glut2 promoter chromatin in wild-type but not hnf1-α−/− islets (lanes 1 and 5 and 9 and 13, panel B). HNF1-α also contacts glut2 promoter chromatin in hepatocytes (lane 1, panel C). The pklr exon 1 sequence is similarly enriched in HNF1-α immunoprecipitated from both islets and liver cells (lane 17, panel B, and lane 5, panel C). The pah promoter is occupied by HNF1-α in hepatocytes (lane 13, panel C). Only trace amounts of these promoter fragments were precipitated with preimmune serum (PI). Diluted input DNA (In) samples were assayed in parallel to illustrate the amplification profile when all promoter fragments are present in equimolar amounts. The data shown here represent at least two PCR amplification assays from two independent immunoprecipitations for islets and three for hepatocytes, yielding essentially identical results.
PCRs were performed in a 15-μl reaction volume containing 2 μl of immunoprecipitate or diluted input samples and primer mixtures designed to coamplify segments located in the transcription initiation site region of selected promoters. PCR conditions were adjusted to ensure nonsaturation kinetics and similar amplification efficiencies for all amplicons within a reaction mixture. Primer mix 1 consisted of glut2 promoter primers at 1 μM, pah promoter primers at 0.3 μM, and ins2 promoter primers at 0.6 μM. Mix 2 consisted of pah promoter primers at 0.3 μM and pklr exon 1 and ins2 promoter primers at 0.6 μM. Mix 3 consisted of glut2 promoter primers at 0.5 μM and sur1 promoter primers at 1 μM. Mix 4 consisted of glut2 promoter primers at 0.75 μM and myoD1 promoter primers at 1 μM. Mix 5 consisted of pah promoter primers at 0.2 μM and myoD1 promoter primers at 1 μM. Mix 6 consisted of pklr exon 1 primers at 0.5 μM and myoD1 promoter primers at 1 μM. Amplification conditions for multiplex PCR were 95°C for 30 s, 60°C for 30 s, and 68°C for 1 min for 27 cycles. PCR products were run on a 9% acrylamide gel, stained with ethidium bromide, and analyzed using ScionImage software (Scion). Oligonucleotide sequences of the primer pairs used are detailed in Table 1.
TABLE 1.
Primer sequences and locations relative to reported major transcription start sites corresponding to the PCR products used in the chromatin immunoprecipitation experiments
| Gene | Amplimer location | Primer sequence |
|---|---|---|
| glut2 | −19 to +123 | 5′CACTCTGGCTGGTCAGCTATTCAT |
| 5′TAGATTCCCAACCTCCTCAAAACC | ||
| pah | −133 to +60 | 5′CATTGCCAGGCCTGTCTGAGC |
| 5′GTTGCCCTGACGTAGCAGTGGA | ||
| ins2 | −227 to +2 | 5′AGGGCCCCTTGTTAAGACTCTAA |
| 5′ACTGGGTCCCCACTACCTTTAT | ||
| pklr | +11 to +139 | 5′TCTTGCAGCCCCAGTCCCACACTT |
| 5′CCTGGAGCCCCACTTAAAGCAGAC | ||
| sur1 | −326 to −101 | 5′TTGGGGGAAAGATCGGGACAG |
| 5′CGAGTGCGTGGATTTGGGATTC | ||
| myoD1 | −151 to +4 | 5′CCGCCCTACTACACTCCTATTG |
| 5′TGGTGAAGAAAGCAGTCGTGTC |
RESULTS
HNF1-α is not essential for insulin mRNA transcription.
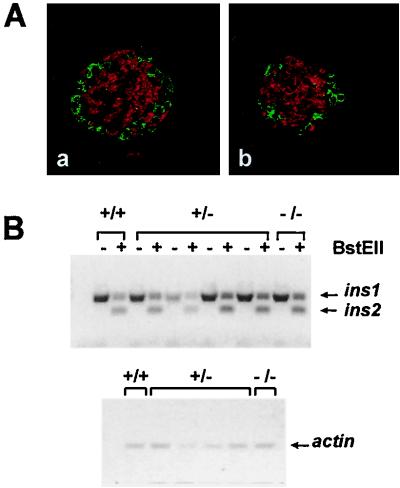
In keeping with previously reported data (23, 30), hnf1-α−/− mice develop mild diabetes at an early stage of postnatal life (data not shown). Immunofluorescence analysis of islet hormones in the pancreas of 2-week-old hnf1-α−/− mice showed normal insulin, glucagon, and somatostatin staining intensity, a normal density of insulin-positive and glucagon-positive cells per field, and a conserved distribution of glucagon-positive cells in the periphery and insulin cells in the core of islet structures (Fig. 1A and data not shown). Although immunofluorescence analysis did not suggest a major decrease in insulin content, previous studies performed with cultured cells suggested that HNF1-α may regulate insulin gene transcription (16, 42). We therefore sought to determine if a lack of HNF1-α resulted in a selective decrease of either insulin I or II mRNA in young animals which have still not developed overt diabetes. A single pair of oligonucleotides with 100% homology to the two nonallelic genes was designed to simultaneously amplify both sequences, and the gene products were distinguished by selective BstEII restriction of insulin II. Amplification at different cycle numbers revealed that the two sequences were being amplified at similar efficiencies. Under these conditions no major differences were encountered for insulin I and II mRNAs between purified islets from 4-week-old wild-type and null mice (Fig. 1B). This suggests that HNF1-α is not a major regulator of insulin gene transcription.
FIG. 1.
Microscopic islet structure and insulin mRNA content are not altered in pancreas tissue of hnf1-α−/− mice. (A) Representative paraffin-embedded pancreatic sections from 2-week-old control (a) and hnf1-α−/− (b) mice, immunostained with anti-insulin (red) and anti-glucagon (green) antisera. (B) Semiquantitative RT-PCR analysis of insulin mRNA levels in freshly isolated islets of 4-week-old control (+/+ and +/−) and hnf1-α−/− mice. Primers with full homology to the two mouse insulin genes were used for PCR. The two amplification products were distinguished by selective restriction of the insulin II gene with BstEII (upper panel). Insulin I and II mRNA content of hnf1-α−/− islets was not different from that of control islets. The same samples were simultaneously analyzed by RT-PCR for β-actin as a control for RNA loading (lower panel). PCRs were carried out at different numbers of cycles to ensure lineal amplification rates (not shown). These results are from a representative experiment from the analysis of four HNF1-α null mice, which yielded analogous results.
HNF1-α controls tissue-specific gene expression.
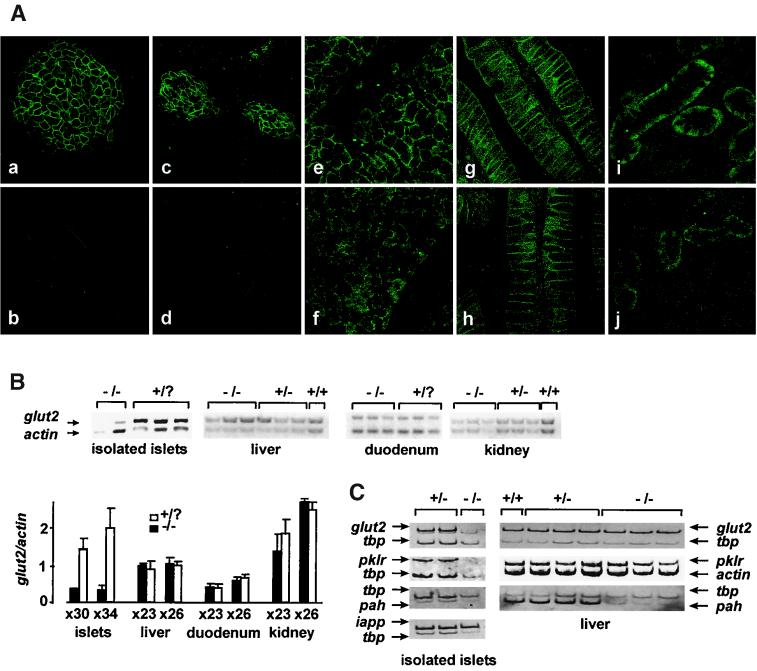
We next assessed the expression of the glut2 glucose transporter gene in hnf1-α−/− mice. glut2 mRNA has a very similar tissue distribution to that of hnf1-α (36). Immunofluorescence costaining with glut2 and insulin antisera revealed that β-cells from 2-week-old hnf1-α−/− mice have undetectable amounts of the sugar carrier (Fig. 2A). Similarly, RT-PCR from freshly isolated islets detected only trace amounts of the normally abundant glut2 RNA (Fig. 2B and C). This result was not due to differences in the purity of pancreatic islets between control and null mice, as shown by the analysis of islet amylin polypeptide, an islet-restricted transcript. To ensure that the reduction is not a secondary phenomenon resulting from diabetes, which is known to cause reduced expression of glut2 in islets (37), we analyzed pancreata from embryonic day 20 (E20) fetal hnf1-α−/− mice obtained from normoglycemic heterozygous mothers. These animals also displayed absent glut2 staining in islets (Fig. 2A).
FIG. 2.
HNF1-α is essential for glut2 expression in differentiated β-cells but not other tissues. (A) Paraffin-embedded sections were immunostained with glut2 antiserum (green) and costained with insulin to identify β-cells (not shown). Pancreatic glut2 expression was readily apparent and restricted to β-cells in 2-week-old wild-type mice (a) but was absent in hnf1-α−/− mice (b). HNF1-α dependence of glut2 expression is already apparent in prenatal pancreas at E20 (c and d). However, no significant decrease in glut2 expression was detected in null mouse liver (f), duodenum (h), and kidney (j) tissues as compared with their respective wild-type controls (e, g, and i). (B) glut2 mRNA content was analyzed by semiquantitative multiplex RT-PCR from tissues of 4-week-old mice. Coamplification of actin and glut2 shows a marked decrease in glut2 mRNA content in hnf1-α−/− mouse purified islets but not in liver, duodenum, and kidney tissues (upper panel). The bar chart below shows a densitometric analysis comprising the results of two independent experiments. The experiment was performed at two different cycle numbers (23 and 26, or 30 and 36, as indicated under the bar graph) to ensure similar amplification rates for both products. (C) The fall of glut2 expression in islets parallels that of pklr and mirrors the loss of pah expression in liver tissue. Semiquantitative multiplex RT-PCR analysis was performed as described above for isolated islets and liver tissue from wild-type and hnf1-α-null mice. Coamplification with tbp as an internal control shows that both glut2 and pklr mRNA levels were decreased in hnf1-α−/− islets but were unaffected in liver tissue. pah showed a marked reduction of expression in liver tissue but no major decrease in islets from mutant mice. Islet amyloid polypeptide (iapp) mRNA levels were unaffected in hnf1-α−/− islets, indicating a comparable islet purity of the different samples.
As expected, glut2 staining was also readily observed in wild-type liver, kidney, and duodenum tissue. Surprisingly, hnf1-α mutants showed no clear reduction of glut2 expression in these organs (Fig. 2A). Likewise, glut2 mRNA levels were not decreased in liver, duodenum, and kidney tissue of hnf1-α−/− mice (Fig. 2B). Thus, HNF1-α is required for expression of the glut2 gene in insulin-producing cells but not in other tissues which coexpress HNF1-α and glut2.
We next assessed whether tissue-specific dependence on HNF1-α could be extended to other HNF1-α targets. The L-type pyruvate kinase gene (pklr) is a key glycolytic enzyme whose tissue distribution also partially overlaps that of hnf1-α and glut2 (13, 25). Furthermore, it has been shown to be regulated by HNF1-α in cell lines (13, 25, 41), thus constituting a suitable in vivo HNF1-α candidate target gene. In analogy to the results obtained for glut2 gene expression, pklr mRNA levels were dramatically decreased in pancreatic islets but not in liver tissue of hnf1-α−/− mice (Fig. 2C). We also analyzed tissue-specific, HNF1-α-dependent expression of a gene which has already been previously shown to be entirely dependent on HNF1-α for its expression in liver tissue, phenylalanine hydroxylase (pah). Our experiments confirmed previous findings that the gene is nearly extinguished in liver tissue in the absence of HNF1-α (Fig. 2C) (28) and extend these in showing that pah, normally expressed at substantially lower levels in pancreas tissue (24), is not significantly reduced in islets from hnf1-α−/− mice (Fig. 2C). In summary, these results show that HNF1-α is essential for glut2 and pklr expression only in islet cells, whereas it is required for pah expression specifically in the liver. This indicates that different HNF1-α target genes establish distinct tissue-specific requirements for HNF1-α.
Lack of glut2 expression in β-cells of hnf1-α−/− mice is not a consequence of reduced expression of pdx1.
In light of the tissue-specific dependence of glut2 expression on HNF1-α, we hypothesized that cell-specific factors which regulate glut2 could act as downstream β-cell targets of HNF1-α. pdx1/idx1 (also known as IPF1 or stf1), the product of a gene which is also capable of causing early-onset monogenic diabetes (33), is a potential candidate intermediary target, as this protein has been shown to regulate glut2 in different experimental models (1, 40). However, immunofluorescence analysis indicated that β-cells from young hnf1-α null mice who have not yet developed full-blown diabetes have intense pdx1/idx1 nuclear staining (Fig. 3A) and do not display significant changes in pancreatic islet pdx1/idx1 mRNA content relative to control littermates (Fig. 3B).
FIG. 3.
Loss of glut2 is not associated with decreased expression of pdx1. (A) Paraffin-embedded pancreatic sections immunostained with anti-pdx1 (green color, a and b) antiserum do not display differences among hnf1-α−/− (b) and control (a) 2-week-old mice. Samples were coimmunostained for insulin to detect β-cells (shown in red). (B) Semiquantitative multiplex RT-PCR analysis was performed for freshly isolated islets to compare the mRNA levels of pdx1 in three wild-type and three null mice. Results were analyzed by densitometry, yielding pdx1/β-actin ratio values of 0.60 ± 0.1 versus 051 ± 0.07 arbitrary units (not significant).
HNF1-α directly interacts with glut2 and pklr promoter chromatin templates in both liver and islet cells.
We next sought to determine whether tissue-specific dependence on HNF1-α for gene activity correlated with differences in the ability of HNF1-α to effectively bind to the endogenous glut2 promoter chromatin in pancreatic islets and liver. Occupation of the glut2 promoter by HNF1-α was assessed by in vivo cross-linking and sonication of chromatin from freshly isolated islets and hepatocytes (Fig. 4A), followed by immunoprecipitation of HNF1-α-bound chromatin. Immunoprecipitates were then analyzed by PCR for enrichment of promoter segments cross-linked to HNF1-α. To ensure that results correspond to promoter-selective enrichment as opposed to random fluctuations in the efficiency of the PCR or variability in the amount of unselected DNA, semiquantitation of specific DNA fragments was carried out by PCR coamplification of a control promoter segment and nonsaturation cycling conditions. A threefold dilution of input DNA was amplified in parallel to assess the expected amplification pattern when coamplified gene segments are present in equimolar amounts as well as to ascertain nonsaturation amplification kinetics. Results were also compared to an immunoprecipitation with preimmune serum. These precautions are critical given that the small amount of tissue material used to analyze chromatin from isolated mouse pancreatic islets is orders-of-magnitude lower than that commonly used in chromatin immunoprecipitation procedures (5, 21, 26). As shown in Fig. 4, immunoprecipitations with anti-HNF1-α from pancreatic islet and hepatocyte chromatin resulted in a selective enrichment of glut2 promoter DNA relative to the results obtained with preimmune serum. Furthermore, as compared to the internal control amplification product in each reaction mixture, the relative amount of glut2 DNA in the immune serum sample markedly differs from that observed when amplifying input DNA (Fig. 4B and C). Thus, in contrast to what was observed for glut2 DNA, neither the sulfonylurea receptor promoter (sur1), which regulates a β-cell gene which is not modified in HNF1-α-deficient mice and does not have a consensus HNF1-α binding site (data not shown), nor the myoD1 promoter (myoD1), which regulates a gene which is not expressed in islets or liver tissue, was enriched in the precipitated sample in either tissue (Fig. 4B and C). As a further control to assess the specificity of the experiment, hnf1-α−/− islets were also analyzed to confirm that the observation of glut2 DNA immunoprecipitated with anti-HNF1-α is indeed specifically dependent on the presence of HNF1-α (Fig. 4B). Likewise, a pklr 5′ genomic sequence was enriched in the HNF1-α immunoprecipitates from pancreatic islets and hepatocytes compared with the myoD1 promoter (Fig. 4B and C). These data indicate that HNF1-α occupies the 5′-flanking region of the glut2 and pklr genes in both islets and liver. The phenylalanine hydroxylase promoter was also clearly enriched in liver chromatin immunoprecipitated with anti-HNF1-α immunoglubulins (Fig. 4C), while only weak association with HNF1-α could be seen in pancreatic islet chromatin (data not shown).
Therefore, HNF1-α is not acting exclusively through intermediary transcriptional regulators forming part of a transcriptional cascade. Furthermore, the observed differences in tissue-specific requirements for HNF1-α for the expression of glut2 and pklr cannot be explained by the existence of hnf1-α promoter occupancy in only certain cell types.
Tissue-specific HNF1-α-dependent gene expression correlates with the effects of HNF1-α on localized nucleosomal hyperacetylation.
We then initiated studies to assess the mechanisms involved in HNF1-α-dependent transcription of tissue-specific target genes. Experiments were designed to address the possible role of gene-selective nucleosomal histone acetylation in transcriptional activation by HNF1-α in vivo. We hypothesized that while HNF1-α interacts with target promoters in diverse tissues, its presence may be required to recruit HAT activity-containing coactivators exclusively to promoters that display HNF1-α-dependent transcription. Chromatin immunoprecipitations from mouse tissues were carried out with antisera recognizing histone H3 acetylated at lysines 9 and 14 (AcH3) and tetraacetylated histone H4 (AcH4). Selective enrichment of specific promoters in the hyperacetylated chromatin fraction was tested by multiplex PCR. In particular, 5′ genomic segments corresponding to ins2, pah, glut2, and pklr were tested, four genes for which we have established distinct tissue-specific expression patterns in wild-type and hnf1-α-deficient mice (see Fig. 1 and 2).
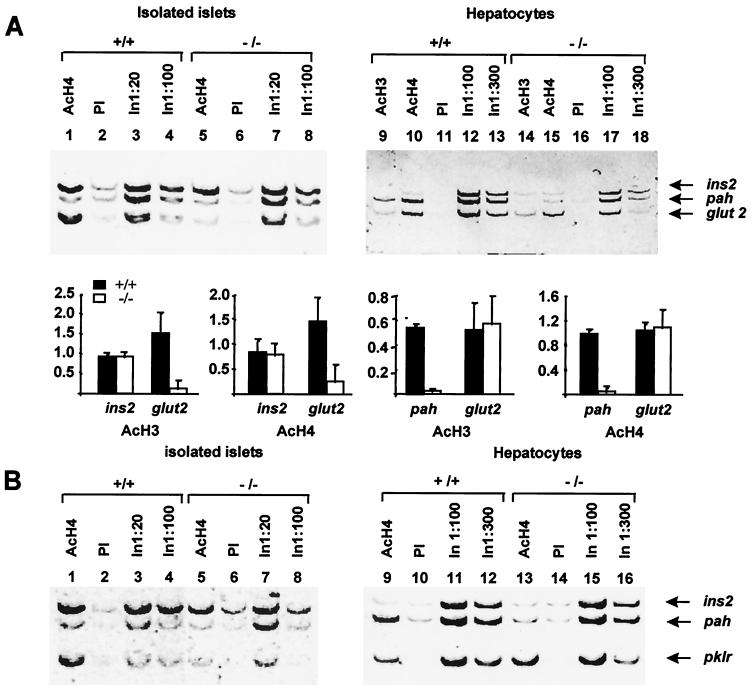
Analysis of hepatocytes from wild-type mice revealed enrichment of glut2 and pah promoter DNA in the AcH3 and AcH4 immunoprecipitates but not of insulin 5′-flanking DNA (Fig. 5A, lanes 9 and 10). Amplification of multiple dilutions of input DNA confirmed that all three amplicons were being amplified at comparable efficiencies when the target sequences were present at equimolar amounts (Fig. 5A, lanes 12 and 13). These findings indicate that insulin gene promoter chromatin is hypoacetylated in liver tissue, whereas transcriptionally active glut2 and pah promoter chromatin is hyperacetylated. In hnf1-α−/− hepatocytes glut2 promoter nucleosomes appeared to be hyperacetylated to an extent similar to that in wild-type cells (Fig. 5A, lanes 14 and 15). Similarly, pklr exon 1 showed high levels of histone H4 hyperacetylation in normal mice (Fig. 5B, lane 9) as well as in hnf1-α mutants (Fig. 5B, lane 13). However, histone acetylation of the pah 5′-flanking region was drastically reduced (Fig. 5A, lanes 14 and 15). Thus, HNF1-α-dependent histone acetylation results closely paralleled the observation that in liver tissue, pah but not glut2 and pklr expression is altered in the absence of HNF1-α.
FIG. 5.
HNF1-α is essential for nucleosomal hyperacetylation of its tissue-specific transcriptional targets. Chromatin immunoprecipitation assays using anti-acetylated histone H3 and H4 antibodies were performed with wild-type (+/+) and hnf1-α−/− pancreatic islets and hepatocytes. The precipitated samples were analyzed by multiplex PCR using primers for the insulin (ins2), pah, pklr, and glut2 promoters. (A) Anti-acetylated histone H4 selectively precipitated ins2 and glut2 promoter DNA but not pah DNA in islets from wild-type mice (lane 1). In hnf1-α−/− islets anti-histone H4 immunoprecipitate was depleted of glut2 DNA with no major changes in ins2 and pah (lane 5). Wild-type hepatocytes showed high levels of histone H3 and H4 acetylation of pah and glut2 promoter chromatin but not of the insulin II promoter (lanes 9 and 10). In liver from mice lacking hnf1-α, pah chromatin was hypoacetylated while glut2 acetylation was unaffected (lanes 14 and 15). PI, preimmune serum; In, input DNA. The bar chart shows the densitometric analysis of the chromatin immunoprecipitation experiments described. Values for each promoter are densitometry results of PCR products expressed as the following equation: (immune serum − PI)/input DNA. (B) Anti-acetylated histone H4 selectively precipitated pklr chromatin in islets of wild-type but not hnf1-α−/− mice (lane 1 versus lane 5). In contrast, pklr chromatin remained acetylated in liver tissue in the absence of HNF1-α (lane 9 versus lane 13). The data represent two to three independent immunoprecipitations with at least two PCR experiments from each, yielding essentially identical results, except for anti-AcH3 and pklr in islets, which represent two PCRs from a single immunoprecipitation.
In wild-type pancreatic islets, immunoprecipitation with anti-AcH4 and anti-AcH3 showed enrichment of glut2 and insulin promoter sequences relative to preimmune serum (Fig. 5A, lane 1 versus lane 2). pklr exon 1 was also clearly enriched in the AcH4 immunoprecipitated samples (Fig. 5B, lane 1 versus lane 2). In hnf1-α−/− mice glut2 promoter DNA and pklr exon 1 were selectively depleted from the acetylated histone immunoprecipitates, whereas the insulin II gene promoter was not significantly modified (Fig. 5A and B, lane 5). These results indicate that HNF1-α is necessary for histone hyperacetylation of glut2 and pklr promoter chromatin in islets. Thus, HNF1-α is required for the acetylation of nucleosomes of target promoters exclusively in those tissues in which it is indispensable for gene activity.
DISCUSSION
HNF1-α plays an essential role in tissue-specific gene expression.
Human genetic studies have revealed that heterozygous mutations in the gene encoding HNF1-α cause pancreatic β-cell dysfunction, with no conspicuous phenotype in other tissues where HNF1-α is expressed (8, 44). This finding strongly suggests that HNF1-α plays a previously unsuspected but crucial role in β-cell-specific transcription. We intend to understand how this is accomplished in the context of whole animal models. Our first step was thus to search for pancreatic islet-specific genetic targets of HNF1-α by analyzing the expression of candidate genes in hnf1-α null mice. We initiated this analysis with genes for which studies performed in cultured cell lines indicated that HNF1-α is able to regulate its expression (16, 42). Our studies indicated that in mice, glut2 glucose transporter gene expression is fully dependent on HNF1-α in pancreatic β-cells but not in other tissues which normally coexpress both genes, including liver, gut, and kidney tissue. Furthermore, glut2 expression already requires HNF1-α in insulin-producing β-cells immediately prior to birth, thus suggesting that glut2 silencing is not secondary to the development of diabetes in the adult mouse. Studies performed with E12.5 embryonic pancreas cells suggest that HNF1-α dependence for glut2 expression is not present in early pancreatic epithelial cells (M. A. Maestro and J. Ferrer, unpublished results), suggesting that it may represent a feature of mature insulin-producing cells. Tissue-specific dependence on HNF1-α is not restricted to the glut2 gene. Thus, HNF1-α is required for the expression of the liver-type pyruvate kinase gene (pklr) in pancreatic islets but not in liver cells, and this is mirrored by the liver-specific dependence on HNF1-α for the expression of pah. Thus, HNF1-α plays tissue-specific transcriptional regulatory roles in both pancreatic islets and liver tissue.
This study has focused on the glut2 and pklr promoters as tools to study β-cell-specific transcriptional regulation by HNF1-α. Therefore, the role that the loss of glut2 and pklr gene expression can play in the β-cell secretory defects present in hnf1-α−/− mice has not been specifically addressed here. Inactivation of glut2 in mice results in a severe diabetic phenotype ascribed to loss of glucose-sensing capabilities secondary to defective β-cell glucose transport (19), although the defect can be rescued by reexpression of very small amounts of this carrier (35). The glucose transport capacity of islets of hnf1-α−/− animals was not quantified in our study to assess if it is likely to be rate limiting for glycolytic flux, but detailed analysis of stimulus-secretion coupling in islet cells of hnf1-α−/− mice suggests the existence of defective glycolytic metabolism distal to glyceraldehyde-3-phosphate (14), while analysis in clonal β-cells overexpressing dominant-negative HNF1 revealed both glycolytic and mitochondrial defects (41, 42). Both types of evidence argue against glucose uptake being the sole cause of abnormal glucose sensing. On the other hand, while pyruvate kinase plays a key role in aerobic glucose metabolism, null mutations of the L-type pyruvate kinase gene in humans result in hemolytic anemia but not diabetes (3). The data presented here, along with recently reported studies, clearly point to the existence of pleiotropic effects of inhibition of HNF1-α function (14, 30, 41, 42). Thus, defective glucose-stimulated insulin release in HNF1-α-deficient mice is likely to result from an aggregate β-cell gene expression defect rather than from a single target abnormality. If the primary role of HNF1-α is to regulate a genetic program involved in glucose sensing, which represents a highly specialized function of differentiated β-cells, the glut2 and pklr genes can be regarded as paradigms to dissect the intimate mechanisms involved. The observation that HNF1-α targets have tissue-specific requirements for HNF1-α is conceptually relevant to the development of diabetes in humans with heterozygous mutations in the gene encoding HNF1-α (8). Thus, selective pancreatic β-cell dysfunction could indicate that a genetic program required for glucose sensing in human β-cells is vulnerable to HNF1-α haploinsufficiency.
We were unable to elicit an analogous dependence on HNF1-α for the expression of insulin I and II mRNA. This suggests that HNF1-α is not essential for insulin gene expression in mice, and therefore lack of insulin gene transcription is unlikely to be a major factor in the pathophysiology of HNF1-α-deficient diabetes in humans. Previous studies based on the overexpression of HNF1-α in cultured cells showed transactivation of rat insulin I promoter reporter minigenes, while expression of high concentrations of dominant-negative HNF1 mutants results in decreased insulin mRNA in cultured tumor β-cell lines (16, 41, 42). One potential explanation to reconcile these experimental results with the data presented here is that at the endogenous concentrations present in mice, HNF1-α plays no role at all in the regulation of chromatinized insulin gene templates from mature native β-cells. However, taken together, the data are also consistent with other explanations, such as the existence of islet-specific redundant mechanisms which are inhibited by the dominant-negative mutant experiments but which remain intact in the HNF1-α null mice, the possibility that HNF1-α-dependent insulin transcription represents a minor fraction of the total gene activity which is not reflected in our assays of mRNA abundance and histone acetylation, or that it is involved in physiological regulatory conditions not examined here. This scenario is not entirely unlike that encountered previously with liver HNF1-α targets. The mRNA content of some genes previously thought to be regulated by HNF1-α, such as the phosphoenolpyruvate carboxy kinase (PEPCK) and albumin genes, was described as either normal (for PEPCK) or only slightly reduced (for albumin) in hnf1-α−/− mice (28). This consideration emphasizes the value of recognizing glut2 and pklr as major β-cell HNF1-α targets in a null mutant animal model as opposed to studies based exclusively on cultured cell systems.
HNF1-α regulates glut2 expression in pancreatic β-cells by a direct mechanism.
One model to explain the cellular specificity of HNF1-α in regulating gene expression in β-cells is that it is achieved indirectly through the regulation of cell-specific factors acting downstream of HNF1-α. Studies performed in hepatoma cell lines suggest that the transcription of HNF1-α in hepatocytes may be controlled by HNF4-α (20), while both appear to be positioned downstream of HNF3-α and HNF3-β in an endodermal regulatory cascade (15). Mutations in the gene encoding HNF4-α in humans result in a very similar β-cell phenotype to those with mutations in the gene encoding HNF1-α (7), supporting the possibility that both are indeed involved in a common regulatory circuit which is likely operative in pancreatic β-cells. It is conceivable that such a transcriptional regulatory circuit may incorporate as-yet unidentified cell-specific factors in different cell types. We hypothesized that pdx1/idx1 could represent such a factor given that pdx1/idx1 has been shown to transactivate glut2 promoter minigene constructs (40), β-cell-specific inactivation of this gene results in β-cells which express insulin but not glut2 (1), and humans with heterozygous mutations in this gene also have a monogenic diabetic phenotype (33). Moreover, the murine pdx1 5′-flanking region reported in the GenBank database contains a perfect HNF1-α binding consensus site (data not shown). Our studies, however, show that there is not a major decrease in pdx1/idx1 content in mice with disruption of the hnf1-α gene. Although functional interactions between pdx1/idx1 and HNF1-α in regulating the glut2 gene need to be investigated, these results argue against a simple transcriptional hierarchy model, in which pdx1 is located dowstream of hnf1-α, to explain the cell-specific effects of HNF1-α on the glut2 gene.
An alternate model is that HNF1-α regulates glut2 transcription at least in part through direct interactions with the glut2 promoter. HNF1-α is able to bind in vitro to conserved AT-rich elements present on the rat and murine glut2 promoters and to stimulate transcription (10; M. Parizas, M. A. Maestro, and J. Ferrer, unpublished data). However, this does not necessarily predict the situation observed in the in vivo chromatin template context. A related issue is whether in live cells HNF1-α could effectively establish interactions with target promoters selectively in tissues where it is required for gene activity. We have been able to address these types of questions for the first time in a whole-animal model by implementing a DNA cross-linking and chromatin immunoprecipitation procedure to study transcription factor occupancy in different mouse tissues. The data presented here indicate that HNF1-α occupies the glut2 and pklr promoters in pancreatic islets, where it acts as an indispensable transactivator for these two genes, as well as in the liver, where it is not required. This suggests that different tissue-specific promoter contexts determine that HNF1-α is essential for glut2 and pklr transcription only in pancreatic β-cells.
Differences in HNF1-α-dependent nucleosome hyperacetylation, rather than in vivo binding, explain the tissue-specific requirements for HNF1-α to regulate glut2 and pklr.
Gene activity has been correlated with hyperacetylation of lysine residues at the N-terminal tail of histones H3 and H4 located in nucleosomes spanning transcriptionally active loci (for a review, see reference 34). The studies presented here reveal that in mice, HNF1-α is required to maintain hyperacetylation of histones H3 and H4 of the glut2, pklr, and pah promoters exclusively in those tissues in which it is essential for activity of these genes. This effect of HNF1-α on histone acetylation is locus specific, as opposed to a global effect on histone acetylation in either tissue. Taken together, the results show that HNF1-α binds the glut2 and pklr promoters independently of its requirement for transcription, whereas the tissue-specific dependence on HNF1-α for transcription of distinct genes is likely to be at least in part mediated by the role of HNF1-α in inducing localized hyperacetylation of chromatin.
The results are therefore consistent with a model whereby HNF1-α is a functionally obligate component of the set of activator-coactivators forming complexes in vivo at the glut2 and pklr promoters in islets. In contrast, in liver tissue it is present as a dispensable component, presumably due to the presence of redundant positive factors or the lack of tissue-specific repressive elements. In either tissue, the activating complex is necessary to target histone acetyltransferase complexes which result in the hyperacetylation of nucleosomal histones and subsequent conformational changes which are instrumental in transcriptional activation. This is, in fact, consistent with prevailing models which indicate that one of the mechanisms whereby transcriptional activators can induce gene activity consists in the recruitment of histone acetylase activity to its target sites. However, existing evidence for this concept stems primarily from yeast genetics, reconstituted in vitro systems, and more recently from cultured mammalian cell systems analyzing hormonal and viral induction of transcription (see, for example, references 6, 11, 22, 27 and 38). Thus, the bearing on the diversity of eukaryotic promoters in live mammals of the notion that transactivators target localized nucleosomal hyperacetylation in order to activate transcription remains untested. The results presented here fortify its physiological relevance, inasmuch as they represent a demonstration in mammals of the essential role of a single activator in inducing gene-selective histone hyperacetylation and provide evidence that this hyperacetylation process is linked to gene expression of distinct promoters.
ACKNOWLEDGMENTS
We are indebted to Frank Gonzalez and D. LeRoith for providing us with hnf1-α null mice and B. Thorens, J. Habener, and C. Van Schravendijk, who provided us with antisera. We are grateful to M. Vallejo for critical reading of the manuscript, J. Habener for helpful comments, P. Farnham for providing us with a chromatin immunoprecipitation protocol, and E. Vilardell for valuable support.
This work was supported by SAF98-0005 (CICYT), Fundació Marató TV3, Eli Lilly/European Association for the Study of Diabetes, and QLRT1999-546 (European Commission) (J.F.). M.P. is the recipient of a postdoctoral fellowship from the CICYT.
REFERENCES
- 1.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry M N, Friend D S. High yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969;43:506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutler E, Baronciani L. Mutations in pyruvate kinase. Hum Mutat. 1996;7:1–6. doi: 10.1002/(SICI)1098-1004(1996)7:1<1::AID-HUMU1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 4.Blumenfeld M, Maury M, Chouard T, Yaniv M, Condamine H. Hepatic nuclear factor 1 (HNF1) shows a wider distribution than products of its known target genes in developing mouse. Development. 1991;113:589–599. doi: 10.1242/dev.113.2.589. [DOI] [PubMed] [Google Scholar]
- 5.Boyd K E, Farnham P J. Coexamination of site-specific transcription factor binding and promoter activity in living cells. Mol Cell Biol. 1999;19:8393–8399. doi: 10.1128/mcb.19.12.8393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brownell J E, Zhou J, Ranalli T, Kobayashi R, Edmondson D G, Roth S Y, Allis C D. Tetrahymena histone acetyltransferase A: a homolog to yeast Gen5p linking histone acetylation to gene activation. Cell. 1996;84:843–851. doi: 10.1016/s0092-8674(00)81063-6. [DOI] [PubMed] [Google Scholar]
- 7.Byrne M M, Sturis J, Fajans S S, Ortiz F J, Stoltz A, Stoffel M, Smith M J, Bell G I, Halter J B, Polonsky K S. Altered insulin secretory responses to glucose in subjects with a mutation in the MODY1 gene on chromosome 20. Diabetes. 1995;44:699–704. doi: 10.2337/diab.44.6.699. [DOI] [PubMed] [Google Scholar]
- 8.Byrne M M, Sturis J, Menzel S, Yamagata K, Fajans S S, Dronsfield M J, Bain S C, Hattersley A T, Velho G, Froguel P, Bell G I, Polonsky K S. Altered insulin secretory responses to glucose in diabetic and nondiabetic subjects with mutations in the diabetes susceptibility gene MODY3 on chromosome 12. Diabetes. 1996;45:1503–1510. doi: 10.2337/diab.45.11.1503. [DOI] [PubMed] [Google Scholar]
- 9.Cereghini S, Blumenfeld M, Yaniv M. A liver-specific factor essential for albumin transcription differs between differentiated and dedifferentiated rat hepatoma cells. Genes Dev. 1988;2:957–974. doi: 10.1101/gad.2.8.957. [DOI] [PubMed] [Google Scholar]
- 10.Cha J Y, Kim H, Kim K S, Hur M W, Ahn Y. Identification of transacting factors responsible for the tissue-specific expression of human glucose transporter type 2 isoform gene. Cooperative role of hepatocyte nuclear factors 1alpha and 3beta. J Biol Chem. 2000;275:18358–18365. doi: 10.1074/jbc.M909536199. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Lin R J, Xie W, Wilpitz D, Evans R M. Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell. 1999;98:675–686. doi: 10.1016/s0092-8674(00)80054-9. [DOI] [PubMed] [Google Scholar]
- 12.Courtois G, Morgan J G, Campbell L A, Fourel G, Crabtree G R. Interaction of a liver-specific nuclear factor with the fibrinogen and alpha 1-antitrypsin promoters. Science. 1987;238:688–692. doi: 10.1126/science.3499668. [DOI] [PubMed] [Google Scholar]
- 13.Cuif M H, Cognet M, Boquet D, Tremp G, Kahn A, Vaulont S. Elements responsible for hormonal control and tissue specificity of L-type pyruvate kinase gene expression in transgenic mice. Mol Cell Biol. 1992;12:4852–4861. doi: 10.1128/mcb.12.11.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dukes I D, Sreenan S, Roe M W, Levisetti M, Zhou Y P, Ostrega D, Bell G I, Pontoglio M, Yaniv M, Philipson L, Polonsky K S. Defective pancreatic beta-cell glycolytic signaling in hepatocyte nuclear factor-1alpha-deficient mice. J Biol Chem. 1998;273:24457–24464. doi: 10.1074/jbc.273.38.24457. [DOI] [PubMed] [Google Scholar]
- 15.Duncan S A, Navas M A, Dufort D, Rossant J, Stoffel M. Regulation of a transcription factor network required for differentiation and metabolism. Science. 1998;281:692–695. doi: 10.1126/science.281.5377.692. [DOI] [PubMed] [Google Scholar]
- 16.Emens L A, Landers D W, Moss L G. Hepatocyte nuclear factor 1 alpha is expressed in a hamster insulinoma line and transactivates the rat insulin I gene. Proc Natl Acad Sci USA. 1992;89:7300–7304. doi: 10.1073/pnas.89.16.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrer J, Gomis R, Fernandez A J, Casamitjana R, Vilardell E. Signals derived from glucose metabolism are required for glucose regulation of pancreatic islet GLUT2 mRNA and protein. Diabetes. 1993;42:1273–1280. doi: 10.2337/diab.42.9.1273. [DOI] [PubMed] [Google Scholar]
- 18.Frain M, Swart G, Monaci P, Nicosia A, Stampfli S, Frank R, Cortese R. The liver-specific transcription factor LF-B1 contains a highly diverged homeobox DNA binding domain. Cell. 1989;59:145–157. doi: 10.1016/0092-8674(89)90877-5. [DOI] [PubMed] [Google Scholar]
- 19.Guillam M T, Hummler E, Schaerer E, Yeh J I, Birnbaum M J, Beermann F, Schmidt A, Deriaz N, Thorens B, Wu J Y. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat Genet. 1997;17:327–330. doi: 10.1038/ng1197-327. [DOI] [PubMed] [Google Scholar]
- 20.Kuo C J, Conley P B, Chen L, Sladek F M, Darnell J E J, Crabtree G R. A transcriptional hierarchy involved in mammalian cell-type specification. Nature. 1992;355:457–461. doi: 10.1038/355457a0. [DOI] [PubMed] [Google Scholar]
- 21.Kuo M H, Allis C D. In vivo cross-linking and immunoprecipitation for studying dynamic protein:DNA associations in a chromatin environment. Methods. 1999;19:425–433. doi: 10.1006/meth.1999.0879. [DOI] [PubMed] [Google Scholar]
- 22.Kuo M H, Zhou J, Jambeck P, Churchill M E, Allis C D. Histone acetyltransferase activity of yeast Gen5p is required for the activation of target genes in vivo. Genes Dev. 1998;12:627–639. doi: 10.1101/gad.12.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee Y H, Sauer B, Gonzalez F J. Laron dwarfism and non-insulin-dependent diabetes mellitus in the Hnf-1α knockout mouse. Mol Cell Biol. 1998;18:3059–3068. doi: 10.1128/mcb.18.5.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lichter-Konecki U, Hipke C M, Konecki D S. Human phenylalanine hydroxylase gene expression in kidney and other nonhepatic tissues. Mol Genet Metab. 1999;67:308–316. doi: 10.1006/mgme.1999.2880. [DOI] [PubMed] [Google Scholar]
- 25.Miquerol L, Lopez S, Cartier N, Tulliez M, Raymondjean M, Kahn A. Expression of the L-type pyruvate kinase gene and the hepatocyte nuclear factor 4 transcription factor in exocrine and endocrine pancreas. J Biol Chem. 1994;269:8944–8951. [PubMed] [Google Scholar]
- 26.Orlando V, Strutt H, Paro R. Analysis of chromatin structure by in vivo formaldehyde cross-linking. Methods. 1997;11:205–214. doi: 10.1006/meth.1996.0407. [DOI] [PubMed] [Google Scholar]
- 27.Parekh B S, Maniatis T. Virus infection leads to localized hyperacetylation of histones H3 and H4 at the IFN-beta promoter. Mol Cell. 1999;3:125–129. doi: 10.1016/s1097-2765(00)80181-1. [DOI] [PubMed] [Google Scholar]
- 28.Pontoglio M, Barra J, Hadchouel M, Doyen A, Kress C, Bach J P, Babinet C, Yaniv M. Hepatocyte nuclear factor 1 inactivation results in hepatic dysfunction, phenylketonuria, and renal Fanconi syndrome. Cell. 1996;84:575–585. doi: 10.1016/s0092-8674(00)81033-8. [DOI] [PubMed] [Google Scholar]
- 29.Pontoglio M, Faust D M, Doyen A, Yaniv M, Weiss M C. Hepatocyte nuclear factor 1α gene inactivation impairs chromatin remodeling and demethylation of the phenylalanine hydroxylase gene. Mol Cell Biol. 1997;17:4948–4956. doi: 10.1128/mcb.17.9.4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pontoglio M, Sreenan S, Roe M, Pugh W, Ostrega D, Doyen A, Pick A J, Baldwin A, Velho G, Froguel P, Levisetti M, Bonner-Weir S, Bell G I, Yaniv M, Polonsky K S. Defective insulin secretion in hepatocyte nuclear factor 1 alpha-deficient mice. J Clin Investig. 1998;101:2215–2222. doi: 10.1172/JCI2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rollini P, Fournier R E. The HNF-4/HNF-1alpha transactivation cascade regulates gene activity and chromatin structure of the human serine protease inhibitor gene cluster at 14q32.1. Proc Natl Acad Sci USA. 1999;96:10308–10313. doi: 10.1073/pnas.96.18.10308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soutoglou E, Papafotiou G, Katrakili N, Talianidis I. Transcriptional activation by hepatocyte nuclear factor-1 requires synergism between multiple coactivator proteins. J Biol Chem. 2000;275:12515–12520. doi: 10.1074/jbc.275.17.12515. [DOI] [PubMed] [Google Scholar]
- 33.Stoffers D A, Ferrer J, Clarke W L, Habener J F. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet. 1997;17:138–139. doi: 10.1038/ng1097-138. [DOI] [PubMed] [Google Scholar]
- 34.Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 35.Thorens B, Guillam M T, Beermann F, Burcelin R, Jaquet M. Transgenic reexpression of GLUT1 or GLUT2 in pancreatic beta cells rescues GLUT2-null mice from early death and restores normal glucose-stimulated insulin secretion. J Biol Chem. 2000;275:23751–23758. doi: 10.1074/jbc.M002908200. [DOI] [PubMed] [Google Scholar]
- 36.Thorens B, Sarkar H K, Kaback H R, Lodish H F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988;55:281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- 37.Thorens B, Wu Y J, Leahy J L, Weir G C. The loss of GLUT2 expression by glucose-unresponsive beta cells of db/db mice is reversible and is induced by the diabetic environment. J Clin Investig. 1992;90:77–85. doi: 10.1172/JCI115858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Utley R T, Ikeda K, Grant P A, Cote J, Steger D J, Eberharter A, John S, Workman J L. Transcriptional activators direct histone acetyltransferase complexes to nucleosomes. Nature. 1998;394:498–502. doi: 10.1038/28886. [DOI] [PubMed] [Google Scholar]
- 39.Vaxillaire M, Boccio V, Philippi A, Vigouroux C, Terwilliger J, Passa P, Beckmann J S, Velho G, Lathrop G M, Froguel P. A gene for maturity onset diabetes of the young (MODY) maps to chromosome 12q. Nat Genet. 1995;9:418–423. doi: 10.1038/ng0495-418. [DOI] [PubMed] [Google Scholar]
- 40.Waeber G, Thompson N, Nicod P, Bonny C. Transcriptional activation of the GLUT2 gene by the IPF-1/STF-1/IDX-1 homeobox factor. Mol Endocrinol. 1996;10:1327–1334. doi: 10.1210/mend.10.11.8923459. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Antinozzi P A, Hagenfeldt K A, Maechler P, Wollheim C B. Molecular targets of a human HNF1alpha mutation responsible for pancreatic beta-cell dysfunction. EMBO J. 2000;19:4257–4264. doi: 10.1093/emboj/19.16.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H, Maechler P, Hagenfeldt K A, Wollheim C B. Dominant-negative suppression of HNF-1alpha function results in defective insulin gene transcription and impaired metabolism-secretion coupling in a pancreatic beta-cell line. EMBO J. 1998;17:6701–6713. doi: 10.1093/emboj/17.22.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Workman J L, Kingston R E. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu Rev Biochem. 1998;67:545–579. doi: 10.1146/annurev.biochem.67.1.545. [DOI] [PubMed] [Google Scholar]
- 44.Yamagata K, Oda N, Kaisaki P J, Menzel S, Furuta H, Vaxillaire M, Southam L, Cox R D, Lathrop G M, Boriraj V V, Chen X, Cox N J, Oda Y, Yano H, Le Beau M M, Yamada S, Nishigori H, Takeda J, Fajans S S, Hattersley A T, Iwasaki N, Hansen T, Pedersen O, Polonsky K S, Bell G I. Mutations in the hepatocyte nuclear factor-1alpha gene in maturity-onset diabetes of the young (MODY3) Nature. 1996;384:455–458. doi: 10.1038/384455a0. [DOI] [PubMed] [Google Scholar]