Abstract
Background:
Intense therapeutic ultrasound (ITU) is an innovative ultrasound-based therapy where sound waves are concentrated into select musculoskeletal tissue. These focused waves generate thermal coagula at a controlled depth and space while preserving surrounding tissues. A multicenter study was conducted evaluating the efficiency, safety, and patient tolerance of ITU for the treatment of chronic plantar fasciitis (CPF) pain.
Methods:
Seventy-four CPF patients, having failed conservative and/or minimally invasive treatment, participated in the study. Randomized participants either received 2 ITU treatments or 2 sham ITU treatments in addition to standard-of-care therapy. Plantar fascia pain was assessed pretreatment and at 4, 8, 12, and 26 weeks after treatment. Diagnostic ultrasonographic images were analyzed to examine hypoechoic, perifascial lesions whose volumes were calculated until week 12. Function and patient satisfaction were measured using self-reported outcome measures.
Results:
The treated group reported significant average pain reduction (–26%, –33%, –43%) and hypoechoic lesion volume (–33%, –53%, –68%) at weeks 4, 8, and 12 compared to baseline. Although the control/sham group reported insignificant pain changes at the same time points (–5%, +8%, and +2%) and increased hypoechoic lesion volume (+15%, +28%, +58%). Treated patients reported a significant increase in daily living activities (+28%, +42%, +47%, +40%) compared to the sham/control group (+0.12%, +12%, +3%, +21%). Patient satisfaction remained more than 80% at weeks 8, 12, and 26 for all treatment groups.
Conclusion:
ITU is an effective pain relief treatment for CPF, which is refractory to either conservative measures or minimally invasive treatments.
Level of Evidence:
Level II.
Keywords: intense therapeutic ultrasound (ITU), plantar fasciitis, plantar fasciopathy, hypoechoic lesion, musculoskeletal pain relief, FAAM, plantar fascia therapy
Introduction
Chronic plantar fasciitis (CPF) is a degenerative condition in which the plantar fascia, an aponeurosis connecting the calcaneus to the flexor mechanism of the lesser toes, becomes thickened and painful at the insertion onto the calcaneus secondary to chronic overload. Recent studies using diagnostic ultrasound have shown a high percentage of patients experiencing plantar fascia pain also have perifascial hypoechoic lesions. 15,17 -19 These may represent a weakness in the support structure, resulting in pain. Ten percent of the adult population will experience some form of plantar fasciopathy, usually between the ages of 40 and 60 years. 16 Traditional treatment consisting of stretching, anti-inflammatory medication, and in shoe orthosis leads to symptomatic resolution in greater than 90% of patients but requires 3 to 6 months. The remaining 10% of patients usually progress through a series of more aggressive treatment options, including corticosteroid injections, platelet-rich plasma injections, amniotic stem cell injections, shockwave treatment, tenotomy, and surgery. 11 At some point during the course of treatment, the patient and their doctor may choose to forego further intervention and the patient adapts to a lifestyle that minimizes pain, in other words—they learn to live with it.
Intense therapeutic ultrasound (ITU) is a newly developed ultrasound-based therapy in which sound waves are focused into select musculoskeletal tissue. These waves produce precise thermal coagulative changes over a small area of interest while adjacent tissue is left unaffected and without causing any damage to superficial structures or the dermis. 20 The depth is focused at 13 to 15 mm below the skin. 16 These minimally ablative changes are known to begin the body’s tissue response cascade and promote new collagen growth in the targeted anatomy. 12,14 ITU has been used clinically for treating the subcutaneous connective tissue since 2008 when it received CE Mark and FDA 510(k) clearance to market for non-surgical brow, submental tissue and Décolletage lifting. Over 4 million patients worldwide have been treated using ITU technology. Clinical studies have shown more than 85% of patients receiving the treatment on subcutaneous tissue showed marked improvement in skin lifting with little to no pain, erythema, inflammation or scarring 1 Histologically, it has been shown that ITU encourages the production of dermal collagen with thickening of the dermis and straightening of the elastic fibers in the reticular dermis. 6 Laboratory studies have shown that ITU therapy can improve healing of damaged Achilles tendons in both a rabbit and rat models. 12,14 These animal study results showed an increase in precursor markers for collagen regeneration (eg, tumor growth factor β1, vascular endothelial growth factor α, tumor necrosis factor α, and interleukin-1β) and consequent upsurge in collagen generation in injured rabbit tendons treated with ITU compared to injured, untreated rabbit tendons. 12,14 The goal of this research was to evaluate the potential of ITU to speed healing and reduce pain in patients with CPF.
Materials and Methods
Study Design
Two separate studies were conducted. The first was a double-blinded, sham controlled study conducted at the University of Arizona (Tucson, AZ) where 41 subjects were randomly assigned to either the active or sham treatment groups. All subjects received physical assessments of both feet, diagnostic ultrasonographic imaging of both feet, and treatment (either experimental or sham) of the affected foot. Both groups received 2 treatments, 2 to 4 weeks apart, either at treatment-level energy settings or with energy levels set at 0 power (sham treatment). Reviewers were blinded to the subject’s ID at each visit, to the treatment group (experimental or sham), to the treated foot, and to the individual’s responses to self-reported questionnaires. All subjects were also instructed to follow standard-of-care post-treatment regimens, including the use of orthotics as well as stretching and strength-building exercises.
The second study was a single blinded, pivotal study of 33 patients conducted at the University Foot & Ankle Institute (Santa Monica, CA) who received 2 treatments 2 to 4 weeks apart. Each subject received physical assessments, diagnostic ultrasonographic imaging and experimental treatment to the affected foot only. Reviewers were blinded to the subject’s identity and responses to the physical assessment and self-reported questionnaires. All were instructed to follow a different standard-of-care regimen that included wearing a walking boot for a few weeks after each treatment, use of orthotics, and stretching and strength-building exercises.
Subjects
Seventy-four subjects between 18 and 85 years of age, with unilateral CPF and who were experiencing pain (>90 days) despite the use of conservative “standard of care” regimens, and in some cases more aggressive minimally invasive therapies, were included in the study. Previous minimally invasive therapies included corticosteroid, platelet-rich plasma, amniotic fluid and hyaluronic acid injections, and in a few cases shockwave and tenotomy. Subjects were excluded from the studies if they experienced current bilateral plantar fascia pain, local infections, previous foot surgery, other foot/ankle pathologies and pregnancy, or were unwilling to complete the posttreatment standard-of-care regimen as prescribed by the principal investigators. Patients were also excluded if they were currently enrolled in any other non-conservative, device, or Investigational New Drug clinical trial, or had participated in a clinical study involving the plantar fascia, within 30 days before study initiation Subjects provided both written and verbal consent as well as willingness to complete treatment and post-treatment regimen as prescribed.
The study population consisted of 50 females and 24 males. The range of age for the study group was 31-73 years, with a median of 56 years. A number of patients were lost to follow-up over the course of the study. The remaining patients—69 patients at week 4, 65 patients at week 8, 59 patients at week 12, and 48 patients at week 26 (Table 1)—were evaluated.
Table 1.
Demographics.
| Patient Demographics | Baseline | Week 4 | Week 8 | Week 12 | Week 16 |
|---|---|---|---|---|---|
| Number of subjects: treatment (control) | 62 (12) | 59 (10) | 54 (11) | 51 (8) | 40 (8) |
| Age range, y, median | 31-73 (56) | ||||
| Average reported symptomatic months | 19.2 | ||||
| Gender, male/female | 24/50 | ||||
Treatments
Treatments were applied using the Actisound System–Intense Therapeutic Ultrasound (Ardent Sound, Inc, Mesa, AZ). Diagnostic ultrasound images were collected using the Spark High Frequency Imaging System (Ardent Sound, Inc). All diagnostic imaging and therapeutic ultrasound treatments were administered by physicians, registered diagnostic medical sonographers, or trained personnel.
Subjects underwent 2 treatments: the first at their initial visit and the final treatment at either 2 or 4 weeks after the baseline visit. During each treatment session, a series of ITU pulses, ranging from 350 to 1000 in total (depending on the size of the foot and relative thickness of the fat pad), with an energy level up to 5 J for the treatment group and 0 J for the control/sham-treatment group were administered to the plantar fascia, approximately 13-15 mm below the skin in the region of the medial and central bands just distal to the calcaneus up to the midfoot. Patients were either positioned in the supine or prone position for the duration of treatment which lasts between 5 and 8 minutes. Ultrasound coupling gel/lotion was applied and allowed to soak into the treatment area. No anesthetic was used before or during the treatment. Treatment pain was monitored by periodic pauses in treatment administration and asking patients to rate their current pain level associated with the treatment. If the patients felt the treatment pain was momentarily intolerable, the treatment was paused, cold coupling lotion was applied to the area, when the patient was comfortable, the treatment continued.
Between treatments, subjects were instructed to complete both gastrocnemius and plantar fascia specific stretching exercises 3 times per day. The patients in the pivotal study also used a walking boot for 2 to 4 weeks after each treatment. Patients were evaluated at 2 weeks after each treatment. Depending on the subject’s progress they were directed to either stop wearing or continue wearing the boot until their next visit. Patients continued to perform standard of care stretching exercises beyond the 2 ITU treatments and for the remainder of the study as prescribed by the study principal investigators.
Outcome measures
Pain
Patients reported an overall pain score relating to plantar fascia pain using the visual analog scale (VAS) pain score of 0 to 10, with 0 indicating no pain and 10 indicating the worst imaginable pain. 2,7 Patients self-reported this pain score at baseline and at each follow-up visit and phone calls at 4, 8, 12, and 26 weeks after initial treatment.
Foot Function Index (FFI) pain subscale
At each visit (weeks 0, 2, 4, 8, and 12) and phone call (week 26), patients answered a series of 9 questions concerning their experience with pain and activities for the plantar fascia during the week prior to completing the questionnaire. Each question was scaled from 0 to 5, indicating a range of no difficulty to unable to do, respectively. Patient scores were calculated by adding the individual question scores together, with a possible score range of 0 to 45. Scores of individual questions as well as total scores at subsequent visits and calls were compared to the initial visit pretreatment for all patients.
Foot Ankle Ability Measure (FAAM) activities of daily living subscale
Patients also self-reported the FAAM questionnaire in the same process as for the FFI pain subscale. 5 This questionnaire focused more on specific tasks patients likely experience in everyday life. Each question was scored based on difficulty, with 0 indicating unable to do and 4 indicating no difficulty. Comparisons for each question as well as total scores were analyzed in similar fashion to FFI patient scores.
Subject satisfaction: subject-reported outcome measure (SROM)
Subjects self-reported their satisfaction with the treatment at each follow-up time point by selecting one of 4 choices: satisfied, satisfied with minor reservations, satisfied with major reservations, and not satisfied. The studies considered a selection of the first 2 listed choices, successful satisfaction criteria. Percentages of patients meeting satisfaction criteria were calculated for each time point and for both treatment and control/sham groups.
Hypoechoic lesion size
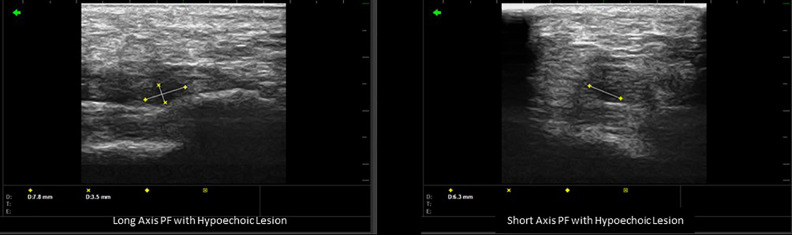
Images of hypoechoic lesions were collected, and their volumes calculated at the initial visit and each follow-up visit by measuring the inferior-to-superior and posterior-to-anterior radii in the long axis and the medial-to-lateral radius of the transverse axis and applying the following formula for the volume of an ellipse:
with r1, r2, and r3 representing the 3 radii detailed above (Figure 1). 9,15,17 -19 Hypoechoic lesions were also seen in 15% of asymptomatic, collateral feet.
Figure 1.
Long- and short-axis plantar fascia with hypoechoic lesion measurements 170 × 50 mm.
Pain/lesion volume reduction correlation
In addition to comparing patients’ pain scores and lesion sizes at each follow-up to baseline, correlation of corresponding average pain scores and lesion size reductions for weeks 4, 8, and 12 following treatment was performed. Linear regression analysis was performed for both treatment and control groups to determine the strength of relationship between these 2 measurements.
Statistical analysis
Pain scores, lesion volumes, FFI scores, FAAM, and SROM scores reported at each follow-up visit were compared to each subject’s baseline scores. Change to lesion volumes were recorded by dividing the volume of the lesion at each follow-up visit with the volume of the same lesion prior to the first treatment. For the total scores of the ability questionnaires, Student paired t tests were calculated to determine statistically significant differences between pretreatment and subsequent follow-up measurements. Error displayed in the text as “±X.X%” are standard error. The level of significance (α) was set to 0.05.
Results
Pain Reduction
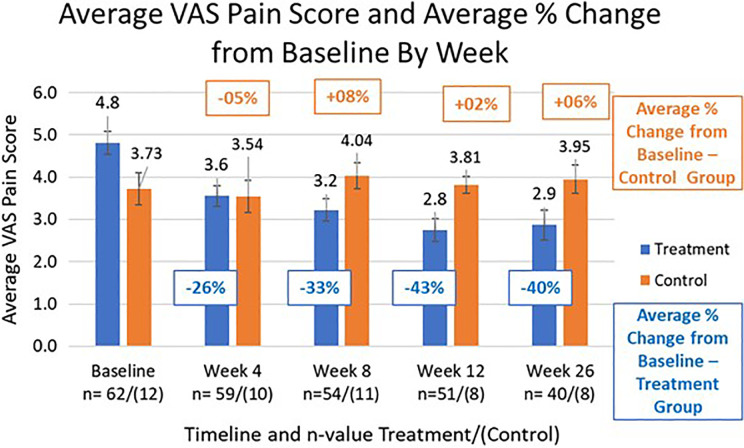
Patients in the treatment group showed significant reduction in pain at all follow-up visits whereas the control group did not show significant changes. The treatment group had an average pain reduction of 26% (VAS: 3.56, ±5.6%) at week 4, and that reduction improves to 43% (VAS: 2.75, ±4.7%) and 40% (VAS: 2.87, ±6.2%) at weeks 12 and 26, respectively compared to baseline (Figure 2, P < .01 for all weeks). The control/sham group average was not significantly different from the average baseline score at any follow-up time point, starting with week 4 at a reduction of 5% (±3.3%) and actually increasing for every other time point, reaching an average percentage increase of 6% (±11.7%) by week 26 compared to baseline (P > .25 for all weeks).
Figure 2.
Average visual analog scale (VAS) pain score and percentage average pain score change by visit.
Hypoechoic Lesion Size/Volume
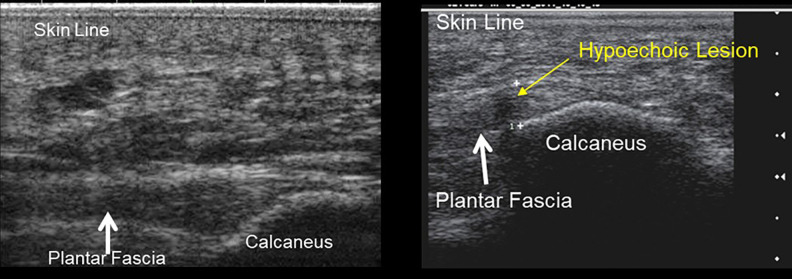
Hypoechoic lesions (Figure 3) were noted on >90% of patients. These hypoechoic lesions were measured, volumes calculated, and compared to baseline (Figure 4). After treatment, the volume of the hypoechoic lesions significantly decreased for the treatment group, starting at 33% (±6.8%) at week 4 and further to 68% (±9.8%) at the last measurement week 12 (Figure 5). For the control/sham group, the average size increased over time, averaging 57% increase (±36.7%) at week 12. Hypoechoic lesions were noted in 13% of the contralateral feet, without knowledge of the presence or absence of diagnosed bilateral disease.
Figure 3.
Ultrasonographic image comparison: normal plantar fascia (left), plantar fascia with hypoechoic lesion (right).
Figure 4.
Long axis plantar fascia ultrasound images measuring hypoechoic lesions at baseline (left = 3.6 × 3.4 mm) and at 12 weeks (right = 1.6 × 1.5 mm).
Figure 5.
Average hypoechoic lesion size/volume change over time.
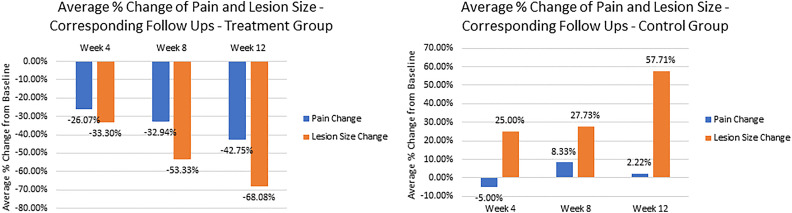
Pain Score/Lesion Size % Reduction Correlation
Results of linear regression analysis (Figure 6) show a strong linear relationship between percentage pain score reduction and percentage lesion size reduction for the treatment group, with r = 0.982. The control group did not share the same correlation, with r = 0.336, indicating a weak relationship between the 2 measurements.
Figure 6.
Correlation of average pain score change compared to baseline: treated and control groups.
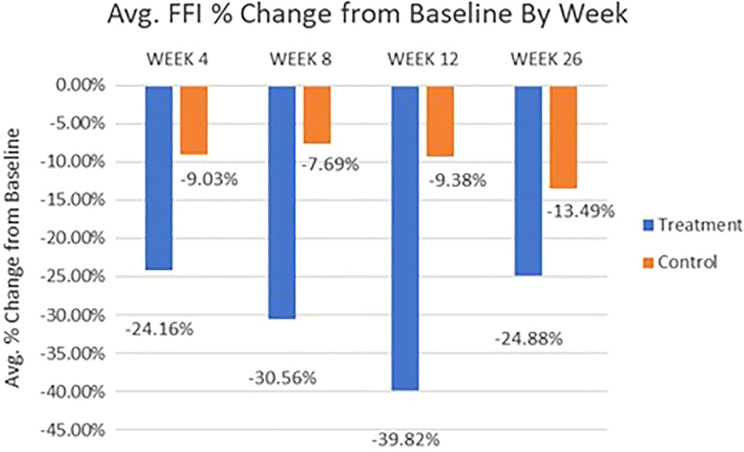
FFI Pain Subscale Score
Results of FFI pain subscale total score (Figure 7) show that the treatment group had highly significant differences between baseline and all follow-up time points (P < .005), resulting in a 24% (±3.8%) average score reduction at week 4 and peaking at 39% (±5.7%) at week 12, indicating rapid and sustaining plantar fasciitis ability improvement in daily activities. However, the control group did not show a significant difference at the 4-, 12-, and 26-week follow-up time points, compared to baseline.
Figure 7.
Average Foot Function Index (FFI) pain subscale score percentage change by visit for treatment vs control patients.
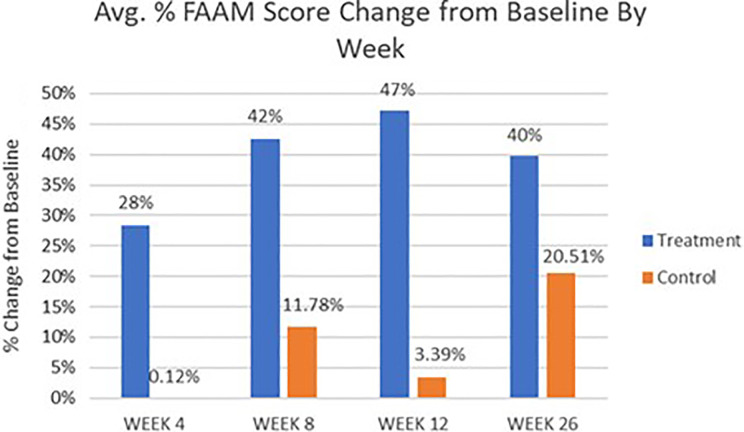
FAAM Score
FAAM total score percentage change results (Figure 8) correlate well with the results seen for the FFI questionnaire; the treatment group showed highly significant differences between baseline and all follow-up time points (P < .001) starting at week 4 with a 28% (±6.3%) score improvement and peaking at week 12 with a 47% (±8.1%) score improvement, indicating a significant increase in daily living ability with everyday tasks. However, the control group did not show a significant difference at any follow-up time points compared to baseline, even at week 26 with a 21% (±29.7%) change from baseline.
Figure 8.
Average Foot Ankle Ability Measure (FAAM) score percentage change results by visit for treatment vs control patients.
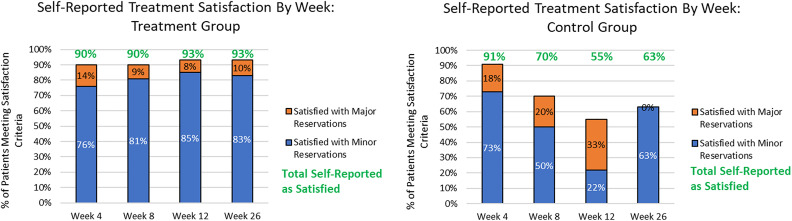
Patient Satisfaction
Based on satisfactory criteria, the treatment group had a ≥70% success rate for every follow-up time point (either completely satisfied or satisfied with minor reservations; Figure 9). Patient satisfaction raises to ≥90% for every time point if “satisfied with major reservations” is added. Comparing these results with the control group, the satisfaction percentages are significantly higher from 8 to 26 weeks after the first treatment.
Figure 9.
Patient satisfaction percentages by visit for treatment and sham treatment/control patients.
Discussion
The goal of these studies was to validate our hypothesis of reduced pain in patients with CPF when treated with ITU. There are 3 main findings of this collaboration: first, patients reported outcomes in pain and level of daily activity improved between treatment and first follow-up and continued to improve or maintain over the duration of the study; second, intra- and perifascial hypoechoic lesion volume reduced alongside pain scores and correlated strongly with this patient self-reported outcome. Hypoechoic lesions as viewed on diagnostic ultrasonography represent areas of noncontinuous fibers, suggesting lack of continuity of the plantar fascia and disruption or edema in the surrounding tisse 15,17 -19 ; third, patients were largely satisfied with the ITU treatment they received, with no adverse effects occurring from treatment. Based on these results and findings, the original hypothesis has support.
Use of ITU for pain reduction for CPF is a unique treatment option that falls somewhere between noninvasive standard of care and other minimally invasive treatment options. The treatment, which works through the skin but does not break the skin, creates microthermal coagula in and around the plantar fascia, beginning the body’s tissue healing cascade, leading to pain reduction. 4
The recalcitrant nature of CPF to standard-of-care treatment modalities generally requires invasive treatments to achieve pain relief and healing of the affected tissue. 13 The goal of these invasive options is often the same: activate the body’s natural wound-healing cascade by damaging the affected tissue and promoting the regrowth of new collagen. By causing microscale damage to the affected tissue, the goal of more aggressive noninvasive or minimally invasive treatment options is to change the local circulation of blood and initiate an inflammatory response. 13 Though the goal is the same, the different treatment options available to the patient vary in treatment pain, recovery time, and long-term effectiveness.
Corticosteroid injections are a widely used treatment for plantar fasciitis. Studies have shown these injections are painful, have a moderate symptom recurrence rate, and may be associated with postinjection plantar fasciitis rupture. 13 Some Level I trials have seen short-term success with corticosteroid injections at 4 weeks over a placebo, but the significant differences disappeared after 3-month follow-ups. 17
Platelet-rich plasma injections is similar to corticosteroid injections but uses the patient’s own centrifuged blood to deliver healing factors to the damaged tissue. One case series of 50 patients showed significant reduction of pain and high satisfaction rates by the patients, but no controlled study has been performed yet. 14
Extracorporeal shockwave therapy (ESWT) works similarly to ITU by directing energy through the skin and focused to the treatment area, but ESWT is also a painful procedure often accompanied with nerve blocks for patient comfort. 17 A study of 160 patients by Buchbinder et al showed no significant difference between the ESWT group over the placebo group at either 6- or 12-week follow-ups. 3 A review performed by Lareau et al showed that 50% of selected ESWT studies showed no significant differences over the placebo group, and one study showed even traditional stretching exercises to be more effective. 11
Another noninvasive energy source treatment option is low-level laser therapy. A prospective study was conducted of 30 patients undergoing 6 treatments of low-level laser therapy with various follow-ups up to 12 months. Both self-reported measurements of pain and daily activity improved significantly, starting at 2 weeks after the initial procedure and continuing to improve overall at 12 months. However, a limitation of that study was the lack of a control group. 9
Surgery is often used as a last resort in treatment of CPF, and though alleviation of patient pain and physiological defects is around 80% to 90%, this option is highly invasive and calls for recovery times as high as 3 months. 10
This study was not without its limitations. First, each study group was relatively small, despite meeting statistical justification requirements at the onset. Treatment groups were pooled from each study location while the control group was enrolled from one center only. Because of attrition, the control group was too small to provide a valid comparison of results between the groups at 12 and 24 weeks. Because of the chronic nature of CPF and extended periods of daily pain, patient compliance is the most challenging obstacle for studies with long follow-up time points. The Low-Level Laser Therapy study also acknowledges the difficulty of expecting patients to refrain from outside treatment if placed in a placebo group. 8 However, additional studies should be developed to include larger populations and a traditional 50/50 ratio of treatment to control groups to provide further evidence of ITU’s effectiveness.
Conclusion
These studies have shown the effectiveness of ITU for pain relief of patients suffering from CPF. Furthermore, hypoechoic lesion size reduction, patient self-reported daily living ability increase, and self-reported satisfaction for the treated area provides additional evidence for the great potential for ITU as a noninvasive, short treatment resulting in pain reduction over the course of the 6-month follow-up period, when compared to the control group.
Supplemental Material
Supplemental Material, FAO862228-ICMJE for Intense Therapeutic Ultrasound for Pain Relief in the Treatment for Chronic Plantar Fasciopathy by Michael H. Slayton, Bob Baravarian, Richard C. Amodei, Keegan B. Compton, Dallin Neil Christensen, Ashley McNelly and L. Daniel Latt in Foot & Ankle Orthopaedics
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Michael H. Slayton, PhD, reports a patent European Patent #2081646- Method and System for Treating Muscle, Tissue, Tendon, Ligament and Cartilage Tissue issued, a patent US Application #13/136,541-Methods and Systems for treating plantar fascia pending, a patent US Application #15/978,651- Systems and Methods for Treating Acute and/or Chronic Injuries in Soft Tissue pending, and a patent European publication #EP20600937- Systems and Methods for Treating Acute and/or Chronic Injuries in Soft Tissue pending. Bob Baravarian, DPM, and L. Daniel Latt, MD, PhD, report grants from Guided Therapy Systems, Inc., during the conduct of the study. Michael H. Slayton, PhD, Richard C. Amodei, RDMS, and Keegan B. Compton, MS, are affiliated with Guided Therapy Systems. ICMJE forms for all authors are available online.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Guided Therapy Systems provided funding for this study. No direct financial support was received by the authors for the work completed in this submission.
ORCID iD: Richard C. Amodei, RDMS,  https://orcid.org/0000-0003-0630-4374
https://orcid.org/0000-0003-0630-4374
L. Daniel Latt, MD, PhD,  https://orcid.org/0000-0002-9925-3440
https://orcid.org/0000-0002-9925-3440
References
- 1. Alam M, White LE, Martin N, Witherspoon J, Yoo S, West DP. Ultrasound tightening of facial and neck skin: a rater-blinded prospective cohort study. J Am Acad Dermatol. 2009;62(2):262–269. [DOI] [PubMed] [Google Scholar]
- 2. Bijur PE, Silver W, Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med. 2001;8:1153–1157. [DOI] [PubMed] [Google Scholar]
- 3. Buchbinder R, Ptasznik R, Gordon J, Buchanan J, Prabaharan V, Forbes A. Ultrasound-guided extracorporeal shock wave therapy for plantar fasciitis: a randomized controlled trial. JAMA. 2002;288(11):1364–1372. [DOI] [PubMed] [Google Scholar]
- 4. Cardinal E, Chhem RK, Beauregard CG, Aubin B, Pelletier M. Plantar fasciitis: sonographic evaluation. Radiology. 1996;201(1):257–259. [DOI] [PubMed] [Google Scholar]
- 5. DiGiovanni BF, Nawoczenski DA, Lintal ME. et al. Tissue-specific plantar fascia-stretching exercise enhances outcomes in patients with chronic heel pain. A prospective, randomized study. J Bone Joint Surg Am. 2003;85(7):1270–1277. [DOI] [PubMed] [Google Scholar]
- 6. Gliklich RE, White WM, Slayton MH, Barthe PG, Makin IR. Clinical pilot study of intense ultrasound therapy to deep dermal facial skin and subcutaneous tissues. Arch Facial Plast Surg. 2007;9(2):88–95. [DOI] [PubMed] [Google Scholar]
- 7. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual analog scale for pain (VAS Pain), numeric rating scale for pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63(suppl 11): S240–S252. [DOI] [PubMed] [Google Scholar]
- 8. Jastifer JR, Catena F, Doty JF, Stevens F, Coughlin MJ. Low-level laser therapy for the treatment of chronic plantar fasciitis: a prospective study. Foot Ankle Int. 2014;35(6):566–571. [DOI] [PubMed] [Google Scholar]
- 9. Kane D, Greaney T, Shanahan M. et al. The role of ultrasonography in the diagnosis and management of idiopathic plantar fasciitis. Rheumatology (Oxford). 2001;40(9):1002–1008. [DOI] [PubMed] [Google Scholar]
- 10. Kudo P, Dainty K, Clarfield M, Coughlin L, Lavoie P, Lebrun C. Randomized, placebo-controlled, double-blind clinical trial evaluating the treatment of plantar fasciitis with an extracorporeal shockwave therapy (ESWT) device: a North American confirmatory study. J Orthop Res. 2005;24(2):115–123. [DOI] [PubMed] [Google Scholar]
- 11. Lareau CR, Sawyer GA, Wang JH, DiGiovanni CW. Plantar and medial heel pain: diagnosis and management. J Am Acad Orthop Surg. 2014;22(6):372–380. [DOI] [PubMed] [Google Scholar]
- 12. Laubach HA, Makin IR, Barthe PG, Slayton MH, Manstein D. Intense focused ultrasound: evaluation of a new treatment modality for precise microcoagulation within the skin. Dermatol Surg. 2008;34:727–734. [DOI] [PubMed] [Google Scholar]
- 13. Miller DL, Smith NB, Bailey MR, Czarnota GJ, Hynynen K, Makin IR; Bioeffects Committee of the American Institute of Ultrasound in Medicine. Overview of therapeutic ultrasound applications and safety considerations. J Ultrasound Med. 2012;31(4): 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Slayton M, Barton J. Feasibility of modulating healing tissue response by ITU (intense therapy ultrasound) in musculoskeletal tissue. Paper presented at: 2014 IEEE International Ultrasonics Symposium; Chicago, IL; September 3–6, 2014. [Google Scholar]
- 15. Slayton MH, Amodei RC, Compton KB, Cicchinelli LD. Retrospective analysis of plantar fascia by ultrasound imaging in patients with plantar fasciitis. J Am Podiatr Med Assoc. 2018;108:349–354. [DOI] [PubMed] [Google Scholar]
- 16. Tahririan MA, Motififard M, Tahmasebi MN, Siavashi B. Plantar fasciitis. J Res Med Sci. 2012;17(8):799–804. [PMC free article] [PubMed] [Google Scholar]
- 17. Tsai WC, Chiu MF, Wang CL, Tang FT, Wong MK. Ultrasound evaluation of plantar fasciitis. Scand J Rheumatol. 2000;29(4):255–259. [DOI] [PubMed] [Google Scholar]
- 18. Vohra PK, Kincaid BR, Japour CJ, Sobel E. Ultrasonographic evaluation of plantar fascia bands. A retrospective study of 211 symptomatic feet. J Am Podiatr Med Assoc. 2002;92(8):444–449. [DOI] [PubMed] [Google Scholar]
- 19. Wall JR, Harkness MA, Crawford A. Ultrasound diagnosis of plantar fasciitis. Foot Ankle. 1993;14(8):465–470. [DOI] [PubMed] [Google Scholar]
- 20. White MW, Makin IR, Barthe PB, Slayton MH, Gliklich RE. Selective creation of thermal injury zones in the superficial musculoaponeurotic system using intense ultrasound therapy: a new target for noninvasive facial rejuvenation. Arch Facial Plast Surg. 2007;9(1):22–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, FAO862228-ICMJE for Intense Therapeutic Ultrasound for Pain Relief in the Treatment for Chronic Plantar Fasciopathy by Michael H. Slayton, Bob Baravarian, Richard C. Amodei, Keegan B. Compton, Dallin Neil Christensen, Ashley McNelly and L. Daniel Latt in Foot & Ankle Orthopaedics