Abstract
The processing method for Chinese traditional herbal medicine is “Pao Zhi” in Chinese. This study examined the efficacy of the Pao Zhi on the preparations of Gardeniae Fructus (GF) on a mitochondrial respiratory function in rats. To determine the efficacy of Pao Zhi, we investigated the effects of GF heat processing on mitochondrial respiratory function. To test the GF components, the rats were randomly divided into a geniposide-alone group, crocin-alone group, and combination groups and treated with geniposide and crocin at different ratios. The results showed that a high dose, raw GF was more effective in improving the neurological function, mitochondrial respiratory function, and activities of Na+-K+-ATPase and Ca2+-Mg2+-ATPase than the preparations that underwent heating. Moreover, mitochondrial ROS production was the lowest in the raw GF-treated group. In addition, treatments with crocin and GC3 were more effective than geniposide in improving the functional deficit in MCAO rats. In conclusion, our results suggest that raw GF is the most suitable preparation for the treatment of cerebral ischemia, and its underlying mechanisms may be associated with the improvement of mitochondrial respiratory function, increased activities of Na+-K+-ATPase and Ca2+-Mg2+-ATPase, and reduced oxidative stress in mitochondria. Our findings suggest that raw GF, especially crocin, could be an ideal therapeutic agent for ischemic stroke.
Introduction
Pao Zhi refers to the processing of raw Chinese medicines using techniques, such as simple heat treatment, various adjuvant treatments, and steaming.1,2 Based on the theory of traditional Chinese medicine (TCM), Pao Zhi can enhance the efficacy, reduce toxicity, alleviate adverse effects, and change the pharmacological properties of a particular herb for different clinical purposes.3 For example, patients administered with raw Panax ginseng C.A. Meyer (Ren-shen in Chinese) experience adverse effects such as insomnia, hypertension, and anxiety.4 These adverse effects decrease when the herb is processed using steam.5 Additionally, raw Polygonum multiflorum (He-shou-wu) can cause hepatotoxicity; processing this herb increases its ability to scavenge free radicals, while decreasing the hepatotoxicity.6,7 Excessive use of raw Pinelliae rhizoma (Ban-xia)8 and Euphorbia kansui (Gan-sui)9 may lead to acute organ damage; processing these herbs with ginger juice or alum reduces their organ toxicity effects.10
Gardeniae Fructus (GF; Zhi Zi in Chinese), which is derived from the dried ripe fruit of Gardenia jasminoides Ellis, is a well-known, pharmacopeia-recorded herb used in China in food or medicine. GF exhibits anti-inflammatory, anti-hypertensive, hepatoprotective, and cholagogue effects11 and is commonly used as an antipyretic and diuretic agent. Excessive use of GF can lead to spleen and stomach injuries due to its bitter taste and coldness properties.12,13 These adverse properties can be neutralized using a stir-baking process. Currently, there are three GF preparations, namely, raw GF (Zhi-zhi in Chinese), stir-baked until brown (GFP, Jiao Zhi-zhi in Chinese), and fried until carbonized (GFC ,Tan Zhi-zhi in Chinese). Then, each serves its own purpose in clinical practice. According to TCM theory, processing can change the properties of Chinese medicines. GF has stronger coldness properties with heat removal and detoxification effects than those of the other two products,14 whereas GFC has a better hemostatic effect and is continually applied clinically for the treatment of hemorrhage and brown stool.15 Previous research has shown that the water extract of GFC may shorten the plasma clotting time in mice.16 On the other hand, GFP has demonstrated hemostatic, antipyretic, and anxiolytic effects in addition to the hepatoprotective effect observed in a carbon tetrachloride (CCl4) rat model.12 Although significant research has been conducted on GF, few studies have reported the differences in the pharmacological effects of raw and processed GF products.
GF exerts neuroprotective effects, particularly in the treatment of nervous system diseases, including cerebral ischemia,17−19 and several lines of evidence suggest that geniposide and crocin largely contribute to the clinical efficacy of GF. Geniposide shows neuroprotective effects by inhibiting inflammation, ameliorating amyloid pathology, and improving cognition.20 Meanwhile, crocin also shows neuroprotective effects against hydrogen peroxide- and l-glutamic-acid-induced SH-SY5Y cell injuries.21 Cerebral ischemia induces mitochondrial dysfunction and adenosine triphosphate (ATP) depletion.22 Mitochondria are the powerhouses of eukaryotic cells, which generate ATP via the mitochondrial electron transport chain (ETC) through oxidative phosphorylation (OXPHOS).23,24 In addition to ATP production, mitochondria also produce ROS as a byproduct of OXPHOS.25 It has been reported that the occurrence of ischemic stroke is often associated with energy metabolism disorder, including mitochondrial functional impairment and imbalance of ion homeostasis.26,27 Na+-K+-ATPase and Ca2+-Mg2+-ATPase are important regulators of Ca2+ homeostasis, which are key indicators of energy metabolism.28−30 For this reason, mitochondria is thought to be an important target of organelles in cerebral ischemia, and the cause of its injury is the key link to cerebral ischemia injury.31 We inferred that GF may play a therapeutic role in cerebral ischemia by affecting mitochondrial respiratory function. As mentioned above, the stir-baking process can improve the curative effect of TCM. However, whether the three stir-baking processes of GF also lead to different curative effects in cerebral ischemia remains largely unknown, with only a few studies exploring this.
The aims of this study were to determine the effects of the three different GF preparations and their components on brain mitochondrial respiratory function and energy metabolism-related enzyme activities, and to preliminarily investigate the mechanisms of the GF neuroprotective effects against cerebral ischemia in rats.
Results
High-Performance Liquid Chromatography (HPLC) Profiles of GF Preparations
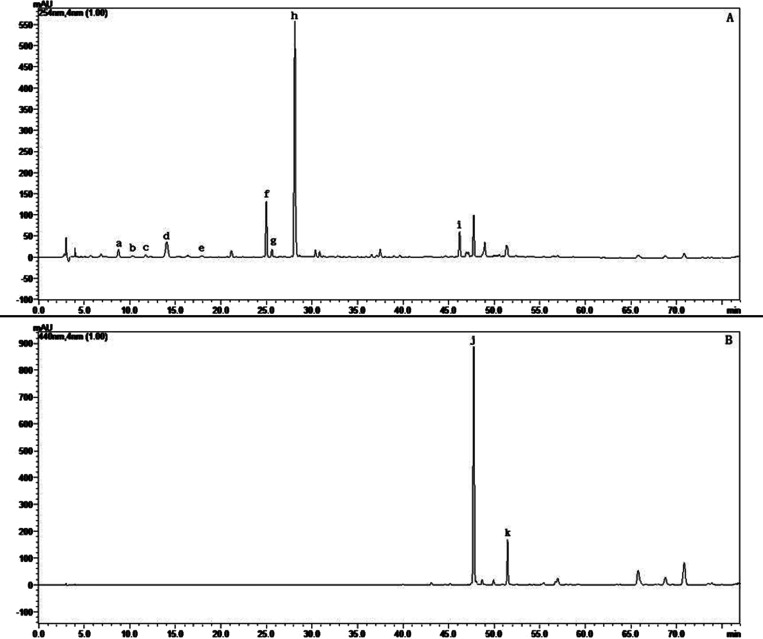
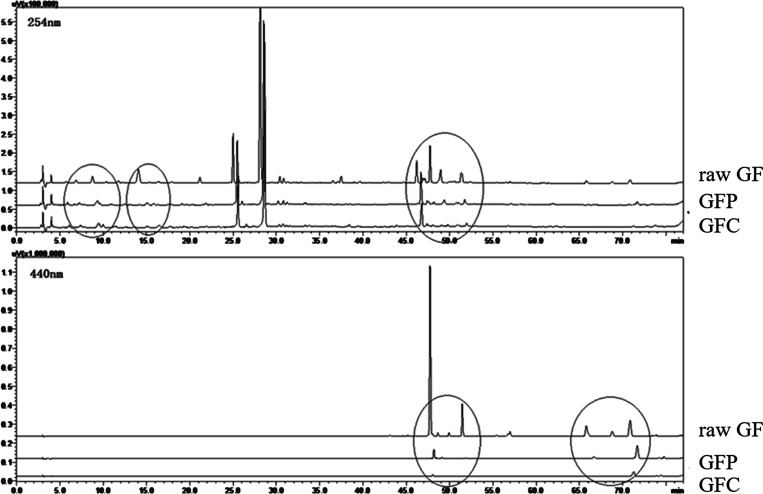
Samples of three GF preparations (raw GF, GFP, and GFC) were analyzed by HPLC to assess their quality and major chemical components. A typical chromatogram of raw GF is presented in Figure 1. Eleven peaks were identified using chemical standards, namely, shanzhiside, gardenoside, genipin-1-β-D-gentiobioside, geniposide, 6″-O-p-coumaroylgenipin gentiobioside, geniposidic acid, deacetyl asperulosidic acid methyl ester, scandoside methyl ester, chlorogenic acid, crocin-I, and crocin-II. As shown in Figure 2, geniposide was the main iridoid component of the three GF preparations, with contents of 57.15, 48.96, and 43.58 mg/g, respectively. Crocin was the main pigment component of the three GF preparations, with contents of 10.97, 0.36, and 0.05 mg/g, respectively. The contents of shanzhiside, deacetyl asperulosidic acid methyl ester, gardenoside, scandoside methyl ester, and total iridoid in raw GF were much higher than those in GFP and GFC. In contrast, compared with raw GF in which the contents of 6″-O-p-coumaroylgenipin gentiobioside and genipin-1-β-D-gentiobioside were increased, they were decreased in GFC, while the content of geniposidic acid was lower in GF than GFP and GFC. Most of iridoid glycosides include a lot of double bonds, phenolic hydroxyl bonds, and glycosidic bonds; the levels of these components were decreased due to instability during the heating process (Tables S1–S4). Diterpene pigments contain many double bonds and glycosidic bonds, and their levels are reduced owing to instability during the heating process.
Figure 1.
Typical chromatograms of raw GF at detection wavelengths of (A) 254 nm and (B) 440 nm: a, shanzhiside; b, geniposidic acid; c, deacetyl asperulosidic acid methyl ester; d, gardenoside; e, scandoside methyl ester; f, genipin-1-β-D-gentiobioside; g, chlorogenic acid; h, geniposide; i, 6″-O-p-coumaroylgenipin gentiobioside; j, crocin-I; and k, crocin-II.
Figure 2.
Typical chromatograms of raw GF and the two heat-processed GF preparations at the two indicated detection wavelengths.
The chemometric methods, hierarchical cluster analysis (HCA), and partial least-squares discriminant analysis (PLS-DA) were used to determine whether it is possible to discriminate between these three different GF preparations solely on the basis of their chemical constituents.
These analyses did not reveal any differences in the chemical constituents of the three GF preparations (Figure S1). The variation in the components could not be discriminated between GFP and GFC by HCA and PLS-DA. The PLS-DA model had R2X, R2Y, and Q2 of 0.929, 0.687, and 0.64, respectively. Hence, we could only distinguish the three GF preparations with the help of pharmacodynamic indices.
Differences in the GF Preparations and Their Effect on the Functional Deficit in Middle Cerebral Artery Occlusion (MCAO) Model Rats
Neurological scores and infarct volume are important markers of brain injury. At 12 h after MCAO, these markers were increased in the MCAO group compared to the sham group.
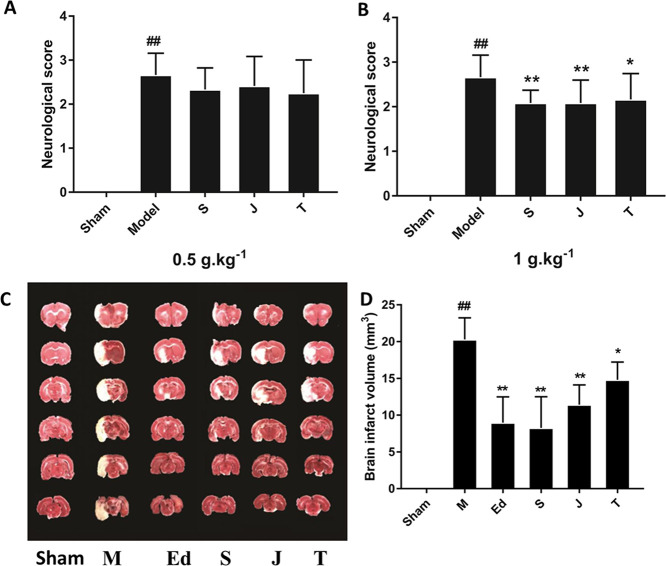
MCAO treatment significantly affected neural behavior, as indicated by a neurological score >3 in the model group, measured 12 h after MCAO (p < 0.01, vs the sham group, Figure 3A). Significant improvements in neurological scores were observed in rats treated with the different GF preparations at a dosage of 1 g·kg–1 (p < 0.05 and p < 0.01, vs the MCAO group). No differences were found among the different GF preparations at a dosage of 0.5 g·kg–1. Among the three GF-treated groups, the raw GF-treated rats showed the lowest score, while the GFC group showed the highest score. The data indicated that at the high dose, the raw GF group was the most effective in improving the neurological function of cerebral ischemic rats. There were significant differences between the GF group and the other two preparations, while the GFP group and the GFC group had no difference (post hoc pairwise comparisons of GF vs GFP or GFC group, p < 0.05 and GFP vs GFC group, p > 0.05).
Figure 3.
Effects of GF, GFP, and GFC on neurological deficits induced by MCAO in rats: (A) at a drug dose of 0.5 g·kg–1 and (B) at a drug dose of 1 g·kg–1. (C) 2,3,5-Triphenyltetrazolium chloride (TTC)-stained coronal sections of rat brains (n = 6). Red and white colors denote the normal and infarcted areas, respectively. The colorless region corresponds to the occluded middle cerebral artery territory. (D) Infarct volumes in different groups, determined by TTC staining and photographic image analysis. Values are expressed as the mean ± standard deviation (SD, n = 6), ##p < 0.01, the model group vs the sham group; **p < 0.05 and *p < 0.01, drug-treated groups vs the model group [one-way analysis of variance (ANOVA)]. S = GF, J = GFP, T = GFC, M = MCAO model group; and Ed = edaravone.
Next, the effects of different GF preparations (1 g·kg–1) on infarct volume were assessed (Figure 3B). At 12 h after MCAO, the infarct volume significantly increased in the model group (p < 0.01, vs the sham group), which showed that the MCAO model was successfully established. The infarct volume in the GF group decreased to 40.7% of that in the model group (p < 0.01). The treatments with GFP and GFC also decreased the infarct volume to 56.4 and 73.0%, respectively, of that in the model group (p < 0.01 and p < 0.05, respectively). The positive control, edaravone, decreased the infarct volume to 43.9% of that in the model group (p < 0.01). Pairwise post hoc comparisons revealed significant differences in the brain infarct volume among the three GF preparations (post hoc pairwise comparisons of GF vs GFP or GFC groups, p < 0.01, and GFP vs GFC groups, p < 0.05).
Effects of GF Preparations on Brain Mitochondrial Respiration in MCAO Rats
Mitochondrial respiratory function consists of five states. Among them, the values of maximal ADP-stimulated respiration (state 3,ST3) and respiration after consumption of ADP (state 4, ST4) are much stronger for the evaluation of mitochondrial respiratory function. The ratio of ST3 to ST4 is termed as the mitochondrial respiratory control ratio (RCR).32
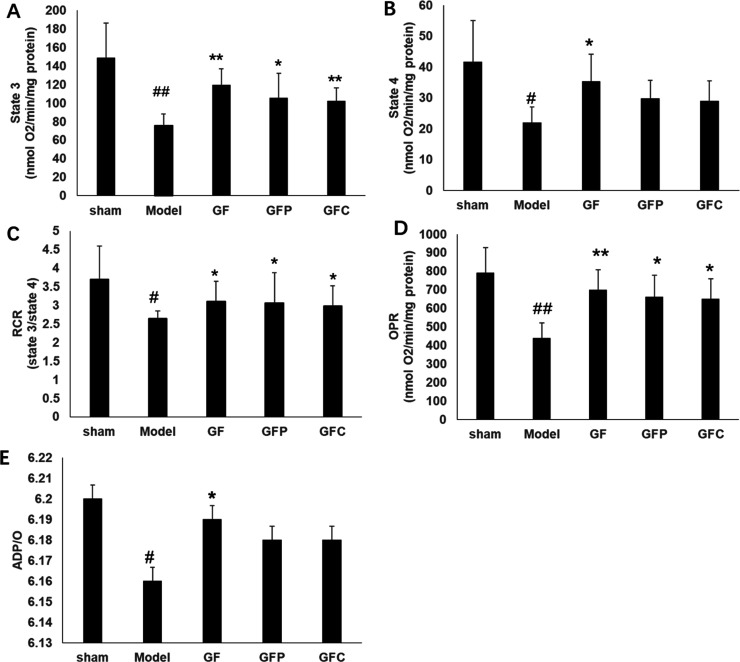
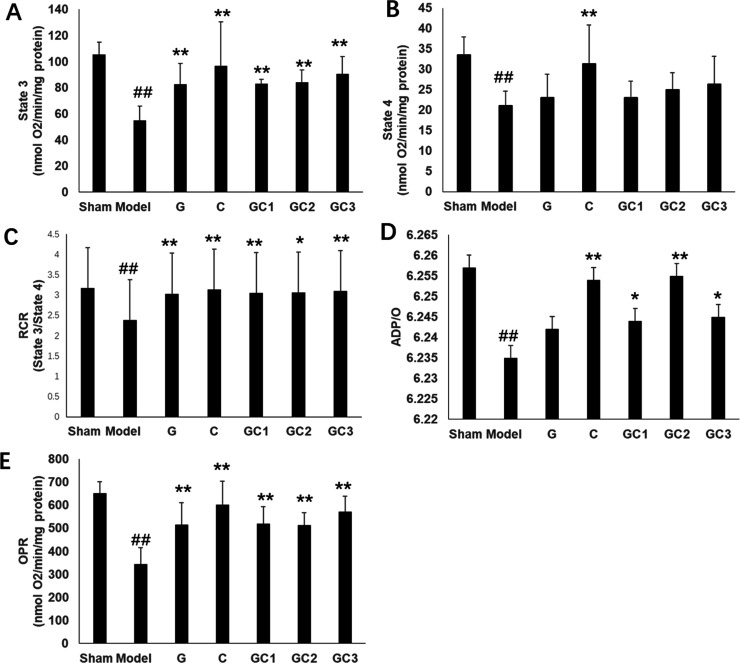
In the MCAO model group, RCR, ST3 respiration, ST4 respiration, phosphate-to-oxygen (P/O), namely, ADP/O ratio, and the oxidative phosphorylation rate (OPR) were significantly decreased compared to the sham group (p < 0.05). GF at a dosage of 1 g·kg–1 significantly improved all the mitochondrial respiration-related indices compared with the model group (p < 0.05). GFP and GFC at a dosage of 1 g·kg–1 significantly improved ST3 respiration, RCR, and OPR compared with the model group (p < 0.05) (Figure 4). GF at a dosage of 0.5 g·kg–1 markedly improved ST4 respiration and OPR compared with the model group. Compared with the model group, GFP at a dosage of 0.5 g·kg–1 significantly improved OPR, while that of GFC at a dosage of 0.5 g·kg–1 was not altered. However, there were no significant differences in other mitochondrial respiratory parameters, including RCR, ST3, and P/O, among GF, GFP, and GFC at a dosage of 0.5 g·kg–1 compared with the model group (Figure S2). Pairwise post hoc comparisons revealed significant differences in the RCR between the GF group and the other two preparations, while the GFP group and GFC group showed no differences (post hoc pairwise comparisons of GF vs GFP or GFC groups, p < 0.001, and GFP vs GFC groups, p > 0.05). Pairwise post hoc comparisons revealed significant differences in the ST3 and OPR between the GFC group and the other two preparations, while the GF group and GFP group showed no differences (post hoc pairwise comparisons of GFC vs GF or GFP groups, p < 0.001, and GF vs GFP groups, p > 0.05). In addition, there were obvious differences in the ST4 between the GFP group and the other two preparations, while the GF group and GFC group showed no differences (post hoc pairwise comparisons of GFP vs GF or GFP groups, p < 0.001, and GF vs GFC groups, p > 0.05).
Figure 4.
Parameters of mitochondrial respiration in each group (GF, GFP, and GFC at a dose of 1 g·kg–1), including (A) state 3 respiration, (B) state 4 respiration, (C) the RCR, (D) OPR, and (E) ADP/O ratio. Glutamate (10 mmol/L) and malate (5 mmol/L) were used as respiration substrates. Oxygen consumption is expressed in nmol O2/min per mg protein (mean ± SD). #p < 0.05 compared with sham; *p < 0.05 compared with the model group.
To investigate the global mitochondrial respiration variations after cerebral ischemia, principal component analysis (PCA) was employed to compare the effects of GF, GFP, and GFC on mitochondrial respiratory function. Consistent with the results of the neural behavior and infarct volume tests, significant differences were observed among the different GF preparations in their effects on mitochondrial respiratory function at a dose of 1 g·kg–1 but not at 0.5 g·kg–1. The results showed that the high-dose GF and GFP groups were both similar to the sham group, although the GF group was more similar, while the GFC group was the most dissimilar among the three. The PCA results indicated that the rats treated with GF showed the best improvement in mitochondrial respiratory function (Figure S3).
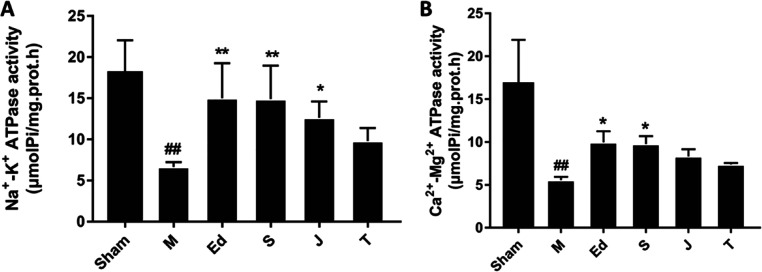
In addition, Na+-K+-ATPase and Ca2+-Mg2+-ATPase activities and mitochondrial ROS generation were explored to compare the mitochondrial respiratory function-improving effects of GF, GFP, and GFC. The Na+-K+-ATPase activity was much lower in the model group than in the sham group (p < 0.01). Treatments with GF (1 g·kg–1) and the positive control, edaravone (3 mg·kg–1), significantly increased the Na+-K+-ATPase activity by 124 and 125.6%, respectively, in the brain homogenate of MCAO rats compared with those in the model group (p < 0.01). Treatment with GFP (1 g·kg–1) also increased the Na+-K+-ATPase activity (p < 0.05); there was no significant difference between the GFC (1 g·kg–1)-treated group and the model group (Figure 5A). The data showed that rats treated with raw GF had higher Na+-K+-ATPase activity than those treated with GFP or GFC.
Figure 5.
Effects of GF, GFP, and GFC on (A) Na+-K+-ATPase activity and (B) Ca2+-Mg2+-ATPase activity in MCAO rats. Data are expressed as the mean ± SD; M = model group, Ed = edaravone, S = GF, J = GFP, and T = GFC; p < 0.01, model group vs the sham group, p < 0.05 and p < 0.05, drug-treated groups vs the model group (one-way ANOVA).
The Ca2+-Mg2+-ATPase activity was much lower in the model group than the sham group (p < 0.01). The treatments with GF (1 g·kg–1) and the positive control, edaravone (3 mg·kg–1), increased the Ca2+-Mg2+-ATPase activity in the brain homogenate of the MCAO rats by 75.4 and 79.0%, respectively, compared with the model group (p < 0.05). Treatment with GFP (1 g·kg–1) or GFC (1 g·kg–1) resulted in no significant difference in the Ca2+-Mg2+-ATPase activity compared with the model group (Figure 5B). The data showed that only raw GF affected the Ca2+-Mg2+-ATPase activity. Pairwise post hoc comparisons revealed significant differences in the Na+-K+-ATPase and Ca2+-Mg2+ ATPase activities among the three GF preparations (post hoc pairwise comparisons of GF vs GFP or GFC groups, p < 0.01, and GFP vs GFC groups, p < 0.05).
As shown in Figure S4, mitochondrial ROS generation was much higher in the model group than the sham group (p < 0.01). Treatments with GF, GFP, GFC (1 g·kg–1), and the positive control, edaravone (3 mg·kg–1), decreased ROS production by 42.0, 41.3, 37.1, and 42.1%, respectively (p < 0.01). Furthermore, the results showed that mitochondrial ROS production was the lowest in the raw GF-treated group. Pairwise post hoc comparisons revealed significant differences in the ROS production among the three GF preparations (post hoc pairwise comparisons of GF vs GFP or GFC groups, p < 0.01, and GFP vs GFC groups, p < 0.05).
Combination Effects of Geniposide and Crocin on the Functional Deficit in MCAO Rats
Next, we explored the effects of geniposide and crocin, as the main bioactive components of GF, on the functional deficits in MCAO rats.
Significant improvements in the neurological scores were observed in the rats treated with geniposide, crocin, and a combination of geniposide and crocin at a ratio of 1:3 (GC3) (p < 0.05 and p < 0.01) (Figure S5). Compared with the MCAO group, the crocin group had the lowest score, whereas the geniposide group had the highest score. The data indicated that the treatments with crocin and GC3 were more effective than that with geniposide in improving the functional deficit in MCAO rats.
Based on the above results, treatment with GC3 was selected to further verify its efficacy using 2,3,5-triphenyltetrazolium chloride (TTC) staining. The results indicated that the infarct volume was 36.8% lower in the GC3 group (p < 0.01) and 55.7% lower in the positive control (edaravone group) (p < 0.01). The results are shown in Figure S6.
Combination Effects of Geniposide and Crocin on Mitochondrial Respiratory Activity in MCAO Rats
In the MCAO model group, RCR, ST3 respiration, ST4 respiration, P/O, and OPR were significantly decreased compared to the sham group (p < 0.05). Treatment with crocin significantly improved all the mitochondrial respiration-related indices compared with those in the model group (p < 0.05). Treatment with geniposide obviously improved RCR, ST3, and OPR compared with those in the model group (p < 0.05). Treatment with combinations GC1, GC2, and GC3 significantly improved RCR, ST3, P/O, and OPR compared with those in the model group (Figure 6). PCA was also employed to compare the integrated improving effects of geniposide or crocin alone and their different combinations on mitochondrial respiratory function. The data indicated that all treatment groups exhibited significantly improved mitochondrial respiratory function. Treatment with crocin was found to be the most effective in improving the functional deficits in MCAO rats. The PCA model had R2X and Q2 values of 0.524 and 0.305, respectively (Figure S7). With an increase in crocin concentration, a stronger effect on mitochondrial respiratory function was observed.
Figure 6.
Parameters of mitochondrial respiration in each group, including (A) state 3 respiration, (B) state 4 respiration, (C) RCR, (D) ADP/O ratio, and (E) OPR. Glutamate (10 mmol/L) and malate (5 mmol/L) were used as respiration substrates. Oxygen consumption is expressed in nmol O2/min per mg protein (mean ± SD). #p < 0.05 compared with sham; *p < 0.05 compared with the model group.
In addition, variable importance in projection (VIP) was employed to analyze the importance of geniposide and crocin treatments on mitochondrial respiration. VIP > 1 was selected as the cutoff value to identify the most important variable (Figure S8). The results showed that treatment with crocin might have had more important effects on mitochondrial respiration than geniposide treatment.
Discussion
The brain is a high-energy-consuming organ in the body, and its cells are rich in mitochondria. Once damage occurs, it can cause a transition in mitochondrial membrane permeability, resulting in a decrease in ATP production.33,34 Thus, energy metabolism disorders have become the key pathogenesis of brain injury in the occurrence of ischemic stroke.35 Mitochondrial respiratory function and membrane-associated ATPases can reflect energy metabolism to a certain extent. We found that geniposide and crocin were the major bioactive components of GF, and crocin was shown to cause a more significant improvement in mitochondrial respiratory function.
Clinically, early neurological functional defects appear to be predictive factors for stroke progression. In rodents, the infarction area and neurological deficit score are employed as the main parameters to estimate the progression of stroke, and neurological scores have been used to correlate with the area of infarction caused by acute stroke.36 Our current data indicated that raw GF, GFP, and GFC can alleviate brain injury caused by stroke, and raw GF was the strongest of the three.
Furthermore, our findings were in accordance with that of a previous study, which reported that when cerebral ischemia occurs, the mitochondrial respiratory activity is reduced.37 A decreased RCR value was obtained in the model group, indicating mitochondrial uncoupling between respiration and OXPHOS. The decreased ST3 and ST4 respiration values obtained in the model group, reflecting the rate of ATP synthesis, were reduced, and the permeability of the mitochondrial membrane was also decreased. Consistent with these observations, the values of P/O were lower in the model group, confirming that OXPHOS efficiency was reduced. In the early phase of cerebral ischemia, blood flow is decreased and glucose and oxygen are deprived, which causes lower production of ATP, decreased H+, initiation of free-radical production by OXPHOS, increased cell Ca2+, and release of glutamate.38
To our knowledge, this is the first study to elucidate the effect of three GF preparations on mitochondrial respiration in ischemic injury. Our findings suggest that the higher the processing temperature is the weaker is the effect of GF preparation on mitochondrial respiratory function. In addition, ST3 respiration is a good indicator for assessing the efficacy of recovery for the brain.39 Thus, we speculate that GF could be used to treat various brain diseases.
The early stage of energy metabolism failure in cerebral ischemia injury can also be studied by measuring the ATPase activity.40 The reduction of ATP, which is usually associated with mitochondrial dysfunction, leads to the reduction of ATP-dependent processes, including Na+-K+-ATPase activity and active Na+ transport.41 Consistent with this, at this stage of research, the activities of Na+-K+-ATPase and Ca2+-Mg2+-ATPase decreased in ischemic brain tissues of rats. Na+-K+-ATPase is the primary enzyme responsible for maintaining the normal gradient equilibrium of Na+ and K+ concentrations and ion homeostasis in both astrocytes and neurons.42,43 It can hydrolyze ATP to ADP in the process of exporting three Na+ ions in exchange for two K+.44 The loss of Na+-K+-ATPase activity could decrease the Na+ gradient required for Na+/Ca2+ exchange and lead to Ca2+ overload.45,46 Moreover, Ca2+-ATPase is the key enzyme responsible for maintaining a low Ca2+ concentration, required to pump Ca2+ ions from the cytosol.30 Decreased Ca2+-Mg2+-ATPase activity also leads to Ca2+accumulation.47 Mitochondrial Ca2+ overload may reduce mitochondrial respiration during traumatic brain injuries.48,49 Treatments with GF (1 g·kg–1) and GFP (1 g·kg–1) may alleviate mitochondrial Ca2+ overload and enhance ATP production by OXPHOS, improve mitochondrial enzyme activity, alleviate the energy metabolism disorder of ischemic brain cells, and protect mitochondria against cerebral ischemia. Another important reason may be that ATPase enzymes have a sulfhydryl structure and are easily damaged by oxidative damage.50 Thus, GF and GFP may reduce the attack of reactive oxygen free radicals on ATPase and improve the enzyme activity so as to play an anticerebral ischemic role.
In addition, mitochondrial ROS production was much higher in the model group than the sham group, while GF, GFP, and GFC significantly decreased ROS production. Increased ROS production is closely related to mitochondrial Ca2+ homeostasis, decreased mitochondrial respiration, and initiation of apoptotic pathways, which may lead to decreased ATP synthesis.51,52 Decreased mitochondrial respiration further results in increased ROS production in pathological states, such as Alzheimer’s disease and diabetes.53,54 Mitochondrial dysfunction has been reported to cause an increase in ROS production and decrease in OXPHOS, thereby reducing ATP synthesis and cellular respiration during aging.55 These results suggest that ROS are involved in mitochondrial dysfunction in MCAO rats, and the three GF preparations show antioxidant activity. GF showing antioxidant activity may be related to increasing the content of superoxide dismutase (SOD) and decreasing the toxicity of nitric oxide synthase (NOS) and activity of acetylcholine esterase (AChE) in ischemic brain injury rats.56
Next, to further clarify the material basis for the differences in the efficacy of GF and its heat-processed products, varying combinations of geniposide and crocin were selected to observe their effects on mitochondrial respiratory function. It has been reported that geniposide and crocin achieve neuroprotective functions by remedying mitochondrial dysfunction, inhibiting apoptosis, and exerting an anti-inflammatory effect.57,58 Previous research showed that genipin, an aglycone of geniposide may protect against cerebral ischemia injury by inhibiting mitochondrial uncoupling protein 2 (UCP2).59 These findings indicate that geniposide and crocin may be the two major active factors of GF that attenuate cerebral ischemic brain injury.
Previous research has shown that crocin can decrease brain ischemia-induced injury and improve the neurological outcomes.19 Moreover, the most effective dose of crocin was 60 mg·kg–1, which remarkably decreased malondialdehyde (MDA) content and increased the activities of SOD and glutathione peroxidase (GPx) in an ischemic stroke rat model.18 Consistent with this, crocin (60 mg·kg–1) improved the neurological function of cerebral ischemic rats. Furthermore, Zheng et al. found that crocin may protect the brain from excessive oxidative stress in an MCAO rat model.60 Huang et al. found that crocin may exert anti-ischemic effects by decreasing the expression of light chain 3 (LC3)-II/I and AMP-activated protein kinase and increasing the expression of p62 and the mammalian target of rapamycin (mTOR) in MCAO rats.61
Our findings support the fact that crocin obviously improves the energy metabolism by preventing the appearance of metabolic disturbances caused by cerebral ischemia. To our knowledge, this is the first study to elucidate the effects of combinations of geniposide and crocin on mitochondrial respiration in ischemic injury.
In addition, treatment with GC3 remarkably improved the energy metabolism disorder of ischemic brain cells, decreased ROS production, and increased Na+-K+-ATPase activity in the brain homogenate of MCAO rats compared with the model group. The results showed that GC3 may enhance ATP production by OXPHOS and alleviate mitochondrial oxidative stress as an analogous antioxidant, causing Ca2+ overload. GC3 combination treatment outperformed the individual use of geniposide, but lower than crocin, in the treatment of ischemia, further confirming that crocin contributed more to the treatment of cerebral ischemia with GF.
This study also showed that crocin and iridoid glycosides levels decreased after the stir-baking of GF, with a more rapid decline in crocin. Another study reported that the content of crocin was the lowest in the GFC and that of geniposide was the highest in raw GF. In GFC, iridoid glycosides were found to be reduced by 30%.63 Moreover, 85% of crocin was found to break down during GF processing by stir-baking.
Hence, the differences in the effects of different GF preparations on mitochondrial respiratory function are closely related to changes in the structures of diterpenoid pigments and the ratios of diterpenoid pigments and iridoid glycosides in raw GF upon heat processing.
Stir-baking changed the properties of GF, mainly by changing the chemical structure of the processed GF and the ratios of the component compounds. Measurement of mitochondrial respiratory function in an MCAO model has the potential to be an effective means for investigating the properties of Chinese medicines and their processed products.
In summary, our results indicate that raw GF is the most suitable preparation for the treatment of cerebral ischemia, and its potential mechanisms of action may be associated with the improvement of mitochondrial respiratory function, increased activities of Na+-K+-ATPase and Ca2+-Mg2+-ATPase, and the reduced oxidative stress in the brain homogenate of MCAO rats. Our findings suggest that raw GF, especially crocin, could be an ideal therapeutic agent for ischemic stroke. However, further research on protein regulation by crocin is needed to reveal the exact mechanism of action of crocin during the development of cerebral ischemia.
Materials and Methods
Chemicals and Reagents
HPLC-grade acetonitrile was purchased from Thermo Fisher Scientific (Fair Lawn, NJ, USA). HPLC-grade formic acid and 2,3,5-triphenylte trazolium chloride (TTC) were purchased from Sigma-Aldrich (St.Louis, MO, USA). Sucrose, KH2PO4, KCl, chloral hydrate, and sodium carboxymethyl cellulose (CMC-Na) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). Tris-base and the BCA protein assay kit were purchased from Applygen Technologies Inc. (Beijing, China). EDTA-Na2 and ADP-Na2 were purchased from Beijing Biodee Biotechnology Co., Ltd. (Beijing, China). ATPase assay kits were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China), and edaravone was purchased from Aladdin Biomedical Technologies Co., Ltd. (Shanghai, China).
Standards, including shanzhiside, gardenoside, genipin-1-β-D-gentiobioside, geniposide, 6″-O-p-coumaroylgenipin gentiobioside, geniposidic acid, deacetyl asperulosidic acid methyl ester, scandoside methyl ester, chlorogenic acid, crocin-I, and crocin-II, were purchased from Chengdu Croma Biological Co., Ltd. (Chengdu, China). The purity of all standard components was more than or equal to 98%. Finally, edaravone was purchased from Aladdin Biomedical Technologies Co., Ltd. (Shanghai, China). Samples of GF, GFP, and GFC were from same batches and processed in Baicaokangshen Pharmaceutical Company (Hebei, China).
Sample Preparation
Preparation of Different Processed Products of GF
Samples of GF, GFP, and GFC were obtained from the same batches and were processed by Baicaokangshen Pharmaceutical Company (Hebei, China). To obtain raw GF, fresh G. jasminoides was put into boiling water (1:8 w/v) for 3–5 min and then dried for 30 min at 60 °C.
Drum-type gas stir-frying machines (Jiangyin Xiangshan Traditional Chinese Medicine Machinery Co., Ltd., Jiangsu, China) were used to prepare GFC and GFP. The instrument parameters were set as follows: power, 3 kW; voltage, 380 V; frequency, 50 Hz; diameter, 90 cm; and speed, 14 rpm. Each time, 10 kg of GF was stir-fried.
To prepare GFP, GF was stir-fried at 168 °C for 11 min, until it became burnt brown outside and brown inside, according to the Chinese Pharmacopeia (2020 Edition). To prepare GFC, GF was stir-fried at 260 °C for 14 min, until it became black on the outside and brown inside, according to the National Processing Standard of Traditional Chinese Medicine (1988 Edition). The dried fruits and processed products of G. jasminoides were authenticated by Prof. Cun Zhang, Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences.
The three GF preparations were boiled in water two times for 1 h each (1:10 and 1:8 w/v, respectively) and then filtered. The filtrates were condensed in vacuum. The final yields were 1 g of liquid extract per 2.62 g of crude herb for GF, 4.88 g of crude herb for GFP, and 3.35 g of crude herb for GFC. Geniposide, crocin, and their combinations (GC1, GC2, and GC3) were prepared in 0.5% CMC-Na.
Sample Preparation for HPLC
The samples were crushed into powder using a pulverizer for 2 min and passed through a 40 μm mesh sieve. A portion of each sample (0.5 g) was accurately weighed and placed into a 50 mL flask, followed by the addition of 25 mL of 50% methanol, and extraction using a KQ-300B ultrasonic extraction device (Kun Shan Ultrasonic Instruments Co., Ltd., Kunshan, Jiangsu, China) for 30 min. The extract was cooled down to room temperature, with the weight loss compensated by the addition of fresh 50% methanol, and filtered through a 0.45 μm microporous membrane.
Reference solutions of shanzhiside (38.14 μg/mL), gardenoside (46.72 μg/mL), genipin-1-β-D-gentiobioside (225.60 μg/mL), geniposide (464.10 μg/mL), 6″-O-p-coumaroylgenipin gentiobioside (65.84 μg/mL), geniposidic acid (6.60 μg/mL), deacetyl asperulosidic acid methyl ester (5.88 μg/mL), scandoside methyl ester (4.19 μg/mL), chlorogenic acid (9.93 μg/mL), crocin-I (99.20 μg/mL), and crocin-II (13.20 μg/mL) were prepared with 50% methanol, filtered through a 0.45 μm microporous membrane, and used for the identification of GF components in HPLC chromatograms.
HPLC of Three Different Processed Products of GF
The chromatography was performed on an SPD-M20A liquid chromatography system (Shimadzu, Kyoto, Japan). The data were acquired and processed using Shimadzu’s Lab-Solutions workstation. Chromatographic separation was performed using a Phenomenex Luna C18(2) 100 Å column (250 mm × 4.6 mm, 5 μm) with a flow rate of 1.0 mL/min at 35 °C. The mobile phase consisted of a 0.5% aqueous solution of formic acid (mobile phase A) and acetonitrile (mobile phase B). The gradient conditions were as follows: 0–10 min, 6% B; 10–18 min, 6–12% B; 18–25 min, 12–17% B; 25–35 min, 17–20% B; 35–45 min, 20–27% B; 45–65 min, 27–32% B; 65–70 min, 32–36% B; 70–72 min, 36–55% B; and 72–77 min, 55–100% B. The injection volume was 10 μL, and the detection wavelengths were set at 254 and 440 nm.62
Animal Model and Treatments
Adult male Sprague–Dawley rats (12 weeks old, 250–270 g) were purchased from Beijing Vital River Laboratory Animal Technology Company (Beijing, China). The rats were group-housed in transparent propylene cages with wood chip bedding. The environment was maintained at 22 ± 2 °C with an air humidity of 50 ± 10% under a 12 h light/dark cycle (lights on at 06:00) for an adaption period of 3 days. The animals were allowed ad libitum access to a commercially available rodent feed (Keaoxieli Co., Ltd., Beijing, China) and tap water. The body weights of rats and food and water intake were monitored daily in the morning for 3 consecutive days before the experiment started by the same animal breeder.
All animal experimental protocols were approved by the Laboratory Animal Care Center of the China Academy of Chinese Medical Sciences, license number SCXK (Beijing) 2019-0008, and were conducted in accordance with the guidelines and regulations for the use and care of animals of the Center for Laboratory Animal Care, China Academy of Chinese Medical Sciences. The study was approved by the Research Ethics Committee on the Welfare of Laboratory Animals of the Institute of Chinese Materia Medica of the China Academy of Chinese Medical Sciences, Beijing, China (no. 20200915).
The animal experiments included testing of the processed GF products and their components. To test the processed products, the rats were randomly divided into the sham group, the MCAO model group, the positive control (edaravone) group, GF (0.5 and 1 g·kg–1) groups, GFP (0.5 and 1 g·kg–1) groups, and GFC (0.5 and 1 g·kg–1) groups. To test the components, the rats were randomly divided into a geniposide-alone group, crocin-alone group, and into three combination groups, treated with geniposide and crocin at ratios of 3:1 (GC1), 1:1 (GC2), and 1:3 (GC3). There were 12 rats in each group. The following dosing regimens were employed: GF 0.5 and 1 g·kg–1 by oral gavage, GFP, 0.5 and 1 g·kg–1 by oral gavage; GFC, 0.5 and 1 g·kg–1 by oral gavage; geniposide-alone, 60 mg·kg–1 by oral gavage; crocin-alone, 60 mg·kg–1 by oral gavage; GC1 combination, the mixture of 45 mg·kg–1 geniposide and 15 mg·kg–1 crocin by oral gavage; GC2 combination, the mixture of 30 mg·kg–1 geniposide and 30 mg·kg–1 crocin by oral gavage; GC3 combination, the mixture of 15 mg·kg–1 geniposide and 45 mg·kg–1 crocin by oral gavage; and edaravone, 3 mg·kg–1 by oral gavage. The sham and MCAO groups were administered by oral gavage with an equivalent volume of 0.9% physiological saline solution. All treatments were administered by oral gavage within 30 min before the MCAO surgery.
MCAO surgery was performed in rats as previously reported with some modifications.18,62 The rats were anesthetized by injection of 10% chloral hydrate (400 mg·kg–1, i.p.), and the common carotid artery (CCA), external carotid artery (ECA), and internal carotid artery (ICA) were dissected. A small incision was made in the ECA after ligation of its distal part. A 4–0 nylon monofilament (20 mm-long) with a rounded tip was inserted into the ICA and advanced until resistance was felt. The ECA was then ligated, and the wound closed. During anesthesia, the rectal temperature was maintained at 37.0 ± 0.5 °C using a thermostat-controlled electrothermal pad (Chengdu Rainbow Electrical Co., Ltd., Chengdu, China). The rats in the sham group received the same surgical treatment without the insertion of the nylon monofilament. The sham and model rats were intragastriclly administered with normal saline, while the other groups were intragastrically administered with their respective GF preparations 15 min before the MCAO. The neurological functional test was performed 12 h after MCAO using the modified neurological severity score. The neurological behaviors were evaluated on a five-point scale as described previously.63 The cerebral infarct volume was assessed using the TTC staining method.64 After neurological examination, the rats were sacrificed by cardiac perfusion. The rat brains were removed and sliced into six coronal sections (2 mm thickness) each, and then stained with a 2% TTC saline solution for 30 min at 37 °C followed by fixation with 4% paraformaldehyde for 2 h. The TTC-stained sections were photographed and analyzed using the Image-Pro Plus 6.0 software. The neurological scores were assessed using Bederson’s method.65
Preparation of Brain Mitochondria
Brain mitochondria were prepared by conventional methods using differential centrifugation, as described previously.66 In brief, rats were sacrificed by cardiac perfusion and the cerebral hemispheres were rapidly isolated into an ice-cold isolation medium containing 0.25 M sucrose, 1 mM EDTA-Na2, and 10 mM Tris–HCl (pH 7.4). The brain tissues were washed three times with the medium and finely cut with scissors. The collected tissues were placed in a Teflon homogenizer with 8 mL of medium and homogenized. The homogenate was centrifuged at 700g for 10 min, and the pellet was discarded. The supernatant was re-centrifuged at 10,000g for 10 min, and the pellet was resuspended in 4 mL of the isolation medium and re-centrifuged at 10,000g for 10 min. The mitochondria were made up to a concentration of 20–25 mg of protein per milliliter of the isolation medium. All procedures were performed at 4 °C.
Measurement of Mitochondrial Respiratory Activity
Mitochondrial respiratory function was determined using a Clark-type oxygen electrode (Strathkelvin 782 2-Channel Oxygen System v1.0, Motherwell, U.K.) as described previously.67,68 The data were analyzed using the 782 Oxygen System software (Strathkelvin Instruments). Reactions were conducted in a 1.5 mL closed thermostatic glass cell (30 °C) with a magnetic stirrer. Mitochondria (1 mg of protein) were placed into a buffer containing 225 mM sucrose, 100 mM KCl, 5 mM K2HPO4, 200 μM EDTA-Na2, and 10 mM Tris (pH 7.4) to a final volume of 800 μL. After a 1 min equilibration period, mitochondrial respiration was activated by adding L-glutamate (20 mM) and malate (5 mM), namely, complex I-dependent respiration. Oxygen consumption (nmol O2/min/mg) was measured in the presence of 625 μM ADP (ST3) and after ADP depletion (ST4). The RCR was calculated as the ST3/ST4 ratio, and the ADP/O ratio was calculated as the ratio of the added ADP concentration to the oxygen consumption during ST3. The OPR was obtained based on the ST3 rate and ADP/O ratio.
Measurement of ROS Production in Mitochondria
The production of intracellular ROS was evaluated using an ROS assay kit (Beyotime, Shanghai, China) according to the manufacturer’s protocol. Mitochondria from different groups (0.5 mg/mL) were incubated with 10 μM 2′,7′-dichlorodihydrofluorescein diacetate at 37 °C for 30 min. Fluorescence intensity was measured at an excitation wavelength of 485 nm and an emission wavelength of 527 nm using a Multiskan plate reader (Thermo Fisher Scientific, Waltham, MA, USA).
Measurement of ATPase Activity in Rat Brain
The ATPase activity was detected using a commercial ultramicro-determination ATPase assay kit from (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocols immediately after the isolation of rat mitochondria. A 10% homogenate of the rat brain in a physiological saline solution was prepared and used to measure ATPase activity at 636 nm.
Statistical Analysis
Data are expressed as the mean ± SD (standard deviation). Statistical significance was determined by one-way ANOVA among multiple groups, and pairwise comparisons were performed using the least significant difference (LSD) test. All statistical analyses were performed using the IBM SPSS Statistics version 20 software (IBM Corp. Armonk, New York). A value of p < 0.05 was considered statistically significant.
The contents of the chemical composition were imported into SIMCA-P (version 14.1, Umetrics, Umea, Sweden) software for multivariate statistical analysis including hierarchical cluster analysis (HCA) and partial least-squares discriminant analysis (PLS-DA). HCA was performed to classify the samples according to the similarities of chemical properties, and the “average linkage between groups” method and cosine applied in the measurements. PLS-DA was used for modeling the group classification in a supervised manner.
The mitochondrial respiratory indexes were also analyzed by principal component analysis (PCA) and PLS-DA using SIMCA (version 14.1, Umetrics, Umea, Sweden) software.
Acknowledgments
This work was supported by grants from the scientific and technological innovation project of the China Academy of Chinese Medical Sciences (nos. CI2021A04204 and CI2021A04203), the National Natural Science Foundation of China (nos. 81873010, 81703708, 81173553, and 81473356), the Fundamental Research Funds for the Central public welfare research institutes of the China Academy of Chinese Medical Sciences (grant numbers zz13-019 and zz13-YQ-050), and the project of Beijing for traditional Chinese Medicine Processing Technology inheritance base.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c03265.
Chemometric analysis of HPLC fingerprints of three GF preparations (Figure S1), parameters of mitochondrial respiration in each group (GF, GFP, and GFC at a dose of 0.5 g·kg–1) (Figure S2), the effects of GF, GFP, and GFC on mitochondrial respiratory function in MCAO rats, evaluated by PCA analysis (Figure S3), GF, GFP, and GFC decreased the mitochondrial ROS production in the MCAO model at the dose of 1 g·kg–1 (Figure S4), geniposide, crocin, and GC3 attenuated neurological deficits induced by MCAO in rats (Figure S5), the effects of GC3 on neurological deficits induced by MCAO in rats (Figure S6), crocin, geniposide, and GC3 improved mitochondrial respiration function in MCAO rats by PCA analysis (Figure S7), crocin was more important than geniposide on mitochondrial respiration function by variable importance in projection (VIP) analysis (Figure S8), the iridoid glycoside content of crude GF (mg/g) (Table S1), the iridoid glycoside content of stir-baked GF (mg/g) (Table S2), the iridoid glycoside content of carbonized GF (mg/g) (Table S3), and the diterpenoid content of processed GF (mg/g) (Table S4) (PDF)
Author Contributions
§ Y.W., P.L., and X.Z. are coauthors.
The authors declare no competing financial interest.
Supplementary Material
References
- Su T.; Yu H.; Kwan H.-Y.; Ma X.-Q.; Cao H.-H.; Cheng C.-Y.; Leung A. K.-M.; Chan C.-L.; Li W.-D.; Cao H.; Fong W.-F.; Yu Z.-L. Comparisons of the chemical profiles, cytotoxicities and anti-inflammatory effects of raw and rice wine-processed Herba Siegesbeckiae. J. Ethnopharmacol. 2014, 156, 365–369. 10.1016/j.jep.2014.09.038. [DOI] [PubMed] [Google Scholar]
- Cui X. B.; Qian X. C.; Huang P.; Zhang Y. X.; Li J. S.; Yang G. M.; Cai B. C. Simultaneous determination of ten flavonoids of crude and wine-processed Radix Scutellariae aqueous extracts in rat plasma by UPLC-ESI-MS/MS and its application to a comparative pharmacokinetic study. Biomed. Chromatogr. 2015, 29, 1112–1123. 10.1002/bmc.3398. [DOI] [PubMed] [Google Scholar]
- Deng X.; Yu J.; Zhao M.; Zhao B.; Xue X.; Che C.; Meng J.; Wang S. Quality assessment of crude and processed ginger by high-performance liquid chromatography with diode array detection and mass spectrometry combined with chemometrics. J. Sep. Sci. 2015, 38, 2945–2952. 10.1002/jssc.201500294. [DOI] [PubMed] [Google Scholar]
- Jia L.; Zhao Y. Current evaluation of the millennium phytomedicine--ginseng (I): etymology, pharmacognosy, phytochemistry, market and regulations. Curr. Med. Chem. 2009, 16, 2475–2484. 10.2174/092986709788682146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M.; Lee H. S.; Hwang M. W.; Jin M. Effects of Korean red ginseng (Panax Ginseng Meyer) on bisphenol A exposure and gynecologic complaints: single blind, randomized clinical trial of efficacy and safety. BMC Complement Altern. Med. 2014, 14, 1472–6882. 10.1186/1472-6882-14-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. H.; Zhang J.; Qiu X. H.; Bai J. Q.; Gao Y. H.; Xu W. Application of Ultra-High-Performance Liquid Chromatography Coupled with LTQ-Orbitrap Mass Spectrometry for the Qualitative and Quantitative Analysis of Polygonum multiflorum Thumb. and Its Processed Products. Molecules 2016, 21, 40. 10.3390/molecules21010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Liu Y.; Chao Z.; Song Z.; Wang C.; Lu A. In vitro antioxidant activities of maillard reaction products produced in the steaming process of Polygonum multiflorum root. Nat. Prod. Commun. 2011, 6, 55–58. [PubMed] [Google Scholar]
- Su T.; Tan Y.; Tsui M. S.; Yi H.; Fu X. Q.; Li T.; Chan C. L.; Guo H.; Li Y. X.; Zhu P. L.; Tse A. K. W.; Cao H.; Lu A. P.; Yu Z. L. Metabolomics reveals the mechanisms for the cardiotoxicity of Pinelliae Rhizoma and the toxicity-reducing effect of processing. Sci. Rep. 2016, 6, 34692. 10.1038/srep34692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B.; Ding J.; Yang Y.; Wu F.; Song F. Systems biochemical responses of rats to Kansui and vinegar-processed Kansui exposure by integrated metabonomics. J. Ethnopharmacol. 2014, 153, 511–520. 10.1016/j.jep.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Yu H.; Pan Y.; Wu H.; Ge X.; Zhang Q.; Zhu F.; Cai B. The alum-processing mechanism attenuating toxicity of Araceae Pinellia ternata and Pinellia pedatisecta. Arch. Pharmacal Res. 2015, 38, 1810–1821. 10.1007/s12272-015-0556-0. [DOI] [PubMed] [Google Scholar]
- Im M.; Kim A.; Ma J. Y. Ethanol extract of baked Gardeniae Fructus exhibits in vitro and in vivo anti-metastatic and anti-angiogenic activities in malignant cancer cells: Role of suppression of the NF-κB and HIF-1α pathways. Int. J. Oncol. 2016, 49, 2377–2386. 10.3892/ijo.2016.3742. [DOI] [PubMed] [Google Scholar]
- Wei C. H.; Shao J.; Luo G. M.; Pharmacy S. O. Comparison Between Protective Effect of Raw and Processed Products of Gardeniae Fructus on Acute Hepatic Injury Induced by CCl_4 in Rats. Chin. J. Exp. Tradit. Med. Formulae 2016, 22, 7–10. [Google Scholar]
- Qi Q.; Mao Y.; Tian Y.; Ke Z.; Zhou X. Geniposide inhibited endothelial-mesenchymal transition via the mTOR signaling pathway in a bleomycin-induced scleroderma mouse model. Am. J. Trans. Res. 2017, 9, 1025–1036. [PMC free article] [PubMed] [Google Scholar]
- Na S. G. G.; Wang Z. D.; Long Z. J. Pharmacological research progress of Gardenia jasminoides Ellis and its active components. Chin. Infor. TCM. 2005, 90–92. [Google Scholar]
- Zhang X.; Wang Y.; Li X.; Dai Y.; Wang Q.; Wang G.; Liu D.; Gu X.; Yu D.; Ma Y.; Zhang C. Treatment Mechanism of Gardeniae Fructus and Its Carbonized Product Against Ethanol-Induced Gastric Lesions in Rats. Front. Pharmacol. 2019, 10, 750. 10.3389/fphar.2019.00750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L.; Meng J.; Zhang C.; Gu X. Z.; Yu D. R.; Ma Y. L.; Huang Q.; Liu H. Content of the Tanning and Absorption Capacity of Carbon in Different Processed Gardenia Slices. Chin. J. Exp. Tradit. Med. Formulae 2014, 20, 45–48. [Google Scholar]
- Li W.; Li P.; Liu Z.; Du Q.; Steinmetz A.; Wang N.; Du H.; Hu J. A Chinese medicine preparation induces neuroprotection by regulating paracrine signaling of brain microvascular endothelial cells. J. Ethnopharmacol. 2014, 151, 686–693. 10.1016/j.jep.2013.11.035. [DOI] [PubMed] [Google Scholar]
- Vakili A.; Einali M. R.; Bandegi A. R. Protective effect of crocin against cerebral ischemia in a dose-dependent manner in a rat model of ischemic stroke. J. Stroke Cerebrovasc. Dis. 2014, 23, 106–113. 10.1016/j.jstrokecerebrovasdis.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Sarshoori J. R.; Asadi M. H.; Mohammadi M. T. Neuroprotective effects of crocin on the histopathological alterations following brain ischemia-reperfusion injury in rat. Iran. J. Basic Med. Sci. 2014, 17, 895–902. [PMC free article] [PubMed] [Google Scholar]
- Lv C.; Wang L.; Liu X.; Yan S. S.; Wang Y.; Zhang W. Multi-faced neuroprotective effects of geniposide depending on the RAGE-mediated signaling in an Alzheimer mouse model. Neuropharmacology 2015, 89, 175–184. 10.1016/j.neuropharm.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Ni Y.; Li L.; Zhang W.; Lu D.; Zang C.; Zhang D.; Yu Y.; Yao X. Discovery and LC-MS Characterization of New Crocins in Gardeniae Fructus and Their Neuroprotective Potential. J. Agric. Food Chem. 2017, 65, 2936–2946. 10.1021/acs.jafc.6b03866. [DOI] [PubMed] [Google Scholar]
- Xu M.; Wang M. M.; Gao Y.; Keep R. F.; Shi Y. The effect of age-related risk factors and comorbidities on white matter injury and repair after ischemic stroke. Neurobiol. Dis. 2019, 126, 13–22. 10.1016/j.nbd.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm A.; Eckert A. Brain aging and neurodegeneration: from a mitochondrial point of view. J. Neurochem. 2017, 143, 418–431. 10.1111/jnc.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agapouda A.; Butterweck V.; Hamburger M.; de Beer D.; Eckert A. Honeybush Extracts (Cyclopia spp.) Rescue Mitochondrial Functions and Bioenergetics against Oxidative Injury. Oxid. Med. Cell. Longevity 2020, 2020, 1948602. 10.1155/2020/1948602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge C.; Dudley S. Mitochondria and arrhythmias. Expert. Rev. Cardiovasc. Ther. 2013, 11, 799–801. 10.1586/14779072.2013.811969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D.; Wang H.; Zhang Y.; Zhang Z. Protective Effects of Chlorogenic Acid on Cerebral Ischemia/Reperfusion Injury Rats by Regulating Oxidative Stress-Related Nrf2 Pathway. Drug Des. Devel. Ther. 2020, Volume 14, 51–60. 10.2147/DDDT.S228751. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wu M. Y.; Yiang G. T.; Liao W. T.; Tsai A. P. Y.; Cheng Y. L.; Cheng P. W.; Li C. Y.; Li C. J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650. 10.1159/000489241. [DOI] [PubMed] [Google Scholar]
- Liang M.; Tian J.; Liu L.; Pierre S.; Liu J.; Shapiro J.; Xie Z. J. Identification of a Pool of Non-pumping Na/K-ATPase. J. Biol. Chem. 2007, 282, 10585–10593. 10.1074/jbc.M609181200. [DOI] [PubMed] [Google Scholar]
- Kasturi S.; Ismail-Beigi F. Effect of thyroid hormone on the distribution and activity of Na, K-ATPase in ventricular myocardium. Arch. Biochem. Biophys. 2008, 475, 121–127. 10.1016/j.abb.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L.; Yu Y.; Li Y.; Yu Y.; Duan J.; Zou Y.; Li Q.; Sun Z. Oxidative Damage and Energy Metabolism Disorder Contribute to the Hemolytic Effect of Amorphous Silica Nanoparticles. Nanoscale Res. Lett. 2016, 11, 57. 10.1186/s11671-016-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.; Hu Z.; Tan J.; Yang S.; Zeng L. Parkin Protects against Oxygen-Glucose Deprivation/Reperfusion Insult by Promoting Drp1 Degradation. Oxid. Med. Cell. Longevity 2016, 2016, 8474303. 10.1155/2016/8474303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. C. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem. J. 1992, 284, 1–13. 10.1042/bj2840001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy C. M.; Nixon J. P.; Butterick T. A. Orexin A attenuates palmitic acid-induced hypothalamic cell death. Mol. Cell. Neurosci. 2016, 75, 93–100. 10.1016/j.mcn.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Giordano S.; Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem. J. 2012, 441, 523–540. 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacek J. C.; Behera J.; George A. K.; Kamat P. K.; Kalani A.; Tyagi N. Tetrahydrocurcumin ameliorates homocysteine-mediated mitochondrial remodeling in brain endothelial cells. J. Cell. Physiol. 2018, 233, 3080–3092. 10.1002/jcp.26145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasner S. E. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006, 5, 603–612. 10.1016/S1474-4422(06)70495-1. [DOI] [PubMed] [Google Scholar]
- Anderson M. F.; Sims N. R. Mitochondrial Respiratory Function and Cell Death in Focal Cerebral Ischemia. J. Neurochem. 1999, 73, 1189–1199. [DOI] [PubMed] [Google Scholar]
- Maalouf M.; Sullivan P. G.; Davis L.; Kim D. Y.; Rho J. M. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience 2007, 145, 256–264. 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.; Perales Villarroel J. P.; Zhang W.; Yin T.; Shinozaki K.; Hong A.; Lampe J. W.; Becker L. B. The Responses of Tissues from the Brain, Heart, Kidney, and Liver to Resuscitation following Prolonged Cardiac Arrest by Examining Mitochondrial Respiration in Rats. Oxid. Med. Cell. Longevity 2016, 2016, 7463407. 10.1155/2016/7463407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J.; Cai T.; Yuan Z.; Wang H.; Liu L.; Haas M.; Maksimova E.; Huang X. Y.; Xie Z. J. Binding of Src to Na+/K+-ATPase Forms a Functional Signaling Complex. Mol. Biol. Cell 2006, 17, 317–326. 10.1091/mbc.e05-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak G.; Clifton G. L.; Godwin M. L.; Bakajsova D. Activation of ERK1/2 pathway mediates oxidant-induced decreases in mitochondrial function in renal cells. Am. J. Physiol. Renal. Physiol. 2006, 291, F840–F855. 10.1152/ajprenal.00219.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taya K.; Marmarou C. R.; Okuno K.; Prieto R.; Marmarou A. Effect of secondary insults upon aquaporin-4 water channels following experimental cortical contusion in rats. J Neurotrauma. 2010, 27, 229–239. 10.1089/neu.2009.0933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y.; Shapiro A. P.; Mopidevi B. R.; Chaudhry M. A.; Liu J. Protein Carbonylation of an Amino Acid Residue of the Na/K-ATPase a1 Subunit Determines Na/K-ATPase Signaling and Sodium Transport in Renal Proximal Tubular Cells. J. Am. Heart Assoc. 2016, 5, e003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko G. Y. P.; Shi L.; Ko M. L. Circadian regulation of ion channels and their functions. J. Neurochem. 2009, 110, 1150–1169. 10.1111/j.1471-4159.2009.06223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. H.; Spees W. M.; Chiang C. W.; Trinkaus K.; Cross A. H.; Song S. K. Diffusion fMRI detects white-matter dysfunction in mice with acute optic neuritis. Neurobiol. Dis. 2014, 67, 1–8. 10.1016/j.nbd.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. T.; Sang K. M.; Maruyama T.; Narumiya S.; Doré S. Prostaglandin FP receptor inhibitor reduces ischemic brain damage and neurotoxicity. Neurobiol. Dis. 2012, 48, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J.; Liu J.; Huang Y.; Zhuo Y.; Chen W.; Duan D.; Tang X.; Lu M.; Hu Z. Olfactory Mucosa Mesenchymal Stem Cells Alleviate Cerebral Ischemia/Reperfusion Injury Via Golgi Apparatus Secretory Pathway Ca2+ -ATPase Isoform1. Front Cell Dev. Biol. 2020, 8, 586541. 10.3389/fcell.2020.586541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love D. T.; Guo C.; Nikelshparg E. I.; Brazhe N. A.; Sosnovtseva O.; Hawkins C. L. The role of the myeloperoxidase-derived oxidant hypothiocyanous acid (HOSCN) in the induction of mitochondrial dysfunction in macrophages. Redox Biol. 2020, 36, 101602. 10.1016/j.redox.2020.101602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. P.; Sullivan P. G.; Pandya J. D.; Rabchevsky A. G. Differential effects of the mitochondrial uncoupling agent, 2,4-dinitrophenol, or the nitroxide antioxidant, Tempol, on synaptic or nonsynaptic mitochondria after spinal cord injury. J. Neurosci. Res. 2009, 87, 130–140. 10.1002/jnr.21814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi A.; Fernandes D.; Bean J. L.; Michaelis M. L. Effects of paraquat-induced oxidative stress on the neuronal plasma membrane Ca(2+)-ATPase. Free Radical Biol. Med. 2009, 47, 1507–1514. 10.1016/j.freeradbiomed.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov D. B.; Juhaszova M.; Sollott S. J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R.; Zhang R.; Yang Y.; Wang X.; Yi Y.; Fan P.; Liu Z.; Chen C.; Chang J. CGA-N12, a peptide derived from chromogranin A, promotes apoptosis of Candida tropicalis by attenuating mitochondrial functions. Biochem. J. 2018, 475, 1385–1396. 10.1042/BCJ20170894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. A.; Larion S.; Mintz J. D.; Belin de Chantemèle E. J.; Fulton D. J.; Stepp D. W. Genetic Deletion of NADPH Oxidase 1 Rescues Microvascular Function in Mice With Metabolic Disease. Circ. Res. 2017, 121, 502–511. 10.1161/CIRCRESAHA.116.309965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q.; Wang Y.; Du F.; Yan S.; Hu G.; Origlia N.; Rutigliano G.; Sun Q.; Yu H.; Ainge J.; Yan S. F.; Gunn-Moore F.; Yan S. S. Overexpression of endophilin A1 exacerbates synaptic alterations in a mouse model of Alzheimer’s disease. Nat Commun. 2018, 9, 018–04389. 10.1038/s41467-018-04389-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X.; Shen W.; Yao K.; Wang H.; Liu B.; Li T.; Song L.; Diao D.; Mao G.; Huang P.; Li C.; Zhang H.; Zou Y.; Qiu Y.; Zhao Y.; Wang W.; Yang Y.; Hu Z.; Auwerx J.; Loscalzo J.; Zhou Y.; Ju Z. Fine-Tuning of PGC1α Expression Regulates Cardiac Function and Longevity. Circ. Res. 2019, 125, 707–719. 10.1161/CIRCRESAHA.119.315529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Lai Q.; Li Y.; Liu Y.; Yang M. Learning and memory improvement and neuroprotection of Gardenia jasminoides (Fructus gardenia) extract on ischemic brain injury rats. J. Ethnopharmacol. 2017, 196, 225–235. 10.1016/j.jep.2016.11.042. [DOI] [PubMed] [Google Scholar]
- Lv S.; Ding Y.; Zhao H.; Liu S.; Zhang J.; Wang J. Therapeutic Potential and Effective Components of the Chinese Herb Gardeniae Fructus in the Treatment of Senile Disease. Aging Dis. 2018, 9, 1153–1164. 10.14336/AD.2018.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F.; Li W.; Li X.; Li F.; Zhang L.; Wang B.; Huang G.; Guo X.; Wan L.; Liu Y.; Zhang S.; Kang S.; Ma J. Geniposide attenuates inflammatory response by suppressing P2Y14 receptor and downstream ERK1/2 signaling pathway in oxygen and glucose deprivation-induced brain microvascular endothelial cells. J. Ethnopharmacol. 2016, 185, 77–86. 10.1016/j.jep.2016.03.025. [DOI] [PubMed] [Google Scholar]
- Zhao B.; Sun L.-k.; Jiang X.; Zhang Y.; Kang J.; Meng H.; Li H.; Su J. Genipin protects against cerebral ischemia-reperfusion injury by regulating the UCP2-SIRT3 signaling pathway. Eur. J. Pharmacol. 2019, 845, 56–64. 10.1016/j.ejphar.2018.12.028. [DOI] [PubMed] [Google Scholar]
- Zheng Y. Q.; Liu J. X.; Wang J. N.; Xu L. Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res. 2007, 23, 86–94. 10.1016/j.brainres.2006.12.064. [DOI] [PubMed] [Google Scholar]
- Huang Z.; Xu J.; Huang X.; Sun G.; Jiang R.; Wu H.; Shan X.; Bao K.; Wu Q.; Wu H.; Tao W. Crocin induces anti-ischemia in middle cerebral artery occlusion rats and inhibits autophagy by regulating the mammalian target of rapamycin. Eur. J. Pharmacol. 2019, 857, 172424. 10.1016/j.ejphar.2019.172424. [DOI] [PubMed] [Google Scholar]
- Lan Y.The study on the Material Basis of Gardenia Jasminoides fried into carbon. Chin. Acad. Chine Med Sci, Beijing, 2014. [Google Scholar]
- Zhang X. H.; Lei H.; Liu A. J.; Zou Y. X.; Shen F. M.; Su D. F. Increased oxidative stress is responsible for severer cerebral infarction in stroke-prone spontaneously hypertensive rats. CNS Neurosci. Ther. 2011, 17, 590–598. 10.1111/j.1755-5949.2011.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longa E. Z.; Weinstein P. R.; Carlson S.; Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989, 20, 84–91. 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- Wu C.; Chen J.; Chen C.; Wang W.; Wen L.; Gao K.; Chen X.; Xiong S.; Zhao H.; Li S. Wnt/β-catenin coupled with HIF-1α/VEGF signaling pathways involved in galangin neurovascular unit protection from focal cerebral ischemia. Sci. Rep. 2015, 5, 16151. 10.1038/srep16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.; Jia L.; Wang J.; Zou W.; Yang H.; Xiao X. Cold/hot pad differentiating assay of property differences of Mahuang and Maxingshigan decoctions. Pharm Biol. 2016, 54, 1298–1302. 10.3109/13880209.2015.1057650. [DOI] [PubMed] [Google Scholar]
- Divakaruni A. S.; Rogers G. W.; Murphy A. N. Measuring Mitochondrial Function in Permeabilized Cells Using the Seahorse XF Analyzer or a Clark-Type Oxygen Electrode. Curr. Protoc. Toxicol. 2014, 60, 1–16. [DOI] [PubMed] [Google Scholar]
- Paital B.; Samanta L. A comparative study of hepatic mitochondrial oxygen consumption in four vertebrates by using Clark-type electrode. Acta Biol. Hung. 2013, 64, 152–160. 10.1556/ABiol.64.2013.2.2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.