Abstract
Malignant transformation of cells is frequently associated with defective HLA class I antigen processing machinery (APM) component expression. This abnormality may have functional relevance, since it may have a negative impact on tumor cell recognition by cognate T cells. Furthermore, HLA class I APM abnormalities appear to have clinical significance, since they are associated with poor prognosis in several malignant diseases and may play a role in the resistance to immune checkpoint inhibitor-based immunotherapy. In this paper, we have reviewed the literature describing abnormalities in HLA class I APM component expression in many types of cancer. These abnormalities have been reported in all types of cancer analyzed with a frequency ranging between a minimum of 35.8% in renal cancer and a maximum of 87.9% in thyroid cancer for HLA class I heavy chains. In addition, we have described the molecular mechanisms underlying defects in HLA class I APM component expression and function by malignant cells. Lastly, we have discussed the clinical significance of HLA class I APM component abnormalities in malignant tumors.
Keywords: NIBIT 2016, HLA class I antigens, Targeted therapy, Immunotherapy, Immune checkpoint
Introduction
It has been known for many years that malignant transformation of human cells may be associated with changes in the expression of HLA class I and HLA class II antigens [1, 2]. The molecular basis of these changes, their functional significance and their clinical relevance have been extensively investigated. Analysis of HLA class I antigens has been emphasized because of their crucial role in the presentation of tumor antigen-derived peptides to cognate T cells which are considered to be the major players in anti-tumor defense [3]. These studies have shown that defects in HLA class I antigen expression are caused by structural and epigenetic mechanisms [1, 4], the latter being much more frequent than the former ones. Defects in HLA class I antigen expression and/or function by tumor cells have a negative impact on their interactions with cognate T cells; as a result, they provide tumor cells with an escape mechanism from immune surveillance [5]. The clinical relevance of these “in vitro” findings is indicated by the association between HLA class I antigen downregulation in malignant tumors and patients’ shorter survival [6].
Analysis of HLA class I antigen defects in malignant cells has been a “hot” research topic at the end of the previous century and at the beginning of the current one. Interest in this topic was stimulated, at least in part, by the potential negative impact of HLA class I APM defects on the therapeutic efficacy of T cell-based immunotherapy for the treatment of malignant diseases [7]. The disappointing clinical results generated by T cell-based immunotherapy which utilized shared tumor antigens as immunogens and/or targets triggered a significant amount of skepticism among tumor immunologists and especially clinical oncologists about the clinical usefulness of this type of immunotherapy for the treatment of malignant diseases [8, 9]. One of the many consequences of this attitude was the waning of the interest in the characterization of HLA class I APM in malignant tumors. This scenario has dramatically changed in recent years because the impressive clinical responses to immunotherapy with checkpoint-specific monoclonal antibodies (mAb)s have restored tumor immunologists’ confidence in the ability of patients’ immune system to recognize and eliminate tumor cells [10]. In addition, these impressive clinical responses have convinced clinical oncologists, even the most skeptical ones that T cell-based immunotherapy can be a very effective strategy to treat malignant diseases in the clinical setting. As a matter of fact, immunotherapy has become a widely used strategy for the treatment of an increasing number of cancers. The mechanism underlying the anti-tumor activity of immunotherapy with checkpoint inhibitors is represented by the targeting of tumor cells by cognate T cells unleashed by checkpoint inhibitors [11]. Crucial to the success of this mechanism is a fully functional HLA class I APM, since the latter plays a major role in the generation and expression on tumor cells of HLA class I antigen-tumor antigen-derived peptide complexes. The latter mediate the interactions of tumor cells with cognate CD8+ T cells. The available information, although scant supports this possibility since loss of β2-microglobulin (β2m) in melanoma tumors and lung tumors has been found to be associated with the development of resistance to immunotherapy with anti-programmed death 1 receptor (PD-1) mAbs [12–14]. Furthermore, HLA class I antigen and checkpoint interactions have been found to result in clinically relevant phenotypes. Specifically, HLA class I defects in combination with Programmed Death-Ligand 1 (PD-L1) expression on tumor cells are associated with poor prognosis in patients with intra-hepatic cholangiocarcinoma, although the two parameters individually are not associated with the clinical course of the disease [15]. Furthermore, clinical responses to immunotherapy with anti-PD-1 mAbs in patients with esophageal cancer have been documented only in those with high HLA class I antigen expression in their tumors [16]. These clinical findings have rekindled interest in HLA class I APM component expression in malignant tumors, since this information may contribute to optimize the use of checkpoint inhibitor-based immunotherapy by guiding the selection of patients to be treated and by suggesting therapies which combine the unleashing of tumor antigen-specific T cells with approaches to upregulate HLA class I APM component expression, to mention a few possibilities. Therefore, in the present paper, we have first reviewed the literature describing abnormalities in HLA class I APM component expression in malignant tumors. Then we have described the molecular mechanisms underlying abnormalities in HLA class I APM component expression in malignant cells and the potential strategies to counteract these abnormalities. Lastly, we have discussed the clinical significance of HLA class I APM component abnormalities in malignant tumors.
Frequency of abnormalities in HLA class I APM component expression
The information presented in this section is derived from the review of 186 papers which are listed as references 35, 39, 40, and 46–233 in the chapter by Cai et al. [17]. These papers have analyzed over 16,000 surgically removed primary malignant tumors for the expression of HLA class I APM components. The malignancies with the largest number of samples analyzed are colorectal cancer (5276) and breast cancer (1872); those with the lowest number of samples analyzed are cholangiocarcinoma and thyroid cancer [17]. Information about defects in HLA class I APM component expression and/or function in metastases has not been included in this review, since the number of samples analyzed is not sufficient to draw any significant conclusions. Furthermore, most studies have analyzed lymph node metastases rather than distant metastases. Here it suffices to say that comparison of a small number of primary tumors and metastases has shown that the frequency of HLA class I APM component abnormalities in metastases is higher than that found in primary tumors [18, 19]. This difference has been suggested to reflect a mechanism by which metastasizing cells escape immune surveillance.
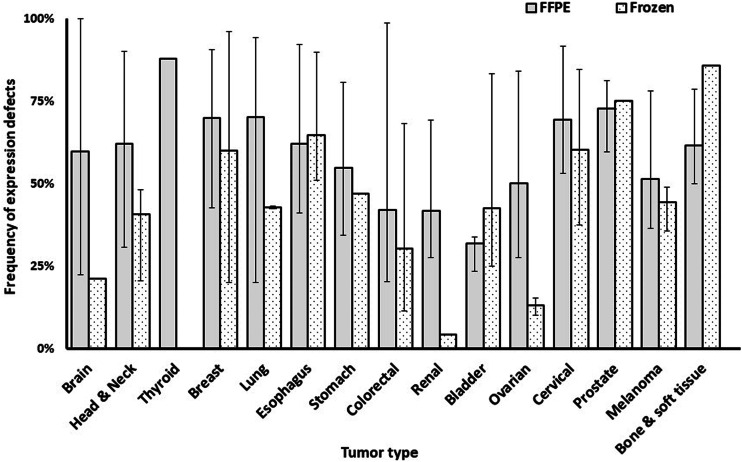
The results presented in Figs. 1, 2, 3, 4 are derived from the analysis of tissue sections of formalin-fixed, paraffin-embedded tumors. The immunohistochemical (IHC) staining performed with this substrate appears to be less sensitive than that which utilizes frozen tissue sections as a substrate (Fig. 5). Therefore, it is likely that the frequency of abnormalities detected with the latter substrate is lower than that detected with the former one. It is also noteworthy that IHC staining of formalin-fixed, paraffin-embedded tissue sections provides information about the expression of the subunits of HLA class I antigens, but provides no information about the expression of HLA class I heavy chain-β2m-tumor antigen-derived peptide complexes since this complex is disrupted by the fixation procedure. As a result, IHC staining of formalin-fixed, paraffin-embedded tissue sections provides no information about the expression of HLA class I alleles since the expression of the conformational epitopes which define HLA class I alleles requires the association of HLA class I heavy chain with β2m and with peptide. Information about the expression of HLA class I alleles in tissues requires the use of frozen tissue sections as substrates. Because of the practical difficulties to obtain and use frozen tissues as substrates in IHC tests, only a limited number of studies have analyzed the expression of HLA class I alleles in malignant tumors. In addition, these studies have analyzed only a small number of HLA class I alleles, since only a few of the available mouse HLA class I allele-specific mAbs yield reliable results when used in IHC assays. Therefore, the information about loss or downregulation of HLA class I alleles in malignant tumors has not been included in this review, since the number of tumor samples analyzed for this abnormality is too small to draw definitive conclusions.
Fig. 1.
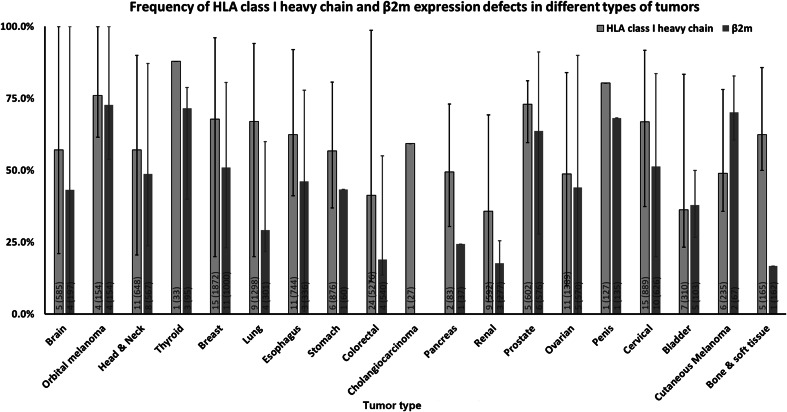
Frequency of HLA class I heavy chain and β2m expression defects by tumor type. The number of included studies (number of cases) for each tumor type is presented within each bar. Error bars represent the maximum and minimum expression defect frequency reported
Fig. 2.
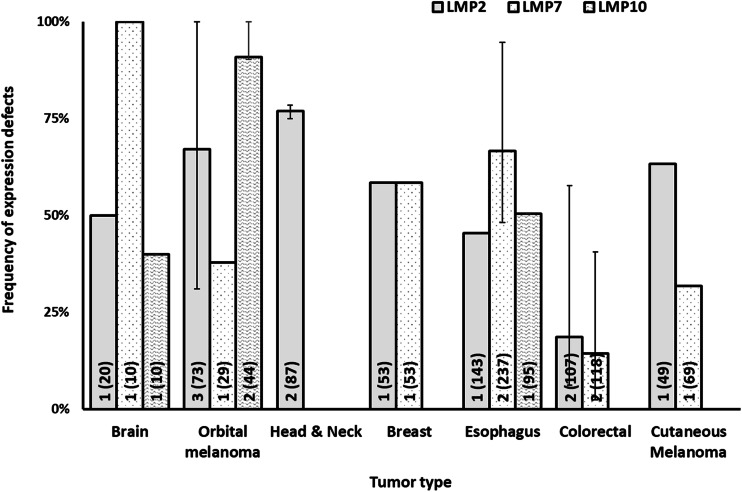
Frequency of defects in HLA class I APM immunoproteasome subunit expression by tumor type. The number of included studies (number of cases) for each tumor type is presented within each bar. Error bars represent the maximum and minimum expression defect frequency reported
Fig. 3.
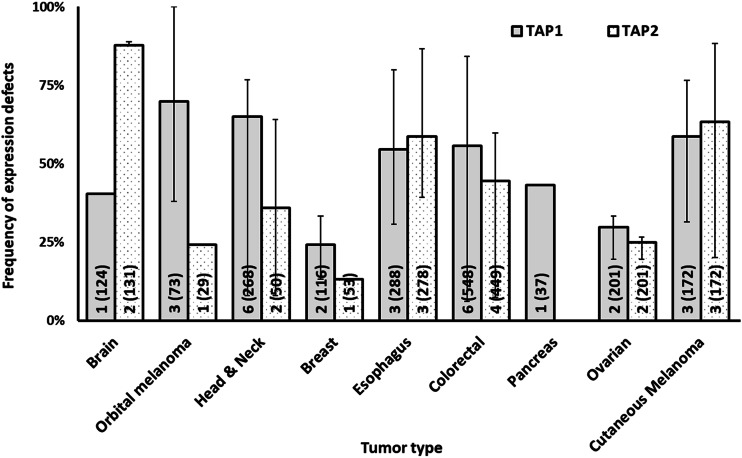
Frequency of defects in HLA class I APM transporter molecule expression by tumor type. The number of included studies (number of cases) for each tumor type is presented within each bar. Error bars represent the maximum and minimum expression defect frequency reported
Fig. 4.
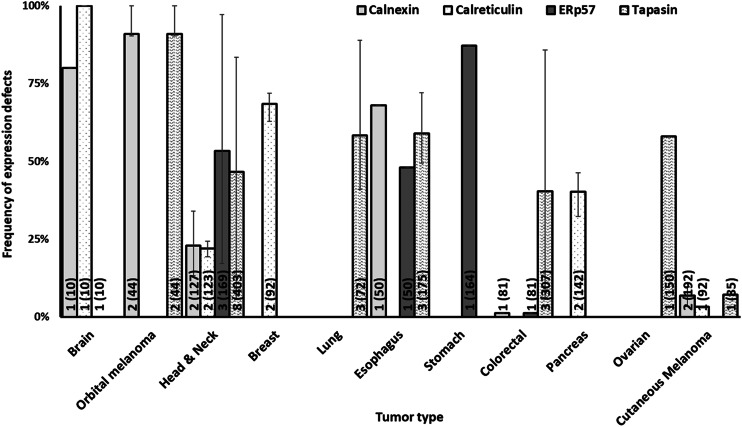
Frequency of defects in HLA class I APM chaperone expression by tumor type. The number of included studies (number of cases) for each tumor type is presented within each bar. Error bars represent the maximum and minimum expression defect frequency reported
Fig. 5.
Higher frequency of HLA class I expression defects in formalin-fixed paraffin embedded (FFPE) tissue sections than in and frozen tissue sections, by tumor type. The number of included studies (number of cases) for each tumor type is presented within each bar. Error bars represent the maximum and minimum expression defect frequency reported
It is also noteworthy that the mAbs utilized to monitor the expression of the gene products of HLA-A, B and C loci recognize epitopes selectively expressed on the gene products of each of the loci. IHC staining with these mAbs does not have the discriminatory power to detect the selective downregulation or lack of expression of the gene products of one of the loci encoded in a tumor cell. This result is not unique of IHC staining of formalin-fixed, paraffin-embedded tissue sections and of mAbs which recognize epitopes expressed in these substrates. A similar result has been generated by studies performed with mAbs which recognize framework epitopes of HLA class I antigens utilizing flow cytometric analysis of viable cells stained with mAbs or IHC staining of frozen tissue sections [20, 21].
HLA class I heavy chain and β2m expression have been investigated in a significantly higher number of tumor samples than that of the other APM components. Among the latter ones, transporter associated with antigen processing 1 (TAP1) expression (36 studies, 2487 cases) has been tested in a significantly higher number of tumor samples than that of other APM components.
The data summarized in Figs. 1, 2, 3 and 4 clearly indicate that abnormalities in the expression of HLA class I APM components have been found in all the solid tumors analyzed. The frequency of defects in HLA class I heavy chains is higher than that of β2m in all the tumors analyzed but bladder carcinoma and cutaneous melanoma. In the latter, the frequency of defective β2m expression is higher than that of HLA class I heavy chain, while in the former one, the frequency of defects is similar for the two subunits of HLA class I antigens. The frequency of defects ranges between a minimum of 35.8% in renal cancer and a maximum of 87.9% in thyroid cancer for HLA class I heavy chain and between a minimum of 16.7% in bone and soft tissue cancer and a maximum of 71.6% in thyroid cancer for β2m. The three subunits of immunoproteasome, low molecular weight polypeptides (LMP)s—LMP2, LMP7 and LMP10 have a frequency of defective expression of at least 40% in all the tumors analyzed with the exception of ovarian carcinoma in which LMP2 and LMP7 have a defective expression of about 20% of the tumor samples analyzed. The subunits of the transporter associated with antigen processing, TAP1 and TAP2 have a defective expression of at least 40% in all the tumors analyzed with the exception of orbital melanoma in which TAP2 has a frequency of defective expression of about 25%, and of breast and ovarian cancer in which TAP1 and TAP2 have a frequency of defective expression of less than 30%. Lastly, the chaperone molecules calnexin, calreticulin, endoplasmic reticulum protein 57 (ERp57) and tapasin have a defective expression of at least 40% in all the tumors analyzed except for head and neck cancer in which calnexin and calreticulin have a frequency of defective expression of about 25%, colorectal cancer in which calnexin and ERp57 have a frequency of defective expression of less than 2% and cutaneous melanoma in which calnexin, calreticulin and tapasin have a defective expression of less than 10%.
Generation of tumors with defective HLA class I APM component expression
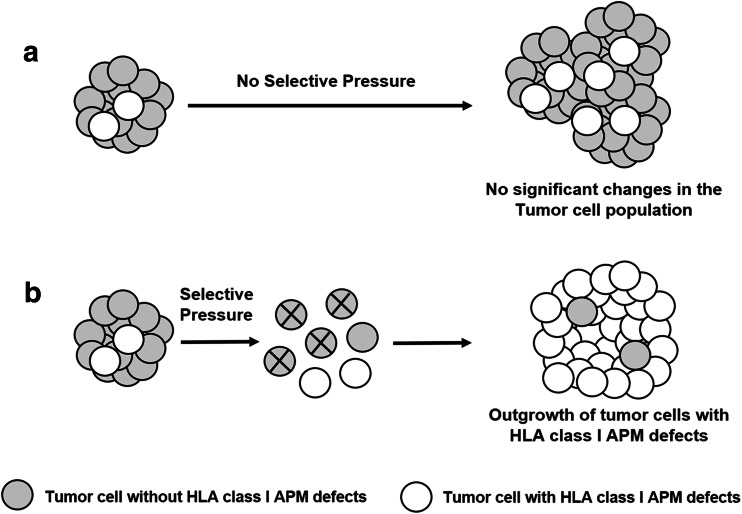
Abnormalities in HLA class I APM component expression and/or function do not appear to have an effect on the growth of tumor cells in an immunologically naïve environment both in vitro and in vivo (Fig. 6a). The most convincing evidence supporting this conclusion is the lack of detectable changes in the growth of cells in which the expression and/or function of a HLA class I APM component(s) have been restored by replacing a structurally mutated gene(s) with a wild type gene(s). This result implies that the formation of tumors with a defective HLA class I APM component expression requires two events. The first one is represented by the introduction because of a structural mutation or more likely an epigenetic mechanism in at least one tumor cell of a defect which interferes with the function of HLA class I APM and provides tumor cells with a potential escape mechanism if exposed to selective pressure. The second event is represented by the development of a tumor antigen-specific T cell-dependent immune response which imposes selective pressure on the targeted tumor cell population and facilitates the outgrowth of tumor cells with the ability to escape from immune recognition because of HLA class I APM defects (Fig. 6b). If this interpretation is correct, one can assume that the presence of a malignant tumor with defects in HLA class I APM component expression in a patient is an indication of the development in the patient under investigation of a tumor antigen-specific immune response which imposes selective pressure on tumor cell population. On the other hand, the lack of detection in a patient of tumors with defective HLA class I APM component expression may reflect two distinct additional mechanisms. One is represented by the patient under investigation’s inability to develop an effective tumor antigen-specific immune response which can facilitate the outgrowth of tumor cells with HLA class I APM defects. An alternative mechanism is represented by the too short time interval between the development of a tumor antigen-specific immune response and the time of observation which is not sufficient for tumor cells with HLA class I APM defects to outgrow other tumor cell subpopulations present in tumors in a patient under investigation and to become the major population.
Fig. 6.
Role of selective pressure in the generation of tumors with defective HLA class I APM component expression. a Abnormalities in HLA class I APM component expression and/or function do not appear to have an effect on the growth of tumor cells in an immunologically naïve environment. As a result, in the absence of selective pressure the representation of subpopulations with defective HLA class I APM expression and of those with no detectable defects is not likely to change in the course of the disease. b The selective pressure imposed by a host’s immune response facilitates the outgrowth of tumor cells which can escape recognition by cognate T cells because of defective presentation of tumor antigen-derived peptides caused by abnormality (ies) in HLA class I APM. As a result, tumor cells with defective HLA class I APM become the major population in a tumor
Growing evidence indicates that multiple structural and epigenetic defects may be present in malignant cells in a tumor. For instance, we have found β2m loss, HLA-A2 antigen loss, and HLA-B and -C antigen downregulation in one melanoma cell line [22] and selective HLA-A2 antigen loss in conjunction with a loss-of-function TAP1 mutation near the ATP-binding site in another melanoma cell line [23]. More recently, we have found a selective HLA-A3 antigen loss in association with one HLA haplotype loss and a germline-originated tapasin frame-shift mutation in a metastatic melanoma cell line with HLA class I antigen downregulation derived from a patient with progressive disease [4]. These combinations of HLA class I APM defects in tumor cells are likely to reflect the plasticity of tumor cells and of patient’s immune system. This plasticity provides on one hand a patient’s immune system with the ability to adapt to changes in tumor cells and to change the target of immune response, and on the other hand, tumor cells with the ability to develop multiple escape mechanisms which allow them to avoid a host’s immune responses. From a therapeutic view point, the presence of multiple escape mechanisms in malignant cells makes the development of effective strategies to counteract them more challenging.
Molecular mechanisms underlying HLA class I APM component defects in malignant cells
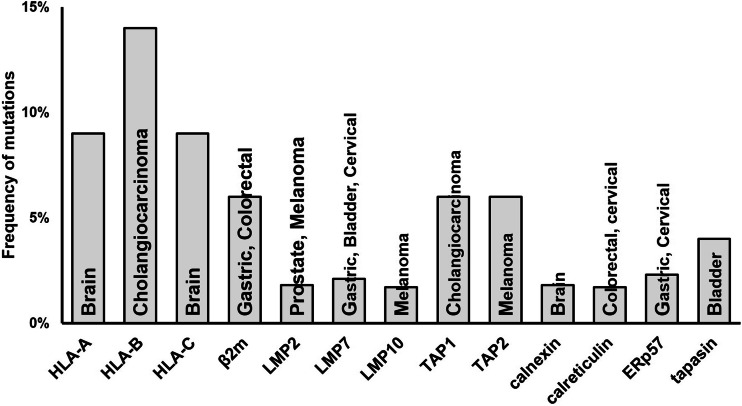
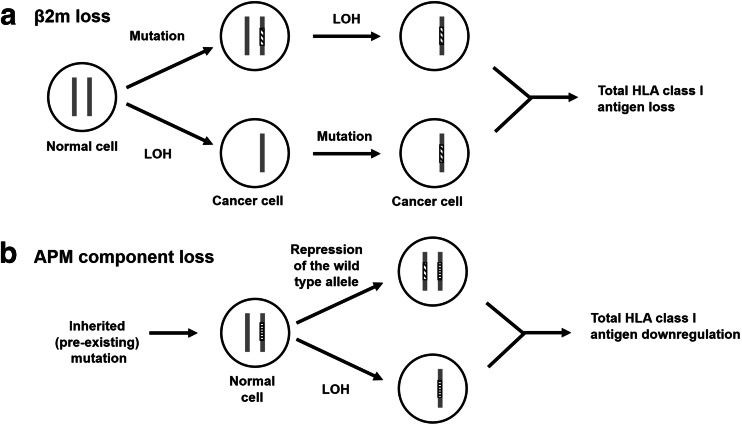
Multiple molecular mechanisms have been shown to underlie defective HLA class I APM component expression and/or function in malignant cells. They include structural mutations in the genes encoding these molecules and epigenetic mechanisms. The frequency of structural mutations in the genes encoding HLA class I APM components is summarized in Fig. 7. This frequency is significantly lower than that of defective expression for all the HLA class I APM components (Figs. 1, 2, 3, 4). As an example, the frequency of defective β2m protein expression ranges from a minimum of 16.7% in bone and soft tissue cancer to a maximum of 71.6% in thyroid cancer. The data generated by the TCGA Research Network (http://cancergenome.nih.gov/) suggest that the frequency of β2m mutation ranges from a minimum of 1% in lung, esophageal, bladder, and ovarian cancer, to a maximum of 6% in gastric and colorectal cancer. However, these values have to be interpreted with caution, since a recent analysis of patient-derived xenografts (PDXs) established from lung cancer reported a markedly higher frequency of β2m mutations [24]. It should also be noted that the loss of β2m and APM component expression requires two events since these molecules such as other HLA class I APM components with the exception of HLA class I heavy chains are encoded by two codominant genes. The mechanism described for β2m loss is represented by inactivation of one gene because of a mutation and loss of the chromosome #15 or of the region which carries the wild-type β2m gene (Fig. 8a) [25]. The chronological order of these two events has not been determined yet. The mechanism underlying APM component loss has been characterized in only a few cases and in all of them it has been found to be different from that described for β2m (Fig. 8b). Loss of TAP1 in a squamous cell lung cancer cell line [26] and of tapasin in a melanoma cell line [22] have been shown to be caused by a cancer-unrelated germline mutation in combination with a cancer-related repression of the other allele. As discussed elsewhere [27], it is not known at present whether the different mechanisms underlying β2m and APM component loss reflect a lower genetic stability of the β2m locus and/or the involvement of APM in crucial alternative functions in malignant cells.
Fig. 7.
Frequency of HLA class I APM component mutations. The tumor type(s) with the highest frequency of mutation for each component is indicated within each bar
Fig. 8.
Events leading to lack of β2m (a) and APM component (b) expression. a β2m loss results from two cancer-related events: inactivation of one β2m encoding gene because of a mutation and LOH on chromosome #15 which carries the wild-type β2m gene. b APM component loss is caused by one germline mutation and one somatic cancer-related mutation, or by one LOH on chromosome #6 and one germline mutation in malignant cells (LOH: loss of heterozygosity)
An additional mechanism which causes irreversible loss of HLA class I heavy chains encoded by HLA-A, HLA-B and HLA-C genes located on the homologous paternal and maternal chromosome 6 is represented by loss of heterozygosity (LOH) in chromosome region 6p21. This region carries the major histocompatibility complex [28]. This mechanism has been described in non-small cell lung cancer with a frequency of about 41% [29]. LOH for β2m which is encoded in chromosome 15 [30] has been described in renal cancer with a frequency of 7% [31]. LOH for HLA and β2m has been described in glioblastoma, laryngeal carcinoma, bladder carcinoma colorectal cancer and melanoma with a frequency of at least 16% [14, 31–34]. LOH for β2m is associated with shorter patients’ survival in melanoma [14], This association has also been found for LOH for HLA which has been shown to be associated with shorter patients’ survival in glioblastoma and non-small cell lung cancer [29, 35, 36]. The association between LOH for HLA and shorter patients’ survival may reflect the increased potential of escape mechanisms available to malignant cells with LOH for HLA because of the markedly reduced number of tumor antigen-derived peptides which can be presented to cognate T cells. This mechanism can also explain the association between LOH for HLA and response to immunotherapy with checkpoint inhibitors [36]. On the other hand, no mechanism is readily available to explain the association between LOH for β2m and shorter patients’ survival in melanoma. This conclusion applies also to the association in melanoma between LOH for β2m and increased frequency of patients who do not respond to immunotherapy with checkpoint inhibitors [14].
Epigenetic mechanisms appear to be the most frequent mechanisms underlying defects in HLA class I APM component expression. These defects have functional relevance, since they reduce the ability of tumor cells to generate peptides from tumor antigens, process them and present them to cognate T cells. Hypermethylation of the HLA-A, B, and C heavy chains, β2m and APM component encoding gene promoter regions [37–39] has been found to underlie defects in HLA class I APM component expression in melanoma [4], nasopharyngeal cancer [40] and esophageal squamous cell carcinoma [37] cell lines. In some cases, these defects have been identified also in the tumors from which the cell lines have been originated [4]. The expression level of the downregulated molecules can be restored by treating cells with demethylating agents such as azacytidine and decitabine. Restoration of the HLA class I APM component expression has been found to be associated with an increased susceptibility of target cells to recognition by cognate T cells in vitro [4, 41]. In addition, demethylating agents have been shown in a mouse model of mammary carcinoma and mesothelioma to markedly enhance the anti-tumor activity of immune checkpoint-specific mAbs [42, 43]. These results argue in favor of the use of demethylating agents in combination with T cell-based immunotherapy for the treatment of patients bearing tumors with HLA class I APM defects caused by hypermethylation.
Another mechanism involved in HLA class I APM component downregulation is unbalanced histone acetylation. Merkel cell carcinoma cells reacquire HLA class I APM component expression following in vitro and in vivo treatment with histone deacetylase (HDAC) inhibitors. At pharmacological level the latter compounds can enhance the in vitro susceptibility of human breast and prostate cancer cell lines to recognition by cognate T cells [44]. These findings have been used as the rationale to suggest the use of HDAC inhibitors in combination with checkpoint inhibitors, to enhance the efficacy of this type of immunotherapy.
Activation of the mitogen-activated protein kinase (MAPK) pathway has been shown to downregulate HLA class I APM component [45, 46] and tumor antigen [47] expression. These changes result in a defective synthesis and expression of HLA class I heavy chain-β2m-tumor antigen-derived peptide complex which lead to lack of recognition of target cells by cognate T cells. This mechanism underlies the lack of recognition of head and neck cancer cells by cognate T cells, although they express HLA class I antigens [48]. Recognition of head and neck cancer cells by cognate T cells could be restored either by treating target cells with IFNɣ which upregulated HLA class I APM component expression or by pulsing target cells with the peptides recognized by cognate T cells. This mechanism appears also to be involved in the disease recurrence in a patient with metastatic melanoma who had initially experienced a complete radiologic complete regression following the T cell-based immunotherapy [49]. Inhibition of the activation of MAPK pathway with BRAF inhibitors in melanoma cells harboring BRAF mutations [45] or with the epidermal growth factor receptor (EGFR)-specific mAb cetuximab in head and neck cancer cells [50] upregulates HLA class I APM component and tumor antigen expression and restores the susceptibility of tumor cells to recognition by cognate T cells both in vitro and in vivo.
Although not proven experimentally, the available evidence suggests that the frequency of defects in HLA class I APM component expression is higher in surgically removed tumors than in the corresponding cell lines. Whether this difference reflects the effect of cytokines and variables present in the tumor microenvironment is not known. In this regard, to the best of our knowledge, no information is available about the effect, if any of hypoxia, a hallmark of solid tumor microenvironment on HLA class I APM component expression by tumor cells [51, 52].
Clinical significance of HLA class I APM component defects in malignant cells
A number of studies have investigated the clinical significance of defects in HLA class I APM component expression and/or function in malignant cells by correlating them with disease-free and overall survival. These studies have shown that downregulation of several HLA class I APM components is associated with the clinical course of the disease in many types of cancer. The following are examples of these associations. TAP1 and TAP2 downregulation has been reported to be associated with the development of metastasis and reduced disease-free and overall survival in melanoma patients [19, 53]. Furthermore, among the HLA class I APM chaperones, calreticulin and tapasin expression has been found to be associated with a favorable 5 year survival rate in colorectal cancer [54, 55], while tapasin downregulation has been found to be a poor prognostic marker in laryngeal squamous cell carcinoma [56]. Lastly, defective HLA class I expression has been reported to be associated with poor overall survival in head and neck cancer [56–59], esophageal cancer [60–64], and melanoma [19, 53].
Conclusions
The evidence in the literature clearly indicates HLA class I APM component defects are present with high frequency in all the solid tumors which have been analyzed. These defects are caused more frequently by epigenetic mechanism than by structural mutations. Furthermore, these defects appear to play a role in the clinical course of many types of cancer. It is likely these defects will be a mechanism of resistance to immune checkpoint inhibitor-based immunotherapy.
Abbreviations
- APM
Antigen processing machinery
- β2m
β2-Microglobulin
- EGFR
Epidermal growth factor receptor
- ERp57
Endoplasmic reticulum protein 57
- HDAC
Histone deacetylase
- HLA
Human leukocyte antigen
- LMP
Low molecular weight polypeptide
- mAb
Monoclonal antibody
- MAPK
Mitogen-activated protein kinases
- PD-1
Programmed death 1 receptor
- PD-L1
Programmed death ligand 1
- PDX
Patient-derived xenograft
- TAP
Transporter associated with antigen processing
Author contributions
All co-authors conceived, wrote and edited the manuscript.
Funding
This study was supported by National Cancer Institute (NCI) R21 (Grant no. CA164756), National Natural Science Foundation of China (Grant no. 81201948) and National Foundation of Chongqing (Grant No. cstc2012jjA10026).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
This paper is a Focussed Research Review based on a presentation given at the Fourteenth Meeting of the Network Italiano per la Bioterapia dei Tumori (NIBIT) on Cancer Bio-Immunotherapy, held in Siena, Italy, 13th–15th October 2016. It is part of a series of Focussed Research Reviews and meeting report in Cancer Immunology, Immunotherapy.
References
- 1.Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008;27(45):5869–5885. doi: 10.1038/onc.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seliger B, Kloor M, Ferrone S. HLA class II antigen-processing pathway in tumors: molecular defects and clinical relevance. Oncoimmunology. 2017;6(2):e1171447. doi: 10.1080/2162402X.2016.1171447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 4.Chang CC, Pirozzi G, Wen SH, Chung IH, Chiu BL, Errico S, Luongo M, Lombardi ML, Ferrone S. Multiple structural and epigenetic defects in the human leukocyte antigen class I antigen presentation pathway in a recurrent metastatic melanoma following immunotherapy. J Biol Chem. 2015;290(44):26562–26575. doi: 10.1074/jbc.M115.676130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrido F, Perea F, Bernal M, Sanchez-Palencia A, Aptsiauri N, Ruiz-Cabello F. The escape of cancer from T cell-mediated immune surveillance: HLA class I loss and tumor tissue architecture. Vaccines (Basel) 2017 doi: 10.3390/vaccines5010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/S0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 7.Hicklin DJ, Marincola FM, Ferrone S. HLA class I antigen downregulation in human cancers: T-cell immunotherapy revives an old story. Mol Med Today. 1999;5(4):178–186. doi: 10.1016/S1357-4310(99)01451-3. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Dudley ME, Restifo NP. Cancer immunotherapy. N Engl J Med. 2008;359(10):1072. doi: 10.1056/NEJMc081511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bot A, Obrocea M, Marincola FM. Cancer vaccines at an inflexion point: what next? J Transl Med. 2011;9:148. doi: 10.1186/1479-5876-9-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 11.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, Torrejon DY, Abril-Rodriguez G, Sandoval S, Barthly L, Saco J, Homet Moreno B, Mezzadra R, Chmielowski B, Ruchalski K, Shintaku IP, Sanchez PJ, Puig-Saus C, Cherry G, Seja E, Kong X, Pang J, Berent-Maoz B, Comin-Anduix B, Graeber TG, Tumeh PC, Schumacher TN, Lo RS, Ribas A. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gettinger S, Choi J, Hastings K, Truini A, Datar I, Sowell R, Wurtz A, Dong W, Cai G, Melnick MA, Du VY, Schlessinger J, Goldberg SB, Chiang A, Sanmamed MF, Melero I, Agorreta J, Montuenga LM, Lifton R, Ferrone S, Kavathas P, Rimm DL, Kaech SM, Schalper K, Herbst RS, Politi K. Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov. 2017;7(12):1420–1435. doi: 10.1158/2159-8290.CD-17-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sade-Feldman M, Jiao YJ, Chen JH, Rooney MS, Barzily-Rokni M, Eliane JP, Bjorgaard SL, Hammond MR, Vitzthum H, Blackmon SM, Frederick DT, Hazar-Rethinam M, Nadres BA, Van Seventer EE, Shukla SA, Yizhak K, Ray JP, Rosebrock D, Livitz D, Adalsteinsson V, Getz G, Duncan LM, Li B, Corcoran RB, Lawrence DP, Stemmer-Rachamimov A, Boland GM, Landau DA, Flaherty KT, Sullivan RJ, Hacohen N. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat Commun. 2017;8(1):1136. doi: 10.1038/s41467-017-01062-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabbatino F, Villani V, Yearley JH, Deshpande V, Cai L, Konstantinidis IT, Moon C, Nota S, Wang Y, Al-Sukaini A, Zhu AX, Goyal L, Ting DT, Bardeesy N, Hong TS, Fernandez-del Castillo C, Tanabe KK, Lillemoe KD, Ferrone S, Ferrone CR. PD-L1 and HLA class I antigen expression and clinical course of the disease in intrahepatic cholangiocarcinoma. Clin Cancer Res. 2016;22(2):470–478. doi: 10.1158/1078-0432.CCR-15-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito S, Okano S, Morita M, Saeki H, Tsutsumi S, Tsukihara H, Nakashima Y, Ando K, Imamura Y, Ohgaki K, Oki E, Kitao H, Mimori K, Maehara Y. Expression of PD-L1 and HLA class I in esophageal squamous cell carcinoma: prognostic factors for patient outcome. Ann Surg Oncol. 2016;23(Suppl 4):508–515. doi: 10.1245/s10434-016-5376-z. [DOI] [PubMed] [Google Scholar]
- 17.Cai L, Michelakos T, Yamada T, Fan S, Schwab JH, Ferrone CR, Ferrone S. HLA class I antigen-processing machinery in cancer. In: Lisa H, editor. Cancer immunotherapy principles and practice. 1. New York: Springer; 2017. pp. 44–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaklamanis L, Leek R, Koukourakis M, Gatter KC, Harris AL. Loss of transporter in antigen processing 1 transport protein and major histocompatibility complex class I molecules in metastatic versus primary breast cancer. Cancer Res. 1995;55(22):5191–5194. [PubMed] [Google Scholar]
- 19.Kageshita T, Hirai S, Ono T, Hicklin DJ, Ferrone S. Downregulation of HLA class I antigen-processing molecules in malignant melanoma: association with disease progression. Am J Pathol. 1999;154(3):745–754. doi: 10.1016/S0002-9440(10)65321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kageshita T, Wang Z, Calorini L, Yoshii A, Kimura T, Ono T, Gattoni-Celli S, Ferrone S. Selective loss of human leukocyte class I allospecificities and staining of melanoma cells by monoclonal antibodies recognizing monomorphic determinants of class I human leukocyte antigens. Cancer Res. 1993;53(14):3349–3354. [PubMed] [Google Scholar]
- 21.Wang Z, Marincola FM, Rivoltini L, Parmiani G, Ferrone S. Selective histocompatibility leukocyte antigen (HLA)-A2 loss caused by aberrant pre-mRNA splicing in 624MEL28 melanoma cells. J Exp Med. 1999;190(2):205–215. doi: 10.1084/jem.190.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang CC, Campoli M, Restifo NP, Wang X, Ferrone S. Immune selection of hot-spot beta 2-microglobulin gene mutations, HLA-A2 allospecificity loss, and antigen-processing machinery component downregulation in melanoma cells derived from recurrent metastases following immunotherapy. J Immunol. 2005;174(3):1462–1471. doi: 10.4049/jimmunol.174.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seliger B, Ritz U, Abele R, Bock M, Tampe R, Sutter G, Drexler I, Huber C, Ferrone S. Immune escape of melanoma: first evidence of structural alterations in two distinct components of the MHC class I antigen processing pathway. Cancer Res. 2001;61(24):8647–8650. [PubMed] [Google Scholar]
- 24.Pereira C, Gimenez-Xavier P, Pros E, Pajares MJ, Moro M, Gomez A, Navarro A, Condom E, Moran S, Gomez-Lopez G, Grana O, Rubio-Camarillo M, Martinez-Marti A, Yokota J, Carretero J, Galbis JM, Nadal E, Pisano D, Sozzi G, Felip E, Montuenga LM, Roz L, Villanueva A, Sanchez-Cespedes M. Genomic profiling of patient-derived xenografts for lung cancer identifies B2M inactivation Impairing Immunorecognition. Clin Cancer Res. 2017;23(12):3203–3213. doi: 10.1158/1078-0432.CCR-16-1946-T. [DOI] [PubMed] [Google Scholar]
- 25.Goodfellow PN, Jones EA, Van Heyningen V, Solomon E, Bobrow M, Miggiano V, Bodmer WF. The beta2-microglobulin gene is on chromosome 15 and not in the HL-A region. Nature. 1975;254(5497):267–269. doi: 10.1038/254267a0. [DOI] [PubMed] [Google Scholar]
- 26.Chen HL, Gabrilovich D, Tampe R, Girgis KR, Nadaf S, Carbone DP. A functionally defective allele of TAP1 results in loss of MHC class I antigen presentation in a human lung cancer. Nat Genet. 1996;13(2):210–213. doi: 10.1038/ng0696-210. [DOI] [PubMed] [Google Scholar]
- 27.Chang CC, Campoli M, Ferrone S. Classical and nonclassical HLA class I antigen and NK Cell-activating ligand changes in malignant cells: current challenges and future directions. Adv Cancer Res. 2005;93:189–234. doi: 10.1016/S0065-230X(05)93006-6. [DOI] [PubMed] [Google Scholar]
- 28.Francke U, Pellegrino MA. Assignment of the major histocompatibility complex to a region of the short arm of human chromosome 6. Proc Natl Acad Sci USA. 1977;74(3):1147–1151. doi: 10.1073/pnas.74.3.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGranahan N, Rosenthal R, Hiley CT, Rowan AJ, Watkins TBK, Wilson GA, Birkbak NJ, Veeriah S, Van Loo P, Herrero J, Swanton C, Consortium TR. Allele-specific HLA loss and immune escape in lung cancer evolution. Cell. 2017;171(6):1259–1271 e11. doi: 10.1016/j.cell.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faber HE, Kucherlapati RS, Poulik MD, Ruddle FH, Smithies O. beta2-microglobulin locus on human chromosome 15. Somat Cell Genet. 1976;2(2):141–153. doi: 10.1007/BF01542627. [DOI] [PubMed] [Google Scholar]
- 31.Maleno I, Aptsiauri N, Cabrera T, Gallego A, Paschen A, Lopez-Nevot MA, Garrido F. Frequent loss of heterozygosity in the beta2-microglobulin region of chromosome 15 in primary human tumors. Immunogenetics. 2011;63(2):65–71. doi: 10.1007/s00251-010-0494-4. [DOI] [PubMed] [Google Scholar]
- 32.Maleno I, Romero JM, Cabrera T, Paco L, Aptsiauri N, Cozar JM, Tallada M, Lopez-Nevot MA, Garrido F. LOH at 6p21.3 region and HLA class I altered phenotypes in bladder carcinomas. Immunogenetics. 2006;58(7):503–510. doi: 10.1007/s00251-006-0111-8. [DOI] [PubMed] [Google Scholar]
- 33.Maleno I, Lopez-Nevot MA, Cabrera T, Salinero J, Garrido F. Multiple mechanisms generate HLA class I altered phenotypes in laryngeal carcinomas: high frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Cancer Immunol Immunother. 2002;51(7):389–396. doi: 10.1007/s00262-002-0296-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maleno I, Cabrera CM, Cabrera T, Paco L, Lopez-Nevot MA, Collado A, Ferron A, Garrido F. Distribution of HLA class I altered phenotypes in colorectal carcinomas: high frequency of HLA haplotype loss associated with loss of heterozygosity in chromosome region 6p21. Immunogenetics. 2004;56(4):244–253. doi: 10.1007/s00251-004-0692-z. [DOI] [PubMed] [Google Scholar]
- 35.Yeung JT, Hamilton RL, Ohnishi K, Ikeura M, Potter DM, Nikiforova MN, Ferrone S, Jakacki RI, Pollack IF, Okada H. LOH in the HLA class I region at 6p21 is associated with shorter survival in newly diagnosed adult glioblastoma. Clin Cancer Res. 2013;19(7):1816–1826. doi: 10.1158/1078-0432.CCR-12-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chowell D, Morris LGT, Grigg CM, Weber JK, Samstein RM, Makarov V, Kuo F, Kendall SM, Requena D, Riaz N, Greenbaum B, Carroll J, Garon E, Hyman DM, Zehir A, Solit D, Berger M, Zhou R, Rizvi NA, Chan TA. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 2017 doi: 10.1126/science.aao4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nie Y, Yang G, Song Y, Zhao X, So C, Liao J, Wang LD, Yang CS. DNA hypermethylation is a mechanism for loss of expression of the HLA class I genes in human esophageal squamous cell carcinomas. Carcinogenesis. 2001;22(10):1615–1623. doi: 10.1093/carcin/22.10.1615. [DOI] [PubMed] [Google Scholar]
- 38.Serrano A, Tanzarella S, Lionello I, Mendez R, Traversari C, Ruiz-Cabello F, Garrido F. Rexpression of HLA class I antigens and restoration of antigen-specific CTL response in melanoma cells following 5-aza-2′-deoxycytidine treatment. Int J Cancer. 2001;94(2):243–251. doi: 10.1002/ijc.1452. [DOI] [PubMed] [Google Scholar]
- 39.Fonsatti E, Sigalotti L, Coral S, Colizzi F, Altomonte M, Maio M. Methylation-regulated expression of HLA class I antigens in melanoma. Int J Cancer. 2003;105(3):430–431. doi: 10.1002/ijc.11077. [DOI] [PubMed] [Google Scholar]
- 40.Dai W, Zheng H, Cheung AK, Lung ML. Genetic and epigenetic landscape of nasopharyngeal carcinoma. Chin Clin Oncol. 2016;5(2):16. doi: 10.21037/cco.2016.03.06. [DOI] [PubMed] [Google Scholar]
- 41.Fonsatti E, Nicolay HJ, Sigalotti L, Calabro L, Pezzani L, Colizzi F, Altomonte M, Guidoboni M, Marincola FM, Maio M. Functional up-regulation of human leukocyte antigen class I antigens expression by 5-aza-2′-deoxycytidine in cutaneous melanoma: immunotherapeutic implications. Clin Cancer Res. 2007;13(11):3333–3338. doi: 10.1158/1078-0432.CCR-06-3091. [DOI] [PubMed] [Google Scholar]
- 42.Maio M, Di Giacomo AM, Robert C, Eggermont AM. Update on the role of ipilimumab in melanoma and first data on new combination therapies. Curr Opin Oncol. 2013;25(2):166–172. doi: 10.1097/CCO.0b013e32835dae4f. [DOI] [PubMed] [Google Scholar]
- 43.Covre A, Coral S, Nicolay H, Parisi G, Fazio C, Colizzi F, Fratta E, Di Giacomo AM, Sigalotti L, Natali PG, Maio M. Antitumor activity of epigenetic immunomodulation combined with CTLA-4 blockade in syngeneic mouse models. Oncoimmunology. 2015;4(8):e1019978. doi: 10.1080/2162402X.2015.1019978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gameiro SR, Malamas AS, Tsang KY, Ferrone S, Hodge JW. Inhibitors of histone deacetylase 1 reverse the immune evasion phenotype to enhance T-cell mediated lysis of prostate and breast carcinoma cells. Oncotarget. 2016;7(7):7390–7402. doi: 10.18632/oncotarget.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabbatino F, Wang Y, Scognamiglio G, Favoino E, Feldman SA, Villani V, Flaherty KT, Nota S, Giannarelli D, Simeone E, Anniciello AM, Palmieri G, Pepe S, Botti G, Ascierto PA, Ferrone CR, Ferrone S. Antitumor activity of BRAF inhibitor and IFNalpha combination in BRAF-mutant melanoma. J Natl Cancer Inst. 2016 doi: 10.1093/jnci/djv435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brea EJ, Oh CY, Manchado E, Budhu S, Gejman RS, Mo G, Mondello P, Han JE, Jarvis CA, Ulmert D, Xiang Q, Chang AY, Garippa RJ, Merghoub T, Wolchok JD, Rosen N, Lowe SW, Scheinberg DA. Kinase regulation of human MHC class I molecule expression on cancer cells. Cancer Immunol Res. 2016;4(11):936–947. doi: 10.1158/2326-6066.CIR-16-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, Ferrone CR, Flaherty KT, Lawrence DP, Fisher DE, Tsao H, Wargo JA. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70(13):5213–5219. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 48.Lopez-Albaitero A, Lee SC, Morgan S, Grandis JR, Gooding WE, Ferrone S, Ferris RL. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol Immunother. 2009;58(11):1853–1864. doi: 10.1007/s00262-009-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donia M, Harbst K, van Buuren M, Kvistborg P, Lindberg MF, Andersen R, Idorn M, Munir Ahmad S, Ellebaek E, Mueller A, Fagone P, Nicoletti F, Libra M, Lauss M, Hadrup SR, Schmidt H, Andersen MH, Thor Straten P, Nilsson JA, Schumacher TN, Seliger B, Jonsson G, Svane IM. Acquired immune resistance follows complete tumor regression without loss of target antigens or IFNgamma signaling. Cancer Res. 2017;77(17):4562–4566. doi: 10.1158/0008-5472.CAN-16-3172. [DOI] [PubMed] [Google Scholar]
- 50.Srivastava RM, Trivedi S, Concha-Benavente F, Hyun-Bae J, Wang L, Seethala RR, Branstetter BF, Ferrone S, Ferris RL. STAT1-induced HLA class I upregulation enhances immunogenicity and clinical response to Anti-EGFR mAb cetuximab therapy in HNC patients. Cancer Immunol Res. 2015;3(8):936–945. doi: 10.1158/2326-6066.CIR-15-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin JD, Fukumura D, Duda DG, Boucher Y, Jain RK. Reengineering the tumor microenvironment to alleviate hypoxia and overcome cancer heterogeneity. Cold Spring Harb Perspect Med. 2016 doi: 10.1101/cshperspect.a027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Semenza GL. The hypoxic tumor microenvironment: a driving force for breast cancer progression. Biochim Biophys Acta. 2016;1863(3):382–391. doi: 10.1016/j.bbamcr.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamarashev J, Ferrone S, Seifert B, Boni R, Nestle FO, Burg G, Dummer R. TAP1 downregulation in primary melanoma lesions: an independent marker of poor prognosis. Int J Cancer. 2001;95(1):23–28. doi: 10.1002/1097-0215(20010120)95:1<23::AID-IJC1004>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 54.Peng RQ, Chen YB, Ding Y, Zhang R, Zhang X, Yu XJ, Zhou ZW, Zeng YX, Zhang XS. Expression of calreticulin is associated with infiltration of T-cells in stage IIIB colon cancer. World J Gastroenterol. 2010;16(19):2428–2434. doi: 10.3748/wjg.v16.i19.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sokol L, Koelzer VH, Rau TT, Karamitopoulou E, Zlobec I, Lugli A. Loss of tapasin correlates with diminished CD8(+) T-cell immunity and prognosis in colorectal cancer. J Transl Med. 2015;13:279. doi: 10.1186/s12967-015-0647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ogino T, Shigyo H, Ishii H, Katayama A, Miyokawa N, Harabuchi Y, Ferrone S. HLA class I antigen downregulation in primary laryngeal squamous cell carcinoma lesions as a poor prognostic marker. Cancer Res. 2006;66(18):9281–9289. doi: 10.1158/0008-5472.CAN-06-0488. [DOI] [PubMed] [Google Scholar]
- 57.Ogino T, Bandoh N, Hayashi T, Miyokawa N, Harabuchi Y, Ferrone S. Association of tapasin and HLA class I antigen downregulation in primary maxillary sinus squamous cell carcinoma lesions with reduced survival of patients. Clin Cancer Res. 2003;9(11):4043–4051. [PubMed] [Google Scholar]
- 58.Weinman EC, Roche PC, Kasperbauer JL, Cha SS, Sargent DJ, Cheville J, Murphy LM, Chen L, Wettstein PJ, Gostout B, Ferrone S, Strome SE. Characterization of antigen processing machinery and survivin expression in tonsillar squamous cell carcinoma. Cancer. 2003;97(9):2203–2211. doi: 10.1002/cncr.11311. [DOI] [PubMed] [Google Scholar]
- 59.Bandoh N, Ogino T, Katayama A, Takahara M, Katada A, Hayashi T, Harabuchi Y. HLA class I antigen and transporter associated with antigen processing downregulation in metastatic lesions of head and neck squamous cell carcinoma as a marker of poor prognosis. Oncol Rep. 2010;23(4):933–939. doi: 10.3892/or_00000717. [DOI] [PubMed] [Google Scholar]
- 60.Hosch SB, Meyer AJ, Schneider C, Stoecklein N, Prenzel KL, Pantel K, Broelsch CE, Izbicki JR. Expression and prognostic significance of HLA class I, ICAM-1, and tumor-infiltrating lymphocytes in esophageal cancer. J Gastrointest Surg. 1997;1(4):316–323. doi: 10.1016/S1091-255X(97)80051-0. [DOI] [PubMed] [Google Scholar]
- 61.Hosch SB, Izbicki JR, Pichlmeier U, Stoecklein N, Niendorf A, Knoefel WT, Broelsch CE, Pantel K. Expression and prognostic significance of immunoregulatory molecules in esophageal cancer. Int J Cancer. 1997;74(6):582–587. doi: 10.1002/(SICI)1097-0215(19971219)74:6<582::AID-IJC4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 62.Mizukami Y, Kono K, Maruyama T, Watanabe M, Kawaguchi Y, Kamimura K, Fujii H. Downregulation of HLA Class I molecules in the tumour is associated with a poor prognosis in patients with oesophageal squamous cell carcinoma. Br J Cancer. 2008;99(9):1462–1467. doi: 10.1038/sj.bjc.6604715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanaka K, Tsuchikawa T, Miyamoto M, Maki T, Ichinokawa M, Kubota KC, Shichinohe T, Hirano S, Ferrone S, Dosaka-Akita H, Matsuno Y, Kondo S. Downregulation of human leukocyte antigen class I heavy chain in tumors is associated with a poor prognosis in advanced esophageal cancer patients. Int J Oncol. 2012;40(4):965–974. doi: 10.3892/ijo.2011.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang X, Lin A, Zhang JG, Bao WG, Xu DP, Ruan YY, Yan WH. Alteration of HLA-F and HLA I antigen expression in the tumor is associated with survival in patients with esophageal squamous cell carcinoma. Int J Cancer. 2013;132(1):82–89. doi: 10.1002/ijc.27621. [DOI] [PubMed] [Google Scholar]