Abstract
The restriction point (R) is defined as the point in G1 after which cells can complete a division cycle without growth factors and divides G1 into two physiologically different intervals in cycling cells, G1-pm (a postmitotic interval with a constant length of 3 to 4 h) and G1-ps (a pre-DNA-synthetic interval with a variable length of 1 to 10 h). Cyclin E is a G1 regulatory protein whose accumulation has been suggested to be critical for passage through R. We have studied cyclin E protein levels in individual cells of asynchronously growing cell populations, with respect to both passage through R and entry into S phase. We found that the postmitotic G1 cells that had not yet reached R were negative for cyclin E accumulation. On the other hand, cells that had passed R were found to accumulate cyclin E at variable times (1 to 8 h) after passage through R and 2 to 5 h before entry into S. These kinetic data rule out the hypothesis that passage through R is dependent on the accumulation of cyclin E but suggest, instead, the converse, that passage through R is a prerequisite for cyclin E accumulation. Furthermore, we found that most of the cyclin E protein is downregulated within 1 to 2 h after entry into S.
In the eukaryotic cell cycle, a reversible growth arrest can be induced in the G1 phase if cells are deprived of growth factors or allowed to grow to confluency (10, 49, 62, 73, 74). Temin (61) showed that chicken cells become independent of external mitogenic growth factors during G1 several hours before entry into S phase. The term restriction point (R) was introduced by Pardee (48) to define the point in G1 after which cells can complete a division cycle independently of mitogenic signals (49). We have previously determined the exact position of R in G1 and its relationship to the previous mitosis and subsequent S phase with time-lapse cinematography (TLC) analysis of mouse and human cells (33, 74, 75, 76, 77). Time-lapse recordings of cells in culture enable analysis of individual cells of an unperturbed, asynchronously growing population. This method is a powerful tool for detailed kinetic analysis of transition events in the cell cycle because, unlike synchronization procedures, it addresses the problem of intercellular variability in cell cycle times, particularly G1 variability. Previous studies using TLC analysis revealed that the G1 phase in cycling cells is separated into two functionally different intervals. During the first part of G1, the G1-pm (postmitosis) period, cell cycle progression is highly dependent on the continuous presence of serum growth factors and on a high rate of protein synthesis. If growth factors are removed from the medium or if protein synthesis is even only moderately inhibited during this period, cells will rapidly (within 30 to 60 min) leave the cell cycle and enter a quiescent state (G0). The G1-pm period has a constant duration of 3 to 4 h in all of the cells studied so far (74, 75, 76). The transition from growth factor-dependent progression to growth factor-independent progression represents passage through R. The part of G1 that follows R, known as the G1-ps (pre-DNA-synthetic) period, is highly variable in duration. Some G1-ps cells initiate DNA replication immediately after passage through R, while others may spend up to 20 h in G1-ps before entering S. This variability in the length of time between R and S implies that even though passage through R is necessary for further progression through the cell cycle, other regulatory events must be completed during G1-ps in order for cells to enter S phase.
The cyclins and their catalytic subunits, the cyclin-dependent kinases (Cdks), control cell cycle progression by regulating events that drive the transitions between cell cycle phases. Cyclins were first identified in clam and sea urchin embryos, where they were observed to accumulate during interphase and to be degraded during mitosis (16). Based on homology to invertebrate and frog embryonic cyclins, human A- and B-type cyclins, essential for progression through S, G2, and M phase, were the first human cyclins to be identified (50, 64). Subsequently, the human G1 cyclins, the D-type cyclins and cyclin E, were identified functionally by screening of human cDNA libraries for sequences that could complement G1 cyclin mutations in Saccharomyces cerevisiae (30, 36, 69). The gene for cyclin D1 is induced in response to mitogenic signals as an early-response gene during the transition from G0 to G1 phase and is associated with the catalytic partner Cdk4 or Cdk6. Cyclin E shows a periodic pattern of expression with accumulation in late G1 and downregulation in S (11, 31, 36; reviewed in reference 54). Cyclin E transcription is activated when the retinoblastoma tumor supressor protein (pRb) is hyperphosphorylated and no longer exerts repression of the cyclin E promoter via E2F-DP transcription factor complexes (see below). Consistent with this, a number of putative E2F binding sites have been identified in the cyclin E promoter (19). E2F-mediated repression was first suggested by experiments showing that the combined mutation of two different E2F sites in the human cyclin E promoter leads to partial derepression of the promoter in G1 (45). Recently, a variant E2F-binding site was found to mediate transcriptional repression by binding of a large E2F4-pRb-containing repressor complex (34, 78). Cyclin E associates specifically with Cdk2, and a number of investigations have demonstrated a requirement for cyclin E-cdk2 activity for the initiation of DNA replication (24, 32, 47). Cyclin E is subjected to ubiquitin-dependent degradation during S phase (7, 58, 68).
Many of the molecular components that are involved in passage through G1 have been identified, but the molecular mechanism underlying R point control still remains to be elucidated. Passage through the R point and phosphorylation-inactivation of pRb have been observed to occur roughly during the same time period in cells entering the cell cycle from G0, but the exact functional relationship between the two events is still unknown. In early G1 phase, pRb is present in an active, hypophosphorylated form, where it is believed to inhibit cell cycle progression by binding to regulatory proteins, including members of the E2F family of transcription factors. Binding of pRb to E2F has been shown to inhibit the transactivation of E2F-dependent genes that are required for cell cycle progression (5, 21, 42). The association between pRb and E2F, as well as other regulatory targets, has been shown to be governed by phosphorylation. pRb is phosphorylated at multiple sites as Cdk activity increases during G1 phase. Hyperphosphorylated pRb first appears during late G1 phase. Although several Cdks have been implicated in pRb phosphorylation in vitro (1, 17, 40, 41), the precise mechanism by which pRb is phosphorylated in vivo is still unclear. By ectopically expressing cyclin D1 or E during early G1, it was demonstrated that expression of either cyclin shortens the G1 phase in rat embryonic fibroblasts but only cyclin D1 expression leads to premature pRb phosphorylation (55). Other evidence suggests that pRb is phosphorylated by both cyclin D- and E-dependent kinases in a sequential manner to achieve hyperphosphorylation (8, 20, 39, 70). Therefore, it has been proposed that the accumulation of either D-type cyclins or cyclin E and the concomitant hyperphosphorylation/inactivation of pRb constitute progression through the R point (9, 53, 77). Consistent with this idea, it has been demonstrated that passage through the R point is dependent on the accumulation of a labile protein (48), a characteristic of both D-type cyclins and cyclin E (11, 36, 37, 47).
The aim of the present study was to perform a detailed analysis of cyclin E expression in relation to passage through R and entry into S phase, in order to determine whether cyclin E could be the labile R-associated protein. In order to determine the exact timing of cyclin E accumulation and downregulation, we carried out an analysis of individual cells which allowed us to consider the variability of cell behavior within the population. We found that cells younger than 3.5 h after mitosis, i.e., cells that had not yet passed R, were negative for cyclin E accumulation. After passage through R, cyclin E begins to accumulate as a cell approaches S, however, with a high degree of temporal variability. These data indicate that passage through R cannot be dependent on the accumulation of cyclin E, as has been proposed, and suggest that since R occurs prior to the accumulation of cyclin E, passage through R may be a prerequisite for cyclin E accumulation. Furthermore, the temporal variability of cyclin E accumulation after passage through R suggests that another late-G1 event(s) controls the precise timing of cyclin E accumulation.
MATERIALS AND METHODS
Cells and media.
Early-passage human diploid fibroblasts (HDF) from embryo lungs and hTERT-BJ cells (Clontech) were maintained in a 5% CO2–95% air mixture in a humidified incubator at 37°C. HDF were cultured in a 1:1 mixture of modified Eagle's medium (MEM) and Ham's F-12 medium supplemented with 10% (vol/vol) fetal calf serum (Life Technologies, Inc.), 2 mM glutamine, and 50 U each of penicillin and streptomycin per ml. hTERT-BJ cells were cultured in a 4:1 ratio of Dulbecco's MEM (Sigma catalog no. D6421) containing Medium 199 (Sigma catalog no. M4530) with 10% fetal bovine serum (FBS; Life Technologies, Inc.), 4 mM l-glutamine, 1 mM sodium pyruvate (Sigma), and 50 U each of penicillin and streptomycin per ml. Cells were never allowed to reach confluence. For transfer, cells were treated with 0.25% (wt/vol) trypsin in Tris-buffered saline containing 0.5 mM EDTA. For immunocytochemistry, 3,000 to 4,000 cells/cm2 were seeded in petri dishes onto a glass coverslip (hemacytometer coverslip, 0.4 mm thick). For time-lapse analysis, cells were seeded onto CELLocate coverslips (Eppendorf).
Recombinant adenovirus procedures.
A recombinant adenovirus containing the human cyclin E cDNA was provided by John Cogswell and Susan Neill at Glaxo-Wellcome, Research Triangle Park, N.C. For transduction experiments, hTERT-BJ cells were incubated with the recombinant adenovirus diluted appropriately in Dulbecco's MEM and 2% FBS for 2 h. After incubation in fresh medium for an additional 24 h, cells were either recorded by time-lapse video microscopy (TLV) for approximately 4 h and then fixed in methanol and stained for cyclin E immunofluorescence assay or harvested for immunoblotting.
Time-lapse analysis.
Ages of individual cells were determined by two methods: time-lapse photography and TLV. For time-lapse photography, every 30 min, a petri dish with cells growing on a coverslip was removed from the incubator and placed under a Zeiss inverted microscope (476100-9901; 10× phase-contrast lens) equipped with a charge-coupled device (CCD) camera (Sony). Three or four marked fields were photographed (approximately 10 to 15 cells per field). For TLV an inverted microscope (Zeiss) equipped with a monochrome, cooled CCD camera (COHU model 2152-2000) was placed in an incubator (ASSAB T 303GF) with temperature and CO2 regulation. Humidity was omitted to protect the instruments. A petri dish with cells growing on a CELLocate coverslip was placed under the microscope, and a field of 20 to 50 separated cells was chosen for recording. Images were captured every 4 to 10 min by a framegrabber card (PX-510-25E Parameter AB) and stored in a personal computer.
After the final photograph was taken, cells were rinsed with phosphate-buffered saline (PBS) and fixed either for 5 min in methanol, followed by 2 min in acetone, at −20°C or for 20 min in 4% fresh paraformaldehyde at room temperature. Methanol-acetone-fixed cells were stored in 70% ethanol at 4°C until they were used for immunocytochemistry analysis. Paraformaldehyde-fixed cells were stained immediately.
Antibodies.
The following primary antibodies were used: an anti-cyclin E mouse monoclonal antibody (HE12; Santa Cruz Biotechnology) and two different affinity-purified anti-cyclin A rabbit polyclonal antibodies (gifts from G. Draetta and J. Pines). The secondary antibodies used in this study included a biotinylated donkey anti-mouse immunoglobulin G (IgG), a fluorescein isothiocyanate (FITC)-conjugated donkey anti-rabbit IgG (Jackson Immunoresearch Laboratories), and a horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Amersham Life Science).
Immunoperoxidase cytochemistry.
Fixed cells were immersed in TBS1 (0.05 M Tris-HCl [pH 7.6] in PBS)–0.5% Tween 20 for 15 min at room temperature before endogenous peroxidase was blocked by soaking in 0.5% H2O2 for 15 min at room temperature. Incubation with the primary antibody was performed overnight at 4°C. After washing in TBS1–0.02% Tween 20 for 3 × 10 min and blocking with 4% normal donkey serum for 30 min at room temperature, the primary antibody was detected using Dako StreptABComplex/HRP Duet according to the manufacturer's instructions. Diaminobenzidine was used as a chromogen, and nuclei were counterstained with Meyer's hematoxylin.
Immunofluorescence cytochemistry.
Fixed cells were treated with blocking buffer (1% bovine serum albumin and 0.5% Tween 20 in PBS) for 15 min at room temperature to block out unspecific interactions prior to addition of the primary antibody. This buffer was also used for antibody dilutions. Following all antibody incubations, cells were washed for 3 × 15 min in washing buffer (0.05 mM Tris-HCl [pH 7.6], 0.3 mM NaCl, 0.02% Tween 20). Cells were incubated with the primary antibody, anti-cyclin E and/or anti-cyclin A (1:1,000), overnight (16 to 18 h) at 4°C, washed, and then treated with 4% normal donkey serum for 15 min at room temperature prior to addition of the secondary antibody. For detection of cyclin E primary antibody, cells were incubated with biotinylated donkey anti-mouse IgG (1:300) for 30 min at room temperature and then incubated with streptavidin-conjugated CY3 (1:2,000; Amersham). For detection of anti-cyclin A primary antibody, cells were incubated with FITC-conjugated donkey anti-rabbit IgG (1:100). Cells were mounted with Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (DAPI) (H-1200; Vector Laboratories Inc.).
Quantitative absorption cytometry.
The equipment used for quantitation of immunoperoxidase signal intensity consisted of a microscope (Zeiss Axioskop) coupled to a CCD camera (COHU model 2152-2000) and a personal computer. The amount of immunoperoxidase material was determined from light absorption at a wavelength of 480 nm after subtraction of the counterstain intensity of immunoperoxidase-negative cells.
Immunoblot analysis.
Cells were lysed on ice for 10 min in lysis buffer (50 mM Tris-HCl [pH 7.5], 0.5% Nonidet P-40, 250 mM NaCl, 0.5 mM phenylmethylsulfonyl flouride, 10 μM each leupeptin, pepstatin, and aprotinin), sonicated for 10 s, and centrifuged at 15,000 × g for 5 min. Total protein concentration was determined by Bio-Rad Protein Assay (Bio-Rad) and read at 595 nm. A 21-μg sample of total protein was loaded per lane, electrophoresed through a sodium dodecyl sulfate–11% polyacrylamide gel, and transferred to an Immobilon-P membrane (Millipore). To monitor equal loading of samples, total protein was visualized by amido black staining (data not shown). The membrane was blocked for 1 h at room temperature in 150 mM NaCl–20 mM Tris-HCl (pH 7.5; TBS2)–5% nonfat dry milk (NFDM) and then incubated overnight at 4°C with anti-cyclin E antibody diluted 1:1,000 in TBS2–5% NFDM–0.05% Tween 20. The membrane was washed in TBS2–5% NFDM–5% Tween 20 for 3 × 15 min at room temperature, incubated with an HRP-conjugated goat anti-mouse IgG secondary antibody diluted 1:5,000 in TBS2–5% NFDM–0.05% Tween 20 for 1 h at room temperature and then washed again as described above. The blot was developed using Super Signal West Pico enhanced-chemiluminescence kit (Pierce).
Fluorescence microscopy.
Data were collected and analyzed with the Delta Vision system (Applied Precision Inc., Issaquah, Wash.) equipped with a Zeiss Axiovert microscope (Zeiss Plan-Neofluar 40×/NA 1.30 oil immersion lens). A mercury lamp with a fiber optic illumination system, conventional microscope optics, and selective filters for excitation and emission was used. A cooled CCD camera was used to record three colors (CY3, FITC, and DAPI). Cells were defined as negative when they had a nuclear fluorescence intensity lower than the background fluorescence of the surrounding cytoplasm. The value of the nonnegative cells (positive cells) was determined in a microfluorimeter after subtraction of the background.
Kinetics of R point passage in the presence of roscovitine.
HDF (IMR-90) on glass coverslips were serum starved for 60 h and then incubated in medium with 10% FBS with or without 10 μM roscovitine. This medium was replaced at 6, 9, or 15 h postrelease from G0 with medium without serum but with 20 μM bromodeoxyuridine (BrdU). To control for recovery from roscovitine treatment, duplicate roscovitine-treated samples received BrdU-containing medium with 10% FBS at 6, 9, and 15 h, respectively. Additional controls were continuous treatment in serum-free medium and continuous treatment with roscovitine in the presence of 10% FBS. Cells not treated with roscovitine were fixed at 22 h postrelease from G0, when none of the cells in the population had reached M phase, based on visual observation. Cells treated with roscovitine were fixed at 34 h postrelease from G0 in order to allow recovery from roscovitine treatment when, similarly, none of the cells in the population had reached M phase. All fixed cells were immunostained for BrdU reactivity by incubation with FITC-conjugated anti-BrdU IgG (Becton Dickinson) according to the manufacturer's instructions. DAPI was used for counterstaining of nuclei. The percentage of cells containing BrdU-positive nuclei was determined in triplicate using a Nikon Eclipse E800 fluorescence microscope with a 40× objective. Since there is some time-dependent toxicity associated with roscovitine treatment (2), experimental data (roscovitine-treated cells from which serum was withdrawn) were normalized to control data (roscovitine-treated cells from which serum was not withdrawn) for all time points.
RESULTS
Cyclin E is not expressed in G1-pm.
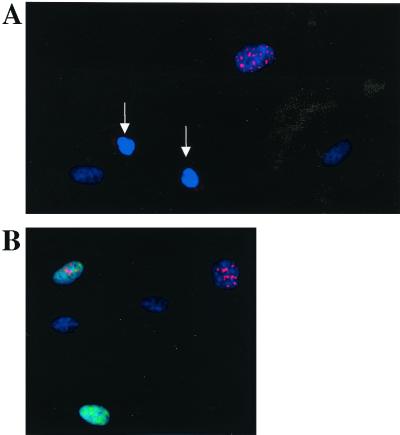
We have previously reported the kinetics of passage through the R point based on TLC analysis of individual continuously cycling HDF and mouse 3T3 cells. In the current study, we have used TLV analysis in combination with immunocytochemistry analysis to investigate the relationship between (i) cyclin E protein accumulation and (ii) passage through the R point and entry into S phase. We used two different methods for immunocytochemical staining to obtain semiquantitative measurements of protein levels: immunoperoxidase staining and immunofluorescence staining. For immunofluorescence staining, two methods of fixation were used: methanol-acetone and paraformaldehyde (see Materials and Methods). The reactivity of the anti-human cyclin E mouse monoclonal antibody used was limited to the nucleus in almost all cyclin E-positive cells. The nuclear localization of cyclin E is in agreement with previous reports describing cyclin E immunolocalization (6, 47). Furthermore, the nuclear staining pattern was primarily punctate throughout the nucleus, with the intensity varying from cell to cell within the cyclin E-positive population. The reactivity of the anti-human cyclin A antibody used in this study was also limited to the nucleus. Also, for the cyclin A antibody, the nuclear staining pattern was primarily punctate throughout the nucleus, with the intensity varying from cell to cell within the cyclin A-positive population. Figure 1A shows a clearly cyclin E-positive cell and two postmitotic, early-G1 cells (G1-pm cells) that are negative for cyclin E immunoreactivity. Figure 1B shows asynchronously growing HDF immunofluorescence stained for both cyclins E and A. Cells found to be positive for both cyclins E and A by immunofluorescence staining exhibited different staining patterns (Fig. 1B; see below for a quantitative analysis).
FIG. 1.
Detection of cyclin E and A expression in HDF. (A) Asynchronously growing HDF were stained for cyclin E by immunofluorescence (red, CY3 fluorescence). DNA was counterstained with DAPI (blue). Newly divided cells (white arrows) are negative for cyclin E. (B) Double-immunofluorescence staining of cyclins E (red, CY3) and A (green, FITC) in asynchronously growing HDF. The cells were counterstained for DNA with DAPI (blue).
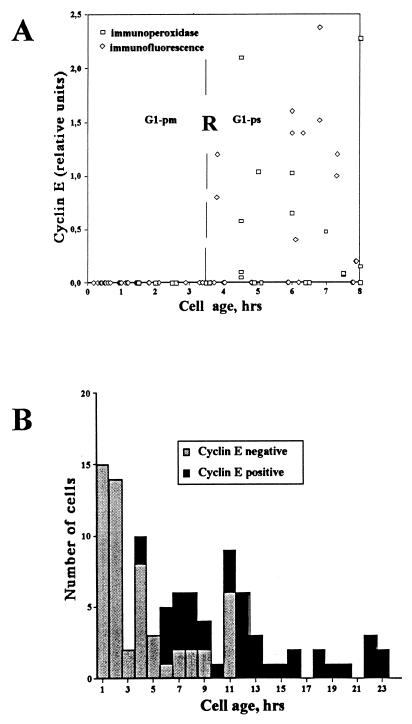
In order to determine the precise timing of cyclin E accumulation in individual cells, continuously growing HDF were video recorded for approximately 35 h or time-lapse recorded using a CCD camera for 8 h and subsequently fixed in methanol and immunostained for cyclin E. The intensity of immunoperoxidase or immunofluorescence staining was measured as described in Materials and Methods and correlated to cell age (time elapsed after the last mitosis). Out of 176 cells analyzed, 43 (24%) were found to be in the G1-pm stage of the cell cycle, as previously defined (33, 74), i.e., having a cell age of less than 3.5 h. None of these G1-pm cells were found to be positive for cyclin E (Fig. 2A). It can therefore be concluded that cyclin E accumulates not before or at R but, instead, after R. To rule out possible fixation artifacts, a similar experiment was carried out except that cells were fixed using paraformaldehyde rather than methanol. Identical results were obtained (data not shown).
FIG. 2.
Kinetics of cyclin E accumulation. (A) Cyclin E protein levels in individual cells. The cyclin E level in individual TLV-analyzed HDF was determined after immunoperoxidase or immunofluorescence staining. Relative staining intensity (ordinate) is plotted against cell age (time elapsed after the last mitosis; abscissa). Immunoperoxidase-stained cells are represented by open squares, whereas immunofluorescence-stained cells are represented by open diamonds. The approximate time of R is shown as a vertical dashed line. The measurement procedures, including background subtraction, are described in Materials and Methods. (B) Cyclin E accumulates during G1-ps. The number of TLV-analyzed HDF negative for cyclin E immunofluorescence (open bars) versus the number of those positive (solid bars) is plotted against cell age (time elapsed after last mitosis). All cells shown are in G1, based on cyclin A negativity (see below).
Cyclin E accumulates at different times after the R point.
Among cells older than 3.5 h, G1-ps cells, both cyclin E-positive and cyclin E-negative cells were observed. The kinetic analysis of individual cells in the population indicated that the timing of cyclin E accumulation after passage through R was highly variable (Fig. 2A). Some cells were found to accumulate cyclin E shortly (within 1 h) after passage through the R point, while other cells accumulated cyclin E considerably later in G1 (up to 8 h after passage through R). As a cell progresses through G1, the probability that it will be cyclin E negative decreases gradually (Fig. 2B). Twelve hours after mitosis, i.e., approximately 8 h after passage through R, no cyclin E-negative G1 cells could be detected. These data are consistent with previous reports showing that cyclin E accumulates late in G1 (11, 31).
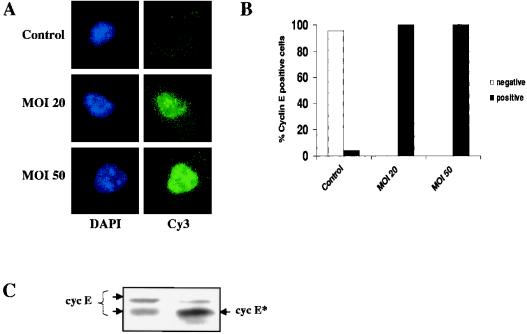
To test whether the inability to detect cyclin E during G1-pm could reflect differential epitope availability rather than absence of protein, we ectopically expressed cyclin E by transducing cells with a recombinant adenovirus containing a human cyclin E cDNA. Transduced cells were recorded by the time-lapse method, fixed, and stained for cyclin E as described above. In virtually all of the transduced cells, cyclin E could be detected during G1-pm, even immediately after mitosis. The level of expression of ectopic cyclin E was shown to be dose dependent, with increasing staining intensity correlating with increasing multiplicity of infection (MOI) (Fig. 3A and B). As expected, cyclin E was not detected in G1-pm in untransduced control cells (Fig. 3A and B). To confirm that the ectopic cyclin E level was not highly elevated above the endogenous level of expression, we performed an immunoblot assay of total protein from asynchronously growing cells transduced at an MOI of 50 and of untransduced control cells (Fig. 3C). As can be seen in Fig. 3C, the level of ectopically expressed cyclin E is only slightly elevated compared to the level in control cells. Note that the recombinant cyclin E corresponds to a more rapidly migrating splice variant. It should also be taken into account that in a population of asynchronously growing cells, the level of endogenous cyclin E, relative to ectopically expressed cyclin E, is underestimated, as its expression is highly periodic while the expression of the ectopic cyclin E is constitutive. These results suggest that the inability to detect cyclin E during G1-pm truly reflects the absence or low abundance of cyclin E protein during this period.
FIG. 3.
Detection of ectopically expressed cyclin E during G1-pm. Asynchronously growing hTERT-BJ cells were transduced with a recombinant adenovirus containing the human cyclin E cDNA. (A) At 24 h posttransduction, cells were TLV recorded for approximately 4 h, fixed in methanol, and immunofluorescence stained for cyclin E. At the top is a control cell with an age of 62 min (after the last mitosis); at the center is a cell transduced at an MOI of 20 with an age of 52 min (after the last mitosis), and at the bottom is a cell transduced at an MOI of 50 with an age of 68 min (after the last mitosis). (B) Same experiment as in panel A. A quantitative analysis of cells positive for cyclin E immunofluorescence is shown. A total of 23 control cells, 14 cells transduced at an MOI of 20, and 33 cells transduced at an MOI of 50 were analyzed. All of the cells analyzed were younger than 3 h (8 to 144 min). (C) Immunoblot analysis of cells transduced with cyclin E adenovirus. The level of endogenously expressed cyclin E (cyc E) in control cells (left lane) is compared to the level of ectopically expressed cyclin E (cyc E∗) in cells transduced at an MOI of 50 (right lane).
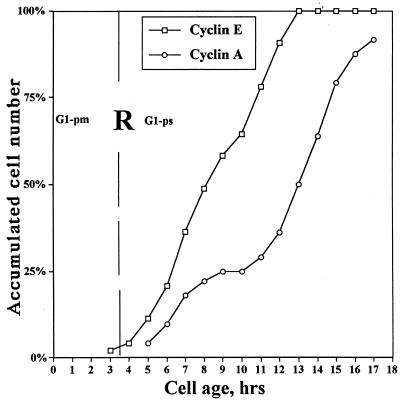
In order to study the temporal relationship between cyclin E accumulation in G1 cells and entry into S phase, TLV-analyzed cells were double immunofluorescence stained for cyclins E and A. Cyclin A positivity can be used as a marker for S phase entrance, based on recent data showing a very high correlation between cyclin A immunostaining and BrdU incorporation (15). Cells were found to enter S phase (as determined by cyclin A positivity) during an interval of 17 h, 5.5 to 22.5 h after mitosis (Fig. 4). In Fig. 4, the immunostaining data are plotted as the cumulative number of cyclin E- and A-positive cells as a function of cell age. It is clear that the curve representing cyclin E-positive cells precedes the curve representing cyclin A-positive cells (entry into S phase) by 2 to 5 h. The most rapidly proliferating cells, i.e., the cells with the shortest G1 phase, enter S phase 1 to 2 h after becoming cyclin E positive, while in cells with a longer G1 phase, e.g., 10 to 20 h, cyclin E accumulation precedes entry into S by approximately 5 to 6 h. Thus, for cells destined to enter S phase, cyclin E accumulates at a variable interval after passage through R but at a relatively fixed interval prior to entry into S phase. However, a substantial proportion of cells accumulates cyclin E without entering S phase or entering S phase after very long delays (Fig. 4). From 15 to 25 h after mitosis, very few cells entered S (Fig. 4). This subpopulation of cyclin E-positive, old G1 cells most likely represents cells that are entering into senescence or have become senescent. This finding is in agreement with previous studies showing that senescent cells are cyclin E positive (60).
FIG. 4.
Temporal relationship between (i) cyclin E expression and (ii) passage through R and entry into S. Continuously cycling, TLV-analyzed HDF were double immunofluorescence stained for cyclins E and A. The cumulative number of cells positive for cyclin E or A immunofluorescence was plotted against cell age (time elapsed after the last mitosis). Cyclin A positivity is used as a marker for S phase. The approximate time of R is shown as a vertical dashed line. Curves were smoothed according to the three-point moving-average method. Percentages refer to percentages of total cyclin E- and A-positive cells, respectively.
Cyclin E-Cdk2 activity is not required for passage through the R point.
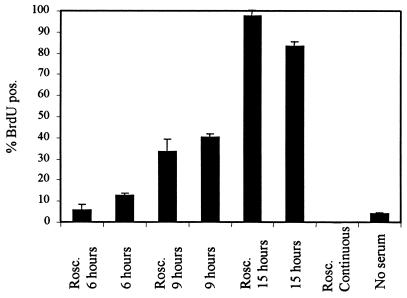
The data presented above show that cyclin E does not accumulate to easily detectable levels prior to R. However, these data cannot exclude the possibility that a low level of cyclin E during G1-pm, undetectable by immunofluorescence, is sufficient for passage through R. To address this issue at the functional level, we determined whether cells could pass through R in the presence of the potent Cdk2 inhibitor roscovitine (2). Cells were synchronized by serum starvation and then released by addition of serum either in the presence or in the absence of roscovitine. The concentration of roscovitine used (10 μM) was sufficient to completely prevent cells from entering S phase in the presence of serum (Fig. 5), and based on other criteria, such as the degree of hyperphosphorylation of pRb, completely inhibits Cdk2 kinase activity in vivo (2). Roscovitine and serum were removed as a function of time, at 6 h postrelease from G0, when few, if any, cells are expected to have traversed R, at 9 h postrelease from G0, when 30 to 50% of the cells are expected to have traversed R, or at 15 h postrelease from G0, when virtually all of the cells should be past R (76). Note that under these experimental conditions, the position of R cannot be fixed as precisely as in continuously cycling cells, since the time of exit from G0 is somewhat variable (76). Cells were then scored for incorporation of BrdU as a measure of passage through R at the time of serum withdrawal (Fig. 5). Whereas approximately 3% of the roscovitine-treated cells for which serum was withdrawn at 6 h were capable of entering into S phase and approximately 30% if serum was withdrawn at 9 h, both comparable to non-roscovitine-treated controls, nearly 100% of the population could enter S phase if serum was withdrawn at 15 h, regardless of the presence of roscovitine. Thus, direct inhibition of cyclin E-Cdk2 does not prevent cells from passing R. These results are consistent with the idea that cyclin E does not accumulate to a functional level prior to R and that, therefore, accumulation of cyclin E is not a component of the R point mechanism.
FIG. 5.
Inhibition of Cdk2 does not affect passage through R. HDF were synchronized by serum starvation and then released in the presence of the Cdk2 inhibitor rescovitine (Rosc.). Roscovitine was removed at various times, and passage through the R point was measured by subsequent serum-independent incorporation of BrdU. Roscovitine-treated samples were normalized for recovery from roscovitine by comparison to samples treated with roscovitine for the same intervals but in the continuous presence of serum during the experiment. Controls untreated with roscovitine were also assayed for R point passage by serum removal after the same intervals. Other controls shown are BrdU incorporation during continuous treatment with roscovitine and in the absence of both serum and roscovitine. The y axis represents the percentage of cells that had incorporated BrdU because they entered S phase during the course of the experiment. Error bars represent 1 standard deviation from the mean of three independent determinations.
Cyclin E levels are downregulated early in S phase.
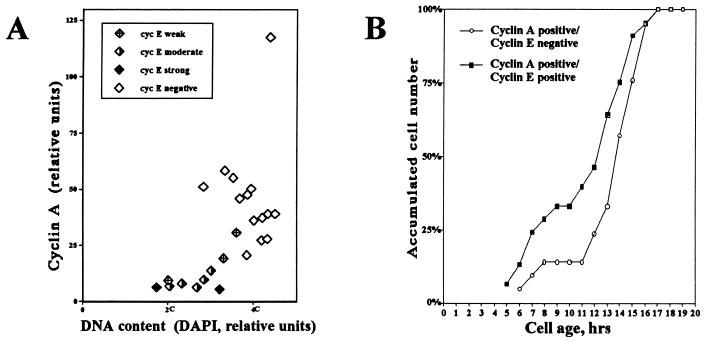
We used three different methods to determine the timing of cyclin E downregulation. First, the time interval in S phase during which cells express cyclin E was calculated by double immunofluorescence staining of cyclins E and A. Cyclin A immunofluorescence positivity was used as a marker for cells collectively in S, G2, and M phase. Simultaneous staining of cells for both cyclins E and A allowed us, therefore, to draw inferences concerning the temporality of cyclin E expression. We estimated the time interval in S phase where cells expressed significant levels of cyclin E by calculating the fraction of cyclin A-positive cells that were also cyclin E positive (Table 1). We have previously determined that for HDF, S phase takes 6 h and G2 and M phase combined take 2 h. Therefore, the cyclin A-positive interval can be estimated as being 8 h in duration. We found that 30% of the cyclin A-positive cells were also cyclin E positive, including cells weakly positive for cyclin E, which represents a 2.4-h interval in S phase prior to complete downregulation of cyclin E. However, if cells with very weak cyclin E staining are excluded, the fraction of cyclin A-positive cells concurrently positive for cyclin E drops to 10%. This suggests that most of the cyclin E is downregulated during the first hour of S phase. This conclusion is further supported by the data presented in Fig. 6A, where cyclin A and E immunofluorescence levels were correlated to the relative DNA content, based on DAPI fluorescence. As S phase proceeded, represented by a doubling of the DNA content from 2C to 4C, cyclin A immunofluorescence levels were found to gradually increase, while cyclin E immunofluorescence levels were found to be higher in late G1 and early S phase (2C to 3C) and to decline as S phase proceeded (3C to 4C). In a much larger sample, intensity of cyclin E staining exhibited an inverse relationship to intensity of cyclin A staining, consistent with downregulation of cyclin E during S phase (data not shown). Examples of cells doubly stained for cyclins E and A and demonstrating these trends are presented in Fig. 1B.
TABLE 1.
Cyclin E in S phase
| Parameter | No. of cells | % of total |
|---|---|---|
| DAPI staining | 1,134 | 100 |
| Cyclin A positivity | 176 | 15.5 |
| Cyclin A + E positivity | 53 | 4.7 |
HDF were fixed and double stained for cyclins E and A. A total of 1,134 cells were analyzed. The proportion of cyclin A-positive cells represents cells in S, G2, and M phase (mean time of 6 + 1 + 1 = 8 h). The fraction of cyclin A-positive cells that are also positive for cyclin E corresponds to the duration of cyclin E protein expression in S phase (30% of 8 h = 2.4 h).
FIG. 6.
Cyclin E is downregulated during the first half of S phase. Continuously cycling HDF were double immunofluorescence stained for cyclins E and A. (A) The relative cyclin A level and cyclin E positivity were plotted against DNA content, as determined by intensity of DAPI fluorescence, for individual cells. Cyclin E positivity is subdivided into four intensity classes, ranging from strong to negative. The 2C and 4C DNA content levels are estimates based on minimal and maximal DAPI staining intensities, respectively. (B) Continuously cycling, TLV-analyzed HDF were double immunofluorescence stained for both cyclins E and A. Cyclin A-positive cells were divided into two classes: those that were also positive for cyclin E and those in which cyclin E had been downregulated (cyclin E-negative cells). Both classes of cyclin A-positive cells were plotted as a function of relative cell age (time elapsed after the last mitosis). Cyclin A positivity is used as a marker for entry into S phase. The curve corresponding to cyclin E-negative cells is shifted by 1 to 4 h compared to that corresponding to cyclin E-positive cells, suggesting that cyclin E downregulation occurs shortly after entry into S phase. Curves were smoothed according to the three-point moving-average method. Percentages refer to the percentage of the total of each class.
In the third method used to determine the timing of cyclin E downregulation, we correlated cyclin A and E immunofluorescence to cell age (Fig. 6B). In Fig. 6B, the curve for the percentage of cells positive for both cyclins E and A demonstrates the kinetics by which cells enter S phase and the curve for the percentage of cells positive for cyclin A but negative for cyclin E demonstrates the kinetics by which cells downregulate cyclin E. In cells with G1 periods of 6 to 15 h, cyclin E was found to be completely downregulated 1 to 4 h after onset of S phase. In cells with G1 periods of more than 15 h, cyclin E is downregulated at a considerably slower rate.
DISCUSSION
Relationship of cyclin E accumulation to known cell cycle landmarks.
Progression through G1-pm is dependent on serum or growth factors in the cell culture medium. The duration of G1-pm is relatively constant (3 to 4 h) in most of the cells studied to date (74, 75). Transition from G1-pm to G1-ps reflects passage through R. The duration of G1-ps, i.e., the time period from R to entry into S phase, is highly variable among the cells in a population, ranging from less than 1 h to more than 20 h. Most of the variation in cell cycle length observed when comparing cells in a population is believed to be a reflection of G1-ps variability (74, 75, 77). The basis for G1-ps variability remains to be determined, but it has been suggested that adjustment of cell size or cellular protein content could be a factor (28, 71, 72, 75).
We have used time-lapse analysis in combination with immunocytochemistry analysis to estimate cyclin E levels in individual unperturbed cells of an asynchronously growing population. This strategy, by focusing on individual cells, allowed us to determine the variability of cyclin E accumulation behavior within the population. In addition, it allowed us to correlate the kinetics of cyclin E accumulation and downregulation with passage through the R point and entry into S phase. This is a critical issue, since cyclin E accumulation and concomitant activation of Cdk2 have been proposed to constitute the molecular basis for the R point phenomenon (9, 53, 77). Previous studies have shown that cyclin E and its associated kinase activity accumulate with kinetics consistent with a functional relationship to the R point. The periodicity of cyclin E accumulation in the cell cycle was first observed by Dulic et al. (11) and has since been verified by a number of other investigators. Using biochemical methods to determine mRNA and protein levels, it was demonstrated that cyclin E protein is induced late in G1, peaks at the G1/S transition, and is subsequently targeted for degradation during S phase (31, 36, 47). However, the methodologies employed in all of these studies, because they rely on analysis of populations, only allow comparison of population averages for any parameter. Information potentially useful for establishing kinetic and functional relationships is lost in this averaging process. Thus, it was not possible to draw strong inferences from these studies concerning the relationship of cyclin E accumulation to passage through the R point or entry into S phase.
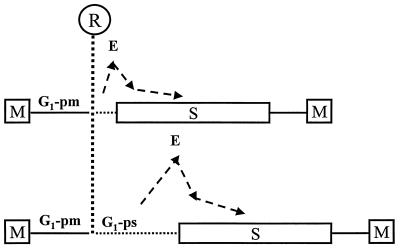
We showed that cyclin E protein begins to accumulate during G1-ps. All cells younger than 3.5 h, i.e., G1-pm cells, were found to be negative for cyclin E immunostaining. This finding argues against the hypothesis that passage through the R point is dependent on the accumulation of cyclin E but suggests that passage through the R point is a prerequisite for accumulation of cyclin E. The fact that cells are cyclin E negative when they pass through R indicates that cyclin E is not the labile R point protein defined by Pardee (48). One might argue that low levels of cyclin E, undetectable by the methods employed, are necessary and sufficient for passage through the R point. However, this is unlikely, since chemical inhibition of Cdk2 did not significantly affect the kinetics of passage through the R point (Fig. 5), although it completely blocked entry into S phase, consistent with a replication initiation role for cyclin E. Furthermore, we found that cyclin E accumulates at variable times after passage through the R point, 2 to 5 h prior to entry into S phase, and is rapidly downregulated after entry into S. Although most of the cyclin E decline occurs during the first hour of S phase, a residual level persists in the nucleus for a significantly longer time. These observations suggest that cyclin E accumulation is likely to be closely linked to the mechanism that sets the length of G1-ps and triggers the G1/S phase transition and that cyclin E downregulation is activated upon entry into S phase. In Fig. 7, we have incorporated this view of cyclin E accumulation and downregulation into a schematic model of the cell cycle.
FIG. 7.
Schematic representation of cyclin E accumulation and downregulation and the relationship to R for serum dependence and initiation of S phase. Analysis of individual cells suggests that cyclin E accumulation correlates much more with entry into S phase than with passage through R. M, M phase.
The physiological substrate(s) of cyclin E-dependent kinase activity is still unknown. Cyclin E has been implicated in the phosphorylation of pRb during late G1 phase and yet, it has also been shown that cyclin E regulates a rate-limiting step in entry into S phase that is distinct from pRb phosphorylation. By using rat fibroblasts ectopically expressing the G1 cyclins in a controlled manner, it was shown that premature expression of cyclin D1 resulted in the immediate appearance of hyperphosphorylated pRb, while the premature expression of cyclin E did not (56). In addition, using the same system, cyclin D1 expression led to rapid release of free E2F (38). On the other hand, the phosphorylation of pRb that occurs during G1 and results in the release of E2F activity has been proposed to be performed in a sequential manner, first by cyclin D1-Cdk4 complexes and then by cyclin E-Cdk2 complexes, during late G1 and early S phase. Consistent with this, it was recently shown by inhibiting endogenous G1 cyclin-dependent kinase activity, that cyclin D1-Cdk4,6 kinase activity alone, although capable of partially phosphorylating pRb, is unable to fully inactivate pRb in vivo. Additional phosphorylation by Cdk2 kinase was shown to be necessary for complete inactivation of pRb and release of E2F activity, although that study did not distinguish between requirements for cyclins E and A (39). Recent findings suggest a model for pRb inactivation in which sequential phosphorylation, first by cyclin D-Cdk4,6 and later by cyclin E-cdk2, results in progressive loss of pRb functions as cells move through G1 (20). According to this model, the first step in pRb inactivation involves phosphorylation of the C-terminal region of pRb by cyclin D-Cdk4,6, resulting in displacement of histone deacetylase from the pocket and inhibition of active transcriptional repression exerted by the pocket. In the next step, a serine residue in the pocket region is phosphorylated by cyclin E-Cdk2, resulting in pocket structure disruption and blocking of E2F inactivation (20). Our data argue that cyclin E protein induction is more closely linked to initiation of DNA replication than to passage through R. If cyclin E-associated kinase activity is required for pRb inactivation, this could imply that pRb inactivation, like cyclin E accumulation, is coupled to the variability of G1-ps and not the molecular basis for passage through R. However, if cyclin E accumulation is critical for functional inactivation of pRb, it must also be required for another aspect of G1/S regulation, since Rb-negative cells have been shown to require cyclin E (47). Furthermore, it has been shown that ectopic expression of cyclin E can drive cells with constitutively active pRb into S phase independent of E2F release (38). These data, in the context of the current work, suggest an essential role for cyclin E downstream of passage through R and more closely related to events directly involved in initiation of DNA replication. How pRb phosphorylation fits into this scheme is not yet clear.
Possible mechanisms of cyclin E accumulation.
We show here that cyclin E protein accumulates rapidly late in G1 phase, approximately 2 h before entry into S phase. These results are consistent with previous data showing that both cyclin E mRNA and protein accumulate near the G1/S transition (11, 36). Whether accumulation of cyclin E protein occurs in response to transcriptional induction or posttranscriptional regulation is not clear. The rapid induction of cyclin E protein in late G1 has been suggested to be the result of a positive transcriptional feedback loop. According to this model, the cyclin E gene is under E2F transcriptional control. Thus, cyclin E activity would stimulate induction of cyclin E transcription by phosphorylation of pRb and the concomitant release of E2F activity. Consistent with this, Rb−/− mouse embryo fibroblasts prematurely induce cyclin E mRNA (22). However, cells with constitutively inactive pRb, such as HeLa cells, exhibit periodic transcription of cyclin E mRNA, suggesting alternative transcriptional regulation (36). It was shown by Oda et al. (44) that cyclin E mRNA synthesis levels are fairly constant, even in G0, and that protein levels are induced as a result of mRNA stabilization at the G1/S transition. Possibly, cyclin E is regulated by two mechanisms, both by pRb and other factors at the level of transcription and by an additional mechanism that mediates mRNA stabilization at the G1/S transition.
Cyclin E downregulation.
Cyclin E has previously been shown to be downregulated during S phase, but it has not been possible to determine the precise kinetics of this process using biochemical approaches. We have used the following three independent methods to study the downregulation of cyclin E protein levels: (i) calculation of the fraction of cyclin A-positive cells that are also positive for cyclin E, (ii) correlation of cyclin E protein levels with DNA content, and (iii) correlation of cyclin A and E positivity and exclusively cyclin A positivity with cell age. Our finding that cyclin E is downregulated 1 to 2 h after entry into S phase is generally consistent with previous investigations (11, 31, 47) but provides a surprisingly narrow window for cyclin E persistence during S phase. The mechanism of downregulation of cyclin E is, however, not fully understood. The periodic accumulation of cyclin E mRNA (11), coupled with the short half-life of cyclin E protein (47, 68), is certainly likely to be a factor. In this regard, cyclin E has been shown to be a target of the ubiquitin-proteasome pathway (68). Unlike mitotic cyclins, which contain a destruction box, or yeast G1 cyclins, which contain distinct PEST sequences and multiple phosphorylation sites (35, 67) targeting them for ubiquitin-mediated degradation, cyclin E appears to be targeted for degradation by autophosphorylation on a specific residue, Thr380 (68). This pathway mediates the degradation of cyclin E that is unbound to Cdk2. It has been proposed that the striking periodicity of cyclin E accumulation is the result of a self-regulated negative feedback loop in which activation of cyclin E-Cdk2 complexes at the G1/S transition leads to prompt proteolytic targeting of cyclin E (68). Our data, which show an inverse relationship between the cyclin E and A proteins in individual cells, suggest that cyclin A accumulation and cyclin A-controlled Cdk2 activity might have a role in the degradation of cyclin E, possibly by phosphorylation of cyclin E on Thr380. Additionally, we have observed that cyclins E and A are colocalized during a short period of S phase, implying that the two protein complexes interact physically (data not shown). Thus, cyclin E might be targeted for degradation by both autophosphorylation and cyclin A-dependent phosphorylation.
Cyclin E and cancer.
The periodic appearance and rapid degradation of cyclin E suggest that strict regulation of the cyclin E protein level may be important. Deregulation of cyclin E protein levels has been suggested to be involved in the development of human cancers. Cyclin E was found to be overexpressed in breast carcinomas and breast tumor cell lines (26, 27), as well as in other cancers (15). Additionally, a high cyclin E level was shown to be a prognostic marker for poor outcome in breast cancer, particularly when correlated with a low p27 level (52). p27 is an inhibitor of cyclin E-Cdk2 kinase activity (56, 63). We have recently observed that cyclin E protein is present during a longer period of the cell cycle in transformed cells (unpublished data). Thus, persistence of cyclin E into S phase or later in the cell cycle may lead to impairment of normal regulatory functions, possibly leading to genetic instability and, ultimately, malignancy. Consistent with this idea, ectopic expression of cyclin E induced significant levels of aneuploidy in both fibroblasts and epithelial cells (59).
Advantages and disadvantages of single-cell analysis.
Time-lapse recordings of cells in culture enable analysis of individual cells of an unperturbed, asynchronously growing population. This method is a powerful tool for detailed kinetic analysis of transition events in the cell cycle because, unlike synchronization procedures, it addresses the problem of intercellular variability in cell cycle times, particularly G1 variability. The major methodological drawback in the use of immunocytochemistry for determination of protein levels is that it is only semiquantitative. Although there is a rough correlation between protein content and immunostaining intensity, epitope availability, on which immunostaining is highly dependent, is often sensitive to variation in fixation and other parameters of sample preparation. However, the fact that cyclin E could be detected during G1-pm when cyclin E was ectopically expressed makes it unlikely that epitope masking is a factor in this analysis. We cannot rule out the possibility that cyclin E is expressed at functional levels during G1-pm but below the limit of detection. However, the fact that cyclin E accumulates to detectable levels with kinetics linked to the initiation of DNA replication rather than passage through R argues against a close mechanistic relationship.
We have made a semiquantitative estimate of protein content based on measurements of immunostaining intensity using the following two different methods: (i) cytometric measurement of immunoperoxidase-stained cells and (ii) determination of immunofluorescence intensity. For immunofluorescence staining, we used two different methods of fixation: methanol-acetone and paraformaldehyde. The results obtained by these different methods were in good agreement, supporting the validity of the approach when samples of sufficient size are analyzed.
ACKNOWLEDGMENTS
We thank Julio Draetta and Jonathon Pines for kindly providing cyclin A antibodies and Mia Olsson, Yvonne Lindell, Kerstin Nyström, and Martha Henze for excellent technical assistance.
This project was supported by grants to Anders Zetterberg from the Swedish Cancer Society (0046-B99-33XB) and the Stockholm Cancer Society and by U.S. Public Health Service grant CA 78343 to Steven Reed.
REFERENCES
- 1.Akiyama T, Ohuchi T, Sumida S, Matsumoto K, Toyoshima K. Phosphorylation of the retinoblastoma protein by cdk2. Proc Natl Acad Sci USA. 1992;89:7900–7904. doi: 10.1073/pnas.89.17.7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi F, Quarta S, Savio M, Riva F, Rossi L, Stivala L A, Scovassi A I, Meijer L, Proseperi E. The cyclin-dependent kinase inhibitors olomoucine and roscovitine arrest human fibroblasts in G1 phase by specific inhibition of CDK2 kinase activity. Exp Cell Res. 1998;245:8–18. doi: 10.1006/excr.1998.4216. [DOI] [PubMed] [Google Scholar]
- 3.Baldin V, Lukas J, Marcote M J, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 4.Bartek J, Bartkova J, Lukas J. The retinoblastoma protein pathway and the restriction point. Curr Opin Cell Biol. 1996;8:805–814. doi: 10.1016/s0955-0674(96)80081-0. [DOI] [PubMed] [Google Scholar]
- 5.Beijersbergen R L, Bernards R. Cell cycle regulation by the retinoblastoma family of growth inhibitory proteins. Biochim Biophys Acta. 1996;1287:103–120. doi: 10.1016/0304-419x(96)00002-9. [DOI] [PubMed] [Google Scholar]
- 6.Bresnahan W A, Boldogh I, Ma T, Albrecht T, Thompson E A. Cyclin E/Cdk2 activity is controlled by different mechanisms in the G0 and G1 phases of the cell cycle. Cell Growth Differ. 1996;7:1283–1290. [PubMed] [Google Scholar]
- 7.Clurman B E, Sheaff R J, Thress K, Groudine M, Roberts J M. Turnover of cyclin E by the ubiquitin-proteasome pathway is regulated by cdk2 binding and cyclin phosphorylation. Genes Dev. 1996;10:1979–1990. doi: 10.1101/gad.10.16.1979. [DOI] [PubMed] [Google Scholar]
- 8.Connell-Crowley L, Harper J W, Goodrich D W. Cyclin D1/Cdk4 regulates retinoblastoma protein-mediated cell cycle arrest by site-specific phosphorylation. Mol Biol Cell. 1997;8:287–301. doi: 10.1091/mbc.8.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dou Q P, Levin A H, Zhao S, Pardee A B. Cyclin E and cyclin A as candidates for the restriction point protein. Cancer Res. 1993;53:1493–1497. [PubMed] [Google Scholar]
- 10.Dulbecco R, Elkington J. Conditions limiting multiplication of fibroblastic and epithelial cells in dense cultures. Nature. 1973;246:197–199. doi: 10.1038/246197a0. [DOI] [PubMed] [Google Scholar]
- 11.Dulic V, Lees E, Reed S I. Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 1992;257:1958–1961. doi: 10.1126/science.1329201. [DOI] [PubMed] [Google Scholar]
- 12.Dulic V, Drullinger L F, Lees E, Reed S I, Stein G H. Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive cyclin E-Cdk2 and cyclin D1-Cdk2 complexes. Proc Natl Acad Sci USA. 1993;90:11034–11038. doi: 10.1073/pnas.90.23.11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duronio R J, Brook A, Dyson N, O'Farrell P H. E2F-induced S phase requires cyclin E. Genes Dev. 1996;10:2505–2513. doi: 10.1101/gad.10.19.2505. [DOI] [PubMed] [Google Scholar]
- 14.Erlandsson F, Linnman C, Ekholm S, Bengtsson E, Zetterberg A. A detailed analysis of cyclin A accumulation at the G(1)/S border in normal and transformed cells. Exp Cell Res. 2000;259:86–95. doi: 10.1006/excr.2000.4889. [DOI] [PubMed] [Google Scholar]
- 15.Erlanson M, Portin C, Linderholm B, Lindh J, Roos G, Landberg G. Expression of cyclin E and the cyclin-dependent kinase inhibitor p27 in malignant lymphomas—prognostic implications. Blood. 1998;92:770–777. [PubMed] [Google Scholar]
- 16.Evans T, Rosenthal E T, Youngblom J, Distel D, Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983;33:389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- 17.Ewen M E, Sluss H K, Sherr C J, Matsushime H, Kato J, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 18.Fang F, Orend G, Watanabe N, Hunter T, Ruoslahti E. Dependence of cyclin E-CDK2 kinase activity on cell anchorage. Science. 1996;271:499–502. doi: 10.1126/science.271.5248.499. [DOI] [PubMed] [Google Scholar]
- 19.Geng Y, Eaton E N, Picon M, Roberts J M, Lundberg A S, Gifford A, Sardet C, Weinberg R A. Regulation of cyclin E transcription by E2Fs and retinoblastoma protein. Oncogene. 1996;12:1173–1180. [PubMed] [Google Scholar]
- 20.Harbour J W, Luo R X, Dei Santi A, Postigo A A, Dean D C. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell. 1999;98:859–869. doi: 10.1016/s0092-8674(00)81519-6. [DOI] [PubMed] [Google Scholar]
- 21.Helin K, Harlow E, Fattaey A. Inhibition of E2F-1 transactivation by direct binding of the retinoblastoma protein. Mol Cell Biol. 1993;13:6501–6508. doi: 10.1128/mcb.13.10.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrera R E, Sah V P, Williams B O, Mäkelä T P, Weinberg R A, Jacks T. Altered cell cycle kinetics, gene expression, and G1 restriction point regulation in Rb-deficient fibroblasts. Mol Cell Biol. 1996;16:2402–2407. doi: 10.1128/mcb.16.5.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hola M, Howard M, Nawaz F N, Castleden S, Brooks R F. Individual nuclei differ in their sensitivity to the cytoplasmic inducers of DNA synthesis: implications for the origin of cell cycle variability. Exp Cell Res. 1996;229:350–359. doi: 10.1006/excr.1996.0380. [DOI] [PubMed] [Google Scholar]
- 24.Jackson P K, Chevalier S, Philippe M, Kirschner M W. Early events in DNA replication require cyclin E and are blocked by p21CIP1. J Cell Biol. 1995;130:755–769. doi: 10.1083/jcb.130.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato J, Matsushime H, Hiebert S W, Ewen M E, Sherr C J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 26.Keyomarsi K, Conte D, Jr, Toyofuku W, Fox M P. Deregulation of cyclin E in breast cancer. Oncogene. 1995;11:941–950. [PubMed] [Google Scholar]
- 27.Keyomarsi K, Pardee A B. Redundant cyclin overexpression and gene amplification in breast cancer cells. Proc Natl Acad Sci USA. 1993;90:1112–1116. doi: 10.1073/pnas.90.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Killander D, Zetterberg A. A quantitative cytochemical investigation of the relationship between cell mass and initiation of DNA synthesis in mouse fibroblasts in vitro. Exp Cell Res. 1965;40:12–20. doi: 10.1016/0014-4827(65)90285-5. [DOI] [PubMed] [Google Scholar]
- 29.Knudsen E S, Buckmaster C, Chen T T, Feramisco J R, Wang J Y. Inhibition of DNA synthesis by RB: effects on G1/S transition and S-phase progression. Genes Dev. 1998;12:2278–2292. doi: 10.1101/gad.12.15.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koff A, Cross F, Fisher A, Schumacher J, Leguellec K, Philippe M, Roberts J M. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell. 1991;66:1217–1228. doi: 10.1016/0092-8674(91)90044-y. [DOI] [PubMed] [Google Scholar]
- 31.Koff A, Giordano A, Desai D, Yamashita K, Harper J W, Elledge S, Nishimoto T, Morgan D O, Franza B R, Roberts J M. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992;257:1689–1694. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- 32.Krude T, Jackman M, Pines J, Laskey R A. Cyclin/Cdk-dependent initiation of DNA replication in a human cell-free system. Cell. 1997;88:109–119. doi: 10.1016/s0092-8674(00)81863-2. [DOI] [PubMed] [Google Scholar]
- 33.Larsson O, Latham C, Zickert P, Zetterberg A. Cell cycle regulation of human diploid fibroblasts: possible mechanisms of platelet-derived growth factor. J Cell Physiol. 1989;139:477–483. doi: 10.1002/jcp.1041390305. [DOI] [PubMed] [Google Scholar]
- 34.Le Cam L, Polanowska J, Fabbrizio E, Olivier M, Philips A, Ng Eaton E, Classon M, Geng Y, Sardet C. Timing of cyclin E gene expression depends on the regulated association of a bipartite repressor element with a novel E2F complex. EMBO J. 1999;18:1878–1890. doi: 10.1093/emboj/18.7.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leone G, DeGregori J, Sears R, Jakoi L, Nevins J R. Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F. Nature. 1997;387:422–426. doi: 10.1038/387422a0. [DOI] [PubMed] [Google Scholar]
- 36.Lew D J, Dulic V, Reed S I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991;66:1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- 37.Lukas J, Pagano M, Staskova Z, Draetta G, Bartek J. Cyclin D1 protein oscillates and is essential for cell cycle progression in human tumour cell lines. Oncogene. 1994;9:707–718. [PubMed] [Google Scholar]
- 38.Lukas J, Herzinger T, Hansen K, Moroni M C, Resnitzky D, Helin K, Reed S I, I. S, Bartek J. Cyclin E-induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997;11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- 39.Lundberg A S, Weinberg R A. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsushime H, Ewen M E, Strom D K, Kato J Y, Hanks S K, Roussel M F, Sherr C J. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992;71:323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- 41.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nevins J R. E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science. 1992;258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- 43.Norbury C, Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- 44.Oda S, Nishida J, Nakabeppu Y, Sekiguchi M. Stabilization of the cyclin E and cdk2 mRNA at G1/S transition in Rat-1A cells emerging from the G0 state. Oncogene. 1995;7:1343–1351. [PubMed] [Google Scholar]
- 45.Ohtani K, DeGregori J, Nevins J R. Regulation of the cyclin E gene by transcription factor E2F1. Proc Natl Acad Sci USA. 1995;92:12146–12150. doi: 10.1073/pnas.92.26.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohtsubo M, Roberts J M. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 1993;259:1908–1912. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- 47.Ohtsubo M, Theodoras A M, Schumacher J, Roberts J M, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pardee A B. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci USA. 1974;4:1286–1290. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pardee A B. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 50.Pines J, Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle. Cell. 1989;58:833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- 51.Pines J, Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990;346:760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- 52.Porter P L, Malone K E, Heagerty P J, Alexander G M, Gatti L A, Firpo E J, Daling J R, Roberts J M. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat Med. 1997;2:222–225. doi: 10.1038/nm0297-222. [DOI] [PubMed] [Google Scholar]
- 53.Reed S I, Dulic V, Lew D J, Richardson H E, Wittenberg C. G1 control in yeast and animal cells. Ciba Found Symp. 1992;170:7–19. doi: 10.1002/9780470514320.ch2. [DOI] [PubMed] [Google Scholar]
- 54.Reed S I. Control of the G1/S transition. Cancer Surv. 1997;29:7–23. [PubMed] [Google Scholar]
- 55.Resnitzky D, Reed S I. Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Mol Cell Biol. 1995;15:3463–3469. doi: 10.1128/mcb.15.7.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reynisdottir I, Polyak K, Iavarone A, Massague J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 1995;9:1831–1845. doi: 10.1101/gad.9.15.1831. [DOI] [PubMed] [Google Scholar]
- 57.Sherr C J. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- 58.Singer J D, Gurian-West M, Clurman B, Roberts J M. Cullin-3 targets cyclin E for ubiquitination and controls S phase in mammalian cells. Genes Dev. 1999;13:2375–2387. doi: 10.1101/gad.13.18.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spruck C H, Won K A, Reed S I. Deregulated cyclin E induces chromosome instability. Nature. 1999;16:297–300. doi: 10.1038/45836. [DOI] [PubMed] [Google Scholar]
- 60.Stein G H, Dulic V. Molecular mechanisms for the senescent cell cycle arrest. J Investig Dermatol Symp Proc. 1998;3:14–18. [PubMed] [Google Scholar]
- 61.Temin H M. Stimulation by serum of multiplication of stationary chicken cells. J Cell Physiol. 1971;78:161–170. doi: 10.1002/jcp.1040780202. [DOI] [PubMed] [Google Scholar]
- 62.Todaro G J, Lazar G K, Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965;66:325–333. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]
- 63.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 64.Wang J, Chenivesse X, Henglein B, Brechot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990;343:555–557. doi: 10.1038/343555a0. [DOI] [PubMed] [Google Scholar]
- 65.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;8:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 66.Weinberg R A. Regulation of cyclin E transcription by E2Fs and retinoblastoma protein. Oncogene. 1996;12:1–8. [PubMed] [Google Scholar]
- 67.Willems A R, Lanker S, Patton E E, Craig K L, Nason T F, Mathias N, Kobayashi R, Wittenberg C, Tyers M. Cdc53 targets phosphorylated G1 cyclins for degradation by the ubiquitin proteolytic pathway. Cell. 1996;86:453–463. doi: 10.1016/s0092-8674(00)80118-x. [DOI] [PubMed] [Google Scholar]
- 68.Won K A, Reed S I. Activation of cyclin E/CDK2 is coupled to site-specific autophosphorylation and ubiquitin-dependent degradation of cyclin E. EMBO J. 1996;15:4182–4193. [PMC free article] [PubMed] [Google Scholar]
- 69.Xiong Y, Connolly T, Futcher B, Beach D. Human D-type cyclin. Cell. 1991;65:691–699. doi: 10.1016/0092-8674(91)90100-d. [DOI] [PubMed] [Google Scholar]
- 70.Zarkowska T, Mittnacht S. Differential phosphorylation of the retinoblastoma protein by G1/S cyclin-dependent kinases. J Biol Chem. 1997;272:12738–12746. doi: 10.1074/jbc.272.19.12738. [DOI] [PubMed] [Google Scholar]
- 71.Zetterberg A. Synthesis and accumulation of nuclear and cytoplasmic proteins during interphase in mouse fibroblasts in vitro. Exp Cell Res. 1966;42:500–511. doi: 10.1016/0014-4827(66)90264-3. [DOI] [PubMed] [Google Scholar]
- 72.Zetterberg A. Nuclear and cytoplasmic growth during interphase in mammalian cells. In: Prescott D M, Goldstein L, McConkey E, editors. Advances in cell biology. Vol. 1. New York, N.Y: Appleton-Century-Crofts; 1970. pp. 211–232. [Google Scholar]
- 73.Zetterberg A, Auer G. Proliferative activity and cytochemical properties of nuclear chromatin related to local cell density of epithelial cells. Exp Cell Res. 1970;62:262–270. doi: 10.1016/0014-4827(79)90527-5. [DOI] [PubMed] [Google Scholar]
- 74.Zetterberg A, Larsson O. Kinetic analysis of regulatory events in G1 leading to proliferation or quiescence of Swiss 3T3 cells. Proc Natl Acad Sci USA. 1985;82:5365–5369. doi: 10.1073/pnas.82.16.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zetterberg A, Larsson O. Coordination between cell growth and cell cycle transit in animal cells. Cold Spring Harbor Symp Quant Biol. 1991;56:137–147. doi: 10.1101/sqb.1991.056.01.018. [DOI] [PubMed] [Google Scholar]
- 76.Zetterberg A, Larsson O. Cell cycle progression and cell growth in mammalian cells: kinetic aspects of transition events. In: Hutchinson C, Glover D M, editors. Cell cycle control. New York, N.Y: Oxford University Press; 1995. pp. 206–227. [Google Scholar]
- 77.Zetterberg A, Larsson O, Wiman K G. What is the restriction point? Curr Opin Cell Biol. 1995;7:835–842. doi: 10.1016/0955-0674(95)80067-0. [DOI] [PubMed] [Google Scholar]
- 78.Zhang H S, Gavin M, Dahiya A, Postigo A A, Ma D, Luo R X, Harbour J W, Dean D C. Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell. 2000;101:79–89. doi: 10.1016/S0092-8674(00)80625-X. [DOI] [PubMed] [Google Scholar]