Abstract
Theoretical calculation of the reactivity of α-imino thioesters indicates that they are very reactive substrates for Umpolung N-alkylation. In fact, treatment of α-aldimino thioesters with dialkylzinc reagents in the presence of aldehydes or imines gives three-component coupling products in good yields.
Treatment of α-aldimino thioesters with dialkylzinc reagents in the presence of aldehydes or imines gives three-component coupling products in good yields with good to high anti-selectivities.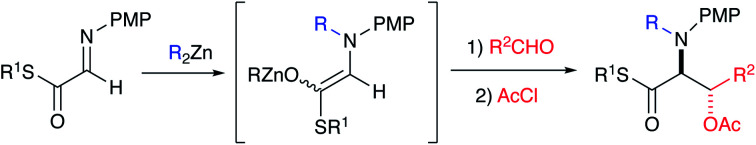
1. Introduction
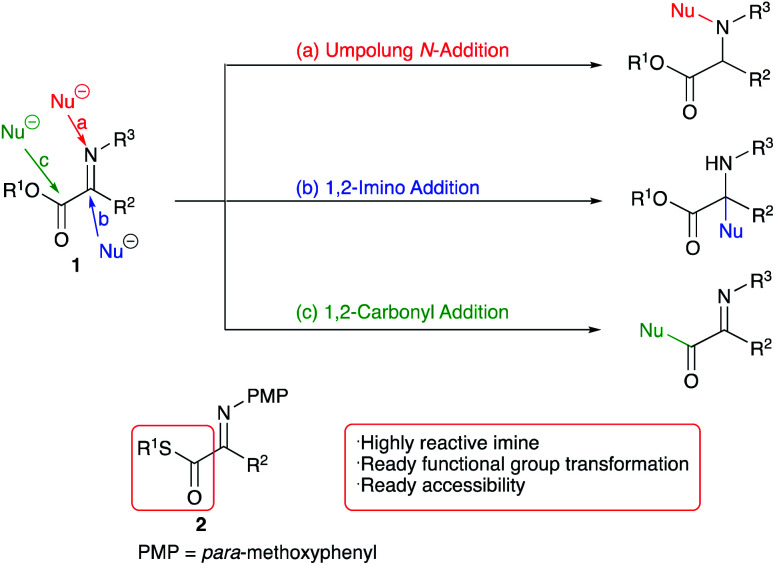
The widespread existence of 1,2-amino alcohol moieties in natural products and pharmaceuticals1 has prompted us to explore efficient synthetic methods for these important materials. Various synthetic 1,2-amino alcohol derivatives have also been employed as drugs for therapeutic purposes, chiral auxiliaries, and metal ligands in catalytic asymmetric synthesis.2 We have reported new approaches to this class of compound via asymmetric reduction of 1,2-imino ketones,3 diastereoselective addition to chiral imines,4 titanium tetraiodide-mediated reductive addition reactions,5 and crossed pinacol-type reductive coupling of aldehydes with imines.6 In addition to these studies, we have been interested in the Umpolung reactions using α-imino esters 1 and related compounds, and have disclosed several interesting features.7,8 Under certain reaction conditions an unusual Umpolung N-addition reaction proceeds to give N-alkylated products in good yields (Scheme 1, path a).
Scheme 1. Reactions of α-imino ester 1 and α-imino thioester 2.
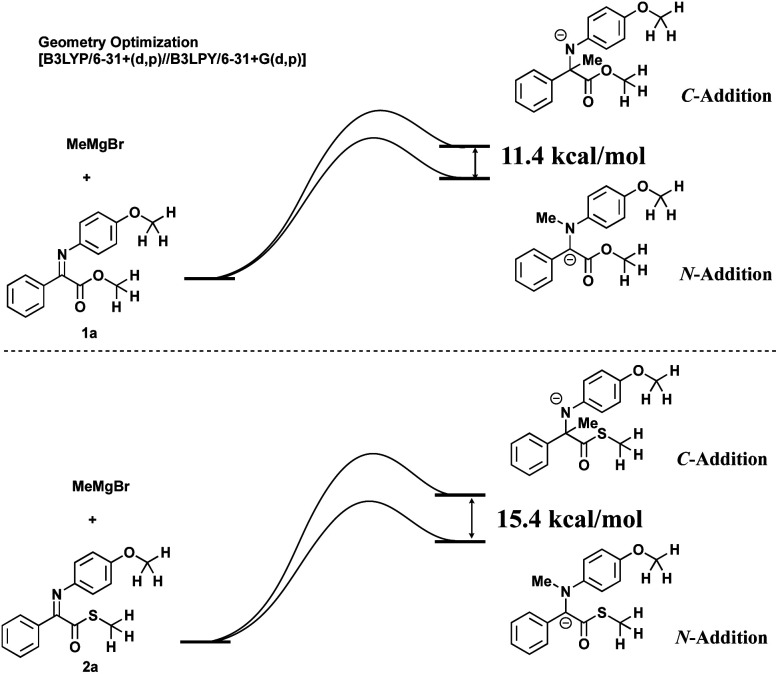
During these investigations, the thioester analogues 2 have intrigued us, since a simple calculation using the model substrates 1a and 2a with the Gaussian 03 program9 indicates that in the addition reactions of a methyl anion to normal ester 1a and thioester 2a, the energy difference between the C- and the N-additions is larger for thioester 2a than for normal ester 1a (Scheme 2). This means that the Umpolung N-addition reaction would be easier for thioesters than for normal esters. Among α-imino thioesters α-aldimino thioester 3 has attracted our attentions since α-aldimino thioesters are expected to be more reactive than α-ketimino analogues and the subsequent reactions at the aldimino moieties would proceed to give interesting and useful products. This paper describes three-component coupling reactions consisting of N-alkylation/aldol reaction10 of α-aldimino thioesters (Scheme 3).
Scheme 2. Model substrates 1a and 2a for energy calculation.
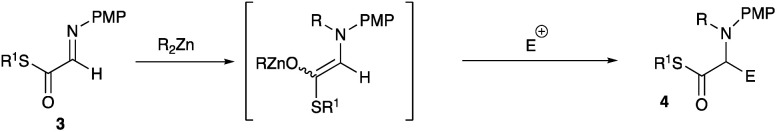
Scheme 3. The present study using α-aldimino thioester 3.
2. Results and discussion
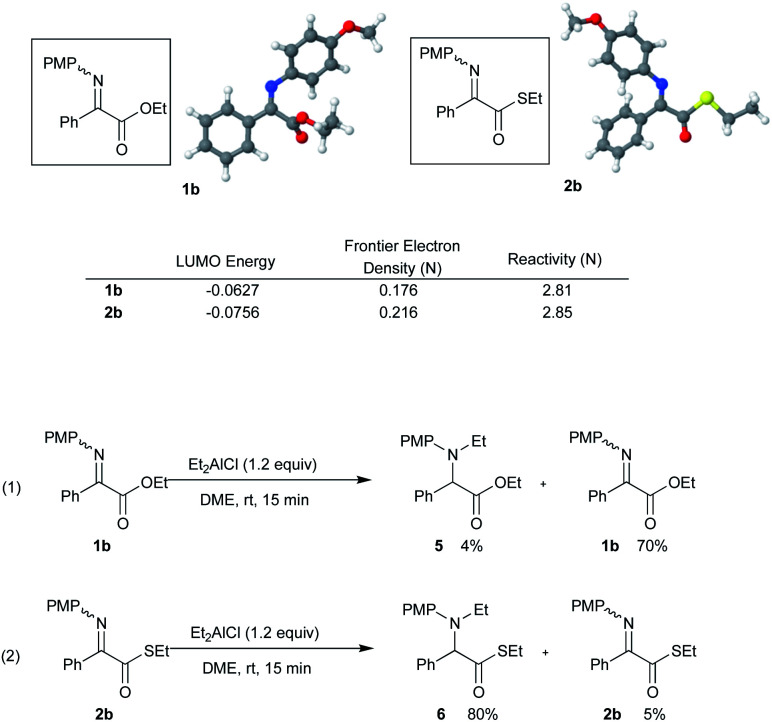
A further calculation using the Gaussian 03 program9 indicates that the LUMO energies of the ethyl ester 1b and its thio analogue are −0.0627 and −0.0756, respectively, which means that the reactivity of the α-imino thioester 2b is higher than that of the α-imino ester 1b. The structures 1b and 2b were fully optimized followed by frequency calculation on the stationary point performed using 6-31G(d) basis set for all atoms, which was employed a B3LYP density functional theory.11 We have also found that the reactivity of the imino nitrogen atom of α-imino thioester 2b is higher than that of α-imino ester 1b since the Frontier electron densities are 0.176 and 0.216, respectively, leading to the increased reactivity of nitrogen atom (Scheme 4). In fact, the reaction of the α-imino ester 1b with Et2AlCl in DME at rt for 15 min gave the N-ethylation product 5 in only 4% yield, whereas the α-imino thioester 2b underwent a similar N-ethylation reaction to give the product 6 in 80% yield. (Scheme 4, eqn (1) and (2)).
Scheme 4. Reactivity difference between α-imino ester 1b and α-imino thioester 2b.
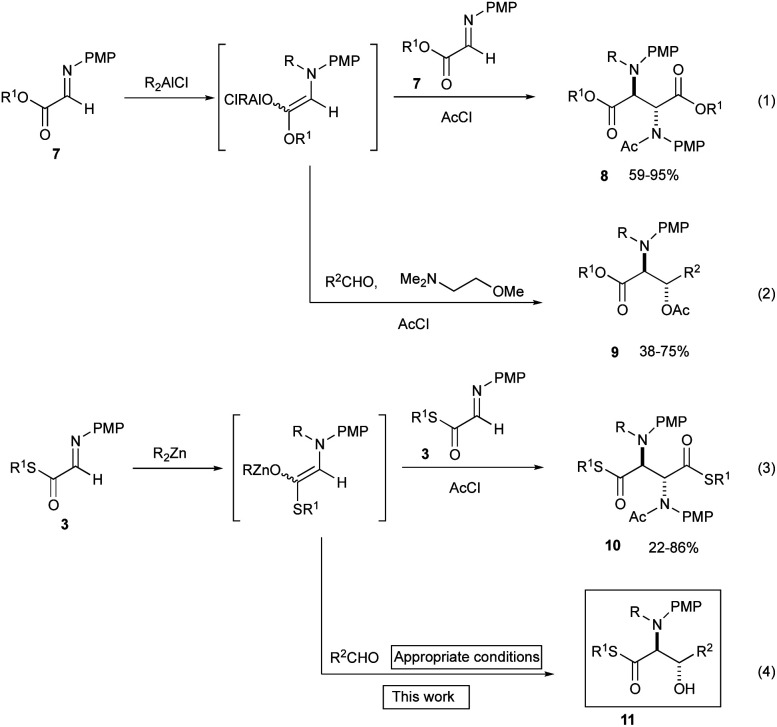
Our previous investigations revealed that N-alkylation proceeded well both with the α-aldimino ester 77a and the α-aldimino thioester 37q to give the intermediary aluminum and the zinc enolates, respectively. However, the subsequent addition reaction with the parent imines 7 and 3 proceeded very rapidly to give homo-coupling products 8 and 10, respectively in good yields (Scheme 5, eqn (1) and (3)), and therefore, it was not trivial to carry out a cross-coupling reaction with another electrophile (eqn (2) and (4)). When the α-aldimino ester 7 was used as a substrate, the presence of an added additive (Me2N(CH2)2OMe) facilitated a cross-coupling reaction with aldehydes to give 1,2-amino alcohols 9 in good yields (eqn (2)).7j In strong contrast to the cases with the α-aldimino ester 7, however, the thioester 3 underwent only a homo-coupling reaction even in the presence of an additive (Me2N(CH2)2OMe) to give the adduct 10 in good yield, and the cross-coupling product 11 was not obtained at all, presumably due to the enhanced electrophilicity of the imine moiety of the α-aldimino thioester 3. We screened various organometallics (RMgBr, R2Mg, R3Al, R2AlCl, RAlCl2, R2Zn and RZnBr), and among them the use of dialkylzinc recorded an acceptable yield of the homo-coupling product 10 (up to 86% yield, eqn (3)).
Scheme 5. Previous investigations using α-aldimino ester 7 and α-aldimino thioester 3.
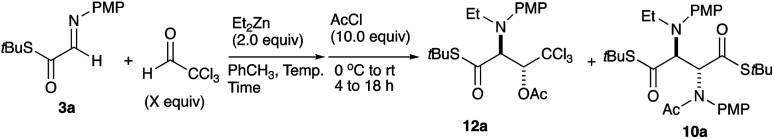
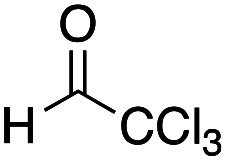
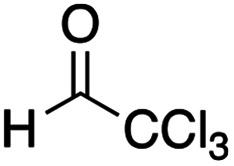
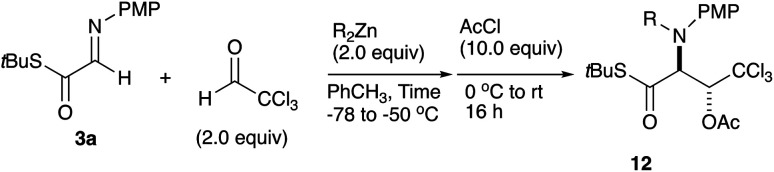
We further examined the reaction conditions for cross-coupling using a series of aldehydes under various conditions. Among the aldehydes tested (pClC6H4CHO, PhCHO, PhCH = CHCHO, nPrCH = CHCHO, nBuCHO, EtOCOCHO, CCl3CHO, etc.) only chloral, a reactive aldehyde gave a cross-coupling product 11. Since the three-component coupling product, the β-hydroxy α-amino thioester obtained was unstable under air due to the oxidative cleavage previously reported,12 it was isolated as the acetate 12a after acetylation with AcCl at the hydroxy moiety. Table 1 summarizes the results.
Three-component coupling reaction: Examination of the reaction conditions.
| ||||||
|---|---|---|---|---|---|---|
| Entry | Chloral (equiv.) | Temp (°C) | Time (h) | 12aa (%) | anti/synb | 10aa (%) |
| 1 | 1.0 | −60 to −30 | 2.0 | 19 | 63 : 37 | 29 |
| 2 | 1.0 | −78 to −50 | 1.0 | 45 | 70 : 30 | 26 |
| 3 | 1.5 | −78 to −50 | 2.0 | 59 | 64 : 36 | 8 |
| 4 | 2.0 | −78 to −50 | 2.0 | 79 | 62 : 38 | 6 |
| 5 | 2.5 | −78 to −50 | 2.0 | 76 | 59 : 41 | 5 |
| 6c | 2.1 | −78 to −50 | 1.0 | 37 | 65 : 35 | 20 |
Isolated yield.
Determined by 1H NMR and/or HPLC.
3a was slowly added to a mixture of chloral and Et2Zn.
As shown in Table 1, the three-component coupling reaction proceeded with the α-imino thioester 3a, diethylzinc, and chloral in toluene to give the adduct 12a together with the homo-coupling product 10a as a major byproduct (entry 1). In an effort to avoid the undesirable formation of the homo-coupling product 10a, a solution of diethylzinc was added to a mixture of the α-imino thioester 3a and chloral at −78 °C (entry 2). An increase in the formation of the cross-coupling product 12a was observed when 1.5 equivalents of chloral were used. The best result was obtained when the reaction was carried out with 2.0 equivalents of chloral, and in this case the desired product was obtained in 79% yield (entry 4). An additional increase in the amount of chloral did not lead to a further improvement of the product yield (entry 5). Since diethylzinc did not react with chloral at −78 °C, a solution of the thioester 3a was added to a mixture of choral and diethylzinc. In this case, however, an increased amount of the homo-coupling product 10a was obtained (entry 6). Under the best conditions found for the cross-coupling reaction, various aldehydes and imines were subjected to the three-component coupling reaction, and Table 2 summarizes the results.
Three-component coupling reaction.
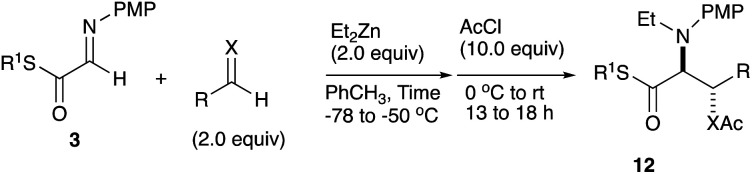
| |||||
|---|---|---|---|---|---|
| Entry | 3 : R1 | Electrophile | Time (h) | 12 : Yielda (%) | anti : synb |
| 1 | 3a : tBu |
|
2.0 | 0 | — |
| 2 | tBu |
|
1.0 | 12a : 79 | 62 : 38 |
| 3 | 3b : Et |
|
1.0 | 12b : 54 | 83 : 17 |
| 4 | 3c : Cy |
|
1.0 | 12c : 47 | 69 : 31 |
| 5 | tBu |
|
2.0 | 12d : 43 | 50 : 50 |
| 6 | tBu |
|
3.0 | 12e : 26 | 50 : 50 |
| 7 | tBu |
|
4.0 | 12f : 32 | 62 : 38 |
| 8 | tBu |
|
2.0 | 0 | — |
| 9 | tBu |
|
3.0 | 0 | — |
| 10 | tBu |
|
1.0 | 12g : 45c | 100 : 0 |
| 11 | Et |
|
1.0 | 12h : 64c | 100 : 0 |
Isolated yield.
Determined by 1H NMR and/or HPLC.
1.0 equiv of the electrophile (imine) was used.
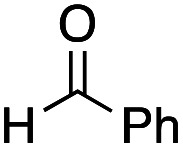
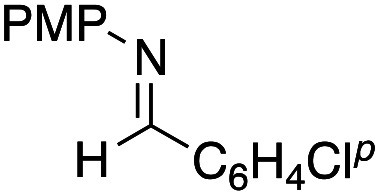
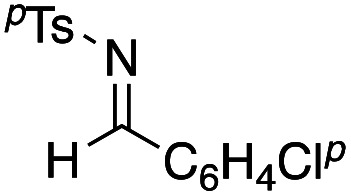
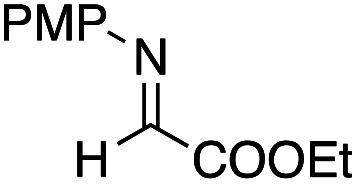
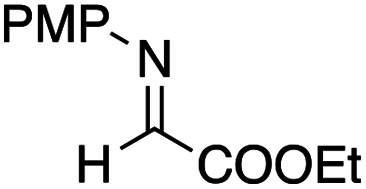
Benzaldehyde did not react with the intermediary zinc enolate, but only the homo-coupling product 10a was obtained (entry 1). The use of the cyclohexylthio ester recorded a slightly better diastereoselectivity of 69 : 31, whereas in the case of the ethylthio ester the ratio of the diastereomers was improved to 83 : 17, although the product yields were moderate (entry 3 and 4). Bromal also reacted with the enolate to give the three-component coupling product 12d in moderate yield, presumably due to the decreased reactivity of bromal compared with that of chloral (entry 5). Ethyl glyoxylate and acrolein did not serve as a good electrophile in this three-component coupling reaction, and the addition reaction gave moderate yields of the products (entries 6 and 7). Regarding the use of imines, a simple imine derived from para-chlorobenzaldehyde and anisole did not give the addition product, nor did its para-tosyl derivative, a relatively reactive imine (entries 8 and 9). The imine derived from ethyl glyoxylate is a better electrophile in this three-component coupling reaction to give the anti-coupling adduct as a sole product, in which the ethylthio ester served as a better substrate (entries 10 and 11). We further examined the use of other dialkylzinc reagents as the initial N-alkylation reagent. Table 3 summarizes the results.
Comparison of dialkylzinc reagents.
| ||||
|---|---|---|---|---|
| Entry | R | Time (h) | 12a (%) | anti/synb |
| 1 | Et | 1.0 | 12a : 79 | 62 : 38 |
| 2 | iso-Pr | 1.5 | 12i : 75 | 56 : 44 |
| 3 | Ph | 3.5 | 0 | — |
Isolated yield.
Determined by 1H NMR and/or HPLC.
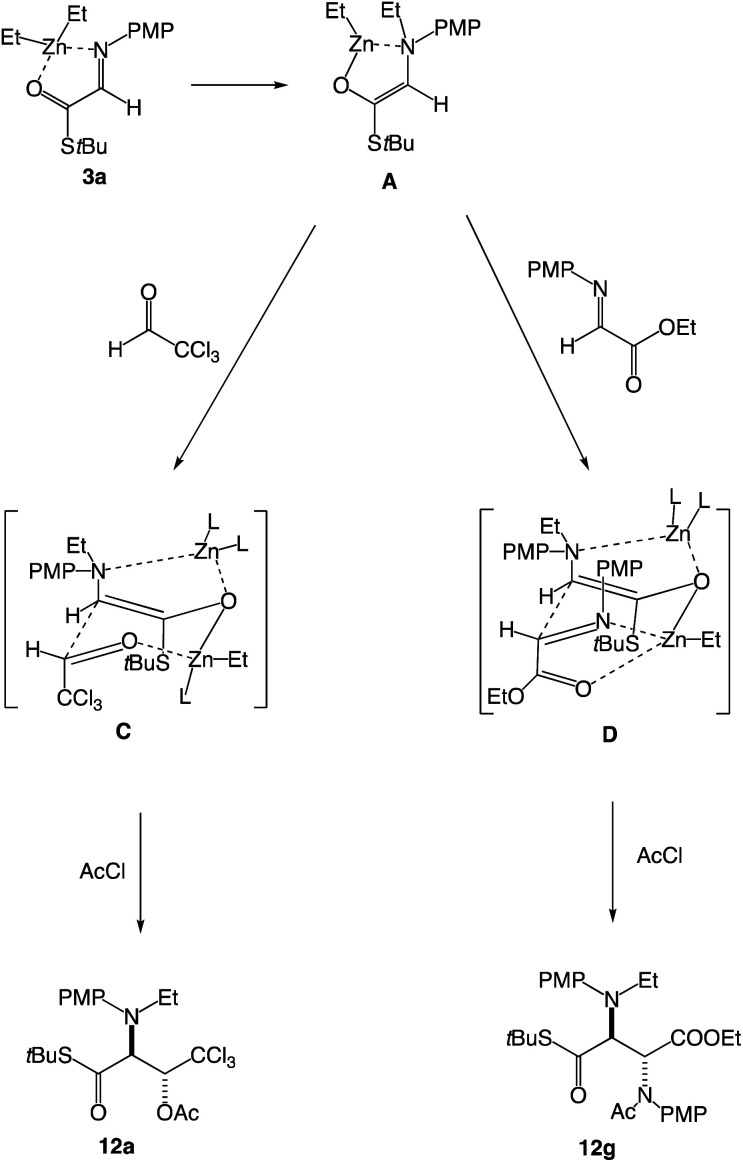
The use of diisopropylzinc and diphenylzinc was examined besides diethylzinc. Diisopropylzinc underwent a similar N-alkylation reaction to give the three-component coupling product 12i in 75% yield, whereas diphenylzinc did not add to the α-imino thioester 3a, but only the starting thioester 3a was recovered (entries 2 and 3). Regarding the reaction mechanisms the following Scheme 6 shows possible pathways.
Scheme 6. Plausible reaction pathways.
First, diethylzinc coordinates with the α-imino thioester 3a and N-ethylation proceeds to give the zinc enolate A, which reacts with chloral to form the β-hydoroxy α-amino thioester 12avia a six-membered transition state C. In the case of the reaction with an imine, the addition also proceeds via a six-membered transition state D to give the α,β-diamino thioester 12g. Since there exist three chelated metallacycles in the TS D, a relatively fixed intermediate would be involved in this model having an N,O-chelated electrophilic imine, leading to a formation of the anti-adduct 12g with high diastereoselectivity. In contrast, in the TS C for the addition with chloral, since there are only two metllacycles and no chelation is available between the aldehyde substituent (CCl3) with the zinc atom, the anti-selectivity would be affected compared with the case of the imine as an electrophile.
3. Conclusions
In conclusion, α-imino thioesters are useful substrates for the Umpolung N-addition of dialkylzinc reagents to form the zinc enolates. A simple theoretical calculation explains the high reactivity of α-imino thioesters.9,13 Since the intermediary zinc enolates formed upon the N-alkylation rapidly react with the parent α-imino thioesters to give homo-coupling products, cross-coupling reactions are not readily carried out. In the present study, we found that the addition of dialkylzinc to a mixture of α-imino thioester and reactive aldehydes or imines at low temperature gave three-component coupling products in good yields with high anti-selectivity. The formed β-hydroxy α-amino thioesters and α,β-diamino thioesters are potentially useful intermediates for further functional group interconversions with respect to the thioester moieties.14 Since the present procedure offers a rapid assembly of three components efficiently, this methodology is useful in addition to the existing precedents for the synthesis of 1,2-hydroxy amines1,2 and 1,2-diamines.15
4. Experimental
General aspects
Infrared spectra were determined on a JASCO FT/IR-460 plus spectrometer. 1H NMR (400 and 500 MHz) and 13C NMR (100 and 125 MHz) spectra were recorded with a JEOL ECX-400P, or a JEOL A-500 spectrometer using tetramethylsilane as an internal standard. Mass spectra were recorded on a JEOL MS-700D spectrometer. High-performance liquid chromatography (HPLC) was carried out on a Hitachi L-4200 detector and a Hitachi L-6200 pump. Toluene (PhCH3), was dried over CaH2, distilled, and stored over molecular sieves 4 Å. 1,2-Dimethoxyethane (DME) was distilled from calcium hydride and then cupper(i) chloride, and stored over sodium. Purification of products was performed by column chromatography on silica gel (Kanto Silica Gel 60N) and/or preparative TLC on silica gel (Merck Kiesel Gel GF254 or Wako Gel B-5F). The staring materials 1b, 2b, 3a–c were prepared as reported.7d,q,s
Ethyl 2-[ethyl(4-methoxyphenyl)amino]-2-phenylacetate 5
Under an argon atmosphere, a suspension of the α-imino ester 1b (42.5 mg, 0.15 mmol) in DME (0.50 mL) was stirred at rt for 5 min, and to it was slowly added Et2AlCl in nhexane (0.17 mL, 0.18 mmol, 1.05 M). After the mixture was stirred for 15 min, it was quenched with an aq. solution of Rochelle's salt (5.0 mL, 30.0 g in 75 mL H2O), and the mixture was stirred at rt for 30 min. The whole mixture was extracted with ethyl acetate (5.0 mL × 5). The combined extracts were washed with an aq. solution of Rochelle's salt, dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified on silica gel TLC (nhexane : ethyl acetate = 5 : 1) under an argon atmosphere to give the title compounds 5 (1.84 mg, 4%) and the recovered 1b (30.5 mg, 70%). The spectral data were identical with those reported.7d
Yield 4% (1.84 mg); a yellow oil; 1H NMR (400 MHz, CDCl3) δ 0.88 (t, 3H, J = 6.9 Hz), 1.17 (t, 3H, J = 7.3 Hz), 3.03–3.29 (m, 2H), 3.78 (s, 3H), 4.06–4.23 (m, 2H), 5.26 (s, 1H), 6.76–6.94 (m, 4H), 7.15–7.48 (m, 5H); 13C NMR (125 MHz, CDCl3) δ 13.0, 14.1, 43.9, 55.5, 60.8, 68.7, 114.3, 119.8, 128.0, 128.4, 128.7, 136.6, 142.6, 153.7, 172.0; IR (neat) 2950, 1755, 1520, 1260, 1190, 1040, 825, 700 cm−1; HRMS (EI) calcd for C19H23NO3 (M)+ 313.1678, found 313.1692.
S-Ethyl 2-[ethyl(4-methoxyphenyl)amino]-2-phenylethanethioate 6
Under an argon atmosphere, a suspension of the α-imino thioester 2b (44.9 mg, 0.15 mmol) in DME (0.50 mL) was stirred at rt for 5 min, and to it was slowly added Et2AlCl in nhexane (0.17 mL, 0.18 mmol, 1.05 M). After the mixture was stirred for 15 min. The reaction was quenched with an aq. solution of Rochelle's salt (5.0 mL, 30.0 g in 75 mL of H2O), and the mixture was stirred at rt for 30 min. The whole mixture was extracted with ethyl acetate (5.0 mL × 5). The combined extracts were washed with an aq. solution of Rochelle's salt, dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified on silica gel TLC (nhexane : ethyl acetate = 5 : 1) under an argon atmosphere to give the title compounds 6 (40.5 mg, 80%) and the recovered 2b (2.3 mg, 5%).
Yield 80% (40.5 mg, 80%); a yellow oil; Rf = 0.80 (nhexane : ethyl acetate = 10 : 1, developed twice); 1H NMR (500 MHz, CDCl3) δ 0.92 (dd, J = 7.0, 7.0 Hz, 3H), 1.22 (dd, J = 7.3, 7.3 Hz, 3H), 2.91–2.81 (m, 2H), 3.05 (dq, J = 7.0, 14.0 Hz, 1H), 3.15 (dq, J = 7.0, 14.0 Hz, 1H), 3.76 (s, 3H), 6.83–6.80 (m, 2H), 5.27 (s, 1H), 6.91–6.88 (m, 2H), 7.32–7.26 (m, 5H); 13C NMR (125 MHz, CDCl3) δ 12.7, 14.5, 23.3, 44.2, 55.5, 76.4, 114.3, 120.5, 128.0, 128.3, 129.3, 135.8, 142.0, 154.0, 201.9; IR (neat) 2971, 2931, 2833, 1684, 1510, 1452, 1375, 1244, 1180, 1038, 966, 819, 761, 702 cm−1; HRMS (EI) calcd for C19H23NO2S(M)+ 329.1450, found 329.1447.
(2R*,3S*)-4-(tert-Butylthio)-1,1,1-trichloro-3-[ethyl(4-methoxyphenyl)amino]-4-oxobutan-2-yl acetate 12a: general procedure for the three-component coupling reaction
In a 30 mL two-necked round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and an argon balloon were placed S-(tertbutyl) 2-[(4-methoxyphenyl)imino]ethanethioate 3a (37.7 mg, 0.15 mmol), and chloral (0.029 mL, 0.30 mmol) in toluene (3.0 mL) at −78 °C, and to it was slowly added Et2Zn (0.30 mL, 1.00 M, 0.30 mmol). After the mixture was stirred for 2 h at −78 to −50 °C, to it was added AcCl (0.11 mL, 1.50 mmol) and stirred for 15.5 h at 0 °C. The mixture was quenched with aq. NaHCO3 (10.0 mL), and the whole mixture was extracted with ethyl acetate (5.0 mL × 3). The combined extracts were washed with brine, dried over anhydrous Na2SO4, and concentrated in vacuo. The crude product was purified on silica gel TLC (toluene : ethyl acetate = 8 : 1) to give the title compound 12a (55.5 mg, 79%) and S,S-di-tert-butyl (2S*,3R*)-2-[ethyl(4-methoxyphenyl)amino]-3-[N-(4-methoxyphenyl)acetamido]butanebis(thioate) 10a (5.2 mg, 6%).
12a
Yield 79% (55.5 mg, anti : syn = 62 : 38); a yellow oil; Rf = 0.63 (toluene : ethyl acetate = 7 : 1); 1H NMR (400 MHz, CDCl3) δ 1.02 (dd, J = 6.9, 6.9 Hz, 1.11H), 1.06 (dd, J = 6.9, 6.9 Hz, 1.89H), 1.44 (s, 5.67H), 1.45 (s, 3.33H), 1.99 (s, 1.89H), 2.12 (s, 1.11H), 3.20 (dq, J = 6.9, 7.2 Hz, 1.26H), 3.29 (dq, J = 6.9, 7.2 Hz, 1.26H), 3.40 (dq, J = 7.0, 7.1 Hz, 0.74H), 3.94 (dq, J = 7.0, 7.1 Hz, 0.74H), 3.77 (s, H), 3.78 (s, H), 4.61 (d, J = 7.6, 0.63H), 4.78 (d, J = 8.7, 0.37H), 6.02 (d, J = 8.7, 0.37H), 6.34 (d, J = 7.6, 0.63H), 6.83–6,94 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 12.6, 13.2, 20.5, 20.6, 29.5, 29.6, 40.7, 42.1, 48.7, 49.1, 55.5, 55.5, 71.4, 71.4, 76.4, 77.2, 98.5, 98.7, 114.4, 114.5, 118.1, 119.1, 140.0, 141.1, 153.2, 153.5, 168.2, 168.7, 194.3, 195.5; IR (neat) 2965, 2930, 2835, 1767, 1676, 1511, 1368, 1246, 1205, 1040, 805, 768 cm−1; HRMS (EI) calcd for C19H26Cl3NO4S (M)+ 469.0648, found 469.0645.
10a
Yield 6% (5.2 mg, anti : syn = 100 : 0); a yellow oil; Rf = 0.40 (nhexane : ethyl acetate = 3 : 1); 1H NMR (400 MHz, CDCl3) δ 1.04 (dd, J = 6.8, 6.8 Hz, 3H), 1.40 (s, 9H), 1.43 (s, 9H), 1.78 (s, 3H), 3.15–3.32 (m, 2H), 3.74 (s, 3H), 3.83 (s, 3H), 4.39 (d, J = 11.3 Hz, 1H), 6.05 (d, J = 11.3 Hz, 1H), 6.75–7.12 (m, 8H); 13C-NMR (100 MHz, CDCl3) δ 13.1, 23.2, 29.5, 29.8, 40.1, 48.4, 48.6, 55.4, 60.8, 70.9, 114.0, 114.4, 119.6, 131.0, 131.9, 140.8, 153.4, 159.4, 170.8, 195.1, 196.5; IR (neat) 2965, 1674, 1511, 1456, 1365, 1293, 1250, 1036, 731, 647 cm−1; HRMS (EI) calcd for C30H42N2O5S2 (M)+ 574.2535, found 574.2517.
(2R*,3S*)-1,1,1-Trichloro -4-(ethylthio)-3-[ethyl(4-methoxyphenyl)amino]-4-oxobutan-2-yl acetate 12b
Yield 54% (36.1 mg, anti : syn = 83 : 17); a yellow oil; Rf = 0.65 (toluene : ethyl acetate = 8 : 1); 1H NMR (400 MHz, CDCl3) δ 1.04 (dd, J = 7.1 Hz, 2.49H), 1.09–1.13 (m, 0.51H), 1.23 (dd, J = 7.3 Hz, 0.51H), 1.23 (dd, J = 7.3 Hz, 2.49H), 1.98 (s, 0.51H), 2.12 (s, 2.49H), 2.81–2.92 (m, 2H), 3.26–3.54 (m, 2H), 3.77 (s, 0.51H), 3.77 (s, 2.49H), 4.69 (d, J = 6.9 Hz, 0.17H), 4.85 (d, J = 8.2 Hz, 0.83H), 6.06 (d, J = 8.2 Hz, 0.83H), 6.41 (d, J = 6.4 Hz, 0.17H), 6.78–6.96 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 12.6, 13.1, 14.5, 14.7, 20.6, 21.0, 23.7, 23.7, 41.2, 43.1, 55.5, 55.5, 71.8, 71.8, 76.5, 77.2, 98.5, 98.8, 114.4, 114.7, 118.0, 119.3, 140.0, 140.8, 153.2, 153.6, 168.3, 168.6, 193.9, 196.2; IR (neat) 2971, 2932, 1772, 1679, 1511, 1371, 1247, 1202, 1039, 797, 764 cm−1; HRMS (EI) calcd for C17H22Cl3NO4S(M)+ 441.0335, found 441.0347.
(2R*,3S*)-1,1,1-Trichloro-4-(cyclohexylthio)-3-[ethyl(4-methoxyphenyl)amino]-4-oxobutan-2-yl acetate 12c
Yield 47% (35.3 mg, anit : syn = 69 : 31); a yellow oil; Rf = 0.58 (nhexane : ethyl acetate = 3 : 1); 1H NMR (400 MHz, CDCl3) δ 1.03 (dd, J = 6.87 Hz, 2.07H), 1.09 (dd, J = 6.87 Hz, 0.93H), 1.19–1.62 (m, 6H), 1.66–1.69 (m, 2H), 1.85–1.95 (m, 2H), 1.99 (s, 0.93H), 2.12 (s, 2.07H), 3.23–3.38 (m, 1H), 3.44–3.53 (m, 2H), 3.77 (s, 0.93H), 3.78 (s, 2.07H), 4.67 (d, J = 6.87 Hz, 0.31H), 4.81 (d, J = 8.24 Hz, 0.69H), 6.05 (d, J = 8.24 Hz, 0.69H), 6.39 (d, J = 6.87 Hz, 0.31H), 6.79–6.96 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 12.6, 13.2, 20.6, 20.6, 25.4, 25.4, 25.8, 25.9, 32.6, 32.7, 32.8, 33.0, 41.0, 42.8, 42.9, 55.4, 55.5, 71.7, 71.8, 76.4, 76.5, 98.5, 98.7, 114.4, 114.4, 118.1, 119.4, 140.0, 141.0, 153.2, 153.6, 168.3, 168.7, 193.6; IR (neat) 2933, 2854, 1770, 1675, 1511, 1371, 1247, 1203, 1039, 910, 797, 764, 732 cm−1; HRMS (EI) calcd for C21H28Cl3NO4S(M)+ 495.0805, found 495.0805.
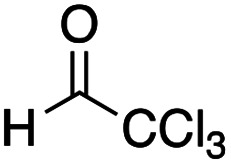
(2R*,3S*)-1,1,1-Tribromo-4-(tert-butylthio)-3-[ethyl(4-methoxyphenyl)amino]-4-oxobutan-2-yl acetate 12d
Yield 43% (38.7 mg, anti : syn = 50 : 50); a yellow oil; Rf = 0.63 (toluene : ethyl acetate = 7 : 1); 1H NMR (400 MHz, CDCl3) δ1H NMR (400 MHz, CDCl3) δ 1.04–1.07 (m, 3H), 1.45 (s, 9H), 2.03 (s, 1.5H), 2.14 (s, 1.5H), 3.22–3.25 (m, 1H), 3.47 (dq), 3.60 (dq), 3.78 (s, 3H), 4.55 (d, J = 6.9 Hz, 0.5H), 4.75 (d, J = 7.8 Hz, 0.5H), 5.96 (d, J = 7.8 Hz, 0.5H), 5.34 (d, J = 7.6 Hz, 0.5H), 6.82–6.96 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 12.6, 13.2, 20.7, 20.8, 29.5, 29.6, 41.0, 41.1, 42.1, 48.7, 49.1, 55.4, 55.5, 72.4, 72.7, 77.2, 77.5, 77.5, 114.3, 114.4, 118.4, 119.0, 140.0, 140.9, 153.1, 153.3, 168.2, 168.7, 194.3, 195.7; IR (neat): 2964, 2929, 1763, 1676, 1511, 1366, 1246, 1205, 1039, 696, 648 cm−1; HRMS (EI) calcd for C19H26Br3NO4S (M)+ 600.9133, found 600.9128.
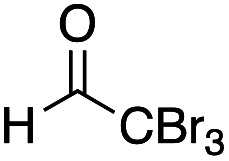
Ethyl (2R*,3S*)-2-acetoxy-4-(tert-butylthio)-3-[ethyl(4-methoxyphenyl)amino]-4-oxobutanoate 12e
Yield 26% (16.3 mg, anti : syn = 50 : 50); a yellow oil; Rf = 0.65 (toluene : ethyl acetate = 8 : 1); 1H NMR (400 MHz, CDCl3) δ1H NMR (400 MHz, CDCl3) δ 1.02–1.22 (m, 6H), 1.45 (s, 4.5H), 1.46 (s, 4.5H), 2.09 (s, 1.5H), 2.11 (s, 1.5H), 3.18–3.46 (m, 2H), 3.76 (s, 1.5H), 3.77 (s, 1.5H), 3.99–4.15 (m, 2H), 4.63 (d, J = 7.3H, 0.5H), 4.84 (d, J = 5.3 Hz, 0.5H), 5.44 (d, J = 7.3 Hz, 0.5H), 5.71 (d, J = 4.6 Hz, 0.5H), 6.79–6.90 (m, 3H), 7.07–7.11 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 10.8, 10.8, 13.1, 14.2, 24.5, 24.5, 29.6, 29.8, 39.8, 39.8, 48.5, 48.6, 55.2, 55.4, 60.4, 65.2, 69.1, 72.3, 76.4, 77.6, 113.4, 114.1, 119.9, 119.9, 130.2, 130.2, 137.7, 141.2, 153.4, 156.8, 173.5, 173.5, 194.9, 197.8; IR (neat) 2964, 1734, 1675, 1510, 1463, 1244, 1179, 1038, 995, 834, 755 cm−1; HRMS (EI) calcd for C21H31NO6S (M)+ 425.1872, found 425.1875.
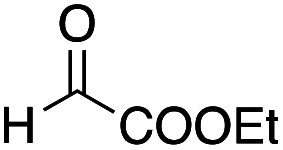
(3S*,4S*)-5-(tert-Butylthio)-4-[ethyl(4-methoxyphenyl)amino]-5-oxopent-1-en-3-yl acetate 12f
Yield 32% (18.1 mg, anti : syn = 62 : 38); a yellow oil; Rf = 0.62 (toluene : ethyl acetate = 8 : 1); 1H NMR (400 MHz, CDCl3) δ 0.99–1.07 (m, 3H), 1.44 (s, 3.42H), 1.44 (s, 5.58H), 1.95 (s, 1.14H), 2.02 (s, 1.86H), 3.27 (dq, J = 7.0, 7.0 Hz, 0.76H), 3.32 (dq, J = 7.0, 7.2 Hz, 1.24H), 3.77 (s, 3H), 4.22 (d, J = 8.2 Hz, 0.38H), 4.25 (d, J = 9.2 Hz, 0.62H), 5.18–5.25 (m, 1H), 5.28–5.37 (m, 1H), 5.80–5.92 (m, 2H), 6.75–6.88 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 13.1, 13.2, 20.9, 29.7, 40.6, 41.2, 48.3, 48.3, 55.5, 71.7, 71.9, 73.7, 74.8, 114.4, 118.3, 118.6, 118.8, 118.9, 133.5, 133.8, 141.3, 142.0, 153.2, 153.3, 169.4, 169.5, 197.6, 197.8; IR (neat) 2965, 2930, 2867, 2834, 1749, 1675, 1511, 1365, 1230, 1039, 936, 817 cm−1; HRMS (EI) calcd for C20H29NO4S (M)+ 379.1817, found 379.1822.
S,S-Di-tert-butyl (2S*,3R*)-2-[ethyl(4-methoxyphenyl)amino]-3-[N-(4-methoxyphenyl)acetamido]butanebis(thioate) 12g
Yield 45% (35.9 mg, anti : syn = 100 : 0); an orange oil; Rf = 0.25 (nhexane : ethyl acetate = 2 : 1); 1H NMR (500 MHz, CDCl3) δ 1.01 (dd, J = 6.9, 6.9, 3H), 1.17 (dd, J = 7.3, 7.3 Hz, 3H), 1.40 (s, 9H), 1.78 (s, 3H), 3.17 (dq, J = 6.9, 7.0 Hz, 1H), 3.24 (dq, J = 6.9, 7.0 Hz, 1H), 3.74 (s, 3H), 3.83 (s, 3H), 4.03 (dq, J = 7.3, 10.8 Hz, 1H), 4.12 (dq, J = 7.3, 10.8 Hz, 1H), 4.44 (d, J = 11.3 Hz, 1H), 5.53 (d, J = 11.3 Hz, 1H), 6.60–7.58 (m, 8H); 13C NMR (125 MHz, CDCl3) δ 13.0, 13.9, 23.0, 29.7, 40.1, 48.4, 55.4, 58.2, 61.3, 70.9, 114.2, 114.5, 118.9, 131.2, 132.9, 141.0, 153.3, 159.4, 171.2, 171.6, 195.2; IR (neat) 2965, 2934, 2870, 1735, 1668, 1511, 1463, 1377, 1296, 1248, 1183, 1036, 986, 840, 755 cm−1; HRMS (EI) calcd for C28H38N2O6S (M)+ 530.2451, found 530.2432.
Ethyl (2R,3S)-3-[ethyl(4-methoxyphenyl)amino]-4-(ethylthio)-2-[N-(4-methoxyphenyl)acetamido]-4-oxobutanoate 12h
Yield 64% (48.2 mg, anti : syn = 100 : 0); a pale yellow oil; Rf = 0.28 (toluene : ethyl acetate = 7 : 1); 1H NMR (500 MHz, CDCl3) δ 1.00 (dd, J = 7.1, 7.1 Hz, 3H), 1.18 (dd, J = 7.3, 7.3 Hz, 3H), 1.20 (t, J = 7.5 Hz, 3H), 1.78 (s, 3H), 2.80 (q, J = 7.5 Hz, 2H), 3.17 (dq, J = 7.1, 14.3 Hz, 1H), 3.21 (dq, J = 7.1, 14.3 Hz, 1H), 3.74 (s, 3H), 3.82 (s, 3H), 4.02–4.17 (m, 2H), 4.55 (d, J = 11.0 Hz, 1H), 5.57 (d, J = 11.0 Hz, 1H), 6.75–7.48 (m, 8H); 13C NMR (125 MHz, CDCl3) δ 12.9, 13.9, 14.7, 23.0, 23.3, 40.5, 55.4, 58.6, 61.3, 70.9, 114.2, 114.4, 119.2, 131.1, 132.8, 140.9, 153.5, 159.4, 170.8, 171.6, 195.1; IR (neat): 2934, 2837, 1736, 1667, 1511, 1376, 1297, 1247, 1183, 1034, 839, 754 cm−1; HRMS (EI) calcd for C26H34N2O6S (M)+ 502.2138, found 502.2160.
(2R*,3S*)-4-(tert-Butylthio)-1,1,1-trichloro-3-(isopropyl(4-methoxyphenyl)amino)-4-oxobutan-2-yl acetate 12i
Yield 75% (54.4 mg, anti : syn = 56 : 44); a yellow oil; Rf = 0.53 (nhexane : ethyl acetate = 5 : 1); 1H NMR (400 MHz, CDCl3) δ 0.99 (d, J = 6.4 Hz, 1.32H), 1.17 (d, J = 6.9 Hz, 1.64H), 1.22 (d, J = 6.9 Hz, 1.32H), 1.32 (d, J = 6.4 Hz, 1.64H), 1.41 (s, 5.04H), 1.52 (s, 3.96H), 1.97 (s, 1.68H), 2.09 (s, 1.32H), 3.72–3.83 (m, 0.56H), 3.76 (s, 1.68H), 3.79 (s, 1.34H), 3.94 (dq, J = 6.7, 6.6 Hz, 0.44H), 4.45 (d, J = 7.3 Hz, 0.56H), 4.46 (d, J = 6.9 Hz, 0.44H), 5.91 (d, J = 7.3 Hz, 0.44H), 6.46 (d, J = 6.9 Hz, 0.56H), 6.76–7.21 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 20.5, 20.7, 21.3, 21.6, 22.1, 23.8, 29.5, 29.7, 48.3, 48.9, 50.4, 50.8, 55.3, 55.4, 70.1, 72.4, 76.0, 78.2, 99.0, 99.4, 113.5, 113.9, 122.8, 129.5, 138.4, 139.6, 154.3, 156.5, 168.2, 168.8, 197.5, 197.9; IR (neat) 2966, 2835, 1768, 1679, 1513, 1367, 1244, 1205, 1039, 808, 766, 734 cm−1; HRMS (EI) calcd for C20H28Cl3NO4S(M)+ 483.0805, found 483.0822.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (B) and on Innovative Areas “Organic Synthesis Based on Reaction Integration. Development of New Methods and Creation of New Substances” from JSPS and MEXT.
Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra02000e
Notes and references
- (a) Ito H. Taguchi T. Hanzawa Y. Tetrahedron Lett. 1992;33:4469–4472. doi: 10.1016/S0040-4039(00)60112-0. [DOI] [Google Scholar]; (b) Guijarro D. Yus M. Tetrahedron. 1993;49:7761–7768. doi: 10.1016/S0040-4020(01)87249-5. [DOI] [Google Scholar]; (c) Clerici A. Clerici L. Porta O. Tetrahedron Lett. 1995;36:5955–5958. doi: 10.1016/00404-0399(50)11517-. [DOI] [Google Scholar]; (d) Ding C. Z. Tetrahedron Lett. 1996;37:945–948. doi: 10.1016/0040-4039(95)02335-6. [DOI] [Google Scholar]; (e) Machrouhi F. Namy J.-L. Tetrahedron Lett. 1999;40:1315–1318. doi: 10.1016/S0040-4039(98)02673-2. [DOI] [Google Scholar]; (f) Bergmeier S. C. Tetrahedron. 2000;56:2561–2576. doi: 10.1016/S0040-4020(00)00149-6. [DOI] [Google Scholar]; (g) Lee B. W. Lee J. H. Jang K. C. Kang J. E. Kim J. H. Park K.-M. Park K. H. Tetrahedron Lett. 2003;44:5905–5907. doi: 10.1016/S0040-4039(03)01394-7. [DOI] [Google Scholar]; (h) Breuer M. Ditrich K. Habicher T. Hauer B. Keßeler M. Stürmer R. Zelinski T. Angew. Chem., Int. Ed. 2004;43:788–824. doi: 10.1002/anie.200300599. [DOI] [PubMed] [Google Scholar]; (i) Maekawa H. Yamamoto Y. Shimada H. Yonemura K. Nishiguchi I. Tetrahedron Lett. 2004;45:3869–3872. doi: 10.1016/j.tetlet.2004.03.109. [DOI] [Google Scholar]; (j) Jung D. Y. Kang S. Chang S. Kim Y. H. Synlett. 2006:86–90. [Google Scholar]; (k) Barbazanges M. Meyer C. Cossy J. Org. Lett. 2007;9:3245–3248. doi: 10.1021/ol0711725. [DOI] [PubMed] [Google Scholar]; (l) Skucas E. Zbieg J. R. Krische M. J. J. Am. Chem. Soc. 2009;131:5054–5055. doi: 10.1021/ja900827p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Karjalainen O. K. Koskinen A. M. P. Org. Biomol. Chem. 2012;10:4311–4326. doi: 10.1039/C2OB25357G. [DOI] [PubMed] [Google Scholar]; (n) Ghislieri D. Turner N. J. Top. Catal. 2014;57:284–300. doi: 10.1007/s11244-013-0184-1. [DOI] [Google Scholar]; (o) Sehl T. Maugeri Z. Rother D. J. Mol. Catal. B: Enzym. 2015;114:65–71. doi: 10.1016/j.molcatb.2014.12.005. [DOI] [Google Scholar]; (p) Schwarz J. L. Kleinmans R. Paulisch T. O. Glorius F. J. Am. Chem. Soc. 2020;142:2168–2174. doi: 10.1021/jacs.9b12053. [DOI] [PubMed] [Google Scholar]
- For reviews, see: ; (a) Ager D. J. Prakash I. Schaad D. R. Chem. Rev. 1996;96:835–876. doi: 10.1021/cr9500038. [DOI] [PubMed] [Google Scholar]; (b) Fache F. Schulz E. Tommasino M. L. Lemaire M. Chem. Rev. 2000;100:2159–2232. doi: 10.1021/cr9902897. [DOI] [PubMed] [Google Scholar]; (c) Sibi M. P. Manyem S. Tetrahedron. 2000;56:8033–8061. doi: 10.1016/S0040-4020(00)00618-9. [DOI] [Google Scholar]; (d) Corey E. J. Angew. Chem., Int. Ed. 2002;41:1650–1667. doi: 10.1002/1521-3773(20020517)41:10<1650::AID-ANIE1650>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]; (e) Alcaide B. Almendros P. Eur. J. Org. Chem. 2002:1595–1601. doi: 10.1002/1099-0690(200205)2002:10<1595::AID-EJOC1595>3.0.CO;2-M. [DOI] [Google Scholar]; (f) Nakano H. Owolabi I. A. Chennapuram M. Okuyama Y. Kwon E. Seki C. Tokiwa M. Takeshita M. Heterocycles. 2018;97:647–667. doi: 10.3987/REV-18-SR(T)3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Shimizu M. Tsukamoto K. Fujisawa T. Tetrahedron Lett. 1997;38:5193–5196. doi: 10.1016/S0040-4039(97)01125-8. [DOI] [Google Scholar]; (b) Shimizu M. Tsukamoto K. Matsutani T. Fujisawa T. Tetrahedron. 1998;54:10265–10274. doi: 10.1016/S0040-4020(98)00483-9. [DOI] [Google Scholar]
- (a) Shimizu M. Kawamoto M. Niwa Y. Chem. Commun. 1999:1151–1152. doi: 10.1039/A902386K. [DOI] [Google Scholar]; (b) Shimizu M. Hayashi Y. Hamanaka R. Hachiya I. Heterocycles. 2007;73:191–195. doi: 10.3987/COM-07-S(U)25. [DOI] [Google Scholar]
- (a) Shimizu M. Takeuchi Y. Sahara T. Chem. Lett. 2001;30:1196–1197. doi: 10.1246/cl.2001.1196. [DOI] [Google Scholar]; (b) Shimizu M. Sahara T. Chem. Lett. 2002;31:888–889. doi: 10.1246/cl.2002.888. [DOI] [Google Scholar]
- Shimizu M. Iwata A. Makino H. Synlett. 2002:1538–1540. doi: 10.1055/s-2002-33510. [DOI] [Google Scholar]
- For N-alkylation to α-iminoesters in our laboratory: ; (a) Shimizu M. Niwa Y. Tetrahedron Lett. 2001;42:2829–2832. doi: 10.1016/S0040-4039(01)00302-1. [DOI] [Google Scholar]; (b) Niwa Y. Takayama K. Shimizu M. Tetrahedron Lett. 2001;42:5473–5476. doi: 10.1016/S0040-4039(01)01022-X. [DOI] [Google Scholar]; (c) Niwa Y. Takayama K. Shimizu M. Bull. Chem. Soc. Jpn. 2002;75:1819–1825. doi: 10.1246/bcsj.75.1819. [DOI] [Google Scholar]; (d) Niwa Y. Shimizu M. J. Am. Chem. Soc. 2003;125:3720–3721. doi: 10.1021/ja029639o. [DOI] [PubMed] [Google Scholar]; (e) Shimizu M. Itou H. Miura M. J. Am. Chem. Soc. 2005;127:3296–3297. doi: 10.1021/ja042330f. [DOI] [PubMed] [Google Scholar]; (f) Mizota I. Tanaka K. Shimizu M. Tetrahedron Lett. 2012;53:1847–1850. doi: 10.1016/j.tetlet.2012.01.133. [DOI] [Google Scholar]; (g) Hata S. Maeda T. Shimizu M. Bull. Chem. Soc. Jpn. 2012;85:1203–1205. doi: 10.1246/bcsj.20120195. [DOI] [Google Scholar]; (h) Shimizu M. Takao Y. Katsurayama H. Mizota I. Asian J. Org. Chem. 2013;2:130–134. doi: 10.1002/ajoc.201200174. [DOI] [Google Scholar]; (i) Shimizu M. Kurita D. Mizota I. Asian J. Org. Chem. 2013;2:208–211. doi: 10.1002/ajoc.201300010. [DOI] [Google Scholar]; (j) Sano T. Mizota I. Shimizu M. Chem. Lett. 2013;42:995–997. doi: 10.1246/cl.130396. [DOI] [Google Scholar]; (k) Mizota I. Matsuda Y. Kamimura S. Tanaka H. Shimizu M. Org. Lett. 2013;15:4206–4209. doi: 10.1021/ol401934x. [DOI] [PubMed] [Google Scholar]; (l) Tanaka H. Mizota I. Shimizu M. Org. Lett. 2014;16:2276–2279. doi: 10.1021/ol5007983. [DOI] [PubMed] [Google Scholar]; (m) Shimizu M. Tateishi M. Mizota I. Chem. Lett. 2014;43:1752–1754. doi: 10.1246/cl.140763. [DOI] [Google Scholar]; (n) Mizota I. Maeda T. Shimizu M. Tetrahedron. 2015;71:5793–5799. doi: 10.1016/j.tet.2015.04.111. [DOI] [Google Scholar]; (o) Tanaka T. Mizota I. Umezu K. Ito A. Shimizu M. Heterocycles. 2017;95:830–843. doi: 10.3987/COM-16-S(S)27. [DOI] [Google Scholar]; (p) Kawanishi M. Mizota I. Aratake K. Tanaka H. Nakahama K. Shimizu M. Bull. Chem. Soc. Jpn. 2017;90:395–403. doi: 10.1246/bcsj.20160402. [DOI] [Google Scholar]; (q) Mizota I. Nakajima Y. Higashino A. Shimizu M. Arabian J. Sci. Eng. 2017;42:4249–4261. doi: 10.1007/s13369-017-2615-y. [DOI] [Google Scholar]; (r) Nakahama K. Suzuki M. Ozako M. Mizota I. Shimizu M. Asian J. Org. Chem. 2018;7:910–913. doi: 10.1002/ajoc.201800186. [DOI] [Google Scholar]; (s) Mizota I. Ueda C. Tesong Y. Tsujimoto Y. Shimizu M. Org. Lett. 2018;20:2291–2296. doi: 10.1021/acs.orglett.8b00639. [DOI] [PubMed] [Google Scholar]; (t) Mizota I. Tadano Y. Nakamura Y. Haramiishi T. Hotta M. Shimizu M. Org. Lett. 2019;21:2663–2667. doi: 10.1021/acs.orglett.9b00654. [DOI] [PubMed] [Google Scholar]; (u) Shimizu M. Mushika M. Mizota I. Zhu Y. RSC Adv. 2019;9:23400–23407. doi: 10.1039/C9RA04889H. [DOI] [PMC free article] [PubMed] [Google Scholar]; (v) Mizota I. Mori M. Shimizu M. J. Heterocycl. Chem. 2020;57:3002–3010. doi: 10.1002/jhet.4015. [DOI] [Google Scholar]; (w) Shimizu M. Furukawa Y. Mizota I. Zhu Y. New J. Chem. 2020;44:152–161. doi: 10.1039/C9NJ05114G. [DOI] [Google Scholar]; (x) Mizota I. Maeda M. Imoto K. Shimizu M. Org. Lett. 2020;22:3079–3083. doi: 10.1021/acs.orglett.0c00824. [DOI] [PubMed] [Google Scholar]; (y) Shimizu M. Morimoto T. Yanagi Y. Mizota I. Zhu Y. RSC Adv. 2020;10:9955–9963. doi: 10.1039/D0RA01152E. [DOI] [PMC free article] [PubMed] [Google Scholar]; (z) Ota K. Fukumoto S. Iwase T. Mizota I. Shimizu M. Hachiya I. Synlett. 2020;31:1930–1936. doi: 10.1055/s-0040-1707265. [DOI] [Google Scholar]
- For reviews and accounts, see: ; (a) Shimizu M. Pure Appl. Chem. 2006;78:1867–1876. [Google Scholar]; (b) Dickstein J. S. Kozlowski M. C. Chem. Soc. Rev. 2008;37:1166–1173. doi: 10.1039/B709139G. [DOI] [PubMed] [Google Scholar]; (c) Shimizu M. Hachiya I. Mizota I. Chem. Commun. 2009:874–889. doi: 10.1039/B814930E. [DOI] [PubMed] [Google Scholar]; (d) Nishi T. Mizota I. Shimizu M. Pure Appl. Chem. 2012;84:2609–2617. [Google Scholar]; (e) Mizota I. Shimizu M. Chem. Rec. 2016;16:688–702. doi: 10.1002/tcr.201500267. [DOI] [PubMed] [Google Scholar]; (f) Eftekhari-Sis B. Zirak M. Chem. Rev. 2017;117:8326–8419. doi: 10.1021/acs.chemrev.7b00064. [DOI] [PubMed] [Google Scholar]
- Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Montgomery Jr J. A., Vreven T., Kudin K. N., Burant J. C., Millam J. M., Iyengar S. S., Tomasi J., Barone V., Mennucci B., Cossi M., Scalmani G., Rega N., Petersson G. A., Nakatsuji H., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Klene M., Li X., Knox J. E., Hratchian H. P., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Ayala P. Y., Morokuma K., Voth G. A., Salvador P., Dannenberg J. J., Zakrzewski V. G., Dapprich S., Daniels A. D., Strain M. C., Farkas O., Malick D. K., Rabuck A. D., Raghavachari K., Foresman J. B., Ortiz J. V., Cui Q., Baboul A. G., Clifford S., Cioslowski J., Stefanov B. B., Liu G., Liashenko A., Piskorz P., Komaromi I., Martin R. L., Fox D. J., Keith T., Al-Laham M. A., Peng C. Y., Nanayakkara A., Challacombe M. P., Gill M. W., Johnson B., Chen W., Wong M. W., Gonzalez C. and Pople J. A., Gaussian 03, Revision C.02, Gaussian, Inc., Wallingford CT, 2004 [Google Scholar]
- For aldol reaction, see: ; (a) Mukaiyama T. Org. React. 1982;28:203–331. [Google Scholar]; (b) Evans D. A. Aldrichimica Acta. 1982;15:23–32. [Google Scholar]; (c) Paterson I., in Comprehensive Organic Synthesis, ed. B. M. Trost and I. Fleming, Pergamon Press, New York, 1991, vol. 2, pp. 301–319 [Google Scholar]; (d) Carreira E. M. Fettes A. Martl C. Org. React. 2006;67:1–216. [Google Scholar]; (e) Trost B. M. Brindle C. S. Chem. Soc. Rev. 2010;39:1600–1632. doi: 10.1039/B923537J. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zhang H. Han M. Chen T. Xu L. Yu L. RSC Adv. 2017;7:48214–48221. doi: 10.1039/C7RA09412D. [DOI] [Google Scholar]; (g) Yamashita Y. Yasukawa T. Yoo W.-J. Kitanosono T. Kobayashi S. Chem. Soc. Rev. 2018;47:4388–4480. doi: 10.1039/C7CS00824D. [DOI] [PubMed] [Google Scholar]
- (a) Lee C. Yang W. Parr R. G. Phys. Rev. B. 1988;37:785–789. doi: 10.1103/PhysRevB.37.785. [DOI] [PubMed] [Google Scholar]; (b) Becke A. D. Phys. Rev. A. 1988;38:3098–3100. doi: 10.1103/PhysRevA.38.3098. [DOI] [PubMed] [Google Scholar]
- Shimizu M. Makino H. Tetrahedron Lett. 2001;42:8865–8868. doi: 10.1016/S0040-4039(01)01945-1. [DOI] [Google Scholar]
- Springborg M., Methods of Electronic-Structure Calculations from Molecules to Solids, John Wiley & Sons, New York, 2000 [Google Scholar]
- (a) Mcgrath N. A. Raines R. T. Acc. Chem. Res. 2011;44:752–761. doi: 10.1021/ar200081s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Burke H. M. McSweeney L. Scanlan E. M. Nat. Commun. 2017;8:15655. doi: 10.1038/ncomms15655. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hirschbeck V. Gehrtz P. H. Fleischer I. Chem.–Eur. J. 2018;24:7092–7107. doi: 10.1002/chem.201705025. [DOI] [PubMed] [Google Scholar]
- (a) Lucet D. Le Gall T. Mioskowski C. Angew. Chem., Int. Ed. 1998;37:2580–2627. doi: 10.1002/(SICI)1521-3773(19981016)37:19<2580::AID-ANIE2580>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]; (b) Viso A. Fernández de la Pradilla R. García A. Flores A. Chem. Rev. 2005;105:3167–3196. doi: 10.1021/cr0406561. [DOI] [PubMed] [Google Scholar]; (c) Kotti S. S. R. S. Timmons C. Li G. Chem. Biol. Drug Des. 2006;67:101–114. doi: 10.1111/j.1747-0285.2006.00347.x. [DOI] [PubMed] [Google Scholar]; (d) Cardona F. Goti A. Nat. Chem. 2009;1:269–275. doi: 10.1038/nchem.256. [DOI] [PubMed] [Google Scholar]; (e) Viso A. Fernández de la Pradilla R. Tortosa M. García A. Flores A. Chem. Rev. 2011;111:PR1–PR42. doi: 10.1021/cr100127y. [DOI] [PubMed] [Google Scholar]; (f) Muñiz K. Barreiro L. Romero R. M. Martínez C. J. Am. Chem. Soc. 2017;139:4354–4357. doi: 10.1021/jacs.7b01443. [DOI] [PubMed] [Google Scholar]; (g) Lee S. Jang Y. J. Phipps E. J. T. Lei H. Rovis T. Synthesis. 2020;52:1247–1252. doi: 10.1055/s-0040-1707600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.