Abstract
BACKGROUND
The prevalence of type 2 diabetes in youth is increasing, but little is known regarding the occurrence of related complications as these youths transition to adulthood.
METHODS
We previously conducted a multicenter clinical trial (from 2004 to 2011) to evaluate the effects of one of three treatments (metformin, metformin plus rosiglitazone, or metformin plus an intensive lifestyle intervention) on the time to loss of glycemic control in participants who had onset of type 2 diabetes in youth. After completion of the trial, participants were transitioned to metformin with or without insulin and were enrolled in an observational follow-up study (performed from 2011 to 2020), which was conducted in two phases; the results of this follow-up study are reported here. Assessments for diabetic kidney disease, hypertension, dyslipidemia, and nerve disease were performed annually, and assessments for retinal disease were performed twice. Complications related to diabetes identified outside the study were confirmed and adjudicated.
RESULTS
At the end of the second phase of the follow-up study (January 2020), the mean (±SD) age of the 500 participants who were included in the analyses was 26.4±2.8 years, and the mean time since the diagnosis of diabetes was 13.3±1.8 years. The cumulative incidence of hypertension was 67.5%, the incidence of dyslipidemia was 51.6%, the incidence of diabetic kidney disease was 54.8%, and the incidence of nerve disease was 32.4%. The prevalence of retinal disease, including more advanced stages, was 13.7% in the period from 2010 to 2011 and 51.0% in the period from 2017 to 2018. At least one complication occurred in 60.1% of the participants, and at least two complications occurred in 28.4%. Risk factors for the development of complications included minority race or ethnic group, hyperglycemia, hypertension, and dyslipidemia. No adverse events were recorded during follow-up.
CONCLUSIONS
Among participants who had onset of type 2 diabetes in youth, the risk of complications, including microvascular complications, increased steadily over time and affected most participants by the time of young adulthood. Complications were more common among participants of minority race and ethnic group and among those with hyperglycemia, hypertension, and dyslipidemia. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others; ClinicalTrials.gov numbers, NCT01364350 and NCT02310724.)
The incidence of youth-onset type 2 diabetes has increased in parallel with the rise in the number of children with obesity in the United States.1,2 In the period from 2002 to 2012, the incidence of type 2 diabetes increased by 4.8% each year.3 Pathologic processes associated with diabetes, including the development of insulin resistance and the deterioration of beta-cell function, progress more rapidly in youth-onset type 2 diabetes than in adult-onset diabetes. These factors result in worse glycemic control and an increased risk of early diabetes-related complications.1,4–7
The SEARCH for Diabetes in Youth registry study and a registry study in the Canadian province of Manitoba showed a higher prevalence of diabetic kidney disease, hypertension, retinal disease, and peripheral nerve disease among youths with type 2 diabetes than among those with type 1 diabetes.4,8 Both studies were cross-sectional; the SEARCH study reported post hoc diagnoses, and the Canadian study identified outcomes in an administrative database. In addition, the burden of complications at 21 years of age in the cohort in the SEARCH study was estimated with the use of modeling. After calculation of a standardized mortality ratio, data from the Australian National Death Index and the Australian National Diabetes Services Scheme showed an inverse relationship between the age at onset of type 2 diabetes and a long-term risk of end-stage kidney disease.1,9
The lack of prospective longitudinal data has challenged the development of evidence-based guidelines for prevention and management of complications in youth-onset type 2 diabetes. We hypothesized that longitudinal follow-up of participants in the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) clinical trial would show a rapid accumulation of diabetes-related complications, including the emergence of potentially life-threatening endorgan damage. We report here the overall incidence and clustering of complications. Subsequent analyses will provide a deeper exploration of the particular risk factors, treatments, and associations of each complication.
Methods
Design of the Clinical Trial
The analysis of the primary outcome of the TODAY clinical trial and the protocol (also available with the full text of this article at NEJM.org) have been published previously.10 In brief, 699 participants were enrolled at 15 centers across the United States from 2004 through 2009. Participants were 10 to 17 years of age and had a duration of type 2 diabetes (defined according to American Diabetes Association 2002 criteria10) of less than 2 years, a body-mass index (BMI [the weight in kilograms divided by the square of the height in meters]) at or above the 85th percentile, a fasting C-peptide level of more than 0.6 ng per milliliter, and a negative test for pancreatic antibodies. Eligible participants were randomly assigned to receive metformin monotherapy, metformin plus rosiglitazone, or metformin plus an intensive lifestyle intervention. Participants were followed for an average of 3.9 years (range, 2.0 to 6.5). A total of 319 participants (45.6%) had lack of glycemic control (the primary outcome): 51.7% of the participants who received metformin alone, 38.6% of those who received metformin plus rosiglitazone, and 46.6% of those who received metformin plus a lifestyle intervention.10 Data on adverse events that occurred during the trial were published previously10 and are also reported in Table S1 in the Supplementary Appendix, available at NEJM.org.
Design of the Follow-up Study
In 2011, we enrolled 572 participants (81.8%) from the TODAY trial in the TODAY2 follow-up study, which was conducted in two phases. In the first phase (March 2011 through February 2014), participants received all diabetes care from the study and were treated with metformin with or without insulin to maintain glycemic control; data on adverse events were collected in the same manner as in the TODAY trial. From March 2014 through January 2020, a total of 518 participants were transitioned to the fully observational second phase of the study; there were annual study visits, but medical management was provided by the participants’ health care providers. Because the study offered no treatment or intervention in this phase, only adverse events related to study procedures were evaluated. The two phases of the study provided a combined average duration of follow-up of 10.2 years. Participants who had a positive result from a genetic analysis for maturity-onset diabetes of the young were excluded from all analyses.
Oversight
The study was approved by the institutional review board at each participating center. All participants and their parents or guardians provided written informed consent or assent as appropriate for the age of the participant, according to local guidelines. The study was designed by the TODAY Study Group, which collected and analyzed the data and vouches for the accuracy of the data and analyses. The first draft of the manuscript was written by the manuscript writing group, and the steering committee made the decision to submit the manuscript for publication. No confidentiality agreements were imposed by the main sponsor (the National Institute of Diabetes and Digestive and Kidney Diseases).
Definitions of Complications
Similar algorithms for the classification of diabetes-related complications were used in the clinical trial and in the two phases of the follow-up study, with differences in the frequency of data collection incorporated in the algorithms. All laboratory assays were performed at Northwest Lipids Research Laboratory according to methods described previously.11 A detailed description of the analysis methods and the definitions of complications are provided in the Detailed Research Methods section in the Supplementary Appendix and are described briefly below.
Hypertension
Blood pressure was measured at every visit. Hypertension was defined as a blood pressure in the 95th percentile or higher for age, sex, and height; a systolic blood pressure of at least 130 mm Hg or a diastolic blood pressure of at least 80 mm Hg on three consecutive occasions; or an elevated blood pressure followed by initiation of antihypertensive therapy.12
Dyslipidemia
Fasting lipid profiles were obtained annually. Low-density lipoprotein (LDL) cholesterol dyslipidemia was defined as consecutive levels of at least 130 mg per deciliter (3.37 mmol per liter), calculated with the use of the Friedewald equation. Triglyceride dyslipidemia was defined as consecutive values of triglycerides of at least 150 mg per deciliter (1.69 mmol per liter) or a single elevated lipid value followed by initiation of lipidlowering therapy.13
Albuminuria as a Marker of Diabetic Kidney Disease
Urine samples were collected annually. If abnormal values were found, testing was repeated on the first sample obtained in the morning. Moderate albuminuria was defined as a ratio of urine albumin (in milligrams) to creatinine (in grams) of 30, and severe albuminuria as a ratio of at least 300 on at least two of three determinations.14
Diabetic Nerve Disease
Scores on the Michigan Neuropathy Screening Instrument (MNSI) and the Semmes–Weinstein monofilament examination were performed annually.15 Scores on the MNSI range from 0 to 8, with higher scores indicating more severe symptoms of neuropathy. The MNSI examination was considered abnormal if the score was higher than 2 on at least two consecutive assessments. The Semmes–Weinstein monofilament examination was considered abnormal if fewer than 8 of 10 responses were correct on at least two consecutive examinations.15
Diabetic Eye Disease
Fundus photography was performed twice for research purposes (in 2010 or 2011 and in 2017 or 2018) and graded at a centralized reading center by examiners who were unaware of the participants’ assigned treatments.16 Eye disease was defined as a grade of at least 20 according to criteria in the protocol of the Early Treatment Diabetic Retinopathy Study (grades range from 10 to 85, with higher values indicating a greater degree of retinopathy), or clinically significant macular edema, or both.
Adjudication of Events Occurring Outside the Study
During semiannual follow-up, participants underwent a structured interview to identify relevant events that had occurred since the previous visit. Medical records were obtained for adjudication by the comorbidity assessment committee according to prespecified criteria from national guidelines.
Statistical Analysis
Demographic, metabolic, and nonmetabolic characteristics are presented as means and standard deviations or percentages. The percentages of participants in each phase of the study in whom glycemic control was not achieved or who had hypertension, LDL or triglyceride dyslipidemia, or moderate or severe albuminuria at the end of the clinical trial are reported. Complications were grouped together to obtain global classifications: moderate and severe albuminuria were classified as kidney disease, abnormal MNSI examination and monofilament results as nerve disease, and LDL or triglyceride dyslipidemia as dyslipidemia. The percentage of participants with eye disease was computed. Microvascular events (nerve, retinal, and kidney disease) were aggregated to identify the first occurrence of any event. Kaplan–Meier estimates were used to estimate all cumulative incidences. Separate univariable and multivariable Cox proportionalhazards regression models that included all available data from all participants were used to estimate the overall risk of any microvascular complication for selected covariates. Covariates that were prespecified on the basis of known associations were included in the Cox models as fixed or time-varying covariates, including time-weighted arithmetic mean values for glycated hemoglobin, BMI, and insulin sensitivity (1 ÷ fasting insulin). For participants with a missing covariate value, the previous observed value was carried forward. All enrolled participants, irrespective of length of follow-up, were grouped according to the number and type of complications. The associations between the number of microvascular complications and risk factors were assessed with the use of a proportional-odds cumulative logit model. Adjudicated complications that occurred during the study were tallied, and the corresponding event rates were computed. Analyses were performed with SAS software, version 9.4 (SAS Institute). All analyses were repeated with the use of a sensitivity cohort that included participants who completed an end-of-study visit for which laboratory values were available.
Results
Demographic and Metabolic Characteristics
The characteristics of the cohorts were similar in the TODAY trial and in both phases of the TODAY2 study (Tables 1 and S2). At the end of the study, the mean (±SD) age of the 500 participants who were assessed from March 2014 through January 2020 and were included in the analyses was 26.4±2.8 years, and the mean time since the diagnosis of diabetes was 13.3±1.8 years. A total of 22 participants who had confirmed maturity-onset diabetes of the young were excluded from all the analyses.
Table 1.
Characteristics of the Participants.*
| Characteristic | TODAY Clinical Trial Cohort (N = 677) | TODAY2 Study, Phase 1 Cohort (N = 550) | TODAY2 Study, Phase 2 Cohort (N = 500) |
|---|---|---|---|
| Baseline | |||
| Age — yr | 14.0+2.0 | 13.9+2.0 | 13.8+2.0 |
| Female sex — % | 65.0 | 65.1 | 65.4 |
| Race and ethnic group — %† | |||
| Hispanic | 39.9 | 39.8 | 38.2 |
| Non-Hispanic Black | 33.1 | 33.6 | 34.8 |
| Non-Hispanic White | 19.5 | 19.3 | 19.2 |
| Other | 7.5 | 7.3 | 7.8 |
| Lived in household with income <$25,000 — % | 41.7 | 42.8 | 44.5 |
| Time since diagnosis of type 2 diabetes — mo | 7.8±5.8 | 7.7±5.9 | 7.8±5.9 |
| Glycated hemoglobin | |||
| Percentage | 6.0±0.7 | 6.0±0.7 | 6.0±0.8 |
| Mean level — mmol/mol | 42.0±7.7 | 42.0±7.7 | 42.0±8.7 |
| BMI | 35.1±7.6 | 35.0±7.8 | 35.1±7.8 |
| Treatment assignment at start of clinical trial — % | |||
| Metformin alone | 33.4 | 33.8 | 33.6 |
| Metformin plus rosiglitazone | 33.5 | 32.9 | 33.0 |
| Metformin plus lifestyle intervention | 33.1 | 33.3 | 33.4 |
| End of the clinical trial | |||
| Lack of maintenance of glycemic control — %‡ | 45.6 | 47.3 | 46.8 |
| Complications — % | |||
| Hypertension | 42.4 | 44.9 | 44.6 |
| Low-density lipoprotein dyslipidemia | 12.9 | 13.3 | 12.4 |
| Triglyceride dyslipidemia | 32.9 | 33.8 | 32.0 |
| Moderate or severe albuminuria | 20.5 | 21.8 | 20.8 |
Plus-minus values are means ±SD. Shown are the characteristics of each cohort at entry into the TODAY trial (baseline) or at the end of the trial (February 2011). The second and third columns reflect the characteristics of the participants from the TODAY trial who continued on to phase 1 and to phase 2 of the TODAY2 follow-up study. Data are excluded for 22 participants in the TODAY trial cohort and 18 participants in the TODAY2 study cohorts who had confirmed maturity-onset diabetes of the young. BMI denotes body-mass index.
Race and ethnic group were reported by the participants.
Lack of maintenance of glycemic control was the primary outcome in the TODAY trial.
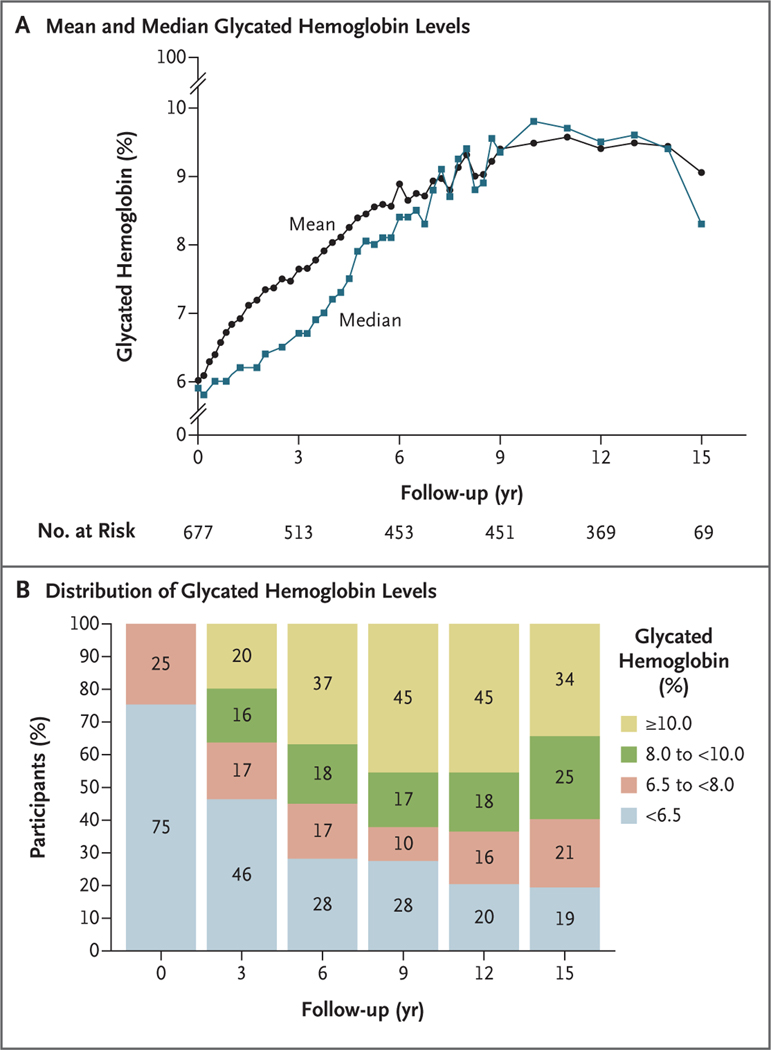
The median glycated hemoglobin level increased over time, and the percentage of participants with a glycated hemoglobin level in the nondiabetes range (<6% [48 mmol per mole]) decreased from 75% at baseline (i.e., the time of entry into the clinical trial) to 19% at 15 years (i.e., the end of the follow-up study), and the percentage with a glycated hemoglobin level of at least 10% (86 mmol per mole) was 0% at baseline and 34% at 15 years (Fig. 1). The BMI increased from baseline (reaching a plateau at 3 to 9 years), followed by a gradual decrease; but the overall median BMI remained in the narrow range of 35.0 to 37.5 (Fig. S1). The diabetes medications that were being taken by the participants at the end of the study remained almost exclusively insulin and metformin, with both being prescribed to nearly 50% of the participants by the time of their final visit; more than a quarter of the participants were taking no medication (Table S3).
Figure 1. Glycated Hemoglobin Level over Time.
Shown are the mean and median glycated hemoglobin levels (Panel A) and the distribution of glycated hemoglobin levels (Panel B) according to year. Percentages may not total 100 because of rounding.
Hypertension and Dyslipidemia
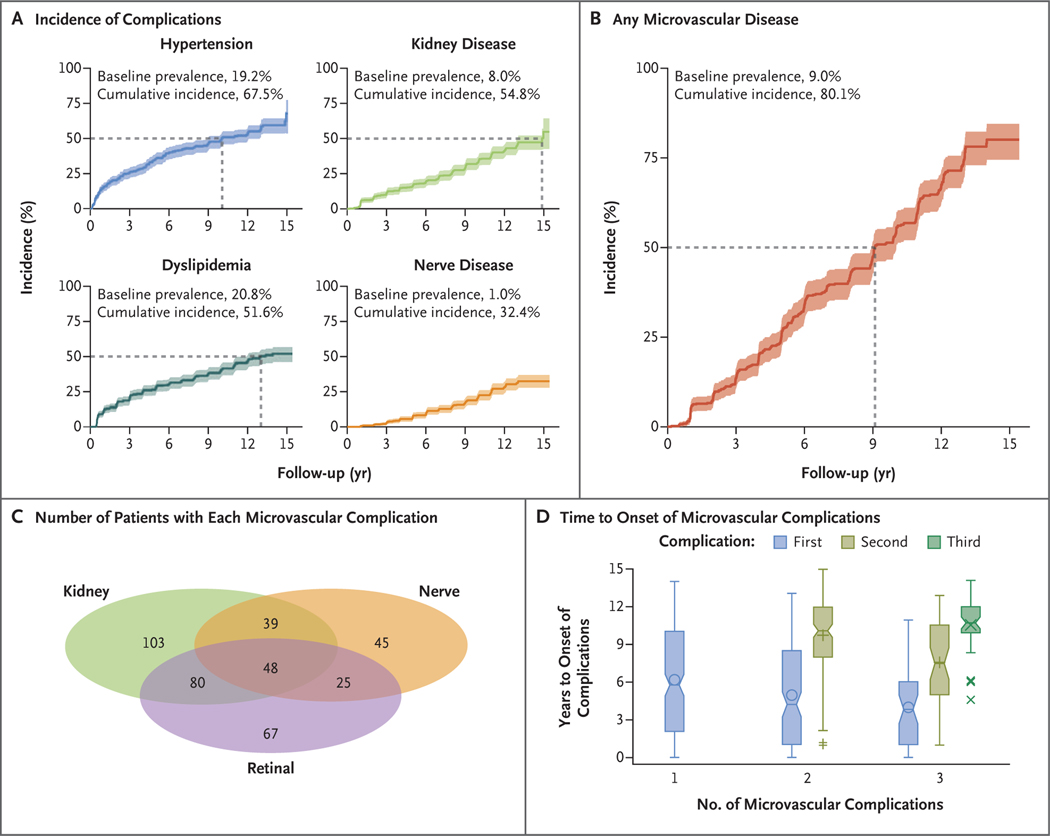
The prevalence of hypertension at baseline was 19.2%, and the cumulative incidence at 15 years was 67.5% (Fig. 2). Dyslipidemia was present in 20.8% of the participants at baseline, and the cumulative incidence at 15 years was 51.6%. There were no differences in the incidences of hypertension or dyslipidemia according to the original treatment assignment.
Figure 2. Diabetes-Related Complications That Occurred during the Study.
Panel A shows the baseline prevalences and cumulative incidences of hypertension, dyslipidemia (low-density-lipoprotein or triglyceride dyslipidemia), kidney disease, and nerve disease. Panel B shows the baseline prevalence and cumulative incidence of any microvascular complication. The dashed lines in Panels A and B indicate the time when the cumulative incidence reached 50%, and the shaded bands represent 95% confidence intervals. Baseline refers to the time of enrollment in the clinical trial. Panel C shows the numbers of patients with kidney, nerve, and retinal complications. Panel D shows the years to onset of the first and subsequent (if applicable) microvascular complications among participants with one, two, or three complications. The bottoms and tops of the boxes represent the first and the third quartiles, respectively, and the horizontal lines inside the boxes indicate medians. The symbols inside the boxes indicate means, and the symbols outside the boxes outliers. I bars indicate the minimum and maximum range, excluding outliers.
Kidney, Nerve, and Eye Disease
The prevalence of kidney disease at baseline was 8.0%, and the cumulative incidence at 15 years was 54.8%. At baseline, 1.0% of the participants had nerve disease, and the cumulative incidence at 15 years was 32.4%. Retinal disease was assessed twice, which prevented determination of cumulative incidence. At the first assessment (in 2010 or 2011), 13.7% had very mild nonproliferative diabetic retinopathy. After an additional 7 years (in 2017 or 2018), 51.0% had eye disease, including 8.8% with moderate to severe retinal changes and 3.5% with macular edema (Table S4).
The cumulative incidence of any microvascular complication was 50.0% by 9 years and 80.1% by 15 years (Fig. 2). In unadjusted models and models adjusted for sex, race and ethnic group, and age at randomization, the factors associated with an increased risk of the development of any microvascular complication were minority race or ethnic group, a high glycated hemoglobin level, low insulin sensitivity, hypertension, and dyslipidemia (Table 2). There were no differences according to the original treatment assignment. A sensitivity analysis that used the same unadjusted and adjusted models yielded similar results (Table S5).
Table 2.
Unadjusted and Minimally Adjusted Models for the Risk of Any Microvascular Complication.*
| Risk Factor | Hazard Ratio (95% CI) |
|---|---|
| Female vs. male | 0.95 (0.78–1.16) |
| Race and ethnic group† | |
| Hispanic vs. non-Hispanic White | 1.50 (1.13–2.00) |
| Hispanic vs. non-Hispanic Black | 1.02 (0.82–1.28) |
| Non-Hispanic Black vs. non-Hispanic White | 1.46 (1.09–1.96) |
| Treatment assignment at start of clinical trial | |
| Metformin plus rosiglitazone vs. metformin | 1.23 (0.97–1.56) |
| Metformin plus lifestyle intervention vs. metformin | 1.17 (0.92–1.48) |
| Metformin plus lifestyle intervention vs. metformin plus rosiglitazone | 0.95 (0.75–1.21) |
| Age at baseline, per each increase of 1 yr of age | 1.05 (1.00–1.10) |
| Duration of type 2 diabetes at baseline, per each increase of 1 mo of duration | 1.01 (0.99–1.02) |
| Unadjusted models | |
| Glycated hemoglobin level, per each increase of 1% or 11 mmol/mol | 1.18 (1.14–1.23) |
| BMI, per each increase of 5 | 1.08 (1.01–1.15) |
| Log insulin sensitivity, per each increase of 1 SD‡ | 0.81 (0.73–0.90) |
| Hypertension | 1.39 (1.12–1.72) |
| Dyslipidemia | 1.28 (1.03–1.59) |
| Adjusted models § | |
| Glycated hemoglobin level, per each increase of 1% or 11 mmol/mol | 1.18 (1.14–1.22) |
| BMI, per each increase of 5 | 1.07 (1.00–1.14) |
| Log insulin sensitivity, per each increase of 1 SD‡ | 0.80 (0.72–0.89) |
| Hypertension | 1.44 (1.16–1.80) |
| Dyslipidemia | 1.45 (1.16–1.82) |
Any microvascular complication was defined as the first occurrence of kidney disease, nerve disease, or retinal disease. Glycated hemoglobin, BMI, hypertension, and dyslipidemia were assessed at baseline and throughout follow-up. Insulin sensitivity was assessed at baseline and through the end of phase 1 of the TODAY2 study, and the assessment was repeated in phase 2 at 6 and 9 years after randomization where applicable. Each variable was analyzed as a time-varying covariate in the model. CI denotes confidence interval.
The “other” category is excluded because of heterogeneity within the group.
Insulin sensitivity was calculated as 1 ÷ fasting insulin.
The models were adjusted for the following preselected covariates: sex, race and ethnic group, and baseline age
Clustering of Complications
At the time of the last visit, 270 of 677 participants (39.9%) had no complications of diabetes, 215 (31.8%) had one complication, 144 (21.3%) had two, and 48 (7.1%) had three (Fig. 2C). The time to the onset of microvascular complications is shown in Figure 2D, and the risk factors for clustering of microvascular complications are shown in Table 3. In univariable analyses, the factors that were strongly associated with an increased risk of accumulation of complications were minority race or ethnic group, hyperglycemia, high BMI, low insulin sensitivity, hypertension, and dyslipidemia. After adjustment for sex, race and ethnic group, age, and diabetes duration at randomization, BMI was no longer a risk factor.
Table 3.
Unadjusted and Minimally Adjusted Models for the Risk of Accumulation of Microvascular Complications.*
| Risk Factors | Number of Complications | Odds Ratio (95% CI) | |||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||
| Female vs. male — % | 34.8 | 31.2 | 34.7 | 54.2 | 0.84 (0.63–1.13) |
| Race and ethnic group, vs. Non-Hispanic White — %† | |||||
| Non-Hispanic Black | 27.4 | 39.1 | 32.6 | 39.6 | 1.80 (1.20–2.68) |
| Hispanic | 38.9 | 38.6 | 43.8 | 39.6 | 1.57 (1.06–2.33) |
| Other | 9.3 | 5.1 | 8.3 | 6.3 | NA |
| Non-Hispanic White | 24.4 | 17.2 | 15.3 | 14.6 | — |
| Treatment, vs. metformin — %‡ | |||||
| Metformin plus rosiglitazone | 35.9 | 32.6 | 31.3 | 31.3 | 0.97 (0.69–1.36) |
| Metformin plus lifestyle intervention | 33.0 | 37.2 | 29.9 | 25.0 | 1.31 (0.93–1.84) |
| Metformin | 31.1 | 30.2 | 38.9 | 43.8 | — |
| Age at baseline, per each increase of 1 yr of age | 14.0±2.0 | 14.0±2.0 | 13.8±2.1 | 14.7±1.9 | 1.02 (0.95–1.09) |
| Duration of type 2 diabetes at baseline, per each increase of 1 mo of duration | 7.4±5.6 | 7.8±5.8 | 8.4±6.4 | 7.8±5.4 | 1.02 (0.99–1.04) |
| Unadjusted models | |||||
| Glycated hemoglobin level, per each increase of 1% or 11 mmol/mol | 7.0±1.7 | 8.2±1.9 | 9.6±1.7 | 10.4±1.3 | 1.78 (1.64–1.93) |
| Mean BMI, per each increase of 5 | 35.5±6.9 | 36.9±8.3 | 35.6±7.4 | 39.4±8.9 | 1.09 (1.00–1.20) |
| Mean log insulin sensitivity, per each increase of 1 SD§ | 0.054±0.030 | 0.046±0.026 | 0.043±0.026 | 0.038±0.017 | 0.65 (0.56–0.74) |
| Hypertension — % | 40.7 | 61.9 | 72.2 | 83.3 | 3.09 (2.31–4.15) |
| Dyslipidemia — % | 40.4 | 54.0 | 70.8 | 66.7 | 2.43 (1.83–3.22) |
| Adjusted model ¶ | |||||
| Glycated hemoglobin level, per each increase of 1% or 11 mmol/mol | 1.80 (1.65–1.95) | ||||
| BMI, per each increase of 5 | 1.09 (0.99–1.19) | ||||
| Natural log insulin sensitivity, per each increase of 1 SD§ | 0.64 (0.56–0.74) | ||||
| Hypertension | 3.18 (2.35–4.30) | ||||
| Dyslipidemia | 2.77 (2.05–3.72) | ||||
Plus-minus values are means ±SD. NA denotes not applicable.
The odds ratio for the “other” category was not calculated because of heterogeneity within the group. The odds ratio for non-Hispanic Black versus Hispanic was −0.13 (95% CI, −0.45 to 0.19).
The odds ratio for metformin plus lifestyle intervention versus metformin plus rosiglitazone was 0.74 (95% CI, 0.53 to 1.04).
Insulin sensitivity was defined as 1 ÷ fasting insulin.
The models were adjusted for the following preselected covariates: sex, race and ethnic group, baseline age, and baseline duration of type 2 diabetes.
Event Rates of Adjudicated Clinically Identified Complications
The rate of all adjudicated heart, vascular, and cerebrovascular events was 3.73 per 1000 person-years; there were 17 serious cardiovascular events (myocardial infarction [4 events], congestive heart failure [6 events], coronary artery disease [3 events], and stroke [4 events]). The rate of all eye disease events, including 60 advanced events, was 12.17 per 1000 person-years. The rate of all liver, pancreas, or gallbladder events was 6.70 per 1000 person-years. The rate of all nerve events was 2.35 per 1000 person-years. For all kidney events, including end-stage kidney disease, the rate was 0.44 per 1000 person-years (Table S6). Six deaths were reported (one each from myocardial infarction, kidney failure, drug overdose, and sepsis, and two from sepsis with multiorgan failure).
Discussion
These prospective, longitudinal data indicate that diabetes-related complications appear early in youth-onset type 2 diabetes and accumulate rapidly; at least one microvascular complication developed in 60.1% of participants in the study. In addition, a clustering of complications was observed, with 28.4% of the participants having two or more diabetes complications at a mean age of 26.4 years; among these participants, the mean time since the diagnosis of diabetes was 13.3 years. Furthermore, serious cardiovascular events, although uncommon, occurred despite the young age of the participants. Taken together, these data illustrate the serious personal and public health consequences of youth-onset type 2 diabetes in the transition to adulthood.
The gravity of these data are underscored by comparison with the risk of microvascular complications reported in type 1 diabetes and adult-onset type 2 diabetes. Microvascular complications, including diabetic kidney disease, affect approximately 25% of young persons with a duration of type 1 diabetes of more than 10 years.17 The Pittsburgh Epidemiology of Childhood-Onset Diabetes Complications Study reported a cumulative risk of 32% for diabetic kidney disease with a duration of type 1 diabetes of 25 years.18 With respect to type 2 diabetes, the U.K. Prospective Diabetes Study (UKPDS) showed a prevalence of 25% for moderately increased albuminuria after 10 years, with an increase in incidence of 2% every year thereafter, thus correlating with an estimated cumulative risk of diabetic kidney disease of 55% at 25 years after the initial diagnosis.19 In the Diabetes Prevention Program (DPP) and DPP Outcome Study, the incidence of the composite microvascular outcome (kidney, nerve, and retinal diseases) was between 12.3% and 14.4% among adult participants with type 2 diabetes.20
The reason for the high incidence of complications in youth-onset type 2 diabetes is unknown, but it is most likely related to the extreme metabolic phenotype (which includes severe insulin resistance and rapid worsening of beta-cell function21–23) and to challenging socioeconomic circumstances.24 In this cohort, the accumulation of complications was tightly associated with hyperglycemia, insulin resistance, hypertension, and dyslipidemia. Unfortunately, youth-onset type 2 diabetes is characterized by a suboptimal response to currently approved medical therapies,10,25,26 which is compounded by major challenges in adherence and management because of age and characteristic socioeconomic factors.27 The only medications approved by the Food and Drug Aministration (FDA) for youth-onset type 2 diabetes are metformin and insulin, with the recent addition of a glucagon-like peptide-1 (GLP-1) receptor agonist.28 Sodium–glucose cotransporter 2 (SGLT2) inhibitors, which impede progression of cardiovascular and kidney disease in patients with adult-onset type 2 diabetes, are not yet approved by the FDA for youth-onset type 2 diabetes, and whether SGLT2 inhibitors and GLP-1 receptor agonists will have cardioprotective and renoprotective effects in patients with youth-onset type 2 diabetes is unknown. Metabolic bariatric surgery, an emerging intervention for youth with type 2 diabetes, results in durable weight loss and improvement in glycemic control in the majority of patients.29,30 Data from a collaboration between the TODAY Study Group and the Teen–Longitudinal Assessment of Bariatric Surgery (Teen-LABS) showed a greater effect of bariatric surgery than medical therapy on both glycemic and nonglycemic outcomes.29 Teen-LABS showed greater regression and earlier attenuation of kidney disease in youth with type 2 diabetes than in adults with type 2 diabetes.31,32
Given the associations of hyperglycemia, hypertension, and dyslipidemia with a high risk for the development of complications, studies exploring early aggressive management of glycemia and risk factors in youth-onset type 2 diabetes are needed. However, until such data are available, primary and secondary prevention strategies based on extrapolation of data from studies in adults, including the use of medications not yet approved for youths, need to be considered. In addition, understanding of health care needs and health care usage patterns of young adults with youth-onset type 2 diabetes is needed so that health care systems in countries with large populations of persons with youth-onset type 2 diabetes are prepared for the anticipated needs.
Strengths of this study include a large sample of participants with youth-onset type 2 diabetes with up to 15 years of prospective, comprehensive, and rigorous phenotyping. The diverse cohort is representative of the general population with youth-onset type 2 diabetes in the United States.2,27 In addition, the assessment of complications was predefined and adjudicated. However, the incomplete follow-up of a quarter of the original cohort may have resulted in underrepresentation of the accumulation and clustering of events, although the findings were consistent in the sensitivity analyses. Also, nicotine use is recognized as an important cardiovascular risk factor; information on participants’ history of nicotine use was collected but was identified as unreliable during analysis. The participants were willing to take part in an intensive clinical trial and received intensive management at no cost as part of the study until 2014; therefore, the incidence of complications might have been even higher in the absence of these interventions. Finally, the lack of longitudinal data on microvascular complications in adolescents without diabetes but with a similar degree of obesity precludes comparison of event rates among youth with and without type 2 diabetes. Of note, the most diabetes-specific complications, such as eye disease, are widely recognized not to occur in populations without diabetes.33
These data show a high burden of diabetes-specific complications in youth-onset type 2 diabetes, with an early and severe effect on persons with this disease, as well as substantial public health implications.
Supplementary Material
Acknowledgments
The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the respective Tribal and Indian Health Service institutional review boards or their members.
Supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (U01-DK61212, U01DK61230, U01-DK61239, U01-DK61242, and U01-DK61254), the National Center for Research Resources (NCRR) General Clinical Research Centers Program (M01-RR00036 [to the Washington University School of Medicine], M01-RR00043-45 [to Children’s Hospital Los Angeles], M01-RR00069 [to the University of Colorado Denver], M01-RR00084 [to Children’s Hospital of Pittsburgh], M01-RR01066 [to Massachusetts General Hospital], M01-RR00125 [to Yale University], and M01-RR14467 [to the University of Oklahoma Health Sciences Center]), and the NCRR Clinical and Translational Science Awards program (UL1-RR024134 [to Children’s Hospital of Philadelphia], UL1RR024139 [to Yale University], UL1-RR024153 [to Children’s Hospital of Pittsburgh], UL1-RR024989 [to Case Western Reserve University], UL1-RR024992 [to Washington University in St. Louis], UL1-RR025758 [to Massachusetts General Hospital], and UL1-RR025780 [to the University of Colorado Denver]).
We thank Becton Dickinson, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, LifeScan, Pfizer, and Sanofi Aventis for donations of medications and supplies; and the American Indian partners associated with the clinical center located at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee tribe, the Cherokee Nation, Chickasaw Nation, Choctaw Nation of Oklahoma, and Oklahoma City Area Indian Health Service, for their participation and guidance.
APPENDIX
The affiliations of the members of the writing committee are as follows: the University of Colorado Anschutz Medical Campus, Children’s Hospital Colorado, Aurora (P.B., P.Z.); George Washington University, Rockville, MD (K.L.D., B.T.); Yale University, New Haven, CT (S.C.); Case Western Reserve University, Rainbow Babies and Children’s Hospital, Cleveland (R.G.-K.); the Massachusetts General Hospital Diabetes Center, Harvard Medical School, Boston (D.M.N.); the University of Oklahoma Health Sciences Center, Oklahoma City (J.T.); and Washington University School of Medicine, St. Louis (N.H.W.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
The members of the writing committee (Petter Bjornstad, M.D., Kimberly L. Drews, Ph.D., Sonia Caprio, M.D., Rose Gubitosi-Klug, M.D., Ph.D., David M. Nathan, M.D., Bereket Tesfaldet, M.S., Jeanie Tryggestad, M.D., Neil H. White, M.D., and Philip Zeitler, M.D., Ph.D.) assume responsibility for the overall content and integrity of this article.
The affiliations of the members of the writing committee are listed in the Appendix.
References
- 1.Al-Saeed AH, Constantino MI, Molyneaux L, et al. An inverse relationship between age of type 2 diabetes onset and complication risk and mortality: the impact of youth-onset type 2 diabetes. Diabetes Care 2016; 39: 823–9. [DOI] [PubMed] [Google Scholar]
- 2.Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 2014; 311: 1778–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer-Davis EJ, Lawrence JM, Dabelea D, et al. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med 2017; 376: 1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA 2017;317: 825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.TODAY Study Group. Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care 2013; 36: 1735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The RISE Consortium. Lack of durable improvements in β-cell function following withdrawal of pharmacological interventions in adults with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2019; 42: 1742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The RISE Consortium. Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2018;41: 1717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dart AB, Martens PJ, Rigatto C, Brownell MD, Dean HJ, Sellers EA. Earlier onset of complications in youth with type 2 diabetes. Diabetes Care 2014; 37: 436–43. [DOI] [PubMed] [Google Scholar]
- 9.Morton JI, Liew D, McDonald SP, Shaw JE, Magliano DJ. The association between age of onset of type 2 diabetes and the long-term risk of end-stage kidney disease: a national registry study. Diabetes Care 2020; 43: 1788–95. [DOI] [PubMed] [Google Scholar]
- 10.TODAY Study Group. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012; 366: 2247–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The TODAY Study Group. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes 2007;8: 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn JT, Kaelber DC, Baker-Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017; 140(3):e20171904. [DOI] [PubMed] [Google Scholar]
- 13.Lozano P, Henrikson NB, Morrison CC, et al. Lipid screening in childhood and adolescence for detection of multifactorial dyslipidemia: evidence report and systematic review for the US Preventive Services Task Force. JAMA 2016; 316: 63444. [DOI] [PubMed] [Google Scholar]
- 14.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020; 98(4S): S1S115. [DOI] [PubMed] [Google Scholar]
- 15.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994; 17: 1281–9. [DOI] [PubMed] [Google Scholar]
- 16.Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991; 98: Suppl 5: 823–33. [PubMed] [Google Scholar]
- 17.Amin R, Widmer B, Prevost AT, et al. Risk of microalbuminuria and progression to macroalbuminuria in a cohort with childhood onset type 1 diabetes: prospective observational study. BMJ 2008; 336: 697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 2006; 55:1463–9. [DOI] [PubMed] [Google Scholar]
- 19.Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 2003;63: 225–32. [DOI] [PubMed] [Google Scholar]
- 20.Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol 2015; 3: 866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The RISE Consortium. Effects of treatment of impaired glucose tolerance or recently diagnosed type 2 diabetes with metformin alone or in combination with insulin glargine on β-cell function: comparison of responses in youth and adults. Diabetes 2019; 68:1670–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The RISE Consortium. Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: II. Observations using the oral glucose tolerance test. Diabetes Care 2018; 41: 1707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The RISE Consortium. Metabolic contrasts between youth and adults with impaired glucose tolerance or recently diagnosed type 2 diabetes: I. Observations using the hyperglycemic clamp. Diabetes Care 2018; 41: 1696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadeau KJ, Anderson BJ, Berg EG, et al. Youth-onset type 2 diabetes consensus report: current status, challenges, and priorities. Diabetes Care 2016; 39: 1635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeitler P, Chou HS, Copeland KC, Geffner M. Clinical trials in youth-onset type 2 diabetes: needs, barriers, and options. Curr Diab Rep 2015; 15: 28. [DOI] [PubMed] [Google Scholar]
- 26.Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and management of youth-onset type 2 diabetes: a position statement by the American Diabetes Association. Diabetes Care 2018; 41:2648–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Copeland KC, Zeitler P, Geffner M, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab 2011; 96: 159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamborlane WV, Barrientos-Pérez M, Fainberg U, et al. Liraglutide in children and adolescents with type 2 diabetes. N Engl J Med 2019; 381:637–46. [DOI] [PubMed] [Google Scholar]
- 29.Inge TH, Laffel LM, Jenkins TM, et al. Comparison of surgical and medical therapy for type 2 diabetes in severely obese adolescents. JAMA Pediatr 2018; 172: 45260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olbers T, Beamish AJ, Gronowitz E, et al. Laparoscopic Roux-en-Y gastric bypass in adolescents with severe obesity (AMOS): a prospective, 5-year, Swedish nationwide study. Lancet Diabetes Endocrinol 2017; 5: 174–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjornstad P, Hughan K, Kelsey MM, et al. Effect of surgical versus medical therapy on diabetic kidney disease over 5 years in severely obese adolescents with type 2 diabetes. Diabetes Care 2020; 43: 187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bjornstad P, Nehus E, Jenkins T, et al. Five-year kidney outcomes of bariatric surgery differ in severely obese adolescents and adults with and without type 2 diabetes. Kidney Int 2020;97: 995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilpatrick ES, Bloomgarden ZT, Zimmet PZ. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes: response to the International Expert Committee. Diabetes Care 2009;32(12): e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.